
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci., 06 September 2021
Sec. Animal Conservation
Volume 2 - 2021 | https://doi.org/10.3389/fcosc.2021.719981
 Michael I. Grant1*
Michael I. Grant1* William T. White2,3
William T. White2,3 Yolarnie Amepou4
Yolarnie Amepou4 Sharon A. Appleyard2,3
Sharon A. Appleyard2,3 Leontine Baje5
Leontine Baje5 Floriaan Devloo-Delva2,6
Floriaan Devloo-Delva2,6 Pierre Feutry2
Pierre Feutry2 Dotty Ibana7
Dotty Ibana7 Dick J. Jogo8
Dick J. Jogo8 Stanley Jogo8
Stanley Jogo8 Peter M. Kyne9
Peter M. Kyne9 Ralph Mana10
Ralph Mana10 Nigel Mapmani10
Nigel Mapmani10 Anthony Nagul11
Anthony Nagul11 Darcy Roeger1
Darcy Roeger1 Colin A. Simpfendorfer1
Colin A. Simpfendorfer1 Andrew Chin1
Andrew Chin1The conservation of threatened elasmobranchs in tropical regions is challenging due to high local reliance on aquatic and marine resources. Due primarily to fishing pressure, river sharks (Glyphis) and sawfishes (Pristidae) have experienced large population declines in the Indo-Pacific. Papua New Guinea (PNG) may offer a refuge for these species, as human population density is low, and river shark and sawfish populations are thought to persist. However, few data are available on these species in PNG, and risk posed by small-scale fishers is poorly understood. This study observed elasmobranch catches in small-scale fisheries in riverine and coastal environments in the East Sepik (northern region), Gulf, and Western Provinces (southern region) of PNG. Surveys were conducted over a period of weeks to months in each region, during the dry season across seven field trips from 2017 to 2020. We observed a total of 783 elasmobranchs encompassing 38 species from 10 families. River sharks made up 29.4% of observations in the southern region, while sawfishes made up 14.8 and 20.3% in the northern and southern regions, respectively. River sharks were commonly caught by small-scale fishers in lower riverine environments in southern PNG, while sawfishes were generally less common and mainly observed through dried rostra. The primary threat to river shark and sawfish populations is their capture by small-scale fishers targeting teleosts for swim bladder. Persisting populations of river sharks and sawfishes indicate that PNG is the second known nation with viable populations of multiple species in the Indo-Pacific. However, populations are declining or at high risk of decline, and fisheries management and conservation are required to realize the potential of PNG as a long-term refuge.
Across the Indo–Pacific there is mounting concern for the conservation status of elasmobranchs (sharks and rays) (White and Kyne, 2010). The major threats of fishing pressure and habitat degradation are generally concentrated in riverine and inshore environments (Compagno and Cook, 1995). Consequently, elasmobranchs that require access to shallow coastal or riverine environments during their life history have been most affected (Dulvy et al., 2014; Grant et al., 2019). Elasmobranchs generally have slow population growth rates resulting in high vulnerability to anthropogenic pressures, and protracted population recovery times (Cortés, 1998). Conservation of elasmobranch species within riverine and inshore environments of the Indo–Pacific is extremely challenging. Most tropical nations are considered “developing” and are characterized by having high human population density, low economic stability, and often high reliance on aquatic resources (Cheung and Sumaila, 2008). Elasmobranchs have become important to the livelihoods of an increasing amount of people for food security (e.g., Vieira et al., 2017) or sale to Asian markets (Blaber et al., 2009). Furthermore, artisanal and subsistence fisheries (hereafter “small-scale fisheries”) dominate developing Indo–Pacific nations. Data on these small-scale fisheries are often lacking due to limited capacity and resources for assessment and monitoring (e.g., catch composition, catch trends, biological characteristics, human livelihood dependence) (Ban et al., 2009; White and Kyne, 2010). These factors create challenging social and cultural considerations for developing sustainable elasmobranch fishing practices in Indo–Pacific nations (White and Kyne, 2010; Booth et al., 2019).
The three Indo–Pacific river shark (genus Glyphis) and four sawfish (family Pristidae) species epitomize the extinction risk of elasmobranchs in this region. The Ganges River shark Glyphis gangeticus, northern river shark Glyphis garricki, green sawfish Pristis zijsron, and the largetooth sawfish Pristis pristis, are listed as Critically Endangered on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species (hereafter “IUCN Red List”), while the speartooth shark Glyphis glyphis, dwarf sawfish Pristis clavata, and narrow sawfish Anoxypristis cuspidata, are listed as Endangered (IUCN 2021). A large factor in the high extinction risk for river sharks and sawfishes are their life history strategies which compound their exposure to anthropogenic pressures (i.e., fisheries and habitat degradation) in both non-marine (freshwater and estuarine environments) and marine environments (Grant et al., 2019).
It is well documented that all sawfish species have experienced dramatic global declines and local extinctions within their historic Indo–Pacific distributions (Dulvy et al., 2016; Yan et al., 2021). In contrast, taxonomic issues and a lack of historic records preclude a clear understanding of the historical distribution of river shark species (Li et al., 2015). Northern Australia is presently the only nation where viable populations of G. garricki (Feutry et al., 2020), G. glyphis (Feutry et al., 2017), and four Indo–Pacific sawfish species (e.g., Peverell, 2005; Morgan et al., 2011) are known to occur. Elsewhere in the Indo–Pacific, distributions of river shark and sawfish species are generally fragmented (e.g., Elhassan, 2018), and reported encounters are infrequent (White et al., 2015; Jabado et al., 2018). However, many Indo–Pacific regions are poorly studied, and there is a need for further investigation into the status of river sharks and sawfishes in these areas. This information will facilitate the implementation of conservation actions at appropriate local and regional scales, helping to alleviate extinction risk of these species.
One nation that has recently emerged as a potential refuge for Indo–Pacific river shark and sawfish species is Papua New Guinea (PNG). A brief survey in PNG's Western Province during 2014 resulted in the scientific rediscovery of both G. garricki and G. glyphis outside of Australia (White et al., 2015). All four Indo–Pacific sawfish species were also observed in this survey (White et al., 2017a), while A. cuspidata and P. pristis were also later observed in the Gulf of Papua Prawn Trawl Fishery (White et al., 2019). Historically, sawfishes have been observed widely throughout PNG (White et al., 2017a) and these recent observations indicate the contemporary presence of all species. Surveys conducted on local knowledge of sawfishes in PNG's north (Leeney et al., 2018) and south (Grant et al., 2021) coasts further substantiate their contemporary presence in small-scale fisheries, although both studies reported declining catch frequency by local fishers. This indicates that conservation initiatives may be required in PNG to prevent similar trends of regional extinction as seen in other Indo–Pacific nations (Dulvy et al., 2016).
Further information is required to assess the viability and conservation potential of river shark and sawfish populations in PNG. While aforementioned studies have provided some preliminary information, conservation assessments and planning are impeded by a lack of data on: (1) contemporary species-specific distributions; and (2) catch frequency and exploitation level by small-scale fishers. There are presently no protection measures in place for river shark or sawfish species in PNG. This raises concern as small-scale fisheries are prominent throughout PNG's coastal and riverine environments (Leeney et al., 2018; Grant et al., 2021), and the level of threat they pose is presently not well understood for most regions where river sharks and sawfishes likely occur (White et al., 2015, 2017a).
This study surveyed small-scale fishing villages throughout riverine and coastal communities in the Western, Gulf, and East Sepik Provinces, to observe elasmobranch catch within small-scale fisheries. Information gathered aims to: (1) inform present level of threat posed by small-scale fishers; (2) provide information to inform the development of conservation initiatives for river sharks and sawfishes in PNG; and, (3) ultimately determine whether PNG has potential to provide a long-term refuge for these species within an Indo–Pacific context.
Surveys of elasmobranch catches were conducted in riverine and coastal areas on the mainland of PNG from 2017–2020. Survey locations were selected based upon historical and contemporary records of river sharks and sawfishes (White et al., 2015, 2017a) (Figure 1). Working closely with the National Fisheries Authority, Provincial Fisheries Authorities, University of Papua New Guinea, and the Piku Biodiversity Network, surveys consisted of visiting village communities and fishing camps in coastal, estuarine, and freshwater environments (Table 1). Surveys coincided with the onset of the dry season when most fishing activity occurs (~September to March), due to safer fishing conditions afforded by calmer whether. Fishers in regions surveyed primarily use gillnets of varied mesh sizes to target croakers (Sciaenidae), barramundi (Lates calcarifer), and elasmobranchs (see Leeney et al., 2018; Grant et al., 2021). With consent from village leaders, local fishermen were invited to present any elasmobranch catch, sawfish rostra, or shark fin. Two or three villages or fishing camps were typically visited each day. In some instances, a camp was set up in villages to observe catch over a period of up to 5 days.
