
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Comput. Neurosci. , 15 July 2022
Volume 16 - 2022 | https://doi.org/10.3389/fncom.2022.946514
This article is part of the Research Topic Neuro-inspired Sensing and Computing: Novel Materials, Devices, and Systems View all 5 articles
Neck pain is a worldwide health problem. Clarifying the etiology and providing effective interventions are challenging for the multifactorial nature of neck pain. As an essential component of cervical spine function, the sensorimotor control system has been extensively studied in both healthy and pathological conditions. Proprioceptive signals generated from cervical structures are crucial to normal cervical functions, and abnormal proprioception caused by neck pain leads to alterations in neural plasticity, cervical muscle recruitment and cervical kinematics. The long-term sensorimotor disturbance and maladaptive neural plasticity are supposed to contribute to the recurrence and chronicity of neck pain. Therefore, multiple clinical evaluations and treatments aiming at restoring the sensorimotor control system and neural plasticity have been proposed. This paper provides a short review on neck pain from perspectives of proprioception, sensorimotor control system, neural plasticity and potential interventions. Future research may need to clarify the molecular mechanism underlying proprioception and pain. The existing assessment methods of cervical proprioceptive impairment and corresponding treatments may need to be systematically reevaluated and standardized. Additionally, new precise motor parameters reflecting sensorimotor deficit and more effective interventions targeting the sensorimotor control system or neural plasticity are encouraged to be proposed.
Neck pain is one of the most commonly reported musculoskeletal disorders, causing a substantial economic burden to healthcare systems, absence from work, and compensations (Kazeminasab et al., 2022). Around 50% of the adult population experience at least one episode of neck pain during their lifetime, and neck pain ranks fourth in the leading causes of global disabilities (Fejer et al., 2006; Hoy et al., 2014). The main challenge in the long-term management of neck pain is to provide accurate diagnosis and effective therapies (Cohen, 2015; Vardeh et al., 2016). Neck pain is a multifactorial disease influenced by many biological, psychological, behavioral and social factors, making it challenging to identify the main contributors and their relevance to the consequences of neck pain (Kazeminasab et al., 2022). A large portion of neck pain patients is classified as non-specific since a clear pathoanatomical etiology of the neck pain is not detected (McLean et al., 2010; Misailidou et al., 2010), which makes therapies tend to focus on addressing the symptoms and the physical impairments of neck pain.
Sensorimotor control system is a very important component of the cervical spine (Figure 1). The impaired proprioception and disturbance of the sensorimotor control system in neck pain have been extensively studied in previous research studies (Reddy et al., 2019; Asiri et al., 2021; Peng et al., 2021), and the long-term sensorimotor alteration and neural plasticity changes due to persistent proprioceptive deficit has been suggested to contribute to the recurrence and chronicity of neck pain (Hodges and Tucker, 2011; Röijezon et al., 2015; Kristjansson et al., 2016; Brumagne et al., 2019). This review presents a short update on proprioception of the cervical spine and impaired proprioception in patients with neck pain. First, the sensorimotor control system of the cervical spine is introduced to evaluate mechanisms underlying normal and neck pain conditions. Then, maladaptive neural plasticity will be discussed in chronic neck pain conditions, and, finally, interventions to manipulate the sensorimotor control system and maladaptive neural plasticity will be proposed.
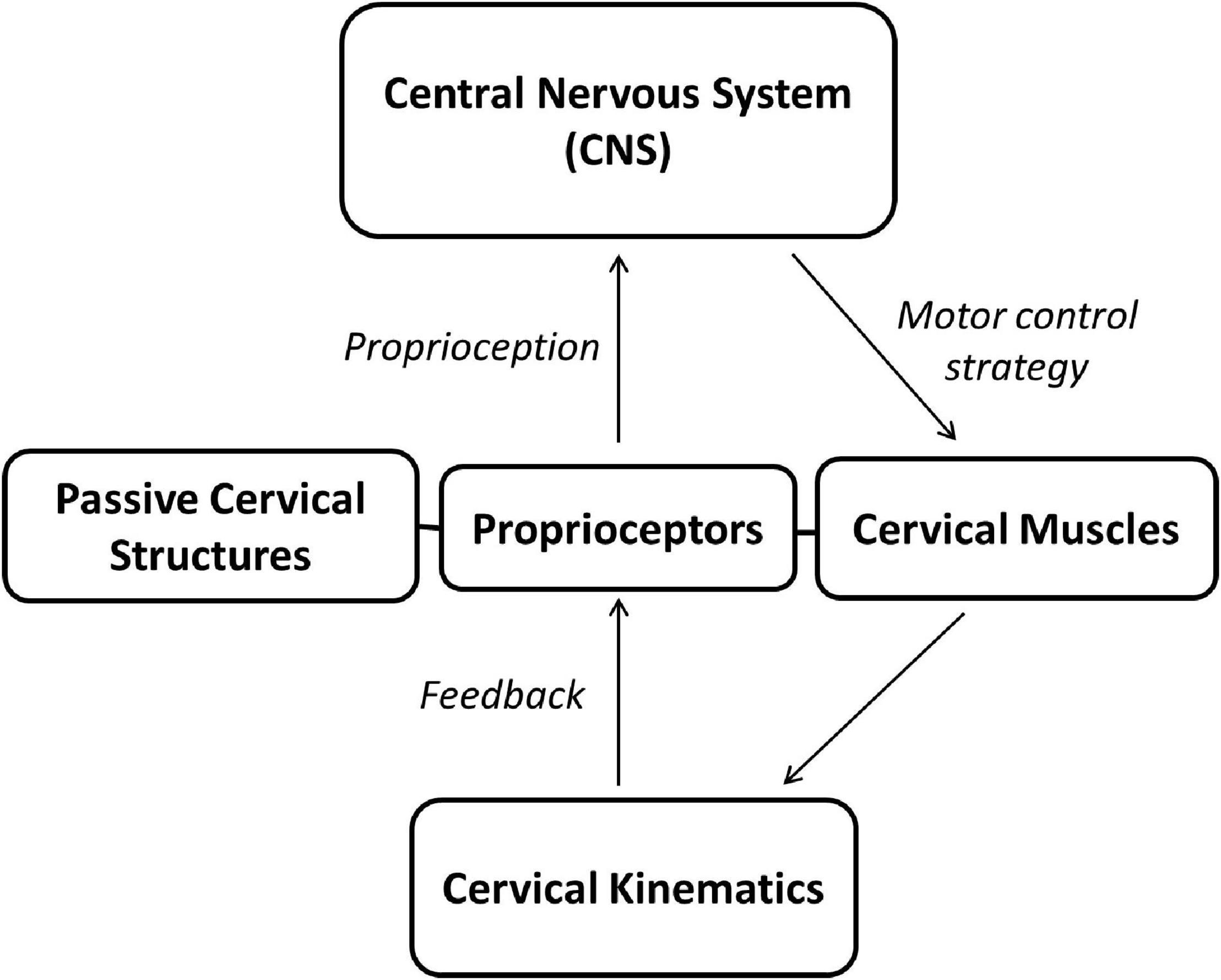
Figure 1. The cervical sensorimotor control system. The central neural system instantly processes proprioception generated from proprioceptors in cervical structures (i.e., cervical muscles and passive structures) and sends motor commends to cervical muscles to complete neck movements. The neck movements, in turn, could affect the proprioception generation.
Cervical proprioception refers to sensory information generated by muscle spindle, Golgi tendon organs (GTOs), joint receptors and cutaneous receptors, which located in muscle, tendon, joint capsules and skin, respectively (Hogervorst and Brand, 1998; Delhaye et al., 2018; Kröger, 2018). The constant sensory information, together with the vestibular and visual systems, ensures coordinated motor functions and rapid reaction of the neck to the surrounding environment (Proske and Gandevia, 2012; Kiehn, 2016). The proprioception plays a crucial role in maintaining posture and stability of the cervical joints during static and dynamic situations (Strimpakos, 2011; Proske and Gandevia, 2018). Extensive literature indicates that GTOs and muscle spindles mainly contribute to neck proprioception, while the contribution of joint and cutaneous receptors are minimal (Armstrong et al., 2008; van der Wal, 2009; Proske and Gandevia, 2012). The density of muscle spindles is distributed diversely across cervical muscles and is particularly high in the small suboccipital muscles, which implies their roles in the fine motor control of the neck (Kulkarni et al., 2001; Boyd-Clark et al., 2002; Liu et al., 2003). The muscle spindles are typically innervated by group Ia and group II afferents, while the GTOs are innervated by group Ib afferents (Jami, 1992; Delhaye et al., 2018). With respect to differences in the anatomical location and type of afferents, the muscle spindles are sensitive to changes in static muscle length and the rate of change in muscle length, while the GTOs are sensitive to the changes in contractile force (Chalmers, 2002; Vincent et al., 2017; Wilkinson, 2022). The core function of the proprioceptors is to transduce mechanical stimulus from muscles and tendons into electrochemical signals and project it via dorsal root ganglia (DRG) to the central neural system (CNS) (Delmas et al., 2011; Bewick and Banks, 2015). As a family of mechanosensitive membrane proteins, Piezo channels have been reported to be the main mechanically activated cation channels during this mechanotransduction process (Coste et al., 2012; Murthy et al., 2017). In particular, the expression of the Piezo2 channel is extremely high in DRG sensory neurons (Coste et al., 2010). Additionally, when conditioned with the deletion of Piezo2 channels in proprioceptive neurons, the experimental mice show severe deficits in movement coordination and sensing limb positions (Florez-Paz et al., 2016). Patients with loss of function mutations in the Piezo2 gene display deficits in producing coordinated movements (Chesler et al., 2016; Szczot et al., 2018). However, the exact molecular mechanism of proprioception still needs further research. Proprioceptive sensory afferents typically interact with monosynaptic motor neurons that control the same muscle or synergistic muscles (Manuel and Zytnicki, 2011; Imai and Yoshida, 2018), and neck pain can impaire cervical proprioception and altered motor control strategy of cervical spine (Meisingset et al., 2015, 2016).
