- 1Department of Speech Pathology and Audiology, National Institute of Mental Health and Neurosciences (NIMHANS), Bengaluru, India
- 2Department of Neurology, National Institute of Mental Health and Neurosciences (NIMHANS), Bengaluru, India
Posterior Cortical Atrophy (PCA) and Logopenic progressive aphasia (LPA) are nonamnestic variants of Alzheimer's disease (AD). Language deficits are a hallmark of LPA but not PCA. Studies have revealed the presence of language deficits in PCA similar to LPA, however it has not been a consistent observation. Only alexia and agraphia are the language deficits listed in the latest consensus criteria for classification of PCA. In this case report we present the clinical, cognitive-linguistic, and neuroimaging features of a patient presenting with an unusual overlap of LPA-PCA. Although the diagnostic characteristics for LPA is fulfilled, the probable progression of the disease to exhibit most of the characteristics similar to PCA is highlighted. Thus, it contributes to the notion of a continuum between the two atypical variants of AD. The possibility of patients with PCA to exhibit language deficits with the progression of the disease similar to that of LPA that primarily involves the phonological short-term memory deficits is emphasized. The need to not undermine the language deficits irrespective of the stage of the disease in PCA is weighed upon for a better identification and management via speech-language intervention programs.
Introduction
Posterior cortical atrophy (PCA) is a form of pre-senile dementia that is primarily characterized by a selective and a progressive decline in cortical visual functioning and posterior cognitive functions. It is characterized by alexia, agraphia, visual agnosia, and few or all features of Balint's syndrome and Gerstmann's syndrome (Benson et al., 1988). Most of the cases are commonly associated with underlying Alzheimer's dementia (AD) pathology while a few are also associated with Dementia of Lewy bodies and Prion disease (Renner et al., 2004; Tang-Wai et al., 2004; Alladi et al., 2007). Unlike those with typical AD, episodic memory, insight, and judgement remain relatively preserved in PCA (Benson et al., 1988). Studies have revealed that in cases of PCA with AD pathology, the distribution of neurofibrillary tangle and, neuritic plaques is higher in density along occipital, posterior parietal and temporo-occipital cortex while fewer pathological changes are observed in the pre-frontal cortex (Ross et al., 1996; Hof et al., 1997). As per the latest consensus on three-level classification of PCA (Crutch et al., 2017), classification level-1 defines the core clinical, cognitive, and neuro-imaging characteristics of PCA and defines pure-PCA as being characterized by insidious onset with gradual progression with prominent early visual disturbance and/other posterior cortical functions. At least three of the several posterior cortical dysfunctions along with relatively spared anterograde memory function, speech and non-visual language functions, personality, behavior, and executive functioning have been described. On neuroimaging, a predominant occipito-parietal or occipito-temporal atrophy/hypometabolism on MRI and PET are reported. Classification level-2 defines PCA-Pure and PCA-Plus i.e., in addition to the features of core PCA, core features of other neurodegenerative conditions can co-occur, like Dementia of Lewy Bodies or Corticobasal syndrome. Level-3 defines the underlying cause of the PCA syndrome based on the pathophysiological biomarker evidence. The major focus in cases with PCA has been on the prominent visual disturbance and the language symptoms are undermined. Alexia and Agraphia are the only language symptoms considered in the consensus criteria (Crutch et al., 2017). Language and visuospatial presentations are primary symptoms of those with non-amnestic variants of probable AD dementia i.e., logopenic progressive aphasia (LPA) and PCA respectively. Language impairments were reported even in the earliest reports of PCA. Transcortical sensory aphasia syndrome was initially described in this cohort (Benson et al., 1988). Studies have revealed the presence of Wernicke's aphasia, Conduction aphasia, and anomic aphasia in some cases with PCA (McMonagle et al., 2006). A “logopenic syndrome” was also reported in cases with PCA and was characterized by fluency impairments and