- 1Program on Statistical and Data Sciences, Smith College, Northampton, MA, United States
- 2Department of Psychiatry, Yale University School of Medicine, New Haven, CT, United States
Objectives: Previous studies identified depression and sleep disturbance as risk factors for diabetes. Sleep disturbance and depression are known to be inter-related. Further, women relative to men are more prone to depression. Here, we investigated how depression and sleep disturbance may jointly influence the risk of diabetes and the effects of sex on these influences.
Methods: Using the data of 21,229 participants from the 2018 National Health Interview Survey, we performed multivariate logistic regression with diabetes diagnosis as the dependent variable, sex, self-reported frequency of weekly depression and nightly sleep duration, and their interactions with sex as independent variables, and age, race, income, body mass index and physical activity as covariates. We employed Bayesian and Akaike Information criteria to identify the best model, evaluated the accuracy of the model in predicting diabetes using receiver operating characteristic analysis, and computed the odds ratios of these risk factors.
Results: In the two best models, depression frequency and sleep hours interact with sex in determining the diagnosis of diabetes, with higher depression frequency and nightly duration of sleep longer or shorter than 7 to 8 hours associated with higher likelihood of diabetes. The two models both predicted diabetes at an accuracy (area under the receiver operating characteristic curve) of 0.86. Further, these effects were stronger in men than in women at each depression and sleep level.
Conclusions: Depression and sleep inter-relatedly rather than independently contributes to diabetes. Depression and sleep hours associate with diabetes more significantly in men than in women. The current findings indicate a sex-dependent relationship between depression, sleep disturbance and diabetes risk and add to a growing body of evidence linking mental and physical health.
1 Introduction
Over 34 million Americans, or more than 1 in 10, are diagnosed with diabetes, one of the most prevalent chronic diseases in the US, according to the Centers for Disease Control and Prevention, National Diabetes Statistics Report, 2020. Diabetes and related health problems have an enormous impact on US health care spending. Individuals with diabetes face an overall 60% greater risk of early death than non-diabetics, underscoring the importance of identifying risk factors for targeted treatment (1).
In addition to diet and physical activity, sleep and mental health have been investigated as modifiable risk factors of diabetes (2). Studies examining the relationship between sleep and diabetes associate both short (<6 h) and long (>8 h) sleep duration with increased diabetes risk as strongly as overweight and family history of diabetes (3). Depression has also been associated with higher risk for diabetes. For instance, a meta-analysis reported that individuals with depression are 37% more likely to develop type 2 diabetes than their depression-free counterparts (4). However, sleep disturbance is one of the core manifestations of depression (5–7). Individuals with depression can demonstrate insomnia or hypersomnia along with other physical symptoms. Thus, whereas sleep and depression have largely been characterized as independent risk factors for diabetes, it is highly likely that depression and sleep disturbance conduce to diabetes in an inter-related manner.
It is known that women relative to men are more vulnerable to depression, as demonstrated in a meta-analysis (8). The latter study showed that sex differences in the risk of depression emerge early in the life span with an odds ratio (OR) peaking at adolescence (OR=3.02) and declining afterwards and remaining stable in adulthood (OR=1.71 to 2.02). Thus, about twice as many female experience depression as male adults. Many studies have investigated the genetic (8), neurobiological (9, 10) and socio-psychological (11) mechanisms underlying sex differences in the prevalence of depression. Some have assessed sex differences in the effect of depression on diabetes. For instance, a study showed significant association between depression and diabetes in women (risk ratio or RR=1.06 to 4.19), but not in men (RR=0.43 to 1.10) (12). Earlier studies have also noted significant association between disturbed sleep patterns and diabetes in women, but not in men (13, 14). In contrast, a meta-analysis showed higher diabetes-depression association in men (RR=1.24-1.99) than in women (RR=0.95-1.67) (15). Further, women have more sleep-related complaints, including insomnia, than men (16) and particularly so in the elderly (17). Thus, it is likely that diabetes may relate to depression and sleep disturbance differently between the sexes (18).
Here, using a large public dataset we investigated how depression and sleep disturbance relate to diabetes and whether the relationships vary between women and men. Based on the literature, we hypothesized that greater severity of depression would be associated with higher likelihood of diabetes and that nightly sleep duration would be associated with diabetes in a U-shaped function, with short and long hours of sleep both associated with higher risk. Further, these relationships would be stronger in females than in males.
2 Methods
2.1 Study Design
This was a cross-sectional study using logistic models to assess factors defining diabetes risk.
2.2 Data Set and Variables
We used the Integrated Public Use Microdata Series (IPUMS) National Health Interview Survey Data, 2018, of 21,229 participants. The data were collected by random sampling of 35,000 U.S. households and random selection of one adult and one child (if any) from each household for interview. We restricted the sample to those 18 years or older as children and adolescents demonstrate sleep patterns and durations different from those of adults. We intended to generalize the current findings to non-institutionalized adult population of the United States.
