- 1Department of Medicine, Adama General Hospital and Medical College, Adama, Ethiopia
- 2Department of Nursing, Adama General Hospital and Medical College, Adama, Ethiopia
- 3Department of Public Health, Adama Hospital Medical College, Adama, Ethiopia
Background: Diabetes comorbidities are a serious public health issue that raises the risk of adverse health effects and complicates diabetes management. It also harms emotional health, medication adherence, self-management, and general quality of life. However, evidence is scarce in Ethiopia, particularly in the study area. Thus, this study aimed to estimate the prevalence of diabetes concordant comorbidities and identify factors associated with the presence of concordant comorbidities among adult diabetic patients in central Ethiopia.
Methods: A health facility-based cross-sectional study was conducted among 398 adult diabetic patients. A computer-generated simple random sampling was used to select study participants. Data were collected using a structured data extraction checklist. The collected data were entered into Epi info version 7.2 and exported to SPSS version 27 for analysis. A binary logistic regression model was used to analyze the association between dependent and independent variables. An adjusted odds ratio with the corresponding 95% confidence interval was used to measure the strength of the association and statistical significance was declared at a p-value < 0.05.
Result: The prevalence of diabetes-concordant comorbidities was 41% (95% CI: 36.2-46.0). The multivariable logistic regression model showed that age 41–60 (AOR = 2.86, 95% CI: 1.60–5.13), place of residence (AOR = 2.22, 95% CI: 1.33–3.70), having type two diabetes (AOR = 3.30, 95% CI: 1.21–8.99), and having positive proteinuria (AOR = 2.64, 95% CI: 1.47–4.76) were significantly associated with diabetes concordant comorbidities.
Conclusion: The prevalence of diabetes-concordant comorbidities was relatively high. Age, place of residence, type of diabetes, and positive proteinuria were factors associated with diabetes-concordant comorbidities. Prevention, early identification, and proper management of diabetes comorbidities are crucial.
1 Introduction
Diabetes mellitus (DM) is a serious public health concern that is on the verge of epidemic proportions, making it a major global threat to the health and well-being of individuals, families, and society. About 537 million adults globally were anticipated to have DM in 2021, and this number is projected to rise to 643 million by 2030 and 783 million by 2045, with low- and middle-income countries holding the greatest burden of the forecasted increase in prevalence. In 2021, 24 million adults in Africa, or 1 in 22 adults, had DM. By 2045, it’s predicted to reach over 55 million (1–3).
With an estimated 1.9 million adults suffering from DM, Ethiopia is among the top five African nations in terms of the number of patients living with DM (3). Despite the lack of national statistics on diabetes prevalence, a systematic review and meta-analysis showed that it ranged from 2% to 6.5%. In Ethiopia, diabetes and its complications constitute the major causes of illness and mortality, with corresponding economic consequences (4).
Comorbidity refers to the presence of two or more chronic diseases or medical conditions in a patient (5). Diabetes comorbidity is the presence of one or more chronic diseases in patients living with DM, which can be divided into concordant and discordant comorbidities. Concordant comorbidities are two or more diseases that have a comparable pathophysiological risk profile and a similar disease treatment plan as DM. whereas, conditions that are not etiologically related to DM and don’t share comparable risk factors are discordant comorbidities (6–8).
Comorbidity harms a DM patient’s overall quality of life. Their emotional health, medication compliance, and self-management are all negatively impacted. It also increases the risk of adverse health outcomes and makes managing DM more demanding (9, 10). A recent study of more than 1.3 million participants found that over 98% had at least one comorbid condition, and nearly 90% had at least two (11). In sub-Saharan Africa (SSA), the prevalence of DM comorbidity ranges from 6% to 64% (12). A study done in Ethiopia also revealed that 55.8% of adults with DM had concordant comorbidities (6).
Previous studies have shown that various factors like sex, age, place of residence, economic status, duration of DM, family history of DM, type of DM, type of treatment, proteinuria, and glycemic control all influence the development of comorbidities in DM patients (6–8, 13–16).
Diabetes comorbidities increase the cost of hospital stays, the requirement for regular checkups, and the demand for healthcare. By being more cognizant of common diabetes comorbidities and related factors, it will be easier to decide on the appropriate course of treatment for DM patients. It has been demonstrated that early detection and effective comorbidity treatment can prolong the patient’s life and improve their quality of life (6, 12, 16, 17). However, there is a dearth of evidence in Ethiopia, particularly in the study area, to show the prevalence and factors associated with diabetes-concordant comorbidities. Hence, this study aimed to estimate the prevalence of diabetes concordant comorbidities and identify factors associated with the presence of concordant comorbidities among adult diabetic patients in central Ethiopia. The findings might be of essential clinical value, aiding in the comprehensive management of DM patients as well as designing policies and suitable intervention strategies. The findings of this study will serve as a basis for further studies as well.
