- Research School of Biology, Australian National University, Canberra, ACT, Australia
The removal of atmospheric carbon dioxide (CO2) is now essential to meet net zero goals and limit the impacts of climate change. Enhanced weathering is a method of sequestering CO2 that involves the distribution of finely ground silicate rocks over agricultural land. The weathering of these silicate rocks releases cations into solution which can balance dissolved inorganic carbon, effectively removing CO2 from the atmosphere. Despite being a promising method of carbon dioxide removal (CDR), enhanced weathering has been limited by uncertainty surrounding the measurement of CO2 sequestration. This study compares current measurement approaches that focus on quantifying inorganic carbon and cations within the soil and leachate. Cation-based calculations of CDR were compared to inorganic carbon-based calculations of CDR and soil results were compared to leachate results. The recovery rate of cations in the soil fraction was also tested. Three different ground silicate minerals/rocks – basalt, olivine and wollastonite, were mixed with two different soils and were allowed to weather over 16 weeks in 320 pots with and without plants under different watering regimes and the application of an acidifying fertiliser. Soil and leachate samples were analysed for cations by ICP-OES and inorganic carbon by direct CO2 analysis after acidification and total alkalinity titration (in leachate only). The results indicate that the soil retains most enhanced weathering products through the cation exchange reactions. CDR estimated by cations is often greater than CDR estimated by inorganic carbon. Measurement approaches to estimate cations are susceptible to incomplete or improper accounting through the under-extraction of cations stored within the soil-exchangeable pool, the activity of non-carbonic acids and CO2 outgassing. Inorganic carbon-based measurements, including direct inorganic carbon and total alkalinity analysis, are also complicated by the potential for CO2 loss through carbonate precipitation and re-equilibration. Therefore, inorganic carbon-based approaches and cation-based approaches should be reconciled to validate the estimation of CDR. The inorganic carbon-based estimation of CDR in leachate should equal the cation-based estimation of CDR in leachate—which will be achieved after quantification or estimation of the natural mechanisms that affect each approach. These findings will support the development of accurate measurement processes for enhanced weathering.
1 Introduction
Climate change is disrupting human society and natural systems. It is a global crisis that threatens to worsen over time without drastic action (IPCC, 2018, 2022a,b). To limit the impacts of climate change, humankind must reach net zero greenhouse gas (GHG) emissions as rapidly as possible. This requires a reduction in emissions, but also an active removal of GHGs, such as carbon dioxide (CO2), from the atmosphere. Carbon dioxide removal (CDR) on the gigatonne (Gt) scale is now crucial to limiting the most severe impacts of climate change (IPCC, 2022a; Smith et al., 2023).
Enhanced Weathering (EW) is a method to capture and store atmospheric CO2. It is based on the natural weathering of calcium (Ca)- and magnesium (Mg)-rich silicate or carbonate minerals in the inorganic carbon cycle, a process which has helped to reduce excessive concentrations of atmospheric CO2 over geological timescales (Walker et al., 1981; Hartmann et al., 2013). The natural weathering of silicate rocks can be accelerated to capture and store atmospheric CO2 on human-relevant timescales. Primarily, this enhancement process involves the crushing of the silicate rocks to particle sizes often below 100 μm and the distribution of the crushed rock in agriculturally productive soils (Hartmann et al., 2013; Rinder and von Hagke, 2021). This drastically increases the surface area of the silicate rocks and the rate of weathering. Chemically, this EW reaction commences with the formation of carbonic acid from water and CO2 (Equation 1), which reacts with Ca- and Mg-rich silicate rocks to form bicarbonate and release the Ca and Mg ions (Equation 2, representing a simplified calcium silicate mineral). The dissolved bicarbonate and cations are transported into the ocean. The bicarbonate may remain in the ocean for hundreds to thousands of years, but will eventually undergo a precipitation reaction (Equation 3), usually biogenic, to produce calcium or magnesium carbonate and release one of the captured CO2 molecules (Hartmann et al., 2013; Renforth and Henderson, 2017). This carbonate precipitate will settle on the ocean floor and will be stored in the Earth’s crust for hundreds of thousands of years (Hartmann et al., 2013). The precipitation of carbonate may also occur in the soil, before the bicarbonate is transported into the ocean. The extent to which carbonate precipitation occurs in soils is not fully known, but depends on rock type and soil factors (Haque et al., 2020b,c; Khalidy et al., 2021, 2024; Holzer et al., 2023b; Clarkson et al., 2024).
Studies suggest that applying the crushed Ca- and Mg-rich silicates to agriculturally productive land as a soil amendment can lead to Gt-scale CDR when applied at a global level (Beerling et al., 2018; Strefler et al., 2018; Goll et al., 2021). The advantage of applying the rocks to agricultural land is in the tight soil pore spaces, in which the biological activity of the plants and soil microbes can increase the concentration of CO2 up to 100× atmospheric levels, which greatly accelerates the CO2 sequestration reaction (Strawn et al., 2015; Renforth and Campbell, 2021; Paessler et al., 2023).
The potential to reach Gt-scale CDR has resulted in a boom of startups with a focus on scaling EW (cdr.fyi, 2024). However, the growth of the EW industry has been limited by two factors: (1) uncertainty around how much CO2 can be removed at scale, and (2) uncertainty in the scientific literature in which measurement approaches most accurately quantify EW CDR (Holzer et al., 2023b; Clarkson et al., 2024). There is huge variation in scientific literature on the potential CDR rate of EW (Table 1), with estimates ranging by over three to four orders of magnitude (Kukla et al., 2024). This is due partly to experimental design differences (e.g., in rock type, particle size, distribution rates) and natural variation (e.g., in soil type, biology and hydrology). However, another factor contributing to the extreme variation in results is the lack of consistency in measurement approaches between scientific studies. Studies measure different EW products: cations (Ca and Mg) or inorganic carbon (IC), in different fractions of the EW system: soil (either the total soil or soil-exchangeable fractions) or leachate (i.e., the water dripping out of the soil) (Table 1) (Almaraz et al., 2022; Clarkson et al., 2024). The focus of this study is on comparing the current measurement approaches shown in Table 1. Ensuring accurate and consistent measurement is vital in order to scale EW CDR, and comes at the behest of repeated calls to look into the current measurement inconsistencies of a notoriously complex system (Dietzen et al., 2018; Vienne et al., 2022; Dietzen and Rosing, 2023; Wood et al., 2023; Clarkson et al., 2024).

Table 1. Literature review of the measurement approaches, calculated CDR rate and important parameters in enhanced weathering trials.
The primary methods to measure the CO2 sequestration from EW focus on two products of the EW reactions – cations (Ca and Mg) and IC (bicarbonate and carbonate). More novel EW measurement techniques that compare Ca/Mg to an immobile tracer such as titanium also rely on cation-based measurements to estimate CDR (Kantola et al., 2023; Reershemius et al., 2023; Beerling et al., 2024; Clarkson et al., 2024; Reershemius and Suhrhoff, 2024; Wolf et al., 2024). These EW products are commonly measured in the soil (either the total soil or soil-exchangeable pool) and/or leachate fraction of the agricultural system (Table 1). The products of Equation 2 are initially dissolved, but may become involved in the complex chemical, biological and physical interactions within the soil (Hartmann et al., 2013). Clay mineral surfaces are predominantly negatively charged and therefore interact primarily with positively charged cations (Sposito, 2004; Blume et al., 2016). These interactions range in strength, including simple electrostatic forces (outer-sphere complexes) but also covalent bonds (inner-sphere complexes/chemisorption) (Blume et al., 2016; Strawn, 2021). Easily reversible cation exchange processes are reflected in the cation exchange capacity (CEC) of soils. Anions in soil either interact with positively charged soil particles directly (although there are few positive surface sites available in most soils), or interact with negatively charged clay minerals, mediated by polyvalent cations such as Ca2+, Mg2+, Fe3+ and Al3+ (Blume et al., 2016; Singh et al., 2018; Strawn, 2021). Equivalent to CEC, a soil anion exchange capacity (AEC) can be measured that is mainly relevant in highly weathered and acidic soils (Blume et al., 2016; Singh et al., 2018; Strawn, 2021). While involved in these complex interactions within the soil, the dissolved IC is not durably stored, and may be converted back into CO2 (West and McBride, 2005; Clarkson et al., 2024). This CDR process is more likely to be durable once the cations and dissolved IC leach out of the soil or precipitate in the soil in the form of solid carbonates. This IC can still be converted back into CO2 through a number of mechanisms, but this usually occurs to a lesser extent over a longer timeframe (Clarkson et al., 2024).
While measurements of cations and IC/alkalinity in soil and leachate have been used interchangeably, this study is one of the first to apply them both to compare their accuracy and consistency with a focus on developing a measurement framework for agricultural EW (te Pas et al., 2023). The aim of this study is to compare cation- and IC-based measurements in soil and leachate to quantify the CDR effect of EW. Studies have already questioned these approaches: Dietzen and Rosing (2023) have shown that simplistic cation-based approaches may overestimate CDR in acidic conditions, for example, while various studies have questioned the accuracy of IC measurements after being unable to measure significant increases in IC despite silicate rock-based soil amendments ranging up to 150 t rock ha−1 (Renforth et al., 2015; Dietzen et al., 2018; Kelland et al., 2020; Larkin et al., 2022). This study tests these measurement approaches through a series of EW pot trials that mimic the application of silicate rock amendments to agricultural land in a more controlled environment. Three highly reactive rocks/minerals commonly used in EW trials are assessed (basalt, olivine, wollastonite) across two different soil types. Different watering rates, the presence of a plant and the effect of an acidifying fertiliser are also assessed. These varied agricultural conditions and soil-rock mixtures allow for a robust assessment of measurement approaches across different conditions. The accuracy of both cation- and IC-based measurement approaches is assessed by the application of known amounts of soluble Ca, Mg, bicarbonate, and carbonate salts instead of silicate rocks to control pots, as performed by ten Berge et al. (2012). The consistency of the cation-and IC-based measurement approaches is assessed by measuring both the Ca and Mg and IC in the leachate and soil fractions of the silicate amended pots, and calculating the CDR estimated by both approaches.
Ultimately, determining the most accurate and robust approaches to measure the CDR effect of EW will be essential in scaling the industry. To reach the Gt levels of CDR required, billions of dollars of investment is required. This investment will not be forthcoming if it is not possible to accurately assess how much CO2 is being removed by the technology.
2 Methods
2.1 Experimental preparation
2.1.1 Soil preparation
Throughout the three trials, two different soil types were used: a sandstone-based clay loam from Kowen Pine Forest, Australian Capital Territory, Australia (Soil A; pH in H2O 5.76; CEC 6.8 cmol+/kg); and a granite-based clay loam from Lerida, New South Wales, Australia (Soil B; pH in H2O 5.82; CEC 2.2 cmol+/kg). Carbonate content of the soils is outlined in Supplementary Table 3. These two soils were selected due to the difference in their parent material, allowing for an assessment of results in different soil conditions. Their pH levels were in line with standard Australian soils, while their exchange capacities were lower than typical agricultural soils (de Caritat and Wilford, 2011; DPI, 2023). Detailed soil analysis was performed by Environmental Analysis Laboratory (Southern Cross University) and is reported in Supplementary Table 1. To prepare for weighing, analysis and potting, soils were passed through a 1 cm sieve to remove large rocks and large pieces of roots, air-dried and then thoroughly mixed.
2.1.2 Rock preparation
Throughout the three trials, three different rock types were used: olivine-dominated dunite from Nelson, New Zealand (hereafter referred to as olivine or olivine rock); basalt from New South Wales, Australia; and wollastonite powder (hereafter referred to as wollastonite or wollastonite rock) (Alpha Chemicals, 325#). Their mineral compositions were determined by X-Ray Diffraction (XRD) (Supplementary Table 2). Basalt was selected due to its global abundance and relative dearth of toxic heavy metals, making it an ideal candidate for large-scale EW (Hartmann et al., 2013; Beerling et al., 2018, 2020; Suhrhoff, 2022; Dupla et al., 2023). Olivine and wollastonite were selected due to their reactivity and likelihood to sequester CO2 rapidly within short timeframes. However, olivine may have less applicability in agricultural systems due to the potential for heavy metal toxicity (Haque et al., 2020a; Suhrhoff, 2022; te Pas et al., 2023). Three different rocks were used in order to assess results across different combinations of soil and rock. Carbonate content of the rocks is outlined in Supplementary Table 3.
Olivine and basalt rocks were crushed in a Jaw Crusher (Jaques Brothers Ltd., Melbourne) to sizes varying from 1 mm to 3 cm. Rocks crushed to sizes below 1 mm were sieved out and discarded to avoid pre-weathered material and contaminants. The rocks were then ground to a powder in a Ring and Puck mill (Gilson Company Inc.) before being sieved to appropriate fractions (basalt, p97: 90 μm, olivine p97: 75 μm, wollastonite p97: 44 μm). Ground rock samples were thoroughly mixed to reduce heterogeneity. These small particle sizes were prepared in order to accelerate weathering and CDR (Rinder and von Hagke, 2021).
2.1.3 Positive control salts
To provide a positive control for the release of Ca, Mg, bicarbonate and carbonate by the weathering of the silicate rocks, known amounts of soluble salts of Ca, Mg, bicarbonate and carbonate were added to soil. This concept, introduced by ten Berge et al. (2012), allows for an assessment of the accuracy of measurement processes by testing their findings on known amounts of cations and IC. In this way, the salts are not intended to mimic the complex silicate rock dissolution process but are intended to represent specifically known amounts of cations and IC that are the products of the silicate dissolution process. Gypsum (calcium sulphate; Bunnings Warehouse, 3,010,181), Epsom salt (magnesium sulphate; Bunnings Warehouse, 2,960,980), sodium bicarbonate (Bunnings Warehouse, 3,090,201) and sodium carbonate (Bunnings Warehouse, 3,090,205) were purchased and used. These salts were chosen due to their high solubility and use as fertilisers in some instances, minimising potential radical effects of the salts on the overall soil system (Shainberg et al., 1989; ten Berge et al., 2012; Martínez-Cuenca et al., 2013; Jezek et al., 2014; Kuttah and Sato, 2015; Araújo et al., 2019; Esteves et al., 2022). The amount of salt added to the soil was determined to be representative of the cations and IC released at the lower end of estimated weathering rates by previous studies (Table 1). Specifically, 2.5 mmol of each ion in salt form was added to the soil in Pot Trial 1, and 8.3 mmol in Pot Trial 2 (4.6 mmol per kg soil).
2.1.4 Negative controls
Negative controls were pots of soil without rock or salt addition. This was designed to provide a measure of the background effects of the experimental conditions, such as watering, on cation and IC levels over the weathering period.
2.1.5 High-watering pot trial set up and sampling regime (PT1)
Pot Trial 1 (PT1) was designed to maximise rock weathering with a high watering regime that reflected semiaquatic agricultural conditions, such as for rice farming.
The three rock types were mixed with two soil types in a 0.15: 0.85 ratio by mass. This is at the higher end of silicate rock distribution rates, equivalent to 105 t ha−1. This was a design choice to increase the CO2 sequestration. Cylindrical, plastic pots with 0.5 L volume were used (diameter 10 cm, surface area 7.85 × 10−7 ha), requiring 550 g of soil in control pots and 467 g of soil with 83 g rock in non-control pots (e.g., Figure 1).