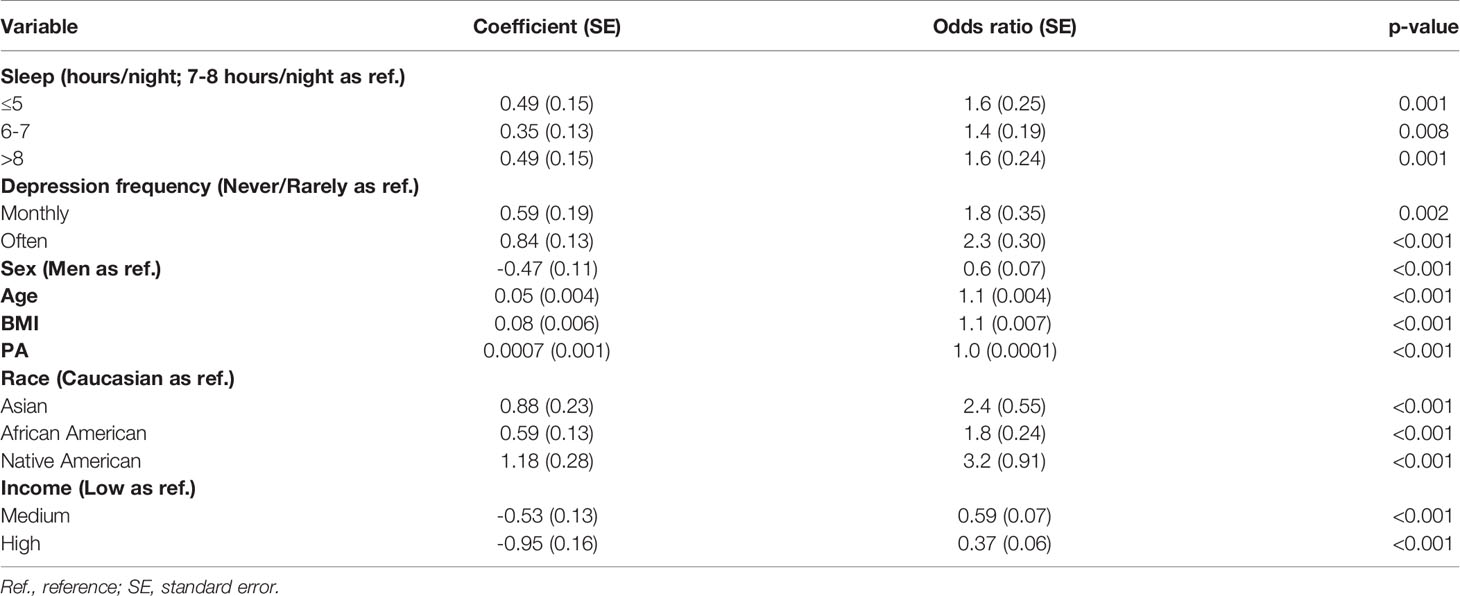
The variables in our analytics included a binary response (dependent) variable indicating whether an individual has diabetes (the IPUMS data set did not distinguish between type I or II of diabetes). Independent variables included 1) the average number of self-reported hours of sleep per night over the prior month in four categories: ≤ 5, 6-7, 7-8, and >8 hours; 2) the frequency an individual experiencing depression over the prior year in three categories: never/rarely, monthly, and often (more often than monthly); and 3) sex: males and females. Covariates included age (in years), race (Caucasian, black/African American, Asian, or Native American/Alaska Native), income, body mass index (BMI), and level of physical activity (PA). Household income was categorized into low (annual family income < $35,000), middle (between $35,000 and $75,000), and high (> $75,000). The variable measuring PA was created using the Oncology Nursing Society formula for weekly leisure activity score, derived from the Godin Leisure-Time Exercise Questionnaire. The data for ‘light exercise’ were not available and thus substituted with the data on strength-related exercise. Table 1 shows the demographic and clinical characteristics of the sample.
2.3 Statistical Analysis
2.3.1 Multivariate Logistic Regression
We used binomial logistic regression to predict binary outcome (presence/absence) of diabetes using an optimized model (19). The variables of interest included sex, depression frequency (depression), and nightly hours of sleep (sleep), and the covariates included age, race, household income (income), BMI, and PA.
We first performed a multivariate logistic regression to include all predictors (sex, frequency of depression, and nightly duration of sleep) and covariates (age, race, BMI, PA, and income) in the model. We checked for the statistical significance of the model; further, the three predictors and five covariates showed a significant relationship with diabetes and thus were all included in the model (see Results).
Next, we examined the continuous variables (age, BMI, and PA) for linearity in relation to the logit of the outcome. The results showed that age, BMI and PA were all linearly related to diabetes in logit scale (Figure 1).
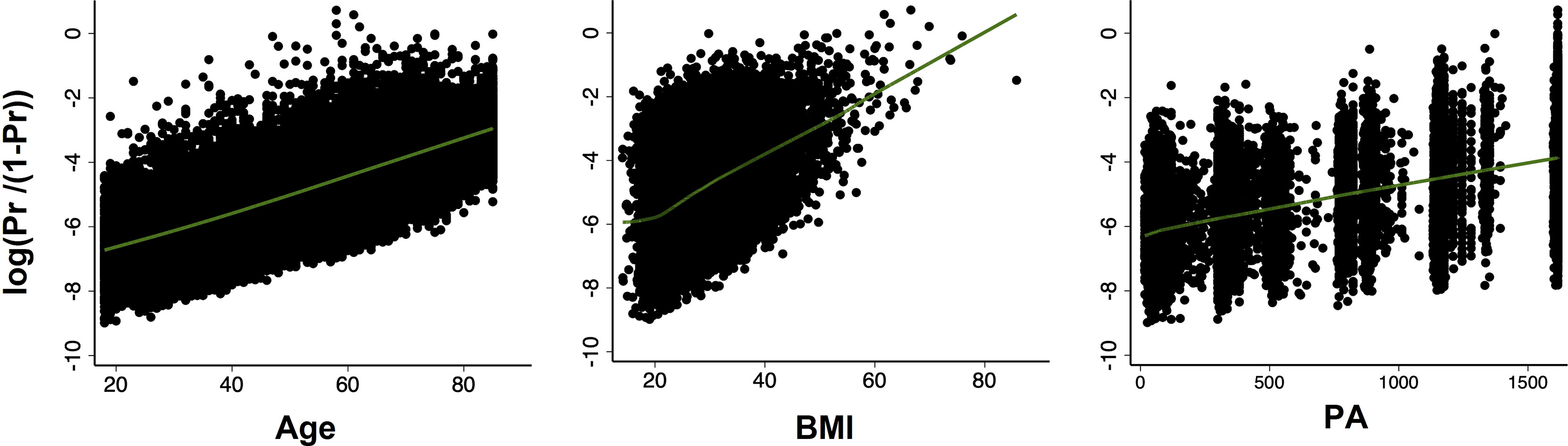
Figure 1 Scatter plots with locally weighted scatter plot smoothing (LOWESS) of the relationship between probability of diabetes (in logit scale) and continuous covariates (age, BMI, PA, respectively). All relationships were linear.
