- 1Department of Pediatrics, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Chinese EQUATOR Centre, Hong Kong Chinese Medicine Clinical Study Centre, Chinese Clinical Trial Registry (Hong Kong), School of Chinese Medicine, Hong Kong Baptist University, Kowloon, Hong Kong SAR, China
- 3Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, China
- 4Clinical Medical School, Beijing University of Chinese Medicine, Beijing, China
The association between gut microbiota and psychiatric disorders has received increasing research attention. Meanwhile, big data analysis has been utilized in many filed including business, human healthcare analysis, etc. The primary objective of this article was to provide insights into Big Data Analytics (BDA) to clarify the association between gut microbiota and TD (Tic disorder). Specifically, we investigated the recent studies related to gut microbiota composition differences in patients with TD compared to health people. We searched on PubMed and Embase (Ovid) databases for relevant published articles until June 15, 2021. A total of 78 TD and 62 health control stool samples were examined. Case-control design was applied in all the studies. No consensus was evident in α-diversity and β-diversity. The abundance of phyla Bacteroidetes and Firmicutes was predominant at the taxa level. Gut microbiota taxonomic differences were found between TD cases and controls, though inconsistently across studies. Further studies are needed to reveal the underlying pathophysiology of TD and correlation between TD and gut microbiota composition.
Introduction
Tic disorder (TD) is characterized by sudden, recurrent, non-rhythmic movement, or phonic tic with childhood onset, ongoing throughout adulthood (Plessen, 2013). According to the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 (American Psychiatric Association., 2013), TD includes Tourette syndrome (TS), chronic motor or vocal tic disorder (CTD), provisional tic disorder (PTD), other specified tonic disorders, and unspecified tic disorders. TD is the most common movement disorder in children, but the reported prevalence of TD varies considerably (Cubo et al., 2011; Yang et al., 2016; Mohammadi et al., 2021) because a significant proportion of patients do not recognize their tics (Ueda and Black, 2021). Children with TD may experience subjective discomfort, sustained social problems, sleep difficulties, and many emotional problems (Conte et al., 2020; Fernández de la Cruz and Mataix-Cols, 2020; Isaacs et al., 2021). TD is commonly associated with obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder (ADHD), and anxiety disorders (Hirschtritt et al., 2015; Eapen et al., 2016). Thus, research to understand the development of TD is receiving increasing attention lately. TD occurs through interactions including but not limited to genetic (Cao et al., 2021), neurobiochemical (Kanaan et al., 2017), inflammation-related (Martino et al., 2021), immunological (Lamothe et al., 2021), and environmental factors (Storch et al., 2017). However, its pathophysiology remains unknown.
Gut microbiota is a variety of microorganisms in the gastrointestinal tract, normally more than 1,000 bacterial species and with more than nine million genes. Gut microbiota is extremely diverse and changeable with the majority of bacteria from the four dominant phyla including Bacteroides, Firmicutes, Proteobacteria, and Actinobacteria, which constitutes more than 98% of all of the human gut microbes. Gut microbiota constitute a very important part in both of the health maintenance and the disease pathogenesis process. It is a known fact that a diverse and stable and gut microbiota is essential to for various normal physiologic functions such as immunology regulation, prevention of bacterial infection, energy harvest and metabolism, and so on. Meanwhile, the gut microbiota is associated with disease is often characterized by a decrease or increase in species richness and proliferation of some specific pathogens. The gut microbiota plays an important role in the extensive reciprocal connections between the gastrointestinal system and human brain, forming the microbiome-gut-brain axis (Cryan et al., 2020). The association between gut microbiota and psychiatric disorders has received increasing research attention (Morais et al., 2021). Over the past decade, many studies have revealed that the gut microbiota is directly involved in the production of various neurotransmitters, such as gamma-aminobutyric acid (GABA), serotonin (5-HT), glutamate, and dopamine (DA) (Bull-Larsen and Mohajeri, 2019; Altaib et al., 2021; Bhatt et al., 2022), which are closely associated with a number of psychiatric disorders, including TD (Kanaan et al., 2017), ADHD (Turna et al., 2020), OCD (Simpson et al., 2021), and anxiety (Ridaura and Belkaid, 2015).
Gastrointestinal symptoms are not common in TD patients (Fernández de la Cruz and Mataix-Cols, 2020). However, studies show that TD patients have a higher risk of metabolic or cardiovascular disease than the general population, which also plays an important role in the pathogenesis and course of TD, suggesting a relationship between TD and microbiota (Brander et al., 2019; Fernández de la Cruz and Mataix-Cols, 2020; Tomasova et al., 2021). Most TD patients have sleep disorder (Hibberd et al., 2020; Isomura et al., 2022) and are sensitive to psychological stress (Tilling and Cavanna, 2020). Meanwhile, gut microbiota can get disrupted under psychological stress (Madison and Kiecolt-Glaser, 2019; McGuinness et al., 2022) and is correlated with the sleep behavior (Qi et al., 2022). Recent studies have shown that the gut microbiota plays an indispensable role in regulating microglial maturation and function (Bairamian et al., 2022). Circulation of microbe-derived neurotransmitters, including acetylcholine, GABA, and 5-HT, can regulate microglial activation (Fung et al., 2017). Interestingly, abnormalities in microglial activation, development, and function in the basal ganglia of TD patients are also widely recognized (Frick and Pittenger, 2016). Some studies have demonstrated that fecal microbiota transplantation (FMT) effectively ameliorates TD symptoms (Zhao et al., 2017, 2020). Animal studies have also shown that microbiota have the potential to improve tic syndromes (Liao et al., 2019). Despite evidence pointing to a connection between gut microbiota and TD, the nature of this relationship remains unclear. Better understanding of which microbiome is associated with TD and its pathophysiological effects will enable researchers to provide new therapeutic and diagnostic avenues of TD in the future.
Thus, the primary objective of this review was to investigate and compare the recent studies relating to gut microbiota composition differences in patients with TD.
Thus, the primary objective of this work is to summarize, investigate and compare recent studies on gut microbiota composition differences in patients with TD.
