- 1Department of Physical Oceanography, Dynamics of Regional Climate Systems, Leibniz Institute for Baltic Sea Research, Warnemünde, Germany
- 2Department of Marine Chemistry, Trace Gas Biogeochemistry, Leibniz Institute for Baltic Sea Research, Warnemünde, Germany
- 3Department of Marine Observations, Integrated Optical Remote Sensing, Leibniz Institute for Baltic Sea Research, Warnemünde, Germany
To achieve carbon neutrality, ocean alkalinity enhancement (OAE) is currently being researched as a marine option for carbon dioxide removal (CDR). The approach of releasing calcite near the sediments and using the effect of enhanced mineral solubility in the pore water for more efficient dissolution may be promising in the Baltic Sea. The Baltic Sea is considered a potential application site for this method, as, in contrast to other seas, it is partly undersaturated in calcite even at shallow depths. However, the possible implications of this method, specifically if applied in coastal settings, are still poorly understood. Therefore, using a coupled hydrodynamic and biogeochemical ocean model of the Baltic Sea, we simulated the release of calcite near the sediment as a possible strategy for OAE. Simulations were run with and without enhanced solubility in the pore water for two release locations, one in shallow coastal water and one in a deep basin. While enhanced solubility by oxic mineralisation did not make a difference for the deep basin, it substantially changed the achievable calcite dissolution rates at the coastal site and therefore the potential CO2 removal. Here, our simulations provide a lower and an upper limit of the effectiveness of calcite dissolution. The release locations differed considerably in magnitude and timescales of CO2 uptake. As the saturation level of calcite appears to be the main limiting factor of the method, the CO2 removal potential of a release location cannot be upscaled infinitely by adding more calcite. Our results demonstrate a potential for OAE using calcite in the Baltic Sea. We used the model results on average and maximum changes in alkalinity and pH to reflect on potential environmental impacts based on a review of the existing literature. However, safe and responsible deployment of this CDR method in the Baltic Sea requires further research on localized dissolution rates, the alkalinity budget of the Baltic Sea and the environmental implications of OAE using calcite.
1 Introduction
Aiming to limit the global temperature increase since the industrial revolution to below 2°C by 2100, the European Union plans to achieve carbon neutrality by 2050 (European Commission, 2019). While this goal primarily focuses on reducing carbon emissions, certain industries such as steel, cement production and aviation inherently produce carbon dioxide making it difficult to lower their emissions (Davis et al., 2018; Keller, 2014; Rogelj et al., 2016). Optimistic predictions estimate these residual annual CO2 emissions to range from 32 to 60 Mt CO2 for Germany (BMU, 2021; Mengis et al., 2022).
The Intergovernmental Panel on Climate Change stated that a transition to renewable energy sources alone will unlikely suffice in achieving net-zero carbon emissions in the future making methods of carbon dioxide removal (CDR) necessary to mitigate any residual emissions (Fridahl et al., 2023; IPCC, 2021, 2022). The oceans have a large capacity to absorb CO2 and have thus substantially buffered the effect of CO2 on climate change to a substantial degree for the last decades (Friedlingstein et al., 2020; Watson et al., 2020).
As atmospheric CO2 enters the sea, it quickly undergoes a chain reaction to form carbonic acid, which dissociates into, primarily, bicarbonate ions and, eventually, carbonate ions, releasing protons in the process (Dickson et al., 2007). With increasing concentrations of anthropogenic CO2, a CO2 partial pressure gradient from air to sea may increase the CO2 uptake under favorable conditions (e.g., Landschutzer et al., 2015; Ford et al., 2024). Consequently, the carbonate system reactions shift direction to buffer the excess release of protons by using carbonate ions to form bicarbonate (Zeebe and Wolf-Gladrow, 2001; Hansson et al., 2010). Bicarbonate is the dominant carbon species in seawater, and together with the carbonate ion, they drive the seawater alkalinity (and buffering capacity), with other seawater elements playing relatively secondary roles depending on the water composition (Zeebe and Wolf-Gladrow, 2001; Kuliński et al., 2022). Still, the resulting higher concentrations of dissolved CO2 in the oceans have led to ocean acidification (i.e., increased hydrogen ion concentration and decreased seawater pH). As a consequence, studies show the surface saturation state of calcium carbonate species (aragonite and calcite) is decreasing, causing stress for marine organisms, particularly calcifiers (Caldeira and Wickett, 2003; Doney et al., 2009; Feely et al., 2004).
The CDR approach of ocean alkalinity enhancement (OAE) can increase the ocean's capacity for further uptake of atmospheric CO2 while potentially countering ocean acidification (Archer, 2005; Kheshgi, 1995; Rau et al., 2013). Introducing alkaline minerals like olivine or limestone to the oceans accelerates the natural weathering process (Kheshgi, 1995), which increases the alkalinity and therefore the buffer capacity of the seawater. The added alkalinity increases the buffer capacity of the seawater, i.e., the ocean's capacity to absorb additional atmospheric CO2 (Eisaman et al., 2023). Thus, the carbonate system is shifted in a way that allows for additional dissolution of atmospheric CO2 which is then bound in bicarbonates which are stored in the ocean for up to 10,000–100,000 years (Archer, 2005; Rau et al., 2013; Renforth and Henderson, 2017).
According to simulations, OAE is also considered one of the marine CDR methods that can be stopped safely (Ilyina et al., 2013; Keller, 2014). As a softer mineral, calcite takes less energy to grind than silicates like olivine, while also bearing a smaller risk of contamination through heavy metals as contained in olivine (Montserrat et al., 2017). In addition, Fuhr et al. (2022) showed that using the silicate forsterite for OAE can catalyze CaCO3 precipitation that would result in a loss of alkalinity. Furthermore, experiments have shown calcite to be the more efficient material for OAE in the Baltic Sea (Fuhr et al., 2024) due to enhanced mineral solubility in the pore water and the superior solubility at colder temperatures of calcite over olivine (Mucci, 1983). As the upper ocean is generally saturated or oversaturated in calcite, most approaches using calcite for alkalinisation suggest releasing the mineral at several hundred meters below the calcite saturation horizon, near upwelling sites where the water is undersaturated in calcite (Harvey, 2008). As this layer is rather deep in most oceans, the time frame until the calcite actively takes up CO2 is linked to the time scales of the upwelling and can thus take several years to take effect. Alternatively, it has been suggested to release ground calcite in the surface ocean where ocean acidification as a result of higher atmospheric CO2 levels has so far progressed that calcite is undersaturated (Harvey, 2008). This approach requires calcite to be ground to a much smaller grain size so that it does not sink before it is dissolved in the surface ocean.
The Baltic Sea, however, is undersaturated in calcite in most areas (Sanders et al., 2020) while also being very shallow with an average depth of 50 m (Schernewski et al., 2021). Hypoxic and anoxic conditions in bottom waters and specifically the sedimentary CO2 release from benthic mineralisation lead to low pH levels (Meier et al., 2019; Melzner et al., 2013) that contribute to the dissolution of calcite. Apart from the permanently anoxic Gotland Deep (Conley et al., 2009), several shallow coastal locations seasonally become anoxic (Bange et al., 2011; Meier et al., 2019; Melzner et al., 2013; Orsi et al., 1996). Locations with permanent anoxia like the Gotland Deep and seasonal anoxia like the Eckernförde Bight are thus potential sites for OAE, where calcite is released near the sediment.
With a bottom depth of 250 m, the Gotland Deep is one of the deepest basins of the brackish Baltic Sea. Dense water with relatively high salinity therefore resides there for longer periods before major inflow events from the North Sea ventilate this location and introduce oxygenated water (Matthäus et al., 1994; Mohrholz et al., 2015). During these stagnation periods, the bottom water becomes increasingly anoxic (Figure 1). Due to the strong haline layering at about 80–150 m depth, there is limited to no exchange with the oxygenated surface waters (Dellwig et al., 2021; Mohrholz et al., 2015). Here, calcite would be continuously exposed to corrosive conditions. However, due to the long surfacing times of bottom water in the Gotland Deep, any alkalinity released by calcite dissolution will not reach the surface for years, preventing it from triggering an atmospheric carbon sink.
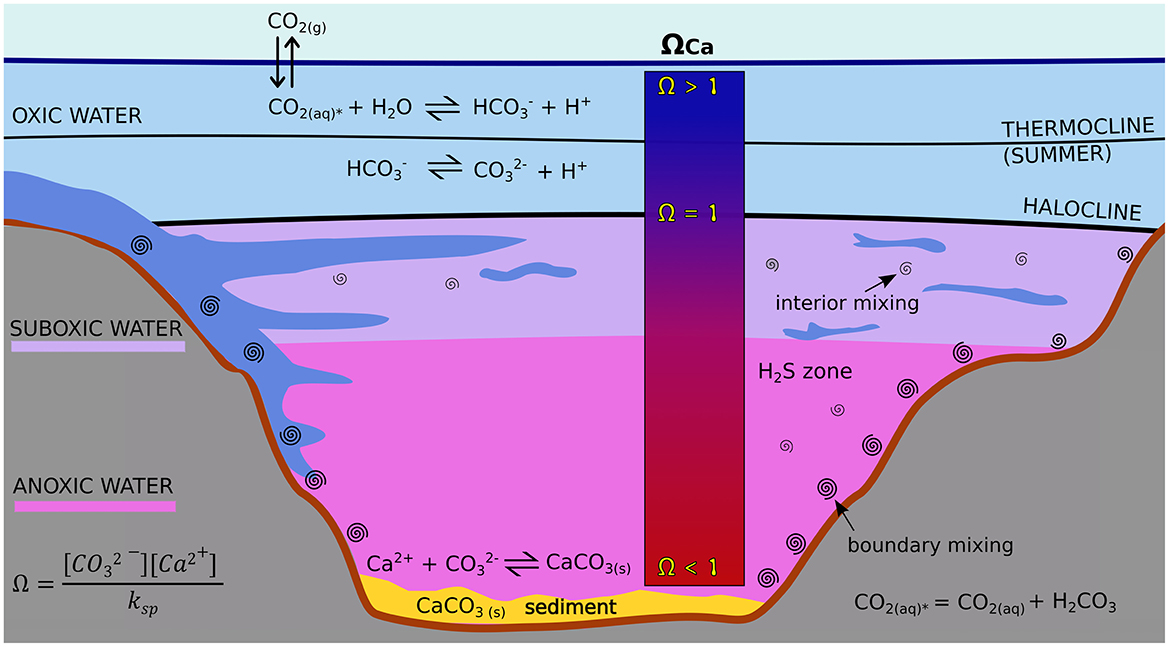
Figure 1. Schematic of the carbonate system in the Gotland Deep in summer. Calcite dissolves when calcite is undersaturated (ΩCa < 1). High CO2 concentrations as they occur with anoxia create acidic conditions that increase the dissolution of calcite.
The Eckernförde Bight is an example of seasonal hypoxia in shallow coastal waters. The surface becomes much warmer than the bottom water in summer and without mixing by strong winds or inflows, the water column becomes stratified and the bottom water anoxic (Orsi et al., 1996). During autumn and winter, inflows from the North Sea introduce colder, more saline and oxygenated water to the bottom of the Eckernförde Bight and the water column becomes mixed by storms (Bange et al., 2011; Orsi et al., 1996). Alkalinity released during the anoxic periods in late summer will thus reach the surface in autumn and winter.
The dissolution of calcite can happen even if the bottom water is already saturated. This effect, also called the benthic weathering engine (BWE), occurs because additional CO2 is produced in the pore water of the sediments, so the dissolved inorganic carbon (DIC) concentration is higher there compared to the overlying bottom water (Meysman and Montserrat, 2017). The type of oxidant for creating DIC makes a difference. If oxygen is used, DIC is created from the particulate organic carbon without the creation of additional alkalinity (Rassmann et al., 2020). This pathway therefore reduces pH and leads to enhanced carbonate dissolution. If sulfate is used, 2 moles of alkalinity are created for each mol of DIC, so pH rises and carbonate dissolution is hampered (Gustafsson et al., 2019). As sulfate reduction is the major oxidation pathway below the oxygen penetration depth, we can assume that the most favorable conditions for dissolution will occur within the oxic zone. Experimental studies in the Eckernförde Bight have shown that the BWE is the key driver for calcite dissolution near the sediment (Fuhr et al., 2024, 2023).
Due to the high variability and seasonality of these benthic fluxes, the potential of OAE per region is difficult to gauge (Fuhr et al., 2023). Fuhr et al. (2024) addressed this issue with an experimental approach, but the results of these localized studies are difficult to extrapolate to larger regions. In our study, we therefore address the question of the potential effectiveness of Baltic Sea carbonate dissolution by a modeling approach. Only the direct effects on the carbonate system can be assessed in our model study. The potential indirect effects on the ecosystem, e.g. through changes in phosphate retention by autigenic apatite formation or influences on marine food webs, should be the subject of future investigations.
2 Methods
2.1 Models
The simulations were run with a hydrodynamic model (MOM5.1) coupled with an ecological model (ERGOM 1.2).
2.1.1 Modular ocean model
We used the MOM5.1 hydrodynamic model system with a hydrodynamic ocean component and a dynamically coupled sea ice model (Griffies, 2012). Vertical turbulence is handled by the k-profile parameterization (KPP, Large et al., 1994). The state variables in this model are defined at z*-levels, which means that the layers are horizontally aligned and the number of vertical layers depends on the local water depth. In contrast to pure z-levels, the cell heights of all cells in a vertical stack change due to a changing sea level elevation. The model is resolved into 152 layers (Neumann et al., 2021). Here, the model was run with a horizontal resolution of 1 n.m. (Neumann et al., 2020) with the atmospheric forcing described in Geyer (2014).
2.1.2 Ecological ReGional Ocean Model
The Ecological ReGional Ocean Model (ERGOM) is a biogeochemical model that simulates the cycles of carbon, nitrogen, phosphorus, oxygen and sulfur (Leibniz Institute for Baltic Sea Research, 2015). The carbonate system is treated in the typical way following (Dickson et al., 2007): DIC and total alkalinity are state variables of the model. The pH value is calculated from these in an iterative procedure. The model assumes a pH-dependent dissociation of several chemical species. In our model, these are water, DIC, phosphate, hydrogen sulfide and boron. In every time step, the pH is calculated in such a way that the sum of the individual alkalinities of these species matches the total alkalinity. The calcite saturation state ΩCa was calculated with CO2SYS (Lewis and Wallace, 1998) based on the alkalinity and DIC parameters, which are state variables in the model. To do so, the formulas from the MatLab version of CO2SYS (van Heuven et al., 2011) were integrated into the ERGOM 1.2 code (Neumann et al., 2022) to calculate the calcite saturation state ΩCa (Equation 1), with the calcite solubility corrected for pressure (kca_corrected):
The calcium ion concentration [mol/kg] which is required for the calculation is assumed to be proportional to the salinity (Riley and Tongudai, 1967):
As the addition of calcium ions via calcite dissolution will be minimal in comparison to the calcium ion concentration already present in the seawater, it was omitted in the model. The carbonate concentration (μmol kg−1) is calculated from the concentrations of DIC and HCO3- according to Dickson et al. (2007), using the acid dissociation constants of CO2 (k1_co2) and HCO3- (k2_co2):
The release of CO32- in practice means a source term for our two state variables DIC and Alkalinity. The model was integrated for 42 years from 1978 to 2020. We chose to simulate the alkalinity enhancement in a hindcast simulation rather than a future scenario since this allows a validation of the model with observational data.
