- Department of Experimental Psychology, Mind, Brain, and Behavior Research Center (CIMCYC), University of Granada, Granada, Spain
Introduction: Spatial interference tasks have been recently used to investigate the supposed uniqueness of gaze processing and attention. For instance, it has been observed that gaze stimuli elicited faster responses when their direction was incongruent with their position (“reversed spatial congruency effect”, RCE), whereas arrows produced faster reaction times (RT) when it was congruent (“standard spatial congruency effect”, SCE). In the present study, we tested whether the RCE is unique to eye-gaze stimuli or can be observed in response to other important social stimuli such as pointing fingers.
Method: To this aim, congruency effects elicited by eye gaze, arrows, and pointing fingers were compared in a spatial interference task.
Results: The RCE was only observed in response to eye-gaze stimuli while pointing fingers and arrows elicited the SCE.
Discussion: This suggests that the RCE reversed congruency effect is specific to gaze stimuli and cannot be generalized to finger-pointing stimuli.
Introduction
The answer to the question “is eye-gaze a special stimulus” is often an obvious one. Routine behaviors like following the gaze direction of a stranger while walking down the street or our attention being caught by an advertisement model's gaze support the notion that eyes are a special type of stimuli. They convey unique information about others' intentions, emotional expressions, and other mental states, which is often beneficial and sometimes critically important for our social interactions and survival (Baron-Cohen et al., 1997; Emery, 2000). However, the extent to which eye gaze uniquely engages human cognition and attention remains an open question in the broader understanding of human social behavior processes (see, for example, Blakemore et al., 2004; Chacón-Candia et al., 2022). This issue has emerged recently in social neuroscience research, given that the available experimental procedures to measure social attention, such as the classical version of the gaze cueing paradigm, have failed to show quantitative differences between the abovementioned scenarios. In the gaze cueing paradigm, a face is presented unpredictably at fixation, gazing either left or right. A target is presented afterwards either in the cued or opposite locations. Participants are typically faster to detect or identify the target when the eye-gaze is directed toward the target location, as compared to when it is directed toward the opposite location (i.e., the so-called gaze cueing effect). There is debate in literature around the question of whether the gaze cueing effect is elicited by the directionality of the gaze cue (e.g., Tipples, 2002) or the social information it carries (e.g., for review, see Birmingham and Kingstone, 2009; Capozzi and Ristic, 2018).
On the one hand, a recent systematic review and meta-analysis of the literature on social attention (Chacón-Candia et al., 2022) showed that, despite generating a large amount of data and a notably increased interest in multiple fields of research, the classical version of the cueing paradigm produces the same attentional effects for social directional cues, such as gaze, and non-social directional cues, such as arrows. This challenges the vastly extended intuition that social stimuli are special in modulating human attention and questions the potential utility of the classic cueing task in revealing social-specific attentional effects.1 On the other hand, several studies have suggested that when other specific variant of the cueing task are used, the processing of eye-gaze direction may rely, at least in part, on the computation of mental states and intentions. For example, it has been observed that attentional orienting in response to eye-gaze direction is reduced when participants believe that the gazer is not able to see a potential target (Nuku and Bekkering, 2008; Teufel et al., 2010) or when its gaze behavior is believed to be controlled by a computer programme (Wiese et al., 2012; Gobel et al., 2018). Moreover, by using a variant of the double-rectangle task, some studies (Marotta et al., 2012; Chacón-Candia et al., 2020) showed that cueing a portion of an object spreads attention across the entire object when arrows are used as cues, while it restricts attention at the specific portion of the cued object when eye-gaze cues are used. These findings are coherent with research showing that when reference objects are presented on the scene, gaze-cues trigger an attentional orienting only to the exact gaze-at object (Vuilleumier, 2002; Wiese et al., 2013). This “special” aspect of gaze attentional orienting may be mediated by theory of mind processes as a consequence of a specific intention automatically attributed to gaze but not arrows. Consistent with this view, Bayliss et al. (2006) adapted the gaze cueing task to investigate to what extent the direction of the gaze can be interpreted as a window into other's intentions. Specifically, in their study, participants had to mark how much they liked target objects after completing a cueing procedure. When eye-gaze was used as a cue, in addition to the classic cueing effect, it was found that objects that other people looked at were likelier than those that did not receive much attention from others. This affective preference for cued objects was not found when arrows were used as cues, despite observing the classic cueing attentional effect. Similarly, combining a traditional gaze cueing paradigm with a visual memory task, Dodd et al. (2012) and Gregory and Jackson (2017) have shown that, despite similar cueing attentional effects, only gaze cues but not arrow cues improved memory accuracy for cued information. This suggests that the eye-gaze stimulus -unlike arrows- is interpreted as an intentional cue that indicates interest and desire.
Taken together all these evidences are difficult to reconcile with the idea that gaze cueing exclusively reflects the operation of mechanisms that only respond to stimulus directionality and it has recently proposed by different authors that both domain-general and mentalizing processes play a crucial role in social orienting (Dalmaso et al., 2015; Capozzi and Ristic, 2020; Chacón-Candia et al., 2022).
All the above studies used variations of the cue-based paradigms where social or nonsocial stimuli were used as cues of the position of an upcoming target in combination with additional manipulations employed to measure extra processes related to target processing such as its selection, learning, memory and likeability.
Recently, target-based paradigms, such as the spatial interference task, have also been used to investigate the supposed uniqueness of gaze processing and attention (i.e., Marotta et al., 2019; Román-Caballero et al., 2021b; Aranda-Martín et al., 2022; Narganes-Pineda et al., 2022). In this task, the critical social or nonsocial stimuli are used as targets instead of as cues. They are presented either to the left or to the right of the fixation point pointing either left or right, and participants are required to respond to the location the stimuli are pointing at. With this type of task, it has been generally observed that non-social stimuli such as words or arrows elicited faster responses when their direction was congruent with their position (e.g., right pointing arrows presented to the right; typical spatial congruency effect, SCE), whereas eye-gaze produced faster reaction times (RT) when it was incongruent (e.g., left looking eye-gaze presented to the right; the “reversed spatial congruency effect”, RCE). This dissociation has been studied and replicated by our and other different research groups and supports the intuition of a unique attentional mechanism for eye gaze stimuli (Torres-Marín et al., 2017; Marotta et al., 2019, 2022; Edwards et al., 2020; Ishikawa et al., 2021; Román-Caballero et al., 2021a,b; Aranda-Martín et al., 2022; Hemmerich et al., 2022; Narganes-Pineda et al., 2022; Tanaka et al., 2022). Moreover, the fact that the RCE is modulated by the emotional expression of the target face (Jones, 2015; Torres-Marín et al., 2017; Marotta et al., 2022) and the finding that only the RCE elicited by eye gaze but not the congruency effect elicited by arrows or words is negatively correlated with social anxiety scores (Ishikawa et al., 2021) emphasize the social nature of this effect. However, which social mechanisms are responsible for the RCE are still under debate. In particular, according to the eye-contact hypothesis, the RCE has been interpreted as resulting from the incongruent gaze trials being misattributed by participants as direct gaze (Cañadas and Lupiáñez, 2012; Marotta et al., 2018). This bias would accelerate reaction times in this condition. On the other hand, according to the joint attention hypothesis (Edwards et al., 2020), participants interpret gaze direction in incongruent trials as directed toward the fixation cross to which they are also looking, facilitating performance in this condition. Conversely, gaze discrimination is not facilitated when the eyes look away from where the participant is looking because joint attention cannot be established. Finally, according to the joint distraction hypothesis (Hemmerich et al., 2022), in congruent trials eyes are directed away from where the participants are looking toward withdrawing attention from the relevant task area, consequently leading to the observed increase in RT in this condition.