Materials and methods
This work has been uploaded and accepted into PROSPERO under the identification number CRD42021265088, performed in accordance with PRISMA guidelines (Page et al., 2021).
Information sources
The databases PubMed and Embase (Ovid) were searched for human studies in English up until June 15, 2021, using the following search strategies (for PubMed): [”tic disorder”(Text Word) OR “tic disorders”(Text Word) OR “tourette syndrome”(Text Word) OR “gilles de la tourette”(Text Word) OR “pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections”(Text Word)] AND [”gut microbiota*”(Text Word) OR “gut microbiome*”(Text Word) OR “intestinal microbiota”(Text Word) OR “intestinal microbiome”(Text Word) OR “gastrointestinal microbiota”(Text Word) OR “gastrointestinal microbiome”(Text Word)] (Supplementary Material 1). Gray literature was included if fulfill the inclusion criteria.
Inclusion and exclusion criteria
Inclusion criteria:
• Original observational studies performed on TD patients diagnosed according to DSM-5 (or IV) or ICD-11 (or 10).
• Detection of gut microbiota composition through high-throughput sequencing techniques.
• Inclusion of a healthy control (HC) group.
• Published in English.
Exclusion criteria:
• Animal studies.
Study selection
Studies were imported into the Mendeley reference manager1 to remove duplicates using its automatic function. Files generated from PubMed and Embase were reviewed and selected using the website: http://syrf.org.uk independently by authors FF and SW based on titles and abstracts, and later the included studies were whole-text reviewed manually. Studies inconsistently agreed upon both reviewers were resolved by a third author, FH.
Outcome measures
Data were extracted from the TD and HC groups using a Microsoft Excel file (Supporting Information 2), focusing on the demographics, microbiota analysis methodology, α- and β-diversity, clinical information, and other relevant findings. A meta-analysis was not performed in the present study.
Risk of bias assessment
The Newcastle-Ottawa Scale (NOS) was used to evaluate the risk of bias in case–control studies. The NOS scale contains three categories comprising total of eight items: selection (four items), comparability (one item), and exposure (three items). Quality score with a maximum of ten was obtained using a rating algorithm: 0–5 (poor), 6–7 (moderate), and 8–10 (high).
Results
Study selection
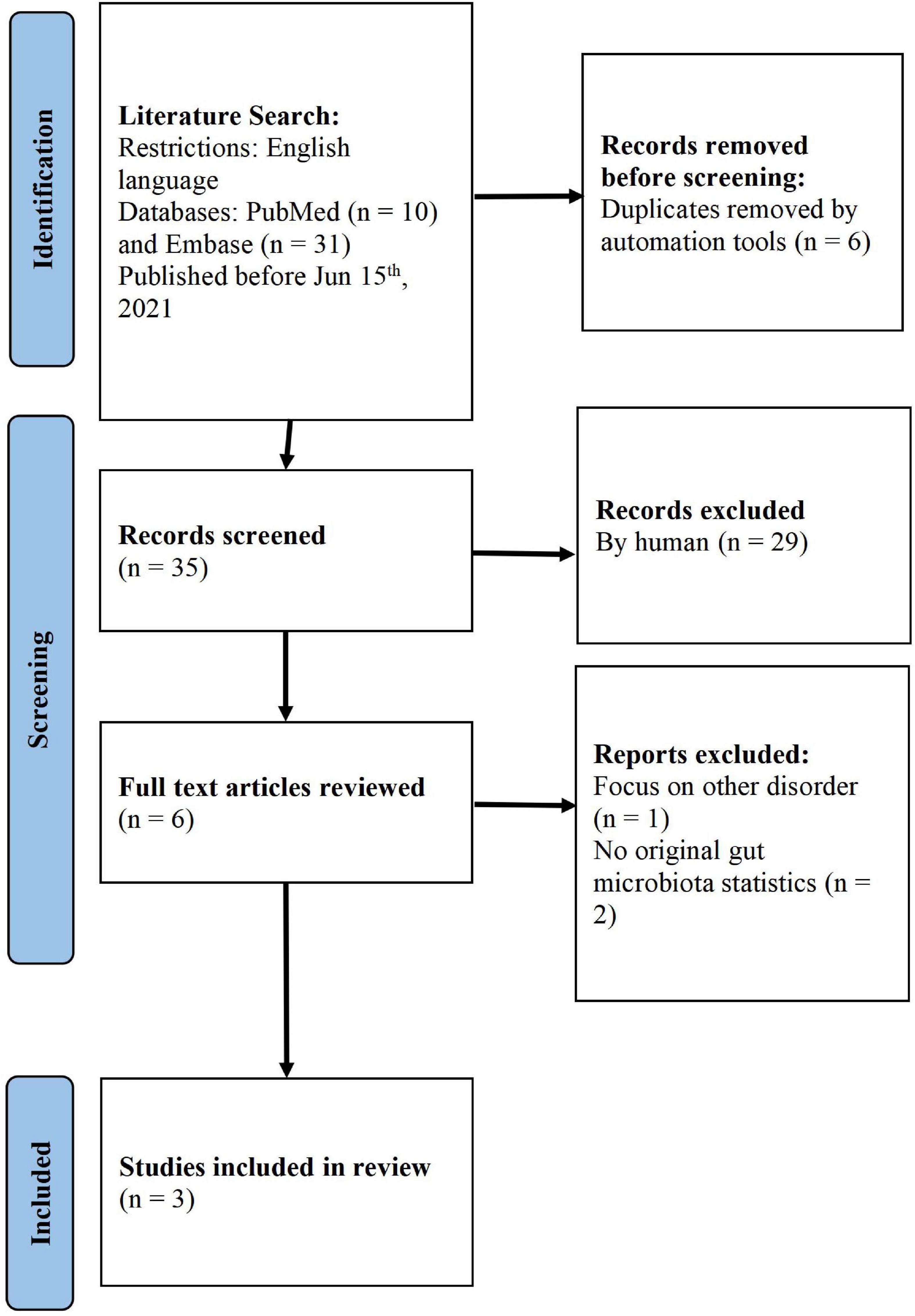
Study selection was conducted using the PRISMA guidelines. Using keywords, we found 41 studies from the literature search. After the automatic removal of duplicates, 35 unique articles were identified. After screening the titles and abstracts of these articles, six were assigned to a full-text assessment, out of which three unqualified articles were removed (one did not focus on TD and two did not have original gut microbiota statistics). Finally, we focused on three articles for further analysis (Lee and Wong, 2018; Zhao et al., 2020; Xi et al., 2021; Figure 1).
