
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Clim. , 07 January 2025
Sec. Carbon Dioxide Removal
Volume 6 - 2024 | https://doi.org/10.3389/fclim.2024.1506181
This article is part of the Research Topic Concept Papers from the World Climate Research Programme: The Future of Climate Research View all 12 articles
The Paris Agreement to limit global warming to well below 2°C requires drastic reductions in greenhouse gas emissions and the balancing of any remaining emissions by carbon dioxide removal (CDR). Due to uncertainties about the potential and durability of many land-based approaches to deliver sufficient CDR, marine CDR options are receiving more and more interest. We present the current state of knowledge regarding the potentials, risks, side effects as well as challenges associated with technical feasibility, governance, monitoring, reporting and accounting of marine CDR, covering a range of biotic and geochemical approaches. We specifically discuss to what extent a comparison with direct injection of CO2 into seawater, which had been proposed decades ago and is now prohibited by international agreements, may provide guidance for evaluating some of the biotic marine CDR approaches.
The ocean represents the largest reservoir of carbon on Earth that is accessible on human timescales, holding more than 50 times as much carbon as the pre-industrial atmosphere and about 20 times as much as all carbon stored in global terrestrial plants and soils (Carlson et al., 2001). The ocean is currently taking up about 10 Gt CO2 per year, i.e., a quarter of anthropogenic CO2 emissions, via air-sea gas exchange that responds to rising atmospheric partial pressure of CO2 (Friedlingstein et al., 2023). Intuitively, it might appear attractive to enhance the uptake of CO2 from the relatively small atmospheric reservoir into the large marine reservoir, where relative changes in the total inventory, and possibly unintended environmental impacts, would be smaller. Marine carbon dioxide removal (CDR) refers to such additional transfer of CO2 from the atmosphere to the ocean by deliberate human activities (e.g., Doney et al., 2024). However, in its recent Advisory Opinion on climate change, the International Tribunal of the Law of the Sea (2024) stated that “anthropogenic GHG emissions into the atmosphere constitute pollution of the marine environment.” And while therefore all measures have to be taken to prevent, reduce and control this pollution, this must not involve transferring damage or hazards from one area to another (International Tribunal of the Law of the Sea, 2024). Accordingly, the simple argument of carbon inventory changes being smaller in the ocean than in the atmosphere does not qualify as justification for additional transfer of carbon from the atmosphere to the ocean, and due diligence must be applied to assess all possible consequences of marine CDR on the ocean per se.
Atmospheric and oceanic carbon pools are in contact via air-sea exchange of CO2 that tends to equalize CO2 partial pressures (pCO2) in the surface ocean and overlying atmosphere, so that an increase in atmospheric pCO2 or a reduction in surface seawater pCO2 induces a net flux of CO2 into the ocean. In seawater, dissolved CO2 forms carbonic acid (H2CO3) and its dissolved salts, bicarbonate (HCO3−) and carbonate (CO32−) ions. All three forms together are referred to as dissolved inorganic carbon (DIC). More than 99% of the ocean’s DIC is in the form of bicarbonate and carbonate ions that buffer any injection or loss of CO2. Which is why most of the carbon in the combined ocean–atmosphere reservoir is held in the ocean, whereas for other gases such as nitrogen or oxygen, the by far dominant portion is in the atmosphere.
While air-sea CO2 fluxes are modulated primarily by surface wind speed (Wanninkhof et al., 2009), the thermodynamic driving force is the pCO2 difference across the sea surface, essentially identical to the fugacity difference, with fugacity fCO2 being identical to pCO2 corrected for the non-ideality of CO2 (Fay et al., 2024) by accounting for solubility effects due to interactions between the molecules. In contrast to atmospheric pCO2, which shows only small spatial and temporal variations of a few μatm on top of the long-term trend from a pre-industrial 280 μatm to 421 μatm in year 2023, substantial spatial and seasonal variations by several tens of μatm are observed for the surface ocean (Fay et al., 2024). In seawater, pCO2 is a function of physical–chemical conditions, with seawater carbonate chemistry and the resulting CO2 buffer capacity being the main chemical control, and temperature-dependent solubility being the main physical control. Air-sea fluxes of anthropogenic CO2 are therefore not simply proportional to increasing atmospheric pCO2, but depend also on the physical–chemical state of the ocean. Photosynthetic production converts CO2 into organic carbon and thereby removes DIC from the water and decreases its pCO2, while respiration adds CO2 back to the water and increases its pCO2. Because respiration happens, on average, deeper in the water column than photosynthesis, biology generates a vertical gradient in DIC and pCO2, which ocean mixing and circulation tend to counteract (e.g., Frenger et al., 2024). Biogeochemical processes such as changes in carbonate production and dissolution, and deoxygenation-mediated changes in carbon, nutrient and alkalinity cycles also impact carbonate chemistry and hence pCO2.
The ocean currently takes up about a quarter of anthropogenic CO2 emissions (Gruber et al., 2023). This fraction is a result of the sluggish ocean circulation that is responsible for a delayed response of the oceanic carbon inventory to rapidly increasing atmospheric CO2. After an eventual cessation of emissions and on ocean overturning timescales of about 1,000 years, the ocean will take up about 80% of the anthropogenic emissions by physical–chemical pCO2 equilibration (Archer and Brovkin, 2008). On timescales of many thousands of years, the CO2 added to the ocean and leading to ocean acidification will dissolve some calcium carbonate primarily in the sediments and thereby neutralize some carbonic acid and reduce the pCO2 of seawater. This process is termed carbonate compensation and will further increase the total oceanic uptake of anthropogenic CO2 emissions to about 90% (Archer and Brovkin, 2008). Note that the effects of marine biota on the oceanic uptake of anthropogenic CO2 is, at least until now, deemed insignificant (Carroll et al., 2022; Frenger et al., 2024), although marine biology can substantially impact atmospheric CO2 in the context of long-term climate swings (e.g., glacial cycles; Khatiwala et al., 2019).
In the geological past, the ocean has repeatedly mitigated large natural increases in atmospheric CO2 and thereby moderated and eventually stabilized climate - on timescales of thousands to hundred thousands of years (Kump et al., 2009). Informed by the geological archive, scientists have put forward ideas aimed at accelerating the natural processes by which the ocean can remove excess CO2 from the atmosphere. These include iron fertilization, which is considered important for reducing atmospheric CO2 during glacial periods with higher atmospheric loads of iron-rich dust (Martin, 1990), and ocean alkalinity enhancement mimicking the natural weathering of marine carbonate deposits on land (Kheshgi, 1995; Rau and Caldeira, 1999). Other proposals for marine CDR (mCDR) extend approaches of terrestrial land-use management to the coastal region in terms of mangroves, seagrasses and salt marshes, often termed blue carbon, and also further offshore in terms of open ocean macroalgae.
The current level of scientific understanding of mCDR is insufficient for backing large-scale deployment of any proposed technology. For some proposed mCDR methods, the balance of scientific knowledge about potential benefits and risks is tilted against large-scale implementation. More research is required to comprehensively assess the diverse portfolio of proposed options in terms of marine CDR potential and environmental and social risks (e.g., GESAMP, 2019; National Academies of Sciences, Engineering, and Medicine, 2021). Increasing the oceanic carbon pool will affect the marine environment and may put additional pressure on marine ecosystems. Modeling practices, policy development, innovation funding, legal governance and reporting should also account for interactions between ocean protection, economy, and climate, in order to avoid overpromises around mCDR (Boettcher et al., 2021).
As a side note we do not consider dumping of terrestrial biomass as marine CDR, because the CO2 would have been removed from the atmosphere on land, constituting terrestrial CDR. For this approach, the ocean serves ‘only’ as a potential storage site of the sequestered carbon. Impacts on the marine environment can be expected to be similar to several of the impacts of macroalgae farming and sinking, an approach that aims at taking up atmospheric CO2 across the sea surface, therefore qualifies as marine CDR and will be discussed below.
Because any mCDR-induced air-sea flux of CO2 will reduce atmospheric pCO2 compared to the counterfactual without mCDR deployment (and possibly increase seawater pCO2 of parts of the ocean), there will be non-local adjustments of global air-sea CO2 fluxes in the coupled ocean–atmosphere system. These effectively lead to a backflux of CO2 from the ocean to the atmosphere and partly counteract the original mCDR-induced air-sea flux (Oschlies, 2009). Even the terrestrial carbon cycle will respond to mCDR-induced changes in atmospheric pCO2, typically by a net carbon loss from the terrestrial system to enhanced ocean uptake, and vice versa (Keller et al., 2018). For CDR methods that move carbon among the continuously exchanging atmospheric, oceanic and terrestrial carbon pools, adjustments in the interconnected Earth system will result in some buffering of the immediate atmospheric carbon removal. This feature is not unique to CDR, and also applies to fossil fuel emissions of which only about half stays in the atmosphere as ‘airborne fraction’ of positive emissions. An airborne fraction smaller than unity is therefore also to be expected for negative emissions. This adjustment among carbon pools including the oceanic one makes carbon accounting for most mCDR methods more challenging than for CDR methods that revert the process of emitting fossil CO2 by moving carbon from the atmosphere back into geological reservoirs that are insensitive to changes in atmospheric pCO2. The metric for comparing mCDR with other CDR approaches should be the equivalent reduction in emissions. It should not be the, for mCDR generally smaller, increase in the oceanic carbon pool. In other words, the success of mCDR is to be measured in how much anthropogenic CO2 it sequesters, not simply by how much atmospheric CO2 it can move from the atmosphere to the ocean in the medium-term.
In the following, we provide an overview over the main concepts, the status of current understanding and key unresolved research questions regarding mCDR. To this extent, we partition the various mCDR approaches proposed by scientists, engineers, and entrepreneurs, into biotic and geochemical methods, an illustration of the main types is given in Figure 1. Other classifications have also been proposed, such as the separation into nature-based and technological approaches. Here, we refrain from the latter because the perception of nature-based and technological may depend on the detailed deployment approach, and because nature-based or natural solutions are not self-evident categories, but ones that are delimited by people acting in social groups (Bellamy and Osaka, 2020).
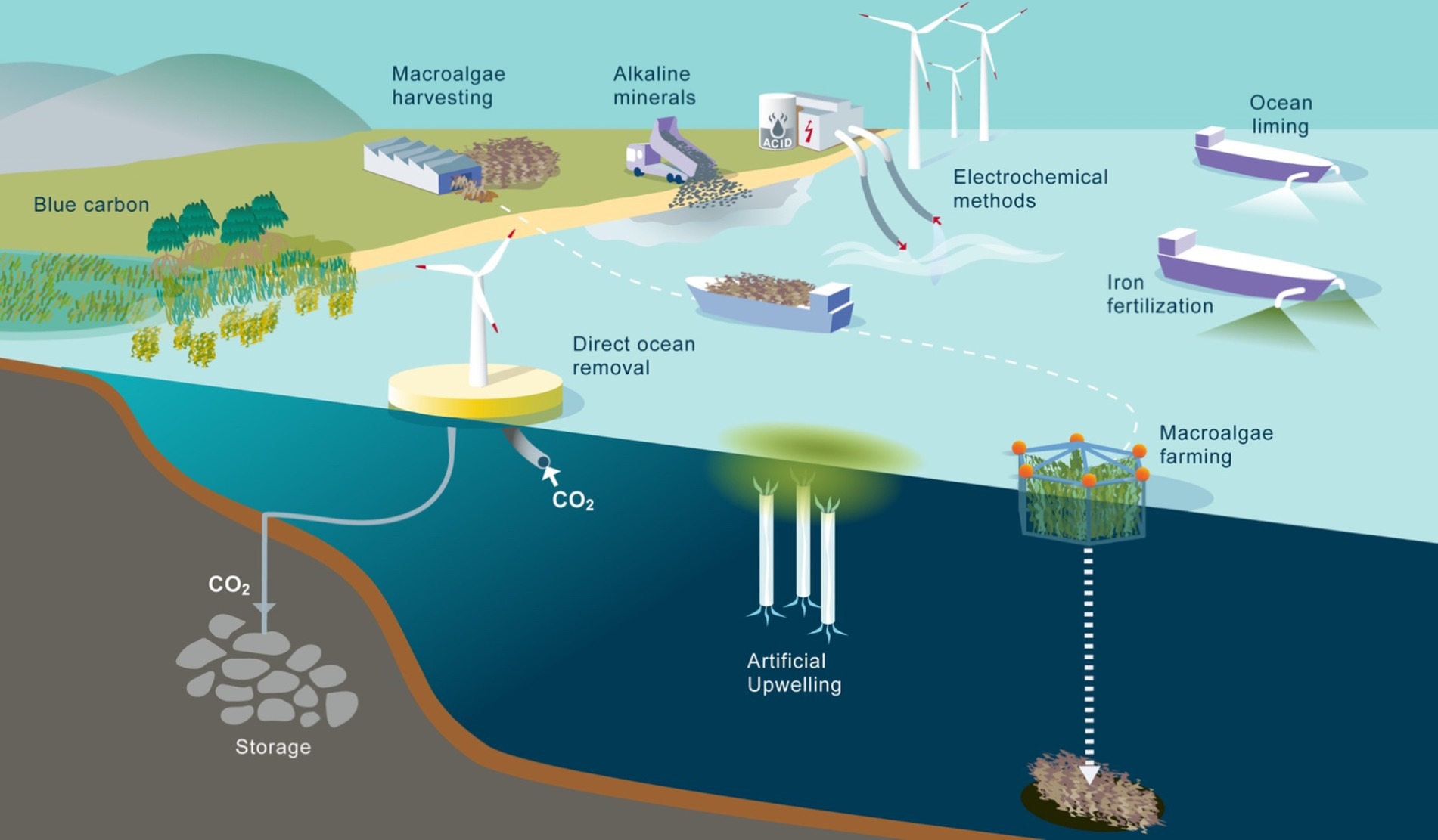
Figure 1. Illustration of various biotic and geochemical methods suggested for marine carbon dioxide removal. Biotic methods include iron fertilization, blue carbon, macroalgae farming, also in combination with artificial upwelling. Geochemical methods include alkalinity enhancement in the form of ocean liming, addition of alkaline minerals, electrochemical methods and direct ocean removal.
