
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Clin. Diabetes Healthc. , 28 July 2022
Sec. Diabetes Clinical Epidemiology
Volume 3 - 2022 | https://doi.org/10.3389/fcdhc.2022.944483
This article is part of the Research Topic Epidemiology of Diabetes in Africa View all 4 articles
Identifying patients with new-onset type 2 diabetes who have insulin deficiency can aid in timely insulin replacement therapy. In this study, we measured fasting C-peptide concentration to assess endogenous insulin secretion and determine the prevalence and characteristics of patients with insulin deficiency in adult Ugandan patients with confirmed type 2 diabetes at presentation.
Methods: Adult patients with new-onset diabetes were recruited from seven tertiary hospitals in Uganda. Participants who were positive for the three islet autoantibodies were excluded. Fasting C-peptide concentrations were measured in 494 adult patients, and insulin deficiency was defined as a fasting C-peptide concentration <0.76 ng/ml. The socio-demographic, clinical, and metabolic characteristics of participants with and without insulin deficiency were compared. Multivariate analysis was performed to identify independent predictors of insulin deficiency.
Results: The median (IQR) age, glycated haemoglobin (HbA1c), and fasting C-peptide of the participants was 48 (39-58) years,10.4 (7.7-12.5) % or 90 (61-113) mmol/mol, and 1.4 (0.8-2.1) ng/ml, respectively. Insulin deficiency was present in 108 (21.9%) participants. Participants with confirmed insulin deficiency were more likely to be male (53.7% vs 40.4%, p=0.01), and had a lower body mass index or BMI [p<0.001], were less likely to be hypertensive [p=0.03], had reduced levels of triglycerides, uric acid, and leptin concentrations [p<0.001]), but higher HbA1c concentration (p=0.004). On multivariate analysis, BMI (AOR 0.89, 95% CI 0.85-0.94, p<0.001), non-HDLC (AOR 0.77, 95% CI 0.61-0.97, p=0.026), and HbA1c concentrations (AOR 1.08, 95% CI 1.00-1.17, p=0.049) were independent predictors of insulin deficiency.
Conclusion: Insulin deficiency was prevalent in this population, occurring in about 1 in every 5 patients. Participants with insulin deficiency were more likely to have high HbA1c and fewer markers of adiposity and metabolic syndrome. These features should increase suspicion of insulin deficiency and guide targeted testing and insulin replacement therapy.
Measurement of fasting, random, or stimulated C-peptide concentrations, as an indicator of pancreatic beta-cell insulin secretion, is recommended in patients with new-onset diabetes to identify specific diabetes subtypes (type 1 and type 2 diabetes and maturity-onset diabetes of the young), patients that require timely insulin replacement therapy, and also to predict response to oral hypoglycaemic agents (1, 2). In patients with type 1 or type 2 diabetes and absolute insulin deficiency, early initiation of insulin therapy at diagnosis helps in achieving early optimal glycaemic control, preserves and improves beta-cell mass and function, in addition to averting diabetic ketoacidosis, and early onset of diabetes complications (3).
While fasting or random C-peptide concentrations are commonly measured to guide the management of diabetes in high-income countries, it remains a less available and expensive test in resource-constrained settings like sub-Saharan Africa (SSA). Initiation of insulin or switching from oral hypoglycaemic agents to insulin therapy in adult patients with new-onset or long-standing diabetes and presumed insulin deficiency in such settings is based on specific clinical suspicion. This has potential limitations, in part because there is little data on the prevalence, characteristics, and clinical correlates of insulin deficiency in adult patients with new-onset type 2 diabetes in SSA.
To address this evidence gap, we undertook this sub-study which was part of the Uganda Diabetes Phenotype (UDIP) study that aimed to investigate the manifestation of diabetes in adult Ugandan patients with recently diagnosed diabetes. In this sub-study, we specifically sought to determine the prevalence, characterisation, and predictors of insulin deficiency in an adult population with newly diagnosed type 2 diabetes in Uganda.
The UDIP study was carried out in seven tertiary public and private hospitals located in Central and Southwestern Uganda between February 2019 and October 2020. These hospitals predominantly serve the surrounding urban, peri-urban, and rural populations and have once weekly outpatient diabetes clinics for long-term management of adult patients with diabetes.
A total of 494 participants were recruited from these outpatient diabetes clinics. The inclusion criteria were patients aged ≥18 years with a recent diagnosis of diabetes (<3 months since diagnosis), initiated on any glucose-lowering treatment or treatment naïve, and tested negative for the three measured islet autoantibodies (defined as a concentration of autoantibodies against glutamic acid decarboxylase-65 [GADA], zinc transporter 8 [ZnT8-A], and tyrosine phosphatase [IA-2A] of ≤34U/ml, ≤67.7 U/ml, and ≤58 U/ml, respectively).
