- 1Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Sciences, Chongqing, China
- 2Southwest Hospital, Third Military Medical University, Chongqing, China
Computer-assisted surgery (CAS) has occupied an important position in modern surgery, further stimulating the progress of methodology and technology. In recent years, a large number of computer vision-based methods have been widely used in surgical workflow recognition tasks. For training the models, a lot of annotated data are necessary. However, the annotation of surgical data requires expert knowledge and thus becomes difficult and time-consuming. In this paper, we focus on the problem of data deficiency and propose a knowledge transfer learning method based on artificial neural network to compensate a small amount of labeled training data. To solve this problem, we propose an unsupervised method for pre-training a Convolutional-De-Convolutional (CDC) neural network for sequencing surgical workflow frames, which performs neural convolution in space (for semantic abstraction) and neural de-convolution in time (for frame level resolution) simultaneously. Specifically, through neural convolution transfer learning, we only fine-tuned the CDC neural network to classify the surgical phase. We performed some experiments for validating the model, and it showed that the proposed model can effectively extract the surgical feature and determine the surgical phase. The accuracy (Acc), recall, precision (Pres) of our model reached 91.4, 78.9, and 82.5%, respectively.
Introduction
Computer-assisted surgery (CAS) emerged in the twentieth century, which means that computer technology is used to guide and assist surgeons. The application (Garg et al., 2005) provides decision-making support and planning tools in the preoperative. Intraoperative computer assistance includes robotic surgical system (Dergachyova, 2018), image guidance and navigation (Peters, 2006), augmented reality and visualization (Kersten-Oertel et al., 2013). Postoperative assistance provides tools to analyze executed procedures and results, as well as to improve and optimize (Schumann et al., 2015). Despite all the advance and valuable assistance, the seamless integration of computer-aided equipment with operating room (OR) and surgical procedures has not yet been achieved. Existing ORs contain a set of unrelated independent systems and devices, most of which appear in isolation, disabling proper communication and interaction (Hübler et al., 2014). Current computer-aided equipment facilitates a number of individual surgical tasks, but their lack of synchronization with the surgical process hampers the work and resource management of the surgical team. It leads to higher stress levels (Agarwal et al., 2006), frequent misunderstandings among surgical staffs, resulting in risks and delays, as well as inefficient surgical groups that incur excessive costs for hospitals (Macario, 2010).
Context-aware Computer-assisted surgery (CA-CAS) has powerful artificial intelligence that understands or perceives the needs of clinicians. It should always be aware of the events that occur, the actions performed, and the current state by tracking the surgical procedure and constantly observing the surgical site. Examples of applications are: optimization of the surgical procedure (Franke et al., 2013; Guédon et al., 2016), prediction of the remaining time of surgery (Bhatia et al., 2007), intraoperative assistance (Nessi et al., 2015; Fard et al., 2016), automatic generation of surgical reports (Agarwal et al., 2006). A large number of studies have focused on IntelliSense intraoperative aids to reduce the pressure on surgeons and facilitate the surgical process (Meng et al., 2021; Liu et al., 2022). Automatic recognition of surgical procedures is an important part of this. Recognizing surgical procedures is a prerequisite for CAS applications. The study on this subject began about 10 years ago. Despite the great progress made, it remains a relatively new area that inspires scientists and clinicians to inspire. Due to the lack of automatic recognition, most applications use manual label of surgical activities, which is a very tedious and time-consuming process.
Today, artificial intelligence and deep learning technologies have developed rapidly (Li et al., 2017; Liu et al., 2020; Zhong et al., 2021; Fan et al., 2022) and have been successfully applied in many different fields, including image labeling, natural language modeling, text generation, image labeling, natural language modeling, text generation, classification (Zheng et al., 2021), medical care (Zhang et al., 2020, 2021), web service QoS prediction (Wu et al., 2022), and risk assessment (Deng et al., 2022). In most cases, their performance is superior to that of traditional machine learning methods. Comprehensive and accurate training data have been playing an important role in machine learning. The quantity and quality of data have become an important factor. The size of the massive data sets that serve as a basis for the training of deep learning model, such as the famous ImageNet (Deng et al., 2009), Microsoft COCO (Deng et al., 2009), the recently released Google’s OpenImages (Krasin et al., 2017; Kuznetsova et al., 2020), and YouTube-8M (Abu-El-Haija et al., 2016; YouTube-8M Dataset, 2018), is self-evident. They contain millions of samples representing thousands of categories. Unfortunately, sometimes learning tasks have to be carried out in an area of interest expressed by a small group of data, such as the field of surgery. A variety of constraints hinder proper data collection: Ethical approvals, the consent of patients and medical personnel, the limited number of cases, the installation of expensive data acquisition equipment, and time-consuming manual annotations that require medical experience. In these cases, the methods of transfer learning may play a role. To a large extent, transfer learning involves the use of methods from resources in other areas of interest, where data may be distributed differently and located in different feature spaces, thus improving the learning of the target task. Depth models make it easy to transfer knowledge of one network to another. Transfer learning is a knowledge transfer technology that is currently widely used with convolution neural networks (CNN) for tasks related to visual content, which benefits from a large number of free datasets. It is also widely used in speech and language processing (Huang et al., 2013), document classification (Dai et al., 2007), sentiment analysis (Glorot et al., 2011), and other sequence analysis tasks.
Therefore, in this paper, we proposed an unsupervised method for training Convolutional-De-Convolutional (CDC) networks to sort surgical workflow frames, which are simultaneously rolled out in space (for semantic abstraction) and temporal convolution (for frame-level resolution). It has unique property in modeling the spatio-temporal interactions between high-level semantics in space and fine-grained action dynamics in time. Specifically, the CDC has to extract features related to understanding the surgical workflow. The knowledge learned from the task is encoded into the weight matrix of the internal parameters of the representation layer. Then the Convolutional-De-Convolution network is fine-tuned to classify the surgical phase.
The contributions of this paper are summarized as follows:
• We proposed a model that can solve the problem of annotating data deficiency in medical field by using the transfer learning method.
• We used a CDC network to recognize the surgical workflow because of its property of spatio-temporal interactions in training.
• We try to achieve intelligent detection of surgical video phase at a low cost. Finally, based on M2CAI 2016 challenge dataset, we performed experiments for validating the model. It shows a good performance compared with other methods.
This paper is organized as follows: Section II presents related work. We summarize methodology and the proposed models in section III. In section IV, we present the experiment and result of our method. In section V, we discuss conclusions and suggestions for future research.
Related work
The OR’s understanding of surgical activities is a new field of research. Surgical workflow identification is closely related to multi-target tracking. Wang et al. (2022) proposed a General Recurrent Tracking Unit (RTU++), which can be flexibly plugged into other trackers, to score track proposals by capturing long-term information. And the experiments showed the generalization ability of RTU++ trained by simulated data in various scenarios. Under the specific limitations and difficulties implied by the surgical environment, only a few jobs deal directly with the application. Since the problem of surgical process identification is a multidisciplinary problem, we have decided to propose different related fields. Surgical phase recognition is similar to time action recognition. We start with a brief introduction to literatures on temporal action recognition. Then, we will focus on the internal approval of the operation.
