
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Clin. Diabetes Healthc. , 30 January 2024
Sec. Diabetes Nephropathy
Volume 5 - 2024 | https://doi.org/10.3389/fcdhc.2024.1336896
This article is part of the Research Topic Pathophysiology of Diabetic Kidney Disease View all 5 articles
Introduction: Kidney transplantation is associated with an increased risk of posttransplant diabetes mellitus (PTDM), impacting recipient and graft survivals. The incidence of PTDM ranges from 15% to 30%, with most cases occurring in the first year post-transplant. Some clinical and laboratory characteristics pre- and post-transplant may be associated with a higher PTDM incidence in a more extended follow-up period. This study aimed to analyze the prevalence of PTDM among renal transplant recipients without previous DM diagnosis during a five-year post-transplant follow-up, as well as clinical and laboratory characteristics associated with a higher incidence of PTDM during this period.
Material and methods: Single-center retrospective cohort including kidney transplant recipients older than 18 years with a functioning graft over six months of follow-up between January and December 2018. Exclusion criteria were recipients younger than 18 years at kidney transplantation, previous diabetes mellitus diagnosis, and death with a functioning graft or graft failure within six months post-transplant.
Results: From 117 kidney transplants performed during the period, 71 (60.7%) fulfilled the inclusion criteria, 18 (25.3%) had PTDM diagnosis, and most (n=16, 88.9%) during the 1st year post-transplant. The need for insulin therapy during the hospital stay was significantly higher in the PTDM group (n=11, 61.1% vs. n=14, 26.4%, PTDM vs. non-PTDM). Other PTDM risk factors, such as older age, high body mass index, HLA mismatches, and cytomegalovirus or hepatitis C virus infections, were not associated with PTDM occurrence in this series. During 5-year post-transplant follow-up, the graft function remained stable in both groups.
Conclusion: The accumulated incidence of PTDM in this series was similar to the reported in other studies. The perioperative hyperglycemia with the need for treatment with insulin before hospital discharge was associated with PTDM.
Kidney transplant improves long-term outcomes and survival of chronic kidney disease patients compared with dialysis (1). However, kidney transplant is associated with an increased risk of posttransplant diabetes mellitus (PTDM), which potentially impacts patient and graft survivals and healthcare costs (2–5). PTDM is characterized as a new diabetes mellitus (DM) diagnosed after organ transplantation in patients on a stable maintenance immunosuppressive regimen and absence of acute infection (6). The PTDM incidence ranges from 15% to 30% during the first year, varying according to the study design, diagnostic criteria, follow-up period, risk factors, and immunosuppressive therapy (7). After, the annual incidence of PTDM is around 6% per year, like patients on the waiting list (8). Diagnostic criteria are based on the World Health Organization (WHO) that specify fasting plasma glucose (FPG) of ≥126 mg/dl (7 mmol/L), two-hour glucose after a 75g oral glucose tolerance test (OGTT) ≥ 200 mg/dL (11.1 mmol/L), or symptomatic hyperglycemia with random plasma glucose of ≥ 200 mg/dl (11,1 mmol/L) (9). However, glycated hemoglobin testing (HbA1c) is not recommended for diagnosis during the first three months after a kidney transplant because of the risk of false low levels due to anemia caused by operative blood loss and reduced red cell production secondary to chronic kidney disease (CKD) (9, 10). According to the American Diabetes Association (ADA), if a patient has discordant results from two different tests, the one that presents an abnormality must be repeated (11).