2.2 Model validation
Observational data on oxygen, salinity and temperature were extracted between 1978 and 2020 from the databases Pangaea (Bange and Malien, 2015; Lennartz et al., 2014) and SHARK (SMHI 2023)1 for the field stations Boknis Eck in the Eckernförde Bight and the station BY15 in the Gotland Deep (Table 1), respectively. The acquired data were used to verify the models' ability to reproduce the conditions at the release sites over the simulated period.
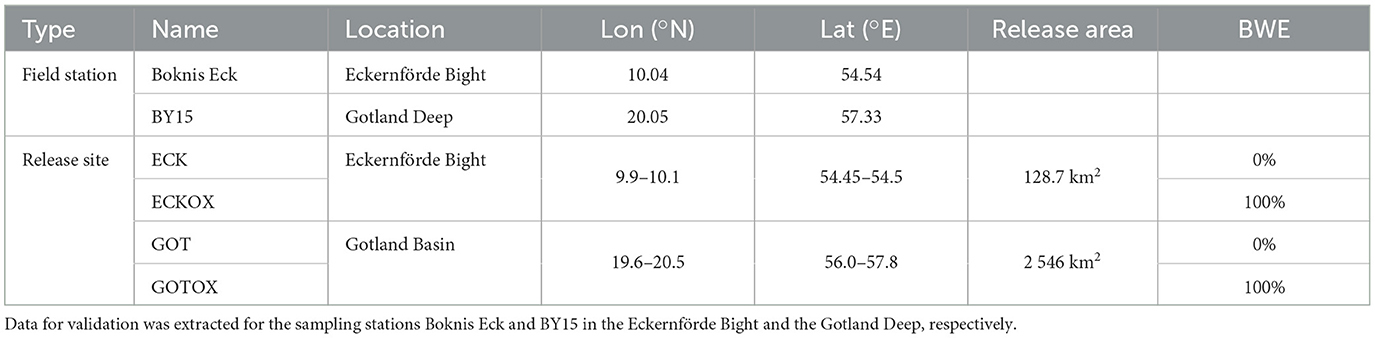
Table 1. Coordinates and area size of the release sites for CaCO3 and assumed benthic weathering engine (BWE) efficiency in the experiments in the Eckernförde Bight and the Gotland Deep.
2.3 Model experiments
We performed one control run and considered two different potential release sites for the experiment runs (Table 1, Figure 2): the Eckernförde Bight (ECK & ECKOX) and the Gotland Deep (GOT & GOTOX). Calcite was released yearly at the sea floor in the whole of the Eckernförde Bight and the area below 160 m in the Gotland Basin, respectively.
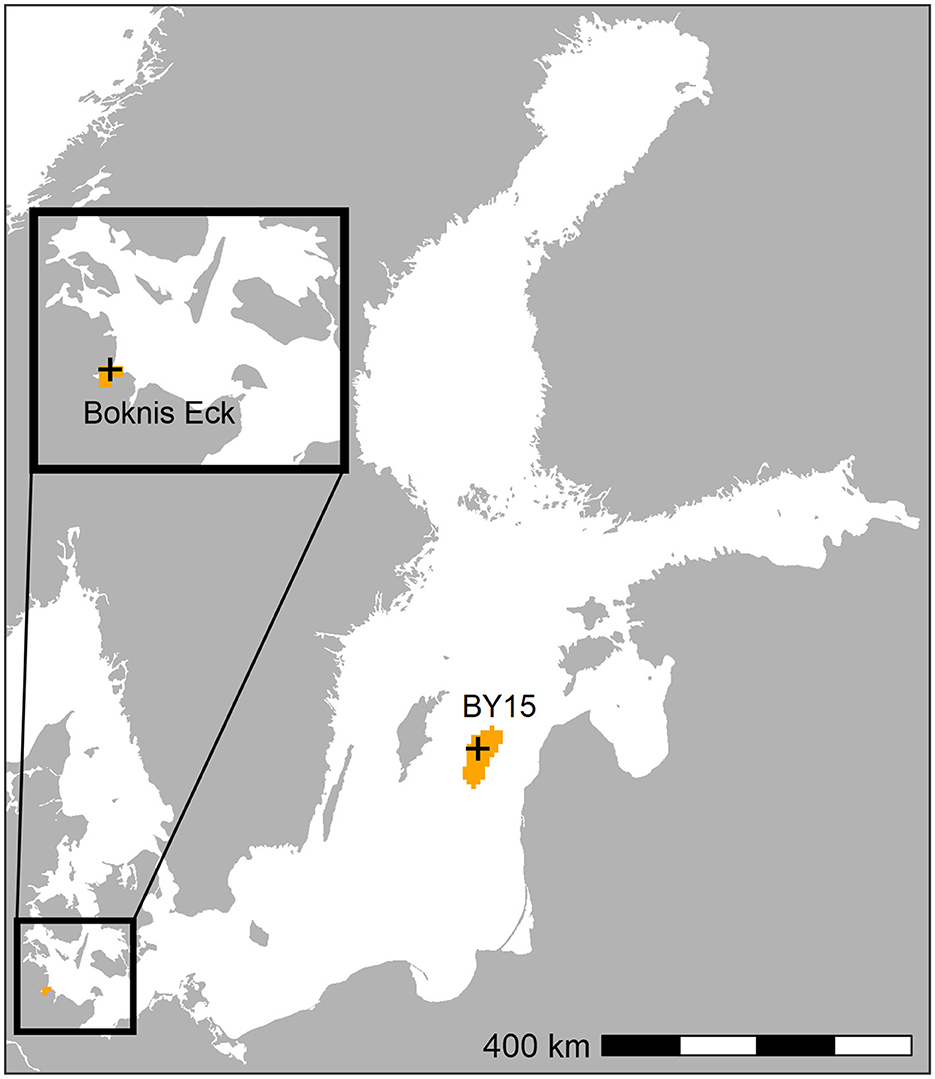
Figure 2. Crosses: field sampling stations for which data was retrieved from the SHARK database for oxygen, temperature, salinity, pH, and alkalinity. Orange areas: release sites for calcite in the Eckernförde Bight and the Gotland Deep in the experiment simulations (ECK, ECKOX, GOT, and GOTOX).
Regarding the efficiency of the benthic weathering machine, we made a pessimistic and optimistic assumption to define lower and upper limits for the potential CO2 capture. In the ECK and GOT experiments, we pessimistically assume that calcite only dissolves if the calcite saturation state ΩCa of the bottom water is below 1. In the ECKOX and GOTOX experiments, oxygen diffuses into the pore water and oxidizes the organic matter in the sediment, producing aqueous CO2. The resulting increase in CO2 concentration can then lower the saturation state.
For these experiments, we optimistically assume that 100% of the oxygen from the water column, which is transported into the sediments, is used to oxidize particulate organic carbon. Under this assumption CO2 is produced in the pore water, contributing solely to DIC, without generating additional alkalinity. This is in contrast to DIC addition with other oxidation pathways like sulfide reduction (Rassmann et al., 2020). Only this benthic DIC produced with oxygen helps to lower the pH and dissolve calcite. The concentration of this DIC originating from oxygen-driven mineralisation of organic matter must obviously be largest at the bottom of the oxic zone since there is no source of it below this zone. To calculate the ΩCa values in this most corrosive location in the pore water, we, therefore, need to assume a higher DIC concentration than in the bottom water, one that is elevated by the benthic DIC that was produced with oxygen as an oxidant (Equation 4). Now we need to estimate this concentration. We find that the maximum concentration of benthic DIC produced with oxygen is equal to the concentration of available oxygen in the bottom water. The reason is that, assuming a similar diffusivity, the oxygen and the DIC gradient in the pore water should exhibit the same steepness at the same flux. So, in practice, to get the elevated DIC in the most corrosive location of the pore water, the concentration of oxygen was added to the concentration of DIC in calculations of the carbonate system (Equations 4–6). The iterative calculation of the carbonate system parameters that follow Dickson et al. (2007) is then based on the bottom-water alkalinity and the corrected DIC. A modified saturation state is derived (ΩCa_corrected, Equation 7). If this is below 1 (undersaturated), calcite will dissolve.
We need to prescribe a rate at which the material goes into solution if the corresponding ΩCa < 1 indicates undersaturation. In this case, we assume that enough calcite was added in fine enough grains to sustain a desired dissolution rate and thus omitted a term to describe grain sizes. For consistency with other model experiments within the same project (CDRmare-RETAKE), which consider locations other than the Baltic Sea, we aimed at releasing a quantity of calcite whose alkalinising effect is expected to extract 5 Mt of CO2 per year. According to the conversion factor of 0.85 (Montserrat et al., 2017), this requires approximately 134 Gmol/year of calcite to dissolve. As the results will show, this prescribed maximum dissolution rate was in practice irrelevant, as saturation occurred at a far lower rate, thereby defining the upper limit of the method irrespective of the amount of material that could be added to the sea floor.
3 Results
3.1 Field data vs. model control run
The locations that were chosen for the simulated release of CaCO3 are close to routinely sampled stations with the long-time series station Boknis Eck in the Eckernförde Bight and station BY15 in the Gotland Deep. As CaCO3 is released near the sediment in the model, the accurate representation of water conditions near the sediment by the model is crucial for the assessment of the calcite dissolution potential per site. Alkalinity, pH, oxygen, salinity, and temperature from the deepest layer of the model at the stations Boknis Eck and BY15 are compared with observational data below 20 m (Boknis Eck) and 200 m (BY15), respectively (Figure 3).
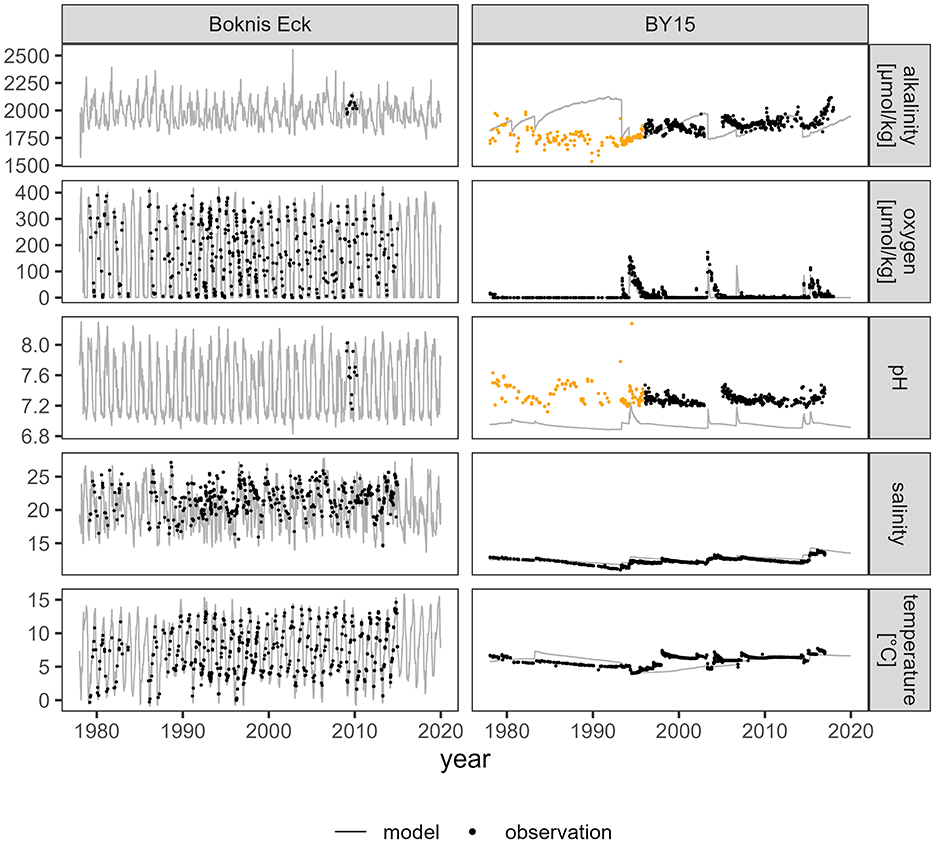
Figure 3. Model data (control run) from the lowermost grid cell and observational data near the bottom at the stations Boknis Eck (observational data > 20 m) and BY15 in the Gotland Deep (observational data > 200 m). The observational data was retrieved from PANGAEA for Boknis Eck and from the SHARK database and Leibniz Institute for Baltic Sea Research Warnemünde (Germany) for BY15. Data in orange derive from measurements before 1996 (Müller et al., 2016). For a property property plot of the data at Boknis Eck see Supplementary material.
Data are scarce for alkalinity and pH at the Boknis Eck station and there is a 6-year sampling gap at BY15 between 1999 and 2005 (Figure 3). Where observational data was available, the model results closely resembled the measured conditions in both the average value and the temporal pattern. At Boknis Eck, the scarce data on alkalinity from 2002 to 2004 are matched by the model with concentrations ranging from 1990–2020 μmol kg-1. The model also follows the pH pattern with minimum and maximum values of 7 to 8, except at station BY15 in the Gotland Deep. The pH levels in the Gotland Deep were underestimated by the model by approximately 0.5 units. The oxygen concentrations in both the model and the sampled data are close to zero almost every summer and reach maximum values of 350 to 420 μmol kg-1 in winter at Boknis Eck. The temperature range between 0 and 15°C is also captured by the model. While the seasonal salinity fluctuation matches the sampling data, the model slightly underestimates the salinity minima in summer.
3.2 OAE in model simulations
To depict the effects of the calcite addition, the control run was subtracted from the calcite experiments for all the parameters shown in the following subsections.