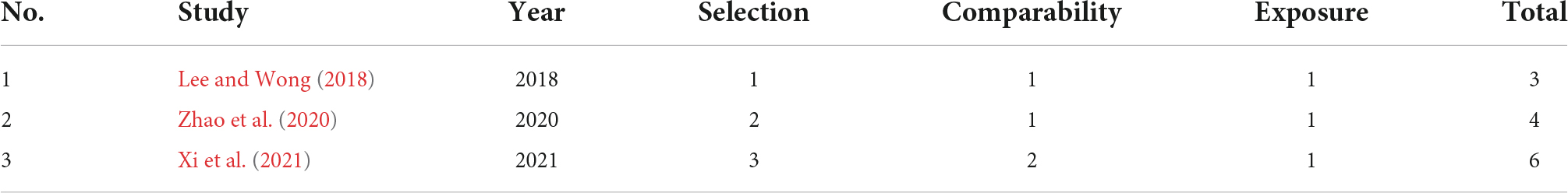
Assessment of study quality/bias
Estimates of bias were obtained for the three studies that compared patients with TD with HCs using the NOS, as indicated in Table 1. One study (Xi et al., 2021) received a score of six (moderate) because the interview was not blinded to the status. The second study received a score of four (low) (Zhao et al., 2020) due to the HC being only one child and thus the resulting potential biases, and the last study received three (low) (Lee and Wong, 2018) due to inadequate description of the study.
Characteristics of studies
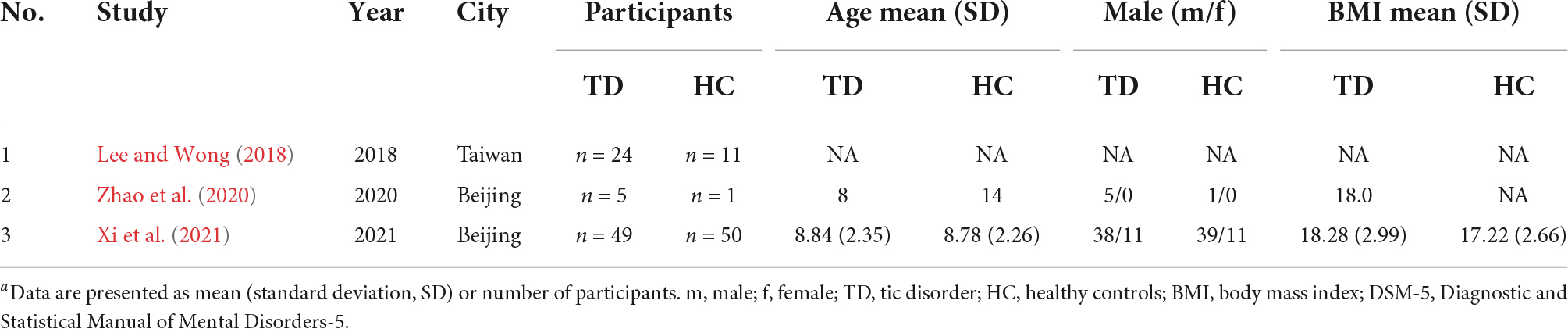
Demographic data of the three studies are shown in Table 2. Two out of three studies were conducted in Beijing, including a total of 54 patients diagnosed with TD and 51 HCs (Zhao et al., 2020; Xi et al., 2021). The other study was conducted in Taiwan, which included 24 TD patients and 11 HCs (Lee and Wong, 2018). The total sample size of the selected studies ranged from 6 to 99, with the number of cases ranging from 5 to 49, and the number of controls ranging from 1 to 50. With these three studies combined, a total of 78 cases and 62 controls were investigated and included TD patients and HCs younger than 18 years. Moreover, the study design of two studies was cross-sectional and compared gut microbiota in TD patients with that in a HC group (Lee and Wong, 2018; Xi et al., 2021).
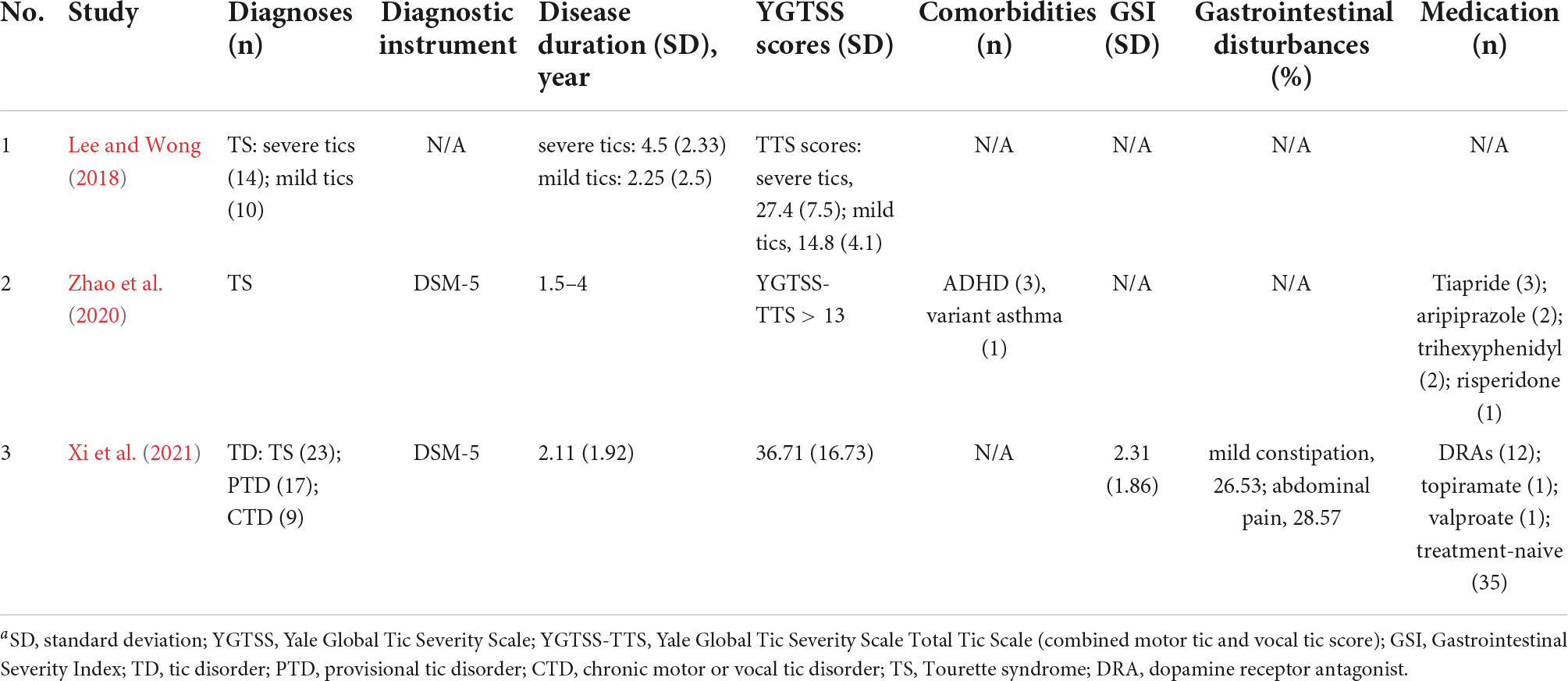
In two studies (Zhao et al., 2020; Xi et al., 2021), patients were assessed according to the DSM-5 criteria. We found that only one study (Xi et al., 2021) mentioned gastrointestinal disturbances (mild constipation and abdominal pain), and provided gastrointestinal severity index (GSI) scores. Two studies (Zhao et al., 2020; Xi et al., 2021) included cases that received dopamine receptor antagonists (DRA) and other medications, while the rest (Zhao et al., 2020; Xi et al., 2021) did not mention these criteria. In addition, only one study (Xi et al., 2021) excluded antibiotics/probiotics taken within 4 weeks prior to sample collection and any infective or other severe disease conditions that may influence the gut microbiota. The ability to compare or interpretation of individual studies is limited by the extensive variability of different aspects of the studies (Table 3).
Microbiota analysis
There were some differences in the sample analysis with respect to the diversity of results in the included studies, as shown in Table 4. Two out of three studies (Zhao et al., 2020; Xi et al., 2021) used shotgun metagenomic sequencing and analyzed the α-diversity and β-diversity of their samples without mentioning the exact index.
Microbiota findings
The gut microbiota of TD patients was compared to that of HCs to assess changes in different individuals’ bacterial abundances. The findings are presented in Table 5 and a more comprehensive listing in Supplementary Material 2. A study by Lee and Wong (2018) stated that the Prevotellaceae family and Prevotella genus were decreased and Ruminococcus genus was increased in TD patients. In the study by Zhao et al. (2020), Bifidobacterium, Catenibacterium, Collinsella, and Dorea genera were decreased in TD patients. In another study by Xi et al. (2021), the species Bacteroides plebeius, Ruminococcus lactaris, Prevotella stercorea, and Streptococcus lutetiensis were decreased in TD patients. Moreover, Xi et al. (2021) found that Bacteroides eggerthii, Bacteroides dorei, and Bacteroides thetaiotaomicron species were positively correlated with the Yale Global Tic Severity Scale (YGTSS) scores (as with the severity of tics). Genus Prevotella was negatively correlated with the severity of tics in another study (Lee and Wong, 2018).
