- Global Diabetes Institute, Diabetes Research Center, Albert Einstein College of Medicine, Bronx, NY, United States
During pregnancy, insulin resistance and impaired insulin secretion may lead to the development of Gestational Diabetes Mellitus (GDM). Although a higher Body Mass Index (BMI) is often cited as a risk factor for the development of GDM, lean pregnant women are also at risk of developing GDM based on evidence from several studies. It is proposed that insulin deficiency (more than insulin resistance) leads to the development of GDM in women with low BMI (BMI <18.5 kg/m2). Neonates of these women are more at risk of preterm birth and small-for-gestational-age. Given this unique pathophysiology and phenotype, this entity needs a modified management approach. This article aims to raise awareness of GDM in lean women to encourage more research on this topic and create a modified management approach.
Introduction
Gestational Diabetes Mellitus (GDM) is a type of diabetes with an onset or initial diagnosis during pregnancy, either due to inadequate insulin production or insulin resistance (1). During pregnancy, the placenta secretes hormones such as human placental lactogen, estrogen, progesterone, and cortisol to reduce insulin sensitivity in pregnant women and increase nutrient availability for the fetus (2). In addition, reduced pancreatic β cell mass, reduced β cell number, β cell dysfunction, or a combination of all three factors (which can be either preexistent or directly related to pregnancy, depending on the individual) may contribute to insufficient insulin secretion in pregnancy (3). Thus, insulin resistance and reduced or impaired insulin secretion in pregnancy lead to the development of GDM. Studies have identified multiple potential risk factors for GDM, with higher Body Mass Index (BMI) commonly identified as a risk factor. The association of maternal obesity with insulin resistance and GDM is well established (4, 5), but it is essential to note that there is also evidence suggesting that GDM can occur in lean pregnant women (BMI<18.5 kg/m2). Lean pregnant women may have a distinct pathophysiology responsible for the development of GDM, which warrants a modified management approach focused on managing the weight of the mother and fetus as well as achieving the glycemic targets. This article aims to raise awareness of GDM in lean women as a separate entity needing modified management and encourage further scientific research into this field to create specific guidelines for the same.
Discussion
Evidence from several studies worldwide suggests that GDM is a significant problem among lean women. In a prospective study of pregnant women attending antenatal clinics in the Jammu region of India, the prevalence of GDM in underweight women (BMI <18.5 kg/m2) was 3% (6). Yong et al. conducted a retrospective cohort study in 1951 pregnant women registered in the antenatal clinics of Seremban district, Malaysia, to identify the effect of BMI in the first antenatal visit on the risk of GDM. It is interesting to note that in this study, the adjusted Odds Ratio of GDM among pregnant women with BMI <18.5 kg/m2 was 0.78 with a confidence interval between 0.47 and 1.31 – thus, even though the overall Odds ratio was <1, the variability within the confidence interval indicates that some women with lower BMI had a higher risk of GDM. Consistent with this intriguing finding, a few studies showed evidence of comparable or even higher risk of GDM in lean women relative to women with normal or higher BMI. Yachi et al. conducted a study to examine the association of BMI at 20 years of age with subsequent risk of GDM in pregnant Japanese women. They found that women who had been lean (with BMI<18 kg/m2) at 20 years of age had a 6.3-fold (2.26-17.59) increased risk of developing GDM relative to women with normal BMI at 20 years of age (7). In another study by Inoue et al., in 30 lean Japanese women, nine women (30%) were diagnosed as having GDM (8). A study conducted in Rwanda found that underweight non-diabetic women had 1-hour and 2-hour blood glucose levels comparable to overweight women and higher than women with normal BMI, consistent with the findings in Japan (9). Furthermore, although these studies reported a lower overall prevalence of GDM in lean women, this could still translate into a significant health problem globally, given the absolute number of women at risk of GDM.
