- 1Quantitative Biosciences and Engineering, Colorado School of Mines, Golden, CO, United States
- 2Chemical and Biological Engineering, Colorado School of Mines, Golden, CO, United States
Oleaginous microalgae have become a focus for large-scale biofuel production due to their ability to accumulate large quantities of lipids. However, production is currently limited by cost and predation. At present, algal biofuel cultivation is optimized through starvation, supplementing media with nutrients, or genetic engineering; these methods can often be costly with little to no increase in lipid production or the culture’s defense. Investigating the phycosphere of algal-bacterial interactions may overcome these current barriers to large-scale production. The phycosphere of algal-bacterial interactions have formed over millions of years through mutualistic and symbiotic relationships and can provide a more direct source of nutrients compared to adding the nutrients in bulk. The most promising of these interactions include the production of phytohormones and quorum signaling compounds that alter the behaviors of the consortia. Phytohormones can improve algal growth rates, lipid production, and stress resistance. Quorum signaling could create consortia capable of warding off invaders—such as rotifers—while self-regulating and altering behavior based on population density. Mechanisms within the algal phycosphere present many opportunities for the development of novel engineering strategies to further improve algal lipid production and operational costs. This review outlines previous preliminary phycosphere research as well as posing possible opportunities to be pursued in future biofuel production.
1 Introduction
The United Nations’ goal of arresting global temperature rise at 2.0°C or below by 2030 had led to substantial investment across the globe in research to develop sustainable, carbon neutral (or even negative) resources to replace current fossil fuels. To enable a rapid shift away from fossil fuels, these carbon neutral fuels must be compatible with our current energy infrastructure. Oleaginous micro-algae have great potential to serve as a source of sustainable, drop-in fuel as they directly convert carbon dioxide into unsaturated fatty acids which are chemically similar to petroleum diesel (EIA, 2023). Algae have fast growth rates, high lipid contents and more efficient photosynthetic light conversion (Li et al., 2008) than crop plants. Despite these advantages, there are several outstanding challenges which need to be addressed to enable large-scale production. Current economic drawbacks include nutrient cost, lipid yield, downstream processing, infrastructure, and energy efficiency ratio (Saad et al., 2019). The most economical large scale production method is growth in open raceway ponds (Smith et al., 2010; Davis et al., 2017), but this introduces yet another challenge to be solved. In these open systems, cultures are subject to predation and/or invading species which can lead to culture crashes. The use of more complex consortia may be an approach to make cultures more robust and productive. Algal phycospheres are a diverse and complex milieu of different genera; here, we specifically focus on how we can leverage the presence of bacteria to manipulate inter-cell interactions and increase algal productivity for the production of biofuels. This review focuses specifically on potential avenues of phycosphere-related research development. We will describe the phycosphere, discuss the important signaling molecules that are found there, and explain how development in this field could replace fossil fuels with biofuels.
2 The phycosphere
The phycosphere is defined as the diffuse region immediately surrounding phytoplankton (single cell or colony) that is nutrient rich and contains numerous signaling molecules (Figure 1). It is an integral part of algae-bacteria interactions and is uncharted territory for engineering applications (Mugnai et al., 2023). It is commonly observed that algal cells secrete organic carbon in various forms into their environment, creating a carbon rich area which attracts heterotrophic bacteria (Seymour et al., 2017). While this relationship benefits the bacteria, it can also benefit the algal cell via a mutualistic relationship (Kazamia et al., 2012; Zhou et al., 2016; Mugnai et al., 2023). Many algae require vitamin B12 to function properly, which is provided by bacteria in the phycosphere (Fuentes et al., 2016; Yao et al., 2019); different bacteria can provide a variety of vitamins including B1 and B7 (Lutzu and Turgut Dunford, 2018; Zhu et al., 2023). Bacteria present in the phycosphere can contribute to algal defense by releasing compounds that impede invasive species, such as the production of antibiotics and toxins (Kizhakkekalam and Chakraborty, 2020). These characteristics of the phycosphere can be linked back to two main forms of algal-bacterial interactions: metabolite exchange and signal transduction (Jiang et al., 2021; Li et al., 2021). This leads to signaling compounds that will shift gene expression in one or both organisms. This process of signal transduction is how an algae-bacteria consortia (ABC) self-regulates and can ward off invasive species through bacterial nutrient and antibiotic production, respectively. These two aspects of ABC present novel directions for consortia design in biofuel engineering.