2 Materials and methods
2.1 Study design, setting, and period
A health facility-based cross-sectional study was conducted at Adama Hospital Medical College (AHMC), Ethiopia. This study was conducted to retrieve five years of data, from January 2018 to December 2022. AHMC is the sole public teaching hospital in Adama town. It has more than 1300 staff, a yearly outpatient flow of 226,000 with an average outpatient flow of 853, 500 beds, and an admittance rate of 173 patients per week. Adama Town is 99 kilometers southeast of Addis Ababa, with a total area of 29.86 square kilometers and a population of over 500,000 people. The hospital collectively provides services to catchment areas with over five million people and acts as a referral hub for zones and regions nearby.
2.2 Population and eligibility criteria
All adults living with DM who were on follow-up at AHMC were considered the source population, whereas all adult diabetic patients who were on follow-up at AHMC from January 2018 to December 2022 were taken as the study population. Patients with an uncertain comorbidity status and/or an incomplete transfer-out history were excluded from this study.
2.3 Sample size determination and sampling procedure
The sample size for this study was determined by using the single population proportion formula by considering the assumptions of a 95% confidence level (the critical value Zα/2 = 1.96), a 5% margin of error (d = 0.05), and a 55.8% (p = 0.558) proportion of diabetes concordant comorbidity from a study done in Hiwot Fana Specialized University Hospital (6). By adding 10% contingency for incomplete data, the final sample size of the study becomes 421.
Using the medical record number of diabetes patients who were on follow-up from January 2018 to December 2022 as a sampling frame, participants who fulfilled the inclusion criteria were selected by a simple random sampling technique using computer-generated random numbers.
2.4 Study variables
2.4.1 Dependent variable
Diabetes concordant comorbidities
2.4.2 Independent variables
2.4.2.1 Socio-demographic factors
Sex, age, place of residence, marital status, and occupational status.
2.4.2.2 Clinical factors
Type of DM, type of treatment, proteinuria, glycemic control, body mass index (BMI), family history of DM, duration of DM, history of smoking, and follow-up miss.
2.5 Operational definitions
2.5.1 Concordant comorbidity
the existence or absence of at least one chronic condition among individuals with diabetes. These include hypertension(HTN), obesity, dyslipidemia, chronic vascular disease (CVD), and/or chronic kidney disease (CKD) (6, 18, 19).
2.5.2 Hypertension
a systolic blood pressure (SBP) of ≥ 140 mmHg or diastolic blood pressure (DBP) of ≥ 90mmHg (at least two recordings on separate days or four hours apart in a single day) or being treated for physician-diagnosed hypertension (20, 21).
2.5.3 Obese
a body mass index (BMI) exceeding 30 kg/m2.
2.5.4 Dyslipidemia
the presence of at least one of those conditions: high triglyceride levels (>150 mg/dl), high low-density lipoprotein cholesterol (LDL-C) levels (>130 mg/dl), low high-density lipoprotein cholesterol (HDL-C) levels (< 40 mg/dl in men or < 50 mg/dl in women), and high plasma total cholesterol levels (>200 mg/dl) or being treated for physician-diagnosed dyslipidemia (22).
2.5.5 Chronic vascular disease
the presence of at least one of those conditions: an electrocardiogram (ECG) finding indicating ischemic heart disease, an echocardiography finding of ischemic heart disease, or being on treatment after being diagnosed by a physician with stroke or ischemic heart disease (23).
2.5.6 Chronic kidney disease
the presence of at least one of those conditions: urine dipstick protein +1 at least twice in the past three months; serum creatinine level ≥ 1.2 mg/dl in males and ≥ 1.0 mg/dl in females at least twice in the last three months; or being on treatment after being diagnosed by a physician with CKD (24).
2.5.7 Glycemic control
hemoglobin A1C (HgA1C) < 7%, or the last fasting blood sugar (FBS) level ≤ 130 mg/dl in the absence of documented HgA1C determined in less than three months, was regarded as good glycemic control; otherwise, it was considered poor glycemic control (19).
2.6 Data collection procedure and quality control
Data were collected using a structured and pre-tested checklist devised by reviewing patients’ charts, follow-up cards, DM registration books, electronic information databases, and previous similar studies. The checklist includes socio-demographic, clinical-related characteristics, and comorbidity histories. After two days of training on the data collection process, three trained nurses collected the data under the supervision of two public health officers.
Before actual data collection, the checklist was pre-tested on 5% of the projected sample size (n = 21) on records earlier than January 2018, and then adjustments and corrections were made accordingly. Moreover, the supervisors and principal investigator constantly monitored the completeness and consistency of the data during the data collection, and all gathered data were cross-checked during data entry to clarify any missing data.
2.7 Data processing and analysis
Following data coding and entry into Epi-Info Vision 7, the data were exported to Statistical Package for Social Sciences (SPSS) Version 27 for cleaning and analysis. Descriptive statistics were employed to describe the study population in light of relevant characteristics. The Shapiro-Wilk test was used to validate the normality assumptions for continuous variables. Binary logistic regression was used to model the association between the independent and the outcome variables. The statistical assumptions of the model i.e., independence of residuals, multi-collinearity, normality, linearity, and outliers, were examined, and no significant violations were found. A p-value of 0.25 was used as a cut-off value in the bivariable logistic regression model to select variables for multivariable logistic regression analysis to control the possible effects of confounders. The standard model-building approach was used to fit the model. The model’s fitness was assessed using Hosmer and Lemeshow’s goodness-of-fit test, and the result was not significant with a p-value of 0.16 (> 0.05), indicating that the model fits the data well. Using the variance inflation factor and tolerance, the multicollinearity between the explanatory variables was also examined, and it was found to be within a tolerable range. In the multivariable logistic regression, the adjusted odds ratio (AOR) with a 95% confidence interval (CI) was used to determine factors independently associated with diabetes concordant comorbidity. At this level, variables with a p-value less than 0.05 were considered statistically significant.
