- Institute for Culture and Society, Western Sydney University, Penrith, NSW, Australia
This article explores the impact that synthetic biology approaches may have on Negative Emissions Technologies (NETs). Synthetic biology has both altered and created biological pathways inspired by nature to develop new NETs that sequester greenhouse gases into industrially useful chemicals, such as biomass and calcium carbonate. However, synthetic biology continues to encounter difficulties when implementing and scaling up production due to a combination of hard limits (within biology) and ‘soft’ limits (of social and economic costs). Additionally, NETs, along with Ecosystem Technologies in general, operate as climate technofixes, wherein insufficient thought is given to the ethical quandaries arising from releasing designed organisms into the environment, even under controlled conditions. In this paper, we provide a technological and ethical appraisal of synthetic biology approaches to NETs, in the context of climate change mitigation through Ecosystem Technology.
Introduction
In 2009, 2 weeks before the 15th conference of the United Nations Framework Convention on Climate Change (UNFCCC), Secretary-General Ban Ki-moon declared to the World Climate Conference that “our foot is stuck on the accelerator and we are heading towards an abyss” (Ban, 2009). 13 years later, at the opening of the 27th UNFCCC conference, Secretary-General António Guterres reprised this analogy, declaring to the audience that “we are on a highway to climate hell with our foot still on the accelerator” (Guterres, 2022). Evidently, it ceased to be sufficient, long ago, to merely take one’s foot off the accelerator as that merely slows down the movement towards an abyss. Namely, what is required is to actually put the brake on.
The question then posed by this article then, is: what does putting the brake on entail for synthetic biology? We argue that using synthetic biology to substitute fossil fuels for biofuels amounts to merely taking our foot off the accelerator. Whereas, putting the brake on requires sequestering existing carbon dioxide (CO2) pollution from the atmosphere and hydrosphere, through Negative Emissions Technologies (NETs). In turn, this article offers a technological and ethical appraisal of synthetic biology approaches to NETs, in the context of climate change mitigation through Ecosystem Technology.
We first provide an overview of central issues in exploiting natural systems and devising systems that are new-to-nature through synthetic biology. We then address the potential viabilities of NETs, to outline the hard limits posed by the immediate constraints of any biological system, such as scaling up production, before concluding with a discussion of so-called ‘soft’ limits, being the social and ethical issues raised by such radical and risky technoscientific interventions in the climate crisis.
Synthetic biology is a multi-disciplinary field of research for engineering biology, which regards complex interconnected biological systems as predictable abstracted engineerable parts that operate within systems similar to logic gates and electrical systems (Kitney and Freemont, 2012). Synthetic biology applies novel technologies such as DNA synthesis, whole cell analysis techniques (genomics, proteomic, metabolomics), genetic assembly and editing tools (CRISPR, Gibson Assembly, Golden Gate Assembly), standardized parts, predictability, and new Public-Private partnership models inspired by the Information Technology Boom (Kitney and Freemont, 2012; Liu et al., 2013; Agapakis, 2014; Carbonell et al., 2016; Si and Zhao, 2016; Lv et al., 2022).
Synthetic biology has only recently been recognized as a potential climate change technoscientific intervention with the IPCC and the US government beginning policy research towards synthetic biology approaches (Symons et al., 2024). Most climate mitigation research in the field is largely premised on replacing broad petrochemical-based industries with biological-based industries, though this amounts to taking one’s foot off the accelerator, rather than putting on the brakes, to reprise Ban and Guterres’ analogy.
In contrast, this article addresses a growing section of the field, which is researching the functional equivalent to ‘putting on the brakes’ as it addresses direct carbon sequestration. Nevertheless, both endeavors utilize the same advantage of using biology as a replacement industry, which is that natural evolutionary processes can, under the right conditions, allow beneficial mutations to accumulate in a host organism to fix bottlenecks in a particular engineered process which cannot be rationally designed for under the Design-Build-Test-Learn cycle.