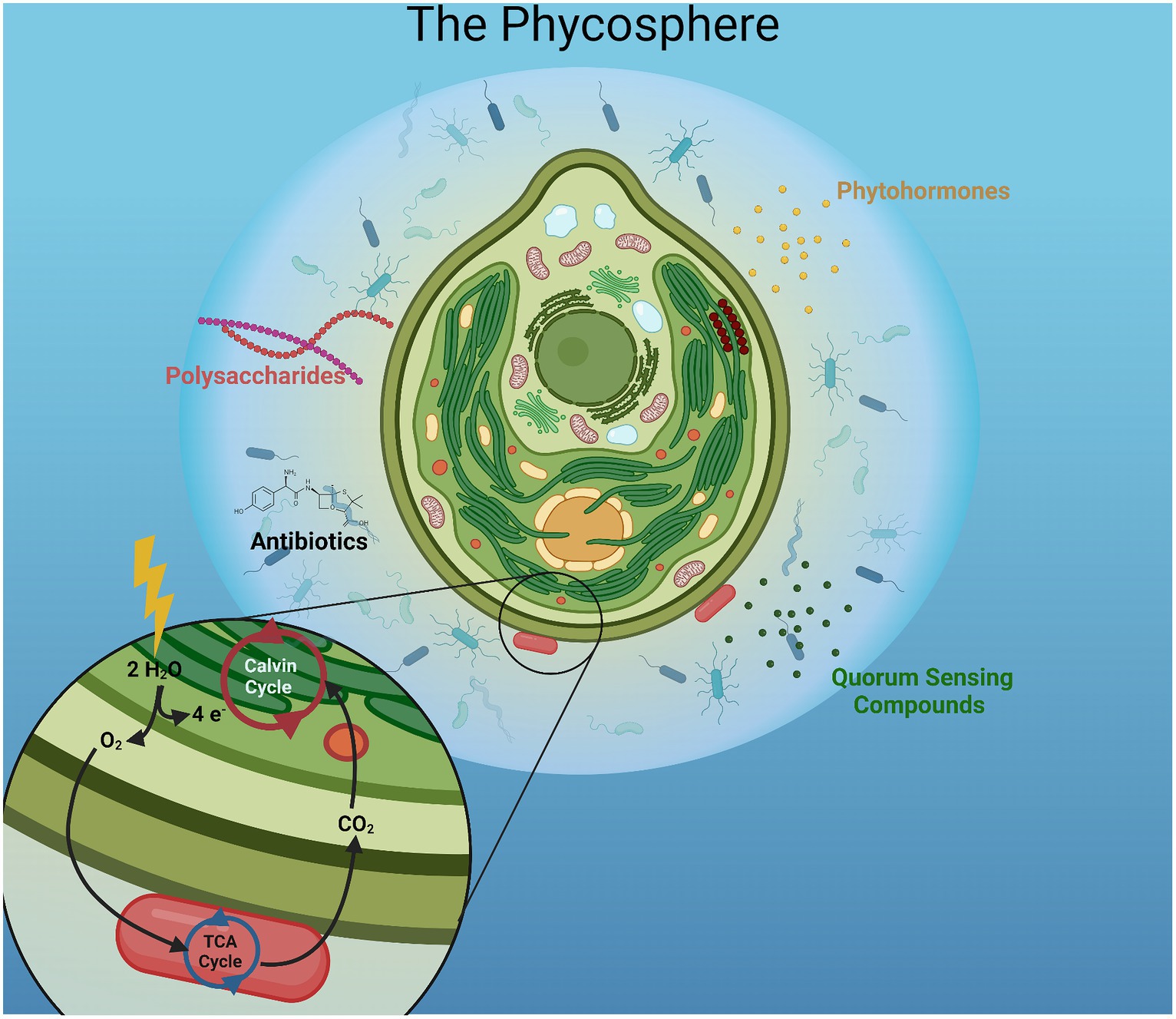
Figure 1. The algal phycosphere. The area immediately surrounding algal cells is rich in oxygen and organic carbon, which attract heterotrophic microbes, such as bacteria. Bacteria can secrete hormones and other signaling molecules to modulate the growth of algae and other microbes to promote or impede growth.