Participants who were critically ill and required urgent hospital admission were not immediately recruited into the study. Enrolment into the study was done later, at least two weeks after discharge from the hospital (but within three months of diagnosis), when they re-attended the outpatient diabetes clinic in a more clinically stable state. Pregnant women with new-onset diabetes were excluded from the study.
Participants were recruited in the study after an overnight fast of a minimum of eight hours. Using pre-tested case report forms, we collected information on relevant socio-demographic and clinical characteristics (age at diagnosis, gender, residence, smoking and alcohol ingestion habits, history of admission at the time of diagnosis, presence of urine or serum ketosis on admission, co-existing medical conditions, and glucose-lowering diabetes therapies initiated).
This was followed by resting blood pressure and anthropometric measurements (weight, height, waist circumference or WC, hip circumference or HC, body mass index or BMI, waist: hip circumference ratio or WHR, and waist circumference: height ratio or WHtR).
Following standardised procedures, a fasting venous blood sample was drawn for measurement of blood glucose (FBG), glycated haemoglobin (HbA1c), insulin, C-peptide, lipid profile, uric acid, leptin, and three islet autoantibodies (GADA, ZnT8-A, and IA-2A). This was followed by a 75-gram oral glucose tolerance test (OGTT), with venous blood samples being drawn again 30 and 120 minutes following glucose solution ingestion to determine the serum glucose, insulin, and C-peptide concentrations at those two time-points.
All the above tests were performed at the ISO-certified clinical chemistry laboratory at the Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit, Entebbe Uganda using electro-chemiluminescence immunoassays manufactured by Roche diagnostics Limited, Germany on a Cobas 6000 C-model SN 14H3-15 machine (Hitachi High Technologies Corporation, Tokyo Japan). Pancreatic autoantibody testing was done using autoantibody ELISA kits from RSR Limited (Cardiff CF14 5DU, UK).
To determine the insulin resistance (homeostatic model assessment-2 [HOMA2] insulin resistance or HOMA2-IR) and the pancreatic beta-cell function (HOMA2-%B), we used the online HOMA2 calculator by the Diabetes Trial Unit of the University of Oxford, Oxford UK (4). Pancreatic beta-cell function was also assessed using an optimal marker of beta-cell function- the oral insulinogenic index (IGI) using the formula: IGI = δ insulin (30 min insulin - 0 min insulin in µU/ml)/δ glucose (30 min glucose - 0 min glucose in mmol/l) (5). We used the online quantitative insulin sensitivity check index (QUICKI) calculator to calculate the QUICKI using the fasting serum glucose and insulin concentrations (6).
Insulin deficiency was defined as a fasting C-peptide concentration <0.76 ng/ml (equivalent to 0.25 nmol/l or 250 pmol/l). Absolute insulin deficiency or requirement was defined as a fasting C-peptide concentration <0.24 ng/ml (equivalent to 0.08 nmol/l or 80 pmol/l) (1, 2).
The categorical and continuous variables were expressed as proportions and medians with inter-quartile range (IQR), respectively. The prevalence of insulin deficiency was expressed as a frequency. Differences in the socio-demographic, clinical, anthropometric, and metabolic characteristics of participants with and without insulin deficiency were analysed using the x2 test for categorical data and the Kruskal Wallis test for continuous data. Multivariate analysis was performed to identify predictors of insulin deficiency. All analyses were done using STATA statistical software version 15 College Station, TX: StataCorp LLC.
This study was approved by the Research Ethics Committee of Uganda Virus Research Centre, Entebbe Uganda (GC/127/18/05/650) and the Uganda National Council of Science and Technology (HS 2431). Administrative clearance was also obtained from all participating study sites. All enrolled study participants provided written informed consent to participate in the study.
The socio-demographic, clinical, anthropometric, and metabolic characteristics of all study participants are summarised in Table 1.
