
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Clin. Diabetes Healthc. , 30 October 2023
Sec. Diabetes Cardiovascular Complications
Volume 4 - 2023 | https://doi.org/10.3389/fcdhc.2023.1258792
This article is part of the Research Topic Diabetes and Cardiovascular Complications: Synergistic Treatment Approaches View all 4 articles
Background: Type 2 diabetes mellitus (T2DM) patients with coronary artery disease (CAD) have an increased risk of recurrent cardiovascular events. These patients require optimal glucose control to prevent the progression of atherosclerotic cardiovascular disease (ASCVD). Contemporary guidelines recommend an HbA1c ≤7% to mitigate this risk. The aim of this study was to evaluate HbA1c control in T2DM patients with angiographically proven ASCVD.
Methods: We conducted a cross-sectional, retrospective study on consecutive T2DM patients with acute and chronic coronary syndromes managed in a tertiary academic hospital in South Africa. Glycaemic control was assessed by evaluating the glycated haemoglobin (HbA1c) level measured at index presentation with acute and chronic coronary syndromes and during the most recent follow-up visit.
Results: The study population comprised 262 T2DM patients with a mean age of 61.3 ± 10.4 years. At index presentation, 110 (42.0%) T2DM patients presented with ST-segment elevation myocardial infarction, 69 (26.3%) had non-ST-segment elevation myocardial infarction, 43 (16.4%) had unstable angina, and 40 (15.3%) had stable angina. After a median duration of 16.5 months (IQR: 7-29), 28.7% of the study participants had an HbA1c ≤7%. On multivariable logistic regression analysis, females were less likely to have poor glycaemic control (HbA1c above 7%) [odds ratio (OR): 0.42, 95% confidence interval (CI): 0.19-0.95, p=0.038]. Also, T2DM patients prescribed metformin monotherapy (OR: 0.34, 95% CI: 0.14-0.82, p=0.017) and patients with ST-segment depression on the electrocardiogram (OR: 0.39, 95% CI: 0.16-0.96, p=0.041) were less likely to have poor glycaemic control.
Conclusion: After a median duration of 16.5 months, only 28.7% of T2DM patients with CAD had an HbA1c ≤7%. This finding underscores the substantial unmet need for optimal diabetes control in this very high-risk group.
Type 2 diabetes mellitus (T2DM) is a global pandemic affecting approximately 537 million adults between 20-79 years (1). This figure is expected to rise to 643 million by the year 2030 and to rise even further to 784 million by the year 2045 (1). In South Africa, 10.1% of individuals older than 15 years have diabetes (2). This high prevalence poses significant health and socio-economic consequences (3). Diabetes mellitus leads to many diabetic-related complications, classified as micro and macrovascular complications. Microvascular complications include retinopathy, nephropathy, and neuropathy, while macrovascular complications encompass coronary artery disease (CAD), peripheral artery disease, and cerebrovascular disease (4).
Diabetes mellitus is a significant risk factor for CAD, causing accelerated atherosclerosis, resulting in severe and diffuse atherosclerotic cardiovascular disease (ASCVD) (5). In a meta-analysis involving 27,049 T2DM patients with at least one risk factor for cardiovascular disease, intensive glycaemic control reduced the risk of major adverse cardiovascular events by 9%, and the risk reduction was driven by myocardial infarction, while the risk of heart failure and stroke was unaffected (6). Furthermore, optimal glycaemic control has been found to slow the progression of coronary artery calcification and, therefore, atherosclerosis in asymptomatic diabetic patients without a history of CAD or stroke (7).
Multiple cardiovascular outcome trials evaluating the cardiovascular safety of novel anti-diabetic therapy have identified new diabetic therapeutic agents with proven cardiovascular benefits (8–12). It is now recommended that these agents with proven cardiovascular benefits be used as first-line therapy in T2DM patients with high and very high cardiovascular risk or established ASCVD (13). Many T2DM patients in low and middle-income countries (LMICs) are unable to access these organ-protective agents despite most diabetics in these regions failing to achieve adequate glycated haemoglobin (HbA1c) control on traditional agents alone (14). The aim of the study was to evaluate HbA1c control in T2DM patients with acute and chronic coronary syndromes, describe the management of these very high-risk patients using currently available therapies and determine the predictors of poor glycaemic control.
A cross-sectional retrospective study was conducted on 1000 consecutive patients who presented with acute and chronic coronary syndromes in the Division of Cardiology at the Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) between April 2017 and December 2019. The CMJAH is a state-owned tertiary academic hospital in Johannesburg, South Africa. Data was collected from the CMJAH catheterisation laboratory patient registry, which captures demographic data of all patients who undergo coronary angiography, and from the electronic health record system, which stores admission data of patients hospitalised in the cardiac intensive care unit and general cardiology wards.
Patients 18 years of age and older with T2DM and angiographically confirmed CAD were included in the study. Clinical parameters captured and analyzed as part of the study included patient demographics, risk factors for CAD, coronary angiography findings, clinical examination findings, laboratory parameters, electrocardiogram (ECG) and echocardiogram parameters, and medication. These clinical parameters reflected the patient’s status at the time of admission. To evaluate HbA1c control among T2DM patients, we assessed the baseline HbA1c measured when T2DM patients presented with coronary syndromes and the most recent HbA1c available on the National Health Laboratory Service electronic database. We defined optimal glycaemic control as an HbA1c ≤7% and poor glycaemic control as an HbA1c >7%. After excluding patients who did not meet the study inclusion criteria and those without baseline and follow-up HbA1c levels, the final cohort consisted of 262 T2DM patients (Figure 1).
