- 1Department of Family Medicine and Primary Care, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
- 2Department of Family Medicine and Primary Care, Protestant University of Congo, Kinshasa, Democratic Republic of Congo
Introduction: Diabetes is a significant problem in sub-Saharan Africa and achieving glycaemic control poses a health challenge among patients living with type 2 diabetes. There are limited data on glycaemic control in Kinshasa, Democratic Republic of the Congo. This study assessed the prevalence and factors associated with glycaemic control to inform potential interventions to improve glycaemic control in Kinshasa.
Methods: This was a cross-sectional study conducted between November 2021–September 2022 among patients recruited from 20 randomly selected health facilities in Kinshasa. Participants were asked to complete a structured questionnaire and to provide two millilitres of blood for Hb1AC assay. Poor glycaemic control was defined as HbA1c ≥7%. Univariate and multivariable logistic regressions were performed to identify factors associated with poor glycaemic control.
Results: A total of 620 participants were recruited for this study. Study participants had a median age of 60 (IQR=53.5-69) years with the majority being female (66.1%), unemployed (67.8%), having income below the poverty line (76.4%), and without health insurance (92.1%). About two-thirds of the participants (420; 67.6%) had poor glycaemic control. Participants on monotherapy with insulin (AOR=1.64, 95%CI [1.10-2.45]) and those on a treatment duration ≥7 years (AOR=1.45, 95%CI [1.01-2.08]) were associated with increased odds of poor glycaemic control while being overweight (AOR= 0.47, 95%CI [0.26-0.85]) and those with uncontrolled blood pressure (AOR=0.65, 95% CI [0.48-0.90]) were protective for poor glycaemic control.
Conclusion: Poor glycaemic control is prevalent among patients with type 2 diabetes in Kinshasa, DRC. Being on insulin alone and a duration of diabetes treatment equal or more than 7 years predisposed to poor glycaemic control. By contrary, having uncontrolled blood pressure and being overweight had protective effect against poor glycaemic control. These links between uncontrolled blood pressure and overweight on the one hand, and glycaemic control on the other are unusual. These reflect, among other things, the specific characteristics of diabetes in sub Saharan Africa.
Introduction
Type 2 diabetes is increasing worldwide (1) – it is expected that the greatest increase in diabetes prevalence will take place in low- and middle-income countries (2). On the African Continent, type 2 diabetes is progressing rapidly due to modifiable risk factors, such as obesity and urbanisation (2).
In sub-Saharan Africa (SSA), diabetes care faces numerous challenges leading to unmet needs and a greater impact on morbidity and mortality (3, 4). In the Democratic Republic of the Congo (DRC), the prevalence of diabetes is estimated to be 5.8% for adults aged 20 to 79 years (4), with higher proportions of persons living with diabetes found in urban areas and the western part of the country (5, 6).
Good glycaemic control is the cornerstone of diabetes management, as it delays the onset of complications, reduces the cost of care and improves persons with diabetes quality of life. Nevertheless, the control of diabetes remains a challenge worldwide, with only about 50% of the person with diabetes controlled (7). In SSA, it is estimated that less than one-third of persons with type 2 diabetes achieve target glycaemic levels (8, 9). A recent systematic review of the studies on glycaemic control found: age, sex, poor socio-economic conditions, place of residence, positive family history of diabetes, longer duration of diabetes, treatment modalities and effects, alcohol consumption, smoking, presence of comorbidities or complications, and poor management were associated with poor glycaemic control (9). Contrarily, high diabetes health literacy, positive perception of family support, adequate coping strategies, dietary adherence, physical activity, adherence to follow−up appointments and medications, were associated with good glycaemic control (9).
An accurate knowledge of factors driving glycaemic control in a particular setting is essential to developing an intervention package to improve glycaemic control. Multiple factors drive glycaemic control in SSA, differing across settings (8). In the DRC, the prevalence of poor glycaemic control among persons with type 2 diabetes was reported as high as 86% and 79.9% have been reported in the nearby province of Kwilu and Kinshasa, respectively (10, 11). In these studies, Sagastume et al. (11) found that persons with diabetes older than 40 years of age had higher odds of achieving good glycaemic control than those younger while Blum et al. (10) found that abdominal obesity and having a body mass index (BMI) > 25 Kg/m2 were associated with poor glycaemic control. The study by Blum et al. (10) was conducted in a single site while Sagastume et al. (11) proceeded to a retrospective analysis of Kinshasa Primary HealthCare Network. Thus very few studies have been devoted to the factors of glycaemic control in DRC leading to a very rudimentary data on the issue and poor understanding of glycaemic control. In anticipation of the building of an intervention package to deal with the issue in Kinshasa, and in an effort to expand knowledge about blood glucose control factors, we designed a mixed-method cross-sectional study. Studies with mixed methodology are appropriate to explore complex phenomena in a broad way. In this article, we present the results of the quantitative phase.
Materials and methods
Study design
This was a cross sectional study, a component of a bigger research project on glycaemic control among persons with type 2 diabetes in Kinshasa, DRC, for which the study protocol was previously published (12). Routinely, type 2 diabetes is defined by a bundle of epidemiological and clinical arguments: onset of diabetes at more than 40 years, history of diabetes in the family, presence of autoimmune pathology, association with metabolic syndrome: hypertension, upper BMI, dyslipidaemia, presence of ketone bodies and favourable response of treatment to oral hypoglycaemic agents (OHA).
Study setting
Our study was multisite within Kinshasa, a city of about 15 million inhabitants spread over an area of 9,965 km2 (13). The participants were recruited from 20 randomly selected health facilities in Kinshasa, DRC. The study was conducted in the health facilities belonging to the Catholic Church and the Salvation Army. With a total of 66 health facilities (1 referral hospital and 65 health centres) distributed across 24 health districts, these organisations own most of the facilities that have integrated diabetes care in primary care in Kinshasa.
Study population
The study population consisted of persons with type 2 diabetes attending health centres that offer diabetes care in the Kinshasa Primary Care Network, with about 7326 persons with diabetes registered in 2020. The inclusion criteria were age ≥18 years, receiving diabetes treatment for at least six months and consenting to the study. The exclusion criteria were pregnancy, and having difficulty communicating due to mental disability.