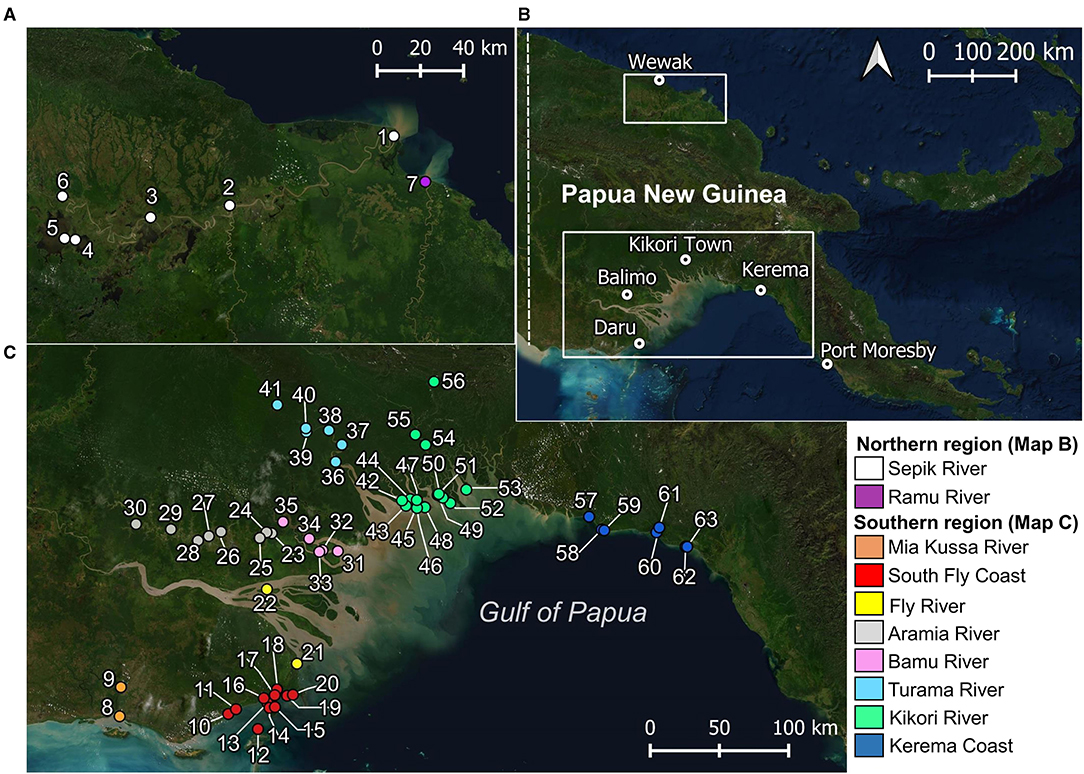
Figure 1. Locations where elasmobranchs (including fins and sawfish rostra) were encountered during surveys. (A) Papua New Guinea mainland, showing major trading centers for regions surveyed; (B) northern region surveyed; and, (C) southern region surveyed. Village names corresponding to each location in (B) and (C) are provided in Supplementary Table 1.
For each whole animal specimen encountered, stretched total length (TL) was recorded for all sharks and shark-like-rays (i.e., guitarfishes, sawfishes, and wedgefishes) and disc width (DW) was recorded for other rays. Maturity was determined by inspection of clasper calcification in males, and uteri and ovaries (presence and size of ova) in females (e.g., White et al., 2001). In most instances it was not possible to dissect specimens to determine maturity status from inspection of internal organs as catch often had to be transported to market, was on sale at market, or was quickly portioned and consumed. For small specimens, the presence of an umbilical scar (indicating recent birth) was also noted. For all specimens, gear type used in their capture was recorded and mesh size (inches) for gillnets used was noted when possible (Supplementary Tables 7, 8). When possible, tissue samples were taken from specimens for species verification.
When dried fins were encountered (sharks and shark-like-rays), the first dorsal fin (D1) from each individual present was identified and photographed. Measurements taken for D1 included, length, height, and anterior margin length (Appleyard et al., 2018). Data collected from sawfish rostra included: photographs, rostral teeth counts (left/right), total rostrum length, and standard rostrum length (these rostrum length measurements followed those described in Whitty et al., 2014). It was also noted when possible what gear type was used, and an approximate date (usually given as month/year) of capture.
In addition to our surveys, cameras and basic data sheets were left with fishers at various locations so they could enumerate shark and ray landings (hereafter referred to as “enumerators”) (Table 2). These enumerators were instructed to take photographs and record date of capture, TL (sharks and shark-like-rays), DW (other rays), sex, fishing gear used, and any other information that may be of interest (e.g., presence of embryos, litter size). It was carefully communicated to enumerators to record catch during their routine fishing operations and not to target any specific species (i.e., river sharks or sawfishes).
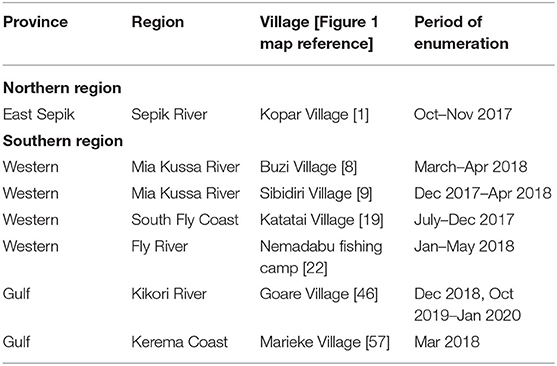
Table 2. Location of enumerators and the time period when they collected data on elasmobranch landings.
For specimens where tissue could not be taken for genetic species identification (ID) (mainly enumerator observations and sawfish rostra), species were identified either using photographs or tissue taken during observation (see Supplementary Material, “Species identification”).
Location data for all species encountered were pooled into two regions, “northern” and “southern” (Figure 1). Due to low sample sizes of maturity observations, maturity was assigned where appropriate using length-at-maturity estimations given by White et al. (2017b). Length measurements taken of sawfish rostra and dried fin (all sharks and shark-like-rays) were used to estimate TL from available relationships (Supplementary Material; Supplementary Tables 2, 3, 4).
A total of 783 elasmobranchs were observed during surveys and by enumerators across locations visited (Figure 1). Observations included 552 (70.5%) whole animals, 117 sawfish rostra (15.0%), 101 dried fins (each from a separate individual) (12.9%), 12 heads (1.5%), and one ray tail (0.1%).
In the northern region, 176 individuals were observed comprising the families Carcharhinidae (57.4%), Sphyrnidae (25.5%), Pristidae (14.8%), Glaucostegidae (1.1%), Aetobatidae (0.6%), and Rhinidae (0.6%) (Supplementary Table 7). In the southern region, 607 individuals were observed comprising the families Carcharhinidae (56.7%), Pristidae (20.3%), Sphyrnidae (11.2%), Dasyatidae (7.4%), Rhinidae (1.6%), Glaucostegidae (1.5%), Hemiscylliidae (0.5%), Aetobatidae (0.3%), Hemigaleidae (0.3%), and Orectolobidae (0.2%). Most observations in the northern (67.6%) and southern (83.4%) regions were immature size classes (Supplementary Table 8).
Threatened species comprised a large proportion of the observed catch. In the northern and southern regions, 44.3% (eight species) and 70.7% (16 species) of observations, respectively, were from species assessed as threatened with extinction on the IUCN Red List (Critically Endangered, Endangered, or Vulnerable) (Figure 2). Anoxypristis cuspidata (7.4%) and P. pristis (7.4%) accounted for 14.8% of observations in the northern region, while no river sharks were recorded in that region. Glyphis garricki (23.1%), G. glyphis (6.3%), and two Glyphis sp. (0.3%) accounted for 29.7% of observations in the southern region, with G. garricki being the most encountered species overall (n = 140). All four Indo–Pacific sawfishes, A. cuspidata (8.6%), Pristis clavata (1.5%), Pristis pristis (9.9%), Pristis zijsron (0.2%), and one Pristidae sp. (0.2%) accounted for 20.5% of observations in the southern region. Collectively, river sharks and sawfishes accounted for over half of observations (50.2%) in the southern region. However, it should be noted that sawfish rostra were more likely to be observed as they have a bias for being kept longer than shark fin or meat, as they are mostly used for decoration rather than sale when retained (Grant et al., 2021). Likewise, shark fin is sold in batches, and species that are finned were more likely to be observed than those retained only for meat (e.g., small sharks and rays that are quickly consumed or sold whole). Therefore, present observations (excluding enumerator data) should be interpreted carefully for use in relative catch rate inferences between species.