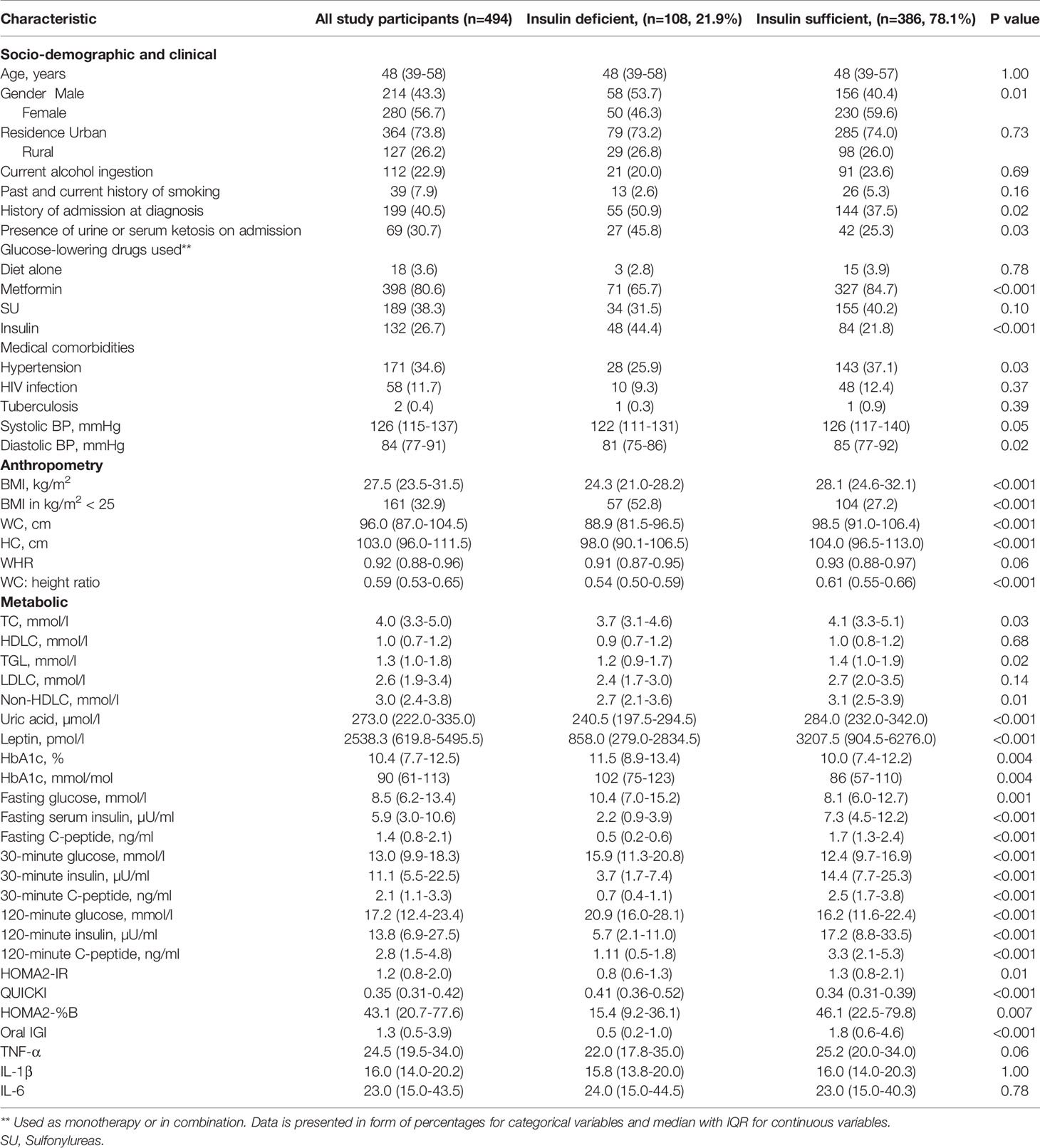
Table 1 Socio-demographic, clinical, anthropometric, and metabolic characteristics of the study participants with and without insulin deficiency.
The median (IQR) age, BMI, HbA1c, and fasting C-peptide of the participants was 48 (39-58) years, 27.5 (23.5-31.5) kg/m2, 10.4 (7.7-12.5) % or 90 (61-113) mmol/mol, and 1.4 (0.8-2.1) ng/ml, respectively, with 56.7% of participants being female. About 41% of participants reported a history of admission at the time of diagnosis with 30.7% of those admitted presenting with urine or serum ketosis. The majority of the participants were initiated on metformin, either as monotherapy or in combination with other therapies (80.6%), with only 26.7% initiated on insulin therapy.
One hundred and eight participants had insulin deficiency, corresponding to a prevalence of 21.9%. Absolute insulin deficiency or requirement was noted in 26 (5.3%) participants.
The socio-demographic, clinical, anthropometric, and metabolic characteristics of participants with and without insulin deficiency are summarised in Table 1.
Compared with those who were insulin sufficient, participants with confirmed insulin deficiency were more likely to be male (53.7% vs 40.4%, p=0.01), to be admitted at the time of diagnosis (50.9% vs 37.5%, p=0.02), to present with urine or serum ketosis on admission (45.8% vs 25.3%, p=0.03), and to be initiated on insulin at diagnosis (44.4% vs 21.8%, p<0.001). No statistically significant differences were noted in the age at diagnosis between the two groups.
