- Trent School of the Environment, Trent University, Peterborough, ON, Canada
There is considerable uncertainty when quantifying carbon dioxide removal (CDR) from enhanced rock weathering (ERW). Faster CDR rates mean ERW may significantly impact climate change mitigation, and more carbon credits will financially benefit private companies. However, overestimating CDR risks undermining ERW if meaningless carbon credits are counted. Here, we aim to contribute to the discussion of CDR quantification by describing three potential pitfalls relating to the geochemical and mineralogical compositions of rock powders. First, rock powders used for ERW are often mineralogically complex and may initially exhibit fast dissolution rates due to reactive surfaces and phases, leading to overestimating long-term CDR rates. Second, the dissolution of accessory carbonates within ERW rock powders will tend to dominate cation and dissolved inorganic carbon fluxes, which, if not identified, can be misconstrued as silicate weathering and overestimate CDR. Third, methods that rely on measuring cations may be prone to misinterpretation as cations will often not be balanced with dissolved inorganic carbon, e.g., during strong acid weathering. As another example, mineral dissolution during solid-phase testing (e.g., cation exchange) is also unrelated to carbonic acid weathering and, thus, may overestimate CDR rates. To avoid these pitfalls, we recommend (1) incorporating high-dosage test plots into ERW trials that avoid reapplication of rock powders that replenish initially fast reactivity, (2) screening rock powders for carbonate minerals using sensitive techniques and distinguishing carbonate and silicate weathering, and (3) measuring carbon to verify carbon dioxide removal. High-quality carbon credits must be durable, additional, and not overestimated.
1 Introduction
Determining carbon dioxide removal (CDR) rates by enhanced rock weathering (ERW) is challenging due to slow silicate dissolution rates, storage of CO2 as either a mineral or soluble phase, and spatially and temporally variable background weathering in complex open systems (Sandalow et al., 2021; Clarkson et al., 2024). Faster CDR rates are desirable as ERW will more significantly impact climate change mitigation, and there will be more interest and development in ERW. There is also financial incentive, as faster rates mean more carbon credits for private ERW companies. However, overestimating CDR rates may undermine the credibility of ERW, as has happened for forest-based carbon credits (Badgley et al., 2022; The Guardian, 2023). The Carbon Direct buyer’s guide for ERW notes that significant accounting uncertainty may lead to a loss of trust amongst various parties (Kyker-Snowman et al., 2024). Furthermore, the guide highlights the potential for over-crediting due to uncertainty, particularly when many CDR quantification methods use indirect measurements rather than directly measuring carbon. Clarkson et al. (2024) review many of these quantification approaches, describing their advantages, disadvantages, and uncertainties. Furthermore, protocols are being established to provide requirements and procedures for quantifying CDR in ERW applications (Sutherland et al., 2024—Isometric). Yet, there remains no universally accepted method for quantifying CDR from ERW.
There are numerous sources of uncertainty when determining CDR rates in ERW applications. Abdalqadir et al. (2024) review many of the factors that affect ERW rates in agricultural soils, and Santos et al. (2023) discuss various pathways, roundabouts, roadblocks and shortcuts to deploying ERW more widely, including a discussion on verification methods. Calabrese et al. (2022) describe nano- to global-scale uncertainties and highlight the discrepancies between theoretical predictions and models, and lab and field observations in assessing geochemical reactions related to ERW. Furthermore, CarbonPlan and Frontier have developed tools for mapping key uncertainties for several CDR technologies, including ERW (CarbonPlan, n.d.). They correctly identify mineral weathering as a component with high uncertainty. Weathering rates are broadly controlled by the reacting fluid composition that is influenced by numerous factors (e.g., soil composition and biological processes), fluid-rock ratios affected by climate and soil moisture, and the rock powder characteristics (e.g., grain size, mineralogical composition); the latter being the focus of this Perspective.
Amongst considerable uncertainty and variable rates being reported, we raise the question: Are ERW rates being overestimated? Our aim is not to answer this question but to contribute to the discussion of CDR quantification and make recommendations to alleviate some of the uncertainty. In this Perspective, we briefly review CDR rates in basalt ERW applications for context and then discuss three potential pitfalls relating to the geochemical and mineralogical compositions of rock powders that can lead to overestimating CDR rates: (1) initially fast dissolution rates of reactive mineral surfaces and labile phases, (2) weathering of accessory carbonates, and (3) quantifying cations rather than carbon using solid-phase testing as an example. We acknowledge that these potential issues are not new but suggest they warrant greater consideration along with other sources of uncertainty.
In addition to examples from the literature, we use four potential ERW feedstocks to support our perspectives: (1) pulverized basalt from Mauna Loa, Hawaii, (2) wollastonite skarn amendment from Canadian Wollastonite, Canada, (3) olivine powder purchased from OCL Industrial Materials Ltd., and (4) kimberlite residues from the Gahcho Kué Diamond Mine, Canada. Characterization methods and data are provided in Supplementary material.
2 Some CDR rates for perspective
Here, we briefly review some CDR rates from ERW studies that used basalt, the leading rock type for ERW, given its mafic composition and global abundance (Figure 1). Buckingham et al. (2022) measured CDR rates of 0.01 t CO2/ha/yr using column experiments amended with basalt; however, their experiments were criticized by West et al. (2023) and later defended by Buckingham et al. (2023). Larkin et al. (2022) found that CO2 removal via alkalinity generation in amended soils was similar to untreated plots; however, they stated that one field plot had an increased removal rate of ∼0.4 t CO2/ha/yr Lewis et al. (2021) estimated CDR rates of 0.09–0.57 t CO2/ha/yr using 1-D reactive transport modelling. Reershemius et al. (2023) determined initial CDR rates of 1.44 ± 0.27 t CO2/ha over 235 days—equal to 2.24 t CO2/ha/yr—with column experiments (50 t basalt/ha) using mass-balance-based methods. Similarly, Beerling et al. (2024) determined time-integrated cumulative CDR potentials of 10.5 ± 3.8 t CO2/ha over 4 yr, an average of 2.6 t CO2/ha/yr, based on mass losses relative to an immobile tracer in field trials that received 50 t metabasalt powder/ha/yr for 4 yr Modelling by Jerden et al. (2024) using the data provided by Beerling et al. (2024) underestimated field rates by ~3 times, requiring either a 10× increase to the basalt rate constant or a reduction in average grain size from 267 to 27 μm to match the measured data. For the same field trial, Kantola et al. (2023) had estimated a CDR rate of 3.7 t CO2/ha/yr using rare earth elements; however, Reershemius and Suhrhoff (2024) wrote that their quantification method was flawed. Amann et al. (2022) measured rates of 2.2 and 4.4 t CO2/ha/yr in column experiments with pure basanite and basanite mixed with oxisol (1:1), respectively, exposed to tropical rainfall rates of 8,000 mm/yr. While these basalt studies vary in application rates, basalt compositions, conditions, and CDR quantification methods, the wide range of CDR rates warrants critical evaluation beyond the scope of this Perspective. For example, Kukla et al. (2024) analyzed a broader range of ERW studies that included numerous rock types and found that CDR rates ranged by four orders of magnitude, with some studies reporting zero removal. In their analysis, they discuss several factors that may explain this wide variability (e.g., climate and environmental conditions) but highlight that these studies (32 in total) ranged substantially in their analytical and operational approaches, including rock type and dosage, study type (lab experiment, field trial, modelling), and CDR quantification method.