Any injuries to cervical structures affect the proprioceptive system, as clearly demonstrated in whiplash-associated and chronic neck patients (De Pauw et al., 2016; Mazaheri et al., 2021). Aside from injuries, cervical structural degeneration that occurs with aging could also lead to proprioceptive deficits (Ferlinc et al., 2019). It has been demonstrated that aged subjects show much fewer intrafusal fibers and denervation of muscle spindles from different parts of the body when compared with young subjects (Swash and Fox, 1972). Studies have confirmed the decline of cervical proprioceptive function in elderly participants (Vuillerme et al., 2008; Landelle et al., 2018), and patients with muscular dystrophy also show spindle morphology changes and corresponding impairment of the proprioception, manifested as postural instability and poor coordination (Kararizou et al., 2007; Troise et al., 2014). Based on literature reviews (Peng et al., 2021), many tests have been applied to measure the sensorimotor control system in neck pain patients, among which the joint position error (JPE) is the most commonly used, reflecting the impairment in joint position sense. Patients with neck pain, in general, show greater JPE when compared with healthy subject, although conflicting results exist between studies due to differences in methodologies (Stanton et al., 2016; de Zoete et al., 2017). In a recent review, the JPE does not differ between patients with traumatic neck pain and non-traumatic neck pain, but both show proprioceptive deficits compared with healthy controls (de Vries et al., 2015). Further, previous studies have also found that cervical JPE was not different between young and old subjects with chronic neck pain (Alahmari et al., 2017). These results indicate that pain itself may have a major influence on the proprioceptive system over degeneration with aging and structural damage to the neck. This point was proved in abundant experimental and clinical neck pain research studies (Malmström et al., 2013; Gizzi et al., 2015; Zaproudina et al., 2015). Furthermore, some previous studies reported that the cervical JPE was positively correlated with neck pain intensity in subjects with cervical spondylosis (Reddy et al., 2019).
Although still unclear, pain may affect cervical proprioception at any stage during the signal transduction process according to the complex neurological pathway (Röijezon et al., 2015). The evidence indicated that the activation of nociceptors (type III and type IV afferents) could inhibit the activity of gamma motor neurons, which leads to proprioceptive disturbance (Riemann and Lephart, 2002; Bennell et al., 2003). Moreover, the cellular bodies of nociceptors are embedded in the dorsal root ganglion as well, and the proprioceptive signals could be competitively suppressed by nociceptive signals in higher CNS centers (Schomburg et al., 1999). Abnormal proprioception from the peripheral cervical structures could cause cortical neuroplastic changes, modify the sensorimotor control system and eventually result in altered motor outputs (Woodhouse et al., 2010; Meisingset et al., 2015; DePauw et al., 2017).
Three interactive systems are involved in the sensorimotor control of neck movements: the active system (cervical muscles), the passive system (vertebrae, intervertebral disks, ligaments, joint capsules and facet joints) and the central nervous system (Panjabi, 1992a; Izzo et al., 2013). It has been estimated that the mechanical stability of the cervical spine is 20% from the osseoligamentous structures and 80% from the musculature structures (Panjabi et al., 1998). Cervical muscles are the direct performers of the sensorimotor control system, and the coordination between cervical muscles ensures the dynamic stability of the cervical spine during neck movements (Panjabi, 1992b; McGill et al., 2003). More than 20 pairs of cervical muscles surround the cervical spine column, including deep and superficial muscles (Blouin et al., 2007). The deep cervical muscles, typically attached to the cervical vertebrae directly with a small moment during neck movements, are supposed to control individual cervical joint motion (e.g., longus colli, longus capitis, and multifidus muscles) (Blouin et al., 2007; Schomacher and Falla, 2013). By contrast, superficial cervical muscles cross several cervical vertebrae or the entire cervical spine and work as the posture maintainer and movement initiator (e.g., sternocleidomastoid and trapezius muscles) (Blouin et al., 2007; Schomacher and Falla, 2013). Cervical ligaments are traditionally supposed to have only mechanical properties, limiting the cervical joint motion at the extremes of neck movements (Hartman et al., 2016). The ligaments are important passive stabilizers but functionally connected to the surrounding muscles by the ligamento-muscular reflex (Dyhre-Poulsen and Krogsgaard, 2000; Chu et al., 2003; Hendershot et al., 2011). Paraspinal muscles (such as multifidus muscle) could be activated by stimulus in ligaments and restrict the segmental cervical joint motion during neck movements (Solomonow et al., 1998). With respect to a specific movement, the central nervous system continuously collects proprioception feedback and adjusts the motor command to regulate muscle activities and achieve dynamic balance, movement acuity and coordination (Strimpakos, 2011; Röijezon et al., 2015; Qu et al., 2019b).
Neck pain is associated with disturbance in cervical sensorimotor control (Woodhouse and Vasseljen, 2008; Gizzi et al., 2015). The motor control strategy of the cervical spine has been most commonly studied by measuring electromyographic (EMG) activity of the cervical muscles involved in a specific motor task (Falla and Farina, 2008). The structural complexity of the cervical spine reflects its potential compensatory mechanism under pathologic conditions (Vasavada et al., 2002; Falla and Farina, 2008). In experimental neck pain studies, the same submaximal-load motor task could be accomplished in the presence of pain by reorganizing the activation of the cervical muscles involved (Tucker et al., 2009; Muceli et al., 2014; Abboud et al., 2016). This kind of reorganization strategy exists between different parts of the same muscle or muscle groups involved in the task (Falla et al., 2007b; Falla and Farina, 2008; Samani et al., 2009). In principle, the CNS explores control strategies to complete the same motor task by minimizing the use of the painful muscle in order to reduce further pain or injuries (Falla, 2004; Falla et al., 2007a). Therefore, the painful muscle generally shows decreased EMG activity during the motor task, together with redistribution of activation among the synergist and antagonist muscles (Falla and Farina, 2007, 2008; Falla et al., 2007a). The altered motor control strategy, in consequence, is often task-specific and direction-specific due to the role of the painful muscles (agonist or antagonist) in the task (Falla et al., 2007a).
Patients with neck pain are typically associated with decreased activity of deep cervical muscles and increased activity of superficial cervical muscles (Schomacher and Falla, 2013; Tsang et al., 2014). In addition, enhanced cervical muscle co-activation has also been demonstrated in previous studies, which is considered to be a strategy to increase the stiffness of the cervical spine (Cheng et al., 2014). This finding aligns with previous studies showing that the cervical spine is controlled in a more stiffening pattern with neck pain (Meisingset et al., 2015). Delayed onset of activation, prolonged activation and reduced resting periods are the other manifestations of deep cervical muscles in patients with neck pain (Falla et al., 2004a,b).