Capturing carbon using biology
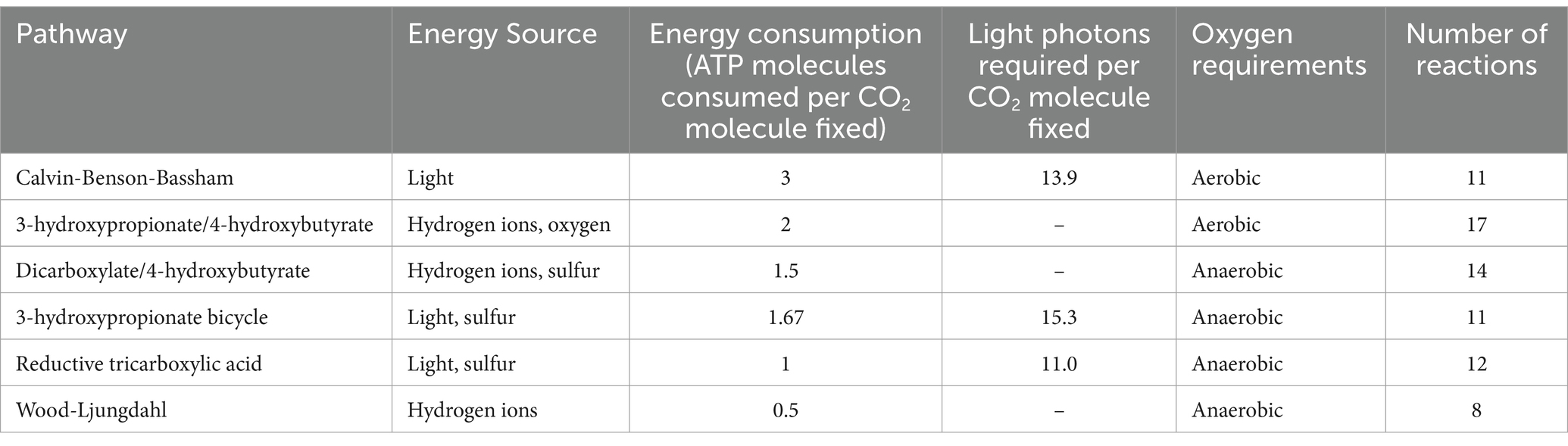
Some microorganisms naturally capture CO2 as part of their lifecycle, primarily through photosynthesis. Within bacteria, complex Carbon Capture (CC) pathways have been described which utilize either CO2 or bicarbonate ions as a carbon source to create more complex molecules which are crucial for the cell lifecycle. There are six canonical pathways known but additional biological mechanisms have been described and continue to be discovered (Buchanan and Arnon, 1990; Hügler and Fuchs, 2005; Hügler et al., 2005; Huber et al., 2008; Zarzycki et al., 2009; Poehlein et al., 2012; Biel and Fomina, 2015; Gong et al., 2016; Loder et al., 2016; Santos Correa et al., 2023). These canonical pathways are summarized in Table 1. While all these processes are superficially similar, they take different pathways and chemical intermediaries to get to their end product. Comparing CC pathways to one another is not straightforward due to the diverse chemical intermediaries and energy requirements, as seen in Table 1.
Within a cell, the metabolic flux—the turnover of molecules—is a zero-sum game (Solomon and Prather, 2011). Cellular energy is finite and intensive biological processes redirect energy away from essential cell growth pathways, creating a hard biological limit to productive capacity. While these pathways exist and are abundant in nature, transferring and optimizing them for a more familiar bacterial host is essential as most research and industrial systems are optimized for a handful of species, such as Escherichia coli. However, E. coli is not optimized to use CO2 as a primary ingredient in its diet.
To overcome this constraint, recent work modified E. coli to directly utilize CO2 as a novel method towards CC. Initial attempts at CO2 utilization in E. coli involved giving them CC enzymes from other species and allowing the cells to evolve in the presence of CO2, by linking CO2 to the cell’s survival and growth (Antonovsky et al., 2016). These E. coli were able to synthesize various sugar compounds from CO2 as their source. However, the CO2 used was only a small part of the cell’s larger carbon diet.
More expansive work later modified E. coli to add new proteins within the CC pathways while also removing native proteins utilizing alternative carbon sources, forcing E. coli to subsist on CO2 (Gleizer et al., 2019; Nissan et al., 2024). While successfully utilizing CO2 for growth, the resulting bacteria was incredibly unhealthy; E. coli populations normally double in 30 min, whereas this strain took 18 h.