Figure 1. Pot trial (PT) set up. PT1 and PT3 consisted of plastic pots containing soil-rock mixture on collection trays. PT2 (pictured) also included soybean plants in half of the pots.
Overall, PT1 contained 112 pots, divided into treatments as follows:
• Rock (3 types) × Soil (2 types) × 12 repeats: 72 pots
• No-rock negative control (1 type) × Soil (2 types) × 12 repeats: 24 pots
• Salt positive control (4 types) × Soil (2 types) × 2 repeats: 16 pots
These pots were arranged in a randomised block design across two greenhouses with the following settings: 25°C day temperature and 23°C night temperature; 14-h days, 10-h nights. These warm temperatures were partly selected to accelerate silicate weathering (Edwards et al., 2017). The pots were watered daily at a rate of 1.4 L per pot per week (3,080 mm in total, equivalent to 10,000 mm per year). The high watering rate was designed to accelerate the weathering process, which is dependent on water (Cipolla et al., 2021) and led to continuous periods of standing water in the pots, reflecting semiaquatic agricultural conditions. The irrigation water used was measured for its cation and IC concentration, to assess whether it was likely to contribute to the final cation and IC budget at the conclusion of the pot trial. The IC in the irrigation water was negligible (below detection threshold), while the cation concentrations were non-negligible (Ca 11.57 mg L−1, Mg 1.03 mg L−1). However, the experimental design of the pot trials ensured that background effects of watering were excluded from analysis of the EW effect, as discussed later and represented in Figure 2.

Figure 2. Process for calculating EW-attributable change in IC. EW-attributable change in Ca + Mg was calculated the same way.
Leachate was collected after 1 week, 8.5 weeks and 16 weeks. The 1-week time-point was selected to observe an initial flux in cations and IC (Taylor et al., 2021). The (rock-amended) soil was allowed to weather for 16 weeks before termination. Soil was sampled upon termination.
2.1.6 Agriculturally representative pot trial set up and sampling regime (PT2)
Pot Trial 2 (PT2) was designed to reflect agricultural conditions, through the addition of a soybean plant, and the use of a more moderate watering regime than PT1.
As with PT1, the three rock types were mixed with two soil types in a 0.15: 0.85 ratio by mass, equivalent to 153 t ha−1. Cylindrical, plastic pots with a volume of 1.7 L were used (diameter 15 cm, surface area 1.77 × 10−6 ha) to allow a plant to grow, requiring 1.8 kg of soil in control pots and 1.53 kg of soil with 270 g of rock in non-control pots (e.g., Figure 1). The mass ratio of rock to soil was kept constant between all pot trials, while the distribution rate in tonnes per hectare differed due to the heights of the pots.
Soybeans were selected due to their ability to fix nitrogen, eliminating the need for a nitrogen fertiliser, and their ability to accelerate silicate rock weathering (Haque et al., 2020b,c; Ciampitti et al., 2021).
Overall, PT2 contained 160 pots, divided into treatments as follows:
• Rock (3 types) × Soil (2 types) × Plant (2 types, soybean or no soybean) × 8 repeats: 96 pots
• No rock negative control (1 type) × Soil (2 types) × Plant (2 types, soybean or no soybean) × 8 repeats: 32 pots
• Salt positive control (4 types) × Soil (2 types) × Plant (2 types, soybean or no soybean) × 2 repeats: 32 pots
These pots were arranged in a randomised block design across two greenhouses with the same settings as PT1. These warm temperatures were also partly chosen to accelerate soybean growth (Agriculture Victoria, 2023). These pots were watered twice a week while soybeans were in vegetative stages of growth. Once they reached reproductive stages, watering increased to four times a week, as per guidance on optimal watering for soybean plants (Matcham, 2022; Agriculture Victoria, 2023). On average, the pots received 400 mL per week (400 mm in total, equivalent to 1,300 mm per year), reflecting standard agricultural practice for soybeans, pasture, potatoes, onions and other bulk crops (Armstrong and Giblin, 2001). Note that this trial will be referred to as agriculturally representative watering, as it reflects standard agricultural practices for many Australian crops. This is opposed to PT1, with a high watering rate that is standard for semi-flooded agriculture, but not Australian cropping.
Leachate was collected after 16 weeks, and the experiment was terminated immediately afterwards. Soil was sampled upon termination.
2.1.7 Fertiliser pot trial set up and sampling regime (PT3)
Pot Trial 3 (PT3) was designed to test the effect of an acidifying fertiliser, ammonium sulphate, on the release of cations and IC in an EW system.
In PT3, only olivine and wollastonite were used, prioritising the fast-weathering rocks. These two rock types were mixed with the two soil types in a 0.15: 0.85 ratio by mass, equivalent to 105 t ha−1. Cylindrical pots with a volume of 0.5 L were used (diameter 10 cm, surface area 7.85 × 10−7 ha), with 450 g of soil in control pots and 383 g of soil with 67 g of rock in non-control pots. Ammonium sulphate was added to half the pots. Ammonium sulphate is a highly acidifying fertiliser (DPIRD, 2021) used in a large range of agricultural practices, 5.9% of global nitrogen fertiliser use (S&P Global, 2022), and was added at a rate equivalent to 150 kg nitrogen ha−1, at the upper limit of agricultural use (DPIRD, 2021).
The day after pot preparation and watering, the ammonium sulphate was added to the “Fertiliser” pots in solution. An equivalent volume of water was added to the “No Fertiliser” pots.
Overall, PT3 contained 48 pots, divided into treatments as follows:
• Rock (2 types) × Soil (2 types) × Fertiliser (2 types, fertiliser or no fertiliser) × 4 repeats: 32 pots
• No rock negative control (1 type) × Soil (2 types) × Fertiliser (2 types, fertiliser or no fertiliser) × 4 repeats: 16 pots
These pots were arranged in a randomised block design across two greenhouses with the same settings as PT1 and 2. These pots were watered, daily, at a rate equal to PT1: 1.4 L per pot per week (1,920 mm in total, equivalent to 10,000 mm per year). Leachate was collected after 10 weeks, as described below. The whole pot trial was terminated after 10 weeks and soil was sampled.
2.2 Sampling
2.2.1 Leachate sampling and preparation
Leachate was collected after 1, 8.5 and 16 weeks from PT1, after 16 weeks from PT2 and after 10 weeks from PT3. Leachate sampling protocol is outlined in the Supplementary material. In brief, leachate accumulated and evaporated on collection trays before re-dissolution for collection. This process introduced a susceptibility to CO2 outgassing, discussed in the Supplementary material.
2.2.2 Soil sampling and preparation
Soil was collected after termination of each of the pot trials. Soil sampling protocol is outlined in the Supplementary material. In brief, the entire soil mixture of each pot was thoroughly mixed, dried and sampled for analysis.
2.3 Measurements and processing data
2.3.1 Soil-exchangeable cation extraction
To extract the soil-exchangeable cations from the soil samples, 1.5 g of soil (between 1.45 g and 1.55 g) was weighed into a 50 mL Falcon tube. 15 mL of 1 M ammonium acetate (pH 7.0) was added, as per the ratio outlined in Rayment and Lyons (2010), and the tubes were shaken horizontally for 1 h at 200 rpm. Following this, they were centrifuged for 30 min at 3,000 rpm. The supernatant was then sampled for ICP-OES analysis.
2.3.2 Inductively coupled plasma – optical emission spectroscopy
ICP-OES is a measurement of cation concentrations in the parts per million (ppm) range which was used to measure the cation concentrations in the leachate and soil, as has been employed in a variety of EW studies (Almaraz et al., 2022). The analysis and processing protocol for ICP-OES is outlined in the Supplementary material. Measurements of 1 ppm quality control (QC) solutions were used to assess precision and accuracy. Standard deviation (i.e., precision) of Ca QC was 2.1% and Mg QC was 2.2%. Measurement error (i.e., inaccuracy) was within 1% for both Ca and Mg.
2.3.3 IC analysis
A PRIMACS Carbon Analyser SNC-100 (Skalar Analytica) was used to measure the total IC content in the soil and leachate samples as per the user manual (Skalar Analytical B.V, 2021). The Carbon Analyser acidified samples in phosphoric acid and measured all liberated CO2 through infrared analysis. Further details are provided in the Supplementary material. Measurements of three primary standard NaHCO3 solutions (0.00050% IC, 0.00100% IC, and 0.00250% IC) were used to assess precision and accuracy. Standard deviation and measurement error depended on %IC. Standard deviation ranged from 3 to 9% (Supplementary Table 5). Measurement error ranged from 3% at 0.00250% IC to 15% at 0.00050% IC.
2.3.4 Total alkalinity titration
TA is a measure of the sum of alkaline substances in a solution, including bicarbonate and carbonate. It is often used to measure EW (ten Berge et al., 2012; Kelland et al., 2020; Larkin et al., 2022; Paessler et al., 2023; Holzer et al., 2023a). By subtracting the TA from soil-only controls, the alkalinity contributed by bicarbonate and carbonate is isolated under the assumption that the flux of all other charged species that comprise alkalinity are the same for the controls as for the silicate amended pots. Therefore, TA was measured to validate the IC analysis. TA measures carbonate as two moles of alkalinity, and therefore “double counts” carbonate molecules and produces a higher estimate of IC than direct IC analysis, which measures both carbonate and bicarbonate as one mole of IC. TA was measured by titration of the alkaline substances in 10 mL of leachate with dilute HCl until a pH of 4.2 (Thermo Scientific, 2019). Further details are provided in the Supplementary material. Measurements of primary standard NaHCO3 solution (0.00251% IC) were used to assess precision and accuracy. Standard deviation was 5.2%; measurement error was 4.8%.
2.3.5 pH measurements
A pH probe (SI analytics) was introduced into >15 mL of leachate solution in a 50 mL Falcon tube. The pH reading was recorded after 4 min, by which time it had stabilised. The pH of the same solution in 50 mL was then calculated.
2.3.6 X-ray diffraction
XRD was carried out as per the methodology in Buss et al. (2023) to determine the mineral composition of the three rock types. XRD details are outlined in the Supplementary material.
2.4 Data analysis
2.4.1 Calculating total IC and cations
The total accumulation of EW products in a single system/pot was calculated by summing the leachate and soil fractions (total soil IC and soil-exchangeable cations) (Equations 4–7). This is common practice for cation-based measurements from ICP-OES (Kelland et al., 2020; te Pas et al., 2023).
With a new direct method for measuring IC from leachate and total soil samples, the validity of summing leachate and soil results was assessed by quantifying the standard deviation of the total soil and leachate IC measurements (Supplementary Table 5). The IC analysis method produced consistent standard deviation as a percentage of the IC in the sample across aqueous sample types (leachate) and solid sample types (soil and rock). Summing leachate and soil samples provided a measure of all sequestered IC, as soybean plants (relevant only to PT2) do not take up IC through their roots (Stolwijk and Thimann, 1957; Poschenrieder et al., 2018; Majlesi et al., 2019). This calculation also provided a measure for the majority of Ca and Mg, as although plants do intake Ca and Mg dissolved in the soil, previous studies have suggested that for soybeans and other crops this is <5% total Ca and Mg (Kelland et al., 2020; Buss et al., 2023; Kantola et al., 2023).
2.4.2 Determining process accuracy from positive control measurements
In order to assess the appropriateness of the processes used to extract and measure cations and IC in EW systems, a known amount of cations and IC were added to the positive control pots. The differencebetween the average total IC in the bicarbonate and carbonate salt treated pots () and the non-salt, soil-only control pots () was calculated (Equation 8). could then be divided by the expected difference , which was equivalent to the amount of cation or IC anion added (2.5 mmol in PT1 and 8.3 mmol in PT2) to calculate a recovery rate (RR) (Equation 9). The same process was applied to Ca + Mg.
2.4.3 Differentiating EW and non-EW background effects
The EW-attributable change in IC was calculated by subtracting the initial IC and the non-EW background effects from the total IC for each rock-treated pot. (Equations 10–12; Figure 2).
Where is the EW-attributable change in IC that represents the actual EW signal that must be isolated and measured, is the initial IC measured in the unweathered soil-rock mixture at time = 0 and represents the background, non-EW-attributable effects on IC, calculated in Equation 12.
is the total IC measured in the soil-only negative controls pots and is the initial IC measured in the unweathered soil of the negative control pots at time = 0. Because the rock amended pots contained 85% of the soil in the control pots, the background effect was multiplied by 0.85 to adjust accordingly. Equation 11 isolated the EW-attributable change, which could be averaged over soil-rock treatment and used to calculate CDR. Again, the same process was applied to Ca + Mg values to determine the EW effect on Ca + Mg.
2.4.4 Estimating CDR from IC and cation results
Total CDR was estimated from cation results (Equation 13).
is the cation-based estimate of CDR and is the EW-attributable change in cations, calculated for Ca + Mg by Equation 11. is the molar mass of CO2 (44.01) and is the molar ratio of CO2 to divalent cations sequestered during enhanced weathering (Renforth, 2019). If each divalent cation were balanced by two bicarbonate molecules, would equal 2. Renforth (2019) indicates that due to carbonate buffering, is between 1.4 and 1.7 for typical ocean chemistry, pCO2 and temperature. As per Renforth (2019), = 1.5 is chosen as a conservative estimate (suggesting 50% bicarbonate, 50% carbonate). Therefore, the cation-based CDR estimate is conservative.
Direct IC analysis does not differentiate between bicarbonate and carbonate. Therefore, the following equations calculate IC-based CDR, irrespective of the form of IC sequestered (Equation 14–16).
Note that the IC-based CDR in soil is multiplied by 2. This is because the heating of the soil samples to 105°C overnight converted all IC in bicarbonate form into carbonate, losing a CO2 molecule in the process (Hills, 1968; Guarini et al., 1995; Hartman et al., 2013). Therefore, half the IC was lost from the soil samples, requiring the factor of 2 multiplication. Note that this assumes all bicarbonate has been converted into carbonate, which may not always be the case. The IC-based CDR calculation is therefore a “maximum” estimate, whereas the cation-based CDR is a “conservative” estimate.
2.4.5 Statistical analyses
Statistical analyses are outlined in detail in the Supplementary material and all tests employed are also outlined in figure or table captions.
Statistical analyses of data and presentation of plots were performed in rStudio. The following packages were used: ggplot2, ggpubr, gridExtra, tidyr, Hmisc and dplyr.
Once applied at farm-scale, measuring EW is bound to produce outliers from inaccurate measurements and unrepresentative sampling. Further, the complexities of natural soil systems ensure a large degree of variation within treatments, and it can be difficult to accurately assess whether data are outliers or reflective of natural variation and abnormalities. To reflect these difficulties, outliers were not removed from the experimental data.
3 Results
3.1 Cations and IC in EW systems were underestimated
The recovery of cation and IC measurement techniques was assessed in PT2 by the addition of known amounts of Ca, Mg and IC salts (8.3 mmol). The soybean plant had no significant effect on the recovery of Ca, Mg or IC, and therefore the results were aggregated for each soil-rock combination across plant treatment.