Sample size estimation
The estimated minimum sample size was computed using Epi info version 7.2.2.2. Assuming that the prevalence of poor glycaemic control was 68% (14), a 95% confidence level and a power of 80%, 59,2% of persons with diabetes who had a diabetes duration ≤7 years (unexposed) presented with poor glycaemic control, and 74,4% of those who had a diabetes duration >7 years (exposed) presented with poor glycaemic control (15). The unexposed to exposed ratio is 0.47 (15). The minimum estimated sample size was 368. Adjusting for a design effect of 1.5, the calculated sample size of 552 was determined. To account for an estimated 10% non-response rate, the minimum required sample size was 614 persons with diabetes, rounded up to 620.
Sampling of participants
Participant selection was a two-stage process. The first stage was the random selection of 20 out of 48 healthcare facilities. As the healthcare facilities have an unequal number of persons with diabetes, the participants were selected by probability proportional to the patient population size. The second stage consisted of the selection of the participants; 31 patients were selected from each selected healthcare facility using systematic sampling. The research assistant was taking the record of the first patient on a clinic day to assess eligibility and subsequently was taking the record of every third patient for questionnaire administration. If the first patient was not eligible or if the third patient selected in the next step was not eligible, the research assistant selected the next patient(s) until an eligible patient was obtained, then continued with the selection of each third patient. This process ensured each patient had the same probability of selection.
Data collection
The data collection process lasted from November 2021–September 2022. For each participant, the research team performed physical and anthropometric measurements. These measurements were taken once by trained staff members on the same portable equipment at all the health facilities. The questionnaire consisted of pre-existing standardised tools translated from English into French and Lingala. The questionnaire was pre-tested before data collection, no changes were necessary on the tools for use in our study. At the end of the interview, 2 millilitres of venous blood was collected in a tube with EDTA from the participant. The tube was identified and put in a fridge at 2-8 °C—when the centre has a fridge—or directly in the isotherm box prepared. At the end of the visit, all the samples were transferred in the laboratory using the isotherm box. The questionnaire was administered using REDCap (Research Electronic Data Capture) on a tablet or smartphone (16). Information captured during the interview on the history of diabetes was verified with what is recorded in the medical records.
Variables of the study
Outcome
The main outcome variable was poor glycaemic control, defined as HbA1c ≥7% (17–19), and obtained from the blood sample assayed at the laboratory of the School of Medicine at the Protestant University of Congo in Kinshasa. The assay was performed using an automated Genuis WP 21B with antibody-based immunoassay method of Cypress Diagnostics (20).
Exposures
The possible determinants for glycaemic control considered in this study were sociodemographic parameters (age, sex, marital status, educational attainment, occupation, income, use of health insurance, access to food, distance from place of residence to health centre), lifestyle parameters (smoking, problematic alcohol consumption), clinical parameters (duration of diabetes, height, weight, body mass index, waist circumference, presence of comorbidities, blood pressure, treatment, duration of treatment), and psychological parameters (adherence to treatment, depression, diabetes distress, social support, self-management, knowledge). Supplementary file 1 detailed the exposures, their measurements, operational definitions, reliability, and references.
Data analysis
All the analyses were performed using survey data analysis with STATA 17 (21) to account for the study design characteristics. We expressed age as median with interquartile range (IQR), as it was not normally distributed. The other variables were analysed as categorical variables and expressed as frequency (n) and percentage (%). Bivariate analysis was performed to compare uncontrolled versus controlled participants in terms of glycosylated haemoglobin using the Chi-square/Fisher exact test for categorical variables. We further carried out multivariable logistic regression to assess factors associated with glycaemic control. Age, sex, duration of treatment, and food security were included in the regression model a priori. Other variables with a p-value <0.2 in univariate analysis were also included in the model. The p-value of <0.05 was considered statistically significant.
Results
A total of 620 participants were included in the study out of a total of 627 invited, accounting for a non-response rate of 1.1%. The participants had a mean age of 60.21 ± 12.44 years, a mean BMI of 23.60 ± 5.21 Kg/m2, a mean waist circumference of 89.95 ± 13.49 centimetres, a mean duration of diabetes disease of 83.17 ± 75.18 months, and a mean duration of 82.17 ± 75.02 months. Table 1 summarises the sociodemographic, lifestyle and clinical characteristics of the participants. Fewer than two-thirds of the participants (64.68%) of the participants presented a complication or a comorbidity. The most common complications or comorbidities were hypertension (53.88%), diabetic retinopathy (15.79%), erectile dysfunction (14.04%), and cataract (3.51%). A slight more than half of the participants (53.87%) were on insulin alone while 38.23% and 7.9% of the participants on Oral Hypoglycaemic agents (OHA) and Mixed treatment (Insulin-OHA). The most common OHA were: Metformin (69.29%), Glibenclamide (15.04%), Gliclazide (11.04%), and Glimepiride (3.15%).
