
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Clin. Diabetes Healthc., 01 April 2025
Sec. Diabetes and Pregnancy
Volume 6 - 2025 | https://doi.org/10.3389/fcdhc.2025.1566577
This article is part of the Research TopicHighlights in Diabetes and PregnancyView all articles
This meta-analysis aimed to evaluate the benefits of prenatal exercise on neonatal outcomes in women with gestational diabetes mellitus (GDM). Systematic searches were conducted in PubMed, EMBASE, Cochrane Library, Web of Science, and Scopus from their inception to September 9, 2023. ClinicalTrials.gov was also searched to ensure comprehensive coverage. We included studies that investigated the association between prenatal exercise and at least one adverse neonatal outcome of interest. A total of 4,268 publications were retrieved, and 3,060 records remained after removing duplicates. After screening abstracts, 107 studies were selected for full-text assessment, and ultimately, 17 articles (including 4 identified through manual searching) were included for data extraction. Extracted information included the first author, publication year, study design, geographical location, sample size, participants’ demographic characteristics, intervention characteristics, and relevant outcome variables.Pooled results from random-effects models showed that prenatal exercise significantly reduced the risk of adverse neonatal outcomes, including: Cesarean delivery (OR = 0.91, 95% CI: 0.88–0.94), Premature birth (OR = 0.49, 95% CI: 0.27–0.90), Macrosomia (OR = 0.58, 95% CI: 0.40–0.83), Fetal growth restriction (OR = 0.21, 95% CI: 0.08–0.52), and Birth trauma (OR = 0.26, 95% CI: 0.13–0.54). Subgroup analyses indicated that single-component exercise programs were more effective than multi-component programs in reducing the risk of macrosomia (P = 0.06). In conclusion, prenatal exercise substantially reduces the risk of multiple adverse neonatal outcomes in women with GDM, including macrosomia, preterm birth, cesarean delivery, fetal growth restriction, and birth trauma. These findings highlight the outstanding benefits of antenatal exercise for fetal health, supporting its inclusion as a key component of prenatal care for women with GDM. This meta-analysis is registered with PROSPERO (Registration Number: CRD42023485375).
Gestational diabetes mellitus (GDM), defined as glucose intolerance with onset or first recognition during pregnancy, is one of the most common complications among pregnant women. Depending on the population and diagnostic criteria used, the prevalence of GDM ranges from 1% to over 30%, posing a significant global public health challenge (1–3). Women with GDM are at an elevated risk of experiencing other pregnancy complications, as well as developing type 2 diabetes, cardiovascular and cerebrovascular diseases, and metabolic syndrome later in life (4–6). Maternal hyperglycemia leads to fetal hyperglycemia through facilitated glucose transport mediated by glucose transporter 1 (GLUT1) (7). This fetal hyperglycemia triggers hyperinsulinemia, which promotes excessive fetal adiposity and accelerated growth, resulting in macrosomia and a range of associated complications, including preterm birth, cesarean delivery, and birth trauma (8–10). Poor maternal glucose control further exacerbates the risk of adverse pregnancy outcomes (10). Additionally, offspring of mothers with GDM are more likely to develop long-term metabolic and cardiovascular disorders (11). Given these risks, timely and effective interventions to mitigate adverse pregnancy and neonatal outcomes associated with GDM, along with continuous monitoring of maternal and child health postpartum, are of critical clinical importance (4).
Lifestyle interventions remain the cornerstone of gestational diabetes mellitus (GDM) management. Upon diagnosis, pregnant women with GDM are advised to engage in at least 150 minutes of moderate-intensity physical activity each week. Additionally, they are encouraged to follow a diet that provides adequate macronutrients and micronutrients to support fetal growth, minimize postprandial glucose fluctuations, and promote appropriate gestational weight gain throughout pregnancy (12). Numerous randomized controlled trials (RCTs) and meta-analyses have demonstrated that exercise can delay the progression of glucose intolerance and improve maternal outcomes in women with GDM, leading to its recommendation for all women with GDM without contraindications to physical activity (13, 14). Despite robust evidence supporting the maternal benefits of prenatal exercise, there is limited research on its effects on neonatal outcomes. A recently published meta-analysis reported that exercise significantly reduces the rates of cesarean delivery, macrosomia, premature rupture of membranes, and neonatal hypoglycemia among women with GDM, but showed no impact on preterm birth rates (15). However, other adverse neonatal outcomes, such as intrauterine growth restriction and birth trauma, were not evaluated in this analysis. It is important to acknowledge that current literature often lacks detailed information regarding key exercise parameters, including the specific type of exercise, its duration, intensity, and the trimester(s) during which the interventions were implemented. This limits our ability to fully understand the nuanced relationship between exercise and pregnancy outcomes. The question of whether prenatal exercise truly benefits neonatal outcomes in women with GDM, and the extent to which these risks can be mitigated, remains insufficiently explored. Furthermore, many studies do not provide statistically normalized results adjusted for potential confounders such as maternal age, pre-pregnancy body weight, or weight gain during pregnancy, which could influence the observed outcomes. The potential development of adverse events such as gestational hypertension related to workload, type of exercise or other demographics of the volunteers is also often not reported. Moreover, evidence suggests that awareness of the potential benefits of exercise for fetal health is a key enabler of pregnant women’s participation in prenatal physical activity (16). In light of these considerations, we conducted a systematic review and meta-analysis to explore whether prenatal exercise can reduce the risk of additional adverse neonatal outcomes, such as preterm birth, intrauterine growth restriction, and birth trauma, specifically in women with a confirmed diagnosis of GDM.
