- Department of Experimental Medicine, Sapienza University of Rome, Rome, Italy
An emerging research niche has focused on the link between social determinants of health and diabetes mellitus, one of the most prevalent non-communicable diseases in modern society. The aim of the present mini-review is to explore and summarize current findings in this field targeting high-income countries. In the presence of disadvantaged neighborhood factors (including socioeconomic status, food environment, walkability and neighborhood aesthetics), diabetes prevention and care are affected at a multidimensional level. The vast majority of the included studies suggest that, besides individual risk factors, aggregated neighborhood inequalities should be tackled to implement effective evidence-based policies for diabetes mellitus.
Introduction
According to the most recent global data, from 1990 to 2021 the estimated annual percentage in diabetes mellitus prevalence rose from 3.2% to 6.1%. Between-country analysis revealed that a rise in diabetes incidence and prevalence has occurred globally, independent of country-level income (1). A relevant research niche, focusing on neighborhood disparities, has been growing in interest with interventional studies showing encouraging results in terms of diabetes prevalence reduction (2). Besides disease prevention, a number of domains of diabetes care, from glycemic control to complications and economic burden, may be affected by social determinants of health at the residential level. The aim of the present review is to assess to which extent neighborhood factors are associated with diabetes mellitus.
Methods
We searched the Pubmed/MEDLINE database for relevant studies published in the last 10 years. Our search strategy combined Medical subject heading (MeSH) terms (“Diabetes mellitus”, “social determinants of health”, “Residence Characteristics”) with relevant keywords (diabetes, neighborhood, neighbourhood, income, "economic status", social, old, aged, handgrip, grip, strength) and truncated words (function*, dynap*, sarcop*, frail*, elder*) connected by Boolean operators (AND, OR, NOT). Exclusion criteria were: low- or middle-income countries, languages other than English. The latest search was performed on August 3, 2023. The search retrieved 2177 records; after screening by title and abstract, 49 reports were searched for retrieval, of which 33 articles were selected. Furthermore, 2 additional reports were obtained through cross-citation, leading to the final inclusion of 35 articles in the present study. Since our search was limited to one database, included only high-income countries and aimed to present a brief state-of-the-art summary on the topic, the mini-review format was adopted.
Neighborhood characteristics influencing diabetes incidence and prevalence
Neighborhood socioeconomic disadvantage
Several neighborhood aspects have been identified and their association with the risk of diabetes has been explored. Among these factors, socioeconomic disadvantage is the most studied. In Bilal et al. (3) 199,621 residents (mean age: 57.6 years; 56% women) of Spanish neighborhoods undergoing greater transformation processes (e.g., new housing and improved socioeconomic status, SES) showed a lower hazard ratio (HR) of diabetes risk than people living in stable areas. In another study, neighborhoods with a high level of disadvantage showed an increased risk of obesity and diabetes, as well as higher odds ratios (ORs) for hypertension and fatty liver (4). Furthermore, in a longitudinal study on 11,035 Australians self-reporting a set of NCDs (min-max age: 40-65 years; 55% women), residents of disadvantaged neighborhoods showed higher ORs of Type 2 Diabetes Mellitus (T2DM) onset (OR: 2.21), heart disease (OR: 1.72), and comorbidity (OR: 4.38) compared to districts with higher socioeconomic status (5). In an elegant Swedish study, 61,386 refugees (min-max age: 25-50 years; 53% women) were randomly assigned to different SES neighborhoods at their entrance into the country; high-deprivation areas increased the refugees’ risk of diabetes and prolonged exposure to high-deprivation neighborhoods (up to 5 years) was associated with a 9% increase in diabetes risk (6). Similar findings emerged from a US study, revealing a higher diabetes prevalence (24.5%) in urban settings compared to either suburban or small-town areas (18.5%), suggesting that individual lifestyle and other neighborhood attributes could also affect diabetes prevalence in high-density residential areas (7). Moreover, the rate of unemployment was associated with an increased risk of T2DM (HR: 1.72) in a German cohort (n = 7,250; min-max age: 45-74 years; 51% women), regardless of any individual characteristic (8).
