- 1College of Veterinary Medicine, University of Minnesota, St. Paul, MN, United States
- 2Notre Dame of Maryland University, Baltimore, MD, United States
- 3The Raptor Center, College of Veterinary Medicine, University of Minnesota, St. Paul, MN, United States
One of the most concerning threats to Galápagos bird populations, including some critically endangered species, is the invasive parasitic fly Philornis downsi. While long-term sustained solutions are under study, immediate actions are needed to reduce the impacts of this fly. Application of permethrin to birds's nests has been successfully done, but there might be potential long-term reproductive effects to birds. Cyromazine, an insect growth regulator, has been proposed as an alternative, but its risks and effectiveness are unknown. The goal of this study was to assist managers to determine which combination of chemical (permethrin or cyromazine) and mode of application (injection, spray, and self-fumigation) was likely to be most effective to control P. downsi while minimizing toxicity to small land birds in Galápagos, given data available and high levels of uncertainty in some cases. This study is presented as a semi-quantitative risk assessment employing the use of a multi-criteria decision analysis (MCDA) model. For the six potential alternatives resulting from the combination of chemical and mode of application, the criteria were given a score from 1 to 6 supported by available evidence from the literature and from expert opinion. In addition, three different scenarios with different sets of weights for each criterion were assessed with stakeholder's input. Considering the scenario with higher weight to effectiveness of the method against P. downsi while also weighing heavily to minimize the toxicity to birds, cyromazine spray followed by permethrin injection were the preferred strategies. Self-fumigation was the mode of application with highest uncertainty but with much potential to be further explored for its feasibility. The approach taken here to evaluate mitigation strategies against an important threat for avian species in Galápagos can also be used in other conservation programs when making real time decisions under uncertainty.
Introduction
Land bird species (passerines, cuckoos, and doves) populations in the Galápagos Islands (Ecuador) are declining. According to the International Union for Conservation of Nature (IUCN), at least 14 out of 28 native small land bird species across the archipelago are threatened, and the situation is even worse within individual islands (Fessl et al., 2018). Two critically endangered finches, the Mangrove Finch (Camarhynchus heliobates) and the Medium-tree Finch (Camarhynchus pauper), and the Little Vermilion Flycatcher (Pyrocephalus nanus), categorized as “vulnerable,” are some of the main species of concern.
Threats to land birds in Galápagos include introduced vertebrate species such as rodents and cats, habitat degradation, diseases, and disease vectors (e.g., avian pox and avian malaria), roadkill, and the invasive parasitic muscid fly Philornis downsi (Fessl et al., 2017). This fly is the most concerning threat for many land birds (Cunninghame et al., 2012; Fessl et al., 2017). Originally from the Caribbean Islands and South America, P. downsi was first detected in the Galápagos during the 1960's (Fessl et al., 2018), and first observed in the nests of a woodpecker finch (Camarhynchus pallidus) in 1997 (Fessl and Tebbich, 2002). Currently, this parasitic fly is present on all large Galápagos Islands except for Genovesa, Española, Darwin, and Wolf (Fessl et al., 2018).
The lifecycle of this parasitic fly has been described in detail elsewhere (Fessl et al., 2006). Briefly, adult flies lay their eggs in bird nests, and when the fly larvae hatch, the first stage larvae migrate to feed in the nares and ear canals of bird nestlings (Fessl et al., 2006; O'Connor et al., 2010). The second and third larval stages are usually found in the base of the nests (Fessl et al., 2017) and emerge at night to feed on the nestlings blood. The third stage larvae usually remain in the nest until they pupate. Infestation by P. downsi leads to clinical manifestations in the nestlings such as anemia, reduction in hemoglobin concentration, wounds, and deformed nasal openings (Dudaniec et al., 2006; Fessl et al., 2006). This leads to inhibited nestling growth, increased mortality, and reduced fledging success, ultimately affecting the reproductive success of bird species (O'Connor et al., 2010; Fessl et al., 2018). At least 19 endemic and two native land bird species have been affected by P. downsi to date, including the critically endangered Mangrove Finch (Fessl et al., 2018; McNew and Clayton, 2018; Anchundia and Fessl, 2020; Coloma et al., 2020). Furthermore, the fly has the potential to drive several endemic species to extinction in Galápagos (McNew and Clayton, 2018).
Mid to long-term solutions to control P. downsi are under study, including strategies such as biological control. Recent field and laboratory experiments using Conura annulifera, a parasitoid wasp that attacks P. downsi in its native range, suggested that this can be an ecologically safe option. However, more studies are needed to understand potential risks to native and endemic species in Galápagos (Boulton et al., 2019). Biochemical control is another strategy under study, such as the use of the endemic Guayabillo tree (Psidium galapageium) which has natural insect repelling properties, and the use of bio-pyrethrin (6.6% pure extract of natural pyrethrum Chrysanthemum cinerariaefolium) (Causton et al., 2019).
