
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Conserv. Sci., 19 August 2021
Sec. Animal Conservation
Volume 2 - 2021 | https://doi.org/10.3389/fcosc.2021.703358
This article is part of the Research TopicAnimal Behavior After Translocation into Novel EnvironmentsView all 11 articles
 Petra Kaczensky1,2,3*
Petra Kaczensky1,2,3* Albert Salemgareyev4
Albert Salemgareyev4 John D. C. Linnell1,3
John D. C. Linnell1,3 Steffen Zuther4,5
Steffen Zuther4,5 Chris Walzer2,6
Chris Walzer2,6 Nikolaus Huber2
Nikolaus Huber2 Thierry Petit7
Thierry Petit7Asiatic wild ass, or kulan (Equus hemionus kulan) were once a key species of the Eurasian steppes and deserts. In Kazakhstan they went extinct by the 1930s. Early reintroductions have reestablished the species in two protected areas, but the species has reclaimed <1% of their former range and remained absent from central Kazakhstan. To initiate restoration in this vast region, we captured and transported a first group of nine wild kulan to a large pre-release enclosure in the Torgai region in 2017, and two more in 2019. We used direct observations and post-release movement data of four kulan equipped with GPS-Iridium collars to document their adaptation process in a vast novel habitat without conspecifics. For comparison with movements in the source populations, we additionally equipped two kulan in Altyn Emel National Park and six in Barsa Kelmes State Nature Reserve. The nine transported kulan formed a cohesive group with very high movement correlation in the enclosure. After release, the group initially stayed tightly together but started to break up by mid-May and all kulan travelled independently by mid-August. With 48,680–136,953 km2, the 95% Autocorrelated Kernel Density Estimation ranges of the reintroduced kulan were huge and about 10–100 times larger than those in the source populations. The reintroduced mares never reconnected, there was no evidence of successful reproduction, and two of the four collared mares were killed by poachers and one died of natural causes. At least one stallion survived in the wild, but the fate of the other uncollared animals remains unclear. We speculate that the fission-fusion dynamics and low movement correlation of kulan societies and the need for migratory movements harbours the risk that animals released into a novel environment loose contact with each other. This risk is likely enhanced in steppe habitats where movement constraining factors are absent. Further kulan reintroductions to the steppes and deserts of central Kazakhstan should aim to release larger groups and build up the free-ranging population quickly to reach a critical mass, increasing the chance of kulan encountering conspecifics to successfully breed and increase their chances of survival.
Old-growth steppe ecosystems store large amounts of carbon and support a great diversity of plant and animal taxa (Török et al., 2016; Wesche et al., 2016; Nerlekar and Veldman, 2020), including a diversity of charismatic ungulates, many of which are migratory (Kauffman et al., 2021). However, land conversion, degradation, and fragmentation has made temperate grasslands one of the most endangered terrestrial biomes. Most of the remaining near-natural grasslands are found in Central Asia, especially in Kazakhstan (Kamp et al., 2016). With the dissolution of the Soviet Union, Kazakhstan experienced dramatic socio-economic changes. While some of these changes have been negative for species conservation (e.g., through the breakdown of management structures that prevented overhunting or controlled poaching; (Milner-Gulland et al., 2003), others, such as large scale rural-urban migration of the human population, have created new opportunities for landscape-level biodiversity conservation and species recovery (Baumann et al., 2020; Freitag et al., 2021).
Large herbivores are important species for the functioning of steppe ecosystems but require large areas to accommodate their seasonal movements (Kauffman et al., 2021). Asiatic wild ass, or kulan (Equus hemionous), were once a key species in the assemblage of large herbivores [along with saiga antelope (Saiga tatarica), several gazelle species and Przewalski's horses (Equus ferus przewaskii)] that ranged the Eurasian steppes and deserts, stretching from the eastern shores of the Mediterranean to China (Bennett et al., 2017). Overhunting and habitat conversion decimated their populations and today they are only found on less than 3% of their historic global distribution range. While it is still possible to see large herds of kulan (E. h. hemionus) in the Gobi Desert of Mongolia (Buuveibaatar et al., 2017), the species only persists in small, isolated fragments in the rest of Central Asia. Consequently, the Central Asian subspecies or ecotypes (E. h. kulan and E. h. onager) are listed as Endangered on the IUCN Red List (Kaczensky et al., 2015).
In Kazakhstan, kulan became extinct in the 1930s, but reintroduction initiatives already started in the early 1950s. Today, kulan are again found in two separate locations, in Barsa Kelmes State Nature Reserve (SNR) in south-western Kazakhstan and in Altyn Emel National Park (NP) in south-eastern Kazakhstan. Two additional reintroduction attempts at different localities (Akatau-Buzachy and Andassay Sanctuary) were not successful (Kaczensky et al., 2016). With an estimated 500 and 3,000 kulan in Barsa Kelmes SNR and Altyn Emel NP respectively, Kazakhstan has become the most important stronghold for this endangered subspecies/ecotype of the Asiatic wild ass (Kaczensky et al., 2018a). Nevertheless, kulan have only reclaimed a tiny fraction of their former range in Kazakhstan and remain absent from the vast steppe and desert regions of central Kazakhstan, so that plans for further kulan reintroductions have been ongoing for decades.
The early reintroduction of kulan to Barsa Kelmes island was well documented (Rashek, 1966; Bannikov, 1981). Subsequent reintroductions, which lacked the confining borders of an island, were less well documented and radio collars were not used on kulan in Kazakhstan. Hence, there is very limited understanding of the factors which lead to the success of a kulan reintroduction (Kaczensky et al., 2016). There is also a total lack of baseline data on kulan movements and range sizes from Kazakhstan against which to evaluate the movements of translocated kulan. Historic observations from central Kazakhstan suggest that kulan migrated between the steppe regions in the north in spring and the desert-steppe regions in the south in fall (Bannikov, 1981, Bekenov and Fadeev, 1984). The same movement strategy is still employed by the Betpak-Dala saiga population to track greenery in summer and avoiding deep snow in winter (Bekenov et al., 1998; CMS, 2019).
In Mongolia range sizes of kulan are huge, and animals move in a nomadic fashion, travelling cumulative annual distances of 6,000–7,000 km and roaming over areas of 7,000–70,000 km2, which makes them one of the most mobile terrestrial mammal species (Kaczensky et al., 2011a; Tucker et al., 2018; Joly et al., 2019; Nandintsetseg et al., 2019). The large scale-movements of kulan make them particularly vulnerable to habitat fragmentation and linear infrastructure with high traffic volume or fences constitute serious barriers to their movements (Linnell et al., 2016; CMS, 2020). Other factors known to negatively influence kulan range use are human activities (Buuveibaatar et al., 2016), and steep terrain (Kaczensky et al., 2011a). Kulan live in fission-fusion groups of frequently changing membership (Rubenstein et al., 2015; Renan et al., 2018) and individual kulan seem to show little overall movement coordination (Calabrese et al., 2018). However, kulan movements are strongly influenced by their need to access water, restricting pasture use to within commuting distances of waterpoints, but also resulting in kulan converging at these localities (Nandintsetseg et al., 2016; Payne et al., 2020) facilitating fission-fusion group dynamics and potentially allowing for social learning, including movement strategies (Brakes et al., 2021).
Little data is available on individual post-release movements of reintroduced kulan, but experiences with other large mammals suggest that “soft release” helps create social bonds and reduces the likelihood of homing and large-scale exploratory movements (Mertes et al., 2019; Resende et al., 2021) in line with natal habitat preference induction (NHPI, Stamps and Swaisgood, 2007). Captive-bred kulan reintroduced in Israel following a soft release approach, initially settled in a ca. 200 km2 area close to the release site where water and good food were available, although bachelor males were observed as far as 70 km away (Saltz et al., 2000). However, reintroduced kulan in Israel seem to generally have small home ranges closely associated with artificial water points (Giotto et al., 2015) and more cohesive societies due to the scarcity of resources (Rubenstein et al., 2007).
Reintroducing ungulates into habitats where they showed migratory movement in the past - as kulan did along the steppe to desert gradient in central Kazakhstan - is even more challenging as there is a lack of understanding if, under what circumstances, and how quickly migratory or far-ranging behaviour can be restored with naïve animals (Kauffman et al., 2021, in press). Evidence from bighorn sheep reintroductions in North America suggest that learning and cultural transmission are the key mechanisms behind their migratory behaviour and it took generations for reintroduced populations to resume this behaviour (Jesmer et al., 2018). Hence, movement behaviour in the early stage of a reintroduction may not yet reflect the original and most adaptive movement behaviour, potentially also because reintroduced animals may initially key in on cues from their natal range rather than track seasonal food availability (Stamps and Swaisgood, 2007).
The Altyn Dala Conservation Initiative aims to conserve and recover nationally and internationally important flagship species and their habitats in the steppe and semi desert zones of Kazakhstan (Zuther et al., 2018). Although the main focus has been on the conservation of the autochthonous saiga populations, the initiative also aims to reintroduce kulan and Przewalski's horse to re-establish the original large ungulate assemblage along the steppe, desert steppe, and desert habitats of Kazakhstan, particularly along the steppe to desert gradient south of Torgai (subsequently referred to “Torgai region”) (Kaczensky, 2011).
The three-year pilot phase of the kulan reintroduction anticipated the transport of 16-18 kulan per year in 2017, 2018, and 2019 using a large transport helicopter to quickly build up a breeding population (Kaczensky et al., 2017). However, we were met by a series of logistical problems which in the end only allowed us to airlift nine wild captured kulan from Altyn Emel NP in October 2017 and transport two additional wild captured kulan by truck from Barsa Kelmes in October 2019 (Kaczensky et al., 2018b, 2020). The first group of kulan transported in 2017, was kept in a large pre-release enclosure in the Torgai region over the winter and was released in April 2018, while the two kulan from Barsa Kelmes have remained in the enclosure since arrival in 2019.