The deficit in the sensorimotor control system alters the kinematic characteristics of the cervical spine in patients with neck pain, including both the quantitative and qualitative aspects, which have been widely reported in previous studies (Ylinen et al., 2004; Sjölander et al., 2008; Sarig Bahat et al., 2010; Tsang et al., 2013). The quantitative measurements reflect the ability of the neck to achieve a specific motor task, such as maximal voluntary contraction (MVC) and cervical range of motion (ROM), which are reported to be reduced in patients with neck pain if beyond the compensatory capacity of the cervical spine (Lindstroem et al., 2012; Rudolfsson et al., 2012). On the other hand, the qualitative parameters indicate the quality of the motor task execution and more representatively reflect the altered motor control strategy during the motion process with neck pain. The velocity, acceleration, smoothness, accuracy, conjunct motion, and ROM-variability of neck movements have been demonstrated to be different between patients with neck pain and healthy controls (Sjölander et al., 2008; Sarig Bahat et al., 2010). However, the quantitative and qualitative measurements both showed conflicting results in previous studies or reviews, which may result from methodologic differences and sample bias et al. (Kauther et al., 2012; Franov et al., 2022). The above-mentioned parameters are gross motor outputs and cannot reflect the individual cervical joint impairment. Meanwhile, the motor deficit of an individual joint will be compensated by the other joints due to the compensative mechanism within the cervical spine resulting in unchanged motor outputs (Schwab et al., 2006; Lan et al., 2014). Theoretically, the altered motor control strategy during pain could change tissue loading, the direction and magnitude of joint forces and contributes to the altered cervical joint motion patterns (Yoganandan et al., 2001). The motor impairments are sometimes subtle and cannot be detected by traditional physical examination (Oddsdottir and Kristjansson, 2012). New dynamic motion parameters, such as anti-directional joint motion or joint motion variability, are needed to precisely capture this motor alteration at individual cervical joints (Qu et al., 2019a,b, 2020).
The ability of neurons to change function, form and number is called neural plasticity (Citri and Malenka, 2008). Adaptive neural plasticity results in changes in the synaptic connection strength between neurons under physiological conditions, and it is a critical process for improving brain functioning (Citri and Malenka, 2008). It is, for example, an essential neuronal substrate for learning and memory (Pascual-Leone et al., 1994). Maladaptive neural plasticity is the pathological side of adaptative neural plasticity and is caused by an imbalance in the synaptic activity of the nervous system (Kuner and Flor, 2017). The effect of maladaptive neural plasticity is a loss of nervous system coordination and function, resulting in impairment and deterioration in the quality of life. Maladaptive neural plasticity during prolonged and persistent pain has been suggested in recent years, and it has been proposed that sustained nociceptive inputs from an injured tissue might result in dysfunctional neural plasticity changes (Kuner and Flor, 2017). Based on various neurophysiological and neuroimaging studies, dysfunctional nervous system activity (Tsao et al., 2011), coupled with structural remodeling (Mansour et al., 2013; Baliki and Apkarian, 2015), has been reported in individuals suffering from persistent musculoskeletal pain, including neck pain (DePauw et al., 2017).
Clinically, somatosensory, proprioceptive and neuromuscular impairments are commonly reported in patients with chronic neck pain. Some of these impairments include cold and mechanical pain hyperalgesia in the neck region (Johnston et al., 2009; Walton et al., 2011), forward head posture (Mahmoud et al., 2019), altered joint motion pattern (Qu et al., 2020), and dysfunction of the deep cervical flexor muscles (Falla et al., 2004b). Patients with chronic neck pain also tend to show unsuitable emotional and cognitive factors associated with pain, such as pain catastrophizing and fear of movement (Dimitriadis et al., 2015; Lee et al., 2015), and nociceptive pain episodes increase the probability of becoming chronic pain when various psychosocial variables exacerbate maladaptive processes triggered by pathophysiological factors (Kuner and Flor, 2017). Since, in many patients with neck pain, particularly those with chronic symptoms, a clear pathophysiological origin explaining the experience of pain is lacking (Elliott et al., 2009), or the nociceptive source is not significant enough to justify the neck pain reported by patients, researchers have moved the focus away from abnormal musculoskeletal tissue explanations and started exploring the role of the nervous system, such as central sensitization (Latremoliere and Woolf, 2009; Peirs and Seal, 2016). Central sensitization mainly occurs due to persistent peripheral nociceptive stimulation, is reported to contribute to the chronic pain and mainly depends on neuronal changes in the CNS (Ji et al., 2018; Bonanni et al., 2022). In some of those patients, there is frequently clinical evidence of maladaptive pain neural plasticity (Van Oosterwijck et al., 2013), a general term used to indicate an alteration in the function of neurons and circuits in nociceptive pathways (Lefaucheur et al., 2014). In the last few decades, the involvement of the nervous system in chronic pain conditions has been widely explored using electrophysiological and imaging techniques (Kuner and Flor, 2017). For instance, from a sensory perspective, reorganization of the primary somatosensory cortex has been examined in patients affected by chronic low back pain using magnetoencephalography (Flor et al., 1997). Motor-evoked potentials (MEPs) to transcranial magnetic stimulation (TMS) have also demonstrated a smudging of corticospinal excitability of specific muscles (overlap of motor cortical maps and centers of gravity) in individuals affected by persistence/recurrence of low back pain compared to healthy control (Tsao et al., 2008; Schabrun et al., 2017). These results may indicate that the primary somatosensory cortex and motor corticospinal excitability show maladaptive neural plasticity in people affected by musculoskeletal pain, including chronic neck pain. Furthermore, neuroimaging studies have also demonstrated that emotional and cognitive regions of the brain, such as the medial prefrontal cortex, amygdala and hippocampus (Mutso et al., 2014; Baliki and Apkarian, 2015), are altered in chronic musculoskeletal pain patients, suggesting that these regions may also be critically involved in the abatement of chronic neck pain.
Exploring the effective treatment of neck pain has long been a challenge. For the importance of the sensorimotor control system, treatments aiming to restore sensorimotor function have been proposed as important managements of neck pain, including balance exercise, joint position and movement sense training, gaze direction recognition exercise, sensory discrimination training, and coordinative exercises (Beinert and Taube, 2013; Kälin et al., 2016; Duray et al., 2018; Saadat et al., 2019). These treatments, in essence, either enhance position/motion sense by repeatedly provoking the proprioceptors or correct motor patterns by increasing the targeted muscle activity (Peng et al., 2021). Abundant evidence has revealed that the proprioceptive training and motor control exercises could improve the joint reposition accuracy and neck disability, and reduce the pain intensity in patients with neck pain, although treatment methods vary among studies (Beinert and Taube, 2013; Sarig Bahat et al., 2015; Duray et al., 2018; Saadat et al., 2019). In a balance exercise, subjects typically need to keep their head upright when standing by a single leg or on a wobble board with/without visual feedback. Beinert and Taube et al. found that the balance exercise can reduce pain intensity and improve the JPE in patients with neck pain (Beinert and Taube, 2013). Gaze direction recognition exercise is able to enhance the beneficial effect of conventional physical therapy on pain reduction, functionality recovery and balance performance (Duray et al., 2018). Deep cervical flexor and extensor training are reported to reduce pain intensity and functional disability in patients with chronic mechanical neck pain, but the effect on strength and endurance remain conflicting (Blomgren et al., 2018; Suvarnnato et al., 2019). Coordination exercises, aiming to restore the active neck movements and retrain the fine movement control of the cervical spine, are reported to reduce pain and alter motor control strategy between deep and superficial cervical muscles (Rudolfsson et al., 2014). With the development of virtual reality (VR) techniques, the VR-based kinematic training on patients with neck pain shows improvements in range of motion, accuracy, velocity, smoothness, fine motor control and coordination of the cervical spine (Nusser et al., 2021). It is believed that the VR-based kinematic training could motivate the visual systems, vestibular systems and sensorimotor control system simultaneously in patients with neck pain (Sarig Bahat et al., 2015). Furthermore, the VR-based training method shows an effect on overcoming kinesiophobia in patients with neck pain (Tejera et al., 2020). However, no conclusion could be made that the sensorimotor therapy is better than other kinds of treatments since there is no unification in terms of interventions, therapy time, populations and variety of control groups across research studies (McCaskey et al., 2014). The beneficial effects of proprioceptive training could be augmented when combined with other therapy exercises, such as physical exercises and biofeedback (Sielski et al., 2017; Saadat et al., 2019; Tsiringakis et al., 2020). Therefore, more large samples of randomized controlled trials are needed to provide robust evidence on sensorimotor control system training. Evidence has shown that proprioceptive training is associated with reorganization within the sensorimotor cortex (Aman et al., 2014). Previous studies indicate that the sensorimotor therapies may reverse the pain-induced cortical changes to a normal level based on the plasticity property of the nervous system, which partially explains the symptoms relief and functions recovery in patients with neck pain (Moseley and Flor, 2012).
Based on electrophysiological and neuroimaging findings in chronic pain patients, treatments that reverse maladaptive neural plasticity, such as non-invasive brain stimulation techniques, have been proposed as a substantial potential for improving future rehabilitation processes (Schabrun and Chipchase, 2012). Non-invasive brain stimulation techniques utilize electromagnetic principles to modulate neural activity non-invasively by generating cortical electrical fields (Rossini et al., 2015). Two main classes of non-invasive brain stimulation are currently applied for research and clinical purposes: repetitive transcranial brain stimulation (rTMS) and transcranial electrical stimulation (tES). Both techniques have generally been shown to be partially effective in reducing pain for some non-musculoskeletal pain conditions, such as peripheral neuropathic pain and migraine, and musculoskeletal pain conditions, such as low back pain (Lefaucheur et al., 2014). However, the clinical evidence for non-invasive brain stimulation in chronic neck pain is still lacking, although some preliminary modulatory effects on motor cortex excitability and analgesic effects have been proven in chronic low back pain (Schabrun et al., 2014; Ambriz-Tututi et al., 2016).
