- 1U.S. Geological Survey, San Diego, CA, United States
- 2Natural History Museum of Los Angeles County, Los Angeles, CA, United States
- 3Cotsen Institute of Archaeology at the University of California, Los Angeles, CA, United States
- 4Merkel & Associates, Inc., San Diego, CA, United States
- 5San Diego Natural History Museum, San Diego, CA, United States
Non-native species having high per capita impacts in invaded communities are those that modulate resource availability and alter disturbance regimes in ways that are biologically incompatible with the native biota. In areas where it has been introduced by humans, American beaver (Castor canadensis) is an iconic example of such species due to its capacity to alter trophic dynamics of entire ecosystems and create new invasional pathways for other non-native species. The species is problematic in several watersheds within the Southern California-Northern Baja California Coast Ecoregion, a recognized hotspot of biodiversity, due to its ability to modify habitat in ways that favor invasive predators and competitors over the region's native species and habitat. Beaver was deliberately introduced across California in the mid-1900s and generally accepted as non-native to the region up to the early 2000s; however, articles promoting the idea that beaver may be a natural resident have gained traction in recent years, due in large part to the species' charismatic nature rather than by presentation of sound evidence. Here, we discuss the problems associated with beaver disturbance and its effects on conserving the region's native fauna and flora. We refute arguments underlying the claim that beaver is native to the region, and review paleontological, zooarchaeological, and historical survey data from renowned field biologists and naturalists over the past ~160 years to show that no evidence exists that beaver arrived by any means other than deliberate human introduction. Managing this ecosystem engineer has potential to reduce the richness and abundance of other non-native species because the novel, engineered habitat now supporting these species would diminish in beaver-occupied watersheds. At the same time, hydrologic functionality would shift toward more natural, ephemeral conditions that favor the regions' native species while suppressing the dominance of the most insidious invaders.
Introduction
Ecologists generally agree that biological invasions now rival other factors like climate change, pollution, nutrient loading, and habitat conversion in determining contemporary ecosystem structure and function (Vitousek, 1990; Strayer, 2012; Koel et al., 2019; Liu et al., 2020). Whereas, traditional approaches to studying such integration have focused on understanding shifts in the diversity and abundance of native species, newer perspectives are tackling the problem from the standpoint of the invaders by examining how and at what level they disrupt trophic dynamics, and the degree to which those disruptions have cascading effects in native ecosystems (Crooks, 2002; Ruscoe et al., 2006; Jackson et al., 2017). Some of the best-known examples of invasive species driving shifts in trophic dynamics are from mammals (Clout and Russell, 2007; Doherty et al., 2016), including the arctic fox Alopex lagopus in the Aleutian archipelago (Croll et al., 2005), multiple invasions by a variety of species of mammals in native beech forests (Nothofagus sp.) of New Zealand (Veblen and Stewart, 1982; Ruscoe et al., 2006; Anderson et al., 2011), and the American beaver Castor canadensis in the Fuegan Archipelago of South America (Anderson and Rosemond, 2010).
Invaders with the highest per capita impacts are those that modulate the acquisition of resources and alter disturbance regimes in ways that are evolutionarily and ecologically incompatible with the resident biota (Vitousek, 1990; Crooks, 2002; Strayer, 2012). The so-called ecosystem engineer, a keystone species that influences resource availability by creating, modifying, or destroying habitat, fulfills these criteria and is particularly effective at influencing trophic dynamics, with beaver representing an iconic example of such species (Jones et al., 1994; Wright and Jones, 2006). The cascading effects of beaver disturbance are mainly tied to dam construction, which impounds surface flow, dissipates stream energy, elevates water temperature, reduces canopy cover, and increases nutrient productivity (Naiman et al., 1988; Alexander, 1998; Lizzaralde et al., 2004; Rosell et al., 2005; Anderson and Rosemond, 2007). This ability to shape trophic structure and function through physical, biochemical and geomorphological modifications to the habitat is overwhelmingly positive for areas in which beaver has evolved as a natural component of the ecosystem (Snodgrass and Meffe, 1999; Wright et al., 2002; Kemp et al., 2012; Levine and Meyer, 2019). However, impacts of beaver disturbance are wide-ranging and complex, and the degree to which they are beneficial requires site-level context and history (Johnson and van Riper, 2014).
The Problem With a High-Performance Ecosystem Engineer at the Wrong Job Site
Theory predicts that when an eco-engineer creates patches that support species that would otherwise not exist within the landscape, species richness will increase due to greater habitat complexity and productivity (Jones et al., 1997; Wright et al., 2002; Martell et al., 2006). This process has been demonstrated empirically for beaver in different parts of the world, where beaver-engineered reaches show equivalent species richness at the patch scale, but increased richness at the watershed scale due to differences in species composition among patches that are differentially modified by beaver (Wright et al., 2002; Nummi et al., 2019). Even in the introduced range, trophic shifts are similar in the direction and magnitude of those in the native range (Anderson et al., 2009). Beaver dams can also maintain surface water or wetlands in stream reaches that might otherwise dry out during certain parts of the year, which in turn favors survival of species requiring perennial water (Albert and Trimble, 2000; McKinstry et al., 2001). Even short-lived beaver dams, as expected in xeric environments, can increase microhabitat complexity that ultimately leads to elevated species richness and abundances (Billman et al., 2013).
While the propagating effects of beaver activity can be beneficial, careful consideration must be given to whether the history of a given watershed is naturally “tuned” to beaver ecology. For example, in areas where beaver has been introduced, trees often lack defensive mechanisms and reproductive strategies that occur in forests that are regularly subject to beaver activity because they do not share a common evolutionary history (Basey et al., 1988; Martínez Pastur et al., 2006; Anderson et al., 2009). Introduced beaver may be particularly devastating for systems invaded by other non-native species, as novel disturbance has the potential to drive feedback loops that favor survivorship of other invaders, promote new introductions or spread, and overwhelm any biotic resistance that might be perpetrated by native species (i.e., invasional meltdown: Simberloff and Von Holle, 1999; Crooks, 2002; Maret et al., 2006; Braga et al., 2018). For example, introduced beaver in Tierra del Fuego have positive effects on the growth of non-native Brown trout (Salmo trutta) (Arismendi et al., 2020), a voracious predator known for its top-down effects on trophic structure and capacity to become invasive (Hansen et al., 2019). In this same system, beaver also promote invasion pathways for non-native plants because native forests (Nothofagus sp.) are unable to regenerate in the aftermath of beaver disturbance (Anderson et al., 2006; Martínez Pastur et al., 2006).
Effects of Novel Ecological Disturbance on Native Fauna and Flora
The presence of beaver has implications for protecting native aquatic species and riparian woodland in the Southern California/Northern Baja California Coast Ecoregion of North America (hereafter SC-NBC: Griffith et al., 2016; Figure 1). This recognized “hotspot” of biodiversity is occupied by numerous at-risk species, many of which are endemic (Stebbins and Major, 1965; Dobson et al., 1997; Myers et al., 2000; Howard et al., 2015). Threats to the aquatic fauna and riparian flora arise from novel conditions created by human influence, either directly or indirectly, that favor the establishment and spread of invasive species while repressing native species that have evolved under the region's natural hydrology and climate (Kats and Ferrer, 2003; Moyle et al., 2015).
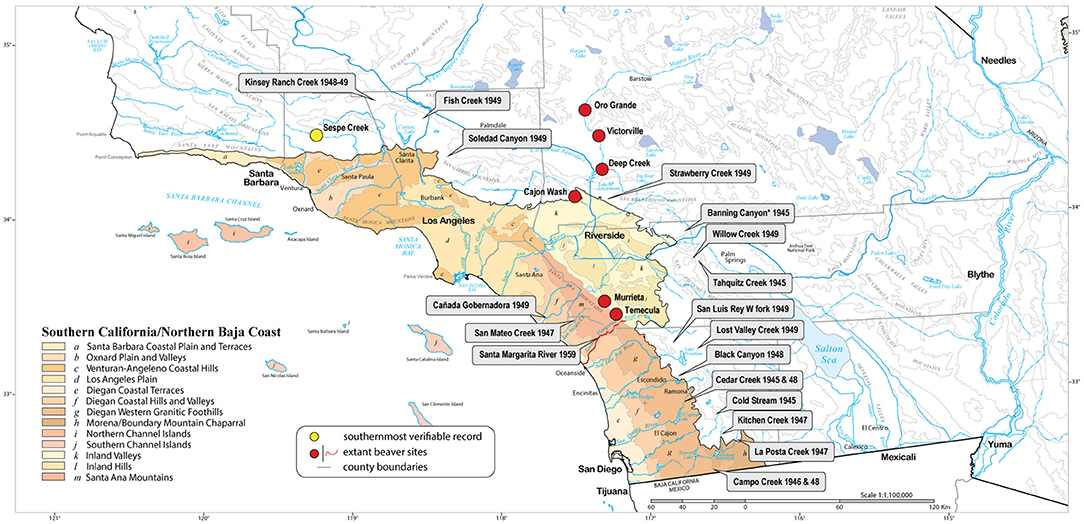
Figure 1. Southern California/Northern Baja California Coast Ecoregion and subregions (a–m); Baja California portion not shown (map adapted from Griffith et al., 2016). Callouts indicate the place/year of known beaver introductions [records provided by the California Department of Fish and Wildlife (CDFW); Hensley, 1946; U.S. Fish and Wildlife Service, 1998]; red bullets indicate sites currently occupied by beaver. Beaver is well-established but discontinuously distributed over ~48 km of the Santa Margarita River (red line), including the Temecula and Murrieta creek tributaries. *We suspect that the locality identified as “Banning Creek” in the original CDFW records refers to the San Gorgonio River in Banning Canyon, Riverside Co. The original CDFW records also include an introduction site at “Caresito Creek” in San Diego County (transplanted in 1944), but no creek exists by that name, and we suspect that it may refer to Carrista Creek near Lake Henshaw.
As is typical of xeric environments, the SC-NBC ecoregion experiences sequential bouts of deluge and drought (Levick et al., 2008; Hershkovitz and Gasith, 2013). Perennial water is limited, and apart from a few natural basins, deep water ponds and reservoirs were historically non-existent (Ferren and Fiedler, 1993; Stephenson and Calcarone, 1999). Episodic winter storms produce flashy flows (i.e., rapid flow rate with high volume but short duration), changing by orders of magnitude over a few hours, followed by dry periods that extend for many months to years (Mount, 1995; Gasith and Resh, 1999). Flood-drought cycles occur at seasonal and multi-year scales, with multi-year variation driven largely by the El Niño/La Niña Southern Oscillation in the Pacific Ocean (Cayan et al., 1999; Patricola et al., 2020). Broad coastal terraces backed by steeper mountain ranges further influence the dynamics of these systems, with heavy precipitation events generating lotic flows that etch out step-pool networks in the uplands, sporadic pool-riffle systems and braided cobble streams in the terraces, and lagoons along the coastline (Mount, 1995; Stephenson and Calcarone, 1999). As a result, geomorphology and physical disturbance were key determinants of habitat heterogeneity prior to the twentieth century.