3 Results
3.1 Socio-demographic characteristics
A total of 398 DM patient records were reviewed and included in the final analysis, giving a response rate of 94.5%. The median age of the participants was 48 (IQR= 37-60), with a minimum age of 18 and a maximum of 85 years. One hundred fifty-one (54.02%) of the participants were male, 293 (73.62%) were urban residents and 334 (83.92%) were married (Table 1).
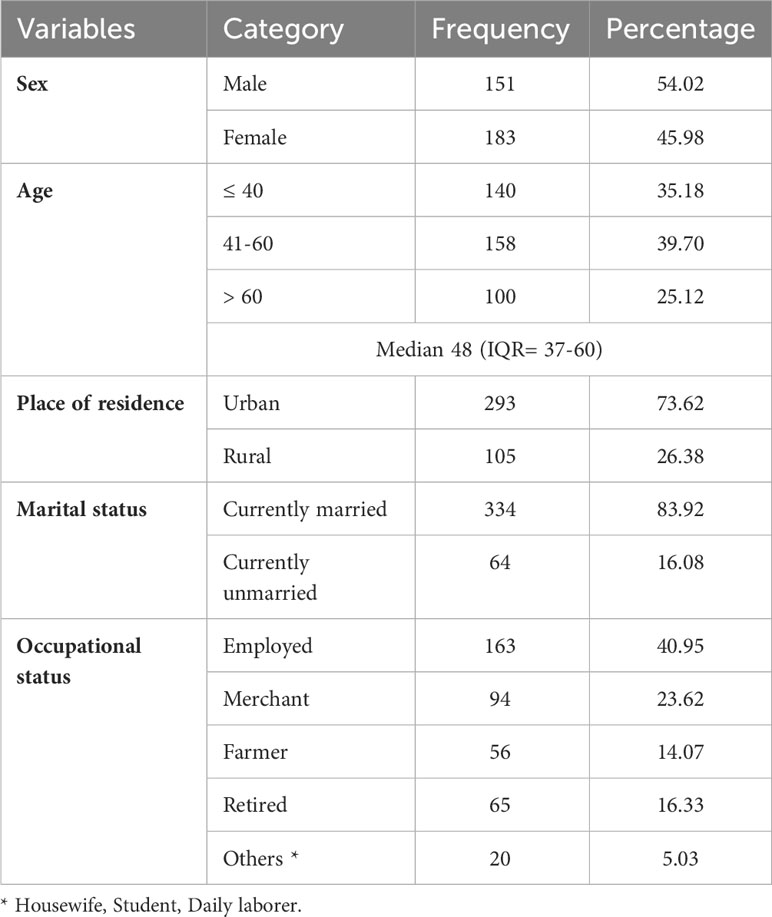
Table 1 Sociodemographic characteristics of diabetic patients at Adama Hospital Medical College, Central Ethiopia (n=398).
3.2 Clinical characteristics
Among the participants included in this study, 341 (85.68%) were type 2 DM patients, and 172 (43.22%) were taking oral hypoglycemic agents (OHAs). The majority of DM patients, 346 (86.9%), had poor glycemic control. In this study, the median duration of DM was 3.8 (IQR = 1.8–6.1), and of the participants, 280 (70.35%) had a history of missed follow-up (Table 2).
