
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SPECIALTY GRAND CHALLENGE article
Front. Cognit., 17 October 2022
Sec. Perception
Volume 1 - 2022 | https://doi.org/10.3389/fcogn.2022.1004576
This article is part of the Research TopicInsights in Perception: 2022View all 8 articles
This contribution highlights the complex role of sexual and genetic differences, behavioral habits, and learning of new skills in shaping the circuitry and the neuroanatomy of the perceptual and sensory human brain. Research in recent years has shown that the brain does not necessarily develop in a predetermined way, and that certain structures and circuits observed in different types of individuals may appear differently in others. In the last 20 years, cognitive neuroscience has made great progress in the identification of the neural circuits underlying the ability to perceive sensory stimuli, recognize them perceptually, categorize them, and imagine them, in the various sensory modalities. A major impetus for the development of this knowledge was given by the development of increasingly sophisticated neuroimaging techniques such as cellular microscopy, Positron Emission Tomography (PET), functional Magnetic Resonance Imaging (fMRI, reaching 7-tesla, e.g., Allen et al., 2022), MEG, diffusion tensor imaging (DTI, Rouw and Scholte, 2007), optogenetics (Day-Cooney et al., 2022). It proved particularly fruitful to compare data obtained with different techniques both in order to overcome their individual technical limitations, and to approach the subject of investigation from different points of view, as in Peelen and Downing's (2007) paper on visual body perception, for example. There are four aspects of the perceptual brain that, in our opinion, need to be investigated from a more dynamic and flexible perspective, to provide a more realistic view of the neural mechanisms under investigation:
It is known that perceptual and sensory processes are not prefixed and stereotyped, but strongly affected by learning, familiarity, and the development of new skills. The examples are innumerable. For example, musical education results in macroscopic and well-documented neuroplastic changes in the processing of linguistic and musical sounds at the level of the primary and secondary auditory areas. Music skills also change the way brainstem processes auditory information, by strengthening oscillatory mechanisms designed for processing the temporal structure of speech and music (Doelling and Poeppel, 2015). Again, musicianship can alter the way the superior temporal area processes audiovisual information (as in the McGurk effect, e.g., Proverbio et al., 2016). Musical expertise can modify the neural circuitry of word recognition, through the development of a right-sided visual-word form area for reading notes and words (Proverbio et al., 2013). In the same way, motion perception is enhanced in deaf native signers (Quandt et al., 2021), subcortical encoding of sound is enhanced in bilinguals (Krizman et al., 2012), visual recognition of actions is more accurate in skilled dancers or athletes (Proverbio et al., 2012; Orlandi and Proverbio, 2019; Orlandi et al., 2020), and so far and so on. These behavioral changes are accompanied by changes in neuronal networks, connectivity, and brain volume, all referable to neuroplasticity so that there is no single way of perceiving sensory stimuli in the human brain.
Of course, also pathological factors can affect cerebral neuroplasticity at molecular, cellularm, and structural levels, thus resulting in severe disruptions in emotional, cognitive, and social behavior. As the population ages, an extraordinary effort would be needed to more satisfactorily treat the genetic and molecular causes of dementia and neurodegenerative diseases, thanks also to the recent identification of many reliable biomarkers (Gatto, 2020).
Recent findings have provided evidence of an early filter of sensory information at thalamic and amygdala levels: ~40–70 ms of post-stimulus latency. Kastner et al. (2020) have highlighted the role of the pulvinar thalamic nucleus in gating sensory inputs within and between cortical areas, synchronizing cortical activity across brain regions, and controlling cortical excitability. Consistently, through a simultaneous EEG-fMRI study, Müller-Bardorff et al. (2018) have shown how the amygdala nuclei modulate visual response to facial expressions thus rapidly biasing stimulus processing as a function of its emotional valence. Again, converging neurophysiological and neuroimaging findings suggest that thalamus and pulvinar, and cortico-striatal projections might serve early attention and alertness modulation of visual processing (regulated by fronto-parietal top-down control), active as early as 30 ms post-stimulus latency, and as reflected by N40 response of visual evoked potentials (Proverbio et al., 2021).