The etiology of PTDM is multifactorial, including general nonmodifiable and modifiable risk factors like the immunocompetent population and some specific conditions associated with the transplantation. PTDM shares many characteristics with type 2 diabetes mellitus (T2DM), such as impaired insulin release and impaired suppression of glucagon release (12). Predisposing PTDM factors common to T2DM include non-Caucasian ethnicity, age over 40, family history of DM, and central obesity (12). These conditions are also associated with the upregulation of pro-inflammatory pathways, with a higher tumor necrosis factor (TNF) expression, aggravating metabolic dysfunction (12). Genetic PTDM susceptibility has been reported, with single nucleotide polymorphisms in genes that encode proteins involved in β-cell apoptosis, ATP-sensitive potassium channels, adiponectin, and leptin (13). The presence of specific human leukocyte antigens (HLA) such as HLA A30, B27, and B42 may also be related (14). Data on HLA antigens associated with PTDM in Brazilian kidney transplant recipients are scarce, although previous studies in this population have identified HLADR13 as a possible PTDM risk factor (4). Central obesity is strongly associated with PTDM, and patients presenting as overweight or obese on the waiting list can be at a higher PTDM risk. The weight gain after transplantation caused by an improvement of a previous uremic condition can also be associated with the PTDM occurrence (12).
Specific risk factors for PTDM include immunosuppressive drugs, acute rejection, cytomegalovirus (CMV) infection, and hepatitis C virus (HCV) infection (3, 7, 14). The incidence of PTDM shows a biphasic curve, with the first peak occurring in the first few months after transplantation, followed by a second peak over 2-3 years post-transplant (12). The first peak is likely associated with the effect of the immunosuppressive treatment in predisposed recipients. The second peak is usually related to recipients’ age and the evolution of classic risk factors for DM combined with specific risk factors related to organ transplantation (12). Recipients of kidney transplantation are exposed to higher doses of steroids during induction of immunosuppression at surgery, followed by oral steroid treatment in tapering doses. Steroids induce peripheral insulin resistance and inhibit the pancreatic insulin production (14). Therefore, in patients at high risk for PTDM, strategies with steroid minimization have been used according to their immunological risk (15, 16). Calcineurin inhibitors (CNI) and mammalian target of rapamycin (mTOR) inhibitors, commonly used for maintenance immunosuppression, are associated with increased insulin resistance, decreased insulin release through direct toxicity to pancreatic beta cells, and impaired insulin-mediated suppression of hepatic glucose production (4, 5, 17–20). Acute rejection episodes can increase the level of insulin antagonist (21), and the drugs used for treating these events can also impact glucose metabolism.
Although the highest incidence of PTDM previously described in the literature occurs in the first year post-transplant, some clinical and laboratory characteristics pre- and post-transplant may also be associated with a higher PTDM incidence in a more extended follow-up period. The primary outcome of this study was to analyze the prevalence of PTDM among renal transplant recipients without previous DM diagnosis during a five-year post-transplant follow-up. Secondary endpoints were clinical and laboratory characteristics associated with a higher incidence of PTDM during this period.
Single-center retrospective cohort including kidney transplant recipients older than 18 years with a functioning graft over six months of follow-up between January and December 2018. Recipients younger than 18 years at kidney transplantation and those with previous DM diagnosis were excluded. The cases of death with a functioning graft or graft failure in the six months post-transplant were also excluded. The University of Campinas Ethics Committee approved the study (CAAE 70994523.0.0000.5404).
Patients diagnosed with stage 5 CKD, with an estimated glomerular filtration rate ≤ 10 ml/min/1.73m2 or under renal replacement therapy, were evaluated on the kidney transplant waiting list. These patients were investigated for general DM risk factors, such as previous DM diagnosis, current or prior use of hypoglycemic medications or insulin, previous viral infections, and body mass index. Laboratory pre-transplant assessment included FPG, associated with OGTT in some cases. All kidney transplant recipients were submitted to HLA typing and screening of anti-HLA antibodies with solid-phase tests, according to the methodology previously described (22). All kidney transplant recipients presented negative consecutive T and B cell complement-dependent cytotoxicity (CDC) crossmatches at transplant. Induction of immunosuppression consisted of monoclonal interleukin-2 receptor antibody or IV anti-thymocyte globulin 3 mg/kg in cases of standard kidney donors (23) and recipients with panel reactive antibody (PRA) lower than 30% and absence of donor-specific anti-HLA antibodies (DSA). For recipients from expanded criteria deceased donors (23), recipients with PRA > 50% or preformed DSA, and cold ischemia time higher than 24 hours, induction of immunosuppression consisted of pre-transplant IV anti-thymocyte globulin 4.5 to 6 mg/kg. All patients received IV methylprednisolone 500 mg on the day of transplant. Steroids were tapered to IV methylprednisolone 250 mg on the 1st day post-transplant, 125 mg on the 2nd day post-transplant, and oral prednisone 20 mg/day thereafter, with a progressive reduction to achieve a maintenance dose of 5-10mg/day within the 3rd month post-transplant. Furthermore, an ICN (tacrolimus or cyclosporine) was associated with an antiproliferative agent (mycophenolate or azathioprine). The patient’s weight guided the initial dose, then the target level for tacrolimus was 5-10 ng/mL and 800 – 1,000 ng/mL for cyclosporine after 2 hours. Immunosuppression was reduced or withdrawn in opportunistic infections depending on the etiological agent, the patient’s clinical status, and immunological risk.