The pathophysiology of GDM among lean women
GDM in lean women may be more related to insulin deficiency than to insulin resistance. Furukawa and Kobayashi examined the correlation between leanness and impaired insulin secretion in pregnant Japanese women. They divided the GDM women in their study into two subgroups based on HOMA-IR (Homeostasis Model Assessment Insulin Resistance): LIR (Low insulin resistance) and HIR (High insulin resistance). After a 75-gram OGTT test, plasma glucose concentrations at 60 and 120 minutes did not differ significantly in the LIR group. In contrast, in the HIR group, the post-glucose load glucose levels were significantly lower at 120 minutes compared to 60-minute levels. The persistent high glucose levels in the LIR group were suggestive of impaired insulin secretion (10). The study found a positive linear correlation between BMI and HOMA-β (Homeostasis model assessment of β-cell function) in women with GDM (10). Also, the authors reported that the HOMA-β of the LIR group was significantly lower than the HOMA-β of the HIR group (10). Based on these findings, they concluded that impaired insulin secretion was strongly related to the onset of GDM in women from the LIR group (10). Additionally, they reported that as the lean Japanese women progressed to worsening glucose tolerance, their insulin secretion was more abnormal relative to insulin resistance. Thus, there may be two distinct causes of GDM in pregnant women at the two extremes of BMI: insulin resistance in overweight and obese women and impaired insulin secretion in lean women (10). Also, Inoue et al. suggested a similar mechanism for the development of GDM in lean Japanese women with BMI < 18.5 kg/m2, in whom islet cell antibodies were not detectable (8). The absence of antibodies in these women would be helpful to rule out type 1 DM.
Diabetes in people with low BMI
The growing body of literature on the metabolic characteristics of diabetes mellitus (DM) among people with low BMI could also help to shed light on the pathophysiology of GDM in lean women. To understand the metabolic characteristics of lean individuals with DM, Lontchi-Yimagou et al. conducted extensive metabolic studies in Vellore, India (11). Participants with low BMI and DM (in whom type 1 DM autoantibodies positive and monogenic forms of diabetes were carefully excluded) were compared to participants with type 1 DM, type 2 DM, and BMI-matched individuals without DM. Investigators used anthropometric measurements, hyperinsulinemic-euglycemic clamp studies (by maintaining the plasma glucose at ~90mg/dl and titrating optimal insulin infusion rates), and a standard Mixed-Meal Tolerance Test (MMTT) (to measure glucose, insulin, glucagon, and C-peptide levels after a standard meal followed by overnight fasting) to carefully phenotype these individuals. The researchers determined that the insulin secretory response in lean individuals with DM was markedly impaired; indeed, it was substantially lower than in subjects with type 2 DM, though higher than in subjects with type 1 DM. The indices for insulin resistance showed lower insulin resistance in lean DM participants compared to those with type 2 DM, but it did not differ significantly in type 1 DM patients (11). Consistent with these findings, Kibirige et al. used HOMA-IR measures to determine the metabolic characteristics of 568 Ugandan adults with newly diagnosed DM. The authors reported that leaner participants had significantly lower pancreatic β cell function than non-lean participants but did not demonstrate substantial insulin resistance (12). There have been dozens of articles (including India, Ethiopia, Japan, and Uganda) over many decades attesting to the persistent presence of diabetes among lean populations from different countries (13–23). Additionally, a recent meta-analysis based on nationally representative surveys that together totaled more than 0.5 million participants estimated the prevalence of low BMI diabetes to be 15 million in 29 low and middle-income countries (24) (Derived from the data as shown in Figure 1). Given this large projected number of people with low BMI diabetes, it is crucial to focus on this group of patients and dedicate further attention and research to this topic. This would help in understanding why lean women can also be susceptible to GDM.