3 Metabolite exchange
A key feature of the phycosphere is the mutualistic exchange of metabolites that occurs between algae and bacteria; this interaction can significantly increase the production of lipids, carbohydrates, pigments, and proteins (González-González and De-Bashan, 2021). Photosynthetic water-splitting results in a high concentration of oxygen around the algal cell. Through cellular respiration, bacteria consume the surrounding oxygen and release carbon dioxide. Then, to facilitate this exchange, bacteria attach themselves to the algae’s surface (Samo et al., 2018) and serves as a carbon sink to “pull” more carbon into photosynthesis. This conformation also lends itself to vitamin transfer. As discussed earlier, many algae rely on bacteria to supply essential vitamins in exchange for secreted organic carbon (Kazamia et al., 2012). For instance, the green algae Auxenochlorella protothecoides requires vitamin B1 (thiamine) to grow (Tandon et al., 2017). This can be resolved by co-culturing with E. coli that produces thiamine derivatives and co-factors capable of alleviating this metabolic bottleneck for the algae (Higgins et al., 2016). Bacteria can also convert other minerals such as nitrogen, phosphorus, and sulfur into more accessible forms to support algal metabolism in exchange for dissolved organic carbon (DOC) (Yao et al., 2019). This exchange of DOC for nutrients and antibiotics drives ABC interactions.
4 Signaling
ABC interactions are strongly influenced by inter-cellular signaling. Here, we focus on the two different types of signaling compounds which have the greatest potential for engineering: phytohormones and quorum sensing (QS) compounds (Zhou et al., 2016; Yu et al., 2017). Engineering these pathways can optimize lipid production and protect algae from competing species, respectively. We will discuss each below in more detail.
4.1 Phytohormones
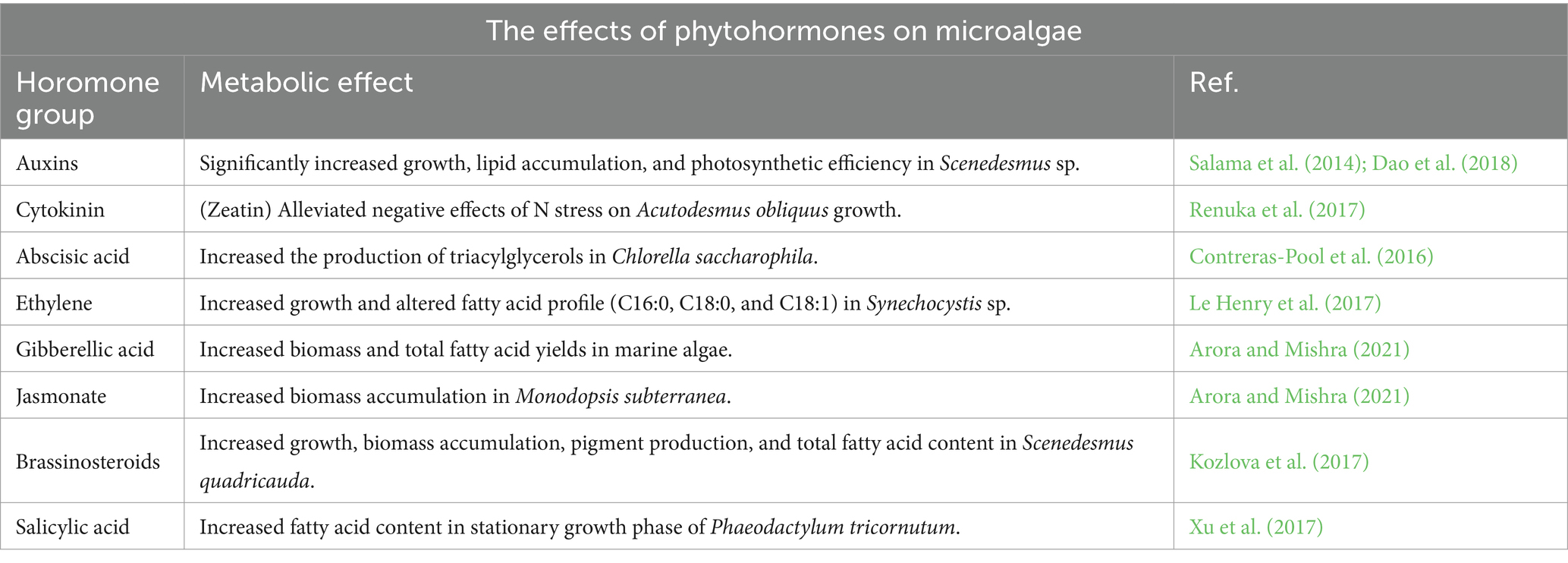