After transplantation, patients had their blood glucose strictly monitored by measuring capillary blood glucose at least every six hours or whenever necessary. All recipients had the FPG test performed daily during their hospital stay. Patients who presented hyperglycemia during their hospital stay received subcutaneous regular insulin, with dose adjustment according to the measured capillary blood glucose values. In the case of persistent hyperglycemia, treatment with NPH (neutral protamine Hagedorn) insulin was administered in one or two daily subcutaneous applications. After hospital discharge, recipients' clinical and laboratory parameters were evaluated at least monthly, with drug dose adjustments according to their clinical conditions. Among the laboratory parameters routinely evaluated, FPG was performed in all assessments. The HbA1c was performed after the 3rd month post-transplant and repeated every six months. Patients who presented high blood glucose levels in the FPG had a faster reduction in steroid and ICN doses, according to the immunological risk presented. Switching from tacrolimus to cyclosporine was also considered for persistently high values despite other measures. Kidney transplant recipients receiving stable immunosuppressive therapy and more than three months of post-transplant follow-up were diagnosed with PTDM according to the WHO criteria. Patients with an increase in serum creatinine higher than 20% from baseline level or new-onset proteinuria were considered suspected for acute rejection. For these cases, a solid-phase anti-HLA antibody screening was made for detecting donor-specific antibodies (DSA) and a percutaneous graft biopsy, with confirmation of rejection according to Banff 2013 criteria (24). Acute T-cell mediated rejection were treated with IV methylprednisolone 500 mg during three consecutive days or anti-thymocyte globulin 6 mg/kg. Acute antibody-mediated rejection treatment consisted of 5 sessions of plasmapheresis associated with IV intravenous immunoglobulin 2g/kg.
Clinical and laboratory data were retrospectively collected from medical records. Clinical data included the cause of CKD, modality and length of dialysis, donor and receptor characteristics, cold ischemia time, HLA mismatches, hospital length of stay, delayed graft function, and immunosuppressive therapy. Laboratory data included immunological status for hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), cytomegalovirus (CMV), mononucleosis, toxoplasmosis, syphilis, and Chagas disease; serum creatinine (mg/dL), proteinuria (urinary protein-to-creatinine ratio, UPCR), fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), and pre-transplant oral glucose tolerance test (OGTT), if available. Furthermore, the incidence of opportunistic infections, including cytomegalovirus, polyomavirus, bacterial and fungal infections, was evaluated. Clinical and laboratory data were collected during hospital discharge and 1st, 3rd, and every sixth month until five years post-transplant.
For analysis, recipients were divided into two groups, according to the PTDM diagnosis: PTDM and non-PTDM. Statistical analysis was performed using the GraphPad Prisma 9.5.1 program (La Jolla CA, USA), with an unpaired t-test for parametric continuous variables and a Chi-square test for categorical variables. Statistical significance was considered at a value of p < 0.05. PTDM prevalence curve was generated using Kaplan-Meier’s model.