A recent meta-analysis demonstrated that non-invasive brain stimulation increased pain thresholds across all modalities, including mechanical and thermal, in healthy individuals when pooling studies of rTMS and tES of the primary motor cortex (Giannoni-Luza et al., 2020). A recent randomized controlled trial in individuals with chronic low back pain looked at the efficacy of rTMS (Ambriz-Tututi et al., 2016), and by the third week of treatment, 41 patients who received 20-Hz rTMS stimulation over the primary motor cortex showed an 80% reduction in pain from baseline, which was considerably lower than those who received sham rTMS. Pressure pain thresholds also increased in healthy individuals following daily sessions of rTMS on the left dorsolateral prefrontal cortex (De Martino et al., 2019a). In a sham-controlled design study, daily rTMS sessions targeting the left dorsolateral prefrontal cortex reduced long-term pain intensity induced by intramuscular nerve growth factor injections, as well as reversing pain-induced pressure hyperalgesia, altered cortical somatosensory excitability, and corticomotor excitability (Seminowicz et al., 2018; De Martino et al., 2019b). Similar analgesic findings were observed following rTMS to the primary motor cortex in a similar long-term pain paradigm (Cavaleri et al., 2019). This proof of concept demonstrates the use of rTMS in larger musculoskeletal pain studies, and, with more research and a stronger focus on clinical outcomes, it is possible that rTMS may become an integral part of the treatment arsenal for therapists for chronic neck pain in the future.
Using non-invasive brain stimulation to the primary motor cortex has also been shown to augment motor training-induced plasticity by producing a rapid and powerful after-effect in facilitating or depressing the motor cortex excitability, outlasting the stimulation period (Bolognini et al., 2009). Although the mechanics are still unclear, non-invasive brain stimulation techniques may cause different patterns of calcium influx to postsynaptic neurons through N-methyl-D-aspartate channels and gamma-aminobutyric acid receptors, resulting in long-term potentiation and long-term depression in the motor cortex (Huang et al., 2017). There is preliminary evidence that therapies aimed at motor control can improve motor cortex excitability and alleviate pain in chronic musculoskeletal disorders. For example, in the case of chronic low back pain, interventions targeting the primary motor cortex representation of lumbar multifidus muscles or the primary somatosensory cortex of the low back could alleviate pain symptoms (Flor et al., 2001; Moseley et al., 2008; Tsao et al., 2010, 2011). Because sensorimotor skill training changes the motor cortex excitability (Pascual-Leone et al., 1995, 2005), therapeutic techniques targeting the primary motor cortex may be able to restore optimal muscle function. However, to date, no studies have investigated the effect of rTMS on the cervical motor output and it is still unknown whether rTMS can produce changes in the cervical motor control strategy.
Altered cervical sensorimotor control system and maladaptive neural plasticity are likely to play a major role in chronic neck pain, and, consequently, various clinical assessments and treatments have been proposed. However, previous research has found conflicting results when these assessments or treatments have been applied to patients with neck pain, likely due to no established standardization. The molecular mechanism underlying proprioception needs to be clarified in the future, which may help to develop mechanism-based therapies for neck pain. New precise motor parameters reflecting sensorimotor deficit and more effective interventions targeting sensorimotor control system or neural plasticity are encouraged to be proposed.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abboud, J., Nougarou, F., and Descarreaux, M. (2016). Muscle activity adaptations to spinal tissue creep in the presence of muscle fatigue. PLoS One 11:e0149076. doi: 10.1371/journal.pone.0149076
Alahmari, K. A., Reddy, R. S., Silvian, P., Ahmad, I., Nagaraj, V., and Mahtab, M. (2017). Influence of chronic neck pain on cervical joint position error (JPE): comparison between young and elderly subjects. J. Back Musculoskelet Rehabil. 30, 1265–1271. doi: 10.3233/bmr-169630
Aman, J. E., Elangovan, N., Yeh, I. L., and Konczak, J. (2014). The effectiveness of proprioceptive training for improving motor function: a systematic review. Front. Hum. Neurosci. 8:1075. doi: 10.3389/fnhum.2014.01075
Ambriz-Tututi, M., Alvarado-Reynoso, B., and Drucker-Colín, R. (2016). Analgesic effect of repetitive transcranial magnetic stimulation (rTMS) in patients with chronic low back pain. Bioelectromagnetics 37, 527–535. doi: 10.1002/bem.22001
Armstrong, B., McNair, P., and Taylor, D. (2008). Head and neck position sense. Sports Med. 38, 101–117. doi: 10.2165/00007256-200838020-200838022
Asiri, F., Reddy, R. S., Tedla, J. S., ALMohiza, M. A., Alshahrani, M. S., Govindappa, S. C., et al. (2021). Kinesiophobia and its correlations with pain, proprioception, and functional performance among individuals with chronic neck pain. PLoS One 16:e0254262. doi: 10.1371/journal.pone.0254262
Baliki, M. N., and Apkarian, A. V. (2015). Nociception, pain, negative moods, and behavior selection. Neuron 87, 474–491. doi: 10.1016/j.neuron.2015.06.005
Beinert, K., and Taube, W. (2013). The effect of balance training on cervical sensorimotor function and neck pain. J. Mot. Behav. 45, 271–278. doi: 10.1080/00222895.2013.785928
Bennell, K. L., Hinman, R. S., Metcalf, B. R., Crossley, K. M., Buchbinder, R., Smith, M., et al. (2003). Relationship of knee joint proprioception to pain and disability in individuals with knee osteoarthritis. J. Orthop. Res. 21, 792–797. doi: 10.1016/s0736-0266(03)00054-58
Bewick, G. S., and Banks, R. W. (2015). Mechanotransduction in the muscle spindle. Pflugers. Arch. 467, 175–190. doi: 10.1007/s00424-014-1536-1539
Blomgren, J., Strandell, E., Jull, G., Vikman, I., and Röijezon, U. (2018). Effects of deep cervical flexor training on impaired physiological functions associated with chronic neck pain: a systematic review. BMC Musculoskelet. Disord. 19:415. doi: 10.1186/s12891-018-2324-z
Blouin, J. S., Siegmund, G. P., Carpenter, M. G., and Inglis, J. T. (2007). Neural control of superficial and deep neck muscles in humans. J. Neurophysiol. 98, 920–928. doi: 10.1152/jn.00183.2007
Bolognini, N., Pascual-Leone, A., and Fregni, F. (2009). Using non-invasive brain stimulation to augment motor training-induced plasticity. J. Neuroeng. Rehabil. 6:8. doi: 10.1186/1743-0003-6-8
Bonanni, R., Cariati, I., Tancredi, V., Iundusi, R., Gasbarra, E., and Tarantino, U. (2022). Chronic pain in musculoskeletal diseases: do you know your enemy? J. Clin. Med. 11:2609. doi: 10.3390/jcm11092609
Boyd-Clark, L. C., Briggs, C. A., and Galea, M. P. (2002). Muscle spindle distribution, morphology, and density in longus colli and multifidus muscles of the cervical spine. Spine 27, 694–701. doi: 10.1097/00007632-200204010-200204015
Brumagne, S., Diers, M., Danneels, L., Moseley, G. L., and Hodges, P. W. (2019). Neuroplasticity of sensorimotor control in low back pain. J. Orthop. Sports Phys. Ther. 49, 402–414. doi: 10.2519/jospt.2019.8489
Cavaleri, R., Chipchase, L. S., Summers, S. J., and Schabrun, S. M. (2019). Repetitive transcranial magnetic stimulation of the primary motor cortex expedites recovery in the transition from acute to sustained experimental pain: a randomised, controlled study. Pain 160, 2624–2633. doi: 10.1097/j.pain.0000000000001656
Chalmers, G. (2002). Do Golgi tendon organs really inhibit muscle activity at high force levels to save muscles from injury, and adapt with strength training? Sports Biomech. 1, 239–249. doi: 10.1080/14763140208522800
Cheng, C. H., Cheng, H. Y., Chen, C. P., Lin, K. H., Liu, W. Y., Wang, S. F., et al. (2014). Altered co-contraction of cervical muscles in young adults with chronic neck pain during voluntary neck motions. J. Phys. Ther. Sci. 26, 587–590. doi: 10.1589/jpts.26.587
Chesler, A. T., Szczot, M., Bharucha-Goebel, D., Èeko, M., Donkervoort, S., Laubacher, C., et al. (2016). The role of PIEZO2 in human mechanosensation. N. Engl. J. Med. 375, 1355–1364. doi: 10.1056/NEJMoa1602812
Chu, D., LeBlanc, R., D’Ambrosia, P., D’Ambrosia, R., Baratta, R. V., and Solomonow, M. (2003). Neuromuscular disorder in response to anterior cruciate ligament creep. Clin. Biomech. 18, 222–230. doi: 10.1016/s0268-0033(03)00002-0
Citri, A., and Malenka, R. C. (2008). Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33, 18–41. doi: 10.1038/sj.npp.1301559
Cohen, S. P. (2015). Epidemiology, diagnosis, and treatment of neck pain. Mayo Clin. Proc. 90, 284–299. doi: 10.1016/j.mayocp.2014.09.008