2.3.2 Identification of Prediction Model With the Best Fit
The findings of multivariate logistic regression showed that all 3 predictors and 5 covariates showed significant relationships with diabetes (Table 2). The goal of the study was to investigate how depression frequency, sleep duration and sex may interact in the contribution to diabetes. Thus, we employed Bayesian Information Criterion (BIC) and Akaike Information Criterion (AIC) to determine which of the following models showed the best fit:
Model 1: Diabetes = depression + sleep + sex + sex × depression + age + BMI + PA + race + income
Model 2: Diabetes = depression + sleep + sex + sex × sleep + age + BMI + PA + race + income
Model 3: Diabetes = depression + sleep + sex + depression × sleep + age + BMI + PA + race + income
Model 4: Diabetes = depression + sleep + sex + sex × depression × sleep + age + BMI + PA + race + income
Model 5: Diabetes = depression + sleep + sex + sex × depression + sex × depression × sleep + age + BMI + PA + race + income
Model 6: Diabetes = depression + sleep + sex + sex × sleep + sex × depression × sleep + age + BMI + PA + race + income
Model 7: Diabetes = depression + sleep + sex + depression × sleep + sex × depression × sleep + age + BMI + PA + race + income
Model 8: Diabetes = depression + sleep + sex + sex × depression + sex × sleep + depression × sleep + sex × depression × sleep + age + BMI + PA + race + income
Model fit according to BIC and AIC was assessed using Hosmer and Lemeshow’s goodness-of-fit test (20).
2.3.3 Consideration of Influential Observations
Logistic regression is highly sensitive to the presence of influential observations (outliers). We assessed potential outliers and their impact on the model. We computed model’s standardized Pearson’s residuals, adjusted for the number of observations that shared the same covariate pattern, to detect potential outliers (i.e., data points with large deviations between observed and fitted values) (21). We employed Pregibon delta beta statistics and Pregibon leverage to evaluate the influence of a data point on estimated coefficients of the model (22). Pregibon delta beta measures the extent to which inclusion or exclusion of an observation sharing the same covariate pattern changes the beta estimates (coefficient). It is presented in the form of standardized difference in betas, with larger values indicating larger influence on model estimates. Pregibon leverage computes the diagonal elements of the hat matrix adjusted for the number of observations that share the same covariate pattern. Large values indicate covariate patterns farther from the average and a larger effect on the fitted model.
2.3.4 Discriminatory Power of the Model
We evaluated the discriminatory power of the best model using Receiver Operating Characteristic (ROC) analysis (23). We showed the sensitivity and specificity vs. probability cut-off points and the ROC curve in a plot of sensitivity vs. (1 – specificity).
3 Results
3.1 Multivariate Logistic Regression
Table 2 shows the results of multivariate logistic regression. For nightly hours of sleep, we used the category of 7-8 hours as the baseline; for depression frequency, we used “never/rarely” as the baseline; for sex, we used men as the baseline; for race, we used Caucasian as baseline; and, finally, for income, we used “low” income as the baseline. Depression reported at a higher frequency and sleep duration either longer or shorter than 7-8 hours both significantly contributed to the odds of a diagnosis of diabetes.
3.2 Model Fit According to BIC and AIC
Although the BIC and AIC values were close across models, the two best models were Model 1 and 2, according to both criteria (Table 3):
Model 1: Diabetes = depression + sleep + sex + sex × depression + age + BMI + PA + race + income, with a BIC value of 3443.46 and AIC value of 3308.09
Model 2: Diabetes = depression + sleep + sex + sex × sleep + age + BMI + PA + race + income, with a BIC value of 3454.89 and AIC value of 3311.56
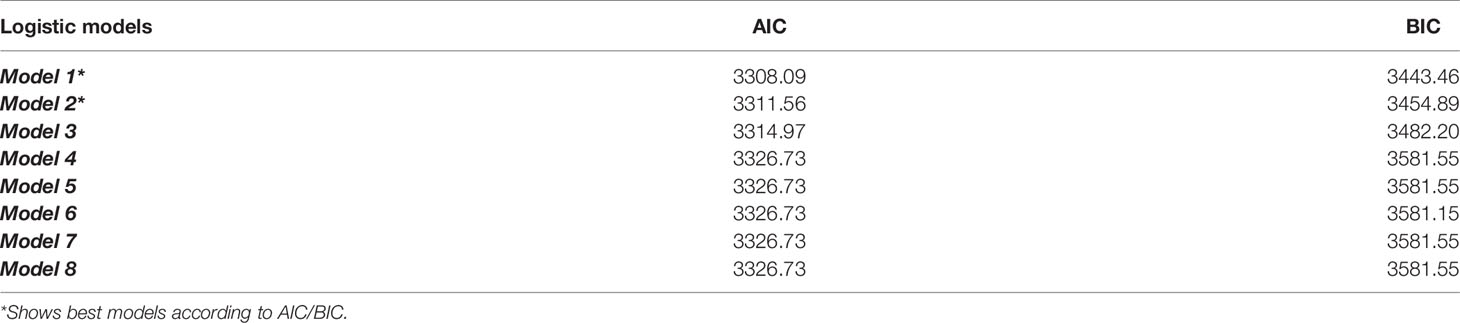
Table 3 Bayesian Information Criterion (BIC) and Akaike Information Criterion (AIC) values of Model 1 – Model 8.
Hosmer and Lemeshow’s goodness of fit revealed non-significant difference between predicted and observed frequencies (Model 1: p=0.792; Model 2: p= 0.342) with Hosmer-Lemeshow χ2 = 4.68 (Model 1) and 9.00 (Model 2), respectively, indicating a good fit for both models.
3.3 Influential Observations
We show the scatter plot of standardized Pearson’s residual versus Pregibon leverage in Supplementary Figure S1. The scatter plot combined three diagnostic values and revealed 10 data points to be potential outliers according to all three statistics (residuals >2 AND delta beta >0.01 AND leverage >0.01). We thus repeated logistic regression excluding these 10 data points. As shown in Supplementary Table S1, the exclusion of these subjects did not materially affect the variable coefficients; thus, we retained and presented findings based on the entire sample.
3.4 Discriminatory Power of Model 1 and 2
In ROC analysis both model 1 and 2 demonstrated excellent discriminatory power distinguishing the presence vs. absence of diabetes, with an area under curve (AUC) = 0.86 (Figure 2). In the plot of sensitivity and specificity versus probability cut-offs, the sensitivity and specificity curves crossed at a point close to the vertical axis, indicating adequate sensitivity and specificity of the fitted models (23).