To document the fate of the first group of reintroduced kulan and inform future translocations to the Torgai region, we analysed the movement data of reintroduced kulan and compared it with those of kulan in the two source populations. We expected reintroduced kulan to:
(1) Show a fission-fusion group dynamics and low movement coordination, but re-connection of animals in places they had previously explored together or in the vicinity of the pre-release area.
(2) Show exploratory movements followed by establishing a movement routine and decreasing proportions of new areas visited.
(3) Show the following movement characteristics:
• Initially, show movements similar to those in the source populations, especially given the abundance of pasture and water at the release site.
• Over time, re-establish migratory behaviour as described for historic kulan or seen in the Betpak-Dala saiga population.
• Be constrained in their movements by topography, rivers, linear infrastructure and human presence.
The Torgai region is located in central Kazakhstan in the south part of the Kostanay province. The release site with the 55-ha pre-release enclosure is located at the abandoned village of Alibi on the Uly-Zhylanshyk river about 80 km southeast of the town of Torgai. Alibi is strategically located in the Torgai region within a ca. 40,000 km2 network of protected areas (Altyn Dala and Irgiz-Torgai SNR), ecological corridors, and two hunting areas managed for conservation (Figure 1).
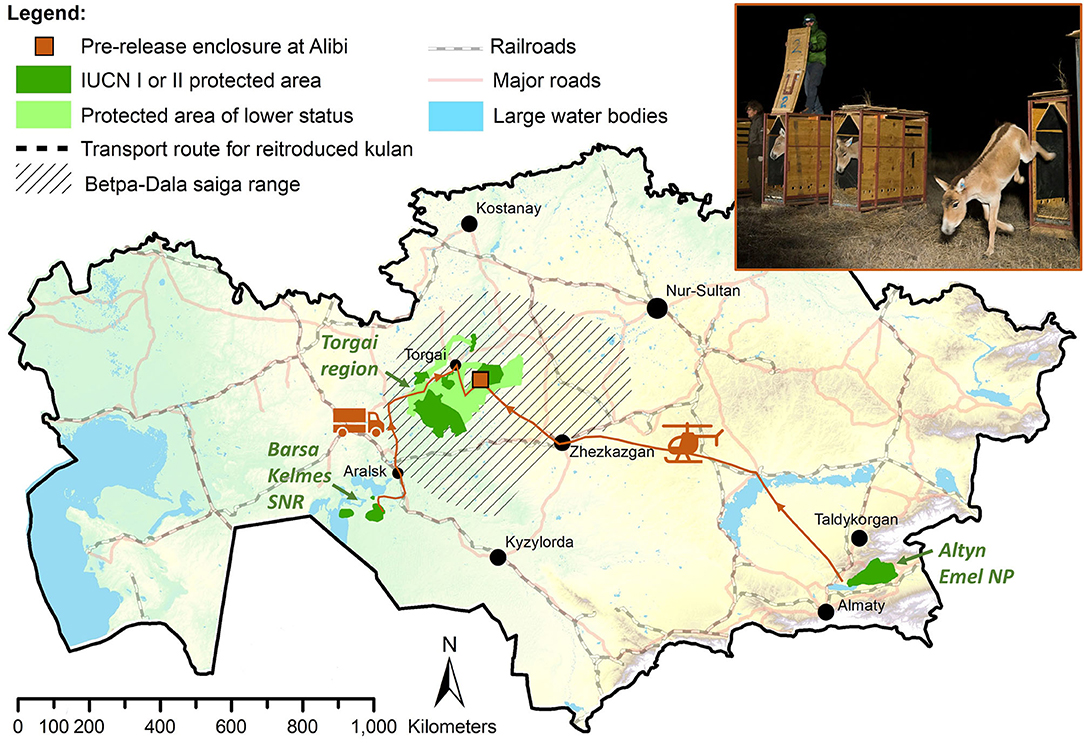
Figure 1. Study areas of kulan in Kazakhstan. Nine kulan were reintroduced to the Torgai region in central Kazakhstan from Altyn Emel National Park (NP) in 2017 and two kulan from Barsa Kelmes State Nature Reserve (SNR) in 2019. Photo: Daniel Rosengren, Frankfurt Zoological Society.
The climate is strongly continental with hot summers and cold winters, the average annual temperature is 7°C, annual precipitation averages 200 mm and is relatively evenly distributed throughout the year, with slight peaks in spring and fall/winter. About 25% of the precipitation falls as snow, with snow cover lasting on average from December through March and reaching up to 20 cm but varying considerably among years (from 7 to 30 cm; all climate data from the meteorological station in Torgai).
The Torgai region is located at the transition zone between the steppe and the semi-desert zone. Geologically it is part of the Torgai basin, which stretches towards the Aral Sea basin in the south. The terrain is flat, and elevations rarely exceed 200 m. Two larger perennial rivers flow through the area; the Torgai river in the north and the Uly-Zhylanshyk river in the centre. There are also several permanent large lakes, a multitude of small ones, and many artificial ponds which were created as livestock watering places during the Soviet era (and most of which retain water year-round).
There is a clear north-south gradient in precipitation and pasture productivity. While in the north steppe plant communities dominate, there is a gradual shift towards desert communities, typical for semi-desert and desert vegetation, towards the south. The vegetation cover in the area is not homogeneous, and in many places sharp boundaries between vegetation communities can be found, caused by the meso- and microrelief in combination with varying soil conditions and the influence of wildfires. The richest vegetation communities are along the Uly-Zhylanshyk river valley.
Human population density in the region is extremely low, ranging from 0.05 to 0.32 inhabitants/km2, and is declining. Many settlements have become abandoned (Lenk, 2008). No major roads cross the Torgai region, but a new, unfenced, single-track railway (Zhezkazgan-Saksaulskiy corridor) was built in 2013–2014 near the southern edge and has been operational since 2015 (Olson, 2014).
The Torgai region is an important core habitat for the Betpak Dala saiga apopulation, which is currently recovering after a Pasteurella multocida induced mass-die off in 2015 (Kock et al., 2018). Other large mammals found in the Torgai region include Siberian roe deer (Capreolus pygargus), wild boar (Sus scrofa), and grey wolf (Canis lupus).
Altyn Emel NP is located in south-eastern Kazakhstan in Almaty province. The NP was established in 1996 and covers 5,700 km2. Its southern boundary is the Illy river and the Kapchagai reservoir and its northern boundary is made up of the Sholak mountains, a spur of the Dzungarian Alatau mountain range. Elevations range from 470 to 2,900 m, the average annual temperature on the plains is 8°C and average annual precipitation is 370 mm with peaks in May and October. The habitat in the plains is characterised by semi-desert and desert vegetation dominated by shrubs and semi-shrubs. The NP is subdivided by a central valley with irrigation agriculture and a string of small villages. The eastern part is extremely dry with only minimal water sources. The western part has several oases on the plains and springs in the foothills of the Sholak mountains. The Ily river is flanked by large reed beds, but the shore of the Kapchagai reservoir allows relatively easy and open access to the water for plains ungulates (Figure 1; Supplementary Figure 5).
Kulan were reintroduced to Altyn Emel NP from 1982–1994 with 32 founders coming from the reintroduced population on Barsa Kelmes island in the Aral Sea (Kaczensky et al., 2018a). Besides kulan, the large ungulate community consists of goitered gazelle (Gazella subgutturosa), wild boar, a few Przewalski's horse (from a failed reintroduction) and grey wolf on the plains, and Siberian ibex (Capra sibirica), argali (Ovis ammon), and snow leopard (Panthera uncia) in the mountains. The kulan population is estimated to number ca. 3,000 animals (Kaczensky et al., 2018a).
Barsa Kelmes SNR is located in Kyzylorda province in south-western Kazakhstan. It used to be a 133 km2 island in the Aral Sea, established as a wildlife refuge in 1939. In the early 1950s, wild kulan from Badhyz, in south-eastern Turkmenistan, saiga antelopes and goitered gazelles were released on the island. In the 1980s, the water level of the Aral Sea started to drop rapidly, and salinity increased dramatically (Edelstein et al., 2012). Without access to suitable drinking water, kulan started to leave the island and found a new home in and around Kaskakulan, another former island with three man-made artesian springs. In 2006, the SNR was expanded to its current size of 1,601 km2 which includes Kaskakulan and the surrounding area. The SNR includes no human inhabitation and is only accessible via unmaintained dirt roads.
Elevations range from 35 to 108 m, average annual temperature is 9°C and average annual precipitation is 132 mm with peaks in April and October–December. Vegetation is dominated by desert shrub vegetation, but also includes parts of the former seabed largely devoid of vegetation or covered by sand (Dimeyeva et al., 2012). Water is extremely scarce and largely limited to the artesian springs on Kaskakulan. Towards the west and north, outside the SNR, there are several lakes and irrigation channels, but these areas are also used by livestock herders, primarily for free-ranging domestic horses and cattle (Figure 1; Supplementary Figure 6). Besides kulan, the large mammal community consists of small populations of goitered gazelle, wild boar, saiga antelope, and grey wolf. The kulan population was estimated at ca. 500 animals (Kaczensky et al., 2018a).
Kulan were captured by chasing them into large capture corrals at night using several jeeps and strong hand-held lights to guide their movements following methods described in Levanov et al. (2013) or by darting them with a CO2-powered rifle from a pursuing jeep as described by Walzer et al. (2007); for further capture details see Kaczensky et al. (2018b, 2020). Kulan were anaesthetised for collaring and loading into the transport boxes with Etorphine in combination with Butorphanol and Detomidine as described in Walzer (2014).