Coste, B., Mathur, J., Schmidt, M., Earley, T. J., Ranade, S., Petrus, M. J., et al. (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. doi: 10.1126/science.1193270
Coste, B., Xiao, B., Santos, J. S., Syeda, R., Grandl, J., Spencer, K. S., et al. (2012). Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181. doi: 10.1038/nature10812
De Martino, E., Fernandes, A. M., Galhardoni, R., De Oliveira Souza, C., Ciampi, De Andrade, D., et al. (2019a). Sessions of prolonged continuous theta burst stimulation or high-frequency 10 Hz stimulation to left dorsolateral prefrontal cortex for 3 days decreased pain sensitivity by modulation of the efficacy of conditioned pain modulation. J. Pain 20, 1459–1469. doi: 10.1016/j.jpain.2019.05.010
De Martino, E., Seminowicz, D. A., Schabrun, S. M., Petrini, L., and Graven-Nielsen, T. (2019b). High frequency repetitive transcranial magnetic stimulation to the left dorsolateral prefrontal cortex modulates sensorimotor cortex function in the transition to sustained muscle pain. Neuroimage 186, 93–102. doi: 10.1016/j.neuroimage.2018.10.076
De Pauw, R., Coppieters, I., Kregel, J., De Meulemeester, K., Danneels, L., and Cagnie, B. (2016). Does muscle morphology change in chronic neck pain patients? - a systematic review. Man. Ther. 22, 42–49. doi: 10.1016/j.math.2015.11.006
de Vries, J., Ischebeck, B. K., Voogt, L. P., van der Geest, J. N., Janssen, M., Frens, M. A., et al. (2015). Joint position sense error in people with neck pain: a systematic review. Man. Ther. 20, 736–744. doi: 10.1016/j.math.2015.04.015
de Zoete, R. M. J., Osmotherly, P. G., Rivett, D. A., Farrell, S. F., and Snodgrass, S. J. (2017). Sensorimotor control in individuals with idiopathic neck pain and healthy individuals: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 98, 1257–1271. doi: 10.1016/j.apmr.2016.09.121
Delhaye, B. P., Long, K. H., and Bensmaia, S. J. (2018). Neural basis of touch and proprioception in primate cortex. Compr. Physiol. 8, 1575–1602. doi: 10.1002/cphy.c170033
Delmas, P., Hao, J., and Rodat-Despoix, L. (2011). Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. 12, 139–153. doi: 10.1038/nrn2993
DePauw, R., Coppieters, I., Meeus, M., Caeyenberghs, K., Danneels, L., and Cagnie, B. (2017). Is traumatic and non-traumatic neck pain associated with brain alterations? - a systematic review. Pain Phys. 20, 245–260.
Dimitriadis, Z., Kapreli, E., Strimpakos, N., and Oldham, J. (2015). Do psychological states associate with pain and disability in chronic neck pain patients? J. Back Musculoskelet Rehabil. 28, 797–802. doi: 10.3233/bmr-150587
Duray, M., Şimşek, Ş, Altuğ, F., and Cavlak, U. (2018). Effect of proprioceptive training on balance in patients with chronic neck pain. Agri 30, 130–137. doi: 10.5505/agri.2018.61214
Dyhre-Poulsen, P., and Krogsgaard, M. R. (2000). Muscular reflexes elicited by electrical stimulation of the anterior cruciate ligament in humans. J. Appl. Physiol. 89, 2191–2195. doi: 10.1152/jappl.2000.89.6.2191
Elliott, J. M., Noteboom, J. T., Flynn, T. W., and Sterling, M. (2009). Characterization of acute and chronic whiplash-associated disorders. J. Orthop. Sports Phys. Ther. 39, 312–323. doi: 10.2519/jospt.2009.2826
Falla, D. (2004). Unravelling the complexity of muscle impairment in chronic neck pain. Man. Ther. 9, 125–133. doi: 10.1016/j.math.2004.05.003
Falla, D., Bilenkij, G., and Jull, G. (2004a). Patients with chronic neck pain demonstrate altered patterns of muscle activation during performance of a functional upper limb task. Spine 29, 1436–1440. doi: 10.1097/01.brs.0000128759.02487.bf
Falla, D. L., Jull, G. A., and Hodges, P. W. (2004b). Patients with neck pain demonstrate reduced electromyographic activity of the deep cervical flexor muscles during performance of the craniocervical flexion test. Spine 29, 2108–2114. doi: 10.1097/01.brs.0000141170.89317.0e
Falla, D., and Farina, D. (2007). Neural and muscular factors associated with motor impairment in neck pain. Curr. Rheumatol. Rep. 9, 497–502. doi: 10.1007/s11926-007-0080-84
Falla, D., Farina, D., Dahl, M. K., and Graven-Nielsen, T. (2007a). Muscle pain induces task-dependent changes in cervical agonist/antagonist activity. J. Appl. Physiol. 102, 601–609. doi: 10.1152/japplphysiol.00602.2006
Falla, D., Farina, D., and Graven-Nielsen, T. (2007b). Experimental muscle pain results in reorganization of coordination among trapezius muscle subdivisions during repetitive shoulder flexion. Exp. Brain Res. 178, 385–393. doi: 10.1007/s00221-006-0746-746
Falla, D., and Farina, D. (2008). Neuromuscular adaptation in experimental and clinical neck pain. J. Electromyogr. Kinesiol. 18, 255–261. doi: 10.1016/j.jelekin.2006.11.001
Fejer, R., Kyvik, K. O., and Hartvigsen, J. (2006). The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur. Spine J. 15, 834–848. doi: 10.1007/s00586-004-0864-864
Ferlinc, A., Fabiani, E., Velnar, T., and Gradisnik, L. (2019). The importance and role of proprioception in the elderly: a short review. Mater. Sociomed. 31, 219–221. doi: 10.5455/msm.2019.31.219-221
Flor, H., Braun, C., Elbert, T., and Birbaumer, N. (1997). Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci. Lett. 224, 5–8. doi: 10.1016/s0304-3940(97)13441-13443
Flor, H., Denke, C., Schaefer, M., and Grüsser, S. (2001). Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet 357, 1763–1764. doi: 10.1016/s0140-6736(00)04890-x
Florez-Paz, D., Bali, K. K., Kuner, R., and Gomis, A. (2016). A critical role for Piezo2 channels in the mechanotransduction of mouse proprioceptive neurons. Sci. Rep. 6:25923. doi: 10.1038/srep25923
Franov, E., Straub, M., Bauer, C. M., and Ernst, M. J. (2022). Head kinematics in patients with neck pain compared to asymptomatic controls: a systematic review. BMC Musculoskelet. Disord. 23:156. doi: 10.1186/s12891-022-05097-z
Giannoni-Luza, S., Pacheco-Barrios, K., Cardenas-Rojas, A., Mejia-Pando, P. F., Luna-Cuadros, M. A., Barouh, J. L., et al. (2020). Noninvasive motor cortex stimulation effects on quantitative sensory testing in healthy and chronic pain subjects: a systematic review and meta-analysis. Pain 161, 1955–1975. doi: 10.1097/j.pain.0000000000001893
Gizzi, L., Muceli, S., Petzke, F., and Falla, D. (2015). Experimental muscle pain impairs the synergistic modular control of neck muscles. PLoS One 10:e0137844. doi: 10.1371/journal.pone.0137844
Hartman, R. A., Tisherman, R. E., Wang, C., Bell, K. M., Lee, J. Y., Sowa, G. A., et al. (2016). Mechanical role of the posterior column components in the cervical spine. Eur. Spine J. 25, 2129–2138. doi: 10.1007/s00586-016-4541-4541
Hendershot, B., Bazrgari, B., Muslim, K., Toosizadeh, N., Nussbaum, M. A., and Madigan, M. L. (2011). Disturbance and recovery of trunk stiffness and reflexive muscle responses following prolonged trunk flexion: influences of flexion angle and duration. Clin. Biomech. 26, 250–256. doi: 10.1016/j.clinbiomech.2010.09.019
Hodges, P. W., and Tucker, K. (2011). Moving differently in pain: a new theory to explain the adaptation to pain. Pain 152, (3 Suppl), S90–S98. doi: 10.1016/j.pain.2010.10.020
Hogervorst, T., and Brand, R. A. (1998). Mechanoreceptors in joint function. J. Bone. Joint. Surg. Am. 80, 1365–1378. doi: 10.2106/00004623-199809000-199809018
Hoy, D., March, L., Woolf, A., Blyth, F., Brooks, P., Smith, E., et al. (2014). The global burden of neck pain: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 73, 1309–1315. doi: 10.1136/annrheumdis-2013-204431
Huang, Y. Z., Lu, M. K., Antal, A., Classen, J., Nitsche, M., Ziemann, U., et al. (2017). Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin. Neurophysiol. 128, 2318–2329. doi: 10.1016/j.clinph.2017.09.007
Imai, F., and Yoshida, Y. (2018). Molecular mechanisms underlying monosynaptic sensory-motor circuit development in the spinal cord. Dev. Dyn. 247, 581–587. doi: 10.1002/dvdy.24611
Izzo, R., Guarnieri, G., Guglielmi, G., and Muto, M. (2013). Biomechanics of the spine. Part I: spinal stability. Eur. J. Radiol. 82, 118–126. doi: 10.1016/j.ejrad.2012.07.024
Jami, L. (1992). Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol. Rev. 72, 623–666. doi: 10.1152/physrev.1992.72.3.623
Ji, R. R., Nackley, A., Huh, Y., Terrando, N., and Maixner, W. (2018). Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 129, 343–366. doi: 10.1097/aln.0000000000002130
Johnston, V., Jimmieson, N. L., Jull, G., and Souvlis, T. (2009). Contribution of individual, workplace, psychosocial and physiological factors to neck pain in female office workers. Eur. J. Pain 13, 985–991. doi: 10.1016/j.ejpain.2008.11.014
Kälin, S., Rausch-Osthoff, A. K., and Bauer, C. M. (2016). What is the effect of sensory discrimination training on chronic low back pain? a systematic review. BMC Musculoskelet. Disord. 17:143. doi: 10.1186/s12891-016-0997-998
Kararizou, E. G., Manta, P., Kalfakis, N., Gkiatas, K. A., and Vassilopoulos, D. (2007). Morphologic and morphometrical study of the muscle spindle in muscular dystrophy. Anal. Quant. Cytol. Histol. 29, 148–152.