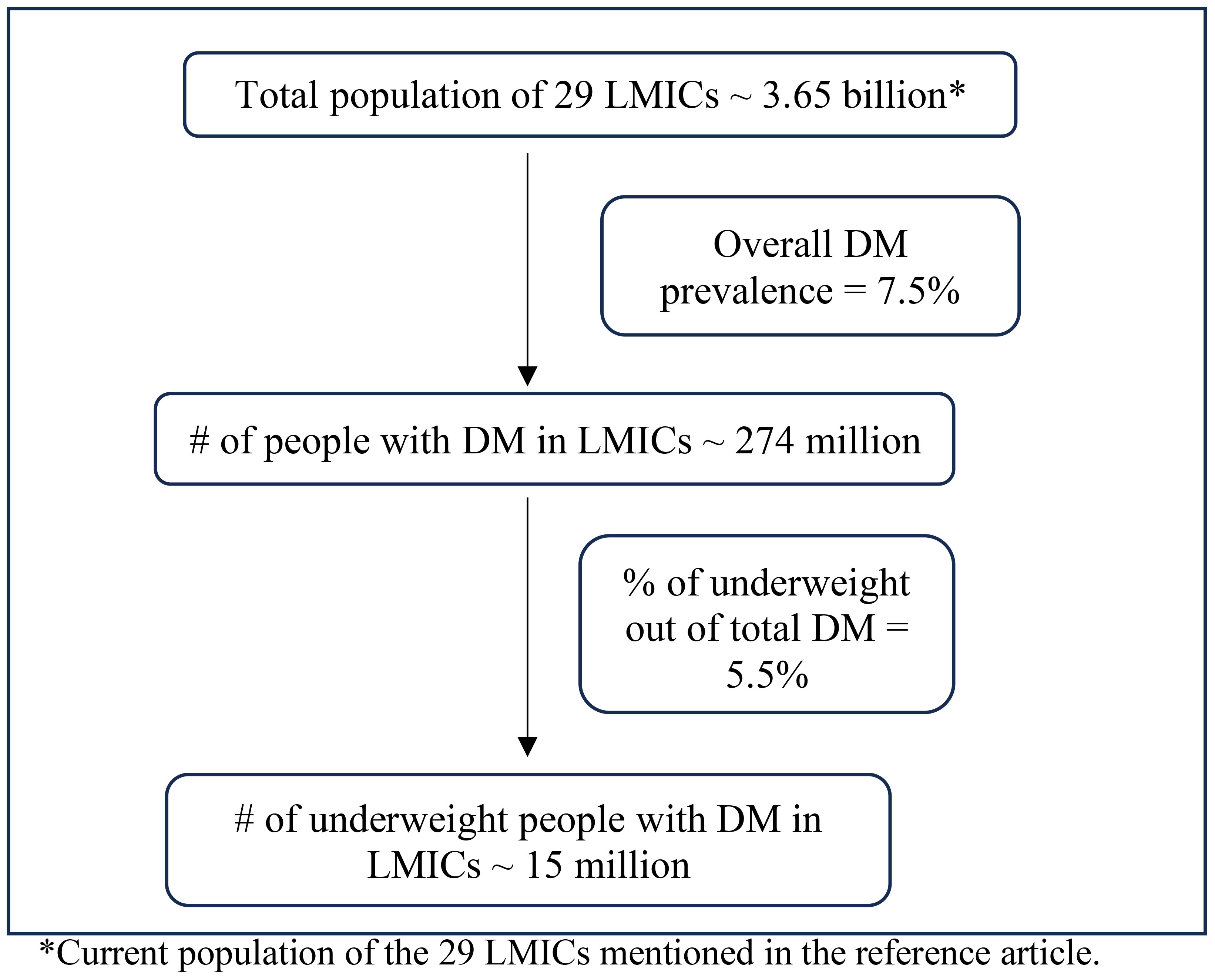
Figure 1 Prevalence of low BMI diabetes in low-middle-income countries. *Current population of the 29 LMICs mentioned in the reference article.