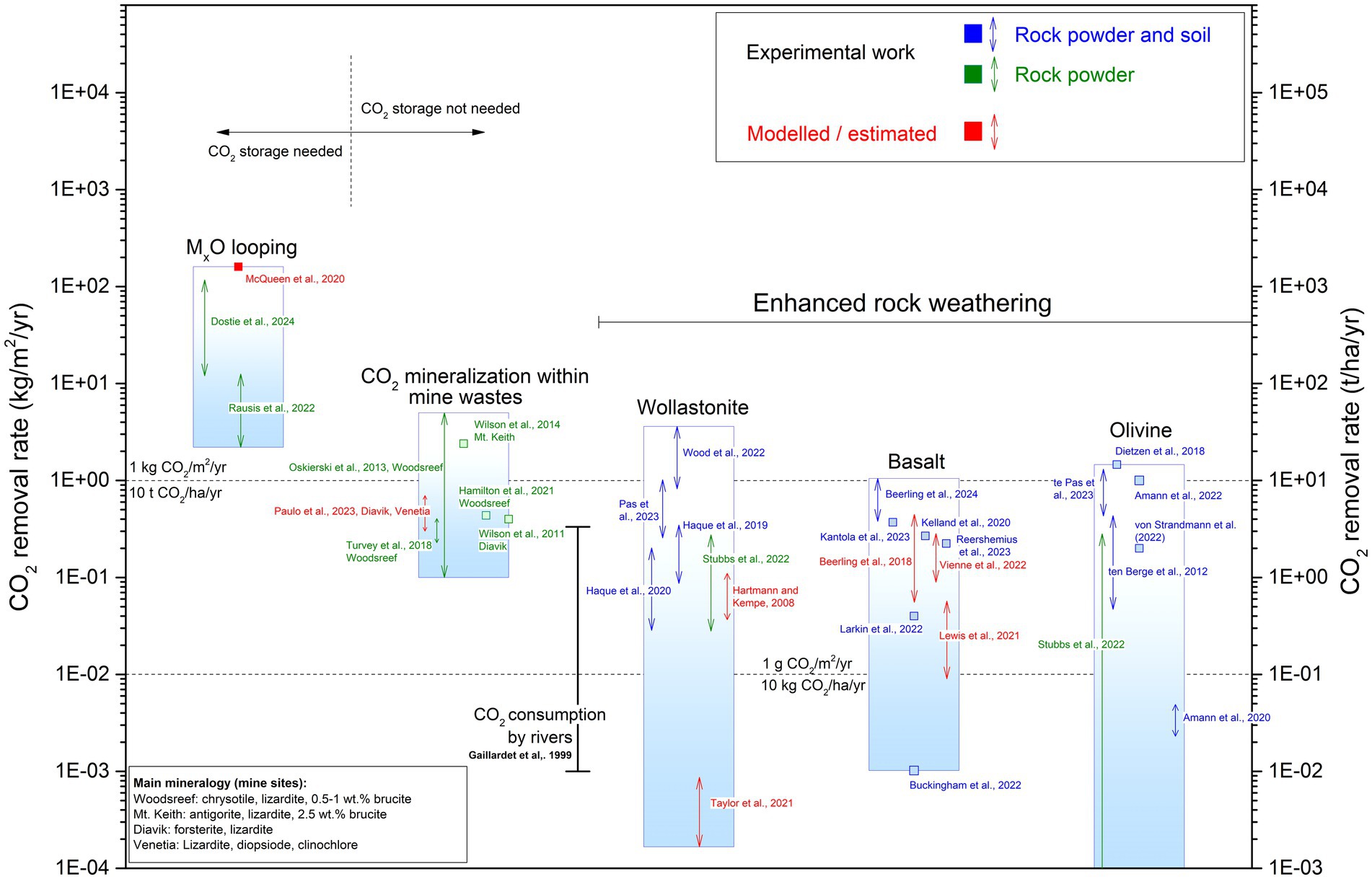
Figure 1. Comparison of CO2 removal rates for oxide mineral looping (McQueen et al., 2020; Rausis et al., 2022; Dostie et al., 2024), CO2 mineralization within mine wastes (Oskierski et al., 2013; Turvey et al., 2018; Hamilton et al., 2021), and enhanced rock weathering using wollastonite (Hartmann and Kempe, 2008; Haque et al., 2019; Taylor et al., 2021; Stubbs et al., 2022; te Pas et al., 2023; Wood et al., 2023), basalt (Beerling et al., 2018; Kelland et al., 2020; Vienne et al., 2022), and olivine (ten Berge et al., 2012; Amann et al., 2022; von Strandmann et al., 2022). Studies are grouped by (1) experimental work using rock powder and soil, (2) experimental work using rock powder, and (3) modelled or estimated rates. Studies not listed here are cited in the manuscript.
For comparison, CDR rates of CO2 mineralization and weathering of ultramafic mine wastes include (Figure 1): 3.7–4.2 t CO2/ha/yr for the Diavik Diamond Mine (Wilson et al., 2011), 3–7 t CO2/ha/yr for the Venetia Diamond Mine (Paulo et al., 2023), and 24 t CO2/ha/yr for the Mount Keith Nickel Mine (Wilson et al., 2014). For context, the mine wastes at Mount Keith were composed of >80 wt.% serpentine group minerals [Mg3Si2O5(OH)4] and ~ 2.5 wt.% brucite [Mg(OH)2], one of the most reactive minerals with CO2 (e.g., Harrison et al., 2015). While these rates range, they may be upper limits for CDR via weathering as these are for pure ultramafic rock powder, i.e., the highest possible dosage for an ERW application. Paulo et al. (2023) found that these rates were comparable to CO2 consumption rates in river basins dominated by either basalt or carbonate lithologies. For example, CO2 consumption rates estimated for basalt-dominated river basins include 0.16 t CO2/ha/yr for the Deccan Traps (Das et al., 2005), 0.16 t CO2/ha/yr for the Columbia Plateau, 0.29 t CO2/ha/yr for Hawaii (Dessert et al., 2003), and 0.57–1.94 t CO2/ha/yr for Iceland (Louvat and Allègre, 1997). Some ERW rates estimated for basalt applications are considerably faster than these natural basalt weathering rates. While the high surface area of rock powders enhances weathering, it seems unlikely that dispersing relatively minor quantities of rock powder on soils would result in CO2 consumption rates that are an order of magnitude faster than in basins that are almost entirely composed of basalt sediments and bedrock. For instance, Linke et al. (2024a) investigated South Iceland soils that have received fine-grained basaltic dust over 3,300 yr (5–8 t/ha/yr), a natural analogue for long-term ERW. The authors estimated a CDR rate of 0.62 t CO2/ha/yr due to alkalinity generation (Linke et al., 2024b), considerably slower than CDR rates estimated for some ERW studies that receive far less amendment.
Some of the variability in CDR rates likely relates the timeframe of the measurements (e.g., weeks vs. months vs. years), feedstock compositional variability even if rock types are the same (e.g., basalt), and the methodologies for carbon quantification (e.g., cation vs. carbon measurements). In the following sections, we discuss three geochemical and mineralogical pitfalls relating to these issues that can result in overestimating CDR rates.
3 Initially fast mineral dissolution rates
ERW rock powders are often mineralogically complex, meaning that many mineral dissolution rates—often varying by orders of magnitude—contribute to an overall CDR rate. Consequently, cation release from the weathering of rock powders greatly depends on their mineralogical compositions and will substantially decrease as reactive surfaces and phases are consumed. For example, in flow-through mineral dissolution experiments, Mg release from serpentine-rich (~94.9 wt.%) tailings containing brucite (0.6 wt.%) decreased substantially from ~60 to 2 mg/L after ~12 h of leaching (Power et al., 2020). The initial rapid release of Mg was attributed to the reaction of serpentine surfaces and the complete dissolution of brucite. Lu et al. (2022) conducted similar experiments on several rock powders and mine tailings. They also observed two stages of dissolution, which they describe as a fast, transient (or labile) stage and a slow, stoichiometric stage. The former stage was attributed to the initially fast release of cations from non-stoichiometric surface reactions of major minerals (e.g., serpentine) and highly reactive phases (e.g., brucite) if present. They found that serpentine minerals dissolved ~10× faster and olivine ~3× faster during this initial phase compared to published steady-state dissolution rates (Lu et al., 2022 and references therein).