The overall health status and condition of the animals were assessed visually and based on blood chemistry values (Gerritsmann et al., 2016) and leucocyte coping capacity [LCC; a proxy for stress (Huber et al., 2019)] obtained on-site with a portable VetScan® (VS2, Abaxis) on equine settings (Equine Profile Plus) and a high sensitivity chemiluminometer (Junior LB 9509, Berthold Technologies, Germany) respectively. During anaesthesia, all kulan were marked with coloured ear tags and fully grown adult kulan were equipped with GPS satellite collars (Vertex Lite or Vertex Plus, Vectronics Aerospace, Berlin, Germany; Supplementary Table 1). Kulan selected for transport also received long-acting neuroleptics (LANs; a mixture of Haloperidol and Perphenazine-decanoate) to keep them calm and stable during transport and in the initial release phase into the pre-release enclosure (Walzer, 2014).
In October 2017, the first group of nine wild kulan (four adult mares, four foals, one subadult stallion) were captured and airlifted by helicopter over 1300 km from Altyn Emel NP to a 55-ha pre-release enclosure at Alibi in the Torgai region (Figure 1), where they were held for 5 months over the winter until release in early April 2018 (Kaczensky et al., 2018b; Gliga et al., 2020). In 2019, two additional wild kulan (one adult mare, one subadult stallion) were successfully transported via truck over 850 km from Barsa Kelmes SNR to the pre-release enclosure (Figure 1; Supplementary Table 1; Kaczensky et al., 2020). In the pre-release enclosure, body condition and behaviour were observed twice a day. From late fall to early spring, kulan were provided with hay, and water in troughs during periods when the oxbow lake in the enclosure was frozen and there was no snow on the ground (Gliga et al., 2020).
With only 11 kulan transported 2017–2019, we fell short of the original plan to transport 16-18 kulan per year. Logistical problems during capture in 2017 resulted in the transport of only a small number of the kulan captured (Kaczensky et al., 2018a). A five-fold increase of the price for the transport helicopter in 2018 forced us to aim for a mixed truck–aeroplane–truck transport the following year, which only allowed for a narrow transport window, which we failed to meet because drought conditions made kulan capture in Altyn Emel NP very challenging. In 2019, we aimed for a truck transport from Barsa Kelmes SNR, which is much closer than Altyn Emel NP, but capture was hindered by issues with the capture corral and the treacherous terrain on the former Aral Sea seabed. Furthermore, with a transport time of 23 h non-stop driving under ideal conditions, the animals and crew were clearly coming to the limits of their endurance, with little safety margin left in case of problems with the transport vehicle or road conditions (Kaczensky et al., 2020).
In the Torgai region we monitored the movement of four adult mares reintroduced from Altyn Emel NP in October 2017 and released from the pre-release enclosure in April 2018. We additionally monitored the movements of one adult mare reintroduced from Barsa Kelmes in 2019 in the pre-release enclosure, where she still is today and gave birth to a foal on 2 June 2021. We also monitored the movements of kulan in the two source populations: two kulan in Altyn Emel NP collared in 2017 (they had to be released back into the wild due to their exited behaviour when loaded into transport boxes) and of six kulan in the source population in Barsa Kelmes SNR collared in 2019 (Table 1; Supplementary Table 1; for further details see Kaczensky et al., 2018b, 2020). All collars were programmed to take 1 GPS location per hour and were equipped with pre-programmed drop-offs (CR-2A, Telonics, Mesa, AZ, USA).
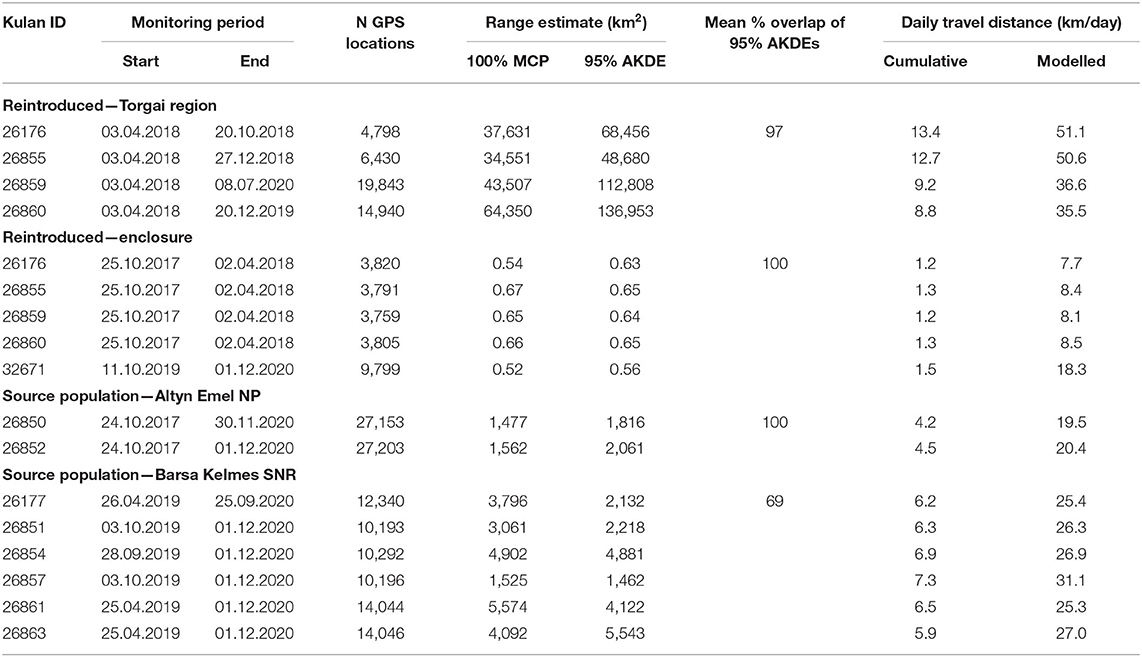
Table 1. Movement characteristics of kulan reintroduced to the Torgai region as compared to those in the source populations in Altyn Emel NP and Barsa Kelmes SNR (for full model results and CI see Supplementary Table 2).
After release from the pre-release enclosure, rangers attempted to locate and observe the collared kulan every two months. However, long-flight distances only made it possible to see collars whereas ear tags were largely invisible and numbers impossible to read. Once a collar became stationary, ACBK rangers checked the location for a dropped collar or a deceased kulan (for details on ground monitoring see: Kaczensky et al., 2020).
To assess how synchronised kulan movements were pre- and post-release, we calculated movement correlation, deconstructed into a drift (directional), a diffusive (social), and a summarised overall component with R package corrMove as described in Calabrese et al. (2018). The different models tested for were: (1) uncorrelated drift and uncorrelated diffusion (UU), (2) correlated drift and uncorrelated diffusion (CU), (3) uncorrelated drift and correlated diffusion (UC), and (4) correlated drift and correlated diffusion (CC). The algorithm further calculates the change date where movement correlation changes from one type to another.
To identify when kulan separated and whether they re-connected again, we calculated the pair-wise daily distances between all kulan pairs. To check for kulan association with the release site, we calculated the straight-line distance (net displacement—NSD) of locations to the pre-release enclosure.
To calculate range sizes, average distances travelled per day, and average range overlap among kulan, we used variograms and continuous-time movement models (ctmms) in the ctmmweb interface (Calabrese et al., 2021) of the R package ctmm (Calabrese et al., 2016). The ctmmweb interface allows for automated model fitting after visual inspection and calculates autocorrelated lifetime kernel density estimation (AKDE) home-range estimators and associated movement parameters with confidence intervals. For visualisation of movements, we used the R package MoveVis (Schwalb-Willmann et al., 2020).
To allow for comparison with conventional home-range estimates, we also calculated the minimum convex polygon around all GPS locations (100% MCPs) and also used this approach to visualise how much new area was incorporated into each kulan's range as consecutive weeks of GPS locations were included in the calculation.
Behavioural observations over the winter 2017/18 documented that the nine kulan in the pre-release enclosure formed a cohesive group (Figure 2; Gliga et al., 2020) which resulted in a very high level of movement correlation [97% correlated diffusion (UC) indicative of social correlation]. After release in early April, the group initially stayed tightly together (100% correlated diffusion UC) but started to split up in mid-May (mare 26860 on 21.05.2018, mare 26859 on 02.06.2018, and mare 26855 on 19.08.2018), which resulted in a drop of the UC to only 15%; the drift correlation remained 0% suggesting that there was no tendency for the kulan to move in the same direction. After the spilt-up, kulan rarely came within ≤ 10 km of each other again (Figure 3). Movement coordination among kulan in the source populations was similar in Barsa Kelmes SNR (UC = 13% with a change point on 20.05.2020) but was constantly higher among the two kulan in Altyn Emel NP (UC = 31%; see Supplementary Figure 1).

Figure 2. Strong group cohesion and high movement correlation characterised the movement of kulan in the adaptation enclosure. Image taken by mare 26176's camera collar.