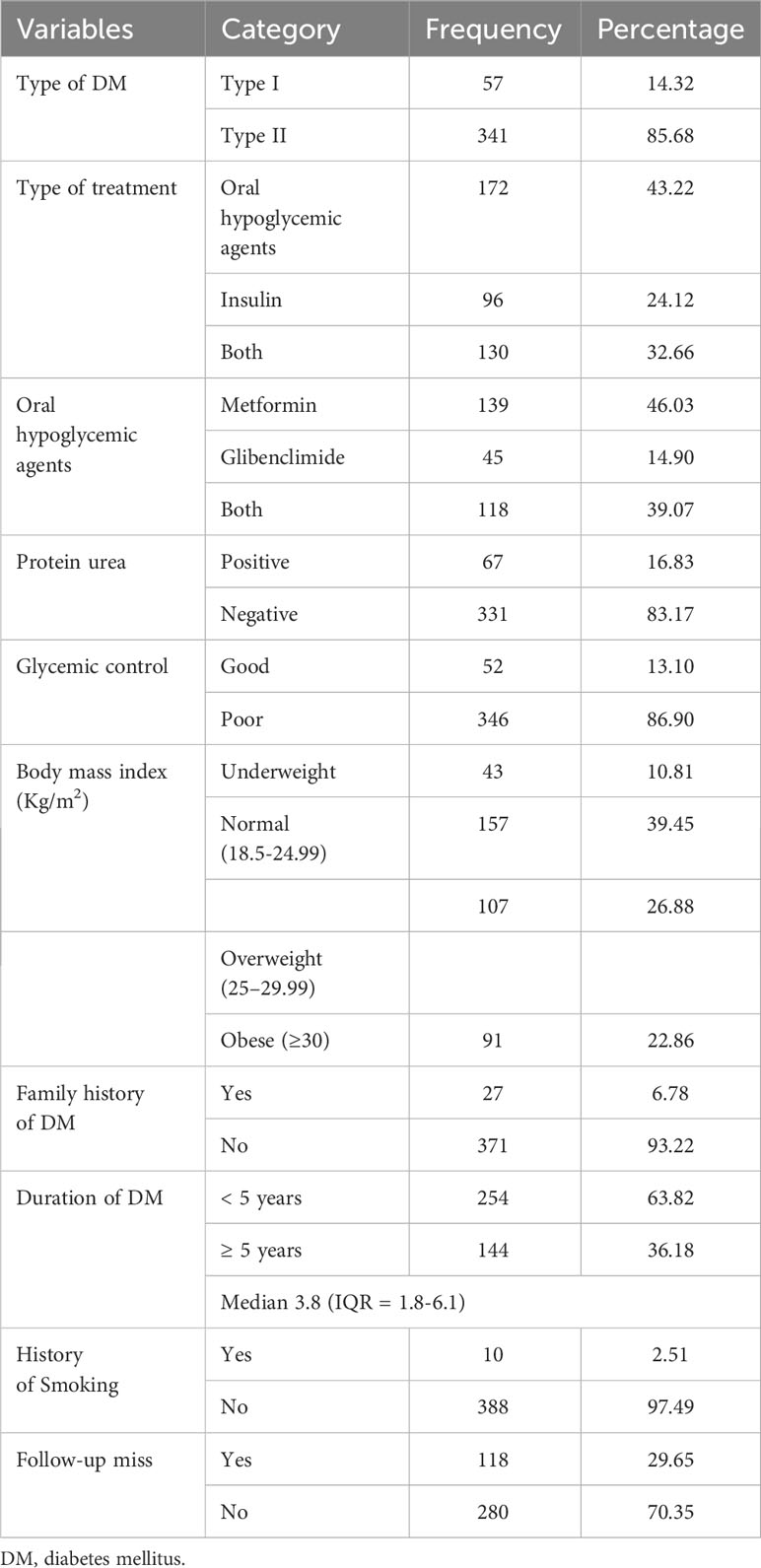
Table 2 Clinical characteristics of diabetic patients at Adama Hospital Medical College, Central Ethiopia (n=398).
3.3 The prevalence of concordant comorbidity
The overall prevalence of diabetes concordant comorbidities was 41% (95% CI: 36.2-46.0). In a separate analysis, it was 14% (95% CI: 5.3-22.8) among type 1 and 45.5% (95% CI: 40.2-50.7) among type 2 DM patients. From this, 79 (48.5%) participants had HTN, 29 (17.8%) had obesity, 6 (3.7%) had dyslipidemia, 12 (7.4%) had CVD, 18 (11%) had CKD, and 19 (11.7%) had multi-comorbidity.
3.4 Factors associated with concordant comorbidities
In the bivariable analysis, age, place of residence, type of DM, type of treatment, proteinuria, and duration of DM showed significant association. After adjusting for potential confounders using multivariable binary logistic regression analysis age, place of residence, type of DM and proteinuria demonstrated a statistically significant association with concordant comorbidities.
Accordingly, compared to diabetic patients who are 40 years of age or younger, those patients in the age range of 41 to 60 had 2.86 times the odds of having concordant comorbidities (AOR = 2.86, 95% CI: (1.60-5.13). The odds of having concordant comorbidities were 2.22 times higher among urban residents compared to those who were rural residents (AOR = 2.22, 95% CI: 1.33-3.70). Type 2 DM patients had 3.3 times greater odds of having concordant comorbidities compared to type one diabetes patients (AOR = 3.30, 95% CI: 1.21-8.99). Moreover, DM patients with positive proteinuria had 2.64 times the odds of having concordant comorbidities than their counterparts (AOR = 2.64, 95% CI: 1.47-4.76) (Table 3).
