
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci. , 17 September 2021
Sec. Global Biodiversity Threats
Volume 2 - 2021 | https://doi.org/10.3389/fcosc.2021.742036
This article is part of the Research Topic Ladybirds: Conservation, Ecology and Interactions with Other Organisms View all 12 articles
 Louis S. Hesler*
Louis S. Hesler* Eric A. Beckendorf
Eric A. BeckendorfLady (= ladybird) beetles (Coleoptera: Coccinellidae) provide agroecosystem services as major predators of aphids and other pests of field crops. Several native coccinellids in North America have declined in association with the introduction of invasive species of lady beetles. In particular, populations of three native species declined drastically (Coccinella transversoguttata richardsoni) or effectively disappeared (Coccinella novemnotata, Adalia bipunctata) from agricultural landscapes in eastern South Dakota, U.S.A., following establishment of an invasive coccinellid (Coccinella septempunctata) in the 1980s. Since then, two other non-native coccinellids (Harmonia axyridis and Hippodamia variegata) have established in eastern South Dakota, but long-term analysis of their impact on the aphidophagous coccinellid guild is lacking. This paper summarizes long-term results from 14 years (2007–2020) of sampling coccinellids by sweepnet and timed searches in five field crops and restored prairie in eastern South Dakota. In all, 17,338 aphidophagous coccinellids comprising 10 species were sampled. Two invasive species (Coc. septempunctata, Har. axyridis) were the third- and fourth-most abundant species, respectively. The seven most abundant species constituted 99% of all coccinellids sampled and were recorded from all six habitats. However, coccinellid species ranged considerably in their evenness of habitat use, resulting in differences in rank abundance among habitats. Coccinellid assemblages were similar for alfalfa and winter wheat, but not for other habitats, which possessed distinct coccinellid assemblages based on rank abundance. Annual abundance of coccinellids varied considerably within habitats, but declining trends were evident from significant negative regressions in annual abundance for adult and immature coccinellids in corn and adults in soybean. As a group, native adult coccinellids showed a significant declining trend in corn but not in other habitats, whereas trends for non-native adult coccinellids were non-significant in all habitats. Sample rates of coccinellids in alfalfa, spring grains, and corn in this study were 74, 26, and 6%, respectively, compared to that of a previous study from the region, further indicating substantial decreases in coccinellid abundance. Possible explanations and implications for observed patterns in coccinellid diversity and individual species abundances in field crops and restored prairie of eastern South Dakota are discussed with respect to prey, agronomic trends, and landscape factors.
Eastern South Dakota, USA, was historically part of the tallgrass prairie portion of the North American Great Plains before conversion of a large majority of land to agriculture in the mid-1800s (Maizel et al., 1998). Less than 14% of tallgrass prairie remains in North America (Samson et al., 2004). In its place, field crops—mainly corn, soybeans, alfalfa, and small grains such as wheat and oats—have become a major component of the landscape (Dumke and Dobbs, 1999; Rashford et al., 2011; Wright and Wimberly, 2013).
Each of the field crops in eastern South Dakota is colonized annually by its own complex of arthropod pests that includes aphids, defoliators, and stem borers (Bing et al., 1999; Hesler et al., 2000, 2005, 2018; Hutchinson et al., 2010; Lundgren et al., 2013; Supplementary Table 1). In turn, these pests have a complex of natural enemies, including a guild of predacious lady (=ladybird) beetles, or coccinellids (Elliott and Kieckhefer, 1990a; Elliott et al., 1996; Lundgren et al., 2013; Hesler, 2014). Coccinellids contribute substantially to pest suppression, with both adults and larvae preying upon pests (Obrycki and Kring, 1998; Michaud, 2012). Thus, their conservation is vital to sustainable agriculture, and long-term studies on their effectiveness and preservation are important (Obrycki and Kring, 1998; Iperti, 1999; Honek et al., 2014).
Extensive surveys of coccinellids of east-central South Dakota commenced in the 1970s in small grains, alfalfa, and corn (Kieckhefer et al., 1992). Six species—Hippodamia convergens, Hippodamia parenthesis, Hippodamia tredecimpunctata tibialis, Coleomegilla maculata lengi, Coccinella transversoguttata richardsoni, and Cycloneda munda—were common to all three crops, whereas Adalia bipunctata was only sampled from corn. Coccinella novemnotata, a species of current conservation concern (Harmon et al., 2007), was only sampled in low numbers on adhesive traps adjacent to fields.
However, coccinellid assemblages of eastern South Dakota cropland have been subjected to various changes since the Kieckhefer et al. (1992) survey. These changes include establishment of new coccinellid species, introduction of an invasive pest, and changes in vegetational composition of the landscape. For instance, three non-native coccinellids have been detected in east-central South Dakota. The first species, Coccinella septempunctata, established in the region in 1987 and quickly became prevalent in alfalfa, spring grains, and corn (Elliott et al., 1996). Moreover, its establishment was associated with significant change in native coccinellid community structure that related to reduced abundances of A. bipunctata and Coc. transversoguttata richardsoni in those crops (Elliott et al., 1996). Two other non-native coccinellids, Harmonia axyridis and Hippodamia variegata, were detected in eastern South Dakota in 1996 (Hesler et al., 2001) and in 2010 (Hesler and Lundgren, 2011), respectively, but long-term analysis of their impact on coccinellid assemblages is lacking. Both species are generalists that compete with native coccinellids in various habitats (Koch, 2003; Gardiner et al., 2011). Harmonia axyridis is considered invasive and has often become dominant in coccinellid assemblages in North America, whereas Hip. variegata establishes relatively low abundance (Koch, 2003; Lucas et al., 2007; Gardiner et al., 2009; Lamb et al., 2019). Coccinella septempunctata has typically remained relatively abundant in various North American habitats following the addition of other invasive coccinellids (Brown, 2003; Alyohkin and Sewell, 2004; Lucas et al., 2007; Gardiner et al., 2009).
Before 2000, soybeans in South Dakota and other parts of the northern Great Plains were characterized by a set of arthropod pests that caused only sporadic economic damage (Lambert and Tyler, 1999; Ragsdale et al., 2011). Consequently, field studies of coccinellids in eastern South Dakota deliberately omitted soybean due to its lack of pests (Kieckhefer et al., 1992; Hesler et al., 2000). However, in 2000, establishment of an invasive pest, the soybean aphid (Aphis glycines), greatly increased the risk of economic injury to soybean and led to enormous increases in insecticide application in northern production areas (Ragsdale et al., 2011). Despite widespread insecticide use against soybean aphid, a large complex of aphid natural enemies, including both native and non-native coccinellids, became associated with soybean (Schmidt et al., 2008; Gardiner et al., 2009; Lundgren et al., 2013; Hesler, 2014).
Finally, regional cropping patterns changed substantially over the last 40 years. Notably, plantings of corn and soybean continue to steadily increase at the expense of spring grains and winter wheat and by additional conversion of grassland and wetland [Johnston, 2013; Wright and Wimberly, 2013; NASS (National Agricultural Statistics Service), 2021]. Such changes in land use may impact coccinellid assemblages and influence biological control of insect pests in cropping systems (Bianchi et al., 2007; Landis et al., 2008; Gardiner et al., 2009; Cox et al., 2014). In particular, increasing dominance by a crop such as corn simplifies agricultural landscape diversity, which reduces the supply of natural enemies available in an area (Landis et al., 2008).
Consequently, only limited amounts of tallgrass prairie have been restored (Samson et al., 2004). Some researchers have hypothesized that tallgrass prairie and other natural areas may serve as alternative, refuge habitats for coccinellids (Hesler and Petersen, 2008; Diepenbrock and Finke, 2013). To date, relatively modest numbers of coccinellids have been documented in tallgrass prairie (Hesler et al., 2005; Hesler and Petersen, 2008; Diepenbrock and Finke, 2013), but further comparison of their utilization by coccinellids vs. field-crop habitats is warranted.
New studies on coccinellid assemblages in eastern South Dakota are sorely needed in light of these major changes in the agricultural landscape. Long-term sampling conducted at the same location is necessary to reliably delineate trends in fauna that may change relatively slowly but exhibit high temporal variability (Elliott et al., 1996; Strayer et al., 2006; Honek et al., 2016). In particular, we would expect long-term monitoring of coccinellids in eastern South Dakota to establish the following outcomes, namely that (1) Coc. septempunctata would maintain a relatively moderate to high abundance, (2) Har. axyridis would become one of the dominant species in coccinellid assemblages, (3) Hip. variegata would establish low (i.e., 1–5%) relative abundance, (4) at least one native coccinellid would show a significant decline in abundance over the course of study, and (5) species in corn and soybean would be favored by the expanding land use for these two crops. To this end, we sampled five field crops and restored prairie over a 14-year period to assess coccinellid assemblages amid the changing landscape in eastern South Dakota. We also compared the sampling rate and composition of coccinellids in our study with earlier, long term studies from eastern South Dakota in order to assess our findings within a recent historical context.