Postpartum risk of diabetes
Type 1 DM
Approximately 10% of women with GDM show autoantibody positivity during pregnancy or even for the first time after delivery (25). Clinically significant antibody levels (varying as per the ethnicity of the woman and type 1 DM risk in the background population) during pregnancy and postpartum can predict the development of type 1 DM postpartum (26, 27). The risk increases in women aged ≤ 30 years, those with more than two previous pregnancies, and those who required insulin during pregnancy (28, 29). Such women are at high risk of developing type 1 DM during pregnancy or postpartum or may develop a few years after delivery and evolve into Latent Autoimmune Diabetes of Adulthood (LADA) (25). So, women with GDM from high-risk populations should be screened for antibodies during pregnancy and followed up after delivery for early diagnosis of type 1 diabetes (30). It is reported that combined screening with three antibody markers [glutamic acid decarboxylase antibodies (GADA), islet cell antibodies (ICA), and antibodies to tyrosine phosphatase ICA512/IA-2 (IA2As)] increases the sensitivity to 82% compared to screening with only one or two antibodies (30).
Type 2 DM
Along with a progressive increase in the risk of type 2 DM in women with high BMI, intriguingly, lean women (with BMI<18 kg/m2) also show a relatively higher incidence of development of type 2 DM than women with normal BMI (31). Previous studies have reported that women with GDM have a ten times higher risk of developing type 2 DM than women without a history of GDM (32). The reason for the development of DM in these women is not yet fully understood; however, it is suggested to be due to pancreatic β-cell dysfunction in the presence of preexisting insulin resistance, unmasked during pregnancy (33).
It should be noted that most of the studies examining the association of GDM with the risk of type 1 or type 2 DM have focused on women with normal or high BMI rather than on lean women with GDM. So, it is crucial to conduct extensive longitudinal studies, potentially including such measures as fasting and postprandial glucose levels, C-peptide levels, and HOMA-IR to assess the risk in the latter population for early diagnosis and management.