Temporal action recognition
Gaidon et al. (2011, 2013) introduced temporally action recognition in untrimmed videos, focusing on limited actions such as “drinking and smoking” (Calder and Siegel, 2009) and “opening the door to sit down” (Laptev and Perez, 2007). Later, researchers worked on building large datasets, including complex action categories such as THUMOS (Mexaction2, 2013), as well as datasets focused on fine-grained actions (Sigurdsson et al., 2016a,b) or high-level semantics activities (Heilbron et al., 2015). Recently, deep learning methods have shown better performance in localizing action instances. Franke et al. (2013) presented a temporal action proposal system based on Long-Short Term Memory (LSTM); Yeung et al. (2018) provided the MultiTHUMOS dataset of each frame multi-label annotations, and a LSTM network is defined to model multiple input and output connections; Shou et al. (2016) introduced a 3D CNN framework (S-CNN) based on end-to-end segmentation, which is superior to other RNN-based methods by capturing spatio-temporal information simultaneously. However, S-CNN lacks the ability to accurately predict time resolution and localize the exact time boundary of an action instance. In Shou et al. (2017), they proposed a CDC network for precise temporal action localization of untrimmed video, which provides a new CDC filter that can simultaneously perform spatial down-sampling (for spatio-temporal semantic abstraction) and temporal up-sampling (for precise time positioning). In this paper, we will use the CDC network structure to recognize the surgical phase by transfer learning. Details are described in the next section. Yang et al. (2018) proposed a Frame Segmentation Network (FSN), which placed a temporal CNN on top of the 2D spatial CNNs, and can make dense predictions at frame-level for a video clip using both spatial and temporal context information.
Surgical phase recognition
Mackenzie et al. (2001) were among the first to propose the creation of a process model. In Mackenzie et al. (2001), it is based on structured multi-level decomposition that describes the surgical action performed during surgery. In the same year, Jannin et al. (2001) also proposed a neural process model based on Uniform Mark-up Language decomposition. Subsequently, the concept of surgical workflow was introduced. Neumuth et al. (2006) proposed the concept of the general methodology described in the acquisition process from surgical intervention, clinical and technical analysis, and automatic processing of workflow schemes can drive a workflow management system as the future of OR process control. Klank et al. (2008) used the evolutionary reinforcement learning to classify the laparoscopic cholecystectomy into 6 stages for the first time, with an Acc rate of about 50%. Klank et al. (2008) presented a method that based on Hidden Markov Model (HMM) and dynamic time warping algorithm (DTW) to perform a dimensionality reduction on image features by using additional information about tool usage for recognition of surgical workflow of laparoscopic video, the Acc of phase detection is 76.8%. Dergachyova et al. (2016) proposed a machine learning method. Specifically, they firstly described the input image by extracting the color, shape, and texture features of the image, and then they used several AdaBoost cascades for intermediate classification. Finally, a definite phase label is given by using the hidden semi-Markov Model. Based on visual features, the Acc of the model is close to 68%, and the Acc of fusion surgical instruments is close to 90%.The recent study in Dergachyova et al. (2016) is a method based on deep learning. The time smoothing convolution neural network and the classical HMM were used for phase recognition. The proposed network challenge is based on the residual network-200 pre-trained ImageNet, where the last layer is replaced by a new fully connected output layer, corresponding to 8 possible surgical phases. It was then fine-tuned on the M2CAI dataset using online data augmentation. The logarithmic probability output vector of the network was processed by temporal smoothing, and then passed to the HMM to correct possible classification errors for previously recognized frames. Twinanda et al. (2016) also proposed a method of deep learning based on pre-trained AlexNet, called PhaseNet, and they replaced the output layer and fine-tuned it using the M2CAI training dataset. At the second last layer of the PhaseNet, one-vs.