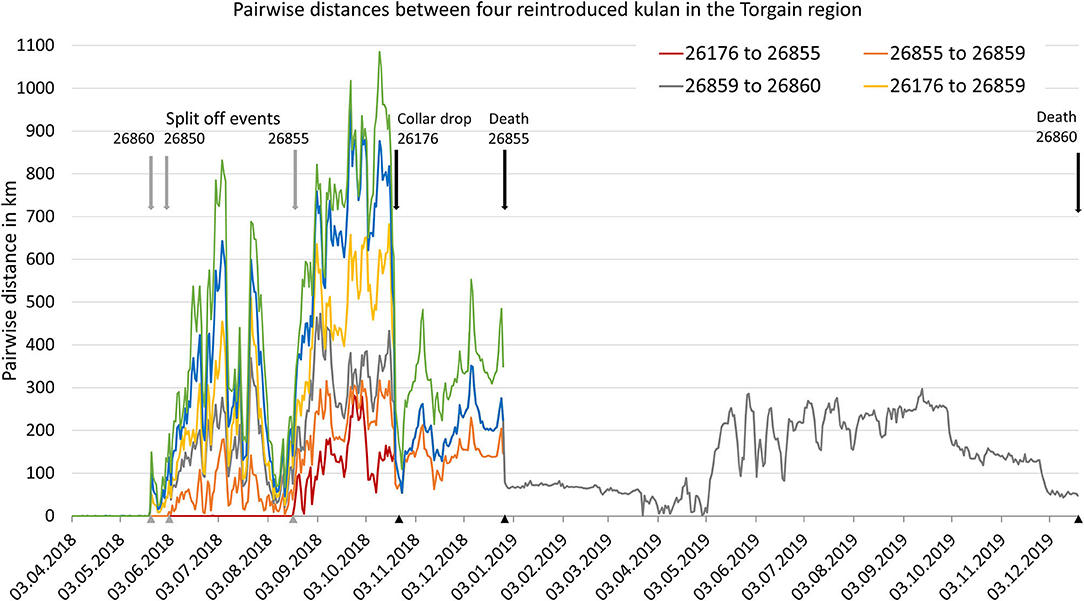
Figure 3. Pairwise distances between reintroduced kulan in the Torgai region after release from the pre-release enclosure on 03.04.2017.
Ground monitoring of reintroduced kulan showed that after the split-up, mare 26860 and 26855 were travelling alone, mare 26859 with the subadult stallion, and mare 26176 with two yearlings (Figure 4). We did not document the presence of a new foal for any of the reintroduced mares during the monitoring period. Monitoring of the four collared mares successively ended with the pre-programmed drop of the collar of mare 26176 on 20.10.2018, and the subsequent deaths of mare 26855 on 27.12.2018 (poached), mare 26860 on 20.12.2019 (poached), and mare 26859 on 08.07.2020 (natural mortality). On 27.05.2021, the (formally subadult) stallion with ear tag #12 who had been seen travelling with mare 26859 in 2020 appeared outside the pre-release enclosure.

Figure 4. Image from mare 26176's camera collar showing two yearlings feeding in the Torgai region after release and after split-up from the other three GPS collared mares.
The first 7 days after release in April 2018, the group stayed within 20 km of the pre-release area, but then went on two exploration trips (10 April−5 May 2018) towards the desert ca. 140 km to the south-east of the pre-release area, both times returning to the vicinity of the pre-release enclosure. After the return from the second trip, the group immediately went to the desert-steppe and steppe north and east where they stayed until mare 26859 split off. After splitting up, kulan continued to range far separately, but also kept returning to the vicinity of the pre-release area (Figure 5).
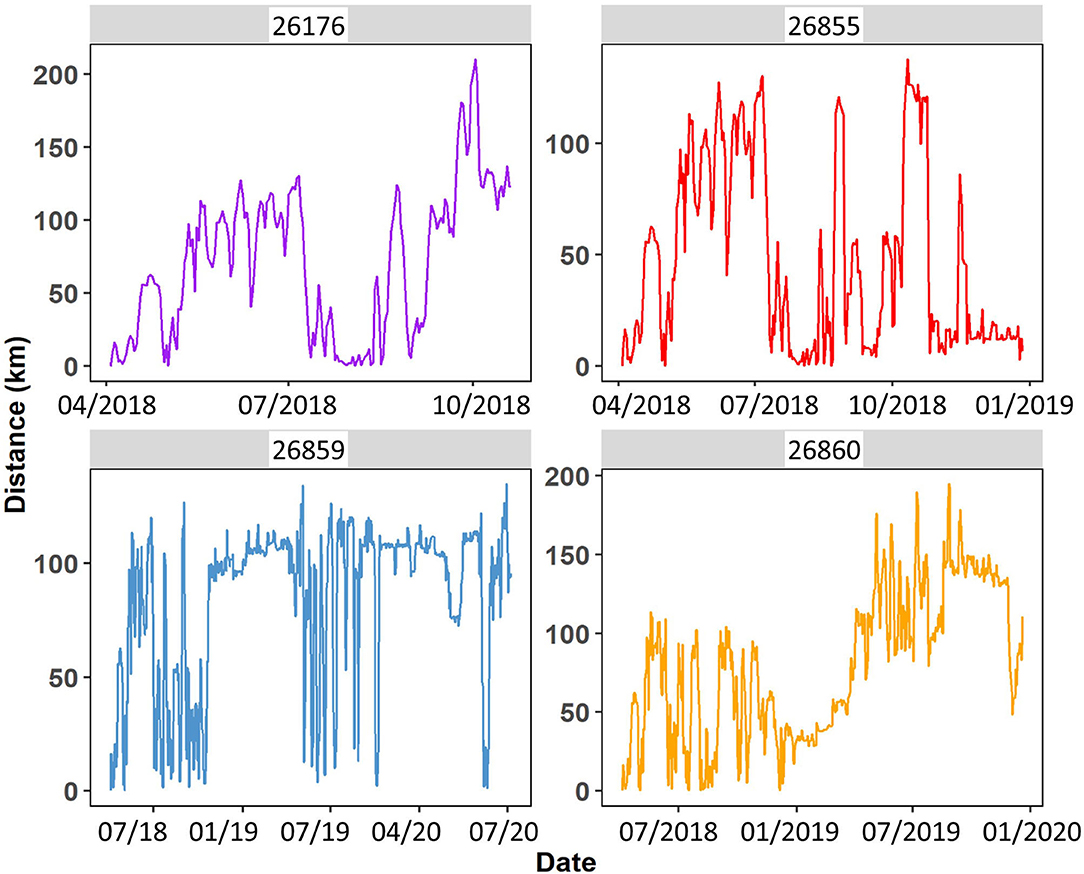
Figure 5. Distance to pre-release enclosure by four reintroduced kulan in the Torgai region April 2018 until July 2020.
The ctmms supported a range-resident movement model for all kulan (both reintroduced and in the source populations). The best fitting model for all kulan was the general OUF model indicative of a home-range, and autocorrelated positions and velocities indicated an anisotrophic (non-circular) home range shape (Supplementary Table 2). The variograms for the ctmms showed an initial steep increase but reached a plateau within 1–4 months (Supplementary Figure 2).
Modelled daily distances travelled were 36–51 km for reintroduced kulan, which is about 2–3 times larger than for kulan in the source populations (Table 1). The 95% AKDEs of reintroduced kulan were huge covering 48,680–136,953 km2, which is 10–100 times larger than those of kulan in the source populations (Table 1 and Figure 6; Supplementary Table 2). The total area covered by the combined 95% AKDEs of the four kulan in the Torgai region was 152,875 km2 (Supplementary Figure 4).
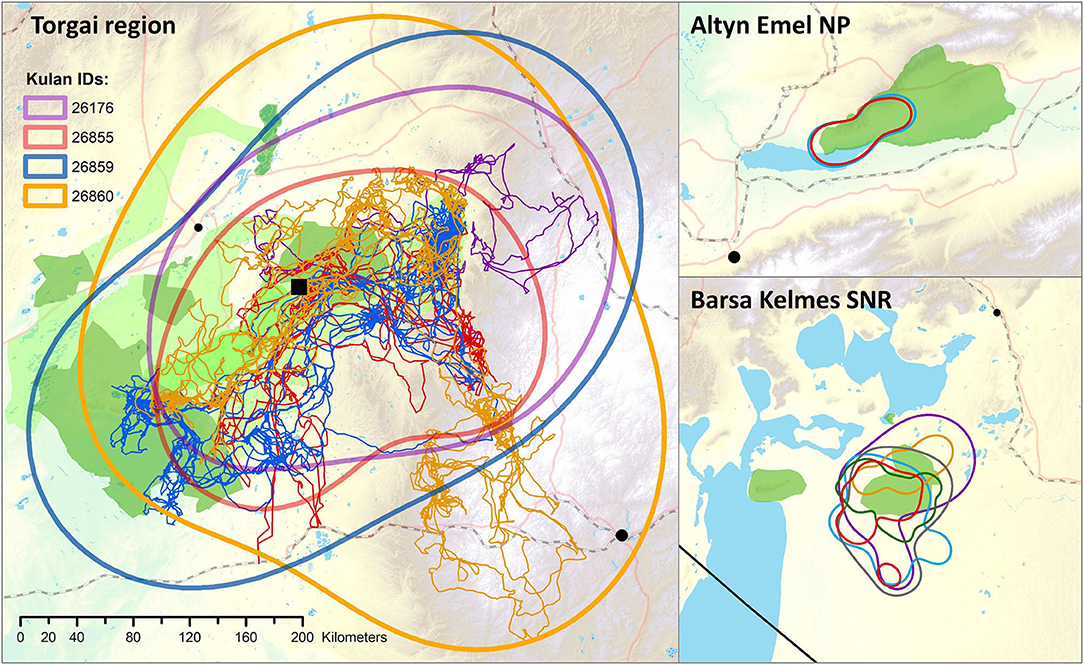
Figure 6. Movement paths and 95% Autocorrelated Kernel Density Estimates (AKDE) of reintroduced kulan in the Torgai region (April 2018 to July 2020). The smaller panels show the 95% ADKEs of kulan in the source populations in Altyn Emel National Park (NP; October 2017–01.12.2020) and Barsa Kelmes State Nature Reserve (SNR; April 2019–01.12.2020). All maps are on the same scale and share the scalebar for the Torgai region. For an overview of the study areas and the legend of landscape features see Figure 1. The black line at the south-western corner in Barsa Kelmes is the international border between Kazakhstan and Uzbekistan.