In the agriculturally representative PT2, there was a statistically significant increase in the Ca and Mg (soil-exchangeable + leachate pool) levels in salt treated pots compared to control pots, but the recovery of both Ca and Mg was incomplete. In Soil A, 5.4 out of 8.3 mmol of Ca were measured, equating to a recovery rate of 65% (Figure 3A). In Soil B, only about 30% of the added Ca was recovered. Mg recovery was greater, ranging from 77% in Soil A to 95% in Soil B (Figure 3B). While PT1 contained too few independent repeats to statistically analyse the difference between salt and control pots, the results were consistent with PT2 – Ca recovery ranged from 56–65%, Mg recovery ranged from 88–100% (Supplementary Figure 1).
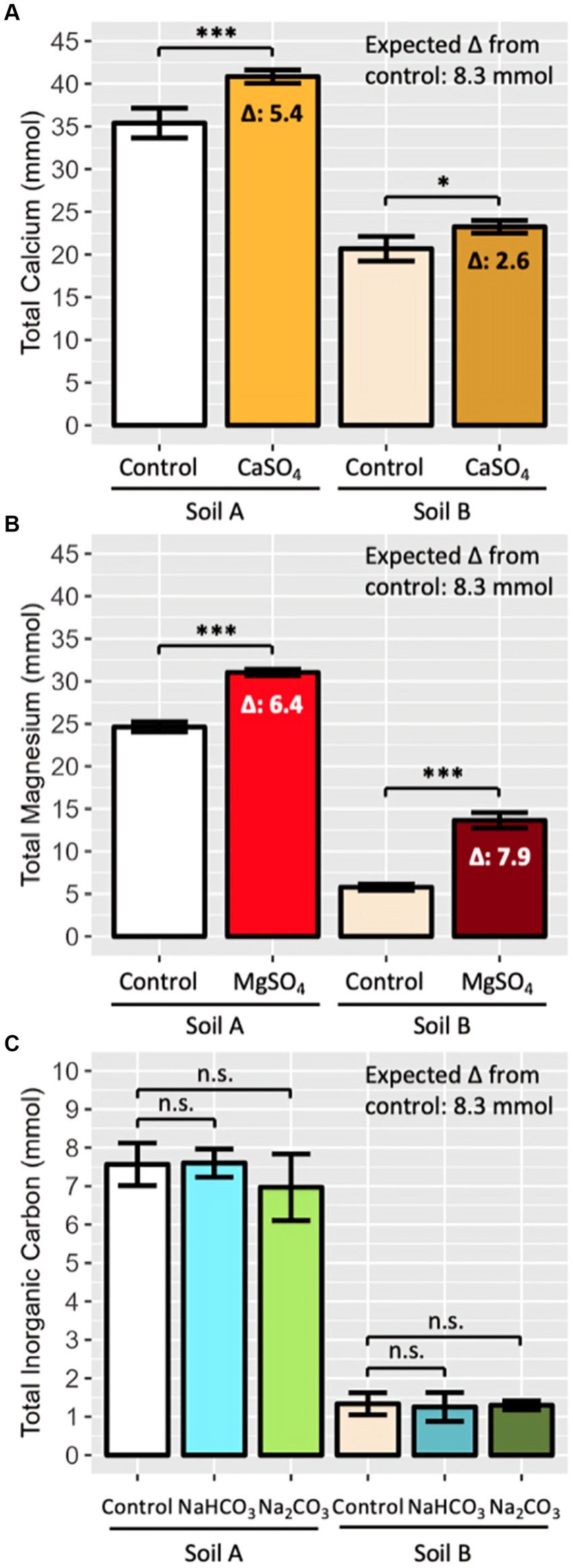
Figure 3. Recovery of known quantities of added (A) Ca, (B) Mg and (C) IC in agriculturally representative enhanced weathering pots (PT2). Minerals (8.3 mmol) were added in salt form (gypsum, Epsom, sodium bicarbonate and sodium carbonate) and recovery was compared to soil-only control pots. Four salt amended pots per soil and salt, 16 soil only pots per soil. Total Ca, Mg, and IC was determined by summing leachate and soil measurements after 16 weeks. Soil A represents sandstone-based soil from Kowen, ACT; Soil B represents granitic soil from Lerida, NSW. Bars represent mean ± SD. Mean difference (Δ) between control and salt amended pots is displayed when significant. Statistical analysis performed by two-way ANOVA (soil and salt effect) and Tukey’s HSD post-hoc test, n.s. p > 0.05, *p < 0.05, ***p < 0.001.
In PT2, there was statistically insignificant recovery of IC across both bicarbonate and carbonate salts in the total soil pool of both soils, despite 8.3 mmol of IC initially added to the salt treated pots (Figure 3C). PT1 results also reflected the very low/negligible recovery rate, with <25% of the added IC extracted in any soil (Supplementary Figure 1C).
3.2 Cation-based estimates of CDR were greater than IC-based estimates
The consistency of cation- and IC-based measurement processes was assessed by applying them both to the pot trials. After subtracting the initial baseline of exchangeable Ca and Mg and total IC present in the soil (Supplementary Tables 3, 4), the change in Ca + Mg and IC over the 16 weeks in each soil-rock treatment was determined. In both PT1 and PT2, the wollastonite treated systems had significantly higher Ca + Mg and IC than the non-rock control systems, indicating a significant CDR effect by both metrics (Figure 4). Generally, rock amended Soil A combinations had a significant increase in cations over their respective soil-only control systems, which was less apparent for Soil B. Meanwhile, IC results also showed no significant change from the soil-only controls in Soil B, while Soil A treated pots saw a statistically significant loss of IC with olivine amendments.
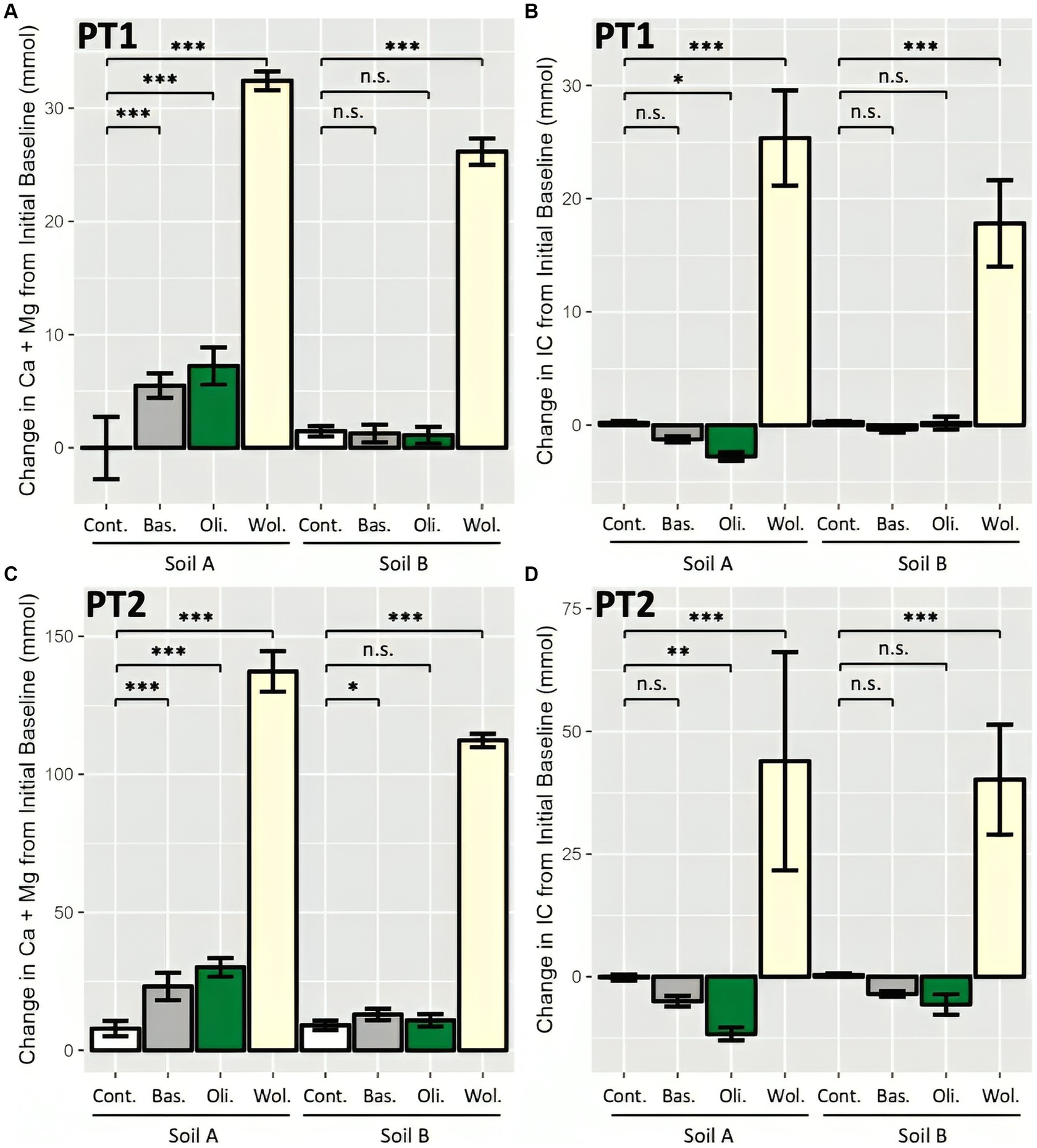
Figure 4. Enhanced weathering effect as measured by sum of leachate and soil cations and IC in two pot trials. (A) Cation-based measurements of weathering in high water PT1; (B) IC-based measurements in PT1; (C) Cation-based measurements in agriculturally representative PT2; and (D) IC-based measurements in PT2. 12 (PT1) and 16 (PT2) pots prepared per soil-rock combination with 15 wt% rock amendment in sandstone-based Soil A and granite-based Soil B, and allowed to weather for 16 weeks. Change from Initial Baseline calculated as per Figure 2. Bars represent mean ± SD. Statistical analysis by two-way ANOVA (soil and rock effect) and Tukey’s HSD post-hoc test, n.s. p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001.
The CDR effect of each soil-rock combination was calculated based on the difference between the soil-only control and rock amended systems, in order to compare the IC-based CDR with the cation-based CDR (Figure 2). Leachate and soil measurements were presented separately, which also allowed for a comparison of the accumulation of leachate and soil weathering products (Table 2). Across soil and leachate, cation-based estimates of CDR were often significantly higher than total-IC-based estimates. The only exception to this was in the leachate in PT2, where the amounts of IC and cations were small enough to show no significant difference between cation- and IC-based estimates. When considering the wollastonite systems, both leachate and soil results showed that cation-based CDR ranged from equivalency to over 200% greater than the IC-based CDR estimates (Table 2).
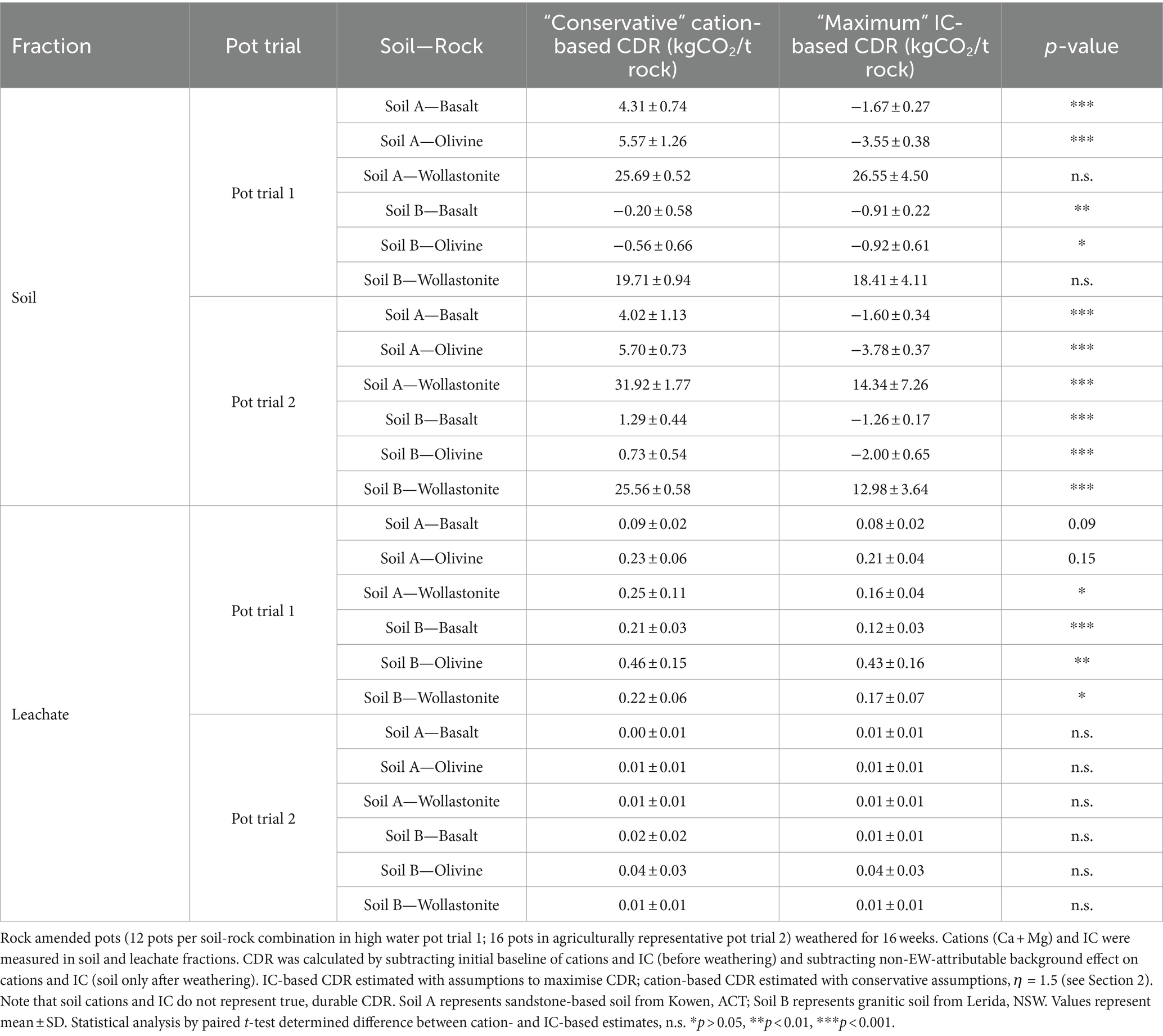
Table 2. Comparison of enhanced weathering CDR estimated across soil-rock treatments by cation-based (Ca + Mg) approach and inorganic carbon-based approach.
The greatest signal for CDR based on an increase in total IC (in total soil and leachate) was in wollastonite amended soils, while basalt and olivine amended soils showed a decrease in total IC. CDR was estimated at between 20 and 32 kgCO2 t−1 wollastonite according to cation-based measurements in soil or between 13 and 27 kgCO2 t−1 wollastonite according to IC-based measurements (Table 2).
In summary, most rock treatments resulted in a significant change in Ca + Mg and/or IC compared to the controls. Cation-based estimates of CDR were significantly greater than IC-based CDR estimates in soil of both pot trials and the leachate of PT1.