From 117 kidney transplants performed between January and December 2018, 71 (60.7%) fulfilled the inclusion criteria. Forty-six patients were excluded, being 23 (50%) with previous DM diagnosis, 8 (17%) younger than 18 years, 7 (15%) deaths before six months post-transplant, 6 (13%) graft failure before six months post-transplant, and 2 (4%) loss of follow-up. The patients were distributed into two groups according to the PTDM diagnosis (Figure 1). Most of the included patients were male (n=42, 59.1%), with a mean age of 46.3 ± 11.7 years. The main CKD etiology was unknown (n=20, 28.3%), followed by hypertensive nephrosclerosis (n=17, 23.9%) and autosomal dominant polycystic kidney disease (n=9, 12.7%). None of the included patients presented chronic HBV infection, 4.2% presented serologic HCV infection, and there was one case of HIV infection. Four (5.6%) recipients presented CMV infection susceptibility (IgG and IgM negatives at transplantation). All the included patients received a kidney from a deceased donor, with a mean age of 42.3 ± 12.0 years and a mean serum creatinine of 1.73 ± 1.77 mg/dL. Thirty-four (47.9%) donors were classified as expanded criteria. Most patients receive induction of immunosuppression with anti-thymocyte globulin (n=68, 95.8%), with doses ranging from 3.0 mg/kg to 6.0 mg/kg, according to the recipient’s immunological risk assessment and donor’s characteristics. In all cases, the initial immunosuppressive therapy includes tacrolimus, associated with an antiproliferative drug (mycophenolate sodium or azathioprine). The mean cold ischemia time was 19.2 ± 4.7 hours, with a delayed graft function (DGF) rate of 35.2% in the general population (Table 1).
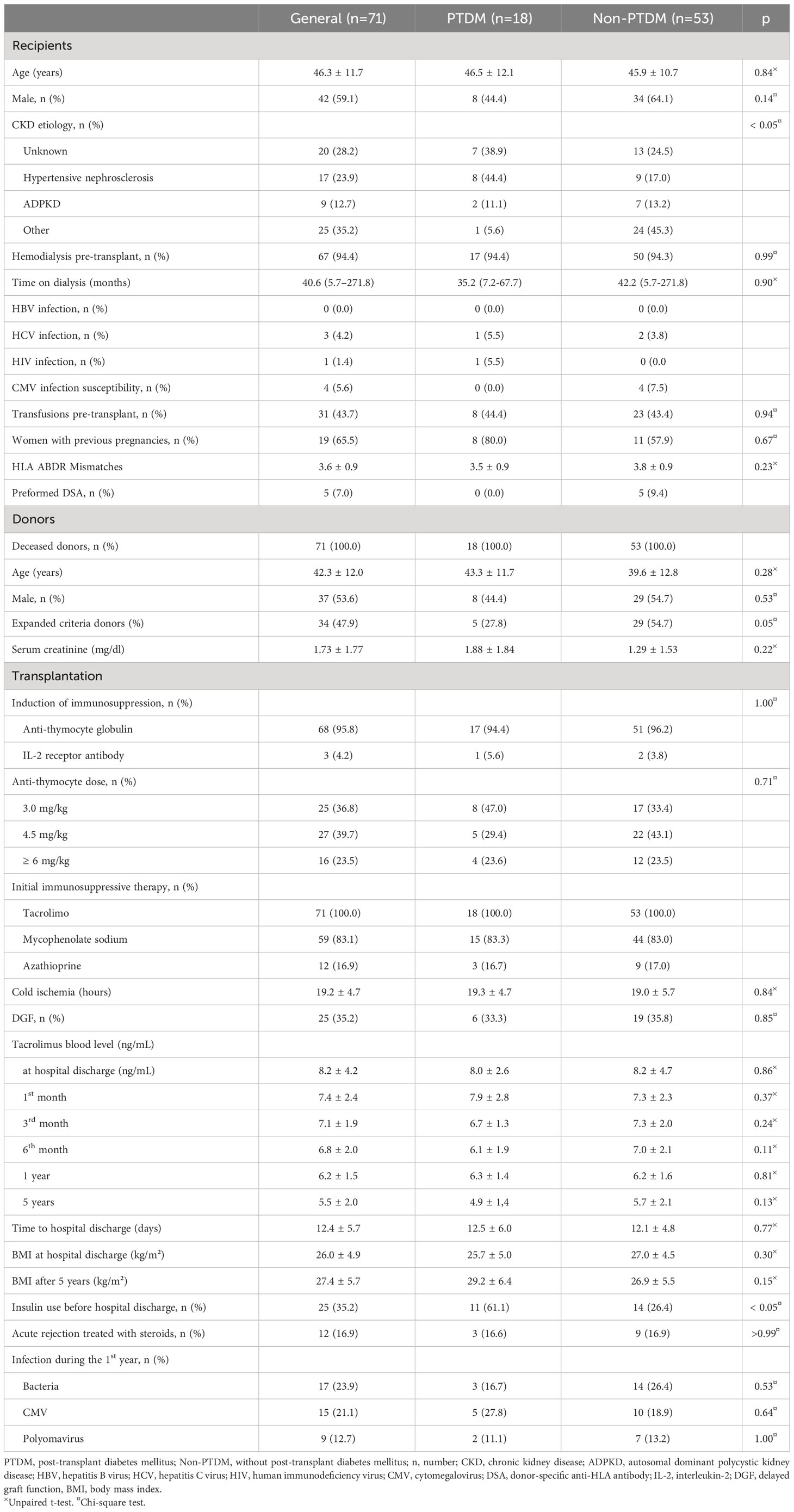
Table 1 General characteristics of kidney transplant recipients according to the post-transplant diabetes mellitus (PTDM) diagnosis.
Eighteen (25.3%) patients presented PTDM diagnosis, most (n=16, 88.9%) during the first year post-transplant, with half of these cases diagnosed during the first three months post-transplant (Figure 2). Most patients of the PTDM group presented CKD secondary to hypertensive nephrosclerosis (n=8, 44.4%), while the mean CKD etiology of the non-PTDM group was unknown (n=13, 24.5%). Hemodialysis was the main previous kidney replacement therapy in both groups. The donors' characteristics and the immunosuppressive treatment were similar between the groups. There was no difference between the groups in the cold ischemia time and DGF rate. There was no difference between the groups in the tacrolimus blood level and the body mass index (BMI) at hospital discharge and throughout follow-up. The need for insulin therapy during the hospital stay was significantly higher in