-all linear SVM is obtained by using the image features extracted by CNN as input. Based on the Support Vector Machine classifier, the hierarchical HMM was introduced to reinforce the temporal constraint. The method was still based on two large datasets of laparoscopic cholecystectomy (Cholec 80 and EndoVis), which achieves better performance. The average Acc of offline analysis was highest, at 92.2% (Cholec80) and 86% (EndoVis), respectively. Shi et al. (2021) proposed a label-efficient Surgical workflow recognition method with a two-stage semi-supervised learning, named as SurgSSL which progressively leverages the inherent knowledge held in the unlabeled data to a larger extent. The SurgSSL method surpasses the state-of-the-art semi-supervised methods by a large margin.
Materials and methods
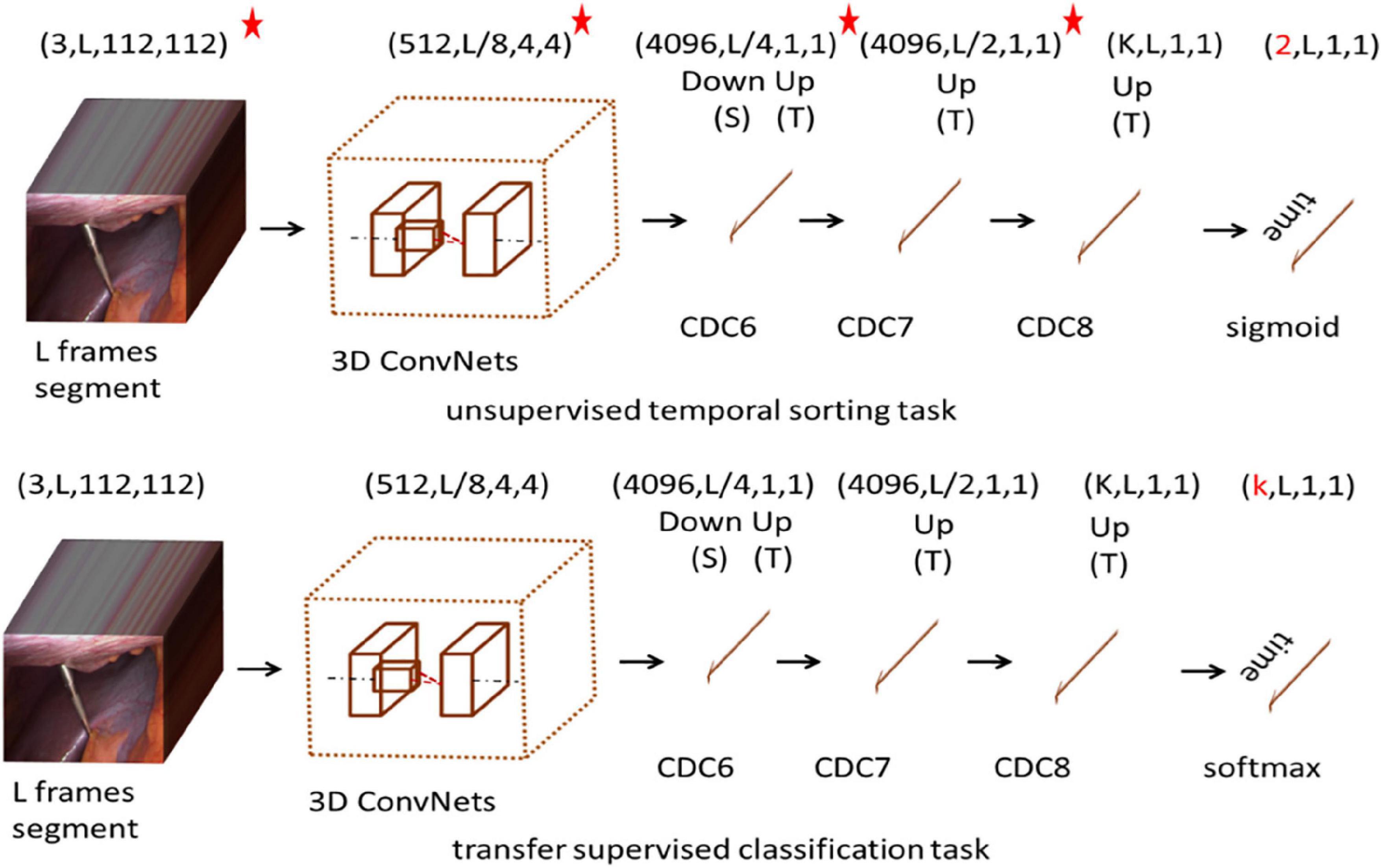
In this paper, we proposed a model for recognizing surgical workflow, as shown in Figure 1. Specifically, the top is an unsupervised time sorting task based on the CDC network, and the bottom is based on the top of the transfer supervised surgical phase classification task. The weights of the layers marked with a star can be passed. The first row shows the shape of the output data of each layer. First, the surgical video clip is fed into 3D ConvNets, and the temporal length is reduced from L to L/8. CDC6 has kernel size (4, 4, 4), Stride (2, 1, 1), padding (1, 0, 0), so the height and width are reduced to 1, while the temporal length increases from L/8 to L/4. CDC7 and CDC8 kernel size (4 1 1), Step (2, 1, 1), padding (1, 0, 0), so CDC7 and CDC8 further perform up-sampling in time by a factor of 2, so the temporal length is back to L in the unsupervised temporal sorting task, sigmoid layer is added on top of CDC8 to determine whether is correct for the order of the given L frames. In the transfer supervised classification task, a frame-wise softmax layer is added on top of CDC8 to obtain confidence scores for every frame. Each channel stands for one class, obtaining confidence scores for every frame.
Unsupervised spatio-temporal context learning
In this section, we describe how to train the CDC network using unmarked video. We do this by addressing a task that requires the CDC to sort L given frames in the correct temporal order. For this, a large dataset from multiple surgical intervention is used. We assume that solving such a task requires CDC to learn to extract visual cues that describe the temporal flow of the surgical workflow.
The CDC (Shou et al., 2017) network is based on 3D convolution C3D network, which simultaneously carries out spatial convolution (for semantic abstraction) and temporal convolution (for frame-level resolution). It has a unique property in the spatio-temporal interactions between joint modeling and summarizing. The CDC network uses from conv1a to conv5b as the first part of the C3D network. For the remaining layers in the C3D, CDC keeps pool5 to perform max pooling in height and width by a factor of 2 but keeps the temporal length. The CDC sets the height and width of the network input as 112 × 112. Given an input video segment with a temporal length L, the output data shape of the pool5 is (512, L/8, 4, 4). To maintain the original temporal resolution (frame level), the CDC makes up-sampling in time (back to L from L/8) and down-sampling in space (from 4 × 4 to 1 × 1). More information is described in Shou et al. (2017).
Our CDC training tasks are shown in Figure 2: Given the same surgical video input for a video clip of temporal length L, what is the most relative order of L frames? That is, is the order of the given L frames correct? We uniformly sample L random frames from the video of the surgical intervention at the moment of transfer and enter them into our CDC. The transfer moment is shown in Figure 1. The CDC must calculate the relative order of L frames in the original video. That is, determines whether the given L frame is in the correct order? That is, in the last layer of the network, we have two categories of L frames, the correct order is positive, otherwise it is negative. We assume that solving this task requires the CDC to extract visual cues related to the surgical process in order to understand the temporal flow of surgical intervention. At the same time, the learning of temporal information is carried out in this process.
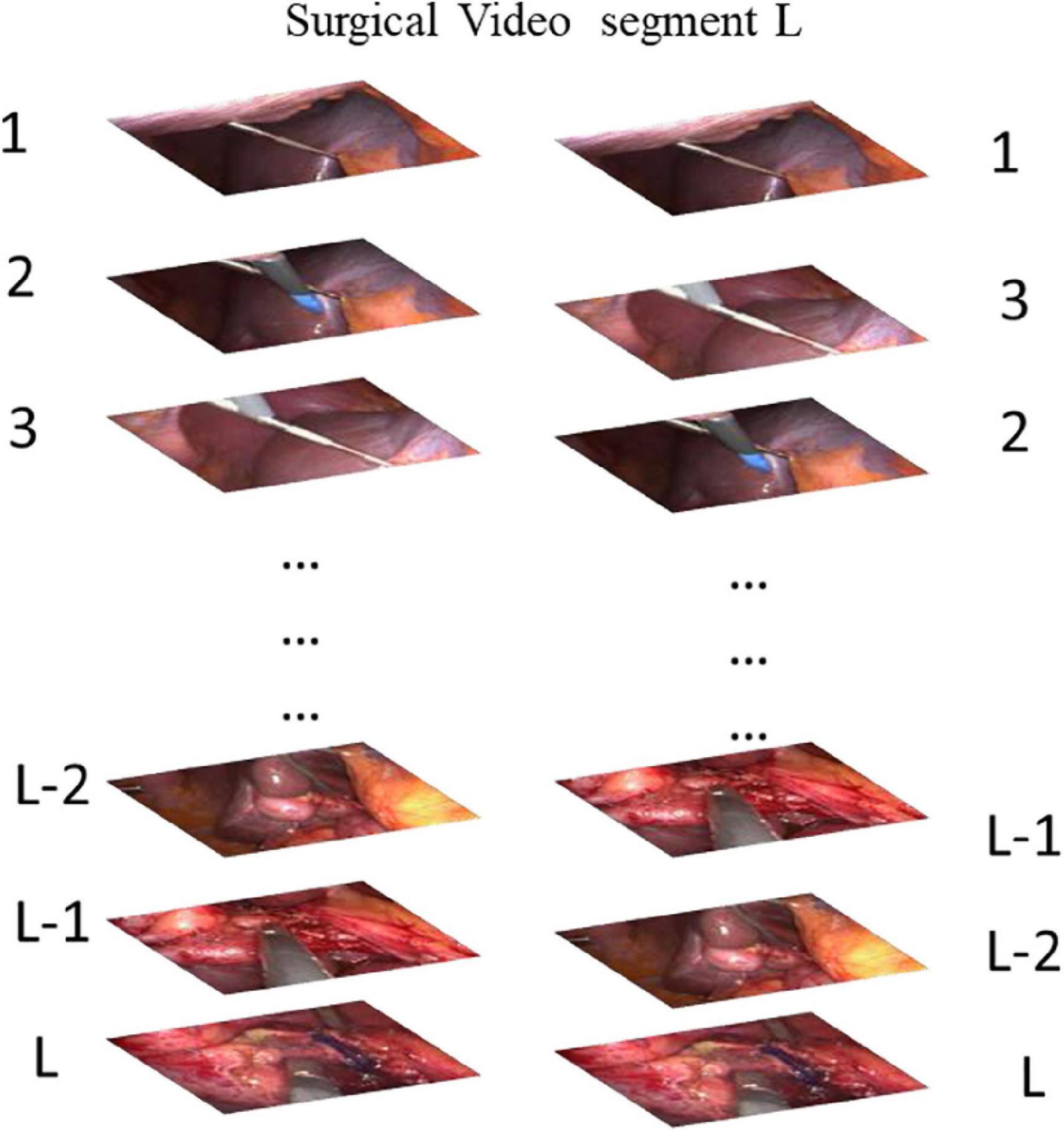
Figure 2. Our task for pretraining a CDC whether is the order of the given L frames correct? (Answer: The Left is correct).