3.3 EW products accumulated to a much larger extent in soil compared to leachate
The exchangeable Ca + Mg and total IC results were much larger in the soil fraction than the leachate fraction (Table 2). This was particularly evident for Ca + Mg results, with the soil recording a significantly higher accumulation of Ca + Mg than the leachate in most soil-rock combinations. This was less visibly evident in IC results, as the total soil fraction lost IC in basalt and olivine amended soils, leading to a negative change in IC. However, the magnitude of the soil signal (regardless of whether it was positive or negative) was much larger than the leachate signal in most soil-rock combinations.
As expected, the accumulation of weathering products (Ca + Mg and IC) in the leachate compared to the soil depended on watering regime. The higher watering regime of PT1 saw much greater leachate Ca + Mg and IC than PT2 leachate. In PT2, leachate Ca + Mg was generally <1% of the soil Ca + Mg, whereas in PT1, it mostly formed over 3% of the soil Ca + Mg.
Leachate from high-water PT1 was also sampled at two time-points before the 16-week termination in order to assess the change in leachate chemistry over time. While there was clearly an initial flux of cations and IC in the first week, the release of EW products did not reflect the much larger accumulation of products within the soil, particularly for wollastonite amendments (Supplementary Figure 2).
3.4 Acidifying fertiliser released cations without forming IC
Non-carbonic acid is hypothesised to release cations from silicate rock amendments without sequestering CO2. In PT3, the acidifying fertiliser had a strongly significant effect on the EW-attributable Ca + Mg release measured in the whole system (Figure 5A). In particular, the effect was significant in increasing EW-attributable exchangeable Ca + Mg in olivine amended soils. By comparison, the fertiliser did not have a significant impact on EW-attributable total IC in any soil-rock mixture tested (Figure 5B). The acidifying effect of the fertiliser was also observed in the pH measurements of the leachate (Supplementary Figure 3).
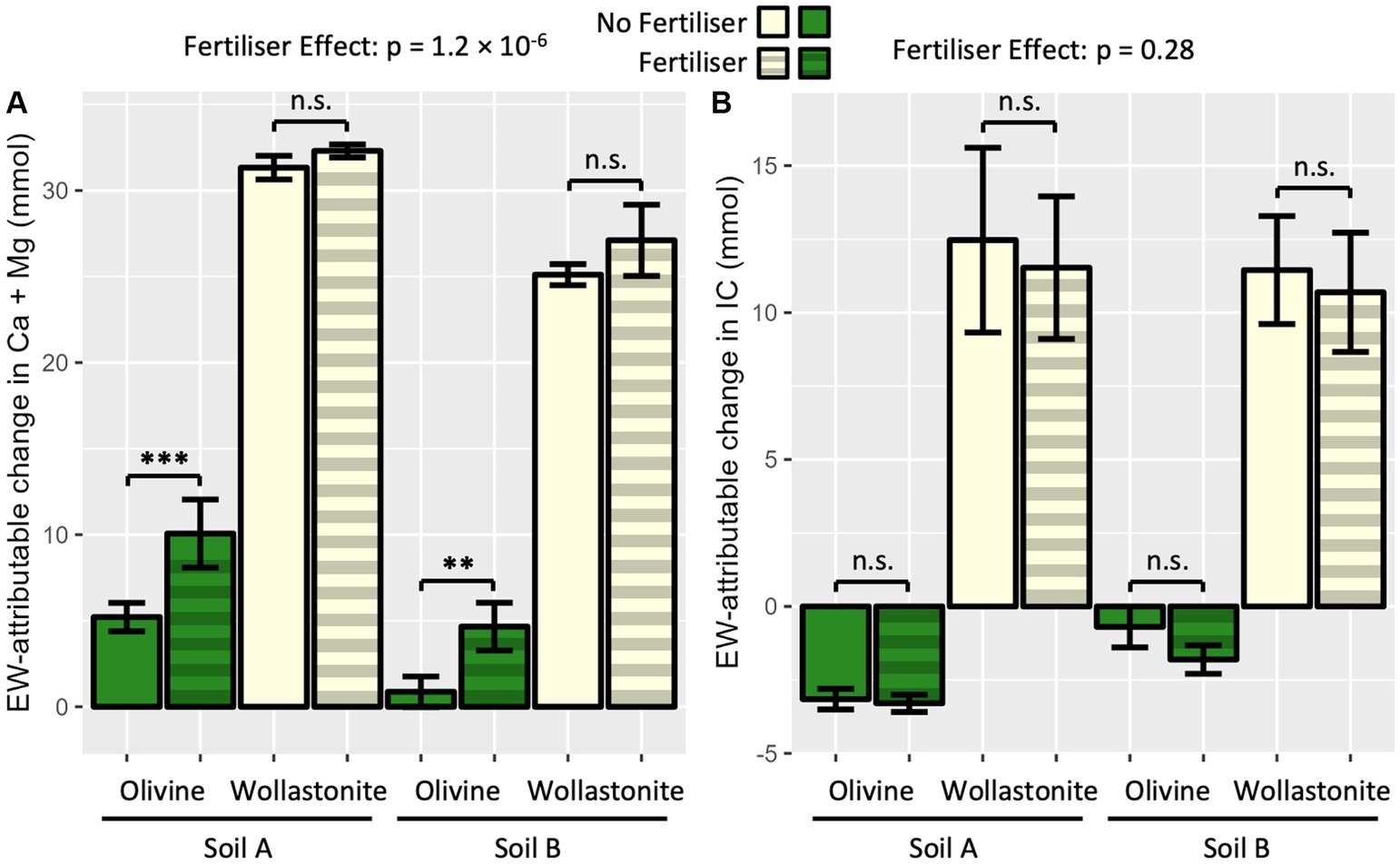
Figure 5. Effect of acidifying fertiliser on enhanced weathering products in high water pot trial (PT3). (A) EW-attributable Ca + Mg; and (B) EW-attributable IC. Four pots prepared per soil-rock-fertiliser combination with 15 wt% rock amendment in sandstone-based Soil A and granite-based Soil B, and allowed to weather for 10 weeks. Ammonium sulphate applied at 150 kgN ha−1. EW-attributable effect calculated by subtracting initial baseline and soil-only control values (Figure 2). Soil A represents sandstone-based soil from Kowen, ACT; Soil B represents granitic soil from Lerida, NSW. Bars represent mean ± SD. Statistical analysis by three-way ANOVA (soil, rock and fertiliser effect) and Tukey’s HSD post-hoc test, n.s. p > 0.05, **p < 0.01, ***p < 0.001.
3.5 EW-attributable cations and IC correlated strongly within leachate but loosely within soil
Averaging the results across soil-rock treatments showed that Ca + Mg measurements led to higher estimates of CDR than IC measurements. This relationship was examined further on the individual pot level, comparing the EW-attributable Ca + Mg to the EW-attributable IC within each individual pot. EW-attributable Ca + Mg and IC were correlated in soil measurements in both PT1 and PT2, with strongly significant linear relationships displaying goodness of fit coefficients of 0.88 in PT1 (Figure 6A) and 0.80 in PT2 (Figure 6B). However, while these relationships were evident across aggregated soil-rock mixtures, they were not present between the pots of each of the individual soil-rock mixtures, which displayed very different trends. There was no significant relationship between EW-attributable IC and cations within each specific soil-rock mixture (i.e., within each individual marker style in Figure 6). The wollastonite amended pots, for example, showed a consistent EW-attributable change in Ca + Mg, but a highly variable EW-attributable change in IC. The variability of the measured IC in the total soil pool for these wollastonite amended pots was much greater than the standard deviation of the direct IC analysis process, which was tested and shown to be <10% of the %IC and <5% at higher IC concentrations (Supplementary Table 5).
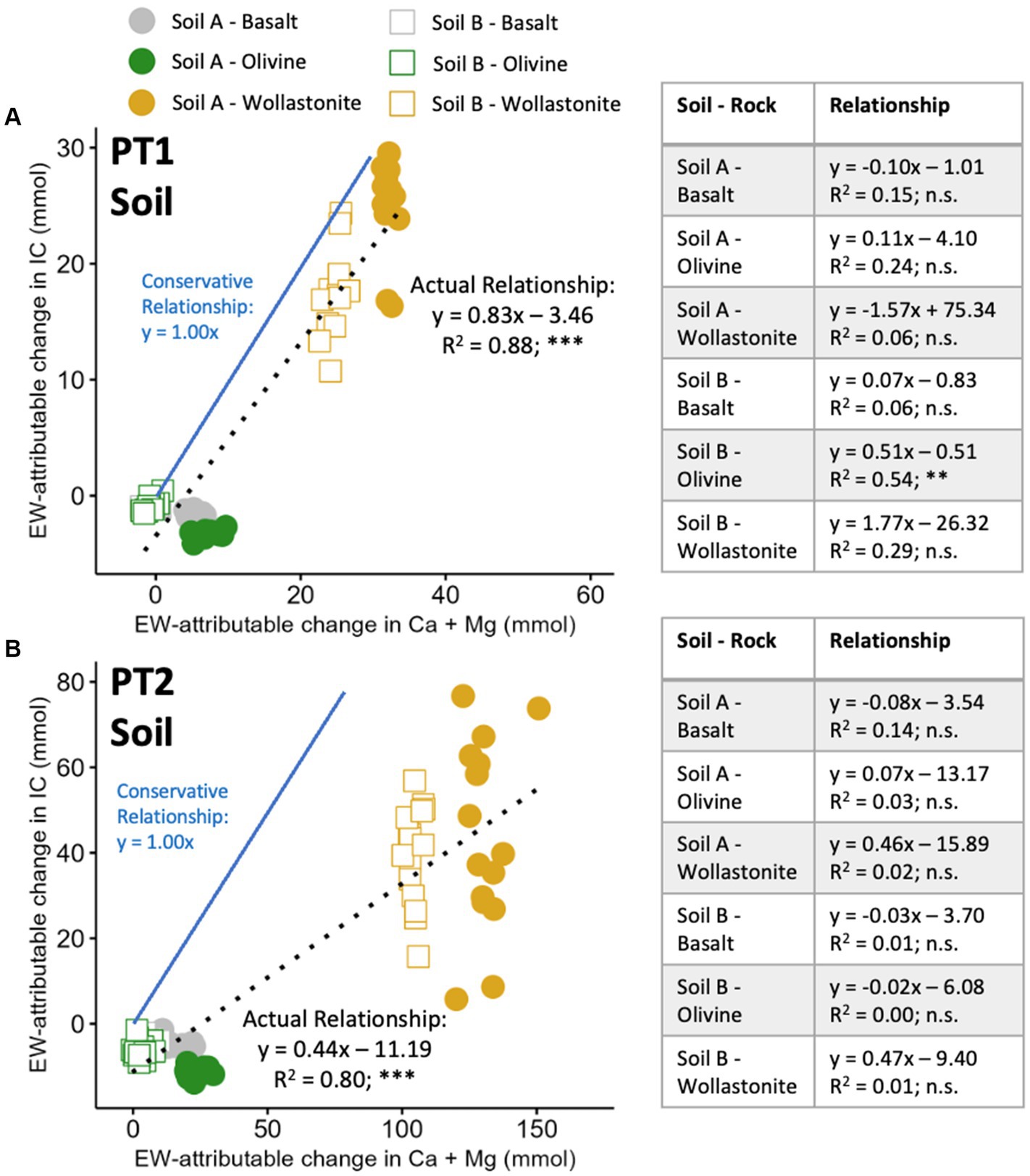
Figure 6. Relationship between soil Ca + Mg and IC released by the enhanced weathering effect, across and within soil-rock mixtures. (A) High water pot trial 1; and (B) Agriculturally representative pot trial 2. Aggregated relationship presented at the top of each plot; individual relationship within each soil-rock treatment presented in Table. 12 (PT1) and 16 (PT2) pots prepared per soil-rock combination with 15 wt% rock amendment in sandstone-based Soil A and granite-based Soil B, and allowed to weather for 16 weeks. EW-attributable effect calculated by subtracting initial baseline and soil-only control values (Figure 2). Conservative relationship assumes all IC existed as bicarbonate and was converted to carbonate during drying process (see Section 2). Maximum relationship (not plotted) assumes no thermal decomposition (). Points represent individual pots. Statistical analysis by linear regression modelling, n.s. p > 0.05, **p < 0.01, ***p < 0.001.
In comparison, EW-attributable cations and IC within leachate displayed much stronger correlation within soil-rock combinations (Figure 7). Across soil-rock combinations, PT1 demonstrated a strongly significant relationship with an R2 of 0.79 (Figure 7A), while the PT2 relationship had an R2 of 0.80 (Figure 7B). Within individual treatments, all relationships were shown to be significant and within a range of to , apart from Soil A – Wollastonite in PT1. These relationships were much more consistent in gradient and y-intercept than the relationships in the soil fraction. The deviations from the conservative and maximum relationships, in both soil and leachate, supported the previous finding that cation-based estimates of CDR produced greater results than IC-based estimates.

Figure 7. Relationship between leachate Ca + Mg and IC released by the enhanced weathering effect, across and within soil-rock mixtures. (A) High water pot trial 1; and (B) Agriculturally representative pot trial 2. Aggregated relationship presented at the top of each plot; individual relationship within each soil-rock treatment presented in table. 12 (PT1) and 16 (PT2) pots prepared per soil-rock combination with 15 wt% rock amendment in sandstone-based Soil A and granite-based Soil B, and allowed to weather for 16 weeks. EW-attributable effect calculated by subtracting initial baseline and soil-only control values (Figure 2). Maximum relationship assumes all IC exists as bicarbonate ( = 2), conservative relationship assumes = 1.5 (see Section 2). Points represent individual pots. Statistical analysis by linear regression modelling, n.s. p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001.
3.6 EW-attributable IC correlated with alkalinity in leachate
Leachate from PT1 was also measured for total alkalinity (TA), a proxy for bicarbonate and carbonate (IC). This was performed to confirm the results of IC analysis. TA includes IC as well as other forms of alkalinity, but by subtracting the TA from soil-only controls, EW-attributable alkalinity can be used as a measure of IC, assuming that the other alkaline species are equal in the control and silicate amended pots.
The relationship between EW-attributable TA and IC was strong (R2 = 0.90) and very close to a 1:1 ratio, as expected (Figure 8).