the PTDM group (n=11, 61.1% vs. n=14, 26.4%, PTDM vs. non-PTDM, p<0.05). There was no significant difference between the groups’ occurrence of bacterial, CMV, or Polyomavirus infection rates during the first year post-transplant. At 5-year follow-up, the graft function remained stable in both groups, without significant differences in the serum creatinine or the UPCR compared to the initial values. There were five cases of death with a functioning graft, without significant difference between the groups (n=1, 5.5% vs. n=4, 7.5%, PTDM vs. non-PTDM, p=0.99). The incidence of graft failure was similar between the groups (n=2, 11.1% vs. n=4, 7.5%, PTDM vs. non-PTDM, p=0.63).
In this series, the accumulated incidence within five years of posttransplant follow-up was 25.3%. As previously described, the prevalence of PTDM is variable, with values ranging from 10% to 74%, according to region, population characteristics, and diagnostic criteria (25). A multicenter observational study in South Korea found an incidence of 11.8% within the first year post-transplant (26), while another trial from Slovak Republic showed an incidence of 38,3% in the same period (27). Considering the time post-transplant for PTDM diagnosis, most reported cases occurred during the first year post-transplant, which is probably related to high doses of immunosuppression in this period (8, 28). In this series, most PTDM cases occurred within the first 12 months, supporting previous studies.
All patients received induction and maintenance of immunosuppression according to the protocol at the center. There was no difference in doses of thymoglobulin between the groups. The CNI drugs, especially tacrolimus, play an essential role in the PTDM pathophysiology, increasing insulin resistance and decreasing insulin release (19). A previous study from Saudi Arabia showed that patients with tacrolimus trough level >10 ng/mL during the first three months after the procedure were at higher risk of PTDM, especially in the elderly or overweight recipients (29). In this series, however, the mean tacrolimus blood level was lower than 10 ng/mL throughout follow-up in both groups. Most patients showed a low immunological risk, with less than 10% having preformed DSA, none in the PTDM group. This could have affected the lower dose of immunosuppressive medications used in this series. The current recommended target trough level of tacrolimus for patients with low immunological risk is lower than that used previously, which potentially explains these findings (26). Although the high dose of steroids used to treat acute rejection may be associated with the development of PTDM, there was no difference in the number of acute rejection cases treated in this series.