Neighborhood environmental attributes: walkability, green space and air pollution
Walkability is a physical environmental characteristic of neighborhood and urban design, which has the potential to influence different types of physical activity, such as walking and cycling. Neighborhood walkability is composed of three factors: street connectivity, residential density, and land use mix (9). A Canadian longitudinal study on 958,567 adults (age > 18 years) found that neighborhoods with comparable walkability levels, even when located in different regions, showed similar diabetes incidence rates, regardless of immigration status and income level. Neighborhoods with high walkability were associated with a lower incidence of diabetes among adults (8.2 vs. 9.2 per 1000; HR: 0.85) but not among the elders (20.7 vs. 19.5 per 1000; HR: 1.01) (10). Another Canadian study analyzed 8,777 neighborhoods across 7 cities with a Walkability Index ranging from 10.1 to 35.2. Over a 12-year period, areas with low walkability showed an increased prevalence of overweight and obesity (absolute changes: 5.4% in quintile 1, 6.7% in quintile 2 and 9.2% in quintile 3). On the other hand, high walkability areas displayed a reduction in diabetes incidence: from 7.7 to 6.2 per 1000 persons in quintile 5 (absolute change: −1.5) and from 8.7 to 7.6 in quintile 4 (absolute change: −1.1) (11). Nonetheless, a Swedish study found an inverse association between T2DM and neighborhood walkability, but statistical significance was not reached after adjusting for individual socio-demographic factors (9). Conversely, a German cohort study with a 9-year follow-up (n = 16,008; mean age: 53 years; 50.4% women) found no significant association between walkability and change in diabetes prevalence (12).
Similar results were observed for the relationship between green space/neighborhood aesthetics and risk of diabetes onset. Green space quantity is usually assessed within a radius of 1-3 km surrounding each participant’s home. In the study by Astell-Burt et al. (13) including 267,072 Australians (age > 45 years), residents in areas with 20% or less green space showed a higher rate of T2DM (9.1%) than those living in neighborhoods with 40% or more green space (8%). Furthermore, a study conducted in the UK on 10,746 adult participants (mean age: 59 years; 47% women; 21% Non-White ethnicity) found a negative correlation between green space and T2DM prevalence. Compared to the lowest quartile, diabetes ORs for increasing quartiles of green space were 0.97 (95% CI: 0.80 to 1.17), 0.78 (95% CI: 0.62 to 0.98) and 0.67 (95% CI: 0.49 to 0.93) respectively, after adjusting for social deprivation, urban status and individual-level covariates (14). Analogous findings were reported by Dalton et al. (15): 23,865 residents (mean age: 59 years; 55.1% men) in areas in the highest quartile of green space showed a 19% lower risk of developing diabetes than other quartiles, even after adjustment for demographic factors and SES. Interestingly, air pollution (i.e., high levels of NO2, PM2.5 and PM10) was associated with an increased risk of T2DM in the city of Leicester, UK (16).
Neighborhood food environment
A research body of limited amplitude has investigated the role of neighborhood food store distribution, with emphasis on the density of food outlets selling unhealthy foods in residential areas. A large longitudinal cohort study conducted on 4,100,650 US veterans without T2DM (mean age: 59.4 years; 92.2% males; 76.3% Non-Hispanic White ethnicity) investigated diabetes incidence rate over a follow-up period of 5.5 person-years, taking into account variation in neighborhood food environment. A positive, moderate association was observed between fast-food density and T2DM risk, whereas supermarket density was associated with a lower T2DM risk only in suburban and rural contexts (17). In Mezuk et al. (18) a large cohort of 4,718,583 adult Swedes (min-max age= 35-80 years) was prospectively followed from 2005 to 2010: neighborhoods with ≥30% unhealthy food stores had the highest T2DM rate. Moreover, participants who moved to worse food environments showed increased diabetes risk. Similar observations revealed that a healthy food environment, combined with neighborhood physical activity resources, diminished the number of T2DM diagnosis among 5,124 US participants (mean age: 60.7 years; 53.6% women; 57.7% Non-White ethnicity) over a 8.9-year follow-up (19).
Glycemic control and neighborhood characteristics
Neighborhood characteristics have been found to affect glycated hemoglobin (HbA1c) levels in different ways. A cross-sectional study (20) on 615 patients with T2DM (age > 18; 61.6% men; 65.7% Non-Hispanic Black ethnicity) showed that neighborhood aesthetics had a direct effect and access to healthy foods an indirect effect (i.e., mediated by self-care behaviors) on HbA1c % (β = 0.12, z = 2.19, p = 0.03 and β = - 0.17, z = -2.95, p = 0.003 respectively).