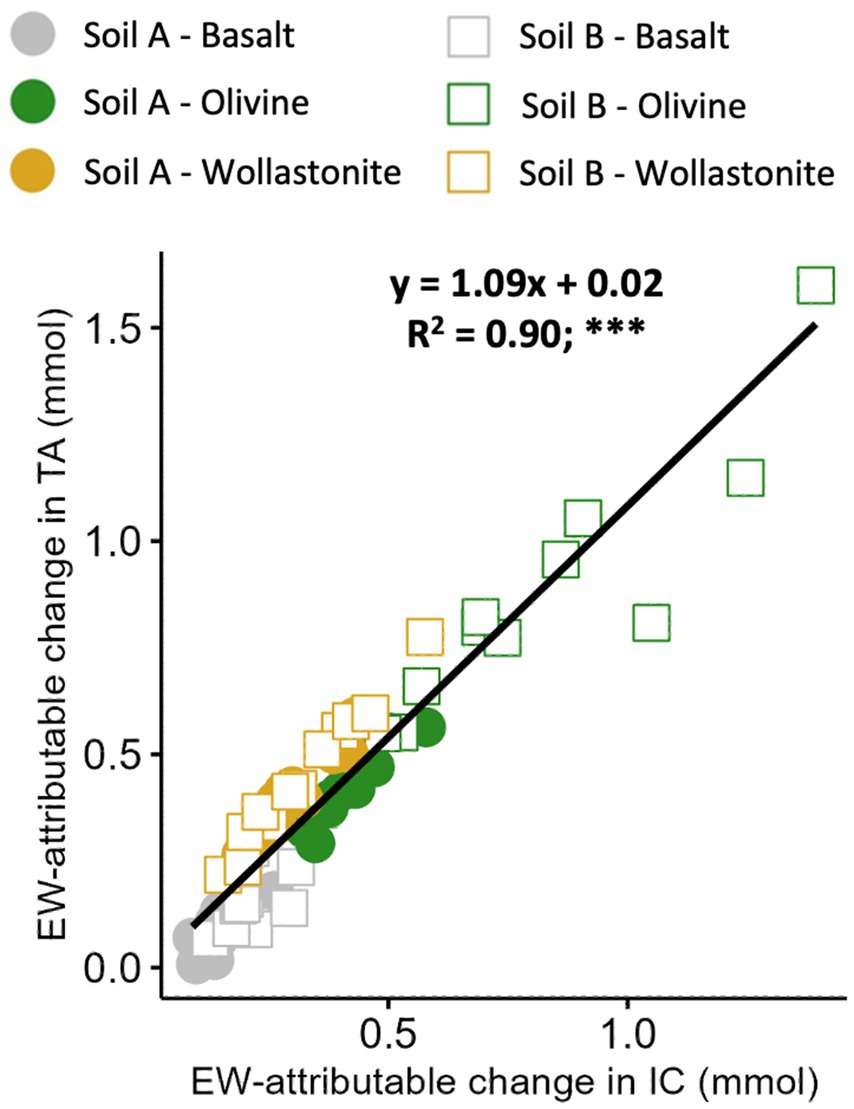
Figure 8. Relationship between total alkalinity and IC in the leachate from the enhanced weathering effect. Aggregated relationship presented at the top of each plot. A total of 12 pots prepared per soil-rock combination with 15 wt% rock amendment in sandstone-based Soil A and granite-based Soil B, and allowed to weather for 16 weeks in high water (PT1) conditions. EW-attributable effect calculated by subtracting initial baseline and soil-only control values (Figure 2). Points represent individual pots. Statistical analysis by linear regression modelling, ***p < 0.001.
4 Discussion
4.1 Comparison of soil and leachate results
The results from this study showed that the exchangeable cations and total IC in the soil fraction generally far exceeded the cations and IC measured in the leachate (Table 2). These findings are consistent with studies that have found limited significant changes in leachate chemistry after one to two years of basalt applications ranging from 50 to 400 t ha−1 (Kelland et al., 2020; Larkin et al., 2022; Paessler et al., 2023). As the soil fractions frequently hold the majority of the EW products, it is important to sample and quantify this pool of cations and IC.
4.1.1 Cation exchange capacity may delay CDR
The most likely explanation for the retention of cations and IC in the soil is the activity of the cation and anion exchange reactions, preventing the Ca, Mg and IC from entering the leachate and delaying the durable CDR process (Beerling et al., 2024; Clarkson et al., 2024; Kanzaki et al., 2024). Kelland et al. (2020) found soil-exchangeable cation concentrations over 100× leachate concentrations, and other studies showed significant exchangeable cation interactions (Pogge von Strandmann et al., 2019, 2021; Buss et al., 2023; Dietzen and Rosing, 2023; Wood et al., 2023). This is supported by the fact that Soil A with a CEC of 6.8 cmol+ kg−1 had a higher proportion of cations and IC in the soil and a lower proportion in the leachate than Soil B with a CEC of 2.3 cmol+ kg−1.
The exchangeable cations extracted from most soil-rock treatments across both PT1 and PT2 at the end of the weathering period were much higher than the effective CEC of the soils (Supplementary Table 1). This was likely due to the increase in soil CEC caused by silicate rock amendments, through the provision of mineral surfaces and mitigation of acidity (Gillman, 1980; te Pas et al., 2023). Gillman (1980) showed that CEC could increase by 2 or 3× with the application of silicate amendments with particle sizes <100 μm and in the range of 100 t ha−1, very similar to the particle sizes and distribution rates in this study. It is important to note that the retention of EW products was observed in soils of very low CEC before rock amendment (6.8 cmol+ kg−1 and 2.3 cmol+ kg−1 compared to agriculturally productive soils ranging from 10 to 30 cmol+ kg−1) with high watering rates (in PT1), indicating that the CEC of most soils may be sufficient to delay the leaching of EW products and the durable CDR effect (DPI, 2023).
The CEC of the soils stored the majority of the cations from silicate weathering. Over time, however, as sorption sites become saturated, weathering products may run through the soil into the leachate (Blume et al., 2016; Strawn, 2021; Paessler, 2023a). Temporal leachate measurements in PT1 showed no visible increase in leachate cations or IC above the non-rock controls after the first week, except for Soil B – Olivine (Supplementary Figure 2). The saturation of sorption sites may take months to decades, and therefore 16 weeks was not long enough to see the large quantities of cations or IC from the weathering reactions entering the leachate at a similar magnitude (Paessler et al., 2023; Clarkson et al., 2024; Kanzaki et al., 2024).
Carbonate precipitation may also be responsible for the retention of cations and IC in the soil phase. The ammonium acetate extraction would likely have dissolved calcite and dolomite and therefore soil carbonates may have contributed to the soil-exchangeable cation results (Rayment and Lyons, 2010). While carbonate precipitation was not quantified, wollastonite trials induce particularly large amounts of carbonate precipitation, which may partly contribute to the significant IC signal in total soil (Haque et al., 2020b,c; Khalidy et al., 2021, 2024).
The retention of EW products within the soil-exchangeable phase, despite low CEC and high watering rates, confirms that the permanent CDR effect may be delayed across time-scales ranging from weeks to years (Kelland et al., 2020; Paessler et al., 2023; Clarkson et al., 2024; Kanzaki et al., 2024). Due to this leaching delay, and the evidence that suggests there is no consistent relationship between exchangeable cations and soil IC produced from silicate weathering (Figure 6), it seems likely that exchangeable cations do not represent durable CDR and should not be quantified as such. Considering the high degree of cation retention in the soil-exchangeable reactions, this may reduce investment and focus on EW as a solution. Therefore, both the measurement of EW products in the exchangeable soil phase and the development of models will be important in understanding the pipeline of future CDR, the speed of leaching and the degree to which the initial alkalinity produced results in durable CDR, which could be vital for the deployment of EW globally (Dietzen and Rosing, 2023; Kanzaki et al., 2024).
4.1.2 Incomplete extraction may affect exchangeable cation quantification
While the ICP-OES analyses were shown to be accurate, the low recovery of known amounts of Ca and Mg suggested that the process by which cations were extracted did not fully account for the actual release of cations, with a greater underestimation of Ca than Mg (Figure 3).
Ten Berge et al. (2012) also produced evidence that cation extraction from soil may underestimate the true cation value, recovering only 30% of a known addition of Mg in soil. Underestimation may be a result of incomplete extraction of exchangeable cations. Our study used 1 M ammonium acetate (buffered to pH 7.0) as an extractant with one hour of shaking, allowing the reactive ammonium ions to displace the exchangeable cations from their sorption reactions (Rayment and Lyons, 2010). However, Dietzen and Rosing (2023) and Borge (1997) showed that 1 M ammonium nitrate (unbuffered) extracted significantly more Ca and Mg than 1 M ammonium acetate. More broadly, many different extractant solutions may be used depending on the soil properties and CEC (Borge, 1997; Rayment and Lyons, 2010; Rogers et al., 2019; Purnamasari et al., 2021). This suggests that the extraction protocol used in this study may have been unable to displace all sorbed cations, leading to a consistent underestimate of cations. This may have been due to the adjustment of the ammonium acetate extractant to a pH of 7.0, well above the initial pH of the soils (5.76 and 5.82), which would have led to a higher CEC in the soil due to the deprotonation of functional groups on organic matter and clay minerals (Sparks et al., 2023). This hypothesis would also explain the difference between Ca and Mg results, as Ca sorbs more strongly in soil (Thompson, 2012). The degree to which this mechanism underestimates cation release could be quantified by comparing different extraction protocols.
An alternative explanation for the low recovery rate of the soil-exchangeable measurements could be the precipitation of Mg and Ca into insoluble compounds. Neither carbonate or non-carbonate precipitation was quantified, but would require XRD analysis or sequential extraction (Tessier et al., 1979). In our study, Mg and Ca salts were applied in solid form and may have remained partly undissolved and un-measured, although a significant excess of water was added for complete dissolution. If this were a major factor, it would be expected that the higher watering regime in PT1 would result in a higher recovery rate than PT2, which was not observed.
The results suggest that exchangeable cations may be underestimated due to incomplete extraction. As previously established, exchangeable cations should not be considered durable CDR. However, many EW trials continue to measure the exchangeable fraction and this will remain important in improving our understanding of the CEC and its effect on EW. Therefore, the potential for incomplete extraction will affect these measurements.
4.2 Comparison of cation and IC results
4.2.1 Cation and IC-based estimates of CDR
Across both pot trials, and across the soil and leachate fractions, the cation (Ca + Mg)-based estimates of CDR were very frequently greater than the IC-based estimates (Table 2; Figures 6, 7). This occurred despite methodological choices to estimate a “conservative” cation-based CDR and a “maximum” IC-based CDR.
As explained in Section 4.1.1, soil-exchangeable cations are not a valid measure of durable CDR. However, if they were, the soil measurements would suggest that the wollastonite treatments had captured between 20 and 32 kgCO2 t−1 wollastonite based on exchangeable Ca + Mg measurements (or 2.1–4.9 tCO2 ha−1), or between 13 and 27 kgCO2 t−1 wollastonite based on total IC measurements (or 1.9–2.8 tCO2 ha−1) (Table 2). These results were smaller than the wollastonite CDR rates estimated by Haque et al. (2019, 2020b) which ranged between 80 and 217 kgCO2 t−1 rock over 2 to 5 months, but more consistent with the CDR rates from wollastonite estimated by te Pas et al. (2023), which varied from 4.9 to 23.6 kgCO2 t−1 rock over 2 months, depending on measurement approach (Table 1).
Only a limited number of EW studies have measured both cations and IC to calculate CDR. These studies have consistently shown that cation measurements produce higher CDR estimates than IC measurements (Taylor et al., 2021; te Pas et al., 2023). For example, Taylor et al. (2021), found that measuring Ca in leachate produced a CDR estimate 5× the estimate from bicarbonate measurements. Other studies have measured significant increases in cation release after silicate rock amendments without finding any significant change in IC (Renforth et al., 2015; Kelland et al., 2020; Vienne et al., 2022). So why do the results of this, and other studies, suggest that cation-based estimates of EW CDR are greater than IC-based estimates?
It is important to differentiate between discrepancies in the two measurement approaches in soil and leachate. In leachate, the cation release should be charge balanced by anions, predominantly bicarbonate in EW systems, and should therefore follow an idealised relationship (Figure 7) (Larkin et al., 2022). In soil, while cations and bicarbonate should be released in defined stoichiometric ratios (Equation 2), it is not fully understood how that relationship is maintained as cations enter the soil-exchangeable phase and whether soil cations and IC should follow an idealised relationship (Figure 6) (te Pas et al., 2023; Kanzaki et al., 2024).
It is also worth noting that the soil-based measurements for cations and IC quantified different pools. The cation measurements quantified the soil-exchangeable pool, while direct IC analysis quantified the total soil pool. Therefore, despite only measuring a subset of the total soil pool, the cation-based EW effect was still larger than the IC-based EW estimate and therefore, the impact of using two different measurement approaches can be dismissed.
In summary, cation-based approaches produced a greater estimate of CDR than IC-based approaches. This is likely due to one or more of the following mechanisms:
1. Non-carbonic acids release cations from silicate rocks without sequestering CO2.
2. CO2 outgassing causes a loss of IC.
3. Measurements systematically underestimate IC.
4.2.2 The role of non-carbonic acid weathering
Non-carbonic acidity has become increasingly recognised as a potential issue in measuring cations for EW CDR (Dietzen et al., 2018; Taylor et al., 2021; Holzer et al., 2023b; Clarkson et al., 2024). Strong acids (e.g., nitric acid) from fertiliser application can react with silicates through Equation 17. This releases cations but does not sequester CO2 (Figure 5). Organic acids can also reduce CDR efficiency (Taylor et al., 2021). CO2 is only captured when CO2-derived carbonic acid reacts with the silicates to form bicarbonate and carbonate (Equation 2).
It should be noted that the neutralisation of strong acid in Equation 17 increases pH, which favours the dissolution of atmospheric CO2 into solution, through the equilibria in Equations 18–20. A loss of H+ causes all the reactions to move to the right, and therefore CO2 is sequestered.
However, while carbonic acid weathering introduces cations that are charge balanced by bicarbonate, thereby sequestering atmospheric CO2, non-carbonic acid weathering releases anions such as nitrate that balance the cations instead (Equation 17). Until our understanding of the carbonate equilibria in soil and ocean waters improves, the silicate-based neutralisation of non-carbonic acids should not be considered as CDR (Dietzen et al., 2018; Taylor et al., 2021; Dietzen and Rosing, 2023; Holzer et al., 2023b; Clarkson et al., 2024). However, in some EW systems, nitrate produced from non-carbonic acid weathering may undergo denitrification, forming N2 (and often N2O) and allowing the cations to again couple with, and sequester, bicarbonate. In these instances, fertilisers may have the ability to accelerate weathering while only reducing CDR efficiency in the near term, although N2O emissions are also a consideration (Val Martin et al., 2023).
Dietzen and Rosing (2023) performed a study which suggested that in soils with a pH above 6.3 (measured in 1:5 H2O), non-carbonic acid weathering does not need to be accounted for, while soils below a pH of 5.2 may not be suitable for EW, due to the dominance of this non-carbonic acid weathering pathway. With a pH of 5.16, the acidity of the soil used by te Pas et al. (2023) may account for the significantly higher estimate of CDR from cation-based methods compared to IC-based methods. The Soil A (pH 5.76) and Soil B (pH 5.82) used in this study were also susceptible to non-carbonic acid weathering and the release of cations without CO2 sequestration, although the extent of this weathering pathway is unknown. While non-carbonic acid weathering was likely a cause of cation release overestimating CDR, the source of these acids is difficult to determine, as nitrogen and sulphur levels in initial soil were low (Supplementary Table 1). This study was limited in its ability to quantify this effect.
The addition of an acidifying fertiliser, ammonium sulphate, to olivine and wollastonite amended pots showed that acidity could increase cation release without increasing IC formation (Figure 5). The ammonium sulphate had an acidifying effect due to the release of protons in the microbial-mediated nitrification of ammonium into nitrate (Norton and Ouyang, 2019). The restoration of leachate pH in the presence of acidifying fertiliser in olivine and wollastonite amended systems supports the occurrence of Equation 17 (Supplementary Figure 3). Fertiliser was added at a rate of 150 kgN ha−1 which could have, at maximum, produced 3.43 mmol of Ca + Mg with complete conversion into strong acid and subsequent weathering. Therefore, the majority of the difference between EW-attributable cations in fertiliser and non-fertiliser olivine pots could be due to strong acid weathering (4.9 mmol in Soil A, 3.8 mmol in Soil B).