Other traditional PTDM risk factors, such as age, gender of receptor and donor, ethnicity, BMI, HLA mismatches, acute rejection, and CMV or HCV infection, were not associated with PTDM in this series. One of the reasons that may justify such findings is the relatively small PTDM group. In this series, the need for insulin before hospital discharge was associated with PTDM occurrence. According to the literature, perioperative hyperglycemia is associated with the development of PTDM, mainly when treatment with insulin is required. In a previous retrospective study including 377 kidney transplant recipients, the requirement of insulin therapy during hospitalization posttransplant was associated with a 4-fold increase in PTDM, with 30% of patients treated with insulin before hospital discharge developing PTDM, while 18% of patients who had hyperglycemia without insulin treatment during this period presented this diagnosis (30).
The PTDM management after hospital discharge include oral hypoglycemic drugs alone or associated with insulin (25). Some authors consider metformin as first-line therapy for PTDM (31). However, this drug must be used carefully, especially in patients with impaired renal function, due to the risk of side effects occurrence, such as lactic acidosis (32). Sulfonylureas are also usually prescribed for PTDM, with scarce data on its efficacy and safety (25). New drugs are under study, such as Dipeptidyl-peptidase-4 (DPP-4) inhibitors, which are considered well tolerated, efficacious, and safe in stable kidney transplant recipients with PTDM (7). Observational studies suggest that sodium-glucose cotransporter-2 (SGLT2) inhibitors can be used in this population, with benefits beyond glycemic control (33, 34). In this cohort, the classes of hypoglycemic drugs used were metformin, sulfonylureas, and insulin. One reason for this choice is its availability on the Brazilian public health system, and the higher costs of other drugs.
This study has some limitations, which suggest a cautious interpretation of the data. First, the included patients were from a single hospital in Southeast Brazil, with regional population and dietetics characteristics. Furthermore, each transplant center has its characteristics, such as immunosuppression protocols, and these variations may affect the incidence of PTDM. The study’s retrospective design could also impact the results because of the lack of some information and the possible inclusion of patients with undiagnosed DM before transplantation. Finally, PTDM incidence was lower than other multicenter studies despite the maintenance of steroid therapy during follow-up, which may interfere with statistical strength. However, the study provides valuable information about the effects of perioperative hyperglycemia on PTDM incidence on the long-term post-transplant follow-up.
PTDM is a condition with a potential impact on the patient and graft survival. In this series, the accumulated incidence within a five-year post-transplant follow-up was 25.3%, most cases occurring during the first year. This can be associated with high doses of immunosuppressive drugs in this period, especially steroids and tacrolimus. Other well-established risk factors for PTDM, such as older age, donor’s and recipient’s age, high BMI, HLA mismatches, and CMV or HCV infections, were not associated with PTDM in this series. The perioperative hyperglycemia with the need for insulin therapy before hospital discharge was associated with PTDM diagnosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Comitê de Ética em Pesquisa da UNICAMP (CEP-UNICAMP). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MR: Data curation, Investigation, Writing – original draft. MM: Conceptualization, Formal analysis, Methodology, Supervision, Writing – review & editing. MS: Conceptualization, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Augustine J. Kidney transplant: New opportunities and challenges. Cleve Clin. J. Med. (2018) 85(2):138–44. doi: 10.3949/ccjm.85gr.18001
2. Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, et al. Incidence and cost of new onset diabetes mellitus among U.S. Wait-listed and transplanted renal allograft recipients. Am. J. Transplantation. (2003) 3(5):590–8 doi: 10.1034/j.1600-6143.2003.00082.x.