However, a subsequent analysis on the same sample (21) did not observe a significant effect of built environment features (namely neighborhood characteristics, neighborhood participation index and neighborhood problems index) on glycemic homeostasis in the fully-adjusted hierarchical model, whereas psychosocial factors (i.e., self-efficacy and social support) and comorbidities retained significance. Another cross-sectional study (22) on 424 TD2M patients (mean age: 60.5 ± 11.5 years; 52.8% women; 63.9% White ethnicity) reported that HbA1c % values were positively associated with higher scores of neighborhood social disorganization (B = 0.47, p = 0.003), a composite index including economic disadvantage, residential instability and ethnic heterogeneity. This effect was still significant (B = 0.39, p = 0.01) after controlling for demographic (race, age, sex, educational level) and psychosocial/clinical covariates (disease duration, diabetes distress, diabetes empowerment, selfcare, comorbidities). In a study conducted between 2013 and 2014 and stemming from the Heart Healthy Hoods project (23), which involved 269,942 electronic health records (EHR) of people aged ≥ 40 years living in Madrid (median age: 56.5 years, IQR age: 47.4- 69.8; 54.9% women), a composite neighborhood socio-economic status (SES) index was created (combining education, wealth, occupation and living conditions) and its association with HbA1c % levels among patients with T2DM was assessed. Using low SES neighborhoods as the reference group, and controlling for age and sex in the regression analysis, medium and high SES neighborhoods displayed decreased HbA1c % mean levels (mean change: -0.05, 95% CI: -0.01 to -0.10 and -0.11, 95% CI: -0.06 to -0.15, respectively) and a reduced prevalence ratio (PR) of uncontrolled diabetes (PR=0.95, 95% CI: 0.91 to 0.99 and PR= 0.91, 95% CI: 0.87 to 0.95, respectively). Within the Heart Healthy Hoods project, a sequential study (24) on 113,265 patients affected by T2DM (mean age: 62.7 ± 8.8 years; 56.8% men) confirmed a significant effect of neighborhood SES on glycemic control target (HbA1c % < 7.0%): after adjusting by sex and age, the SES index 5th quintile (the most deprived) displayed a higher risk of uncontrolled diabetes (OR= 1.20, 95% CI: 1.10 to 1.32) than the reference group (SES index 1st quintile). In a retrospective, longitudinal study (2007–2013) on 182,756 adults with diabetes living in New York City (mean age= 64 years; 47.4% White ethnicity) (25), a residential composite score (reflecting neighborhood socioeconomic status, food environment and aesthetics) was computed, with residential areas categorized into quintiles (1st quintile= least advantaged, reference group; 5th quintile= most advantaged) and incorporated into a multilevel model. Living in a 5th quintile area was associated with a higher probability of reaching HbA1c % < 7% (OR= 2.59, CI 2.43 to 2.77), a shorter median time to reach glycemic control (median time 9.9 vs. 11.5 months; HR 1.14, CI 1.12 to 1.16) and lower HbA1c % levels (mean difference= - 0.44%). Finally, moving from the 1st to the 5th quintile residential areas was associated with better glycemic control (mean HbA1c % reduction: 0.40%, 95% CI 0.22 to 0.55), while the opposite was evident for those moving from the more advantaged to less advantaged areas (mean HbA1c % rise = 0.33%, 95% CI: 0.24 to 0.44).
Another retrospective longitudinal study on 2,662 patients (mean age: 69.3 ± 9.13 years; 55.3% women; 65.5% Non-Hispanic White ethnicity) (26) with self-reported diabetes investigated the role of four neighborhood factors (i.e., social cohesion, social participation, physical disorder, and perceived everyday discrimination) on glycemic control over time. In the unadjusted model, only social cohesion was significantly associated with HbA1c values (β = -0.05, 95% CI -0.10 to -0.001) among neighborhood factors; however, this relationship became non-significant after adjusting for demographic, psychosocial and financial factors.
A third retrospective longitudinal study (27) on EHR data of 15,308 patients affected by T2DM (mean age = 57.8 ± 11.9 years; 45.8% women; 96.4% Non-Hispanic White ethnicity) assessed the relationship between four community factors (namely: community socioeconomic deprivation, CSD; food availability; fitness and recreational assets; utilitarian physical activity favorability), HbA1c levels and pharmacological therapy intensification (TI), after stratifying by community type (townships, boroughs and city census tracts). Over a 6-month period, the mean HbA1c reduction was 0.07% lower in townships in the 3rd CSD quartile vs. 1st CSD quartile and 0.10% higher in townships in the 4th food availability quartile (i.e., best food availability) vs. 1st food availability quartile (i.e., worst food availability). In townships and boroughs, TI was associated with a smaller 6-month HbA1c reduction in the 4th vs. 1th CSD quartile, which was confirmed in the 24-month analysis only for census tracts. Finally, one prospective study (28) investigated the role of neighborhood walkability on 1-year glycemic control in a cohort of 1,230 patients with T2DM (mean age: 68.9 ± 9.0 years; 62.6% men) of the Diabetes Care System Cohort. Neither objective walkability (based on geographic information system) nor subjective walkability (questionnaire-based) showed a significant main association with changes in HbA1c and fasting glycemia; likewise, after adjusting for the mediator (total physical activity or moderate-vigorous physical activity, in turn) no direct effect was apparent.