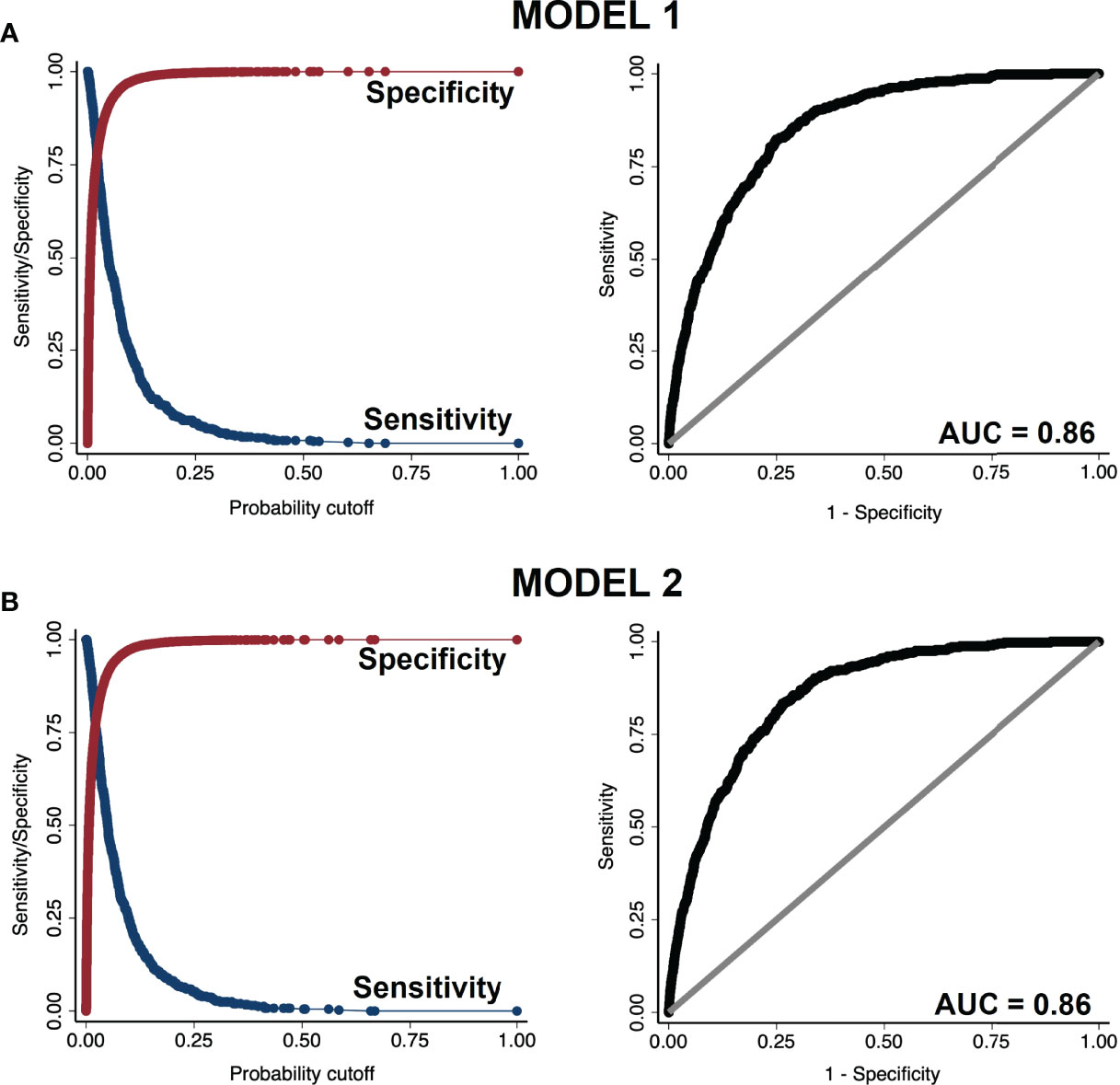
Figure 2 Sensitivity vs. specificity plot (left panels) and receiver operating characteristic (ROC) curve (right panels) of (A) model 1 and (B) model 2.
3.5 The Effects of Depression and Sleep on the Probability of Diabetes and Their Interaction With Sex
To visualize the effects of depression frequency and nightly sleep duration on the probability of diabetes, we plotted predictive margins of response (outcome: diabetes) versus predictor in Figures 3A, B. Overall, as revealed by logistic regression (Table 2), more frequent experience of depression was positively related to diabetes risk. On the other hand, nightly sleep hours were associated with diabetes risk in a U function; duration more or less than 7-8 hours was both associated with a higher risk.
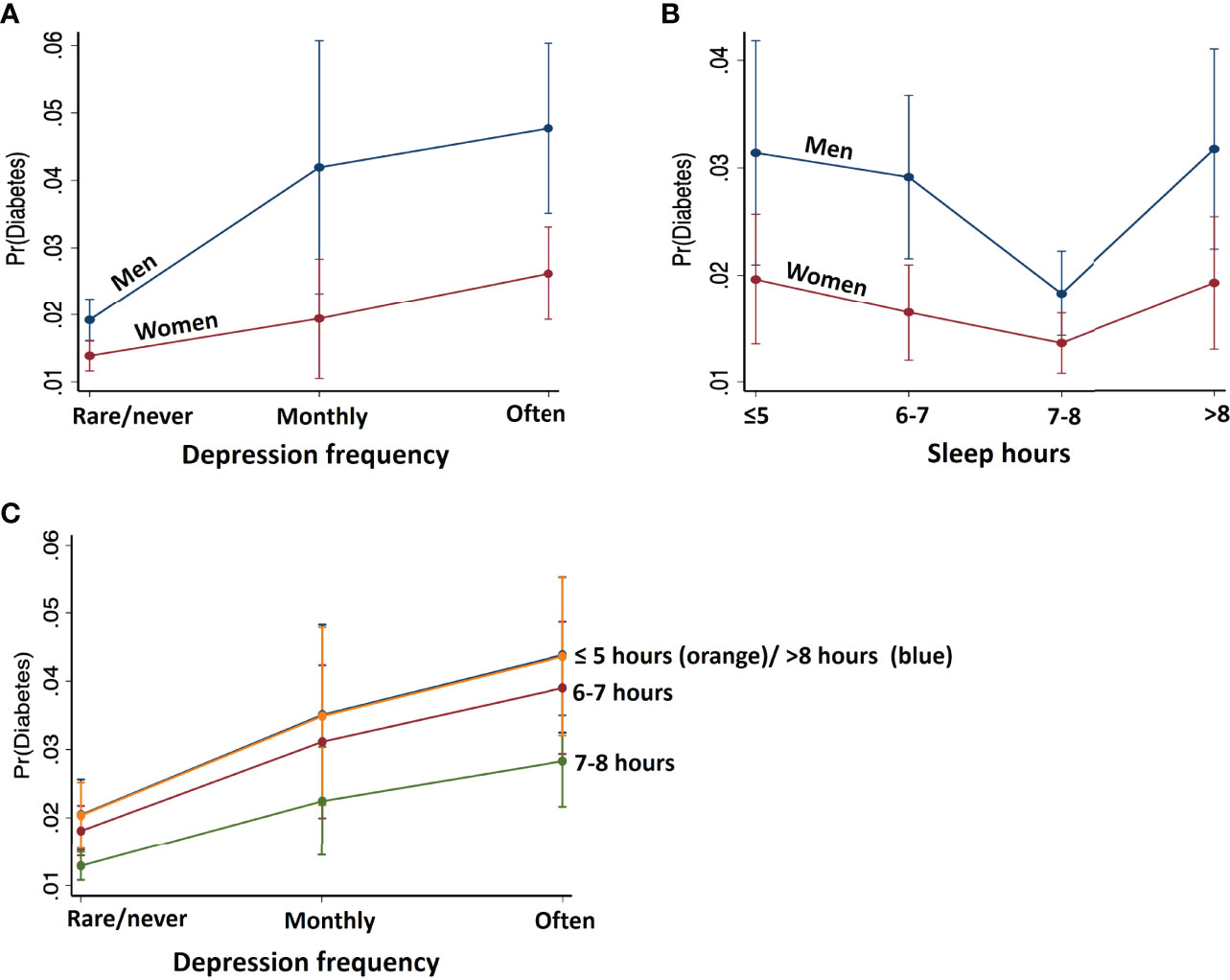
Figure 3 Predicted margins of diabetes with 95% confidence intervals with respect to (A) depression frequency, (B) nightly sleep hours, shown separately for males and females, and (C) depression at each level of sleep. Men relative to women showed stronger associations between depression frequency and nightly sleep hours with the probability of diabetes. Further, reduced/increased sleep hours in synergy with higher frequency of depression predicts higher probability of diabetes.