Both joint attention and joint distraction hypotheses underline the importance of the sharing attention and theory of mind mechanisms on the emergence of RCE. This raises the critical issue of whether the RCE observed with eye-gaze stimuli might be generalized to other socio-biological stimuli, such as pointing fingers. Developmental research has shown that young infants display evidence of interpreting pointing fingers and gaze direction in referential terms and are very sensitive to the communicative situations in which these actions occur (Csibra, 2003). Moreover, both stimuli are crucial to developing language understanding (Tomasello et al., 2007). Other studies have shown that in healthy participants finger pointing cues elicit attentional orienting effects similar to those generally produced by eye-gaze cues (Langton and Bruce, 2000; Belopolsky et al., 2008; Sato et al., 2009). Interestingly, some authors have found that gaze but not finger-pointing cues influence how objects are later valued (Ulloa et al., 2015). Specifically, in Ulloa et al. (2015) study, participants had to mark how much they liked target objects after completing a cueing procedure. When eye-gaze was used as a cue, in addition to the classic attentional orienting effect, it was found that objects that other people looked at were likelier than those that did not receive much attention from others (liking effect; see also Tipples and Pecchinenda, 2019 for different results). This affective preference for cued objects was not found when pointing fingers were used as cues, despite the observation of the classic cueing attentional effect. Of relevance, the presence of a typical attentional orienting but the absence of a liking effect has also been observed in response to arrow cues (Bayliss et al., 2006). This may suggest that only eye-gaze stimuli - unlike finger-pointing or arrows - are interpreted as an intentional cue that indicates interest and desire. The effects of finger-pointing and eye gaze on attention have also been compared in several studies with clinical populations. For example, reduced early attentional orienting has been observed in patients with anorexia nervosa in response to gaze and arrow cues but not pointing gestures (Dalmaso et al., 2015). On the other hand, impairment in attentional orienting was observed in patients with schizophrenia only in response to gaze but not in response to finger-pointing and arrows (Dalmaso et al., 2013). However, it is noteworthy that these cues show similar patterns of orienting attention on simple cueing tasks measured by reaction times in healthy controls (Sato et al., 2010; Dalmaso et al., 2013, 2015). Therefore, pointing gestures represent a crucial comparison tool to evaluate the nature of the RCE elicited by eye-gaze. Indeed, it is a powerful referential cue that we use to draw attention to objects or persons, like gaze direction (Langton and Bruce, 2000; Belopolsky et al., 2008; Sato et al., 2009). However, it does not reflect the same higher cognitive systems, such as the theory of mind mechanisms (Ulloa et al., 2015). For this reason, this study examines how spatial interference effects triggered by eye-gaze stimuli differ from those elicited by finger-pointing gestures and typical non-social stimuli such as arrows. As mentioned above, previous studies have shown that in spatial interference tasks, the RCE is observed when a face with averted eyes or eye gaze alone is used as target stimuli, while the SCE is observed when non-social stimuli such as words or arrows are used. However, it remains unclear whether the RCE is unique to the eye-gaze stimuli or generalizes to other socio-biological stimuli, including the pointing finger. If the RCE is mediated by a common foundation that processes several socio-biological cues (e.g., orienting attention and signaling objects in the environment), then finger-pointing stimuli, like eye gaze, should elicit the RCE. In contrast, if RCE is mediated by mechanisms only elicited by eye-gaze stimuli (e.g., theory of mind mechanisms), finger-pointing should produce, as arrows, the SCE.
Experiment 1
Participants
The study included 24 participants (17 women, 7 men) with a mean age of 23.13 years; they were all students from the University of Granada and received partial course credit for participating. All of them had self-reported normal or corrected-to-normal vision, and they were naïve as to the purpose of the experiment. We estimated the required sample size assuming a significance level of .05 and a power of .9, taking as a reference the effect size obtained in Narganes-Pineda et al. (2022, Experiment 1).
Apparatus and stimuli
Stimuli presentation, timing, and data collection were controlled by a program written using E-prime 2.0 (Schneider et al., 2002) run on a standard Pentium 4 PC. Stimuli were presented on a 17′′ widescreen monitor with a 1024 × 768 pixel resolution. They consisted of two black arrows display, two full color cropped eyes, or two fingers subtending a 1° × 4° degrees of visual angle at a viewing distance of 57 cm. Cropped eyes were obtained by manipulating an original face (taken from the MacBrain Face Stimulus Set; https://www.macbrain.org/resources.htm)2 with Adobe Photoshop CS.
Procedure
After expressing their consent to participate in the study, participants filled in a short version of Autism-Spectrum Quotient (AQ-10) by Baron-Cohen et al. (2001). It is a 10 items self-report measure of autistic spectrum-related traits in adults with normal intelligence. For each item, participants had to respond on a 4-points Likert scale ranging from definitely disagree to definitely agree. Due to previous works showing that the degree of autistic traits is inversely related to the ability to draw mentalistic inferences from the eyes (Baron-Cohen et al., 2001), the attentional cueing from eye-gaze (Bayliss and Tipper, 2005; Bayliss et al., 2005) and the processing of eye-gaze direction in spatial interference task (Marotta et al., 2022), in the present study we used the AQ-10 to exclude participants with a score of 6 or higher. According to the test, this cut-off is indicative of autism or a significant number of autistic traits. No participant was excluded from both Experiments 1 and 2.