Permission to conduct the study was obtained from the University of the Witwatersrand Human Research Ethics Committee (clearance certificate number: M191191). Patients were not required to provide informed consent as this was a retrospective study.
Stata (version 17, College Station, Texas) was used for the statistical analysis. Categorical data is summarized as frequencies and percentages, and a Pearson’s chi-square test was used to compare categorical variables. Normally distributed continuous data is summarized using the mean and standard deviation (SD), and non-normally distributed continuous data is presented as the median and interquartile range (IQR). The Student’s t-test was used to compare normally distributed continuous variables, and the Wilcoxon rank sum test was used to compare continuous data with a non-normal distribution. Univariable and multivariable logistic regression analyses were performed to determine clinical variables associated with poor glycaemic control (HbA1c above 7%), and the results are presented as odds ratios (OR) with their corresponding 95% confidence intervals (CI). To select candidate variables for inclusion in the univariable logistic regression model, we selected variables with a p-value less than 0.1 after conducting the Student’s t-test, Pearson’s chi-square and Wilcoxon rank sum tests. We subsequently included variables in the multivariable logistic regression model with a p-value of less than 0.05 on the univariable analysis. A p-value of less than 0.05 represented statistical significance.
The final study population comprised 262 T2DM patients, of which 188 (71.8%) were males. The mean age was 61.3 ± 10.4 years. At presentation with an acute or chronic coronary event, there were 148 (75.6%) to 196 (74.8%) T2DM patients with poor glycaemic control (HbA1c above 7%). Patients with a baseline HbA1c ≤7% were older than those with poor glycaemic control [63.6 ± 10.1 years (95% CI: 61.1-66.1) vs 60.5 ± 10.4 years (95% CI: 59.0-61.9); p-value= 0.0332]. The median systolic blood pressure was higher in patients with an HbA1c ≤7%, compared to those with an HbA1c above 7% [136 mmHg (IQR: 117-151) vs 124 mmHg (IQR: 112-142), p-value= 0.0121]. The median diastolic blood pressure was also higher in patients with an HbA1c ≤7%, compared to those with an HbA1c above 7% [85 mmHg (IQR: 75-97) vs. 78 mmHg (IQR: 71-88), p-value= 0.0205].
Among the 262 T2DM patients, 204 (77.9%) presented with Killip class 1 symptoms. Of these patients with Killip class 1 symptoms, 45 (68.2%) had an HbA1c ≤7% and 159 (81.1%) had an HbA1c above 7%, p-value= 0.029. Regarding the acute and chronic coronary syndrome presentation, 110 (42.0%) patients presented with ST-segment elevation myocardial infarction (STEMI). Of these patients with STEMI, 89 (80.9%) had an HbA1c above 7%. There were 69 (26.3%) patients with non-ST-segment elevation myocardial infarction, 43 (16.4%) with unstable angina and 40 (15.3%) with stable angina.
On the resting ECG at the time of admission into the hospital, 41 (15.7%) patients had ST-segment depression and 19 (46.3%) of these patients had an HbA1c ≤7%, while 22 (53.7%) had an HbA1c above 7%, p-value= 0.001. Coronary angiography revealed single vessel disease in 107 (40.8%) patients, double vessel disease in 92 (35.1%) and triple vessel disease in 63 (24.1%). Among the 63 patients with triple vessel disease, 51 (80.9%) had an HbA1c above 7%. The rest of the baseline demographics and clinical characteristics are depicted in Table 1.