The 100% MCP covered over time by the reintroduced kulan showed a steep increase at the onset, a short temporary plateau after 15 weeks, followed by further increases. The two kulan monitored the longest reached a plateau in the summer of the second year, while the two kulan monitored over <1 year did not reach a plateau while being monitored (Figure 7).
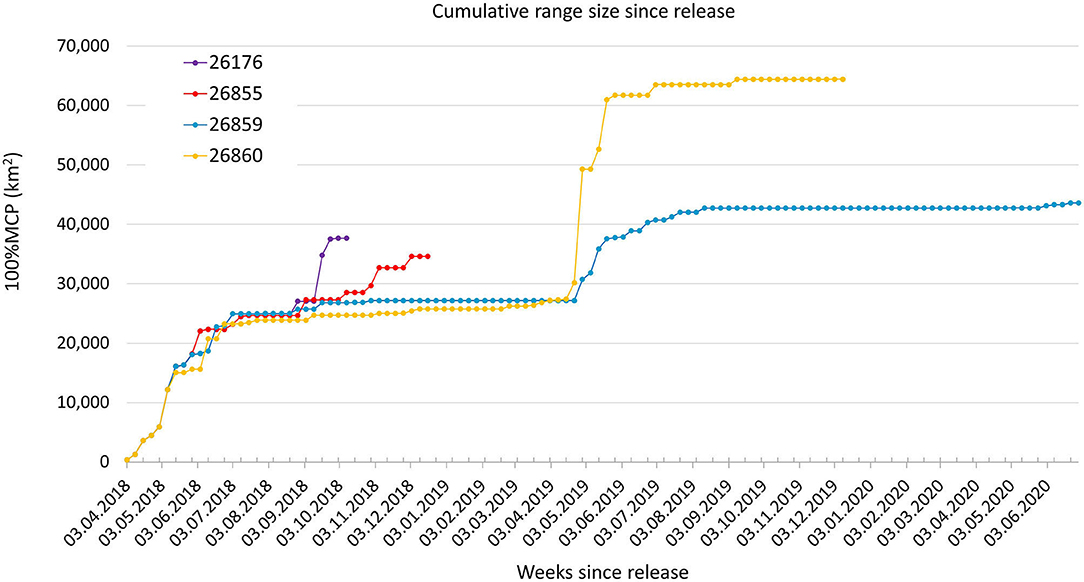
Figure 7. Range size development of reintroduced kulan in the Torgai region since release. MCP100% were re-calculated at weekly intervals.
Reintroduced kulan in the Torgai region did not spend the winter in the same general location and there was no evidence for a southward migration in winter. Only mare 26855 spent her first winter ca. 100 km to the south of the pre-release enclosure into the desert but was then killed by poachers. Mares 26859 and 26860 spent both winters in adjacent, non-overlapping areas ca. 100 km to the north and north-east of the pre-release enclosure in the steppe, and mare 26176 was also in this general area when her collar dropped in October 2018 (Supplementary Figure 3).
The ranges of reintroduced kulan overlapped almost entirely, and movements of individual kulan extended up to ca. 300 km away from the pre-release area to the southeast, ca. 200 km to the east and west, and ca. 60 km to the north (Table 1, Figure 6). In the east, mare 27176 crossed a railway twice (back and forth within 24 h), and a nearby connective road four times. In the south, mare 26860 crossed the new Zhezkazgan-Saksaulskiy railway 8 times and a nearby connecting road 11 times, but mare 26855 appeared to have “bounced off” the same railway track at two locations further to the west (Figure 6). All kulan crossed the Uly-Zhylanshyk river to the north of the pre-release enclosure, but none crossed the larger Torgai river further north and consequently no kulan came close to the connecting road between Torgai and Arkalyk (Figure 6; for 1-years animation of movements see Torgai region).
In Altyn Emel NP, the two collared kulan only used the western part of the NP and did not cross the central valley with its string of villages and irrigated agricultural land. Kulan also hardly ever went beyond the western boundary of the PA and stayed away from the steeper and higher reaches of the Sholak mountains. In the south the large Ily river and the Kapchagai reservoir were never crossed (Supplementary Figure 5; for a 1-years animation of movements see Altyn Emel)
In Barsa Kelmes SNP, kulan never ventured onto the most recently exposed part of the former seabed and none of the collared kulan travelled to Barsa Kelmes island. Kulan also did not venture far beyond the eastern SNR boundary into irrigated land or land used for livestock grazing (Supplementary Figure 6; for a 1-years animation of movements see Barsa Kelmes).
The reintroduced kulan behaved like a group of horses in the pre-release enclosure, forming a tightly knit and extremely coordinated group with animals staying together virtually the entire time and with little signs of aggression (Gliga et al., 2020). After release the kulan initially stayed together and were highly synchronised in their movements, but the group broke up starting in mid-May. This point-in-time coincided with the normal birthing and mating period of kulan in the Altyn Emel source population, and the two mares which split off first had faecal oestrogen and progesterone levels which suggested that they were pregnant (Kaczensky et al., 2020). It is therefore quite possible that they left the group or stayed behind to give birth (Estep et al., 1995; Kaczensky et al., 2019).
The remaining two mares stayed together with three yearlings for another 2 months, but then also separated. We can only speculate what triggered the separation, but it could well have been the lack of a mating partner for the adult mares. Although kulan don't form stable groups, females are mostly encountered in groups and seem to profit from the combined vigilance of multiple members in the group to avoid predators (Wang et al., 2016; Buuveibaatar et al., 2017). Being alone, and especially being alone in an unknown area, is risky and this may explain why we did not observe any foals of the year in the two pregnant mares (they most likely were lost to predation) in 2017, while the lack of a mating partner during the mating season which immediately follows the birthing season did not allow for a foal the following year. However, with only the adult mares equipped with radio collars, our ability to follow up on the fate of unmarked individuals was very limited.
Given their fission-fusion society, kulan likely do not hesitate to leave a social group, especially if they originate from a large population where overall cohesion may be lower than in smaller populations (Rubenstein et al., 2007). In Altyn Emel, ca. 3000 kulan roam over a relatively small area where water and pasture are limited and which they have known all their lives, making it easy to find conspecifics. The reintroduced kulan in the Torgai region lacked all these advantages and once separated most likely did not find each other again, pointing towards the importance of social learning in the context of reintroduction projects (Brakes et al., 2021). That kulan were seeking company became apparent from an observation of mare 26860 grazing together with a group of domestic horses in February 2019 (Kaczensky et al., 2020).
The soft release approach may not have dampened post-release exploratory behaviour, but at least kulan did stay in the vicinity of the pre-release enclosure during the first week post-release and made the first exploratory movements as a group. NHPI predicts that dispersing or translocated (basically “assisted dispersal”) animals are more likely to settle in areas which are similar to their natal habitat. Translocated into novel habitats, they may embark on long-distance movements in search for familiar cues or to avoid unknown cues (Stamps and Swaisgood, 2007). We can only speculate about the motivation for the initial long-distance excursions, but we doubt they were forage, water, or predator induced. The kulan had thrived on the pasture in the pre-release enclosure and hardly touched the hay, water was abundant in the release area, and wolves were a known predator also present in Altyn Emel NP. However, what was missing were any cues of other kulan, which are plentiful in Altyn Emel NP. With the foaling and subsequent mating season coming up in a couple weeks in June, kulan may have explored the area for the presence of other kulan.
Despite showing long-distance movements, kulan seemed to have bonded with the area as all animals kept coming back to the pre-release area and their home ranges were centred around the pre-release enclosure. Unfortunately, kulan did not return at the same time to allow them to re-connect. The small number of kulan, heightened by the loss of two adult mares to poaching early in the reintroduction certainly did not help and may have resulted in too few or too faint cues for kulan to find each other. However, if kulan capture in 2018 had been successful, the presence of new kulan in the enclosure might have acted as an attraction for free ranging kulan, thus increasing the chance that free ranging kulan stay around and re-connect. In the Przewalski's horse reintroduction project in the Mongolian Gobi, the pre-release enclosure has continuously attracting free-ranging Przewalski's horses (mainly bachelor males), some of which even jumped the fence to join a captive group (P. Kaczensky unpubl. data).
And as proof of concept, in late May 2021, the stallion released as a subadult in 2018 (and recognisable by a blue ear tag with #12) suddenly showed up outside the pre-release enclosure, just days before the birth of a foal by mare 32671 on 02.06.2021. The stallion may well have been attracted by the upcoming post-partum estrous. This recent observation is very encouraging as it not only shows that kulan can survive in the Torgai region over multiple seasons, but they are able to re-connect with conspecifics and that the pre-release enclosure may act as a strong attraction point in a landscape otherwise largely devoid of cues of other kulan.
Reintroduced kulan in the Torgai region were much more mobile than their respective peers with whom they were captured in Altyn Emel NP in 2017. Thus, the more productive pasture and the abundance of water did not result in lower mobility. Although initial exploratory movements were to be expected, the two reintroduced kulan which we monitored over two winters showed little indication of restricting their movements, but rather kept exploring some new areas to the south-east (mare 26860) and south-west (mare 26859). The range sizes of the reintroduced kulan in the Torgai region are in the same order of magnitude of those of kulan from Mongolia's South Gobi Region (Kaczensky et al., 2011b; Payne et al., 2020), but contrary to kulan in the Gobi, which seem to be primarily nomadic (Noonan et al., 2020), the ctmm analysis indicated a clear home-range for the reintroduced kulan.