anomia (Magnin et al., 2013). LPA is a variant of Primary Progressive Aphasia (PPA), which is characterized by prominent language deficits. Deficits in word retrieval in spontaneous speech and confrontation naming and sentence repetition deficits are the hallmark features of LPA. Based on the International consensus criteria for classification of PPA (Gorno-Tempini et al., 2011), the clinical diagnosis of LPA requires the presence of the following core features: impaired word retrieval in confrontation naming and spontaneous speech along with impaired repetition of phrases and sentences. At least three of the following four features need to be present: phonological errors in naming and spontaneous speech, spared object knowledge and word comprehension, spared motor speech and absence of frank agrammatism. The clinical diagnosis should be further supported by neuro-imaging findings which include predominant left posterior perisylvian or parietal atrophy/hypometabolism on MRI and PET respectively. PCA can also frequently involve atrophy of the left parietal and posterior temporal cortices (Magnin et al., 2013). Considerable overlap in the language profiles of PCA and LPA has been reported with respect to auditory input processing, digit span, and repetition tasks with a relatively spared performance in comprehension and spontaneous speech task (Crutch et al., 2013). A recent case study reported a case presenting with overlapping (clinical, anatomical, and cognitive) features of both PCA and LPA (Fitzpatrick et al., 2019). The possibility of either conditions to occur along a spectrum depending upon the stage of the disease rather than fulfilling the strict diagnostic criteria for a particular condition, i.e., PCA or LPA, was considered. Identifying such cases with significant overlap of the two conditions is crucial for early identification and planning intervention. Although there are no definitive management options for visual disturbances in such overlap conditions, speech and language intervention plays an important role in language deficit (Louis et al., 2001; Henry et al., 2019). The current case study illustrates the importance of comprehensive cognitive-linguistic evaluation of a case presenting with characteristics of PCA and LPA at the initial stages of disease onset. The need to not underestimate the language deficits (at the outset) is emphasized for facilitating diagnosis and intervention by a multi-disciplinary team.
Clinical history
A 50-year-old, right-handed, Tamil, Kannada, and English-speaking engineer presented with the complaint of difficulty in recalling information for the past 2.5 years. He was normal until he noticed a gradual difficulty in recollecting things that he wanted to convey to his colleagues at work, losing the information conveyed after a while, searching for letters while typing on the keyboard, making spelling mistakes in written notes, change in handwriting and orientation of text while writing on a paper, and problems with calculation. He further reported having difficulty in framing sentences while speaking and conveying things cohesively in a conversation. His spouse gave a history of slowness in recollecting names of people, reading difficulty especially when having to shift to the next phrase in a paragraph, interchanging letters of a word while reading, and difficulty in locating his belongings in the wardrobe, i.e., a tendency to reach the target object by exhibiting movements surrounding it and not immediately reaching it. She reported the symptoms as having occurred over the last 2.5 years but having worsened in the past 5 months. She also reported having noticed prominent difficulty in word retrieval in naming and during general conversation as the initial symptoms, followed by a gradual onset of visuoperceptual impairments. He had taken a sabbatical from his job due to difficulty in coping with the roles and responsibilities assigned to him. He was partially dependent on her for his activities of daily life, which was attributed primarily to his visual disturbances. The nature and course of the condition were insidious in onset and progressive, respectively.
There was no history of way finding difficulty, dressing apraxia, visual neglect, behavioral disturbances, or weakness of limbs. There was no h/o hypertension, diabetes, ischemic heart disease or chronic illness, or any family history of similar illness.