Kauther, M. D., Piotrowski, M., Hussmann, B., Lendemans, S., and Wedemeyer, C. (2012). Cervical range of motion and strength in 4,293 young male adults with chronic neck pain. Eur. Spine J. 21, 1522–1527. doi: 10.1007/s00586-012-2369-x
Kazeminasab, S., Nejadghaderi, S. A., Amiri, P., Pourfathi, H., Araj-Khodaei, M., Sullman, M. J. M., et al. (2022). Neck pain: global epidemiology, trends and risk factors. BMC Musculoskelet. Disord. 23:26. doi: 10.1186/s12891-021-04957-4954
Kiehn, O. (2016). Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 17, 224–238. doi: 10.1038/nrn.2016.9
Kristjansson, E., Björnsdottir, S. V., and Oddsdottir, G. L. (2016). The long-term course of deficient cervical kinaesthesia following a whiplash injury has a tendency to seek a physiological homeostasis. a prospective study. Man. Ther. 22, 196–201. doi: 10.1016/j.math.2015.12.008
Kröger, S. (2018). Proprioception 2.0: novel functions for muscle spindles. Curr. Opin. Neurol. 31, 592–598. doi: 10.1097/wco.0000000000000590
Kulkarni, V., Chandy, M. J., and Babu, K. S. (2001). Quantitative study of muscle spindles in suboccipital muscles of human foetuses. Neurol. India 49, 355–359.
Kuner, R., and Flor, H. (2017). Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 18:113. doi: 10.1038/nrn.2017.5
Lan, H. C., Chen, H. Y., Kuo, L. C., You, J. Y., Li, W. C., and Wu, S. K. (2014). The shift of segmental contribution ratio in patients with herniated disc during cervical lateral bending. BMC Musculoskelet. Disord. 15:273. doi: 10.1186/1471-2474-15-273
Landelle, C., El Ahmadi, A., and Kavounoudias, A. (2018). Age-Related impairment of hand movement perception based on muscle proprioception and touch. Neuroscience 381, 91–104. doi: 10.1016/j.neuroscience.2018.04.015
Latremoliere, A., and Woolf, C. J. (2009). Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain 10, 895–926. doi: 10.1016/j.jpain.2009.06.012
Lee, H., Hübscher, M., Moseley, G. L., Kamper, S. J., Traeger, A. C., Mansell, G., et al. (2015). How does pain lead to disability? a systematic review and meta-analysis of mediation studies in people with back and neck pain. Pain 156, 988–997. doi: 10.1097/j.pain.0000000000000146
Lefaucheur, J. P., André-Obadia, N., Antal, A., Ayache, S. S., Baeken, C., Benninger, D. H., et al. (2014). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 125, 2150–2206. doi: 10.1016/j.clinph.2014.05.021
Lindstroem, R., Graven-Nielsen, T., and Falla, D. (2012). Current pain and fear of pain contribute to reduced maximum voluntary contraction of neck muscles in patients with chronic neck pain. Arch. Phys. Med. Rehabil. 93, 2042–2048. doi: 10.1016/j.apmr.2012.04.014
Liu, J. X., Thornell, L. E., and Pedrosa-Domellöf, F. (2003). Muscle spindles in the deep muscles of the human neck: a morphological and immunocytochemical study. J. Histochem. Cytochem. 51, 175–186. doi: 10.1177/002215540305100206
Mahmoud, N. F., Hassan, K. A., Abdelmajeed, S. F., Moustafa, I. M., and Silva, A. G. (2019). The relationship between forward head posture and neck pain: a systematic review and meta-analysis. Curr. Rev. Musculoskelet Med. 12, 562–577. doi: 10.1007/s12178-019-09594-y
Malmström, E. M., Westergren, H., Fransson, P. A., Karlberg, M., and Magnusson, M. (2013). Experimentally induced deep cervical muscle pain distorts head on trunk orientation. Eur. J. Appl. Physiol. 113, 2487–2499. doi: 10.1007/s00421-013-2683-y
Mansour, A. R., Baliki, M. N., Huang, L., Torbey, S., Herrmann, K. M., Schnitzer, T. J., et al. (2013). Brain white matter structural properties predict transition to chronic pain. Pain 154, 2160–2168. doi: 10.1016/j.pain.2013.06.044
Manuel, M., and Zytnicki, D. (2011). Alpha, beta and gamma motoneurons: functional diversity in the motor system’s final pathway. J. Integr. Neurosci. 10, 243–276. doi: 10.1142/s0219635211002786
Mazaheri, M., Abichandani, D., Kingma, I., Treleaven, J., and Falla, D. (2021). A meta-analysis and systematic review of changes in joint position sense and static standing balance in patients with whiplash-associated disorder. PLoS One 16:e0249659. doi: 10.1371/journal.pone.0249659
McCaskey, M. A., Schuster-Amft, C., Wirth, B., Suica, Z., and de Bruin, E. D. (2014). Effects of proprioceptive exercises on pain and function in chronic neck- and low back pain rehabilitation: a systematic literature review. BMC Musculoskelet. Disord. 15:382. doi: 10.1186/1471-2474-15-382
McGill, S. M., Grenier, S., Kavcic, N., and Cholewicki, J. (2003). Coordination of muscle activity to assure stability of the lumbar spine. J. Electromyogr. Kinesiol. 13, 353–359. doi: 10.1016/s1050-6411(03)00043-49
McLean, S. M., May, S., Klaber-Moffett, J., Sharp, D. M., and Gardiner, E. (2010). Risk factors for the onset of non-specific neck pain: a systematic review. J. Epidemiol. Community Health 64, 565–572. doi: 10.1136/jech.2009.090720
Meisingset, I., Stensdotter, A. K., Woodhouse, A., and Vasseljen, O. (2016). Neck motion, motor control, pain and disability: a longitudinal study of associations in neck pain patients in physiotherapy treatment. Man. Ther. 22, 94–100. doi: 10.1016/j.math.2015.10.013
Meisingset, I., Woodhouse, A., Stensdotter, A. K., Stavdahl, Ø, Lorås, H., Gismervik, S., et al. (2015). Evidence for a general stiffening motor control pattern in neck pain: a cross sectional study. BMC Musculoskelet. Disord. 16:56. doi: 10.1186/s12891-015-0517-512
Misailidou, V., Malliou, P., Beneka, A., Karagiannidis, A., and Godolias, G. (2010). Assessment of patients with neck pain: a review of definitions, selection criteria, and measurement tools. J. Chiropr. Med. 9, 49–59. doi: 10.1016/j.jcm.2010.03.002
Moseley, L. G., Zalucki, N. M., and Wiech, K. (2008). Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain 137, 600–608. doi: 10.1016/j.pain.2007.10.021
Moseley, G. L., and Flor, H. (2012). Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabil. Neural Repair. 26, 646–652. doi: 10.1177/1545968311433209
Muceli, S., Falla, D., and Farina, D. (2014). Reorganization of muscle synergies during multidirectional reaching in the horizontal plane with experimental muscle pain. J. Neurophysiol. 111, 1615–1630. doi: 10.1152/jn.00147.2013
Murthy, S. E., Dubin, A. E., and Patapoutian, A. (2017). Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 18, 771–783. doi: 10.1038/nrm.2017.92
Mutso, A. A., Petre, B., Huang, L., Baliki, M. N., Torbey, S., Herrmann, K. M., et al. (2014). Reorganization of hippocampal functional connectivity with transition to chronic back pain. J. Neurophysiol. 111, 1065–1076. doi: 10.1152/jn.00611.2013
Nusser, M., Knapp, S., Kramer, M., and Krischak, G. (2021). Effects of virtual reality-based neck-specific sensorimotor training in patients with chronic neck pain: a randomized controlled pilot trial. J. Rehabil. Med. 53:jrm00151. doi: 10.2340/16501977-16502786
Oddsdottir, G. L., and Kristjansson, E. (2012). Two different courses of impaired cervical kinaesthesia following a whiplash injury. a one-year prospective study. Man. Ther. 17, 60–65. doi: 10.1016/j.math.2011.08.009
Panjabi, M. M. (1992a). The stabilizing system of the spine. Part I. function, dysfunction, adaptation, and enhancement. J. Spinal Disord. 5, 383–389; discussion 397. doi: 10.1097/00002517-199212000-199212001.