Synthetic biology also creates unnatural metabolic pathways. These are pathways where individual enzymes are chosen from databases for their unique activities, combined, and then go on to create chemicals and pathways not seen in nature (Lin et al., 2019). In the two key unnatural pathways designed for CC metabolism, multiple enzymes with defined functions were taken, optimized, and combined to transform CO2 into intermediaries, such as propionyl-CoA, relevant for cell growth through unique and more efficient pathways (Schwander et al., 2016; McLean et al., 2023).
Unlike research using natural CC pathways, these are computer designed pathways which can overcome production bottlenecks through unique enzyme choices divorced from biological constraints, i.e., not being limited to the same genus or kingdom (Bernhardsgrütter et al., 2021). This dramatically increases the rate of CO2 sequestration. For example, the CETCH cycle fixes 1.5–5 times more CO2 while requiring 20% less energy inputs than the globally dominant Calvin-Benson-Bassham cycle responsible for photosynthesis (Osmanoglu et al., 2021; Diehl et al., 2023).
While most CC metabolic engineering transfers these pathways into more industrially useful organisms, CETCH and HOPAC are examples of in vitro expression, where the purification of key enzymes and the chemical reactions themselves occur within a separate non-biological receptacle. In vitro expression has key advantages over in vivo (biological) expression, such as separating cell growth from designed functions, as engineered enzymes can have toxic effects on cell survival.
However, in vitro expression removes the key advantage of biological systems: adaptive evolution, which can help optimize a designed metabolic pathway by linking pathway function to cell survival. Unlocking these unnatural pathways has been made possible by the vast enzymatic databases that are elemental to synthetic biology design processes.
Hard limits: the challenges of scale in biology
While both natural and unnatural metabolic pathways have shown new ways of removing CO2 from the environment, all biotechnologies contend with the hard limit of our ability to grow cells. That is: to generate biomass. This hard limit presents the major challenge for mitigating climate change through synthetic biology.
To be efficacious, any biotechnology requires the large-scale production of pure biomass to have enough material for the intended purposes. In the case of synthetic biology approaches to NETs, it is not only titer (product per volume) that is crucial, but also the rate and yield of the process, as that defines the economic cost and usefulness of any microbial cell factory (Konzock and Nielsen, 2024). Any synthetic biology innovations first occur in laboratory scale liquid media volumes of <1 L. However, at this scale the metabolic dynamics of growing cells are extremely different from those at 2–2,000 L, with, for instance, E. coli growing up to 20% less biomass at a higher culture volume compared to a lab-scale system (Hewitt and Nienow, 2007).
There are many challenges in scaling up cell production, especially on a commercially viable level. The first major shift is transitioning from lab-scale volumes to bioreactors, which are crucial for growing the required biomass. As a result, the cost scale increase is disproportionate to the corresponding volume scale-up (Mahdinia et al., 2019). Laboratory experiments use higher quality chemicals and substrates, but these are cost prohibitive for large scale cultivations, requiring lower purity industrial grade materials further altering the cellular growth dynamics (Cardoso et al., 2020).
To optimize biomass manufacturing, any scale-up must go through a series of established steps. These steps require small bioreactors (2–20 L), a pilot scale (50–10,000 L), and a plant scale (>10,000 L) as the kinetics and performance of cells are different at each volume and the result of each step informs the following step (Mahdinia et al., 2019). While these industrial approaches are still immature, driving biomass production purely through CO2 utilization could provide a useful carbon sink mechanism which would interact well with existing Biomass Carbon Removal and Storage (BiCRS) protocols, especially with the growing research into bacterial hydrogen production processes (King et al., 2022; Rosa and Mazzotti, 2022).
When growing cells long term, the evolutionary adaptation process key to CC pathway design optimization, can be a risk to engineered functions. Since non-native processes interrupt the streamlined growth of an organism by diverting metabolic flux, DNA mutations which remove engineering interventions provide the mutated organism with a growth advantage, such that they will eventually dominate the population (Sleight et al., 2011; Nikolados et al., 2019). As a result, any engineered microbes are turned back to a more wild-type version.