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (1). The study was prospectively registered in the international database of systematic reviews (PROSPERO; registration number: CRD42023485375).
To identify relevant studies, we systematically searched English-language databases, including PubMed, EMBASE, the Cochrane Library, Web of Science, and Scopus, from their inception to September 9, 2023. Our search utilized a combination of terms targeting participants and interventions, such as: *gestational diabetes mellitus OR GDM OR gestational diabetes OR pregnancy-induced diabetes* AND *sports OR exercise OR activit* OR physical activit* OR exercise training*. Full details of the search strategy are provided in online Supplementary Table S1.
Additionally, we searched ClinicalTrials.gov, though no relevant studies were identified through this source. To ensure comprehensive coverage, we also manually reviewed the reference lists of included articles and incorporated any appropriate studies identified during this process.
Study selection Studies were deemed eligible for inclusion if they met the following criteria: participants were definitively diagnosed with GDM; a complete exercise intervention program was implemented, specifying exercise duration, frequency, and intensity; The criteria used to categorize exercise intensity (mild, moderate, high) were based on the guidelines provided by the American College of Sports Medicine (ACSM) and were defined as follows: Mild intensity was defined as 30-39% HRR or VO2R, Moderate intensity as 40-59% HRR or VO2R, and High intensity as 60-89% HRR or VO2R. When studies did not directly report HRR or VO2R, we used METs (Metabolic Equivalents) as an alternative, with Mild intensity corresponding to 1.5-3 METs, Moderate intensity to 3-6 METs, and High intensity to >6 METs. the control group did not undergo any exercise intervention; and at least one outcome of interest was reported, such as cesarean delivery, preterm birth, or macrosomia. Studies with combined interventions (diet + exercise) were excluded because we aimed to isolate the specific effects of exercise on GDM outcomes and minimize confounding factors. The potential interaction between diet and exercise could obscure the true impact of exercise alone. Studies were excluded if they involved additional interventions beyond physical exercise, lacked original data (e.g., reviews, editorials, or comments), or were published in languages other than English. Study selection was conducted in two phases: an initial screening of titles and abstracts, followed by a full-text review of potentially eligible articles. Two independent reviewers (Hangyu Cui and Hua Li) assessed the eligibility of studies, with any discrepancies resolved by consultation with a third investigator (Mingzi Li).
The risk of bias for intervention studies was assessed using the Cochrane Risk of Bias version 2 (RoB2) tool, following the guidelines outlined in the Cochrane Handbook. This tool evaluates five domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of outcomes, and bias in the selection of the reported results (2). For observational cohort studies, the Newcastle-Ottawa Scale (NOS) was employed to assess methodological quality.(3) The NOS evaluates eight categories related to study quality, with each study receiving a final score out of a maximum of nine points.Two authors (Hangyu Cui and Hua Li) independently conducted the evaluations, with any discrepancies resolved through discussion with a third investigator (Mingzi Li).
Two authors (Hangyu Cui and Hua Li) independently extracted data from eligible studies using pretested, Excel-based data extraction sheets. The extracted information included the first author, publication year, study design, country, sample size, participants’ demographic characteristics, study setting, details of the intervention (type, frequency, intensity, and duration), and outcome variables of interest.
The adverse neonatal outcomes assessed in this study included cesarean delivery, macrosomia, preterm birth, neonatal complications, fetal growth restriction, birth trauma, low birth weight, neonatal hypoglycemia, premature rupture of membranes, large-for-gestational-age infants, gestational age at delivery, birth asphyxia, stillbirth, fetal distress, and congenital malformations.