Discussion
Due to the limited treatment methods for tic disorder at present, and the effectiveness of some treatment methods is not so effective, or the effectiveness is limited, so the exploration of its pathogenesis is particularly important, which will guide the better diagnosis and treatment of tic disorder in the future. In recent 10 years, in addition to finding better drug treatments, there are more and more studies on the influences of both hereditary and environmental factors on the occurrence and development of tic disorders (TD). Understanding the microbiome associated with TD has the potential to further research on TD pathophysiology and provide individual treatment options. Although many microbiome infections appear to be correlated with TD (Müller et al., 2004; Mell et al., 2005; Prasad, 2021), to our knowledge, so far no study has revealed the fine-grain pathophysiology. In this work, we attempt to assess whether individuals with TD had a distinct gut microbiota composition compared to HCs. Notably, all the studies identified that the gut microbiota of individuals with TD were distinguishable from that of HCs, although the results of each study varied. The fine structure of the gut microbiota varies greatly among cases (Caporaso et al., 2011).
Main findings
Overall, no consensus regarding α-diversity and β-diversity was found. Xi et al. (2021) found no significant differences in diversity. However, Zhao et al. (2020) found some possible differences, but this was not described in detail. At the taxa level, the abundance of phyla Bacteroidetes and Firmicutes was the predominant difference between TD patients and HCs. One family, one genus, and three species of Bacteroidetes were found to be decreased, while two species were found to be increased in patients with TD. Two genera and eight species of Firmicutes were found to be decreased, while one genus and one species were found to be increased in TD patients. A study by Lee and Wong (2018) found that the proportion of genus Prevotella was negatively correlated with the severity of tics. Meanwhile, Xi et al. (2021) found that the species Bacteroides eggerthii, Bacteroides dorei, and Bacteroides thetaiotaomicron were positively correlated with severity. Bacteroidetes and Firmicutes phyla are also the most dominant gut microbiota in normal people (Jandhyala et al., 2015) and are correlated with inflammatory conditions such as inflammatory bowel disease (Stojanov et al., 2020). The establishment of the gut microbiota has been shown to be a progressive process, and the ratio of Firmicutes to Bacteroidetes is significantly correlated with human age (Ley et al., 2006). The Firmicutes/Bacteroidetes ratio increases from birth to adulthood and further changes with age (Mariat et al., 2009). Reports have shown that changes in the ratio of Firmicutes/Bacteroidetes are significant factors affecting childhood diseases childhood obesity (Indiani CMDSP et al., 2018), autism spectrum disorders (ASD) (Strati et al., 2017), and others (Quagliariello et al., 2016; Valentini et al., 2020). TD typically begins in childhood and often improves in early adulthood, but the reason remains unknown (Hartmann et al., 2020). Current studies link age correlation with TD and the ratio of Firmicutes to Bacteroidetes, although the result is still not definitive. Further studies should focus on this ratio to reveal more comparable results.