Participants with insulin deficiency had statistically significant higher glycaemic indices (FBG-10.4 [7.0-15.2] vs 8.1 [6.0-12.7] mmol/l, p=0.001, HbA1c- 11.5 [8.9-13.4] vs 10 [7.4-12.2] % or 102 [75-123] vs 86 [57-110] mmol/mol, p=0.004 and 30-minute glucose- 15.9 [11.3-20.8] vs 12.4 [9.7-16.9] mmol/l, p<0.001) and a lower HOMA2-IR (0.8 [0.6-1.3] vs 1.3 [0.8-2.1], p=0.01). In addition, these participants had statistically significant lower markers of pancreatic beta cell function like HOMA2-B%, oral IGI, 30-and 120-minute C-peptide concentrations (p<0.001).
Compared with those who were insulin sufficient, participants with insulin deficiency were less likely to have hypertension comorbidity (25.9% vs 37.1%, p=0.03) and had statistically significant lower median BMI (24.3 [21.0-28.2] vs 28.1 [24.6-32.1] kg/m2, p<0.001), WC (88.9 [81.5-96.5] vs 98.5 [91.0-106.4] cm, p<0.001), triglyceride (1.2 [0.9-1.7] vs 1.4 [1.0-1.9] mmol/l, p=0.02), non-high-density lipoprotein cholesterol or non-HDLC (2.7 [2.1-3.6] vs 3.1 [2.5-3.9] mmol/l, p=0.01), uric acid (240.5 [197.5-294.5] vs 284 [232-342] µmol/l, p<0.001), and leptin (858 [279-2834.5] vs 3207.5 [904.5-6276], p=0.001) concentrations.
Table 2 shows the independent predictors of insulin deficiency. On multivariate analysis, BMI (AOR 0.89, 95% CI 0.85-0.94, p<0.001), non-HDLC concentration (AOR 0.77, 95% CI 0.61-0.97, p=0.026), and HbA1c level (AOR 1.08, 95% CI 1.00-1.17, p=0.049) were noted to independently predict insulin deficiency in this study population.
To our knowledge, this is the first study to robustly investigate the frequency, characterisation, and predictors of insulin deficiency in an adult African population with newly diagnosed confirmed type 2 diabetes. We demonstrate that the prevalence of insulin deficiency is relatively high, occurring in about 1 in every 5 adult patients with newly diagnosed type 2 diabetes. Absolute insulin deficiency or requirement, similar to what is described in most patients with type 1 diabetes, was also prevalent in our study population. The documented high prevalence of insulin deficiency in these participants with newly diagnosed type 2 diabetes is less likely to be due to pancreatic beta-cell exhaustion related to the natural disease progression, seen in patients with long-standing diabetes.
Studies that have evaluated pancreatic beta-cell function using fasting or random C-peptide measurement in adult populations with newly diagnosed type 2 diabetes in SSA are limited. The few available studies have been conducted in adult patients with long-standing type 2 diabetes, reporting a wide variation in the documented prevalence of insulin deficiency ranging from 7% to 73% (7–11). Except for one study which used a lower cut-off of fasting C-peptide concentration of <0.2 nmol/l (0.6 ng/ml) to define low C-peptide levels (7), the rest of the above studies used a cut-off of <0.3 nmol/l (0.9 ng/ml).
Varying degrees of insulin deficiency have been reported in Asian and Caucasian adult populations with new-onset type 2 diabetes, further underscoring phenotypic differences across ethnicities. Despite using varied parameters to define insulin deficiency (fasting and stimulated C-peptide, HOMA2-B%, and insulin sensitivity or HOMA2-S) and fasting C-peptide thresholds, a lower prevalence of insulin deficiency was reported in an adult South Korean (4.4%) (12) and Danish population (9.6%) (13) with new-onset presumed type 2 diabetes. Similar to our study findings, the Swedish All New Diabetics in Scania (ANDIS) cohort (14) and the India-Scotland Partnership for precision medicine in diabetes (INSPIRED) Indian cohort (15) also reported a relatively high frequency of insulin deficiency of 17.5% and 26.2%, respectively. Only the study performed on 500 adult patients with new-onset diabetes in Yemen reported a higher prevalence of insulin deficiency of 43.6%, based on HOMA2-B% and S (16).
In this study, participants who were insulin-deficient were more likely to be of the male gender, with a history of being admitted with ketosis and initiated on insulin therapy at the time of diagnosis of diabetes. A higher prevalence of insulin deficiency in male patients with type 2 diabetes has also been reported in two large studies conducted in Indian populations with long-standing type 2 diabetes (15, 17). Similar to what we observed in our study, male participants had a lower mean BMI compared to their female counterparts in one study that investigated novel diabetes subgroups in Indians with young-onset diabetes (17). The reasons to explain this clinical observation are not well-known and ought to be investigated.