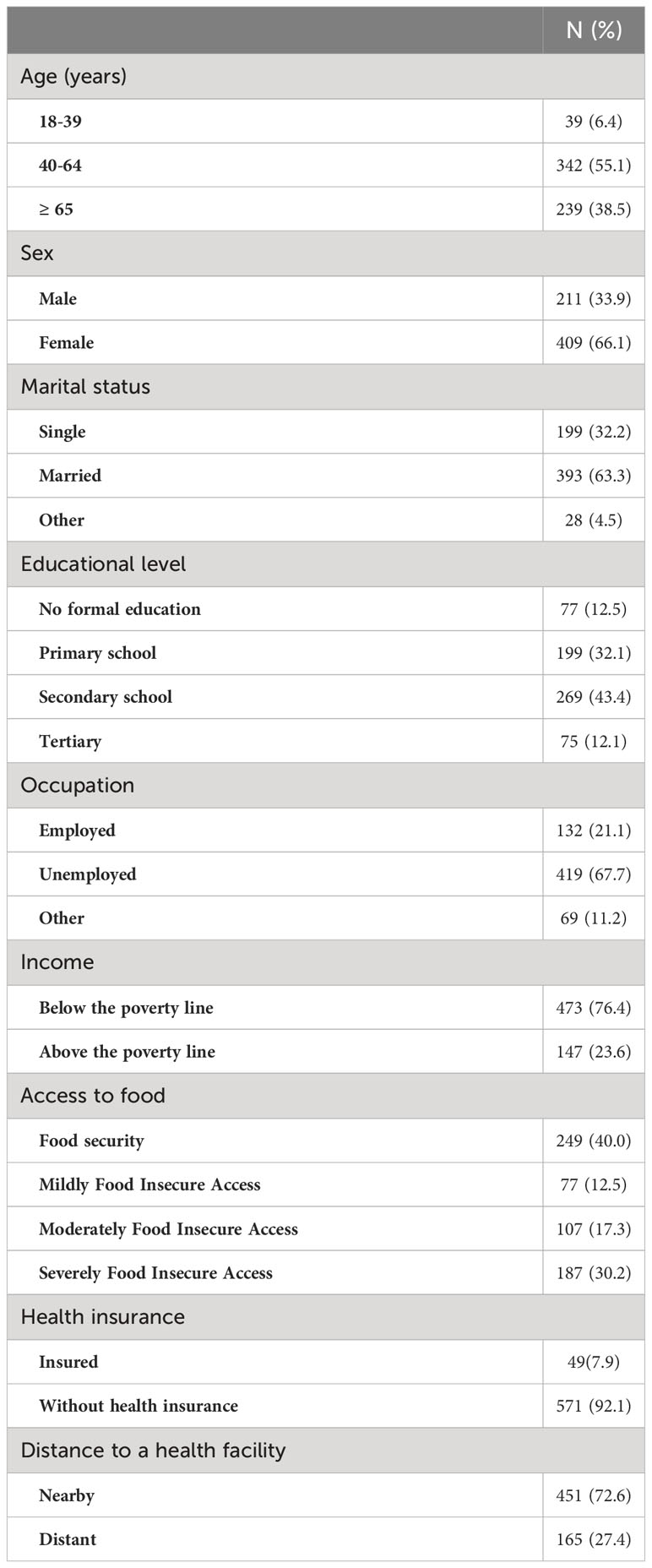
Table 1 Sociodemographic characteristics of participants with type 2 diabetes in Kinshasa, n=620 (2021-2022).
Prevalence of glycaemic control and participants’ characteristics
About two-thirds of the participants (67.8%; n=420) had poor glycaemic control.
There was no statistically significant difference between controlled and uncontrolled participants in terms of sociodemographic characteristics (Table 2).
However, controlled participants differed significantly from uncontrolled participants in terms of BMI (p=0.005), control of blood pressure (p=0.027), and treatment regimens (p=0.002) (Table 2).
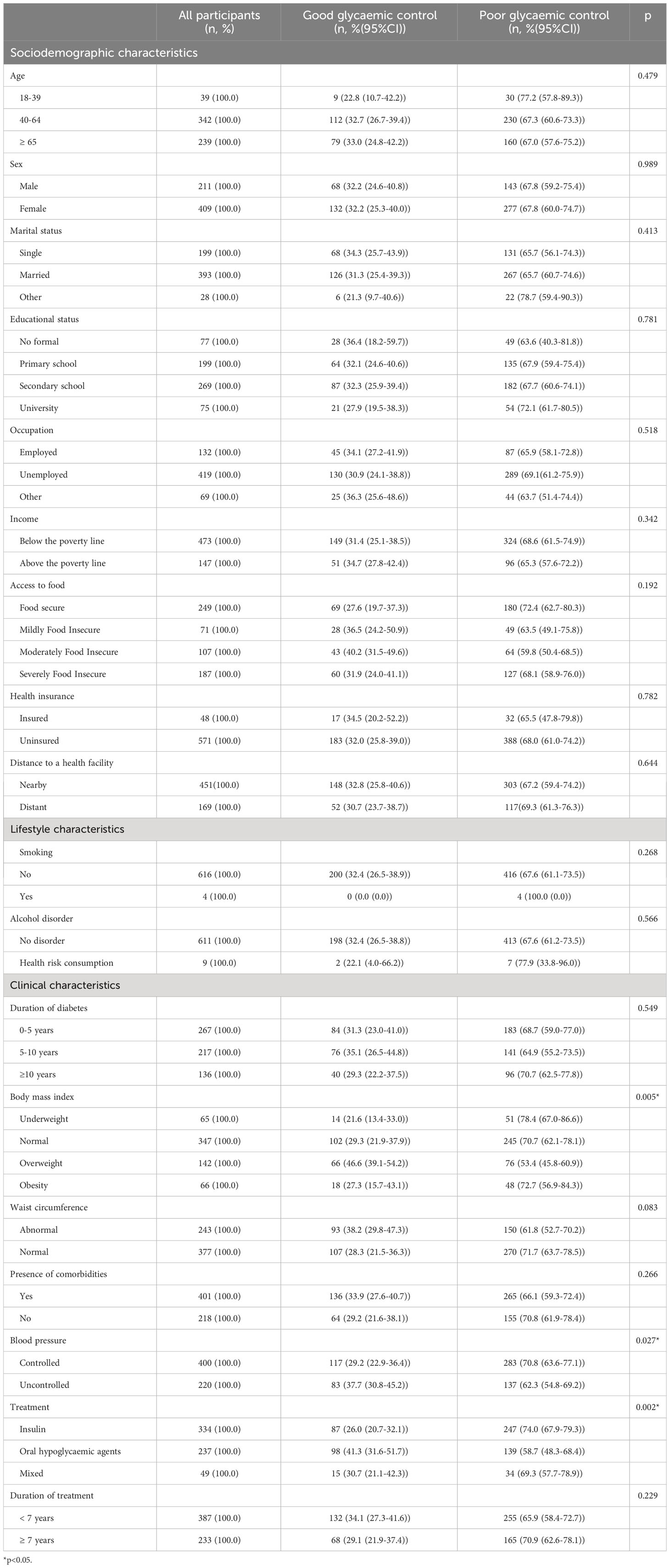
Table 2 Sociodemographic, lifestyle and clinical characteristics in relation with glycaemic control among participants with type 2 diabetes in Kinshasa, n=620 (2021-2022).