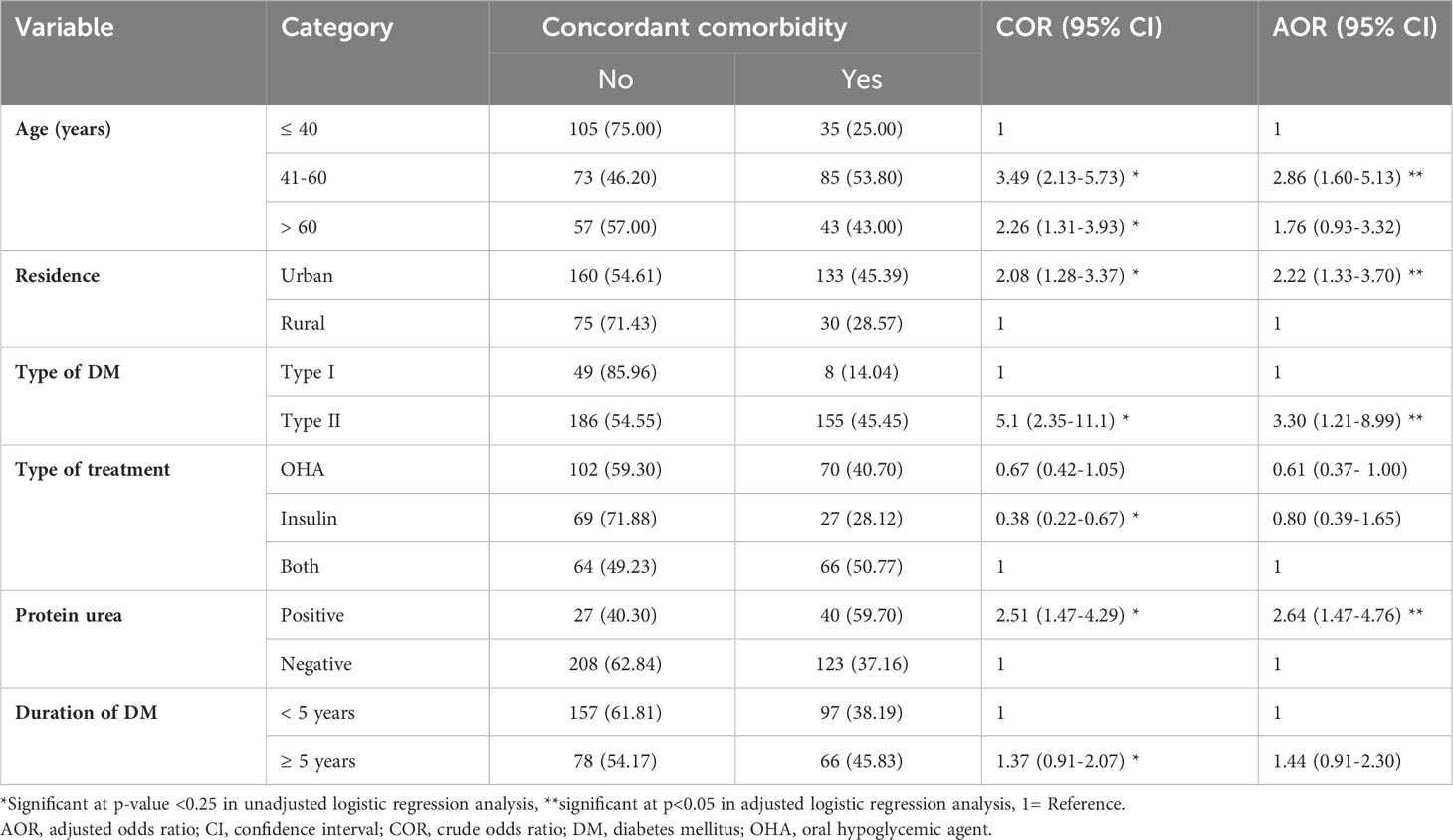
Table 3 Factors associated with diabetes concordant comorbidities among diabetic patients at Adama Hospital Medical College, Central Ethiopia (n=398).
4 Discussion
The present study sought to assess the prevalence of concordant comorbidities and identify factors associated with the presence of concordant comorbidities among adult diabetic patients in central Ethiopia. The prevalence of diabetes concordant comorbidities was relatively high. Age 41 to 60, being an urban resident, having type 2 DM, and having positive proteinuria were factors significantly associated with diabetes concordant comorbidities.
In this study, the overall prevalence of diabetes concordant comorbidities was 41% (95% CI: 36.2-46.0). This finding is in agreement with studies conducted in Germany(43%) (25) and Scotland (42.2%) (26). The prevalence of concordant comorbidities in this study is higher than studies done in the United States of America (7%) (27), Israel (16.6%) (7), and Australia (18.9%) (28). Conversely, the finding of the current study is lower than those of studies done in the United States of America (USA) (88.5%) (11), Spain (82%) (29), India (66%) (8) and Ethiopia (55.8%) (6). This disparity may be explained by differences in the study population; most earlier studies focused exclusively on type 1 or type 2 DM, whereas the current study included both types of DM (7, 8, 27, 29). Further, differences in sample sizes, socio-demographic, and study period disparities might be another possible explanation for the observed variations.
The odds of having concordant comorbidities were 2.86 times higher among DM patients in the age range of 41 to 60 compared with those who are 40 years of age or younger. this finding is supported by studies done in Ethiopia (6), Israel (7), Basque country (15) and Saudi Arabia (30). This can be explained by the fact Arteries tend to constrict and harden with age, resulting in a condition called arterial stiffening. This process can lead to blocked arteries, which can raise the risk of various complications, including hypertension and cardiovascular problems. People tend to have more sedentary lives as they get older, which causes difficulty managing cholesterol and increases the risk of dyslipidemia. Furthermore, age-related kidney alterations, such as a decrease in the number of filtering units (nephrons) and diminished kidney tissue, might also lead to hypertension and renal complications (31–33).