These natural hydrologic processes have been altered since the early 1940s, as the growing human population of southern California led to the construction of flood control infrastructure to avoid loss of life and property (Orsi, 2004). Some of the most heavily modified habitats include estuaries, lakes, and streams, with non-native predatory fish species thriving where these habitats have either been created or altered from their natural state (Power, 1990; Moyle and Marchetti, 2006; Francis and Chadwick, 2012). These include bass (Micropterus sp.); bullhead (Ameiurus sp.); channel catfish Ictalurus punctatus; carp (Cyprinus sp., Ctenopharyngodon sp.); sunfish (Lepomis sp.); mosquito fish (Gambusia affinis); and fathead minnow (Pimephales promelas) (Moyle and Marchetti, 2006; Moyle et al., 2011). The combined effects of reduced stream energy, greater availability of permanent water, and loss of riparian vegetation have been linked to higher density of these non-native fish species (Swift et al., 1997; Moyle and Marchetti, 2006; Rinne and Miller, 2006), with many tolerating broad ranges of temperature, salinity, turbidity, and dissolved oxygen (Holland and Swift, 2000; Braig and Johnson, 2003; Marchetti et al., 2004a,b; Ribeiro et al., 2007; Moyle et al., 2013).
Other predominant invasives include American bullfrog (Lithobates catesbeianus), red swamp crayfish (Procambarus clarkii), African clawed frog (Xenopus laevis), and Emydid turtles [e.g., red-eared slider (Trachemys scripta elegans) and others in the pet trade], with bullfrog and red swamp crayfish being two of the worst and most common invaders (Kats and Ferrer, 2003). Introduced bullfrog prey upon on a variety of native species, and the larger an individual grows the large the prey item it can consume (Rosen and Schwalbe, 1995; Casper and Hendricks, 2005; Maret et al., 2006; Nicholson et al., 2020). The presence of invasive centrarchid fish species has been shown to increase the abundance and spread of bullfrog in systems where they co-occur, as the non-native fish consume native macroinvertebrates that would otherwise prey on larval bullfrog (Adams et al., 2003). Invasive red swamp crayfish, commonly introduced as bait for sport fishing (Lodge et al., 2000), readily prey on the eggs and larvae of native amphibians and will even attack adult newts (genus Taricha) despite a powerful neurotoxin that makes Taricha lethal to most predators (Brodie, 1968; Williams et al., 2010).
Stabilization of aquatic habitat that reduces disturbance by natural flood-drought cycles can lead to invasions by the non-native species described above because most require slow moving, permanent water for survival (Meffe, 1984; Moyle and Light, 1996; Gasith and Resh, 1999; Adams, 2000; Marchetti et al., 2004c). In contrast, native species in the region have evolved traits for coping with ephemeral habitat or promoting resilience once hydrologic stress has subsided. For example, the endangered steelhead (Oncorhynchus mykiss) and Pacific lamprey (Entosphenus tridentatus) rely on winter and spring rainfall events as cues for dispersal and to avoid becoming stranded during dry parts of the year (National Marine Fisheries Service, 2012; Goodman et al., 2015). Others like Santa Ana sucker (Catostomus santaanae) have high fecundity, rapid maturity, and a long spawning period that allows rapid rebound in demography after drought-induced declines (Swift et al., 1993; Moyle, 2002). Miller et al. (2012) showed that the arroyo toad was able to recolonize ephemeral sites more rapidly than non-native species, and periodic flood events cause sufficient bullfrog and crayfish mortality to enable the threatened California red-legged frog (Rana draytonii) and other native amphibians to persist in areas where these species have invaded (Doubledee et al., 2003; Kats et al., 2013).
Beaver also drive successional shifts in vegetation communities through selective harvesting of trees and by altering floodplain dynamics (Naiman et al., 1988; Anderson et al., 2006; Martínez Pastur et al., 2006). Despite their preference for cottonwood, aspen, and willow (Populus or Salix spp.; McGinley and Whitham, 1985; Johnston and Naiman, 1990), beaver harvest a wide range of trees and shrubs and move up to ~30 m from a stream body to do so (Allen, 1983; Baker and Hill, 2003; Gallant et al., 2004; Anderson et al., 2006). Newly introduced individuals are known to harvest beyond their needs and impacts to riparian habitat persist long after beaver vacate an area (Tappe, 1942; Naiman et al., 1994; Wright et al., 2004; Anderson et al., 2009). In Tierra del Fuego, invasive beaver has converted extensive tracts of riparian forest to meadows; regeneration of the former is suppressed and altered succession favors invasion by exotic plants (Anderson et al., 2006; Pastur et al., 2006; Papier et al., 2019). Altered succession is particularly problematic in areas where old growth riparian woodland is a rare habitat type, as it tends to be in xeric systems (Levick et al., 2008; Gibson and Olden, 2014; Johnson and van Riper, 2014), and a larger proportion of the total habitat area is exposed to potential beaver disturbance. The problem is exacerbated if many beavers are present within the system (Anderson et al., 2009).
There is also evidence that beaver alters the competitive hierarchy between native and invasive plant species. For example, growth rates for introduced Russian olive (Elaeagnus angustifolia) and saltcedar (Tamarix spp.) can be substantially higher where beaver reduce the cottonwood canopy cover (Lesica and Miles, 2004), with light being a probable limiting resource for these invasives in mesic environments. Beaver is therefore suspected of lowering competitive stress through felling of the taller, dominant cottonwoods (Sher et al., 2000; Lesica and Miles, 2004; Mortenson et al., 2008). In Tierra del Fuego, elimination of Nothofagus forests and associated seedling banks by introduced beaver has similarly led to invasions by non-native plants, which now comprise much of the species richness and abundance in converted habitat (Anderson et al., 2006; Martínez Pastur et al., 2006).
Native Fauna and Flora Under Threat
A variety of native species in the SC-NBC coast ecoregion have the potential to be negatively impacted by beaver activity. Of greatest concern are those that are threatened or endangered, and for many, the earliest life history stages are the most vulnerable (Kats and Ferrer, 2003). Below, we highlight several species due to their conservation status and unique circumstances surrounding interactions with beaver. A list of other examples is provided in the Supplementary File.
Steelhead
The range of the endangered steelhead overlaps broadly with the native range of beaver in the Pacific Northwest, and previous work indicates that beaver-engineered habitat can improve conditions for steelhead (Bouwes et al., 2016; Wathen et al., 2019). However, the “rules of coexistence” vary with geography, and little attention has been devoted to beaver-steelhead interactions in xeric systems (Gibson and Olden, 2014). In the Southern California Steelhead (SCS) Recovery Planning Area (within the SC-NBC ecoregion), steelhead habitat exists in chaparral ecosystems that differ markedly from the snow-fed and/or mixed conifer ecosystems in the Sierra Nevada or North and Central Coasts of California, Oregon, and Washington (Boughton et al., 2009). Surface flow is intermittent and considerably more ephemeral, and steelhead exploit different habitats between the mountains and coastal terraces during different life history stages (Boughton et al., 2007; Moyle et al., 2008). A consensus exists that populations within SCS Recovery Planning Area have experienced serial extirpations and recolonizations by anadromous migrants during wet-dry cycles throughout the species' history, and that the region may have supported steelhead populations only sporadically (Boughton et al., 2005; National Marine Fisheries Service, 2012).
It is noteworthy that the SCS Recovery Plan does not mention beaver as an important component to the habitat, improving habitat, or creating habitat for steelhead in this part of the species' range (National Marine Fisheries Service, 2012). If it were, this would likely be discussed. The Plan instead emphasizes that steelhead are preyed upon by and cannot compete with invasive fish species (e.g., bass, sunfish, carp, and bullhead), with the highest vulnerability occurring during the earliest life history stages. So, even if large adult steelhead can tolerate conditions in occupied beaver habitat, the eggs and juvenile fish remain at risk. Trout densities also negatively correlate with aquatic macrophyte densities (Douglas, 1995), and beaver ponds can lead to significant increases in macrophytic diversity through reduction of dominant species cover (Ray et al., 2001; Parker et al., 2007; Law et al., 2014).
Riparian Birds
Obligate riparian bird species in the SC-NBC ecoregion may also be at risk from beaver activity, but preference for similar habitat by certain birds [i.e., the endangered southwestern willow flycatcher (Empidonax trailii extimus) and Least Bell's Vireo (Vireo bellii pusillus)] and beaver have made the interaction difficult to study in arid ecosystems. This subject was addressed by Johnson and van Riper (2014) in a field study along the San Pedro River in southeastern Arizona (United States) and northern Sonora (México), where beaver was re-introduced to the system 5–6 years prior to the study (beaver was common on the San Pedro >100 years ago; Webb et al., 2007). Although increased bird abundance and species richness were associated with the presence of beaver, Johnson and van Riper (2014) were unable to rule out that beaver had selected habitat that already contained high bird abundance and species richness, given that similar factors predicted the presence of both birds and beaver. They further state that these confounding effects are likely to be more prevalent in the desert southwest, where riparian vegetation provides resources and facilitates trophic opportunities that benefit both birds and beaver.
Some have argued more forcefully, but speculatively, that introduced beaver improve habitat for riparian bird species while at the same time suppressing stands of invasive saltcedar (Longcore et al., 2007). While there may be truth to the bird claim where beaver is native, it is not a reasonable argument for systems in which beaver has no evolutionary history. The notion of protecting a non-native ecosystem engineer for the purpose of benefitting one or even two native species, or as a potential control for saltcedar, warrants careful consideration by managers given the potential for wide-ranging collateral damage to other native species and habitat. Other research also indicates that the loss of native riparian hardwoods opens the potential for invasion by saltcedar (Lesica and Miles, 2004; Mortenson et al., 2008), alters avian communities, and reduces the quality of migratory habitat (Cohan et al., 1978; Olson and Knopf, 1986; Fischer et al., 2015). However, we acknowledge uncertainty about the functional role of saltcedar in supporting riparian bird species (see reviews in Bateman and Paxton, 2010) and the degree to which non-native beaver influence the spread of saltcedar in the region (Longcore et al., 2007).
Arroyo Toad
Beaver activity in the SC-NBC ecoregion also threatens populations of the endangered arroyo toad; impoundments created by beaver dams inundate low flow, shallow stream reaches required for toad breeding while providing deeper pooled habitat for invasive red swamp crayfish, American bullfrog, and various predatory fish (U.S. Fish and Wildlife Service, 2009; Miller et al., 2012). Using 16 years of field data (2003–2018) collected by the U.S. Geological Survey (USGS) in three watersheds on Marine Corps Base Camp Pendleton, at least one of which supports introduced beaver (Santa Margarita: Ervin, 2017), we developed occupancy models to identify factors that influence breeding habitat occupancy and colonization-extinction dynamics of arroyo toad in coastal San Diego County (Supplementary Table 1). A single best-fit model showed that the presence of non-native aquatic species was a significant predictor of site occupancy, colonization of a previously unoccupied site, and local extinction of arroyo toad (see Supplementary File for further details on the analysis). Site occupancy declined, colonization was suppressed, and local extinction increased in the presence of invasive species, with the magnitude-of-effect being amplified when more than one invasive occurred within a 250 m reach (Figure 2). The presence of a beaver dam was also a significant predictor of extirpation, with the probability of local extinction being 2.5 (95% CI 0.9–3.4) times higher when a dam was present. The probability of extinction grew to nearly 0.70 when a beaver dam and all three non-native species were present, nearly seven times higher than in reaches without any invasive species (Figure 2A). Data averaged across years further showed that a non-native species index (NNI: the number of non-native aquatic species recorded at a site, ranging from 0–3 and consisting of American bullfrog, red swamp crayfish, and or large predatory fish [e.g., Micropterus sp., treated as a single species]) and beaver dam presence were significantly correlated (Spearman's ρ = 0.62, P < 0.001). The presence of a beaver dam also increased the probability of a higher NNI score, with the estimated NNI being nearly three times greater (mean = 2.9; 95% credible interval = 2.1–3.9) at sites where beaver dams were present versus sites where they were absent yet still contained water (See Supplemental File for further details). Thus, beaver dams were not only a top predictor of occupancy dynamics in the arroyo toad, their presence was also associated with increased richness of nonnative aquatic predators.