Perceived support from significant others (p=0.005), perceived family support (p=0.020), treatment regimen distress (p=0.029), and adherence to physical activity (p=0.017) were significantly different between controlled and uncontrolled participants (Table 3).
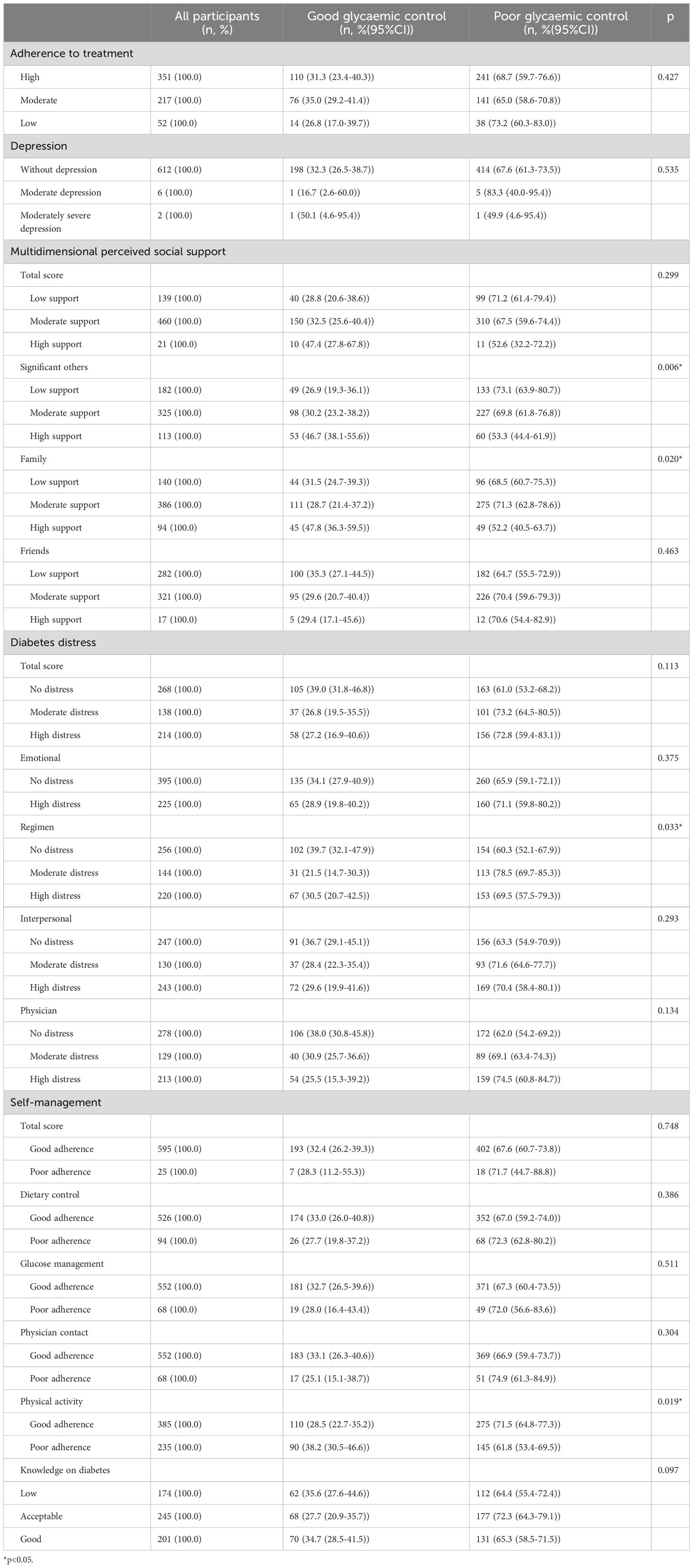
Table 3 Psychological characteristics and glycaemic control among participants with type 2 diabetes in Kinshasa (n=620).
Determinants of glycaemic control
Being on monotherapy with insulin (AOR=1.64, 95%CI [1.10-2.45]) and having a treatment duration ≥7 years (AOR=1.45, 95%CI [1.01-2.08]) increased the odds of poor glycaemic control. On the other hand, being overweight (AOR= 0.47, 95%CI [0.26-0.85]) and having uncontrolled blood pressure (AOR=0.65, 95% CI [0.48-0.91]) decreased the odds of poor glycaemic control (Table 4). An analysis of the relation between BMI and poor glycaemic control in two separated groups as non-insulin and insulin users found that only in non-insulin users, being overweight was protective against poor glycaemic control ((AOR: 0.28,95%CI [0.10-0.80],p:0.020) versus (AOR:0.55, 95%CI [0.25-1.17], p:0.115) for non-insulin users and insulin users respectively).