The finding of this study indicated that the odds of having concordant comorbidities were higher among urban residents compared to those who were rural residents. which is supported by the study done in China (34). This may be attributable to a sedentary lifestyle, unhealthy eating habits, less physical activity, and an increased rate of obesity that are common in urban settings, all of which raise the risk of concordant comorbidities (35, 36). Conversely, different studies have produced contradictory findings about the association between residence and concordant comorbidities (37). Uncertainty exists regarding the nature of the association between the place of residence and concordant comorbidities, and further studies are needed to draw that conclusion.
Type of DM was another significant factor associated with concordant comorbidities. Type 2 DM patients had 3.3 times greater odds of having concordant comorbidity compared to type 1 DM patients. This finding is similar to studies conducted in Ethiopia (6), Israel (7), and Italy (38). This might be explained by shared risk factors, insulin resistance, atherogenesis, and chronic inflammation, as well as the increased incidence of obesity and autonomic neuropathy among Type 2 DM patients (39, 40).
This study also showed a statistically significant association between proteinuria and DM concordant comorbidities. In line with studies conducted in Bangladesh (14), India (41), and Korea (42) DM patients with positive proteinuria had higher odds of having concordant comorbidity. This could be because proteinuria impairs the kidneys’ capacity to remove waste products. Consequently, proteins like albumin that should stay in the body seep into the urine, raising the risk of cardiovascular and renal problems. Salt and water retention due to damaged kidneys may raise blood volume and, as a result, blood pressure. Persistently high blood sugar levels also potentially damage the blood vessels in the retina, resulting in vision problems (43, 44).
5 Limitations of the study
Given the study’s cross-sectional design, determining whether independent and dependent variables are causally connected is difficult. There may be a potential selection bias in conducting this study in a third-tier reference center. Moreover, due to the retrospective nature of the study, it was not possible to investigate the impact of certain sociodemographic and behavioral factors, as well as perform an in-depth analysis of cardiovascular comorbidities.
6 Conclusions
The prevalence of diabetes-concordant comorbidities was relatively high. Age, place of residence, type of DM, and positive proteinuria were factors associated with diabetes-concordant comorbidity. Thus, patients with diabetes who have co-morbid conditions or are at risk of getting them, such as those who are older, have type 2 DM, or have positive proteinuria, should receive due attention. Furthermore, prospective studies are recommended.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval for the study was received from Adama Hospital Medical College's Institutional Review Board (IRB), and authorization to access data from medical records was secured from the hospital's medical director. The need for consent was waived by the IRB. Data confidentiality was maintained at all stages of study processes and was stored on a secure password-protected system. All the study's procedures were conducted in line with the principles of the Helsinki Declaration (45).
Author contributions
YN: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MG: Writing – review & editing. NB: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors like to acknowledge Adama Hospital Medical College for providing all necessary assistance. We also are thankful for the data collectors and supervisors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AHMC, Adama Hospital Medical College; AHR, Adjusted Hazard Ratio; CI, Confidence Interval, CKD, Chronic Kidney Disease; DM, Diabetes Mellitus; FBS, Fasting Blood Sugar; HgA1C, Hemoglobin A1C; HTN, hypertension; OHA, Oral Hypoglycemic Agent.
References
1. Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The growing epidemic of diabetes mellitus. Curr. Vasc. Pharmacol. (2020) 18(2):104–9. doi: 10.2174/1570161117666190405165911
2. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Practice. (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
3. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Practice. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
4. Bishu KG, Jenkins C, Yebyo HG, Atsbha M, Wubayehu T, Gebregziabher M. Diabetes in Ethiopia: A systematic review of prevalence, risk factors, complications, and cost. Obes. Med. (2019) 15, 15:100132. doi: 10.1016/j.obmed.2019.100132
5. Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann. Fam Med. (2009) 7(4):357–63. doi: 10.1370/afm.983
6. Ejeta A, Abdosh T, Hawulte B, Lamessa A, Belete Fite M, Fekadu G. Diabetes concordant comorbidities and associated factors among adult diabetic out-patients at hiwot fana specialized university hospital, harar, eastern Ethiopia: a cross-sectional study. Diabetes Metab. Syndr. Obes. (2021) 14:2281–9. doi: 10.2147/DMSO.S308553
7. Eilat-Tsanani S, Margalit A, Golan LN. Occurrence of comorbidities in newly diagnosed type 2 diabetes patients and their impact after 11 years’ follow-up. Sci. Rep. (2021) 11(1):11071. doi: 10.1038/s41598-021-90379-0
8. Soji DJ, Lordson J, Mini GK. Multimorbidity patterns among rural adults with Type-2 diabetes mellitus: A cross-sectional study in Kerala, India. WHO South East Asia J. Public Health (2021) 10(1):32–6. doi: 10.4103/WHO-SEAJPH.WHO-SEAJPH_73_21
9. Pati S, Pati S, van den AM, Schellevis FFG, Jena S, Burgers JS. Impact of comorbidity on health-related quality of life among type 2 diabetic patients in primary care. Prim Health Care Res. Dev. (2020) 21:e9. doi: 10.1017/S1463423620000055
10. Beverly EA, Wray LA, Chiu CJ, Weinger K. Perceived challenges and priorities in co-morbidity management of older patients with Type 2 diabetes: Co-morbidity and Type 2 diabetes self-management. Diabetic Med. (2011) 28(7):781–4. doi: 10.1111/j.1464-5491.2011.03282.x
11. Iglay K, Hannachi H, Joseph Howie P, Xu J, Li X, Engel SS, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr. Med. Res. Opin. (2016) 32(7):1243–52. doi: 10.1185/03007995.2016.1168291
12. Ekoru K, Doumatey A, Bentley AR, Chen G, Zhou J, Shriner D, et al. Type 2 diabetes complications and comorbidity in Sub-Saharan Africans. EClinicalMedicine. (2019) 16:30–41. doi: 10.1016/j.eclinm.2019.09.001
13. Kengne AP, Echouffo-Tcheugui JB, Sobngwi E, Mbanya JC. New insights on diabetes mellitus and obesity in Africa-part 1: prevalence, pathogenesis and comorbidities. Heart. (2013) 99(14):979–83. doi: 10.1136/heartjnl-2012-303316
14. Yokota F, Ahmed A. Differences in relationships and risk factors associated with hypertension, diabetes, and proteinuria among urban and rural adults in Bangladesh: -findings from portable health clinic research project 2013–2018, in: HCI international 2019 – late breaking papers (2019). Cham: Springer International Publishing (Accessed 2023 Oct 2).