Common to all biotic CDR approaches is that they intentionally modify ecosystems with the aim to generate an additional net carbon removal from the atmosphere. Additionality with respect to the situation without CDR deployment is critical and requires quantification of carbon fluxes of the pre-existing unmodified ecosystem that will be modified or even destroyed by the biotic CDR deployment (Bach et al., 2024). In principle, biotic CDR can be achieved either by an additional photosynthetic uptake of CO2 from the atmosphere or by an additional avoidance of respiratory CO2, i.e., CO2 resulting from the respiration of organic carbon, release back to the atmosphere. Regarding marine CDR, biotic approaches aim to shift the balance between photosynthetic CO2 uptake and respiration towards more uptake and less respiration in the surface waters in immediate contact with the atmosphere. Almost all biotic mCDR approaches proposed consider enhanced photosynthetic CO2 uptake, one exception being the idea of converting more labile into more refractory dissolved organic carbon and thereby inhibiting release of respiratory CO2 to the atmosphere (Jiao et al., 2014). When applied in the open ocean system, both categories can be framed as modifications of the biological carbon pump. In coastal regions, enhancement of vegetated ecosystems is also considered as a biotic mCDR approach. The different approaches are discussed in more detail in the following subsections.
The biological carbon pump (BCP) describes the biotically mediated storage of carbon in the ocean interior. It results from photosynthetic conversion of DIC to organic carbon by phytoplankton or macroalgae floating in the sunlit surface ocean and subsequent respiration of organic matter back to DIC that can occur everywhere in the water column. Organic carbon is operationally partitioned into two fractions: dissolved organic carbon (DOC) defined as passing through a filter with a pore size of typically half a micron, and particulate organic carbon (POC) that does not pass through the filter. DIC and DOC are transported with the flow, whereas POC also moves relative to the water via gravitational settling. In consequence, there is a net downward transport of organic carbon, the injection of remineralized DIC in the ocean interior that reduces pCO2 in the surface ocean and increases it at depth (Figure 2).
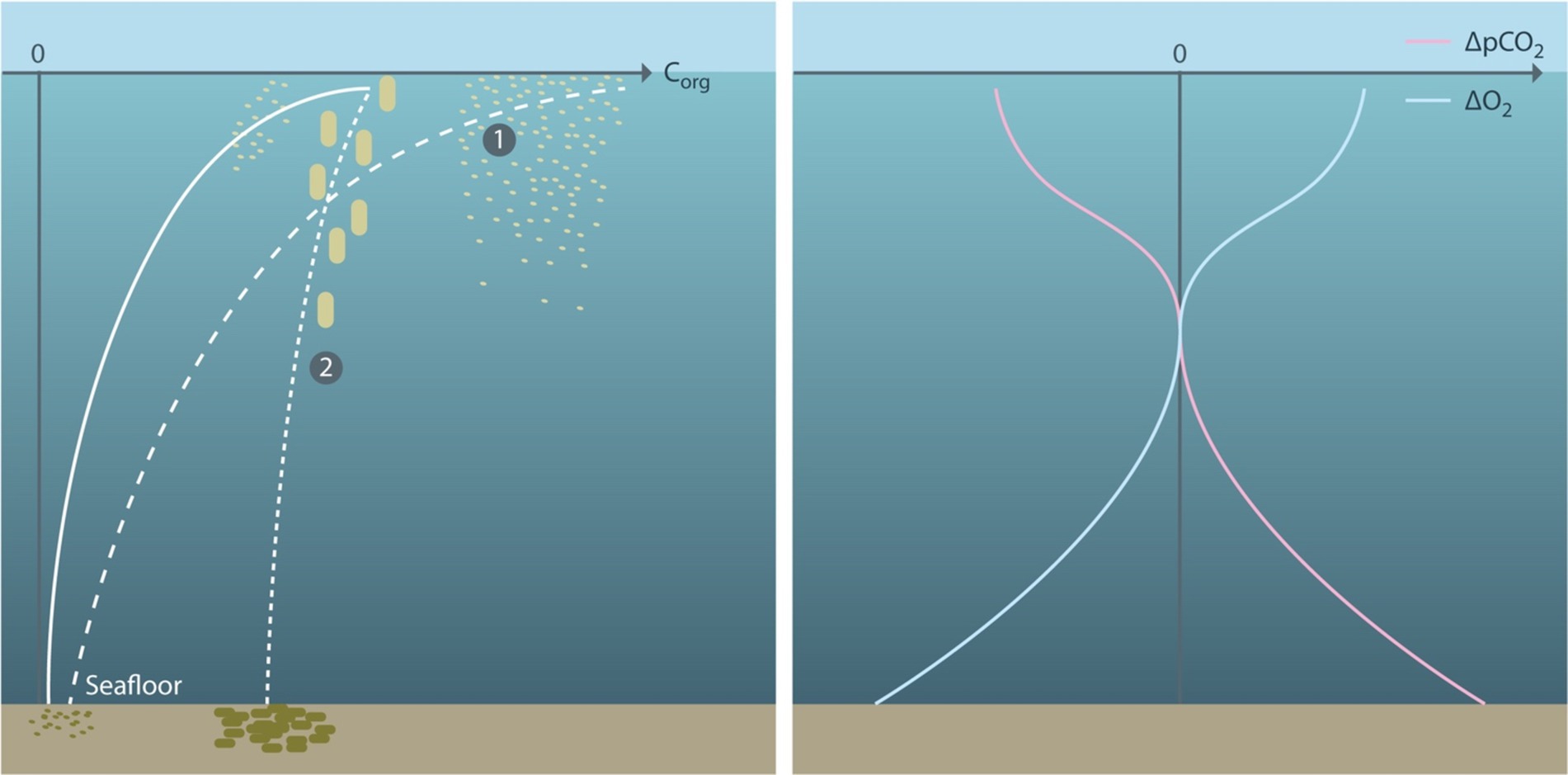
Figure 2. (Left) Schematic vertical profile of organic carbon produced photosynthetically near the sea surface, transported downward via a range of physical (e.g., sinking, mixing) and biotic (e.g., vertical migration) processes (solid white line). Globally, almost all organic matter is respired in the water column, with only a small portion (<1%) being buried in the sediment at the seafloor. The vertical profile of organic carbon, Corg, is maintained against respiratory loss by a continuous resupply of Corg from the sea surface. Biotic marine CDR can be achieved by (1) enhancing the photosynthetic production of organic carbon in the surface layer, or (2) generating a steeper vertical profile of organic carbon in the water column, e.g., via enhanced sinking speed or reduced remineralization rate. (Right) Directions of change in the vertical distributions of pCO2 (rose) and dissolved oxygen (blue) resulting from an enhanced biological carbon pump. Direction of changes in nutrients will generally follow those of pCO2. Air-sea fluxes of oxygen and CO2 will act to compensate mCDR induced changes in surface waters, with equilibration of CO2 being significantly slower than that of oxygen because of the buffering effect of carbonate chemistry (Jones et al., 2014).
In the ocean, the total amount of organic carbon is, at any time, about 600 GtC and thus much smaller than the 37,000 GtC of DIC (Friedlingstein et al., 2023). Conversions among inorganic and organic carbon, as well as gravitational settling of POC to depth provide a leverage by which the biotic cycling of organic carbon is responsible for more than two thirds of the vertical DIC gradient in the ocean (DeVries, 2022). The remaining third is due to the solubility pump that originates from the primarily temperature-dependent gas solubility and the lateral-vertical temperature gradient resulting from deep waters being formed at high latitudes.
While the BCP has substantial impact on the distribution of carbon, nutrients and oxygen in the ocean, it does, in steady state, not lead to a net CO2 uptake or release by the ocean because the downward flux of organic carbon is balanced by an upward return flux of remineralized DIC via ocean mixing and circulation (Frenger et al., 2024). Only a small portion of organic carbon leaving the surface layer (<1%) enters the sediment where storage times can be much longer than the millennial-scale overturning timescale of the ocean. Only about 15% of this burial occurs in water depths deeper than 1,000 m (Siegel et al., 2023). Most of the carbon burial happens on the continental shelves, where durability of storage can be affected by human activities such as bottom trawling (Atwood et al., 2024). Timescales on which DIC that is remineralized in the water column will eventually return to the ocean surface depend on location and depth where remineralization occurs, generally increasing with increasing depth, and typically amounting to decades to centuries (DeVries et al., 2012; Siegel et al., 2021; Ricour et al., 2023).
Changes in ocean carbon storage due to the BCP result from the net effect of changes in the downward flux of organic carbon and in the upward return flux of remineralized DIC (Frenger et al., 2024). Proposals to intentionally enhance the BCP with the aim to sequester additional CO2 from the atmosphere can be grouped into two categories: (1) increasing the amount of organic carbon produced in the surface ocean, and (2) changing the shape of the vertical organic carbon profile by accelerating the downward transport of organic carbon or reducing its remineralization rate (Figure 2). Assuming that ocean circulation is unchanged, both mechanisms would expand the duration of carbon storage away from the atmosphere and thereby lead to an additional, at least temporal, net removal of CO2 from the atmosphere. It is obvious that any modification of marine ecosystems will likely affect the endemic flora and fauna of the ocean (Boyd et al., 2022). In particular, an additional flux or storage of organic carbon will be associated with an additional flux or storage of nutrients, whereas respiration of the additional organic matter will lead to additional oxygen consumption (Figure 2).
In the following we will discuss (micro)nutrient fertilization as representative of category (1) and macroalgae cultivation and sinking or harvesting of category (2).
Microalgae are unicellular photosynthesizing organisms floating near-neutrally buoyant in the surface ocean. Ocean fertilization aims to increase photosynthetic carbon fixation through the addition of macronutrients (primarily nitrogen and/or phosphorus) or micronutrients (in particular iron) to the surface ocean where the added nutrient(s) would otherwise be limiting. Artificial upwelling of deep-water nutrients does not add nutrients to the ocean, but merely redistributes nutrients within the ocean. It has been considered in the context of microalgae and macroalgae fertilization and will be discussed in subsection 2.1.3 below.