Many unnatural metabolic pathways, like CETCH and HOPAC, are designed around cell-free protein reactions. Such reactions can be fed proteins through two main methods, direct protein purification or secretion from cell-free protein synthesis, with both requiring large biomass sources (Gregorio et al., 2019; Garenne et al., 2021). While cell-free protein synthesis has some key advantages over purification as protein production is separated from cell survival, cell-free production yields are relatively low, production values are hard to predict, and industrial scale-up is still an immature technology (Borkowski et al., 2020; Garenne et al., 2021). Such challenges majorly undermine the efficacy of methods such as CETCH and HOPAC.
While most synthetic biology approaches to CC are still at the laboratory stage, there are some extant bioindustrial approaches to mitigating climate change. The exemplary approach to date is by LanzaTech (USA/NZ), who use autotrophic bacteria to carry out gas fermentation from waste gases, in order to produce industrial relevant chemicals where CO2 is a carbon source via the native Wood-Ljundahl pathway (Liew et al., 2016). It should be noted that Lanzatech utilizes carbon instead of removing carbon from polluting industrial processes, however, they remain a useful touchstone for bacterial gas fermentation processes utilizing pollution. While companies such as LanzaTech are successful, two of the major synthetic biology companies—Amyris and Gingko Bioworks—recently either declared bankruptcy or conducted mass layoffs due to issues translating their products into profit growth (Babu, 2023; Reporter, 2024).
All of these outlined issues are all downstream from another monumental complexity to overcome, which is the physical removal of carbon dioxide from the atmosphere. Currently most bacterial processes requiring CO2 as a source involve enriching the growth conditions with concentrated CO2 due to the efficient uptake of CO2 into the cell and the relative inefficiency of carboxylase enzymes. This obviously would severely limit the application of synthetic biology NETs in environmental use. However, approaches to overcoming this issue have begun through a process known as Carbon Concentration Mechanisms (CCM) where transport enzymes concentrate CO2 near carbon utilization enzymes allowing bacteria to utilize atmospheric CO2 concentrations (Flamholz et al., 2020; Flamholz et al., 2022).
‘Soft’ limits: social and ethical issues in synthetic biology approaches to NETs
The commercial imperatives of such companies highlight just how entrenched normative responses to climate change mitigation are. According to such normative responses, technoscientific interventions in the climate crisis, such as NETs, must operate according to prevailing market conditions. Wherein, attempts to mitigate climate change are considered within the prevailing economic framework of cost-competitiveness, rather than the actual context in question: an existential challenge of incomparable imminence, urgency and consequence. To recognize the context as such would entail that the planet is a global commons (where humans hold no exceptional status over any other lifeform), wherein, ameliorating the negative effects of human industrial activity constitutes a ‘public good’, which should then no longer be assessed through a restrictive economic lens.
Social and ethical issues in synthetic biology approaches to NETs are further compounded by how NETs constitute climate technofixes (Fulvi and Wodak, 2024a,b). Namely—NETs seek to ameliorate climate change consequences, while, admittedly not seeking to address the causes (such as capitalism and consumerism). This is not a criticism of technofixes per se, which literally denote ‘technological fixes.’ Nor is it a criticism of climate technofixes, as it has now become physically impossible to avert runaway climate change without the implementation of NETs, at scale, and in time (Fulvi and Wodak, 2024a,b). Hence the analogy Ban and Guterres’ make to ineffable notions of ‘putting on the brake’, and the seemingly intractable difficulties that confront attempts to find, let alone activate this ‘brake.’
By applying this analogy to the case of synthetic biology, this article has outlined scientists’ attempts to circumvent the hard limits toward activating this ‘brake.’ However, it is important to note that there is a growing corpus that is outrightly dismissive of the ability of any NETs to be sufficiently efficacious, and deployed at scale, and in time. This is exemplified in a response from climatologist Kevin Anderson, a delegate at the 2015 UNFCCC conference in Paris.
Following the conference, Anderson penned a commentary in Nature, lamenting how the Paris Agreement “rests on the assumption that the world will successfully suck the carbon pollution it produces back from the atmosphere in the longer term” via NETs. While such “exotic Dr. Strangelove options were discussed only as last-ditch contingencies” until just a few years ago, now, he decried, “they are Plan A.” As a result, Anderson surmised that “the world has just gambled its future on the appearance in a puff of smoke of a carbon-sucking fairy godmother” (Anderson, 2015).