The total loss is defined as:
where labeli is the ground truth for i-th segment, probi is predictions for i-th segment.
When an unsupervised dataset is generated, data generation is primarily performed randomly at the time of conversion. Each phase is randomly sampled according to the ratio column, and the main sampling point is the transfer point. The specific sampling is related to the experimental dataset.
Knowledge transfer for recognition of surgical phase
The phase sequence indicating a surgical process encodes some form of abstract knowledge about a given procedure. The knowledge can be extracted and utilized to improve various operations on surgical process data, including analysis, recognition and prediction. It is particularly assumed that the knowledge gained from one procedure can improve the prediction of the surgical phase of another procedure. The knowledge involved may include dependencies between phases in a sequence, relationships between elements in an activity, and connections between individual elements of different activities. In view of the difficulty of formalizing the concealment of knowledge, the CDC network can extract features from time and space at the same time, so the CDC network is chosen as a method to extract and transfer knowledge.
Deep neural networks have an interesting property that enables networks to store extracted information in a distributed hierarchical manner. It means that the basic information that is more common for many areas stored separately from the features that describe the characteristics of a particular domain. It also means that this information can be shared with other learning goal (e.g., other training task or area). In the deep model, the knowledge learned from the data is encoded into the weight matrix of the internal parameters of the representation layer. In order to establish the value of internal parameters, the domain containing a large number of training samples is first trained. Then, depending on the quantity and quality of data in the actual target domain, there are three transfer options. First, if the new data is close enough to the data used for training, and the task has not changed, we can use the same training model directly for the new data. The second option is to use the weights (in whole or in part) of the training model as the initialization of the new model. This applies where a reasonable amount of new data is available for training use. The third option, called fine-tuning, is typically used when the new domain contains only a small number of examples. It includes importing the trained weight matrix into the new model, but “freezes” some layers that usually contain more basic features during training. The weight setting of pre-training on other data is usually more optimized than random initialization. The network can benefit from what has been learned, thus, we should focus its “attention” on the specific characteristics of the new data. This section is based on the CDC time sorting network for knowledge transfer learning. Modify the final output layer of the CDC network to be L and classify each surgical step. In the transfer supervision classification task, the Softmax output is the vector of the K-value. Note that for the i-th class:
The total loss L is defined as:
Where zn is the ground truth class label for the n-th segment.
Experiment and result
Dataset and data sampling
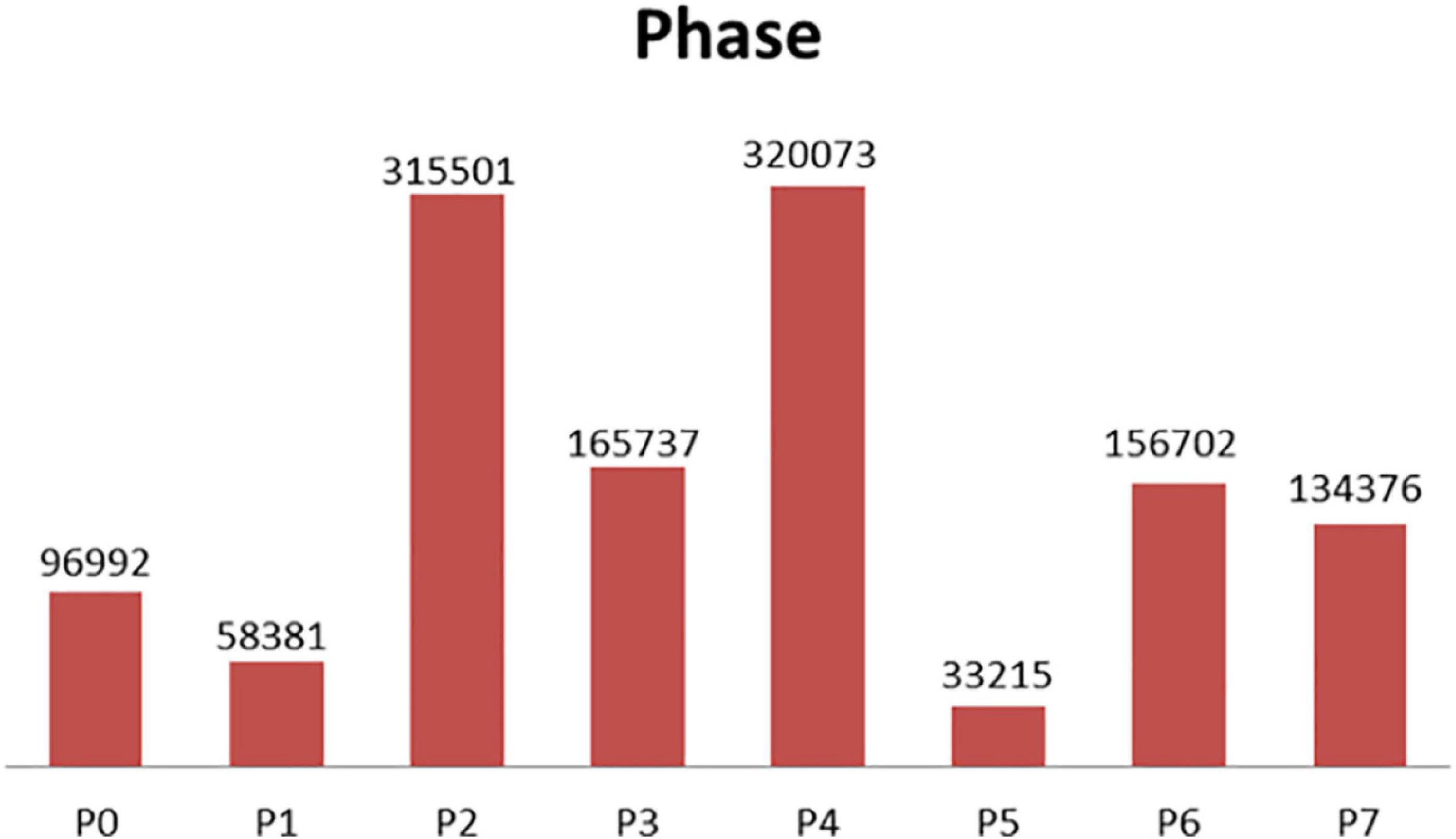
The experiment in this paper is based on the M2CAI16-workflow dataset, which is available from http://camma.u-strasbg.fr/m2cai2016/. It contains videos of 41 cholecystectomy processes from the University Hospital of Strasbourg/IRCAD (Strasbourg, France) and Klinikum Rechts der Isar Hospital (Munich, Germany). The datasets are divided into two parts: the training subset (containing 27 videos) and the testing subset (14 videos). The videos are recorded at 25 fps. All the frames are fully annotated with 8 defined phases: (1) trocarplacement, (2) preparation, (3) calot triangle dissection, (4) clipping and cutting, (5) gallbladder dissection, (6) galbladder packaging, (7) cleaning and coagulation, and (8) gallbladder retraction. The list of phases in the dataset is shown in Table 1. The distribution of the phases in dataset is shown in Figure 3.