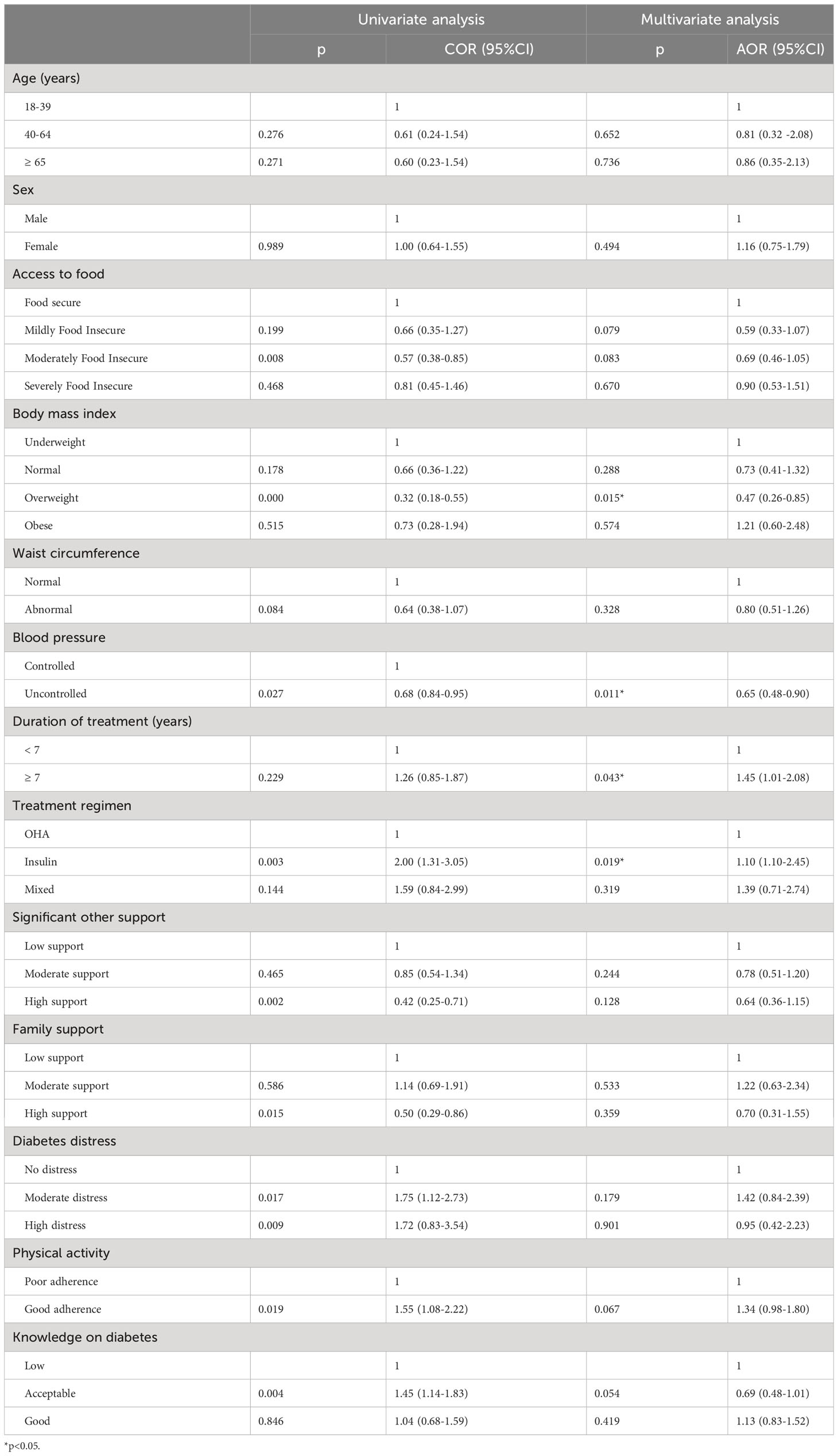
Table 4 Results of survey logistic regression estimating the odds for poor glycaemic control in Kinshasa, 2021-2022.
Discussion
This study was designed to assess the extent of poor glycaemic control among persons living with type 2 diabetes in Kinshasa, including its driving factors. The study found that poor glycaemic control is very prevalent (67.8%) and no sociodemographic or lifestyle characteristics were associated with glycaemic control. While monotherapy with insulin and having a treatment duration ≥7 years increased the odds of poor glycaemic control, being overweight and having uncontrolled blood pressure reduced its odds.
That more than two-thirds of the study participants have poor glycaemic control corroborates the findings of other studies in SSA (8, 9). Furthermore, this study found a lower prevalence of poor glycaemic control than that found by Blum et al. in the nearby rural province of Kwilu in the DRC. In rural areas in the Democratic Republic of Congo, there are fewer centers that offer care to persons with diabetes, and the access to medicines and the range of foods are limited. Health system planners must work to ensure equitable geographical distribution of diabetes care centers, and regular and extensive supply of medications. Glycaemic control in our study was poorer than that found in the European or North American studies (22, 23), and indicated poor diabetes care in Kinshasa. Our result also translate the issues in SSA in general, where diabetes care faces multiple barriers, such as lack of funding for non-communicable diseases, lack of accurate guidelines directed to the specificities of diabetes in the population, lack of availability of medications and other supplies, and inequity between public and private sector diabetes care (3, 24). Moreover, self-management in SSA is poor and represents a threat to the health of individuals and capacity of the health system (25). An effective preparation of the health care systems to face diabetes burden is crucial and including effective financing, training of the healthcare providers, and provision of required materials and medicines (3, 26).
In this study, participants on monotherapy with insulin were 1,64 times more likely to have poor glycaemic control than those on oral hypoglycaemic drugs. Studies have shown that only around one-fourth of persons with diabetes on insulin could achieve glycaemic targets because they might be erroneously taking an insufficient daily dose and incorrectly titrating insulin (27). One may also hypothesize that as most of the persons with diabetes were unemployed and not covered by health insurance, they could have been unable to adequately follow the prescribed regimen when they lack money to pay for their medicines or food. The psychological resistance to insulin, prevalent in our setting according to the study by Rita et al. (28), could also be another explanation for poor glycaemic control among persons with type 2 diabetes in our study. In the diabetes attitudes, wishes and needs second study (DAWN), participants reported low confidence in the efficacy of insulin, with 26.9% of participants abstaining from insulin because they thought insulin unfeasible or impracticable to manage their diabetes (29). Healthcare providers must ensure that psychological resistance to initiating insulin is adequately addressed, and effectively train the persons with diabetes to correctly follow their prescriptions.
Type 2 diabetes is a lifestyle disease, and all guidelines recommend that insulin therapy should accompany lifestyle modification and oral hypoglycaemic drugs. The propensity of clinicians to use insulin may reflect the lack of appropriate guidelines or poor clinicians’ adherence to evidence-based clinical guidelines. There is also no system of safeguards to regulate medical prescriptions, especially since these are mostly provided in private pharmacies. Clear management guidelines must also be adapted for the use of available medicines and efforts must be made to offer new hypoglycaemic agents at affordable prices. The technical supervision of health facilities by the national programme is crucial to ensure the proper management of diabetes in accordance with standards.