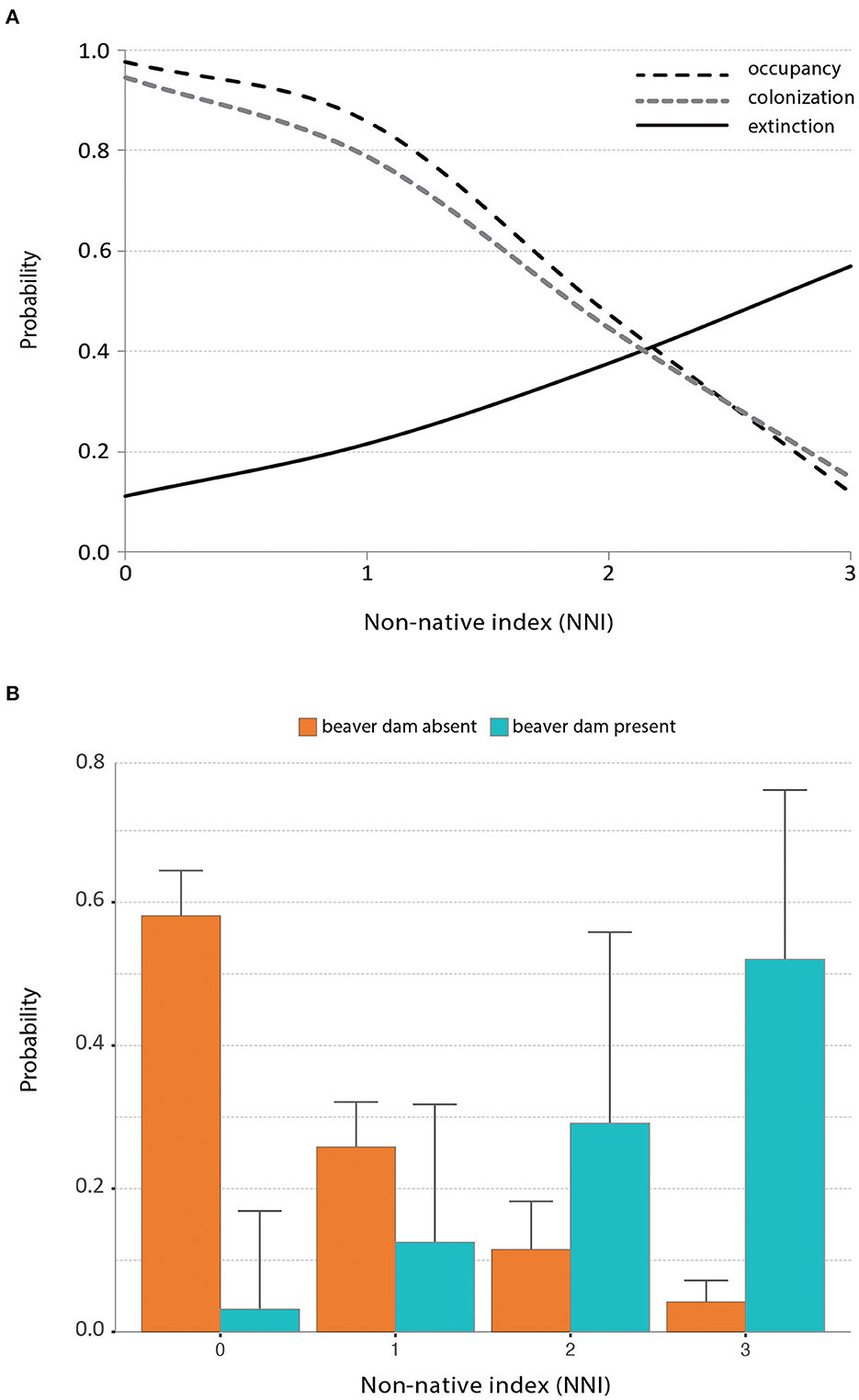
Figure 2. (A) Effect of the non-native aquatic species index (NNI: the number of non-native aquatic species observed at a site, ranging from 0–3 and consisting of American bullfrog, red swamp crayfish, and or large predatory fish [e.g., Micropterus sp., treated as a single species]) on the probability of occupancy, colonization, and local extinction of arroyo toad in coastal San Diego county from 2003–2018. (B) Results of the categorical regression showing the probability of the NNI score relative to beaver dam presence/absence (error bars represent the upper 95% credible intervals of the posterior probability distributions: see Supplemental File for further details).
Indigenous Evaluation of the Ecosystem Engineer—How Strong Is the Evidence?
Discussions about protecting beaver in SC-NBC ecoregion have led to questions about its historic distribution, abundance, and impacts on protected species and habitat (Longcore et al., 2007; Gibson and Olden, 2014). That beaver is native to coastal southern California has been proposed by previous authors (Longcore et al., 2007; Lanman et al., 2013), with supporting arguments based on dubious documented (e.g., first person observations) and so-called “supplementary” evidence (e.g., ethnolinguistics, geographic place names, habitat suitability, etc.), but no physical evidence except for a beaver skull collected in 1906 in Sespe Creek, a tributary of the Santa Clara River in Ventura County (Museum of Vertebrate Zoology:Mamm:4918). This skull represents the southernmost verifiable record for beaver in coastal southern California, well-north of the Los Angeles basin (Figure 1).
In Lanman et al. (2013), the authors build their case by citing a newspaper article in the San Diego Union in 1889 that references a beaver on exhibition in the downtown area that was purportedly the largest beaver ever trapped in this “section” (unclear what “section” refers to, or where the beaver was trapped). This suggested that others must have been trapped previously to call this one the largest. However, this article simply tells us that a beaver was on display in downtown San Diego at the time it was written—we have no way of knowing where it was trapped, and the observation itself says nothing about the ancestral origins of the species in southern California1.
A second piece of evidence from Lanman et al. (2013) is an article published in a medical journal by Dr. D. B. Hoffman, a physician reporting on the suitability of San Diego Bay as a site for military barracks and a hospital. The article includes numerous anecdotes ranging from Native American rituals to disease, geology, and climatology, and includes beaver on a list of resident mammals in San Diego County (Hoffman, 1864). However, Hoffman provides no source for any observations of beaver, no information about numbers of observations, no specific location data, and no specimens—readers are simply left with “beaver” in a short list of mammals, so one cannot even verify if the listing is a first-person observation. It is possible that Hoffman was referencing beaver on the Colorado River (Ervin, 2017), as San Diego County extended from the coast to Arizona at the time the article was published. However, we believe this is unlikely given that his focus was on montane and coastal San Diego County.
A sense of Hoffman's credibility as a zoologist can be gained from other accounts in the same article. For example, in describing the fish fauna he states, “mountain brooks are well-filled with trout (Gila elegans), mullet, and minnows.” However, Gila elegans is not a trout, but the bony tailed chub native to the Colorado River basin, “minnow” could refer to any of a number of small cyprinid fish, and mullet occur in coastal waters or lower reaches of coastal streams, not mountain brooks. In this same section devoted to fish, he states that “whale” were taken in large numbers, apparently not understanding that whales are mammals. Hoffman was not a zoologist, but by the mid-nineteenth century the scientific community had reached consensus that whales are mammals and not fish (Linnaeus, 1758; Hunter, 1787; Cooper, 1868). For reptiles, he stated that the “chameleon, the horned toad, and several varieties of lizard are common.” However, chameleons are native to the Old World, not southern California, and a zoologist would know that horned toads are in fact lizards and would report them as such. In the “Reptiles” section, he includes scorpions and states that they are “non-venomous” and that their “bite” is innocuous. Of course, scorpions are not reptiles, and the tip of the tail contains venom glands and a hypodermic barb that stings rather than bites. This is all to say that despite Dr. Hoffman's talents as a respected surgeon and community member, his zoological accounts of the area appear unreliable.
A third argument by Lanman et al. (2013) involves the existence of a word for beaver in Yuman–Cochimí linguistics, which is spoken by the Kumeyaay of southern San Diego County and northern Baja California. The authors imply that if the word exists in the local native American language, this somehow means that beaver is native to the area. Instead, we argue that the existence of a word for beaver in the Yuman–Cochimí linguistics is best explained by the geographic distribution of the language itself, which spans from the lower Colorado River Basin into coastal and northern Baja California (Laylander, 2010). We know, based on verifiable evidence, that beaver is indigenous to the lower Colorado Basin (Cooper, 1869; Stephens, 1906; Tappe, 1942). Like gene flow, language flow occurs with population connectivity, so it makes sense that a word for beaver would exist throughout the geographic distribution of the language.
A fourth argument involves a 5.2 km non-perennial tributary to the Sweetwater River known as Beaver Hollow in San Diego County. Lanman et al. (2013) point out there is often a correspondence between places named after animals and those that occur there (citing Cox et al., 2002), and Beaver Hollow was named before the California Department of Fish and Game began stocking beaver (see “Origins of the Engineer in the SC-NBC Ecoregion” below for details about stocking). We agree with both points, but the name Beaver Hollow says nothing about the ancestry of beaver that may or may not have been there, and no verifiable records exist from the site. We contacted the great grandson of William A. Sloane, the namesake of Sloane Canyon into which Beaver Hollow drains and original patent owner of the Sloane Ranch estate (established in 1891), who stated that “there were no family stories about beavers ever being present” (W. Bretz, pers. comm. email dated 23 February 2021). His mother (granddaughter of W. A. Sloane) spent considerable time in the area during her youth and became an educated biologist with a master's degree. Bretz stated that she “would have been interested in and become well-informed about beavers there, if it were true.” The feature was named sometime between 1901 and 1903 (see the Supplementary File for further discussion), but for all we know a felled tree snag could have resembled a beaver dam to the cartographers.
A fifth and final argument presented by Lanman et al. (2013) is that because suitable habitat exists for beaver in coastal southern California, the species must have always occurred there. They criticize Grinnell et al. (1937) and Tappe's (1942) suggestion that the region lacks beaver-preferred habitat and raise the question of how beaver could now occur there if the habitat was unsuitable. However, beaver currently survive only in river systems that have been hydrologically modified by humans. Without controlled discharge from reservoirs or other upstream areas, these drainages would largely dry out during the latter parts of the year. Tappe and Grinnell were surely referring to habitat prior to a time when this infrastructure existed, as major construction began only in the early 1940s (Orsi, 2004). In fact, beaver were unable to persist at sites in upper San Mateo Creek, San Diego County following releases in 1947 because surface water was too sporadic, forcing them to move ~32 km downstream to the lagoon at the mouth of the creek (Ervin, 2017). The species is now presumed to be extirpated from this drainage, as no dams have been documented in ~18 years of monitoring by the USGS.