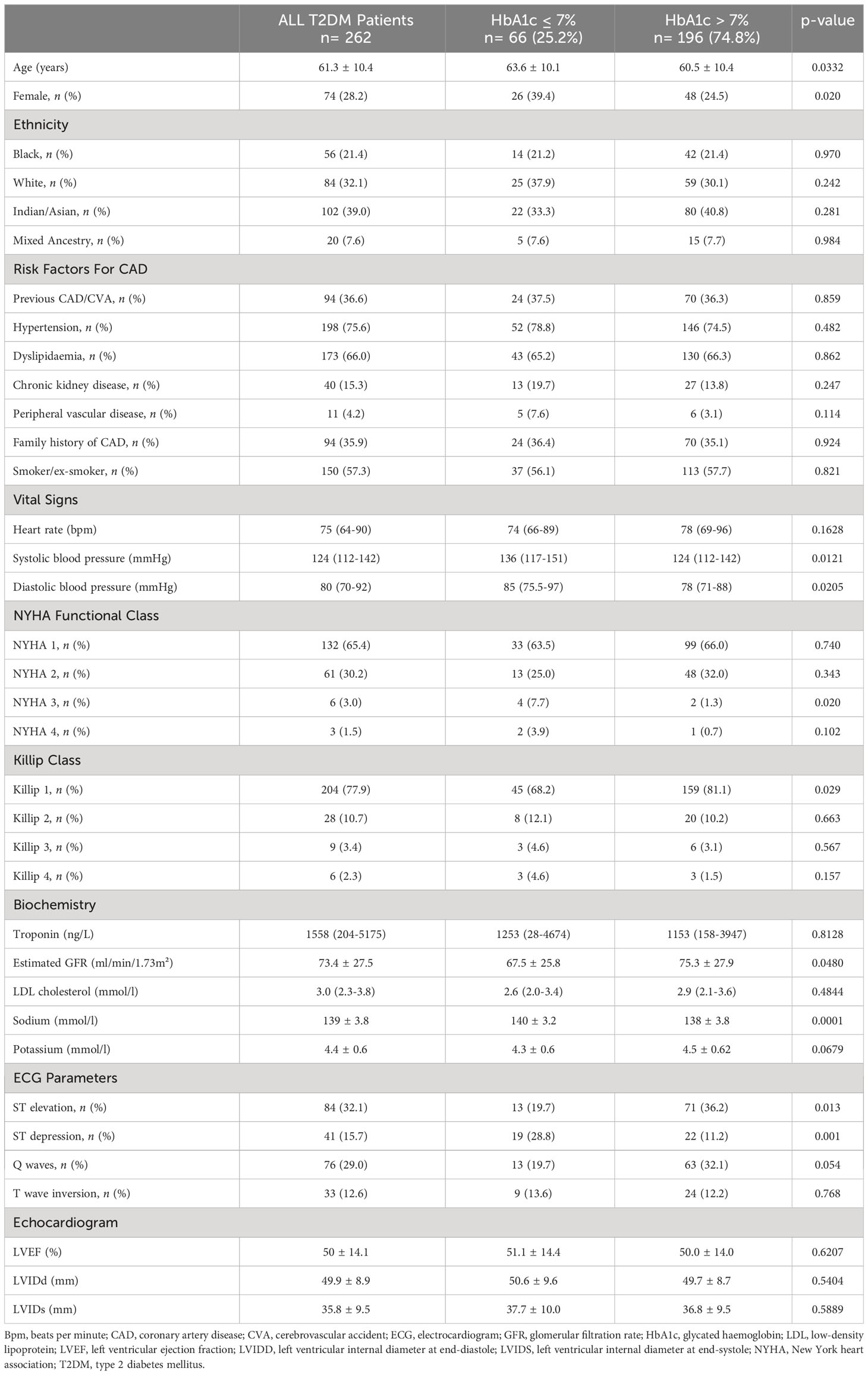
Table 1 Baseline characteristics of patients with Type 2 Diabetes Mellitus and coronary artery disease at index presentation with coronary syndromes.
Metformin was prescribed to 224 (85.5%) patients with T2DM. Among these patients, 140 (53.4%) were on metformin monotherapy, 41 (15.6%) patients were on metformin and insulin combination therapy, 40 (15.3%) were on metformin and sulphonylurea and three (1.1%) patients were prescribed metformin, sulphonylurea and insulin. Table 2 depicts the rest of the medications prescribed to the T2DM patients.
The proportion of diabetic patients with a baseline HbA1c ≤7% was 25.2% (95% CI: 18.4-31.5), and 74.8% (95% CI: 68.4-81.6) of T2DM had an HbA1c above 7%. After a median duration of 16.5 months (IQR: 7-29), 28.7% (95% CI: 22.2- 36.1) of T2DM patients had an HbA1c ≤7% and 71.3% (95% CI: 63.9-77.8) of patients had an HbA1c level above 7% (Figure 2).
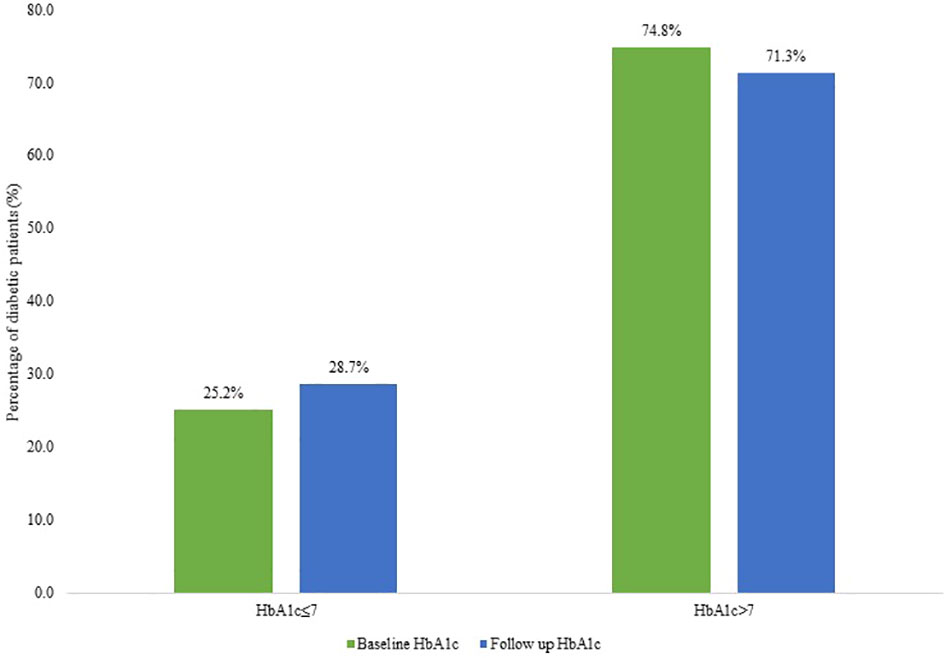
Figure 2 Graph showing baseline versus follow-up HbA1c in diabetic patients categorized as controlled diabetes mellitus (HbA1c ≤7) and poorly controlled diabetes mellitus (HbA1c >7%).