In summary, cation-based estimates of CDR were higher than IC-based estimates, likely due in part to non-carbonic acids present within the two soil types that released Ca and Mg from the silicate rock without forming IC. This highlights a flaw in cation-based measurements. The implications of these findings are clear: large-scale application of silicate rock amendments must account for non-carbonic acid weathering, particularly in acidic soils or when acidifying fertilisers are used. This can be partly achieved by measuring IC as well as cations (Section 4.3). With 50% of Australian agricultural soils below a pH of 5.5 and significant use of acidifying fertilisers, there is susceptibility to significant non-carbonic acid weathering (de Caritat and Wilford, 2011; Dietzen and Rosing, 2023).
4.2.3 The potential for CO2 outgassing
Two pieces of evidence support a hypothesis that some CO2 outgassing occurred throughout this study. Firstly, IC measurements were consistently lower than cation results. Secondly, across PT1, 2 and 3, basalt and olivine amended Soil A consistently showed a net reduction in total IC (soil + leachate) across the weathering period, which was not observed in the control pots (Figure 4). However, this was only statistically significant for Soil A – olivine treatments. There are four natural mechanisms by which outgassing of CO2 and therefore loss of IC may occur: (1) bicarbonate may precipitate as carbonate, releasing a CO2 molecule (Equation 3), (2) solid IC, either in the form of pedogenic or lithogenic carbonate, may react with a strong acid to outgas CO2 (Hartmann et al., 2013), (3) dissolved IC in the soil may react with protons to release CO2 (West and McBride, 2005; Cho et al., 2019) or (4) authigenic clay precipitation may release CO2 from the leachate (Schuiling and de Boer, 2010; Oelkers et al., 2018; Renforth and Campbell, 2021; Fuhr et al., 2022; Clarkson et al., 2024). The extent to which these processes occurred were not quantified for this study. Note, however, that cation-based approaches will not account for mechanisms 2 or 3, and will overestimate the net CDR effect as a result.
Beyond natural CO2 outgassing, it should be noted that the methodological choice of leachate sampling process may have been susceptible to CO2 outgassing through two mechanisms. Firstly, the carbonate equilibrium in leachate was initially established in pore spaces with high pCO2 and was then subjected to standard atmospheric pCO2 in the leachate collection trays. This may have led to outgassing of CO2, which was not quantified, but this is a common problem for all EW trials that collect leachate without controlling atmospheric conditions. Further, in agricultural deployments of EW, leachate will eventually be exposed to atmospheric pCO2 in rivers or oceans, and therefore this leachate collection process reflects this. The second mechanism that may have resulted in CO2 outgassing was the evaporation and re-dissolution of leachate solutes. As discussed in Supplementary material Section 1.1, the evaporation-redissolution process was tested and found to reduce IC by 5–10% without impacting TA. This is likely due either to the loss of dissolved CO2 or the precipitation and subsequent re-dissolution of carbonate from bicarbonate, losing IC without affecting TA. Importantly, the close relationship between TA and IC in leachate (Figure 8) also suggests that this evaporation-redissolution process did not reduce IC by more than 10%, and the discrepancy between cation-based and IC-based estimates of CDR in the leachate was often >10%, ranging from 7 to 75%.
In summary, the observed loss of IC in olivine and basalt amended Soil A may have been partly due to a low degree of CO2 outgassing by one or multiple of the natural and methodological-induced mechanisms suggested above. It is important to note that the amendment of agricultural soils with silicate rocks often includes the addition of IC in the form of magnesium and calcium carbonates (Supplementary Table 3). The potential emissions of CO2 that can be caused by the interactions of this lime with acidity in the soil, particularly strong acids from fertiliser use, should be considered, even if it is minimal and may be outweighed by the CDR process (IPCC, 2006; EPA, 2016; Raza et al., 2021). Importantly, cation measurements do not capture this outgassing process, while IC measurements will account for the reduction in net CDR.
4.2.4 Accuracy of IC quantification
Previously, many EW studies have been unable to quantify significant changes in IC (Renforth et al., 2015; Kelland et al., 2020; Larkin et al., 2022; Vienne et al., 2022; Wood et al., 2023). This has raised concerns around the accuracy of techniques to quantify IC. In our study, direct IC analysis was used, involving sample acidification in 30% phosphoric acid to convert the IC into CO2 and infra-red measurement of the released CO2 (Skalar Analytical B.V, 2021). This process measured all IC in the leachate, and in the soil, including precipitated IC and dissolved IC within the soil complex. In comparison to direct IC analysis, the standard approach to measuring soil IC in EW trials has been a combustion-based method, in which all inorganic and organic carbon (OC) is combusted into CO2 and IC is measured by subtracting OC from total carbon after acid-based treatment to remove IC and a second combustion (Loeppert and Suarez, 1996; Nelson and Sommers, 1996; Chatterjee et al., 2009; Almaraz et al., 2022; te Pas et al., 2023). The combustion process has a limited resolution of IC measurement, due partly to the number of steps required, each of which is able to introduce further uncertainty. In a previous EW trial that amended soils with a basalt-granite mix, the direct IC analysis method was compared to the combustion-based method. While the combustion process was often unable to measure IC, the direct IC analysis was able to measure IC to a much greater resolution and provided results much more consistent with rock vs. control treatments (Supplementary Figure 4), reflecting the improvement of the direct IC analysis over the combustion-based measurements. The direct IC analysis also produced a fairly consistent standard deviation across leachate and soil measurements, as a proportion of the %IC in the sample (Supplementary Table 5). The evidence suggests that direct IC analysis employed in this study is an improvement over the standard two-step combustion process for quantifying IC in EW studies.
While the direct IC analysis was shown to be accurate, the use of positive controls to assess the entire IC sampling, extraction and measurement process was invalidated by the potential for the applied bicarbonate and carbonate salts to escape the system through CO2 outgassing.
However, the IC sampling and measurement process in leachate was still partly assessed through a comparison of EW-attributable alkalinity (ΔTAEW) and IC results (ΔICEW). As described in Equations 21, 22, alkalinity and IC would be expected to produce similar results, with the majority of dissolved IC present as bicarbonate from a pH of 7–9 (Rohling, 2023). Therefore, the observed relationship of 1.09 mol alkalinity to 1 mol IC with strong correlation (R2 = 0.90), suggests that IC analysis in leachate was generally accurate (Figure 8). Note that this relationship suggests = 1.9.
Finally, a systematic measurement error generated through the baseline measurement process was considered. Quantifying the baselines involved a separate measurement of soil and rock IC and then a calculation of the overall baseline for each pot given the 0.85: 0.15 ratio. This approach was chosen to avoid extra variability from incomplete mixing (potentially due to the density separation of rock and soil) that was more likely to invalidate a measurement of a combined soil-rock sample. We still measured the IC content in pre-mixed rock-soil baseline samples and did not find any consistent over- or under-estimation of the IC values when comparing the two baseline approaches (Supplementary Table 6). This highlights that measurement of the mixed soil-rock samples did not cause any matrix effect, thereby validating the chosen baseline measurement technique. Another systematic error, which can always impact EW trials, may have arisen from heterogeneity of IC in ground rock samples, which could have led to an over-estimate of baseline IC.
In summary, the results suggested that IC can be measured in leachate with reasonable accuracy using direct IC analysis or TA titration, and that direct IC analysis is an improvement over the two-step combustion process. However, the recovery rate of IC measurements in the total soil fraction was not clearly assessed. Overall, inaccuracy in IC measurements is unlikely to have caused the discrepancy between cation-based and IC-based CDR estimates.
4.3 The potential for complementary IC and cation-based measurements
This study has shown the potential for natural mechanisms to cause incomplete and improper accounting of cations in EW systems (Figure 9). Incomplete extraction may lead to an underestimation of soil-exchangeable cations, while the activity of non-carbonic acids and CO2 outgassing may lead to overestimates of CDR from leachate cation measurements. These processes generally lead to the release of cations without an equivalent formation of bicarbonate (carbonic acid weathering of existing carbonates, non-carbonic acid weathering of silicates and existing carbonates), or the outgassing of IC as CO2 without an associated reduction in cations (dissolved bicarbonate reacting with acidity, non-carbonic acid outgassing of existing carbonates) (Figure 9). More novel cation-based methods that use an immobile, often titanium, tracer element are also susceptible to the limitations of cation-based measurements examined in this study (Reershemius et al., 2023; Beerling et al., 2024; Clarkson et al., 2024). IC-based approaches, including direct IC analysis and TA titration, can overcome these problems with cation measurements. IC-based approaches will not quantify cations released from non-carbonic acid weathering and will account for loss of CO2 that reduces the net CDR effect. Therefore, EW measurement should not rely solely on cations, without comparing results to IC measurements and quantifying any non-carbonic acid weathering or CO2 outgassing.
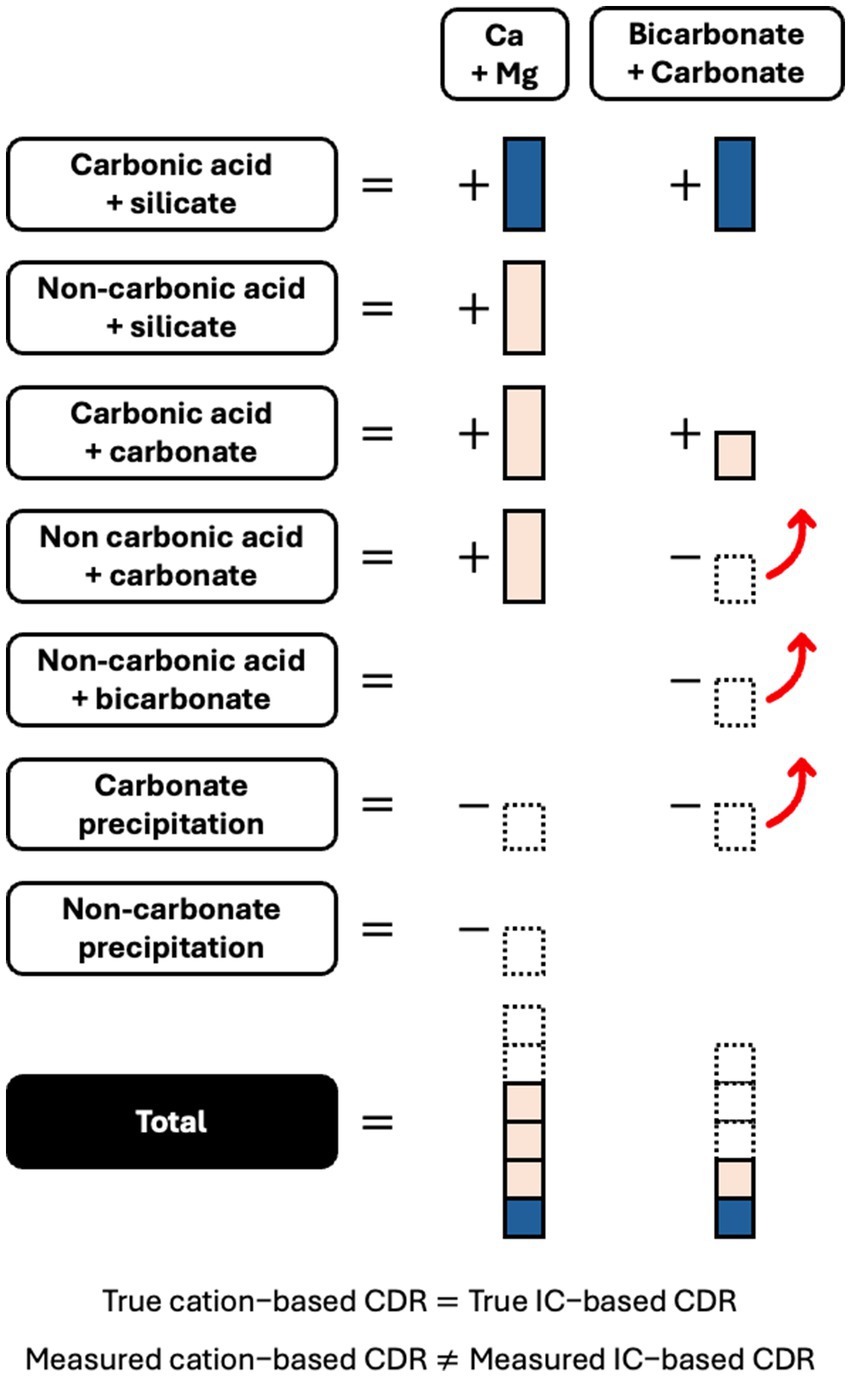
Figure 9. Schematic representing how the “true” Ca and Mg silicate EW CDR effect (blue) is masked by complex soil processes that produce EW products (tan) or remove dissolved EW products (dashed border) through precipitation or outgassing (red arrow). The “true” CDR will consist of stoichiometrically balanced cations and inorganic carbon (IC). However, the soil processes will cause measured cations and measured IC to be stoichiometrically unbalanced.
Meanwhile, the causes of improper accounting and inaccuracy in IC-based estimates of EW CDR are well known: outgassing of CO2 due to re-equilibration or carbonate precipitation can occur downstream in rivers and oceans (Figure 9) (Renforth and Henderson, 2017; Knapp and Tipper, 2022; Zhang et al., 2022; Harrington et al., 2023; Holzer et al., 2023b), or during the sampling and measuring process itself (i.e., as may have occurred from leachate in this study). Cation-based approaches are not affected by these mechanisms and can therefore complement and increase the accuracy of IC-based approaches.
Therefore, to improve the accuracy of EW quantification, cation and IC measurements from leachate should be used together (te Pas et al., 2023). Leachate cation and IC measurements can be used complementarily, because the relationship between EW-attributable cations and IC in leachate is mostly consistent (Figure 7). In comparison, this relationship is much more complicated in soil, and the IC is not durably stored at that stage (Figure 6). To reconcile leachate cation and IC measurements, natural mechanisms that cause a discrepancy between cation and IC results should be individually quantified or estimated. Non carbonic acid weathering, for example, may be quantifiable through anion measurement or discount factors (Taylor et al., 2021; Dietzen and Rosing, 2023). Khalidy et al. (2021, 2024) have investigated processes to measure pedogenic carbonate precipitation. Once these individual mechanisms are accounted for, a valid and accurate approach to quantifying EW will show the same cation-based and IC-based CDR estimate (Figure 9).
4.4 Soil, rock and plant effects on CDR and measurements
The final key finding of this study was the importance of specific soil and rock factors in determining overall CDR and affecting measurement approaches. Soil and rock type affected weathering rate, retention of EW products in soil and led to specific soil-rock relationships between EW-attributable IC and cation release (Figures 6, 7). The effect of plants was not a major focus and has been excluded. However, the presence of the soybean plant in PT2 led to statistically significant increases in IC and decreases in Ca + Mg measurements (Supplementary Figure 5).