Other arguments suggesting beaver are indigenous to the region are found in Longcore et al. (2007, p. 466). The authors state that “During the Holocene, beavers were certainly found in southern California, and their apparent restriction to the northern, central, and southeastern portions of the state is either the result of recent climate change or overexploitation,” and “The flora of California, and indeed southern California, coexisted for thousands of years with beavers.” However, there are no citations for these statements, and there is no evidence that indicates either is true (see next section). They go on to say “Furthermore, the natural predators of beavers such as coyotes (Canis latrans) are found in southern California,” insinuating that the presence of a predator validates the geographic origin of its potential prey. However, coyote is a generalist predator that occurs from coast to coast in North America and as far south as Costa Rica, so it is unclear how its presence is relevant to the origin of beaver in the region.
Finally, our own searches for the word “beaver” at the San Diego History Center and the associated San Diego History Journal (https://sandiegohistory.org) turned up the account of Dr. D. B. Hoffman, references to a name, town or other irrelevant use of the word beaver, and numerous accounts of beaver pelts brought into San Diego from other areas. San Diego was an important port of commerce in the 1800s and furs and hides were transported down the coast from northern California and up from México during a time when beaver was abundant in the San Joaquin Valley, northern California, and parts of the lower Colorado River (Smith, 1908). The only other references to beaver were exhibits in the 1915–1916 International Exposition at Balboa Park and at the San Diego Zoo in the 1920s and 1940s.
An Engineer That Leaves No Trace
There is no evidence for beaver in the zooarchaeological or Pleistocene fossil record from coastal southern California (Jefferson, 1991; NeotomaDB.org, Castor canadensis, accessed 2/16/21). The only reported fossil specimens from the southern part of the state are both from the San Joaquin Valley, the southern portion of California's Great Central Valley; a single Castor californicus molar from the Miocene/Pliocene boundary site in the Kettleman Hills in Kings County (Kellogg, 1911), and the Late Pleistocene (c. 38 kya) Castor canadensis from Dudley Ridge (Jefferson, 1991), just east of the Kettleman Hills.
Fossils of numerous large and small mammals, as well as amphibian, reptile and rodent bones, insects, mollusks, leaves, seeds, wood, and pollen grains, have been extricated from tar pits at McKittrick in Kern County (Schultz, 1937), Carpinteria in Santa Barbara County (Hoffmann et al., 1927), and Rancho La Brea in the Los Angeles Basin (Stock, 1992[1930]). However, no beaver specimens are reported from Late Pleistocene deposits at any of these sites. Likewise, no specimens have been recovered from Pleistocene dune deposits in Huntington Beach (Wake and Roeder, 2009) or anywhere else in the Los Angeles Basin (Jefferson, 1991), and none are among the Middle and Late Holocene archaeofaunas reported from the Ballona wetlands (Lev-Tov et al., 2016). Late Holocene (Late Prehistoric) faunal assemblages from coastal sites on Marine Corp Base Camp Pendleton in San Diego County mirror these findings. Site CA-SDI-13325 within the San Mateo Creek coastal flood plain produced 36 different taxa, including California sea lion (Zalophus californianus), sea otter (Enhydra lutris), American badger (Taxidea taxus), ringtail (Bassariscus astutus), and coyote (Wake, 1999). A second site, a rock shelter midden just above the Santa Margarita River (CA-SDI-21240), produced 84 different taxa, including Guadalupe fur seal (Arctocephalus townsendi), California sea lion, sea otter, coyote, bobcat, and American badger. However, neither site revealed any beaver. An exhaustive search of gray literature zooarchaeology reports from southern California has also found no reported beaver specimens.
It is difficult to envisage how a rodent the size of beaver would leave no trace in the fossil or zooarchaeological record in the low gradient areas of the Los Angeles Basin, lower San Mateo Creek floodplain, or other coastal terraces of southern California, where suitable beaver habitat would have most likely existed. We recognize of course that absence of fossil or zooarchaeological evidence is not proof of absence, but one also cannot argue that a species existed someplace historically when there is no evidence to indicate that it did.
Historic Surveys of the Mammal Fauna—Still No Sign of the Engineer
As far back as 1769, members of the famous Serra and Portolá expedition conducting reconnaissance for nascent Spanish missions along coastal California noted that in San Diego, Kumeyaay blankets, shawls, and other garments were made of deer, hare, rabbit, otter, and fox skin, with hare and rabbit consistently cited as the predominant resources in accounts dating to the eighteenth and nineteenth centuries (Maximin Piette, 1946; Carrico, 1977). If present, beaver would have surely made this list as well.
Field studies by one of the most prolific mid-nineteenth century collectors of mammal specimens on the west coast of North America, James Graham Cooper MD, also cannot be overlooked. Cooper was a pioneering contributor to the fields of mammalogy, ornithology, and botany, and began his career as a physician-naturalist for the Pacific Railroad Survey in Washington state from 1853 to 1855 (Taylor, 1919). From 1860 to 1874, he worked for the California Geological Survey as a zoologist and was the first to record five species of mammal, 16 species of bird, and three species of reptile for California (Cooper, 1861; Grinnell, 1902). He was active at the California Academy of Sciences in various official capacities, including being the Director of the Museum from 1887 to 1891, and published a wealth of material on the natural history of California and Oregon. Cooper was well-versed in beaver ecology due to considerable time spent on the lower Colorado River at Fort Mojave in Arizona, and at various locations in Washington, Oregon, and northern California. Yet never once does he document beaver from coastal southern California, despite his attention to detail regarding location data, species identities, habitat notes, weather, specimen collections, etc. (Taylor, 1919).
The survey work of Frank Stephens (1849–1937), a ground-breaking naturalist of the Southwest and premiere director of the San Diego Natural History Museum, also deserves attention. He first moved to Campo (San Diego County, CA) in 1876, then traveled to various parts of southern California and Arizona collecting specimens of mammals, birds, and reptiles and amphibians before settling in the city of San Diego in 1897. His contributions in ornithology, mammalogy, herpetology, and vertebrate paleontology are renowned, and his personal collection of some 2,000 bird and mammal specimens represents the foundation of the San Diego Natural History Museum's Birds & Mammals Department. Stephens wrote the celebrated California Mammals (Stephens, 1906) and discusses in detail the two subspecies of beaver in the state, emphasizing the large size of their lodges, physical signatures of tree cutting and harvesting, and conspicuous tail slapping behavior on the surface of the water when individuals become startled. His descriptions underscore the fact that beaver is easily detectable when present, yet he never collected any specimens nor mentioned beaver from any of his field surveys of coastal southern California.
The following accounts also make no mention of any beaver records for coastal southern California or northern Baja California, even though each review the region's natural history and discuss extirpated species such as the California grizzly bear (Ursus arctos californicus) and pronghorn (Antilocapra americana). The first is from Holder (1906), an avid sportsman and author of the book “Life in the Open,” which details his forays around southern California for ~20 years preceding the book. He discusses all larger wildlife that could be seen and or hunted, including places where beaver exist today (e.g., lower Deep Creek and the Temecula Creek tributary to the Santa Margarita River in Riverside County). However, he describes systems without physical evidence of dams or felled trees caused by beaver activity. He further discusses other creeks around southern California in relation to trout and steelhead, but nowhere does he mention beaver as a constituent of this fauna.
The next account is from Grinnell (1908) who studied the San Bernardino Mountains for three summers, including the upper watershed of the Mojave River in Holcomb Valley and throughout the Santa Ana Watershed (although not explicitly in the lower Deep Creek area). He documented 35 species of mammals during these surveys but reported no evidence of beaver, nor was the species even addressed. In this same account he relays the observations of various people that lived in the mountains, none of whom mentioned beaver but did discuss other megafauna known from the range.
Then Stephenson (1931) published the classic book “Shadows of Old Saddleback, tales of the Santa Ana Mountains,” an account of the history and biology of the range that brings forward stories from the first settlers about the wildlife they encountered. This includes pronghorn and Mexican wolf (Canis lupus baileyi) and a detailed discussion of the last known California grizzlies, which were killed during Stephenson's time in the region. He discusses frogs, turtles, and myriad wildlife but neither from his first-hand experience in the Santa Ana Mountains between the 1880s until the book was published, nor from first-hand accounts of settlers living in the mountains since the 1860s, was beaver ever mentioned. Stephenson's more narrative work was followed by Pequegnat (1951), whose account spans the region from the Santa Ana River in the north to the Temecula River (Santa Margarita River) in the south, beginning in the 1930s. His thorough coverage of the fauna (with emphasis on the aquatic fauna) and flora makes no mention of beaver over this large part of southern California.
There are many other relevant mammal papers from southern California and northern Baja California from the same period, reporting prior to the known introductions of beaver in the state (see below). For example, Grinnell and Swarth (1913) studied the fauna of the San Jacinto Mountains and found no evidence of beaver from this range. Vaughn (1954) reviewed the mammals of the San Gabriel Mountains and makes no mention of beaver as part of the mammal fauna during that time or historically. Huey (1964) published the “The Mammals of Baja California, México” and discusses beaver from the Colorado delta and its occurrence in the canals in northeastern Baja California, but there is no discussion of any coastal populations of beaver. As the Tijuana watershed is one of the largest in Baja and southern California, this would be a logical drainage where beaver might naturally exist in the region, yet there is no evidence that the species ever occurred there.
Origins of the Engineer in the SC-NBC Ecoregion
In addition to the absence of any verifiable records for beaver in the SC-NBC ecoregion, it is well-known that the species was introduced across California as a fur resource and as an aid in water conservation and erosion control (Figure 1). The U.S. Forest Service and California Department of Fish and Game made efforts to extend the range and increase the number of populations by transplanting live beaver to selected places that were not inhabited by them (Tappe, 1942; Hensley, 1946). From 1923 to 1949, only one out of 274 transplantations show that beaver were harvested from any county in southern California—eastern Riverside County on the lower Colorado River, where beaver is unquestionably native (Hensley, 1946). Otherwise, beaver was never harvested anywhere in coastal southern California, only transplanted there (N = 22 plants in Los Angeles, Orange, Riverside, and San Diego counties; Figure 1). The most successful introductions, and one in which beaver remain well-established to this day, have been in the Santa Margarita River watershed (U.S. Fish and Wildlife Service, 1998; Ervin, 2017).
Containing the Engineer?
That beaver is non-indigenous to the SC-NBC ecoregion, combined with the effects of novel disturbance and support of other non-native species, suggests that managing beaver in invaded systems has potential to improve habitat and protect native species by shifting hydrologic function toward natural states. Eradicating all non-native aquatic species is not feasible; however, managing a single ecosystem engineer in this case could have broad-ranging, trickle-down effects on the richness and abundance of invasive species because niche opportunities would diminish or perhaps even be eliminated for some areas (Crooks, 2002; Wright et al., 2006; Strayer, 2012). At the same time, hydrologic conditions may revert to more punctuated, flashy dynamics that repress the dominance of the most insidious invaders while tipping the scales back in favor of natives (Stromberg, 2001; Marchetti et al., 2004c).