Univariable and multivariable logistic regression analyses were conducted to identify clinical variables associated with poor glycaemic control (HbA1c >7%). On multivariable logistic regression analysis, females were less likely to have poor glycaemic control [odds ratio (OR): 0.42, 95% CI: 0.19-0.95, p=0.038]. Also, T2DM patients prescribed metformin monotherapy were less likely to have poor glycaemic control (OR: 0.34, 95% CI: 0.14-0.82, p=0.017). Furthermore, T2DM patients with ST-segment depression on the ECG were less likely to have poor glycaemic control (OR: 0.39, 95% CI: 0.16-0.96, p=0.041). However, patients in Killip class one were three times more likely to have poor glycaemic control (OR: 3.39, 95% CI: 1.26-9.12, p=0.015) (Table 3).
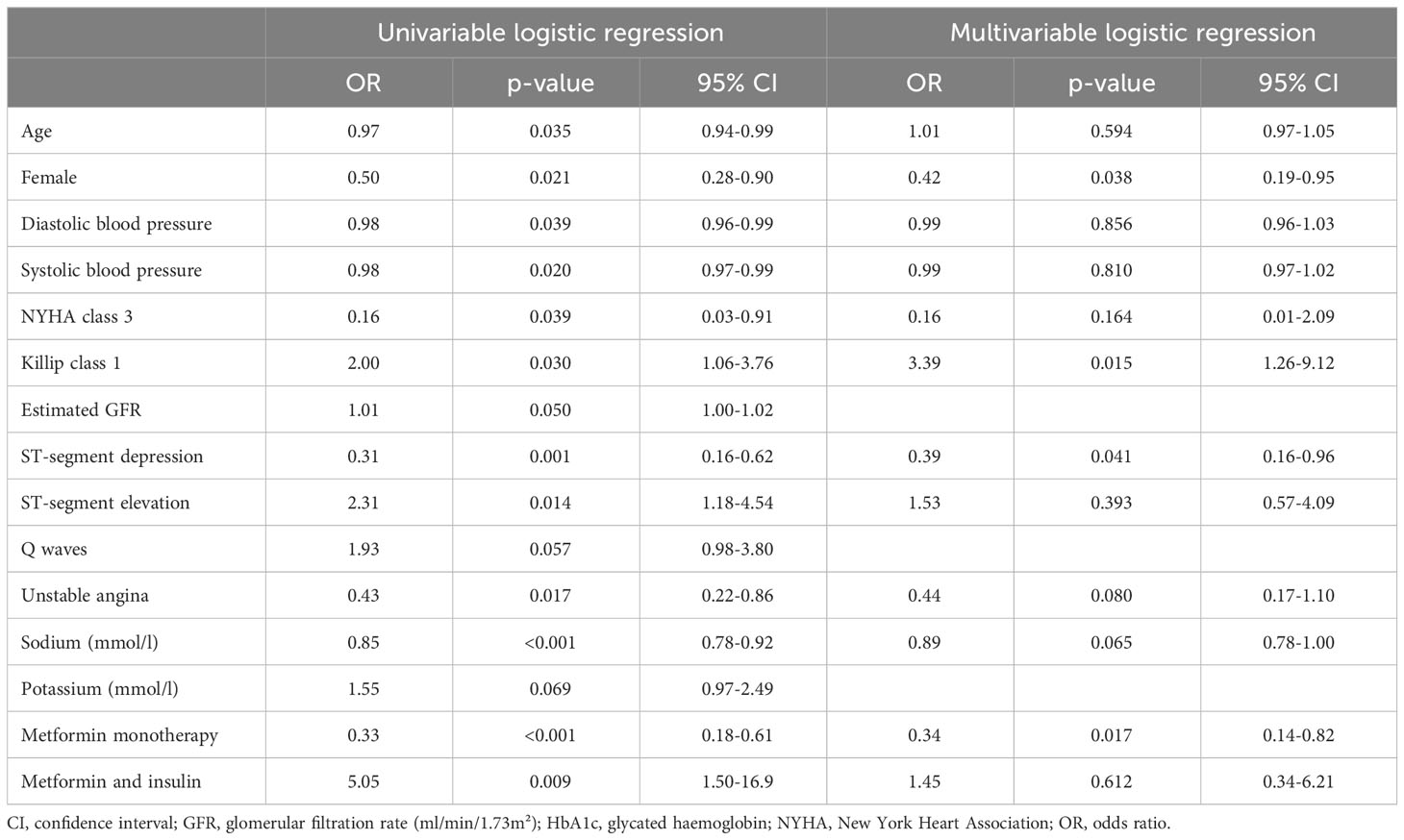
Table 3 Univariable and multivariable logistic regression analysis evaluating factors predisposing diabetic patients to poor glycaemic control (HbA1c above 7%) at index presentation.