15. Alonso-Morán E, Orueta JF, Esteban JIF, Axpe JMA, González MLM, Polanco NT, et al. The prevalence of diabetes-related complications and multimorbidity in the population with type 2 diabetes mellitus in the Basque Country. BMC Public Health (2014) 14(1):1059. doi: 10.1186/1471-2458-14-1059
16. Tripathy JP, Thakur JS, Jeet G, Jain S. Prevalence and determinants of comorbid diabetes and hypertension: Evidence from non communicable disease risk factor STEPS survey, India. Diabetes Metab. Syndr. (2017) 11 Suppl 1:S459–65. doi: 10.1016/j.dsx.2017.03.036
17. Magnan EM, Palta M, Johnson HM, Bartels CM, Schumacher JR, Smith MA. The impact of a patient’s concordant and discordant chronic conditions on diabetes care quality measures. J. Diabetes Complications. (2015) 29(2):288–94. doi: 10.1016/j.jdiacomp.2014.10.003
18. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care (2006) 29(3):725–31. doi: 10.2337/diacare.29.03.06.dc05-2078
20. National High Blood Pressure Education Program. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure (2004). Bethesda (MD: National Heart, Lung, and Blood Institute (US. Available at: http://www.ncbi.nlm.nih.gov/books/NBK9630/ (Accessed 2023 Aug 28).
21. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. International society of hypertension global hypertension practice guidelines. Hypertension (2020) 75(6):1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
22. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J. Am. Coll. Cardiol. (2018) 73(24):e285–350. doi: 10.1016/j.jacc.2018.11.002
23. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation (2012) 126(25):e354–471. doi: 10.1161/CIR.0b013e318277d6a0
24. Gaitonde DY, Cook DL, Rivera IM. Chronic kidney disease: detection and evaluation. Am. Fam Physician. (2017) 96(12):776–83.
25. Du Y, Heidemann C, Gößwald A, Schmich P, Scheidt-Nave C. Prevalence and comorbidity of diabetes mellitus among non-institutionalized older adults in Germany - results of the national telephone health interview survey “German Health Update (GEDA)” 2009. BMC Public Health (2013) 13:166. doi: 10.1186/1471-2458-13-166
26. Luijks H, Schermer T, Bor H, van Weel C, Lagro-Janssen T, Biermans M, et al. Prevalence and incidence density rates of chronic comorbidity in type 2 diabetes patients: an exploratory cohort study. BMC Med. (2012) 10:128. doi: 10.1186/1741-7015-10-128
27. Lin PJ, Pope E, Zhou FL. Comorbidity type and health care costs in type 2 diabetes: A retrospective claims database analysis. Diabetes Ther. (2018) 9(5):1907–18. doi: 10.1007/s13300-018-0477-2
28. Aung E, Donald M, Coll J, Dower J, M. Williams G, Doi SAR. The impact of concordant and discordant comorbidities on patient-assessed quality of diabetes care. Health Expect. (2015) 18(5):1621–32. doi: 10.1111/hex.12151
29. Mata-Cases M, Franch-Nadal J, Real J, Cedenilla M, Mauricio D. Prevalence and coprevalence of chronic comorbid conditions in patients with type 2 diabetes in Catalonia: a population-based cross-sectional study. BMJ Open (2019) 9(10):e031281. doi: 10.1136/bmjopen-2019-031281
30. Alshaya AK, Alsayegh AK, Alshaya HK, Almutlaq BA, Alenazi NSG, Rasheedi HMAA, et al. The common complications and comorbidities among saudi diabetic patients in northern Saudi Arabia. OJEMD. (2017) 07(07):151–61. doi: 10.4236/ojemd.2017.77014
31. Caughey GE, Roughead EE, Vitry AI, McDermott RA, Shakib S, Gilbert AL. Comorbidity in the elderly with diabetes: Identification of areas of potential treatment conflicts. Diabetes Res. Clin. Practice. (2010) 87(3):385–93. doi: 10.1016/j.diabres.2009.10.019
32. Kohn JC, Lampi MC, Reinhart-King CA. Age-related vascular stiffening: causes and consequences. Front. Genet. (2015) 6:112. doi: 10.3389/fgene.2015.00112
33. Kitai Y, Nangaku M, Yanagita M. Aging-related kidney diseases. Contrib Nephrol. (2021) 199:266–73. doi: 10.1159/000517708
34. Zhao Y, Zhang P, Lee JT, Oldenburg B, Heusden AV, Haregu TN, et al. The prevalence of metabolic disease multimorbidity and its associations with spending and health outcomes in middle-aged and elderly chinese adults. Front. Public Health (2021) 9:658706. doi: 10.3389/fpubh.2021.658706
35. Leitzmann M. Physical activity, sedentary behaviour, and obesity, in: Energy balance and obesity (2017). Lyon (FR): International Agency for Research on Cancer. Available at: http://www.ncbi.nlm.nih.gov/books/NBK565813/ (Accessed 2023 Oct 3).