Anderson’s observation surmises what normative responses to climate change mitigation have become. On the one hand, climate technofixes are no longer fringe (and admonished) ideas. Even the Intergovernmental Panel on Climate Change recently deemed NETs to be “unavoidable” in climate change mitigation (IPCC, 2014). On the other hand, critique from the humanities and social sciences, as well as scientists such as Anderson, inveigh against the hubris of such climate technofixes (Friedmann, 2019). After all, what is at stake is planetary scale climatic change through bioengineering the microbial world. While it is beyond the scope of this article to further outline the social and ethical issues in synthetic biology approaches to NETs, we note that a growing corpus of humanities and social sciences research is exploring these issues (Preston, 2018).
Discussion and conclusion
Arguably, one of the major constraints to mitigating climate change persists due to conflating hard and soft limits. For instance—the second law of thermodynamics is a hard limit, wherein any human attempts to modify, let alone circumvent, this law, will be impeded by the specific properties of applicable limits. In contrast, the social and ethical constraints to mitigating climate change are, in principal, soft limits, with their attendant temporal and spatial scales, which do not ‘map’ on to the time and spatial efficiencies of the corresponding ‘hard’ limits. For instance—the audiences that Ban and Guterres addressed in their respective United Nations conferences of 2009 and 2022 would have included numerous politicians, diplomats, policy makers, business executives and investors. In these forums, the soft limits of, for instance, social and economic policies, confront the hard limits of climate tipping points.
Wherein, constraints to reducing GHG emissions through policies such as carbon taxation are ‘soft’ limits. Namely: limits arise through phenomena such as the social license or political mandate to make GHG emissions so prohibitively expensive, as to facilitate the update of renewable energies. These limits are ‘soft’, in that their intrinsic limits are that which is deemed socially acceptable.
The efficacy and uptake of so-called climate technofixes, such as Ecosystem Technology and NETs, could be facilitated by disambiguating between soft and hard limits. Rather than treat such (regrettably) necessary climate technofixes as having to operate according to conventional economic frameworks, such as cost-competitiveness, such technologies could, and should, be regarded as a risky, uncertain and nevertheless essential subject of research and development. Currently, scientists using synthetic biology for NETs must not only contend with the applicable hard limits, but they are also tasked with inventing and implementing means of removing CO2 according to normative soft limits. The latter limits only serve to compound the seemingly intractable limits of the former.
For instance, one of the first scientific articles to outline how synthetic biology could be used for climate change mitigation stated that a decade of fundamental scientific research would be required, to comprehensively know the efficacy of such mitigation (DeLisi, 2019). Given the seismic scientific breakthroughs that would be required to develop the commensurate efficacy, such as those we have outlined in this article, such research is undermined when it is compelled to function in an analogous manner to incomparable issues that face contemporary society.
While we argue against the largely unsubstantiated hype and promissory rhetoric that all too frequently dominates these fields of technoscience, the potential efficacy of synthetic biology approaches to NETs will continue to be undermined, if said approaches are considered in overwhelmingly market-driven frameworks. As the saying goes: there is no business to be done on a dead planet. The real ‘business’ then, amounts to exploring all remaining options, including morally abhorrent climate technofixes, without conflating hard limits with soft limits. The functional equivalent to ‘putting on the brakes’ will require interdisciplinary research, across both the natural sciences and the human sciences, amidst their respective hard and soft limits. Failure to do so merely hastens the hard limit of the “abyss” that “we are heading towards.”
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DL: Writing – original draft, Writing – review & editing. JW: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Australian Research Council Centre of Excellence in Synthetic Biology (CE200100029). The views expressed herein are those of the author and are not necessarily those of the Australian Government or the Australian Research Council.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agapakis, C. M. (2014). Designing synthetic biology. ACS Synth. Biol. 3, 121–128. doi: 10.1021/sb4001068
Anderson, K. (2015). Talks in the city of light generate more heat. Nature 528:437. doi: 10.1038/528437a
Antonovsky, N., Gleizer, S., Noor, E., Zohar, Y., Herz, E., Barenholz, U., et al. (2016). Sugar synthesis from CO2 in Escherichia coli. Cell 166, 115–125. doi: 10.1016/j.cell.2016.05.064
Ban, K. (2009). Press conference by secretary-general Ban Ki-moon at international conference Centre, Geneva. United Nations Press.