Kulan 26860 and 26859 both returned to the same area in the second winter which further suggests the animals had settled, but also points towards a possible re-emergence of migratory behaviour as documented for a zebra population in Botswana (Bartlam-Brooks et al., 2011). However, contrary to our expectation and reports from the past (Bannikov, 1981) these two kulan spent the winter in the steppe and roamed both north and south during the reminder of the year. However, re-establishment of the most adaptive movement behaviour may take time (Jesmer et al., 2018). At least exploratory behaviour had brought all kulan in contact with the full gradient of the Torgai region from desert habitats in the south to steppe habitat in the north and this knowledge may eventually enable surviving kulan to fine-tune their movements to avoid deep snow and access the seasonally most nutritional pastures. Given our very small sample and short monitoring period relative to the potential lifespan of a kulan, which is well over 20 years (Lkhagvasuren et al., 2017), these preliminary results should not be over-interpreted.
The huge differences in daily movements and range size between the reintroduced kulan and those in the source populations in Altyn Emel NP and Barsa Kelmes SNR are puzzling. Pasture and water are more abundant in the steppe, so the question emerges as to why we see these large-scale movements of reintroduced kulan in the Torgai region. Alternatively, the question can be framed as to why we see these small-scale movements in the semi-desert of Altyn Emel NP and Barsa Kelmes SNR when kulan in the Mongolian Gobi also have huge ranges in a similar semi-desert habitat?
The movement of kulan in the Torgai region suggested that smaller rivers do not act as significant barriers, but larger ones, especially those associated with a broad band of dense vegetation probably do. The reintroduced kulan moved so far that two reached the newly constructed Zhezkazgan-Saksaulskiy railway in the south and another railway in the east and also encountered some larger connective roads. These structures have a barrier effect if traffic volume is high, but as our GPS data and experience from Mongolia showed, kulan can cross them - as long as they are not fenced (Batsaikhan et al., 2014).
In Altyn Emel NP, kulan are constrained in their movements by the Sholak mountains in the north and the Ily river and Kapchagai reservoir in the south. Movement to the west is discouraged by protected area staff with the help of a ditch dug along the western boundary and by actively chasing kulan groups back into the NP if encountered near or outside the western border (M. Sydygaliev pers. comm, 2017). This is done to protect kulan from poaching outside the NP. That kulan do not move further east may have to do with the presence of humans, livestock, and irrigated land along the central valley and the general lack of water in the eastern part of the NP. However, data from more kulan will be needed to confirm this assumption.
In Barsa Kelmes, no large infrastructure, rivers or topography restrict kulan movements. However, to the west the recently exposed seabed of the Aral sea constitutes a barrier as it has a treacherous substrate, is almost devoid of vegetation, and the remaining water in the basin is too saline to drink (Edelstein et al., 2012). Other than the Island of Barsa Kelmes, which has no drinking water, there is no suitable habitat to the west. We do not know why kulan do not expand further north, east, and south, but this may well have to do with the lack of protection. Anywhere outside the SNP where there is water and pasture there are small villages or livestock farms. Poaching also seems to be a problem and anti-poaching control is one of the main tasks of the SNR rangers (G. Satekeyev pers. comm. 2017).
It appears that in both Altyn Emel and Barsa Kelmes a combination of natural and anthropogenic factors restrict kulan movement. In contrast, in the Torgai region there are few features other than large rivers and salt lakes that restrict movement and human and livestock presence is minimal, which was one of the main reasons for selecting this area as a reintroduction site. We therefore speculate that kulan will naturally exhibit very high mobility in a landscape like the Torgai steppe when freed from anthropogenic constraints (Tucker et al., 2018). However, the downside is that there are few features which guide movements. This makes finding a highly mobile species like a kulan similar to the proverbial quest for a needle in the haystack. Even with GPS collars, rangers had a hard time to find the animals due to the kulan's high mobility, the time delay in the transmission of GPS fixes, and the lack of cell phone coverage. This difficulty to regularly check on released animals, makes them vulnerable to poaching as confirmed by the killing of two of our four GPS tagged kulan.
We believe that the main reason for the poor success of the three-year pilot phase of the kulan reintroduction to the Torgai region was the small number of kulan released, which was well below the minimum of 30 animals of past successful wild ass reintroductions (Kaczensky et al., 2016). We speculate that the fission-fusion dynamics and low movement correlation of kulan societies harbours the risk that animals released into a novel environment lose contact with each other. We believe, that this risk is particularly high if only a small number of animals is released and that it is further enhanced in steppe habitats where topographic features constraining movements are largely absent and where forage and water are more abundant and widely available than in desert-steppe or desert habitats.
Future kulan reintroductions into the Torgai region of central Kazakhstan should aim to release larger groups of kulan as originally planned. Some losses and initially lower reproductive success must be expected (Saltz and Rubenstein, 1995; Kaczensky et al., 2016) and it is therefore crucial to build up the free-ranging population as quickly as possible to reach a critical mass to increase the chance of kulan encountering conspecifics to successfully breed and increase their chance of survival.
We see the large ranges and high mobility of the reintroduced kulan as a sign of success as kulan along the steppe desert gradient should not be expected to stay only in the steppe or only in the desert habitat, but rather migrate between those two on a seasonal basis. Large ranges and high mobility are the best adaptation to highly variable climatic conditions or extreme events and can lower the risk of mass mortality due to droughts or extreme winters (“dzud”) as has been shown for kulan in Mongolia (Kaczensky et al., 2011b).
The use of a pre-release enclosure seems to result in animals settling in the wider area and having kulan in the enclosure has shown to act as an attraction point for free-ranging animals, making it easier for them to re-connect with other free-ranging conspecifics. Future releases should aim to keep kulan in the pre-enclosure until after foals are born and mating has happened in the hope that this will lower the mortality risk for foals and increase the chances of new foals in the following year. However, such an approach needs to be carefully monitored (Gliga et al., 2020) as kulan held in captivity can show highly aggressive behaviour to conspecifics and infanticide is a known phenomenon in equids (Cameron et al., 2003) both of which could easily result in losses and welfare issues in the enclosure if animals are not released before things escalate.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Committee of Forestry and Wildlife of the Ministry of Ecology, Geology, and Natural Resources of Kazakhstan (permit 17-2-18/1613 dated 17.10.2017 and permit KZ28VDY00000016 dated 29.08.2019) and by the transfer agreements with Kostanay oblast (permit 17-2-18/1613 dated from 17.10.2017 and unnumbered permit dated from 07.08.2019) with Altyn Emel NP and Barsa Kelmes SNR, in 2017 and 2019, respectively and the Altyn Dala SNR. Captures, animal handling, and transport were performed in accordance with relevant guidelines and regulations. The ethic commission at the University of Veterinary Medicine Vienna was informed and provided general consent. The IUCN Equid and Reintroduction Specialist reviewed the feasibility study and provided letters of support for the reintroduction project.
PK designed and supervised the work, wrote the first draft of the manuscript, and did most of the data analysis and visualisation. AS and SZ planned and organised field work in Kazakhstan and CW, TP, and NH oversaw the veterinary aspects. All authors were involved in field work and all reviewed and revised the manuscript.
Main funding came from the Fondation Segré, Nürnberg Zoo, Taipei Zoo, and Association of the Friends of Nürnberg Zoo. Additional small funds came from Wroclaw Zoo and La Passerelle Conservation. Salaries for PK and JL were largely covered by the Research Council of Norway grant 251112 and those of the international veterinarians by their respective employers: LaPalmyr Zoo, University of Veterinary Medicine Vienna, Wildlife Conservation Society, Zoo Budapest, and Frankfurt Zoological garden.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Many people have committed much time and effort to driving this project forward over the last 2 years. We are particularly grateful for ACBK staff Baurzhan Iskakov, Sayat Mukhtarov, Alexandr Putillin, Roman Alexandrovich, and Renat Eskazyuly for help with all aspects of field work and to the caretakers Kishkentay Ordabayev, Kairzhan Zhusupbekov, Gani Sadvakasov, Aidar Erzhanov at Alibi for looking after the reintroduced kulan. Vitaly Levanov and rangers and personnel from the Committee of Forestry and Wildlife, Okhotzooprom, Altyn Emel NP, Barsa Kelmes SNR, Altyn Dala SNR, and the ACBK hunting reserves were instrumental for capture and monitoring. Directors Kalyk Bayadilov and Zauresh Alimbetova made work possible in Altyn Emel NP and Barsa Kelmes SNR, respectively. Endre Sos and Christina Geiger oversaw the kulan transport in 2019, Vera Voronova, Sergey Sklyarenko, Fariza Fariza Adilbekova, Dag Enke, Stephanie Ward, Michael Brombacher, and Mark Day helped steer the project, and Stephanie Kramer-Schadt provided some valuable late night R advice.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcosc.2021.703358/full#supplementary-material
Bannikov, A. G. (1981). The Asian Wild Ass. Transl. by M. Proutkina in English. Moscow: Zoological Society of San Diego.