Data analyses were performed using Stata Statistical Software version 18.0 (StataCorp, TX, USA) and R statistical software version 4.2.2. A p-value of <0.05 was considered statistically significant. Due to variations in study designs and the limited number of studies, a random-effects model was employed to calculate the estimated effect sizes and their respective 95% confidence intervals. Event counts and overall participant numbers were initially extracted, and odds ratios (ORs) were used for dichotomous variables. Heterogeneity across studies was assessed using the I² statistic, with thresholds of 0–25%, 25–50%, and >50% indicating low, moderate, and high heterogeneity, respectively. Sensitivity analyses were conducted to test the robustness of the results by sequentially removing individual studies. Publication bias was evaluated using funnel plots (for datasets with ≥10 studies) and the results of Egger’s and Begg’s tests.Given the broad publication time span and varying study designs, subgroup analyses were conducted to explore subgroup differences and potential sources of heterogeneity. Studies were stratified by study design (RCTs or observational studies), economic level of the study location (developed or developing countries), publication year (before or after 2018), type of exercise program (single-component or multi-component), and exercise mode (aerobic, resistance, or mixed exercise). The p-value for differences between subgroups was calculated, with a threshold of <0.1 considered statistically significant.
A total of 4,268 studies were identified through the database search. After removing duplicate records, 3,060 unique publications remained for the initial screening. Based on a review of titles and abstracts, 2,953 studies were excluded. Subsequently, a full-text review of the remaining articles led to a final inclusion of 17 studies (13 identified through database searches and 4 through manual searching). These included 15 randomized controlled trials (RCTs) (4–18) and 2 retrospective cohort studies (Figure 1) (19, 20). According to the RoB2 assessment, for studies analyzed using the intention-to-treat approach, 1 study (25%) demonstrated a low risk of bias, 2 studies (50%) had some concerns, and 1 study (25%) indicated a high risk of bias (online Supplementary Figure S1). When using the per-protocol analysis, 1 study (9.1%) showed a low risk of bias, 9 studies (81.8%) demonstrated some concerns, and 1 study (9.1%) indicated a high risk of bias. The two observational studies were of high quality, receiving scores of 8 and 9 points, respectively, on the Newcastle-Ottawa Scale (online Supplementary Table S2).
Characteristics of enrolled studies are presented in Table 1. 2200 and 77910 women with GDM were consisted in the 15 RCTs and 2 cohort studies, respectively. Exercise types comprised aerobic exercise, resistance exercise and composited exercise program. Duration of exercise ranged from 15 to more than 60 minutes with a frequency fluctuating between 2 and 7 times per week. The adverse pregnancy outcomes which were assessed in at least 2 publications so as to be pooled contained premature birth, macrosomia, cesarean section, fetal growth restriction, birth trauma, neonatal hypoglycemia, low birth weight, premature rupture of membranes, large for gestational age, birth asphyxia, stillbirth, fetal distress and congenital malformation.
The pooled results showed that exercise during pregnancy of women with GDM significantly decreased the incident rate of cesarean section (OR = 0.91, 95% CI: 0.88, 0.94), premature birth (OR = 0.49, 95% CI: 0.27, 0.90), macrosomia (OR = 0.58, 95% CI: 0.40, 0.83), fetal growth restriction (OR = 0.21, 95% CI: 0.08, 0.52), birth trauma (OR = 0.26, 95% CI: 0.13, 0.54). Heterogeneity of cesarean section (I2 = 0.00%), fetal growth restriction (I2 = 0.00%) and birth trauma (I2 = 0.00%) was all very low. Heterogeneity of macrosomia (I2 = 29.27%) was moderate, while that of premature birth (I2 = 77.71%) was substantial (Figure 2, Supplementary Figures S2-S6).
No significant association was observed between exercise during pregnancy and several neonatal outcomes in women with GDM. These outcomes included neonatal hypoglycemia (OR = 0.78, 95% CI: 0.26–2.33, P = 0.66), low birth weight (OR = 0.70, 95% CI: 0.07–7.09, P = 0.76), premature rupture of membranes (OR = 0.86, 95% CI: 0.27–2.74, P = 0.78), large-for-gestational-age infants (OR = 0.32, 95% CI: 0.07–1.54, P = 0.15), birth asphyxia (OR = 0.13, 95% CI: 0.01–2.14, P = 0.15), stillbirth (OR = 0.51, 95% CI: 0.12–2.06, P = 0.34), fetal distress (OR = 0.81, 95% CI: 0.37–1.76, P = 0.59), and congenital malformation (OR = 0.39, 95% CI: 0.09–1.77, P = 0.22) (Figure 2, Supplementary Figures S7-S14). Heterogeneity was low for congenital malformation, fetal distress, stillbirth, premature rupture of membranes, and neonatal hypoglycemia. In contrast, heterogeneity was high for birth asphyxia, large-for-gestational-age infants, and low birth weight (Figure 2).
The sensitivity analysis, performed by sequentially removing each study, confirmed the robustness of the pooled estimates for preterm birth, cesarean delivery, and macrosomia (online Supplementary Figure S15). However, the robustness of other outcomes was not assessed due to the limited number of studies or the lack of statistically significant associations. No evidence of publication bias was detected based on the visualization of funnel plots or the results of Egger’s and Begg’s tests (Figure 2, Supplementary Figure S16).