These data suggest that the distinction between early visual processing (objective, photographic analysis) and late perceptual analysis (modulated by top-down factors) is more subtle than previously thought, and perhaps limited to pre-thalamic visual processing.
A paradigmatic case of this relationship is the hypo-activation of the fusiform face area (FFA) observed in people with Autistic Spectrum Disorder (ASD). Sometimes this is accompanied by a reduced volume and hypo-activation of other brain areas involved in face processing, such as the right amygdala, the inferior frontal cortex, the superior temporal sulcus, and the premotor cortex (mirror neurons) (Hadjikhani et al., 2007). These neuroanatomical anomalies of ASD individuals are believed to be causally related to their deficits in social behavior, including the abnormal visual processing of human faces, the tendency of avoiding people's faces and eyes, and the lack of imitative behavior and reciprocity. However, persistent and early social avoidance might be the cause of the hypo-activation of the face and the social areas of the brain. In this regard, a recent study has shown the possibility to train participants with ASD to achieve up-regulation of the FFA activation using neurofeedback based on real-time fMRI (Pereira et al., 2019). This data is quite relevant in suggesting how specific training can reduce the anatomical alterations, which would therefore not be the cause but the effect of some of these deficits.
A similar case is the magnocellular theory of dyslexia (Stein, 2001), according to which certain forms of developmental dyslexia would be caused by an anomalous development of the visual magnocellular system, also resulting in: deficits in contrast sensitivity, motion processing, binocular control, and visuospatial attention. This deficit would also determine a hypo-activation of the parietal cortex (V5/MT) during motion processing. Using fMRI, it was more recently demonstrated that when dyslexics are matched to younger inexperienced readers, no metabolic differences emerge, thus suggesting that the hypoactivation of V5/MT might not be the real cause of dyslexia. In a further test, dyslexics underwent a phonological-based reading intervention followed by new fMRI scanning, and it was found that V5/MT activity increased along with intervention-driven reading skills (Olulade et al., 2013). These findings provide strong evidence that visual magnocellular dysfunction is not the cause of developmental dyslexia but may instead be consequential to impoverished reading and insufficient visuomotor and ocular training.
These are two very strong examples of how neuroplastic changes induced by behavioral habits can modify genetically determined functional neuroanatomy and produce new deficits (e.g., the hypoactivation of FFA or of MT) or reduce previous shortcomings (e.g., as in the case of neuro-rehabilitation or neuro-training).
After many influential papers (e.g., see Cahill and Aswad, 2015; Gur and Gur, 2017), editorials, and special topics devoted to this issue (e.g., P, rager2017), the time has come that sex differences should finally be taken into consideration in neuroscientific research. This paragraph regards sex differences in hemispheric functional lateralization. As is well known, some mental functions are more lateralized in one of the two hemispheres in humans (e.g., memory encoding vs. retrieval, language, spatial attention, music processing (etc…). Nevertheless, lateralization seems less pronounced in women especially for language (e.g., Kansaku et al., 2000). This sexual dimorphism might explain the lower incidence of aphasia in women (Sharma et al., 2019), and, more in general, the many sex differences in neurological and psychiatric disorders (Young and Pfaff, 2014).
The most paradigmatic example of sex differences in perception concerns face processing, which appears differently wired in the two sexes (Proverbio, 2021a). Much evidence has shown that the Fusiform Face Area (FFA) is active bilaterally in women and mainly over the right side in men (e.g., Proverbio et al., 2006, 2010; Liu et al., 2020). This leads to obvious consequences in the case of brain injuries and prosopagnosia. A recent meta-analysis, conducted from an initial database of 250 Event-related potentials (ERP) and MEG articles, dated between 1985 and 2020, and involving strictly right-handed and healthy participants aged 18–35 years (from Asia, Europe, and America continents), found a marked right-sided asymmetry of the N170 bioelectrical activity in males, and a bilateral or left-sided activity in females, during the processing of neutral upright faces (Proverbio, 2021b). Only female participants showed significantly larger left hemispheric responses compared to right (in the right visual field stimulation), whereas male participants always showed a right-sided activation. Consistently, a systematic meta-analysis of functional neuroimaging data (Liu et al., 2020) using two independent structural-neuroimaging datasets (n > 2,000), has reported a sex difference in the concentration of human gray matter volume (GMV) within brain regions subserving face processing, with a statistically significant male-minus-female GMV over the right hemisphere in face areas. Overall, it seems that FFA is not just randomly more active over the right side or bilaterally depending on the study or the experimental setting, but it is consistently more active over the right hemisphere in men, and bilaterally in women during face perception, due to biological sex. Indeed, by imaging-transcriptomic analyses, Liu et al. (2020) showed that GMV sex differences in face areas were specifically coupled to regional expression of sex-chromosome genes.