Research on ocean fertilization has mainly focused on ocean iron fertilization (OIF), which is economically and logistically more attractive than macronutrient fertilization (but see Harrison, 2017) because relatively small amounts of the micronutrient iron added in regions with excess macronutrients may have the potential for a relatively large impact on the BCP (Martin, 1990). Research on OIF has progressed via, so far, 13 open ocean experiments since 1994, where in each experiment up to 4 tons of iron fertilizer was added in iron-deplete and macronutrient-replete regions such as the North Pacific or the Southern Ocean (Boyd et al., 2007; Yoon et al., 2018). These experiments have shown that OIF enhances marine photosynthetic CO2 fixation, which immediately reduces surface water pCO2 and thereby drives an additional flux of atmospheric CO2 into the ocean (Bakker et al., 2001). However, an unknown, and presumably large, fraction of the photosynthetically fixed carbon is converted back to CO2 via respiration already in the surface layer and may escape back into the atmosphere shortly after the iron fertilization (but possibly after the end of the experiments). Because export flux and the depth of remineralization and eventual deposition of additional CO2 depend on properties of the local ecosystem, the durability of the CO2 storage in the ocean after the deployment of ocean fertilization remains difficult to constrain, which is considered one of the biggest limitations of the approach (National Academies of Sciences, Engineering, and Medicine, 2021). Idealized modeling studies estimated that OIF, when continuously applied over the entire Southern Ocean or at ocean-basin to global scales, could sequester about 2–4 Gt CO2/year on a centennial timescale (Aumont and Bopp, 2006; Fu and Wang, 2022; Oschlies et al., 2010a; Tagliabue et al., 2023; Zahariev et al., 2008). As with other (including terrestrial) methods that enhance biological production and hence remineralization and recycling of nutrients, OIF is expected to enhance nitrification and the associated production of nitrous oxide, N2O, which is also produced as a by-product of denitrification occurring in oxygen-deficient areas. Enhanced production of N2O and its release to the atmosphere may offset a significant fraction of the CDR potential of OIF and other biotic approaches (Jin and Gruber, 2003).
Because of the removal of macronutrients from the fertilized surface areas and the associated reduced nutrient availability downstream, annual sequestration rates are expected to decrease over time and would approach zero after a few hundred years when a new steady-state of the BCP is reached that accounts for continuous OIF with iron-fertilized biological production balancing the return flux of remineralized CO2 to the surface ocean and hence atmosphere (Figure 3). Stopping OIF at any time would lead to a rearrangement of the BCP, a reduction of downward flux of organic matter and net outgassing of a substantial portion of the previously sequestered and remineralized CO2 (Figure 3; Keller et al., 2014). Ocean circulation models indicate that the durability of CDR via fertilization-induced injection of remineralized CO2 into the deep ocean can be a few hundred years (Siegel et al., 2021). Deployment of OIF would also lead to side-effects such as shifting geographical patterns of macronutrient concentrations (‘nutrient robbing’) and hence primary production, changes in local upper-ocean ecosystem structure, and additional deep ocean oxygen loss (Gnanadesikan et al., 2003).
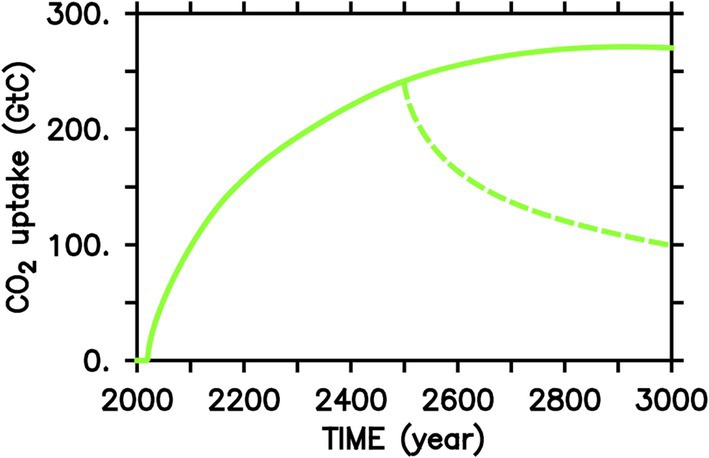
Figure 3. Cumulative air-sea ocean CO2 flux due to simulated continuous Southern Ocean iron fertilization (solid) started in year 2020 for a high-emission (RCP8.5) scenario until year 2,100, as in Keller et al. (2014) and a subsequent linear decrease to zero emissions in year 3,000 and thereafter. The dashed line shows the results of a simulation where iron fertilization is stopped in year 2,500. OIF-induced CO2 uptake saturates after a few hundred years.
Macroalgae are macroscopic, multicellular marine algae, such as seaweed or kelp. They generally require an attachment point and therefore commonly grow naturally in shallow coastal waters particularly of rocky shores, with free floating Sargassum fluitans and natans being two noteworthy exceptions. Farming of macroalgae has been proposed as mCDR approach via farming along floating structures providing attachment points, in the coastal and even open ocean, sometimes called ocean afforestation (N’Yeurt et al., 2012). By now several initiatives are exploring macroalgae cultivation and sinking. There are two fundamental differences with respect to CDR approaches based on microalgae discussed above: (i) Because of cellulose- and lignin-rich structural biomass, the carbon-to-nutrient ratios (carbon-to-nitrogen and carbon-to-phosphorus, but questioned for carbon-to-iron, see Paine et al., 2023) of macroalgae can be several-fold higher than that of microalgae, implying an excess production of organic carbon per units of nutrients available (Sheppard et al., 2023), i.e., contributing to category (1) in Figure 2. (ii) Once macroalgae sink (or are actively sunk via engineered processes in macroalgae farms), sinking velocities can be much faster and remineralization rates are slower than for microalgae, yielding deeper export and longer carbon sequestration times and thereby contributing to category (2) in Figure 2. Harvesting of macroalgae and possible safe storage on land or usage as feedstock for durable products can be viewed as an extreme case of (2) where no remineralization occurs in the water column, and hence no additional deep-ocean deoxygenation and acidification. Still, additional nutrient consumption in the surface ocean would be an issue.
Expected side effects of macroalgae farming are qualitatively similar to those of microalgae fertilization: the net removal of nutrients from the surface layer (‘nutrient robbing’) and, if macroalgae are sunk, enhanced oxygen consumption at depth, even though remineralization of at least structural parts of macroalgae may be slower than those of microbially produced organic matter. Modeling studies have shown that the availability of macronutrients poses limits to the overall CDR potential of macroalgae, which may be partly relaxed when combined with artificial upwelling to enhance the CDR potential of macroalgae farms (Berger et al., 2023; Wu et al., 2023; see subsection 2.1.3 below).
Investigations of natural occurrences of macroalgae have, in the meantime, raised concerns that initial assumptions about their CDR potential might have been too optimistic by neglecting effects such as nutrient-robbing, shading and partial offsetting of photosynthetic pCO2 reduction by calcification within macroalgae and their epibionts (Bach et al., 2021). Moreover, particularly high requirements of at least some macroalgae for dissolved iron may make it challenging to grow them in open-ocean waters remote from coastal areas (Paine et al., 2023). Macroalgae are also known for producing and emitting halocarbons (Leedham et al., 2013), which may impact atmospheric chemistry and eventually radiative forcing.
There are still fundamental research questions that need to be resolved before macroalgae afforestation can be considered a viable mCDR approach. These include the types and quantities of nutrients required to sustain offshore growth of macroalgae, and the quantification of the amount and durability of the additional CO2 removed. The durability of macroalgae carbon sunk into the deep ocean depends on poorly known respiration rates, but could be >1,000 years if the organic carbon is buried in the sediment (Krause-Jensen et al., 2018; Ortega et al., 2019). Microbial degradation of thick macroalgae mats on the seafloor may create anoxic regions where potent greenhouse gases like methane and N2O may be produced which, when degassing to the atmosphere, would diminish or offset the intended benefits for climate. Modeling studies indicate that naturally occurring upper ocean nutrient levels will, with the exception of few high-latitude regions of intermediate and deep-water formation with significant levels of unutilized nutrients (Carter et al., 2021), not be able to sustain large amounts of additional macroalgae (or microalgae) production (Berger et al., 2023; Wu et al., 2023). As with other mCDR approaches, the monitoring and verification of carbon removed is extremely challenging (Hurd et al., 2024).
Artificial upwelling (AU) of nutrient-rich waters from below the thermocline was first suggested as a means to enhance the biological carbon pump and the drawdown of atmospheric CO2 by Lovelock and Rapley (2007). Because respired CO2 is also upwelled together with the remineralized nutrients, modeling studies have found limited impact of artificial upwelling on the net marine CO2 uptake via stimulation of microalgae growth for a constant nutrient-to-carbon stoichiometry (Yool et al., 2009). The dominant effect of artificial upwelling was instead a temporary cooling of surface waters and hence the atmosphere, associated with a slowdown of terrestrial respiration and hence a temporary increase/decrease in the terrestrial/atmospheric carbon pool (Oschlies et al., 2010b; Keller et al., 2014). A more detailed modeling analysis by Jürchott et al. (2023) demonstrated that of AU-induced additional oceanic carbon uptake is strongly dependent on the emission scenario, with lower emission scenarios yielding lower AU-induced carbon uptake. This sensitivity is primarily due to the solubility pump that even yields CO2 outgassing by AU in low or zero emission scenarios (Jürchott et al., 2023). Further inspection of biogeochemical mechanisms showed that AU will lead to enhanced ocean carbon uptake by the biological pump only if supported by simultaneous iron fertilization (Jürchott et al., 2024). AU has also been suggested for enhancing the growth of carbon-rich macroalgae, where model studies show some potential when iron replete conditions are assumed (Berger et al., 2023; Wu et al., 2023).
A major concern of AU is that it also affects temperature and salinity, and hence ocean physics and will, in particular, weaken ocean stratification, which counteracts the ambition to keep deep-ocean carbon away from the atmosphere. Moreover, despite an initial cooling effect due to the upwelling of generally colder subsurface waters, altered thermal stratification and feedbacks on ocean circulation and the planet’s radiative balance could even lead to additional global warming (Oschlies et al., 2010a; Kwiatkowski et al., 2015). AU impacts on thermal stratification may be reduced by specially designed upwelling pipes that allow for the exchange of heat, but not salt. Such devices first suggested by Stommel et al. (1956) can under certain conditions lead to self-propelled upwelling while reducing some immediate impacts on the ocean’s thermal stratification compared to adiabatic upwelling (Kemper et al., 2023), but net effects of such an approach are still poorly understood.
While the use of renewable energy, e.g., from waves, has been suggested to drive artificial upwelling pumps, the technical challenges of pumping water up from several tens to a few hundred meters depth are substantial, and the few practical field trials so far did not last longer than a few days in the energetic marine environment (e.g., White et al., 2010).
Mangroves, salt marshes, and seagrass beds are vegetated coastal ecosystems that capture significant amounts of organic carbon in their soils and sediments, with decomposition processes inhibited by low oxygen availability and high salinity. Known as coastal blue carbon ecosystems, these environments are highly efficient at sequestering carbon per unit area (McLeod et al., 2011; Duarte et al., 2013), despite covering only about 0.5% of the ocean’s surface (Macreadie et al., 2021). Effective management of coastal blue carbon ecosystems (CBCEs) can enhance climate mitigation efforts in two primary ways: (1) conserving habitats to prevent the release of stored greenhouse gases, thereby avoiding emissions, and (2) enhancing CO2 removal through the restoration or creation of habitats.
The following discussion focuses on the restoration and creation of CBCEs, highlighting three key approaches that collectively contribute to increased atmospheric CO2 uptake by enhancing carbon burial and storage (Williamson and Gattuso, 2022). Firstly, resource management initiatives can be employed to enhance local environmental conditions, thereby promoting the natural functioning of these ecosystems. This can include restoring natural water dynamics to improve freshwater inflows, tidal exchanges, and sediment deposition; reducing pollution, particularly nutrient pollution; and reinstating natural predators to control organisms that hinder carbon storage. Secondly, efforts can be made to re-establish habitats that have been lost due to land-use changes or other coastal development. This typically involves planting seagrass or mangrove seedlings in appropriate coastal zones, either subtidal or intertidal. Thirdly, there is the creation of entirely new habitats, such as deliberately flooding areas to encourage the formation of salt marshes. Although not strictly restoration, this creation of habitats is often regarded as such in policy discussions.