Phytohormones are natural signaling compounds capable of altering and regulating metabolic behaviors in higher plants (Voß et al., 2014) and have been reported to have similar effects in microalgae. They have a variety of regulatory roles including increasing stress tolerance, promoting cell division, and improving photosynthetic efficiency (Wang et al., 2021). The most studied phytohormones in algae are auxins, cytokinins, abscisic acid, ethylene, and gibberellin whereas brassinosteroids, jasmonate, and salicylic acid are less studied. While the physiological roles of these compounds are well documented in vascular plants, their specific roles in regulating microalgae are still not fully understood (Lu and Xu, 2015). There is also evidence of phytohormone synthesis pathways in algae (Tarakhovskaya et al., 2007), implying algae utilize similar signaling compounds. In general, these compounds have been reported to have a positive effect, contributing to improved growth, metabolic activity, and stress tolerance (Table 1).
Whether improved symbiosis is a product of co-evolution or gene transfer, ABC interactions improve the efficiency of their photosynthetic alga through DOC transfer (Peng et al., 2021). Much of the increased robustness and performance of ABC can be attributed to phytohormones altering or supporting algal metabolism. Research of the specific metabolic impacts of these compounds on microalgae has only begun recently but present a promising avenue of increasing biofuel yields from algae (Wang et al., 2021).
In terms of design and implementation, the use of phytohormones to manipulate algal growth is relatively simple. Before rationale engineering can be performed, however, more research is needed to identify the impact of phytohormones on different strains and to identify phytohormone receptors. Engineering bacteria to secrete specific phytohormones will also require providing the necessary metabolite precursors; for example, most auxins are synthesized by bacteria from tryptophan (Lin et al., 2022), therefore, strains with high flux through amino acid pathways should be used. It could also be beneficial to focus on engineering the chloroplast genome due to the simple genetics to prioritize phytohormone production (precursors included) as a means of improving consortia performance. Regardless of the chosen implementation method, phytohormones are a relatively straight-forward and highly effective way of improving algal growth and lipid synthesis.