Another perceptual domain where the effect of sex and sex hormones seems to affect performance and hemispheric lateralization is the visuo-spatial rotation of 3D shapes, which is the ability to identify how a tridimensional object would appear if rotated in space. Studies have investigated the hormonal basis in males favoring spatial abilities and have suggested a role for testosterone, both at the intrauterine level, during neurodevelopment (e.g., in puberty, Vuoksimaa et al., 2012) and in adult life. Meta-analysis of individuals with congenital adrenal hyperplasia (exposing the fetus to excessive amounts of testosterone), evidenced sex-specific effects of testosterone on visuospatial abilities (Puts et al., 2008). Again, females with adrenal hyperplasia have better spatial abilities than control females. Interestingly, a study on twins reported significantly better mental rotation test performance in females with a male twin compared to females with a female twin (Vuoksimaa et al., 2010). This pattern of results might be due to prenatal exposure to higher levels of testosterone in females with a male twin. These sex differences in performance seem to be associated with an anatomo-functional difference. For example, Hahn et al. (2010) found a bilateral parietal activation during 3D rotation in preschool boys, and a left-sided activation in girls. Partly in agreement, the fMRI study by Hugdahl et al. (2006) reported a bilateral activation of the superior parietal cortex in men and a right-sided parietal activation in women.
If neuroscientists have found a sexual dimorphism in the anatomy of FFA, structural MRI studies have also reported sexual dimorphism in the parietal cortex anatomy (Gur et al., 1999). For example, Koscik et al. (2009) found that men compared to women had proportionately greater parietal lobe surface area (especially over the left hemisphere), and this morphologic difference was associated with a performance advantage on mental rotation tasks. However, the literature is not homogeneous on this matter, since, for example, Hänggi et al. (2010) reported that in women visuospatial cognition was correlated with left parietal GMV differences, while in men with right parietal white matter volume differences. In general, males seem to generally outperform females on 3D shape rotation tasks (e.g., Astur et al., 2004), and females usually outperform males on facial expression recognition tasks (Proverbio, 2017). In both cases, this might possibly be associated with a bilateral (vs. unilateral) recruitment of devoted brain areas, due to genetic and/or hormonal factors, but this hypothesis deserves further investigation.
The cases of sexual dimorphism in hemispheric lateralization for processing faces and 3D shapes are emblematic of how genetics can differently shape the perceptual brain in human individuals.
In conclusion, new and exciting research is needed and sought to better comprehend how the perceptual and sensory brain is modulated and shaped in various individuals, in the multiple possible ways allowed by our adaptive and plastic brain. In this regard, Figure 1 offers a simplified model of neuroplasticity in perception (not including, for example, epigenetics), showing how perceptual processes are not prefixed abilities in humans but depend on the complex interaction between genetic and “environmental” factors during an individual's lifetime.