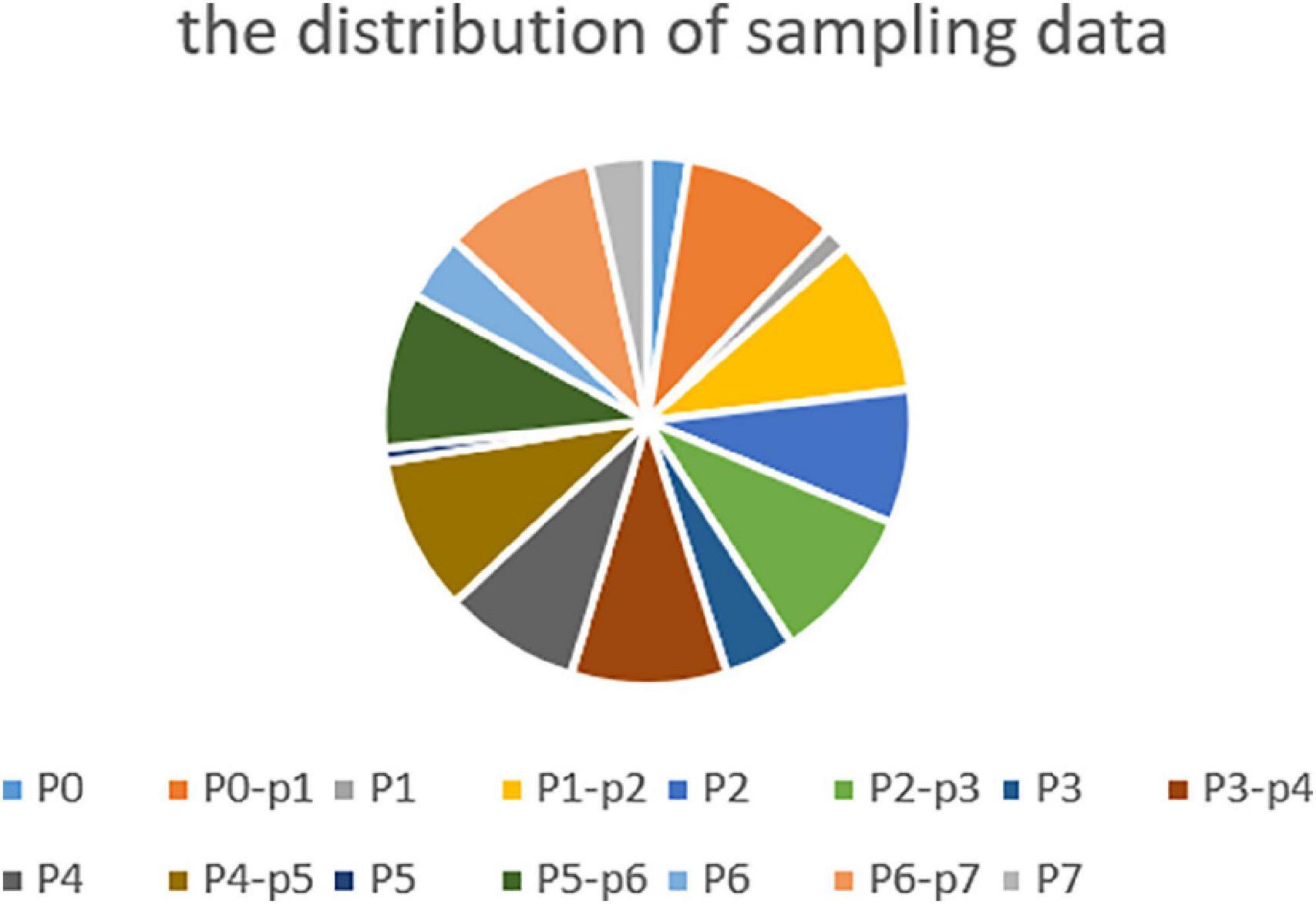
In the case of a frame rate of 1, a total of 1.3 million frames are available. Depending on the distribution of the surgical phase, we randomly collected 250,000 surgical video clips from different surgical phases, 500,000 surgical video clips for the transition period, and 750,000 surgical video clips for unsupervised temporal learning. The sampling data for each stage and transition time is shown in Figure 4.
Comparison algorithms
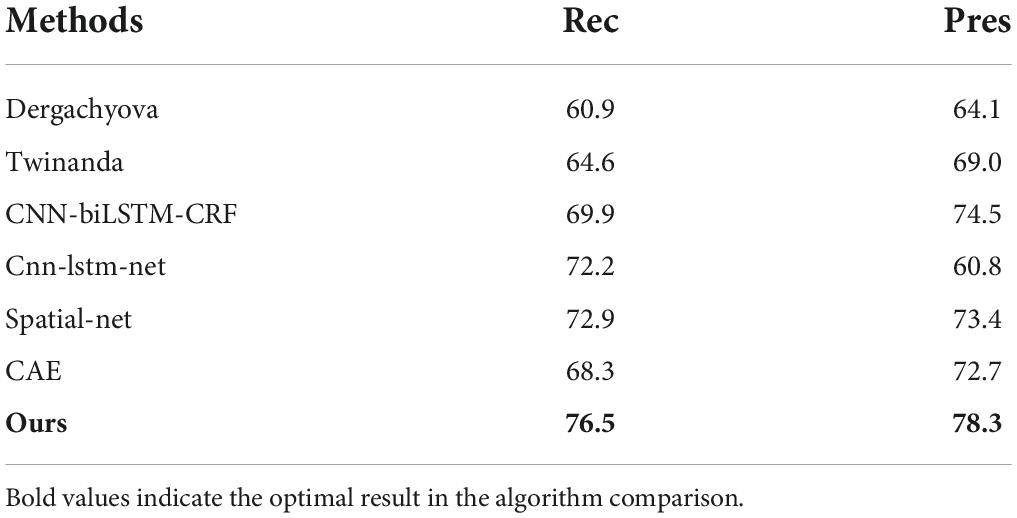
We compared our method with several state-of-the-art method. Dergachyova et al. (2016) and Twinanda et al. (2016) are two of the methods submitted to the M2CAI 2016 challenge. CNN-biLSTM-CRF (Yu et al., 2019) is a semi-supervised method with 12 labeled vides and 15 unlabeled videos. The cnn-lstm-net and spatial-net are temporal and spatial models depicted in Chen et al. (2018). In the CAE method (Qi et al., 2020), a convolutional auto-encoder network is trained first, and then surgical process segmentation is performed.