Bacteria with increased abundance were found in the gut microbiota of patients with various inflammatory diseases (Zhang et al., 2015; Mondot et al., 2016), suggesting a potential pro-inflammatory effect. Moreover, other studies suggest that decreased abundance of genus Bifidobacterium (Plaza-Díaz et al., 2017) and species Holdemanella biformis (Pujo et al., 2021), which also decreased in this study, had an anti-inflammatory effect. Zhao et al. (2020) analyzed a wide range of inflammatory markers associated with the gut microbiota. Several studies have confirmed this mechanism, and reported elevated levels of pro-inflammatory cytokines [including IL-12 and TNF-α (Leckman et al., 2005)] and decreased levels of anti-inflammatory cytokines (including IL-13) in TD patients (Parker-Athill et al., 2015). In addition, the decreased levels of Prevotella copri, Prevotella stercorea, and Roseburia faecis also determine short-chain fatty acid (SCFA) levels (Louis et al., 2010; Liu et al., 2021). SCFAs play an anti-inflammatory and antimicrobial role in various interactions between gut microbiome and host metabolism (Tan et al., 2014; Sanna et al., 2019). Additionally, microbial metabolites can affect central neurotransmitters by activating afferent nerve fibers. SCFAs can stimulate the release of central neurotransmitters (including 5-HT) in the intestine (Yano et al., 2015). Bifidobacterium is a key member of the human gut microbiota affecting GABA production (Barrett et al., 2012). High levels of Ruminococcus lactaris (Dan et al., 2020) and low levels of the genera Collinsella and Dorea (Strati et al., 2017) have also been found in ASD patients with constipation symptoms, further explaining the potential role and related symptoms of Ruminococcus lactaris in the pathological mechanism of neurodevelopmental disorders.
Treatment and diet
Although there have studies that attempted to utilize FMT (Zhao et al., 2017, 2020) in the treatment of TS (the most severe type of TD), the results have been limited. Zhao et al. (2020) found that FMT might reduce fecal lipopolysaccharide levels in TD patients and increase Bacteroides coprocola and Dialister succinatiphilus abundance and decrease Bacteroides vulgatus abundance. In the study by Xi et al. (2021), DRA-treated patients showed enrichment of Bacteroides dorei, Escherichia coli, Bacteroides caccae, and Ruminococcus gnavus. These enterotypes also seem to have some functional relevance to diet. The genus Bacteroides is associated with high-fat or high-protein diets and Prevotella with high-carbohydrate diets (Wu and Hui, 2011).
Risk of bias
Of the three studies, Xi et al. (2021) displayed age and BMI information as mean and SD, and Zhao et al. (2020) included mean age and BMI. It has been reported that age and BMI are related to the composition of the gut microbiota (Haro et al., 2016; Odamaki et al., 2016). The study by Zhao et al. (2020) was the only study with all-male cases. This actually made the samples more homogeneous because gut microbiota composition has also been shown to differ according to sex (Haro et al., 2016). Lee and Wong (2018) study had scarce demographic data. Although all included studies reported YGTSS scores, there was a lack of consistent diagnostic criteria for the case definition. The reliability and accuracy of microbiome studies depend largely on the molecular biology techniques used, and differences in databases can affect the results of microbiome data (Haro et al., 2016). The studies in this review lack such information, and it is recommended that all studies use uniform classification criteria and databases to obtain more comparable results.
Limitation
However, there are several limitations that should be acknowledged. First, this review included only three studies and a small sample size; thus, more TD patients enrolled from different studies are needed to make our results more reliable and reasonable. Second, in vitro and in vivo experiments were not conducted in the included studies. Finally, differences in the study population, including age, sex, height, weight, genetics, emotion, stress, and environmental factors, were not analyzed in the included studies.
Conclusion
Emerging scientific data support the significant role of the gut microbiota in the regulation of the central nervous system. The results of the included studies show that the gut microbiota in children with TD is significantly different from healthy children. There is variability in microbial diversity as well as the abundance of taxa in patients with TD, which suggesting the complicity of the phenomenon. Furthermore, pro-inflammatory cytokines and central neurotransmitters may both play an important role in the pathophysiology of the gut microbiota in TD.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
FF and SW contributed to the study conception. HW designed the project. ZB and XZ collected the data and performed the formal analysis of finding. QW and SZ organized and integrated the data. FF drafted the manuscript. FF and FH critically reviewed the manuscript. ZB contributed to the visualization. FH acquired the funding source. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded through a grant from the Fundamental Research Funds for the Central Public Welfare Research Institutes, Beijing, China (grant numbers: ZZ13-024-5 and ZZ15-XY-PT-03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncom.2022.986591/full#supplementary-material
Supplementary Material 1 | Methodology.
Supplementary Material 2 | Microbiota analysis.
Supplementary Material 3 | Study quality of case-control studies.
Abbreviations
TD, Tic disorder; DSM, Diagnostic and Statistical Manual of Mental Disorders; TS, Tourette syndrome; CTD, chronic motor or vocal tic disorder; PTD, provisional tic disorder; OCD, obsessive-compulsive disorder; ADHD, attention-deficit/hyperactivity disorder; GABA, gammaaminobutyric acid; 5-HT, serotonin; DA, glutamate and dopamine; FMT, fecal microbiota transplantation; HC, healthy control; NOS, Newcastle-Ottawa Scale; GSI, gastrointestinal severity index; DRA, dopamine receptor antagonists; YGTSS, Yale Global Tic Severity Scale; ASD, autism spectrum disorders; SCFA, short-chain fatty acid.