While these long-term solutions are being evaluated, immediate solutions to mitigate P. downsi are needed. Current control strategies include the application of permethrin to nests. Permethrin is a synthetic, broad-spectrum pyrethroid which kills the adults and larvae of many invertebrates by acting directly on their nervous system (Cox, 1999; Causton and Lincango, 2014). Permacap® and permethrin EC (emulsifiable concentrate) injected into the base of the nests in Galápagos have shown to be effective against P. downsi. However, an analysis conducted on the risks of permethrin to birds as used in Galápagos found no short-term risks but uncovered unknown long-term effects to birds (Causton and Lincango, 2014). Furthermore, a multi-generational experimental study using Zebra finches (Taeniopygia guttata) found negative sub-lethal effects consisting of decreased breeding success in the second finch generation as well as decreased body weight in the nestlings after nests were sprayed with low concentrations (1%) of permethrin EC (Bulgarella et al., 2020). Cyromazine, an insect growth regulator, has been tested as an alternative to permethrin in both laboratory and field settings with effective results against P. downsi (Causton et al., 2019).
Cyromazine is a selective triazine that affects the molting process of dipteran insects, inhibiting larval growth and development. It is primarily used to control nuisance flies in livestock farms either directly (e.g., wetting in sheep), or indirectly by spray application to manure, or by mixing it into feed (e.g., in poultry farms to control flies in manure). It is also applied to crops and plants to control diptera and coleoptera (EFSA, 2008). To the best of the authors' knowledge, cyromazine has not been used to date in conservation programs to control parasitic flies, except for the recent pilot trials conducted in Galápagos (Causton et al., 2019). Despite promising results, the risks to birds have yet to be evaluated, a step that is required by the Galápagos authorities, prior to extending the use of this chemical more broadly in the Galápagos Islands.
For both permethrin and cyromazine, there are three modes of application to bird nests in Galápagos either being used or under evaluation: injection, spray, and self-fumigation (Knutie et al., 2014; Causton et al., 2019; Tebbich et al., 2019). Injection and spray require climbing up to the nest to apply the chemical, which is a challenge as some of the bird nests are found up to 25 m high. Self-fumigation (birds taking nest materials previously impregnated with the chemical from a dispenser back to the nest) would be more feasible, but there is higher uncertainty about the effectiveness of this mode. Given the urgent need to find an effective and safe mitigation strategy for P. downsi in land bird nests in the Galápagos Islands, a comparison of these two chemical candidates was needed. The goal of this study was to find the optimal risk management strategy (combination of chemical and mode of application) to control P. downsi while minimizing toxicity to small land birds in Galápagos, maximizing the feasibility of application, and minimizing the uncertainty. To this end, a semi-quantitative risk assessment using a multi-criteria decision analysis (MCDA) was conducted. Even though potential effects of these two chemicals to other non-target species (beyond small land birds) and the ecosystem at large need to be included in the management decision of a chemical application, these were out of the scope of this assessment.
Materials and Methods
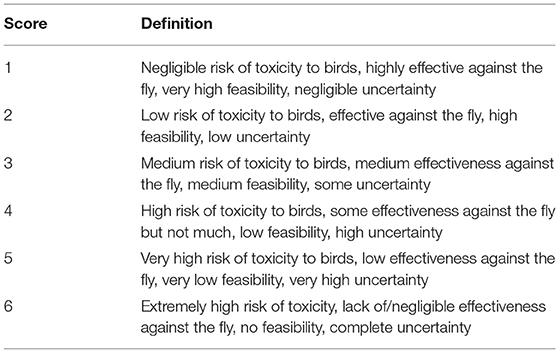
To evaluate the optimal risk management strategy, a semi-quantitative risk assessment was conducted. Risk assessment methods can be divided into qualitative, quantitative, and semi-quantitative approaches depending on the needs of the users and the amount of data available. A qualitative approach produces a descriptive and non-numerical estimate of risk. In contrast, a quantitative risk assessment relies on numerical expressions to characterize risk, either with a deterministic (producing point estimates of risks) or a probabilistic approach (producing probability distributions to estimate risks) (Travis and Smith, 2019; Yoe, 2019). Finally, a semi-quantitative risk assessment assigns numbers in the form of probability ranges, weights, or scores to qualitative estimates and then combines them either by adding or multiplying them (Jakob-Hoff et al., 2014). The semi-quantitative approach used here was a set of scores from 1 to 6 with specific definitions for each one of the numerical categories (Table 1).