Bartlam-Brooks, H. L. A., Bonyongo, M. C., and Harris, S. (2011). Will reconnecting ecosystems allow long-distance mammal migrations to resume? A case study of a zebra Equus burchelli migration in Botswana. Oryx 45, 210–216. doi: 10.1017/S0030605310000414
Batsaikhan, N., Buuveibaatar, B., Chimed, B., Enkhtuya, O., Galbrakh, D., Ganbaatar, O., et al. (2014). Conserving the world's finest grassland amidst ambitious national development. Conserv. Biol. 28, 1736–1739. doi: 10.1111/cobi.12297
Baumann, M., Kamp, J., Pötzschner, F., Bleyhl, B., Dara, A., Hankerson, B., et al. (2020). Declining human pressure and opportunities for rewilding in the steppes of Eurasia. Divers. Distrib. 26, 1058–1070. doi: 10.1111/ddi.13110
Bekenov, A. B., and Fadeev, V. A. (1984). “Kulan—Equus hemionus Pallas, 1775,” in Mammals of Kazakhstan Volume 3, Part 4, eds E. V. Gvozdev, and E. I. Strautman (Nauka KazSSR, Alma-Ata), 189–217. [in Russian]
Bekenov, A. B., Grachevand, I. A., and Milner-Gulland, E. J. (1998). The ecology and management of the Saiga antelope in Kazakhstan. Mamm. Rev. 28, 1–52. doi: 10.1046/j.1365-2907.1998.281024.x
Bennett, E. A., Champlot, S., Peters, J., Arbuckle, B. S., Guimaraes, S., Pruvost, M., et al. (2017). Taming the late Quaternary phylogeography of the Eurasiatic wild ass through ancient and modern DNA. PLoS ONE 12:e0174216. doi: 10.1371/journal.pone.0174216
Brakes, P., Carroll, E. L., Dall, S. R. X., Keith, S. A., McGregor, P. K., Mesnick, S. L., et al. (2021). A deepening understanding of animal culture suggests lessons for conservation. Proc. Biol. Sci. 288:20202718. doi: 10.1098/rspb.2020.2718
Buuveibaatar, B., Mueller, T., Strindberg, S., Leimgruber, P., Kaczensky, P., and Fuller, T. K. (2016). Human activities negatively impact distribution of ungulates in the Mongolian Gobi. Biol. Conserv. 203, 168–175. doi: 10.1016/j.biocon.2016.09.013
Buuveibaatar, B., Strindberg, S., Kaczensky, P., Payne, J., Chimeddorj, B., Naranbaatar, G., et al. (2017). Mongolian Gobi supports the world's largest populations of khulan and goitered gazelles. Oryx 51, 639–647. doi: 10.1017/S0030605316000417
Calabrese, J. M., Fleming, C. H., Fagan, W. F., Rimmler, M., Kaczensky, P., Bewick, S., et al. (2018). Disentangling social interactions and environmental drivers in multi-individual wildlife tracking data. Philos. Trans. B 373:20170007. doi: 10.1098/rstb.2017.0007
Calabrese, J. M., Fleming, C. H., Gurarie, E., and Freckleton, R. (2016). ctmm: anrpackage for analyzing animal relocation data as a continuous-time stochastic process. Methods Ecol. Evol. 7, 1124–1132. doi: 10.1111/2041-210X.12559
Calabrese, J. M., Fleming, C. H., Noonan, M. J., and Dong, X. (2021). ctmmweb: a graphical user interface for autocorrelation-informed home range estimation. Wildlife Soc. Bull. 45, 162–169. doi: 10.1101/2020.05.11.087932
Cameron, E. Z., Linklater, W. L., Stafford, K. J., and Minot, E. O. (2003). Social grouping and maternal behaviour in feral horses (Equus caballus): the influence of males on maternal protectiveness. Behav. Ecol. Sociobiol. 53, 92–101. doi: 10.1007/s00265-002-0556-1
CMS (2019). Central Asian Mammals Migration and Linear Infrastructure Atlas. CMS Technical Series Publication No. 41. CMS
Dimeyeva, L. A., Ogar, N. P., Alimbetova, Z., and Breckle, S. W. (2012). “Nature conservation in the Aral Sea region: Barsa-Kelmes as an example” in Aralkum - A Man-Made Desert: The Desiccated Floor of the Aral Sea (Central Asia). Ecological Studies-Analysis and Synthesis, eds S. W. Breckle, W. Wucherer, L. A. Dimeyeva, and N. P. Ogar (New York, NY: Springer), 315–341.
Edelstein, M. R., Cerny, A., and Gadaev, A. (2012). Disaster by Design: Disappearance of the Aral Sea, Dry Run for the Emerging Climate Crisis. Research in Social Problems and Public Policy, Vol. 20. Bingley: Emerald Group Publishing Limited.
Estep, D. Q., Crowell-Davis, S. L., Earl-Costello, S.-A., and Beatey, S. A. (1995). Changes in the social behaviour of drafthorse (Equus caballus) mares coincident with foaling. Appl. Anim. Behav. Sci. 35, 199–213. doi: 10.1016/0168-1591(93)90137-E
Freitag, M., Kamp, J., Dara, A., Kuemmerle, T., Sidorova, T. V., Stirnemann, I. A., et al. (2021). Post-Soviet shifts in grazing and fire regimes changed the functional plant community composition on the Eurasian steppe. Glob. Chang. Biol. 27, 388–401. doi: 10.1111/gcb.15411
Gerritsmann, H., Stalder, G. L., Kaczensky, P., Buuveibaatar, B., Payne, J., Boldbaatar, S., et al. (2016). Arterial pH and blood lactate levels of anesthetized Mongolian Khulan (Equus hemionus hemionus) in the Mongolian Gobi correlate with induction time. J. Wildl. Dis. 52, 642–646. doi: 10.7589/2015-07-198
Giotto, N., Gerard, J. F., Ziv, A., Bouskila, A., and Bar-David, S. (2015). Space-use patterns of the Asiatic Wild Ass (Equus hemionus): Complementary insights from displacement, recursion movement and habitat selection analyses. PLoS ONE 10:e0143279. doi: 10.1371/journal.pone.0143279
Gliga, D. S., Petrova, N., Linnell, J. D. C., Salemgareyev, A. R., Zuther, S., Walzer, C., et al. (2020). Dynamics of gastro-intestinal strongyle parasites in a group of translocated, wild-captured asiatic wild asses in Kazakhstan. Front.Vet. Sci. Brief Res. Rep. 7:598371. doi: 10.3389/fvets.2020.598371
Huber, N., Marasco, V., Painer, J., Vetter, S. G., Göritz, F., Kaczensky, P., et al. (2019). Leukocyte coping capacity: an integrative parameter for wildlife welfare within conservation interventions. Front. Vet. Sci. 6:105. doi: 10.3389/fvets.2019.00105
Jesmer, B. R., Merkle, J. A., Goheen, J. R., Aikens, E. O., Beck, J. L., Courtemanch, A. B., et al. (2018). Is ungulate migration culturally transmitted? Evidence of social learning from translocated animals. Science 361, 1023–1025. doi: 10.1126/science.aat0985
Joly, K., Gurarie, E., Sorum, M. S., Kaczensky, P., Cameron, M. D., Jakes, A. F., et al. (2019). Longest terrestrial migrations and movements around the world. Sci. Rep. 9:15333. doi: 10.1038/s41598-019-51884-5
Kaczensky, P. (2011). First Assessment of the Suitability of the Altyn Dala and Altyn Emel Region of Kazakhstan for Przewalski's Horse Re-introduction. Report Research Institute of Wildlife Ecology, University of Veterinary Medicine, Vienna.
Kaczensky, P., Doldin, R., Enke, D., Linnell, J. D. C., Lukanovsky, O., Salemgareyev, A. R., et al. (2017). Feasibility study for kulan (Equus hemionus kulan) reintroduction into the central steppe of Kazakhstan. NINA Report 1366.
Kaczensky, P., Ganbataar, O., Altansukh, N., Enkhsaikhan, N., Stauffer, C., and Walzer, C. (2011b). The danger of having all your eggs in one basket—winter crash of the re-introduced Przewalski's horses in the Mongolian Gobi. PLoS ONE 6:e28057. doi: 10.1371/journal.pone.0028057
Kaczensky, P., Hrabar, H., Lukarevski, V., Zimmermann, W., Usukhjargal, D., Ganbaatar, O., et al. (2016). “Reintroduction of Wild Equids” in wild equids—ecology, management, and conservation, eds J. I. Ransom, P. Kaczensky (Baltimore, MA: Johns Hopkins University Press), 196–214.
Kaczensky, P., Khaliun, S., Payne, J., Boldgiv, B., Buuveibaatar, B., and Walzer, C. (2019). Through the eye of a Gobi khulan—application of camera collars for ecological research of far-ranging species in remote and highly variable ecosystems. PLoS ONE 14:e0217772. doi: 10.1371/journal.pone.0217772
Kaczensky, P., Kovtun, E., Habibrakhmanov, R., Hemami, M.-R., Khaleghi, A., Linnell, J. D. C., et al. (2018a). First population-level genetic analysis of free-ranging Asiatic wild ass populations in Central Asia—implications for conservation. Conserv. Genet. 19, 1169–1184. doi: 10.1007/s10592-018-1086-3
Kaczensky, P., Kuehn, R., Lhagvasuren, B., Pietsch, S., Yang, W., and Walzer, C. (2011a). Connectivity of the Asiatic wild ass population in the Mongolian Gobi. Biol. Conserv. 144, 920–929. doi: 10.1016/j.biocon.2010.12.013
Kaczensky, P., Linnell, J. D. C., Zuther, S., Salemgareyev, A., and Doldin, R. (2018b). Reintroduction of Kulan into the Central Steppe of Kazakhstan: Field Report for 2017. NINA Report 1459.
Kaczensky, P., Lkhagvasuren, B., Pereladova, O., Hemami, M.-R., and Bouskila, A. (2015). Equus hemionus. The IUCN Red List of Threatened Species 2015:eT7951A45171204.
Kaczensky, P., Salemgareyev, A. R., Zuther, S., Suttibayev, M., Adilbekova, F., and Linnell, J. D. C. (2020). Reintroduction of Kulan into the Central Steppe of Kazakhstan: Field Report for 2018–2019. NINA report 1782.