We conducted a cross-sectional, retrospective study on consecutive patients with T2DM and angiographically proven ASCVD. Considering that our study population had established ASCVD, glycaemic control should have been individualized based on the diabetes duration, life expectancy and burden of comorbidities (6, 15). It is recommended that in patients with a short life expectancy, such as elderly patients with a terminal illness or multiple comorbidities, the target HbA1c should be <8.5%. In individuals with a longer life expectancy, the target HbA1c should be < 7% (6, 15). In our study, we did not evaluate glycaemic control according to these criteria. We used HbA1c levels measured at the time of presentation with the acute or chronic coronary syndromes and during the most recent follow-up visit.
In our study, T2DM patients were treated with metformin, sulphonylureas and/or insulin therapy. However, despite using these traditional anti-diabetic agents, only 28.7% of these very high-risk T2DM patients achieved optimal HbA1c control after a median duration of 16.5 months. This finding underscores that glycaemic control in our study population is sub-optimal. This is comparable to data from other LMICs, where studies have shown similar results indicative of poor glycaemic control (16, 17).
Multiple landmark trials have demonstrated that intensive glycaemic control reduces microvascular complications in type 1 and type 2 diabetic patients (18, 19). However, strict glycaemic control did not yield a similar reduction in the occurrence of macrovascular complications. Some evidence suggests that the initial tight glycaemic control may reduce macrovascular complications in the long term (18, 20). These results are most likely attributed to the legacy effect or metabolic memory of tight glycaemic control (21). Furthermore, tight glycaemic control may lead to adverse effects. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial evaluated tight glycaemic control in T2DM patients with established CVD. In this trial, patients were assigned to intensive treatment with a target HbA1c of less than 6% versus standard treatment with a target HbA1c between 7.0 and 7.9%. The average HbA1c achieved in the intensive therapy group was 6.4%, which was associated with increased mortality rates, hypoglycaemia and weight gain (22). Similarly, the United Kingdom Prospective Diabetes Study (UKPDS) trial evaluated tight glycaemic control in newly diagnosed T2DM patients and demonstrated similar adverse results (19). These deleterious effects may increase the risk of cardiovascular events and mortality (15, 23). Therefore, it is a first-line recommendation to avoid hypoglycaemia, especially in patients with CVD, to prevent the risk of subsequent events (24).
There is a substantial unmet need to control HbA1c levels and provide organ protection. Novel agents such as the sodium-glucose co-transporter 2 inhibitors (SGLT2-I) have demonstrated a reduction in major adverse cardiovascular events in T2DM patients with ASCVD (8–10). Similar results have been shown with some glucagon-like peptide 1(GLP-1) analogues (12, 25, 26). The current use of these agents in our setting is limited due to their high cost. However, cost-effectiveness analyses investigating the potential impact of SGLT2-I and GLP-1 analogues in preventing ASCVD complications and reducing the overall cost burden on the healthcare systems are still lacking in LMICs (27, 28).
In a systematic review and meta-analysis of 220,689 T2DM patients, diabetes increased the risk of all-cause mortality, cardiovascular death and strokes (29). The risk of all-cause mortality and cardiovascular death has also been found to be higher in diabetic patients with prior myocardial infarction (MI) compared to non-diabetics with and without previous MI (30). Therefore, reducing the risk of subsequent cardiovascular events is imperative by instituting the early use of novel anti-diabetic therapy that provides organ protection (24). The 2023 European Society of Cardiology guidelines for managing CVD in patients with diabetes recommend that in patients with CAD and T2DM, SGLT2-I or GLP-1 analogues should be added to the treatment regimen, irrespective of HbA1c control (10, 31, 32). In those patients not on metformin, it is recommended that these agents should be instituted as first-line therapy (24).
Metformin was the anti-diabetic agent used by 85.5% of T2DM patients included in our study. This is in keeping with current diabetes clinical practice guidelines, which recommend metformin as first-line treatment in T2DM (33). Metformin monotherapy was prescribed to 53.4% of patients, and 15.6% of T2DM patients were on metformin and insulin combination therapy. Among these patients on metformin and insulin combination therapy, 92.7% had poor glycaemic control despite evidence suggesting that adding insulin to metformin monotherapy improves glycaemic control (34). Clinical inertia to intensify diabetic therapy is common in LMICs and significantly contributes to poor glycaemic control (35). Therefore, the poor glycaemic control in our study participants may have resulted from a lack of treatment intensification and a possible low level of commitment to use insulin therapy optimally.
In our study, poor glycaemic control was more common in younger patients. Several reasons could account for this occurrence. Firstly, younger patients are often less likely to commit to medication and self-management of diabetes practices (36). Secondly, younger T2DM patients tend to be less informed about their condition and are more likely to have misconceptions about their susceptibility to disease complications because of their younger age (37, 38). Furthermore, our study population consisted predominantly of male patients, probably because ASCVD is more common in males. This is in contrast with a report by the International Diabetes Federation, which revealed a similar prevalence of diabetes in men and women (1). Of the 196 patients with poor glycaemic control, only 74 (24.5%) were females. Also, this finding was confirmed on the multivariable logistic regression model, where females were less likely to have poor glycaemic control. However, other studies have shown that poor glycaemic control is more likely in women than men (39, 40). There are several hypotheses to explain this observation, including differences among men and women in glucose metabolism, body mass index, hormonal differences, and psychosocial factors (41). Clinicians managing females with T2DM in our setting likely managed the disease more aggressively in light of the emerging evidence suggesting that females are at a higher risk of poor glycaemic control than their male counterparts (39, 40). It is also plausible that in our study, females living with diabetes were more engaged with the optimal treatment and control of their diabetes than their male counterparts. This phenomenon has been noted in Northern Sweden, where men seemed to underestimate problems related to diabetes more than women (42).