The consumption of reactive surfaces and labile cations will substantially reduce CDR rates in ERW applications over time. The formation of amorphous silica layers will passivate silicate surfaces and control dissolution rates past any initially reactive period (Daval et al., 2011; Maher et al., 2016; Mergelsberg et al., 2023). Furthermore, the formation of secondary carbonates and silicate minerals, such as those that may occur during wollastonite weathering (e.g., Daval et al., 2009), may also hinder further dissolution. As a laboratory ERW example, Amann et al. (2020) measured CDR rates of 0.023–0.049 t CO2/ha/yr in mesocosm experiments with olivine powders amended to soils (220 t/ha; 800 mm rainfall/yr). They noted high Mg/Si ratios in waters attributed to preferential Mg leaching, leaving cation-depleted and Si-enriched grain surfaces. While Calabrese et al. (2022) highlight the discrepancies between lab and field mineral dissolution rates and note that the evolution of mineral surfaces in soils is mostly unknown, a relatively reactive initial period is likely after applying unweathered rock powders followed by slower weathering rates.
In addition to consuming reactive surfaces, weathering will eventually completely exhaust entire amendment minerals, which may significantly decrease overall weathering rates. In a previous example from Power et al. (2020), complete brucite dissolution resulted in Mg release decreasing drastically when the system shifted from one dominated by brucite dissolution to one dominated by lizardite dissolution; two minerals with dissolution rates differing by five orders of magnitude (Pokrovsky and Schott, 2004; Daval et al., 2013). As another example, wollastonite skarn is a reactive ERW feedstock containing major abundances of wollastonite and diopside (Supplementary Table S1; e.g., Haque et al., 2020a) that have far-from-equilibrium dissolution rates of approximately 10−9 and 10−11 mol/m2/s at pH 7, respectively (Heřmanská et al., 2022, 2023). Therefore, it would be expected that wollastonite weathering will control the CDR rate until it is consumed. Once reactive phases are removed in the soil, the more recalcitrant phases remain, and the CDR rate will significantly decline.
For reference, Heřmanská et al. (2022, 2023) provide comprehensive reviews of dissolution rates of primary and secondary silicate minerals, many of which are found in ERW rock powders. It is worth noting that single mineral dissolution rates determined under controlled laboratory conditions reported by different studies can vary by approximately an order of magnitude, highlighting the magnitude of variability that should be expected in ERW when using mineralogically complex feedstocks in heterogenous systems.
Compounding the issue of variable mineralogical compositions is variable grain size. ERW rock powders have grain sizes typically ranging from the micron to the millimetre scale (e.g., Lewis et al., 2021). For instance, Calabrese et al. (2022) estimate that 1,000–10,000 yr are required to completely dissolve a 400 μm grain of olivine, depending on the dissolution rate it experiences under field conditions. As another example, the wollastonite skarn material (Supplementary Table S1) had 56 wt.% of its particles ranging from 0.5–4 mm in diameter with a specific surface area of 0.30 m2/g compared to 1.24 m2/g for the material <0.5 mm (44 wt.%). The very finest portions of rock powders will dissolve more quickly (e.g., Harrison et al., 2015), leaving coarse material with lower surface areas that take considerably longer to dissolve.
CDR capacities are calculated based on the geochemical composition of the amendment, assuming that all minerals will completely weather given sufficient time. To be more conservative, calculating these capacities could consider the mineralogical composition of the rock powder. For example, the wollastonite skarn has a CDR capacity of 409–464 kg CO2/t using the Steinour equation (Supplementary Table S1; Steinour, 1959; Renforth, 2012, 2019). However, this capacity decreases to 153–173 kg CO2/t if only considering the 26.9 wt.% wollastonite, which is more likely to react in the short term. This capacity drops further to 102 kg CO2/t if considering calcite (CaCO3) formation from wollastonite weathering instead of solubility trapping, a likely scenario in circumneutral to alkaline soils (Haque et al., 2020b). Consequently, there is a disconnect between the stated CDR capacity based on whole-rock geochemistry and the measurement of initially fast rates resulting from reactivity that may not represent the whole rock.
Regarding basalt, column experiments conducted by Amann et al. (2022) illustrate the loss of reactivity that feedstocks will experience over time. Total alkalinity in leachate waters from basanite columns exponentially declined by approximately an order of magnitude in months, likely caused by reactive surfaces being consumed. While volcanic glasses (amorphous material) in basalts (Lewis et al., 2021) do not have substantially faster dissolution rates compared to plagioclase minerals (Heřmanská et al., 2022), they tend to have greater surface areas due to their irregular morphologies (Richards-Thomas et al., 2021). These high surface areas will contribute labile cations that, once leached, leave the bulk, more recalcitrant material. Furthermore, many basalts being used for ERW have large portions of coarse particles (>1 mm; Lewis et al., 2021) that are undoubtedly far less reactive than the finest portion.
Initial weathering rates likely overestimate long-term CDR. Furthermore, yearly applications of rock powders in ERW trials will perpetuate the issue of initially fast rates, i.e., CDR rates are never estimated past this initial reactive period.
4 Weathering of accessory carbonates
Other reactive phases that may be present in ERW rock powders include carbonate minerals that readily dissolve compared to silicate minerals. Given their much faster dissolution rates, even trace to minor abundances of carbonates will tend to dominate cation release and alkalinity generation in the short term until carbonates have been consumed. As examples, the wollastonite and kimberlite samples contained 0.27 and 0.25 wt.% inorganic C (Supplementary Table S1); equivalent to 2.3 and 2.1 wt.% CaCO3. Cation release and alkalinity generation from carbonate weathering could be misinterpreted as occurring from silicate weathering, leading to overestimating CDR rates. For instance, complete dissolution of the carbonate in the wollastonite skarn and kimberlite amendments in 1 yr would be equivalent to CDR rates of 0.84 and 0.78 t CO2/ha/yr, respectively, if these rock powders were applied at 50 t/ha; relatively high, yet erroneous CDR contributions. While carbonate weathering can remove atmospheric CO2, it is far less efficient than silicate weathering and can be reversed if carbonate minerals reform downstream. If soils are acidic, the weathering of carbonates within ERW rock powders will release CO2 (Kunhikrishnan et al., 2016), which should be quantified and subtracted from the overall CDR rate.
Two potential benefits of ERW are replacing AgLime with alkaline silicate powders that contain nutrients for enhancing plant growth and preventing CO2 release from this amendment when applied to acidic soils (West and McBride, 2005; Dietzen et al., 2018; Swoboda et al., 2022). However, silicate-based rock powders may be insufficient to achieve the desired soil pH in cases of more acidic soils, lower dosages, or less reactive feedstocks. Van der Bauwhede et al. (2024) note that the acid-neutralizing capacities and dissolution rates of silicate rock powders are highly variable. As previously discussed, silicate-based ERW feedstocks may provide initially high buffering capacity that will fade when reactivity declines. Furthermore, adding AgLime along with ERW feedstocks to achieve a desired soil pH (Amoakwah et al., 2023) may confuse CDR quantification, as does the addition of fertilizers (e.g., K excluded by Reershemius et al., 2023). Similarly, the application of ERW feedstocks containing carbonate can potentially complicate solid-phase measurements if using soil inorganic carbon to measure CDR (Haque et al., 2020a).
Given the challenges that accessory carbonates pose, ERW rock powders must be screened for these minerals prior to application using sensitive techniques such as coulometry (Paulo et al., 2021). If necessary, carbonate weathering from ERW rock powders should be quantified to avoid overestimating CDR rates.
5 Quantifying cations not carbon and cation exchange
Cations released from the weathering of mineral amendments may be transported in soil pore waters, precipitated as secondary minerals (e.g., clays and carbonates), absorbed by plants, or adsorbed onto soil particles, becoming part of the exchangeable fraction. The assumption that dissolved inorganic carbon (DIC) balances cations is not always true. For example, Clarkson et al. (2024) highlight the role of non-carbonic acids, including nitric and phosphoric acids formed from fertilizers, and sulphuric acid generated by the oxidation of sulphides in feedstocks. Here, we consider cation exchange testing as another example of how DIC may not balance cations and lead to overestimating CDR rates.