To conduct the semi-quantitative risk assessment, a MCDA model was used. This structured approach is useful to support decision-making under uncertainty when there are multiple criteria involved. In this process, the alternatives to be evaluated are compared against a set of explicitly defined criteria (Adem Esmail and Geneletti, 2018). Depending on the importance (i.e., weight) given to each one of the criteria, the ranking among the alternatives under consideration will vary (OIE, 2017; Yoe, 2019). This approach is iterative, so as more data become available, the model can be updated and thus provide more precise outputs for decision-making.
Two different chemicals (permethrin and cyromazine) and three modes of application (injection to the nests, spray to the nests, and self-fumigation) were evaluated. Specifically, the six alternatives to be evaluated by the MCDA were: permethrin injection, permethrin spray, permethrin self-fumigation, cyromazine injection, cyromazine spray, and cyromazine self-fumigation. The criteria used to assess each one of the alternatives, which were based on stakeholder feedback, were: toxicity to birds, effectiveness against P. downsi, feasibility, and uncertainty (i.e., lack of evidence). Each one of these criteria was informed by a set of sub-criteria and factors (Table 2).

Table 2. Criteria, sub-criteria, and factors considered to evaluate the six alternatives in the risk assessment model.
Available evidence for each one of the factors, sub-criteria, and criteria was obtained through a literature search and through expert opinion. Search engines (PubMed, Web of Science, Google Scholar, Google), different agencies (Food and Drug Administration, Environmental Protection Agency, European Food Safety Authorization, European Medicines Agency, Food and Agriculture Organization), and consultation with companies selling cyromazine and permethrin helped capture both peer-reviewed and gray literature. Within the Environmental Protection Agency, the databases CompTox (https://comptox.epa.gov/dashboard) and ECOTOX (https://cfpub.epa.gov/ecotox/) were used to extract data from available studies. The time frame considered for the main search was from inception through September 2020; however, additional searches were conducted as needed through the end of December 2020. The following were the main keywords that were used in the search, which were used both in English and Spanish: “cyromazine,” “permethrin,” “toxicity,” “bird,” “avian,” “wildlife,” “invasive fly,” “injection,” “spray,” “impregnated cotton,” “nest,” “pesticide,” “insecticide,” “growth regulator,” “poultry,” “chicken,” “turkey,” “chitin synthesis inhibitor,” “melamine effects to birds.”
Additionally, a range of experts were contacted to obtain information about cyromazine, permethrin, and other chemical control methods against parasitic flies in avian species. These experts were chosen based on their field of expertise (ecotoxicology, avian health, avian biology, entomology, as well as previously recognized experience using insecticides in bird nests in the field). A previous analysis of the risks of permethrin for birds in Galápagos had already been conducted (Causton and Lincango, 2014), so the literature and expert elicitation was more heavily focused on cyromazine compared to permethrin.
Supported by the evidence available and stakeholder input, each one of the sub-criteria was scored on a scale of 1–6, with 1 being the most preferred option and 6 being the least preferred (Table 1). Then, all sub-criteria that made up one criterion were totaled and averaged to obtain a single final score for each criterion. Criteria scores for each of the six alternatives (chemical/mode of application combination) were used as input into a decision matrix to build a MCDA using Logical Decisions software version 7.2 (Smith, 2021).
Three scenarios (A, B, and C) were created assigning different weights to each one of the criteria. Weights are values assigned to each criterion that indicate its relative importance with respect to the other criteria being considered (Malczewski, 1999). The weights here ranged from 0 to 1, with 0 being the lowest importance and 1 being the maximum importance. In Scenario A, the criterion “toxicity to birds” received a weight of 0.8, while in Scenarios B and C, this criterion received a weight of 0.5. Based on stakeholder's feedback, this criterion could not receive a weight below 0.5. For the other criteria, in Scenario B, “effectiveness against P. downsi” received a weight of 0.4, and in Scenario C, “feasibility” received a weight of 0.4. “Uncertainty” received a low weight (0.05) in all three scenarios (Table 3). Given the criteria scores and the weights, the multi-attribute utility theory (MAUT) algorithm was used to transform the criteria into one common scale of utility or value (Linkov et al., 2006). The final output consisted of a ranking of the six alternatives for each one of the three scenarios.
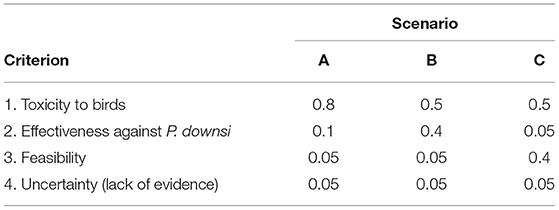
Table 3. Weights for each one of the criteria for each of the three scenarios considered in the risk assessment.