4.2 Quorum sensing
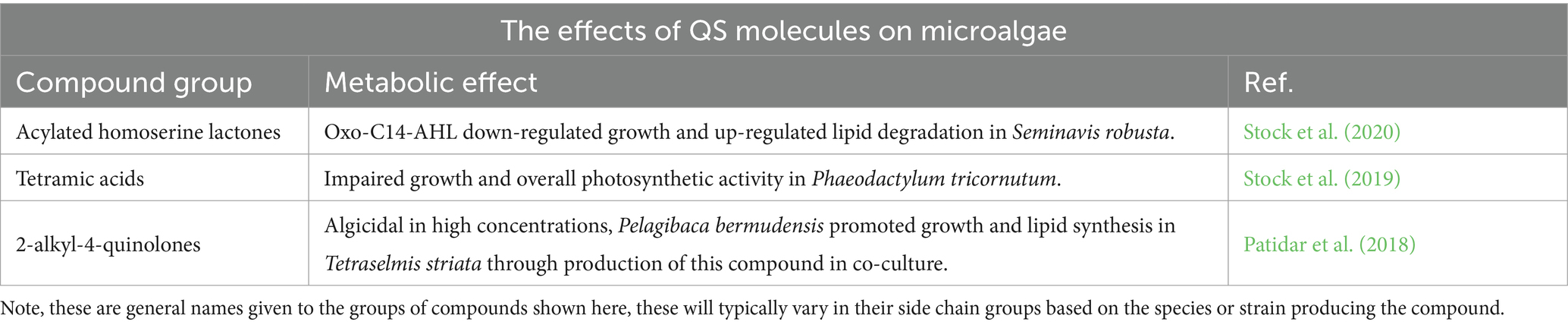
Quorum sensing (QS) is the process by which bacteria in a colony communicate with one another, regulate gene expression, and limit population growth (Tong et al., 2023). Bacteria exude compounds that accumulate with increasing cell density, signaling the initiation of lag phase (Miller and Bassler, 2001). Alternatively, bacteria can interrupt or modulate this process in adjacent colonies through quorum quenching (QQ) (Mugnai et al., 2023). The processes involved in QQ can vary; bacteria can produce enzymes that degrade QS molecules, or they can produce QS compounds which resemble non-self-bacterium (Rolland et al., 2016). Bacteria use these signals for more than just bacterial (self and non-self) regulation; QS enzymes secreted by bacteria can also impact the cell density of microalgae in marine environments (Zhou et al., 2016). Unmitigated growth of bacteria within the phycosphere can lead to cell lysis, ultimately providing additional nutrients to the bacteria and further increases in growth (Dow, 2021). Not all bacteria acquire nutrients from algae through this algacidal route, some bacteria regulate algal growth to receive a sustained source of DOC. Zhu et al. (2022) used bacterial colonies to induce artificial algal blooms and used metagenomic approaches to monitor QS-related gene productions (synthesizers and receptors) among the consortia. They found the dominant bacterial strains were those most active in QS and QQ within the ABC (Zhu et al., 2022). The bacterial gene most regulated by their QS/QQ molecules were those controlling algal production of toxoflavin. Toxoflavin has been shown to be phytotoxic against monocots and dicots and has antibiotic activity against bacteria and fungi (Kim et al., 2013; Philmus et al., 2015). In response to the presence of metabolites needed by the bacteria, they will secrete an enzyme that degrades toxoflavin lyase—a QS enzyme to protect the algae from this toxin. This ultimately provides an environment for bacterial growth, as well as increased cell density of algal cultures.
Many QS molecules have been shown to have a deleterious impact on microalgae growth (Table 2), but some algae have evolved to resist the effects or interrupt QS signaling. Chlamydomonas reinhardtii and other soil-based algae strains were found to produce QS-mimicking compounds that inhibited growth in soil-based bacteria (Teplitski et al., 2004). Although QS interfering behavior is less documented and understood in marine algae, participation in QS regulation may indicate a possibility of its existence (Rolland et al., 2016). The QS system within the ABC can be weaponized against algae and bacteria foreign to the consortia. For instance, if domestic bacteria were selected and genetically engineered to produce QS compounds that protected domestic algae while attacking foreign algae and bacteria, QS compounds would then serve as a natural protective agent in the growth environment.