Bernhardsgrütter, I., Stoffel, G. M. M., Miller, T. E., and Erb, T. J. (2021). CO2-converting enzymes for sustainable biotechnology: from mechanisms to application. Curr. Opin. Biotechnol. 67, 80–87. doi: 10.1016/j.copbio.2021.01.003
Biel, K., and Fomina, I. (2015). Benson-Bassham-Calvin cycle contribution to the organic life on our planet. Photosynthetica 53, 161–167. doi: 10.1007/s11099-015-0112-7
Borkowski, O., Koch, M., Zettor, A., Pandi, A., Batista, A. C., Soudier, P., et al. (2020). Large scale active-learning-guided exploration for in vitro protein production optimization. Nat. Commun. 11:1872. doi: 10.1038/s41467-020-15798-5
Buchanan, B. B., and Arnon, D. I. (1990). A reverse KREBS cycle in photosynthesis: consensus at last. Photosynth. Res. 24, 47–53. doi: 10.1007/BF00032643
Carbonell, P., Gök, A., Shapira, P., and Faulon, J. L. (2016). Mapping the patent landscape of synthetic biology for fine chemical production pathways. Microb. Biotechnol. 9, 687–695. doi: 10.1111/1751-7915.12401
Cardoso, V. M., Campani, G., Santos, M. P., Silva, G. G., Pires, M. C., Gonçalves, V. M., et al. (2020). Cost analysis based on bioreactor cultivation conditions: production of a soluble recombinant protein using Escherichia coli BL21 (DE3). Biotechnol. Rep. (Amst.) 26:e00441. doi: 10.1016/j.btre.2020.e00441
DeLisi, C. (2019). The role of synthetic biology in climate change mitigation. Biol. Direct 14, 1–5.
Diehl, C., Gerlinger, P. D., Paczia, N., and Erb, T. J. (2023). Synthetic anaplerotic modules for the direct synthesis of complex molecules from CO2. Nat. Chem. Biol. 19, 168–175. doi: 10.1038/s41589-022-01179-0
Flamholz, A. I., Dugan, E., Blikstad, C., Gleizer, S., Ben-Nissan, R., Amram, S., et al. (2020). Functional reconstitution of a bacterial CO2 concentrating mechanism in Escherichia coli. eLife 9:e59882. doi: 10.7554/eLife.59882
Flamholz, A. I., Dugan, E., Panich, J., Desmarais, J. J., Oltrogge, L. M., Fischer, W. W., et al. (2022). Trajectories for the evolution of bacterial CO2-concentrating mechanisms. Proc. Natl. Acad. Sci. 119:e2210539119. doi: 10.1073/pnas.2210539119
Friedmann, S. J. (2019). Engineered CO2 removal, climate restoration, and humility. Front. Climate 1:3. doi: 10.3389/fclim.2019.00003
Fulvi, D., and Wodak, J. (2024a). Gambling on unknown unknowns: risk ethics for a climate change technofix. Anthropocene Rev. 11, 285–301. doi: 10.1177/20530196231204324
Fulvi, D., and Wodak, J. (2024b). Using synthetic biology to avert runaway climate change: a consequentialist appraisal. Ethics Policy Environ. 27, 89–107. doi: 10.1080/21550085.2023.2215147
Garenne, D., Haines, M. C., Romantseva, E. F., Freemont, P., Strychalski, E. A., and Noireaux, V. (2021). Cell-free gene expression. Nat. Rev. Methods Primers 1:49. doi: 10.1038/s43586-021-00046-x
Gleizer, S., Ben-Nissan, R., Bar-On, Y. M., Antonovsky, N., Noor, E., Zohar, Y., et al. (2019). Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell 179, 1255–1263.e12. doi: 10.1016/j.cell.2019.11.009
Gong, F., Cai, Z., and Li, Y. (2016). Synthetic biology for CO2 fixation. Sci. China Life Sci. 59, 1106–1114. doi: 10.1007/s11427-016-0304-2
Gregorio, N. E., Levine, M. Z., and Oza, J. P. (2019). A User's guide to cell-free protein synthesis. Methods Protoc 2:24. doi: 10.3390/mps2010024
Guterres, A. (2022). Secretary-General’s remarks to high-level opening of COP27 : United Nations. United Nations (un.org).