While coastal blue carbon ecosystems offer potential for climate mitigation in terms of additional removal of CO2 from the atmosphere, the effectiveness and reliability to achieve quantified and secure carbon removal through these ecosystems remain uncertain, largely due to the following biogeochemical and socio-economic issues (Williamson and Gattuso, 2022):
• Additionality has to be demonstrated, i.e., all carbon fluxes must be evaluated with respect to the system that would be in place without the mCDR intervention.
• Carbon burial rates are difficult to measure reliably and are highly variable among different CBCEs. They are influenced by factors like sedimentation, microbial decomposition, and primary production. This complicates the estimation of their carbon sequestration potential, posing significant challenges for reliable carbon accounting.
• Lateral carbon transport: it is critical to identify the origin of the carbon stored in coastal sediments, differentiating between local (autochthonous) and non-local (allochthonous) sources. This distinction is crucial as non-local carbon does not contribute directly to atmospheric CO2 reduction, and its long-term storage might be incidental rather than a result of active carbon sequestration practices.
• CBCEs are known to emit methane (CH4) and nitrous oxide (N2O), both potent greenhouse gases. Such emissions can potentially negate the benefits of carbon burial, introducing additional complexity to the assessment of the overall climate impact of CBCE restoration efforts (Rosentreter et al., 2021). This factor must be carefully considered in evaluating the net effectiveness of restoration projects in these environments.
• The processes of carbonate formation and dissolution play a critical role in influencing CO2 levels, with the potential to release CO2 depending on how these processes are balanced. Gaining a deep understanding of these carbonate dynamics is crucial for accurately assessing the overall climatic effects of CBCEs.
• The susceptibility of CBCEs to both climatic factors such as sea-level rise, increased storminess, global warming, and ocean acidification, as well as non-climatic influences, poses a significant concern. These elements can compromise the structural integrity and functional capacity of CBCEs, thereby affecting their ability to store carbon over the long term.
• The cost-effectiveness of carbon removal varies greatly. Although the associated costs can be significant, the wider socio-economic advantages—including biodiversity conservation, coastal defense, and improved fisheries—often provide strong justification for these financial outlays.
The potential effectiveness of restoring coastal blue carbon ecosystems in terms of additional annual CO2 capture is estimated to range from 0.06 to 2.1 Gt CO2/yr (Williamson and Gattuso, 2022), which corresponds to about 0.02–6.6% of global CO2 emissions for 2020 (Friedlingstein et al., 2023). These figures underline the significant uncertainties listed above. Some estimates represent theoretical upper limits that may not be practical for policy formulation and implementation. For instance, historical losses of CBCEs, such as a 50% loss of mangroves since the 1940s, 29% of seagrass since 1879, and 25% of saltmarshes since the 1800s, cannot always be reversed. This is largely because much of the area previously occupied by these ecosystems has been developed in ways that make restoration either technically infeasible, economically prohibitive, or socially undesirable—for example, areas now used for urban development, port facilities, or tourism infrastructure.
The effectiveness of coastal vegetation restoration in mitigating global warming and its associated impacts has been evaluated as relatively low (Gattuso et al., 2018, 2021). However, it is crucial to recognize that restoring coastal blue carbon ecosystems offers significant benefits beyond climate mitigation. These include, if done appropriately, enhancing coastal protection, reducing pollution, supporting biodiversity, and improving food security (Macreadie et al., 2021). Such multifaceted benefits underscore the importance of these restoration efforts even if their direct impact on climate change is modest. Restoration of CBCEs is therefore considered a “low regret” or “no regrets” mitigation strategy (Gattuso et al., 2021).
In conclusion, while CBCEs offer potential benefits for climate mitigation through enhanced carbon sequestration, their practical application in national and international climate strategies must be approached with caution. The various challenges and uncertainties surrounding these ecosystems demand rigorous scientific research, dependable monitoring, and thorough evaluations to verify that they can provide the anticipated benefits to the climate.
Geochemical methods that have been suggested so far aim to increase the air-sea CO2 flux by altering the chemistry, and thereby reducing pCO2, of the surface waters in contact with the atmosphere. This can be achieved by reducing the DIC concentration of the surface waters via CO2 extraction from the sea water, also called direct ocean removal, or by enhancing the alkalinity and thereby shifting the DIC species from dissolved CO2 towards bicarbonate and carbonate ions. We will first discuss various approaches of alkalinity enhancement that mimic the naturally occurring chemical weathering of rocks and have already been studied for a few decades, before looking at direct ocean removal, which is a newly emerging field that has no direct natural analog.
Alkalinity is a metric that determines how much CO2 can be durably stored in seawater. The idea behind ocean alkalinity enhancement (OAE) is to enhance the transformation of dissolved CO2 into bicarbonate and carbonate ions and thereby reduce the partial pressure of CO2 by dissolving alkaline materials or via electrochemical methods. If this happens at the ocean surface, it will result in enhanced air-sea flux of CO2 into the ocean.
The concept of OAE is inspired by natural alkalinity production by weathering of silicate or carbonate rocks on land that removes atmospheric CO2 and, on geological timescales, compensates for CO2 emissions from volcanic activity. Weathering rates generally increase with temperature and thereby act as a natural thermostat that has been of critical importance to keeping the Earth a habitable planet for almost 4 billion years. Natural weathering is expected to reduce atmospheric CO2 to values close to pre-industrial, but only on timescales of order 105 years (Archer and Brovkin, 2008). The issue regarding OAE is thus not one of capacity, but rather of how to accelerate alkalinity input and storage of additional carbon in the ocean in a safe and efficient way (Renforth and Henderson, 2017). Durability of OAE-induced carbon storage is considered high and corresponds to the 105 years response timescale of the natural carbonate cycle. Main loss processes of alkalinity, and hence of potentially sequestered carbon, are spontaneous precipitation of carbonates at high carbonate saturation levels that may be reached upon addition of alkalinity (Fuhr et al., 2022; Moras et al., 2022; Hartmann et al., 2023) and the reduced dissolution of already existing carbonates relative to a baseline without OAE. Several ideas have been proposed and are being proposed to enhance the alkalinity of ocean surface waters (Eisaman et al., 2023), organized into 3 categories in the subsections below.
In this approach, first suggested by Kheshgi (1995), an enhancement of alkalinity is achieved by the addition of quick lime (CaO) or hydrated lime (Ca(OH)2) to the surface ocean. In contrast to naturally occurring limestone (CaCO3), lime readily dissolves in water in an exothermic reaction. Lime would have to be sourced through mining of limestone followed by an energy-intensive, industrial process that involves the calcination of limestone at high temperatures (Eisaman et al., 2023). Lime is already produced at large scale for use in steel and paper production, and in the building industry. However, even if no fossil fuel is used in the calcination of limestone, the process itself produces CO2. Thus, CO2 generated in the production of lime would have to be captured and stored in geological reservoirs to result in net carbon negativity (Renforth et al., 2013; Caserini et al., 2019). Life cycle analyses (i.e., the full accounting of carbon emissions and removal throughout all aspects of the overall process) suggest that it is feasible to accomplish net carbon negativity (Foteinis et al., 2022).
The high reactivity of lime can give rise to high pH and high calcite and aragonite oversaturation (Ωcalcite> > 1, Ωaragonite> > 1) in seawater which has been shown to trigger spontaneous precipitation of calcium carbonates (Moras et al., 2022, Hartmann et al., 2023). Such precipitation must be avoided because the process removes alkalinity and thus can greatly reduce or even eliminate the intended enhancement of seawater alkalinity and resulting carbon removal from the atmosphere. The appropriate dosing and dispersal of lime is thus an important research and operational question. One suggested option is deployment from ships that, by moving around enhance dispersal compared to fixed installations, and that has recently been found to be economically competitive with direct air capture of CO2 (Kowalczyk et al., 2024).
A more recent idea to realize ocean liming is via the conversion of limestone, typically occurring in the crystal forms of calcite and aragonite, into a more soluble crystal form such as ikaite (Renforth et al., 2022). As for classic ocean liming, calcite/aragonite would have to be mined and converted into ikaite in a facility in an energy-intense process, but without calcination and hence without process emissions of CO2. The ikaite is, however, not stable and needs to be added relatively quickly after its production into the ocean. Nevertheless, the process appears to have potential to be competitive with other OAE methods (Renforth et al., 2022).
Relevant in this context is that liming of acidic soils, rivers or lakes refers to the addition of calcium carbonate, i.e., limestone rather than lime (Henrikson and Brodin, 1995). Fossil fuel burning and fertilizer application on land have long lowered pH and alkalinity in rivers and lakes (Rotteveel et al., 2022), to levels at which limestone dissolves, in contrast to most ocean regions that are oversaturated. This has inspired river and freshwater liming programs in North America and in Europe for at least two decades (Henrikson and Brodin, 1995; Clair and Hindar, 2005), with the purpose of the restoration of degraded ecosystems and conservation of anadromous fishes, e.g., salmon (Mant et al., 2013). Continuous dosing of limestone into rivers has been shown to raise pH and alkalinity to within the critical ranges for healthy ecosystems (Henrikson and Brodin, 1995). The same process may be applicable for CDR because the added alkalinity eventually enters the ocean, where a carbonate chemistry different from that of freshwater systems may impact the stability of the added alkalinity. The CDR potential of river liming is just beginning to be explored (Sterling et al., 2023).
As alternative to lime with its energetically demanding calcination process, the addition of pulverized silicate or carbonate rocks, anthropogenic rock material such as concrete or steel slag, or their dissolution products has been proposed to enhance the alkalinity of the surface ocean (Rau and Caldeira, 1999; Caldeira and Rau, 2000; Renforth and Henderson, 2017; Renforth, 2019). The idea is to accelerate the slow, naturally occurring weathering of alkaline material (Archer et al., 2009) by increasing their effective surface area, and thus dissolution rate, before direct dispersion in the surface ocean or by inducing dissolution in reactors on land or on ships followed by dispersion of dissolved feedstock in the ocean (National Academies of Sciences, Engineering, and Medicine, 2021). For example, Rau and Caldeira (1999) demonstrated a process in which exposure of carbonate minerals to concentrated CO2, which could come from an industrial point source, and seawater led to rapid dissolution. Such reactor-based methods have some advantage regarding the quantification of CO2 removed, but would only count as CDR if the CO2 is taken from the atmosphere, e.g., via biomass.
In the open ocean with its much larger contact area and dilution and storage capacity, solid alkaline materials generally dissolve relatively slowly, despite their pulverization, although dissolution rates vary greatly across considered alkaline feedstocks (e.g., olivine, slag, see Table 1 in Renforth and Henderson, 2017). For application in the open ocean, the materials would have to dissolve before sinking out of the surface layer which exchanges CO2 with the atmosphere. This requires small particles, which have lower sinking rates and larger effective surface area and thus dissolution rates than larger particles, but grinding requires energy (Strefler et al., 2018). The economically optimal particle size will depend on the specific material and its dissolution rate but also the oceanographic regime where particles are to be added (Yang and Timmermans, 2024).
In coastal regions where the water column is seasonally mixed all the way down to the sediments, it may be less crucial for particles to dissolve in the mixed layer because the alkalinity resulting from particles that dissolve below the mixed layer or on the seabed will be exposed to the surface ocean within a year or less. However, since seabed sediments can be a natural source of alkalinity questions arise about accurate quantification of the sediment-water flux of alkalinity and how much of this flux is the direct result of added feedstock (Bach, 2024).
Some coastal regions such as the Baltic Sea or eastern boundary upwelling regions include regions with very low or zero oxygen levels at relatively shallow depths and exchanging with the atmosphere on annual or multi-annual to multi-decadal timescales. The oxygen deficit results from respiration of organic matter that also releases CO2 and can make these water undersaturated in CaCO3 (Harvey, 2008). In these regions, limestone added to the sea floor dissolves relatively quickly, as observed for naturally occurring erosion of carbonate-bearing sedimentary rocks (Wallmann et al., 2022). Incubation experiments reveal a substantial potential for marine CDR by adding calcite to the seafloor (Fuhr et al., 2024; Dale et al., 2024).