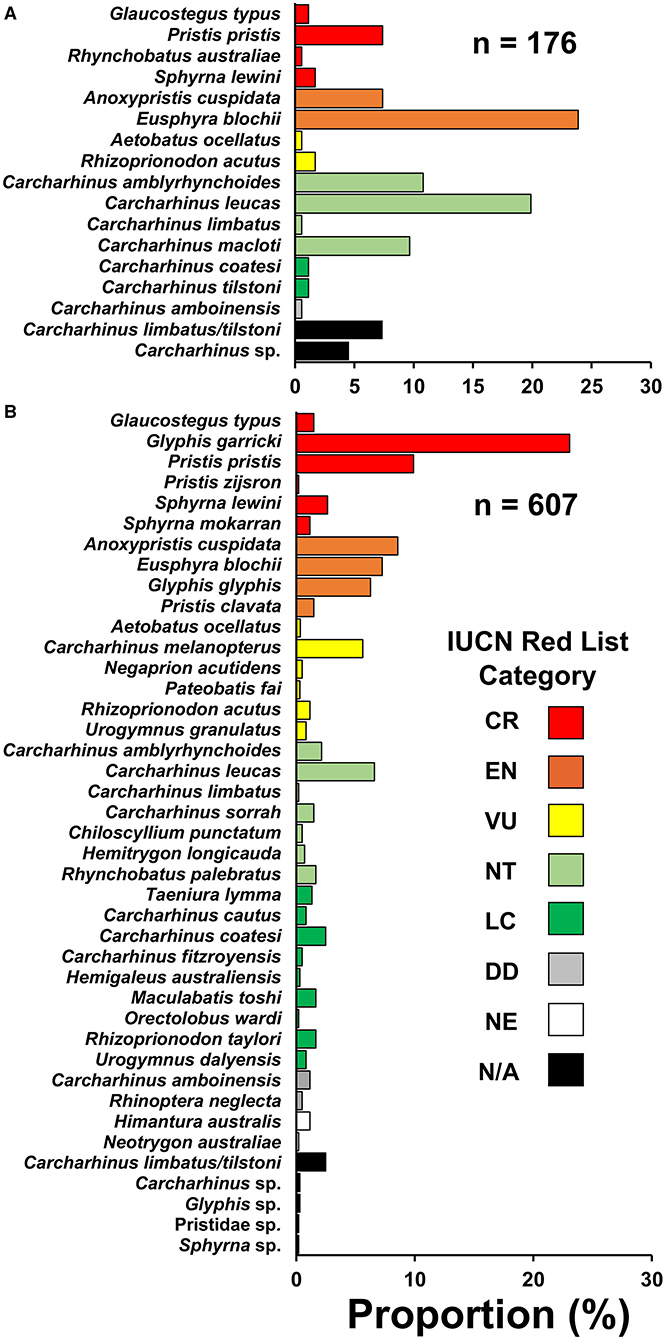
Figure 2. Species compositions encountered from small-scale fisheries in the (A) northern region and (B) southern region of Papua New Guinea. Species are categorized into their current IUCN Red List category (IUCN 2021). CR, critically endangered; EN, endangered; VU, vulnerable; NT, near threatened; LC, least concern; DD, data deficient; NE, not evaluated; N/A, not applicable.
Enumerators caught a total of 409 elasmobranchs, comprising 52.2% of the total 783 observations (Figure 3). Most enumerator observations came from Katatai Village (Figure 1, location 19), with 128 records encompassing 26 different species. Enumerators in delta regions of the Fly and Kikori Rivers (Figure 1, locations 22 and 46, respectively) recorded very high proportions of river sharks (84.9 and 62.1%, respectively). Sawfishes were recorded in low numbers at all enumerator locations except the Fly River, with the highest catch abundance (10.6%) occurring in the Mia Kussa River (Figure 1, location 9). The enumerator at Marieke Village (Figure 1, location 57) only recorded one specimen. The enumerator at Sibidiri Village (Figure 1, location 8) recorded only eight specimens due to a change in fishing gear for seasonal targeting of mud crab.
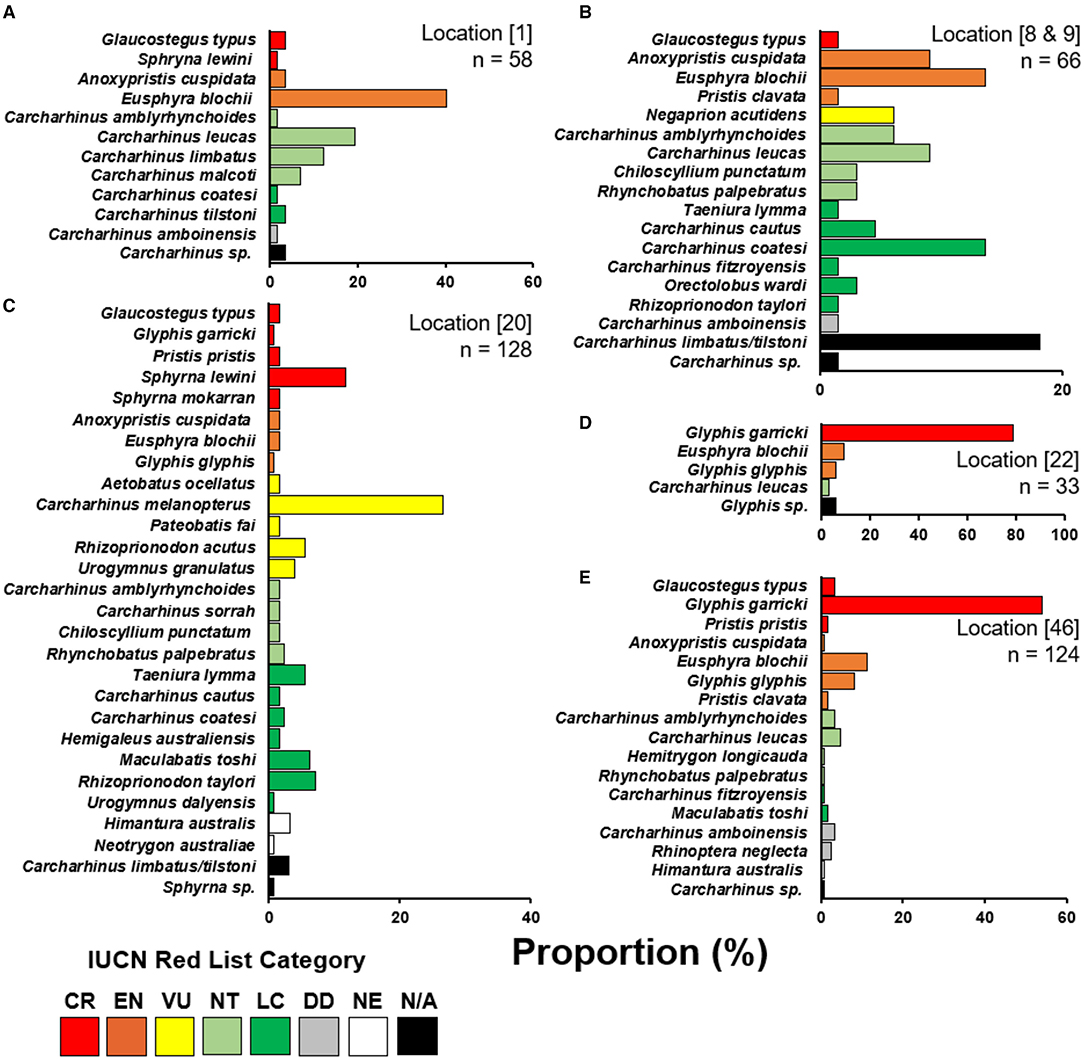
Figure 3. Species composition of enumerator recorded catch. (A) Kopar Village, Sepik River; (B) Sibidiri and Buzi Village combined, Mia Kussa River; (C) Katatai Village, South Fly Coast; (D) Nemadabu fishing camp, Fly River; and, (E) Goare Village, Kikori River. Location numbers from Figure 1 are provided in square parentheses with number of specimens (n) observed by each enumerator. Species are categorized into their current IUCN Red List category (IUCN 2021). CR, critically endangered; EN, endangered; VU, vulnerable; NT, near threatened; LC, least concern; DD, data deficient; NE, not evaluated; N/A, not applicable.
In the northern region, two sawfish species were observed. Anoxypristis cuspidata (n = 13) were observed from the mouth of both the Sepik and Ramu Rivers (Figure 4). Size classes ranged from 100.0 to 300.0 cm TL at the Sepik River (all whole specimens), while two mature sized specimens, 231.4 and 276.5 cm TL, were observed at the Ramu River mouth from dried rostra caught three months prior. Pristis pristis (n = 13) were observed from the mouth of the Sepik River, upstream to Korogu Village and Chambri Lake (Figure 1, locations 4–6). Size classes ranged from 270.4–486.9 cm TL at the mouth, while all specimens upstream were <90 cm TL. All observations were made from dried rostra except one smoked whole specimen measuring 49 cm (distorted body length from smoking). All sawfish records from examined rostra were reported to have been caught within the last two years at the time of surveys (2017).