Panjabi, M. M. (1992b). The stabilizing system of the spine. Part II. neutral zone and instability hypothesis. J. Spinal Disord. 5, 390–396; discussion 397. doi: 10.1097/00002517-199212000-199212002.
Panjabi, M. M., Cholewicki, J., Nibu, K., Grauer, J., Babat, L. B., and Dvorak, J. (1998). Critical load of the human cervical spine: an in vitro experimental study. Clin. Biomech. 13, 11–17. doi: 10.1016/s0268-0033(97)00057-50
Pascual-Leone, A., Grafman, J., and Hallett, M. (1994). Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science 263, 1287–1289. doi: 10.1126/science.8122113
Pascual-Leone, A., Nguyet, D., Cohen, L. G., Brasil-Neto, J. P., Cammarota, A., and Hallett, M. (1995). Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J. Neurophysiol. 74, 1037–1045. doi: 10.1152/jn.1995.74.3.1037
Pascual-Leone, A., Amedi, A., Fregni, F., and Merabet, L. B. (2005). The plastic human brain cortex. Annu. Rev. Neurosci. 28, 377–401. doi: 10.1146/annurev.neuro.27.070203.144216
Peirs, C., and Seal, R. P. (2016). Neural circuits for pain: recent advances and current views. Science 354, 578–584. doi: 10.1126/science.aaf8933
Peng, B., Yang, L., Li, Y., Liu, T., and Liu, Y. (2021). Cervical proprioception impairment in neck pain-pathophysiology, clinical evaluation, and management: a narrative review. Pain Ther. 10, 143–164. doi: 10.1007/s40122-020-00230-z
Proske, U., and Gandevia, S. C. (2012). The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 92, 1651–1697. doi: 10.1152/physrev.00048.2011
Proske, U., and Gandevia, S. C. (2018). Kinesthetic senses. Compr. Physiol. 8, 1157–1183. doi: 10.1002/cphy.c170036
Qu, N., Lindstrøm, R., Graven-Nielsen, T., and Hirata, R. P. (2019a). Experimental cervical interspinous ligament pain altered cervical joint motion during dynamic extension movement. Clin. Biomech. 65, 65–72. doi: 10.1016/j.clinbiomech.2019.04.002
Qu, N., Lindstrøm, R., Hirata, R. P., and Graven-Nielsen, T. (2019b). Origin of neck pain and direction of movement influence dynamic cervical joint motion and pressure pain sensitivity. Clin. Biomech. 61, 120–128. doi: 10.1016/j.clinbiomech.2018.12.002
Qu, N., Graven-Nielsen, T., Lindstrøm, R., Blogg Andersen, Dc, V., and Hirata, R. P. (2020). Recurrent neck pain patients exhibit altered joint motion pattern during cervical flexion and extension movements. Clin. Biomech. 71, 125–132. doi: 10.1016/j.clinbiomech.2019.10.026
Reddy, R. S., Tedla, J. S., Dixit, S., and Abohashrh, M. (2019). Cervical proprioception and its relationship with neck pain intensity in subjects with cervical spondylosis. BMC Musculoskelet. Disord. 20:447. doi: 10.1186/s12891-019-2846-z
Riemann, B. L., and Lephart, S. M. (2002). The sensorimotor system, part I: the physiologic basis of functional joint stability. J. Athl. Train 37, 71–79.
Röijezon, U., Clark, N. C., and Treleaven, J. (2015). Proprioception in musculoskeletal rehabilitation. Part 1: basic science and principles of assessment and clinical interventions. Man. Ther. 20, 368–377. doi: 10.1016/j.math.2015.01.008
Rossini, P. M., Burke, D., Chen, R., Cohen, L. G., Daskalakis, Z., Di Iorio, R., et al. (2015). Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. an updated report from an I.F.C.N. committee. Clin. Neurophysiol. 126, 1071–1107. doi: 10.1016/j.clinph.2015.02.001
Rudolfsson, T., Björklund, M., and Djupsjöbacka, M. (2012). Range of motion in the upper and lower cervical spine in people with chronic neck pain. Man. Ther. 17, 53–59. doi: 10.1016/j.math.2011.08.007
Rudolfsson, T., Djupsjöbacka, M., Häger, C., and Björklund, M. (2014). Effects of neck coordination exercise on sensorimotor function in chronic neck pain: a randomized controlled trial. J. Rehabil. Med. 46, 908–914. doi: 10.2340/16501977-16501869
Saadat, M., Salehi, R., Negahban, H., Shaterzadeh, M. J., Mehravar, M., and Hessam, M. (2019). Traditional physical therapy exercises combined with sensorimotor training: the effects on clinical outcomes for chronic neck pain in a double-blind, randomized controlled trial. J. Bodyw. Mov. Ther. 23, 901–907. doi: 10.1016/j.jbmt.2019.02.016
Samani, A., Holtermann, A., Søgaard, K., and Madeleine, P. (2009). Experimental pain leads to reorganisation of trapezius electromyography during computer work with active and passive pauses. Eur. J. Appl. Physiol. 106, 857–866. doi: 10.1007/s00421-009-1083-1089
Sarig Bahat, H., Weiss, P. L., and Laufer, Y. (2010). The effect of neck pain on cervical kinematics, as assessed in a virtual environment. Arch. Phys. Med. Rehabil. 91, 1884–1890. doi: 10.1016/j.apmr.2010.09.007
Sarig Bahat, H., Takasaki, H., Chen, X., Bet-Or, Y., and Treleaven, J. (2015). Cervical kinematic training with and without interactive VR training for chronic neck pain - a randomized clinical trial. Man. Ther. 20, 68–78. doi: 10.1016/j.math.2014.06.008
Schabrun, S. M., and Chipchase, L. S. (2012). Priming the brain to learn: the future of therapy? Man. Ther. 17, 184–186. doi: 10.1016/j.math.2011.12.001
Schabrun, S. M., Jones, E., Elgueta Cancino, E. L., and Hodges, P. W. (2014). Targeting chronic recurrent low back pain from the top-down and the bottom-up: a combined transcranial direct current stimulation and peripheral electrical stimulation intervention. Brain Stimul. 7, 451–459. doi: 10.1016/j.brs.2014.01.058
Schabrun, S. M., Elgueta-Cancino, E. L., and Hodges, P. W. (2017). Smudging of the motor cortex is related to the severity of low back pain. Spine 42, 1172–1178. doi: 10.1097/brs.0000000000000938
Schomacher, J., and Falla, D. (2013). Function and structure of the deep cervical extensor muscles in patients with neck pain. Man. Ther. 18, 360–366. doi: 10.1016/j.math.2013.05.009
Schomburg, E. D., Steffens, H., and Kniffki, K. D. (1999). Contribution of group III and IV muscle afferents to multisensorial spinal motor control in cats. Neurosci. Res. 33, 195–206. doi: 10.1016/s0168-0102(99)00006-1
Schwab, J. S., Diangelo, D. J., and Foley, K. T. (2006). Motion compensation associated with single-level cervical fusion: where does the lost motion go? Spine 31, 2439–2448. doi: 10.1097/01.brs.0000239125.54761.23
Seminowicz, D. A., de Martino, E., Schabrun, S. M., and Graven-Nielsen, T. (2018). Left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation reduces the development of long-term muscle pain. Pain 159, 2486–2492. doi: 10.1097/j.pain.0000000000001350
Sielski, R., Rief, W., and Glombiewski, J. A. (2017). Efficacy of biofeedback in chronic back pain: a meta-analysis. Int. J. Behav. Med. 24, 25–41. doi: 10.1007/s12529-016-9572-9579
Sjölander, P., Michaelson, P., Jaric, S., and Djupsjöbacka, M. (2008). Sensorimotor disturbances in chronic neck pain–range of motion, peak velocity, smoothness of movement, and repositioning acuity. Man. Ther. 13, 122–131. doi: 10.1016/j.math.2006.10.002