To examine whether the association between exercise and selected adverse pregnancy outcomes was influenced by subgroup differences, subgroup analyses were conducted, with a p-value of <0.1 considered indicative of potential interactions. Given the growing popularity of exercise interventions and improved exercise compliance in recent years, subgroup analyses were stratified by publication year. The results revealed a significant difference in the association between exercise and cesarean delivery based on the year of publication (P = 0.05). Studies published after 2018 demonstrated a more pronounced effect of exercise interventions on reducing cesarean delivery rates compared to those published earlier (Figure 3A). Additionally, there was a consistent trend indicating that single-component exercise programs were more effective in reducing the risk of cesarean delivery, preterm birth, and macrosomia compared to multi-component programs that combined different types of exercise. However, it is important to note that this conclusion requires caution, as no studies directly compared single-component and multi-component interventions within the same trial. This observed trend is based on indirect comparisons across different studies. Similarly, randomized controlled trials (RCTs) showed better results than observational studies, although statistical significance was observed only for macrosomia. Furthermore, subgroup analyses suggested that aerobic exercise specifically conferred benefits in reducing adverse neonatal outcomes (Figure 3).
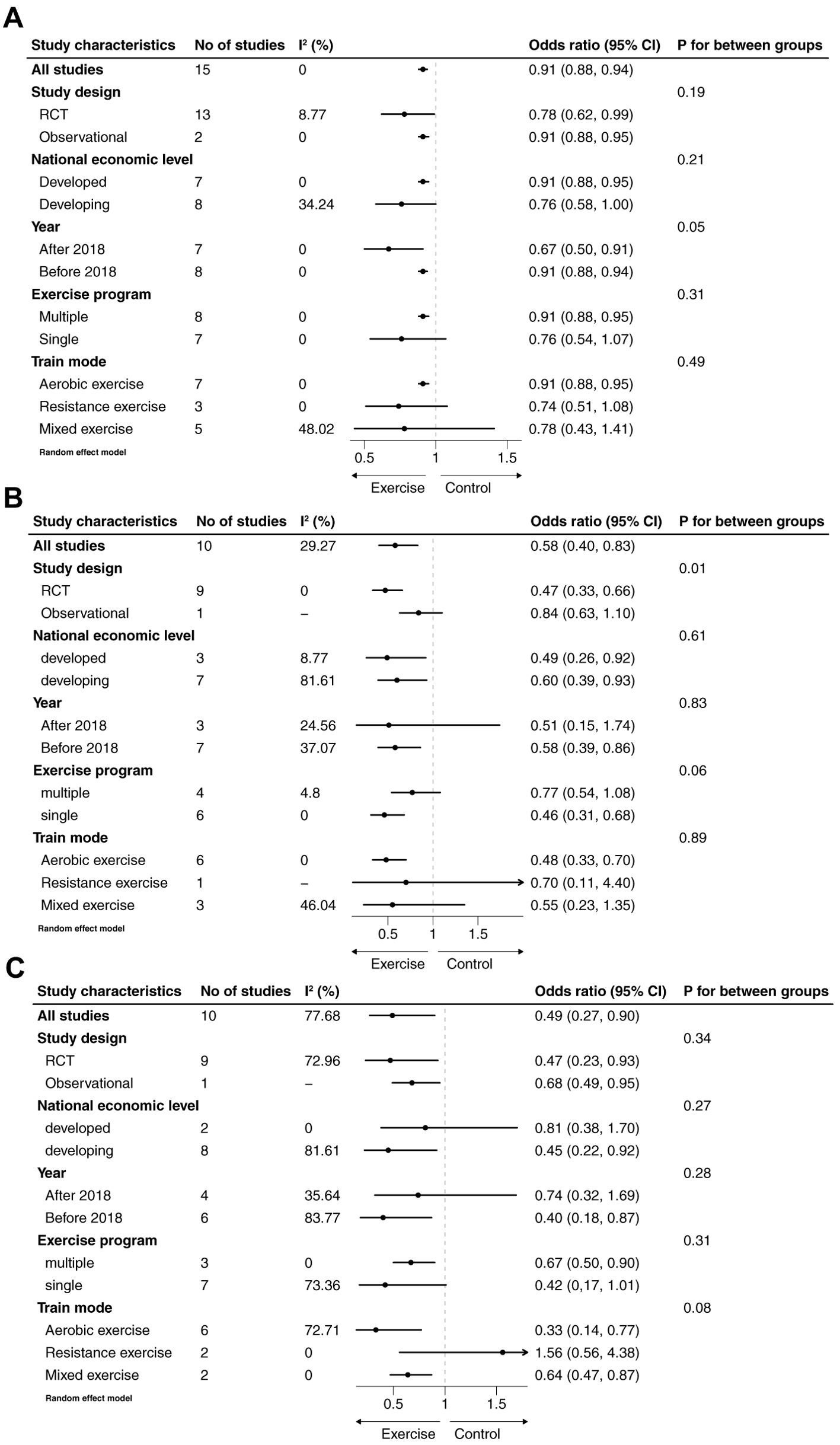
Figure 3. Subgroup analyses of association between exercise and cesarean section (A), premature birth (B) and macrosomia (C).