Access and adherence to therapy, access to healthcare services and other health outcomes
A number of studies investigated to which extent neighborhood characteristics affect self-care behaviors in the context of different diseases, showing a relevant impact on psycho-physical well-being. Concerning the relationship between environmental factors and diabetes, some authors focused on the interference of community and neighborhood on healthcare access and process (e.g., medication adherence).
A study on a cohort of 615 adults with T2DM (29) (South-Eastern US, one third White ethnicity, approximately two third were men), observed significant associations between neighborhood activities and some self-care behaviors, such as exercise (β= - 0.104), diet (β= - 0.072) and foot care (β= - 0.114). Exercise was also negatively associated with walking environment (β= -0.040), whereas medication adherence was negatively associated with food insecurity (β = -0.147). Based on data from 179 patients enrolled in a prospective randomized controlled trial (30) (of whom two third were women and one third was White), the adherence to oral hypoglycemic agents was examined according with some neighborhood-related characteristics, namely: social affluence, residential stability 12.91, 95% CI: 2.20-75.80). In addition and neighborhood advantage. Patients living in a neighborhood with all the above-mentioned factors had a better adherent pattern than residents living in neighborhoods with low indicators (adjusted OR: 12.91, 95% CI: 2.20-75.80). In addition to adherence patterns, some authors investigated the access to different medication categories based on neighborhood features. In a large cohort of 1,203,317 Australian individuals with T2DM, Morton et al. examined diabetes medication dispensing based on remoteness.
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) were less prescribed in patients living in regional areas compared to their counterparts living in major cities, though this difference was blunted over time (31). When considering remote areas, a delay was observed in the access to any new glucose- lowering drug, requiring from five to seven years since medication release (for Dipeptidyl peptidase-4 inhibitors, DPP4i) and GLP-1RAs, respectively) to reduce the magnitude of the difference compared to major cities. As for SGLT2 inhibitors, patients were less likely to receive those medications in remote areas compared to major cities. Kowitt et al. (22) found that among 450 patients with T2DM, living in neighborhoods with high economic disadvantage was positively associated with the use of acute/emergency health care services, compared to the medium economic disadvantage as the reference group (β = 0.60, 95% CI: 0.10 to 1.09). However, when other neighborhood indicators of social disorganization were taken into account and considered as a single composite measure (namely, neighborhood economic disadvantage, residential instability, as ethnic heterogeneity), no association emerged with self-reported use of acute/emergency health care services.
A Canadian study examined non-drug related healthcare costs among 698,112 adults with diabetes, revealing that individuals in the young age class (20-39 years) exhibited an increase of costs up to 41.3% (32) in the lowest quintile of neighborhood socioeconomic status compared to Ontarians with diabetes in the highest quintile.
In another Canadian study (33) involving a cohort of immigrants and long-term residents with diabetes (n = 175,414), individuals living in urban areas had a significantly reduced risk for cardiovascular events or death (fully adjusted HR: 0.85, 95% CI: 0.76-0.95), underscoring the relevant impact of the neighborhood of settlement.
According to data from the 2014 Health Center Patient Survey (34), unstable housing (37% out of 1,087 participants) was related to a five-fold increase of the risk of diabetes-related emergency department and hospital use in the past year (OR: 5.17, 95% CI: 2.08 to 12.87) compared to stable housing, after adjustment for potential confounders. A whole-population longitudinal study (35) conducted in England investigated the changes in the slope indices of inequality (SIIs) between neighborhoods of least versus greatest deprivation (for a total of 32,482 neighborhoods). From 2004/2005 to 2011/2012 emergency hospitalizations for diabetes increased especially in neighborhoods of greater deprivation (SII change: +19.59 admissions for diabetes per 100 000, 95% CI: 16.00 to 23.17). Conversely diabetes-related amenable mortality decreased at a faster rate in neighborhoods of greater deprivation, with the SII reduced by 2.68 (95% CI: 1.93 to 3.43).