Results
A total of 52 references from the literature were used to inform the MCDA (Supplementary Material). In addition, 11 experts contributed their expertise about chemical control of invasive species in avian nests (n = 4), ecotoxicology (n = 2), entomology (n = 2), pesticides (n = 2), and environmental chemistry (n = 1). While there were more data on the effects of permethrin to birds (Causton and Lincango, 2014, Bulgarella et al., 2020), there was a paucity of data for cyromazine, and available information was derived from poultry studies.
Briefly, no acute toxic effects have been reported for permethrin in birds (Causton and Lincango, 2014), and no mortality has been observed after application of permethrin to bird nests in Galápagos (Causton et al., 2019). The LD50 (Lethal Dose 50 or dose at which 50% of the animals are expected to die) reported for permethrin was >11,275 mg/kg in Mallard ducks (Anas platyrhynchos) and >13,500 mg/kg in Japanese quails (Coturnix japonica). These values classify permethrin as practically non-toxic (EPA, 2015). For long-term, in an experimental study with Zebra finches (T. guttata), negative effects consisting of decreased fledging success and lower body mass in hatchlings were reported-especially in the second generation of finches-after their nesting material had been exposed to 1% permethrin solution (Bulgarella et al., 2020).
For cyromazine, no short-term effects or mortality has been observed from preliminary field trials in Galápagos where the base of finch nests was injected or sprayed with cyromazine at 0.2 or at 0.4 g/L (Causton et al., 2020). Experimental data from other avian species showed that cyromazine can be considered practically non-toxic to slightly toxic (EPA, 2015) with a LD50 for Northern bobwhite quails (Colinus virginianus) of 1,785 mg/kg (NCBI, 2020) and a LD50 for Mallard ducks (A. platyrhynchos) of >2,510 mg/kg (NCBI, 2020). Data from poultry studies, which are based on high oral doses (ranging from 50 to 10,000 mg/kg) often given for several weeks or months, showed low to negligible acute toxicity. With these dosing parameters, some of these poultry studies reported sublethal effects consisting of weight loss and a reduction in the hatching of normal chicks.
In regard to the effectiveness of the chemicals against P. downsi, permethrin application increased finch fledging survival and was effective at reducing the number of P. downsi larvae in avian nests in Galápagos (Causton et al., 2019; Cimadom et al., 2019), as well as against other nest parasites in conservation programs elsewhere (Alves et al., 2020). Cyromazine preliminary field applications have also led to finch's reproductive success as well as reduction in fly development in Galápagos (Causton et al., 2019, 2020).
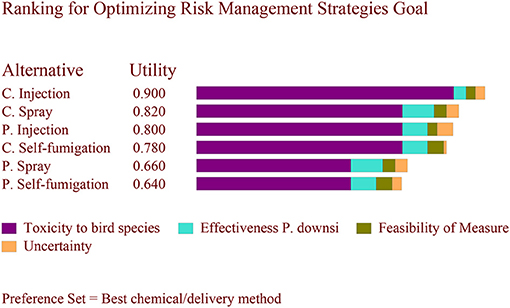
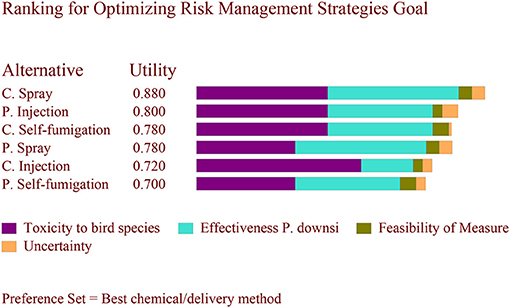
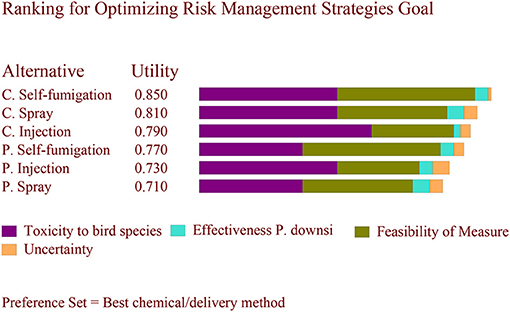
The final risk scores assigned to each criterion for each alternative are summarized in Table 4. Based on the set of three different weights, the results differed. Using the weights from Scenario A, where a high weight was assigned to minimizing toxicity to birds (0.8), the preferred alternative was cyromazine injection followed by cyromazine spray (Figure 1). Using the weights from Scenario B, where higher weight (0.4) was assigned to effectiveness against P. downsi while minimizing toxicity to birds was set to 0.5, the preferred alternative was cyromazine spray followed by permethrin injection (Figure 2). Finally, using the weights from Scenario C, where a weight of 0.4 was assigned to feasibility and minimizing toxicity to birds was set to 0.5, the best alternative was cyromazine self-fumigation followed by cyromazine spray (Figure 3). Across all three scenarios, the highest uncertainty among the six alternatives was cyromazine self-fumigation followed by cyromazine injection, and permethrin self-fumigation.