To examine whether depression frequency and nightly sleep duration may interact to determine diabetes risk, we obtained predictive margins of the interaction effect on diabetes probability using Model 1 (Figure 3C, Supplementary Table S2). For each level of sleep hours, diabetes probability increases with more frequent depression in the same manner. Sleep levels ≤5 hours and >8 hours shared similar, highest probability of diabetes at each level of depression, followed by the probability associated with 6-7 and 7-8 hours of sleep, the latter with the least probability of diabetes.
Further, the effects of depression frequency and nightly hours of sleep interacted with sex in predicting diabetes. The effects of depression and sleep disturbance contributed to diabetes more significantly in men than in women (Table 4). Specifically, at each level of depression, diabetes probability was higher in men than in women: rarely/never depression [coefficient (SE), odds-ratio (SE), p-value in women with men as reference: -0.35 (0.13), 0.70 (0.09), 0.006], monthly depression [-0.86 (0.37), 0.42 (0.15), 0.019], “often” depression [-0.68 (0.21), 0.50 (0.11), 0.001].
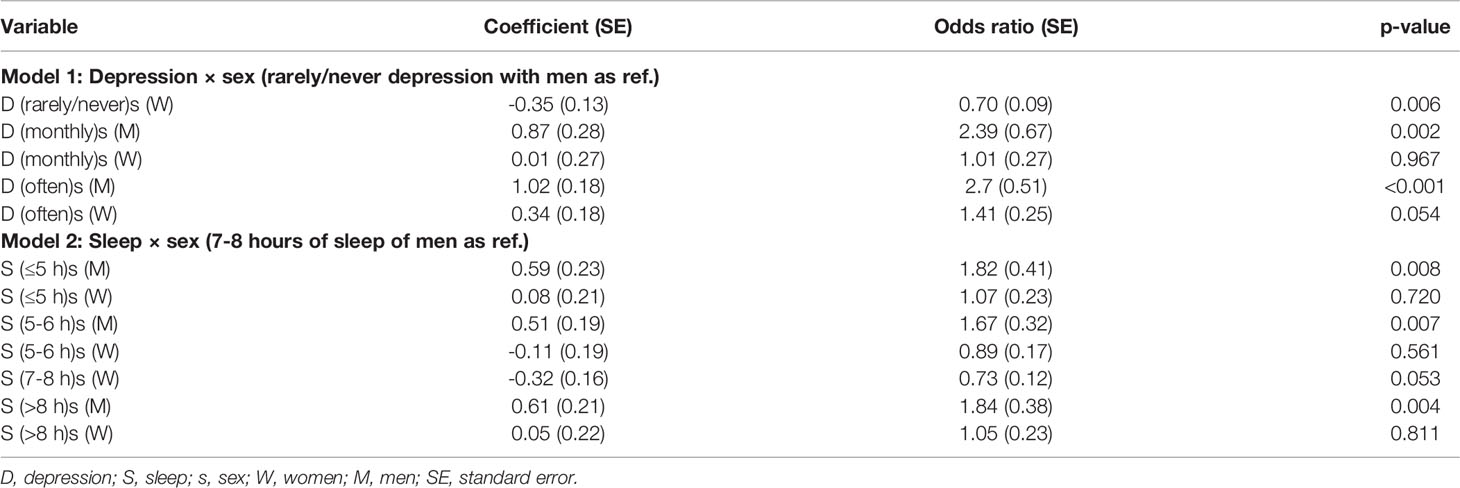
Table 4 The effects of depression and sleep interaction with sex on the probability of diabetes (Model 1 and 2).
Similarly, at each level of sleep hours, probability of diabetes was greater in men compared to women: sleep < 5 hours [coefficient (SE), odds-ratio (SE), p-value: -0.52 (0.25), 0.59 (0.15), 0.042], 6-7 hours [-0.62 (0.21), 0.53 (0.11), 0.003)], 7-8 hours [-0.32 (0.16), 0.73 (0.12), 0.053], and >8 hours [-0.55 (0.24), 0.57 (0.14), 0.023].