Exchangeable leaches quantify cations bound to negatively charged surfaces, including clays, micas, and organic matter in soils. From an ERW perspective, one assumption is that exchangeable cations in excess of a control soil have dissolved from mineral feedstocks (Dietzen and Rosing, 2023), and are part of an enhanced weathering signal. However, some of these cations may be released from the ERW rock powders during cation exchange testing of amended soils and, thus, are not related to carbonic acid weathering.
The solid:fluid ratios imposed by leach tests are high. As an example, consider a hypothetical square-metre soil plot with a weathering depth of 20 cm and soil bulk density of 1 g/cm3 that receives 1,000 mm of precipitation per year. Therefore, the solid:fluid ratio is 1 g soil to 5 mL of precipitation per year (200 kg:1,000 L/yr). The methods used for determining the exchangeable fraction vary in terms of the solid:fluid ratio, e.g., 1:10 to 1:40 (Paulo et al., 2020, 2021; Zeyen et al., 2022) and the leaching solution composition (e.g., Dietzen and Rosing, 2023). Extrapolating cation data means applying these leaches to the entirety of the hypothetical soil, requiring 2,000–8,000 L of leachate solution or 2–8× the volume of precipitation per year, depending on the ratio used. Although the fluid compositions of precipitation and leach solutions differ, the potential for amendment dissolution during exchangeable cation testing should not be considered negligible, given the substantial volume of solution required.
Here, we conducted ammonium acetate leaches (NH4OAc; 1.0 M) using basalt, olivine, kimberlite, and wollastonite rock powders (Supplementary Table S1) at pH 7 and 9 to compare different mineral dissolution rates. We used modified methods by ASTM International (2018; D7503-18) and a solid:fluid ratio of 1:10. Soils amended with rock powder could contain approximately 2.5 wt.% amendment assuming a soil bulk density of 1 g/cm3, dosage of 50 t/ha, and mixing depth of 20 cm (2,000 t soil over 1 ha). Tests were conducted using pure soils and amendments, and mixtures of amendment (2.5 wt.%) with soil from Haliburton Forest, Ontario, Canada, a site of an ongoing ERW trial. Detailed methods are in Supplementary material.
Releases of Ca, Mg, Na, K and Si (mg/kg) from the amendments, amendment-soil mixtures, and soil are summarized in Supplementary Tables S1, S2. All data is available in Supplementary material. The erroneous CDR contributions were calculated using a modified version of the Steinour equation (Steinour, 1959; ω = 1.7) and assuming a 50 t/ha dosage. Leaching of the basalt, olivine, kimberlite, and wollastonite amendments at pH 7 gave erroneous CDR contributions of 0.13, 0.52, 1.27, and 1.31 t CO2/ha/yr, respectively. While testing the amendments alone removes the background soil leaching, it also facilitates mineral dissolution as there is no release of cations from soil that would inhibit amendment dissolution. However, these leaches demonstrate that the amendments release cations during testing that may be misinterpreted. Paulo et al. (2020, 2021) used similar leaches to assess the reactivity of various rock powders and tailings. Leaching of the basalt, olivine, kimberlite, and wollastonite at pH 9 mainly gave lower erroneous CDR contributions of 0.13, 0.35, 0.82, and 1.00 t CO2/ha/yr, respectively, due to the more alkaline pH of the NH4OAc solution.
Leaching of the mixtures of soil with basalt, olivine and kimberlite at pH 7 gave erroneous CDR contributions of 0.03, 0.22, and 0.20 t CO2/ha/yr, respectively. These contributions are within the range of CDR rates for ERW previously discussed. To be conservative, these rates were calculated using only Ca, Mg, Na, and K values that did not have overlapping standard deviations when comparing the average mixtures to average control values (values bolded in Table 1); otherwise, leach values were considered to be nonsignificant. The wollastonite-soil mixture did not leach any cations that were greater than variability. While this may appear favourable, it is worth noting that Ca accounts for ~90% of the cations leached from the soil controls and may obscure or inhibit any amendment leaching. The variability of the Ca leaching data for the control soils alone results in CDR contribution variability of ±0.33 t CO2/ha/yr. Leaching of the soil mixtures with basalt, olivine and kimberlite mixtures at pH 9 gave erroneous CDR contributions of 0.05, 0.03, and 0.08 t CO2/ha/yr, respectively, substantial reductions for the olivine and kimberlite. The erroneous CDR contribution for basalt was greater at pH 9 than 7 because the leached Mg was considered significant compared to the soil control.
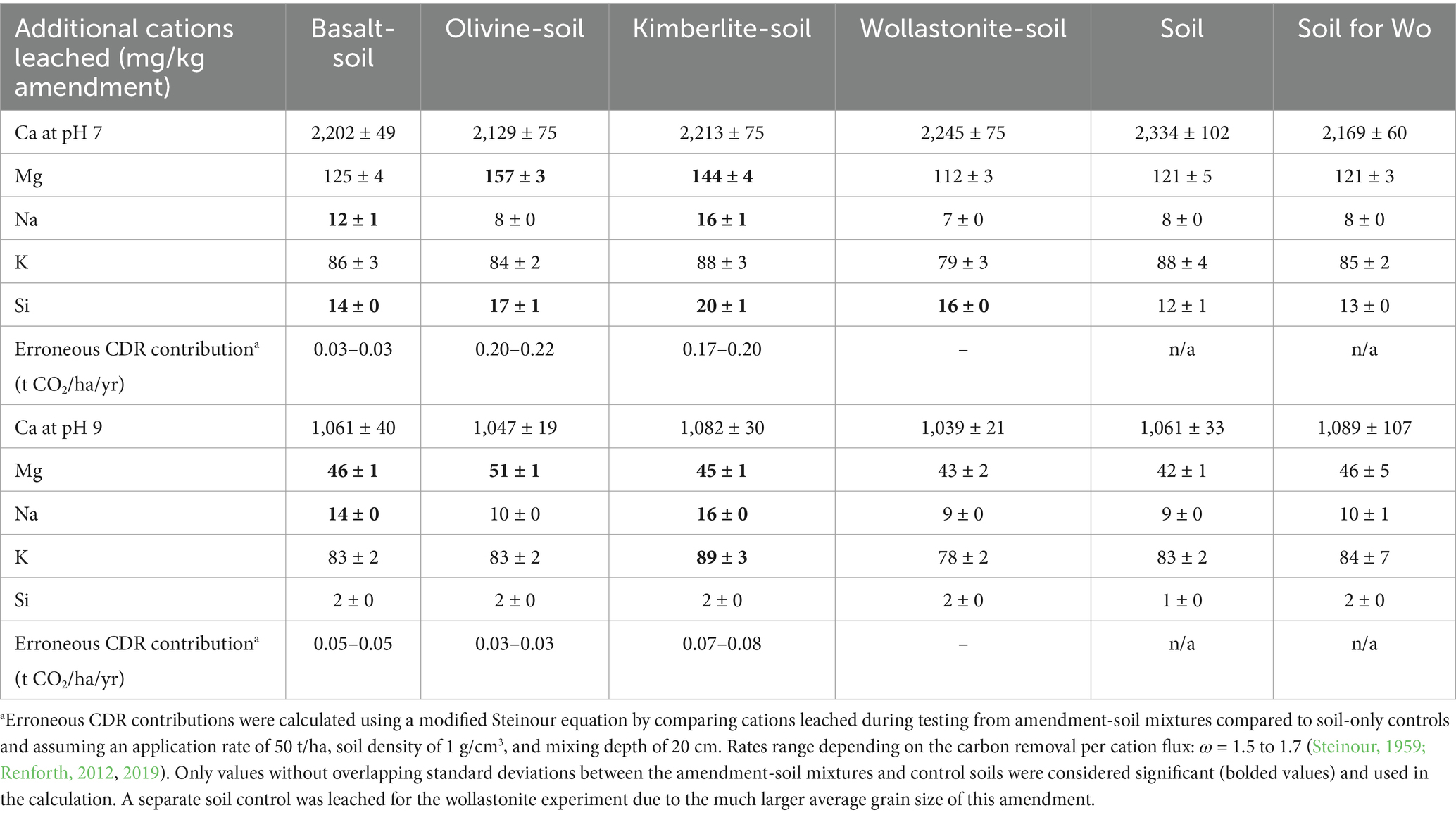
Table 1. Cations released from the four amendment-soil mixtures during cation exchange testing compared to soil-only controls at pH 7 and 9 using ammonium acetate (1.0 M).