Discussion about best practices for control of beaver populations is outside the scope of this paper but would be part of a comprehensive management plan to remove them. Efforts to control beaver can be conducted in a humane manner and in consultation with experts who have previously engaged in the practice (Pollock et al., 2015). Studies on human–beaver interactions in the United States have shown resistance to lethal management and advocacy for beaver (Jonker et al., 2006; Morzillo and Needham, 2015), suggesting that plans to restore beaver-impacted habitat through management requires consideration of human sociological factors as well as ecological benefits (Rees, 2001; Baker and Hill, 2003; Kemp et al., 2012; Anderson et al., 2017; Pilliod et al., 2018). Proper communication about the rationale for management, combined with an understanding of stakeholder concerns, is more likely to lead to positive outcomes than if managers were to plan and act alone (Longcore et al., 2007; Yarmy and Hood, 2020). Educators could also be encouraged to teach about the impacts of beaver ecology in both the native and introduced range, as the ecological benefits in the native range do not necessarily translate to areas outside of it.
In California, beaver management falls under the jurisdiction of multiple branches of the California Department of Fish and Wildlife (CDFW; formerly California Department of Fish and Game). The agency provides guidance to mitigate any conflict involving human/beaver interactions and is responsible for Lake and Streambed Alteration (LSA) agreements (https://wildlife.ca.gov/Conservation) and depredation permits (https://nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=114087&inline). Some concern may be raised that beaver management in the SC-NBC ecoregion could affect recreational freshwater fisheries, as numerous non-native fishes are also favorite food and game fishes (Dill and Cordone, 1997). However, these fish species survive best in lakes, ponds, and reservoirs, which is where most of the freshwater sport fishing occurs in the region (Swift et al., 1993; Moyle, 2002). It is therefore unlikely that managing introduced beaver in natural stream systems would negatively impact recreational fisheries.
Conclusion
Best practices suggest that beaver management in the SC-NBC ecoregion could be employed when beaver ecology negatively impacts a variety of indigenous species and habitat, including but not limited to those that are of conservation concern. Humane treatment of beaver is of paramount concern, and the outcome of any management directive may not include eradication—population control could be appropriate under certain circumstances, although beaver is known to readily re-invade areas where they have been extricated (Houston et al., 1995; Wilson and McEwen, 1998).
Perspectives on beaver management in xeric regions range from protection (e.g., introduction as a means for habitat restoration: Pollock et al., 2014) to eradication (e.g., to protect riparian vegetation; Mortenson et al., 2008; Anderson et al., 2017). Given the interest in using beaver in stream restoration efforts (DeVries et al., 2012; Pollock et al., 2014; Bouwes et al., 2016), managers may first want to consider whether the focal system is dominated by invasive species, and whether beaver is native to that system. Facilitating beaver reintroductions to sites that were occupied by beaver historically, so long as they are not overrun with invasives and/or if invasives can be removed prior to reintroduction, may be prudent. Ultimately any restoration value provided by beaver disturbance could depend on, among other factors, the trade-off between benefits for native species and the cost of promoting non-natives.
Data Availability Statement
Data for the arroyo toad habitat occupancy analysis and R code for the Poisson generalized linear mixed effects model (GLM) and categorical regression analyses can be downloaded from the journal website.
Author Contributions
All authors contributed to the writing and review of the manuscript.
Funding
Funding for this research was provided by the San Diego Association of Governments (SANDAG) and the USGS Ecosystems Mission Area.
Conflict of Interest
EE is affiliated with Merkel & Associates, Inc., but participated in an independent capacity toward this research.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank K. Cripe, K. Hoffman, and M. Meshriy for assistance with records of beaver introduction. We thank W. Bretz, K. Phillips, and T. Myers for assistance with tracing the origin of the name Beaver Hollow in San Diego County. D. Decker uncovered the history of the Beaver Hollow feature on official USGS topographical maps. We thank S. Arter for summarizing the faunal assemblages of late Holocene zooarchaeological sites on MCB Camp Pendleton. K. Wilson and C. Hitchcock provided helpful comments on the manuscript. This is Contribution Number 815 of the USGS Amphibian Research and Monitoring Initiative (ARMI). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2021.752400/full#supplementary-material
Footnotes
1. ^Efforts to locate the article, aided by librarians at San Diego State University, were unsuccessful despite obtaining a copy of the newspaper edition cited in Lanman et al. (2013).
References
Adams, M. J. (2000). Pond permanence and the effects of exotic vertebrates on anurans. Ecol. Appl. 10, 559–568. doi: 10.1890/1051-0761(2000)010[0559:PPATEO]2.0.CO;2
Adams, M. J., Pearl, C. A., and Bury, R. B. (2003). Indirect facilitation of an anuran invasion by non-native fishes. Ecol. Lett. 6, 343–351. doi: 10.1046/j.1461-0248.2003.00435.x
Albert, S., and Trimble, T. (2000). Beavers are partners in riparian restoration on the Zuni Indian Reservation. Ecol. Restor. 18, 87–92. doi: 10.3368/er.18.2.87
Alexander, M. D. (1998). Effects of beaver (Castor canadensis) impoundments on stream temperature and fish community species composition and growth in selected tributaries of Miramichi River, New Brunswick. Can. Tech. Rep. Fish. Aquat. Sci. 2227:53.
Allen, A. W. (1983). Habitat Suitability Index Models: Beaver. FWS/OBS-82/10.30, US Fish and Wildlife Service, Fort Collins, CO. Available online at: http://pubs.er.usgs.gov/publication/fwsobs82_10_30 (accessed March 04, 2021).
Anderson, C. B., Griffith, C. R., Rosemond, A. D., Rozzi, R., and Dollenz, O. (2006). The effects of invasive North American beavers on riparian plant communities in Cape Horn, do exotic beavers engineer differently in sub-Antarctic ecosystems? Biol. Conserv. 128, 467–474. doi: 10.1016/j.biocon.2005.10.011
Anderson, C. B., Martínez Pastur, G., Lencinas, M. V., Wallem, P. K., Moorman, M. C., and Rosemond, A. D. (2009). Do introduced North American beavers Castor canadensis engineer differently in southern South America? An overview with implications for restoration. Mamm. Rev. 39, 33–52. doi: 10.1111/j.1365-2907.2008.00136.x
Anderson, C. B., and Rosemond, A. D. (2007). Ecosystem engineering by invasive exotic beavers reduces in-stream diversity and enhances ecosystem function in Cape Horn, Chile. Oecologia 154, 141–153. doi: 10.1007/s00442-007-0757-4
Anderson, C. B., and Rosemond, A. D. (2010). Beaver invasion alters terrestrial subsidies to subantarctic stream food webs. Hydrobiologia 652, 49–361. doi: 10.1007/s10750-010-0367-8
Anderson, C. B., Roulier, C., and Pizarro, J. C. (2017). Perspectivas de actores clave respecto del acuerdo binacional entre Argentina y Chile sobre la erradicación del castor norteamericano y la restauración de los ecosistemas afectados. Bosque 38, 555–562. doi: 10.4067/S0717-92002017000300013
Anderson, S. H., Kelly, D., Ladley, J. J., Molloy, S., and Terry, J. (2011). Cascading effects of bird functional extinction reduce pollination and plant density. Science 331, 1068–1071. doi: 10.1126/science.1199092
Arismendi, I., Penaluna, B. E., and Jara, C. G. (2020). Introduced beaver improve growth of non-native trout in Tierra del Fuego, South America. Ecol. Evol. 10, 9454–9465. doi: 10.1002/ece3.6636
Baker, B. W., and Hill, E. P. (2003). “Beaver (Castor canadensis),” in Wild Mammals of North America: Biology, Management, and Conservation, eds G. A. Feldhamer, B. C. Thompson, and J. A. Chapman (Baltimore, MD: The Johns Hopkins University Press), 288–310.
Basey, J. M., Jenkins, S. H., and Busher, P. E. (1988). Optimal central-place foraging by beavers: Tree-size selection in relation to defensive chemicals of quaking aspen. Oecologia 76, 278–282. doi: 10.1007/BF00379963
Bateman, H. L., and Paxton, E. H. (2010). “Saltcedar and Russian olive interactions with wildlife,” in Saltcedar and Russian Olive Control Demonstration Act Science Assessment, eds P. B. Shafroth, C. A. Brown, and D. M. Merritt (Fort Collins, CO: U.S. Geological Survey Scientific Investigations Report 2009–5247), 49–64.
Billman, E. J., Kreitzer, J. D., Creighton, J. C., Habit, E., McMillan, B., and Belk, M. C. (2013). Habitat enhancement and native fish conservation: can enhancement of channel complexity promote the coexistence of native and introduced fishes? Environ. Biol. Fish. 96, 555–566. doi: 10.1007/s10641-012-0041-2
Boughton, D. A., Adams, P. B., Andersen, E., Fusaro, C., Keller, E., Kelley, E., et al. (2007). Viability Criteria for Steelhead of the South-Central and Southern California Coast. National Oceanic and Atmospheric Administration Technical Memorandum, National Marine Fisheries Service NMFS-SWFSC 407. Available online at: https://repository.library.noaa.gov/view/noaa/3948 (accessed March 10, 2021).
Boughton, D. A., Fish, H., Pipal, K., Goin, J., Watson, F., Casagrande, J., et al. (2005). Contraction of the Southern Range Limit for Anadromous Oncorhynchus mykiss. National Oceanic and Atmospheric Administration Technical Memorandum, National Marine Fisheries Service NMFS-SWFSC 380. Available online at: https://repository.library.noaa.gov/view/noaa/3433 (accessed March 10, 2021).
Boughton, D. A., Fish, H., Pope, J., and Holt, G. (2009). Spatial patterning of habitat for Oncorhynchus mykiss in a system of intermittent and perennial streams. Ecol. Freshw. Fish 18, 92–105. doi: 10.1111/j.1600-0633.2008.00328.x
Bouwes, N., Weber, N., Jordan, C. E., Saunders, W. C., Tattam, I. A., et al. (2016). Ecosystem experiment reveals benefits of natural and simulated beaver dams to a threatened population of Steelhead (Oncorhynchus mykiss). Sci. Rep. 6:28581. doi: 10.1038/srep28581
Braga, R. R., Gómez-Aparicio, L., Heger, T., Vitule, J. R. S., and Jeschke, J. M. (2018). Structuring evidence for invasional meltdown: broad support but with biases and gaps. Biol. Invasions 20, 923–936. doi: 10.1007/s10530-017-1582-2
Braig, E. C., and Johnson, D. L. (2003). Impact of black bullhead (Ameiurus melas) on turbidity in a diked wetland. Hydrobiologia 490, 11–21. doi: 10.1023/A:1023405823216
Brodie, E. D. (1968). Investigations on the skin toxin of the adult rough-skinned newt, Taricha granulosa. Copeia 1968, 307–313. doi: 10.2307/1441757
Carrico, R. L. (1977). Portola's 1769 edition of coastal native villages of San Diego County. J. Calif. Anthropol. 4, 30–41.
Casper, G. S., and Hendricks, R. (2005). “Rana catesbeiana Shaw, 1802,” in Amphibian Declines: The Conservation Status of United States Species, ed M. Lannoo (Berkeley, CA: University of California Press), 540–546.