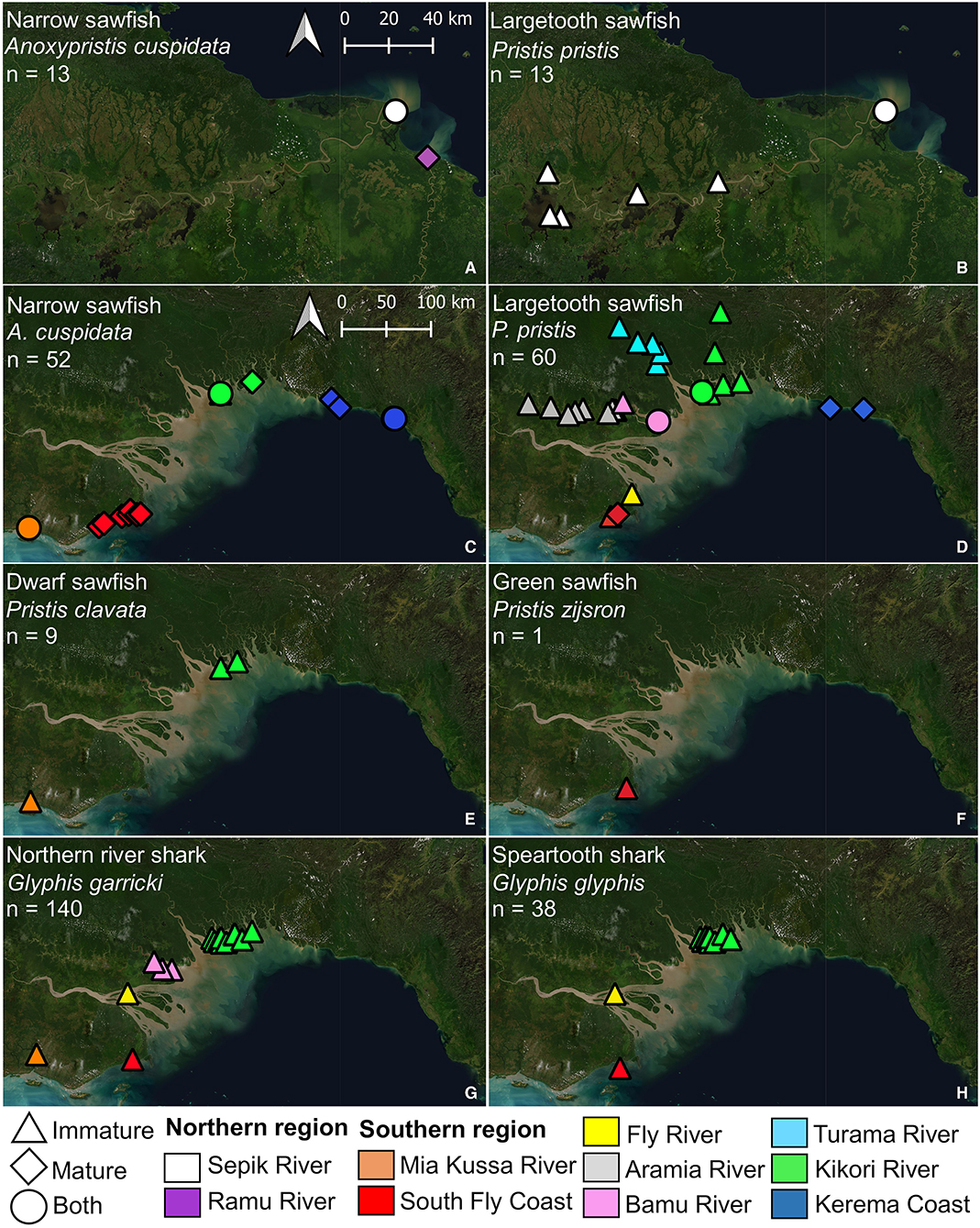
Figure 4. Locations where sawfish (A–F) and river shark (G,H) species were observed during surveys of northern (A,B) and southern Papua New Guinea (C–H). Estimated maturity of each specimen observed is indicated, and abundance (n) for each species is given.
In the southern region, all four Indo–Pacific sawfish species were observed (Figure 4). Anoxypristis cuspidata (n = 52) was observed from Mia Kussa River to the Kerema Coast. Most specimens (82.7%) observed from dried rostra (n = 46) were estimated to be mature (228.8–309.4 cm TL). Of the whole specimens observed, the enumerator at the Mia Kussa River mouth caught five immature specimens (55.0–134.0 cm TL), while the enumerator in the Kikori River Delta caught one 101.0 cm TL specimen. Pristis clavata (n = 9) was observed from the Mia Kussa River and Kikori River Delta. All were immature (103.0–248.4 cm TL). Two specimens were caught in the Kikori River Delta (203.0 and 248.4 cm TL), and one was caught at the Mia Kussa River mouth (203.9 cm TL). Other observations came from dried fin (n = 3) and rostra (n = 3) reported to be from recent catch during each of the 2018 and 2019 survey trips. Pristis pristis (n = 60) was observed from the South Fly Coast to the Kerema Coast. Observations were made from rostra (n = 54), fin (n = 3), and whole specimens (n = 3). Of the 32 rostra observed where a capture date (usually month/year) could be provided, 30 were reported to have been caught since 2016. All fins with capture date information had been caught within the month of observation. Specimens observed upstream (n = 30) from river delta environments ranged from 72.9–207.1 cm TL, although sizes were generally small with only six specimens >100 cm TL (three in each of the Aramia and Turama Rivers). Specimens observed in river deltas and coastal environments (n = 30) ranged from 99.0–561.8 cm TL, although only four of these specimens were <200 cm TL (all in the Kikori River Delta). Only one P. zijsron was observed from a historic rostrum on the South Fly Coast (352 cm TL).
Glyphis garricki (n = 140) was encountered from Mia Kussa River to Kikori River Delta (Figure 4). Glyphis glyphis was encountered from the South Fly Coast to Kikori River Delta. Specimens encountered of both species were all immature ranging from 49.0–117.0 cm TL for G. garricki, and 46.4–122.0 cm TL for G. glyphis. Glyphis garricki specimens encountered included 102 (72.9%) whole specimens and 38 (27.1%) from dried fin. Glyphis glyphis specimens encountered included 26 (68.4%) whole specimens, six (15.8%) dried fin, and six (15.8%) from heads/carcasses.
Gillnets were the most used gear by small-scale fishers in both northern and southern regions accounting for 709 (90.5%) of total catch observations, and was the only gear used in capture of elasmobranchs in the northern region (176; 100%). In the southern region, hook and line additionally accounted for 23 (2.9%) of total catch observations, while poison root was reported to be used in capture of five (0.6%) mangrove whipray Urogymnus granulatus. No information on gear type was available for 46 (5.8%) specimens. It was not always possible to record gillnet mesh size for each specimen as multiple nets of a range of mesh sizes were usually checked by fishers each fishing expedition before catch was recorded back on shore. Furthermore, many sawfish rostra and fin records were from previous catch, and fishers could not recall mesh size used. Of the 233 specimens that mesh size could be recorded for (32.9% of total gillnet catch), mesh sizes ranged from 2–8 inches (″) with a median of 6″ (Figure 5). For sawfishes, 22 specimens (18.5% of sawfish gillnet catch) had mesh size recorded ranging from 3″ and 5″-7″, with a median of 6″, and 130 Glyphis spp. (73.4% of river shark gillnet catch) had mesh size reported ranging from 4″-6″ to 8″, with a median of 6″ (Figure 5).
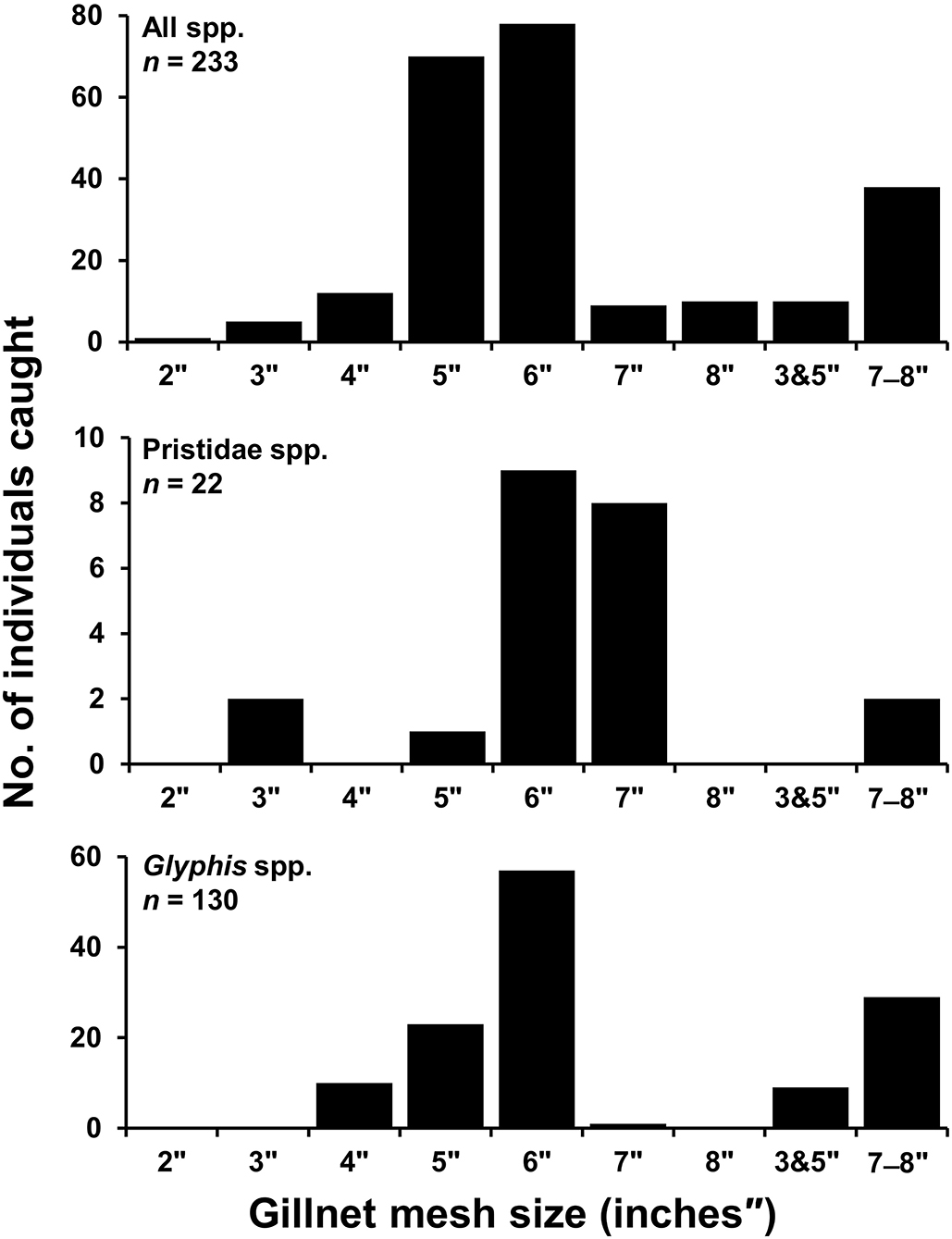
Figure 5. Number of specimens caught where records of gillnet mesh size (inches) was reported in small-scale fisheries of Papua New Guinea. All species (Top), Pristidae spp. (Middle), Glyphis spp. (Bottom). 3″ and 5″ refers to mesh sizes overlaid in a single net, 7″-8″ were records reported from either 7″ or 8″ nets though not discernible per specimen caught.