Cation release from amendment minerals during these tests may have occurred due to the dissolution of the silicate and carbonate minerals, the latter being more easily dissolved, and cation exchange with clay and mica minerals present in the amendments. Importantly, neither of these processes relates to carbonic acid weathering. In an ERW pot experiment using basalt, olivine and wollastonite, Hasemer et al. (2024) suggested that soils retained most of the ERW products through cation exchange and found that CDR estimates using cations were often greater than measurements using inorganic carbon. The authors questioned the efficiency of their cation exchange methods and concluded that exchangeable cations should not be considered durable CDR. It may also be that cation exchange testing overestimated CDR rates due to cation release during testing, particularly when considering that these soils contained 15 wt.% amendment.
While our tests used an amendment abundance of 2.5 wt.% of the total mass with soil, successive annual applications would likely result in greater amendment dissolution during testing as the soil would contain a greater proportion of amendment (e.g., 200 t/ha over 4 yr ≈ 10 wt.%). Furthermore, the leaches at pH 7 and 9 demonstrate how sensitive cation release is to pH (Oorts et al., 2004), which generates considerable variability and likely error, particularly if comparing soils that have different pH values, e.g., an amended versus control soil, or soils from different locations in the field.
In contrast to the Mauna Loa basalt used in our tests, the six basalts being used in large-scale ERW field trials characterized by Lewis et al. (2021) may be more susceptible to CDR overestimation due to cation exchange, carbonate weathering, and initially fast surface reactions. Five of these basalts (Blue Ridge, Cragmill, Hillhouse, Oregon, Tawau) contained clay minerals, such as chlorite and smectite group minerals, which are common in altered and weathered basalts (e.g., Ehlmann et al., 2012). Chlorite can be particularly abundant in basalts that have metamorphosed to greenschist facies (e.g., 34 wt.% in Blue Ridge metabasalt; Beerling et al., 2024). Consequently, cation exchange from these during testing could be considerable. For instance, Zeyen et al. (2022) proposed using cation exchange to access labile cations from clay minerals within kimberlite residues for carbonation. Furthermore, these basalts had calcite abundances ranging from 0.17 (Oregon) to 1.25 wt.% (Cragmill). Carbonate weathering from these basalts could provide erroneous CDR contributions of 0.065–0.47 t CO2/ha/yr if applied at 50 t/ha and assuming complete carbonate dissolution in 1 yr or release CO2 at rates of 0.04–0.28 t CO2/ha/yr if applied to acidic soils. Two of these basalts had considerably greater specific surface areas (Oregon = 14.54 m2/g and Tichum = 10.30 m2/g) than the others, which would facilitate faster weathering but also make them more susceptible to cation release during leach testing or provide initial rates that are much faster than long-term rates due to rapid surface reactions. Furthermore, these high surface areas indicate high abundances of clays.
There are numerous reasons why quantifying cations does not equate to quantifying carbon (e.g., strong acid weathering), with amendment dissolution during solid-phase testing being one more example. Therefore, verifying CDR rates using carbon-based measurements should be compulsory for CDR quantification. At the very least, ERW practitioners and researchers should establish a clear correlation between carbon and proxy measurements to demonstrate that the latter quantifies CDR effectively. Regarding fast CDR rates, directly measuring carbon in combination with cation fluxes or losses (proxy analyses) should be straightforward. For example, a CDR rate of 2 t CO2/ha/yr would result in a highly significant increase of DIC in near-surface soil pore waters, assuming solubility trapping. This rate equals 0.28 kg HCO3/m2/yr. Given a square-meter plot receiving 1,000 mm (1,000 L) of precipitation per year with half of this water evaporating (500 L), soil waters should exhibit an increase in DIC of 560 mg HCO3/L, which can easily be measured and would confirm CDR estimations by cation accounting. While there are numerous analytical techniques for measuring CDR (Campbell et al., 2023), coulometry is an overlooked technique to measure inorganic carbon in solids and waters (Rausis et al., 2022; Dostie et al., 2024). Accurate quantification of carbon is a hallmark of successful geochemical negative emissions technologies and should be a priority for ERW.
6 Conclusions and recommendations
Overestimating CDR rates risks weakening confidence in ERW if erroneous carbon credits are counted and, more importantly, does not aid in combating climate change. While there are numerous sources of uncertainty, we highlighted three potential pitfalls that relate to the geochemical and mineralogical composition of ERW rock powders, which may contribute to overestimating CDR rates. First, fast rates due to the dissolution of reactive surfaces and labile phases will not be maintained long-term. Second, trace and minor abundances of carbonate minerals can dominate cation release and alkalinity generation, which can be misconstrued as silicate weathering. Third, quantifying cations that DIC may not balance will overestimate CDR. In addition to strong acid dissolution, amendment dissolution and cation exchange during solid-phase testing release cations unrelated to carbonic acid weathering. Considering these geochemical and mineralogical pitfalls, we recommend:
(1) Incorporating high-dosage areas that avoid reapplication of rock powders to measure long-term CDR rates. While successive annual applications increase overall CDR (e.g., Beerling et al., 2024), they interfere with measuring CDR rates past the initially fast reactivity period. However, high dosage plots may ensure an ERW signal is detected and can be used to assess high cumulative dosages, e.g., 50 t/ha year after year.
(2) Using sensitive techniques to measure accessory carbonate and then identifying and quantifying carbonate weathering if necessary. Differentiating carbonate from silicate weathering may involve measuring silicon concentrations or subtracting the CDR potentials of carbonates present in rock powders to be conservative. Furthermore, any CO2 release from carbonates must be included in overall CDR quantification.
(3) Measuring carbon for carbon dioxide removal to build confidence in carbon accounting. Proxy measurements (e.g., cation quantification) should be verified using carbon measurements for carbon crediting purposes.
Large-scale field trials by interdisciplinary teams that produce complete and open-access datasets are necessary to alleviate uncertainty. Careful and detailed characterization of a feedstock used in multiple trials in different climates and environments would be advantageous for comparing datasets more easily. Furthermore, multiple carbon quantification methods, including cation- and carbon-based techniques, should be used. Reporting conservative CDR rates that consider all sources of uncertainty will ensure carbon credits are verifiable and additional.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
IP: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. VH: Investigation, Methodology, Writing – original draft, Writing – review & editing. MG: Investigation, Writing – review & editing. ZS: Investigation, Writing – review & editing. KR: Visualization, Writing – review & editing. HK-H: Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by a Natural Sciences and Engineering Research Council of Canada Discovery grant (RGPIN-2024-03734) and Canada Research Chair (CRC-2021-00338) to I.M. Power.