In this study, we also demonstrated that insulin deficiency is associated with high glycaemic levels and reduced markers of adiposity and metabolic syndrome. An increase in BMI reduced the odds of insulin deficiency by 11%. A similar direct relationship between fasting C-peptide, as a measure of pancreatic beta-cell secretory function, and BMI has been reported in similar studies investigating pancreatic beta-cell function in African and Caucasian patients with long-standing and recently diagnosed diabetes (7–9, 18, 19). In addition to its role in regulating glucose homeostasis, insulin is an anabolic hormone that promotes lipogenesis and protein synthesis through the tyrosine kinase receptor pathway (20). Insulin deficiency, therefore, would result in an increased lipolysis, diffuse muscle wasting, and a low BMI phenotype as clinical hallmarks.
The association between an increase in HbA1c concentrations and insulin deficiency as shown in our study has been replicated in most studies, further highlighting the key role of insulin in glucose homeostasis (8, 9, 19, 21).
To our knowledge, non-HDLC concentration, as an indicator of increased cardiovascular disease risk, has not been reported in any study as a predictor of reduced pancreatic beta-cell secretory dysfunction. Conversely, an increase in non-HDLC concentrations reduced the odds of insulin deficiency in our study population. Most studies have reported a close relationship between increased TC, LDLC, TGL, and low HDLC concentrations and reduced insulin secretion by the pancreatic beta-cells (19, 22–24). This variation in study findings may be related to differences in diabetes phenotypes across populations. Participants with confirmed insulin deficiency in our study had a favourable lipid profile pattern (lower TC, LDLC, TGL, and non-HDLC concentrations).
Disorders in lipid metabolism have been implicated in pancreatic beta-cell dysfunction through their adverse effects of inhibiting glucokinase activity, glucose-stimulated insulin secretion, pancreatic beta-cell proliferation, in addition to inducing beta-cell apoptosis, and suppressing transcription of the pre-proinsulin gene (25, 26).
The reasons explaining the high frequency of insulin deficiency in our study population are unknown but may be related to structural pancreatic defects (presence of a naturally existing small beta-cell mass), local environmental (in-utero and early childhood chronic infections and malnutrition), and genetic factors that influence the capacity for beta-cell expansion (replication or neogenesis) or secretory function (27–30). The developmental origins of health and disease (DOHaD) concept emphasises the direct influence of chronic infections and undernutrition on structural development and physiologic function of most critical body organs during the early stages of life and development, hence increasing the future risk of metabolic conditions like type 2 diabetes (31). Undernutrition and chronic infections remain common in SSA and could explain the high rates of pancreatic beta-cell dysfunction and insulin deficiency noted in adult African patients with type 2 diabetes (32–36).
The African continent also has diverse populations with marked genetic heterogeneity. Black Africans may have susceptibility genes that directly affect pancreatic beta-cell mass and secretory function hence increasing the risk of developing type 2 diabetes. Two significant susceptibility genes that have been discovered to be highly prevalent in Western and Eastern African patients with type 2 diabetes and are worth mentioning are the Zinc Finger RANBP2-Type Containing 3 (ZRANB3) gene, which increases apoptosis resulting in reduced pancreatic beta-cell mass (37) and the transcription factor 7-like 2 (TCF7L2) gene, known to affect pancreatic beta-cell secretory function (38).
Because our study population did not include participants positive for islet autoantibodies and participants with insulin deficiency had a favourable lipid profile pattern (low TC, TGL, LDLC, and non-HDLC concentrations) and low circulating pro-inflammatory cytokines (IL-6, TNF-α, and IL-1β), we hypothesise that the observed insulin deficiency may be related to a structural defect (inadequate functional pancreatic beta-cell mass) either due to prior early-life environmental insults or genetic influences affecting the pancreatic beta-cell growth or regeneration and insulin secretion, as opposed to increased pancreatic beta-cell apoptosis due to lipotoxicity, increased endoplasmic reticulum stress, or inflammation.
In the context of SSA, communicable diseases like tuberculosis and HIV infection have been implicated in causing acute and chronic pancreatitis, hence reduced pancreatic beta-cell mass and consequent dysglycaemia (39, 40). Cases of brittle diabetes due to tuberculosis of the pancreas have been reported in SSA, a region with one of the highest burden of TB globally (39). In our study, pre-existing HIV and tuberculosis infections were only reported in 11.7% and 0.4% of participants, respectively with no differences between those who were insulin deficient and sufficient.
Our study had several strengths. It recruited a substantially large number of patients with incident adult-onset diabetes without markers of islet autoimmunity from seven tertiary hospitals. Recruiting newly diagnosed adult patients rules out the possibility that the insulin deficiency observed in this study population is due to pancreatic beta-cell mass exhaustion which is part of the natural progressive nature of type 2 diabetes. We performed additional metabolic tests to further evaluate pancreatic beta-cell function in this study population like 30-and 120-minute C-peptide measurement, oral insulinogenic index, and HOMA2-%B calculation. To further characterise insulin deficiency in our study population, we performed additional relevant metabolic and immunological tests like leptin, uric acid, and pro-inflammatory cytokines. Few studies have performed these tests as part of their metabolic and immunologic characterisation.