Cayan, D. R., Redmond, K. T., and Riddle, L. G. (1999). ENSO and hydrologic extremes in the western United States. J. Clim. 12, 2881–2893. doi: 10.1175/1520-0442(1999)012<2881:EAHEIT>2.0.CO;2
Clout, M. N., and Russell, J. C. (2007). The invasion ecology of mammals: a global perspective. Wildl. Res. 35, 180–184. doi: 10.1071/WR07091
Cohan, D. R., Anderson, W., and Ohmart, R. D. (1978). Avian Population Responses to Saltcedar Along the Lower Colorado River. Washington, DC: U.S. Forest Service, U.S. Department of Agriculture General Technical Report WO-12.
Cooper, J. G. (1868). Some recent additions to the fauna of California. Proc. Calif. Acad. Sci. 4, 3–13.
Cooper, J. G. (1869). The naturalist in California. Am. Nat. 3, 182–189, 470–481. doi: 10.1086/270475
Cox, J. J., Maehr, D. S., and Larkin, J. L. (2002). The biogeography of faunal place names in the United States. Conserv. Biol. 16, 1143–1150. doi: 10.1046/j.1523-1739.2002.01202.x
Croll, D. A., Maron, J. L., Estes, J. A., Danner, E. M., and Byrd, G. V. (2005). Introduced predators transform subarctic islands from grassland to tundra. Science 307, 1959–1961. doi: 10.1126/science.1108485
Crooks, J. A. (2002). Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97, 153–166. doi: 10.1034/j.1600-0706.2002.970201.x
DeVries, P., Fetherston, K. L., Vitale, A., and Madsen, S. (2012). Emulating riverine landscape controls of beaver in stream restoration. Fisheries 37, 246–255. doi: 10.1080/03632415.2012.687263
Dill, W. A., and Cordone, A. J. (1997). History and Status of Introduced Fishes in California, 1871–1996. Sacramento, CA: California Department of Fish and Game Fish Bulletin, 178.
Dobson, A. P., Rodriguez, J. P., Roberts, W. M., and Wilcove, D. S. (1997). Geographic distribution of endangered species in the United States. Science 275, 550–553. doi: 10.1126/science.275.5299.550
Doherty, T. S., Glen, A. S., Nimmo, D. G., Ritchie, E. G., and Dickman, C. R. (2016). Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. U.S.A. 113, 11261–11265. doi: 10.1073/pnas.1602480113
Doubledee, R. A., Muller, E. B., and Nisbet, R. M. (2003). Bullfrogs, disturbance regimes, and the persistence of California red-legged frogs. J. Wildl. Manage. 67, 424–438. doi: 10.2307/3802783
Douglas, P. L. (1995). Habitat relationships of oversummering rainbow trout (Oncorhynchus mykiss) in the Santa Ynez drainage (Master's thesis). University of California, Santa Barbara, CA, United States.
Ervin, E. L. (2017). “North American Beaver Castor canadensis,” in San Diego County Mammal Atlas, eds S. Tremor, D. Stokes, W. Spencer, J. Diffendorfer, H. Thomas, and S. Chivers (San Diego, CA: Sunbelt Publications), 46–47.
Ferren, W. R. Jr., and Fiedler, P. L. (1993). “Rare and threatened wetlands in coastal central and southern California.” in Interface between Ecology and Land Development in California, ed J. Keeley (Los Angeles, CA: The Southern California Academy of Sciences), 119–131.
Fischer, R. A., Valente, J. J., and Guilfoyle, M. P. (2015). Spring migrant use of native and saltcedar-dominated riparian areas along the lower Colorado River in Arizona. Southwest. Nat. 60, 6–14. doi: 10.1894/MCG-06.1
Francis, R. A., and Chadwick, M. A. (2012). “Invasive alien species in freshwater ecosystems: a brief overview,” in A Handbook of Global Freshwater Invasive Species, ed R. A. Francis (New York, NY: Earthscan), 3–24. doi: 10.4324/9780203127230
Gallant, D., Bérubé, C., Tremblay, E., and Vasseur, L. (2004). An extensive study of the foraging ecology of beavers (Castor canadensis) in relation to habitat quality. Can. J. Zool. 82, 922–933. doi: 10.1139/z04-067
Gasith, A., and Resh, V. H. (1999). Streams in Mediterranean climate regions: abiotic influences and biotic responses to predictable seasonal events. Annu. Rev. Ecol. Syst. 30, 51–81. doi: 10.1146/annurev.ecolsys.30.1.51
Gibson, P. P., and Olden, J. D. (2014). Ecology, management, and conservation implications of North American beaver (Castor canadensis) in dryland ecosystems. Aquat. Conserv. 24, 391–409. doi: 10.1002/aqc.2432
Goodman, D. H., Reid, S. B., Som, N. A., and Poytress, W. R. (2015). The punctuated seaward migration of Pacific lamprey (Entosphenus tridentatus): environmental cues and implications for stream management. Can J. Fish. Aquat. Sci. 72, 1817–1828. doi: 10.1139/cjfas-2015-0063
Griffith, G. E., Omernik, J. M., Smith, D. W., Cook, T. D., Tallyn, E., Moseley, K., et al. (2016). Ecoregions of California. U.S. Geological Survey Open-File Report 2016–1021. doi: 10.3133/ofr20161021
Grinnell, J. (1902). The ornithological writings of Dr. J. G. Cooper. Condor 4, 103–105. doi: 10.2307/1361648
Grinnell, J., Dixon, J. S., and Linsdale, J. M. (1937). Fur-Bearing Mammals of California: Their Natural History, Systematic Status, and Relations to Man. Berkeley, CA: University of California Press.
Grinnell, J., and Swarth, H. S. (1913). An account of the birds and mammals of the San Jacinto Area of southern California with remarks upon the behavior of geographic races on the margins of their habitats. Univ. Calif. Publ. Zool. 10, 197–406. doi: 10.5962/bhl.title.15778
Hansen, M. J., Guy, C. S., Budy, P., and McMahon, T. E. (2019). “Trout as native and nonnative species: a management paradox,” in Trout and Char of the World, eds J. L. Kershner, J. E. Williams, R. E. Gresswell, and J. Lobón-Cerviá (Bethesda, MD: American Fisheries Society), 645–684.
Hensley, A. L. (1946). A progress report on beaver management in California. Calif. Fish Game 32, 87–99.
Hershkovitz, Y., and Gasith, A. (2013). Resistance, resilience, and community dynamics in Mediterranean-climate streams. Hydrobiologia 719, 59–75. doi: 10.1007/s10750-012-1387-3
Hoffman, D. B. (1864). Sanitary Report – New San Diego Barracks, California, Vol. 6. San Francisco Medical Press, 145–158.
Hoffmann, R., Stock, C., Miller, L., Chaney, R. W., and Mason, H. L. (1927). The finding of Pleistocene material in an asphalt pit at Carpinteria, California. Science 66, 155–157. doi: 10.1126/science.66.1702.155.a
Holder, C. F. (1906). Life in the Open; Sport with Rod, Gun, Horse and Hound in Southern California. New York, NY; London: G.P. Putnam's Sons. doi: 10.5962/bhl.title.29458
Holland, D. C., and Swift, C. C. (2000). “Exotic aquatic species on MCB Camp Pendleton, California: Control and Management,” in Prepared for AC/S Environmental Security Wildlife Management Branch, Marine Corps Base Camp Pendleton, Contract Number M 00681-97-P-1687, Fallbrook, CA.
Houston, A. E., Pelton, M. R., and Henry, R. (1995). Beaver immigration into a control area. South J. Appl. For. 19, 127–130. doi: 10.1093/sjaf/19.3.127
Howard, J. K., Klausmeyer, K. R., Fesenmyer, K. A., Furnish, J., Gardali, T., Grantham, T., et al. (2015). Patterns of freshwater species richness, endemism, and vulnerability in California. PLoS ONE 10:e0130710. doi: 10.1371/journal.pone.0130710
Huey, L. M. (1964). The mammals of Baja California, Mexico. Trans. San Diego Soc. Nat. Hist. 13, 85–168.
Hunter, J. (1787). Observation on the structure and oeconomy of whales. Philos. T. R. Soc. 77, 371–450. doi: 10.1098/rstl.1787.0038
Jackson, M. C., Wasserman, R. J., Grey, J., Ricciardi, A., Dick, J. T. A., and Alexander, M. E. (2017). “Novel and disrupted trophic links following invasion in freshwater ecosystems,” in Advances in Ecological Research, Vol. 57, eds D. A. Bohan, A. J. Dumbrell, and F. Massol (Oxford: Academic Press), 55–97. doi: 10.1016/bs.aecr.2016.10.006
Jefferson, G. T. (1991). A Catalogue of Late Quaternary Vertebrates from California: Mammals. Part Two, Volume 2. Los Angeles, CA: Natural History Museum of Los Angeles County.
Johnson, G. E., and van Riper, C. III. (2014). Effects of Reintroduced Beaver (Castor canadensis) on Riparian Bird Community Structure Along the Upper San Pedro River, Southeastern Arizona and Northern Sonora, Mexico. U.S. Geological Survey Open-File Report 2014–1121, 98. doi: 10.3133/ofr20141121
Johnston, C. A., and Naiman, R. J. (1990). Aquatic patch creation in relation to beaver population trends. Ecology 71, 1617–1621. doi: 10.2307/1938297
Jones, C. G., Lawton, J. H., and Shachak, M. (1994). Organisms as ecosystem engineers. Oikos 69, 373–386. doi: 10.2307/3545850
Jones, C. G., Lawton, J. H., and Shachak, M. (1997). Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78, 1946–1957. doi: 10.1890/0012-9658(1997)078[1946:PANEOO]2.0.CO;2
Jonker, S. A., Muth, R. M., Organ, J. F., Zwick, R. R., and Siemer, W. F. (2006). Experiences with beaver damage and attitudes of Massachusetts residents toward beaver. Wildl. Soc. Bull. 34, 1009–1021. doi: 10.2193/0091-7648(2006)34[1009:EWBDAA]2.0.CO;2
Kats, L. B., Bucciarelli, G. M., Vandergon, T. L., Honeycutt, R. L., Mattiasen, E., Sanders, A., et al. (2013). Effects of natural flooding and manual trapping on the facilitation of invasive crayfish-native amphibian coexistence in a semi-arid perennial stream. J. Arid Environ. 98, 109–112. doi: 10.1016/j.jaridenv.2013.08.003
Kats, L. B., and Ferrer, R. P. (2003). Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers. Distrib. 9, 99–110. doi: 10.1046/j.1472-4642.2003.00013.x
Kellogg, L. (1911). A fossil beaver from the Kettleman Hills, California. Bull. Dept. Geol. Univ. Calif. 6, 401–402.
Kemp, P. S., Worthington, T. A., Langford, T. E., Tree, A. R., and Gaywood, M. J. (2012). Qualitative and quantitative effects of reintroduced beavers on stream fish. Fish Fish. 13, 158–181. doi: 10.1111/j.1467-2979.2011.00421.x
Koel, T. M., Tronstad, L. M., Arnold, J. L., Gunther, K. A., Smith, D. W., Syslo, J. M., et al. (2019). Predatory fish invasion induces within and across ecosystem effects in Yellowstone National Park. Sci. Adv. 5:eaav1139. doi: 10.1126/sciadv.aav1139
Lanman, C. W., Lundquist, K., Perryman, H., Eli Asarian, J., Dolman, B., Lanman, R. B., et al. (2013). The historical range of beaver (Castor canadensis) in coastal California: an updated review of the evidence. Calif. Fish Game 99, 193–221.