This systematic review and meta-analysis included data from 17 studies, comprising 15 randomized controlled trials (RCTs) and 2 retrospective cohort studies. The findings indicate that exercise during pregnancy significantly reduces the risks of adverse pregnancy outcomes, including cesarean delivery, preterm birth, macrosomia, fetal growth restriction, and birth trauma. Notably, the analysis underscores the critical role of regular, sustained, and moderate-intensity exercise in mitigating these risks. Furthermore, single-component exercise programs demonstrated greater effectiveness in improving adverse neonatal outcomes compared to multi-component programs, emphasizing the value of targeted exercise interventions during pregnancy.
Premature birth and macrosomia are common neonatal complications among women with GDM, often accompanied by an increased incidence of cesarean delivery (21, 22). While it is widely acknowledged that exercise can delay the progression of glucose intolerance by enhancing insulin secretion and improving maternal outcomes for women with GDM, few studies have specifically investigated the association between exercise and adverse neonatal outcomes (23–25). Previous meta-analyses focusing on pregnant women, in general, reported that exercise reduced the risk of macrosomia, with risk reductions ranging from 4% to 61%. However, specific data concerning the impact of exercise on macrosomia in women with GDM remained unavailable (26, 27). A meta-analysis published in 2022, which included 9 studies, found that exercise reduced the incidence of macrosomia (RR = 0.57, P = 0.03) and cesarean delivery (RR = 0.83, P = 0.02), while outcomes such as preterm birth, premature rupture of membranes, and neonatal hypoglycemia were not significantly affected (28). Despite these findings, the relationship between exercise and certain adverse neonatal outcomes in women with GDM remains inconclusive, leaving significant gaps in understanding and a need for further research on this topic.
In our systematic review and meta-analysis, we comprehensively evaluated adverse neonatal outcomes associated with GDM. The pooled results demonstrated that exercise during pregnancy significantly reduced the incidence of macrosomia, cesarean delivery, preterm birth, fetal growth restriction, and birth trauma. Additionally, we explored the association of exercise with other adverse neonatal outcomes, including neonatal hypoglycemia, low birth weight, premature rupture of membranes, large-for-gestational-age infants, stillbirth, birth asphyxia, fetal distress, and congenital malformations. However, none of these associations were found to be statistically significant. This study offers several advantages over previous meta-analyses. First, it included a larger number of studies, enabling a comprehensive and systematic assessment of the effects of exercise on adverse neonatal outcomes in women with GDM, thereby providing a more holistic understanding of the association. Second, the results are robust and reliable, supported by the absence of publication bias and minimal heterogeneity across the included studies. Third, the study’s strengths include its large sample size and representation across diverse countries, enhancing the generalizability of the findings to broader populations.
In the subgroup analyses, the associations between exercise and specific adverse neonatal outcomes varied based on study design, publication year, exercise program, and exercise mode. The association between exercise and outcomes such as cesarean delivery, preterm birth, and macrosomia was attenuated to varying degrees in multiple-component exercise programs compared to single-component programs, although statistical significance was observed only for macrosomia (P = 0.06). Among the included studies, 7 implemented multiple-component exercise interventions, while 9 employed single-component interventions. Additionally, the retrospective study by Wang et al. did not provide details about the specific types of exercise involved; however, the exercise program was categorized as multiple-component due to the systematic cluster sampling method used to recruit participants (36). None of the included studies directly compared the effects of single- versus multiple-component exercise programs. To address this gap, we analyzed the differences by dividing the studies into two subgroups. A plausible explanation for the observed variation is that single-component exercise programs may be easier for pregnant women with GDM to adhere to. This is particularly relevant as the gravid uterus can obstruct the aorta and inferior vena cava, reducing cardiac output and potentially discouraging physical activity as pregnancy progresses (42–44). However, it’s also important to consider that differences in program adherence or participant characteristics, such as baseline fitness levels, motivation, or access to resources, could also contribute to this finding. For example, participants in single-component programs might have had higher overall adherence rates or may have been more motivated due to the simpler nature of the intervention. Further investigation is needed to determine whether these factors influenced the observed trend.