Across all regions, gillnet fishing activity was usually oriented around the tides, with nets often remaining in the water 24 h a day, only being checked and re-set if needed on low tides. On spring tide cycles fishermen generally did not fish due to increased chance of floating debris damaging nets, and a general consensus that fishing was not as productive. In freshwater environments, nets were observed soaking both night and day, although there was less tendency for fishers to leave nets soaking 24 h a day. In freshwater environments, fishing activity was mainly subsistence in nature, likely due to a lack of market access to sell excess catch (excluding the Kikori River where fishers sell catch at Kikori Town market). Gillnets were most commonly set from the bank stretching out into the channel, or perpendicular to coast, where they were secured by tying to large sharpened sticks or bamboo that were stuck into the substrate. This generally restricted fishing activity to shallow water where the high tide mark did not exceed ~5 m.
Thirty-eight elasmobranch species (22 sharks, 16 rays) were observed in PNG's small-scale fisheries. This represents 29.2% of known PNG elasmobranch diversity (130 species; White et al., 2017b) which is a similar level to the commercial Gulf of Papua prawn trawl (31% of elasmobranch diversity; White et al., 2019) and higher than the former shark long-line fishery (13.8% of elasmobranch diversity; White et al., 2020). The highest species diversity was observed in PNG's southwest (South Fly Coast and Mia Kussa River), encompassing 81.6% of species observed in this study. This diversity is likely due to fishers in this region accessing a range of estuarine, inshore, and reef habitats. In the Gulf of Papua, outflow from several major river systems results in high turbidity and lowered salinity in much of the inshore region (e.g., sediment plume evident in Figure 1). Consequently, catch was dominated by river sharks, bull sharks Carcharhinus leucas, and sawfishes, which all have increased tolerance for such conditions (Grant et al., 2019). Lower species diversity observed in the northern region was likely due to: (1) smaller spatial and temporal scale of surveys; (2) lack of estuary habitat in the Sepik and Ramu Rivers; and, (3) narrow continental shelf along the Bismarck coastline limiting inshore habitat availability (Coates, 1987).
Considering the majority of fishing activity observed in this study was in riverine and inshore environments, it is unsurprising that catch mainly comprised immature size classes. Shallow coastal environments are generally used as nurseries by inshore marine elasmobranchs (Heupel et al., 2007), with some species having a preference for river outflow areas (e.g., Heupel et al., 2019). Meanwhile, estuarine or freshwater environments are preferentially used as nurseries by euryhaline and estuarine generalist elasmobranchs (Grant et al., 2019).
It is difficult to quantify the extent of any population trends over time for river sharks in regions surveyed. The best available data are from observations by Haines (1979) in the Purari and Kikori Rivers, where river sharks (reported as Carcharhinus gangeticus or Carcharhinus glyphis) were reported to be rare. Present enumerator data in Kikori River indicates both species are commonly caught relative to other elasmobranchs. It is difficult to make interpretations about population trends in river sharks as the validity of species identifications by Haines (1979) cannot be certain due to poorly resolved taxonomy at the time (Compagno and Cook, 1995), and it is not possible to examine differences in gillnet fishing methods used between historic and present studies. However, the large number of smaller sized individuals of both species observed in this study is an encouraging indicator that breeding adults are clearly present.
The estuarine delta systems of rivers throughout the Gulf of Papua appear to be important nursery habitat for both river shark species. Despite extensive surveys in upstream freshwater environments during this study, neither species occurred far from the estuary. Similar habitat use patterns have been observed for G. glyphis (Dwyer et al., 2020) and G. garricki (Pillans et al., 2009) in northern Australia, suggesting that neither species penetrates far into freshwater reaches of rivers like other euryhaline species do (e.g., C. leucas, Dwyer et al., 2020). Meanwhile, the absence of larger size classes is likely because fishers in the Gulf of Papua remain within rivers and delta environments. Mature sized G. garricki and G. glyphis (some reportedly with near-term pups) were observed offshore (~3 km) on the South Fly Coast (Figure 1, location 19) in October 2014 by White et al. (2015). Congruently, neonates (with open umbilical scars) of both species were observed in October during this study. The absence of river sharks in the northern region can likely be attributed to the lack of estuarine environment in the Sepik River and limited coastal shelf habitat along the Bismarck coastline (Coates, 1987).
Declines in sawfish catch have recently been reported in the northern (Leeney et al., 2018) and southern (Grant et al., 2021) regions surveyed. Present enumerator data suggest a small number of interactions with A. cuspidata (excluding northern PNG), P. clavata, and P. pristis, and rostra from recently caught animals (e.g., <1 month) were not commonly encountered in surveys. Collectively, this indicates that while sawfish populations are persisting, efforts to prevent further declines and rebuild populations are required.
In southern PNG, juvenile P. pristis were observed in freshwater reaches of the Aramia, Bamu, Turama, and Kikori Rivers. Small sawfish in upstream freshwater environments were additionally reported by locals from each of the Mia Kussa and Fly Rivers in Western Province, and Purari, Vailala, and Tiamura Rivers in Gulf Province. There are numerous historic reports of P. pristis throughout rivers of southern PNG (White et al., 2017a) and this study indicates they still occur in rivers presently surveyed. Furthermore, P. pristis is typically philopatric to natal river systems (Feutry et al., 2015; Phillips et al., 2017), and their presence in multiple rivers may indicate several populations within southern PNG. However, aside from the Turama River, P. pristis did not appear common. Declines in sawfish catch frequency and in sizes caught were recently reported by fishers throughout southern PNG in freshwater reaches of the same rivers as the present study (Grant et al., 2021). While comparable historical data of P. pristis in southern PNG is limited, fisheries surveys by Haines (1979) indicate that P. pristis was commonly caught in comparison to other elasmobranchs in the Kikori River during the 1970s. Present observations and enumerator data in the Kikori River however, suggest that while P. pristis is persisting, they are not caught commonly relative to other elasmobranchs. Elsewhere in southern PNG, declines of P. pristis have been noted in the Fly River (Storey et al., 2009). Collectively, declines can be inferred for P. pristis throughout southern PNG, although given populations are persisting, declines appear less severe than in other Indo–Pacific regions, excluding northern Australia (Yan et al., 2021).
In northern PNG, P. pristis was reported to be very common in the Sepik River during surveys in the 1930s, while abundance appears to have reduced by the 1980s (Coates, 1987). Most rostra observed in present surveys were reportedly caught 1 or 2 years prior to our arrival indicating P. pristis are not presently common, as supported by Leeney et al. (2018). Pristis pristis has historically been observed upstream to Ambunti Village (White et al., 2017a) and it is possible this species occurs much further upstream and in floodplain areas not accessible in present surveys.
For the other sawfish species, comparable historic data mainly include anecdotal observations (White et al., 2017a). On the South Fly Coast, P. clavata were reported to be “common” ~100 km east of the Mia Kussa River at the mouth of the Bensbach and Morehead Rivers in the early 1970s (White et al., 2017a). In the present study, P. clavata was caught just once over the respective enumeration periods in the Mia Kussa and Kikori Rivers. While it is difficult to infer any population trend, the present enumerator observations and lack of dried rostra in fishing communities indicate this species is not commonly caught within the small-scale fishery observed.
Anoxypristis cuspidata was the most commonly encountered sawfish species in coastal and riverine delta environments. Most observations came from the Mia Kussa River mouth and Kerema Coast. Both of these regions receive significantly less river outflow than locations surveyed in the north and western Gulf of Papua, and fishing pressure was also noted to be lighter. It is unclear if the higher presence of A. cuspidata around the Mia Kussa River and Kerema Coast is a function of the environmental preferences (salinity and turbidity) of this species, or lower local fishing pressure. Anoxypristis cuspidata occurs around river and creek outflows in northern Australia suggesting tolerance to estuarine conditions (e.g., Thorburn et al., 2003), however rivers within the Gulf of Papua have considerably higher outflow volumes. On the Kerema Coast, most observations of A. cuspidata were rostra from mature sizes in coastal villages. Village leaders cited concerns about men using sawfish rostra to fight (Supplementary Material; Supplementary Figure 1) suggesting a bias for fishers to retain larger rostra. Meanwhile, only juveniles were observed at the Mia Kussa River mouth indicating it is a nursery area. In northern PNG, a single specimen of A. cuspidata was collected near the mouth of the Ramu River in 1965, although no other data is available to compare present observations. Nine specimens were caught in just 5 days of observation at Kopar Village, Sepik River mouth, with two more specimens during November 2017. Anoxypristis cuspidata likely occurs in along the coastline of the adjacent Murik lakes region to the west, and in Broken Water Bay and around the Ramu River to the east, due to very similar environmental conditions.