Acknowledgments
We are grateful to our colleagues and collaborators in the enhanced rock weathering field who indirectly contributed to this Perspective through countless conversations and discussions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fclim.2024.1510747/full#supplementary-material
References
Abdalqadir, M., Hughes, D., Gomari, S. R., and Rafiq, U. (2024). A state of the art of review on factors affecting the enhanced weathering in agricultural soil: strategies for carbon sequestration and climate mitigation. Environ. Sci. Pollut. Res. 31, 19047–19070. doi: 10.1007/s11356-024-32498-5
Amann, T., Hartmann, J., Hellmann, R., Pedrosa, E. T., and Malik, A. (2022). Enhanced weathering potentials—the role of in situ CO2 and grain size distribution. Front. Clim. 4:21. doi: 10.3389/fclim.2022.929268
Amann, T., Hartmann, J., Struyf, E., Garcia, W. D., Fischer, E. K., Janssens, I., et al. (2020). Enhanced weathering and related element fluxes – a cropland mesocosm approach. Biogeosciences 17, 103–119. doi: 10.5194/bg-17-103-2020
Amoakwah, E., Shim, J., Kim, S., Lee, Y. H., Kwon, S., Sangho, J., et al. (2023). Impact of silicate and lime application on soil fertility and temporal changes in soil properties and carbon stocks in a temperate ecosystem. Geoderma 433:116431. doi: 10.1016/j.geoderma.2023.116431
ASTM International (2018). Standard test method for measuring the exchange complex and cation exchange capacity of inorganic fine-grained soils (D7503-18). West Conshohocken, PA: ASTM International.
Badgley, G., Freeman, J., Hamman, J. J., Haya, B., Trugman, A. T., Anderegg, W. R. L., et al. (2022). Systematic over-crediting in California's forest carbon offsets program. Glob. Change Biol. 28, 1433–1445. doi: 10.1111/gcb.15943
Beerling, D. J., Epihov, D. Z., Kantola, I. B., Masters, M. D., Reershemius, T., Planavsky, N. J., et al. (2024). Enhanced weathering in the US Corn Belt delivers carbon removal with agronomic benefits. Proc. Natl. Acad. Sci. USA 121:e2319436121. doi: 10.1073/pnas.2319436121
Beerling, D. J., Leake, J. R., Long, S. P., Scholes, J. D., Ton, J., Nelson, P. N., et al. (2018). Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 4, 138–147. doi: 10.1038/s41477-018-0108-y
Buckingham, F. L., Henderson, G. M., Holdship, P., and Renforth, P. (2022). Soil core study indicates limited CO2 removal by enhanced weathering in dry croplands in the UK. Appl. Geochem. 147:105482. doi: 10.1016/j.apgeochem.2022.105482
Buckingham, F. L., Henderson, G. M., and Renforth, P. (2023). Response to Comment from West et al. on, Soil core study indicates limited CO2 removal by enhanced weathering in dry croplands in the UK. Appl. Geochem. 152:4. doi: 10.1016/j.apgeochem.2023.105622
Calabrese, S., Wild, B., Bertagni, M. B., Bourg, I. C., White, C., Aburto, F., et al. (2022). Nano-to global-scale uncertainties in terrestrial enhanced weathering. Environ. Sci. Technol. 56, 15261–15272. doi: 10.1021/acs.est.2c03163
Campbell, J., Bastianini, L., Buckman, J., Bullock, L., Foteinis, S., Furey, V., et al. (2023). Measurements in geochemical carbon dioxide removal. Heriot-Watt University.
CarbonPlan CDR Verfication framework [online]. CarbonPlan and Frontier. Available at: https://carbonplan.org/research/cdr-verification/enhanced-weathering (accessed November 16, 2024).
Clarkson, M. O., Larkin, C. S., Swoboda, P., Reershemius, T., Suhrhoff, T. J., Maesano, C. N., et al. (2024). A review of measurement for quantification of carbon dioxide removal by enhanced weathering in soil. Front. Clim. 6. doi: 10.3389/fclim.2024.1345224
Das, A., Krishnaswami, S., Sarin, M. M., and Pande, K. (2005). Chemical weathering in the Krishna Basin and Western Ghats of the Deccan traps, India: rates of basalt weathering and their controls. Geochim. Cosmochim. Acta 69, 2067–2084. doi: 10.1016/j.gca.2004.10.014
Daval, D., Hellmann, R., Martinez, I., Gangloff, S., and Guyot, F. (2013). Lizardite serpentine dissolution kinetics as a function of pH and temperature, including effects of elevated pCO2. Chem. Geol. 351, 245–256. doi: 10.1016/j.chemgeo.2013.05.020
Daval, D., Martinez, I., Corvisier, J., Findling, N., Goffé, B., and Guyot, F. (2009). Carbonation of ca-bearing silicates, the case of wollastonite: experimental investigations and kinetic modeling. Chem. Geol. 265, 63–78. doi: 10.1016/j.chemgeo.2009.01.022
Daval, D., Sissmann, O., Menguy, N., Saldi, G. D., Guyot, F., Martinez, I., et al. (2011). Influence of amorphous silica layer formation on the dissolution rate of olivine at 90 °C and elevated pCO2. Chem. Geol. 284, 193–209. doi: 10.1016/j.chemgeo.2011.02.021
Dessert, C., Dupré, B., Gaillardet, J., François, L. M., and Allègre, C. J. (2003). Basalt weathering laws and the impact of basalt weathering on the global carbon cycle. Chem. Geol. 202, 257–273. doi: 10.1016/j.chemgeo.2002.10.001
Dietzen, C., Harrison, R., and Michelsen-Correa, S. (2018). Effectiveness of enhanced mineral weathering as a carbon sequestration tool and alternative to agricultural lime: an incubation experiment. Int. J. Greenh. Gas Control 74, 251–258. doi: 10.1016/j.ijggc.2018.05.007
Dietzen, C., and Rosing, M. T. (2023). Quantification of CO2 uptake by enhanced weathering of silicate minerals applied to acidic soils. Int. J. Greenh. Gas Control 125:103872. doi: 10.1016/j.ijggc.2023.103872
Dostie, L., Rausis, K., and Power, I. M. (2024). Passive direct air capture using calcium oxide powder: the importance of water vapor. J. Clean. Prod. 457:142394. doi: 10.1016/j.jclepro.2024.142394
Ehlmann, B. L., Bish, D. L., Ruff, S. W., and Mustard, J. F. (2012). Mineralogy and chemistry of altered Icelandic basalts: application to clay mineral detection and understanding aqueous environments on Mars. J. Geophys. Res. Planets 117:27. doi: 10.1029/2012je004156
Hamilton, J. L., Wilson, S., Turvey, C. C., Morgan, B., Tait, A. W., McCutcheon, J., et al. (2021). Carbon accounting of mined landscapes, and deployment of a geochemical treatment system for enhanced weathering at Woodsreef chrysotile mine, NSW, Australia. J. Geochem. Explor. 220:106655. doi: 10.1016/j.gexplo.2020.106655
Haque, F., Santos, R. M., and Chiang, Y. W. (2020a). CO2 sequestration by wollastonite-amended agricultural soils – an Ontario field study. Int. J. Greenh. Gas Control 97:103017. doi: 10.1016/j.ijggc.2020.103017
Haque, F., Santos, R. M., and Chiang, Y. W. (2020b). Optimizing inorganic carbon sequestration and crop yield with wollastonite soil amendment in a microplot study. Front. Plant Sci. 11:12. doi: 10.3389/fpls.2020.01012
Haque, F., Santos, R. M., Dutta, A., Thimmanagari, M., and Chiang, Y. W. (2019). Co-benefits of wollastonite weathering in agriculture: CO2 sequestration and promoted plant growth. ACS Omega 4, 1425–1433. doi: 10.1021/acsomega.8b02477
Harrison, A. L., Dipple, G. M., Power, I. M., and Mayer, K. U. (2015). Influence of surface passivation and water content on mineral reactions in unsaturated porous media: implications for brucite carbonation and CO2 sequestration. Geochim. Cosmochim. Acta 148, 477–495. doi: 10.1016/j.gca.2014.10.020
Hartmann, J., and Kempe, S. (2008). What is the maximum potential for CO2 sequestration by "stimulated" weathering on the global scale? Naturwissenschaften 95, 1159–1164. doi: 10.1007/s00114-008-0434-4
Hasemer, H., Borevitz, J., and Buss, W. (2024). Measuring enhanced weathering: inorganic carbon-based approaches may be required to complement cation-based approaches. Front. Clim. 6. doi: 10.3389/fclim.2024.1352825
Heřmanská, M., Voigt, M. J., Marieni, C., Declercq, J., and Oelkers, E. H. (2022). A comprehensive and internally consistent mineral dissolution rate database: part I: primary silicate minerals and glasses. Chem. Geol. 597:120807. doi: 10.1016/j.chemgeo.2022.120807
Heřmanská, M., Voigt, M. J., Marieni, C., Declercq, J., and Oelkers, E. H. (2023). A comprehensive and consistent mineral dissolution rate database: part II: secondary silicate minerals. Chem. Geol. 636:121632. doi: 10.1016/j.chemgeo.2023.121632
Jerden, J., Mejbel, M., Zamuner, A. N., Carroll, M., and Campe, J. (2024). The impact of geochemical and life-cycle variables on carbon dioxide removal by enhanced rock weathering: development and application of the Stella ERW model. Appl. Geochem. 167:18. doi: 10.1016/j.apgeochem.2024.106002
Kantola, I. B., Blanc-Betes, E., Masters, M. D., Chang, E., Marklein, A., Moore, C. E., et al. (2023). Improved net carbon budgets in the US Midwest through direct measured impacts of enhanced weathering. Glob. Change Biol. 29, 7012–7028. doi: 10.1111/gcb.16903
Kelland, M. E., Wade, P. W., Lewis, A. L., Taylor, L. L., Sarkar, B., Andrews, M. G., et al. (2020). Increased yield and CO2 sequestration potential with the C4 cereal Sorghum bicolor cultivated in basaltic rock dust-amended agricultural soil. Glob. Change Biol. 26, 3658–3676. doi: 10.1111/gcb.15089
Kukla, T., Suhrhoff, T. J., Loeffler, S., Martin, K., and Chay, F. (2024). Does enhanced weathering work? We’re still learning [online]. CarbonPlan. Available: at: https://carbonplan.org/research/enhanced-weathering-fluxes (accessed November 16, 2024).