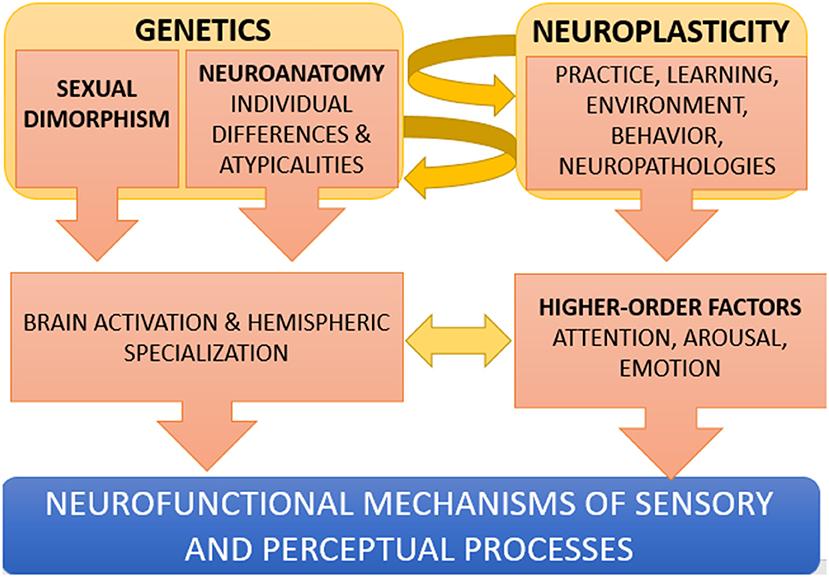
Figure 1. Simplified model of the interplay between genetic and environmental factors in perception. Genetic factors (such as for example, being a female, or having an atypical connectivity or functional lateralization) may result in a bilateral activation of FFA during face processing, or to an insufficient activation of the left VWFA during letter processing. The development of new skills, due to an extensive practice (e.g., studying music) or to neuro-rehabilitation (e.g., a phonological-based reading intervention) can in turn modify neuroanatomy (neuroplasticity interacts with genetics). Higher order mental functions such as emotion, motivation, attention and arousal also modulate the way sensory and perceptual areas of the brain process the incoming inputs.
The author confirms being the sole contributor of this work and has approved it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Allen, E. J., St-Yves, G., Wu, Y., Breedlove, J. L., Prince, J. S., Dowdle, L. T., et al. (2022). A massive 7T fMRI dataset to bridge cognitive neuroscience and artificial intelligence. Nat. Neurosci. 25, 116–126. doi: 10.1038/s41593-021-00962-x
Astur, R. S., Tropp, J., Sava, S., Constable, R. T., and Markus, E. J. (2004). Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav. Brain Res. 151, 103–115. doi: 10.1016/j.bbr.2003.08.024
Cahill, L., and Aswad, D. (2015). Sex influences on the brain: an issue whose time has come. Neuron. 88, 1084–1085. doi: 10.1016/j.neuron.2015.11.021
Day-Cooney, J., Cone, J. J., and Maunsell, J. H. R. (2022). Perceptual weighting of V1 spikes revealed by optogenetic white noise stimulation. J. Neurosci. 42, 3122–3132. doi: 10.1523/JNEUROSCI.1736-21.2022
Doelling, K. B., Poeppel, D. (2015). Cortical entrainment to music and its modulation by expertise. Proc. Natl. Acad. Sci. U. S. A. 112, E6233–E6242. doi: 10.1073/pnas.1508431112
Gatto, R. G. (2020). Molecular and microstructural biomarkers of neuroplasticity in neurodegenerative disorders through preclinical and diffusion magnetic resonance imaging studies. J. Integr. Neurosci. 30;19, 571–592. doi: 10.31083/j.jin.2020.03.165
Gur, R. C., and Gur, R. E. (2017). Complementarity of sex differences in brain and behavior: From laterality to multimodal neuroimaging. J. Neurosci. Res., 2 95.,189–199. doi: 10.1002/jnr.23830
Gur, R. C., Turetsky, B. I., Matsui, M., Yan, M., Bilker, W., Hughett, P., et al. (1999). Sex differences in brain gray and white matter in healthy young adults: Correlations with cognitive performance. J. Neurosci. 19, 4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999
Hadjikhani, N., Joseph, R. M., Snyder, J., Tager-Flusberg, H. (2007). Abnormal activation of the social brain during face perception in autism. Hum. Brain. Mapp. 28, 441–449. doi: 10.1002/hbm.20283
Hahn, N., Jansen, P., and Heil, M. (2010). Preschoolers' mental rotation: sex differences in hemispheric asymmetry. J. Cogn. Neurosci. 22, 1244–1250. doi: 10.1162/jocn.2009.21236
Hänggi, J., Buchmann, A., Mondadori, C. R., Henke, K., Jäncke, L., Hock, C., et al. (2010). Sexual dimorphism in the parietal substrate associated with visuospatial cognition independent of general intelligence. J. Cogn. Neurosci. 22, 139–155. doi: 10.1162/jocn.2008.21175