Complications and management
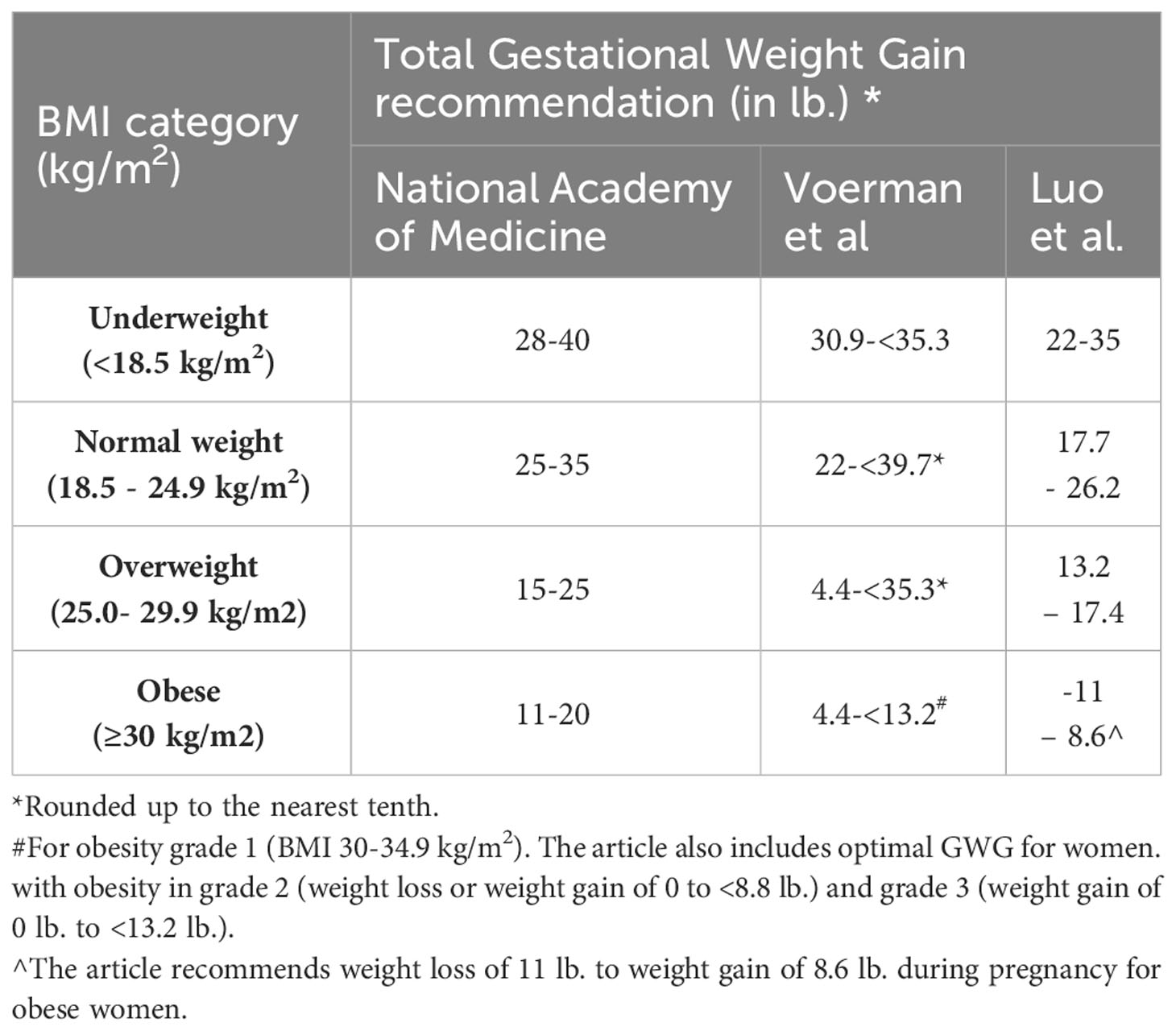
Given the unique clinical, epidemiological, and pathophysiological features of lean DM, the current treatment guidelines for overweight/obese women with GDM may not be helpful (or even potentially harmful) to lean women with GDM. For example, while large-for-gestational-age neonates are considered to be the most common adverse outcome in women with GDM, lean women with GDM are five times more likely to have small-for-gestational-age (SGA) neonates than normal or overweight women with GDM (34). Intrauterine protein deficiency has been clearly established to contribute to low birth weight and other neonatal development issues, as has been confirmed by many studies in animals (35). In addition, recommendations from Scandinavia have proposed that adequate protein intake is essential for the appropriate development of both fetal and maternal tissues during pregnancy (36). Management guidelines focused on carbohydrate and calorie limitation may further increase SGA and low birth weight. These conditions have long-term metabolic consequences for the neonate. Additionally, metformin, which is commonly used as a first-line pharmacologic therapy in GDM and increases maternal insulin sensitivity, may have limited efficacy in women whose primary deficit is insulin deficiency. Finally, insulin therapy may be dangerous in lean women because of food insecurity. Despite this, there is limited literature and recommendations focused on the specific management of GDM in lean women. Although the National Academy of Medicine (previously Institute of Medicine) (37), Voerman, et al. (38), and Luo et al. (39) do recommend greater gestational weight gain in underweight women than in women with normal/overweight/obese BMI (as shown in Table 1), additional research is needed to determine optimal protein and calorie intake for underweight women with GDM (40) as well as strategies to prevent postpartum diabetes in these individuals.