Despite these strengths, the study had some limitations. Participants were recruited only from tertiary hospitals, which may introduce a selection bias and limit the generalisability of the findings to the adult Ugandan population with diabetes. Despite this limitation, it is important to note that the majority of patients self-refer to these tertiary hospitals for long-term diabetes management. Being cross-sectional in design, the study offers no evidence of a temporal relationship between type 2 diabetes and insulin deficiency.
The relatively high prevalence of insulin deficiency in this Ugandan population with recently diagnosed adult-onset type 2 diabetes has significant clinical practice and policy implications in Uganda. It underscores the need for targeted assessment of pancreatic beta-cell function at diagnosis of diabetes, especially in patients with high glycaemic levels and reduced features of adiposity and metabolic syndrome. This will be important in identifying patients that require immediate insulin replacement therapy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Uganda Virus Research Centre, Entebbe Uganda and the Uganda National Council of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
DK oversaw the entire data collection process and wrote the initial draft of the manuscript. IS performed the statistical analysis and reviewed all the versions of the manuscript. WL contributed to the discussion and reviewed all the versions of the manuscript. MN supervised this work, collectively contributed to the research idea, and reviewed all the versions of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement for the Medical Research Council/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit, Entebbe Uganda (Project Reference: MC_UP_1204/16), and the National Institute for Health Research (NIHR) (17/63/131).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to express our gratitude to the UDIP study research team at the different participating hospitals, the entire staff of the clinical chemistry unit of the Clinical Diagnostics Laboratory Services at the Medical Research Council/Uganda Virus Research Institute, and the London School of Hygiene and Tropical Medicine Uganda Research Unit, Entebbe, Uganda and all the study participants who consented to join the study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2022.944483/full#supplementary-material
1. Jones AG, Hattersley AT. The Clinical Utility of C-Peptide Measurement in the Care of Patients With Diabetes. Diabetes Med (2013) 30(7):803–17. doi: 10.1111/dme.12159
2. Leighton E, Sainsbury CA, Jones GC. A Practical Review of C-Peptide Testing in Diabetes. Diabetes Ther. (2017) 8(3):475–87. doi: 10.1007/s13300-017-0265-4
3. Owens DR. Clinical Evidence for the Earlier Initiation of Insulin Therapy in Type 2 Diabetes. Diabetes Technol. Ther. (2013) 15(9):776–85. doi: 10.1089/dia.2013.0081
4. The Homeostasis Model Assessment (Homa) 2 Calculator. Available at: https://www.dtu.ox.ac.uk/homacalculator/ (Accessed 01 June 2020).
5. Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of Pancreatic beta-Cell Function: Review of Methods and Clinical Applications. Curr. Diabetes Rev. (2014) 10(1):2–42. doi: 10.2174/1573399810666140214093600
6. Insulin Sensitivity Quicki Calculator. Available at: https://www.mdapp.co/insulin-sensitivity-quicki-calculator-324/ (Accessed 1 June 2020).
7. Siraj ES, Reddy SSK, Scherbaum WA, Abdulkadir J, Hammel JP, Faiman C. Basal and Postglucagon C-Peptide Levels in Ethiopians With Diabetes. Diabetes Care (2002) 25(3):453. doi: 10.2337/diacare.25.3.453
8. Oputa RN, Ekwueme C, Amah JN, Chinenye S, Nwaokoro CJ. Connecting Peptide (C-Peptide) and the Duration of Diabetes Mellitus Amongst Patients, at the Federal Medical Centre (Fmc), Owerri, Southeast, Nigeria. Res. J. Health Sci. (2016) 4(1):9–14.