Metrics and result
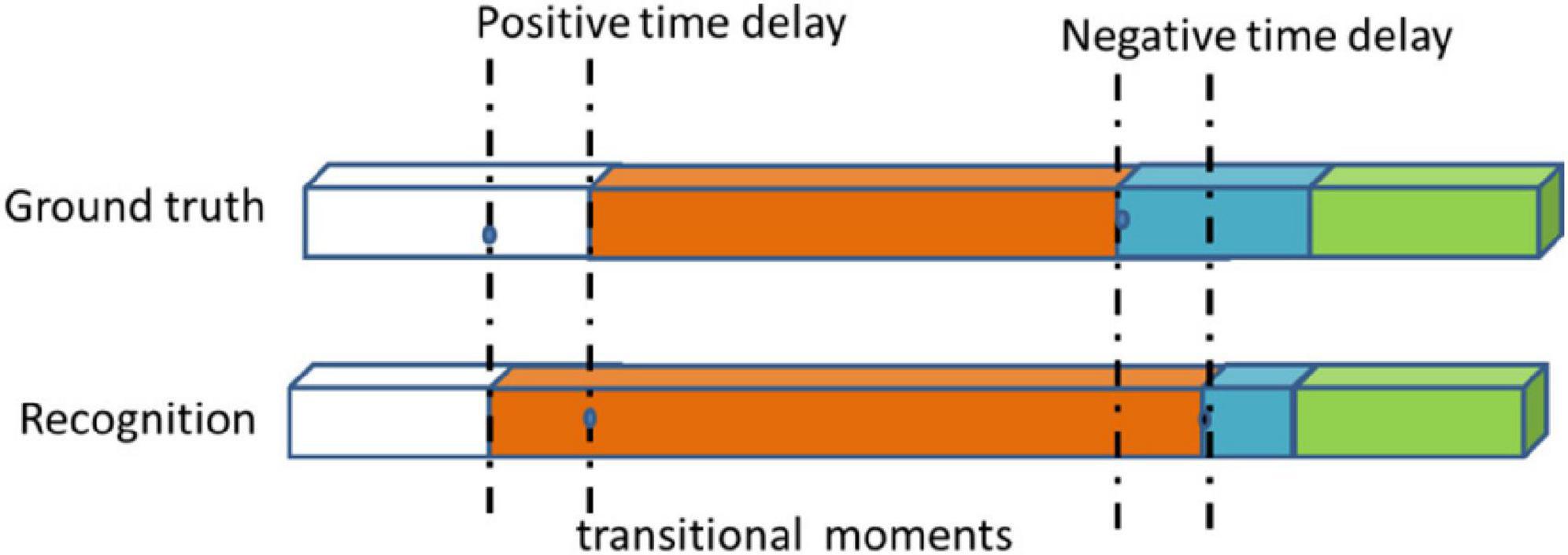
As described in other literatures (Chen et al., 2018; Qi et al., 2020; Shi et al., 2021), the metrics includes standard accuracy (Acc), recall rate (Rec), precision (Pres), average conversion delay (ATD), and real transition ratio (TRR). Some applications do not require a frame-by-phase identification. They may tolerate a certain time delay, but have no fundamental impact on the assistance provided. We introduced the concept of a transition window that a time interval centered on a real transitional moment, at both ends, authorizing an acceptable delay d. If the time moment being checked is in the transition window and occurs because of a delay, it is considered true. In this experiment, we set up different delay time d to calculate the Acc, Rec, and Pres of the model. We called it a time delay standard score. ATD measures the latency generated during all conversions of all available interventions in order to make an average estimate of the delay (see Figure 5). The negative and positive delays are measured separately and used to define the range of values for the average transition delay. A negative delay indicates that the transition between phases is detected in a delayed manner with regards to the ground truth. Conversely, positive delay means that the system decides to switch phases prematurely before the actual transition, details in Dergachyova (2018). The TRR Metric calculates the actual TRR detected between numbers. It is an indicator of system stability and reflects the robustness of the system, as systems with high TRR may have a lower tolerance for intrinsic changes in input data. This ratio also provides a simple and intuitive idea of how many incorrect transfer moments are detected with the number and actual number of transitional moments that they actually detect (see Equation 4).
where the s is the real transfer moment, the s’ is transfer moment detected by the model.
Based on the data collected randomly, we first carry out unsupervised temporal task learning, pre-training, and then use the transfer learning method to carry out phase supervision classification. The corresponding results are shown in Tables 2, 3.
As can be seen from the results in Table 2, our approach has the shortest transition delay [−15s; 30s]. As can be seen from the results in Table 3, the standard Acc, Rec, and Pres of our model reach 89.2, 76.5, and 78.3%, respectively. Based on these results, this is why our model improves Acc less than other usage time delay standard scores. Our approach is more suitable for applications that require rapid system response. However, it makes too many incorrect conversions between phases (6 times more than it should be). On the other hand, the Dergachyova method provides greater delays recognition, but less incorrect phase change peaks (TRR = 2.7). Compared with our method, its recognition is more consistent. The Twinanda method also has a lower TRR. This shows that our model is more suitable for online use, while the Twinanda method and the Dergachyova method are suitable for offline use. The results in Table 3 show how to use the delay transition window to improve performance scores. This helps to make a clearer estimate of how close these methods are actually to clinical applications in specific applications. From the above analysis, it is also important that we do not use a single indicator to distinguish and objectively compare these surgical phases of the identification model. In Table 4, the experimental results of Rec and Pres with no time delay are compared. The results show that our method outperform the comparison methods.
Conclusion
The automatic recognition of the current surgical phase can provide the correct computer assistance at the right time, which is the basis of realizing the context-aware OR system. However, the lack of clinical data in this area is a well-known problem. This creates obstacles to the recognition and analysis of surgical workflow tasks that require significant amounts of data. In this paper, an unsupervised CDC network method is proposed, which simultaneously carries out spatial convolution (for semantic abstraction) and temporal convolution (for visual resolution) of surgical workflow frame sequences. Then through the transfer learning, the CDC network is fine-tuned to classify the operative stage. Based on M2CAI 2016 challenge dataset, experiments and comparisons have been made, and good results have been obtained. The transparency is a very important attribute of the medical system. In this paper, we use a deep learning method has been criticized for the nature of its learning process that is poorly understood. This can cause distrust among doctors. In the future work, we want to visualize the learning processes of deep networks in order to understand exactly what they have learned.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://mldta.com/dataset/m2cai-2016-challenge/.
Author contributions
Y-WC and JZ: study concept and design. K-HZ, Y-WC, and PW: analysis and interpretation of data. Y-WC, Z-YH, and PW: technical support. Y-WC: obtain funding. Y-WC, K-HZ, and Z-YH: writing original manuscript. K-HZ and Y-WC: revision of manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (No. 2018YFC0116704 to Y-WC) and the Youth Innovation Promotion Association of Chinese Academy of Sciences (No. 2020377 to Y-WC).