Footnotes
References
Altaib, H., Nakamura, K., Abe, M., Badr, Y., Yanase, E., Nomura, I., et al. (2021). Differences in the Concentration of the Fecal Neurotransmitters GABA and Glutamate Are Associated with Microbial Composition among Healthy Human Subjects. Microorganisms 9:378. doi: 10.3390/microorganisms9020378
American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th Edn. Washington, DC: American Psychiatric Association. doi: 10.1176/appi.books.9780890425596
Bairamian, D., Sha, S., Rolhion, N., Sokol, H., Dorothée, G., Lemere, C. A., et al. (2022). Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer’s disease. Mol. Neurodegener. 17:19. doi: 10.1186/s13024-022-00522-2
Barrett, E., Ross, R. P., O’Toole, P. W., Fitzgerald, G. F., and Stanton, C. (2012). γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 113, 411–417. doi: 10.1111/j.1365-2672.2012.05344.x
Bhatt, S., Kanoujia, J., Mohanalakshmi, S., Patil, C. R., Gupta, G., Chellappan, D. K., et al. (2022). Role of Brain-Gut-Microbiota Axis in Depression: Emerging Therapeutic Avenues. CNS Neurol. Disord. Drug Targets [Epub Online ahead of print]. doi: 10.2174/1871527321666220329140804
Brander, G., Isomura, K., Chang, Z., Kuja-Halkola, R., Almqvist, C., Larsson, H., et al. (2019). Association of Tourette Syndrome and Chronic Tic Disorder With Metabolic and Cardiovascular Disorders. JAMA Neurol. 76, 454–461. doi: 10.1001/jamaneurol.2018.4279
Bull-Larsen, S., and Mohajeri, M. H. (2019). The Potential Influence of the Bacterial Microbiome on the Development and Progression of ADHD. Nutrients 11:2805. doi: 10.3390/nu11112805
Cao, X., Zhang, Y., Abdulkadir, M., Deng, L., Fernandez, T. V., Garcia-Delgar, B., et al. (2021). Whole-exome sequencing identifies genes associated with Tourette’s disorder in multiplex families. Mol. Psychiatry 26, 6937–6951. doi: 10.1038/s41380-021-01094-1
Caporaso, J. G., Lauber, C. L., Costello, E. K., BergLyons, D., Gonzalez, A., Stombaugh, J., et al. (2011). Moving pictures of the human microbiome. Genome Biol. 12:R50. doi: 10.1186/gb-2011-12-5-r50
Conte, G., Valente, F., Fioriello, F., and Cardona, F. (2020). Rage attacks in Tourette syndrome and chronic tic disorder: a systematic review. Neurosci. Biobehav. Rev. 119, 21–36. doi: 10.1016/j.neubiorev.2020.09.019
Cryan, J. F., O’Riordan, K. J., Sandhu, K., Peterson, V., and Dinan, T. G. (2020). The gut microbiome in neurological disorders. Lancet Neurol. 19, 179–194. doi: 10.1016/S1474-4422(19)30356-4
Cubo, E., Gabriel, Y., Galán, J. M., Villaverde, V. A., Velasco, S. S., Benito, V. D., et al. (2011). Prevalence of tics in schoolchildren in central Spain: a population-based study. Pediatric neurology 45, 100–108. doi: 10.1016/j.pediatrneurol.2011.03.003
Dan, Z., Mao, X., Liu, Q., Guo, M., Zhuang, Y., Liu, Z., et al. (2020). Altered gut microbial profile is associated with abnormal metabolism activity of Autism Spectrum Disorder. Gut Microbes 11, 1246–1267. doi: 10.1080/19490976.2020.1747329
Eapen, V., Cavanna, A. E., and Robertson, M. M. (2016). Comorbidities, Social Impact, and Quality of Life in Tourette Syndrome. Front. Psychiatry 7:97. doi: 10.3389/fpsyt.2016.00097
Fernández de la Cruz, L., and Mataix-Cols, D. (2020). General health and mortality in Tourette syndrome and chronic tic disorder: A mini-review. Neurosci. Biobehav. Rev. 119, 514–520. doi: 10.1016/j.neubiorev.2020.11.005
Frick, L., and Pittenger, C. (2016). Microglial Dysregulation in OCD, Tourette Syndrome, and PANDAS. J. Immunol. Res. 2016:8606057. doi: 10.1155/2016/8606057
Fung, T. C., Olson, C. A., and Hsiao, E. Y. (2017). Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155. doi: 10.1038/nn.4476
Haro, C., Rangel-Zúñiga, O. A., Alcalá-Díaz, J. F., Gómez-Delgado, F., Pérez-Martínez, P., Delgado-Lista, J., et al. (2016). Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PloS One 11:e0154090. doi: 10.1371/journal.pone.0154090
Hartmann, A., Worbe, Y., and Black, K. J. (2020). Tourette syndrome research highlights from 2019. F1000Res. 9:1314. doi: 10.12688/f1000research.27374.2
Hibberd, C., Charman, T., Bhatoa, R. S., Tekes, S., Hedderly, T., Gringras, P., et al. (2020). Sleep difficulties in children with Tourette syndrome and chronic tic disorders: a systematic review of characteristics and associated factors. Sleep 43:zsz308. doi: 10.1093/sleep/zsz308
Hirschtritt, M. E., Lee, P. C., Pauls, D. L., Dion, Y., Grados, M. A., Illmann, C., et al. (2015). Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry 72, 325–333. doi: 10.1001/jamapsychiatry.2014.2650
Indiani CMDSP, Rizzardi, K. F., Castelo, P. M., Ferraz, L. F. C., Darrieux, M., and Parisotto, T. M. (2018). Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child. Obesity 14, 501–509. doi: 10.1089/chi.2018.0040
Isaacs, D. A., Riordan, H. R., and Claassen, D. O. (2021). Clinical Correlates of Health-Related Quality of Life in Adults With Chronic Tic Disorder. Front. Psychiatry 12:619854. doi: 10.3389/fpsyt.2021.619854
Isomura, K., Sidorchuk, A., Sevilla-Cermeño, L., Åkerstedt, T., Silverberg-Morse, M., Larsson, H., et al. (2022). Insomnia in Tourette Syndrome and Chronic Tic Disorder. Move. Disord. 37, 392–400. doi: 10.1002/mds.28842
Jandhyala, S. M., Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M., and Nageshwar Reddy, D. (2015). Role of the normal gut microbiota. World J. Gastroenterol. 21, 8787–8803. doi: 10.3748/wjg.v21.i29.8787
Kanaan, A. S., Gerasch, S., García-García, I., Lampe, L., Pampel, A., Anwander, A., et al. (2017). Pathological glutamatergic neurotransmission in Gilles de la Tourette syndrome. Brain 140, 218–234. doi: 10.1093/brain/aww285
Lamothe, H., Tamouza, R., Hartmann, A., and Mallet, L. (2021). Immunity and Gilles de la Tourette syndrome: A systematic review and meta-analysis of evidence for immune implications in Tourette syndrome. Eur. J. Neurol. 28, 3187–3200. doi: 10.1111/ene.14983
Leckman, J. F., Katsovich, L., Kawikova, I., Lin, H., Zhang, H., Krönig, H., et al. (2005). Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in Tourette’s syndrome. Biol. Psychiatry 57, 667–673. doi: 10.1016/j.biopsych.2004.12.004
Lee, W. T., and Wong, L. C. (2018). Alterations of the intestinal microbiota were correlated with the severity of Tourette syndrome in children. Mov. Disord. 33:S275–S275.