9. Young EE, Chinenye S, Unachukwu CN. Beta Cell Response to a Mixed Meal in Nigerian Patients With Type 2 Diabetes. BMC Endocr. Disord. (2012) 12(1):11. doi: 10.1186/1472-6823-12-11
10. Gill GV, Tekle A, Reja A, Wile D, English PJ, Diver M, et al. Immunological and C-Peptide Studies of Patients With Diabetes in Northern Ethiopia: Existence of an Unusual Subgroup Possibly Related to Malnutrition. Diabetologia (2011) 54(1):51–7. doi: 10.1007/s00125-010-1921-7
11. Rheeder P, Stolk RP, Grobbee DE. Ethnic Differences in C-Peptide Levels and Anti-Gad Antibodies in South African Patients With Diabetic Ketoacidosis. QJM: Int. J. Med. (2001) 94(1):39–43. doi: 10.1093/qjmed/94.1.39
12. Ha KH, Park CY, Jeong IK, Kim HJ, Kim SY, Kim WJ, et al. Clinical Characteristics of People With Newly Diagnosed Type 2 Diabetes Between 2015 and 2016: Difference by Age and Body Mass Index. Diabetes Metab. J. (2018) 42(2):137–46. doi: 10.4093/dmj.2018.42.2.137
13. Stidsen JV, Henriksen JE, Olsen MH, Thomsen RW, Nielsen JS, Rungby J, et al. Pathophysiology-Based Phenotyping in Type 2 Diabetes: A Clinical Classification Tool. Diabetes Metab. Res. Rev. (2018) 34(5):e3005. doi: 10.1002/dmrr.3005
14. Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel Subgroups of Adult-Onset Diabetes and Their Association With Outcomes: A Data-Driven Cluster Analysis of Six Variables. Lancet Diabetes Endocrinol. (2018) 6(5):361–9. doi: 10.1016/s2213-8587(18)30051-2
15. Anjana RM, Baskar V, Nair ATN, Jebarani S, Siddiqui MK, Pradeepa R, et al. Novel Subgroups of Type 2 Diabetes and Their Association With Microvascular Outcomes in an Asian Indian Population: A Data-Driven Cluster Analysis: The Inspired Study. BMJ Open Diabetes Res. Care (2020) 8(1):e001506. doi: 10.1136/bmjdrc-2020-001506
16. Gunaid AA, Al-Kebsi MM, Bamashmus MA, Al-Akily SA, Al-Radaei AN. Clinical Phenotyping of Newly Diagnosed Type 2 Diabetes in Yemen. BMJ Open Diabetes Res. Care (2018) 6(1):e000587. doi: 10.1136/bmjdrc-2018-000587
17. Prasad RB, Asplund O, Shukla SR, Wagh R, Kunte P, Bhat D, et al. Subgroups of Patients With Young-Onset Type 2 Diabetes in India Reveal Insulin Deficiency as a Major Driver. Diabetologia (2022) 65(1):65–78. doi: 10.1007/s00125-021-05543-y
18. Thunander M, Törn C, Petersson C, Ossiansson B, Fornander J, Landin-Olsson M. Levels of C-Peptide, Body Mass Index and Age, and Their Usefulness in Classification of Diabetes in Relation to Autoimmunity, in Adults With Newly Diagnosed Diabetes in Kronoberg, Sweden. Eur. J. Endocrinol. (2012) 166(6):1021–9. doi: 10.1530/eje-11-0797
19. Russo GT, Giorda CB, Cercone S, Nicolucci A, Cucinotta D. On Behalf of BetaDecline Study G. Factors Associated With Beta-Cell Dysfunction in Type 2 Diabetes: The Betadecline Study. PloS One (2014) 9(10):e109702. doi: 10.1371/journal.pone.0109702
20. Rahman MS, Hossain KS, Das S, Kundu S, Adegoke EO, Rahman MA, et al. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. (2021) 22(12):6403. doi: 10.3390/ijms22126403
21. Hou X, Liu J, Song J, Wang C, Liang K, Sun Y, et al. Relationship of Hemoglobin A1c With beta-Cell Function and Insulin Resistance in Newly Diagnosed and Drug Naive Type 2 Diabetes Patients. J. Diabetes Res. (2016) 2016:8797316. doi: 10.1155/2016/8797316
22. Zheng S, Xu H, Zhou H, Ren X, Han T, Chen Y, et al. Associations of Lipid Profiles With Insulin Resistance and beta Cell Function in Adults With Normal Glucose Tolerance and Different Categories of Impaired Glucose Regulation. PloS One (2017) 12(2):e0172221. doi: 10.1371/journal.pone.0172221
23. Shimodaira M, Niwa T, Nakajima K, Kobayashi M, Hanyu N, Nakayama T. Serum Triglyceride Levels Correlated With the Rate of Change in Insulin Secretion Over Two Years in Prediabetic Subjects. Ann. Nutr. Metab. (2014) 64(1):38–43.