3.2.1 Calcite dissolution
In the Eckernförde Bight without the benthic weathering machine (ECK), calcite dissolved at pH levels of 7.5 and 8.4 (Figure 4). Calcite dissolution rates were a bit higher in hypoxic to anoxic conditions than during oxic conditions but lower at colder temperatures (0–2°C) than at higher temperatures (14°C). When the benthic weathering machine was included (ECKOX), calcite dissolution occurred at bottom water pH levels of up to 9. Peak dissolution rates of over 40 μmol cm-2 d-1 occurred under high oxygen concentrations and at around 3°C, but overall, there was no discernible trend in dissolution rates in response to oxygen or temperature. The inclusion of the benthic weathering machine made little difference in the calcite dissolution dependencies in the Gotland Deep (GOT vs. GOTOX). Without the benthic weathering machine, calcite dissolution started at a pH of 7 and decreased toward a pH of 8. With the benthic weathering machine calcite dissolved at a pH up to 8.5. Calcite only dissolved in response to oxygen concentrations of up to 200 μmol kg-1 and temperatures between 4 and 8°C. The maximum dissolution rate in both experiments was 14.4 μmol cm-2 d-1.
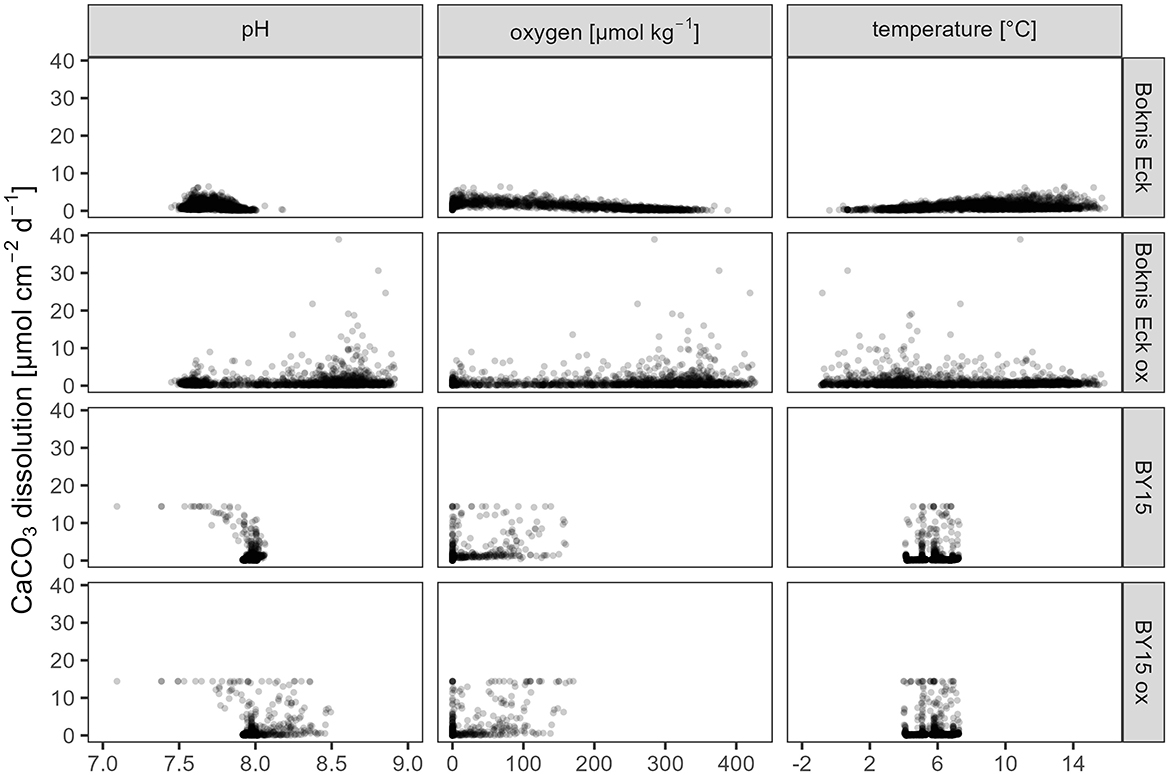
Figure 4. Daily CaCO3 dissolution rates per m2 near the sediment and their dependence on pH, oxygen, and temperature. Shown are the model experiments with and without the benthic weathering engine at the release sites in the Eckernförde Bight (Boknis Eck) and the Gotland Deep (BY15). Shown are the calcite dissolution rates in the bottom grid cell at the Boknis Eck and BY15 stations (Table 1). The dots have some transparency to show high densities of data.
In both experiments without benthic weathering (GOT and ECK), the average calcite dissolution rate ranged between 1 to 2 μmol cm-2 d-1 (Figure 5). In the ECKOX experiment, calcite dissolution varied over the year on average between 3.3 and 22.6 μmol cm-2 d-1 with the lowest rates in late summer and the highest rates in winter. The average rate of calcite dissolution was 10.2 μmol cm-2 d-1 in ECKOX.

Figure 5. Seasonal variation of calcite dissolution rates climatologically averaged over the simulation period (42 years). Shown are the experiments with and without the benthic weathering engine at the release sites in the Eckernförde Bight (ECK, ECKOX) and the Gotland Deep (GOT, GOTOX). The calcite dissolution rates of the whole area were divided by the area size.
3.2.2 Alkalinity and pH change
The two release sites differ substantially regarding the local changes in net pH and alkalinity in the water column as a result of calcite dissolution (Figure 6). In the ECK experiment (Eckernförde Bight, no benthic weathering), the average increase in alkalinity and pH occurred mostly from June to December. During August, the changes in pH and alkalinity are seen closest to the surface at 10 m depth. The average net change in pH and alkalinity did not exceed 0.75 units and 500 μmol kg-1, respectively.
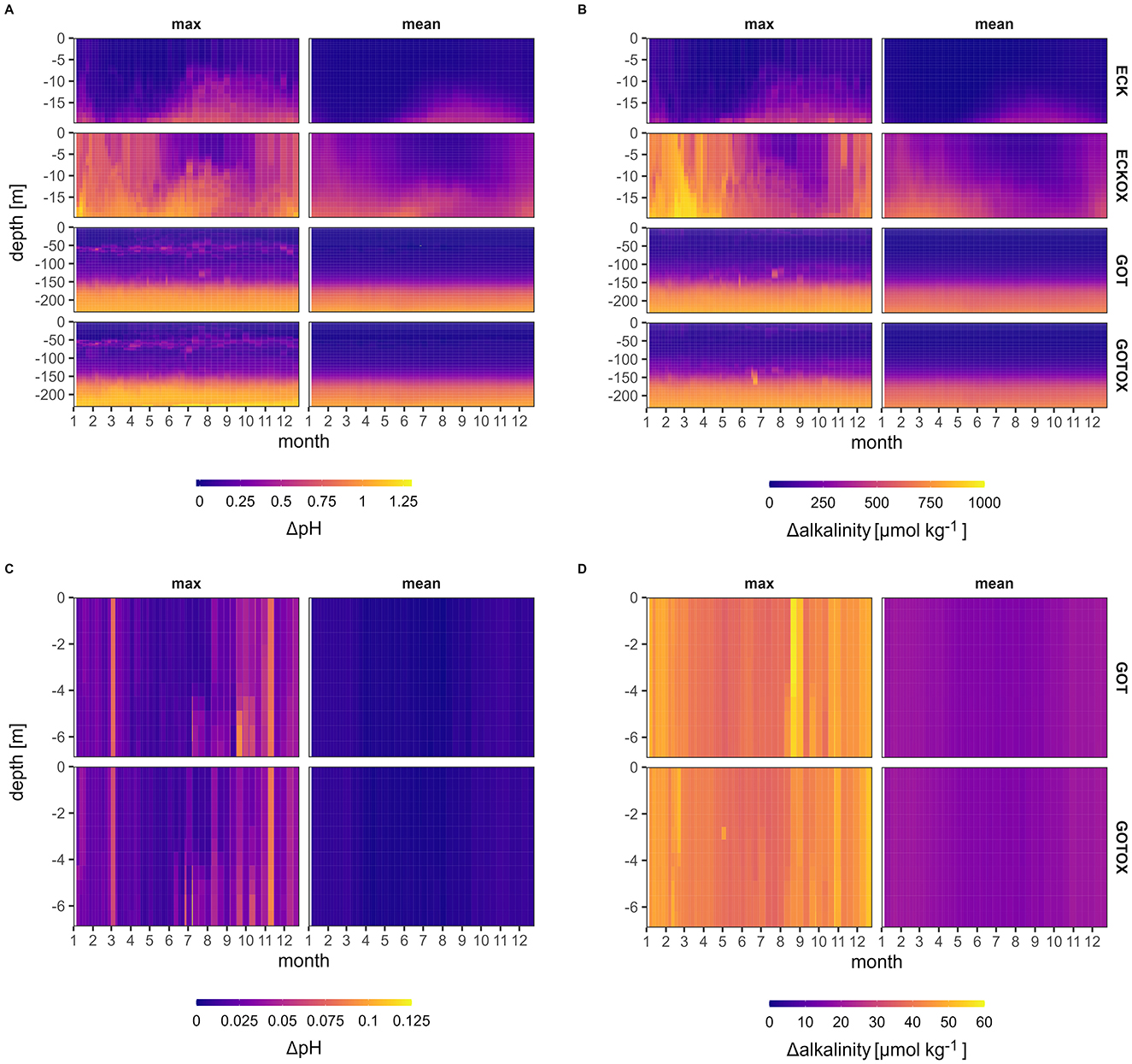
Figure 6. Net pH (A) and alkalinity (B) change throughout the water column over a year at the stations Boknis Eck for the Eckernförde Bight and BY15 for the Gotland Deep experiments. Shown are both the maximum values that occurred at each depth and time in 42 years and the average net change. The control was subtracted from the experiments' results for pH and alkalinity change. (C, D) Show the net pH and alkalinity change on the East coast of Sweden (16.8°E, 57.8°N), where most of the CO2 uptake took place in the GOT and GOTOX experiments. Note the different scales between the locations.
However, according to the maximum values, changes in alkalinity and pH can occur throughout the water column from early summer to winter. In the ECKOX experiment (Eckernförde Bight with benthic weathering), pH changed on average mainly in June and December and thus both earlier and later than in the ECK experiment. The main changes in alkalinity and pH were temporally slightly separated as the average alkalinity change occurred mainly during late winter and early spring before pH began to change. On average, pH and alkalinity did not change by more than 1 unit and 750 μmol kg-1. These changes occur throughout the water column from late autumn to early summer. During summer, the upper 10 m of the water column remain largely unaffected by changes in both pH and alkalinity. In the ECKOX experiments, pH and alkalinity may increase at maximum by 1.25 units and 1,000 μmol kg-1.
In both experiments in the Gotland Deep (GOT and GOTOX), pH and alkalinity were changed continuously throughout the year. Below 150 m, the average change in pH and alkalinity were 0.75 units and 750 μmol kg-1, respectively. Near the sediment, pH and alkalinity increased at maximum by 1.25 units and around 850 μmol kg-1, respectively. On average, the water column above 150 m did not experience changes in pH and alkalinity. At 50 m, maximum changes in pH of <0.5 units are possible. The benthic weathering did not affect pH and alkalinity change.
Regarding the spread of the released alkalinity, there were differences between the ECK and ECKOX experiments, but not between the experiments in the Gotland Deep (Figure 7). The integration of the net alkalinity over depth and the simulation period revealed that <4% of the alkalinity produced in the Eckernförde Bight remained close to the release site regardless of including the benthic weathering machine. Still, the location with the highest accumulation of alkalinity was the Eckernförde Bight in the ECK experiment. With benthic weathering, the alkalinity concentrated near the Eckernförde Bight, the Gotland Deep and the Landsort Deep. The maximum added alkalinity per m2 over the simulation period of 42 years was 1.5 mmol m2 for both experiments. In both experiments in the Gotland Deep, the majority of the alkalinity remained close to the release site in the deep basin with a small proportion of it spreading to the north of Gotland and the Landsort Deep. The maximum change in alkalinity over 42 years was 81 mmol m-2 in the Gotland Basin.
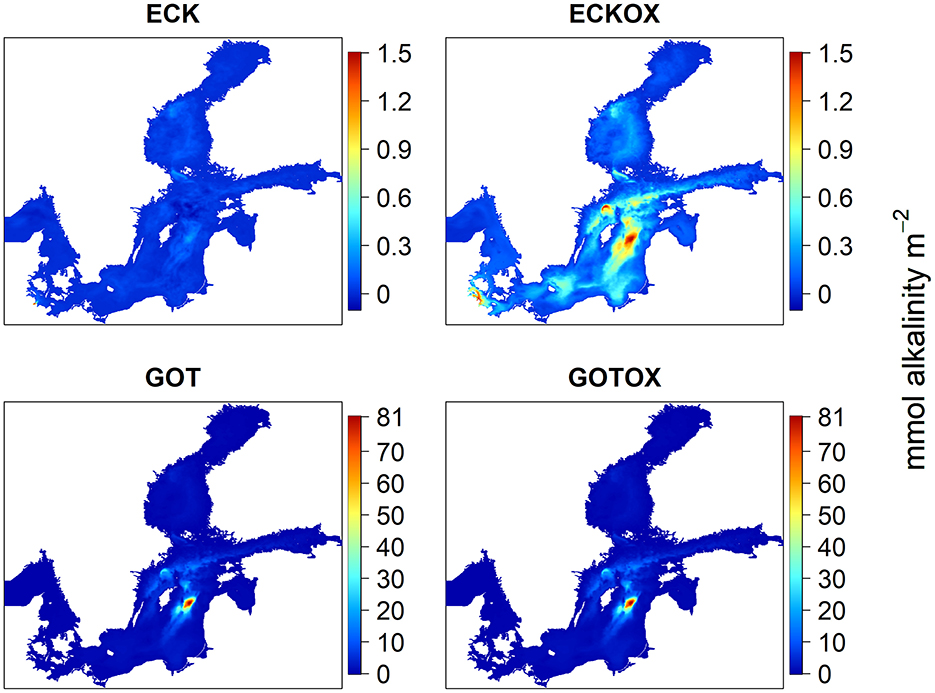
Figure 7. Net alkalinity change integrated over depth, after the model was integrated over 42 years. In ECK the proportion of alkalinity present in the Eckernförde Bight (9.8–10.5°E, 54.3–55°N) compared with the total study area is 3.27%. In ECKOX the proportion is 3.69%.
3.2.3 Net removal of atmospheric CO2
The original target of annually capturing 5 Mt CO2 was not met for either release site regardless of the assumptions made for the benthic weathering machine (Figure 8A). For calcite released in the Gotland Deep, the annual CO2 capture increased for 12 years until it reached a plateau of around one Mt CO2 per year (Figure 8A). The assumption made regarding the benthic weathering machine made no difference in the annual capture rates for this release site. The annual rates varied around 0.01 Mt yr-1 for ECK and 0.18 Mt yr-1 for ECKOX. In the Eckernförde Bight, the average annual CO2 capture was 18 times higher (0.18 Mt yr-1) in the ECKOX experiment than in the ECK experiment (0.01 Mt yr-1). In both experiments, the annual capture for the Eckernförde Bight did not increase as for the Gotland Deep. The release sites differed greatly in area size with the Gotland Deep being almost 20 times larger than the Eckernförde Bight (Table 1). Dividing the annual capture rates by the area size of the release sites shows that the annual capture per m2 for the Gotland Deep (GOT & GOTOX) is about the same as for the ECK experiment with an average rate of 0.1 and 0.2 kg CO2 m2 yr-1, respectively (Figure 8B). At the same time, the annual capture per m2 in the ECKOX experiments exceeded this rate by roughly an order of magnitude (1.4 kg CO2 m2 yr-1).