Another major question regarding material addition to seawater concerns the effects of silicate and trace metals, which will be present in alkaline materials in varying quantities, on primary producers, higher trophic level species, and the ecosystem (Guo et al., 2024). Application of silicate favors diatoms (Ferderer et al., 2024), an important group of primary producers, that is generally considered desirable and may contribute to additional carbon export via the biological carbon pump. Some trace metals like iron and magnesium may also stimulate productivity, while others including nickel, copper, cadmium, and chromium may pose risks to ecosystems and humans, especially if they accumulate in higher trophic level species (Garai et al., 2021). The high nickel content of olivine is in particular focus (Montserrat et al., 2017).
In a nutshell, electrochemical OAE methods enhance seawater alkalinity by removing acids from seawater while leaving bases behind (House et al., 2007; Rau, 2008; Rau et al., 2013). Technically, seawater is pumped into an electrochemical reactor where seawater (or brine derived from seawater through previous desalination) is converted into a strong acid (mostly hydrochloric acid, HCl) and a strong base (mostly sodium hydroxide, NaOH). Alkaline NaOH is discharged back into the oceans, constituting OAE and increasing seawater pH, transforming dissolved CO2 into bicarbonate and carbonate ions and causing a decline in surface ocean pCO2.
Electrochemical conversion of seawater into acid and base requires (renewable) energy and can be accomplished via electrolysis or electrodialysis with the former generating higher concentration acid and base but also being more energy-intensive (Eisaman et al., 2023). Electrolysis essentially uses the well-known chlor-alkali process, whereas electrodialysis relies on bipolar membranes and ion-selective separation to enhance the natural dissociation of water into H+ and OH− (Eisaman et al., 2023).
The primary by-products of electrolytic alkalinity generation are Cl2 and H2 gases. Scaling up this process would likely require the development of more efficient technology and production of O2 instead of Cl2 (La Plante et al., 2023) or a strategy to use or dispose of the highly reactive chlorine gas produced. The primary by-product of electrodialysis is strong acid, e.g., HCl. Producing enough alkalinity for CDR on gigaton scales would require significant upscaling of currently available industrial capacity for processing and finding a use or safe disposal method for the large quantities of acid produced as a by-product (Eisaman et al., 2023). One possibility is to react HCl with silicate rocks, mimicking the naturally occurring weathering (House et al., 2007).
The addition of the electrochemically generated alkalinity back into the ocean requires similar considerations as ocean liming. Specifically, the addition rate must be sufficiently low to avoid high pH and CaCO3 oversaturation that could trigger spontaneous precipitation of calcium carbonate or magnesium hydroxides (Moras et al., 2022; Hartmann et al., 2023). Partial pre-equilibration of alkalinity with atmospheric CO2, in effect converting some hydrogen ions (OH−) into safe and stable carbonate ions (CO32−), has been proposed to mitigate this risk (Stolaroff et al., 2008). Evidence available so far suggests that plankton communities show comparatively little response to strong perturbations to OAE via NaOH (Ferderer et al., 2022; Marin-Samper et al., 2024).
Direct ocean removal (DOR) aims to extract CO2 from seawater in a designated facility, e.g., via electrochemistry powered by renewable energy. The CO2 extracted from seawater is sequestered in geological storages underground or utilized in a way that durably stores the carbon. The CO2-depleted seawater is released out of the facility back into the surface ocean, where it can absorb atmospheric CO2 via gas exchange through the air-sea interface. Because of ocean mixing and circulation, contact times of the CO2-depleted seawater with the atmosphere will be finite and equilibration incomplete. Should the CO2 extracted via DOR be used for short-lived products such as synthetic fuels, the net result would be an increase in atmospheric CO2 on the expense of the depletion of the ocean carbon reservoir. Storage durability of the extracted CO2 is key, as the ocean already represents a durable storage system, and DOR must not lead to a net reduction in the durability of CO2 storage. It is important to note that DOR itself does not represent CDR, but only the subsequent air-sea flux of CO2 is climatically relevant and counts as CDR. This has implications for monitoring, reporting and verification (see below).
Technically, DOR utilizes electrochemical methods that are mostly identical to electrochemical methods used for OAE discussed above. However, instead of sequestering the acid and maintaining the base in seawater as in OAE, for DOR the acid is used to acidify seawater and convert (bi)carbonate into CO2 which is extracted via vacuum. After CO2 extraction, the acidified seawater is neutralized with the base, thereby leaving the chemical composition (including its alkalinity) of outflowing seawater unaltered except for the removal of CO2. Electrochemical procedures are generally more energy-efficient under higher salinity, which is why DOR is often planned in association with seawater desalination plants which provide brines.
A recent variety for DOR is one where solar energy is used to extract CO2 from seawater (Saha et al., 2024). Here, molecules that increase their acidity upon the provision of light (photoacids), are used to acidify seawater and transform (bi)carbonate into CO2. As for electrochemical methods, CO2 is extracted, captured and stored, while the CO2 depleted seawater is released into the surface ocean after recovery of the photoacids.
DOR has the advantage that the removal of CO2 from seawater can precisely be quantified since all major steps occur in a fully engineered facility, i.e., a closed system. However, the removal of CO2 from the atmosphere, constituting CDR, occurs outside of the facility in the open system. The durability of mCDR via DOR can be very high (> > 1,000 years) if the CO2 is subsequently stored in appropriate underground reservoirs and if the CO2 deficit in the surface water is topped up by CO2 from the atmosphere.
With regard to environmental effects, the method extracts CO2 and leaves lower-CO2 seawater behind, essentially the opposite process of ocean acidification. As such, thresholds on surface-water CO2 levels can be defined so that immediate changes are in the direction of pre-industrial conditions and that environmental effects on surface ocean biota will likely be small. Effects are likely more extreme for organisms that may be drawn into the DOR facility and associated with the necessary pumping of large volumes of water through the system components and considerable acid–base swings within the facility. For installations on the coast there will also be environmental impacts on terrestrial ecosystems associated with the footprint of the facility itself and the required energy supply/CO2 disposal chains.
A fundamental development over the last years was the recognition that mCDR, regardless of its scale, must be monitored, reported, and ultimately verified by independent parties. Monitoring, reporting, and verification (MRV) is needed to constrain the uncertainty with which the implementation of a mCDR method has led to additional removal of CO2 from the atmosphere compared to a baseline without the implementation (Doney et al., 2024). Particularly attractive from an MRV viewpoint would be closed-system approaches where input and output of CO2 can be directly measured, e.g., in greenhouses or chemical reactors. However, these do not readily exist for mCDR methods that use the sea surface as an absorber for CO2 from the atmosphere. Even apparent closed-system approaches such as alkalinity addition from chemical reactors or direct ocean removal of CO2 in specific facilities require the open-system air-sea flux of CO2 to realize the reduction in atmospheric CO2 compared to a situation without this activity, and only then count as mCDR. In fact, a main argument in favor of mCDR is the large area of the CO2-absorbing sea surface in contact with the atmosphere. Because of the slow CO2 gas equilibration of typically several months (Jones et al., 2014), which in the moving ocean translate into large regions in space, mCDR-induced air-sea flux is challenging to measure directly and its quantification will most likely include estimates involving numerical models (Ho et al., 2023).
The challenge of estimating the mCDR-induced air-sea CO2 flux is essentially the same for most biotic and all geochemical methods that aim to reduce pCO2 in the ocean’s surface waters, the only exception being mangroves and salt marshes that extend above the sea surface and take up CO2 directly from the air. However, different methods differ in the way they reduce surface ocean pCO2, in the way additionality with respect to a situation without mCDR deployment can be determined, and in the durability of the sequestration of CO2. Therefore, the need for MRV challenges different mCDR methods differently: Arguably, methods associated with relatively high physico-biogeochemical complexity are harder to monitor, report, and verify than those where complexity is low (Bach et al., 2024). For example, biotic mCDR with iron fertilization driven by a series of processes in the marine food web and involving downward transport of organic matter will likely be associated with higher uncertainty than a simpler and more local geochemical method like electrodialytical alkalinity enhancement, which is based upon well understood processes in inorganic carbonate chemistry. As such, MRV requirements may well have an influence on the potential upscaling of the different mCDR methods, possibly favoring those with fewer MRV challenges and uncertainties.
In practice, MRV for mCDR requires budgeting the net climatic benefit of individual mCDR deployments and data integration under a globally consistent MRV protocol. Currently, MRV for mCDR is comparatively underdeveloped with respect to some other CDR methods such as direct air capture or afforestation (Arcusa and Sprenkle-Hyppolite, 2022), thereby providing a unique window of opportunity for the marine science community to help build MRV protocols that are less fragmented than in other CDR sectors. This effort is unprecedented and requires international coordination, collaboration and standardization. Practical needs involve the development of common standards, for example: Does MRV require to be focused on CDR or on changes in radiative forcing? Or: how far into the future must carbon be monitored to be deemed “durably removed from the atmosphere”? Furthermore, open availability of data and common standards for their generation is needed to allow verification by independent parties. Globally coordinated remote sensing programs like Argo are an ideal basis for such efforts but need to be expanded to become more useful for mCDR, where perturbations of the carbon cycle are at least initially very small scale (Boyd et al., 2023). Accessible observational data will be crucial for setting baselines before a potential mCDR deployment and also to improve numerical models (Fennel et al., 2023). Eventually, and should mCDR be upscaled to larger scales, observations may also be used to directly detect environmental perturbations associated with mCDR. However, in the medium term, it is likely that observations have their biggest value in helping to develop MRV modeling tools since detection limits of analytical tools and insufficient spatio-temporal measurements will impede MRV through direct observations (Ho et al., 2023).
While MRV of additional CO2 taken up by individual mCDR deployments would be a necessary requirement for accurate accounting and certification schemes, specific MRV may also be required for possible environmental side effects. Expected side effects can be of different quality for different mCDR methods, and assessing these will require definition of a baseline. With ocean warming, acidification, deoxygenation and other natural and anthropogenic environmental changes under way, the assumption of a static baseline will not be adequate and global ocean observation systems will be required to reliably monitor environmental change across the global ocean. Attribution of observed changes to natural variability, fossil fuel emissions or mCDR will be challenging, will require appropriate observing systems and likely have to involve numerical models in order to interpolate information across different observations and space- and time-scales, and also to estimate uncertainties.
The main arguments in favor of considering mCDR are the large contact area of the ocean surface with the atmosphere and the knowledge that the ocean has, in the geological past of the planet, repeatedly removed huge quantities of CO2 from the atmosphere, albeit on timescales of thousands to hundred thousands years. On the other hand, any mCDR deployment would present an intervention into the marine environment that is often regarded as deserving -and requiring- specific protection (with fisheries sometimes treated as one exception). This is also expressed in the recent and internationally binding Agreement under the United Nations Convention on the Law of the Sea (UNCLOS) on the Conservation and Sustainable Use of Marine Biological Diversity of Areas Beyond National Jurisdiction (BBNJ, Ricard, 2023).
Marine CDR is, until now, explicitly governed only with respect to ocean fertilization activities in the International Regime for the control of dumping of wastes and other matter into the marine environment (London Protocol). This represents a first international governance effort in response to the early activities on ocean iron fertilization about two decades ago, where scientific research quickly inspired private enterprises to utilize the method for CDR. According to the London Protocol only legitimate scientific research may be allowed for ocean fertilization (Brent et al., 2019). In 2022, parties of the London Protocol started a process considering whether and how to regulate four emerging marine geoengineering techniques, namely enhancing ocean alkalinity, macroalgae cultivation and other biomass for sequestration including artificial upwelling, and two ocean-based solar radiation management approaches. Enhancement of coastal vegetated ecosystems generally falls within national jurisdiction.
It is interesting to compare the current state of mCDR governance with that of direct injection of fossil CO2 directly into deep ocean waters. Direct injection had been suggested several decades ago in a paper that introduced this as ‘geoengineering’ (Marchetti, 1977). At that time, the proposed activity had CO2 from emissions-avoiding fossil-fuel carbon-capture installations in mind, but one could, in principle, imagine combining CDR approaches like direct air capture, biomass energy and carbon capture, or direct ocean removal with direct injection. Concerns about initial research efforts and proposals for field trials, specifically regarding possible harm to deep-ocean ecosystems led to international prohibition of direct injection, implicitly under the 2006 amendment of the London Protocol and explicitly by the Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR; Leung et al., 2014).