Poor glycaemic control is often associated with high blood pressure (43). However in our study, patients with poor glycaemic control had lower systolic and diastolic blood pressure. These patients likely had extensive ASCVD, since poorly controlled diabetes is associated with more severe CAD (44). Furthermore, we found that 24.0% of T2DM patients had triple vessel disease. Among these patients, 80.9% had an HbA1c above 7%. Diabetes mellitus is often associated with more complex CAD with multi-vessel involvement. Increased HbA1c levels result in extensive coronary artery involvement (44). These patients generally require surgical revascularisation, translating to higher healthcare costs (45). This further underscores the need to optimise HbA1c control and offer medication that is proven to delay the progression of ASCVD, thereby decreasing the prevalence of complex CAD and the need for surgical interventions, ultimately leading to decreased total healthcare costs.
We also found that 42.0% of T2DM patients presented with STEMI, and 80.9% had poor glycaemic control. Also, 15.7% of T2DM patients had ST-segment depression on the ECG at the time of hospitalisation. Type 2 diabetes mellitus patients with poor glycaemic control are more likely to have severe atherosclerotic disease characterized by vulnerable plaques. Therefore, these patients are more likely to have acute plaque rupture and present with STEMI (44, 46). Furthermore, we found that ST-segment depression on ECG was independently associated with optimal HbA1c control. This is likely because patients with controlled diabetes have less atherosclerotic burden and more stable plaques than those with poor glycaemic control (47).
Given the poor HbA1c control found in our study population, other strategies, besides the early use of novel anti-diabetic agents, must be implemented. Patients with T2DM should be educated about critical aspects of self-management of their condition. These aspects include adherence to medication, dietary advice, physical activity, and self-monitoring of glucose levels. Self-management of diabetes is inadequate in most individuals residing in sub-Saharan Africa (48). Other strategies to optimise glycaemic control include frequent follow-up visits, decentralisation of care and implementation of nurse practitioner or community health care worker home-based visits.
This study highlights the complex interplay between glycaemic control, cardiovascular health, and various clinical factors in T2DM patients with established cardiovascular disease. Our findings emphasize the importance of personalised treatment strategies that consider gender, compliance to medication, and cardiovascular symptoms since newer agents that offer organ protection are not readily available in state-owned healthcare facilities. Furthermore, we provided valuable information on the burden of T2DM in patients with ASCVD and clinical parameters associated with poor glycaemic control. Also, our findings provide a framework for further research into factors influencing glycaemic control in this population.
Our study had several limitations. Patient data was reviewed retrospectively, and we excluded T2DM patients who did not have baseline and follow-up HbA1c results. This reduced the study sample size. Furthermore, this was a single-center study. Therefore, the generalisability of the findings may not apply to other centers. However, most patients seen in our hospital were referred from primary and secondary-level hospitals to our hospital for further specialist-driven management. Another limitation is that our study did not consider psychosocial factors and the duration of T2DM. Both these factors may have played a role in the glycaemic control of our study participants. We also attempted to evaluate patient compliance to anti-diabetic therapy. However, such information was rarely documented in patient files. Furthermore, although we assessed glycaemic control using the most recent HbA1c level, we did not collect and analyze other clinical parameters gathered during the corresponding follow-up outpatient visit. Despite these limitations, our study data provides real-world insights into the standard of diabetic control in a South African state-owned tertiary hospital for a very high-risk population of T2DM patients with established ASCVD.
In our study, only 28.7% of T2DM patients with CAD achieved an HbA1c ≤7% after a median follow-up duration of 16.5 months. The small proportion of T2DM with optimal glycaemic control highlights the importance of an individualized treatment approach and the need for better management strategies beyond HbA1c control in this very high-risk group of patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study involved humans and was approved by the University of the Witwatersrand Human Research Ethics Committee (clearance certificate number: M191191). The study was conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study entailed a retrospective review of patient records.
LM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. DM: Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. NT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank staff members in the catheterisation laboratory at the Division of Cardiology at the CMJAH for assisting with retrieving patient data.