Different exercise modes also exhibited varying effects, with aerobic exercise specifically reducing the incidence of adverse neonatal outcomes. This aligns with current recommendations from the American College of Obstetricians and Gynecologists (ACOG), which advocate for regular aerobic exercise during pregnancy (45). However, a study comparing the effects of resistance versus aerobic exercise on blood glucose control found that resistance exercise was more effective in reducing 2-hour postprandial blood glucose levels (14). This difference may be attributed to the distinct physiological mechanisms by which aerobic and resistance exercise impact glucose metabolism. Aerobic exercise primarily enhances insulin sensitivity and glucose uptake in skeletal muscles through increased blood flow and energy expenditure. Resistance exercise, on the other hand, promotes muscle hypertrophy and increases basal metabolic rate, leading to improved long-term glucose control and insulin sensitivity. Furthermore, resistance exercise may directly stimulate glucose disposal independent of insulin, offering a unique benefit for managing GDM. It is important to interpret these findings with caution, as there is limited evidence directly comparing the effects of these two exercise modes. Further research is needed to clarify their relative benefits for pregnant women with GDM.
There was a consistent trend indicating that exercise had a greater impact on reducing adverse neonatal outcomes in randomized controlled trials (RCTs) compared to observational studies. This is likely because, in interventional studies, exercise is implemented through structured programs with scheduled activities and timely guidance, ensuring better adherence and standardized practices (29). Additionally, the pooled effect of studies published before 2018 was weaker compared to those published in more recent years. This difference may reflect advancements in intervention strategies, with more rational and evidence-based approaches being adopted over time. Moreover, increased awareness and efforts to address perceived barriers to physical activity in pregnant women may have contributed to improved exercise compliance and outcomes in recent studies (30).
In summary, exercise during pregnancy serves as an effective intervention to reduce the incidence of various neonatal complications in women with GDM, without increasing the risk of adverse events (34, 48). The subgroup differences observed in this study suggest that simpler and more accessible forms of exercise yield greater benefits, highlighting the importance of tailoring exercise interventions to enhance adherence among pregnant women. Based on current guidelines and the findings of this review, we recommend that women with GDM engage in at least 150 minutes of moderate-intensity aerobic exercise per week, spread across at least three days. This could include activities such as brisk walking, swimming, or stationary cycling. Each session should ideally last for at least 30 minutes. Furthermore, the inclusion of resistance exercise, performed 2-3 times per week, can offer additional benefits for glucose control. However, it is crucial to emphasize the importance of professional supervision, especially for resistance exercises, to ensure proper form and technique, minimize the risk of injury, and optimize adherence. A qualified healthcare professional or certified exercise specialist can provide personalized guidance and monitor progress throughout the pregnancy. However, the potential risk of bias underscores the need for high-quality research to further clarify the association between exercise and neonatal outcomes. Future studies should focus on improving study design, ensuring broader geographical representation, and adequately controlling for confounding factors to address existing evidence gaps and provide more robust and generalizable conclusions.
This study has several limitations that should be acknowledged. First, as with all meta-analyses, the quality of the included studies inherently constrains the overall validity of the findings. While most of the RCTs included exhibited either some concerns or a high risk of bias, the low overall heterogeneity and absence of significant publication bias suggest that the results remain statistically reliable. Second, the two observational studies included in this analysis had relatively large sample sizes, which gave them greater weight in the pooled results and may have influenced the overall outcomes. However, through subgroup analyses and sensitivity testing—where individual studies were removed one by one—the pooled findings remained consistent and robust, supporting the reliability of the conclusions. Finally, the association between exercise and certain adverse neonatal outcomes was evaluated using only a limited number of studies. As a result, these findings should be interpreted with caution, and further research is required to validate these associations and strengthen the evidence base. Specifically, we acknowledge the limitations stemming from the lack of detailed information in the included studies regarding crucial aspects of the exercise interventions. We often lacked data on the specific type of exercise, duration, intensity, and the gestational trimester(s) of participation. This makes it difficult to determine the optimal exercise prescription for women with GDM. Furthermore, there was often a lack of information regarding statistical normalization for potential confounders like maternal age, pre-pregnancy BMI, or weight gain during pregnancy across the included studies. This absence of standardized reporting limits our ability to isolate the independent effect of exercise. Finally, we note that information regarding the development of adverse events, such as gestational hypertension related to exercise workload, type of exercise, or other demographics, was often missing. This prevents us from fully assessing the safety and tolerability of exercise interventions in this population. These data gaps highlight the need for future studies to incorporate more comprehensive and standardized reporting of exercise parameters, potential confounders, and adverse events.