Coccinellids were sampled from mid-May through early September during the years 2007 through 2020 within a roughly 8-km2 area of Brookings Plat, Brookings County, in east-central South Dakota, USA. This area was within a triangle bounded by the three sampling sites in east-central South Dakota used by Kieckhefer et al. (1992) and Elliott et al. (1996) for previous long-term monitoring of coccinellid populations. Our study sampled six habitats, i.e., five types of field crops and restored prairie. Sample sites for field crops consisted of 0.5 to 2-ha plots that were located at the north end of the 8-km2 area and roughly 1km N of the city of Brookings, South Dakota. The five field crops included a perennial crop, i.e., alfalfa, and four annual row crops: spring-seeded small grains (wheat in 2007, wheat and oats in 2008, and oats in following years), winter wheat (fall-planted), corn, and soybean. Alfalfa plots ranged from 2 to 6-years-old. The crops were grown using standard agronomic practices for the region (Pikul et al., 2008) that included fertilization of annual crops and one or more herbicide applications in the first half of each growing season. None of the plots received foliar insecticide spray, but corn seed was typically treated with a neonicotinoid insecticide, which had become routine practice since 2004 [USGS (U.S. Geological Survey), 2014]. Three replicate plots of each field crop were typically sampled, but in 2007–2009 only 1 or 2 plots of a particular crop were available (Table 1, Supplementary Table 2).
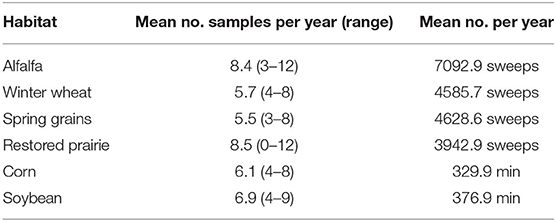
Table 1. Mean number of sampling occasions per year in alfalfa, winter wheat, spring grains and restored prairie (sweepnet samples) and minutes sampled in corn and soybeans (timed visual searches) from 2007 to 2020.
Two restored prairie tracts that consisted of native grasses and forbs were sampled over the course of the study. A list of dominant plants at the prairies are listed in Supplementary Table 3. The first, “Brookings Prairie,” was a 16-ha tract that was sampled from 2007 through 2013. It was the nearest, suitable native prairie tract during that period and located at the south end of the 8-km2 area. However, by 2014, the first prairie tract had accumulated excessive dry plant residue due to lack of burning and grazing. In 2014, sampling was switched to a 2-ha tract of restored prairie established in 2000 at the north end of the 8-km2 area (“North Prairie”) and that tract was spring burned every 2–3 years.
Sampling of coccinellids was conducted by sweepnetting spring grains, winter wheat, alfalfa, and prairie and by timed searching of corn and soybean. Searches and sweeps were conducted between 09:00 and 16:00 h when sunny to mostly sunny and wind speed <32 km h−1 (Kieckhefer et al., 1992). Sampling was conducted in a manner that avoided casting a shadow on the sample area to avoid startling and dispersing coccinellids (Hesler and Kieckhefer, 2008). Sweepnetting consisted of 180°-sweeps with a 38-cm (diam.), mesh net along 50-m transects within individual plots of spring grain, winter wheat, alfalfa, and restored prairie. Three hundred sweeps were made per plot on each sampling date in the field crops, whereas sampling consisted of 300 (2007–2008) or 900 sweeps per date (other years) at Brookings Prairie and 300 sweeps at North Prairie. We made 900 sweeps in the Brookings Prairie (16 ha) in later years to equate to the 300 sweeps × 3 replicates in other habitats, but the smaller size of the North Prairie (2 ha) constrained us to 300 sweeps per date. Sweep samples from each plot were placed into a plastic bag and stored in a freezer until coccinellids could be identified and counted at a later date. Adults were identified to species based on descriptions in Gordon (1985) and Gordon and Vandenberg (1991). Immature coccinellids (larvae and pupae) were tallied without species identification.
Timed sampling consisted of a 20-min search per plot while walking between inner rows of corn or soybean plots and tallying all coccinellids by species seen on plants. Our sampling was biased toward searching for lady beetles in the mid- to upper canopy of corn and soybean, where a majority of aphids tends to be in these crops (Bing et al., 1999; McCornack et al., 2008; Prescott and Andow, 2016). As the season progresses, lady beetles can become increasingly difficult to see in the uppermost canopy of corn (over 2.5 m height) and the mid-canopy of soybean (density of foliage), thereby reducing sampling efficiency by an undetermined amount (Prescott and Andow, 2016). Identifications of adults were made in the field based on familiarity with species commonly encountered in our area.
Sampling among the different habitats varied during the season, similar to the timing of sampling in Kieckhefer et al. (1992) and Elliott et al. (1996). Alfalfa and winter wheat were the first habitats to be sampled each year, with first sample dates ranging from mid-May to early June. These were followed by availability of spring grains and prairie in early to mid-June. Alfalfa was often available for sampling throughout the summer, but its cutting and baling precluded sampling during some weeks. Dry conditions that prevented adequate regrowth of alfalfa occasionally delayed or prevented sampling it later in summer. Winter wheat and spring grains were sampled until their respective grain maturities in mid-summer. Corn and soybean were sampled in basically the latter half of summer, ranging from late June to early September, when prey of coccinellids were available in these crops.
Efforts were made to sample habitats weekly, and from one to four habitats may have been sampled in any given week. However, various abiotic conditions and logistical considerations imposed limitations on sampling frequency. For example, periods of excessive wind, extensive cloud cover, and rain, herbicide application, and competing workload occasionally precluded sampling within a week, resulting in different numbers of sample dates among habitats each year (Table 1, Supplementary Table 2).
The numbers of adults with individual species and immature coccinellids in aggregate were summed across habitats, replicates, and years to determine their relative abundance. In addition, annual abundances by habitat were summed across species for adult and immature coccinellids and used to test whether annual abundance was at equilibrium within each habitat (Lamb et al., 2019). Trends in abundance over the 14-year sampling period were tested by using the slopes of a linear regression model relating annual abundance by time in years, with a significant positive or negative relationship between abundance and years indicating a lack of equilibrium in coccinellids within a particular habitat (Lamb et al., 2019). Tests of multiple species from a habitat were not considered independent, and therefore adjusted α values were used in individual tests (Hochberg, 1988).
Abundance was also averaged across all sampling dates for each habitat, and measurements were standardized by sampling method to account for unevenness in sampling frequency among habitats and years. The standardized measures of abundance were used to determine the breadth of habitat use for each species (Southwood and Henderson, 2000; Lamb et al., 2019). Breadth of habitat use was calculated by using the reciprocal Simpson-Yule index (Southwood and Henderson, 2000):
where pi is the proportion of species i in each of the m ≥ 2 habitats. The value of D ranges from 1 when a species is present in only one of the habitats sampled and reaches a maximum of m when a species is distributed equally among the m habitats. Breadth in habitat use among coccinellids was calculated individually for adult species and for immatures across species and reported separately for the four habitats sampled by sweeping (alfalfa, winter wheat, spring small grains, and restored prairie) and the two sampled by timed searching (corn and soybean). As such, D could range from 1 to 4 for the group of habitats sampled by sweeping and from 1 to 2 for corn and soybean.
Average abundance of coccinellids over the 14-year period was also used to determine their overall rank in abundance by habitat. Rank abundance of species may be used as a measure to test for similarity, or concordance, in the structure of coccinellid assemblages among habitats (Southwood and Henderson, 2000; Legendre, 2005). Concordance was determined by calculating Kendall's coefficient (W, Kendall and Gibbons, 1990; Legendre, 2005; Zar, 2010) and accounted for zero abundance of a species and ties in rank abundance within a particular habitat (Legendre, 2005). The value of W may range from 0, when there is no correlation in ranks among habitats, to 1, when ranks completely agree. Calculation of Friedman's χ2 = M(n – 1)W was used to determine if the concordance value differed significantly from zero (Zar, 2010). W is related to the pairwise Spearman correlations in rank abundance (Legendre, 2005). Hence, following a significant outcome for W, a Spearman correlation matrix was computed among the ranks for all habitat pairs using an adjusted α (Hochberg, 1988; Legendre, 2005), and the Spearman correlations were interpreted as similarity indices of rank abundance of coccinellids among habitats (Legendre, 2005). Spearman correlations were used as thresholds for developing a dendrogram to delineate clusters of habitats with concordant ranks of coccinellid assemblages (Southwood and Henderson, 2000; Legendre, 2005; Honek et al., 2014).