Then participants were conducted in a sufficiently lit room where they seated ~60 cm from the computer screen; they were instructed to put on headphones. They were required to perform a discrimination task in which they had to respond as fast and accurately as possible to the direction (left or right) indicated by the eye gaze, arrows or fingers. The experiment consisted of three experimental blocks (one for each target type), each composed of 15 practice trials followed by 72 experimental trials. The order of blocks was counterbalanced across participants.
Each trial (see Figure 1) began with a fixation cross presented in the center of a white screen for 1 s. Participants were instructed to fixate on the cross. Then a pair of eyes, arrows, or fingers looking/pointing to the right or the left was presented to either the left or the right of the fixation cross until the participant's response or for 2 s.3 The distance from the center of the lateral stimulus to the central fixation cross subtended 4.8° of visual angle. Participants were instructed to press the “Z” key in response to targets indicating the left and the “M” key in response to targets indicating the right, independent of the target's location. Feedback was provided when the participants did not respond to the trial and for incorrect responses. In the latter case, a 220 Hz tone was presented for 1,500 ms. Visual feedback of the same duration was provided in the center of the screen when no response was detected. Importantly, this design produced congruent (e.g., a right-indicating target presented on the right) or incongruent trials (e.g., a left-indicating target presented on the right). An equal number of congruent and incongruent trials were presented throughout the experiment.
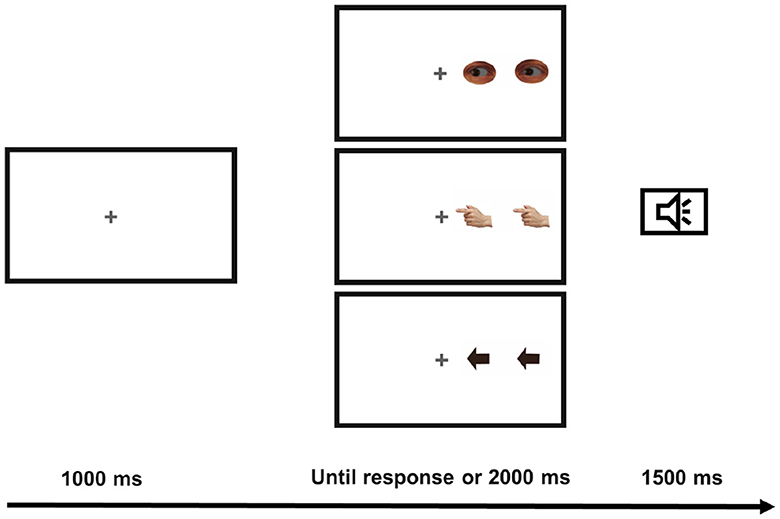
Figure 1. Schematic view of a trial sequence from the left to right. One of the three types of target were used: gaze, finger and arrow targets. The example represents incongruent trials. The speaker icon represents the given auditory feedback.
Design
The experiment had a two-factor repeated measures design, with 36 observations per experimental condition. Data were submitted to a 3 (Target type) × 2 (Congruency) repeated measures ANOVA. Target type had three levels: gaze, arrow and finger. Congruency had two levels: congruent and incongruent trials. Post-hoc tests were conducted to analyze the interactions. For each participant, median RTs and accuracy (as mean percent errors) were calculated for each experimental condition. Original data to this study can be found online at: https://osf.io/trzbk/
Results
Incorrect responses (2% of the trials) were excluded from the RT analysis. Table 1 shows the median (± SDs) of the RTs and percentages of errors for each experimental condition.
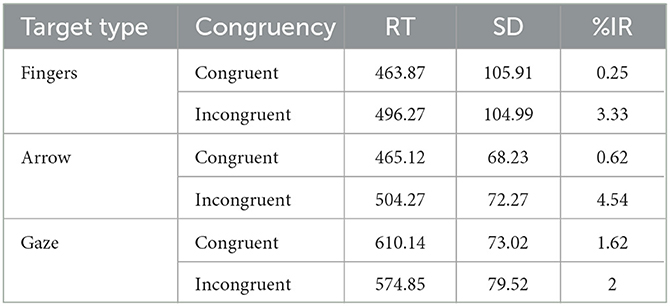
Table 1. Medians (RT in milliseconds), standard deviation (SD) and percentage of incorrect responses (%IR) for each experimental condition.
Reaction times
The ANOVA performed on RTs showed a main effect of target type, F(2,46) = 50.87, p < 0.001, η2p = 0.69, with slower RTs for the gaze targets (592 ms) compared to both arrows (485 ms; F1,23 = 118.15, p < 0.001) and finger pointing targets (480 ms; F1,23 = 67.05, p < 0.001); RTs were not significantly different between arrow targets and fingers pointing targets (F1,23 < 1). The main effect of congruency was also significant, F(1,23) = 8.76, p = 0.007, η2p = 0.27, with slower RTs for incongruent than congruent trials (524 ms vs. 513 ms). Importantly, the critical target type × congruency interaction was significant, F(2,46) = 23.77, p < 0.001, = 0.51 (Figure 2). Post-hoc tests on each target type showed that RTs were significantly longer on incongruent than on congruent trials when both arrows and fingers were used as the targets, F(1,23) = 39.07, p < 0.001, = 0.63 and F(1,23) = 67.83, p < 0.001, = 0.75, respectively; in contrast, RTs were significantly faster on incongruent than on congruent trials when eye gaze was used as the target, F(1,23) = 9.03, p = 0.006, = 0.28.
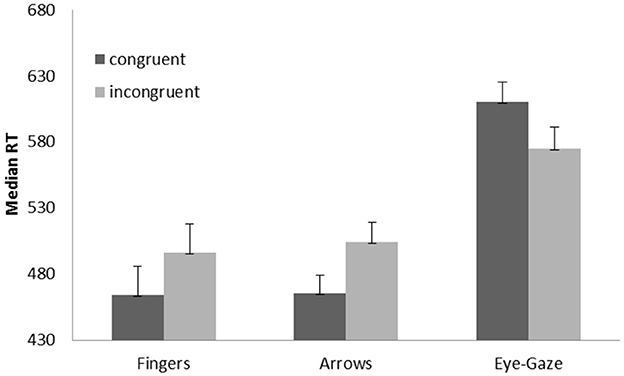
Figure 2. Medians (RT in milliseconds) for each type of target and experimental condition. Error bars represent standard errors of the means.