4.5 Limitations
A primary limitation in this EW study and many other empirical EW trials is the use of imperfect controls. In this study and in the broader EW field, CDR is calculated by subtracting a non-rock amended control from a rock amended treatment to isolate the specific EW effect. However, due to high levels of natural variability, this control may not accurately reflect the background effects within the rock amended soils. In this study, results have repeatedly shown minimal change in the IC and Ca + Mg of the control treatments over time, indicating limited background effects on IC and cations, supporting this approach. Another significant limitation that exists in most EW studies is the difficulty of accounting for downstream changes in EW CDR over long periods of time due to the ongoing potential for CO2 outgassing from precipitation and re-equilibration in the rivers and ocean. There are many complex ocean factors that cause the net CDR of EW to change over time, including the lag by which ocean alkalinity causes uptake of atmospheric CO2 which may range from months to years, the impact of local temperature and salinity on the carbonate equilibrium, the changing atmospheric concentration of CO2, and the changing dynamics of marine carbonate precipitation and dissolution with depth (Harvey, 2008; Feng et al., 2017; Renforth and Henderson, 2017; Bach et al., 2019; Middelburg et al., 2020; Rohling, 2023). There were other potential errors introduced by improper or non-representative sampling and mixing. Clarkson et al. (2024) explores these errors in greater detail.
5 Conclusion and future directions
EW may play a significant role in humanity’s pathway to a net zero future. This, however, requires accurate and robust measurement of its CDR effect.
This study has examined the accuracy and consistency of some existing measurement approaches. It has concluded that there are key causes of incomplete accounting and inaccuracy in the commonly employed cation-based measurement approaches. These include the incomplete extraction of exchangeable cations within soils, non-carbonic acid weathering and CO2 outgassing. Similarly, IC-based measurements, including direct IC analysis and TA titration are limited by the complexity of the IC equilibria and potential for downstream CO2 loss.
However, complementary use of the cation- and IC-based measurement approaches may mitigate these issues. In particular, reconciling leachate cation and IC measurements through quantification of the natural soil mechanisms may validate and increase the accuracy of the EW CDR estimation. This study has also shown that soil contains the majority of EW products within its complex exchange reactions and should be measured for an understanding of the durable CDR effect in the pipeline.
In conclusion, this article has shown a potential pathway for the development of increasingly accurate, robust and reproducible EW measurement approaches.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. JB: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. WB: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Research School of Biology, ANU.
Acknowledgments
We deeply appreciate the assistance of James Latimer, Brett Knowles, Robin Grun, Yang Wu, Sonali Varma, Andi Wibowo, Sophia Cain, Emma John, Rebecca Lyons, Christine Larsen, Leah Moore, Danielle Way, and Andrew Scafaro.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fclim.2024.1352825/full#supplementary-material
References
Agriculture Victoria. (2023). Growing soybean in Victoria. Agriculture Victoria. Available at: https://agriculture.vic.gov.au/crops-and-horticulture/grains-pulses-and-cereals/growing-grains-pulses-and-cereals/growing-soybean-in-victoria (Accessed October 22, 2023).
Almaraz, M., Bingham, N. L., Holzer, I. O., Geoghegan, E. K., Goertzen, H., Sohng, J., et al. (2022). Methods for determining the CO2 removal capacity of enhanced weathering in agronomic settings. Front. Clim. 4:970429. doi: 10.3389/fclim.2022.970429
Amann, T., Hartmann, J., Struyf, E., de Oliveira Garcia, W., Fischer, E. K., Janssens, I., et al. (2020). Enhanced weathering and related element fluxes – a cropland mesocosm approach. Biogeosciences 17, 103–119. doi: 10.5194/bg-17-103-2020
Araújo, L. G., de Sousa, D. M. G., de Figueiredo, C. C., Rein, T. A., de Souza Nunes, R., dos Santos, J. D. D. G., et al. (2019). How does gypsum increase the organic carbon stock of an Oxisol profile under sugarcane? Geoderma 343, 196–204. doi: 10.1016/j.geoderma.2019.02.029
Armstrong, D., and Giblin, M. (2001). Assessing your water resources, wise watering irrigation management course notes. Hobart, TAS: Department of Primary Industry, Parks, Water and the Environment.
Bach, L. T., Gill, S. J., Rickaby, R. E. M., Gore, S., and Renforth, P. (2019). CO2 removal with enhanced weathering and ocean alkalinity enhancement: potential risks and CO-benefits for marine pelagic ecosystems. Front. Clim. 1:7. doi: 10.3389/fclim.2019.00007
Beerling, D. J., Epihov, D. Z., Kantola, I. B., Masters, M. D., Reershemius, T., Planavsky, N. J., et al. (2024). Enhanced weathering in the US Corn Belt delivers carbon removal with agronomic benefits. Proc. Natl. Acad. Sci. U. S. A. 121:e2319436121. doi: 10.1073/pnas.2319436121
Beerling, D. J., Kantzas, E. P., Lomas, M. R., Wade, P., Eufrasio, R. M., Renforth, P., et al. (2020). Potential for large-scale CO(2) removal via enhanced rock weathering with croplands. Nature 583, 242–248. doi: 10.1038/s41586-020-2448-9
Beerling, D. J., Leake, J. R., Long, S. P., Scholes, J. D., Ton, J., Nelson, P. N., et al. (2018). Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 4, 138–147. doi: 10.1038/s41477-018-0108-y
Blume, H.-P., Brümmer, G. W., Fleige, H., Horn, R., Kandeler, E., Kögel-Knabner, I., et al. (2016). “Chemical properties and processes” in Scheffer/Schachtschabel soil science. eds. H.-P. Blume, G. W. Brümmer, H. Fleige, R. Horn, E. Kandeler, and I. Kögel-Knabner (Berlin: Springer), 123–174.
Borge, A. (1997). A comparison of buffered and unbuffered amonium salts to determine exchanged base cations in acid soils. Commun. Soil Sci. Plant Anal. 28, 1421–1428. doi: 10.1080/00103629709369884
Buss, W., Hasemer, H., Ferguson, S., and Borevitz, J. (2023). Stabilisation of soil organic matter with rock dust partially counteracted by plants. Glob. Chang. Biol. 30:e17052. doi: 10.1111/gcb.17052
cdr.fyi. (2024). Carbon removal map. Available at: https://www.cdr.fyi/carbon-removal-map (Accessed July 20, 2024).
Chatterjee, A., Lal, R., Wielopolski, L., Martin, M. Z., and Ebinger, M. H. (2009). Evaluation of different soil carbon determination methods. Crit. Rev. Plant Sci. 28, 164–178. doi: 10.1080/07352680902776556
Cho, S. R., Jeong, S. T., Kim, G. Y., Lee, J. G., Kim, P. J., and Kim, G. W. (2019). Evaluation of the carbon dioxide (CO2) emission factor from lime applied in temperate upland soil. Geoderma 337, 742–748. doi: 10.1016/j.geoderma.2018.10.007
Ciampitti, I. A., de Borja Reis, A. F., Córdova, S. C., Castellano, M. J., Archontoulis, S. V., Correndo, A. A., et al. (2021). Revisiting biological nitrogen fixation dynamics in soybeans. Front. Plant Sci. 12:727021. doi: 10.3389/fpls.2021.727021
Cipolla, G., Calabrese, S., Noto, L. V., and Porporato, A. (2021). The role of hydrology on enhanced weathering for carbon sequestration I. Modeling rock-dissolution reactions coupled to plant, soil moisture, and carbon dynamics. Adv. Water Resour. 154:103934. doi: 10.1016/j.advwatres.2021.103934
Clarkson, M. O., Larkin, C. S., Swoboda, P., Reershemius, T., Suhrhoff, T. J., Maesano, C. N., et al. (2024). A review of measurement for quantification of carbon dioxide removal by enhanced weathering in soil. Front. Clim. 6:1345224. doi: 10.3389/fclim.2024.1345224
de Caritat, P. C. M., and Wilford, J. (2011). The pH of Australian soils: field results from a national survey. Soil Res. 49, 173–182. doi: 10.1071/SR10121
Dietzen, C., Harrison, R., and Michelsen-Correa, S. (2018). Effectiveness of enhanced mineral weathering as a carbon sequestration tool and alternative to agricultural lime: an incubation experiment. Int. J. Greenhouse Gas Control 74, 251–258. doi: 10.1016/j.ijggc.2018.05.007
Dietzen, C., and Rosing, M. T. (2023). Quantification of CO2 uptake by enhanced weathering of silicate minerals applied to acidic soils. Int. J. Greenhouse Gas Control 125:103872. doi: 10.1016/j.ijggc.2023.103872
DPI. (2023). Cation exchange capacity. Department of Primary Industries, NSW Government. Available at: https://www.dpi.nsw.gov.au/agriculture/soils/guides/soil-nutrients-and-fertilisers/cec (Accessed October 23, 2023).
DPIRD. (2021). Boosting winter pasture growth with nitrogen fertiliser – Western Australia. Department of Primary Industries and Regional Development. Available at: https://www.agric.wa.gov.au/pasture-management/boosting-winter-pasture-growth-nitrogen-fertiliser-%E2%80%93-western-australia (Accessed October 22, 2023).
Dupla, X., Möller, B., Baveye, P. C., and Grand, S. (2023). Potential accumulation of toxic trace elements in soils during enhanced rock weathering. Eur. J. Soil Sci. 74:e13343. doi: 10.1111/ejss.13343
Edwards, D. P., Lim, F., James, R. H., Pearce, C. R., Scholes, J., Freckleton, R. P., et al. (2017). Climate change mitigation: potential benefits and pitfalls of enhanced rock weathering in tropical agriculture. Biol. Lett. 13:20160715. doi: 10.1098/rsbl.2016.0715
EPA (2016). Inventory of US greenhouse gas emissions and sinks: 1990–2015, EPA 430-P-17-001. Washington, DC: US Environmental Protection Agency.
Esteves, E., Kadyampakeni, D. M., Zambon, F., Ferrarezi, R. S., and Maltais-Landry, G. (2022). Magnesium fertilization has a greater impact on soil and leaf nutrient concentrations than nitrogen or calcium fertilization in Florida orange production. Nutr. Cycl. Agroecosyst. 122, 73–87. doi: 10.1007/s10705-021-10182-1
Feng, E. Y., Koeve, W., Keller, D. P., and Oschlies, A. (2017). Model-based assessment of the CO2 sequestration potential of Coastal Ocean Alkalinization. Earth's Future 5, 1252–1266. doi: 10.1002/2017EF000659
Fuhr, M., Geilert, S., Schmidt, M., Liebetrau, V., Vogt, C., Ledwig, B., et al. (2022). Kinetics of olivine weathering in seawater: an experimental study. Front. Clim. 4:831587. doi: 10.3389/fclim.2022.831587
Gillman, G. P. (1980). The effect of crushed basalt scoria on the cation exchange properties of a highly weathered soil. Soil Sci. Soc. Am. J. 44, 465–468. doi: 10.2136/sssaj1980.03615995004400030005x
Goll, D. S., Ciais, P., Amann, T., Buermann, W., Chang, J., Eker, S., et al. (2021). Potential CO2 removal from enhanced weathering by ecosystem responses to powdered rock. Nat. Geosci. 14, 545–549. doi: 10.1038/s41561-021-00798-x
Guarini, G. G. T., Dei, L., and Sarti, G. (1995). The thermal decomposition of NaHCO3 powders and single crystals. J. Therm. Anal. 44, 31–44. doi: 10.1007/BF02547131
Haque, F., Chiang, Y. W., and Santos, R. M. (2020a). Risk assessment of Ni, Cr, and Si release from alkaline minerals during enhanced weathering. Open Agric. 5, 166–175. doi: 10.1515/opag-2020-0016
Haque, F., Santos, R. M., and Chiang, Y. W. (2020b). CO2 sequestration by wollastonite-amended agricultural soils – an Ontario field study. Int. J. Greenhouse Gas Control 97:103017. doi: 10.1016/j.ijggc.2020.103017
Haque, F., Santos, R. M., and Chiang, Y. W. (2020c). Optimizing inorganic carbon sequestration and crop yield with Wollastonite soil amendment in a microplot study. Front. Plant Sci. 11:1012. doi: 10.3389/fpls.2020.01012
Haque, F., Santos, R. M., Dutta, A., Thimmanagari, M., and Chiang, Y. W. (2019). Co-benefits of Wollastonite weathering in agriculture: CO(2) sequestration and promoted plant growth. ACS Omega 4, 1425–1433. doi: 10.1021/acsomega.8b02477
Harrington, K. J., Hilton, R. G., and Henderson, G. M. (2023). Implications of the riverine response to enhanced weathering for CO2 removal in the UK. Appl. Geochem. 152:105643. doi: 10.1016/j.apgeochem.2023.105643
Hartman, M., Svoboda, K., Pohořelý, M., and Šyc, M. (2013). Thermal decomposition of sodium hydrogen carbonate and textural features of its calcines. Ind. Eng. Chem. Res. 52, 10619–10626. doi: 10.1021/ie400896c
Hartmann, J., West, A. J., Renforth, P., Köhler, P., De La Rocha, C. L., Wolf-Gladrow, D. A., et al. (2013). Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Rev. Geophys. 51, 113–149. doi: 10.1002/rog.20004
Harvey, L. D. D. (2008). Mitigating the atmospheric CO2 increase and ocean acidification by adding limestone powder to upwelling regions. J. Geophys. Res. Oceans 113:C04028. doi: 10.1029/2007JC004373
Hills, A. W. D. (1968). The mechanism of the thermal decomposition of calcium carbonate. Chem. Eng. Sci. 23, 297–320. doi: 10.1016/0009-2509(68)87002-2
Holzer, I. O., Nocco, M. A., and Houlton, B. Z. (2023a). Direct evidence for atmospheric carbon dioxide removal via enhanced weathering in cropland soil. Environ. Res. Commun. 5:101004. doi: 10.1088/2515-7620/acfd89
Holzer, I. O., Sokol, N., Slessarev, E., Martin, K., and Chay, F. (2023b). Quantifying enhanced weathering (non-peer reviewed blog post). Available at: https://carbonplan.org/research/cdr-verification/enhanced-weathering (Accessed January 23, 2024).
IPCC (2018). Global warming of 1.5°C. An IPCC special report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate povertu. Geneva: IPCC.
IPCC (2022a). Climate change 2022: impacts, adaptation, and vulnerability. Contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change. Geneva: IPCC.
IPCC (2022b). Climate change 2022: mitigation of climate change. Contribution of working group III to the sixth assessment report of the intergovernmental panel on climate change. Geneva: IPCC.
Jezek, M., Geilfus, C. M., Bayer, A., and Mühling, K. H. (2014). Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 5:781. doi: 10.3389/fpls.2014.00781
Kantola, I. B., Blanc-Betes, E., Masters, M. D., Chang, E., Marklein, A., Moore, C. E., et al. (2023). Improved net carbon budgets in the US Midwest through direct measured impacts of enhanced weathering. Glob. Chang. Biol. 29, 7012–7028. doi: 10.1111/gcb.16903
Kanzaki, Y., Planavsky, N., Zhang, S., Jordan, J., and Reinhard, C. T. (2024). Soil cation storage as a key control on the timescales of carbon dioxide removal through enhanced weathering. ESS Open Archive. doi: 10.22541/essoar.170960101.14306457/v1
Kelland, M. E., Wade, P. W., Lewis, A. L., Taylor, L. L., Sarkar, B., Andrews, M. G., et al. (2020). Increased yield and CO2 sequestration potential with the C4 cereal Sorghum bicolor cultivated in basaltic rock dust-amended agricultural soil. Glob. Chang. Biol. 26, 3658–3676. doi: 10.1111/gcb.15089
Khalidy, R., Chiang, Y. W., and Santos, R. M. (2024). Tracking pedogenic carbonate formation and migration in agricultural soils amended with crushed wollastonite ore- evidence from field trials. Res. Square. doi: 10.21203/rs.3.rs-3851689/v1
Khalidy, R., Haque, F., Chiang, Y. W., and Santos, R. M. (2021). Monitoring pedogenic inorganic carbon accumulation due to weathering of amended silicate minerals in agricultural soils. JoVE 172:e61996. doi: 10.3791/61996
Knapp, W. J., and Tipper, E. T. (2022). The efficacy of enhancing carbonate weathering for carbon dioxide sequestration. Front. Clim. 4:928215. doi: 10.3389/fclim.2022.928215
Kukla, T., Suhrhoff, T. J., Loeffler, S., Martin, K., and Chay, F. (2024). Does enhanced weathering work? We’re still learning (non-peer reviewed blog post). Available at: https://carbonplan.org/research/enhanced-weathering-fluxes (Accessed July 20, 2024).