3. Gomes MB, Cobas RA. Post-transplant diabetes mellitus. Diabetol. Metab. Syndr. (2009) 1(1):14. doi: 10.1186/1758-5996-1-14
4. Mazali F, Lalli C, Alves-Filho G, Mazzali M. Posttransplant diabetes mellitus: incidence and risk factors. Transplant. Proc. (2008) 40(3):764–6. doi: 10.1016/j.transproceed.2008.03.018
5. Chakkera HA, Weil EJ, Pham PT, Pomeroy J, Knowler WC. Can new-onset diabetes after kidney transplant be prevented? Diabetes Care (2013) 36(5):1406–12. doi: 10.2337/dc12-2067
6. Sharif A, Hecking M, de Vries APJ, Porrini E, Hornum M, Rasoul-Rockenschaub S, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am. J. Transplantation. (2014) 14(9):1992–2000. doi: 10.1111/ajt.12850
7. Lim SW, Jin JZ, Jin L, Jin J, Li C. Role of dipeptidyl peptidase-4 inhibitors in new-onset diabetes after transplantation. Korean J. Intern. Med. (2015) 30(6):759–70. doi: 10.3904/kjim.2015.30.6.759
8. Chakkera HA, Weil EJ, Pham PT, Pomeroy J, Knowler WC. Can new-onset diabetes after kidney transplant be prevented? Diabetes Care (2013) 36(5):1406–12. doi: 10.2337/dc12-2067
9. Yates CJ, Fourlanos S, Colman PG, Cohney SJ. Screening for new-onset diabetes after kidney transplantation. Transplantation (2013) 96(8):726–31. doi: 10.1097/TP.0b013e3182a012f3
10. Rodríguez-Rodríguez AE, Porrini E, Hornum M, Donate-Correa J, Morales-Febles R, Khemlani Ramchand S, et al. Post-transplant diabetes mellitus and prediabetes in renal transplant recipients: an update. Nephron (2021) 145(4):317–29. doi: 10.1159/000514288
11. Ponticelli C, Favi E, Ferraresso M. New-onset diabetes after kidney transplantation. Medicina (B Aires). (2021) 57(3):250. doi: 10.3390/medicina57030250
12. Jenssen T, Hartmann A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat. Rev. Endocrinol. (2019) 15(3):172–88. doi: 10.1038/s41574-018-0137-7
13. McCaughan JA, McKnight AJ, Maxwell AP. Genetics of new-onset diabetes after transplantation. J. Am. Soc. Nephrology. (2014) 25(5):1037–49. doi: 10.1681/ASN.2013040383
14. Pham PT, Pham PM, Pham PC. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab. Syndr. Obes. (2011) 175:175–86. doi: 10.2147/DMSO.S19027
15. Chadban S. New-onset diabetes after transplantation–should it be a factor in choosing an immunosuppressant regimen for kidney transplant recipients. Nephrol. Dialysis Transplantation. (2008) 23(6):1816–8. doi: 10.1093/ndt/gfn052
16. Ghisdal L, Van Laecke S, Abramowicz MJ, Vanholder R, Abramowicz D. New-onset diabetes after renal transplantation. Diabetes Care (2012) 35(1):181–8. doi: 10.2337/dc11-1230
17. Hjelmesaeth J, Hartmann A, Kofstad J, Stenstrøm J, Leivestad T, Egeland T, et al. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation (1997) 64(7):979–83. doi: 10.1097/00007890-199710150-00008
18. Geer EB, Islam J, Buettner C. Mechanisms of glucocorticoid-induced insulin resistance. Endocrinol. Metab. Clin. North Am. (2014) 43(1):75–102. doi: 10.1016/j.ecl.2013.10.005
19. Chakkera H, Kudva Y, Kaplan B. Calcineurin inhibitors: pharmacologic mechanisms impacting both insulin resistance and insulin secretion leading to glucose dysregulation and diabetes mellitus. Clin. Pharmacol. Ther. (2017) 101(1):114–20. doi: 10.1002/cpt.546
20. Soleimanpour SA, Crutchlow MF, Ferrari AM, Raum JC, Groff DN, Rankin MM, et al. Calcineurin signaling regulates human islet β-cell survival. J. Biol. Chem. (2010) 285(51):40050–9. doi: 10.1074/jbc.M110.154955