Acknowledgments
We thank all the people who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-El-Haija, S., Kothari, N., and Lee, J. (2016). YouTube-8M: A large-scale video classification benchmark. arXiv [preprint]. *arXiv:1609.08675v1.
Agarwal, S. K., Joshi, A., and Finin, T. (2006). Context-Aware System to Create Electronic Medical Encounter Records. UMBC, TR-CS-06-05.
Bhatia, B., Oates, T., Xiao, Y., and Hu, P. (2007). “Real-time identification of operating room state from video,” in Proceedings of the 19th Conference on Innovative Applications of Artificial Intelligence (IAAI), 1761–1766.
Calder, A., and Siegel, J. (2009). “Automatic annotation of human actions in video,” in Paper Presented at the IEEE International Conference on Computer Vision.
Chen, Y., Sun, Q., and Zhong, K. (2018). Semi-supervised spatio-temporal CNN for recognition of surgical workflow. EURASIP J. Image Video Proc. 2018:76. doi: 10.1186/s13640-018-0316-4
Dai, W., Yang, Q., Xue, G. R., and Yu, Y. (2007). “Boosting for transfer learning,” in Paper Presented at the International Conference on Machine Learning. doi: 10.1145/1273496.1273521
Deng, J., Dong, W., Socher, R., Li, L. J., Li, K., and Li, F. F. (2009). “ImageNet: A large-scale hierarchical image database,” in Paper Presented at the IEEE Conference on Computer Vision & Pattern Recognition. doi: 10.1109/CVPR.2009.5206848
Deng, S., Zhang, J., Wu, D., He, Y., Xie, X., and Wu, X. (2022). A quantitative risk assessment model for distribution cyber physical system under cyber attack. IEEE Trans. Indust. Inform. doi: 10.1109/TII.2022.3169456
Dergachyova, O. (2018). Knowledge-Based Support For Surgical Workflow Analysis And Recognition Ph. D, Thesis. Rennes University.
Dergachyova, O., Bouget, D., Huaulmé, A., Morandi, X., and Jannin, P. (2016). Automatic data-driven real-time segmentation and recognition of surgical workflow. Int. J. Comput. Assist. Radiol. Surg. 11, 1–9. doi: 10.1007/s11548-016-1371-x
Fan, Q., Zhang, Z., and Huang, X. (2022). Parameter conjugate gradient with secant equation based elman neural network and its convergence analysis. Adv. Theory Simulat. 2022:2200047. doi: 10.1002/adts.202200047
Fard, M. J., Pandya, A. K., Chinnam, R. B., Klein, M. D., and Ellis, R. D. (2016). Distance-based time series classification approach for task recognition with application in surgical robot autonomy. International Journal of Medical Robotics + Computer Assisted Surgery Mrcas 13:3. doi: 10.1002/rcs.1766
Franke, S., Meixensberger, J., and Neumuth, T. (2013). Intervention time prediction from surgical low-level tasks. J. Biomed. Inform. 46, 152–159. doi: 10.1016/j.jbi.2012.10.002
Gaidon, A., Harchaoui, Z., and Schmid, C. (2011). “Actom sequence models for efficient action detection,” in Paper Presented at the IEEE Conference on Computer Vision & Pattern Recognition. doi: 10.1109/CVPR.2011.5995646
Gaidon, A., Harchaoui, Z., and Schmid, C. (2013). Temporal localization of actions with actoms. IEEE Trans. Pattern Analy. Mach. Intelli. 35, 2782–2795. doi: 10.1109/TPAMI.2013.65
Garg, A., Adhikari, N., McDonald, H., Rosas Arellano, M., Devereaux, P. J., Beyene, J., et al. (2005). Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: A systematic review. JAMA J. Am. Med. Assoc. 293, 1223–1238. doi: 10.1001/jama.293.10.1223
Glorot, X., Bordes, A., and Bengio, Y. (2011). “Domain adaptation for large-scale sentiment classification: A deep learning approach,” in Paper Presented at the International Conference on International Conference on Machine Learning.
Guédon, A. C., Paalvast, M., Meeuwsen, F. C., Tax, D. M., van Dijke, A. P., Wauben, L. S., et al. (2016). ’It is time to prepare the next patient’ real-time prediction of procedure duration in laparoscopic cholecystectomies. J. Med. Syst. 40:271. doi: 10.1007/s10916-016-0631-1
Heilbron, F. C., Escorcia, V., Ghanem, B., and Niebles, J. C. (2015). “ActivityNet: a large-scale video benchmark for human activity understanding,” in Proceedings of the 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). 961–970. doi: 10.1109/CVPR.2015.7298698
Huang, J. T., Li, J., Dong, Y., Li, D., and Gong, Y. (2013). “Cross-language knowledge transfer using multilingual deep neural network with shared hidden layers,” in Paper Presented at the IEEE International Conference on Acoustics. doi: 10.1109/ICASSP.2013.6639081
Hübler, A., Hansen, C., Beuing, O., Skalej, M., and Preim, B. (2014). Workflow Analysis for Interventional Neuroradiology Using Frequent Pattern Mining. CURAC Medicine.
Jannin, P., Raimbault, M., Morandi, X., Seigneuret, E., and Gibaud, B. (2001). Design of a neurosurgical procedure model for multimodal image-guided surgery. Int. Cong. 1230, 102–106. doi: 10.1016/S0531-5131(01)00025-5
Kersten-Oertel, M., Jannin, P., and Collins, D. L. (2013). The state of the art of visualization in mixed reality image guided surgery. Comput. Med. Imag. Graph. 37, 98–112. doi: 10.1016/j.compmedimag.2013.01.009
Klank, U., Padoy, N., Feussner, H., and Navab, N. (2008). Automatic feature generation in endoscopic images. Int. J. Comput. Assist. Radiol. Surg. 3, 331–339. doi: 10.1007/s11548-008-0223-8
Krasin, I., Duerig, T., and Alldrin, N. (2017). OpenImages: A Public Dataset for Large-Scale Multi-Label and Multi-Class Image Classification. Available online at: https://storage.googleapis.com/openimages/web/index.html.(accessed March 16, 2022).
Kuznetsova, A., Rom, H., and Alldrin, N. (2020). The open images dataset V4: unified image classification, object detection, and visual relationship detection at scale. arXiv [Preprint]. doi: 10.1007/s11263-020-01316-z
Laptev, I., and Perez, P. (2007). “Retrieving actions in movies,” in Paper Presented at the IEEE International Conference on Computer Vision. doi: 10.1109/ICCV.2007.4409105
Li, J., Xu, K., Chaudhuri, S., Yumer, E., Zhang, H., and Guibas, L. (2017). GRASS: Generative recursive autoencoders for shape structures. ACM Trans. Graph. 36, 1–14. doi: 10.1145/3072959.3073637
Liu, F., Zhang, G., and Lu, J. (2020). Multi-source heterogeneous unsupervised domain adaptation via fuzzy-relation neural networks. IEEE Trans. Fuzzy Syst. 1:3018191. doi: 10.1109/TFUZZ.2020.3018191
Liu, Y., Tian, J., and Hu, R. (2022). Improved feature point pair purification algorithm based on SIFT during endoscope image stitching. Front. Neuror. 2022:840594. doi: 10.3389/fnbot.2022.840594
Macario, A. (2010). What does one minute of operating room time cost? J. Clin. Anesth. 22, 233–236. doi: 10.1016/j.jclinane.2010.02.003
Mackenzie, L., Ibbotson, J. A., Cao, C. G. L., and Lomax, A. J. (2001). Hierarchical decomposition of laparoscopic surgery: A human factor approach to investigating the operating room environment. Minimally Invasive Ther. 10, 121–127. doi: 10.1080/136457001753192222
Meng, Q., Lai, X., Yan, Z., Su, C., and Wu, M. (2021). Motion planning and adaptive neural tracking control of an uncertain two-link rigid-flexible manipulator with vibration amplitude constraint. IEEE Trans. Neural Networks Learn. Syst. 2021, 1–15. doi: 10.1109/TNNLS.2021.3054611
Mexaction2 (2013). Available online at: http://mexculture.cnam.fr/xwiki/bin/view/Datasets/Mex+action+dataset (accessed March 5, 2022).