Discussion
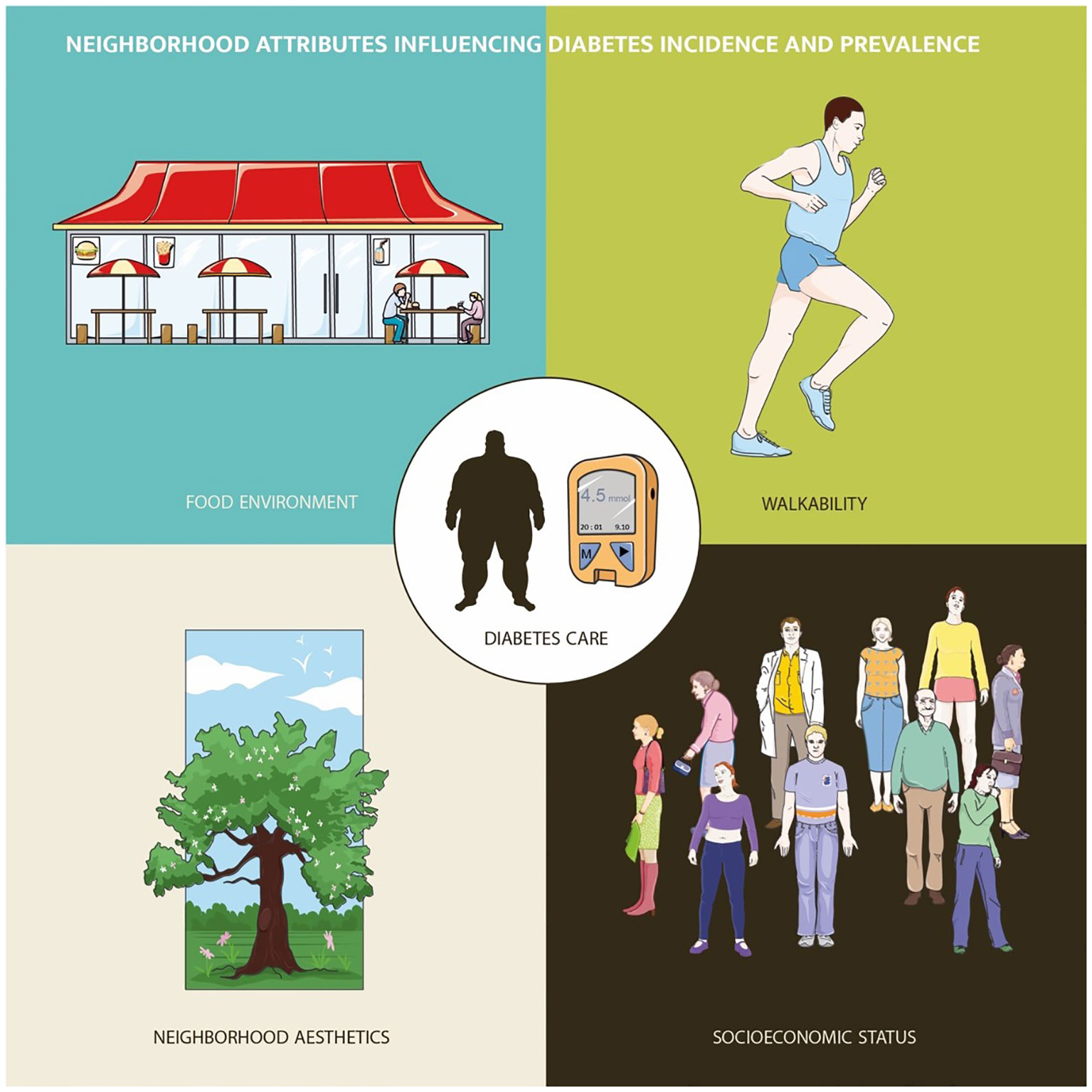
Diabetes-related dimensions – from disease onset to access to medical therapy and glycemic control – have been thoroughly studied during the last decade; in this context, different neighborhood attributes can modulate diabetes epidemiology and care (Figure 1). For instance, walkability, greenspace presence and air quality in neighborhoods were correlated with reduced diabetes incidence and prevalence; as a matter of fact, factors other than neighborhood environmental (including food and beverage retail composition) and geographical characteristics may have mediated the relationship between areas of residence and diabetes mellitus risk.