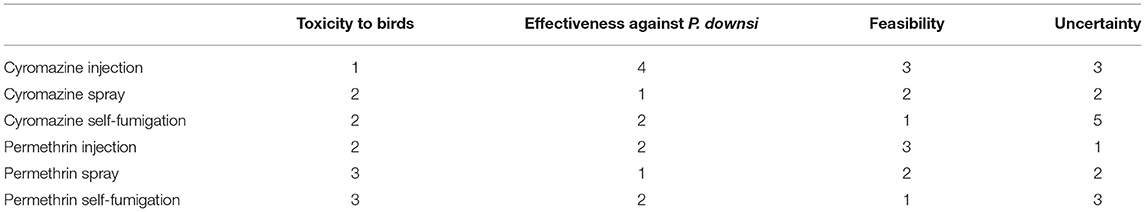
Table 4. Risk scores for each alternative (chemical/mode of application combination) and criterion that informed the Multi-Criteria Decision Approach (MCDA) model.
Discussion
One of the most concerning threats for the survival of small land birds in the Galápagos Islands is the parasitic invasive fly P. downsi. Immediate strategies to mitigate the damage caused by this fly include the use of chemicals such as permethrin and cyromazine. Permethrin has been used in bird nests before to control parasites in different conservation programs around the world, and more evidence is available about the potential risks to birds including long-term reproductive effects (Causton and Lincango, 2014; Bulgarella et al., 2020). Cyromazine, however, has not been used in a conservation program before except for a limited number of pilot field trials in the Galápagos Islands in recent years—where researchers found that using a dose of 0.4 g/L of cyromazine, applying the product close to bird hatching time, and making sure there is contact between the sides and bottom of the bird nest with the product, can be effective at controlling eggs and developing fly larvae with no observable effects on nestling health (Causton et al., 2020). Further to this, a risk assessment was required by authorities to evaluate potential risks to birds. Given these two chemicals were proposed to control P. downsi in bird nests in Galápagos, both chemicals were compared in terms of toxicity to birds, their efficacy against P. downsi, feasibility of application through three delivery methods (injection, spray, and self-fumigation), and on the uncertainty about their effectiveness. Combining the two chemicals and the three modes of application, six alternatives were ranked using a MCDA model.
The MCDA is a transparent process that allows decision-making when multiple criteria are involved (Yoe, 2019). In addition, this is an iterative process that can change depending on stakeholder's feedback as well as improvement of data availability over time. This approach has been used previously in conservation planning (Davies et al., 2013). In fact, Adem Esmail and Geneletti (2018) reviewed the empirical applications of MCDA to nature conservation published in the scientific literature over the last 20 years. They found 86 published studies describing MCDA applied to conservation topics and assessed the best practices and pitfalls of this approach across all these studies. The review found that only a small percentage of MCDA papers engaged stakeholders in alternatives and criteria identification (15 and 25%, respectively), only 22% included rankings according to specific criterion, and most papers conducted some sort of weighting (Adem Esmail and Geneletti, 2018). Our MCDA approach engaged the stakeholders (scientists working on avian conservation in the Galápagos Islands) from the beginning and the process (alternatives, criteria, and the assigned weights) was improved based on their feedback. We also had rankings broken down by criterion for each scenario. Despite MCDA being a transparent process and considering stakeholder input, assignment of weights to criteria is still subjective (Yoe, 2019).
When evaluating the six alternatives to control P. downsi in Galápagos, the preferred option varied depending on the weights assigned to each criterion. According to the available evidence, cyromazine seems to be safe for application to bird nests, and thus it ranked first (specifically cyromazine injection) when weighing toxicity to birds as very high in scenario A. One of the main challenges when applying permethrin or cyromazine as injection or spray to bird nests in Galápagos is nest access. These applications require an experienced person to climb up to the nests for chemical application, and some of these nests are located very high up in the canopy (up to 25 m). Therefore, it would be ideal to have an alternative such as self-fumigation which would decrease human resources and difficulties to access the nests. Further, a more feasible approach would make it possible to treat a higher number of infested nests across the Islands. Self-fumigation with cyromazine however had the highest uncertainty among the six alternatives, as it has not been previously tested. Self-fumigation with permethrin impregnated cotton has been applied in Galápagos before with success in reducing the number of parasites in finches nests (Knutie et al., 2014). This management technique has also been tested in New Zealand to control the fly Passeromyia longicornis, which affects the endangered forty-spotted pardalote (Pardalotus quadragintus). In this case, they used impregnated feathers treated with a combination of permethrin, piperonyl butoxide, and methoprene. The pardalotes not only incorporated the treated feathers into their nests, but the survival of hatchlings was higher in nests that contained treated feathers compared to those that did not (Alves et al., 2020).