4 Discussion
We assessed how sleep duration and depression frequency related to diabetes diagnosis and how these relationships differed between men and women. Both short (< 7 hours/night) and long (> 8 hours/night) sleep durations were associated with a higher probability of a diagnosis of diabetes. Confirming our first hypothesis, this finding aligns with prior studies documenting a U-shaped relationship between sleep duration and diabetes risk (3, 18). Likewise, experiencing depression daily or weekly was associated with higher probability of a diagnosis of diabetes. Importantly, these relationships were consistent with the symptoms of insomnia and hypersomnia in depression and were stronger in men than in women. Further, the addition of an interaction term of depression × sleep or depression × sleep × sex did not result in better model fit. These findings together suggest an inter-related mechanism associating both depression and sleep disturbance to diabetes. Further, we observed a stronger association in men than in women between these risk factors and diabetes, contrary to our hypothesis. Although the latter findings were largely consistent with the literature, the physiological and psychological mechanisms underlying the sex differences remain to be investigated.
4.1 Sleep Disturbance and Depression as Risk Factors of Diabetes
We found that, relative to 7 to 8 hours of nightly sleep, both shorter (< 5 hours) and longer (> 8 hours) sleep were associated with an odds ratio of 1.6 of having a diagnosis of diabetes. A systematic review of 36 studies showed that sleep disturbances significantly impact diabetes risk, with the effects comparable to family history of diabetes and overweight and exceeding the risk associated with physical inactivity (1). A meta-analysis of 13 independent cohorts with a total of 107,756 male and female participants and 3,586 cases of type 2 diabetes reported that both short and long sleep duration was associated with the likelihood of diabetes, although the underlying mechanisms may be different (3). There is a relatively strong consensus in the literature that short sleep duration (< 6 hours) is a significant risk factor of diabetes (1, 24–26). Insufficient sleep and poor sleep quality may contribute to diabetes and/or hamper treatment via physiologic mechanisms such as insulin resistance, decreased leptin/increased ghrelin, and tissue inflammation as well as behavioral mechanisms, including elevated food intake, smoking, drinking and sedentary behavior, all disposing individuals to both obesity and diabetes (27). Indeed, sleep as a crucial survival-related behavior, is regulated through the hypothalamic circuits, and investigators have specifically linked shortened or disturbed sleep to higher cortisol levels, leading to both increased glucose production, decreased glucose utilization, and eventually insulin resistance (24, 28). Short sleep may also increase levels of the inflammatory marker C-reactive protein, which inhibits physiological function of leptin and contributes to weight gain and impaired glycemic control (3, 28). Although less conclusive, a few studies have also shown excessively long sleep (>8 hours) as a risk factor for diabetes (4, 18). In a two-year prospective study individuals whose sleep duration increases ≥2 hours per night are at an enhanced risk of diabetes (29). Prolonged sleep elevates inflammatory markers similarly to shortened sleep, resulting in dysfunctional regulation of leptin and food intake (18). As sleep disturbance is associated with poor glycemic control, optimizing sleep may improve overall diabetes management (30).
On the other hand, some have cautioned that the relationship between sleep and diabetes may be accounted for by other confounding or risk factors. Indeed, both shorter and longer than normal sleep has been documented as core symptoms of depression (31–35). Higher rates of depression have been consistently found among those diagnosed with diabetes and vice-versa, indicating a bidirectional etiological relationship (32, 36, 37). One prospective study of U.S. military members in the Millenium Cohort Study found that, after controlling for baseline covariates, the relationship between depression and diabetes appeared to be insignificant (24). However, distinct demographics and life experiences of enlisted servicemen might have contributed to these contrasting results. Here, we found that both altered sleep duration and depression were associated with greater odds of a diabetes diagnosis. In particular, the regression models with interaction between sleep and depression did not provide a better fit, suggesting that sleep hours and depression frequency contributed to diabetes risk in a concerted manner. Further, as discussed in detail in the next section, although men relative to women demonstrated a more significant association of both depression and altered sleep hours with the diagnosis of diabetes, both showed a similar pattern of the two risk factors in relation to diabetes. The latter findings suggest an inter-related mechanism by which depression and sleep disturbance contribute to diabetes.
4.2 Sex Differences in Sleep Disturbance and Depression as Risk Factors of Diabetes
The effect of sleep on diabetes risk has also been found to vary between sexes. A systematic search of 10 studies found a higher diabetes risk for men sleeping less than 5-6 hours per night, as compared to women (3). The afore-mentioned study of U.S. military service members reported that, whereas non-depressed women were more likely to be diagnosed with diabetes than non-depressed men, this discrepancy became insignificant when both women and men had depression (24). Further, Hein et al., reported that among adults with major depression, only male sex was a significant risk factor for diabetes (32). Thus, the current finding that aberrant sleep duration – whether too short or long – might impact the risk for diabetes in men more significantly than in women is consistent with this literature. On the other hand, because women as compared to men were more likely to experience and report depression (8), we hypothesized a stronger association between depression and diabetes in women than in men. One possibility for the current finding is a ceiling effect of depression masking the relationship between depression and diabetes in women. However, our data did not support this hypothesis, as women did not appear to show more severe depression in the current data set.
The exact mechanism behind this sex-specific association is not clear. But it should be noted that disturbed sleep has been shown to interfere with testosterone production in men with type 2 diabetes. Given the benefits of testosterone on glucose metabolism, deficient sleep might conceivably lead to poorer glycemic control (38). Further, given the nature of self-report in the current data, the relationship between sex, depression, and diabetes remains to be investigated (37, 39). Studies with objective measures of glycemic control and sleep quality and duration would be particularly useful in addressing this question.
4.3 Limitations and Conclusions
A number of limitations should be considered. First, the data comprised self-reported measures, including sleep duration and subjective experience of depression. Previous studies have reported sex differences in self-reported depressive symptoms, with women tending to report more depressive symptoms than men (40, 41), which may introduce a bias to the current findings. In addition, as depression severity data were not available, we used symptom occurrence rather than severity across the analyses. As no medical measure of the severity of diabetes was available, the status of diabetes was investigated as a categorical variable. Further, the prevalence rates of ~2% of self-reported diabetes in the current sample is exceedingly low, as compared to the 13% noted for the US populations 18 years of older (42). The quality of the data set thus presents a major limitation and the current findings would need to be replicated. Second, these were cross-sectional, observational data; thus, we cannot draw any causal inferences from the current findings. Future studies in a controlled and longitudinal setting ought to examine the direction of the relationship between diabetes, sleep, and depression and how the relationships vary between males and females.