Errors
The analysis of errors showed a main effect of congruency, F(1,23) = 17.98, p = 0.001 = 0.44, with more errors for incongruent (3.28%) than congruent trials (0.83%). The main effect was target type was not significant, F(2,46) = 1.15, p = 0.326. The target type × congruency interaction was also significant, F(2,46) = 5.27, p = 0.009 = 0.19. Post hoc tests on each target type showed that participants made more errors on incongruent than on congruent trials when both arrows and fingers were used as the targets, F(1,23) = 24.20, p < 0.001, = 0.51 and F(1,23) = 8.3, p = 0.008, = 0.26, in contrast, no difference between incongruent and congruent trials were observed when eye gaze was used as the target, F < 1.
Experiment 2
The primary aim of this experiment was to replicate and extend the findings of Experiment 1. Importantly we wanted to investigate whether the different congruency effects elicited by the three types of stimuli (eye gaze, arrows, and finger pointing) would also be observed even when they are presented within the same block of trials in a random sequence. The type of target stimuli was manipulated between experimental blocks in the previous experiment. Thus, participants might have adopted different strategies for the different target conditions. Consequently, the different findings observed in the three conditions might not be related to different attentional mechanisms elicited by each stimulus type; instead, they might be determined by the different attentional strategies adopted by participants. In order to control for this possibility and to replicate the main findings obtained in the previous studies, we will conduct the present study using a within-block design. It will replicate Experiment 1, except that the type of stimulus will vary randomly across trials. Such a within-block design will prevent participants from adopting a specific “task set” according to the type of stimuli used as targets.
Method
Stimuli and procedure were nearly identical to those used in Experiment 1, except for the order of targets: trials with eye-gaze, arrows and finger pointing were randomly interspersed in each of the three blocks of trials. A different group of 22 participants (17 women, 5 men) participated in this experiment, with the same characteristics as those of Experiment 1. We estimated the required sample size assuming a significance level of .05 and a power of .9, taking as a reference the effect size obtained in Experiment 1.
Design
Data were submitted to a 3 (Target type) × 2 (Congruency) repeated measures ANOVA. Target type had three levels: gaze, arrow and finger. Congruency had two levels: congruent and incongruent trials. Post hoc tests were conducted to analyze the interactions.
Results
Trials with incorrect responses (3%) were excluded from the RT analysis. Table 2 shows the medians (± SDs) of the RTs and percentages of errors for each experimental condition.4
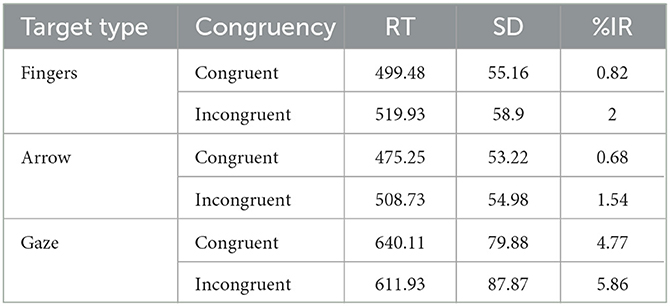
Table 2. Medians (RT in milliseconds), standard deviation (SD), and percentage of incorrect responses (%IR) for each experimental condition.
Reaction times
The ANOVA performed on RTs showed a main effect of target type, F(2,42) = 188.84, p < 0.001, η2p = 0.89, with slower RTs for the gaze targets (626 ms) compared to both arrows (492 ms; F1,21 = 228.40. p < 0.001) and finger pointing targets (510 ms; F1,21 = 171.65, p < 0.001); RTs were significantly slower for finger pointing than for arrows targets (F1,21 = 28.61, p < 0.001). The main effect of congruency was not significant, F(1,21) = 4.06, p = 0.057, η2p = 0.16. Importantly, the critical target type × congruency interaction was significant, F(2,42) = 18.09, p < 0.001, = 0.046 (Figure 3). Post hoc tests on each target type showed that RTs were significantly longer on incongruent than on congruent trials when both arrows and fingers were used as the targets, F(1,21) = 37.64, p < 0.001, = 0.64 and F(1,21) = 19.51, p < 0.001, = 0.48, respectively; in contrast, RTs were significantly faster on incongruent than on congruent trials when eye gaze was used as the target, F(1,21) = 6.62, p = 0.018, = 0.24.
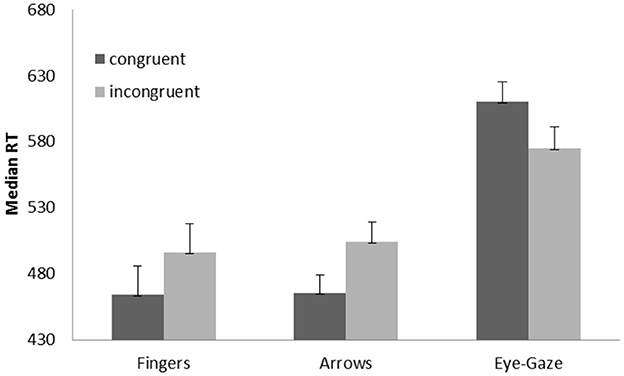
Figure 3. Medians (RT in milliseconds) for each type of target and experimental condition. Error bars represent standard errors of the means.
Errors
Only the main effect of target type was significant, F(2,42) = 27.02, p < 0.001, = 0.56, with more errors for the eye-gaze targets compared to both arrows (F1,21 = 31.65, p < 0.001) and finger pointing targets (F1,21 = 36.05, p < 0.001); no differences were observed between finger and arrow targets (F < 0.1). Neither the main effect of congruency, F(1,21) = 2.39, p = 0.137, nor the target type × congruency interaction was significant, F < 1.
Discussion
The present study aimed to explore if the RCE elicited by eye-gaze stimuli can be generalized to another powerful, social, referential, and attention-orienting stimulus, such as finger-pointing. To this aim, congruency effects elicited by eye gaze, finger-point, and arrows were compared in a context of a spatial interference task. Consistent with previous studies, we observed that the eye gaze and arrow stimuli led to opposite spatial interference effects, with arrows producing the SCE (e.g., faster RTs when the arrow direction was congruent with its position) and eye gaze producing the RCE (faster RTs when eyes direction was incongruent with its position). Of relevance for the present study, we also showed that finger-pointing did not elicit the RCE instead of a robust SCE similar to that produced by arrows. These results indicate that RCE elicited by eye-gaze stimuli is not generalizable to finger-pointing stimuli. This may suggest that the joint attention explanation of the RCE is not the correct one, since pointing with the index finger has been generally considered a crucial tool for referring to the intentions and actions of others. However, the debate about whether the influence of pointing gestures on visual attention reflects higher cognitive systems, such as the theory of mind mechanisms, is still open. Therefore, before acknowledging the possible limitations of this study, we will mention which important aspects could differentiate eye-gaze direction and finger-pointing as referential stimuli. As suggested by Ulloa et al. (2015), although both gaze and finger-pointing are useful stimuli for signaling objects of interest in the environment, an important difference between these two types of stimuli is that eye gaze is intrinsically bearing on other's preferences and intentions (Baron-Cohen et al., 1997; Ulloa and George, 2013). Consistent with this view, they showed that when eye-gaze stimuli were used as cues, a robust attentional orienting effect was observed, and participants liked the objects looked at by others more than non-looked-at objects (liking effect). However, when finger-pointing was used as a cue, only attentional orienting was observed, while the liking effect was absent. Thus, finger-pointing, like arrows, may not communicate information about others' preferences per se. This is a fundamental property of the joint attention and mentalizing processes that may underlie the RCE and would explain why only eye-gaze stimuli elicit it.