It is unclear if P. zijsron persists in PNG. It is hard to determine if the single specimen observed had migrated from Australia to PNG, as Green et al. (2018) suggest a similar movement was made by a male A. cuspidata. Australia to PNG migrations would be most expected on the South Fly Coast due to its closer proximity and homogeneity of adjoining habitat. Historically, P. zisjron has only been recorded in the southeast Gulf of Papua, while a single Sepik River record is uncertain (White et al., 2017a). White et al. (2017a) observed P. zijsron fins at Daru (Western Province) however, due to the presence of the Torres Strait Trade Treaty (see Busilacchi et al., 2014), it is possible that these fins originated in Australian waters.
Threats to populations of sawfishes in PNG have previously been outlined (White et al., 2017a, 2019; Leeney et al., 2018; Grant et al., 2021). Key threats identified include: (1) widespread gillnet use by small-scale fishers; (2) tendency of fishers to kill sawfishes or amputate their rostra to untangle animals from nets; (3) absence of bycatch reduction devices in the Gulf of Papua prawn trawl; (4) commercial and non-commercial markets for sawfish products including meat, fin, and rostra (Supplementary Material; Supplementary Figures 2, 3); (5) lack of enforcement and monitoring of PNG's international shark fin trade; (6) environmental degradation from mining and logging activities (e.g., Storey et al., 2009); and, (7) possible ecological implications resulting from introduced fish species. While many of these threats also apply to river sharks, conservation concern may not be as high. Only juvenile size classes of river sharks appear to be caught in substantial numbers in PNG's small-scale fisheries. Long-lived carcharhinids can withstand relatively high fisheries mortality in instances where fishing pressure is exclusive to young age classes (e.g., Smart et al., 2020). However, essential life history information (age and growth, reproductive parameters etc.) is lacking for these river shark species to make an informed assessment. We caution risk of population declines in the near future if present levels of fisheries mortality are sustained. Conversely for sawfishes, a range of size classes including breeding adults appear to be caught. This suggests that current fishing mortality in PNG's small-scale fishery may carry higher risks to sawfish populations compared to river sharks.
Only juvenile P. pristis occur in PNG's upstream freshwater environments. Very few fishers in these communities sell fin (limited access to shark fin traders) and consumption or sale of meat at local markets is opportunistic when other fish are not caught, or markets can be accessed (Leeney et al., 2018; Grant et al., 2021). The largest issue appears to be the tendency for fishers to kill or remove rostra from P. pristis entangled in gillnets, irrespective of fishers using the animal for consumption or trade (Grant et al., 2021). Therefore, conservation initiatives focused on minimizing non-essential use, coupled with better release practices have potential to be successful for P. pristis in freshwater environments.
Fishers in all coastal and lower riverine environments observed in this study primarily target teleost swim bladder (mainly from barramundi Lates calcarifer and scale croaker Nibea squamosa) (see Grant et al., 2021). Elasmobranchs (including river sharks and sawfishes) are incidentally caught in this fishery. Dried swim bladder (also called “fish maw”) is used mainly as a food or medicine in Asia, and value can be as high as $23,433 USD kg−1 in Asian markets with croakers (Sciaenidae) generally having the highest value (Sadovy de Mitcheson et al., 2019). In PNG, dried swim bladder from large individuals of L. calcarifer and N. squamosa (a sciaenid) are worth 500–1400 Papua New Guinean Kina (PGK) kg−1 (1 PGK = ~$0.28 USD, 04/04/2021) through licensed buyers in Gulf Province (Supplementary Table 9; Ibana, 2020), while in both the Western and Gulf Provinces, fishers reported value up to 10,000 PGK kg1 from non-licensed buyers (mainly for large N. squamosa, this figure was verified several times with fishers throughout 2019–2020). Comparative to the value of shark fin (inclusive of sawfish), ~1–75 PGK kg−1 in southern PNG (Busilacchi et al., 2021; Grant et al., 2021) or 100–350 PGK kg−1 in northern PNG (Leeney et al., 2018), swim bladder has a significantly higher economic incentive for local fishers.
Swim bladder values in the Gulf of Papua appear to be considerably higher than Busilacchi et al. (2021) report for legal (131 PGK kg−1) and illegal (152 PGK kg−1) markets on the South Fly Coast. The differences in value are likely due to the species of origin and weight of swim bladder. For example, in the Gulf Province, Ibana (2020) indicates that swim bladders (<0.2 kg) are worth 50–300 PGK kg−1 from smaller L. calcarifer, N. squamosa, or varied sizes of less-valued species such as catfishes (Siluriformes) and king threadfin salmon Polydactylus macrochir. The Gulf of Papua has extensive riverine habitat availability for L. calcarifer and N. squamosa, and due to less historical fishing effort compared to South Fly Coast (White et al., 2017a), it is likely that a greater availability of larger individuals are present. It is also possible that alternative market chains operate out of Gulf Province (and possibly extend to the eastern South Fly Coast) where the extremely high value of N. squamosa swim bladder in illegal markets was reported. Busilacchi et al. (2021) indicated that end user market prices for swim bladder were 18 times higher than value local South Fly Coast fishers receive. This indicates that: (1) N. squamosa is highly valued by end users, and/or (2) the market chain operating out of the Gulf Province may have more direct links to Asia and does not appear to be subject to the same incremental price increases along its market chain (i.e., an increase of 18 times for N. squamosa based on Gulf Province illegal market value would equal ~$50,400 USD kg−1 in end user markets). It remains unclear why swim bladder buyers would offer such high value to local fishers, assuming they are aware of the comparatively lower value of alternative markets. The apparent presence multiple of local markets does however complicate management of this fishery in southern PNG. Meanwhile, further information is needed on the swim bladder trade in northern PNG, including value and market chains comparative to shark fin.
For river sharks and sawfishes, the concern is that due to their overlapping habitat use with L. calcarifer and N. squamosa (lower riverine and inshore areas), they have increased vulnerability to incidental capture in the swim bladder fishery (e.g., spatial fishing effort indicated by Eisemberg et al., 2015). River sharks and sawfishes unsurprisingly were more commonly caught by large gillnet mesh sizes (5–8 inches), which are mainly used by fishers targeting swim bladder in river mouth and coastal-estuarine environments (see Grant et al., 2021 for further details on small-scale fishery characteristics). Meanwhile, small mesh sizes were observed to mainly be used by fishers in sheltered waters that are protected from tidal currents to target small fish for subsistence purposes. All reports of fishers using gillnets with 7- and 8-inch mesh came from the Fly River, and eastern South Fly Coast. In the Western Province, fishers are permitted to use a maximum mesh size of 6 inches to target L. calcarifer under the Barramundi Management Plan (NFA, 2003). Since the implementation of this management plan, N. squamosa appears to have emerged as an additional target species of local fishers, and larger mesh sizes appear to be used to target this species. This may implicate the effectiveness of 6-inch mesh size gear restriction under the Barramundi Management plan, as N. squamosa has overlapping habitat use in coastal-estuarine environments. Furthermore, we caution that future increases in fishing effort targeting high value swim bladder are likely throughout southern PNG, and this could have severe conservation consequences for river sharks and sawfishes. The disproportionate local economic value of swim bladder has had negative impacts for incidentally captured species in many regions throughout the globe (Sadovy de Mitcheson et al., 2019). Most notably in Mexico, vaquita (Phocoena sinus) faces imminent extinction resulting from illegal targeting of totoaba (Totoaba macdonaldi) for swim bladder (Rojas-Bracho et al., 2006).
Fishers targeting swim bladder (mainly lower riverine and coastal communities) generally retained all incidental elasmobranch catch, further complicating river shark and sawfish conservation approaches. While some consumption of meat occurs (Grant et al., 2021), remote communities with limited market access retain surplus elasmobranch catch for fin only, with carcasses being discarded (Supplementary Figure 3). Because fishers lack access to refrigeration, excess catch either needs to be quickly transported to market or smoked. In remote communities however, the use of fuel to travel to markets precludes its economic viability, and readily available fresh fish means smoked elasmobranch products are less marketable. Dried products such as swim bladder and shark fin are therefore more practical for local fishers (Vieira et al., 2017), notwithstanding their higher economic value than meat. Easily accessible legal and illegal trade markets for dried swim bladder and shark fin likely increase incentive for local fishers to engage in fisheries as a livelihood (Busilacchi et al., 2021; Grant et al., 2021) as other livelihood options such as agriculture are not practical in PNG's river delta environments (Allen et al., 2005). Ultimately, management is required to ensure future sustainability of PNG's inshore teleost and elasmobranch fishery resources, although the complex social and cultural characteristics of the swim bladder fishery, including its high value, present numerous challenges (Busilacchi et al., 2021; Grant et al., 2021).
A concerted effort to examine characteristics of the swim bladder fishery and incidentally caught threatened elasmobranch species is needed by PNG's National Fisheries Authority (NFA) and Conservation and Environment Protection Authority (CEPA), respectively. Furthermore, until there is a spatially broader understanding of market and trade routes, and livelihood aspects to compliment information on the South Fly Coast (Busilacchi et al., 2021), conservation of threatened incidentally caught species will be challenging as their value to local fishers is not well understood. The present lack of fisher livelihood information risks poor engagement, participation, and compliance with conservation initiatives. Better availability of information on the swim bladder fishery would also help improve enforcement efforts for illegal shark fin trade (including contravention of the Convention on the International Trade of Endangered Species of Flora and Fauna Appendix I listing of sawfishes; Grant et al., 2021), as the value of shark fin appears to be supplementary to the swim bladder trade in PNG's legal and illegal markets.