Ley, R. E., Turnbaugh, P. J., Klein, S., and Gordon, J. I. (2006). Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023. doi: 10.1038/4441022a
Liao, J. F., Cheng, Y. F., Li, S. W., Lee, W. T., Hsu, C. C., Wu, C. C., et al. (2019). Lactobacillus plantarum PS128 ameliorates 2,5-Dimethoxy-4-iodoamphetamine-induced tic-like behaviors via its influences on the microbiota-gut-brain-axis. Brain Res. Bull. 153, 59–73. doi: 10.1016/j.brainresbull.2019.07.027
Liu, P., Jiang, Y., Gu, S., Xue, Y., Yang, H., Li, Y., et al. (2021). Metagenome-wide association study of gut microbiome revealed potential microbial marker set for diagnosis of pediatric myasthenia gravis. BMC Med. 19:159. doi: 10.1186/s12916-021-02034-0
Louis, P., Young, P., Holtrop, G., and Flint, H. J. (2010). Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 12, 304–314. doi: 10.1111/j.1462-2920.2009.02066.x
Madison, A., and Kiecolt-Glaser, J. K. (2019). Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 28, 105–110. doi: 10.1016/j.cobeha.2019.01.011
Mariat, D., Firmesse, O., Levenez, F., Guimarães, V., Sokol, H., Doré, J., et al. (2009). The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 9:123. doi: 10.1186/1471-2180-9-123
Martino, D., Schrag, A., Anastasiou, Z., Apter, A., Benaroya-Milstein, N., Buttiglione, M., et al. (2021). Association of Group A Streptococcus Exposure and Exacerbations of Chronic Tic Disorders: A Multinational Prospective Cohort Study. Neurology 96:e1680–e1693. doi: 10.1212/WNL.0000000000011610
McGuinness, A. J., Davis, J. A., Dawson, S. L., Loughman, A., Collier, F., O’Hely, M., et al. (2022). A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 27, 1920–1935. doi: 10.1038/s41380-022-01456-3
Mell, L. K., Davis, R. L., and Owens, D. (2005). Association between streptococcal infection and obsessive-compulsive disorder, Tourette’s syndrome, and tic disorder. Pediatrics 116, 56–60. doi: 10.1542/peds.2004-2058
Mohammadi, M. R., Badrfam, R., Khaleghi, A., Ahmadi, N., Hooshyari, Z., and Zandifar, A. (2021). Lifetime Prevalence, Predictors and Comorbidities of Tic Disorders: A Population-Based Survey of Children and Adolescents in Iran. Child Psychiatry Hum. Dev. doi: 10.1007/s10578-021-01186-7
Mondot, S., Lepage, P., Seksik, P., Allez, M., Tréton, X., Bouhnik, Y., et al. (2016). Structural robustness of the gut mucosal microbiota is associated with Crohn’s disease remission after surgery. Gut 65, 954–962. doi: 10.1136/gutjnl-2015-309184
Morais, L. H., Schreiber, H. L. IV, and Mazmanian, S. K. (2021). The gut microbiota-brain axis in behaviour and brain disorders. Nat.Rev. Microbiol. 19, 241–255. doi: 10.1038/s41579-020-00460-0
Müller, N., Riedel, M., Blendinger, C., Oberle, K., Jacobs, E., and Abele-Horn, M. (2004). Mycoplasma pneumoniae infection and Tourette’s syndrome. Psychiatry Res. 129, 119–125. doi: 10.1016/j.psychres.2004.04.009
Odamaki, T., Kato, K., Sugahara, H., Hashikura, N., Takahashi, S., Xiao, J. Z., et al. (2016). Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016:90. doi: 10.1186/s12866-016-0708-5
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71
Parker-Athill, E. C., Ehrhart, J., Tan, J., and Murphy, T. K. (2015). Cytokine correlations in youth with tic disorders. J. child Adolescent Psychopharmacol. 25, 86–92. doi: 10.1089/cap.2014.0103
Plaza-Díaz, J., Ruiz-Ojeda, F. J., Vilchez-Padial, L. M., and Gil, A. (2017). Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 9:555. doi: 10.3390/nu9060555
Plessen, K. J. (2013). Tic disorders and Tourette’s syndrome. Eur. Child Adolescent Psychiatry 22:S55–S60. doi: 10.1007/s00787-012-0362-x
Prasad, K. M. (2021). Infectious agents as risk factors for psychosis - A time to reconsider and reinvigorate investigations. Schizophrenia Res. 233, 111–113. doi: 10.1016/j.schres.2021.07.007
Pujo, J., Petitfils, C., Le Faouder, P., Eeckhaut, V., Payros, G., Maurel, S., et al. (2021). Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 70, 1088–1097. doi: 10.1136/gutjnl-2020-321173
Qi, X., Ye, J., Wen, Y., Liu, L., Cheng, B., Cheng, S., et al. (2022). Evaluating the Effects of Diet-Gut Microbiota Interactions on Sleep Traits Using the UK Biobank Cohort. Nutrients 14:1134. doi: 10.3390/nu14061134
Quagliariello, A., Aloisio, I., Bozzi Cionci, N., Luiselli, D., D’Auria, G., Martinez-Priego, L., et al. (2016). Effect of Bifidobacterium breve on the Intestinal Microbiota of Coeliac Children on a Gluten Free Diet: A Pilot Study. Nutrients 8:660. doi: 10.3390/nu8100660