Cognitive-linguistic assessment
Cognitive examination revealed significant deficits in general cognition on Addenbrooke's Cognitive Examination-Revised (ACE-R) (Mekala et al., 2020). A total score of 42/100 with the sub-domain scores: attention: 07/18, memory: 07/26, fluency: 07/14, language: 18/26, and visuospatial abilities: 03/16 were obtained. The poor performance on the domains of attention and memory could be primarily attributed to deficits in phonological short-term memory since our case constantly said he knows what to say but is unable to say it immediately and his responses improved when provided with phonemic cues. Speech evaluation revealed normal motor speech abilities. Language assessment using Western Aphasia Battery-Revised (WAB-R) revealed relatively fluent speech with word finding pauses, and adequate content. However, spontaneous speech lacked coherence due to inappropriate pauses, whole phrase repetitions, and interjections. Auditory word recognition was adequate except for fingers and right-left orientation, for which confusion persisted. Comprehension of complex sequential commands was affected owing to their length and spatial nature of the task. Repetition was intact only up to 3-word phrases and naming was fair although marked by phonological paraphasia (ex: “y/eye”, “as/axe”, “pumpin/pumpkin”) and semantic paraphasias (ex: coconut/onion, cockroach/ant). Generative and phoneme fluency were poor. The aphasia quotient (AQ) was 77.5, suggestive of mild language impairment. Performance on the Indian semantic memory battery revealed relatively preserved semantic memory (Paplikar et al., 2022). Due to the lack of coherence in the spontaneous speech task, a sentence production task was administered which revealed difficulty in forming sentences when provided with visual and auditory primes, however, on the anagram task developed for assessment of sentence formation, the performance was adequate, thus suggestive of auditory verbal working memory deficits. Reading was intact at word and phrase level and affected for sentences. Reading comprehension was adequate for simple sentences and writing was characterized by slowness and spatial errors affecting legibility. Spelling mistakes were characterized by substitution, omission, and addition errors (ex: boile/boil, milke/milk, fiter/filter and suger/sugar) (Figure 1), features suggestive of the possibility of a graphemic buffer disorder. Evaluation of construction ability revealed simplification of 3D images to 2D. Assessment of higher cognitive functions revealed dyscalculia. Constructional dyspraxia was present along with right-left confusion and finger agnosia. The Clinical Dementia Rating (CDR) score was 1 suggestive of mild dementia.
Neuroimaging of the brain on Magnetic Resonance Imaging (MRI) (Figure 2) revealed asymmetrical left predominant parietal lobe atrophy. FDG-PET brain (Figure 3) revealed moderate to severe hypometabolism involving the posterior parieto-occipital and temporal cortices with left predominance.
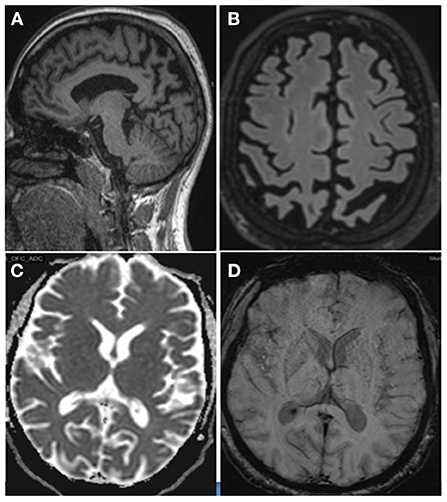
Figure 2. Magnetic Resonance Imaging of the Brain-T1 MPR Sagittal (A) and T2 Flair coronal (B) image showing asymmetrical left predominant parietal lobe atrophy. Axial ADC (C) section showing no diffusion restrictions. Axial SWI (D) sections showing no areas of blooming.
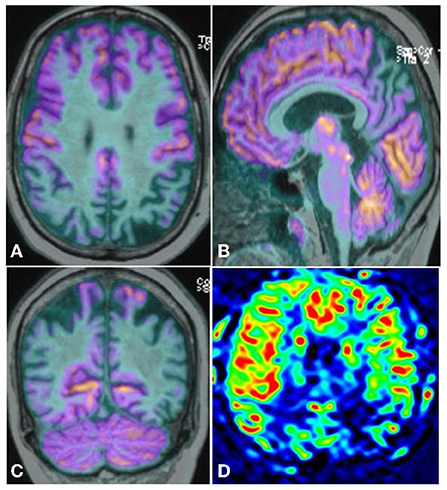
Figure 3. FDG-PET Brain Axial (A), Sagittal (B), and Coronal (C) showing moderate to severe hypo metabolism predominantly involving the posterior parieto-occipital and temporal cortices with left predominance along with posterior cingulate gyrus. ASL image (D) showing reduced perfusion in bilateral parietal and parietotemporal with left affected more than right. Post cingulate gyrus hypoperfusion is also seen.