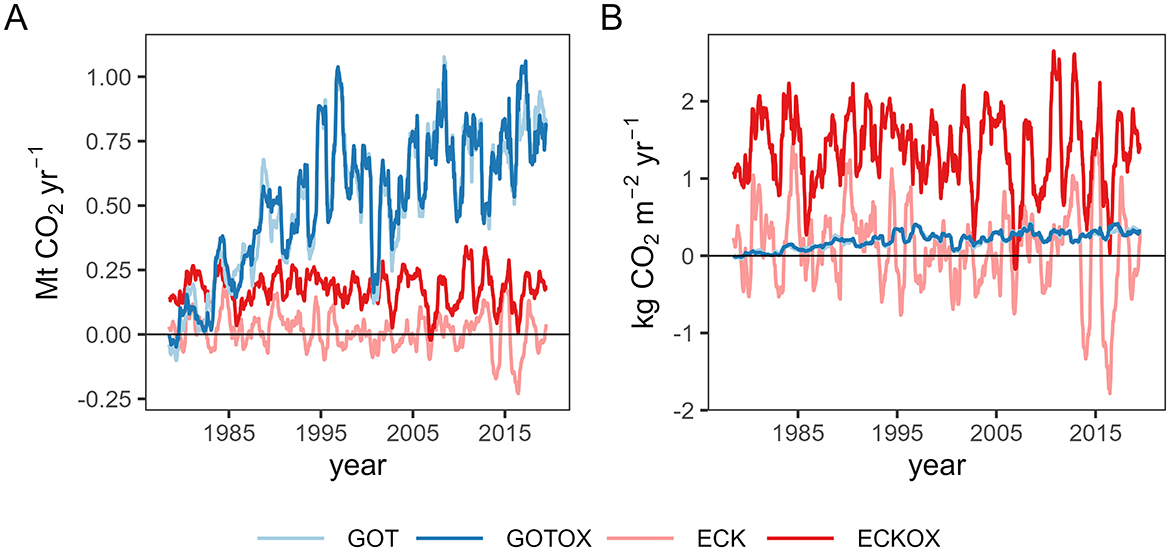
Figure 8. (A) Annual net CO2 capture in the entire Baltic Sea per release site. (B) Net CO2 negative emission as described in (A). As the release sites differ in area, the CO2 flux here is calculated per m2 for comparability of dissolution efficiency (note that random fluctuations occur in the simulations. These generate noise in the time series of the difference between two model runs. This is why these time series may change their sign).
To better compare the potential of CO2 capture based on the dissolved calcite and the actual CO2 capture, the dissolved calcite was converted into CO2 with a factor of 0.85 established by Montserrat et al. (2017). A comparison of the cumulative potential and actual CO2 capture by the release sites shows that calcite dissolution begins linearly in the first year in the Gotland Deep (GOT & GOTOX), while actual CO2 capture does not begin for several years, but then increases exponentially before it becomes linear after almost 15 years after the initial release (Figure 9). Taking 10 Mt CO2 capture as a reference shows that the actual capture crosses that mark 10 years later than the potential capture revealing a substantial delay in the former. For calcite released in the Eckernförde Bight, the actual capture of CO2 begins simultaneously with the dissolution of calcite in both experiments and the cumulative potential CO2 capture per dissolved calcite is equal to the actual captured CO2. By the end of the 42-year simulation period, 22 Mt of CO2 are captured if the CaCO3 is released in the Gotland Deep, which is 3 times the amount captured by the ECKOX experiment (7.5 Mt). The potential CO2 capture for the Gotland Deep reached 29 Mt after 42 years. The total CO2 captured in the ECK experiment does not exceed 0.5 Mt.
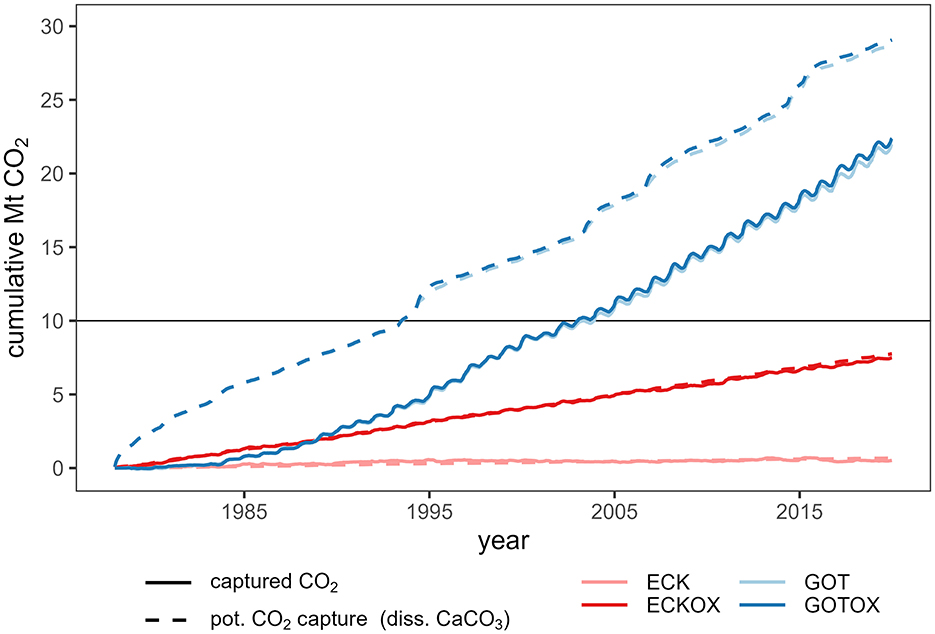
Figure 9. Cumulated change in the CO2 flux over the entire surface area of the Baltic Sea from the atmosphere to the sea over the simulated period per release site. In addition, the potential CO2 uptake per year based on the dissolved CaCO3 was calculated assuming a removal potential of 0.85 Gmol CO2 per Gmol CaCO3 (Montserrat et al., 2017). The line at the arbitrarily chosen 10 Mt mark was introduced as a reference to show the delay between potential and actual CO2 capture for the Gotland Deep (see Section 4).
The release locations differed not only in uptake rates of CO2 but also in the area distribution of the CO2 uptake (Figure 10). In the experiments within the Eckernförde Bight, the majority of total net CO2 capture of 42 years takes place close to the release site, with 71.07% CO2 captured in the experiment without the benthic weathering machine and 49.07 % captured in the experiment with the benthic weathering machine (Figure 10). For calcite released in the Gotland Deep, net CO2 capture took place mainly in the Baltic Proper and the Gulf of Finland with minimal CO2 capture in the Bothnian Bay, the Danish Straits and the Gulf of Riga. The maximum CO2 capture took place in the upwelling region on the East coast of Sweden.
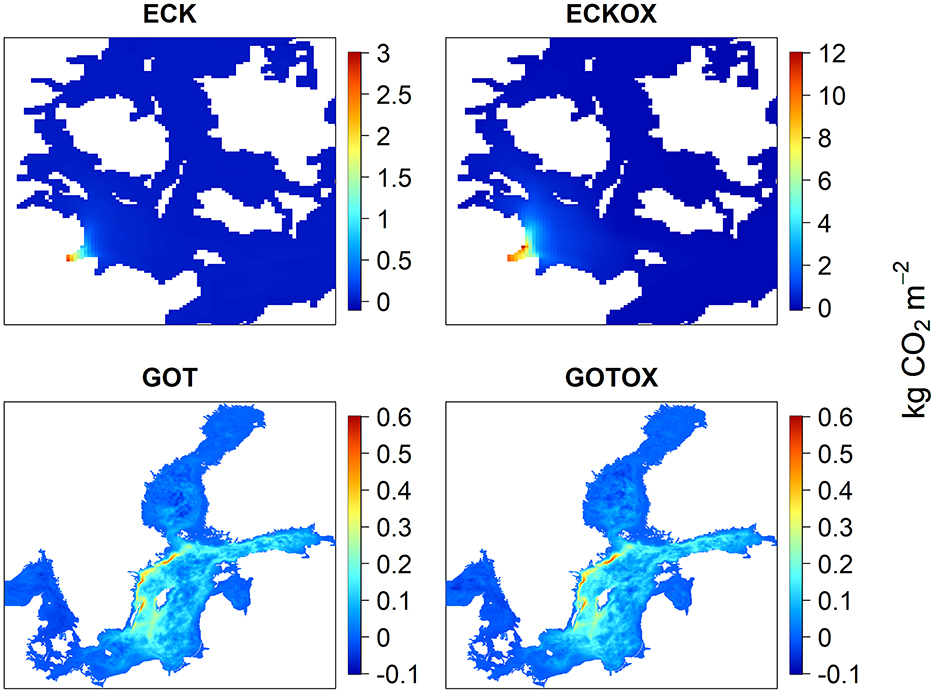
Figure 10. Net change in the sea surface flux of CO2 from the atmosphere into the water integrated over 42 years. In ECK, the proportion of CO2 flux present in Eckernförde Bight (see Figure 2) compared with the total study area is 71.07%. In ECKOX the proportion is 49.07%.
The method's efficiency of CO2 removal is defined as net removed CO2 per net change in alkalinity. Similar efficiencies were reached in all experiments by the end of the simulations with an efficiency of 0.41 in the ECKOX experiment and 0.33 in the other experiments. During the simulation period, the efficiency varied depending on the release location and, in the case of the Eckernförde Bight, on the inclusion of the benthic weathering machine (Figure 11). While the efficiency for both experiments in the Gotland Deep remained close to 0 for several years before it increased and finally reached a plateau, the pattern was almost reversed for the Eckernförde Bight. At the start of the Eck experiment, the efficiency displayed high variability with values ranging from –0.5 to 2.5 that later became more stable and reached the same level as the experiments in the Gotland Deep. In the ECKOX experiment the efficiency increased to 0.41 within a few years and remained steady with minor variances of 0.01.
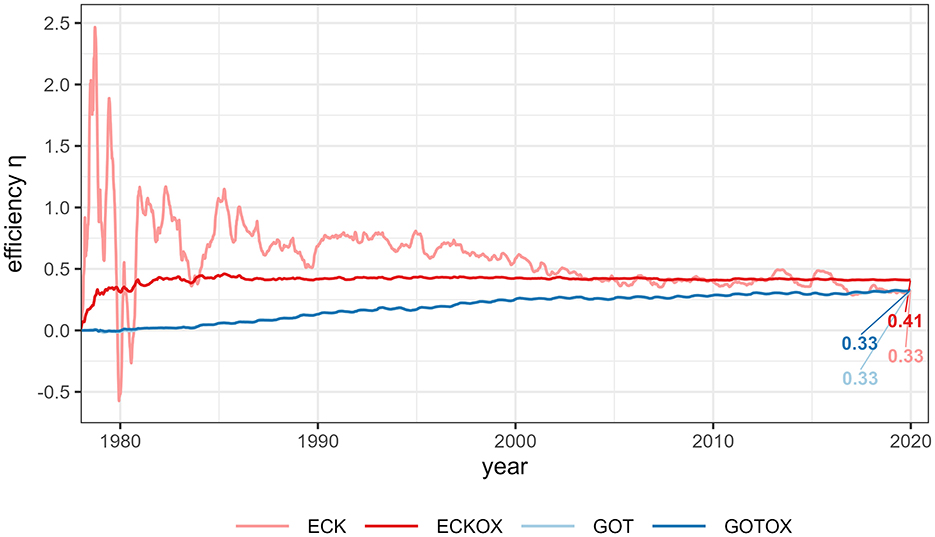
Figure 11. OAE efficiency (ΔDIC/Δalkalinity). Shown is the cumulated change in net DIC uptake at the sea surface of the entire surface of the Baltic Sea divided by the released net alkalinity for each experiment. The alkalinity is derived from the calcite dissolution rates assuming 2 mol of alkalinity added by 1 mol of CaCO3 dissolved. The numbers show the efficiency reached in each experiment by the end of the simulation.
4 Discussion
4.1 Model validation
The comparison of the control run with observational data showed that the model accurately replicates the recorded conditions of oxygen, pH and temperature at Boknis Eck in the Eckernförde Bight and at station BY15 in the Gotland Deep (Figure 3). The lower pH levels in the Gotland Deep in the model mean that the model somewhat overestimates the acidity at this location. As this model bias is approximately half of the size of the pH signal seen in the GOT and GOTOX experiments, this may indicate that the dissolution rates that we simulate in these runs could be overestimated and be a bit lower in reality. At the same time, historic observational data on pH bear a degree of uncertainty due to issues with the validation of the methodology (Noffke et al., 2016; Müller et al., 2016; Álvarez et al., 2020; Carter et al., 2024). Thus, the mismatch between observational and modeling pH data could well be a result of limited accuracy of earlier measurements of the carbonate system (Müller et al., 2016).
A comparison of the oxygen levels showed that the model not only captures the seasonal regularity of oxic and anoxic conditions in the Eckernförde Bight (Bange et al., 2011; Orsi et al., 1996) but also most of the spikes in oxygen in the Gotland Deep that are caused by the highly irregular saltwater inflows from the North Sea. These do not only change the physical and chemical conditions in the bottom water but also, in the long term, cause the transport of bottom water to the surface (Kankaanpää et al., 2023; Mohrholz et al., 2015; Mohrholz, 2018; Myrberg and Andrejev, 2003).
In addition to correctly replicating the dissolution conditions, the model also needs to replicate water inflows, mixing and transport of water in order to accurately simulate the transport of added alkalinity. We compare seasonal temperature and salinity trends. In the Eckernförde Bight, temperature decreases and salinity and oxygen increase in winter due to saltwater inflows and storms. The water from the North Sea brings cold water with high salinity and oxygen down to the bottom of Eckernförde Bight (Bange et al., 2011; Orsi et al., 1996). In summer, the bottom water becomes warmer despite thermal stratification, and anoxic due to thermal stratification and benthic respiration (Orsi et al., 1996). This pattern is well captured by the model (Figure 3). The model is thus reliable in representing the conditions near the sediment at the two study sites that are relevant to calcite dissolution and alkalinity transport.