NT has received consulting and speaker fees from Acino Health Care Group, Boehringer-Ingelheim, Boston Scientific, Eli Lilly, Merck, Novartis Pharmaceuticals, Novo Nordisk, Organon, Pfizer, Phillips, Servier, and Takeda. NT has also received educational grants from Biotronik, Boston Scientific, Medtronic, and Vertice Health Care Group.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Stokes A, Berry KM, McHiza Z, Parker WA, Labadarios D, Chola L, et al. Prevalence and unmet need for diabetes care across the care continuum in a national sample of South African adults: Evidence from the SANHANES-1, 2011-2012. PloS One (2017) 12:e0184264. doi: 10.1371/journal.pone.0184264
3. Erzse A, Stacey N, Chola L, Tugendhaft A, Freeman M, Hofman K. The direct medical cost of type 2 diabetes mellitus in South Africa: a cost of illness study. Global Health action (2019) 12:1636611. doi: 10.1080/16549716.2019.1636611
4. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. (2018) 14:88–98. doi: 10.1038/nrendo.2017.151
5. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. (2020) 21:1–34. doi: 10.3390/ijms21176275
6. Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. (2009) 52:2288–98. doi: 10.1007/s00125-009-1470-0
7. Won KB, Han D, Lee JH, Lee SE, Sung JM, Choi SY, et al. Impact of optimal glycemic control on the progression of coronary artery calcification in asymptomatic patients with diabetes. Int. J. Cardiol. (2018) 266:250–3. doi: 10.1016/j.ijcard.2018.03.112
8. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl. J. Med. (2017) 377:644–57. doi: 10.1056/NEJMoa1611925
9. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl. J. Med. (2019) 380:347–57. doi: 10.1056/NEJMoa1812389
10. McGuire DK, Zinman B, Inzucchi SE, Wanner C, Fitchett D, Anker SD, et al. Effects of empagliflozin on first and recurrent clinical events in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a secondary analysis of the EMPA-REG OUTCOME trial. Lancet Diabetes Endocrinol. (2020) 8:949–59. doi: 10.1016/s2213-8587(20)30344-2
11. Mann JFE, Fonseca V, Mosenzon O, Raz I, Goldman B, Idorn T, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation. (2018) 138:2908–18. doi: 10.1161/CIRCULATIONAHA.118.036418
12. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl. J. Med. (2016) 375:1834–44. doi: 10.1056/NEJMoa1607141
13. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur. Heart J. (2019) 41:255–323. doi: 10.1093/eurheartj/ehz486
14. Soetedjo NN, McAllister SM, Ugarte-Gil C, Firanescu AG, Ronacher K, Alisjahbana B, et al. Disease characteristics and treatment of patients with diabetes mellitus attending government health services in Indonesia, Peru, Romania and South Africa. Trop. Med. Int. Health (2018) 23:1118–28. doi: 10.1111/tmi.13137
15. The International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. (2019) 7:385–96. doi: 10.1016/s2213-8587(18)30315-2
16. Aschner P, Gagliardino JJ, Ilkova H, Lavalle F, Ramachandran A, Mbanya JC, et al. Persistent poor glycaemic control in individuals with type 2 diabetes in developing countries: 12 years of real-world evidence of the International Diabetes Management Practices Study (IDMPS). Diabetologia. (2020) 63:711–21. doi: 10.1007/s00125-019-05078-3
17. Borgharkar SS, Das SS. Real-world evidence of glycemic control among patients with type 2 diabetes mellitus in India: the TIGHT study. BMJ Open Diabetes Res. Care (2019) 7:e000654. doi: 10.1136/bmjdrc-2019-000654
18. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl. J. Med. (2005) 353:2643–53. doi: 10.1056/NEJMoa052187
19. United Kingdom Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet (1998) 352:837–53. doi: 10.1016/S0140-6736(98)07019-6
20. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl. J. Med. (2008) 359:1577–89. doi: 10.1056/NEJMoa0806470
21. Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The diabetes & Aging study). Diabetes Care (2019) 42:416–26. doi: 10.2337/dc17-1144
22. Gerstein HC, Miller ME, Byington RP, Goff DC Jr., Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl. J. Med. (2008) 358:2545–59. doi: 10.1056/NEJMoa0802743
23. Davis SN, Duckworth W, Emanuele N, Hayward RA, Wiitala WL, Thottapurathu L, et al. Effects of severe hypoglycemia on cardiovascular outcomes and death in the veterans affairs diabetes trial. Diabetes Care (2019) 42:157–63. doi: 10.2337/dc18-1144
24. Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. (2023) 44:4043–4140. doi: 10.1093/eurheartj/ehad192
25. Verma S, Bain SC, Buse JB, Idorn T, Rasmussen S, Ørsted DD, et al. Occurence of first and recurrent major adverse cardiovascular events with liraglutide treatment among patients with type 2 diabetes and high risk of cardiovascular events: A post hoc analysis of a randomized clinical trial. JAMA Cardiol. (2019) 4:1214–20. doi: 10.1001/jamacardio.2019.3080
26. Hernandez AF, Green JB, Janmohamed S, D'Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. (2018) 392:1519–29. doi: 10.1016/s0140-6736(18)32261-x
27. Bagepally BS, Chaikledkaew U, Youngkong S, Anothaisintawee T, Thavorncharoensap M, Dejthevaporn C, et al. Cost-utility analysis of dapagliflozin compared to sulfonylureas for type 2 diabetes as second-line treatment in Indian healthcare payer's perspective. Clinicoecon Outcomes Res. (2021) 13:897–907. doi: 10.2147/ceor.S328433