Table 1 describes the consensus criteria for pure- PCA, PPA, and LPA based on the criteria by Mesulam (2001), Gorno-Tempini et al. (2011), and Crutch et al. (2017) respectively and the features observed in our case. Based on the clinical, cognitive-linguistic, and neuro-imaging features, the possibility of an LPA-PCA overlap was considered.

Table 1. Consensus criteria for pure-PCA, PPA, and LPA and the clinical, cognitive, and neuroimaging features of our patient.
Speech and language intervention was initiated for the case with a major focus on intensive word retrieval that emphasized on using the relatively spared semantic system. Caregiver counseling was also done. Informed consent was obtained from the participant for the study.
Discussion
Our case report describes the cognitive-linguistic and neuroimaging features of an individual presenting with features of both PCA and LPA. Our case successfully meets most of the criterion for the diagnosis of LPA with respect to the deficits in language functions as described in Table 1, as well as some of the criteria for PCA, but does not completely fit into the “Pure-PCA” or “PCA-Plus” conditions. A diagnosis of PPA requires language disturbance as a primary symptom at onset and this being a principal cause of impaired daily activities (Mesulam, 2001). In our patient, although deficits in language and visual disturbances were the earliest symptoms, the deficits in word retrieval in general conversation and naming were one of the first symptom reported by the spouse when insisted to recall the timeline of problem onset. The probability that the condition evolved with time from LPA into an overlap of LPA-PCA complex is higher. This finding adds to the notion of a phenotypic continuum between these atypical forms of AD. This also emphasizes the subtle boundary between the posterior syndromes. The progression of symptoms from word retrieval deficits to difficulty in recollecting information to be conveyed to the other posterior cognitive symptoms is quite rapid in our patient. Considering the swiftness in progression of the symptoms, a question arises on whether individuals with “Pure PCA” can also have associated language deficits in addition to the primary higher visual processing disturbances? This question becomes significant considering the latest consensus criteria for the classification of PCA (Crutch et al., 2017). Alexia and agraphia are the only language symptoms reported in the consensus criteria with otherwise spared non-visual language functions. Based on this, there will be a tendency to undermine the language symptoms, if any, at onset or with the progression of the disease. Studies have revealed the presence of language deficits in individuals with PCA. One of the earliest studies by Benson et al. (1988), identified the presence of a transcortical sensory aphasia syndrome in PCA cohort; a profile similar to Wernicke's aphasia, Conduction aphasia, and anomic aphasia was also revealed by another study (McMonagle et al., 2006). The presence of a logopenic syndrome i.e., anomia, deficits in fluency, and length dependent deficits were also observed in individuals with PCA (Magnin et al., 2013). A recent study aimed to evaluate the language profile of PCA (Crutch et al., 2013). A comparison of language profiles between LPA and PCA revealed better performance on all language tasks in PCA over the LPA cohort. But PCA patients had poorer performance in auditory input processing and working memory tasks while those with LPA had poor performance in comprehension, naming, fluency, and spontaneous speech. But the language profile described here for the PCA cohort is similar to the profile observed in our patient. Most of the language deficits were mainly associated with deficits in phonological short-term memory. This in turn highlights the commonalities in the language profile of those with PCA and LPA (with no mutual overlap). The presence of phonological deficits in either condition is possible when associated with atrophy of the posterior temporo-parietal cortex, since the phonological loop is attributed to this area (Baddeley, 2003). Based on the latest models of the articulatory-phonological pathway, the conversions between the motor and auditory phonological codes are linked to the left temporo-parietal cortex (Hickok, 2012). This explains the phonological errors and verbal short-term memory deficits of our patient. The performance on most of the tasks involving auditory input i.e., digit, letter, word, and sentence repetition tasks was significantly affected, suggestive of a deficit in phonological short-term memory and a relatively better performance on sentence formation involving anagram tasks over those involving auditory and visual primes emphasizes on the possibility of working memory deficits (Weintraub et al., 2009; Foxe et al., 2013). The spelling impairment was consistent with graphemic buffer disorder, neuro-anatomical areas implicated for which include left parietal-occipital (Hillis et al., 2002), left temporo-parietal (Tainturier and Rapp, 2003), and left posterior temporal (Kan et al., 2006) regions.