Hewitt, C. J., and Nienow, A. W. (2007). The scale-up of microbial batch and fed-batch fermentation processes. Adv. Appl. Microbiol. Acad. Press 62, 105–135. doi: 10.1016/S0065-2164(07)62005-X
Huber, H., Gallenberger, M., Jahn, U., Eylert, E., Berg, I. A., Kockelkorn, D., et al. (2008). A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis. Proc. Natl. Acad. Sci. USA 105, 7851–7856. doi: 10.1073/pnas.0801043105
Hügler, M., and Fuchs, G. (2005). Assaying for the 3-hydroxypropionate cycle of carbon fixation. Methods Enzymol. 397, 212–221. doi: 10.1016/S0076-6879(05)97012-2
Hügler, M., Wirsen, C. O., Fuchs, G., Taylor, C. D., and Sievert, S. M. (2005). Evidence for autotrophic CO2 fixation via the reductive tricarboxylic acid cycle by members of the ε subdivision of proteobacteria. J. Bacteriol. 187, 3020–3027. doi: 10.1128/JB.187.9.3020-3027.2005
IPCC (2014). "Mitigation of climate change." Contribution of working group III to the fifth assessment report of the intergovernmental panel on climate change.
King, S. J., Jerkovic, A., Brown, L. J., Petroll, K., and Willows, R. D. (2022). Synthetic biology for improved hydrogen production in Chlamydomonas reinhardtii. Microb. Biotechnol. 15, 1946–1965. doi: 10.1111/1751-7915.14024
Kitney, R., and Freemont, P. (2012). Synthetic biology – the state of play. FEBS Lett. 586, 2029–2036. doi: 10.1016/j.febslet.2012.06.002
Konzock, O., and Nielsen, J. (2024). Trying to evaluate production costs in microbial biotechnology. Trends Biotechnol. 42, 1339–1347. doi: 10.1016/j.tibtech.2024.04.007
Liew, F., Martin, M. E., Tappel, R. C., Heijstra, B. D., Mihalcea, C., and Köpke, M. (2016). Gas fermentation—a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Front. Microbiol. 7:694. doi: 10.3389/fmicb.2016.00694
Lin, G.-M., Warden-Rothman, R., and Voigt, C. A. (2019). Retrosynthetic design of metabolic pathways to chemicals not found in nature. Curr. Opin. Syst. Biol. 14, 82–107. doi: 10.1016/j.coisb.2019.04.004
Liu, D., Hoynes-O’Connor, A., and Zhang, F. (2013). Bridging the gap between systems biology and synthetic biology. Front. Microbiol. 4:211. doi: 10.3389/fmicb.2013.00211
Loder, A. J., Han, Y., Hawkins, A. B., Lian, H., Lipscomb, G. L., Schut, G. J., et al. (2016). Reaction kinetic analysis of the 3-hydroxypropionate/4-hydroxybutyrate CO2 fixation cycle in extremely thermoacidophilic archaea. Metab. Eng. 38, 446–463. doi: 10.1016/j.ymben.2016.10.009
Lv, X., Hueso-Gil, A., Bi, X., Wu, Y., Liu, Y., Liu, L., et al. (2022). New synthetic biology tools for metabolic control. Curr. Opin. Biotechnol. 76:102724. doi: 10.1016/j.copbio.2022.102724
Mahdinia, E., Cekmecelioglu, D., and Demirci, A. (2019). Essentials in fermentation technology. Berlin: Springer International Publishing.
McLean, R., Schwander, T., Diehl, C., Cortina, N. S., Paczia, N., Zarzycki, J., et al. (2023). "Exploring alternative pathways for the in vitro establishment of the HOPAC cycle for synthetic CO2 fixation." science. Advances 9:eadh4299.