4.2 Potential for CO2 capture and calcite dissolution
4.2.1 Gotland Deep
With an overall CO2 capture three times larger than the maximum achieved for the Eckernförde Bight (Figure 8A), the Gotland Deep appears to have the larger potential in total as a calcite release site for OAE. The release area in the Gotland Deep is almost 20 times larger than in the Eckernförde Bight (Table 1) and the calcite was thus released on a larger surface than in the Eckernförde Bight. Therefore, a much larger surface area of calcite is exposed for dissolution than in the smaller Bight resulting in a larger amount of dissolved calcite overall. Due to the permanent anoxia and mostly stable conditions, calcite dissolution takes place at a rather continuous rate over the year (Figure 9) in the Gotland Deep. The underestimation of pH values here indicates an overestimation of the dissolution rate of calcite, thus, the potential of the region to capture CO2.
In the Gotland Deep, there is a substantial delay in carbon capture, as water from the bottom takes multiple years to a decade to reach the surface, where atmospheric CO2 can be absorbed (Figures 8A, B, 9). The bottom water in the Gotland Deep and any alkalinity in it moves counter-clockwise in the basin (Holtermann et al., 2014) and surfaces mainly in the upwelling region east of the Swedish Coast and along the Southern Finnish Coast (Lehmann et al., 2012; Myrberg and Andrejev, 2003). According to the model results, the Eastern Swedish Coast is also the site of the largest CO2 uptake per m2 for calcite release in the Gotland Deep (Figure 10). However, most of the net alkalinity change occurs in the Eastern and Northern Gotland Basin (Figure 7). This is because alkalinity change already occurs before surfacing and follows the counter-clockwise path of the water advection.
The “CO2 uptake” that concentrates in the upwelling areas (Figure 10) is just a relative one compared to the reference simulation. In practice, it means less CO2 of the surfacing DIC-rich bottom water with high pCO2 is released into the atmosphere. More of the DIC that was produced mostly by organic matter mineralisation in the sediment can be kept in the upwelled bottom water and less is outgassing as CO2.
4.2.2 Eckernförde Bight
While the overall CO2 capture for the ECKOX experiments is much lower than for the Gotland Deep experiments, the calcite dissolution rates per m2 (Figure 8B) and thus the associated CO2 capture per m2 release site are much higher than in the Gotland Deep.
However, this strongly depends on the assumed BWE efficiency. In the case of the optimistic assumption for the benthic weathering machine, the Eckernförde Bight might be a much more efficient location for calcite dissolution. Enhanced benthic weathering makes all the difference in the Eckernförde Bight. Depending on its impact on calcite dissolution, the CO2 capture potential of the Eckernförde Bight can be as high as 5 times the potential of the Gotland Deep and 11 times the potential of the Eckernförde Bight without the BWE. In contrast to the Gotland Deep which experiences permanent anoxia except for a few inflow events, the Eckernförde Bight shows seasonal patterns of oxic and hypoxic bottom water.
In the ECKOX scenario, calcite dissolution was the highest in winter during which much of the alkalinity enhancement also reached the surface. Since any freshly produced alkalinity surfaces almost immediately in the Eckernförde Bight, due to the shallowness of the location, mixing and inflows, the CO2 capturing effect is almost instant after the dissolution of calcite. Saltwater inflows from the North Sea and storms in autumn and winter cause the bottom water to be pushed out of the Bight and to the surface (Meier et al., 2019; Melzner et al., 2013).
This regime likely “resets” the system's alkalinity and oxygen conditions to the levels before stagnation after every mixing. The newly added oxygen then again oxidizes the organic matter in the sediment leading to enhanced benthic weathering (i.e., the BWE). While the positive effect of the BWE on calcite dissolution is well documented for the Eckernförde Bight in experimental studies that used sediments from the Bight (Fuhr et al., 2024, 2023, 2022), it is unclear how large this effect would be under in situ conditions. The experiments featured a continuous supply of oxygen rather than a seasonal one. As our model results show (Figures 9A, B), the size of the area in which calcite is exposed to dissolution limits the potential for CO2 capture per released calcite amount as well as opportune dissolution kinetics.
Calcite dissolution stops when calcite becomes saturated. As a result, the calcite saturation state limits OAE with calcite at both release locations. Therefore, the potential for CO2 capture at these sites cannot be increased by adding more calcite or enhancing the surface area. The only way to scale up the CO2 capture is by applying calcite in additional regions with similar conditions where calcite is still undersaturated (more coastal bays like Eckernförde Bight or more anoxic basins like Gotland Deep).
An indication for a possible underestimation of the true potential of the BWE in Eckernförde Bight are experimental results published very recently by Fuhr et al. (2024). In benthocosm experiments, the authors found dissolution rates of calcite which exceed our upper-limit estimates. The reason why the solubility limit, which we derived here based on theoretical consideratons, could be exceeded, is still unclear though.
4.2.3 OAE efficiency
Regarding the efficiency in terms of CO2 captured by released alkalinity, the release locations were similar long-term (Figure 11). However, as CO2 capture begins almost immediately after the release of calcite in the Eckernförde Bight, the location has a higher efficiency over the entire deployment period even in the pessimistic scenario. In addition to higher dissolution rates per m2, the smaller Eckernförde Bight may have a better OAE potential short term. The efficiencies of both locations in our simulations exceed the predicted efficiency for calcite of 15%–30% by Renforth and Henderson (2017). What is yet unclear is the level of additionality of OAE in the Baltic Sea, meaning how much of the added alkalinity contributes directly to carbon capture and how much is canceling out natural alkalinity production (Bach, 2024).
4.3 Environmental implications and direct effects
4.3.1 Gotland Deep
The expected changes in alkalinity and pH associated with OAE remain one of the main reasons for concern regarding the method's environmental impact (Dupont and Metian, 2023; Iglesias-Rodríguez et al., 2023; Oschlies et al., 2023). Numerous studies have shown the detrimental effect of ocean acidification on marine communities (Dupont and Metian, 2023; Iglesias-Rodríguez et al., 2023; Thomsen et al., 2010). Iglesias-Rodríguez et al. (2023) caution that ocean acidification experiments have shown species competition to alter with changes in the carbon chemistry and that the trophic states of organisms may increase their susceptibility to such changes. At the same time, research on the environmental impact of enhanced alkalinity and pH remains scarce (Oschlies et al., 2023). It is yet unknown if and how OAE may impact plankton community composition, seasonal succession, trophic transfer, and therefore higher trophic levels (Dupont and Metian, 2023).
Thus, in addition to understanding the ecological response to OAE, reliable estimates of average and maximum changes in pH and alkalinity are important for the assessment of the method's environmental safety. In the Gotland Deep, the net changes in pH and alkalinity over time are not homogeneously distributed over the water column (Figures 6A, B). The largest changes in pH and alkalinity remain below the halocline at 150 m depth and virtually no changes are observed near the surface. As there are no photosynthetically active organisms at this depth, these changes would not impact primary production. The extended periods of hypoxia below the halocline in the Gotland Deep prevent the development of long-term benthic communities of macrofauna (Olenin, 1997; Noffke et al., 2016; Almroth-Rosell et al., 2021) that may be affected by changes in alkalinity and pH. The exception is sulfur bacteria in the sediments (Feistel et al., 2008). Experiments on calcite addition to anoxic sediments of the Eckernförde Bight showed no clear response by bacteria to the alkalinity release by calcite dissolution (Fuhr et al., 2023).
While the Gotland Deep currently experiences extended hypoxia, this state is brought about largely by eutrophication and climate change (Meier et al., 2019, 2012; Wåhlström et al., 2020). One aim of the Baltic Sea Action Plan is to reduce eutrophication in order to shrink the anoxic zones in the Baltic Sea (Wåhlström et al., 2020). The successful reoxygenation of the deep basins would also allow for benthos communities to settle which may then experience stress by OAE.
While the largest changes in alkalinity and pH occurred below the halocline in the Gotland Deep, these parameters were also altered in the surface off the East coast of Sweden, where most of the CO2 uptake took place (Figures 6C, D). Here, the changes in pH reached a maximum of 0.12 units in early spring and late autumn, which are not the peak seasons for plankton blooms in the concerned region around Gotland and south of Finland (Höglander et al., 2004). The average changes in pH and alkalinity throughout the water column over the year are very small. In fact, the maximum ΔpH falls within the range of diurnal pH fluctuations in the surface ocean (Havenhand, 2012; Hofmann et al., 2011; Wootton et al., 2007). Also, there has been an internal alkalinity increase by around 100 μmol kg-1 in the surface waters of the Baltic Sea over the last three decades (Müller et al., 2016). The internal changes in alkalinity in the Baltic Sea thus exceed the purposefully added alkalinity according to our model. Therefore, the release of calcite in the Gotland Deep for OAE is unlikely to impact organisms in the photic zone.
4.3.2 Eckernförde Bight
In comparison to the Gotland Deep, the changes in alkalinity and pH in the Eckernförde Bight were much larger while also reaching the surface. The calcite dissolution changed the local pH and alkalinity levels throughout the water column to a larger degree in both experiments (Figures 6A, B). In the ECKOX experiments, maximum net changes of up to pH 1.12 units and alkalinity of 1,000 μmol kg-1 may occur in spring and early summer, when plankton blooms occur at the surface (Brando et al., 2021). While this was only the case in the summer and autumn months in the ECK experiment, the surface layer was impacted mostly during spring, autumn and winter (Figures 6A, B).
In a neighboring fjord of the Eckernförde Bight, the Kiel fjord, pH was found to vary naturally by around 0.7 daily (Thomsen et al., 2010), which is more than the average net pH change in the ECKOX experiments. Coastal plankton communities experience high fluctuations in pCO2 (Bermúdez et al., 2016) and may therefore be less impacted by changes in pH, alkalinity and pCO2 caused by ocean alkalinisation through calcite addition near the coast. Furthermore, coastal plankton communities may face future stress due to increasing ocean acidification (Bermúdez et al., 2016). As ocean alkalinisation decreases acidification (Keller, 2014), the method may alleviate stress by acidification for plankton communities in the Eckernförde Bight.
The change in pH and alkalinity may also directly impact the physiology of organisms in the vicinity of the release site. Lower CO2 concentrations are known to decrease phytoplankton growth rates across phytoplankton groups (Berge et al., 2010; Langer et al., 2006; Paul and Bach, 2020; Riebesell et al., 1993; Schippers et al., 2004). The main driver here is the lower availability of carbon for photosynthesis, which may decrease and delay their bloom. The question is by how much OAE can decrease pCO2 levels in the surface to impact primary production. Lower carbon sequestration as a result of OAE would make the method less efficient, while delays in phytoplankton blooms could impact higher levels in the food web. Limiting CO2 concentration may also cause changes in the species composition of phytoplankton favoring species with the ability to use bicarbonate or carbon concentrating mechanisms (Nimer et al., 1997). However, based on the expected calcite dissolution rates in our model and experimental results (Fuhr et al., 2024), the resulting lowering of the pCO2 will likely be negligible (see calculations in Supplementary material).
Experiments on the effects of OAE on phytoplankton have shown that bacteria abundances were higher in the alkalinity treatments, while the growth of picoeukaryotes appeared to be more negatively impacted by low CO2 or high pH than microphytoplankton (Ferderer et al., 2022). As primary production is expected to shift toward smaller cells in warming oceans (Morán et al., 2010; Peter and Sommer, 2013), the higher sensitivity to low CO2 and high pH in picoplankton may make the biological carbon pump more vulnerable to OAE. Experiments on OAE in the North Atlantic Subtropical Gyre revealed that OAE may in some cases change the community composition toward smaller cells while lowering net primary production by up to 30% (Subhas et al., 2022). The high net changes in alkalinity and pH predicted by the model may thus in the worst case lead to a localized decrease in biological carbon uptake in the Eckernförde Bight. Current research suggests that a variety of factors, which we have yet to fully understand, affect phytoplankton's susceptibility to changes in carbonate chemistry and, consequently, to OAE (Cyronak et al., 2023; Riebesell et al., 2023; Iglesias-Rodríguez et al., 2023). These include species composition, competition and physiology like the trophic state.
The experiments by Ferderer et al. (2022) further showed lower concentrations of chlorophyll a in the OAE treatments that according to the authors could be explained by increased grazing. Ferderer et al. (2022) found that increased alkalinity changed the succession pattern of phytoplankton, though the mechanisms driving the change remain unclear. In contrast, results from a mesocosm study on plankton from oligotrophic subtropical waters of the Atlantic showed significant changes in protein expression but no cellular stress response to OAE (Ramírez et al., 2024). Changes in species composition in the phytoplankton impact the energy transfer to higher trophic levels (Mallin and Paerl, 1994; Traboni et al., 2021; Wassmann, 1997). However, the oligotrophic open ocean and eutrophic shallow coastal regions with much lower salinities are very different ecosystems. Results cannot be extrapolated from one to the other, further highlighting the need for experiments representative of the target regions.
It is further unclear what effect enhanced alkalinity may have on higher organisms. Experiments on Carcinus maenas showed that enhanced alkalinity with Ca(OH)2 disturbs the crustaceans' acid-base regulation (Cripps et al., 2013) suggesting that OAE may impact the physiology of the benthic macrofauna. However, Goldenberg et al. (2024b) found no direct or indirect effect of OAE on fish larvae in their mesocosm experiments. The authors state that these results may not rule out potential effects on other life stages or habitats. It is thus difficult to foresee what effects localized large changes in alkalinity and pH will have on the wider food web. Apart from the Eckernförde Bight itself, a large portion of the alkalinity produced in the ECKOX experiments also accumulated in the deep basins: the Landsort and the Gotland Deep. As the maximum net change in alkalinity of 1.5 mmol m-2 over the whole water column and simulation period is less than the natural variability in the Baltic Proper which had a natural internal increase in alkalinity over the last 30 years (Müller et al., 2016), it can be expected that it has no impact on the environment here.
Lastly, experiments with OAE on fish larvae showed no changes in their physiology with alkalinity additions of up to 600 μmol kg-1, suggesting a certain resilience toward changes in alkalinity (Goldenberg et al., 2024a). Given that our simulations assuming optimistic calcite dissolution in the Eckernförde Bight showed that maximum increases in alkalinity can reach up to 1,000 μmol kg-1, further research with higher alkalinity changes is needed.
4.4 Environmental implications and indirect effects
In this study, only the potential direct effects of ocean alkalinity enhancement on the carbonate chemistry and ecosystems in the Baltic Sea were addressed by the model. However, OAE may also have indirect effects related to mineral formation such as in the phosphate cycle. Anthropogenic eutrophication has heavily changed the Baltic Sea and increased levels of nitrogen and phosphate led to the spread of hypoxia (Österblom et al., 2007). Under oxic conditions, phosphate adsorbs to iron oxyhydroxides and is released from the sediments again during anoxic conditions (Boesen and Postma, 1988; Conley et al., 2002). Another major sink of phosphate is the authigenic formation of Ca-P under oxic conditions (Conley et al., 2009). This would permanently bind phosphate, leading to reduced primary production. While this would be desirable from the perspective of restoring oxic conditions in the central basins of the Baltic Sea (Carstensen et al., 2014), it would be undesirable from a carbon capture perspective since the biological carbon pump would be decelerated. This points to the general dilemma that eutrophication in coastal waters, though highly undesirable from an ecosystem health consideration, usually enhances organic carbon storage and CO2 uptake. Consequently, combatting coastal eutrophication bears the risk of counteracting climate mitigation.