28. Gu S, Deng J, Shi L, Mu Y, Dong H. Cost-effectiveness of saxagliptin vs glimepiride as a second-line therapy added to metformin in Type 2 diabetes in China. J. Med. Econ. (2015) 18:808–20. doi: 10.3111/13696998.2015.1049542
29. Nwaneri C, Cooper H, Bowen-Jones D. Mortality in type 2 diabetes mellitus: magnitude of the evidence from a systematic review and meta-analysis. Br. J. Diabetes Vasc. Disease (2013) 13:192–207. doi: 10.1177/1474651413495703
30. Schramm TK, Gislason GH, Kober L, Rasmussen S, Rasmussen JN, Abildstrom SZ, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation (2008) 117:1945–54. doi: 10.1161/CIRCULATIONAHA.107.720847
31. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl. J. Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
32. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl. J. Med. (2021) 384:129–39. doi: 10.1056/NEJMoa2030186
33. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. (2022) 65:1925–66. doi: 10.1007/s00125-022-05787-2
34. Paczkowska A, Hoffmann K, Michalak M, Bryl W, Kopciuch D, Zaprutko T, et al. A comparison between the therapeutic effect of metformin alone versus a combination therapy with insulin in uncontrolled, non-adherence patients with type 2 diabetes: six months follow-up. Diabetes Metab. Syndr. Obes. (2021) 14:3243–52. doi: 10.2147/dmso.S317659
35. Wan KS, Moy FM, Mohd Yusof K, Mustapha FI, Mohd Ali Z, Hairi NN. Clinical inertia in type 2 diabetes management in a middle-income country: A retrospective cohort study. PloS One (2020) 15:e0240531. doi: 10.1371/journal.pone.0240531
36. Kirkman MS, Rowan-Martin MT, Levin R, Fonseca VA, Schmittdiel JA, Herman WH, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care (2015) 38:604–9. doi: 10.2337/dc14-2098
37. Xie Z, Liu K, Or C, Chen J, Yan M, Wang H. An examination of the socio-demographic correlates of patient adherence to self-management behaviors and the mediating roles of health attitudes and self-efficacy among patients with coexisting type 2 diabetes and hypertension. BMC Public Health (2020) 20:1–13. doi: 10.1186/s12889-020-09274-4
38. Shiyanbola OO, Unni E, Huang Y-M, Lanier C. The association of health literacy with illness perceptions, medication beliefs, and medication adherence among individuals with type 2 diabetes. Res. Soc. Administrative Pharmacy (2018) 14:824–30. doi: 10.1016/j.sapharm.2017.12.005
39. Duarte FG, da Silva Moreira S, Maria da Conceição CA, de Souza Teles CA, Andrade CS, Reingold AL, et al. Sex differences and correlates of poor glycaemic control in type 2 diabetes: a cross-sectional study in Brazil and Venezuela. BMJ Open (2019) 9:e023401. doi: 10.1136/bmjopen-2018-023401
40. Demoz GT, Gebremariam A, Yifter H, Alebachew M, Niriayo YL, Gebreslassie G, et al. Predictors of poor glycemic control among patients with type 2 diabetes on follow-up care at a tertiary healthcare setting in Ethiopia. BMC Res. notes (2019) 12:1–7. doi: 10.1186/s13104-019-4248-6
41. Kautzky-Willer A, Leutner M, Harreiter J. Sex differences in type 2 diabetes. Diabetologia (2023) 66:986–1002. doi: 10.1007/s00125-023-05891-x
42. Gafvels C, Lithner F, Borjeson B. Living with diabetes: relationship to gender, duration and complications. A survey northern Sweden Diabetes Med. (1993) 10:768–73. doi: 10.1111/j.1464-5491.1993.tb00162.x
43. Wei F. Correlation between glycosylated hemoglobin level of patients with diabetes and cardiovascular disease. Pak J. Med. Sci. (2019) 35:454–8. doi: 10.12669/pjms.35.2.589
44. Hegde SS, Mallesh P, Yeli SM, Gadad VM, Giri Punja M. Comparitive angiographic profile in diabetic and non-diabetic patients with acute coronary syndrome. J. Clin. Diagn. Res. (2014) 8:Mc07–10. doi: 10.7860/jcdr/2014/9072.4851
45. Thuijs D, Kappetein AP, Serruys PW, Mohr FW, Morice MC, Mack MJ, et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet. (2019) 394:1325–34. doi: 10.1016/s0140-6736(19)31997-x
46. Pathak S, Gajurel R, Poudel C, Shrestha H, Thapa S, Koirala P. Angiographic severity of coronary artery disease in diabetic and non-diabetic acute STEMI patients in a tertiary care centre of Nepal. Kathmandu Univ. Med. J. (2021) 19:410–4. doi: 10.3126/kumj.v19i4.49752
47. Engel LC, Landmesser U, Goehler A, Gigengack K, Wurster TH, Manes C, et al. Noninvasive imaging of endothelial damage in patients with different hbA(1c) levels: A proof-of-concept study. Diabetes. (2019) 68:387–94. doi: 10.2337/db18-0239
Keywords: diabetes mellitus, HbA1c, coronary artery disease, glucose control, Africa
Citation: Mhlaba L, Mpanya D and Tsabedze N (2023) HbA1c control in type 2 diabetes mellitus patients with coronary artery disease: a retrospective study in a tertiary hospital in South Africa. Front. Clin. Diabetes Healthc. 4:1258792. doi: 10.3389/fcdhc.2023.1258792
Received: 14 July 2023; Accepted: 16 October 2023;
Published: 30 October 2023.
Edited by:
Belma Pojskic, Cantonal Hospital Zenica, Bosnia and HerzegovinaReviewed by:
Roberta Lamptey, Korle Bu Teaching Hospital, GhanaCopyright © 2023 Mhlaba, Mpanya and Tsabedze. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nqoba Tsabedze, TnFvYmEuVHNhYmVkemVAd2l0cy5hYy56YQ==
†These authors have contributed equally to this work and share senior and last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.