One of the major limitations of the study is the lack of CSF biomarkers in supplementing the clinical, cognitive-linguistic, and neuroimaging findings. But the findings of this case study are important to enhance awareness among clinicians about the possibility of language deficits similar to LPA even among cases with PCA with disease progression. Based on our observation in the current study, we hypothesize that the higher the chances of progression of atrophy from occipito-parietal or occipito-temporal cortices to gradually involve the temporo-parietal cortex with left predominance, the higher the possibility of a logopenic syndrome in a PCA patient. The anticipation of such possibilities can only add to the enhancement of patient care. In our patient, although the visuo-spatial disturbances were prominent, the language deficits were still affecting the psycho-social and vocational aspects of life. Recent studies have evaluated the efficacy of speech-language intervention programs for individuals with PPA in general and LPA in particular. Lexical retrieval programs that aim at self-directing the individual's spared semantic system to facilitate word retrieval have revealed positive outcomes (Louis et al., 2001; Henry et al., 2019). Further, programs to enhance phonological awareness and verbal working memory deficits can also be equally effective.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institute Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
The authors would like to thank the participant and the caregivers for consenting to participate in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alladi, S., Xuereb, J., Bak, T., Nestor, P., Knibb, J., Patterson, K., et al. (2007). Focal cortical presentations of Alzheimer's disease. Brain 130, 2636–2645. doi: 10.1093/brain/awm213
Baddeley, A. (2003). Working memory: looking back and looking forward. Nat. Rev. Neurosci. 4, 829–839. doi: 10.1038/nrn1201
Benson, D. F., Davis, R. J., and Snyder, B. D. (1988). Posterior cortical atrophy. Arch. Neurol 45, 789–793. doi: 10.1001/archneur.1988.00520310107024
Crutch, S. J., Lehmann, M., Warren, J. D., and Rohrer, J. D. (2013). The language profile of posterior cortical atrophy. J. Neurol. Neurosurg. Psychiatry. 84, 460–466. doi: 10.1136/jnnp-2012-303309
Crutch, S. J., Schott, J. M., Rabinovici, G. D., Murray, M., Snowden, J. S., van der Flier, W. M., et al. (2017). Consensus classification of posterior cortical atrophy. Alzheimer's Dement. 13, 870–884. doi: 10.1016/j.jalz.2017.01.014
Fitzpatrick, D., Blanco-Campal, A., and Kyne, L. (2019). A case of overlap posterior cortical atrophy and logopenic variant primary progressive aphasia. Neurologist 24, 62–65. doi: 10.1097/NRL.0000000000000225
Foxe, D. G., Irish, M., Hodges, J. R., and Piguet, O. (2013). Verbal and visuospatial span in logopenic progressive aphasia and Alzheimer's disease. J. Int. Neuropsychol. Soc. 19, 247–253. doi: 10.1017/S1355617712001269
Gorno-Tempini, M. L., Hillis, A. E., Weintraub, S., Kertesz, A., Mendez, M., Cappa, S. F., et al. (2011). Classification of primary progressive aphasia and its variants. Neurology. 76, 1006–1014. doi: 10.1212/WNL.0b013e31821103e6
Henry, M. L., Hubbard, H. I., Grasso, S. M., Dial, H. R., Beeson, P. M., Miller, B. L., et al. (2019). Treatment for word retrieval in semantic and logopenic variants of primary progressive aphasia: Immediate and long-term outcomes. J. Speech, Lang. Hear. Res. 62, 2723–2749. doi: 10.1044/2018_JSLHR-L-18-0144
Hickok, G. (2012). The cortical organization of speech processing: feedback control and predictive coding the context of a dual-stream model. J. Commun. Disord. 45, 393–402. doi: 10.1016/j.jcomdis.2012.06.004