Furthermore, calcite addition may affect the nutrient cycle in the Eckernförde Bight, where anoxic and oxic conditions alternate in close temporal and spatial proximity. While the precipitation of phosphate as Ca-P would decrease eutrophication, it may also lower phytoplankton blooms, which could lead to a decrease in permanent burial of carbon, thus lowering the methods' CO2 removal potential in a secondary pathway (Subhas et al., 2022). Increased phosphate precipitation could increase the nitrogen-to-phosphate ratio.
There is currently no research on how calcite precipitates may impact plankton in the Baltic Sea. Zooplankton may directly or indirectly (bound in aggregates) ingest calcite precipitates or grains (Arendt et al., 2011; Fakhraee et al., 2023; Harvey, 2008). While some species of microplankton and copepods have shown no response in feeding toward suspended sediments (Arendt et al., 2011; Maselli et al., 2023), species that were less discriminatory feeders were negatively impacted (Arendt et al., 2011; Sommaruga, 2015). The dissolution of suspended calcite may therefore be impacted by plankton while also impacting plankton depending on the species. The grain size and method of calcite release in the Baltic Sea may thus be an important factor to consider regarding the methods' environmental impact. Calcifying plankton such as coccolithophores are absent in the Baltic Sea, which has been attributed to the Baltic Sea's undersaturation in calcite (Tyrrell et al., 2008). It is unclear if adding calcite would lead to the establishment of coccolithophores in the Baltic Sea outside the Kattegat.
4.5 Monitoring and attribution
Safe, ethical and reliable implementation of OAE requires reliable protocols for monitoring, reporting and verification (MRV) to confirm carbon capturing from added calcite and account for possible CO2 degassing back into the atmosphere. Carbon removal through anthropogenic calcite addition needs to be differentiated from the baseline carbon sequestration due to natural sources of alkalinity (Bach, 2024; Ho et al., 2023). This question of additionality requires further research on alkalinity sources and sinks in the Baltic Sea for accountability of any OAE measures in the region. Aside from weathering rock, natural sources of alkalinity in the oceans are denitrification, pyrite burial, and in anoxic conditions, the release of hydrogen sulfide (Gustafsson et al., 2014; Gustafsson and Gustafsson, 2020; Gustafsson et al., 2019; Hiscock and Millero, 2006). The Eckernförde Bight, in particular, is surrounded by a lime coast rich in calcite (Fuhr, 2024) which may conflict with calcite additions.
Reliable confirmation of removed atmospheric carbon dioxide also needs to consider background variability of the partial pressure of carbon dioxide (pCO2) (Ho et al., 2023). As seasonal variability in pCO2 is expected to increase (Gallego et al., 2018), a shallow release site for calcite such as the Eckernförde Bight with substantial seasonal fluctuations in environmental conditions may be more challenging to monitor than a more stable location such as the Gotland Deep. But even for the central Baltic Sea, variability and trends in surface pCO2 challenge the identification of an enhanced air to sea flux due to OAE. In addition, tracing Ca2+ as a means of quantification will be challenging to differentiate from natural Ca2+ addition via erosion and riverine inputs in coastal regions (Fuhr et al., 2023; Wallmann et al., 2023, 2022). Aside from accurately estimating alkalinity sources, information is also required on alkalinity sinks and the stability of the added alkalinity to ensure the method's long-term effect (Hartmann et al., 2023). Counteracting effects (i.e., processes enhancing the sea to air CO2 flux) may be caused by reprecipitation of calcite either as a result of calcite oversaturation or biotic calcification (Ho et al., 2023).
Carbon uptake and changes in alkalinity and pH will unlikely be measured at the necessary frequency and spatial scale unless a robust monitoring service with state-of-the-art instrumentation is implemented. This effort is critical to identification of net changes in the near-field of the calcite addition. Yet, these net changes will likely fall below the instrumentation's detection level (especially under anoxic conditions) farther away from the deployment site. As a result, models will be a necessary tool in forthcoming MRV protocols.
As recently pointed out, no stringent protocols or even consensus of parameters needed to be monitored to assess OAE efficiency, as well as potential environmental side effects, have been reached in the scientific literature so far. Additionally, strategic considerations to integrate observations and modeling components of MRV would be required to trace additional CO2 uptake (and thus, potentially accountability). While carbon observations in the Baltic Sea might be more frequent than in other marine regions, the high seasonal and interannual variability in the Baltic Sea would currently make it very challenging to reliably trace the success of the OAE measures investigated in this study.
Finally, looking at the difference between the possible deployment sites, the localized higher net changes in alkalinity and pH and the temporal proximity of release and effect in the Eckernförde Bight make it easier to detect any environmental changes and stop the project sooner in case adverse effects are observed (Figures 6A, B). In the Gotland Deep, where the alkalinity does not reach the upper ocean for several years, aborting the deployment of calcite would only stop possible adverse effects in the surface ocean after about one to two decades.
4.6 Model limitations
A challenge of this study is the balance of resolving the release site to sufficient detail while also covering the entirety of the Baltic Sea. A horizontal resolution of 1 n.m. includes the Eckernförde Bight, but results in a relatively coarse resolution. Highly localized variances in pH, oxygen concentrations, temperature etc. are thus not resolved in the current model and could further impact the estimations made here. However, the validation with data from Boknis Eck showed that the model closely reproduced the conditions measured at the site. Furthermore, the model only captures the conditions and changes in the Baltic Sea. Any export or exchange with the North Sea is not represented. Therefore, it was not possible to track the long-term fate of the added alkalinity when it is transported to the North Sea.
Simplifications were also made regarding the horizontal transport of calcite. If calcite is released at the tested locations as a ground material, the grains will be horizontally transported by turbulence and currents. This factor is currently not considered in the model. It would require assumptions on the grain size and the focus was the potential of calcite dissolution. However, horizontal transport of a substantial amount of the released calcite into areas with less optimal conditions for calcite dissolution could decrease the method's efficiency. This is more likely to be an issue in the relatively shallow Eckernförde Bight, which receives a lot of bottom shear by wind-induced turbulence and frequent saltwater inflows from the North Sea, than in the deep basin of the Gotland Deep.
The effect of the oxygen regime on pyrite formation and manganese precipitation is currently not included in the model as the riverine input of iron and manganese is poorly constrained and thus difficult to represent in the model. Calcifying organisms such as coccolithophores and mussels may be a potential source of biogenic calcite reprecipitation. Such a biogenic calcite sink could lower the efficiency of calcite as a mineral for OAE. With coccolithophores being absent from the Baltic Sea except for the Kattegat, mussels would be the potentially largest biogenic sink of calcite. As mussels are currently not part of the ecosystem model used in this study, the model cannot make any predictions on the scale of the biogenic calcite reprecipitation and its impact on OAE efficiency.
Furthermore, the model currently ignores the effects of changes in pH on any functional organism groups and only considers the air-sea exchange. It is thus not possible to estimate the effect of OAE on higher trophic levels. Also omitted from the model are grain sizes, accumulation of calcite grains and their transport into the sediment by bioturbation. Bioturbation may increase calcite dissolution by working the calcite further into the sediment thereby exposing it to lower pH levels than directly on the sediment. In the same vein, ingestion by benthic organisms may increase calcite dissolution when calcite is exposed to highly acidic conditions inside the gut. There is currently neither sufficient qualitative nor quantitative data for either of these effects to be incorporated into the model. Similarly, it is not possible here to make predictions on how benthic organisms could be impacted by repeated loadings of calcite by being buried or smothered. Lastly, the simulations are based on past climate conditions and pCO2 concentrations between 1978 and 2019 and do not consider future acidification or changes in the eutrophication state.
4.7 Future research
Knowledge of accurate calcite dissolution rates in the target region is essential for reliable estimations of future carbon removal. Calcite dissolution was narrowed down between two limits in this study, based on theoretical considerations. The next step is to harmonize these theoretical limits with the measured calcite dissolution rates for sediments of the Eckernförde Bight published by Fuhr et al. (2024). Under oxic conditions, these can exceed our upper-limit estimates. The cause of this exceedance is not yet understood, which would be required to allow for a mechanistic modeling approach matching with the experimental results. Besides accurate calcite dissolution rates, narrowing down the true CDR potential of OAE with calcite in the Baltic Sea also requires closing any gaps regarding alkalinity and calcite budgets. Measurements of the carbonate system in the Baltic Sea have become more frequent over the past few years, especially with the implementation of underway systems in ships of opportunity (Jacobs et al., 2021). However, whole-basin observational studies still rely on uncertain extrapolations (Bittig et al., 2024). Furthermore, sampling campaigns for the carbonate chemistry of river end members would provide data to revisit estimates.
In this study, OAE was simulated under the climate conditions of the past 40 years. To assess how climate change in this region will affect the CDR potential and OAE efficiency, calcite addition should also be simulated using future climate projections. A potential sink of added calcite and therefore limiting factor to the efficiency of OAE could be calcifying organisms such as coccolithophores and bivalves. The absence of coccolithophores in the Baltic Sea leaves bivalves as the largest potential calcite sink. Thus, research on bivalve distribution and their calcification rates relative to calcite saturation is required. Further unknowns regarding the relationship between OAE and the biosphere are the impact of the macrozoobenthos in the sediments on calcite dissolution through ingestion and the effect of the grain size of added calcite on the ingestion rates of said benthic organism but also microzooplankton.
5 Conclusion
The release locations Eckernförde Bight and Gotland Deep greatly differ in their carbon capture potential, efficiency, time between release and effect, and the potential environmental implications. The larger area of the Gotland Deep results in a larger total carbon capture potential. The Eckernförde Bight is much smaller in area but may provide conditions for much more efficient calcite dissolution. The potential of the Gotland Deep can be gauged with some certainty. As the efficiency of the BWE at the release site in the Eckernförde Bight bears temporal and spacial uncertainty, the carbon capture potential of this location remains difficult to estimate. If the scale of the BWE, in reality, is close to our results in the optimistic scenario, the potential of the Eckernförde Bight could be upscaled by releasing calcite in similar shallow regions along the coast.
Further research is required regarding the rates of benthic weathering as well as the impact on OAE in the Baltic Sea using calcite. The majority of current studies are on marine plankton communities outside the Baltic Sea at much higher salinities. Results from these studies cannot be directly translated to the unique ecosystem of the Baltic Sea.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://hyrax.io-warnemuende.de/opendap/thredds/content_thredds/maintainers/anschuet.html.
Author contributions
A-AA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. JL-A: Methodology, Writing – review & editing. GR: Funding acquisition, Methodology, Project administration, Writing – review & editing. BC: Methodology, Writing – review & editing. TN: Methodology, Software, Writing – review & editing. HR: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the German Federal Ministry of Education and Research (project RETAKE with grant no. 03F0895 and project RETAKE II with grant no. 03F0965D, DAM Mission “Marine carbon sinks in decarbonization pathways”; CDRmare).
Acknowledgments
The simulations were performed with resources provided by the North-German Supercomputing Alliance (HLRN). We sincerely thank Michael Fuhr and Andrew Dale for their criticism and insight to the benthic weathering engine in the Eckernförde Bight that informed our model design.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fclim.2025.1450468/full#supplementary-material
Footnotes
1. ^Swedish Meteorological and Hydrological Institute (SMHI). Swedish Archive for Oceanographic Data (SHARK). Available online at: https://www.smhi.se.
References
Almroth-Rosell, E., Wåhlström, I., Hansson, M., Väli, G., Eilola, K., Andersson, P., et al. (2021). A regime shift toward a more anoxic environment in a Eutrophic Sea in Northern Europe. Front. Mar. Sci. 8:799936. doi: 10.3389/fmars.2021.799936
Álvarez, M., Fajar, N. M., Carter, B. R., Guallart, E. F., Pérez, F. F., Woosley, R. J., et al. (2020). Global Ocean Spectrophotometric pH Assessment: consistent Inconsistencies. Environ. Sci. Technol. 54, 10977–10988. doi: 10.1021/acs.est.9b06932
Archer, D. (2005). Fate of fossil fuel CO2 in geologic time. J. Geophys. Res. Oceans 110, 1–6. doi: 10.1029/2004JC002625
Arendt, K. E., Dutz, J., Jónasdóttir, S. H., Jung-Madsen, S., Mortensen, J., Møller, E. F., et al. (2011). Effects of suspended sediments on copepods feeding in a glacial influenced sub-Arctic fjord. J. Plankton Res. 33, 1526–1537. doi: 10.1093/plankt/fbr054
Bach, L. T. (2024). The additionality problem of ocean alkalinity enhancement. Biogeosciences 21, 261–277. doi: 10.5194/bg-21-261-2024
Bange, H. W., Hansen, H. P., Malien, F., Laß, K., Dale, A., Karstensen, J., et al. (2011). “Boknis Eck Time Series Station (SW Baltic Sea): measurements from 1957 to 2010,” in LOICZ Imprint, 16–12.
Bange, H. W., and Malien, F. (2015). Hydrochemistry from time series station Boknis Eck from 1957 to 2014. PANGEA.
Berge, T., Daugbjerg, N., Andersen, B. B., and Hansen, P. J. (2010). Effect of lowered pH on marine phytoplankton growth rates. Mar. Ecol. Prog. Ser. 416, 79–91. doi: 10.3354/meps08780
Bermúdez, R., Winder, M., Stuhr, A., Almén, A.-K., Engström-Öst, J., and Riebesell, U. (2016). Effect of ocean acidification on the structure and fatty acid composition of a natural plankton community in the Baltic Sea. Biogeosciences 13, 6625–6635. doi: 10.5194/bg-13-6625-2016
Bittig, H. C., Jacobs, E., Neumann, T., and Rehder, G. (2024). A regional pCO2 climatology of the Baltic Sea from in situ pCO2 observations and a model-based extrapolation approach. Earth Syst. Sci. Data 16, 753–773. doi: 10.5194/essd-16-753-2024
BMU (2021). Revised Climate Change Act sets out binding trajectory towards climate neutrality by 2045. Available at: https://www.bundesregierung.de/breg-de/schwerpunkte/klimaschutz/climate-change-act-2021-1936846 (accessed February 28, 2025).