24. Zheng T, Gao Y, Tian H. Relationship Between Blood Lipid Profiles and Pancreatic Islet beta- Cell Function in Chinese Men and Women With Normal Glucose Tolerance: A Cross-Sectional Study. BMC Public Health (2012) 12(1):634. doi: 10.1186/1471-2458-12-634
25. Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct Effect of Cholesterol on Insulin Secretion: A Novel Mechanism for Pancreatic Beta-Cell Dysfunction. Diabetes (2007) 56(9):2328–38. doi: 10.2337/db07-0056
26. Rütti S, Ehses JA, Sibler RA, Prazak R, Rohrer L, Georgopoulos S, et al. Low- and High-Density Lipoproteins Modulate Function, Apoptosis, and Proliferation of Primary Human and Murine Pancreatic Beta-Cells. Endocrinology (2009) 150(10):4521–30. doi: 10.1210/en.2009-0252
27. Weir GC, Gaglia J, Bonner-Weir S. Inadequate beta-Cell Mass Is Essential for the Pathogenesis of Type 2 Diabetes. Lancet Diabetes Endocrinol. (2020) 8(3):249–56. doi: 10.1016/s2213-8587(20)30022-x
28. Donath MY, Halban PA. Decreased Beta-Cell Mass in Diabetes: Significance, Mechanisms and Therapeutic Implications. Diabetologia (2004) 47(3):581–9. doi: 10.1007/s00125-004-1336-4
29. Wysham C, Shubrook J. Beta-Cell Failure in Type 2 Diabetes: Mechanisms, Markers, and Clinical Implications. Postgrad Med. (2020) 132(8):676–86. doi: 10.1080/00325481.2020.1771047
30. Defronzo RA. Banting Lecture. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes (2009) 58(4):773–95. doi: 10.2337/db09-9028
31. Mandy M, Nyirenda M. Developmental Origins of Health and Disease: The Relevance to Developing Nations. Int. Health (2018) 10(2):66–70. doi: 10.1093/inthealth/ihy006
32. Ladwa M, Hakim O, Amiel SA, Goff LM. A Systematic Review of Beta Cell Function in Adults of Black African Ethnicity. J. Diabetes Res. (2019) 2019:7891359. doi: 10.1155/2019/7891359
33. PrayGod G, Filteau S, Range N, Kitilya B, Kavishe BB, Ramaiya K, et al. beta-Cell Dysfunction and Insulin Resistance in Relation to Pre-Diabetes and Diabetes Among Adults in North-Western Tanzania: A Cross-Sectional Study. Trop. Med. Int. Health (2021) 26(4):435–43. doi: 10.1111/tmi.13545
34. Kibirige D, Lumu W, Jones AG, Smeeth L, Hattersley AT, Nyirenda MJ. Understanding the Manifestation of Diabetes in Sub Saharan Africa to Inform Therapeutic Approaches and Preventive Strategies: A Narrative Review. Clin. Diabetes Endocrinol. (2019) 5:2. doi: 10.1186/s40842-019-0077-8
35. Gill GV, Mbanya JC, Ramaiya KL, Tesfaye S. A Sub-Saharan African Perspective of Diabetes. Diabetologia (2009) 52(1):8–16. doi: 10.1007/s00125-008-1167-9
36. Filteau S, PrayGod G, Rehman AM, Peck R, Jeremiah K, Krogh-Madsen R, et al. Prior Undernutrition and Insulin Production Several Years Later in Tanzanian Adults. Am. J. Clin. Nutr. (2021) 113(6):1600–8. doi: 10.1093/ajcn/nqaa438
37. Adeyemo AA, Zaghloul NA, Chen G, Doumatey AP, Leitch CC, Hostelley TL, et al. Zranb3 Is an African-Specific Type 2 Diabetes Locus Associated With Beta-Cell Mass and Insulin Response. Nat. Commun. (2019) 10(1):3195. doi: 10.1038/s41467-019-10967-7
38. Adeyemo AA, Tekola-Ayele F, Doumatey AP, Bentley AR, Chen G, Huang H, et al. Evaluation of Genome Wide Association Study Associated Type 2 Diabetes Susceptibility Loci in Sub Saharan Africans. Front. Genet. (2015) 6:335(335). doi: 10.3389/fgene.2015.00335
39. Panic N, Maetzel H, Bulajic M, Radovanovic M, Löhr JM. Pancreatic Tuberculosis: A Systematic Review of Symptoms, Diagnosis and Treatment. United Eur. Gastroenterol. J. (2020) 8(4):396–402. doi: 10.1177/2050640620902353
Keywords: insulin deficiency, adult, newly diabetes mellitus, Uganda, Sub-Sahara Africa
Citation: Kibirige D, Sekitoleko I, Balungi P, Lumu W and Nyirenda MJ (2022) Apparent Insulin Deficiency in an Adult African Population With New-Onset Type 2 Diabetes. Front. Clin. Diabetes Healthc. 3:944483. doi: 10.3389/fcdhc.2022.944483
Received: 15 May 2022; Accepted: 21 June 2022;
Published: 28 July 2022.
Edited by:
George Praygod, National Institute of Medical Research, TanzaniaReviewed by:
Metin Guclu, Bursa Research and Training Hospital, TurkeyCopyright © 2022 Kibirige, Sekitoleko, Balungi, Lumu and Nyirenda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Davis Kibirige, a2liaXJpZ2VkYXZpc0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.