Conclusion
To conclude, although the prevalence of GDM among lean women is generally lower than in overweight and obese women, it is still a crucial area of study, given the high numbers of pregnancies among lean women globally. Indeed, lean pregnant women and their offspring face a unique set of challenges, including a higher risk of postpartum diabetes, as well as small-for-gestational-age neonates and preterm births. Thus, following the current guidelines might be detrimental to fetal outcomes, suggesting they may require specialized approaches to managing their GDM.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
MH: Writing – review & editing. PW: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases (DK069861) and the Einstein-Mount Sinai Diabetes Research Center (5P30DK020541-41).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Centers for Disease Control and Prevention. Centers for disease control and prevention, in: Gestational Diabetes (2022). Available online at: https://www.cdc.gov/diabetes/basics/gestational.html (Accessed 2023 Oct 9).
2. Dinh Le T, Minh Bui T, Hien Vu T, Phi Thi Nguyen N, Thanh Thi Tran H, Nguyen ST, et al. Insulin resistance in gestational diabetes mellitus and its association with anthropometric fetal indices. Clin. Med. Insights Endocrinol. Diabetes. (2022) 15. doi: 10.1177/11795514221098403
3. Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. (2018) 19. doi: 10.3390/ijms19113342
4. Zhang S, Liu H, Li N, Dong W, Li W, Wang L, et al. Relationship between gestational body mass index change and the risk of gestational diabetes mellitus: a community-based retrospective study of 41,845 pregnant women. BMC Pregnancy Childbirth. (2022) 22. doi: 10.1186/s12884-022-04672-5
5. Yong HY, Mohd Shariff Z, Mohd Yusof BN, Rejali Z, Tee YYS, Bindels J, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci. Rep. (2020) 10. doi: 10.1038/s41598-020-65251-2
6. Wahi P, Dogra V, Jandial K, Bhagat R, Gupta R, Gupta S, et al. Prevalence of gestational diabetes mellitus (GDM) and its outcomes in Jammu region. J. Assoc. Physicians India. (2011) 59:227–30.
7. Yachi Y, Tanaka Y, Nishibata I, Sugawara A, Kodama S, Saito K, et al. Low BMI at age 20years predicts gestational diabetes independent of BMI in early pregnancy in Japan: Tanaka Women’s Clinic Study. Diabetic Med. (2013) 30:70–3. doi: 10.1111/j.1464-5491.2012.03712.x
8. Inoue S, Kozuma Y, Miyahara M, Yoshizato T, Tajiri Y, Hori D, et al. Pathophysiology of gestational diabetes mellitus in lean Japanese pregnant women in relation to insulin secretion or insulin resistance. Diabetol. Int. (2020) 11:269–73. doi: 10.1007/s13340-020-00425-x
9. Meharry PM, Tengera O, Rulisa S, Byambu AK, Nietert PJ, Byiringiro S, et al. Prevalence of gestational diabetes mellitus among women attending antenatal care at public health centers in Rwanda. Diabetes Res. Clin. Pract. (2019) 151:252–9. doi: 10.1016/j.diabres.2019.03.035
10. Furukawa S, Kobayashi Y. Leaner women with impaired insulin secretion accounts for about 40% of gestational diabetes mellitus in Japan. J. Pregnancy. (2019) 2019. doi: 10.1155/2019/7578403
11. Lontchi-Yimagou E, Dasgupta R, Anoop S, Kehlenbrink S, Koppaka S, Goyal A, et al. An atypical form of diabetes among individuals with low BMI. Diabetes Care. (2022) 45:1428–37. doi: 10.2337/dc21-1957
12. Kibirige D, Sekitoleko I, Lumu W, Jones AG, Hattersley AT, Smeeth L, et al. Understanding the pathogenesis of lean non-autoimmune diabetes in an African population with newly diagnosed diabetes. Diabetologia. (2022) 65:675–83. doi: 10.1007/s00125-021-05644-8
13. George AM, Jacob AG, Fogelfeld L. Lean diabetes mellitus: An emerging entity in the era of obesity. World J. Diabetes. (2015) 6:613. doi: 10.4239/wjd.v6.i4.613
14. Hugh-jones PM. Diabetes in Jamaica. Lancet. (1955) 269:891–7. doi: 10.1016/S0140-6736(55)92530-7
15. Akanji AO. Malnutrition-related diabetes mellitus in young adult diabetic patients attending a Nigerian diabetic clinic. Am. J. Trop. Med. Hyg. (1990) 93:35–8.