Solomonow, M., Zhou, B. H., Harris, M., Lu, Y., and Baratta, R. V. (1998). The ligamento-muscular stabilizing system of the spine. Spine 23, 2552–2562. doi: 10.1097/00007632-199812010-199812010
Stanton, T. R., Leake, H. B., Chalmers, K. J., and Moseley, G. L. (2016). Evidence of impaired proprioception in chronic, idiopathic neck pain: systematic review and meta-analysis. Phys. Ther. 96, 876–887. doi: 10.2522/ptj.20150241
Strimpakos, N. (2011). The assessment of the cervical spine. Part 1: range of motion and proprioception. J. Bodyw. Mov. Ther. 15, 114–124. doi: 10.1016/j.jbmt.2009.06.003
Suvarnnato, T., Puntumetakul, R., Uthaikhup, S., and Boucaut, R. (2019). Effect of specific deep cervical muscle exercises on functional disability, pain intensity, craniovertebral angle, and neck-muscle strength in chronic mechanical neck pain: a randomized controlled trial. J. Pain Res. 12, 915–925. doi: 10.2147/jpr.S190125
Swash, M., and Fox, K. P. (1972). The effect of age on human skeletal muscle. studies of the morphology and innervation of muscle spindles. J. Neurol. Sci. 16, 417–432. doi: 10.1016/0022-510x(72)90048-90042
Szczot, M., Liljencrantz, J., Ghitani, N., Barik, A., Lam, R., Thompson, J. H., et al. (2018). PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci. Transl. Med. 10:eaat9892. doi: 10.1126/scitranslmed.aat9892
Tejera, D. M., Beltran-Alacreu, H., Cano-de-la-Cuerda, R., Leon Hernández, J. V., Martín-Pintado-Zugasti, A., Calvo-Lobo, C., et al. (2020). Effects of virtual reality versus exercise on pain, functional, somatosensory and psychosocial outcomes in patients with non-specific chronic neck pain: a randomized clinical trial. Int. J. Environ. Res. Public Health 17:5950. doi: 10.3390/ijerph17165950
Troise, D., Yoneyama, S., Resende, M. B., Reed, U., Xavier, G. F., and Hasue, R. (2014). The influence of visual and tactile perception on hand control in children with Duchenne muscular dystrophy. Dev. Med. Child Neurol. 56, 882–887. doi: 10.1111/dmcn.12469
Tsang, S. M., Szeto, G. P., and Lee, R. Y. (2013). Movement coordination and differential kinematics of the cervical and thoracic spines in people with chronic neck pain. Clin. Biomech. 28, 610–617. doi: 10.1016/j.clinbiomech.2013.05.009
Tsang, S. M., Szeto, G. P., and Lee, R. Y. (2014). Altered spinal kinematics and muscle recruitment pattern of the cervical and thoracic spine in people with chronic neck pain during functional task. J. Electromyogr. Kinesiol. 24, 104–113. doi: 10.1016/j.jelekin.2013.10.011
Tsao, H., Galea, M. P., and Hodges, P. W. (2008). Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain 131(Pt 8), 2161–2171. doi: 10.1093/brain/awn154
Tsao, H., Danneels, L. A., and Hodges, P. W. (2011). ISSLS prize winner: smudging the motor brain in young adults with recurrent low back pain. Spine 36, 1721–1727. doi: 10.1097/BRS.0b013e31821c4267
Tsao, H., Druitt, T. R., Schollum, T. M., and Hodges, P. W. (2010). Motor training of the lumbar paraspinal muscles induces immediate changes in motor coordination in patients with recurrent low back pain. J. Pain 11, 1120–1128. doi: 10.1016/j.jpain.2010.02.004
Tsiringakis, G., Dimitriadis, Z., Triantafylloy, E., and McLean, S. (2020). Motor control training of deep neck flexors with pressure biofeedback improves pain and disability in patients with neck pain: a systematic review and meta-analysis. Musculoskelet Sci. Pract. 50:102220. doi: 10.1016/j.msksp.2020.102220
Tucker, K., Butler, J., Graven-Nielsen, T., Riek, S., and Hodges, P. (2009). Motor unit recruitment strategies are altered during deep-tissue pain. J. Neurosci. 29, 10820–10826. doi: 10.1523/jneurosci.5211-08.2009
van der Wal, J. (2009). The architecture of the connective tissue in the musculoskeletal system-an often overlooked functional parameter as to proprioception in the locomotor apparatus. Int. J. Ther. Massage Bodywork 2, 9–23. doi: 10.3822/ijtmb.v2i4.62
Van Oosterwijck, J., Nijs, J., Meeus, M., and Paul, L. (2013). Evidence for central sensitization in chronic whiplash: a systematic literature review. Eur. J. Pain 17, 299–312. doi: 10.1002/j.1532-2149.2012.00193.x
Vardeh, D., Mannion, R. J., and Woolf, C. J. (2016). Toward a mechanism-based approach to pain diagnosis. J. Pain 17, (9 Suppl), T50–T69. doi: 10.1016/j.jpain.2016.03.001
Vasavada, A. N., Peterson, B. W., and Delp, S. L. (2002). Three-dimensional spatial tuning of neck muscle activation in humans. Exp. Brain Res. 147, 437–448. doi: 10.1007/s00221-002-1275-1276
Vincent, J. A., Gabriel, H. M., Deardorff, A. S., Nardelli, P., Fyffe, R. E. W., Burkholder, T., et al. (2017). Muscle proprioceptors in adult rat: mechanosensory signaling and synapse distribution in spinal cord. J. Neurophysiol. 118, 2687–2701. doi: 10.1152/jn.00497.2017
Vuillerme, N., Pinsault, N., and Bouvier, B. (2008). Cervical joint position sense is impaired in older adults. Aging Clin. Exp. Res. 20, 355–358. doi: 10.1007/bf03324868
Walton, D. M., Macdermid, J. C., Nielson, W., Teasell, R. W., Nailer, T., and Maheu, P. (2011). A descriptive study of pressure pain threshold at 2 standardized sites in people with acute or subacute neck pain. J. Orthop. Sports Phys. Ther. 41, 651–657. doi: 10.2519/jospt.2011.3667
Wilkinson, K. A. (2022). Molecular determinants of mechanosensation in the muscle spindle. Curr. Opin. Neurobiol. 74:102542. doi: 10.1016/j.conb.2022.102542
Woodhouse, A., and Vasseljen, O. (2008). Altered motor control patterns in whiplash and chronic neck pain. BMC Musculoskelet. Disord. 9:90. doi: 10.1186/1471-2474-9-90
Woodhouse, A., Stavdahl, Ø, and Vasseljen, O. (2010). Irregular head movement patterns in whiplash patients during a trajectory task. Exp. Brain Res. 201, 261–270. doi: 10.1007/s00221-009-2033-2039
Ylinen, J., Takala, E. P., Kautiainen, H., Nykänen, M., Häkkinen, A., Pohjolainen, T., et al. (2004). Association of neck pain, disability and neck pain during maximal effort with neck muscle strength and range of movement in women with chronic non-specific neck pain. Eur. J. Pain 8, 473–478. doi: 10.1016/j.ejpain.2003.11.005
Yoganandan, N., Kumaresan, S., and Pintar, F. A. (2001). Biomechanics of the cervical spine Part 2. cervical spine soft tissue responses and biomechanical modeling. Clin. Biomech. 16, 1–27. doi: 10.1016/s0268-0033(00)00074-77
Keywords: neck pain, proprioception, sensorimotor control, neural plasticity, intervention
Citation: Qu N, Tian H, De Martino E and Zhang B (2022) Neck Pain: Do We Know Enough About the Sensorimotor Control System? Front. Comput. Neurosci. 16:946514. doi: 10.3389/fncom.2022.946514
Received: 17 May 2022; Accepted: 24 June 2022;
Published: 15 July 2022.
Edited by:
Hongwei Tan, Aalto University, FinlandReviewed by:
Weiwei Xia, Peking University People’s Hospital, ChinaCopyright © 2022 Qu, Tian, De Martino and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Zhang, YWNrZXIxMUAxMjYuY29t
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.