Finally, to evaluate abundance of coccinellids in our study against that reported from previous studies in the region, we compared the rates at which adult coccinellids were sampled between our study and that of Elliott et al. (1996). Rates at which coccinellids were sampled by Elliott et al. (1996) were derived by using the total number of adult coccinellids sampled in each crop (their Table 2) and dividing that by the total amount of sampling within each crop, which was calculated by multiplying mean number of samples per year by mean number of sweeps per year (alfalfa and spring grains) or minutes of searching per year (corn) from their Table 1.
In all, 17,338 predacious coccinellids comprising 10 species were sampled (Table 2). Hippodamia convergens, Col. maculata lengi, Har. axyridis, Coc. septempunctata, Hip. parenthesis, Hip. tredecimpunctata tibialis, and Cyc. munda collectively constituted nearly all individuals sampled, and each of these species occurred in all habitats. Two of the thee invasive species, Har. axyridis and Coc. septempunctata, ranked third and fourth in abundance, respectively. The non-native Hip. variegata accounted for only nine of all coccinellids sampled, and it was found in alfalfa, winter wheat, and soybean. Two Hippodamia glacialis were sampled from alfalfa, and a single Anisosticta bitriangularis was sampled from restored prairie.
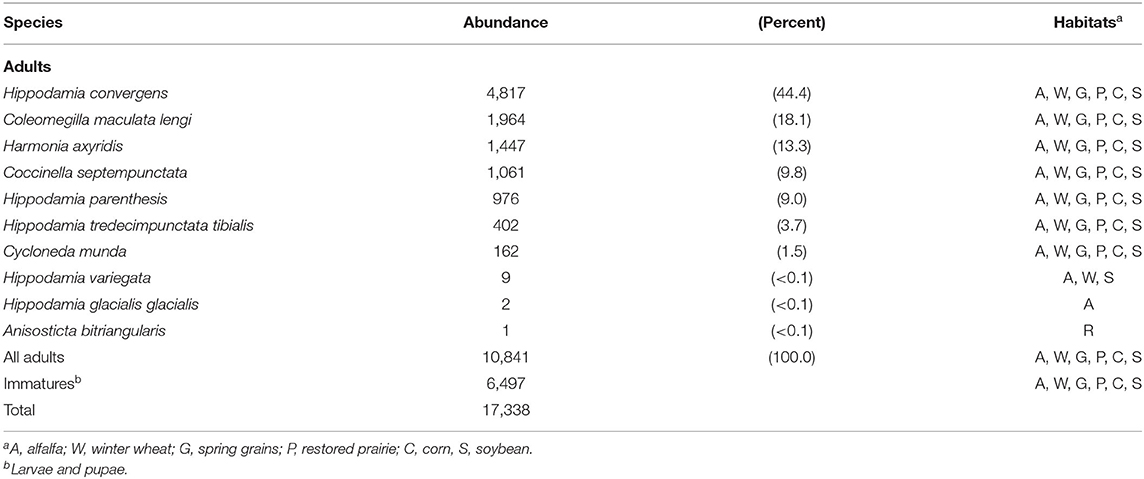
Table 2. Number of predacious coccinellids sampled from 2007 to 2020 in field crops and restored prairie in east-central South Dakota, USA.
As a group, average abundance of coccinellids fluctuated considerably from year to year in each of the six habitats. All habitats were associated with declining trends in annual abundance of total adult coccinellids (i.e., across species) over the 14 years of sampling (Table 3), with significant negative regressions in annual abundance among years for adult and immature coccinellids in corn and adults in soybean (Figure 1). As a group, native adult coccinellids showed a significant declining trend in corn but not in other habitats, whereas trends for non-native adult coccinellids were non-significant in all habitats (Table 3). Individual species of either native or non-native adult coccinellids showed no significant trends in abundance over the years (Table 3).
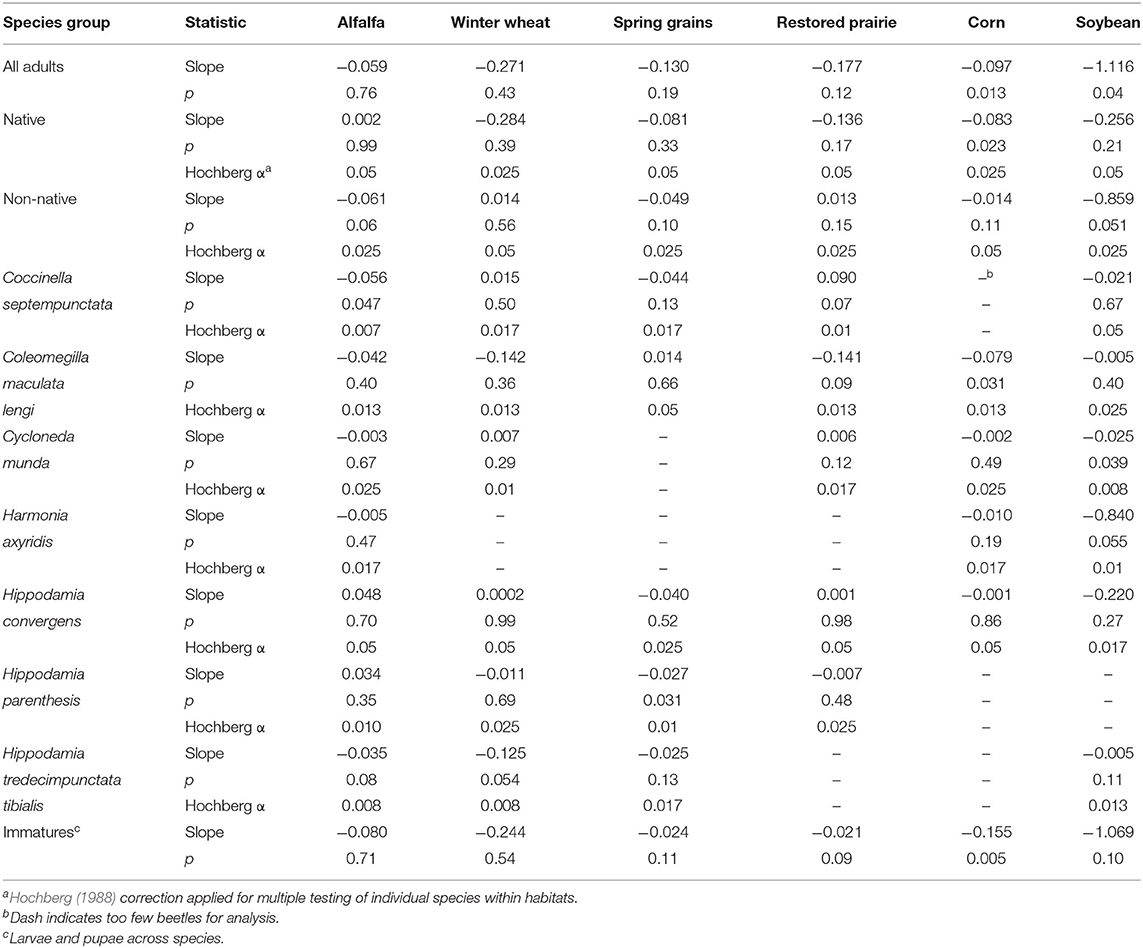
Table 3. Slopes of linear regressions of annual abundance of adult coccinellids by species groups across years of sampling in six habitats.
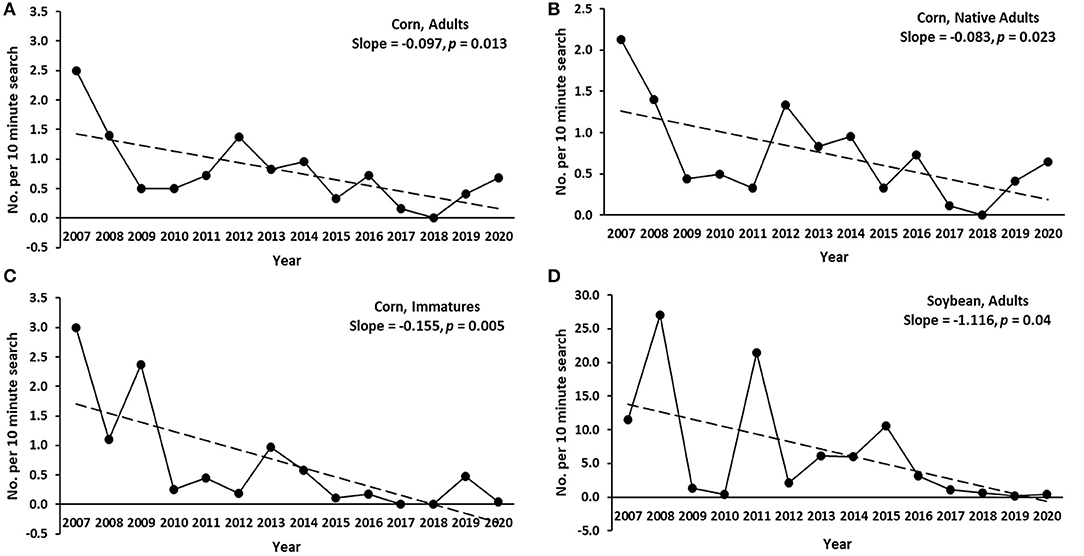
Figure 1. Significant declining trends in annual coccinellid abundance (average no. of coccinellids per 10-min search) in corn and soybean in east-central South Dakota, with slopes and p-values of linear regressions for the respective trend lines. (A) Corn, adults across species. (B) Adults of native species. (C) Corn, immatures (larvae and pupae) across species. (D) Soybean, adults across species.