In this study, the odds of overweight participants having poor glycaemic control were reduced by 53.0%. The sub-analysis also found that being overweight was protective only for non-insulin users. Our finding contrasted the well-known relationship between being overweight and suboptimal glycaemic control and poor glycaemic control (30). One may note that in our context, poor glycaemic patients are receiving, in most instances, insulin. Once they are better controlled, they are put on OHA. Thus, it is possible that current patients receiving OHA gained weight during a previous treatment with insulin phase and are beginning oral treatment with better glycaemic control. Blum et al. (10), in their study near Kinshasa, also found that BMI>25 Kg/m2 and abdominal obesity were protective against poor glycaemic control. The authors stated that this finding could reflect the existence of special features of diabetes in SSA. Weight loss or the prevention of weight gain is an important goal in the management of type 2 diabetes or prediabetes (31). However, increasing weight could also arise in persons with diabetes due to the effect of antidiabetic medication on body weight. Apart from metformin and thiazolidinediones, other antidiabetic agents could lead to weight gain (32). In Kinshasa, insulin is largely used and there has been a limited range of affordable medications for persons with diabetes. Healthcare providers must furthermore ensure that the persons with diabetes are adequately managed to avoid adverse effects (32).
In the study sample, persons with diabetes having uncontrolled blood pressure reduced the odds of having poor glycaemic control by 35.0%. Mobula et al. (33), in a Ghanaian study, also found that systolic blood pressure was significantly higher among persons with diabetes having adequate glycaemic control compared to the group with poor glycaemic control. As discussed by Mobula et al. (33), among persons with diabetes having good glycaemic control, it can be that there was a significantly higher proportion of patients with dual diagnosis—hypertension and diabetes. This observation can also be explained by the fact that health providers give more attention to persons with comorbidity or an increase in healthcare utilisation by the persons with comorbidity (34). Hypertension is frequently associated with diabetes (35), which indicates that more insight into adequate management of hypertension among persons with diabetes in our setting will be required (34).
This study found that a treatment duration ≥7 years increased the odds of poor glycaemic control by 1,45 times. Longer duration of treatment has been linked to poor glycaemic control in SSA (36). As diabetes is a progressive disease with deterioration in the function of the ßeta cells of the pancreas with time, more adjustments in the treatments are required in older persons with diabetes who would generally have had diabetes longer and are more likely to have comorbidities (37). Health providers must be informed of the progression of diabetes and be able to adjust the treatments for persons with diabetes accordingly.
Most of our participants (93.7%) were older than 40 years. This proportion aligns with the classic description of type 2 diabetes, in which the disease appears in individuals older than 40 years most of the time. Female persons with diabetes represented approximately two-thirds of the participants. A retrospective analysis of the Kinshasa Health Network database conducted by Sagastume et al. (11) also found the same-sex prevalence. This high prevalence of type 2 diabetes affects more women than men, due to the higher metabolic risk in the former (38). Furthermore, the health-seeking behaviour of women is better than in men (39). Most of the participants were unemployed, poor and without health insurance, which has been representative of the condition of the general population in Kinshasa. No sociodemographic and lifestyle characteristics were associated with poor glycaemic control. Our finding here has been corroborated by the study of Blum et al. (10), who also found no sociodemographic or lifestyle factors associated with poor glycaemic control in a cross-sectional survey in the nearby province of Bandundu in the DRC. However, we can discuss the efficiency of the assessment of certain characteristics such as income in our environment. The income assessed was the individual’s income and did not take into account the contribution of relatives, which was sometimes substantial. And since most persons with diabetes were not paid employees, those in the informal or liberal sector could not accurately determine their income. In a retrospective study in Kinshasa, Sagastume et al. (11) found that younger persons with diabetes needed prioritised attention to reach glycaemic targets. Nevertheless, interventions for better glycaemic control have to prioritise vulnerable groups, such as younger and older age, women and non-insured persons with diabetes (9). Implementing universal coverage can increase access to care for the aforementioned groups (40).
Limitations of the study
This study estimated the extent of poor glycaemic control among persons with type 2 diabetes in Kinshasa. Because of the cross-sectional nature of the study, it is not possible to ascertain a causal relationship between poor glycaemic control and the determinants. Other potential biases include selection bias as only persons with diabetes who attended the diabetic clinics in the period of the study could be included in the study, recall bias as for some responses, the participants might refer to their history with the possibility to have lost memory or omitted details for some events, interviewer bias since the data collectors could have influenced the participants by how they asked questions or reacted to the answers, and social desirability bias since participants could have given answers that made them look good to respondents and did not talk about their true experiences (41). These biases were minimised by ensuring effective training of the data collectors to make certain that the aim and objectives of the study were clearly stated to the participants, and that questions were asked in a non-judgemental way.
Nonetheless, this study provides an understanding of important factors on which to focus for improved glycaemic control in Kinshasa, DRC or similar settings, particularly in sub-Saharan Africa.
Conclusion
Poor glycaemic control is prevalent among persons with type 2 diabetes in Kinshasa, DRC. Being on insulin alone and a duration of diabetes treatment equal or more than 7 years predisposed to poor glycaemic control. By contrary, having uncontrolled blood pressure and being overweight had protective effect against poor glycaemic control. These links between uncontrolled blood pressure and overweight on the one hand, and glycaemic control on the other are unusual. These reflect, among other things, the specific characteristics of diabetes in sub-Saharan Africa.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Supplementary Information Files.
Ethics approval
The researchers declared that they complied with the conditions under which this study obtained approval from the ethics committees of the Protestant University of Congo (reference number: CEUPC 0067; Date: 05/02/2021) and Human Research Ethics Committee (Medical) of the University of the Witwatersrand (reference number: M210308; Date: 26/08/2021). The study was conducted according to the ethical guidelines of the Declaration of Helsinki. Permission was obtained from the Kinshasa Primary HealthCare Network to conduct the study. Informed consent was obtained from each participant. Data collection was done in strict adherence to local COVID-19 regulations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics committee of the Protestant University of Congo (reference number: CEUPC 0067; Date: 05/02/2021) and Human Research Ethics Committee (Medical) of the University of the Witwatersrand (reference number: M210308; Date: 26/08/2021). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
J-PL, OO, and JF designed the study. J-PL contributed to acquisition of funding and data. J-PL oversaw the research process. J-PL and JF performed the data processing and quality control. J-PL conducted the statistical analyses, drafted the manuscript, and is the guarantor of this work. J-PL, OO, and JF interpreted the data. All authors contributed to the article and approved the submitted version.
Funding
The authors received no specific funding for this work. The Protestant University of Congo provided laboratory reagents and facilitated laboratory assays.