Recently, to explain the dissociation observed between the spatial congruency effects observed with eye-gaze and arrow stimuli, we proposed an integrated framework in which both domain-general attentional and domain-specific social processes contribute to the RCE (Chacón-Candia et al., 2022; Hemmerich et al., 2022). In particular, on the one hand, domain-general attentional processes linked to the stimulus's pointing direction and its spatial location would lead to either congruent or incongruent responses, producing a standard congruency effect. On the other hand, additional “special” processes would take place in the case of eye-gaze, reverting the nature of the spatial conflict. In the context of this framework, our results suggest that only eye-gaze stimuli elicited social-specific unique processes, while the congruency effect elicited by arrows and finger-pointing can only rely on domain-general attentional processes.
Moreover, the fact that finger-pointing produced a robust SCE similar to that observed with arrows suggests that it can function as a symbolic cue acquired through daily experience and learning rather than functioning as a socio-biologically cue, such as a gaze cue. Indeed, infants as young as 3 months attend in the same direction as the eyes of an adult face (Hood et al., 1998), while pointing is acquired at ~12 months (Liszkowski et al., 2004). Consequently, these results reflect the earlier establishment of gaze direction as a cue than the establishment of pointing with a finger. Taken together, our results are consistent with the view that eye gaze has a special status in non-verbal communication and social cognition. However, the possibility that RCE generalizes to other types of social stimuli cannot be excluded. Indeed, from a perspective of cross-cultural study, pointing with hand gestures is not necessarily one of the most powerful, social, referential stimuli since at least some communities prefer face-related stimuli such as the nose or head orientation (Cooperrider et al., 2018). Moreover, our experiment used an image including only hands and fingers as a finger-pointing stimulus. It is possible that this type of impoverished stimulus was not able to communicate the intention and trigger mentalizing processes. Finally, whether the RCE reflects social processing or not is still open. Additional studies must examine whether mentalizing or other social processes mediate this effect. Moreover, consistent with previous studies, responses were generally slower for gaze than arrow stimuli (Vlamings et al., 2005; Hietanen et al., 2006). They were also slower for gaze than finger stimuli. In this study we chose to use realistic eye-gaze stimuli to ensure their approximation to a real social situation. Nevertheless, this may have affected the complexity of stimuli direction detection, being eye gazes direction more difficult as compared to arrows and fingers direction. From our point of view, the slowing of reaction times observed for gaze stimuli may be due to both their social significance and complexity that induces a greater exploration of it. Supporting this view, Vlamings et al. (2005) showed slower reaction times after eye-gaze than arrow stimuli only in typically developed individuals but not in individuals with autism, who are generally referred to as impaired in social attention behavior (Leekam et al., 2000; Werner et al., 2000; Marotta et al., 2012). However, this does not rule out the possibility that the complexity of the three types of stimuli may have partially affect the different congruency effects observed among the three types of stimuli in our study. A previous study showed that when eye-gaze stimuli were compared with equivalent complex non-social stimuli (e.g. inverted triangles) using the spatial interference paradigm, equivalent RTs were observed while preserving the opposite congruency effects observed between eye-gaze and no-social stimuli (Cañadas and Lupiáñez, 2012). This may suggest that differences between gaze and other stimuli are due to his social meaning rather than to his increased complexity. However, further studies manipulating the complexity of both social and non-social stimuli direction are surely necessary to shed light on this issue.
Finally, it is important to know that the interference task we used is a Simon + Spatial Stroop task, in other words, a type 7 dimensional overlap according to Kornblum et al. (1992) taxonomy. Given the compatible mapping between the stimulus direction and the response location (for example, an arrow pointing left always required a left response), two sources of spatial congruency may have contributed to our measure of the congruency effects. In particular, on incongruent trials, there was a stimulus–stimulus (S-S) source of spatial conflict between the irrelevant stimulus location and the relevant stimulus direction, as well as a stimulus–response (S-R) source of spatial conflict between the irrelevant stimulus location and the response location. This second type of spatial congruency is usually referred to as the Simon effect (see Simon and Small Jr, 1969; Simon et al., 1973; Lu and Proctor, 1995, for a review). As such, it is unclear which of these two sources of spatial conflict was reversed by eye-gaze stimuli. In a recent study using an implicit version of the spatial interference task in which participants were required to respond to the color of both directional eyes-gaze and arrow stimuli (Narganes-Pineda et al., 2022; Experiment 2), a compatible response mapping was directly compared with an incompatible response mapping, where participants respond with left keypresses to stimuli pointing right and right keypresses to stimuli pointing left. The results of this study revealed a similar Simon effect (S-R spatial conflict) with both eye-gaze and arrow stimuli. This may suggest that the Simon effect is not modulated by the type of stimuli. However, in this type of implicit task, S-S spatial conflict effects were not observed either with arrows or with eye-gaze. Therefore, it is unknown if the Simon effect can contribute to the congruency effects observed in the explicit version of the task, such as that we used in the present study. On the other hand, in another experiment of the same study (Narganes-Pineda et al., 2022; Experiment 3), it was observed that when the manual Simon effect was eliminated using a verbal task (see Experiment 3) the interaction between Target Type and Congruence (a standard congruency effect with arrows and a reversed congruency effect with eye-gaze) was still observed. This suggests that the manual generation of a spatial response is not responsible for the congruency differences observed between eye gaze and arrows. However, since the Simon effect has been reported also with vocal responses (Wühr, 2006) the possibility that it can contribute to the different congruency effects observed in our study cannot be ruled out. In any case, we humbly consider that the important point of the present study is not whether eye-gaze modulates S-R or S-S source of spatial compatibility, but that it produces opposite congruency effects as compared to other social and non-social stimuli such as directional fingers and arrows.