Kunhikrishnan, A., Thangarajan, R., Bolan, N. S., Xu, Y., Mandal, S., Gleeson, D. B., et al. (2016). Functional relationships of soil acidification, liming, and greenhouse gas flux. Adv. Agron. 139, 1–71. doi: 10.1016/bs.agron.2016.05.001
Kyker-Snowman, E., Federman, S., Friedmann, J., Gifford, L., Hong, A., McCormick, C., et al. (2024). A buyer’s guide to enhanced rock weathering in croplands. Carbon Direct. Available at: https://www.carbon-direct.com/research-and-reports/enhanced-rock-weathering-in-croplands
Larkin, C. S., Andrews, M. G., Pearce, C. R., Yeong, K. L., Beerling, D. J., Bellamy, J., et al. (2022). Quantification of CO2 removal in a large-scale enhanced weathering field trial on an oil palm plantation in Sabah, Malaysia. Front. Clim. 4:20. doi: 10.3389/fclim.2022.959229
Lewis, A. L., Sarkar, B., Wade, P., Kemp, S. J., Hodson, M. E., Taylor, L. L., et al. (2021). Effects of mineralogy, chemistry and physical properties of basalts on carbon capture potential and plant-nutrient element release via enhanced weathering. Appl. Geochem. 132:105023. doi: 10.1016/j.apgeochem.2021.105023
Linke, T., Oelkers, E. H., Dideriksen, K., Möckel, S. C., Nilabh, S., Grandia, F., et al. (2024a). The geochemical evolution of basalt enhanced rock weathering systems quantified from a natural analogue. Geochim. Cosmochim. Acta 370, 66–77. doi: 10.1016/j.gca.2024.02.005
Linke, T., Oelkers, E. H., Möckel, S. C., and Gislason, S. R. (2024b). Direct evidence of CO2 drawdown through enhanced weathering in soils. Geochem. Perspect. Lett. 30, 7–12. doi: 10.7185/geochemlet.2415
Louvat, P., and Allègre, C. J. (1997). Present denudation rates on the island of Reunion determined by river geochemistry: basalt weathering and mass budget between chemical and mechanical erosions. Geochim. Cosmochim. Acta 61, 3645–3669. doi: 10.1016/s0016-7037(97)00180-4
Lu, X. Y., Carroll, K. J., Turvey, C. C., and Dipple, G. M. (2022). Rate and capacity of cation release from ultramafic mine tailings for carbon capture and storage. Appl. Geochem. 140:105285. doi: 10.1016/j.apgeochem.2022.105285
Maher, K., Johnson, N. C., Jackson, A., Lammers, L. N., Torchinsky, A. B., Weaver, K. L., et al. (2016). A spatially resolved surface kinetic model for forsterite dissolution. Geochim. Cosmochim. Acta 174, 313–334. doi: 10.1016/j.gca.2015.11.019
McQueen, N., Kelemen, P., Dipple, G., Renforth, P., and Wilcox, J. (2020). Ambient weathering of magnesium oxide for CO2 removal from air. Nat. Commun. 11:3299. doi: 10.1038/s41467-020-16510-3
Mergelsberg, S. T., Rajan, B. P., Legg, B. A., Kovarik, L., Burton, S. D., Bowers, G. M., et al. (2023). Nanoscale mg-depleted layers slow carbonation of forsterite (Mg2SiO4) when water is limited. Environ. Sci. Technol. Lett. 10, 98–104. doi: 10.1021/acs.estlett.2c00866
Oorts, K., Vanlauwe, B., Pleysier, J., and Merckx, R. (2004). A new method for the simultaneous measurement of pH-dependent cation exchange capacity and pH buffering capacity. Soil Sci. Soc. Am. J. 68, 1578–1585. doi: 10.2136/sssaj2004.1578
Oskierski, H. C., Dlugogorski, B. Z., and Jacobsen, G. (2013). Sequestration of atmospheric CO2 in chrysotile mine tailings of the Woodsreef Asbestos mine, Australia: quantitative mineralogy, isotopic fingerprinting and carbonation rates. Chem. Geol. 358, 156–169. doi: 10.1016/j.chemgeo.2013.09.001
Paulo, C., Power, I. M., Stubbs, A. R., Wang, B. L., Zeyen, N., and Wilson, S. A. (2021). Evaluating feedstocks for carbon dioxide removal by enhanced rock weathering and CO2 mineralization. Appl. Geochem. 129:104955. doi: 10.1016/j.apgeochem.2021.104955
Paulo, C., Power, I. M., Stubbs, A. R., Zeyen, N., and Wilson, S. A. (2020). An analytical tool to assess the carbonation potential of mineral deposits and mining wastes. Conference of Metallurgists, 9.