Populations of river sharks and sawfishes are persisting in PNG primarily due to low historic human population density, which has resulted in lower exposure to intense pressures experienced by these species elsewhere in the Indo–Pacific. PNG appears to be only the second nation with viable populations of both river sharks and sawfishes in the Indo–Pacific, with Australia being the other (e.g., Morgan et al., 2011). However, population pressures in PNG appear to be increasing with increases in human population. This threatens PNG's role as a refuge for remnant populations of these species into the future. The main pressures facing PNG's river sharks and sawfishes include: lack of nationally legislated species-specific protections; lack of riverine and inshore fishery management; widespread use of fishing gears that species are highly susceptible to; the economic value of river sharks and sawfishes to local fishers; and, ongoing environmental impacts from mining and logging. Despite these pressures, PNG also has many positive conservation attributes for these species: limited coastal and riverine development; free-flowing unobstructed rivers; generally low human population density (southern PNG only) relative to other Indo-Pacific nations, making capacity required for outreach and enforcement lower; customary ownership of land and waterways with general awareness and interest in environmental protection and management by local people; and, presence of adult and juvenile size classes observed in this study that currently appear to be at considerably higher population densities relative to other global regions, excluding Australia (e.g., Li et al., 2015; Yan et al., 2021). Collectively, these positive attributes are unique to PNG. Therefore, great potential exists for PNG to play a significant role in global conservation for river sharks and sawfishes as a refuge nation into the future.
Moving forward, conservation and fisheries management actions need to focus on alleviating current population pressures, and safe-guarding PNG's positive attributes where possible. There is also a need for further surveys in regions not covered in the present study where river sharks and sawfishes may also be persisting (e.g., White et al., 2017a). Understanding the extent of population distributions and specific local threats, which may differ to those identified in the present study, will assist in overall population management and development of both national and locally appropriate conservation initiatives for these species. Considerations to sawfish conservation have previously been discussed in detail (including efforts at both government and community level; Leeney et al., 2018; Grant et al., 2021), and we identify that incidental capture in the swim bladder fishery is the primary, and most immediate threat to both river shark and sawfish populations in PNG. Due to the larger expanse of river delta and coastal-estuarine habitat (where target swim bladder species occur), southern PNG has the highest imperative for inshore fishery management initiatives to be developed. Improved management of the swim bladder fishery would help manage incidentally caught elasmobranchs and be a major step forward in securing PNG as a long-term refuge for threatened river shark and sawfish species.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the animal study because all observations of sharks and rays during this study were made on deceased animals that were retained for sale and consumption in small scale fisheries in Papua New Guinea.
MG, WW, YA, LB, PK, CS, and AC developed the study design. MG, YA, LB, DI, DJ, SJ, RM, NM, AN, and DR conducted field work and data collection. MG, WW, YA, SA, LB, FD-D, PF, PK, CS, and AC analyzed data and interpreted results. All authors contributed to writing and editing of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the Save Our Seas Foundation (Keystone project number 388).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This manuscript is dedicated to that Dick J. Jogo who sadly passed away during the completion of this study. The authors wish to thank the Save Our Seas Foundation, and the Piku Biodiversity Network, Gulf Provincial Fisheries, Western Provincial Fisheries, and National Fisheries Authority for facilitating our research. We additionally would like to thank all interviewees, Councilors, and Chiefs for letting us into their community and sharing information, and to Jagara Page for support and assistance to our research since 2014. A special thanks to James Cook University staff Melissa Joyce, Megan Harris, Verona Nobel, and Ben Marriott for valuable assistance in navigating fieldwork in remote regions of Papua New Guinea. Also special mention to Mathew Young, Arthur Georges, Francis Tobias, and to all the crew members who assisted with extensive fieldwork, Samson, Baera Nawia, Obiri Bottu, Nagai Thomas, Aikaru Ba'au, Baibai, Max Aimari, Kenneth Korokai, Councilor Buara Esege (Goare Village), Jerry Mana, and Paul Aipa. We thank Jagara Page (Katatia Village), Sampson (Sibidiri Village), Daniel (Buzi Village), Obiri Bottu, Aikaru Ba'au, and Kenneth Koroku (Goare Village), Ian (Marieke Village), and Jerry (Kopar Village) for enumerating fisheries landings. MG was supported by an Australian Post-Graduate Award and PK was supported by the Marine Biodiversity Hub, a collaborative partnership supported through funding from the Australian Government's National Environmental Science Program. This study was conducted with human ethics approval (H7240) from James Cook University and the project was registered with the Conservation and Environmental Protection Authority (CEPA) of Papua New Guinea.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2021.719981/full#supplementary-material
Allen, B., Bourke, R. M., and Gibson, J. (2005). Poor rural places in Papua New Guinea. Asia Pac. Viewp. 46, 201–217. doi: 10.1111/j.1467-8373.2005.00274.x
Appleyard, S., White, W., Vieira, S., and Sabub, B. (2018). Artisanal shark fishing in Milne Bay Province, Papua New Guinea: biomass estimation from genetically identified shark and ray fins. Sci. Rep. 8, 1–12. doi: 10.1038/s41598-018-25101-8
Ban, N. C., Hansen, G. J. A., Jones, M., and Vincent, A. C. J. (2009). Systematic marine conservation planning in data-poor regions: socioeconomic data is essential. Mar. Policy 33, 794–800. doi: 10.1016/j.marpol.2009.02.011
Blaber, S. J. M., Dichmont, C. M., White, W., Buckworth, R., Sadiyah, L., Iskandar, B., et al. (2009). Elasmobranchs in southern Indonesian fisheries: the fisheries, the status of the stocks and management options. Rev. Fish Biol. Fish. 19, 367–391. doi: 10.1007/s11160-009-9110-9
Booth, H., Squires, D., and Milner-Gulland, E. J. (2019). The neglected complexities of shark fisheries, and priorities for holistic risk-based management. Ocean Coast. Manag. 182:104994. doi: 10.1016/j.ocecoaman.2019.104994
Busilacchi, S., Butler, J. R. A., Skewws, T., Posu, J., Shimada, T., Rochester, W., et al. (2014). Characterization of the traditional fisheries in the Torres Strait Treaty communities, Papua New Guinea. AFMA Torres Strait Research Project No. 2013/0802
Busilacchi, S., Butler, J. R. A., van Putten, I., Cosijn, M., Posu, J., Fitriana, R., et al. (2021). Why does illegal wildlife trade persist in spite of legal alternatives in transboundary regions? Human Dimen. Wildl. 2021, 1–18. doi: 10.1080/10871209.2021.1876963
Cheung, W. W., and Sumaila, U. R. (2008). Trade-offs between conservation and socio-economic objectives in managing a tropical marine ecosystem. Ecol. Econ. 66, 193–210. doi: 10.1016/j.ecolecon.2007.09.001
Coates, D. (1987). Consideration of fish introductions into the Sepik river, Papua New Guinea. Aquac. Res. 18, 231–241. doi: 10.1111/j.1365-2109.1987.tb00143.x
Compagno, L., and Cook, S. (1995). The exploitation and conservation of freshwater elasmobranchs: status of taxa and prospects for the future. J. Aquaricult. Aquat. Sci. 7, 62–91.
Cortés, E. (1998). Demographic analysis as an aid in shark stock assessment and management. Fish. Res. 39, 199–208. doi: 10.1016/S0165-7836(98)00183-0
Dulvy, N. K., Davidson, L. N. K., Kyne, P. M., Simpfendorfer, C. A., Harrison, L. R., Carlson, J. K., et al. (2016). Ghosts of the coast: global extinction risk and conservation of sawfishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 26, 134–153. doi: 10.1002/aqc.2525
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, P. M., Harrison, L. R., et al. (2014). Extinction risk and conservation of the world's sharks and rays. eLife 3:e00590. doi: 10.7554/eLife.00590
Dwyer, R. G., Campbell, H. A., Cramp, R. L., Burke, C. L., Micheli-Campbell, M. A., Pillans, R. D., et al. (2020). Niche partitioning between river shark species is driven by seasonal fluctuations in environmental salinity. Funct. Ecol. 34, 2170–2185. doi: 10.1111/1365-2435.13626
Eisemberg, C. C., Amepou, Y., Rose, M., Yaru, B., and Georges, A. (2015). Defining priority areas through social and biological data for the pig-nosed turtle (Carettochelys insculpta) conservation program in the Kikori Region, Papua New Guinea. J. Nat. Conserv. 28, 19–25. doi: 10.1016/j.jnc.2015.08.003
Elhassan, I. S. (2018). Occurrence of the green sawfish Pristis zijsron in the Sudanese Red Sea with observations on reproduction. Endanger. Species Res. 36, 41–47. doi: 10.3354/esr00873
Feutry, P., Berry, O., Kyne, P. M., Pillans, R. D., Hillary, R. M., Grewe, P. M., et al. (2017). Inferring contemporary and historical genetic connectivity from juveniles. Mol. Ecol. 26, 444–456. doi: 10.1111/mec.13929
Feutry, P., Devloo-Delva, F., Tran Lu, Y. A., Mona, S., Gunasekera, R. M., Johnson, G., et al. (2020). One panel to rule them all: DArTcap genotyping for population structure, historical demography, and kinship analyses, and its application to a threatened shark. Mol. Ecol. Resour.. 20, 1470–1485. doi: 10.1111/1755-0998.13204
Feutry, P., Kyne, P. M., Pillans, R. D., Chen, X., Marthick, J. R., Morgan, D. L., et al. (2015). Whole mitogenome sequencing refines population structure of the Critically Endangered sawfish Pristis pristis. Mar. Ecol. Prog. Ser. 533, 237–244. doi: 10.3354/meps11354
Grant, M. I., Kyne, P. M., Simpfendorfer, C. A., White, W. T., and Chin, A. (2019). Categorising use patterns of non-marine environments by elasmobranchs and a review of their extinction risk. Rev. Fish Biol. Fish. 29, 689–710. doi: 10.1007/s11160-019-09576-w
Grant, M. I., White, W. T., Amepou, Y., Baje, L., Diedrich, A., Ibana, D., et al. (2021). Local knowledge surveys with small-scale fishers indicate challenges to sawfish conservation in southern Papua New Guinea. Aquat. Conserv. Mar. Freshw. Ecosyst. doi: 10.1002/aqc.3678
Green, M. E., Anastasi, B. R., Hobbs, J.-P. A., Feldheim, K., McAuley, R., Peverell, S., et al. (2018). Mixed-marker approach suggests maternal philopatry and sex-biased behaviours of narrow sawfish Anoxypristis cuspidata. Endanger. Species Res. 37, 45–54. doi: 10.3354/esr00912
Haines, A. K. (1979). An ecological survey of the Lower Purari River System, Papua New Guinea, in Purari River (Wabo) Hydroelectric Scheme Environmental Studies, Vol. 6. Konedobu: Office of Environment and Conservation, Waigani and Department of Minerals and Energy
Heupel, M. R., Carlson, J., and Simpfendorfer, C. (2007). Shark nursery areas: concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 337, 287–297. doi: 10.3354/meps337287
Heupel, M. R., Munroe, S. E. M., Lédée, E. J. I., Chin, A., and Simpfendorfer, C. A. (2019). Interspecific interactions, movement patterns and habitat use in a diverse coastal shark assemblage. Mar. Biol. 166:68. doi: 10.1007/s00227-019-3511-7
Ibana, D. (2020). Fish Maw Report. Report prepared for the Gulf Provincial Administration, Fisheries and Marine Resource Division.