In habitats sampled by sweepnetting, abundances of adult coccinellids by individual species were generally highest in alfalfa and winter wheat, the two earliest crops, and followed in abundance by spring grains and then restored prairie, except that Cyc. munda was more abundant in prairie than spring grains (Table 4). This trend was reflected by evenness values that ranged from moderately high for Cyc. munda (D = 2.91) to moderately low for Har. axyridis (D = 2.09). Similarly, immature coccinellids had moderately low evenness among habitats (D = 2.33), reflected by relatively high abundance in alfalfa and winter wheat, moderate abundance in spring grains, and low abundance in restored prairie.
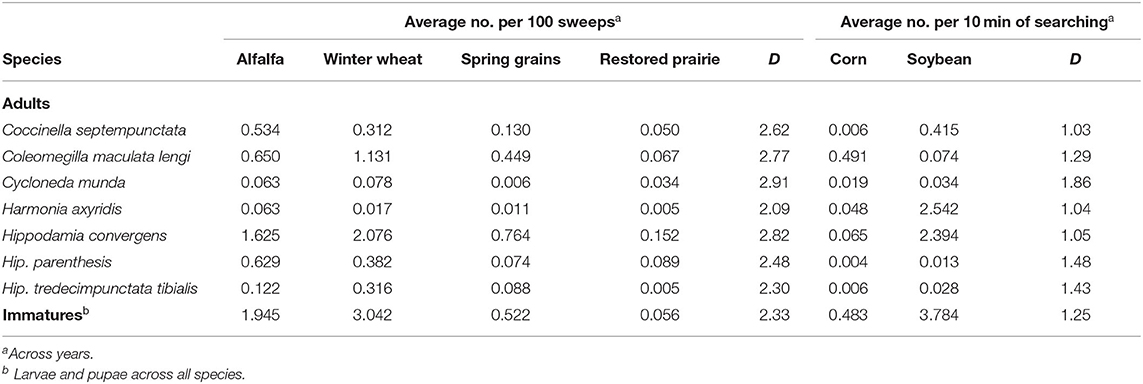
Table 4. Abundance of predacious coccinellids and evenness (D) in their habitat use among selected field crops and restored prairie in east-central South Dakota, 2007–2020.
Some adult coccinellids were particularly uneven (D = 1.03–1.48) between habitats sampled by timed searches, with abundance of most species skewed toward soybean, except that Col. maculata lengi had higher abundance in corn and Cyc. munda (D = 1.86) was sampled roughly equally in both crops (Table 4). Immature coccinellids reflected the general trend of adults, with particularly high abundance in soybean and relatively low abundance in corn (D = 1.25).
The rank abundances of coccinellid species were significantly though only moderately concordant among habitats (W = 0.541; χ2 = 19.5, df = 6, p = 0.003). Post-hoc Spearman correlation tests (Supplementary Table 4) revealed that concordance was derived mainly from significantly high similarity in rank abundances of adult coccinellids between alfalfa and winter wheat (Figure 2). Spring grains had a high but non-significant similarity to the alfalfa-winter wheat group. Likewise, restored prairie had relatively high but non-significant similarity in species composition to the alfalfa-small grains group. Corn and soybean showed a modest, non-significant similarity in rank abundance that was distinct from that of alfalfa, winter wheat, spring grains, and restored prairie.
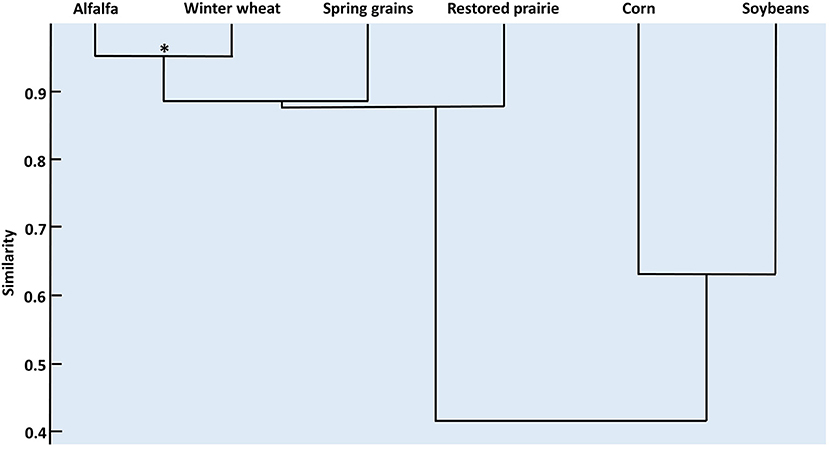
Figure 2. Dendrogram depicting the relationships among coccinellid assemblages in six habitats in east-central South Dakota based on correlations of species rank abundance. *p < 0.001.
A comparison of the rates of at which coccinellids were sampled between our study and that of Elliott et al. (1996) showed that we sampled adult coccinellids at lower rates that varied by crop (Table 5). Specifically, we sampled coccinellids at 73.7% of their rate in alfalfa (adult coccinellids per 100 sweeps), roughly one-quarter of their rate in small grains (adults per 100 sweeps), and only 5.6% of that in corn (per 10 min. search).

Table 5. Sample rates of adult coccinellids among habitats in two studies from east-central South Dakota, USA.
The six habitats in our study shared seven coccinellid species that accounted for >99% of all coccinellids sampled (Table 2). Kieckhefer et al. (1992) also found that seven coccinellid species were predominant in alfalfa, spring grains, and corn in east-central South Dakota. Five species were common to their study and ours (Col. maculata lengi, Cyc. munda, Hip. convergens, Hip. parenthesis, and Hip. tredecimpunctata tibialis), whereas A. bipunctata and Coc. transversoguttata richardsoni were only found in their studies. Elliott et al. (1996) subsequently reported establishment of Coc. septempunctata in alfalfa, spring grains and corn in east-central South Dakota, and detected significant decreases in abundance of A. bipunctata and Coc. transversoguttata richardsoni following its establishment. There have been no subsequent reports of A. bipunctata from eastern South Dakota, and Coc. transversoguttata richardsoni has not been reported there since 1993 (Hesler et al., 2005; Hesler and Kieckhefer, 2008; Hesler and Petersen, 2008). Thus, it is likely that A. bipunctata and Coc. transversoguttata richardsoni have become locally extinct in east-central South Dakota.
Three non-native species (Coc. septempunctata, Har. axyridis, and Hip. variegata) comprised 23.1% of all coccinellids that we sampled. Coc. septempunctata, the first invasive to establish in eastern South Dakota, remained relatively abundant during the 14 years of our study, consistent with our prediction. Harmonia axyridis, first detected in east-central South Dakota in 1996, had become third-most abundant coccinellid overall, which is similar to that reported from earlier surveys following its establishment in east-central South Dakota (Hesler and Petersen, 2008; Hesler, 2014). Thus, abundance of Har. axyridis was also in accord with our predictions, but its abundance pattern depended on habitat (see below). In contrast, only seven Hip. variegata, the latest non-native to arrive in South Dakota (Hesler and Lundgren, 2011), were sampled in our study, contrary to our prediction that it would establish at least low abundance. Many non-native species, such as Hip. variegata currently in eastern South Dakota, persist indefinitely at very low population levels and may be classified as “sleeper populations” due to undetermined biotic or abiotic conditions that limit their populations (Spear et al., 2021).
Hippodamia variegata has been associated with a negative impact on population levels of a native species, Cyc. munda (Lamb et al., 2019). Cycloneda munda accounted for 1.5% of coccinellids sampled in our study, comparable to its previous 1% abundance (Kieckhefer et al., 1992). Furthermore, it showed no significant population trends in any of the six habitats that we surveyed (Table 4). Nonetheless, additional research is needed to monitor the abundance of Hip. variegata and for potential adverse impacts it may have on Cyc. munda and other coccinellids in eastern South Dakota.
Although all six habitats shared the seven most abundant coccinellids, different patterns of their abundance were evident among habitats. Cycloneda munda was consistently sampled at low but even rates among habitats, and four species (Col. maculata lengi, Coc. septempunctata, Hip. convergens, and Hip. parenthesis) were sampled at high to moderately high rates in four of the six habitats, consistent with their generalist use patterns in previous studies (Elliott et al., 1996; Turnock et al., 2003; Hesler and Kieckhefer, 2008). In comparison, Har. axyridis was sampled at high rates mainly in soybean, indicative of a high degree of specialization among the six habitats in this study. This high degree of habitat specialization by Har. axyridis contrasts with other reports of its relatively even (Lamb et al., 2019) and general habitat use (Koch, 2003; Hesler et al., 2004; Hesler and Kieckhefer, 2008).