Furthermore, current metabolic engineering efforts that are limited when scaled up could be overcome by using self-regulating quorum sensing pathways. Gupta et al. (2017) found that by using a cell density dependent cellular switch in Escherichia coli, they achieved a 5.5-fold increase in the production of target compounds myo-inositol and glucaric acid (Gupta et al., 2017). While this finding focuses on bacteria, there is a possibility for similar methods to be used to engineer algal-bacterial interactions. Using a pathway-independent QS circuit could overcome current scale-up limitations of biofuels.
5 Engineering the phycosphere
As discussed, engineering the phycosphere is relatively uncharted territory that has the potential to increase growth rates, lipid accumulation and manipulate the consortium composition in open raceway ponds. Instead of permanently modifying intracellular pathways and regulatory mechanisms, engineering the phycosphere allows the ABC to adapt to environmental changes. This approach takes advantage of naturally occurring processes within the algal and bacterial colonies to optimize growth and lipid productivity.
5.1 Synthetic consortia
Algal monocultures are difficult to maintain in an open environment due to their simple but vulnerable nature; engineered consortia have the potential to boost carbon fixation rates, increase the synthesis rate of lipids and protect against unwanted species. With this in mind, it is pivotal to select the right organisms in a pond design. Polycultures can introduce complex metabolic interactions which allow them to be robust in changing environments, similar to the natural consortia we find in the environment (Nagarajan et al., 2022). It is common in wastewater treatments involving ABC to use activated sludge as the bacterial component, which consists of a plethora of bacteria. In a wastewater treatment study done by Chen et al. (2019), activated sludge pulled from sewage treatments facilities was grown in combination with a strain of Chlorella or Scenedesmus in synthetic wastewater containing glucose. These ABC were found to have increased nutrient uptake and lipid productivity in certain pairings compared to axenic cultures. These improvements were mainly attributed to nutrient exchange and production of indole-3-acetic acid, a known phytohormone of the auxin family (Chen et al., 2019). A point worth noting in this study is that not all pairings saw an increase, indicating a need to study the impact of different phytohormones on different algal species. Focusing on ABC selection and design interactions in the phycosphere can enable algae to be more stable and productive for biofuel production.
5.2 Predation and multi-culture solutions
Earlier, it was discussed that in ABC interactions, algal cultures benefit from the bacterial production of B12. While B12 can increase algal productivity, B12 unfortunately also increases rotifer productivity. Rotifers are a zooplankton that damage and kill algal cells through grazing; currently, algal cultures have productivity loss—and culture crash—due to this invasive species (Cheng et al., 2004). However, through multi-organism interactions, there is a possibility for in-situ rotifer prevention. A study done by Turnau et al. (2021) explored arming a predatory fungus, Stenotrophomonas maltophilia, with bacteria, Bacillus sp., to terminate rotifers within the sample (Turnau et al., 2021). While this research focused on fungal-bacterial interactions in predation maintenance, application into multi-culture algal ponds may prove fortuitous in large-scale biofuel production. Turnau et al. (2021) found that protection against rotifers was achieved in a quadruple microbial trophic interaction. Although this can be difficult to design, the payoffs to this research and design would greatly improve algal cultivation. Thus, multi-culture interactions warrant more research in this field to improve predation protection mechanisms.