21. Xia M, Yang H, Tong X, Xie H, Cui F, Shuang W. Risk factors for new-onset diabetes mellitus after kidney transplantation: A systematic review and meta-analysis. J. Diabetes Investig. (2021) 12(1):109–22. doi: 10.1111/jdi.13317
22. de Sousa MVMV, Gonçalez ACAC, Zollner RLR de L, Mazzali M. Effect of preformed or de novo anti-HLA antibodies on function and graft survival in kidney transplant recipients. Ann. Transplant. (2018) 23:457–66. doi: 10.12659/AOT.908491
23. Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am. J. Transplant. (2003) 3(s4):114–25. doi: 10.1034/j.1600-6143.3.s4.11.x
24. Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am. J. Transplant. (2014) 14(2):272–83. doi: 10.1111/ajt.12590
25. Shivaswamy V, Boerner B, Larsen J. Post-transplant diabetes mellitus: causes, treatment, and impact on outcomes. Endocr. Rev. (2016) 37(1):37–61. doi: 10.1210/er.2015-1084
26. Paek JH, Kang SS, Park WY, Jin K, Park SB, Han S, et al. Incidence of post-transplantation diabetes mellitus within 1 year after kidney transplantation and related factors in Korean cohort study. Transplant. Proc. (2019) 51(8):2714–7. doi: 10.1016/j.transproceed.2019.02.054
27. Dedinská L, Baltesová T, Beňa Ĺ, Čellár M, Galajda P, Chrastina M, et al. Incidence of diabetes mellitus after kidney transplantation in Slovakia: multicentric, prospective analysis. Transplant. Proc. (2016) 48(10):3292–8. doi: 10.1016/j.transproceed.2016.09.041
28. Hjelmesæth J, Hartmann A, Leivestad T, Holdaas H, Sagedal S, Olstad M, et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. (2006) 69(3):588–95. doi: 10.1038/sj.ki.5000116
29. Ajabnoor A, Nasser M, Khan N, Habhab W. Evaluation of tacrolimus trough level in patients who developed post-transplant diabetes mellitus after kidney transplantation: A retrospective single-center study in Saudi Arabia. Transplant. Proc. (2020) 52(10):3160–7. doi: 10.1016/j.transproceed.2020.05.014
30. Chakkera HA, Knowler WC, Devarapalli Y, Weil EJ, Heilman RL, Dueck A, et al. Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus. Clin. J. Am. Soc. Nephrology. (2010) 5(9):1669–75. doi: 10.2215/CJN.09481209
31. Sharif A. Should metformin be our antiglycemic agent of choice post-transplantation? Am. J. Transplant. (2011) 11(7):1376–81. doi: 10.1111/j.1600-6143.2011.03550.x
32. Larsen JL. Potential risks of metformin in transplant patients. Am. J. Transplantation. (2012) 12(3):795. doi: 10.1111/j.1600-6143.2011.03921.x
33. Ujjawal A, Schreiber B, Verma A. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) in kidney transplant recipients: what is the evidence? Ther. Adv. Endocrinol. Metab. (2022) 13:20420188221090000. doi: 10.1177/20420188221090001
Keywords: diabetes mellitus, kidney transplantation, immunosuppressive therapy, graft survival, diabetes complication
Citation: Rossi MR, Mazzali M and de Sousa MV (2024) Post-transplant diabetes mellitus: risk factors and outcomes in a 5-year follow-up. Front. Clin. Diabetes Healthc. 5:1336896. doi: 10.3389/fcdhc.2024.1336896
Received: 11 November 2023; Accepted: 10 January 2024;
Published: 30 January 2024.
Edited by:
Pedro Henrique Franca Gois, The University of Queensland, AustraliaReviewed by:
Erika Bevilaqua Rangel, Albert Einstein Israelite Hospital, BrazilCopyright © 2024 Rossi, Mazzali and de Sousa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcos Vinicius de Sousa, bWFyY29zbmVmcm9AZ21haWwuY29t
†ORCID: Marilda Mazzali, orcid.org/0000-0001-6297-4909
Marcos Vinicius de Sousa, orcid.org/0000-0002-0280-1069
Matheus Rizzato Rossi, orcid.org/0000-0003-4597-4266
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.