While we have no recommendation whether direct injection of CO2 into the water should be done or whether its governance should be changed, we here suggest to consider it as a well-defined example against which other proposed mCDR methods should be judged. From a climate perspective, a hypothetical, perfectly working BCP enhancement would also inject (respired) CO2 into the deep ocean, with potential impacts on the marine carbon chemistry and with issues regarding durability of the storage similar to those that would result from appropriately designed direct injection. In contrast to direct injection of CO2, however, the enhancement of the BCP not only increases deep-ocean CO2 and acidity, but also intentionally manipulates marine ecosystems to drive CDR. In consequence it changes biodiversity as well as nutrient and oxygen concentrations at the ocean surface and at depth (Figure 2). It is interesting that, given the internationally agreed prohibition of direct injection of CO2 for almost 20 years, there is so much, and growing, interest from scientists, industry and policymakers in developing approaches for BCP enhancement that would impact the marine environment in more dimensions than direct injection of CO2. A comprehensive discussion about large-scale deployment of BCP enhancement should also reflect calls for reconsidering direct injection (Keeling, 2009) of CO2 removed from the atmosphere,
The comparison with direct injection of CO2 is different for geochemical mCDR methods. Here, inorganic chemistry drives CDR and biotic processes are not targeted intentionally. Immediate storage of additionally sequestered CO2 does not happen at depth but in the surface waters. Sequestration and storage does not aggravate ocean acidification or ocean deoxygenation, neither at the surface nor at depth. Still, the addition of alkalinity does change ocean chemistry, which can affect marine biota and therefore needs to be studied very carefully before decisions about deployment can be made (Bach et al., 2019). From what is known from theoretical and modeling studies, geochemical methods appear to have a larger CDR potential and longer durability of the stored carbon compared to biotic methods. While geochemical methods may be applied in a way that minimizes impacts on marine biota and ecosystems, biotic methods explicitly impact ecosystems, often, and (particularly in the case of coastal blue carbon ecosystems) with the intention to restore systems that have been recently lost due to climate change or other anthropogenic actions. Despite their apparently larger potential and fewer ecological side effects compared to biotic methods, geochemical approaches are sometimes associated with ‘chemistry’ versus ‘biology’ and framed as ‘technological” versus ‘natural’ approaches. Framing is of high relevance for social and political acceptance, but it is subjective, often inaccurate and it may not always align with what is optimal from a climate viewpoint (Bellamy and Osaka, 2020; Bertram and Merk, 2020). Transparent interdisciplinary scientific assessments of different CDR methods with respect to different indicators can provide unbiased information to society and policymakers that could help to make informed decisions about possible CDR pathways.
Given the urgency of the climate problem and the failure of sufficient emissions reductions over the past decades, scientific evidence now indicates that deployment of CDR will be unavoidable if promised climate targets are to be reached (IPCC, 2022). Scaling up CDR fast enough so that it may allow compensating for residual emissions by mid-century appears extremely challenging (Smith et al., 2023), and it is clear that CDR cannot replace, but only complement, drastic emissions reduction.
The role of mCDR in the global CDR portfolio needs to be clarified in an inter- and transdisciplinary approach. Natural sciences need to quantify the CDR potential and environmental risks, and also develop concepts for reliable MRV and uncertainty assessments. Social sciences and humanities have to investigate appropriate governance schemes, political and social pathways and economic feasibility that could allow for responsible deployment of CDR. In contrast to at least some terrestrial CDR methods, the impacts of mCDR cannot be restricted to small regions or territories of individual states. This makes it mandatory to develop international regimes for responsible governance, but also for transparent monitoring, for which appropriate tools need to be developed. The World Climate Research Program could play an important role in this, including the provision of unbiased scientific understanding of processes and feedbacks in the Earth system, and the development of observing networks required for an appropriate description of the baseline state of the ocean as well as for independent monitoring and verification of possible deployments.
AO: Conceptualization, Writing – original draft, Writing – review & editing. LB: Writing – original draft, Writing – review & editing. KF: Writing – original draft, Writing – review & editing. J-PG: Writing – original draft, Writing – review & editing. NM: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. AO acknowledges funding by the German Federal Ministry of Education and Research (grant no. 03F0965A; project RETAKE, DAM Mission “Marine carbon sinks in decarbonization pathways’, CDRmare) and the European Union’s Horizon Europe research and innovation program (grant no. 101081362; project SEAO2-CDR). LB acknowledges funding from the Australian Research Council via Future Fellowship (grant no. FT200100846) and the Carbon to Sea Initiative.
AO acknowledges helpful comments from Wolfgang Koeve.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Archer, D., and Brovkin, V. (2008). The millennial atmospheric lifetime of anthropogenic CO2. Clim. Chang. 90, 283–297. doi: 10.1007/s10584-008-9413-1
Archer, D., Eby, M., Brovkin, V., Ridgwell, A., Cao, L., Mikolajewicz, U., et al. (2009). Atmospheric lifetime of fossil fuel carbon dioxide. Annu. Rev. Earth Planet. Sci. 37, 117–134. doi: 10.1146/annurev.earth.031208.100206
Arcusa, S., and Sprenkle-Hyppolite, S. (2022). Snapshot of the carbon dioxide removal certification and standards ecosystem (2021–2022). Clim. Pol. 22, 1319–1332. doi: 10.1080/14693062.2022.2094308
Atwood, T. B., Romanou, A., DeVries, T., Lerner, P. E., Mayorga, J. S., Bradley, D., et al. (2024). Atmospheric CO2 emissions and ocean acidification from bottom-trawling. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1125137
Aumont, O., and Bopp, L. (2006). Globalizing results from ocean in situ iron fertilization studies. Glob. Biogeochem. Cycles 20:GB2017. doi: 10.1029/2005GB002591
Bach, L. T. (2024). The additionality problem of ocean alkalinity enhancement. Biogeosciences 21, 261–277. doi: 10.5194/bg-21-261-2024
Bach, L. T., Gill, S. J., Rickaby, R. E. M., Gore, S., and Renforth, P. (2019). CO2 removal with enhanced weathering and ocean alkalinity enhancement: potential risks and CO-benefits for marine pelagic ecosystems. Front. Climate 1:7. doi: 10.3389/fclim.2019.00007
Bach, L. T., Tamsitt, V., Gower, J., Hurd, C. L., Raven, J. A., and Boyd, P. W. (2021). Testing the climate intervention potential of ocean afforestation using the great Atlantic Sargassum Belt. Nat. Commun. 12:2556. doi: 10.1038/s41467-021-22837-2
Bach, L. T., Vaughan, N. E., Law, C. S., and Williamson, P. (2024). Implementation of marine CO2 removal for climate mitigation: the challenges of additionality, predictability, and governability. Elementa 12. doi: 10.1525/elementa.2023.00034
Bakker, D. C. E., Watson, A. J., and Law, C. S. (2001). Southern Ocean iron enrichment promotes inorganic carbon drawdown. Deep-Sea Res. II Top. Stud. Oceanogr. 48, 2483–2507. doi: 10.1016/S0967-0645(01)00005-4
Bellamy, R., and Osaka, S. (2020). Unnatural climate solutions? Nat. Clim. Chang. 10, 98–99. doi: 10.1038/s41558-019-0661-z
Berger, M., Kwiatkowski, L., Ho, D. T., and Bopp, L. (2023). Ocean dynamics and biological feedbacks limit the potential of macroalgae carbon dioxide removal. Environ. Res. Lett. 18:024039. doi: 10.1088/1748-9326/acb06e
Bertram, C., and Merk, C. (2020). Public perceptions of ocean-based carbon dioxide removal: the nature-engineering divide? Front. Climate 2:31. doi: 10.3389/fclim.2020.594194
Boettcher, M., Brent, K., Buck, H. J., Low, S., McLaren, D., and Mengis, N. (2021). Navigating potential hype and opportunity in governing marine carbon removal. Front. Clim. 3:664456. doi: 10.3389/fclim.2021.664456
Boyd, P. W., Bach, L. T., Holden, R., and Turney, C. (2023). Carbon offsets aren’t helping the planet – for ways to fix them. Nature 620, 947–949. doi: 10.1038/d41586-023-02649-8
Boyd, P. W., Bach, L. T., Hurd, C. L., Paine, E., Raven, J. A., and Tamsitt, V. (2022). Potential negative effects of ocean afforestation on offshore ecosystems. Nat. Ecol. Evol. 6, 675–683. doi: 10.1038/s41559-022-01722-1
Boyd, P. W., Jickells, T., Law, C. S., Blain, S., Boyle, E. A., Buesseler, K. O., et al. (2007). Mesoscale iron enrichment experiments 1993-2005: synthesis and future directions. Science 315, 612–617. doi: 10.1126/science.1131669
Brent, K., Burns, W., and McGee, J. (2019). Governance of marine geoengineering. Special Report, Centre for International Governance Innovation.
Caldeira, K., and Rau, G. H. (2000). Accelerating carbonate dissolution to sequester carbon dioxide in the ocean: geochemical implications. Geophys. Res. Lett. 27, 225–228. doi: 10.1029/1999GL002364
Carlson, C. A., Bates, N. R., Hansell, D. A., and Steinberg, D. K. (2001). “Carbon cycle” in Encyclopedia of ocean science. eds. J. Steele, S. Thorpe, and K. Turekian. 2nd ed (Oxford: Academic Press), 477–486.