Acknowledgments
The authors would like to thank all the staff of the Kinshasa Primary Health Network for their support during the study. We thank Mrs Manase Lusuami for performing the laboratory assays, and Christian Mungongo Kifu for his support during data collection and data management. This manuscript has been previously published as a preprint (42).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2023.1241882/full#supplementary-material
Supplementary file 2 | xls — Study dataset. Anonymised database.
Abbreviations
AOR, Adjusted Odds Ratio; BMI, Body mass index; CEUPC, Ethics Committee of the Protestant University of the Congo; COR, Crude Odds Ratio; DAWN, Diabetes Attitudes, Wishes and Needs second study; DRC, Democratic Republic of the Congo; IQR, Inter Quartile Range; OHA, Oral Hypoglycaemic Agents; RedCap, Research Electronic Data Capture; SSA, Sub-Saharan Africa; STATA, Statistics and data.
References
2. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
3. Pastakia S, Pekny C, Manyara S, Fischer L. Diabetes in sub-Saharan Africa – from policy to practice to progress: targeting the existing gaps for future care for diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. (2017) 10:247–63. doi: 10.2147/DMSO.S126314
4. Democratic Republic of the Congo diabetes report 2000 — 2045. (2023). Available at: https://diabetesatlas.org/data/en/country/55/cd.html (Accessed Dec 21, 2022).
5. Katchunga PB, Mirindi P, Baleke A, Ntaburhe T, Twagirumukiza M, M’buyamba-Kabangu J-R. The trend in blood pressure and hypertension prevalence in the general population of South Kivu between 2012 and 2016: Results from two representative cross-sectional surveys-The Bukavu observational study. PLoS One (2019) 14(8):e0219377. doi: 10.1371/journal.pone.0219377
6. Muyer MTMC, Botomba S, Poka N, Mpunga D, Sibongwere DK, Peñalvo JL, et al. Diabetes prevalence and risk factors, underestimated without oral glucose tolerance test, in rural Gombe-Matadi Adults, Democratic Republic of Congo, 2019. Sci. Rep. (2022) 12(1):15293. doi: 10.1038/s41598-022-18658-y
7. Giugliano D, Maiorino MI, Bellastella G, Esposito K. Glycemic control in type 2 diabetes: from medication nonadherence to residual vascular risk. Endocrine (2018) 61(1):23–7. doi: 10.1007/s12020-017-1517-9
8. Sobngwi E, Ndour-Mbaye M, Boateng KA, Ramaiya KL, Njenga EW, Diop SN, et al. Type 2 diabetes control and complications in specialised diabetes care centres of six sub-Saharan African countries: The Diabcare Africa study. Diabetes Res. Clin. Pract. (2012) 95(1):30–6. doi: 10.1016/j.diabres.2011.10.018
9. Lubaki F, Omole OB, Francis JM. Glycaemic control among type 2 diabetes patients in sub − Saharan Africa from 2012 to 2022 : A systematic review and meta − Analysis. Diabetol. Metab. Syndr. (2022) 14(1):134. doi: 10.1186/s13098-022-00902-0
10. Blum J, Chaney M, Mudji J, Mfungwa JAK, Rice T, Labhardt ND. Glycaemic control among patients with type 2 diabetes followed in a rural African primary care setting - A reality check in the Democratic Republic of Congo. Prim Care Diabetes (2020) 14(2):139–46. doi: 10.1016/j.pcd.2019.08.002
11. Sagastume D, Mertens E, Sibongwere DK, Dimbelolo JC, Kabundi JCK, de Man J, et al. A retrospective database study of the demographic features and glycemic control of patients with type 2 diabetes in Kinshasa, Democratic Republic of the Congo. BMC Med. (2022) 20(1):1–14. doi: 10.1186/s12916-022-02458-2
12. Fina Lubaki JP, Omole OB, Francis JM. Protocol: Developing a framework to improve glycaemic control among patients with type 2 diabetes mellitus in Kinshasa, Democratic Republic of the Congo. PLoS One (2022) 17(9):e0268177. doi: 10.1371/journal.pone.0268177
13. Megacity Kinshasa Eyes Climate Resilient Future Through Urban Management. Available at: https://www.worldbank.org/en/news/feature/2021/08/19/why-kinshasa-could-be-in-the-vanguard-of-megacities-climate-resilience (Accessed Mar 2, 2022).
14. Longo-Mbenza B, Kasiam Lasi On’kin JB, Nge Okwe A, KAngola Kabangu N. The metabolic syndrome in a Congolese population and its implications for metabolic syndrome definitions. Diabetes Metab. Syndr. (2011) 5(1):17–24. doi: 10.1016/j.dsx.2010.05.009
15. Chetoui A, Kaoutar K, Elmoussaoui S, Boutahar K, El Kardoudi A, Chigr F, et al. Prevalence and determinants of poor glycaemic control: a cross-sectional study among Moroccan type 2 diabetes patients. Int. Health (2020) 14(4):390–7. doi: 10.1093/inthealth/ihz107
16. REDCap. Available at: https://redcap.core.wits.ac.za/redcap/ (Accessed Nov 4, 2022).
17. Ministry of Public Health of the Democratic Republic of the Congo. Standards and Guidelines for Diabetes Care (2012). Available at: https://extranet.who.int/ncdccs/Data/COD_B6_NV_Draft%20final_Normes%20Diab%C3%A8te_09_11_13final%20%282%29.pdf. Nov 22, 2022
18. King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol. (1999) 48(5):643–8. doi: 10.1046/j.1365-2125.1999.00092.x
19. American Diabetes Association. 6. Glycemic targets: Standards of Medical Care in Diabetes-2021. Diabetes Care (2021) 44(Supplement_1):S73–84. doi: 10.2337/dc21-S006