Alfalfa, winter wheat, and spring grains were similar in rank abundance, but coccinellids were sampled at higher rates in alfalfa and winter wheat. There was a lack of similarity in rank abundance of coccinellids between alfalfa and winter wheat and the other four habitats. Coccinellids were abundant in soybean, but particularly low in abundance in corn and restored prairie. Previous long-term studies of coccinellids in east-central South Dakota found correlations in rank abundance of adult and immature coccinellids were strongest between alfalfa and spring grains, intermediate between spring grains and corn, and weakest between alfalfa and corn (Kieckhefer et al., 1992). However, short-term studies in such habitats within the region have failed to find similarities in coccinellid abundance among crops (Hesler and Kieckhefer, 2008; Prescott and Andow, 2016), which may be indicative of the large year-to-year fluctuations in abundance of coccinellid species and temporal sensitivity in the analysis of coccinellid assemblages (Elliott and Kieckhefer, 1990b; Elliott et al., 1996).
Our results showed declines in coccinellid abundance during the tenure of our study and also decreased abundance when compared to a previous long-term survey in east-central South Dakota (Elliott et al., 1996). Declines in coccinellid abundance during our study were significant for both adult and immature coccinellids in corn and for adults in soybean. Decreased abundance relative to earlier studies was most striking in corn, substantial in spring grains, and notable, though less severe, in alfalfa. Decreased prey availability in various crops might have been responsible for declining trends in coccinellid abundance during our study and for discrepancies in sample rates between our study and Elliott et al. (1996). However, it should be noted that neither study quantified prey, but nonetheless, discussion about the prey within our crop plots and information from recent studies in the various crops may help to explain trends in coccinellid abundance between studies.
Two common pests of alfalfa between our study and Elliott et al. (1996) have been the pea aphid (Acyrthosiphum pisum) and the alfalfa weevil (Hypera postica), which occasionally reach economic levels in eastern South Dakota (Catangui et al., 2002). Both are prey for coccinellids, but aphids are preferred over weevil larvae (Kalaskar and Evans, 2001). We did not find published data regarding recent long-term trends in alfalfa weevil populations in South Dakota, but pea aphid abundance declined from 1 to 30% between 2005 and 2019 as measured by a suction trap network across the Midwestern U.S. that included eastern South Dakota (Crossley et al., 2021). Day and Tatman (2006) suggested that widespread establishment of Aphidius spp. of parasitoid wasps in the U.S. may have reduced pea aphid populations in alfalfa, with subsequent reductions in coccinellids, although they acknowledged this has not been documented by published studies.
In spring grains, various species of cereal aphids (Hemiptera: Aphididae) have been the main pests, primarily as vectors of viruses that are responsible for diseases generically known as barley yellow dwarf (Hesler et al., 2018). However, these aphids rarely build sufficient numbers to cause yield loss directly in east-central South Dakota (Hesler et al., 2000, 2018). Abundance, as measured by suction traps, showed a decline of 1–10% for two common cereal aphids (Rhopalosiphim padi and Schizaphis graminum) but increases ranging up to 29% for a third species (Sitobion avenae) (Crossley et al., 2021).
Early-season colonization of alfalfa, spring grains and winter wheat by coccinellids may be a trade-off between crop phenology and crop extensiveness. In terms of phenology, winter wheat is available for colonization by coccinellids and their prey earlier than spring grains. However, spring grains have historically been roughly six times more extensive than winter wheat in the agricultural landscape of east-central South Dakota, although both types of crops have steadily declined as a proportion of farmland over the last four decades [NASS (National Agricultural Statistics Service), 2021]. Thus, the much greater amount of spring grains may have considerably offset the earlier availability of winter wheat, such that in the study by Elliott et al. (1996), fields of spring grains would have been a major early-season sink for coccinellids and their prey. In contrast, spring grains and winter wheat made up small but comparable land uses at our research site, and thus favored coccinellid colonization of winter wheat due to its earlier availability. Indeed, the combined rate of adult coccinellid capture in spring grains and winter wheat (5.8%) in our study was comparable to that by Elliott et al. (1996) in spring grains alone (5.9%) (Table 5).
The corn leaf aphid (CLA, Rhopalosiphum maidis) and European corn borer (ECB, Ostrinia nubilalis) have historically been common pests of corn in the Midwestern U.S., including South Dakota (Bing et al., 1999; Hutchinson et al., 2010). We infrequently observed CLA and the ECB or its damage in eastern South Dakota corn fields during the period of our study. Corn hybrids with ECB resistance have become increasingly common since 1996 in the Midwestern U.S., including eastern South Dakota (Fernandez-Cornejo et al., 2014, Perry and Moschini, 2020), and the widespread planting of ECB-resistant corn has decreased levels of this pest across the Midwest due to areawide suppression (Hutchinson et al., 2010).
The near absence of CLA in our corn plots was first noticed in 2010, which corresponded temporally to lower annual abundance of coccinellids since 2009 in corn (Figure 1). Concurrently, CLA captures in suction traps declined 11 to 30% in the Midwest between 2005 and 2019 (Crossley et al., 2021). It was not clear why CLA populations decreased, but factors either within or outside of our study area may have been responsible. For instance, the development of corn hybrids highly resistant to CLA could have suppressed populations of this pest, but unlike ECB resistance, we have not found evidence that CLA resistance had been intentionally bred into commercial corn hybrids used in the USA.
Alternatively, factors outside of our study area may have led to decreased CLA infestations of corn in our study. CLA infestations in eastern South Dakota originate from immigrants of populations on crops such as sorghum in the southern United States (Irwin and Thresh, 1988). A recent upsurge in insecticide applications against the invasive sugarcane aphid (Melanaphis sacchari) on sorghum in Mexico and south-central U.S.A. (Bowling et al., 2016) may have concomitantly suppressed co-infestations by CLA and thus diminished migrant populations. Additional research is needed to delineate factors underlying the regional scarcity of CLA.
Soybean aphid had been a major pest of soybean in the Midwestern United States since 2000 (Ragsdale et al., 2011) and a major prey item of coccinellids in that crop (Fox et al., 2004; Schmidt et al., 2008). However, populations of soybean aphid have decreased substantially over the last 10–15 years in the region (Bahlai et al., 2015; Crossley et al., 2021; Hesler and Beckendorf, 2021), and this temporally corresponds to significant declines of adult coccinellids in soybeans in our study (Figure 1). Comparisons with earlier surveys of coccinellids in eastern South Dakota are not available because historically the crop lacked significant arthropod pests and robust predator populations (Ragsdale et al., 2011; Hesler, 2014).
Beyond considerations of prey availability in various crops, markedly lower sampling rates could have been due to factors such as field size and various landscape characteristics. Whereas our crop plots were generally between 0.5 to 2-ha, Elliott et al. (1996) sampled coccinellids from commercial crop fields, which were typically >10 ha in east-central South Dakota [NASS (National Agricultural Statistics Service), 2021]. Literature on the effect of field size on coccinellid abundance is sparse and often confounded with larger landscape factors. Some studies suggest that densities of coccinellids are lower near edges compared to the interior of fields (Olson and Andow, 2008; Caballero-López et al., 2012). Thus, while we avoided sampling from edges of our plots, their greater edge-to-area ratio could have lowered coccinellid densities amid the interiors. Among many tradeoffs that may be considered in designing experiments, plot size may need to be accounted for in research on coccinellid assemblages.
Landscape factors may have influenced coccinellid abundance in our study. For instance, over the last 40 years, winter wheat and spring grains have been steadily replaced by corn and soybeans, and these latter two crops now comprise a large majority of the cropland in eastern South Dakota [Dumke and Dobbs, 1999; Johnston, 2013; Wright and Wimberly, 2013; NASS (National Agricultural Statistics Service), 2021]. The intensified planting of corn and soybean decreases the overall heterogeneity of the landscape, and studies have generally shown that diminished landscape heterogeneity is associated with decreased abundance of natural enemies, and particularly (native) coccinellids (e.g., Elliott et al., 2002; Landis et al., 2008; Grez et al., 2014, 2021; Woltz and Landis, 2014).