Carroll, D., Menemenlis, D., Dutkiewicz, S., Lauderdale, J. M., Adkins, J. F., Bowman, K. W., et al. (2022). Attribution of space-time variability in global-ocean dissolved inorganic carbon. Glob. Biogeochem. Cycles 36:e2021GB007162. doi: 10.1029/2021GB007162
Carter, B. R., Feely, R. A., Lauvset, S. K., Olsen, A., DeVries, T., and Sonnerup, R. (2021). Preformed properties for marine organic matter and carbonate mineral cycling quantification. Glob. Biogeochem. Cycles 35:e2020GB006623. doi: 10.1029/2020GB006623
Caserini, S., Barreto, B., Lanfredi, C., Cappello, G., Ross Morey, D., and Grosso, M. (2019). Affordable CO2 negative emission through hydrogen from biomass, ocean liming, and CO2 storage. Mitig. Adapt. Strateg. Glob. Change 24, 1231–1248. doi: 10.1007/s11027-018-9835-7
Clair, T. A., and Hindar, A. (2005). Liming for the mitigation of acid rain effects in freshwaters: a review of recent results. Environ. Rev. 13, 91–128. doi: 10.1139/a05-009
Dale, A. W., Geilert, S., Diercks, I., Fuhr, M., Perner, M., Scholz, F., et al. (2024). Seafloor alkalinity enhancement as a carbon dioxide removal strategy in the Baltic Sea. Commun. Earth Environment 5:452. doi: 10.1038/s43247-024-01569-3
DeVries, T. (2022). The ocean carbon cycle. Annu. Rev. Environ. Resour. 47, 317–341. doi: 10.1146/annurev-environ-120920-111307
DeVries, T., Primeau, F., and Deutsch, C. (2012). The sequestration efficiency of the biological pump. Geophys. Res. Lett. 39:L13601. doi: 10.1029/2012GL051963
Doney, S. C., Wolfe, W. H., McKee, D. C., and Fuhrman, J. G. (2024). The science, engineering, and validation of marine carbon dioxide removal and storage. Annu. Rev. Mar. Sci. 17. doi: 10.1146/annurev-marine-040523-014702
Duarte, C. M., Losada, I. J., Hendriks, I. E., Mazarrasa, I., and Marbà, N. (2013). The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 3, 961–968. doi: 10.1038/nclimate1970
Eisaman, M.D., Geilert, S., Renforth, P., Bastianini, L., Campbell, J., Dale, A.W., et al. (2023). Assessing the technical aspects of ocean-alkalinity-enhancement approaches, in: Guide to best practices in ocean alkalinity enhancement research, edited by: Oschlies, A., Stevenson, A., Bach, L.T., Fennel, K., Rickaby, R. E.M., Satterfield, T., et al., Copernicus Publications, State Planet, 2-oae2023:3
Fay, A. R., Munro, D. R., McKinley, G. A., Pierrot, D., Sutherland, S. C., Sweeney, C., et al. (2024). Updated climatological mean DfCO2 and net sea–air CO2 flux over the global open ocean regions. Earth System Science Data 16, 2123–2139. doi: 10.5194/essd-16-2123-2024
Fennel, K., Long, M.C., Algar, C., Carter, B., Keller, D., Laurent, A., et al. (2023). Modelling considerations for research on ocean alkalinity enhancement, in: Guide to best practices in ocean alkalinity enhancement research, edited by: Oschlies, A., Stevenson, A., Bach, L.T., Fennel, K., Rickaby, R.E.M., Satterfield, T., et al. Copernicus Publications, State Planet
Ferderer, A., Chase, Z., Kennedy, F., Schulz, K. G., and Bach, L. T. (2022). Assessing the influence of ocean alkalinity enhancement on a coastal phytoplankton community. Biogeosciences 19, 5375–5399. doi: 10.5194/bg-19-5375-2022
Ferderer, A., Schulz, K. G., Riebesell, U., Baker, K. G., Chase, Z., and Bach, L. T. (2024). Investigating the effect of silicate- and calcium-based ocean alkalinity enhancement on diatom silicification. Biogeosciences 21, 2777–2794. doi: 10.5194/bg-21-2777-2024
Foteinis, S., Andresen, J., Campo, F., Caserini, S., and Renforth, P. (2022). Life cycle assessment of ocean liming for carbon dioxide removal from the atmosphere. J. Clean. Prod. 370:133309. doi: 10.1016/j.jclepro.2022.133309
Frenger, I., Landolfi, A., Kvale, K., Somes, C. J., Oschlies, A., Yao, W., et al. (2024). Misconceptions of the marine biological carbon pump in a changing climate: thinking outside the “export” box. Glob. Chang. Biol. 30:e17124. doi: 10.1111/gcb.17124
Friedlingstein, P., O'Sullivan, M., Jones, M. W., Andrew, R. M., Bakker, D. C. E., Hauck, J., et al. (2023). Global carbon budget 2023. Earth System Science Data 15, 5301–5369. doi: 10.5194/essd-15-5301-2023
Fu, W., and Wang, W.-L. (2022). Biogeochemical equilibrium responses to maximal productivity in high nutrient low chlorophyll regions. Journal of geophysical research. Biogeosciences 127:e2021JG006636. doi: 10.1029/2021JG006636
Fuhr, M., Geilert, S., Schmidt, M., Liebetrau, V., Vogt, C., Ledwig, B., et al. (2022). Kinetics of olivine weathering in seawater: an experimental study. Front. Climate 4. doi: 10.3389/fclim.2022.831587
Fuhr, M., Wallmann, K., Dale, A. W., Kalapurakkal, H. T., Schmidt, M., Sommer, S., et al. (2024). Alkaline mineral addition to anoxic to hypoxic Baltic Sea sediments as a potentially efficient CO2-removal technique. Front. Climate 6:1338556. doi: 10.3389/fclim.2024.1338556
Garai, P., Banerjee, P., Modal, P., and Saha, N. C. (2021). Effect of heavy metals on fishes: toxicity and bioaccumulation. J. Clinical Toxicol. 11, 1–10. doi: 10.35248/2161-0495.21.s18.001
Gattuso, J.-P., Magnan, A. K., Bopp, L., Cheung, W. W. L., Duarte, C. M., Hinkel, J., et al. (2018). Ocean solutions to address climate change and its effects on marine ecosystems. Front. Mar. Sci. 5:337. doi: 10.3389/fmars.2018.00337
Gattuso, J.-P., Williamson, P., Duarte, C. M., and Magnan, A. K. (2021). The potential for ocean-based climate action: negative emissions technologies and beyond. Front. Climate 2:37. doi: 10.3389/fclim.2020.575716
GESAMP (2019). “High level review of a wide range of proposed marine geoengineering techniques” in IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UN environment/UNDP/ISA joint Group of Experts on the scientific aspects of marine environmental protection. eds. P. W. Boyd and C. M. G. Vivian (London, UK: International Maritime Organization).
Gnanadesikan, A., Sarmiento, J., and Slater, R. (2003). Effects of patchy ocean fertilization on atmospheric carbon dioxide and biological production. Glob. Biogeochem. Cycles 17:1050. doi: 10.1029/2002GB001940
Gruber, N., Bakker, D. C. E., DeVries, T., Gregor, L., Hauck, J., Landschützer, P., et al. (2023). Trends and variability in the ocean carbon sink. Nat. Rev. Earth Environ. 4, 119–134. doi: 10.1038/s43017-022-00381-x
Guo, J. A., Strzepek, R. F., Swadling, K. M., Townsend, A. T., and Bach, L. T. (2024). Influence of ocean alkalinity enhancement with olivine or steel slag on a coastal plankton community in Tasmania. Biogeosciences 21, 2335–2354. doi: 10.5194/bg-21-2335-2024
Harrison, D. P. (2017). Global negative emissions capacity of ocean macronutrient fertilization. Environ. Res. Lett. 12:035001. doi: 10.1088/1748-9326/aa5ef5
Hartmann, J., Suitner, N., Lim, C., Schneider, J., Marín-Samper, L., Arístegui, J., et al. (2023). Stability of alkalinity in ocean alkalinity enhancement (OAE) approaches – consequences for durability of CO2 storage. Biogeosciences 20, 781–802. doi: 10.5194/bg-20-781-2023
Harvey, L. D. D. (2008). Mitigating the atmospheric CO2 increase and ocean acidification by adding limestone powder to upwelling regions. J. Geophys. Res. Oceans 113:C04028. doi: 10.1029/2007JC004373
Henrikson, L., and Brodin, Y. (1995). Liming of surface waters in Sweden — a synthesis. In liming of acidified surface waters. Heidelberg, Germany: Springer.
Ho, D. T., Bopp, L., Palter, J. B., Long, M. C., Boyd, P. W., Neukermans, G., et al. (2023). “Monitoring, reporting, and verification for ocean alkalinity enhancement” in Guide to best practices in ocean alkalinity enhancement research. eds. A. Oschlies, A. Stevenson, L. T. Bach, K. Fennel, R. E. M. Rickaby, and T. Satterfield, et al. (Copernicus Publications, State Planet).
House, K. Z., House, C. H., Schrag, D. P., and Aziz, M. J. (2007). Electrochemical acceleration of chemical weathering as an energetically feasible approach to mitigating anthropogenic climate change. Environ. Sci. Technol. 41, 8464–8470. doi: 10.1021/es0701816
Hurd, C. L., Gattuso, J.-P., and Boyd, P. W. (2024). Air-sea carbon dioxide equilibrium: will it be possible to use seaweeds for carbon removal offsets? J. Phycol. 60, 4–14. doi: 10.1111/jpy.13405
IPCC (2022). “Climate change 2022: mitigation of climate change,” in Contribution of working group III to the sixth assessment report of the intergovernmental panel on climate change. eds. P. R. Shukla, J. Skea, R. Slade, and A. Al Khourdajie, Van Diemen, R., D. McCollum, et al. (Cambridge, UK and New York, NY, USA: Cambridge University Press).
International Tribunal of the Law of the Sea (2024). Advisory Opinion on a request for an advisory opinion submitted by the commission of small island states on climate change and international law. Available at: https://www.itlos.org/fileadmin/itlos/documents/cases/31/Advisory_Opinion/C31_Adv_Op_21.05.2024_orig.pdf (Accessed December 23, 2024).
Jiao, N., Robinson, C., Azam, F., Thomas, H., Baltar, F., Dang, H., et al. (2014). Mechanisms of microbial carbon sequestration in the ocean – future research directions. Biogeosciences 11, 5285–5306. doi: 10.5194/bg-11-5285-2014
Jin, X., and Gruber, N. (2003). Offsetting the radiative benefit of ocean iron fertilization by enhancing N2O emissions. Geophys. Res. Lett. 30:2249. doi: 10.1029/2003GL018458
Jones, D. C., Ito, T., Takano, Y., and Hsu, W.-C. (2014). Spatial and seasonal variability of the air-sea equilibration timescale of carbon dioxide. Glob. Biogeochem. Cycles 28, 1163–1178. doi: 10.1002/2014GB004813
Jürchott, M., Koeve, W., and Oschlies, A. (2024). The response of the ocean carbon cycle to artificial upwelling, ocean iron fertilization and the combination of both. Environ. Res. Lett., revision submitted 19:114088. doi: 10.1088/1748-9326/ad858d
Jürchott, M., Oschlies, A., and Koeve, W. (2023). Artificial upwelling—a refined narrative. Geophys. Res. Lett. 50:e2022GL101870. doi: 10.1029/2022GL101870
Keller, D. P., Feng, E. Y., and Oschlies, A. (2014). Potential climate engineering effectiveness and side effects during a high carbon dioxide-emission scenario. Nat. Commun. 5:3304. doi: 10.1038/ncomms4304
Keller, D. P., Lenton, A., Littleton, E. W., Oschlies, A., Scott, V., and Vaughan, N. E. (2018). The effects of carbon dioxide removal on the carbon cycle. Curr. Clim. Chang. Rep. 4, 250–265. doi: 10.1007/s40641-018-0104-3
Kemper, J., Mense, J., Graf, K., Kröger, J., and Riebesell, U. (2023). “Towards reliable performance predictions for Stommel’s perpetual salt fountain” in International conference on computational methods in marine engineering. Barcelona, Spain: CIMNE, 1–19.
Khatiwala, S., Schmittner, A., and Muglia, J. (2019). Air-sea disequilibrium enhances ocean carbon storage during glacial periods. Sci. Advances 5:eaaw4981. doi: 10.1126/sciadv.aaw4981
Kheshgi, H. S. (1995). Sequestering atmospheric carbon dioxide by increasing ocean alkalinity. Energy 20, 915–922. doi: 10.1016/0360-5442(95)00035-F
Kowalczyk, K., Amann, T., Strefler, J., Vorrath, M.-E., Hartmann, J., De Marco, S., et al. (2024). Marine carbon dioxide removal by alkalinization should no longer be overlooked. Environ. Res. Lett. 19:074033. doi: 10.1088/1748-9326/ad5192
Krause-Jensen, D., Lavery, P., Serrano, O., Marba, N., Masque, P., and Duarte, C. M. (2018). Sequestration of macroalgal carbon: the elephant in the blue carbon room. Biol. Lett. 14:20180236. doi: 10.1098/rsbl.2018.0236
Kump, L. R., Bralower, T. J., and Ridgwell, A. J. (2009). Ocean acidification in deep time. Oceanography 22, 94–107. doi: 10.5670/oceanog.2009.100
Kwiatkowski, L., Ricke, K. L., and Caldeira, K. (2015). Atmospheric consequences of disruption of the ocean thermocline. Environ. Res. Lett. 10:034016. doi: 10.1088/1748-9326/10/3/034016
La Plante, E. C., Chen, X., Bustillos, S., Bouissonnie, A., Traynor, T., Jassby, D., et al. (2023). Electrolytic seawater mineralization and the mass balances that demonstrate carbon dioxide removal. ACS EST Eng. 3, 955–968. doi: 10.1021/acsestengg.3c00004
Leedham, E. C., Hughes, C., Keng, F. S. L., Phang, S.-M., Malin, G., and Sturges, W. T. (2013). Emission of atmospherically significant halocarbons by naturally occurring and farmed tropical macroalgae. Biogeosciences 10, 3615–3633. doi: 10.5194/bg-10-3615-2013
Leung, D. Y. C., Caramanna, G., and Maroto-Valer, M. M. (2014). An overview of current status of carbon dioxide capture and storage technologies. Renew. Sust. Energ. Rev. 39, 426–443. doi: 10.1016/j.rser.2014.07.093
Lovelock, J. E., and Rapley, C. G. (2007). Ocean pipes could help the earth to cure itself. Nature 449:403. doi: 10.1038/449403a
Macreadie, P. I., Costa, M. D. P., Atwood, T. B., Friess, D. A., Kelleway, J. J., Kennedy, H., et al. (2021). Blue carbon as a natural climate solution. Nat. Rev. Earth Environ. 2, 826–839. doi: 10.1038/s43017-021-00224-1
Mant, R. C., Jones, D. L., Reynolds, B., Ormerod, S. J., and Pullin, A. S. (2013). A systematic review of the effectiveness of liming to mitigate impacts of river acidification on fish and macro-invertebrates. Environ. Pollut. 179, 285–293. doi: 10.1016/j.envpol.2013.04.019
Marchetti, C. (1977). On geoengineering and the CO2 problem. Clim. Chang. 1, 59–68. doi: 10.1007/BF00162777
Marin-Samper, L., Aristegui, J., Hernandez-Hernandez, N., Ortiz, J., Archer, S. D., Ludwig, A., et al. (2024). Assessing the impact of CO2-equilibrated ocean alkalinity enhancement on microbial metabolic rates in an oligotrophic system. Biogeosciences 21, 2859–2876. doi: 10.5194/bg-21-2859-2024
Martin, J. H. (1990). Glacial-interglacial CO2 change: the iron hypothesis. Paleoceanography 5, 1–13. doi: 10.1029/PA005i001p00001
Mcleod, E., Chmura, G. L., Bouillon, S., Salm, R., Björk, M., Duarte, C. M., et al. (2011). A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, 552–560. doi: 10.1890/110004
Montserrat, F., Renforth, P., Hartmann, J., Leermakers, M., Knops, P., and Meysman, F. J. R. (2017). Olivine dissolution in seawater: implications for CO2 sequestration through enhanced weathering in coastal environments. Environ. Sci. Technol. 51, 3960–3972. doi: 10.1021/acs.est.6b05942
Moras, C. A., Bach, L. T., Cyronak, T., Joannes-Boyau, R., and Schulz, K. G. (2022). Ocean alkalinity enhancement – avoiding runaway CaCO3 precipitation during quick and hydrated lime dissolution. Biogeosciences 19, 3537–3557. doi: 10.5194/bg-19-3537-2022
N’Yeurt, A. D. R., Chynoweth, D. P., Capron, M. E., Stewart, J. R., and Hasan, M. A. (2012). Negative carbon via ocean afforestation. Process Saf. Environ. Prot. 90:467–474. doi: 10.1016/j.psep.2012.10.008
National Academies of Sciences, Engineering, and Medicine (2021). A research strategy for ocean-based carbon dioxide removal and sequestration. Washington, DC: The National Academies Press.