Paulo, C., Power, I. M., Zeyen, N., Wang, B. L., and Wilson, S. A. (2023). Geochemical modeling of CO2 sequestration in ultramafic mine wastes from Australia, Canada, and South Africa: implications for carbon accounting and monitoring. Appl. Geochem. 152:105630. doi: 10.1016/j.apgeochem.2023.105630
Pokrovsky, O. S., and Schott, J. (2004). Experimental study of brucite dissolution and precipitation in aqueous solutions: surface speciation and chemical affinity control. Geochim. Cosmochim. Acta 68, 31–45. doi: 10.1016/s0016-7037(03)00238-2
Power, I. M., Dipple, G. M., Bradshaw, P. M. D., and Harrison, A. L. (2020). Prospects for CO2 mineralization and enhanced weathering of ultramafic mine tailings from the Baptiste nickel deposit in British Columbia, Canada. Int. J. Greenh. Gas Control 94:102895. doi: 10.1016/j.ijggc.2019.102895
Rausis, K., Stubbs, A. R., Power, I. M., and Paulo, C. (2022). Rates of atmospheric CO2 capture using magnesium oxide powder. Int. J. Greenh. Gas Control 119:103701. doi: 10.1016/j.ijggc.2022.103701
Reershemius, T., Kelland, M. E., Jordan, J. S., Davis, I. R., D'Ascanio, R., Kalderon-Asael, B., et al. (2023). Initial validation of a soil-based mass-balance approach for empirical monitoring of enhanced rock weathering rates. Environ. Sci. Technol. 57, 19497–19507. doi: 10.1021/acs.est.3c03609
Reershemius, T., and Suhrhoff, T. J. (2024). On error, uncertainty, and assumptions in calculating carbon dioxide removal rates by enhanced rock weathering in Kantola et al., 2023. Glob. Change Biol 30:3. doi: 10.1111/gcb.17025
Renforth, P. (2012). The potential of enhanced weathering in the UK. Int. J. Greenh. Gas Control 10, 229–243. doi: 10.1016/j.ijggc.2012.06.011
Renforth, P. (2019). The negative emission potential of alkaline materials. Nat. Commun. 10:1401. doi: 10.1038/s41467-019-09475-5
Richards-Thomas, T., McKenna-Neuman, C., and Power, I. M. (2021). Particle-scale characterization of volcaniclastic dust sources within Iceland. Sedimentology 68, 1137–1158. doi: 10.1111/sed.12821
Sandalow, D., Aines, R., Friedmann, J., Kelemen, P., McCormick, C., Power, I. M., et al. (2021). Carbon mineralization roadmap.
Santos, R. M., Araujo, F., Jariwala, H., Khalidy, R., Haque, F., and Chiang, Y. W. (2023). Pathways, roundabouts, roadblocks, and shortcuts to safe and sustainable deployment of enhanced rock weathering in agriculture. Front. Earth Sci. 11:7. doi: 10.3389/feart.2023.1215930
Steinour, H. H. (1959). Some effects of carbon dioxide on mortars and concrete-discussion. J. Am. Concr. Inst. 30, 905–907.
Stubbs, A. R., Paulo, C., Power, I. M., Wang, B. L., Zeyen, N., and Wilson, S. A. (2022). Direct measurement of CO2 drawdown in mine wastes and rock powders: implications for enhanced rock weathering. Int. J. Greenh. Gas Control 113:103554. doi: 10.1016/j.ijggc.2021.103554
Sutherland, K., Matlin-Wainer, M., Holme, E., He, J., Savage, R., Marsland, E., et al. (2024). "Isometric: enhanced weathering in agriculture v1.0".
Swoboda, P., Doring, T. F., and Hamer, M. (2022). Remineralizing soils? The agricultural usage of silicate rock powders: a review. Sci. Total Environ. 807:150976. doi: 10.1016/j.scitotenv.2021.150976
Taylor, L. L., Driscoll, C. T., Groffman, P. M., Rau, G. H., Blum, J. D., and Beerling, D. J. (2021). Increased carbon capture by a silicate-treated forested watershed affected by acid deposition. Biogeosciences 18, 169–188. doi: 10.5194/bg-18-169-2021
te Pas, E. E. E. M., Hagens, M., and Comans, R. N. J. (2023). Assessment of the enhanced weathering potential of different silicate minerals to improve soil quality and sequester CO2. Front. Clim. 4. doi: 10.3389/fclim.2022.954064
ten Berge, H. F. M., van der Meer, H. G., Steenhuizen, J. W., Goedhart, P. W., Knops, P., and Verhagen, J. (2012). Olivine weathering in soil, and its effects on growth and nutrient uptake in ryegrass (Lolium perenne L.): a pot experiment. PLoS One 7:8. doi: 10.1371/journal.pone.0042098
The Guardian (2023). Revealed: more than 90% of rainforest carbon offsets by biggest certifier are worthless, analysis shows. Available at: https://www.theguardian.com/environment/2023/jan/18/revealed-forest-carbon-offsets-biggest-provider-worthless-verra-aoe
Turvey, C. C., Wilson, S. A., Hamilton, J. L., Tait, A. W., McCutcheon, J., Beinlich, A., et al. (2018). Hydrotalcites and hydrated mg-carbonates as carbon sinks in serpentinite mineral wastes from the Woodsreef chrysotile mine, New South Wales, Australia: controls on carbonate mineralogy and efficiency of CO2 air capture in mine tailings. Int. J. Greenh. Gas Control 79, 38–60. doi: 10.1016/j.ijggc.2018.09.015
van der Bauwhede, R., Muys, B., Vancampenhout, K., and Smolders, E. (2024). Accelerated weathering of silicate rock dusts predicts the slow-release liming in soils depending on rock mineralogy, soil acidity, and test methodology. Geoderma 441:116734. doi: 10.1016/j.geoderma.2023.116734
Vienne, A., Poblador, S., Portillo-Estrada, M., Hartmann, J., Ijiehon, S., Wade, P., et al. (2022). Enhanced weathering using basalt rock powder: carbon sequestration, co-benefits and risks in a mesocosm study with Solanum tuberosum. Front. Clim. 4:14. doi: 10.3389/fclim.2022.869456
von Strandmann, P., Tooley, C., Mulders, J., and Renforth, P. (2022). The dissolution of olivine added to soil at 4°C: implications for enhanced weathering in cold regions. Front. Clim. 4:11. doi: 10.3389/fclim.2022.827698
West, L. J., Banwart, S. A., Martin, M. V., Kantzas, E., and Beerling, D. J. (2023). Making mistakes in estimating the CO2 sequestration potential of UK croplands with enhanced weathering. Appl. Geochem. 151:105591. doi: 10.1016/j.apgeochem.2023.105591
West, T. O., and McBride, A. C. (2005). The contribution of agricultural lime to carbon dioxide emissions in the United States: dissolution, transport, and net emissions. Agric. Ecosyst. Environ. 108, 145–154. doi: 10.1016/j.agee.2005.01.002
Wilson, S. A., Dipple, G. M., Power, I. M., Barker, S. L. L., Fallon, S. J., and Southam, G. (2011). Subarctic weathering of mineral wastes provides a sink for atmospheric CO2. Environ. Sci. Technol. 45, 7727–7736. doi: 10.1021/es202112y
Wilson, S. A., Harrison, A. L., Dipple, G. M., Power, I. M., Barker, S. L. L., Mayer, K. U., et al. (2014). Offsetting of CO2 emissions by air capture in mine tailings at the mount Keith nickel mine, Western Australia: rates, controls and prospects for carbon neutral mining. Int. J. Greenhouse Gas Control 25, 121–140. doi: 10.1016/j.ijggc.2014.04.002
Wood, C., Harrison, A. L., and Power, I. M. (2023). Impacts of dissolved phosphorus and soil-mineral-fluid interactions on CO2 removal through enhanced weathering of wollastonite in soils. Appl. Geochem. 148:105511. doi: 10.1016/j.apgeochem.2022.105511
Keywords: enhanced rock weathering, carbon dioxide removal (CDR), carbon quantification, soil, carbonate, cation exchange, mineral dissolution
Citation: Power IM, Hatten VNJ, Guo M, Schaffer ZR, Rausis K and Klyn-Hesselink H (2025) Are enhanced rock weathering rates overestimated? A few geochemical and mineralogical pitfalls. Front. Clim. 6:1510747. doi: 10.3389/fclim.2024.1510747
Edited by:
Raffaella Pomi, Sapienza University of Rome, ItalyReviewed by:
Fatima Haque, University of Guelph, CanadaCopyright © 2025 Power, Hatten, Guo, Schaffer, Rausis and Klyn-Hesselink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian M. Power, aWFucG93ZXJAdHJlbnR1LmNh
 Ian M. Power
Ian M. Power Victoria N. J. Hatten
Victoria N. J. Hatten Zivi R. Schaffer
Zivi R. Schaffer Kwon Rausis
Kwon Rausis