Further studies testing this method with cyromazine and permethrin in Galápagos will provide invaluable information about their effectiveness.
Aside from further evaluating the effectiveness of cyromazine via self-fumigation, other outstanding knowledge gaps remain: duration of the effect of cyromazine once applied, potential risks to birds through dermal and inhalation exposure for both chemicals, and variability of effectiveness depending on nest type and size. Further, determining exact volumes of both chemicals that are deemed safe for birds via the three different application methods considering variables such as nest size, type, bird species, and exposure pathway would be very relevant. Upcoming laboratory and field trials will provide insight into some of these knowledge gaps, which will improve the next iteration of the risk assessment. Despite current knowledge gaps and uncertainty, the need to control P. downsi outweighs the caveats that have not been evaluated to date due to lack of data.
Another important consideration when evaluating the optimal chemical to use is the potential risk of the chemicals to other non-target species and the ecosystem at large. The MCDA model presented herein was focused on a very specific question and could not address the potential downstream ecosystem effects of chemical application. Permethrin was already evaluated in relation to the Galápagos ecosystem (Causton and Lincango, 2014), while only a preliminary evaluation of cyromazine has been conducted by our team (unpublished data). Even though risks associated with the Galápagos ecosystem associated with the application of cyromazine to bird nests is perceived as low based on this analysis, a more thorough evaluation that assesses the ecosystem at large is warranted prior to broadly expanding the application of this chemical.
Conclusions
A MCDA approach was a very useful science-based approach to evaluate six alternatives (combination of two chemicals and three modes of application) to mitigate the effects of P. downsi to small land birds in the Galápagos Islands. Maximizing effectiveness against P. downsi while minimizing toxicity to birds, cyromazine spray followed by permethrin injection were the best alternatives. The approach taken here to evaluate mitigation strategies against an important threat for avian species in Galápagos is not only useful and applicable to the Galápagos system but can also be used in other conservation programs when making decisions in the face of uncertainty.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
IB: conceptualization, methodology, analysis, and writing. RS: conceptualization, writing-reviewing, and editing. CY: visualization, conceptualization, methodology, writing-reviewing, and editing. DT: conceptualization, methodology, writing-reviewing, and editing. RP: methodology and writing-reviewing. JP: conceptualization, visualization, writing-reviewing, and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Galápagos Invasive Species Fund and the Galápagos Conservancy. The funding sources did not have any involvement in the study design, analysis, preparation, or submission of this manuscript.
Conflict of Interest
This work was part of a consultancy for the Charles Darwin Foundation on behalf of the Galapagos National Park Directorate. The consultancy was financed with support from the Galapagos Invasive Species Fund and Galapagos Conservancy. Name of the Consultancy: “Consultant to analyse risks of using Cyromazine to control an invasive parasitic fly in bird nests.” https://www.darwinfoundation.org/images/vacantes/emp_con_cyromazina_en.pdf. The funding sources did not have any involvement in the study design, analysis, preparation, or submission of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This research was carried out for the Charles Darwin Foundation on behalf of the Galapagos National Park Directorate and the Galapagos Biosecurity Agency with a grant from the Galapagos Invasive Species Fund. This publication is contribution number 2413 of the Charles Darwin Foundation for the Galapagos Islands. We would like to thank Drs. Charlotte Causton and Birgit Fessl (Charles Darwin Foundation) for their ongoing support and feedback. We would also like to thank all the experts who provided information for this project. We are particularly thankful to Dr. Mark D. Jankowski, ecotoxicologist at the U.S. Environmental Protection Agency (EPA) for his input.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2021.721892/full#supplementary-material
References
Adem Esmail, B., and Geneletti, D. (2018). Multi-criteria decision analysis for nature conservation: a review of 20 years of applications. Methods Ecol. Evol. 9, 42–53. doi: 10.1111/2041-210X.12899
Alves, F., Langmore, N., Heinsohn, R., and Stojanovic, D. (2020). ‘Self-fumigation'of nests by an endangered avian host using insecticide-treated feathers increases reproductive success more than tenfold. Anim. Conserv. 24, 239–245. doi: 10.1111/acv.12627
Anchundia, D., and Fessl, B. (2020). The conservation status of the Galapagos Martin Progne modesta: assessment of historical records and results of recent surveys. Bird. Cons. Inter. 31, 129–138. doi: 10.1017/S095927092000009X
Boulton, R., Bulgarella, M., Ramirez, I., Causton, C., and Heimpel, G. (2019). “Management of an invasive avian parasitic fly in the Galapagos Islands: is biological control a viable option?” in Island Invasives: Scaling Up to Meet the Challenge. Occasional Paper SSC No. 62, eds C. R. Veitch, M. N. Clout, A. R. Martin, J. C. Russell, and C. J. West (Gland: IUCN), p. 360.