Law, A., Jones, K. C., and Willby, N. J. (2014). Medium vs. short-term effects of herbivory by Eurasian beaver on aquatic vegetation. Aquat. Bot. 116, 27–34. doi: 10.1016/j.aquabot.2014.01.004
Laylander, D. (2010). Linguistic prehistory and the archaic-late transition in the Colorado Desert. J. Calif. Great Basin Anthropol. 30, 141–155.
Lesica, P., and Miles, S. (2004). Beavers indirectly enhance the growth of Russian olive and tamarisk along eastern Montana rivers. West. Nort Am. Nat. 64, 93–100.
Levick, L. R., Goodrich, D. C., Hernandez, M., Fonseca, J., Semmens, D. J., Stromberg, J. C., et al. (2008). The Ecological and Hydrological Significance of Ephemeral and Intermittent Streams in the Arid and Semi-Arid American Southwest. U.S. Environmental Protection Agency, Office of Research and Development and USDA/ARS Southwest Watershed Research Center, EPA/600/R-08/134, ARS/233046, 116.
Levine, R., and Meyer, G. A. (2019). Beaver-generated disturbance extends beyond active dam sites to enhance stream morphodynamics and riparian plant recruitment. Sci. Rep. 9:8124. doi: 10.1038/s41598-019-44381-2
Lev-Tov, J., Van Galder, S., Maxwell, D., Reddy, S., Wake, T. A., and Gobalet, K. (2016). “Analysis of vertebrate fauna,” in People in a Changing Land: The Archaeology and History of the Ballona in Los Angeles, California. Vol. 3, Material Culture and Subsistence Practices, eds S. N. Reddy and J. G. Douglass (Tucson, AZ: Technical Series 94, Statistical Research, Inc.), 505–558.
Linnaeus, C. (1758). “Classis I. Mammalia,” in Systema Naturae Per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis, 10th Edn, ed C. Linnaeus (Stockholm: Laurentius Salvius), 14–787. doi: 10.5962/bhl.title.542
Liu, X., Blackburn, T. M., Song, T., Wang, X., Huang, C., and Li, Y. (2020). Animal invaders threaten protected areas worldwide. Nat. Commun. 11:2892. doi: 10.1038/s41467-020-16719-2
Lizzaralde, M., Escobar, J., and Deferrari, G. (2004). Invader species in Argentina: a review about the beaver (Castor canadensis) population situation on Tierra del Fuego ecosystem. Interciencia 29, 352–356.
Lodge, D. M., Taylor, C. A., Holdich, D. M., and Skurdal, J. (2000). Reducing impacts of exotic crayfish introductions: new policies needed. Fisheries 25, 21–23. doi: 10.1577/1548-8446(2000)025<0007:NCTNAF>2.0.CO;2
Longcore, T., Rich, C., and Müller-Schwarze, D. (2007). Management by assertion: beavers and songbirds at Lake Skinner (Riverside County, California). Environ. Manage. 39, 460–471. doi: 10.1007/s00267-005-0204-4
Marchetti, M. P., Light, T., Moyle, P. B., and Viers, J. H. (2004c). Fish invasions in California watersheds: testing hypotheses using landscape patterns. Ecol. Appl. 14, 1507–1525 doi: 10.1890/03-5173
Marchetti, M. P., Moyle, P. B., and Levine, R. (2004a). Invasive species profiling? Exploring the characteristics of exotic fishes across invasion stages in California. Freshw. Biol. 49, 646–661. doi: 10.1111/j.1365-2427.2004.01202.x
Marchetti, M. P., Moyle, P. B., and Levine, R. (2004b). Alien fishes in California watersheds: Characteristics of successful and failed invaders. Ecol. Appl. 14, 587–596. doi: 10.1890/02-5301
Maret, T. J., Snyder, J. D., and Collins, J. P. (2006). Altered drying regime controls distribution of endangered salamanders and introduced predators. Biol. Conserv. 127, 129–138. doi: 10.1016/j.biocon.2005.08.003
Martell, K. A., Foote, A. L., and Cumming, S. G. (2006). Riparian disturbance due to beavers (Castor canadensis) in Alberta's boreal mixed wood forests: implications for forest management. Ecoscience 13, 164–171. doi: 10.2980/i1195-6860-13-2-164.1
Martínez Pastur, G., Lencinas, M. V., Escobar, J., Quiroga, P., Malmierca, L., and Lizarralde, M. (2006). Understorey succession in Nothofagus forests in Tierra del Fuego (Argentina) affected by Castor canadensis. Appl. Veg. Sci. 9, 143–154
Maximin Piette, C. J. G. (1946). The diarios of early California, 1769-1784. Americas 146, 409–422. doi: 10.2307/977712
McGinley, M. A., and Whitham, T. G. (1985). Central place foraging by beavers (Castor canadensis): a test of foraging predictions and the impact of selective feeding on the growth form of cottonwoods (Populus fremontii). Oecologia 66, 558–562. doi: 10.1007/BF00379350
McKinstry, M. C., Caffrey, P., and Anderson, S. H. (2001). The importance of beaver to wetland habitats and waterfowl in Wyoming. J. Am. Water Resour. Assoc. 37, 1571–1577. doi: 10.1111/j.1752-1688.2001.tb03660.x
Meffe, G. K. (1984). Effects of abiotic disturbance on coexistence of predator-prey fish species. Ecology 65, 1525–1534. doi: 10.2307/1939132
Miller, D. A. W., Brehme, C. S., Hines, J. E., Nichols, J. D., and Fisher, R. N. (2012). Joint estimation of habitat dynamics and species interactions: disturbance reduces co-occurrence of non-native predators with an endangered toad. J. Anim. Ecol. 81, 1288–1297. doi: 10.1111/j.1365-2656.2012.02001.x
Mortenson, S. G., Weisberg, P. J., and Ralston, B. E. (2008). Do beavers promote the invasion of non-native Tamarix in the Grand Canyon riparian zone? Wetlands 28, 666–675. doi: 10.1672/07-142.1
Morzillo, A. T., and Needham, M. D. (2015). Landowner incentives and normative tolerances for managing beaver impacts. Hum. Dimens. Wildl. 20, 514–530. doi: 10.1080/10871209.2015.1083062
Mount, J. F. (1995). California Rivers and Streams: The Conflict Between Fluvial Process and Land Use. Berkeley: University of California Press. doi: 10.1525/9780520916937
Moyle, P. B. (2002). Inland fishes of California, 2nd Edn. Berkeley, CA: University of California Press.
Moyle, P. B., Israel, J. A., and Purdy, S. E. (2008). Salmon, Steelhead, and Trout in California: Status of an Emblematic Fauna. Davis, CA: Center for Watershed Sciences, University of California. Available online at: https://watershed.ucdavis.edu/pdf/SOS-Californias-Native-Fish-Crisis-Final-Report.pdf (accessed April 03, 2021).
Moyle, P. B., Katz, J. V. E., and Quinones, R. M. (2011). Rapid decline of California's native inland fishes: a status assessment. Biol. Conserv. 144, 2414–2423. doi: 10.1016/j.biocon.2011.06.002
Moyle, P. B., Kieman, J. D., Crain, P. K., and Quinones, R. M. (2013). Climate change vulnerability of native and alien freshwater fishes of California: a systematic assessment approach. PLoS ONE 8:e63883. doi: 10.1371/journal.pone.0063883
Moyle, P. B., and Light, T. (1996). Fish invasions in California: do abiotic factors determine success? Ecology 77, 1666–1670. doi: 10.2307/2265770
Moyle, P. B., and Marchetti, M. P. (2006). Predicting invasion success: freshwater fishes in California as a model. Trends Ecol. Evol. 56, 515–524. doi: 10.1641/0006-3568(2006)56[515:PISFFI]2.0.CO;2
Moyle, P. B., Quinones, R. M., Katz, J. V., and Weaver, J. (2015). Fish Species of Special Concern in California. Sacramento, CA: California Department of Fish and Wildlife. Available online at: https://wildlife.ca.gov/Conservation/SSC/Fishes (accessed November 11, 2020).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Naiman, R. J., Johnston, C. A., and Kelley, J. C. (1988). Alteration of North American streams by beaver. BioScience 38, 753–762. doi: 10.2307/1310784
Naiman, R. J., Pinay, G., Johnston, C. A., and Pastor, J. (1994). Beaver influences on the long-term biogeochemical characteristics of boreal forest drainage networks. Ecology 75, 905–921. doi: 10.2307/1939415
National Marine Fisheries Service (2012). Southern California Steelhead Recovery Plan. Long Beach, CA: Southwest Region, Protected Resources Division, 563. Available online at: https://www.fisheries.noaa.gov/resource/document/southern-california-steelhead-recovery-plan (accessed February 05, 2021).
Nicholson, E. G., Manzo, S., Devereux, Z., Morgan, T. P., Fisher, R. N., Brown, C. R., et al. (2020). Historical museum collections and contemporary population studies implicate roads and introduced predatory bullfrogs in the decline of western pond turtles. PeerJ 8:e9248. doi: 10.7717/peerj.9248
Nummi, P., Liao, W., Huet, O., Scarpulla, E., and Sundell, J. (2019). The beaver facilitates species richness and abundance of terrestrial and semi-aquatic mammals. Glob. Ecol. Conserv. 20:e00701. doi: 10.1016/j.gecco.2019.e00701
Olson, T. E., and Knopf, F. L. (1986). Naturalization of Russian olive in the western United States. West. J. Appl. For. 1, 65–69. doi: 10.1093/wjaf/1.3.65
Orsi, J. (2004). Hazardous Metropolis: Flooding and Urban Ecology in Los Angeles. Berkeley, CA: University of California Press. doi: 10.1525/california/9780520238503.001.0001
Papier, C. M., Poulos, H. M., and Kusch, A. (2019). Invasive species and carbon flux: the case of invasive beavers (Castor canadensis) in riparian Nothofagus forests of Tierra del Fuego, Chile. Clim. Change 153, 219–234. doi: 10.1007/s10584-019-02377-x
Parker, J. D., Caudill, C. C., and Hay, M. E. (2007). Beaver herbivory on aquatic plants. Oecologia 151, 616–625. doi: 10.1007/s00442-006-0618-6
Pastur, G. M., Lencinas, M. V., Escobar, J., Quiroga, P., Malmierca, L., and Lizarralde, M. (2006). Understory succession in Nothofagus forests in Tierra del Fuego (Argentina) affected by Castor canadensis. Appl. Veg. Sci 9, 143–154. doi: 10.1658/1402-2001(2006)9[143:USINFI]2.0.CO;2
Patricola, C. M., O'Brien, J. P., Risser, M. D., Rhoades, A. M., O'Brien, T. A., Ullrich, P. A., et al. (2020). Maximizing ENSO as a source of western US hydroclimate predictability. Clim. Dyn. 54, 351–372. doi: 10.1007/s00382-019-05004-8
Pilliod, D. S., Rohde, A. T., Charnley, S., Davee, R. R., Dunham, J. B., Gosnell, H., et al. (2018). Survey of beaver-related restoration practices in rangeland streams of the western USA. Environ. Manage. 61, 58–68. doi: 10.1007/s00267-017-0957-6
Pollock, M. M., Beechie, T. J., Wheaton, J. M., Jordan, C. E., Bouwes, N., Weber, N., et al. (2014). Using beaver dams to restore incised stream ecosystems. BioScience 64, 279–290. doi: 10.1093/biosci/biu036
Pollock, M. M., Lewallen, G., Woodruff, K., Jordan, C. E., and Castro, J. M., (eds.). (2015). The Beaver Restoration Guidebook: Working with Beaver to Restore Streams, Wetlands, and Floodplains, Version 1.02. Portland, OR: U.S. Fish and Wildlife Service, 189. Available online at: http://www.fws.gov/oregonfwo/ToolsForLandowners/RiverScience/Beaver.asp (accessed June 08, 2021).