Hillis, A. E., Kane, A., Tuffiash, E., Beauchamp, N. J., Barker, P. B., Jacobs, M. A., et al. (2002). Neural substrates of the cognitive processes underlying spelling: Evidence from MR diffusion and perfusion imaging. Aphasiology 16, 425–438. doi: 10.1080/02687030244000248
Hof, P. R., Vogt, B. A., Bouras, C., and Morrison, J. H. (1997). Atypical form of Alzheimer's disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. Vision Res. 37, 3609–3625. doi: 10.1016/S0042-6989(96)00240-4
Kan, I. P., Biran, I., Thompson-Schill, S. L., and Chatterjee, A. (2006). Letter selection and letter assembly in acquired dysgraphia. Cogn. Behav. Neurol. 19, 225–236. doi: 10.1097/01.wnn.0000213918.18138.f2
Louis, M., Espesser, R., Rey, V., Daffaure, V., Di Cristo, A., and Habib, M. (2001). Intensive training of phonological skills in progressive aphasia: a model of brain plasticity in neurodegenerative disease. Brain Cogn. 46, 197–201. doi: 10.1016/S0278-2626(01)80065-8
Magnin, E., Sylvestre, G., Lenoir, F., Dariel, E., Bonnet, L., Chopard, G., et al. (2013). Logopenic syndrome in posterior cortical atrophy. J. Neurol. 260, 528–533. doi: 10.1007/s00415-012-6671-7
McMonagle, P., Deering, F., Berliner, Y., and Kertesz, A. (2006). The cognitive profile of posterior cortical atrophy. Neurology 66, 331–338. doi: 10.1212/01.wnl.0000196477.78548.db
Mekala, S., Paplikar, A., Mioshi, E., Kaul, S., Divyaraj, G., Coughlan, G., et al. (2020). Dementia Diagnosis in Seven Languages: The Addenbrooke's Cognitive Examination-III in India. Arch. Clin. Neuropsychol. 35, 528–538. doi: 10.1093/arclin/acaa013
Paplikar, A., Vandana, V. P., Mekala, S., Darshini, K. J., Arshad, F., Iyer, G. K., et al. (2022). Semantic memory impairment in dementia: A cross-cultural adaptation study. Neurol. Sci. 43, 265–273. doi: 10.1007/s10072-021-05272-5
Renner, J. A., Burns, J. M., Hou, C. E., McKeel, D. W., Storandt, M., and Morris, J. C. (2004). Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology 63, 1175–1180. doi: 10.1212/01.WNL.0000140290.80962.BF
Ross, S. J., Graham, N., Stuart-Green, L., Prins, M., Xuereb, J., Patterson, K., et al. (1996). Progressive biparietal atrophy: an atypical presentation of Alzheimer's disease. J. Neurol. Neurosurg. Psychiat. 61, 388–395. doi: 10.1136/jnnp.61.4.388
Tainturier, M. J., and Rapp, B. (2003). Is a single graphemic buffer used in reading and spelling? Aphasiology 17, 537–562. doi: 10.1080/02687030344000021
Tang-Wai, D. F., Graff-Radford, N. R., Boeve, B. F., Dickson, D. W., Parisi, J. E., Crook, R., et al. (2004). Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology 63, 1168–1174. doi: 10.1212/01.WNL.0000140289.18472.15
Keywords: logopenic progressive aphasia, posterior cortical atrophy (PCA), cognition, language, intervention - behavioral
Citation: Jeevendra Kumar D, Goyal S, Arshad F, Purushothaman VV, Ramakrishnan S and Alladi S (2022) Posterior cortical atrophy in logopenic progressive aphasia: A case report. Front. Commun. 7:979475. doi: 10.3389/fcomm.2022.979475
Received: 27 June 2022; Accepted: 18 October 2022;
Published: 08 November 2022.
Edited by:
Antonio Benítez-Burraco, University of Seville, SpainReviewed by:
Ruth Ingram, The University of Manchester, United KingdomAaron Meyer, Georgetown University, United States
Copyright © 2022 Jeevendra Kumar, Goyal, Arshad, Purushothaman, Ramakrishnan and Alladi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Darshini Jeevendra Kumar, a2pkYXJzaGluaUBnbWFpbC5jb20=
 Darshini Jeevendra Kumar
Darshini Jeevendra Kumar Sheetal Goyal2
Sheetal Goyal2 Faheem Arshad
Faheem Arshad Subasree Ramakrishnan
Subasree Ramakrishnan Suvarna Alladi
Suvarna Alladi