In conclusion, this systematic review and meta-analysis demonstrated that exercise, particularly single-component exercise programs, can significantly reduce the risk of adverse neonatal outcomes in women with GDM, including macrosomia, preterm birth, cesarean delivery, fetal growth restriction, and birth trauma. These findings emphasize the substantial benefits of antenatal exercise for fetal health, underscoring its importance as a cornerstone intervention for women diagnosed with GDM. To further enhance our understanding, long-term follow-up studies are warranted to evaluate the sustained effects of exercise interventions on the health and development of offspring. However, the conclusions should be interpreted with caution, considering the limitations regarding the quality of information, the normalization of results as well as a comprehensive detailing of the exercise programs.
HC: Writing – review & editing, Writing – original draft. JH: Methodology, Writing – review & editing. HL: Methodology, Writing – review & editing. YW: Supervision, Writing – review & editing. YAW: Conceptualization, Writing – review & editing. ML: Formal Analysis, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2025.1566577/full#supplementary-material
1. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clin Res. Ed). (2021) 372:n71. doi: 10.1016/j.rec.2021.07.010
2. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clin Res. Ed). (2019) 366:l4898. doi: 10.1136/bmj.l4898
3. George AW, Garrett W, Beverly S, Beverly S, O'Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Symposium on Systematic Reviews: Beyond the Basics (2014). doi: 10.1006/bioe.2002.0137
4. Abirami P, Judie A. Integrated approach of yoga therapy on maternal and fetal outcome in gestational diabetes mellitus. Int. J. Pharm. Clin. Res. (2015) 7:377–82.
5. Artal R, Catanzaro RB, Gavard JA, Mostello DJ, Friganza JC. A lifestyle intervention of weight-gain restriction: diet and exercise in obese women with gestational diabetes mellitus. Appl. Physiol. Nutr. Metab. (2007) 32:596–601. doi: 10.1139/H07-024
6. Avery MD, Leon AS, Kopher RA. Effects of a partially home-based exercise program for women with gestational diabetes. Obstet Gynecol. (1997) 89:10–5. doi: 10.1016/S0029-7844(97)84256-1
7. Awad E, Ahmed H, Yousef A, Saab IM. Effect of antenatal exercise on mode of delivery in gestational diabetic females: A single-blind randomized controlled trial. Physiother Q. (2019) 27:1–5. doi: 10.5114/pq.2019.84270
8. Balaji PA, Varne SR. Physiological effects of yoga asanas and pranayama on metabolic parameters, maternal, and fetal outcome in gestational diabetes. NJPPP. (2017) 7:724–8. doi: 10.5455/njppp.2017.7.0306713032017
9. Barakat R, Pelaez M, Lopez C, Lucia A, Ruiz JR. Exercise during pregnancy and gestational diabetes-related adverse effects: a randomised controlled trial. Br. J. Sports Med. (2013) 47:630–6. doi: 10.1136/bjsports-2012-091788
10. Bo S, Rosato R, Ciccone G, Canil S, Gambino R, Poala CB, et al. Simple lifestyle recommendations and the outcomes of gestational diabetes. A 2 × 2 factorial randomized trial. Diabetes Obes. Metab. (2014) 16:1032–5. doi: 10.1111/dom.12289
11. de Barros MC, Lopes MA, Francisco RP, Sapienza AD, Zugaib M. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am. J. Obstet Gynecol. (2010) 203:556.e1–6. doi: 10.1016/j.ajog.2010.07.015
12. Jin Y, Chen Z, Li J, Zhang W, Feng S. Effects of the original Gymnastics for Pregnant Women program on glycaemic control and delivery outcomes in women with gestational diabetes mellitus: A randomized controlled trial. Int. J. Nurs. Stud. (2022) 132: 104271. doi: 10.1016/j.ijnurstu.2022.104271
13. Kokic IS, Ivanisevic M, Biolo G, Simunic B, Kokic T, Pisot R. Combination of a structured aerobic and resistance exercise improves glycaemic control in pregnant women diagnosed with gestational diabetes mellitus. A randomised controlled trial. Women Birth. (2018) 31:E232–E8. doi: 10.1016/j.wombi.2017.10.004
14. Kumar V, Shete S, Agarwal S, Verma A. Effects of exercise on blood glucose level, weight gain and pregnancy outcome in patients with gestational diabetes mellitus. Int. J. Pharm. Clin. Res. (2023) 15:1337–44. doi: 10.1016/j.jdiacomp.2022.108186