Ridaura, V., and Belkaid, Y. (2015). Gut microbiota: the link to your second brain. Cell 161, 193–194. doi: 10.1016/j.cell.2015.03.033
Sanna, S., van Zuydam, N. R., Mahajan, A., Kurilshikov, A., Vich Vila, A., Võsa, U., et al. (2019). Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nature genetics 51, 600–605. doi: 10.1038/s41588-019-0350-x
Simpson, C. A., Diaz-Arteche, C., Eliby, D., Schwartz, O. S., Simmons, J. G., and Cowan, C. S. M. (2021). The gut microbiota in anxiety and depression - A systematic review. Clin. Psychol. Rev. 83:101943. doi: 10.1016/j.cpr.2020.101943
Stojanov, S., Berlec, A., and Štrukelj, B. (2020). The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 8:1715. doi: 10.3390/microorganisms8111715
Storch, E. A., Johnco, C., McGuire, J. F., Wu, M. S., McBride, N. M., Lewin, A. B., et al. (2017). An initial study of family accommodation in children and adolescents with chronic tic disorders. Eur. child Adolescent Psychiatry 26, 99–109. doi: 10.1007/s00787-016-0879-5
Strati, F., Cavalieri, D., Albanese, D., De Felice, C., Donati, C., Hayek, J., et al. (2017). New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5:24. doi: 10.1186/s40168-017-0242-1
Tan, J., McKenzie, C., Potamitis, M., Thorburn, A. N., Mackay, C. R., and Macia, L. (2014). The role of short-chain fatty acids in health and disease. Advances Immunol. 121, 91–119. doi: 10.1016/B978-0-12-800100-4.00003-9
Tilling, F., and Cavanna, A. E. (2020). Relaxation therapy as a treatment for tics in patients with Tourette syndrome: a systematic literature review. Neurol. Sci. 41, 1011–1017. doi: 10.1007/s10072-019-04207-5
Tomasova, L., Grman, M., Ondrias, K., and Ufnal, M. (2021). The impact of gut microbiota metabolites on cellular bioenergetics and cardiometabolic health. Nutr. Metabolism 18:72. doi: 10.1186/s12986-021-00598-5
Turna, J., Grosman Kaplan, K., Anglin, R., Patterson, B., Soreni, N., Bercik, P., et al. (2020). The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age- and sex-matched controls: a pilot study. Acta Psychiatrica Scandinavica 142, 337–347. doi: 10.1111/acps.13175
Ueda, K., and Black, K. J. (2021). A Comprehensive Review of Tic Disorders in Children. J. Clin. Med. 10:2479. doi: 10.3390/jcm10112479
Valentini, F., Evangelisti, M., Arpinelli, M., Di Nardo, G., Borro, M., Simmaco, M., et al. (2020). Gut microbiota composition in children with obstructive sleep apnoea syndrome: a pilot study. Sleep Med. 76, 140–147. doi: 10.1016/j.sleep.2020.10.017
Wu, S. V., and Hui, H. (2011). Treat your bug right. Front. Physiol. 2:9. doi: 10.3389/fphys.2011.00009
Xi, W., Gao, X., Zhao, H., Luo, X., Li, J., Tan, X., et al. (2021). Depicting the composition of gut microbiota in children with tic disorders: an exploratory study. J. Child Psychol. Psychiatry Allied Disciplines 62, 1246–1254. doi: 10.1111/jcpp.13409
Yang, C., Zhang, L., Zhu, P., Zhu, C., and Guo, Q. (2016). The prevalence of tic disorders for children in China: A systematic review and meta-analysis. Medicine 95:e4354. doi: 10.1097/MD.0000000000004354
Yano, J. M., Yu, K., Donaldson, G. P., Shastri, G. G., Ann, P., Ma, L., et al. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276. doi: 10.1016/j.cell.2015.02.047
Zhang, X., Zhang, D., Jia, H., Feng, Q., Wang, D., Liang, D., et al. (2015). The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 21, 895–905. doi: 10.1038/nm.3914
Zhao, H., Shi, Y., Luo, X., Peng, L., Yang, Y., and Zou, L. (2017). The Effect of Fecal Microbiota Transplantation on a Child with Tourette Syndrome. Case Rep. Med. 2017:6165239. doi: 10.1155/2017/6165239
Keywords: tic disorder, gut microbiota, data analysis, bacteroidetes, firmicutes
Citation: Fan F, Bian Z, Zhang X, Wu H, Wang S, Zhang S, Wang Q and Han F (2022) Big data analytics frameworks for the influence of gut microbiota on the development of tic disorder. Front. Comput. Neurosci. 16:986591. doi: 10.3389/fncom.2022.986591
Received: 05 July 2022; Accepted: 29 July 2022;
Published: 25 August 2022.
Edited by:
Deepika Koundal, University of Petroleum and Energy Studies, IndiaReviewed by:
Arvind Dhaka, Manipal University Jaipur, IndiaNeelam Goel, Panjab University, India
Rajit Nair, VIT Bhopal University, India
Copyright © 2022 Fan, Bian, Zhang, Wu, Wang, Zhang, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Fan, ZmFuZmFuc29maWFAaG90bWFpbC5jb20=; Fei Han, aGY0MzgzQDEyNi5jb20=
†These authors have contributed equally to this work
 Fei Fan
Fei Fan Zhaoxiang Bian
Zhaoxiang Bian Xuan Zhang2
Xuan Zhang2 Hongwei Wu
Hongwei Wu Qiong Wang
Qiong Wang