It is unclear whether adequate alternative habitat can be exploited by coccinellids to compensate for the impact of increased corn and soybean plantings with lower pest levels. All of the coccinellids found in our study are considered generalists (Elliott et al., 1996; Hesler and Kieckhefer, 2008), and thus may adapt to alternative habitats, although the spillover of agrobiont species into non-agricultural habitats can impact native non-pest herbivores (Koch, 2003; Rand and Louda, 2006). Some have suggested that alternative habitat such as restored tallgrass prairie may serve as refuges for coccinellids (Hesler and Petersen, 2008; Diepenbrock and Finke, 2013), but we found relatively low numbers of coccinellids in prairie in our study and no evidence that they shifted to prairie as their populations declined in corn and soybean. Thus, the types of alternative, late-season habitat that may be colonized by coccinellids remains undetermined for east-central South Dakota.
Although the six habitats sampled in our study largely shared coccinellid species, stark distinctions in rank abundance of coccinellids among corn and soybean and a group consisting of alfalfa, winter wheat, spring grains and prairie suggest that species may be, or already have been, impacted differentially by the expansion of corn and soybean plantings in east-central South Dakota. The impacts may differ among species. For instance, Har. axyridis was a specialist in soybean and present at relatively low numbers in early-season habitats and corn. Thus, it likely has alternative, perhaps arboreal, early-season habitat, but it will have to find additional late-season habitat. Decreased abundance of Har. axyridis may have benefits in terms of limiting its intra-guild predation within the coccinellid assemblage (Gardiner et al., 2011; Koch and Costamagna, 2017) and by reducing the nuisance of its mass overwintering in homes (Koch, 2003).
Native species made up the majority of coccinellids in corn and experienced significant declines in this crop, whereas trends for non-native species were non-significant in corn. A species such as Col. maculata lengi may be expected to persist in corn irrespective of prey levels because of its ability to complete development solely on corn pollen (Smith, 1960). Nevertheless, its population trend was also negative and marginally significant (p = 0.03, adjusted α = 0.013), and this raises questions about what factors contributed to declines that have affected native coccinellids and whether they are prey-dependent or not.
The significant declines of native species in corn may be especially impactful on Hip. tredecimpunctata tibialis. It was historically one of the three most abundant coccinellids in alfalfa, spring grains, and corn (Olsen, 1971; Kieckhefer et al., 1992; Elliott et al., 1996) but one of the least abundant species across habitats in our study. Numbers of Hip. tredecimpunctata tibialis were particularly low in late season crops, with only three adults sampled from corn (all in 2008) and 15 from soybean in six of the 14 years, suggesting that its decline may have pre-dated our study. We suggest that Hip. tredecimpunctata tibialis is a specific candidate for further monitoring, particularly in late-season alternative habitats, given its very low numbers in corn and soybean in our study.
Long-term declines of native coccinellid populations have now been documented in many regions (Harmon et al., 2007). Often, the declines are associated with the establishment of invasive coccinellids species (Elliott et al., 1996; Turnock et al., 2003; Alyohkin and Sewell, 2004; Honek et al., 2016; Brown and Roy, 2018), though not in all cases (Harmon et al., 2007; Evans et al., 2011). In our study, declines were generally observed for coccinellid assemblages over 14 years in corn and soybean, with significant declines for native species as a group in corn. In addition, sampling rates were lower compared to an earlier study (Elliott et al., 1996). However, direct effects of non-native coccinellids on the declines were not apparent. Although it is unclear what factors may actually underlie the lower coccinellid population trends observed in our study, the trend of increased amounts of corn and soybean could have compounding effects that result in major impacts on coccinellid populations in east-central South Dakota and perhaps elsewhere. First, planting corn and soybean at the expense of crops such as alfalfa and small grains directly diminishes availability of early-season habitat for coccinellids, where they have historically built up populations while providing biocontrol services. Second, while diminished pest levels in corn and soybean undoubtedly benefit production of these crops, vast areas of cropland devoid of suitable prey will decrease late summer populations of coccinellids and subsequently reduce overwintering populations that would emerge the following spring to colonize (early-season) crops. Thus, the annual cycle of coccinellids in South Dakota cropland could be severely disrupted both early and late in the season.
Various measures may be implemented to counter the decrease in landscape diversity due to widespread planting of corn and soybean in east-central South Dakota. Such measures include tailoring economic incentives for farmers to plant a wider diversity of crops and promote preservation of natural and semi-natural areas that may serve as refuges for coccinellids and other beneficial insects (Maisashvili et al., 2020). Measures that increase within-field diversity include leaving patches of plants that provide alternative, non-pest prey and the planting of cover crops that promote coccinellids and other beneficial insects but do not support crop pests (Schellhorn et al., 2015; Lundin et al., 2019). However, much of the cover crop research in northern Great Plains cropping systems has focused on soil health parameters, and additional research is needed to determine cover crops that may specifically provide resource continuity for coccinellids in the latter half of the growing season (Schellhorn et al., 2015). In summary, additional long-term research is needed to determine the factors underlying declines in eastern South Dakota, the extent to which various coccinellid species will adapt to changing conditions within crop fields and the general landscape of east-central South Dakota, and ways to sustain coccinellid populations in and around crop fields.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
LH conceived of the study, analyzed the data, and wrote the manuscript. LH and EB co-led data collection. EB managed the data and contributed to making figures. All authors contributed to the drafts and gave final approval for publication.
This research was supported by base funds from USDA-ARS CRIS Projects 5447-21220-001-00D.and 3080-21220-006-00D.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This article is dedicated to Robert W. Kieckhefer (1933–2019) and Lester E. Ehler (1946–2016), who inspired appreciation for the Coccinellidae, insect ecology, and the science of biological control. Norm Elliott, Karl Roeder, Patrick Ewing, Sharon Schneider, and Lauren Hesler graciously reviewed a draft of this manuscript. Max Pravecek, Chris Nelson, Dave Schneider, and Darrell McKeown maintained research plots. Numerous seasonal workers assisted in sampling coccinellids. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2021.742036/full#supplementary-material
Alyohkin, A., and Sewell, G. (2004). Changes in a lady beetle community following the establishment of three alien species. Biol. Invasions 6, 463–471. doi: 10.1023/B:BINV.0000041554.14539.74
Bahlai, C. A., vander Werf, W., O'Neal, M., Hemerik, L., and Landis, D. A. (2015). Shifts in dynamic regime of an invasive lady beetle are linked to the invasion and insecticidal management of its prey. Ecol. Appl. 25, 1807–1818. doi: 10.1890/14-2022.1
Bianchi, F. J. J. A., Honěk, A., and vander Werf, W. (2007). Changes in agricultural land use can explain population decline in a ladybeetle species in the Czech Republic: evidence from a process-based spatially explicit model. Landsc. Ecol. 22, 1541–1554. doi: 10.1007/s10980-007-9145-z
Bing, J. W., Kieckhefer, R. W., and Reese, J. C. (1999). “Management of aphids,” in Handbook of Corn Insects, eds. K. Steffey, M. Rice, J. All, D. Andow, M. Gray and J. Van Duyn (Lanham, MD: Entomological Society of America), 49.
Bowling, R. D., Brewer, M. J., Kerns, D. L., Gordy, J., Seiter, N., Elliott, N. E., et al. (2016). Sugarcane aphid (Hemiptera: Aphididae): a new pest on sorghum in North America. J. Integr. Pest Manag. 7:12. doi: 10.1093/jipm/pmw011
Brown, M. W. (2003). Intraguild responses of aphid predators on apple to the invasion of an exotic species, Harmonia axyridis. Biocontrol 4, 141–153. doi: 10.1023/A:1022660005948
Brown, P. M. J., and Roy, H. E. (2018). Native ladybird decline caused by the invasive harlequin ladybird Harmonia axyridis: evidence from a long-term field study. Insect Conserv. Divers. 11, 230–239. doi: 10.1111/icad.12266
Caballero-López, B., Bommarco, R., Blanco-Moreno, J. M., Sans, F. X., Pujade-Villar, J., Rundlöf, M., et al. (2012). Aphids and their natural enemies are differently affected by habitat features at local and landscape scales. Biol. Control 63, 222–229. doi: 10.1016/j.biocontrol.2012.03.012
Catangui, M., Deneke, D., Draper, M., Ruden, B., Wilson, J., and Wrage, L. (2002). Crop Profile for Alfalfa and Other Hay Production in South Dakota. Brookings, SD: South Dakota State University Agricultural Experiment Station, p. 17. Available online at: http://openprairie.sdstate.edu/extension_ss/6 (accessed June 29, 2021).