Of note, at times the effects of residential socio-economic disparities were no longer apparent after adjusting for individual characteristics. For example, using glycemic control as the outcome measure, the included studies were concordant on the role of specific residential socioeconomic, food environment and aesthetic characteristics on HbA1c % levels, both at baseline and prospectively. Nonetheless, when considering composite neighborhood scores and after controlling for baseline covariates, this relationship became non-significant in some models. Continuously living in or moving to a more advantaged neighborhood was also associated with a higher probability of reaching glycemic control and reduced time-to-event, in comparison with disadvantaged areas. However, neighborhood walkability and subjective walkability were neither directly nor indirectly associated with glycated hemoglobin values, whereas the availability of fitness assets was positively associated with glycemic control in one work. It is worth highlighting that this lack of significance may be partly explained by the known inaccuracy of self-reported questionnaires regarding individual physical activity levels (36) and by the lack of variability in objective neighborhood walkability, as the authors of the paper suggested (28). Indeed, a homogenous level of exposure to this (putative) risk factor would hinder the statistical power of observational studies to find a significant association (37). Analogous observations underscored the remarkable role of neighborhood deprivation on access to medications, novel medication dispensing, adherence to antidiabetic therapy, and access to healthcare services related to T2DM. On the other hand, medication adherence was negatively associated with food insecurity, indicating that cultural factors (neighborhood-related and/or neighborhood-mediated) can exacerbate the connection. The so-called “causes of incidence” (based on Geoffrey Rose’s theory on the differences in individual and population causes of diseases) stem from the population’s social, cultural, economic and political contexts that make environments detrimental for the promotion of the efficacy of prevention strategies (37). In conclusion, it is crucial that policymakers develop evidence-based policies at national and regional levels based on effective multidimensional treatments. The latter should not solely rely on tackling individual risk factors, which may be particularly inefficient among the most deprived (38), but on population-level interventions in order to display a true impact on diabetes inequalities.
Author contributions
FF: Investigation, Methodology, Writing – original draft. LM: Data curation, Software, Visualization, Writing – original draft. AP: Supervision, Writing – review & editing, Validation. LD: Supervision, Writing – review & editing, Resources, Validation. EP: Conceptualization, Writing – original draft, Methodology, Supervision, Validation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. LD acknowledges the support of grant PE00000003 (decree 1550, 11.10.2022) ON Foods—Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods) from the Italian Ministry of University and Research (CUP D93C22000890001) under the National Recovery and Resilience Plan (NRRP), funded by the European Union-NextGenerationEU.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ong KL, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet (2023) 402:203–34. doi: 10.1016/s0140-6736(23)01301-6
2. Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, et al. Neighborhoods, obesity, and diabetes — A randomized social experiment. New Engl. J. Med. (2011) 365:1509–19. doi: 10.1056/NEJMSA1103216/SUPPL_FILE/NEJMSA1103216_DISCLOSURES.PDF
3. Bilal U, Glass TA, del Cura-Gonzalez I, Sanchez-Perruca L, Celentano DD, Franco M. Neighborhood social and economic change and diabetes incidence: The HeartHealthyHoods study. Health Place (2019) 58:102149. doi: 10.1016/j.healthplace.2019.102149
4. Kivimäki M, Vahtera J, Tabák AG, Halonen JI, Vineis P, Pentti J, et al. Neighbourhood socioeconomic disadvantage, risk factors, and diabetes from childhood to middle age in the Young Finns Study: a cohort study. Lancet Public Health (2018) 3:e365–73. doi: 10.1016/S2468-2667(18)30111-7
5. Rachele JN, Giles-Corti B, Turrell G. Neighbourhood disadvantage and self-reported type 2 diabetes, heart disease and comorbidity: A cross-sectional multilevel study. Ann. Epidemiol. (2016) 26:146–50. doi: 10.1016/j.annepidem.2015.11.008