Conclusion
The present study is the first to examine the effect of different types of social and non-social stimuli on spatial congruency effects. Results indicate that the RCE was only elicited by eye-gaze stimuli while pointing fingers and arrows elicited the SCE. This suggests that the RCE is specific to gaze stimuli and underlie their importance for the human attentional systems.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the University of Granada Ethical Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AM: conceptualization, funding acquisition, analysis and interpretation of results, and draft manuscript preparation. SB: analysis and interpretation of results and draft manuscript preparation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Department of University, Research, and Innovation of the Regional Government of Andalusia and the European Regional Development Fund (FEDER), through research project B-SEJ-572-UGR20 to AM.
Conflict of interest
The author AM declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^Using the so-called counterpredictive cueing paradigm (the target was more likely to appear in the location opposite the one indicated by the cue), Friesen et al. (2004) showed that better performance at the indicated location was only observed when eye gaze was used as cue, but not when the indicated location was cued by an arrow. However, using the same paradigm, Tipples (2008) found that both eye and arrow cues produce similar reflexive shifts of attention, while Guzzon et al. (2010; Experiment 1) observed an early (i.e., from 100 ms) advantage for the predicted, although spatially not signaled, positions for both eye gaze and arrow cues. A recent meta-analysis showed that in counterpredictive paradigms eye gaze and arrow cues produce similar reflexive shifts of attention since a significant and early cueing effect (around 100–200 ms) was observed with both types of cues.
2. ^The face stimulus was drawn from the NimStim Face Stimulus Set, developed by NimTottenham and supported by the John D. and Catherine T. MacArthur Foundation ResearchNetwork on Early Experience and Brain Development. Please contact Nim Tottenham, attott0006@tc.umn.edu, for more information concerning the stimulus set.
3. ^Consistent with the majority of the studies investigating the reversion of the spatial congruency effect, in the present study gaze targets were presented until the behavioural response (Torres-Marín et al., 2017; Marotta et al., 2019, 2022; Edwards et al., 2020; Ishikawa et al., 2021; Hemmerich et al., 2022; Tanaka et al., 2022). However, to our knowledge, the effect of the gaze target duration on congruency effect has never been investigated in the context of a spatial interference task. When eye-gaze stimuli have been used as cues, instead of as targets, it has been shown that from an SOA of 200 ms, the cueing effect with both gaze and arrow decreased progressively as the cue-target interval increased. This reduction in the magnitude of the effect was more pronounced with short cues (≤300 ms of duration) compared to long cues (for a meta-analysis of the cueing literature, see Chacón-Candia et al., 2022). Moreover, some studies have shown that at very long SOAs (2400 ms) responses to targets presented at the gazed location are slower than to targets presented at the ungazed location, leading to the well-known inhibition of return (IOR) effect (Frischen and Tipper, 2004; Frischen et al., 2007; Marotta et al., 2013). However, is important to know that in our task gaze stimuli were used as targets not as cues. Further studies are surely necessary to investigate the effect of target duration in a context of a spatial interference task.
4. ^To check if there was a speed-accuracy trade-off only for some of the 3 types of stimuli, a Pearson correlation was conducted between reaction times and error for each type of stimuli. In Experiment 1 a negative correlation was observed for arrows (r = −0.4502 p = 0.027) and eye-gaze (r = −0.4239 p = 0.039), but not for fingers (r = 0.3458 p = 0.098). In Experiment 2, none of the correlations was significant (arrows: r = −0.0476 p = 0.833; eye-gaze: r = −0.1077 p = 0.633; fingers: r = −0.0512 p = 0.821).
References
Aranda-Martín, B., Ballesteros-Duperón, M. Á., and Lupiáñez, J. (2022). What gaze adds to arrows: changes in attentional response to gaze versus arrows in childhood and adolescence. Br. J. Psychol. 113, 718–738. doi: 10.1111/bjop.12552
Baron-Cohen, S., Wheelwright, S., Hill, J., Raste, Y., and Plumb, I. (2001). The “reading the mind in the eyes” test revised version: a study with normal adults, and adults with asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatr. Allied Disciplines 42, 241–251. doi: 10.1111/1469-7610.00715
Baron-Cohen, S., Wheelwright, S., and Jolliffe, A. T. (1997). Is there a “language of the eyes”? Evidence from normal adults, and adults with autism or Asperger syndrome. Visual Cognit. 4, 311–331. doi: 10.1080/713756761
Bayliss, A. P., Paul, M. A., Cannon, P. R., and Tipper, S. P. (2006). Gaze cuing and affective judgments of objects: i like what you look at. Psychon. Bullet. Rev. 13, 1061–1066. doi: 10.3758/BF03213926
Bayliss, A. P., Pellegrino, G. D., and Tipper, S. P. (2005). Sex differences in eye gaze and symbolic cueing of attention. Q. J. Exp. Psychol. 58, 631–650. doi: 10.1080/02724980443000124
Bayliss, A. P., and Tipper, S. P. (2005). Gaze and arrow cueing of attention reveals individual differences along the autism spectrum as a function of target context. Br. J. Psychol. 96, 95–114. doi: 10.1348/000712604X15626
Belopolsky, A. V., Olivers, C. N., and Theeuwes, J. (2008). To point a finger: attentional and motor consequences of observing pointing movements. Acta Psychol. 128, 56–62. doi: 10.1016/j.actpsy.2007.09.012
Birmingham, E., and Kingstone, A. (2009). Human social attention: A new look at past, present, and future investigations. Annal. Acad. Sci. 1156, 118–140. doi: 10.1111/j.1749-6632.2009.04468.x