To conclude, the study supports a potential sex-related association between depression frequency and nightly sleep duration and diabetes risk and adds to rapidly growing literature linking mental and physical health. Our findings that both short and long sleep and depression elevate the odds of diabetes support an inter-related rather than independent mechanism of depression and sleep disturbance as risk factors of diabetes. This diabetes risk appears to be stronger in men than in women, suggesting the need of more research to understand sex-specific physiological and psychological processes underlying the care and management of those with or at risk of diabetes.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://nhis.ipums.org/nhis/.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CL: study design, data curation, statistical analysis, manuscript writing; RP: study design, data curation, statistical analysis, manuscript writing, SC: statistical analysis, manuscript editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Benjamin Baumer and Dr. Bea Capistrant for their advice on the early phase of the study. The authors declare no competing interests in the current study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcdhc.2022.914451/full#supplementary-material
References
1. Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep Disturbances Compared to Traditional Risk Factors for Diabetes Development: Systematic Review and Meta-Analysis. Sleep Med Rev (2016) 30:11–24. doi: 10.1016/j.smrv.2015.10.002
2. Griggs S, Grey M, Strohl KP, Crawford SL, Margevicius S, Kashyap SR, et al. Variations in Sleep Characteristics and Glucose Regulation in Young Adults With Type 1 Diabetes. J Clin Endocrinol Metab (2022) 107:e1085–95. doi: 10.1210/clinem/dgab771
3. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and Quality of Sleep and Incidence of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Care (2010) 33:414–20. doi: 10.2337/dc09-1124
4. Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, et al. A Prospective Study of Sleep Duration and Coronary Heart Disease in Women. Arch Intern Med (2003) 163:205–9. doi: 10.1001/archinte.163.2.205
5. American Psychiatric Association A. Diagnostic and Statistical Manual of Mental Disorders. 5th Edn. Washington, DC: American Psychiatric Association (2013).
6. Nutt D, Wilson S, Paterson L. Sleep Disorders as Core Symptoms of Depression. Dialogues Clin Neurosci (2008) 10:329–36. doi: 10.31887/DCNS.2008.10.3/dnutt
7. Salk RH, Hyde JS, Abramson LY. Gender Differences in Depression in Representative National Samples: Meta-Analyses of Diagnoses and Symptoms. Psychol Bull (2017) 143:783–822. doi: 10.1037/bul0000102
8. Zhao L, Han G, Zhao Y, Jin Y, Ge T, Yang W, et al. Gender Differences in Depression: Evidence From Genetics. Front Genet (2020) 11:562316. doi: 10.3389/fgene.2020.562316
9. Eid RS, Gobinath AR, Galea LAM. Sex Differences in Depression: Insights From Clinical and Preclinical Studies. Prog Neurobiol (2019) 176:86–102. doi: 10.1016/j.pneurobio.2019.01.006
10. Labaka A, Goñi-Balentziaga O, Lebeña A, Pérez-Tejada J. Biological Sex Differences in Depression: A Systematic Review. Biol Res Nurs (2018) 20:383–92. doi: 10.1177/1099800418776082
11. Piccinelli M, Wilkinson G. Gender Differences in Depression: Critical Review. Br J Psychiatry (2000) 177:486–92. doi: 10.1192/bjp.177.6.486
12. Demmer RT, Gelb S, Suglia SF, Keyes KM, Aiello AE, Colombo PC, et al. Sex Differences in the Association Between Depression, Anxiety, and Type 2 Diabetes Mellitus. Psychosom Med (2015) 77:467–77. doi: 10.1097/PSY.0000000000000169
13. Zheng R, Niu J, Wu S, Wang T, Wang S, Xu M, et al. Gender and Age Differences in the Association Between Sleep Characteristics and Fasting Glucose Levels in Chinese Adults. Diabetes Metab (2021) 47:101174. doi: 10.1016/j.diabet.2020.07.001
14. Sun K, Li F, Qi Y, Lin D, Ren M, Xu M, et al. Sex Difference in the Association Between Habitual Daytime Napping and Prevalence Of Diabetes: A Population-Based Study. Endocrine (2016) 52:263–70. doi: 10.1007/s12020-015-0772-x
15. Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and Type 2 Diabetes Over the Lifespan: A Meta-Analysis. Diabetes Care (2008) 31:2383–90. doi: 10.2337/dc08-0985
16. Mallampalli MP, Carter CL. Exploring Sex and Gender Differences in Sleep Health: A Society for Women’s Health Research Report. J Womens Health (Larchmt) (2014) 23:553–62. doi: 10.1089/jwh.2014.4816
17. Krishnan V, Collop NA. Gender Differences in Sleep Disorders. Curr Opin Pulm Med (2006) 12:383–9. doi: 10.1097/01.mcp.0000245705.69440.6a
18. Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, et al. Sleep Duration and Risk of Type 2 Diabetes: A Meta-Analysis of Prospective Studies. Diabetes Care (2015) 38:529–37. doi: 10.2337/dc14-2073
19. Zhang Z. Model Building Strategy for Logistic Regression: Purposeful Selection. Ann Transl Med (2016) 4:111. doi: 10.21037/atm.2016.02.15
20. Nattino G, Pennell ML, Lemeshow S. Assessing the Goodness of Fit of Logistic Regression Models in Large Samples: A Modification of the Hosmer-Lemeshow Test. Biometrics (2020) 76:549–60. doi: 10.1111/biom.13249
21. Zhang Z. Residuals and Regression Diagnostics: Focusing on Logistic Regression. Ann Transl Med (2016) 4:195. doi: 10.21037/atm.2016.03.36