6. White JS, Hamad R, Li X, Basu S, Ohlsson H, Sundquist J, et al. Long-term effects of neighbourhood deprivation on diabetes risk: Quasi-experimental evidence from a refugee dispersal policy in Sweden. Lancet Diabetes Endocrinol. (2016) 4:517–24. doi: 10.1016/S2213-8587(16)30009-2
7. Uddin J, Malla G, Long DL, Zhu S, Black N, Cherrington A, et al. The association between neighborhood social and economic environment and prevalent diabetes in urban and rural communities: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. SSM Popul Health (2022) 17:101050. doi: 10.1016/j.ssmph.2022.101050
8. Müller G, Wellmann J, Hartwig S, Greiser KH, Moebus S, Jöckel KH, et al. Association of neighbourhood unemployment rate with incident Type 2 diabetes mellitus in five German regions. Diabetic Med. (2015) 32:1017–22. doi: 10.1111/dme.12652
9. Sundquist K, Eriksson U, Mezuk B, Ohlsson H. Neighborhood walkability, deprivation and incidence of type 2 diabetes: A population-based study on 512,061 Swedish adults. Health Place (2015) 31:24–30. doi: 10.1016/j.healthplace.2014.10.011
10. Booth GL, Creatore MI, Luo J, Fazli GS, Johns A, Rosella LC, et al. Neighbourhood walkability and the incidence of diabetes: An inverse probability of treatment weighting analysis. J. Epidemiol. Community Health (1978) (2019) 73:287–94. doi: 10.1136/jech-2018-210510
11. Creatore MI, Glazier RH, Moineddin R, Fazli GS, Johns A, Gozdyra P, et al. Association of neighborhood walkability with change in overweight, obesity, and diabetes. JAMA - J. Am. Med. Assoc. (2016) 315:2211–20. doi: 10.1001/jama.2016.5898
12. Kartschmit N, Sutcliffe R, Sheldon MP, Moebus S, Greiser KH, Hartwig S, et al. Walkability and its association with prevalent and incident diabetes among adults in different regions of Germany: Results of pooled data from five German cohorts. BMC Endocr. Disord. (2020) 20(1):7. doi: 10.1186/s12902-019-0485-x
13. Astell-Burt T, Feng X, Kolt GS. Is neighborhood green space associated with a lower risk of type 2 diabetes evidence from 267,072 Australians. Diabetes Care (2014) 37:197–201. doi: 10.2337/dc13-1325
14. Bodicoat DH, O’Donovan G, Dalton AM, Gray LJ, Yates T, Edwardson C, et al. The association between neighbourhood greenspace and type 2 diabetes in a large cross-sectional study. BMJ Open (2014) 4:e006076. doi: 10.1136/BMJOPEN-2014-006076
15. Dalton AM, Jones AP, Sharp SJ, Cooper AJM, Griffin S, Wareham NJ. Residential neighbourhood greenspace is associated with reduced risk of incident diabetes in older people: a prospective cohort study. BMC Public Health (2016) 16:1–10. doi: 10.1186/s12889-016-3833-z
16. O’Donovan G, Chudasama Y, Grocock S, Leigh R, Dalton AM, Gray LJ, et al. The association between air pollution and type 2 diabetes in a large cross-sectional study in Leicester: The CHAMPIONS Study. Environ. Int. (2017) 104:41–7. doi: 10.1016/j.envint.2017.03.027
17. Kanchi R, Lopez P, Rummo PE, Lee DC, Adhikari S, Schwartz MD, et al. Longitudinal analysis of neighborhood food environment and diabetes risk in the veterans administration diabetes risk cohort. JAMA Netw. Open (2021) 4(10):e2130789. doi: 10.1001/jamanetworkopen.2021.30789
18. Mezuk B, Li X, Cederin K, Rice K, Sundquist J, Sundquist K. Beyond access: Characteristics of the food environment and risk of diabetes. Am. J. Epidemiol. (2016) 183:1129–37. doi: 10.1093/aje/kwv318
19. Christine PJ, Auchincloss AH, Bertoni AG, Carnethon MR, Sánchez BN, Moore K, et al. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). JAMA Intern. Med. (2015) 175:1311–20. doi: 10.1001/jamainternmed.2015.2691
20. Smalls BL, Gregory CM, Zoller JS, Egede LE. Direct and indirect effects of neighborhood factors and self-care on glycemic control in adults with type 2 diabetes. J. Diabetes Complications (2015) 29:186–91. doi: 10.1016/j.jdiacomp.2014.10.008
21. Walker RJ, Smalls BL, Egede LE. Social determinants of health in adults with type 2 diabetes-Contribution of mutable and immutable factors. Diabetes Res. Clin. Pract. (2015) 110:193–201. doi: 10.1016/j.diabres.2015.09.007
22. Kowitt SD, Donahue KE, Fisher EB, Mitchell M, Young LA. How is neighborhood social disorganization associated with diabetes outcomes? A multilevel investigation of glycemic control and self-reported use of acute or emergency health care services. Clin. Diabetes Endocrinol. (2018) 4:19. doi: 10.1186/s40842-018-0069-0
23. Bilal U, Hill-Briggs F, Sánchez-Perruca L, Del Cura-González I, Franco M. Association of neighbourhood socioeconomic status and diabetes burden using electronic health records in Madrid (Spain): The Heart Healthy Hoods study. BMJ Open (2018) 8(9):e021143. doi: 10.1136/bmjopen-2017-021143