15. Nobles C, Marcus BH, Stanek EJ, Braun B, Whitcomb BW, Solomon CG, et al. Effect of an exercise intervention on gestational diabetes mellitus. Obstetrics Gynecology. (2015) 125:1195–204. doi: 10.1097/AOG.0000000000000738
16. Wu Y, Xu M, Zheng G. Application of diversified and quantitative management model of exercise intervention in patients with gestational diabetes mellitus. J. Matern-Fetal Neonatal Med. (2022) 35:5001–7. doi: 10.1080/14767058.2021.1874340
17. Xie Y, Zhao H, Zhao M, Huang H, Liu C, Huang F, et al. Effects of moderate-intensity aerobic exercise on blood glucose levels and pregnancy outcomes in patients with gestational diabetes mellitus: A randomized controlled trial. Diabetes Ther. (2021) 12:2585–98. doi: 10.1007/s13300-021-01135-6
18. Zhao H, Xie Y, Zhao M, Huang H, Liu C, Huang F, et al. Effects of moderate-intensity resistance exercise on blood glucose and pregnancy outcome in patients with gestational diabetes mellitus: A randomized controlled trial. J. Diabetes Complications. (2022) 36: 1056–8727. doi: 10.1016/j.jdiacomp.2022.108186
19. Snapp CA, Donaldson SK. Gestational diabetes mellitus: Physical exercise and health outcomes. Biol. Res. Nurs. (2008) 10:145–55. doi: 10.1177/1099800408323728
20. Wang C, Zhu W, Wei Y, Feng H, Su R, Yang H. Exercise intervention during pregnancy can be used to manage weight gain and improve pregnancy outcomes in women with gestational diabetes mellitus. BMC Pregnancy Childbirth. (2015) 15: 255. doi: 10.1186/s12884-015-0682-1
21. Sweeting A, Wong J, Murphy HR, Ross GP. A clinical update on gestational diabetes mellitus. Endocr. Rev. (2022) 43:763–93. doi: 10.1210/endrev/bnac003
22. Vrijkotte TG, Krukziener N, Hutten BA, Vollebregt KC, van Eijsden M, Twickler MB. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study. J. Clin. Endocrinol. Metab. (2012) 97:3917–25. doi: 10.1210/jc.2012-1295
23. Xie Y, Zhao H, Zhao M, Huang H, Liu C, Huang F, et al. Effects of resistance exercise on blood glucose level and pregnancy outcome in patients with gestational diabetes mellitus: a randomized controlled trial. BMJ Open Diabetes Res. Care. (2022) 10: e002622. doi: 10.1136/bmjdrc-2021-002622
24. Juan J, Yang H. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int. J. Environ. Res. (2020) 17: 9517. doi: 10.3390/ijerph17249517
25. Brankston GN, Mitchell BF, Ryan EA, Okun NB. Resistance exercise decreases the need for insulin in overweight women with gestational diabetes mellitus. Am. J. Obstet Gynecol. (2004) 190:188–93. doi: 10.1016/s0002-9378(03)00951-7
26. Pastorino S, Bishop T, Crozier SR, Granström C, Kordas K, Küpers LK, et al. Associations between maternal physical activity in early and late pregnancy and offspring birth size: remote federated individual level meta-analysis from eight cohort studies. BJOG. (2019) 126:459–70. doi: 10.1111/bjo.2019.126.issue-4
27. Bennett CJ, Walker RE, Blumfield ML, Ma J, Wang F, Wan Y, et al. Attenuation of maternal weight gain impacts infant birthweight: systematic review and meta-analysis. J. Dev. Orig Health Dis. (2019) 10:387–405. doi: 10.1017/S2040174418000879
28. Li X, Luo R, Qiao B, Ou H. Exercise intervention improves blood glucose levels and adverse pregnancy outcomes in GDM patients: A meta-analysis. Comput. Math Method Med. (2022) 2022:1–9. doi: 10.1155/2022/9287737
29. Wang C, Wei Y, Zhang X, Zhang Y, Xu Q, Sun Y, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet Gynecol. (2017) 216:340–51. doi: 10.1016/j.ajog.2017.01.037
Keywords: diabetes, gestational, physical activity, fetus, pregnancy outcome, meta-analysis
Citation: Cui H, Li H, Huang J, Wu Y, Wei Y and Li M (2025) The effect of exercise on the adverse neonatal outcomes related to women with gestational diabetes mellitus: a systematic review and meta-analysis. Front. Clin. Diabetes Healthc. 6:1566577. doi: 10.3389/fcdhc.2025.1566577
Received: 25 January 2025; Accepted: 12 March 2025;
Published: 01 April 2025.
Edited by:
A.Seval Ozgu-Erdinc, Ankara Bilkent City Hospital University, TürkiyeReviewed by:
Halvatsiotis Panagiotis, National and Kapodistrian University of Athens, GreeceCopyright © 2025 Cui, Li, Huang, Wu, Wei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingzi Li, bGltaW5nemlAYmptdS5lZHUuY24=; Yuan Wei, d2VpeXVhbmJ5c3lAMTYzLmNvbQ==
‡ORCID: Jing Huang, orcid.org/0000-0002-5740-1767
Yi Wu, orcid.org/0000-0001-9281-7891
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.