Bulgarella, M., Knutie, S. A., Voss, M. A., Cunninghame, F., Florence-Bennett, B. J., Robson, G., et al. (2020). Sub-lethal effects of permethrin exposure on a passerine: implications for managing ectoparasites in wild bird nests. Conserv. Physiol. 8:coaa076. doi: 10.1093/conphys/coaa076
Causton, C., Fessl, B., Tebbich, S., Lahuatte, P., Pike, C., Cunninghame, F., et al. (2019). Eficacia en el uso de Métodos Para Controlar Philornis downsi en los Nidos de aves Amenazadas de Galápagos. Reporte Técnico No.5. Puerto Ayora, Galápagos, Ecuador. Charles Darwin Foundation.
Causton, C., and Lincango, M. (2014). Review of Chemical Control Methods for Use Against Philornis downsi in Nests of Threatened Galapagos Birds, with an In-Depth Nontarget Risk Assessment of Permethrin. Charles Darwin Foundation for the Galapagos Islands, Puerto Ayora, Galápagos, Ecuador.
Causton, C., Mendes, F., Teschke, I., Tores, T., Wascher, K., Poveda, C., et al. (2020). Evaluación de la Factibilidad de Usar el Inhibidor de Crecimiento de Insectos, Cyromazina, Para Tratar Nidos con las Tecnicas de Autofumigación o Rociador. Reporte Técnico; No. 02-2020. Charles Darwin Foundation for the Galapagos Islands, Puerto Ayora, Galápagos, Ecuador. Available online at: https://researchnow.flinders.edu.au/en/publications/evaluation-of-the-feasibility-of-using-the-insect-growth-inhibito (accessed June 10, 2020).
Cimadom, A., Jäger, H., Schulze, C. H., Hood-Nowotny, R., Wappl, C., and Tebbich, S. (2019). Weed management increases the detrimental effect of an invasive parasite on arboreal Darwin's finches. Biol. Conserv. 233, 93–101. doi: 10.1016/j.biocon.2019.02.025
Coloma, A., Anchundia, D., Piedrahita, P., Pike, C., and Fessl, B. (2020). Observations on the nesting of the Galapagos dove Zenaida galapagoensis in Galapagos, Ecuador. Galapagos Res. 69, 34–38.
Cunninghame, F., Causton, C., and Tapia, W. (2012). Landbird Conservation Plan: Strategies for Reversing the Decline of Passerine Birds on the Galapagos Islands. Secondary Report of the Workshop: Searching for Solutions for the Control of the Avian Parasite, Philornis downsi. Santa Cruz; Puerto Ayora, Galapagos, Ecuador. p. 26.
Davies, A., Bryce, R., and Redpath, S. (2013). Use of multicriteria decision analysis to address conservation conflicts. Conserv. Biol. 27, 936–944. doi: 10.1111/cobi.12090
Dudaniec, R. Y., Kleindorfer, S., and Fessl, B. (2006). Effects of the introduced ectoparasite Philornis downsi on haemoglobin level and nestling survival in Darwin's small ground finch (Geospiza fuliginosa). Austral Ecol. 31, 88–94. doi: 10.1111/j.1442-9993.2006.01553.x
EFSA (2008). Conclusion regarding the peer review of the pesticide risk assessment of the active substance cyromazine. EFSA J. 6:168r. doi: 10.2903/j.efsa.2007.109r
EPA (2015). Technical Overview of Ecological Risk Assessment - Analysis Phase: Ecological Effects Characterization. United States Environmental Protection Agency. Available online at: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/technical-overview-ecological-risk-assessment-0 (accessed November 12, 2020).
Fessl, B., Anchundia, D., Carrion, J., Cimadom, A., Cotin, J., Cunninghame, F., et al. (2017). Galapagos Landbirds (Passerines, Cuckoos, and Doves): Status, Threats, and Knowledge Gaps. Galapagos Report 2015-2016. GNPD, GCREG, CDF, and GC. Puerto Ayora, Galapagos, Ecuador, p. 149–160.
Fessl, B., Heimpel, G. E., and Causton, C. E. (2018). “Invasion of an avian nest parasite, Philornis downsi, to the Galapagos Islands: colonization history, adaptations to novel ecosystems, and conservation challenges” in Disease Ecology, ed. P. Parker (Cham: Springer), 213–266.