Blakemore, S. J., Winston, J., and Frith, U. (2004). Social cognitive neuroscience: where are we heading?. Trends Cognit. Sci. 8, 216–222. doi: 10.1016/j.tics.2004.03.012
Cañadas, E., and Lupiáñez, J. (2012). Spatial interference between gaze direction and gaze location: A study on the eye contact effect. Q. J. Exp. Psychol. 65, 1586–1598. doi: 10.1080/17470218.2012.659190
Capozzi, F., and Ristic, J. (2018). How attention gates social interactions. Annal. Acad. Sci. 1426, 179–198. doi: 10.1111/nyas.13854
Capozzi, F., and Ristic, J. (2020). Attention and mentalizing? Reframing a debate on social orienting of attention. Vis. Cogn. 28, 97–105. doi: 10.1080/13506285.2020.1725206
Chacón-Candia, J. A., Lupiáñez, J., Casagrande, M., and Marotta, A. (2020). Sex differences in attentional selection following gaze and arrow cues. Front. Psychol. 11, 95. doi: 10.3389/fpsyg.2020.00095
Chacón-Candia, J. A., Román-Caballero, R., Aranda-Martín, B., Casagrande, M., Lupiáñez, J., Marotta, A., et al. (2022). Are there quantitative differences between eye-gaze and arrow cues? A meta-analytic answer to the debate and a call for qualitative differences. Neurosci. Biobehav. Rev. 104993. doi: 10.31234/osf.io/5aqde
Cooperrider, K., Slotta, J., and Núñez, R. (2018). The preference for pointing with the hand is not universal. Cognit. Sci. 42, 1375–1390. doi: 10.1111/cogs.12585
Csibra, G. (2003). Teleological and referential understanding of action in infancy. Philosophical Transactions of the Royal Society of London. Biol. Sci. 358, 447–458. doi: 10.1098/rstb.2002.1235
Dalmaso, M., Castelli, L., Franchetti, L., Carli, L., Todisco, P., Palomba, D., et al. (2015). Altered orienting of attention in anorexia nervosa. Psychiatry Res. 229, 318–325. doi: 10.1016/j.psychres.2015.06.044
Dalmaso, M., Galfano, G., Tarqui, L., Forti, B., and Castelli, L. (2013). Is social attention impaired in schizophrenia? Gaze, but not pointing gestures, is associated with spatial attention deficits. Neuropsychology 27, 608. doi: 10.1037/a0033518
Dodd, M. D., Weiss, N., McDonnell, G. P., Sarwal, A., and Kingstone, A. (2012). Gaze cues influence memory… but not for long. Acta Psychologica 141, 270–275. doi: 10.1016/j.actpsy.2012.06.003
Edwards, S. G., Seibert, N., and Bayliss, A. P. (2020). Joint attention facilitates observed gaze direction discrimination. Q. J. Exp. Psychol. 73, 80–90. doi: 10.1177/1747021819867901
Emery, N. J. (2000). The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 24, 581–604. doi: 10.1016/S0149-7634(00)00025-7
Frischen, A., Smilek, D., Eastwood, J. D., and Tipper, S. P. (2007). Inhibition of return in response to gaze cues: The roles of time course and fixation cue. Visual Cognit. 15, 881–895. doi: 10.1080/13506280601112493
Frischen, A., and Tipper, S. P. (2004). Orienting Attention Via Observed Gaze Shift Evokes Longer Term Inhibitory Effects: Implications for Social Interactions, Attention, and Memory. Journal of Experimental Psychology: General. 133, 516–533. doi: 10.1037/0096-3445.133.4.516
Gobel, M. S., Tufft, M. R., and Richardson, D. C. (2018). Social beliefs and visual attention: how the social relevance of a cue influences spatial orienting. Cognitive Science 42, 161–185. doi: 10.1111/cogs.12529
Gregory, S. E., and Jackson, M. C. (2017). Joint attention enhances visual working memory. J. Exp. Psychol. Learning Memory Cognit. 43, 237. doi: 10.1037/xlm0000294
Hemmerich, K., Narganes-Pineda, C., Marotta, A., Martín-Arévalo, E., Jiménez, L., Lupiáñez, J., et al. (2022). Gaze elicits social and nonsocial attentional orienting: an interplay of shared and unique conflict processing mechanisms. J. Exp. Psychol. Hum. Percep. Perf. 48, 824. doi: 10.1037/xhp0001015
Hietanen, J. K., Nummenmaa, L., Nyman, M. J., Parkkola, R., and Hämäläinen, H. (2006). Automatic attention orienting by social and symbolic cues activates different neural networks: an fMRI study. Neuroimage 33, 406–413. doi: 10.1016/j.neuroimage.2006.06.048
Hood, B. M., Willen, J. D., and Driver, J. (1998). Adult's eyes trigger shifts of visual attention in human infants. Psychol. Sci. 9, 131–134. doi: 10.1111/1467-9280.00024
Ishikawa, K., Oyama, T., and Okubo, M. (2021). The malfunction of domain-specific attentional process in social anxiety: attentional process of social and non-social stimuli. Cognit. Emotion 35, 1163–1174. doi: 10.1080/02699931.2021.1935217
Jones, S. (2015). The mediating effects of facial expression on spatial interference between gaze direction and gaze location. J. Gen. Psychol. 142, 106–117. doi: 10.1080/00221309.2015.1009822
Kornblum, S., Hasbroucq, T., and Osman, A. (1992). Dimensional overlap: cognitive basis for stimulus-response compatibility–a model and taxonomy: correction to kornblum et al. Psychol. Rev. 99, 44.
Langton, S. R., and Bruce, V. (2000). You must see the point: automatic processing of cues to the direction of social attention. J. Exp. Psychol. Perf. 26, 747. doi: 10.1037/h0090380
Leekam, S. R., Lopez, B., and Moore, C. (2000). Attention and joint attention in preschool children with autism. Dev. Psychol. 36, 261. doi: 10.1037/0012-1649.36.2.261
Liszkowski, U., Carpenter, M., Henning, A., Striano, T., and Tomasello, M. (2004). Twelve-month-olds point to share attention and interest. Dev. Sci. 7, 297–307. doi: 10.1111/j.1467-7687.2004.00349.x
Lu, C. H., and Proctor, R. W. (1995). The influence of irrelevant location information on performance: A review of the Simon and spatial Stroop effects. Psychon. Bullet. Rev. 2, 174–207. doi: 10.3758/BF03210959
Marotta, A., Aranda-Martín, B., Cono, D. e, Ballesteros-Duperón, M., Casagrande, M., Á, M., and Lupiáñez, J. (2022). Integration of facial expression and gaze direction in individuals with a high level of autistic traits. Int. J. Environ. Res. Public Health 19, 2798. doi: 10.3390/ijerph19052798
Marotta, A., Lupiánez, J., Martella, D., and Casagrande, M. (2012). Eye gaze versus arrows as spatial cues: two qualitatively different modes of attentional selection. J. Exp. Psychol. Perf. 38, 326. doi: 10.1037/a0023959