Power, M. E. (1990). Effects of fish in river food webs. Science 250, 811–814. doi: 10.1126/science.250.4982.811
Ray, A. M., Rebertus, A. J., and Ray, H. L. (2001). Macrophyte succession in Minnesota beaver ponds. Can. J. Bot. 79, 487–499. doi: 10.1139/b01-018
Rees, P. A. (2001). Is there a legal obligation to reintroduce animal species into their former habitats? Oryx 35, 216–223. doi: 10.1046/j.1365-3008.2001.00178.x
Ribeiro, F., Elvira, B., Collares-Pereira, M. J., and Moyle, P. B. (2007). Life-history traits of non-native fishes in Iberian watersheds across several invasion stages: a first approach. Biol. Invasions 10, 89–102. doi: 10.1007/s10530-007-9112-2
Rinne, J. N., and Miller, D. (2006). Hydrology, geomorphology and management: implications for sustainability of native southwestern fishes. Rev. Fish. Sci. Aquac. 14, 91–110. doi: 10.1080/10641260500341379
Rosell, F., Bozser, O., Collen, P., and Parker, H. (2005). Ecological impact of beavers Castor fiber and Castor canadensis and their ability to modify ecosystems. Mamm. Rev. 35, 248–276. doi: 10.1111/j.1365-2907.2005.00067.x
Rosen, P. C., and Schwalbe, C. R. (1995). “Bullfrogs: introduced predators in southwestern wetlands,” in Our Living Resources: A Report to the Nation on the Distribution, Abundance, and Health of U.S. Plants, Animals, and Ecosystems, eds E. T. LaRoe, G. S. Farris, C. E. Puckett, P. D. Doran, and M. J. Mac (Washington, DC: U.S. Department of the Interior, National Biological Service), 452–454.
Ruscoe, W. A., Norbury, G., and Choquenot, D. (2006). “Trophic interactions among native and introduced animal species,” in Biological invasions in New Zealand. Ecological Studies, Vol. 186, eds R. B. Allen and W. G. Lee (Heidelberg: Springer-Verlag), 247–264. doi: 10.1007/3-540-30023-6_16
Schultz, J. R. (1937). A Late Quaternary mammal fauna from the tar seeps of McKittrick, California (Doctoral dissertation). California Institute of Technology, Pasadena, CA, United States.
Sher, A. A., Marshall, D. L., and Gilbert, S. A. (2000). Competition between native Populus deltoides and invasive Tamarix ramosissima and the implications for reestablishing flooding disturbance. Conserv. Biol. 14, 1744–1754. doi: 10.1111/j.1523-1739.2000.99306.x
Simberloff, D., and Von Holle, B. (1999). Positive interactions of nonindigenous species: invasional meltdown? Biol. Invasions 1, 21–32. doi: 10.1023/A:1010086329619
Smith, W. E. (1908). History of San Diego, 1542-1908. San Diego, CA: The History Company. Available online at: https://sandiegohistory.org/archives/books/smythe/ (accessed October 10, 2021).
Snodgrass, J. W., and Meffe, G. K. (1999). Habitat use and temporal dynamics of blackwater stream fishes in and adjacent to beaver ponds. Copeia 3, 628–639. doi: 10.2307/1447595
Stebbins, G. L., and Major, J. (1965). Endemism and speciation in the California flora. Ecol. Monogr. 35, 1–35. doi: 10.2307/1942216
Stephens, F. (1906). California Mammals. San Diego, CA: The West Coast Publishing Co. doi: 10.5962/bhl.title.57008
Stephenson, J. R., and Calcarone, G. M. (1999). Southern California Mountains and Foothills Assessment: Habitat and Species Conservation Issues (General Technical Report GTR-PSW-172). Albany, CA: Pacific Southwest Research Station, U.S. Forest Service, U.S. Department of Agriculture. doi: 10.2737/PSW-GTR-172
Stephenson, T. E. (1931). Shadows of Old Saddleback: Tales of the Santa Ana Mountains. Santa Ana, CA: Rasmussen Press.
Stock, C. (1992[1930]). Rancho La Brea: A Record of Pleistocene life in California, 7th Edn, Transl. by J. M. Harris. Natural History Museum of Los Angeles County, Contributions in Science Series, No. 37.
Strayer, D. L. (2012). Eight questions about invasions and ecosystem functioning. Ecol. Lett. 15, 1199–1210. doi: 10.1111/j.1461-0248.2012.01817.x
Stromberg, J. C. (2001). Restoration of riparian vegetation in the south-western United States: importance of flow regimes and fluvial dynamism. J. Arid Environ. 49,17–34. doi: 10.1006/jare.2001.0833
Swift, C. C., Duangsitti, P., Clemente, C., Hasserd, K., and Valle, L. (1997). Biology and Distribution of the Tidewater Goby, Eucyclogobius newberryi, on Vandenberg Air Force Base, Santa Barbara County, California. Final Report, U.S. National Biological Survey Cooperative Agreement No. 1445-007-94-8129 with Loyola Marymount University, Los Angeles, 121.
Swift, C. C., Haglund, T. R., Ruiz, M., and Fisher, R. N. (1993). The status and distribution of the freshwater fishes of southern California. Bull. South. Calif. Acad. Sci. 92, 101–167.
Tappe, D. T. (1942). The Status of Beavers in California. State of California Department of Natural Resources, Division of Fish and Game, Game Bulletin No. 3, 1–59.
Taylor, W. P. (1919). Notes on the mammals principally in Washington and California between the years 1853 and 1874 by Dr. James Graham Cooper. Proc. Calif. Acad. Sci. 9, 69–121.
U.S. Fish and Wildlife Service (1998). Southern steelhead Oncorhynchus mykiss habitat suitability survey of the Santa Margarita River, San Mateo and San Onofre creeks on Marine Corp Base, Camp Pendleton. Arcata, CA: Coastal California Fish and Wildlife Office, Prepared for Assistant Chief of Staff, Environmental Security.
U.S. Fish and Wildlife Service (2009). Arroyo Toad (Bufo californicus), 5-Year Review: Summary and Evaluation. Ventura, CA: U.S. Fish and Wildlife Service.
Vaughn, T. A. (1954). Mammals of the San Gabriel Mountains of California. Univ. Kans. Publ. Mus. Nat. Hist. 7, 513–582.
Veblen, T. T., and Stewart, G. H. (1982). The effects of introduced wild animals on New Zealand forests. Ann. Am. Assoc. Geogr. 72, 372–397. doi: 10.1111/j.1467-8306.1982.tb01832.x
Vitousek, P. M. (1990). Biological invasions and ecosystem processes – towards an integration of population biology and ecosystem studies. Oikos 57, 7–13. doi: 10.2307/3565731
Wake, T. A. (1999). Temporal variation in vertebrate archaeofaunas from Camp Pendleton Marine Corps Base, San Diego County, California. Pac. Coast Archaeol. Soc. Q. 35, 45–64.
Wake, T. A., and Roeder, M. (2009). A diverse Rancholabrean vertebrate microfauna from southern California includes the first fossil record of Ensatina (Ensatina eschscholtzii: Plethodontidae). Quat. Res. 72, 364–370. doi: 10.1016/j.yqres.2009.06.012
Wathen, G., Allgeier, J. E., Bouwes, N., Pollock, M. M., Schindler, D. E., and Jordan, C. E. (2019). Beaver activity increases habitat complexity and spatial partitioning by steelhead trout. Can. J. Fish. Aquat. Sci. 76, 1086–1095. doi: 10.1139/cjfas-2018-0171
Webb, R. H., Leake, S. A., and Turner, R. M. (2007). The Ribbon of Green—Change in Riparian Vegetation in the Southwestern United States. Tucson, AZ: University of Arizona Press.
Williams, B. L., Hanifin, C. T., Brodie, E. D., and Brodie, I. I. I. E. D. (2010). Tetrodotoxin affects survival probability of rough-skinned newts (Taricha granulosa) faced with TTX-resistant garter snake predators (Thamnophis sirtalis). Chemoecology 20, 285–290. doi: 10.1007/s00049-010-0057-z
Wilson, B. S., and McEwen, G. M. (1998). Will continued monitoring of beaver-damaged resources minimize future damage? Proc. Vertebr. Pest Conf. 18, 213–220. doi: 10.5070/V418110255
Wright, J. P., Gurney, W. S., and Jones, C. G. (2004). Patch dynamics in a landscape modified by ecosystem engineers. Oikos 105, 336–348. doi: 10.1111/j.0030-1299.2004.12654.x
Wright, J. P., and Jones, C. G. (2006). The concept of organisms as ecosystem engineers ten years on: progress, limitations, and challenges. BioScience 56, 203–209. doi: 10.1641/0006-3568(2006)056[0203:TCOOAE]2.0.CO;2
Wright, J. P., Jones, C. G., Boeken, B., and Shachak, M. (2006). Predictability of ecosystem engineering effects on species richness across environmental variability and spatial scales. J. Ecol. 94, 815–824. doi: 10.1111/j.1365-2745.2006.01132.x
Keywords: invasive species, hydrology, ecosystem, ecoregion, Southern California, invasional meltdown
Citation: Richmond JQ, Swift CC, Wake TA, Brehme CS, Preston KL, Kus BE, Ervin EL, Tremor S, Matsuda T and Fisher RN (2021) Impacts of a Non-indigenous Ecosystem Engineer, the American Beaver (Castor canadensis), in a Biodiversity Hotspot. Front. Conserv. Sci. 2:752400. doi: 10.3389/fcosc.2021.752400
Received: 03 August 2021; Accepted: 29 October 2021;
Published: 18 November 2021.
Edited by:
Anthony J. Giordano, Society for the Preservation of Endangered Carnivores and their International Ecological Study (SPECIES), United StatesReviewed by:
Bill Bateman, Curtin University, AustraliaRurik List, Autonomous Metropolitan University, Mexico
Copyright © 2021 Richmond, Swift, Wake, Brehme, Preston, Kus, Ervin, Tremor, Matsuda and Fisher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Q. Richmond, anJpY2htb25kQHVzZ3MuZ292
†Retired
 Jonathan Q. Richmond
Jonathan Q. Richmond Camm C. Swift2†
Camm C. Swift2† Cheryl S. Brehme
Cheryl S. Brehme Barbara E. Kus
Barbara E. Kus Robert N. Fisher
Robert N. Fisher