Boesen, C., and Postma, D. (1988). Pyrite formation in anoxic environments of the Baltic. Am. J. Sci. 288, 575–603. doi: 10.2475/ajs.288.6.575
Brando, V. E., Sammartino, M., Colella, S., Bracaglia, M., Di Cicco, A., D'Alimonte, D., et al. (2021). Phytoplankton bloom dynamics in the Baltic Sea using a consistently reprocessed time series of multi-sensor reflectance and novel chlorophyll-a retrievals. Remote Sens. 13:3071. doi: 10.3390/rs13163071
Caldeira, K., and Wickett, M. E. (2003). Anthropogenic carbon and ocean pH. Nature 425, 365–365. doi: 10.1038/425365a
Carstensen, J., Conley, D. J., Bonsdorff, E., Gustafsson, B. G., Hietanen, S., Janas, U., et al. (2014). Hypoxia in the Baltic Sea: biogeochemical cycles, benthic fauna, and management. Ambio 43, 26–36. doi: 10.1007/s13280-013-0474-7
Carter, B. R., Sharp, J. D., Dickson, A. G., Álvarez, M., Fong, M. B., García-Ibá nez, M. I., et al. (2024). Uncertainty sources for measurable ocean carbonate chemistry variables. Limnol. Oceanogr. 69, 1–21. doi: 10.1002/lno.12477
Conley, D. J., Björck, S., Bonsdorff, E., Carstensen, J., Destouni, G., Gustafsson, B. G., et al. (2009). Hypoxia-related processes in the Baltic Sea. Environ. Sci. Technol. 43, 3412–3420. doi: 10.1021/es802762a
Conley, D. J., Humborg, C., Rahm, L., Savchuk, O. P., and Wulff, F. (2002). Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environ. Sci. Technol. 36, 5315–5320. doi: 10.1021/es025763w
Cripps, G., Widdicombe, S., Spicer, J. I., and Findlay, H. S. (2013). Biological impacts of enhanced alkalinity in Carcinus maenas. Mar. Pollut. Bull. 71, 190–198. doi: 10.1016/j.marpolbul.2013.03.015
Cyronak, T., Albright, R., and Bach, L. T. (2023). Field experiments in ocean alkalinity enhancement research. State Planet 2-oae2023:7. doi: 10.5194/sp-2-oae2023-7-2023
Davis, S. J., Lewis, N. S., Shaner, M., Aggarwal, S., Arent, D., Azevedo, I. L., et al. (2018). Net-zero emissions energy systems. Science 360:eaas9793. doi: 10.1126/science.aas9793
Dellwig, O., Wegwerth, A., and Arz, H. W. (2021). Anatomy of the Major Baltic Inflow in 2014: impact of manganese and iron shuttling on phosphorus and trace metals in the Gotland Basin, Baltic Sea. Cont. Shelf Res. 223:104449. doi: 10.1016/j.csr.2021.104449
Dickson A. G. Sabine C. L. Christian J. R. Bargeron C. P. Organization N. P. M. S. (2007). Guide to Best Practices for Ocean CO2 Measurements. Number no. 3 in PICES Special Publication. North Pacific Marine Science Organization, Sidney, BC.
Doney, S. C., Fabry, V. J., Feely, R. A., and Kleypas, J. A. (2009). Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192. doi: 10.1146/annurev.marine.010908.163834
Dupont, S., and Metian, M. (2023). General considerations for experimental research on ocean alkalinity enhancement. State Planet 2-oae2023:4. doi: 10.5194/sp-2-oae2023-4-2023
Eisaman, M. D., Geilert, S., Renforth, P., Bastianini, L., Campbell, J., Dale, A. W., et al. (2023). Assessing the technical aspects of ocean-alkalinity-enhancement approaches. State Planet 2-oae2023:3. doi: 10.5194/sp-2-oae2023-3-2023
European Commission (2019). Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions: The European Green Deal. COM(2019) 640 Final. The European Commission, Brussels.
Fakhraee, M., Li, Z., Planavsky, N. J., and Reinhard, C. T. (2023). A biogeochemical model of mineral-based ocean alkalinity enhancement: impacts on the biological pump and ocean carbon uptake. Environ. Res. Lett. 18:044047. doi: 10.1088/1748-9326/acc9d4
Feely, R. A., Sabine, C. L., Lee, K., Berelson, W., Kleypas, J., Fabry, V. J., et al. (2004). Impact of anthropogenic CO2 on the CaCO3 system in the Oceans. Science 305, 362–366. doi: 10.1126/science.1097329
Feistel, R., Nausch, G., and Wasmund, N. (2008). State and Evolution of the Baltic Se, 1952–2005: A Detailed 50-Year Survey of Meteorology and Climate, Physics, Chemistry, Biology, and Marine Environment. Hoboken, NJ, USA: John Wiley &Sons, Inc. doi: 10.1002/9780470283134
Ferderer, A., Chase, Z., Kennedy, F., Schulz, K. G., and Bach, L. T. (2022). Assessing the influence of ocean alkalinity enhancement on a coastal phytoplankton community. Biogeosciences 19, 5375–5399. doi: 10.5194/bg-19-5375-2022
Ford, D. J., Shutler, J. D., Blanco-Sacristán, J., Corrigan, S., Bell, T. G., Yang, M., et al. (2024). Enhanced ocean CO2 uptake due to near-surface temperature gradients. Nat. Geosci. 17, 1135–1140. doi: 10.1038/s41561-024-01570-7
Fridahl, M., Schenuit, F., Lundberg, L., Möllersten, K., Böttcher, M., Rickels, W., et al. (2023). Novel carbon dioxide removals techniques must be integrated into the European Union's climate policies. Commun. Earth Environ. 4, 1–5. doi: 10.1038/s43247-023-01121-9
Friedlingstein, P., O'Sullivan, M., Jones, M. W., Andrew, R. M., Hauck, J., Olsen, A., et al. (2020). Global carbon budget 2020. Earth Syst. Sci. Data 12, 3269–3340. doi: 10.5194/essd-12-3269-2020
Fuhr, M. (2024). Experimental assessment of enhanced benthic weathering of calcite and dunite in the south western Baltic Sea. PhD Thesis, Christian-Albrechts-Universität zu Kiel.
Fuhr, M., Geilert, S., Schmidt, M., Liebetrau, V., Vogt, C., Ledwig, B., et al. (2022). Kinetics of olivine weathering in seawater: an experimental study. Front. Clim. 4:831587. doi: 10.3389/fclim.2022.831587
Fuhr, M., Wallmann, K., Dale, A. W., Diercks, I., Kalapurakkal, H. T., Schmidt, M., et al. (2023). Disentangling artificial and natural benthic weathering in organic rich Baltic Sea sediments. Front. Clim. 5:1245580. doi: 10.3389/fclim.2023.1245580
Fuhr, M., Wallmann, K., Dale, A. W., Kalapurakkal, H. T., Schmidt, M., Sommer, S., et al. (2024). Alkaline mineral addition to anoxic to hypoxic Baltic Sea sediments as a potentially efficient CO2-removal technique. Front. Clim. 6:1338556. doi: 10.3389/fclim.2024.1338556
Gallego, M. A., Timmermann, A., Friedrich, T., and Zeebe, R. E. (2018). Drivers of future seasonal cycle changes in oceanic phpCO2. Biogeosciences 15, 5315–5327. doi: 10.5194/bg-15-5315-2018
Geyer, B. (2014). High-resolution atmospheric reconstruction for Europe 1948–2012: coastDat2. Earth Syst. Sci. Data 6, 147–164. doi: 10.5194/essd-6-147-2014
Goldenberg, S. U., Riebesell, U., Brüggemann, D., Börner, G., Sswat, M., Folkvord, A., et al. (2024a). Early life stages of fish under ocean alkalinity enhancement in coastal plankton communities. Biogeosciences 21, 4521–4532. doi: 10.5194/bg-21-4521-2024
Goldenberg S. U. Riebesell U. Brüggemann D. Börner G. Sswat M. Folkvord A. (2024b) Viability of coastal fish larvae under ocean alkalinity enhancement: from organisms to communities. EGUsphere 2024, 1–14. 10.5194/egusphere-2024-286 .
Griffies, S. M. (2012). Elements of the Modular Ocean Model (MOM). Technical Report 7, NOAA/Geophysical Fluid Dynamics Laboratory, Princeton, USA.
Gustafsson, E., and Gustafsson, B. G. (2020). Future acidification of the Baltic Sea-A sensitivity study. J. Marine Syst. 211:103397. doi: 10.1016/j.jmarsys.2020.103397
Gustafsson, E., Hagens, M., Sun, X., Reed, D. C., Humborg, C., Slomp, C. P., et al. (2019). Sedimentary alkalinity generation and long-term alkalinity development in the Baltic Sea. Biogeosciences 16, 437–456. doi: 10.5194/bg-16-437-2019
Gustafsson, E., Wällstedt, T., Humborg, C., Mörth, C.-M., and Gustafsson, B. G. (2014). External total alkalinity loads versus internal generation: the influence of nonriverine alkalinity sources in the Baltic Sea: alkalinity in the Baltic Sea. Global Biogeochem. Cycles 28, 1358–1370. doi: 10.1002/2014GB004888
Hansson, L., Fabry, V., Gattuso, J., Riebesell, U., and European Commission: Directorate-General for Research Innovation (2010). Guide to Best Practices for Ocean Acidification Research and Data Reporting. Publications Office.
Hartmann, J., Suitner, N., Lim, C., Schneider, J., Marín-Samper, L., Arístegui, J., et al. (2023). Stability of alkalinity in ocean alkalinity enhancement (OAE) approaches-consequences for durability of CO2 storage. Biogeosciences 20, 781–802. doi: 10.5194/bg-20-781-2023
Harvey, L. D. D. (2008). Mitigating the atmospheric CO2 increase and ocean acidification by adding limestone powder to upwelling regions. J. Geophys. Res. 113:2007JC004373. doi: 10.1029/2007JC004373
Havenhand, J. N. (2012). How will ocean acidification affect baltic sea ecosystems? An assessment of plausible impacts on key functional groups. Ambio 41, 637–644. doi: 10.1007/s13280-012-0326-x
Hiscock, W. T., and Millero, F. J. (2006). Alkalinity of the anoxic waters in the Western Black Sea. Deep Sea Res. Part II 53, 1787–1801. doi: 10.1016/j.dsr2.2006.05.020
Ho, D. T., Bopp, L., Palter, J. B., Long, M. C., Boyd, P. W., Neukermans, G., et al. (2023). Monitoring, reporting, and verification for ocean alkalinity enhancement. State Planet 2-oae2023:12. doi: 10.5194/sp-2-oae2023-12-2023
Hofmann, G. E., Smith, J. E., Johnson, K. S., Send, U., Levin, L. A., Micheli, F., et al. (2011). High-frequency dynamics of Ocean pH: a multi-ecosystem comparison. PLoS ONE 6:e28983. doi: 10.1371/journal.pone.0028983
Höglander, H., Larsson, U., and Hajdu, S. (2004). Vertical distribution and settling of spring phytoplankton in the offshore NW Baltic Sea proper. Mar. Ecol. Prog. Ser. 283, 15–27. doi: 10.3354/meps283015
Holtermann, P. L., Burchard, H., Gräwe, U., Klingbeil, K., and Umlauf, L. (2014). Deep-water dynamics and boundary mixing in a nontidal stratified basin: a modeling study of the Baltic Sea. J. Geophys. Res. Oceans 119, 1465–1487. doi: 10.1002/2013JC009483
Iglesias-Rodríguez, M. D., Rickaby, R. E. M., Singh, A., and Gately, J. A. (2023). Laboratory experiments in ocean alkalinity enhancement research. State Planet 2-oae2023:5. doi: 10.5194/sp-2-oae2023-5-2023
Ilyina, T., Wolf-Gladrow, D., Munhoven, G., and Heinze, C. (2013). Assessing the potential of calcium-based artificial ocean alkalinization to mitigate rising atmospheric CO2 and ocean acidification. Geophys. Res. Lett. 40, 5909–5914. doi: 10.1002/2013GL057981
IPCC (2021). “Summary for policymakers,” in Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, eds. V. Masson-Delmotte, A. Zhai, S.L. Pirani, C. Connors, S. Péan, N. Berger, et al. (Cambridge: Cambridge University Press), 3–32.
IPCC (2022). Global Warming of 1.5°C: IPCC Special Report on Impacts of Global Warming of 1.5°C above Pre-industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. Cambridge: Cambridge University Press, 1 edition. doi: 10.1017/9781009157940
Jacobs, E., Bittig, H. C., Gräwe, U., Graves, C. A., Glockzin, M., Müller, J. D., et al. (2021). Upwelling-induced trace gas dynamics in the Baltic Sea inferred from 8 years of autonomous measurements on a ship of opportunity. Biogeosciences 18, 2679–2709. doi: 10.5194/bg-18-2679-2021
Kankaanpää, H. T., Alenius, P., Kotilainen, P., and Roiha, P. (2023). Decreased surface and bottom salinity and elevated bottom temperature in the Northern Baltic Sea over the past six decades. Sci. Total Environ. 859:160241. doi: 10.1016/j.scitotenv.2022.160241
Keller, D. P. (2014). Potential climate engineering effectiveness and side effects during a high carbon dioxide-emission scenario. Nat. Commun. 5:11. doi: 10.1038/ncomms4304
Kheshgi, H. S. (1995). Sequestering atmospheric carbon dioxide by increasing ocean alkalinity. Energy 20, 915–922. doi: 10.1016/0360-5442(95)00035-F
Kuliński, K., Rehder, G., Asmala, E., Bartosova, A., Carstensen, J., Gustafsson, B., et al. (2022). Biogeochemical functioning of the Baltic Sea. Earth Syst. Dyn. 13, 633–685. doi: 10.5194/esd-13-633-2022
Landschutzer, P., Gruber, N., Haumann, F. A., Rödenbeck, C., Bakker, D. C. E., Heuven, S. V., et al. (2015). The reinvigoration of the Southern Ocean carbon sink. Science 349, 1221–1224. doi: 10.1126/science.aab2620
Langer, G., Geisen, M., Baumann, K.-H., Kläs, J., Riebesell, U., Thoms, S., et al. (2006). Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem. Geophys. Geosyst. 7:1227. doi: 10.1029/2005GC001227
Large, W. G., McWilliams, J. C., and Doney, S. C. (1994). Oceanic vertical mixing: a review and a model with a nonlocal boundary layer parameterization. Rev. Geophys. 32:363. doi: 10.1029/94RG01872
Lehmann, A., Myrberg, K., and Höflich, K. (2012). A statistical approach to coastal upwelling in the Baltic Sea based on the analysis of satellite data for 1990–2009. Oceanologia 54, 369–393. doi: 10.5697/oc.54-3.369
Leibniz Institute for Baltic Sea Research (2015). ERGOM: Ecological ReGional Ocean Model [code]. Available at: https://ergom.net/ (accessed April 5, 2022).