Marotta, A., Lupiáñez, J., Román-Caballero, R., Narganes-Pineda, C., and Martín-Arévalo, E. (2019). Are eyes special? Electrophysiological and behavioural evidence for a dissociation between eye-gaze and arrows attentional mechanisms. Neuropsychologia 129, 146–152. doi: 10.1016/j.neuropsychologia.2019.03.017
Marotta, A., Pasini, A., Ruggiero, S., Maccari, L., Rosa, C., Lupianez, J., et al. (2013). Inhibition of return in response to eye gaze and peripheral cues in young people with Asperger's syndrome. J. Autism Dev. Disorders 43, 917–923. doi: 10.1007/s10803-012-1636-3
Marotta, A., Román-Caballero, R., and Lupiáñez, J. (2018). Arrows don't look at you: qualitatively different attentional mechanisms triggered by gaze and arrows. Psychon. Bullet. Rev. 25, 2254–2259. doi: 10.3758/s13423-018-1457-2
Narganes-Pineda, C., Chica, A. B., Lupiáñez, J., and Marotta, A. (2022). Explicit vs. implicit spatial processing in arrow vs. eye-gaze spatial congruency effects. Psychol. Res. 8, 1–18. doi: 10.1007/s00426-022-01659-x
Nuku, P., and Bekkering, H. (2008). Joint attention: Inferring what others perceive (and don't perceive). Consciousness Cogni. 17, 339–349. doi: 10.1016/j.concog.2007.06.014
Román-Caballero, R., Marotta, A., and Lupiáñez, J. (2021a). Target–background segregation in a spatial interference paradigm reveals shared and specific attentional mechanisms triggered by gaze and arrows. J. Exp. Psychol. Perf. 47, 1561. doi: 10.1037/xhp0000953
Román-Caballero, R., Marotta, A., and Lupiáñez, J. (2021b). Spatial interference triggered by gaze and arrows. The role of target background on spatial interference. Psicológica J. 42, 192–209. doi: 10.2478/psicolj-2021-0010
Sato, W., Kochiyama, T., Uono, S., and Yoshikawa, S. (2009). Commonalities in the neural mechanisms underlying automatic attentional shifts by gaze, gestures, and symbols. Neuroimage 45, 984–992. doi: 10.1016/j.neuroimage.2008.12.052
Sato, W., Kochiyama, T., Uono, S., and Yoshikawa, S. (2010). Automatic attentional shifts by gaze, gestures, and symbols. Psychologia 53, 27–35. doi: 10.2117/psysoc.2010.27
Schneider, W., Eschman, A., and Zuccolotto, A. (2002). E-Prime: User's Guide. Psychology Software Incorporated.
Simon, J. R., Craft, J. L., and Webster, J. B. (1973). Reactions toward the stimulus source: analysis of correct responses and errors over a five-day period. J. Exp. Psychol. 101, 175. doi: 10.1037/h0035766
Simon, J. R., and Small Jr, A. M. (1969). Processing auditory information: interference from an irrelevant cue. J. Appl. Psychol. 53, 433. doi: 10.1037/h0028034
Tanaka, Y., Ishikawa, K., Oyama, T., and Okubo, M. (2022). Face inversion does not affect the reversed congruency effect of gaze. Psychon. Bullet. Rev. 14, 1–9. doi: 10.3758/s13423-022-02208-8
Teufel, C., Alexis, D. M., Clayton, N. S., and Davis, G. (2010). Mental-state attribution drives rapid, reflexive gaze following. Attention Percep. Psychophy. 72, 695–705. doi: 10.3758/APP.72.3.695
Tipples, J. (2002). Eye gaze is not unique: automatic orienting in response to uninformative arrows. Psychon. Bullet. Rev. 9, 314–318. doi: 10.3758/BF03196287
Tipples, J., and Pecchinenda, A. (2019). A closer look at the size of the gaze-liking effect: a preregistered replication. Cognit. Emot. 33, 623–629. doi: 10.1080/02699931.2018.1468732
Tomasello, M., Carpenter, M., and Liszkowski, U. (2007). A new look at infant pointing. Child Dev. 78, 705–722. doi: 10.1111/j.1467-8624.2007.01025.x
Torres-Marín, J., Carretero-Dios, H., Acosta, A., and Lupiáñez, J. (2017). Eye contact and fear of being laughed at in a gaze discrimination task. Front. Psychol. 8, 1954. doi: 10.3389/fpsyg.2017.01954
Ulloa, J. L., and George, N. (2013). A cognitive neuroscience view on pointing: what is special about pointing with the eyes and hands?. MENTE J. Philos. Stu. 6, 203–228. Available online at: https://www.humanamente.eu/index.php/HM/article/view/156
Ulloa, J. L., Marchetti, C., Taffou, M., and George, N. (2015). Only your eyes tell me what you like: exploring the liking effect induced by other's gaze. Cognit. Emot. 29, 460–470. doi: 10.1080/02699931.2014.919899
Vlamings, P. H., Stauder, J. E., van Son, I. A., and Mottron, L. (2005). Atypical visual orienting to gaze-and arrow-cues in adults with high functioning autism. J. Autism Dev. Disorders 35, 3. doi: 10.1007/s10803-005-3289-y
Vuilleumier, P. (2002). Perceived gaze direction in faces and spatial attention: a study in patients with parietal damage and unilateral neglect. Neuropsychologia 40, 1013–1026. doi: 10.1016/S0028-3932(01)00153-1
Werner, E., Dawson, G., Osterling, J., and Dinno, N. (2000). Brief report: Recognition of autism spectrum disorder before one year of age: a retrospective study based on home videotapes. J. Autism Dev. Disorders 30, 157. doi: 10.1023/A:1005463707029
Wiese, E., Wykowska, A., Zwickel, J., and Müller, H. J. (2012). I see what you mean: How attentional selection is shaped by ascribing intentions to others. PLos ONE 9, 45391. doi: 10.1371/journal.pone.0045391
Wiese, E., Zwickel, J., and Müller, H. J. (2013). The importance of context information for the spatial specificity of gaze cueing. Attention, Percept. Psychophy. 75, 967–982. doi: 10.3758/s13414-013-0444-y
Keywords: eye-gaze, finger pointing, congruency effect, spatial interference task, arrows
Citation: Bonventre S and Marotta A (2023) Is the reversed congruency effect unique to the eye-gaze? Investigating the effects of finger pointing, eye-gaze and arrows stimuli on spatial interference. Front. Cognit. 2:1135435. doi: 10.3389/fcogn.2023.1135435
Received: 31 December 2022; Accepted: 12 May 2023;
Published: 25 May 2023.
Edited by:
Dariusz Asanowicz, Jagiellonian University, PolandReviewed by:
Wanyi Huang, Shanghai Normal University, ChinaAnna Pecchinenda, Sapienza University of Rome, Italy
José Luis Ulloa, University of Talca, Chile
Copyright © 2023 Bonventre and Marotta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Marotta, bWFyb3R0YUB1Z3IuZXM=
 Sofia Bonventre
Sofia Bonventre Andrea Marotta
Andrea Marotta