Nessi, F., Beretta, E., Ferrigno, G., and De, M. E. (2015). “Recognition of user’s activity for adaptive cooperative assistance in robotic surgery,” in Paper Presented at the International Conference of the IEEE Engineering in Medicine & Biology Society. doi: 10.1109/EMBC.2015.7319582
Neumuth, T., Strauß, G., Meixensberger, J., Lemke, H. U., and Burgert, O. (2006). “Acquisition of process descriptions from surgical interventions,” in Paper Presented at the Database & Expert Systems Applications, International Conference. doi: 10.1007/11827405_59
Peters, T. M. (2006). Image-guidance for surgical procedures. Phys. Med. Biol. 51:R505. doi: 10.1088/0031-9155/51/14/R01
Qi, B. L., Zhong, K. H., and Chen, Y. W. (2020). Semi-supervised surgical video workflow recognition based on convolution neural network. Comput. Sci. 47, 172–175.
Schumann, S., Bühligen, U., and Neumuth, T. (2015). Outcome quality assessment by surgical process compliance measures in laparoscopic surgery. Artifi. Intelli. Med. 63, 85–90. doi: 10.1016/j.artmed.2014.10.008
Shi, X., Jin, Y., Dou, Q., and Heng, P. (2021). Semi-supervised learning with progressive unlabeled data excavation for label-efficient surgical workflow recognition. Med. Image Analy. 73:102158. doi: 10.1016/j.media.2021.102158
Shou, Z., Chan, J., Zareian, A., Miyazawa, K., and Chang, S. F. (2017). CDC: Convolutional-de-convolutional networks for precise temporal action localization in untrimmed videos. arXiv [preprint]. *arXiv:1703.01515v2. doi: 10.1109/CVPR.2017.155
Shou, Z., Wang, D., and Chang, S. F. (2016). “Temporal action localization in untrimmed videos via multi-stage CNNs,” in Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). 1049Ű1058. doi: 10.1109/CVPR.2016.119
Sigurdsson, G. A., Russakovsky, O., Farhadi, A., Laptev, I., and Gupta, A. (2016a). Much Ado About Time: Exhaustive Annotation of Temporal Data. arXiv [Preprint]. doi: 10.48550/arXiv.1607.07429
Sigurdsson, G. A., Varol, G., Wang, X., Farhadi, A., Laptev, I., and Gupta, A. (2016b). “Hollywood in homes: Crowdsourcing data collection for activity understanding,” in Paper Presented at the European Conference on Computer Vision. doi: 10.1007/978-3-319-46448-0_31
Twinanda, A. P., Mutter, D., Marescaux, J., De Mathelin, M., and Padoy, N. (2016). Single- and multi-task architectures for tool presence detection challenge at M2CAI 2016. arXiv [Preprint]. doi: 10.48550/arXiv.1610.08851
Wang, S., Sheng, H., Yang, D., Zhang, Y., Wu, Y., and Wang, S. (2022). Extendable multiple nodes recurrent tracking framework with RTU++. IEEE Trans. Image Proc. 2022:319206. doi: 10.1109/TIP.2022.3192706
Wu, D., Zhang, P., He, Y., and Luo, X. (2022). A double-space and double-norm ensembled latent factor model for highly accurate web service QoS prediction. IEEE Trans. Serv. Comput. 2022:3178543. doi: 10.1109/TSC.2022.3178543
Yang, K., Qiao, P., and Wang, Q. (2018). “Frame segmentation networks for temporal action localization,” in Advances in Multimedia Information Processing – PCM 2018. Lecture Notes in Computer Science, eds R. Hong, W. H. Cheng, T. Yamasaki, M. Wang, and C. W. Ngo (Cham: Springer). doi: 10.1007/978-3-030-00767-6_23
Yeung, S., Russakovsky, O., Jin, N., Andriluka, M., Mori, G., and Li, F. F. (2018). Every moment counts: Dense detailed labeling of actions in complex videos. Int. J. Comput. Vision 126, 375–389. doi: 10.1007/s11263-017-1013-y
YouTube-8M Dataset (2018). Available online at: https://research.google.com/youtube8m/index.html (accessed February 9, 2022).
Yu, T., Mutter, D., Marescaux, J., and Padoy, N. (2019). “Learning from a tiny dataset of manual annotations: a teacher/student approach for surgical phase recognition,” in Proceeding of the International Conference on Information Processing in Computer-Assisted Interventions.
Zhang, M., Chen, Y., and Lin, J. (2021). A privacy-preserving optimization of neighborhood-based recommendation for medical-aided diagnosis and treatment. IEEE Int. Things J. 8, 10830–10842. doi: 10.1109/JIOT.2021.3051060
Zhang, M., Chen, Y., and Susilo, W. (2020). PPO-CPQ: A privacy-preserving optimization of clinical pathway query for e-healthcare systems. IEEE Int. Things J. 7, 10660–10672. doi: 10.1109/JIOT.2020.3007518
Zheng, W., Xun, Y., Wu, X., Deng, Z., Chen, X., and Sui, Y. (2021). A comparative study of class rebalancing methods for security bug report classification. IEEE Trans. Reliabili. 70, 1–13. doi: 10.1109/TR.2021.3118026
Keywords: neural networks, convolutional-de-convolutional, transfer learning, surgical workflow, deep learning
Citation: Chen Y-w, Zhang J, Wang P, Hu Z-y and Zhong K-h (2022) Convolutional-de-convolutional neural networks for recognition of surgical workflow. Front. Comput. Neurosci. 16:998096. doi: 10.3389/fncom.2022.998096
Received: 19 July 2022; Accepted: 18 August 2022;
Published: 07 September 2022.
Edited by:
Song Deng, Nanjing University of Posts and Telecommunications, ChinaReviewed by:
Huyong Yan, Chongqing Technology and Business University, ChinaJia Chen, Beihang University, China
Copyright © 2022 Chen, Zhang, Wang, Hu and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun-hua Zhong, emhvbmdrdW5odWFAY2lnaXQuYWMuY24=
 Yu-wen Chen
Yu-wen Chen Ju Zhang1
Ju Zhang1 Kun-hua Zhong
Kun-hua Zhong