Lennartz, S. T., Lehmann, A., Herrford, J., Malien, F., Hansen, H.-P., Biester, H., et al. (2014). Long-term trends at the Boknis Eck time series station (Baltic Sea), 1957–2013: does climate change counteract the decline in eutrophication? Biogeosciences 11, 6323–6339. doi: 10.5194/bg-11-6323-2014
Lewis, E. R., and Wallace, D. W. R. (1998). Program developed for CO2 system calculations. Technical Report cdiac:CDIAC-105, Environmental System Science Data Infrastructure for a Virtual Ecosystem (ESS-DIVE) (United States). doi: 10.2172/639712
Mallin, M. A., and Paerl, H. W. (1994). Planktonic trophic transfer in an estuary: seasonal, diel, and community structure effects. Ecology 75, 2168–2184. doi: 10.2307/1940875
Maselli, M., Meire, L., Meire, P., and Hansen, P. J. (2023). Effects of glacial flour on marine micro-plankton: evidences from natural communities of greenlandic fjords and experimental studies. Protist 174:125928. doi: 10.1016/j.protis.2022.125928
Matthäus, W., Nehring, D., and Nausch, G. (1994). “Effects of the inflows of salt-rich water during 1993 and the early 1994 in the central Baltic Sea,” in ICES CM, 1–17.
Meier, H. E. M., Eilola, K., Almroth-Rosell, E., Schimanke, S., Kniebusch, M., Höglund, A., et al. (2019). Disentangling the impact of nutrient load and climate changes on Baltic Sea hypoxia and eutrophication since 1850. Clim. Dyn. 53, 1145–1166. doi: 10.1007/s00382-018-4296-y
Meier, H. E. M., Hordoir, R., Andersson, H. C., Dieterich, C., Eilola, K., Gustafsson, B. G., et al. (2012). Modeling the combined impact of changing climate and changing nutrient loads on the Baltic Sea environment in an ensemble of transient simulations for 1961–2099. Clim. Dyn. 39, 2421–2441. doi: 10.1007/s00382-012-1339-7
Melzner, F., Thomsen, J., Koeve, W., Oschlies, A., Gutowska, M. A., Bange, H. W., et al. (2013). Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 160, 1875–1888. doi: 10.1007/s00227-012-1954-1
Mengis, N., Kalhori, A., Simon, S., Harpprecht, C., Baetcke, L., Prats-Salvado, E., et al. (2022). Net-Zero CO2 Germany–A Retrospect From the Year 2050. Earth's Fut. 10:2324. doi: 10.1029/2021EF002324
Meysman, F. J. R., and Montserrat, F. (2017). Negative CO2 emissions via enhanced silicate weathering in coastal environments. Biol. Lett. 13:20160905. doi: 10.1098/rsbl.2016.0905
Mohrholz, V. (2018). Major baltic inflow statistics-revised. Front. Mar. Sci. 5:384. doi: 10.3389/fmars.2018.00384
Mohrholz, V., Naumann, M., Nausch, G., Krüger, S., and Gräwe, U. (2015). Fresh oxygen for the Baltic Sea – an exceptional saline inflow after a decade of stagnation. J. Mar. Syst. 148, 152–166. doi: 10.1016/j.jmarsys.2015.03.005
Montserrat, F., Renforth, P., Hartmann, J., Leermakers, M., Knops, P., and Meysman, F. J. R. (2017). Olivine dissolution in seawater: implications for CO2 sequestration through enhanced weathering in coastal environments. Environ. Sci. Technol. 51, 3960–3972. doi: 10.1021/acs.est.6b05942
Morán, X. A. G., López-Urrutia, Á., Calvo-Díaz, A., and Li, W. K. W. (2010). Increasing importance of small phytoplankton in a warmer ocean. Glob. Chang. Biol. 16, 1137–1144. doi: 10.1111/j.1365-2486.2009.01960.x
Mucci, A. (1983). The solubility of calcite and aragonite in seawater at various salinities, temperatures, and one atmosphere total pressure. Am. J. Sci. 283, 780–799. doi: 10.2475/ajs.283.7.780
Müller, J. D., Schneider, B., and Rehder, G. (2016). Long-term alkalinity trends in the Baltic Sea and their implications for CO2 -induced acidification: Baltic Sea alkalinity trends. Limnol. Oceanogr. 61, 1984–2002. doi: 10.1002/lno.10349
Myrberg, K., and Andrejev, O. (2003). Main upwelling regions in the Baltic Sea – a statistical analysis based on three-dimensional modelling. Boreal Env. Res. 8, 97–112.
Neumann, T., Koponen, S., Attila, J., Brockmann, C., Kallio, K., Kervinen, M., et al. (2021). Optical model for the Baltic Sea with an explicit CDOM state variable: a case study with Model ERGOM (version 1.2). Geosci. Model Dev. 14, 5049–5062. doi: 10.5194/gmd-14-5049-2021
Neumann, T., Radtke, H., Cahill, B., Schmidt, M., and Rehder, G. (2022). Non-Redfieldian carbon model for the Baltic Sea (ERGOM version 1.2)-implementation and budget estimates. Geosci. Model Dev. 15, 8473–8540. doi: 10.5194/gmd-15-8473-2022
Neumann, T., Siegel, H., Moros, M., Gerth, M., Kniebusch, M., and Heydebreck, D. (2020). Ventilation of the northern Baltic Sea. Ocean Sci. 16, 767–780. doi: 10.5194/os-16-767-2020
Nimer, N. A., Iglesias-Rodriguez, M. D., and Merrett, M. J. (1997). Bicarbonate utilization by marine phytoplankton species. J. Phycol. 33, 625–631. doi: 10.1111/j.0022-3646.1997.00625.x
Noffke, A., Sommer, S., Dale, A. W., Hall, P. O. J., and Pfannkuche, O. (2016). Benthic nutrient fluxes in the Eastern Gotland Basin (Baltic Sea) with particular focus on microbial mat ecosystems. J. Marine Syst. 158, 1–12. doi: 10.1016/j.jmarsys.2016.01.007
Olenin, S. (1997). Benthic zonation of the Eastern Gotland Basin, Baltic Sea. Netherlands J. Aquatic Ecol. 30, 265–282. doi: 10.1007/BF02085871
Orsi, T. H., Werner, F., Milkert, D., Anderson, A. L., and Bryant, W. R. (1996). Environmental overview of Eckernförde Bay, northern Germany. Geo-Marine Lett. 16, 140–147. doi: 10.1007/BF01204501
Oschlies, A., Stevenson, A., Bach, L. T., Fennel, K., Rickaby, R. E. M., Satterfield, T., et al. (2023). Guide to Best Practices in Ocean Alkalinity Enhancement Research. Technical report, Copernicus GmbH. doi: 10.5194/sp-2-oae2023
Österblom, H., Hansson, S., Larsson, U., Hjerne, O., Wulff, F., Elmgren, R., et al. (2007). Human-induced trophic cascades and ecological regime shifts in the Baltic Sea. Ecosystems 10, 877–889. doi: 10.1007/s10021-007-9069-0
Paul, A. J., and Bach, L. T. (2020). Universal response pattern of phytoplankton growth rates to increasing CO2. New Phytol. 228, 1710–1716. doi: 10.1111/nph.16806
Peter, K. H., and Sommer, U. (2013). Phytoplankton Cell Size Reduction in Response to Warming Mediated by Nutrient Limitation. PLoS ONE 8:e71528. doi: 10.1371/journal.pone.0071528
Ramírez, L., Pozzo-Pirotta, L. J., Trebec, A., Manzanares-Vázquez, V., Díez, J. L., Arístegui, J., et al. (2024). “Ocean Alkalinity Enhancement (OAE) does not cause cellular stress in a phytoplankton community of the sub-tropical Atlantic Ocean,” in EGUsphere, 1–34. doi: 10.5194/egusphere-2024-847-supplement
Rassmann, J., Eitel, E. M., Lansard, B., Cathalot, C., Brandily, C., Taillefert, M., et al. (2020). Benthic alkalinity and dissolved inorganic carbon fluxes in the Rhône River prodelta generated by decoupled aerobic and anaerobic processes. Biogeosciences 17, 13–33. doi: 10.5194/bg-17-13-2020
Rau, G. H., Carroll, S. A., Bourcier, W. L., Singleton, M. J., Smith, M. M., and Aines, R. D. (2013). Direct electrolytic dissolution of silicate minerals for air CO2 mitigation and carbon-negative H2 production. Proc. Nat. Acad. Sci. 110, 10095–10100. doi: 10.1073/pnas.1222358110
Renforth, P., and Henderson, G. (2017). Assessing ocean alkalinity for carbon sequestration: Ocean Alkalinity for C Sequestration. Rev. Geophys. 55, 636–674. doi: 10.1002/2016RG000533
Riebesell, U., Basso, D., Geilert, S., Dale, A. W., and Kreuzburg, M. (2023). Mesocosm experiments in ocean alkalinity enhancement research. State Planet 6, 1–14. doi: 10.5194/sp-2-oae2023-6-2023
Riebesell, U., Wolf-Gladrow, D. A., and Smetacek, V. (1993). Carbon dioxide limitation of marine phytoplankton growth rates. Nature 361, 249–251. doi: 10.1038/361249a0
Riley, J. P., and Tongudai, M. (1967). The major cation/chlorinity ratios in sea water. Chem. Geol. 2, 263–269. doi: 10.1016/0009-2541(67)90026-5
Rogelj, J., den Elzen, M., Höhne, N., Fransen, T., Fekete, H., Winkler, H., et al. (2016). Paris Agreement climate proposals need a boost to keep warming well below 2°C. Nature 534, 631–639. doi: 10.1038/nature18307
Sanders, T., Thomsen, J., Müller, J. D., Rehder, G., and Melzner, F. (2021). Decoupling salinity and carbonate chemistry: low calcium ion concentration rather than salinity limits calcification in Baltic Sea mussels. Biogeosciences 18, 2573–2590. doi: 10.5194/bg-18-2573-2021
Schernewski, G., Radtke, H., Hauk, R., Baresel, C., Olshammar, M., and Oberbeckmann, S. (2021). Urban microplastics emissions: effectiveness of retention measures and consequences for the Baltic Sea. Front. Mar. Sci. 8:594415. doi: 10.3389/fmars.2021.594415
Schippers, P., Lürling, M., and Scheffer, M. (2004). Increase of atmospheric CO2 promotes phytoplankton productivity. Ecol. Lett. 7, 446–451. doi: 10.1111/j.1461-0248.2004.00597.x
Sommaruga, R. (2015). When glaciers and ice sheets melt: consequences for planktonic organisms. J. Plankton Res. 37, 509–518. doi: 10.1093/plankt/fbv027
Subhas, A. V., Marx, L., Reynolds, S., Flohr, A., Mawji, E. W., Brown, P. J., et al. (2022). Microbial ecosystem responses to alkalinity enhancement in the North Atlantic Subtropical Gyre. Front. Clim. 4:784997. doi: 10.3389/fclim.2022.784997
Thomsen, J., Gutowska, M. A., Saphörster, J., Heinemann, A., Trübenbach, K., Fietzke, J., et al. (2010). Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences 7, 3879–3891. doi: 10.5194/bg-7-3879-2010
Traboni, C., Calbet, A., and Saiz, E. (2021). Mixotrophy upgrades food quality for marine calanoid copepods. Limnol. Oceanogr. 66, 4125–4139. doi: 10.1002/lno.11948
Tyrrell, T., Schneider, B., Charalampopoulou, A., and Riebesell, U. (2008). Coccolithophores and calcite saturation state in the Baltic and Black Seas. Biogeosciences 5, 485–494. doi: 10.5194/bg-5-485-2008
van Heuven, S., Pierrot, D., Rae, J., Lewis, E., and Wallace, D. (2011). CO2SYS v 1.1, MATLAB program developed for CO2 system calculations. ORNL/CDIAC-105b. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. DoE, Oak Ridge, TN. doi: 10.3334/CDIAC/otg.CO2SYS_MATLAB_v1.1
Wåhlström, I., Höglund, A., Almroth-Rosell, E., MacKenzie, B. R., Gröger, M., Eilola, K., et al. (2020). Combined climate change and nutrient load impacts on future habitats and eutrophication indicators in a eutrophic coastal sea. Limnol. Oceanogr. 65, 2170–2187. doi: 10.1002/lno.11446
Wallmann, K., Diesing, M., Scholz, F., Rehder, G., Dale, A. W., Fuhr, M., et al. (2022). Erosion of carbonate-bearing sedimentary rocks may close the alkalinity budget of the Baltic Sea and support atmospheric CO2 uptake in coastal seas. Front. Mar. Sci. 9:968069. doi: 10.3389/fmars.2022.968069
Wallmann, K., Geilert, S., and Scholz, F. (2023). Chemical alteration of riverine particles in seawater and marine sediments: effects on seawater composition and atmospheric CO2. Am. J. Sci. 323:87455. doi: 10.2475/001c.87455
Wassmann, P. (1997). Retention versus export food chains: processes controlling sinking loss from marine pelagic systems. Hydrobiologia 363, 29–57. doi: 10.1023/A:1003113403096
Watson, A. J., Schuster, U., Shutler, J. D., Holding, T., Ashton, I. G. C., Landschützer, P., et al. (2020). Revised estimates of ocean-atmosphere CO2 flux are consistent with ocean carbon inventory. Nat. Commun. 11:4422. doi: 10.1038/s41467-020-18203-3
Wootton, E. C., Zubkov, M. V., Jones, D. H., Jones, R. H., Martel, C. M., Thornton, C. A., et al. (2007). Biochemical prey recognition by planktonic protozoa. Environ. Microbiol. 9, 216–222. doi: 10.1111/j.1462-2920.2006.01130.x
Keywords: OAE, calcite dissolution, Baltic Sea, potential, model, hypoxia and anoxia, release locations
Citation: Anschütz A-A, Lencina-Avila JM, Rehder G, Cahill B, Neumann T and Radtke H (2025) Direct effects of ocean alkalinity enhancement in the Baltic Sea–results from in-silico experiments. Front. Clim. 7:1450468. doi: 10.3389/fclim.2025.1450468
Received: 17 June 2024; Accepted: 19 February 2025;
Published: 10 March 2025.
Edited by:
Phil Renforth, Heriot-Watt University, United KingdomReviewed by:
Mim Rahimi, University of Houston, United StatesWil Burns, American University, United States
Copyright © 2025 Anschütz, Lencina-Avila, Rehder, Cahill, Neumann and Radtke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna-Adriana Anschütz, YW5uYS5hbnNjaHVldHpAaW8td2FybmVtdWVuZGUuZGU=
 Anna-Adriana Anschütz
Anna-Adriana Anschütz Jannine M. Lencina-Avila
Jannine M. Lencina-Avila Gregor Rehder
Gregor Rehder Bronwyn Cahill
Bronwyn Cahill Thomas Neumann
Thomas Neumann Hagen Radtke
Hagen Radtke