Fessl, B., Sinclair, B., and Kleindorfer, S. (2006). The life-cycle of Philornis downsi (Diptera: Muscidae) parasitizing Darwin's finches and its impacts on nestling survival. Parasitology 133, 739–747. doi: 10.1017/S0031182006001089
Fessl, B., and Tebbich, S. (2002). Philornis downsi–a recently discovered parasite on the Galápagos archipelago–a threat for Darwin's finches? Ibis 144, 445–451. doi: 10.1046/j.1474-919X.2002.00076.x
Jakob-Hoff, R. M., MacDiarmid, S. C., Lees, C., Miller, P. S., Travis, D., and Kock, R. (2014). Manual of Procedures for Wildlife Disease Risk Analysis. Paris: OIE (World Organisation for Animal Health).
Knutie, S. A., McNew, S. M., Bartlow, A. W., Vargas, D. A., and Clayton, D. H. (2014). Darwin's finches combat introduced nest parasites with fumigated cotton. Curr. Biol. 24, R355–R356. doi: 10.1016/j.cub.2014.03.058
Linkov, I., Satterstrom, F. K., Kiker, G., Seager, T. P., Bridges, T., Gardner, K. H., et al. (2006). Multicriteria decision analysis: a comprehensive decision approach for management of contaminated sediments. Risk Anal. 26, 61–78. doi: 10.1111/j.1539-6924.2006.00713.x
McNew, S. M., and Clayton, D. H. (2018). Alien invasion: biology of Philornis flies highlighting Philornis downsi, an introduced parasite of Galápagos birds. Annu. Rev. Entomol. 63, 369–387. doi: 10.1146/annurev-ento-020117-043103
National Center for Biotechnology Information (NCBI). (2020). PubChem Compound Summary for CID 47866, Cyromazine. Available online at: https://pubchem.ncbi.nlm.nih.gov/compound/Cyromazine (accessed November 10, 2020).
O'Connor, J. A., Sulloway, F. J., Robertson, J., and Kleindorfer, S. (2010). Philornis downsi parasitism is the primary cause of nestling mortality in the critically endangered Darwin's medium tree finch (Camarhynchus pauper). Biodivers. Conserv. 19, 853–866. doi: 10.1007/s10531-009-9740-1
OIE (2017). Training Manual on Wildlife Health Risk Assessment in Support of Decisions and Policies: Workshop for OIE National Focal Points for Wildlife. Paris: OIE. Available online at: http://www.oie.int/en/international-standard-setting/specialists-commissions-groups/working-groups-reports/working-group-on-wildlife-diseases/ (accessed May 13, 2020).
Smith, G. R. (2021). Logical Decisions. Available online at: http://www.logicaldecisionsshop.com/catalog/ (accessed June 02, 2020).
Tebbich, S., Cimadon, A., Cunninghame, F., Anchundia, D., Causton, C., and Fessl, B. (2019). Protocolo Para la Aplicación de Insecticidas en la Base de Nidos de Aves Terrestres Amenazadas en Galápagos. Informe Tecnico No. 03 2019. Charles Darwin Foundation, Galapagos, Ecuador.
Travis, D. A., and Smith, K. (2019). “Risk analysis framework guidance for wildlife health professionals,” in Fowler's Zoo and Wild Animal Medicine Current Therapy, Vol. 9, eds E. Miller, N. Lamberski, and P. P. Calle (Netherlands: Elsevier), 4–10. doi: 10.1016/B978-0-323-55228-8.00002-3
Keywords: Galápagos, risk analysis, multi-criteria decision analysis, cyromazine, birds, nest parasite
Citation: Bueno I, Singer RS, Yoe C, Parrish R, Travis DA and Ponder JB (2021) Optimizing Risk Management Strategies for the Control of Philornis downsi—A Threat to Birds in the Galápagos Islands. Front. Conserv. Sci. 2:721892. doi: 10.3389/fcosc.2021.721892
Received: 07 June 2021; Accepted: 02 August 2021;
Published: 24 August 2021.
Edited by:
Zhang Zejun, China West Normal University, ChinaReviewed by:
Dunwu Qi, Chengdu Research Base of Giant Panda Breeding, ChinaArtur Andriolo, Juiz de Fora Federal University, Brazil
Vanessa Hull, University of Florida, United States
Copyright © 2021 Bueno, Singer, Yoe, Parrish, Travis and Ponder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Irene Bueno, YnVlbm8wMDRAdW1uLmVkdQ==
 Irene Bueno
Irene Bueno Randall S. Singer
Randall S. Singer Charles Yoe
Charles Yoe Rees Parrish
Rees Parrish Dominic A. Travis
Dominic A. Travis Julia B. Ponder
Julia B. Ponder