Cox, R., O'Neal, M., Hessel, R., Schulte, L. A., and Helmers, M. (2014). The impact of prairie strips on aphidophagous predator abundance and soybean aphid predation in agricultural catchments. Environ. Entomol. 43, 1185–1197. doi: 10.1603/EN13129
Crossley, M. S., Smith, O. M., Davis, T. S., Eigenbrode, S. D., Hartman, G. L., Lagos-Kutz, D., et al. (2021). Complex life histories predispose aphids to recent abundance declines. Global Change Biol. 27, 4283–4293. doi: 10.1111/gcb.15739
Day, W. H., and Tatman, K. M. (2006). Changes in abundance of native and adventive Coccinellidae (Coleoptera) in alfalfa fields, in northern New Jersey (1993–2004) and Delaware (1999–2004), U.S.A. Entomol. News 117, 491–502. doi: 10.3157/0013-872X(2006)117491:CIAONA2.0.CO
Diepenbrock, L. M., and Finke, D. L. (2013). Refuge for native lady beetles (Coccinellidae) in perennial grassland habitats. Insect Conserv. Divers. 6, 671–679. doi: 10.1111/icad.12027
Dumke, L., and Dobbs, T. L. (1999). Historical Evolution of Crop Systems in Eastern South Dakota: Economic Influences. Department of Economics Research Reports. Paper 63. Brookings, SD: South Dakota State University, p. 59. Available online at: http://openprairie.sdstate.edu/econ_research/63 (accessed July 4, 2021).
Elliott, N., Kieckhefer, R., and Kauffman, W. (1996). Effects of an invading coccinellid on native coccinellids in an agricultural landscape. Oecologia 105, 537–544. doi: 10.1007/BF00330017
Elliott, N. C., and Kieckhefer, R. W. (1990a). A thirteen-year survey of the aphidophagous insects of alfalfa. Prairie Nat. 22, 87–96.
Elliott, N. C., and Kieckhefer, R. W. (1990b). Dynamics of aphidophagous coccinellid assemblages in small grain fields in eastern South Dakota. Environ. Entomol. 19, 1320–1329. doi: 10.1093/ee/19.5.1320
Elliott, N. C., Kieckhefer, R. W., and Beck, D. A. (2002). Effect of aphids and the surrounding landscape on the abundance of Coccinellidae in cornfields. Biol. Control 24, 214–220. doi: 10.1016/S1049-9644(02)00036-1
Evans, E. W., Soares, A. O., and Yasuda, H. (2011). Invasions by ladybugs, ladybirds, and other predatory beetles. Biocontrol 56, 597–611. doi: 10.1007/s10526-011-9374-6
Fernandez-Cornejo, J., Wechsler, S., Livingston, M., and Mitchell, L. (2014). Genetically Engineered Crops in the United States, ERR-162. Washington, DC: U.S. Department of Agriculture, Economic Research Service. 54.
Fox, T. B., Landis, D. A., Cardoso, F. F., and Difonzo, C. D. (2004). Predators suppress Aphis glycines Matsumura population growth in soybean. Environ. Entomol. 33, 608–618. doi: 10.1603/0046-225X-33.3.608
Gardiner, M. M., Landis, D. A., Gratton, C., Schmidt, N., O'Neal, M., Mueller, E., et al. (2009). Landscape composition influences patterns of native and exotic lady beetle abundance. Divers. Distrib. 15, 554–564. doi: 10.1111/j.1472-4642.2009.00563.x
Gardiner, M. M., O'Neal, M. E., and Landis, D. A. (2011). Intraguild predation and native lady beetle decline. PLoS ONE 6:e23576. doi: 10.1371/journal.pone.0023576
Gordon, R. D. (1985). The Coccinellidae (Coleoptera) of America North of Mexico. J. New York Entomol. Soc. 93, 1–912.
Gordon, R. D., and Vandenberg, N. (1991). Field guide to recently introduced species of Coccinellidae (Coleoptera) in North America, with a revised key to north American genera of Coccinellini. Proc. Entomol. Soc. Washingt. 93, 845–864.
Grez, A. A., Zaviezo, T., Casanoves, F., Oberti, R., and Pliscoff, P. (2021). The positive association between natural vegetation, native coccinellids and functional diversity of aphidophagous coccinellid communities in alfalfa. Insect Conserv. Div. 14, 464–475. doi: 10.1111/icad.12473
Grez, A. A., Zaviezo, T., Hernandez, T., Rodriguez-San Pedro, A., and Acuña, P. (2014). The heterogeneity and composition of agricultural landscapes influence native and exotic coccinellids in alfalfa fields. Agric. For. Entomol. 16, 382–390. doi: 10.1111/afe.12068
Harmon, J., Stephens, E., and Losey, J. (2007). The decline of native coccinellids (Coleoptera: Coccinellidae) in the United States and Canada. J. Insect Conserv. 11, 85–94. doi: 10.1007/s10841-006-9021-1
Hesler, L. S. (2014). Inventory and assessment of foliar natural enemies of the soybean aphid (Hemiptera: Aphididae) in South Dakota. Environ. Entomol. 43, 577–588. doi: 10.1603/EN13210
Hesler, L. S., and Beckendorf, E. A. (2021). Soybean aphid infestation and crop yield in relation to cultivar, foliar insecticide, and insecticidal seed treatment in South Dakota. Phytoparasitica doi: 10.1007/s12600-021-00914-y [Epub ahead of print].
Hesler, L. S., and Kieckhefer, R. W. (2008). Status of exotic and previously common native coccinellids (Coleoptera) in South Dakota landscapes. J. Kansas Entomol. Soc. 81, 29–49. doi: 10.2317/JKES-704.11.1
Hesler, L. S., Kieckhefer, R. W., and Beck, D. A. (2001). First record of Harmonia axyridis (Coleoptera: Coccinellidae) in South Dakota and notes on its activity there and in Minnesota. Entomol. News 112, 264–270.
Hesler, L. S., Kieckhefer, R. W., and Catangui, M. A. (2004). Surveys and field observations of Harmonia axyridis and other Coccinellidae (Coleoptera) in eastern and central South Dakota. Trans. Am. Entomol. Soc. 130, 113–133. www.jstor.org/stable/2507884
Hesler, L. S., Kieckhefer, R. W., and Ellsbury, M. M. (2005). Abundance of coccinellids (Coleoptera) in field-crop and grass habitats in eastern South Dakota. Great Lakes Entomol. 38, 83–96.
Hesler, L. S., Kieckhefer, R. W., and Evenson, P. D. (2000). Cereal aphids and their predators in a spring wheat-alfalfa intercrop under different crop management intensities. Great Lakes Entomol. 33, 17–31.
Hesler, L. S., and Lundgren, J. G. (2011). Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae) found in South Dakota, U.S.A. Coleopts. Bull. 65, 78–79. doi: 10.1649/0010-065X-65.1.78
Hesler, L. S., and Petersen, J. D. (2008). Survey for previously common native Coccinellidae (Coleoptera) in the northern Great Plains. Gt. Lakes Entomol. 41, 86–93.
Hesler, L. S., Sappington, T. W., Luttrell, R. G., Allen, K. C., and Papiernik, S. K. (2018). Selected early-season pests of wheat in the United States and factors affecting their risks of infestation. J. Integ. Pest Mngmt. 9:17. doi: 10.1093/jipm/pmx023
Hochberg, Y. (1988). A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75, 800–802.
Honek, A., Martinkova, Z., Dixon, A. F. G., Roy, H. E., and Pekar, S. (2016). Long-term changes in communities of native coccinellids: population fluctuations and the effect of competition from an invasive nonnative species. Insect Conserv. Divers. 9, 202–209. doi: 10.1111/icad.12158
Honek, A., Martinkova, Z., Kindlmann, P., Ameixa, O. M. C. C., and Dixon, A. F. G. (2014). Long-term trends in the composition of aphidophagous coccinellid communities in Central Europe. Insect Conserv. Divers. 7, 55–63. doi: 10.1111/icad.12032
Hutchinson, W. D., Burkness, E. C., Hellmich, R. L., Kaster, L. V., Hunt, T. E., Wright, R. J., et al. (2010). Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 330, 222–225. doi: 10.1126/science.1190242
Iperti, G. (1999). Biodiversity of predaceous Coccinellidae in relation to bioindication and economic importance. Agric. Ecosys. Environ. 74, 323–342. doi: 10.1016/S0167-8809(99)00041-9
Irwin, M. E., and Thresh, J. M. (1988). Long-range aerial dispersal of cereal aphids as virus vectors in North America. Philos. Trans. R. Soc. Lond. B Biol. Sci. 321, 421–446. doi: 10.1098/rstb.1988.0101
Johnston, C. A. (2013). Wetland losses due to row crop expansion in the Dakota prairie pothole region. Wetlands 33, 175–182. doi: 10.1007/s13157-012-0365-x
Kalaskar, A., and Evans, E. W. (2001). Larval responses of aphidophagous lady beetles (Coleoptera: Coccinellidae) to weevil larvae versus aphids as prey. Ann. Entomol. Soc. Am. 94, 76–81. doi: 10.1603/0013-8746(2001)0940076:LROALB2.0.CO
Kendall, M., and Gibbons, J. D. (1990). Rank Correlation Methods, 5th Edn. London: Edward Arnold 272.
Kieckhefer, R. W., Elliott, N. C., and Beck, D. A. (1992). Aphidophagous coccinellids in alfalfa, small grains, and maize in eastern South Dakota. Great Lakes Entomol. 25, 15–23.