24. Ares-Blanco S, Polentinos-Castro E, Rodríguez-Cabrera F, Gullón P, Franco M, del Cura-González I. Inequalities in glycemic and multifactorial cardiovascular control of type 2 diabetes: The Heart Healthy Hoods study. Front. Med. (Lausanne) (2022) 9:966368. doi: 10.3389/fmed.2022.966368
25. Tabaei BP, Rundle AG, Wu WY, Horowitz CR, Mayer V, Sheehan DM, et al. Associations of residential socioeconomic, food, and built environments with glycemic control in persons with diabetes in New York City from 2007-2013. Am. J. Epidemiol. (2018) 187:736–45. doi: 10.1093/aje/kwx300
26. Walker RJ, Garacci E, Palatnik A, Ozieh MN, Egede LE. The longitudinal influence of social determinants of health on glycemic control in elderly adults with diabetes. Diabetes Care (2020) 43:759–66. doi: 10.2337/dc19-1586
27. Hirsch AG, Durden TE, Nordberg C, Berger A, Schwartz BS. Associations of four community factorswith longitudinal change in hemoglobin A1c levels in patients with type 2 diabetes. Diabetes Care (2018) 41:461–8. doi: 10.2337/dc17-1200
28. Braver NR, Rutters F, Wagtendonk AJ, Kok JG, Harms PP, Brug J, et al. Neighborhood walkability, physical activity and changes in glycemic markers in people with type 2 diabetes: The Hoorn Diabetes Care System cohort. Health Place (2021) 69:102560. doi: 10.1016/j.healthplace.2021.102560
29. Smalls BL, Gregory CM, Zoller JS, Egede LE. Assessing the relationship between neighborhood factors and diabetes related health outcomes and self-care behaviors. BMC Health Serv. Res. (2015) 15:445. doi: 10.1186/s12913-015-1086-7
30. De Vries McClintock HF, Wiebe DJ, O’Donnell AJ, Morales KH, Small DS, Bogner HR. Neighborhood social environment and patterns of adherence to oral hypoglycemic agents among patients with type 2 diabetes mellitus. Fam Community Health (2015) 38:169–79. doi: 10.1097/FCH.0000000000000069
31. Morton JI, Ilomäki J, Magliano DJ, Shaw JE. The association of socioeconomic disadvantage and remoteness with receipt of type 2 diabetes medications in Australia: a nationwide registry study. Diabetologia (2021) 64:349–60. doi: 10.1007/s00125-020-05304-3
32. Isaranuwatchai W, Fazli GS, Bierman AS, Lipscombe LL, Mitsakakis N, Shah BR, et al. Universal drug coverage and socioeconomic disparities in health care costs among persons with diabetes. Diabetes Care (2020) 43:2098–105. doi: 10.2337/dc19-1536
33. Okrainec K, Bell CM, Hollands S, Booth GL. Risk of cardiovascular events and mortality among a population-based cohort of immigrants and long-term residents with diabetes: Are all immigrants healthier and if so, for how long? Am. Heart J. (2015) 170:123–32. doi: 10.1016/j.ahj.2015.04.009
34. Berkowitz SA, Kalkhoran S, Edwards ST, Essien UR, Baggett TP. Unstable housing and diabetes-related emergency department visits and hospitalization: A nationally representative study of safety-net clinic patients. Diabetes Care (2018) 41:933–9. doi: 10.2337/dc17-1812
35. Fleetcroft R, Asaria M, Ali S, Cookson R. Outcomes and inequalities in diabetes bfrom 2004/2005 to 2011/2012: English longitudinal study. Br. J. Gen. Pract. (2017) 67:e1–9. doi: 10.3399/bjgp16X688381
36. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Activity (2008) 5:1–24. doi: 10.1186/1479-5868-5-56/FIGURES/8
37. Rose G. Sick individuals and sick populations. Int. J. Epidemiol. (2001) 30:427–32. doi: 10.1093/IJE/30.3.427
Keywords: diabetes, T2DM, inequalities, social determinants of health, neighborhood, residence characteristics
Citation: Frigerio F, Muzzioli L, Pinto A, Donini LM and Poggiogalle E (2023) The role of neighborhood inequalities on diabetes prevention care: a mini-review. Front. Clin. Diabetes Healthc. 4:1292006. doi: 10.3389/fcdhc.2023.1292006
Received: 10 September 2023; Accepted: 30 October 2023;
Published: 15 November 2023.
Edited by:
Massimiliano Caprio, Università Telematica San Raffaele, ItalyReviewed by:
María José Pérez-Lacasta, University of Rovira i Virgili, SpainCaterina Conte, Università Telematica San Raffaele, Italy
Copyright © 2023 Frigerio, Muzzioli, Pinto, Donini and Poggiogalle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eleonora Poggiogalle, ZWxlb25vcmEucG9nZ2lvZ2FsbGVAdW5pcm9tYTEuaXQ=
†These authors have contributed equally to this work
 Francesco Frigerio
Francesco Frigerio Luca Muzzioli
Luca Muzzioli Alessandro Pinto
Alessandro Pinto Lorenzo Maria Donini
Lorenzo Maria Donini Eleonora Poggiogalle
Eleonora Poggiogalle