Jabado, R. W., Kyne, P. M., Nazareth, E., and Sutaria, D. N. (2018). A rare contemporary record of the Critically Endangered Ganges shark Glyphis gangeticus. J. Fish Biol. 92, 1663–1669. doi: 10.1111/jfb.13619
Leeney, R. H., Mana, R. R., and Dulvy, N. K. (2018). Fishers ecological knowledge of sawfishes in the Sepik and Ramu rivers, northern Papua New Guinea. Endanger. Species Res. 36, 15–26. doi: 10.3354/esr00887
Li, C., Corrigan, S., Yang, L., Straube, N., Harris, M., Hofreiter, M., et al. (2015). DNA capture reveals transoceanic gene flow in endangered river sharks. Proc. Nat. Acad. Sci. 112, 13302–13307. doi: 10.1073/pnas.1508735112
Morgan, D. L., Whitty, J. M., Phillips, N. M., Thorburn, D. C., Chaplin, J. A., and McAuley, R. (2011). North-western Australia as a hotspot for endangered elasmobranchs with particular reference to sawfishes and the northern river shark. J. R. Soc. West. Aust. 2, 345–358.
NFA (National Fisheries Authority) (2003). Barramundi Management Plan, 1–9. Port Moresby, Papua New Guinea: National Fisheries Authority.
Peverell, S. C. (2005). Distribution of sawfishes (Pristidae) in the Queensland Gulf of Carpentaria, Australia, with notes on sawfish ecology. Environ. Biol. Fish. 73, 391–402. doi: 10.1007/s10641-005-1599-8
Phillips, N. M., Chaplin, J. A., Peverell, S. C., and Morgan, D. L. (2017). Contrasting population structures of three Pristis sawfishes with different patterns of habitat use. Mar. Freshw. Res. 68, 452–460. doi: 10.1071/MF15427
Pillans, R. D., Stevens, J. D., Kyne, P. M., and Salini, J. (2009). Observations on the distribution, biology, short-term movements and habitat requirements of river sharks Glyphis spp. in northern Australia. Endanger. Species Res. 10, 321–332. doi: 10.3354/esr00206
Rojas-Bracho, L., Reeves, R. R., and Jaramillo-Legorreta, A. (2006). Conservation of the vaquita Phocoena sinus. Mamm. Rev. 36, 179–216. doi: 10.1111/j.1365-2907.2006.00088.x
Sadovy de Mitcheson, Y., To, A. W.-,l., Wong, N. W., Kwan, H. Y., and Bud, W. S. (2019). Emerging from the murk: threats, challenges and opportunities for the global swim bladder trade. Rev. Fish Biol. Fish. 29, 809–835. doi: 10.1007/s11160-019-09585-9
Smart, J. J., White, W. T., Baje, L., Chin, A., D'Alberto, B. M., Grant, M. I., et al. (2020). Can multi-species shark longline fisheries be managed sustainably using size limits? Theoretically, yes. Realistically, No. J. Appl. Ecol. 57, 1847–1860. doi: 10.1111/1365-2664.13659
Storey, A. W., Yarrao, M., Tenakanai, C., Figa, B., and Lynas, J. (2009). Use of changes in fish assemblages in the Fly River system, Papua New Guinea, to assess effects of the Ok Tedi copper mine. Dev. Earth Environ. Sci. 9, 427–462. doi: 10.1016/S1571-9197(08)00412-6
Thorburn, D., Peverell, S., Stevens, J., Last, P., and Rowland, A. (2003). Status of freshwater and estuarine elasmobranchs in northern Australia. Final Report to Natural Heritage Trust
Vieira, S., Kinch, J., White, W., and Yaman, L. (2017). Artisanal shark fishing in the Louisiade Archipelago, Papua New Guinea: socio-economic characteristics and management options. Ocean Coast. Manag. 137, 43–56. doi: 10.1016/j.ocecoaman.2016.12.009
White, W., Baje, L., Simpfendorfer, C., Appleyard, S., Chin, A., Sabub, B., et al. (2019). Elasmobranch bycatch in the demersal prawn trawl fishery in the Gulf of Papua, Papua New Guinea. Sci. Rep. 9, 1–16. doi: 10.1038/s41598-019-45715-w
White, W. T., Appleyard, S. A., Kyne, P. M., and Mana, R. R. (2017a). Sawfishes in Papua New Guinea: a preliminary investigation into their status and level of exploitation. Endanger. Species Res. 32, 277–291. doi: 10.3354/esr00810
White, W. T., Appleyard, S. A., Sabub, B., Kyne, P. M., Harris, M., Lis, R., et al. (2015). Rediscovery of the threatened river sharks, Glyphis garricki and G. glyphis, in Papua New Guinea. PLoS ONE. 10:e0140075. doi: 10.1371/journal.pone.0140075
White, W. T., Baje, L., Appleyard, S. A., Chin, A., Smart, J. J., and Simpfendorfer, C. A. (2020). Shark longline fishery of Papua New Guinea: size and species composition and spatial variation of the catches. Mar. Freshw. Res. 71, 627–640. doi: 10.1071/MF19191
White, W. T., Baje, L., Sabub, B., Appleyard, S. A., Pogonoski, J. J., and Mana, R. R. (2017b). Sharks and rays of Papua New Guinea. ACIAR Monograph No. 189. Australian Centre for International Agricultural Research, Canberra.
White, W. T., and Kyne, P. M. (2010). The status of chondrichthyan conservation in the Indo-Australasian region. J. Fish Biol. 76, 2090–2117. doi: 10.1111/j.1095-8649.2010.02654.x
White, W. T., Platell, M. E., and Potter, I. C. (2001). Relationship between reproductive biology and age composition and growth in Urolophus lobatus (Batoidea: Urolophidae). Mar. Biol. 138, 135–147. doi: 10.1007/s002270000436
Whitty, J. M., Phillips, N. M., Thorburn, D. C., Simpfendorfer, C. A., Field, I., Peverell, S. C., et al. (2014). Utility of rostra in the identification of Australian sawfishes (Chondrichthyes: Pristidae). Aquat. Conserv. Mar. Freshw. Ecosyst. 24, 791–804. doi: 10.1002/aqc.2398
Keywords: Glyphis, Pristidae, riverine, small-scale fisheries, threatened species, swim bladder
Citation: Grant MI, White WT, Amepou Y, Appleyard SA, Baje L, Devloo-Delva F, Feutry P, Ibana D, Jogo DJ, Jogo S, Kyne PM, Mana R, Mapmani N, Nagul A, Roeger D, Simpfendorfer CA and Chin A (2021) Papua New Guinea: A Potential Refuge for Threatened Indo–Pacific River Sharks and Sawfishes. Front. Conserv. Sci. 2:719981. doi: 10.3389/fcosc.2021.719981
Received: 03 June 2021; Accepted: 09 August 2021;
Published: 06 September 2021.
Edited by:
Oded Berger-Tal, Ben-Gurion University of the Negev, IsraelReviewed by:
Jeff Kinch, National Fisheries College, Papua New GuineaCopyright © 2021 Grant, White, Amepou, Appleyard, Baje, Devloo-Delva, Feutry, Ibana, Jogo, Jogo, Kyne, Mana, Mapmani, Nagul, Roeger, Simpfendorfer and Chin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael I. Grant, bWljaGFlbC5ncmFudDRAbXkuamN1LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.