Nikolados, E.-M., Weiße, A. Y., Ceroni, F., and Oyarzún, D. A. (2019). Growth defects and loss-of-function in synthetic gene circuits. ACS Synth. Biol. 8, 1231–1240. doi: 10.1021/acssynbio.8b00531
Nissan, R. B., Milshtein, E., Pahl, V., de Pins, B., Jona, G., Levi, D., et al. (2024). Autotrophic growth of Escherichia coli is achieved by a small number of genetic changes. eLife 12:RP88793. doi: 10.7554/eLife.88793
Osmanoglu, Ö., Khaled AlSeiari, M., AlKhoori, H. A., Shams, S., Bencurova, E., Dandekar, T., et al. (2021). Topological analysis of the carbon-concentrating CETCH cycle and a Photorespiratory bypass reveals boosted CO2-sequestration by plants. Front. Bioeng. Biotechnol. 9:708417. doi: 10.3389/fbioe.2021.708417
Poehlein, A., Schmidt, S., Kaster, A. K., Goenrich, M., Vollmers, J., Thurmer, A., et al. (2012). An ancient pathway combining carbon dioxide fixation with the generation and utilization of a sodium ion gradient for ATP synthesis. PLoS One 7:e33439. doi: 10.1371/journal.pone.0033439
Preston, C. J. (2018). The synthetic age: Outdesigning evolution, resurrecting species, and reengineering our world. London: The MIT Press.
Reporter, S.. (2024). Ginkgo bioworks to lay off 35 percent of workforce. Available at: https://www.genomeweb.com/business-news/ginkgo-bioworks-lay-35-percent-workforce
Rosa, L., and Mazzotti, M. (2022). Potential for hydrogen production from sustainable biomass with carbon capture and storage. Renew. Sust. Energ. Rev. 157:112123. doi: 10.1016/j.rser.2022.112123
Santos Correa, S., Schultz, J., Lauersen, K. J., and Soares Rosado, A. (2023). Natural carbon fixation and advances in synthetic engineering for redesigning and creating new fixation pathways. J. Adv. Res. 47, 75–92. doi: 10.1016/j.jare.2022.07.011
Schwander, T., Schada von Borzyskowski, L., Burgener, S., Cortina, N. S., and Erb, T. J. (2016). A synthetic pathway for the fixation of carbon dioxide in vitro. Science 354, 900–904. doi: 10.1126/science.aah5237
Si, T., and Zhao, H. (2016). A brief overview of synthetic biology research programs and roadmap studies in the United States. Synth. Syst. Biotechnol. 1, 258–264. doi: 10.1016/j.synbio.2016.08.003
Sleight, S. C., Bartley, B. A., and Sauro, H. M. (2011). Synthetic systems as microbial threats: predictability of loss-of-function mutations in engineered systems. The science and applications of Synthetic and systems biotechnology: Workshop Summary, National Academies Press (US).
Solomon, K. V., and Prather, K. L. (2011). The zero-sum game of pathway optimization: emerging paradigms for tuning gene expression. Biotechnol. J. 6, 1064–1070. doi: 10.1002/biot.201100086
Symons, J., Dixon, T. A., Dalziell, J., Curach, N., Paulsen, I. T., Wiskich, A., et al. (2024). Engineering biology and climate change mitigation: policy considerations. Nat. Commun. 15:2669. doi: 10.1038/s41467-024-46865-w
Keywords: synthetic biology, negative emissions technologies, carbon capture technologies, carbon fixation, environmental ethics
Citation: Logel DY and Wodak J (2025) Synthetic biology approaches to negative emissions technologies: a technological and ethical appraisal. Front. Clim. 6:1516823. doi: 10.3389/fclim.2024.1516823
Edited by:
Claudia Gunsch, Duke University, United StatesReviewed by:
Stephen McCord, University of Michigan, United StatesCopyright © 2025 Logel and Wodak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Josh Wodak, ai53b2Rha0B3ZXN0ZXJuc3lkbmV5LmVkdS5hdQ==
†ORCID: Dominic Y. Logel, orcid.org/0000-0001-7721-8321
 Dominic Y. Logel
Dominic Y. Logel Josh Wodak
Josh Wodak