Ortega, A., Geraldi, N. R., Alam, I., Kamau, A. A., Acinas, S. G., Logares, R., et al. (2019). Important contribution of macroalgae to oceanic carbon sequestration. Nat. Geosci. 12, 748–754. doi: 10.1038/s41561-019-0421-8
Oschlies, A. (2009). Impact of atmospheric and terrestrial CO2 feedbacks on fertilization-induced marine carbon uptake. Biogeosciences 6, 1603–1613. doi: 10.5194/bg-6-1603-2009
Oschlies, A., Koeve, W., Rickels, W., and Rehdanz, K. (2010a). Side effects and accounting aspects of hypothetical large-scale Southern Ocean iron fertilization. Biogeosciences 7, 4017–4035. doi: 10.5194/bg-7-4017-2010
Oschlies, A., Pahlow, M., Yool, Y., and Matear, R. T. (2010b). Climate engineering by artificial ocean upwelling: channelling the sorcerer's apprentice. Geophysical Res. Lett. 37, 1–5. doi: 10.1029/2009GL041961
Paine, E. R., Boyd, P. W., Strzepek, R. F., Ellwood, M., Brewer, E. A., Diaz-Pulido, G., et al. (2023). Iron limitation of kelp growth may prevent ocean afforestation. Commun. Biol. 6:607. doi: 10.1038/s42003-023-04962-4
Rau, G. H. (2008). Electrochemical splitting of calcium iarbonate to in-crease solution alkalinity: implications for mitigation of carbon dioxide and ocean acidity. Environ. Sci. Technol. 42, 8935–8940. doi: 10.1021/es800366q
Rau, G. H., and Caldeira, K. (1999). Enhanced carbonate dissolution: a means of sequestering waste CO2 as ocean bicarbonate. Energy Convers. Manag. 40, 1803–1813. doi: 10.1016/S0196-8904(99)00071-0
Rau, G. H., Carroll, S. A., Bourcier, W. L., Singleton, M. J., Smith, M. M., and Aines, R. D. (2013). Direct electrolytic dissolution of silicate minerals for air CO2 mitigation and carbon-negative H2 production. Proc. Natl. Acad. Sci. USA 110, 10095–10100. doi: 10.1073/pnas.1222358110
Renforth, P. (2019). The negative emission potential of alkaline materials. Nat. Commun. 10:1401. doi: 10.1038/s41467-019-09475-5
Renforth, P., Baltruschat, S., Peterson, K., Mihailova, B. D., and Hartmann, J. (2022). Using ikaite and other hydrated carbonate minerals to increase ocean alkalinity for carbon dioxide removal and environmental remediation. Joule 6, 2674–2679. doi: 10.1016/j.joule.2022.11.001
Renforth, P., and Henderson, G. (2017). Assessing Ocean alkalinity for carbon sequestration. Rev. Geophys. 55, 636–674. doi: 10.1002/2016RG000533
Renforth, P., Jenkins, B. G., and Kruger, T. (2013). Engineering challenges of ocean liming. Energy 60, 442–452. doi: 10.1016/j.energy.2013.08.006
Ricard, P. (2023). The advent of the 2023 “BBNJ” agreement: a preliminary legal analysis. Environmental Policy and Law 53, 427–437. doi: 10.3233/EPL-239014
Ricour, F., Guidi, L., Gehlen, M., DeVries, T., and Legendre, L. (2023). Century-scale carbon sequestration flux throughout the ocean by the biological pump. Nat. Geosci. 16, 1105–1113. doi: 10.1038/s41561-023-01318-9
Rosentreter, J. A., Al-Haj, A. N., Fulweiler, R. W., and Williamson, P. (2021). Methane and nitrous oxide emissions complicate coastal blue carbon assessments. Glob. Biogeochem. Cycles 35:e2020GB006858 35. doi: 10.1029/2020GB006858
Rotteveel, L., Heubach, F., and Sterling, S. M. (2022). The surface water chemistry (SWatCh) database: a standardized global database of water chemistry to facilitate large-sample hydrological research. Earth System Science Data 14, 4667–4680. doi: 10.5194/essd-14-4667-2022
Saha, P., Meeder, J., Singh, S., Ramesh, S., Nippe, M., and Kwabi, D. G. (2024). Enabling light-driven CO2 removal from seawater using metastable photoacids. J. Phys. Chem. C 128, 4914–4923. doi: 10.1021/acs.jpcc.3c08306
Sheppard, E. J., Hurd, C. L., Britton, D. D., Reed, D. C., and Bach, L. T. (2023). Seaweed biogeochemistry: global assessment of C:N and C:P ratios and implications for ocean afforestation. J. Phycol. 59, 879–892. doi: 10.1111/jpy.13381
Siegel, D. A., DeVries, T., Cetinic, I., and Bisson, K. M. (2023). Quantifying the ocean’s biological pump and its carbon cycle impacts on global scales. Annu. Rev. Mar. Sci. 15, 329–356. doi: 10.1146/annurev-marine-040722-115226
Siegel, D. A., DeVries, T., Doney, S., and Bell, T. (2021). Assessing the sequestration time scales of some ocean- based carbon dioxide reduction strategies. Environ. Res. Lett. 16:104003. doi: 10.1088/1748-9326/ac0be0
Smith, S.M., Geden, O., Nemet, G.F., Gidden, M.J., Lamb, W.F., Powis, C., et al. (2023). The state of carbon dioxide removal - 1st edition. Technical report. Available at https://www.stateofcdr.org/ (Accessed December 22, 2024).
Sterling, S. M., Halfyard, E., Hart, K., Trueman, B., Grill, G., and Lehner, B. (2023). Addition of alkalinity to Rivers: a new CO2 removal strategy. Authorea Preprints. doi: 10.22541/essoar.168380809.92137625/v1
Stolaroff, J. K., Keith, D. W., and Lowry, G. V. (2008). Carbon dioxide capture from atmospheric air using sodium hydroxide spray. Environ. Sci. Technol. 42, 2728–2735. doi: 10.1021/es702607w
Stommel, H., Arons, A. B., and Blanchard, D. (1956). An Oceanographical curiosity: the perpetual salt fountain. Deep-Sea Res. 3, 152–153. doi: 10.1016/0146-6313(56)90095-8
Strefler, J., Amann, T., Bauer, N., Kriegler, E., and Hartmann, J. (2018). Potential and costs of carbon dioxide removal by enhanced weathering of rocks. Environ. Res. Lett. 13:034010. doi: 10.1088/1748-9326/aaa9c4
Tagliabue, A., Twining, B. S., Barrier, N., Maury, O., Berger, M., and Bopp, L. (2023). Ocean iron fertilization may amplify climate change pressures on marine animal biomass for limited climate benefit. Glob. Chang. Biol. 29, 5250–5260. doi: 10.1111/gcb.16854
Wallmann, K., Diesing, M., Scholz, F., Rehder, G., Dale, A. W., Fuhr, M., et al. (2022). Erosion of carbonate-bearing sedimentary rocks may close the alkalinity budget of the Baltic Sea and support atmospheric CO2 uptake in coastal seas. Front. Mar. Sci. 9:968069. doi: 10.3389/fmars.2022.968069
Wanninkhof, R., Asher, W. E., Ho, D. T., Sweeney, C., and McGillis, W. R. (2009). Advances in quantifying air-sea gas exchange and environmental forcing. Annu. Rev. Mar. Sci. 1, 213–244. doi: 10.1146/annurev.marine.010908.163742
White, A., Björkman, K., Grabowski, E., Letelier, R., Poulos, S., Watkins, B., et al. (2010). An open ocean trial of controlled upwelling using wave pump technology. J. Atmos. Ocean. Technol. 27, 385–396. doi: 10.1175/2009JTECHO679.1
Williamson, P., and Gattuso, J.-P. (2022). Carbon removal using coastal blue carbon ecosystems is uncertain and unreliable, with questionable climatic cost-effectiveness. Front. Climate 4:853666. doi: 10.3389/fclim.2022.853666
Wu, J., Keller, D. P., and Oschlies, A. (2023). Carbon dioxide removal via macroalgae open-ocean mariculture and sinking: an earth system modeling study. Earth Syst. Dynam. 14, 185–221. doi: 10.5194/esd-14-185-2023
Yang, A. J. K., and Timmermans, M.-L. (2024). Assessing the effective settling of mineral particles in the ocean with application to ocean-based carbon-dioxide removal. Environ. Res. Lett. 19:024035. doi: 10.1088/1748-9326/ad2236
Yool, A., Shepherd, J. G., Bryden, H. L., and Oschlies, A. (2009). Low efficiency of nutrient translocation for enhancing oceanic uptake of carbon dioxide. J. Geophys. Res. Oceans 114:C08009. doi: 10.1029/2008JC004792
Yoon, J.-E., Yoo, K.-C., Macdonald, A. M., Yoon, H.-I., Park, K.-T., Yang, E. J., et al. (2018). Reviews and syntheses: ocean iron fertilization experiments – past, present, and future looking to a future Korean Iron fertilization experiment in the Southern Ocean (KIFES) project. Biogeosciences 15, 5847–5889. doi: 10.5194/bg-15-5847-2018
Keywords: carbon dioxide removal, marine geoengineering, ocean carbon uptake, blue carbon, ocean fertilization, ocean alkalinity enhancement
Citation: Oschlies A, Bach LT, Fennel K, Gattuso J-P and Mengis N (2025) Perspectives and challenges of marine carbon dioxide removal. Front. Clim. 6:1506181. doi: 10.3389/fclim.2024.1506181
Received: 04 October 2024; Accepted: 13 December 2024;
Published: 07 January 2025.
Edited by:
Matthew Collins, University of Exeter, United KingdomReviewed by:
Juliana Monteiro, Netherlands Organisation for Applied Scientific Research, NetherlandsCopyright © 2025 Oschlies, Bach, Fennel, Gattuso and Mengis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Oschlies, YW9zY2hsaWVzQGdlb21hci5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.