Hugdahl, K., Thomsen, T., and Ersland, L. (2006). Sex differences in visuo-spatial processing: an fMRI study of mental rotation. Neuropsychologia 44, 1575–1583. doi: 10.1016/j.neuropsychologia.2006.01.026
Kansaku, K., Yamaura, A., and Kitazawa, S. (2000). Sex differences in lateralization revealed in the posterior language areas. Cereb. Cortex 10, 866–872. doi: 10.1093/cercor/10.9.866
Kastner, S., Fiebelkorn, I. C., and Eradath, M. K. (2020). Dynamic pulvino-cortical interactions in the primate attention network. Curr. Opin. Neurobiol. 65, 10–19. doi: 10.1016/j.conb.2020.08.002
Koscik, T., O'Leary, D., Moser, D. J., Andreasen, N. C., and Nopoulos, P. (2009). Sex differences in parietal lobe morphology: relationship to mental rotation performance. Brain Cogn. 69, 451–459. doi: 10.1016/j.bandc.2008.09.004
Krizman, J., Marian, V., Shook, A., Skoe, E., and Kraus, N. (2012). Subcortical encoding of sound is enhanced in bilinguals and relates to executive function advantages. Proc. Natl. Acad. Sci. U. S. A.15;109, 7877–7881. doi: 10.1073/pnas.1201575109
Liu, S., Seidlitz, J., Blumenthal, J. D., Clasen, L. S., and Raznahan, A. (2020). Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc. Natl. Acad. Sci. U. S. A. 117, 18788–18798. doi: 10.1073/pnas.1919091117
Müller-Bardorff, M., Bruchmann, M., Mothes-Lasch, M., Zwitserlood, P., Schlossmacher, I., Hofmann, D., et al. (2018). Early brain responses to affective faces: A simultaneous EEG-fMRI study. Neuroimage 178, 660–667. doi: 10.1016/j.neuroimage.2018.05.081
Olulade, O. A., Napoliello, E. M., and Eden, G. F. (2013). Abnormal visual motion processing is not a cause of dyslexia. Neuron 10;79, 180–190. doi: 10.1016/j.neuron.2013.05.002
Orlandi, A., Arno, E., and Proverbio, A. M. (2020). The effect of expertise on kinesthetic motor imagery of complex actions. Brain Topogr. 33, 238–254. doi: 10.1007/s10548-020-00760-x
Orlandi, A., and Proverbio, A. M. (2019). Bilateral engagement of the occipito-temporal cortex in response to dance kinematics in experts. Sci. Rep. 9, 1000. doi: 10.1038/s41598-018-37876-x
Peelen, M. V., and Downing, P. E. (2007). The neural basis of visual body perception. Nat. Rev. Neurosci. 8, 636–648. doi: 10.1038/nrn2195
Pereira, J. A., Sepulveda, P., Rana, M., Montalba, C., Tejos, C., Torres, R., et al. (2019). Self-regulation of the fusiform face area in autism spectrum: a feasibility study with real-time fMRI neurofeedback. Front. Hum. Neurosci. 20, 13.446. doi: 10.3389/fnhum.2019.00446
Prager, E. M. (2017). Addressing sex as a biological variable. J Neurosci Res. 95:11. doi: 10.1002/jnr.23979
Proverbio, A. M. (2017). Sex differences in social cognition: The case of face processing. J. Neurosci. Res. 95, 222–234. doi: 10.1002/jnr.23817
Proverbio, A. M. (2021a). Sex differences in the social brain and in social cognition. J. Neurosci. Res. 20. doi: 10.1002/jnr.24787
Proverbio, A. M. (2021b). Sexual dimorphism in hemispheric processing of faces in humans: a meta-analysis of 817 cases. Soc. Cogn. Affect. Neurosci. 16, 1023–1035. doi: 10.1093/scan/nsab043
Proverbio, A. M., Brignone, V., Matarazzo, S., Del Zotto, M., Zani, A. (2006). Gender differences in hemispheric asymmetry for face processing. BMC Neurosci. 7, 44. doi: 10.1186/1471-2202-7-44