16. Maiti S, Sinha NK, Khan MM, Das PK, Chattopadhyay JC. Diabetes in rural individuals of different nutritional status and the alarming situation demands focus more on its under-nutrition association. Arch. Physiol. Biochem. (2015) 121:26–31. doi: 10.3109/13813455.2014.959973
17. Tripathy BB, Samal KC. Protein deficient diabetes mellitus (PDDM) in India. Int. J. Diab. Dev. Countries. (1993) 13:3–13. doi: https://rssdi.in/newwebsite/journal/1993_jan-june/article1.pdf.
18. Samal KC, Kanungo A, Sanjeevi CB. Clinicoepidemiological and biochemical profile of malnutrition-modulated diabetes mellitus. Ann. NY Acad. Sci. (2002) 958:131–7. doi: 10.1111/j.1749-6632.2002.tb02955.x
19. Bajaj JS. Diabetes and malnutrition. World Health. (1988), 22–3. Available at: https://iris.who.int/bitstream/handle/10665/53092/WH-1988-Oct-p22-23-eng.pdf?isAllowed=y&sequence=1.
20. Balasubramanyam A, Yajnik CS, Tandon N. Non-traditional forms of diabetes worldwide: implications for translational investigation. Translational Endocriology & Metabolism (2011) 2(1):43–67. doi: 10.1210/TEAM.9781879225824.ch2
21. Tripathy BB, Kar BC. Observations on clinical patterns of diabetes mellitus in India. Diabetes. (1965) 14:404–12. doi: 10.2337/diab.14.7.404
22. Alemu S, Dessie A, Seid E, Bard E, Lee PT, Trimble ER, et al. Insulin-requiring diabetes in rural Ethiopia: Should we reopen the case for malnutrition-related diabetes? Diabetologia. (2009) 52:1842–5. doi: 10.1007/s00125-009-1433-5
23. Huh KB, Lee HC, Kim HM, Cho YW, Kim YL, Lee KW, et al. Immunogenetic and nutritional profile in insulin-using youth-onset diabetics in Korea. Diabetes Res. Clin. Pract. (1992) 16:63–70. doi: 10.1016/0168-8227(92)90136-F
24. Seiglie JA, Marcus ME, Ebert C, Prodromidis N, Geldsetzer P, Theilmann M, et al. Diabetes prevalence and its relationship with education, wealth, and BMI in 29 low- And middle-income countries. Diabetes Care. (2020) 43:767–75. doi: 10.2337/dc19-1782
25. Lapolla A, Dalfrà MG, Fedele D. Diabetes related autoimmunity in gestational diabetes mellitus: Is it important? Nutrition Metab. Cardiovasc. Dis. (2009) 19:674–82. doi: 10.1016/j.numecd.2009.04.004
26. Beunen K, Vercauter L, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, et al. Type 1 diabetes-related autoimmune antibodies in women with gestational diabetes mellitus and the long-term risk for glucose intolerance. Front. Endocrinol. (Lausanne). (2022) 13. doi: 10.3389/fendo.2022.973820
27. Fujishima A, Onodera Y, Miura H, Terada Y. Anti-glutamic acid decarboxylase antibody-positive gestational diabetes mellitus with autoimmune type 1 diabetes mellitus in the early postpartum period: A case report and literature review. Tohoku J. Exp. Med. (2023) 259:327–33. doi: 10.1620/tjem.2023.J013
28. Löbner K, Knopff A, Baumgarten A, Mollenhauer U, Marienfeld S, Garrido-Franco M, et al. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes. (2006) 55:792–7. doi: 10.2337/diabetes.55.03.06.db05-0746
29. Järvelä IY, Juutinen J, Koskela P, Hartikainen AL, Kulmala P, Knip M, et al. Gestational Diabetes Identifies Women at Risk for Permanent Type 1 and Type 2 Diabetes in Fertile Age Predictive role of autoantibodies. Diabetes Care. (2006) 29:607–12. doi: 10.2337/diacare.29.03.06.dc05-1118