Kamp, J., Koshkin, M. A., Bragina, T. M., Katzner, T. E., Milner-Gulland, E. J., Schreiber, D., et al. (2016). Persistent and novel threats to the biodiversity of Kazakhstan's steppes and semi-deserts. Biodivers. Conserv. 25, 2521–2541. doi: 10.1007/s10531-016-1083-0
Kauffman, M. J., Aikens, E., Esmaeili, S., Kaczensky, P., Middleton, A. D., Monteith, K. L., et al. (2021). Causes, consequences and conservation of ungulate migrations. Annu. Rev. Ecol. Evol. Syst. (in press) 52.
Kock, R. A., Orynbayev, M., Robinson, S., Zuther, S., Singh, N. J., Beauvais, W., et al. (2018). Saigas on the brink: multidisciplinary analysis of the factors influencing mass mortality events. Sci. Adv. 4:eaao2314. doi: 10.1126/sciadv.aao2314
Lenk, M. (2008). Demografische und Sozioökonomischen Situation im Projektgebiet der “Altyn Dala Conservation Initiative” (ADCI) (Kasachstan). Report [in German].
Levanov, V. F., Sokolov, S. V., and Kaczensky, P. (2013). Corral mass capture device for Asiatic wild asses Equus hemionus. Wildlife Biol. 19, 325–334. doi: 10.2981/13-036
Linnell, J. D., Trouwborst, A., Boitani, L., Kaczensky, P., Huber, D., Reljic, S., et al. (2016). Border security fencing and wildlife: the end of the transboundary paradigm in Eurasia? PLoS Biol. 14:e1002483. doi: 10.1371/journal.pbio.1002483
Lkhagvasuren, D., Batsaikhan, N., Fagan, W. F., Ghandakly, E. C., Kaczensky, P., Müller, T., et al. (2017). First assessment of the population structure of the Asiatic wild ass in Mongolia. Eur. J. Wildl. Res. 64, 3. doi: 10.1007/s10344-017-1162-x
Mertes, K., Stabach, J. A., Songer, M., Wacher, T., Newby, J., Chuven, J., et al. (2019). Management background and release conditions structure post-release movements in reintroduced ungulates. Front. Ecol. Evol. 7:470. doi: 10.3389/fevo.2019.00470
Milner-Gulland, E. J., Bukreeva, O. M., Coulson, T., Lushchekina, A. A., Kholodova, M. V., Bekenov, A. B., et al. (2003). Reproductive collapse in saiga antelope harems. Nat. Feature 422:135. doi: 10.1038/422135a
Nandintsetseg, D., Bracis, C., Leimgruber, P., Kaczensky, P., Bayarbaatar, B., Badamjav, L., et al. (2019). Variability in nomadism: environmental gradients modulate the movement behaviors of dryland ungulates. Ecosphere 10:e02924. doi: 10.1002/ecs2.2924
Nandintsetseg, D., Kaczensky, P., Ganbaatar, O., Leimgruber, P., and Mueller, T. (2016). Spatiotemporal habitat dynamics of ungulates in unpredictable environments: the khulan (Equus hemionus) in the Mongolian Gobi desert as a case study. Biol. Conserv. 204, 313–321. doi: 10.1016/j.biocon.2016.10.021
Nerlekar, A. N., and Veldman, J. W. (2020). High plant diversity and slow assembly of old-growth grasslands. Proc. Natl. Acad. Sci. U.S.A. 117, 18550–18556. doi: 10.1073/pnas.1922266117
Noonan, M. J., Fleming, C. H., Tucker, M. A., Kays, R., Harrison, A. L., Crofoot, M. C., et al. (2020). Effects of body size on estimation of mammalian area requirements. Conserv. Biol. 34, 1017–1028. doi: 10.1111/cobi.13495
Olson, K. A. (2014). Saiga Crossing Options Guidelines and Recommendations to Mitigate Barrier Effects of Border Fencing and Railroad Corridors on Saiga Antelope in Kazakhstan. Report for Frankfurt Zoological Society, Association for the Conservation of Biodiversity of Kazakhstan, Fauna and Flora International, Convention on Migratory Species.
Payne, J. C., Buuveibaatar, B., Bowler, D. E., Olson, K. A., Walzer, C., and Kaczensky, P. (2020). Hidden treasure in the Gobi: understanding how water limits range use of khulan in the Mongolian Gobi. Sci. Rep. 10:2989. doi: 10.1038/s41598-020-59969-2
Rashek, V. A. (1966). Kulan ecology and its acclimatisation on the island of Barsa Kelmes (PhD thesis). Moscow, UDSSR.
Renan, S., Speyer, E., Ben-Nun, T., Ziv, A., Greenbaum, G., Templeton, A. R., et al. (2018). Fission-fusion social structure of a reintroduced ungulate: implications for conservation. Biol. Conserv. 222, 261–267. doi: 10.1016/j.biocon.2018.04.013
Resende, P. S., Viana-Junior, A. B., Young, R. J., and Azevedo, C. S. (2021). What is better for animal conservation translocation programs: soft- or hard-release? A phylogenetic meta-analytical approach. J. Appl. Ecol. doi: 10.1111/1365-2664.13873
Rubenstein, D. I., Sundaresan, S., Fischhoff, I., and Saltz, D. (2007). Social networks in wild asses: comparing patterns and processes among populations. Explor. Biol. Resour. Mongolia 10, 159–176. Available online at: https://digitalcommons.unl.edu/biolmongol/index.2.html#year_2007
Rubenstein, D. I., Sundaresan, S., Fischhoff, I., Tantipathananandh, C., and Berger-Wolf, T. Y. (2015). Similar but different: dynamic social network analysis highlights fundamental differences between the fission-fusion societies of two equid species, the Onager and Grevy's Zebra. PLoS ONE 10:e0138645. doi: 10.1371/journal.pone.0138645
Saltz, D., Rowen, M., and Rubenstein, D. I. (2000). The effects of space-use patterns of reintroduced Asiatic wild ass on effective population size. Conserv. Biol. 14, 1852–1861. doi: 10.1111/j.1523-1739.2000.99227.x
Saltz, D., and Rubenstein, D. I. (1995). Population dynamics of a reintroduced asiatic wild ass (Equus Hemionus) herd. Ecol. Appl. 5, 327–335. doi: 10.2307/1942025
Schwalb-Willmann, J., Remelgado, R., Safi, K., and Wegmann, M. (2020). moveVis: animating movement trajectories in synchronicity with static or temporally dynamic environmental data in R. Methods Ecol. Evol. 11, 664–669. doi: 10.1111/2041-210X.13374
Stamps, J. A., and Swaisgood, R. R. (2007). Someplace like home: experience, habitat selection and conservation biology. Appl. Anim. Behav. Sci. 102, 392–409. doi: 10.1016/j.applanim.2006.05.038
Török, P., Ambarl,i, D., Kamp, J., Wesche, K., and Dengler, J. (2016). Step(pe) up! Raising the profile of the Palaearctic natural grasslands. Biodivers. Conserv. 25, 2187–2195. doi: 10.1007/s10531-016-1187-6
Tucker, M. A., Böhning-Gaese, K., Fagan, W. F., Fryxell, J. M., Van Moorter, B., Alberts, S. C., et al. (2018). Moving in the Anthropocene: global reductions in terrestrial mammalian movements. Science 359:466. doi: 10.1126/science.aam9712
Walzer, C. (2014). “Non-domestic equids,” in Zoo Animal and Wildlife Immobilization and Anesthesia, 2nd Edition, eds G. West, D. Heard, and N. Caulkett (Hoboken: Wiley Blackwell), 719–728. doi: 10.1002/9781118792919.ch52
Walzer, C., Kaczensky, P., Ganbaatar, O., Lengger, J., Enkhsaikhan, N., and Lkhagvasuren, D. (2007). Capture and anaesthesia of wild Mongolian equids—the Przewalski's horse (Equus ferus przewalskii) and khulan (E. hemionus). Mongol. J. Biol. Sci. 4, 19–30. doi: 10.22353/mjbs.2006.04.02
Wang, M. Y., Ruckstuhl, K. E., Xu, W. X., Blank, D., and Yang, W. K. (2016). Human activity dampens the benefits of group size on vigilance in Khulan (Equus hemionus) in Western China. PLoS ONE 11:e0146725. doi: 10.1371/journal.pone.0146725
Wesche, K., Ambarl,i, D., Kamp, J., Török, P., Treiber, J., and Dengler, J. (2016). The palaearctic steppe biome: a new synthesis. Biodivers. Conserv. 25, 2197–2231. doi: 10.1007/s10531-016-1214-7
Zuther, S., Wilkins, V., and Salemgareyev, A. (2018). Strategy, successes and challenges of the Altyn Dala Conservation Initiative, a multi-organisation collaboration involving protected area establishment and population level conservation efforts. SCB Conservation Asia conference, Bishkek, 6–10 August 2018.
Keywords: Equus hemionus kulan, kulan, Kazakhstan, reintroduction, social cohesion, soft release, post-release movement
Citation: Kaczensky P, Salemgareyev A, Linnell JDC, Zuther S, Walzer C, Huber N and Petit T (2021) Post-release Movement Behaviour and Survival of Kulan Reintroduced to the Steppes and Deserts of Central Kazakhstan. Front. Conserv. Sci. 2:703358. doi: 10.3389/fcosc.2021.703358
Received: 30 April 2021; Accepted: 28 July 2021;
Published: 19 August 2021.
Edited by:
Oded Berger-Tal, Ben-Gurion University of the Negev, IsraelReviewed by:
David Blank, Research Center for Ecology and Environment of Central Asia, KyrgyzstanCopyright © 2021 Kaczensky, Salemgareyev, Linnell, Zuther, Walzer, Huber and Petit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petra Kaczensky, cGV0cmEua2FjemVuc2t5QGlubi5ubw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.