Proverbio, A. M., Broido, V., Benedetto, D. e, Zani, F. A. (2021). Scalp-recorded N40 visual evoked potential: Sensory and attentional properties. Eur J. Neurosci., 54, 6553–6574. doi: 10.1111/ejn.15443
Proverbio, A. M., Crotti, N., Manfredi, M., Adorni, R., Zani, A. (2012). Who needs a referee? How incorrect basketball actions are automatically detected by basketball players' brain. Sci. Rep. 2, 883. doi: 10.1038/srep00883
Proverbio, A. M., Manfredi, M., Zani, A., and Adorni, R. (2013). Musical expertise affects neural bases of letter recognition. Neuropsychologia 51, 538–549. doi: 10.1016/j.neuropsychologia.2012.12.001
Proverbio, A. M., Massetti, G., Rizzi, E., and Zani, A. (2016). Skilled musicians are not subject to the McGurk effect. Sci. Rep. 6, 30423. doi: 10.1038/srep30423
Proverbio, A. M., Riva, F., Martin, E., and Zani, A. (2010). Face coding is bilateral in the female brain. PLoS ONE. 5, e11242. doi: 10.1371/journal.pone.0011242
Puts, D. A., McDaniel, M. A., Jordan, C. L., Breedlove, S. M. (2008). Spatial ability and prenatal androgens: meta-analyses of congenital adrenal hyperplasia and digit ratio (2D:4D) studies. Arch. Sex. Behav. 37, 100–111. doi: 10.1007/s10508-007-9271-3
Quandt, L. C., Kubicek, E., Willis, A., and Lamberton, J. (2021). Enhanced biological motion perception in deaf native signers. Neuropsychologia 161, 107996. doi: 10.1016/j.neuropsychologia.2021.107996
Rouw, R., and Scholte, H. S. (2007). Increased structural connectivity in grapheme-color synesthesia. Nat. Neurosci. 10, 792–797. doi: 10.1038/nn1906
Sharma, S., Briley, P. M., Wright, H. H., Perry, J. L., Fang, X., Ellis, C., et al. (2019). Gender differences in aphasia outcomes: evidence from the Aphasia Bank. Int. J. Lang. Commun. Disord. 54, 806–813. doi: 10.1111/1460-6984.12486
Stein, J. (2001). The magnocellular theory of developmental dyslexia. Dyslexia 7, 12–36. doi: 10.1002/dys.186
Vuoksimaa, E., Kaprio, J., Eriksson, C. J., and Rose, R. J. (2012). Pubertal testosterone predicts mental rotation performance of young adult males. Psychoneuroendocrinology 37, 1791–1800. doi: 10.1016/j.psyneuen.2012.03.013
Vuoksimaa, E., Kaprio, J., Kremen, W. S., Hokkanen, L., Viken, R. J., Tuulio-Henriksson, A., et al. (2010). Having a male co-twin masculinizes mental rotation performance in females. Psychol. 21, 1069–1071. doi: 10.1177/0956797610376075
Keywords: behavior and cognition, sensory processing, neuroanatomy, cognition and perception, neural circuits, behavioral habits, functional connectivity, attention
Citation: Proverbio AM (2022) Perception: A dynamic interplay between genetics and neuroplasticity. Front. Cognit. 1:1004576. doi: 10.3389/fcogn.2022.1004576
Received: 27 July 2022; Accepted: 28 September 2022;
Published: 17 October 2022.
Edited by:
Kasia M. Bieszczad, Rutgers, The State University of New Jersey, United StatesReviewed by:
Melisa Carolina Monteleone, CONICET Institute of Biotechnological Research (IIB-INTECH), ArgentinaCopyright © 2022 Proverbio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alice Mado Proverbio, bWFkby5wcm92ZXJiaW9AdW5pbWliLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.