36. Bavuma CM, Musafiri S, Rutayisire PC, Ng’ang’a LM, McQuillan R, Wild SH. Socio-demographic and clinical characteristics of diabetes mellitus in rural Rwanda: time to contextualize the interventions? A cross-sectional study. BMC Endocr. Disord. (2020) 20(1):180. doi: 10.1186/s12902-020-00660-y
37. Lynch CP, Gebregziabher M, Axon RN, Hunt KE, Payne E, Egede LE. Geographic and racial/ethnic variations in patterns of multimorbidity burden in patients with type 2 diabetes. J. Gen. Intern. Med. (2015) 30(1):25–32. doi: 10.1007/s11606-014-2990-y
38. Guerrero-Fernández De Alba I, Orlando V, Monetti VM, Mucherino S, Gimeno-Miguel A, Vaccaro O, et al. Comorbidity in an older population with type-2 diabetes mellitus: identification of the characteristics and healthcare utilization of high-cost patients. Front. Pharmacol. (2020) 11:586187. doi: 10.3389/fphar.2020.586187
39. Nowakowska M, Zghebi SS, Ashcroft DM, Buchan I, Chew-Graham C, Holt T, et al. The comorbidity burden of type 2 diabetes mellitus: patterns, clusters and predictions from a large English primary care cohort. BMC Med. (2019) 17(1):145. doi: 10.1186/s12916-019-1373-y
40. Khan A, Uddin S, Srinivasan U. Comorbidity network for chronic disease: A novel approach to understand type 2 diabetes progression. Int. J. Med. Informatics. (2018) 115:1–9. doi: 10.1016/j.ijmedinf.2018.04.001
41. Maheshwari A. Proteinuria, a marker of cardiovascular risks. JDMDC (2018) 5(6):208–210. doi: 10.15406/jdmdc.2018.05.00167
42. Lee H, Park MS, Kang MK, Song TJ. Association between proteinuria status and risk of hypertension: A nationwide population-based cohort study. J. Pers. Med. (2023) 13(9):1414. doi: 10.3390/jpm13091414
43. Haider MZ, Aslam A. Proteinuria, in: StatPearls (2023). Treasure Island (FL: StatPearls Publishing. Available at: http://www.ncbi.nlm.nih.gov/books/NBK564390/ (Accessed 2023 Oct 2).
44. Blaustein MP, Zhang J, Chen L, Hamilton BP. How does salt retention raise blood pressure? Am. J. Physiol. Regul. Integr. Comp. Physiol. (2006) 290(3):R514–523. doi: 10.1152/ajpregu.00819.2005
Keywords: concordant comorbidity, diabetes mellitus, diabetic epidemiology, adama, Ethiopia
Citation: Negussie YM, Getahun MS and Bekele NT (2023) Factors associated with diabetes concordant comorbidities among adult diabetic patients in Central Ethiopia: a cross-sectional study. Front. Clin. Diabetes Healthc. 4:1307463. doi: 10.3389/fcdhc.2023.1307463
Received: 04 October 2023; Accepted: 27 November 2023;
Published: 13 December 2023.
Edited by:
Nikos Pantazis, National and Kapodistrian University of Athens, GreeceReviewed by:
Neftali Eduardo Antonio-Villa, National Institute of Cardiology Ignacio Chavez, MexicoGeorgia Vourli, National and Kapodistriann University of Athens, Greece
Copyright © 2023 Negussie, Getahun and Bekele. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yohannes Mekuria Negussie, eW9oYW5uZXNfbWVrdXJpYUB5YWhvby5jb20=
†ORCID: Yohannes Mekuria Negussie, orcid.org/0000-0002-1332-670X
 Yohannes Mekuria Negussie
Yohannes Mekuria Negussie Mihiret Shawel Getahun2
Mihiret Shawel Getahun2