Koch, R. L. (2003). The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J. Insect Sci. 3, 32. doi: 10.1093/jis/3.1.32
Koch, R. L., and Costamagna, A. C. (2017). Reaping benefits from an invasive species: role of Harmonia axyridis in natural biological control of Aphis glycines in North America. Biocontrol. 62, 331–340. doi: 10.1007/s10526-016-9749-9
Lamb, R. J., Bannerman, J. A., and Costamagna, A. C. (2019). Stability of lady beetle populations in a diverse landscape. Ecosphere 10:e02630. doi: 10.1002/ecs2.2630
Lambert, L., and Tyler, J. (1999). “Appraisal of insect-resistant soybeans,” in Economic, Environmental, and Social Benefits of Insect Resistance in Field Crops, eds B. R. Wiseman and J. A. Webster (Lanham, MD: Thomas Say Publications, Entomological Society of America), 131–148.
Landis, D. A., Gardiner, M. M., vander Werf, W., and Swinton, S. M. (2008). Increasing corn for biofuel production reduces biocontrol services in agricultural landscapes. Proc. Natl. Acad. Sci. U.S.A. 105, 20552–20557. doi: 10.1073/pnas.0804951106
Legendre, P. (2005). Species associations: the Kendall coefficient of concordance revisited. J. Agric. Biol. Environ. Stat. 10, 226–245. doi: 10.1198/108571105x46642
Lucas, E., Vincent, C., Labrie, G., Chouinard, G., Fournier, F., Pelletier, F., et al. (2007). The multicolored Asian ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae) in Quebec agroecosystems ten years after its arrival. Eur. J. Entomol. 104, 737–743. doi: 10.14411/eje.2007.094
Lundgren, J. G., Hesler, L. S., Clay, S. A., and Fausti, S. F. (2013). Insect communities in soybeans of eastern South Dakota: the effects of vegetation management and pesticides on soybean aphids, bean leaf beetles, and their natural enemies. Crop Prot. 43, 104–118. doi: 10.1016/j.cropro.2012.08.005
Lundin, O., Ward, K. L., and Williams, N. M. (2019). Identifying native plants for coordinated habitat management of arthropod pollinators, herbivores, and natural enemies. J. Appl. Ecol. 56, 665–676. doi: 10.1111/1365-2664.13304
Maisashvili, A., Bryant, H., and Jones, J. (2020). Implications of alternative crop insurance subsidies. J. Agric. Appl. Econ. 52, 240–263. doi: 10.1017/aae.2019.46
Maizel, M., White, R. D., Gage, S., Osborne, L., Root, R., Stitt, S., et al. (1998). “Historical interrelationships between population settlement and farmland in the conterminous United States, 1790 to 1992,” in Perspectives on the Land Use History of North America: A Context for Understanding Our Changing Environment, USGS/BRD/BSR-1998-0003 (Washington, DC: U.S. Geological Survey), 5–12.
McCornack, B. P., Costamagna, A. C., and Ragsdale, D. W. (2008). Within-plant distribution of soybean aphid (Hemiptera: Aphididae) and development of node-based sample units for estimating whole-plant densities in soybean. J. Econ. Entomol. 101, 1488–1500. doi: 10.1093/jee/101.4.1488
Michaud, J. P. (2012). “Coccinellids in biological control” in Ecology and Behaviour of the Ladybird Beetles (Coccinellidae), eds I. Hodek, H. F. van Emden, and A. Honěk (Chichester, UK: Wiley-Blackwell), 488–519.
NASS (National Agricultural Statistics Service) (2021). QuickStats Query Tool. Available online at: quickstats.nass.usda.gov (accessed June 23, 2021).
Obrycki, J. J., and Kring, T. J. (1998). Predaceous Coccinellidae in biological control. Annu. Rev. Entomol. 43, 295–321. doi: 10.1146/annurev.ento.43.1.295
Olsen, G. A. (1971). Field Populations and Flight Activity of Three Hippodamia Species in Eastern South Dakota. Master’s thesis, Brookings, SD: South Dakota State University.
Olson, D., and Andow, D. (2008). Patch edges and insect populations. Oecologia 155, 549–558. doi: 10.1007/s00442-007-0933-6
Perry, E. D., and Moschini, G. (2020). Neonicotinoids in U.S. maize: insecticide substitution effects and environmental risk. J. Environ. Econ. Manage. 102:102320. doi: 10.1016/j.jeem.2020.102320
Pikul, J. L. Jr., Johnson, J. M. F., Schumacher, T. E., Vigil, M., and Riedell, W. E. (2008). Change in surface soil carbon under rotated corn in eastern South Dakota. Soil Sci. Soc. Am. J. 72, 1738–1744. doi: 10.2136/sssaj2008.0020
Prescott, K. K., and Andow, D. A. (2016). Lady beetle (Coleoptera: Coccinellidae) communities in soybean and maize. Environ. Entomol. 45, 74–82. doi: 10.1093/ee/nvv154
Ragsdale, D. W., Landis, D. A., Brodeur, J., Heimpel, G. E., and Desneux, N. (2011). Ecology and management of the soybean aphid in North America. Annu. Rev. Entomol. 56, 375–399. doi: 10.1146/annurev-ento-120709-144755
Rand, T. A., and Louda, S. M. (2006). Predator spillover across the agricultural–natural interface: a threat to native insect herbivores in fragmented landscapes? Conserv. Biol. 20, 1720–1729. doi: 10.1111/j.1461-0248.2006.00911.x
Rashford, B. S., Walker, J. A., and Bastian, C. T. (2011). Economics of grassland conversion to cropland in the Prairie Pothole Region. Conserv. Biol. 25, 276–284. doi: 10.1111/j.1523-1739.2010.01618.x
Samson, F. B., Knopf, F. L., and Ostlie, W. R. (2004). Great Plains ecosystems: past, present, and future. Wildlife Soc. Bull. 32, 6–15. doi: 10.2193/0091-7648(2004)326:GPEPPA2.0.CO;2
Schellhorn, N. A., Gagic, V., and Bommarco, R. (2015). Time will tell: resource continuity bolsters ecosystem services. Trends Ecol. Evol. 30, 524–530. doi: 10.1016/j.tree.2015.06.007
Schmidt, N. P., O’Neal, M. E., and Dixon, P. M. (2008). Aphidophagous predators in Iowa soybean: a community comparison across multiple years and sampling methods. Ann. Entomol. Soc. Am. 101, 341–350. doi: 10.1603/0013-8746(2008)101341:APIISA2.0.CO
Smith, B. C. (1960). A technique for rearing coccinellid beetles on dry foods, and influence of various pollens on the development of Coleomegilla maculata lengi Timb. (Coleoptera: Coccinellidae). Can. J. Zool. 38, 1047–1049. doi: 10.1139/z60-109
Southwood, T. R. E., and Henderson, P. A. (2000). Ecological Methods, 3rd Edn. Malden, MA: Blackwell 575.
Spear, M. J., Walsh, J. R., Ricciardi, A., and Vander Zanden, M. J. (2021). The invasion ecology of sleeper populations: prevalence, persistence, and abrupt shifts. Bioscience 71, 357–369. doi: 10.1093/biosci/biaa168
Strayer, D. L., Eviner, V. T., Jeschke, J. M., and Pace, M. L. (2006). Understanding the long-term effects of species invasions. Trends Ecol. Evol. 21, 645–651. doi: 10.1016/j.tree.2006.07.007
Turnock, W. J., Wise, I. L., and Matheson, F. O. (2003). Abundance of some native coccinellines (Coleoptera: Coccinellidae) before and after the appearance of Coccinella septempunctata. Can. Entomol. 135, 391–404. doi: 10.4039/n02-070
USGS (U.S. Geological Survey). (2014). National Water-quality Assessment (NAWQA) Program Annual Pesticide Use Maps. Available online at: https://water.usgs.gov/nawqa/pnsp/usage/maps (accessed March 3, 2021).
Woltz, J. M., and Landis, D. A. (2014). Coccinellid response to landscape composition and configuration. Agric. Forest Entomol. 16, 341–349. doi: 10.1111/afe.12064
Keywords: beetle conservation, invasive species, habitat use, biodiversity, lady beetles
Citation: Hesler LS and Beckendorf EA (2021) Declining Abundance of Coccinellidae (Coleoptera) Among Crop and Prairie Habitats of Eastern South Dakota, USA. Front. Conserv. Sci. 2:742036. doi: 10.3389/fcosc.2021.742036
Received: 15 July 2021; Accepted: 13 August 2021;
Published: 17 September 2021.
Edited by:
Danny Haelewaters, Ghent University, BelgiumReviewed by:
Todd Ugine, Cornell University, United StatesCopyright © 2021 Hesler and Beckendorf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Louis S. Hesler, bG91aXMuaGVzbGVyQHVzZGEuZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.