20. Cypres Diagnostics. HbA1c Turbi. Available at: https://diagnostics.be/fr/product/ht001 (Accessed Nov 22, 2022).
21. StataCorp. Stata Statistical Software: Release 17. College Station in Texas, StataCorp LLC (2021).
22. De Pablos-Velasco P, Parhofer KG, Bradley C, Eschwège E, Gönder-Frederick L, Maheux P, et al. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: Data from the PANORAMA study. Clin. Endocrinol. (Oxf) (2014) 80(1):47–56. doi: 10.1111/cen.12119
23. Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. Adults, 1999–2018. N Engl. J. Med. (2021) 384(23):2219–28. doi: 10.1056/NEJMsa2032271
24. Motala AA, Mbanya JC, Ramaiya K, Pirie FJ, Ekoru K. Type 2 diabetes mellitus in sub-Saharan Africa: challenges and opportunities. Nat. Rev. Endocrinol. (2022) 18(4):219–29. doi: 10.1038/s41574-021-00613-y
25. Stephani V, Opoku D, Beran D. Self-management of diabetes in Sub-Saharan Africa: A systematic review. BMC Public Health (2018) 18:1148. doi: 10.1186/s12889-018-6050-0
26. Kapongo RY, Lulebo AM, Mafuta EM, Mutombo PB, Dimbelolo JC, Bieleli IE. Assessment of health service delivery capacities, health providers' knowledge and practices related to type 2 diabetes care in Kinshasa primary healthcare network facilities, Democratic Republic of the Congo. BMC Health Serv. Res. (2015) 15:9. doi: 10.1186/s12913-015-0679-5
27. Sendekie AK, Belachew EA, Dagnew EM, Netere AK. Rate of glycaemic control and associated factors in patients with type 2 diabetes mellitus treated with insulin-based therapy at selected hospitals in Northwest Ethiopia: a multicentre cross-sectional study. BMJ Open (2022) 12(9):e065250. doi: 10.1136/bmjopen-2022-065250
28. Rita SL, Lubaki FJP, Bompeka LF, Ogunbanjo GA, Ngwala LP. Prevalence and determinants of psychological insulin resistance among type 2 diabetic patients in Kinshasa, Democratic Republic of Congo. Afr. J. Prim Heal Care Fam Med. (2019) 11(1):1–5. doi: 10.4102/phcfm.v11i1.1993
29. Funnell MM, Bootle S, Stuckey HL. The diabetes attitudes, wishes and needs second study. Clin. Diabetes (2015) 33(1):32–6. doi: 10.2337/diaclin.33.1.32
30. Bae JP, Lage MJ, Mo D, Nelson DR, Hoogwerf BJ. Obesity and glycemic control in patients with diabetes mellitus: Analysis of physician electronic health records in the US from 2009-2011. J. Diabetes Complications (2016) 30(2):212–20. doi: 10.1016/j.jdiacomp.2015.11.016
31. Boye KS, Lage MJ, Thieu V, Shinde S, Dhamija S, Bae JP. Obesity and glycemic control among people with type 2 diabetes in the United States: A retrospective cohort study using insurance claims data. J. Diabetes Complications (2021) 35(9):107975. doi: 10.1016/j.jdiacomp.2021.107975
32. Han SJ, Boyko EJ. The evidence for an obesity paradox in type 2 diabetes mellitus. Diabetes Metab. J. (2018) 42(3):179–87. doi: 10.4093/dmj.2018.0055
33. Mobula LM, Stephen F, Carson KA, Burnham G, Arthur L, Ansong D, et al. Translational Metabolic Syndrome Research Predictors of glycemic control in type-2 diabetes mellitus : Evidence from a multicenter study in Ghana. Transl. Metab. Syndr. Res. (2018) 1:1–8. doi: 10.1016/j.tmsr.2018.09.001
34. Schnell O, Crocker JB, Weng J. Impact of HbA1c testing at point of care on diabetes management. J. Diabetes Sci. Technol. (2017) 11(3):611–7. doi: 10.1177/1932296816678263
35. Ekoru K, Doumatey A, Bentley AR, Chen G, Zhou J, Shriner D, et al. Type 2 diabetes complications and comorbidity in Sub-Saharan Africans. EClinicalMedicine (2019) 16:30–41. doi: 10.1016/j.eclinm.2019.09.001
36. Fekadu G, Bula K, Bayisa G, Turi E, Tolossa T, Kasaye HK. Challenges and factors associated with poor glycemic control among type 2 diabetes mellitus patients at Nekemte referral hospital, western Ethiopia. J. Multidiscip Healthc (2019) 12:963–74. doi: 10.2147/JMDH.S232691
37. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care (2018) 41(12):2669–701. doi: 10.2337/dci18-0033
38. Goedecke JH, Olsson T. Pathogenesis of type 2 diabetes risk in black Africans: a South African perspective. J. Intern. Med. (2020) 288(3):284–94. doi: 10.1111/joim.13083
39. Yeatman S, Chamberlin S, Dovel K. Women’s (health) work: A population-based, cross-sectional study of gender differences in time spent seeking health care in Malawi. PLoS One (2018) 13(12):3–4. doi: 10.1371/journal.pone.0209586
40. Jackson Y, Lozano Becerra JC, Carpentier M. Quality of diabetes care and health insurance coverage: a retrospective study in an outpatient academic public hospital in Switzerland. BMC Health Serv. Res. (2016) 16(1):1–7. doi: 10.1186/s12913-016-1801-z
41. Bowling A. Research Methods in Health. 4rd Edition. Oxford: Oxford University Press-McGraw Hill Education (2014).
Keywords: diabetes mellitus, type 2, factors, glycaemic control, cross-sectional study, sub-Sahara Africa
Citation: Fina Lubaki J-P, Omole OB and Francis JM (2023) Poor glycaemic control: prevalence, factors and implications for the care of patients with type 2 diabetes in Kinshasa, Democratic Republic of the Congo: a cross-sectional study. Front. Clin. Diabetes Healthc. 4:1241882. doi: 10.3389/fcdhc.2023.1241882
Received: 17 June 2023; Accepted: 24 October 2023;
Published: 20 November 2023.
Edited by:
Dr. Sanjith Saseedharan, S.L. Raheja Hospital, IndiaReviewed by:
Jean Claude Katte, Université de YaoundéI, CameroonMasoud Mohammadnezhad, University of Bradford, United Kingdom
Copyright © 2023 Fina Lubaki, Omole and Francis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean-Pierre Fina Lubaki, amVhbnBpZXJyZWZpbmFAeWFob28uZnI=
 Jean-Pierre Fina Lubaki
Jean-Pierre Fina Lubaki Olufemi Babatunde Omole1
Olufemi Babatunde Omole1 Joel Msafiri Francis
Joel Msafiri Francis