30. Fiichtenbusch M, Ferber K, Standl E, Ziegler AG. Prediction of type 1 diabetes postpartum in patients with gestational diabetes mellitus by combined islet cell autoantibody screening: A prospective multicenter study. Diabetes. (1997) 46:1459–67. doi: 10.2337/diabetes.46.9.1459
31. Perämäki R, Gissler M, Ollila MM, Hukkanen J, Vääräsmäki M, Uotila J, et al. The risk of developing type 2 diabetes after gestational diabetes: A registry study from Finland. Diabetes Epidemiol. Management. (2023) 10. doi: 10.1016/j.deman.2022.100124
32. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ. (2020) 369. doi: 10.1136/bmj.m1361
33. Diaz-Santana MV, O’brien KM, Park YMM, Sandler DP, Weinberg CR. Persistence of risk for type 2 diabetes after gestational diabetes mellitus. Diabetes Care. (2022) 45:864–70. doi: 10.2337/dc21-1430
34. Kim M, Hur KY, Choi SJ, Oh SY, Roh CR. Influence of pre-pregnancy underweight body mass index on fetal abdominal circumference, estimated weight, and pregnancy outcomes in gestational diabetes mellitus. Diabetes Metab. J. (2022) 46:499–505. doi: 10.4093/dmj.2021.0059
35. Alćazar MAA, Dinger K, Rother E, Östreicher I, Vohlen C, Plank C, et al. Prevention of early postnatal hyperalimentation protects against activation of transforming growth factor-β/bone morphogenetic protein and interleukin-6 signaling in rat lungs after intrauterine growth restriction. J. Nutr. (2014) 144:1943–51. doi: 10.3945/jn.114.197657
36. Nordic Council of Ministers 2014. Integrating nutrition and physical activity. In: Nordic Nutrition Recommendations 2012. Copenhagen: Nordic Council of Ministers, 2014. (2014). p. 627–7. Available at: https://scholar.google.com/scholar_lookup?title=Nordic+Nutrition+Recommendations+2012&publication_year=2014&.
37. Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines INSTITUTE OF MEDICINE (2009). Available online at: www.iom.edu/pregnancyweightgain.
38. Voerman E, Santos S, Inskip H, Amiano P, Barros H, Charles MA, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. (2019) 321:1702–15. doi: 10.1001/jama.2019.3820
39. Luo X, Gao J, He Z, Ji J, Zhang W, Wu P, et al. What is an appropriate gestational weight gain for women with gestational diabetes mellitus: based on the adverse pregnancy outcomes of over 12 thousand participants? Diabetol. Metab. Syndr. (2022) 14. doi: 10.1186/s13098-022-00940-8
Keywords: gestational diabetes, lean women, BMI, insulin secretion, GDM, insulin deficiency
Citation: Wadivkar P and Hawkins M (2024) Is gestational diabetes mellitus in lean women a distinct entity warranting a modified management approach? Front. Clin. Diabetes Healthc. 5:1338597. doi: 10.3389/fcdhc.2024.1338597
Received: 14 November 2023; Accepted: 02 April 2024;
Published: 07 May 2024.
Edited by:
Jyoti Mathad, Cornell University, United StatesReviewed by:
Shira B. Eytan, Md face, New York University, United StatesCopyright © 2024 Wadivkar and Hawkins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meredith Hawkins, bWVyZWRpdGguaGF3a2luc0BlaW5zdGVpbm1lZC5lZHU=
 Pradnyashree Wadivkar
Pradnyashree Wadivkar Meredith Hawkins*
Meredith Hawkins*