22. Pregibon D. Logistic Regression Diagnostics. Ann Stat (1981) 9:705–24. doi: 10.1214/aos/1176345513
23. Meurer WJ, Tolles J. Logistic Regression Diagnostics: Understanding How Well a Model Predicts Outcomes. JAMA (2017) 317:1068–9. doi: 10.1001/jama.2016.20441
24. Boyko EJ, Seelig AD, Jacobson IG, Hooper TI, Smith B, Smith TC, et al. Sleep Characteristics, Mental Health, and Diabetes Risk: A Prospective Study of U.S. Military Service Members in the Millennium Cohort Study. Diabetes Care (2013) 36:3154–61. doi: 10.2337/DC13-0042
25. Holliday EG, Magee CA, Kritharides L, Banks E, Attia J. Short Sleep Duration Is Associated With Risk of Future Diabetes But Not Cardiovascular Disease: A Prospective Study and Meta-Analysis. PLos One (2013) 8:e82305. doi: 10.1371/journal.pone.0082305
26. Lou P, Chen P, Zhang L, Zhang P, Yu J, Zhang N, et al. Relation of Sleep Quality and Sleep Duration to Type 2 Diabetes: A Population-Based Cross-Sectional Survey. BMJ Open (2012) 2:e000956. doi: 10.1136/bmjopen-2012-000956
27. Grandner MA, Seixas A, Shetty S, Shenoy S. Sleep Duration and Diabetes Risk: Population Trends and Potential Mechanisms. Curr Diab Rep (2016) 16:106. doi: 10.1007/s11892-016-0805-8
28. Barone MTU, Menna-Barreto L. Diabetes and Sleep: A Complex Cause-and-Effect Relationship. Diabetes Res Clin Pract (2011) 91:129–37. doi: 10.1016/j.diabres.2010.07.011
29. Song Q, Liu X, Zhou W, Wang X, Wu S. Short-Term Changes in Sleep Duration and Risk of Type 2 Diabetes: Kailuan Prospective Study. Med (Baltimore) (2016) 95:e5363. doi: 10.1097/MD.0000000000005363
30. Griggs S, Hickman RL, Strohl KP, Redeker NS, Crawford SL, Grey M. Sleep-Wake Characteristics, Daytime Sleepiness, and Glycemia in Young Adults With Type 1 Diabetes. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med (2021) 17:1865–74. doi: 10.5664/jcsm.9402
31. Fang H, Tu S, Sheng J, Shao A. Depression in Sleep Disturbance: A Review on a Bidirectional Relationship, Mechanisms and Treatment. J Cell Mol Med (2019) 23:2324–32. doi: 10.1111/jcmm.14170
32. Hein M, Lanquart J-P, Loas G, Hubain P, Linkowski P. Prevalence and Risk Factors of Type 2 Diabetes in Major Depression: A Study on 703 Individuals Referred for Sleep Examinations. Psychosomatics (2018) 59:144–57. doi: 10.1016/j.psym.2017.11.003
33. Li L, Wu C, Gan Y, Qu X, Lu Z. Insomnia and the Risk of Depression: A Meta-Analysis of Prospective Cohort Studies. BMC Psychiatry (2016) 16:375. doi: 10.1186/s12888-016-1075-3
34. Morssinkhof MWL, van Wylick DW, Priester-Vink S, van der Werf YD, den Heijer M, van den Heuvel OA, et al. Associations Between Sex Hormones, Sleep Problems and Depression: A Systematic Review. Neurosci Biobehav Rev (2020) 118:669–80. doi: 10.1016/j.neubiorev.2020.08.006
35. Riemann D, Krone LB, Wulff K, Nissen C. Sleep, Insomnia, and Depression. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol (2020) 45:74–89. doi: 10.1038/s41386-019-0411-y
36. Campayo A, Gómez-Biel CH, Lobo A. Diabetes and Depression. Curr Psychiatry Rep (2011) 13:26–30. doi: 10.1007/s11920-010-0165-z
37. Roy T, Lloyd CE. Epidemiology of Depression and Diabetes: A Systematic Review. J Affect Disord (2012) 142 Suppl:S8–21. doi: 10.1016/S0165-0327(12)70004-6
38. Wang J, Kwok MK, Au Yeung SL, Li AM, Lam HS, Leung JYY, et al. Sleep Duration and Risk of Diabetes: Observational and Mendelian Randomization Studies. Prev Med (Baltim) (2019) 119:24–30. doi: 10.1016/j.ypmed.2018.11.019
39. Aschner P, Gagliardino JJ, Ilkova H, Lavalle F, Ramachandran A, Mbanya JC, et al. High Prevalence of Depressive Symptoms in Patients With Type 1 and Type 2 Diabetes in Developing Countries: Results From the International Diabetes Management Practices Study. Diabetes Care (2021) 44:1100–7. doi: 10.2337/dc20-2003
40. Frazier L, Yu E, Sanner J, Liu F, Udtha M, Cron S, et al. Gender Differences in Self-Reported Symptoms of Depression Among Patients With Acute Coronary Syndrome. Nurs Res Pract (2012) 2012:109251. doi: 10.1155/2012/109251
41. Sigmon ST, Pells JJ, Boulard NE, Whitcomb-Smith S, Edenfield TM, Hermann BA, et al. Gender Differences in Self-Reports of Depression: The Response Bias Hypothesis Revisited. Sex Roles (2005) 53:401–11. doi: 10.1007/s11199-005-6762-3
42. Centers for Disease Control and Prevention. National Diabetes Statistics Report (2020). Available at: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
Keywords: diabetes, risk factors, sleep, depression, sex differences
Citation: Li CS, Porta R and Chaudhary S (2022) Sex Differences in Depression and Sleep Disturbance as Inter-Related Risk Factors of Diabetes. Front. Clin. Diabetes Healthc. 3:914451. doi: 10.3389/fcdhc.2022.914451
Received: 06 April 2022; Accepted: 07 June 2022;
Published: 19 July 2022.
Edited by:
Nikos Pantazis, National and Kapodistrian University of Athens, GreeceReviewed by:
Ding-Lieh Liao, Bali Psychiatric Center, TaiwanGeorgia Vourli, National and Kapodistrian University of Athens, Greece
Copyright © 2022 Li, Porta and Chaudhary. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clara S. Li, Y3NsaUBzbWl0aC5lZHU=; Shefali Chaudhary, c2hlZmFsaS5jaGF1ZGhhcnlAeWFsZS5lZHU=
†These authors have contributed equally to this work
 Clara S. Li1*†
Clara S. Li1*† Shefali Chaudhary
Shefali Chaudhary