Kuttah, D., and Sato, K. (2015). Review on the effect of gypsum content on soil behavior. Transp. Geotech. 4, 28–37. doi: 10.1016/j.trgeo.2015.06.003
Larkin, C. S., Andrews, M. G., Pearce, C. R., Yeong, K. L., Beerling, D. J., Bellamy, J., et al. (2022). Quantification of CO2 removal in a large-scale enhanced weathering field trial on an oil palm plantation in Sabah, Malaysia. Front. Clim. 4:959229. doi: 10.3389/fclim.2022.959229
Loeppert, R. H., and Suarez, D. L. (1996). Carbonate and gypsum. Methods Soil Anal. 5, 437–474. doi: 10.2136/sssabookser5.3.c15
Majlesi, S., Juutilainen, J., Kasurinen, A., Mpamah, P., Trubnikova, T., Oinonen, M., et al. (2019). Uptake of soil-derived carbon into plants: implications for disposal of nuclear waste. Environ. Sci. Technol. 53, 4198–4205. doi: 10.1021/acs.est.8b06089
Manning, D. A. C., Renforth, P., Lopez-Capel, E., Robertson, S., and Ghazireh, N. (2013). Carbonate precipitation in artificial soils produced from basaltic quarry fines and composts: an opportunity for passive carbon sequestration. Int. J. Greenhouse Gas Control 17, 309–317. doi: 10.1016/j.ijggc.2013.05.012
Martínez-Cuenca, M.-R., Iglesias, D. J., Forner-Giner, M. A., Primo-Millo, E., and Legaz, F. (2013). The effect of sodium bicarbonate on plant performance and iron acquisition system of FA-5 (Forner-Alcaide 5) citrus seedlings. Acta Physiol. Plant. 35, 2833–2845. doi: 10.1007/s11738-013-1317-7
Matcham, E. C. S. P. (2022). Soybean irrigation during reproductive growth. Madison, WI: University of Wisconsin-Madison.
Middelburg, J. J., Soetaert, K., and Hagens, M. (2020). Ocean alkalinity, buffering and biogeochemical processes. Rev. Geophys. 58:e2019RG000681. doi: 10.1029/2019RG000681
Nelson, D. W., and Sommers, L. E. (1996). Total carbon, organic carbon, and organic matter. Methods Soil Anal. 9, 961–1010. doi: 10.2136/sssabookser5.3.c34
Norton, J., and Ouyang, Y. (2019). Controls and adaptive management of nitrification in agricultural soils. Front. Microbiol. 10:1931. doi: 10.3389/fmicb.2019.01931
Oelkers, E. H., Declercq, J., Saldi, G. D., Gislason, S. R., and Schott, J. (2018). Olivine dissolution rates: a critical review. Chem. Geol. 500, 1–19. doi: 10.1016/j.chemgeo.2018.10.008
Paessler, D. (2023a). Quantification of ERW: suggesting the concept of “scopes” for MRV. Available at: https://www.carbon-drawdown.de/blog/2023-8-7-quantification-of-erw-with-scopes-for-mrv (Accessed March 13, 2024).
Paessler, D., Steffens, R. H., and Smet, I. (2023). Monitoring CO2 concentrations in soil gas: a novel MRV approach for cropland-based ERW? (non-peer reviewed blog post). Available at: https://www.carbon-drawdown.de/blog/2023-5-17-monitoring-co2-concentrations-in-soil-gas-a-novel-mrv-approach-for-cropland-based-erw (Accessed May 17, 2023).
te Pas, E. E., Hagens, M., and Comans, R. N. J. (2023). Assessment of the enhanced weathering potential of different silicate minerals to improve soil quality and sequester CO2. Front. Clim. 4:954064. doi: 10.3389/fclim.2022.954064
Pogge von Strandmann, P. A. E., Fraser, W. T., Hammond, S. J., Tarbuck, G., Wood, I. G., Oelkers, E. H., et al. (2019). Experimental determination of Li isotope behaviour during basalt weathering. Chem. Geol. 517, 34–43. doi: 10.1016/j.chemgeo.2019.04.020
Pogge von Strandmann, P. A. E., Renforth, P., West, A. J., Murphy, M. J., Luu, T.-H., and Henderson, G. M. (2021). The lithium and magnesium isotope signature of olivine dissolution in soil experiments. Chem. Geol. 560:120008. doi: 10.1016/j.chemgeo.2020.120008
Poschenrieder, C., Fernández, J. A., Rubio, L., Pérez, L., Terés, J., and Barceló, J. (2018). Transport and use of bicarbonate in plants: current knowledge and challenges ahead. Int. J. Mol. Sci. 19:1352. doi: 10.3390/ijms19051352
Purnamasari, L., Rostaman, T., Widowati, L. R., and Anggria, L. (2021). Comparison of appropriate cation exchange capacity (CEC) extraction methods for soils from several regions of Indonesia. IOP Conf. Series Earth Environ. Sci. 648:012209. doi: 10.1088/1755-1315/648/1/012209
Rayment, G., and Lyons, D. (2010). Soil chemical methods-Australasia. Clayton, VIC: CSIRO Publishing.
Raza, S., Zamanian, K., Ullah, S., Kuzyakov, Y., Virto, I., and Zhou, J. (2021). Inorganic carbon losses by soil acidification jeopardize global efforts on carbon sequestration and climate change mitigation. J. Clean. Prod. 315:128036. doi: 10.1016/j.jclepro.2021.128036
Reershemius, T., Kelland, M. E., Jordan, J. S., Davis, I., D’Ascanio, R., Kalderon-Asael, B., et al. (2023). Initial validation of a soil-based mass-balance approach for empirical monitoring of enhanced rock weathering rates. Environ. Sci. Technol. 57, 19497–19507. doi: 10.1021/acs.est.3c03609
Reershemius, T., and Suhrhoff, T. J. (2024). On error, uncertainty, and assumptions in calculating carbon dioxide removal rates by enhanced rock weathering in Kantola et al., 2023. Glob. Chang. Biol. 30:e17025. doi: 10.1111/gcb.17025
Renforth, P. (2019). The negative emission potential of alkaline materials. Nat. Commun. 10:1401. doi: 10.1038/s41467-019-09475-5
Renforth, P., and Campbell, J. S. (2021). The role of soils in the regulation of ocean acidification. Philos. Trans. Royal Soc. B Biol. Sci. 376:20200174. doi: 10.1098/rstb.2020.0174
Renforth, P., and Henderson, G. (2017). Assessing ocean alkalinity for carbon sequestration. Rev. Geophys. 55, 636–674. doi: 10.1002/2016RG000533
Renforth, P., Pogge von Strandmann, P. A. E., and Henderson, G. M. (2015). The dissolution of olivine added to soil: implications for enhanced weathering. Appl. Geochem. 61, 109–118. doi: 10.1016/j.apgeochem.2015.05.016
Rinder, T., and von Hagke, C. (2021). The influence of particle size on the potential of enhanced basalt weathering for carbon dioxide removal - insights from a regional assessment. J. Clean. Prod. 315:128178. doi: 10.1016/j.jclepro.2021.128178
Rogers, C. W., Dari, B., and Schroeder, K. L. (2019). Comparison of soil-test extractants for potassium, calcium, magnesium, sulfur, and micronutrients in Idaho soils. Agrosyst. Geosci. Environ. 2, 1–9. doi: 10.2134/age2019.08.0067
Rohling, E. J. (2023). Marine methods for carbon dioxide removal: fundamentals and myth-busting for the wider community. Oxford Open Clim. Change 3:kgad004. doi: 10.1093/oxfclm/kgad004
Schuiling, R. D., and de Boer, P. L. (2010). Coastal spreading of olivine to control atmospheric CO2 concentrations: a critical analysis of viability. Comment: nature and laboratory models are different. Int. J. Greenhouse Gas Control 4, 855–856. doi: 10.1016/j.ijggc.2010.04.012
Shainberg, I., Sumner, M. E., Miller, W. P., Farina, M. P. W., Pavan, M. A., and Fey, M. V. (1989). “Use of gypsum on soils: a review” in Advances in soil science. ed. B. A. Stewart, vol. 9 (New York, NY: Springer US), 1–111.
Singh, M., Sarkar, B., Sarkar, S., Churchman, J., Bolan, N., Mandal, S., et al. (2018). “Chapter two-stabilization of soil organic carbon as influenced by clay mineralogy” in Advances in agronomy. ed. D. L. Sparks, vol. 148 (Cambridge, MA: Academic Press), 33–84.
Skalar Analytical B.V (2021). User manual: Primacs. In (SNAccess1.0.42 ed.). Breda: Skalar Analytical B.V.
Smith, S. M., Geden, O., Nemet, G., Gidden, M., Lamb, W. F., Powis, C., et al. (2023). The State of Carbon Dioxide Removal - 1st Edition. The State of Carbon Dioxide Removal. doi: 10.17605/OSF.IO/W3B4Z
Sparks, D. L., Singh, B., and Siebecker, M. G. (2023). “Chapter 6- ion exchange processes” in Environmental soil chemistry. eds. D. L. Sparks, B. Singh, and M. G. Siebecker. 3rd ed (Cambridge, MA: Academic Press), 283–304.
Stolwijk, J. A., and Thimann, K. V. (1957). On the uptake of carbon dioxide and bicarbonate by roots, and its influence on growth. Plant Physiol. 32, 513–520. doi: 10.1104/pp.32.6.513
Strawn, D. G. (2021). Sorption mechanisms of chemicals in Soils. Soil Syst. 5:13. doi: 10.3390/soilsystems5010013
Strawn, D. G., Bohn, H. L., and O'Connor, G. A. (2015). Soil chemistry. New York, NY: John Wiley & Sons.
Strefler, J., Amann, T., Bauer, N., Kriegler, E., and Hartmann, J. (2018). Potential and costs of carbon dioxide removal by enhanced weathering of rocks. Environ. Res. Lett. 13:034010. doi: 10.1088/1748-9326/aaa9c4
Suhrhoff, T. J. (2022). Phytoprevention of heavy metal contamination from terrestrial enhanced weathering: can plants save the day? Front. Clim. 3:820204. doi: 10.3389/fclim.2021.820204
Taylor, L. L., Driscoll, C. T., Groffman, P. M., Rau, G. H., Blum, J. D., and Beerling, D. J. (2021). Increased carbon capture by a silicate-treated forested watershed affected by acid deposition. Biogeosciences 18, 169–188. doi: 10.5194/bg-18-169-2021
ten Berge, H. F. M., van der Meer, H. G., Steenhuizen, J. W., Goedhart, P. W., Knops, P., and Verhagen, J. (2012). Olivine weathering in soil, and its effects on growth and nutrient uptake in ryegrass (Lolium perenne L.): a pot experiment. PLoS One 7:e42098. doi: 10.1371/journal.pone.0042098
Tessier, A., Campbell, P. G. C., and Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 51, 844–851. doi: 10.1021/ac50043a017
Thermo Scientific (2019). Thermo scientific orion star T900 series potentiometric Titrators: user manual. Waltham, MA: Thermo Scientific.
Thompson, A. G. K. W. (2012). Introduction to the sorption of chemical constituents in soils. Nature education knowledge. Available at: https://www.nature.com/scitable/knowledge/library/introduction-to-the-sorption-of-chemical-constituents-94841002/ (Accessed October 15. 2023).
Val Martin, M., Blanc-Betes, E., Fung, K. M., Kantzas, E. P., Kantola, I. B., Chiaravalloti, I., et al. (2023). Improving nitrogen cycling in a land surface model (CLM5) to quantify soil N2O, NO, and NH3 emissions from enhanced rock weathering with croplands. Geosci. Model Dev. 16, 5783–5801. doi: 10.5194/gmd-16-5783-2023
Vienne, A., Poblador, S., Portillo-Estrada, M., Hartmann, J., Ijiehon, S., Wade, P., et al. (2022). Enhanced weathering using basalt rock powder: carbon sequestration, co-benefits and risks in a mesocosm study with Solanum tuberosum. Front. Clim. 4:869456. doi: 10.3389/fclim.2022.869456
Walker, J. C. G., Hays, P. B., and Kasting, J. F. (1981). A negative feedback mechanism for the long-term stabilization of earth's surface temperature. J. Geophys. Res. Oceans 86, 9776–9782. doi: 10.1029/JC086iC10p09776
Washbourne, C. -L., Lopez-Capel, E., Renforth, P., Ascough, P. L., and Manning, D. A. C. (2015). Rapid removal of atmospheric CO2 by urban soils. Environ. Sci. Technol. 49, 5434–5440. doi: 10.1021/es505476d
West, T. O., and McBride, A. C. (2005). The contribution of agricultural lime to carbon dioxide emissions in the United States: dissolution, transport, and net emissions. Agric. Ecosyst. Environ. 108, 145–154. doi: 10.1016/j.agee.2005.01.002
Wolf, A., Chang, E., Kantola, I. B., Blanc-Betes, E., Masters, M. D., Marklein, A., et al. (2024). Validating assumptions in calculating carbon dioxide removal by enhanced rock weathering in Kantola et al., 2023. Glob. Chang. Biol. 30:e17031. doi: 10.1111/gcb.17031
Wood, C., Harrison, A. L., and Power, I. M. (2023). Impacts of dissolved phosphorus and soil-mineral-fluid interactions on CO2 removal through enhanced weathering of wollastonite in soils. Appl. Geochem. 148:105511. doi: 10.1016/j.apgeochem.2022.105511
Keywords: enhanced weathering, carbon dioxide removal, carbon sequestration, negative emissions technologies, measurement, MRV, inorganic carbon, cations
Citation: Hasemer H, Borevitz J and Buss W (2024) Measuring enhanced weathering: inorganic carbon-based approaches may be required to complement cation-based approaches. Front. Clim. 6:1352825. doi: 10.3389/fclim.2024.1352825
Edited by:
Melissa Cook, Lorax Environmental Services Ltd., CanadaReviewed by:
James Campbell, Heriot-Watt University, United KingdomPhil Renforth, Heriot-Watt University, United Kingdom
Copyright © 2024 Hasemer, Borevitz and Buss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wolfram Buss, d29sZnJhbS5idXNzQGFudS5lZHUuYXU=
 Heath Hasemer
Heath Hasemer Justin Borevitz
Justin Borevitz Wolfram Buss
Wolfram Buss