5.3 Biocontainment
The main concern with any large-scale production of genetically engineered organisms is their release into the environment. This is exacerbated by the use of open raceway ponds which preclude the use of physical barriers, which have been proven to be the most effective method of containment (Miller and Bergmann, 1993). There are several different approaches to minimize the spread of genetically engineered systems, which include the use of inducible systems, auxotrophy and cellular circuits. We will briefly describe these methods here, but for more complete review of these techniques, please refer to the review by Arnolds et al. (2021). Both inducible systems and auxotrophy engineer the cell to require either the presence of an inducer gene or an organic compound that is (hopefully) not plentiful in the environment. When the inducer or molecule are not present, the cell is unable to grow. Auxotrophy for specific amino acids is common in laboratory strains, but amino acids are not rare in the natural environment. Another challenge with auxotrophy is that horizontal gene transfer can enable cells to regain the function that was removed, thus bypassing the need for supplementation. An approach to ensure this does not happen is to recode essential enzymes to require non-natural amino acids. For example, Mandell et al. engineered the UAG stop codon in E. coli to encode for a non-natural amino acid; they also redesigned the cores of essential enzymes to encode for non-natural amino acids. This makes it very hard for the cell to bypass the need for these non-natural aminos acids since there are several different proteins that require them. They showed that normal amino acid auxotrophs were able to circumvent their auxotrophy at a frequency of 2.2 × 10−12 escapees per colony forming unit but the recoded synthetic auxotrophs were unable to bypass their need for non-natural amino acids in their studies. Advances in synthetic biology has enabled the ability to engineer much more complex cellular circuits into the cell, the most popular for biocontainment being kill switches. Kill switches are a mechanism by which an environmental trigger expresses the promoter of a toxin gene. Stirling et al. (2017) presents an example of a cryodeath kill switch, a cold-inducible promotor which expresses a toxin (Stirling et al., 2017). This technique can be applied for biocontainment and it can also be used as a crop protectant. In this context, bacteria would be engineered to monitor growth of known predators and if it reached a critical level, they would start the production of a known toxin.
5.4 Rewriting the rules
Inter-species communications between bacteria have been seen to improve bacterial relationships as they rarely grow axenically in the wild and can recognize non-self QS compounds (Teplitski et al., 2004; Wellington and Greenberg, 2019). This creates possibilities for consortia design and engineering as it implies that bacterium have natural mechanisms to identify and respond to exocellular triggers. As mentioned earlier, some bacteria will produce enzymes that degrade non-self QS compounds, inhibiting the growth of invaders through QQ. Genetically engineering or selecting a type of bacteria to only recognize specific QS compounds such 121 as N-(heptanoyl)-homoserine lactone (Choudhary and Schmidt-Dannert, 2010), to be recognized as non-self would create a strong environmentally defensive bacterium. Another direction for this concept would be to focus these disruptive tendencies on invasive algae. Perhaps the bacteria produce algicidal QS compounds that the desired algae are naturally or have been altered to be resistant to. Using these innate defensive mechanisms as a basis for creating a self-protecting consortia or co-culture could drastically improve pond stability.
As discussed previously, QS is mainly a process by which bacteria self-regulate according to population density. This process also provides an avenue for timed release of compounds that alter genetic expression that could be redesigned for alternative purposes. It is common in algal cultivation to engage in a two-stage process; stage 1 focuses on accumulation of biomass and stage 2 on the accumulation of the desired product. Cell density-sensitive genetic activation can have numerous applications. A couple are mentioned above and those only focus on the bacteria present detecting self and non-self-bacteria related compounds. However, the bacteria are capable of recognizing compounds from algae as well. A condensation of multiple studies by Rolland et al. (2016) on the interactions of Phaeobacter gallaeciencis BS107 with Emiliana huxleyi shows that bacteria will produce phytohormones (auxins) in the early stages of an algal bloom to support growth and the accumulation of sulfur compounds by the algae. In the later to last stages of the bloom, bacteria will react to compounds released by decaying algal cells and transition to producing algicidal compounds likely as a means of increasing available nutrients (Lin et al., 2022). This demonstrates the ability of bacteria to alter their behavior depending on the state of their algal companion. This could be used as a means of timed release in a pond environment, the bacteria promote growth early with phytohormones and then shifts to producing lysing compounds once algal growth reaches a certain density. Thus, decreasing harvesting costs (Ubeda et al., 2017) (Figure 2). Engineering these means of cell communication, identification, and controlled genetic expression and using them as a means of innate timed release to regulate the interactions within an ABC stands to present immense potential as an engineering tool.
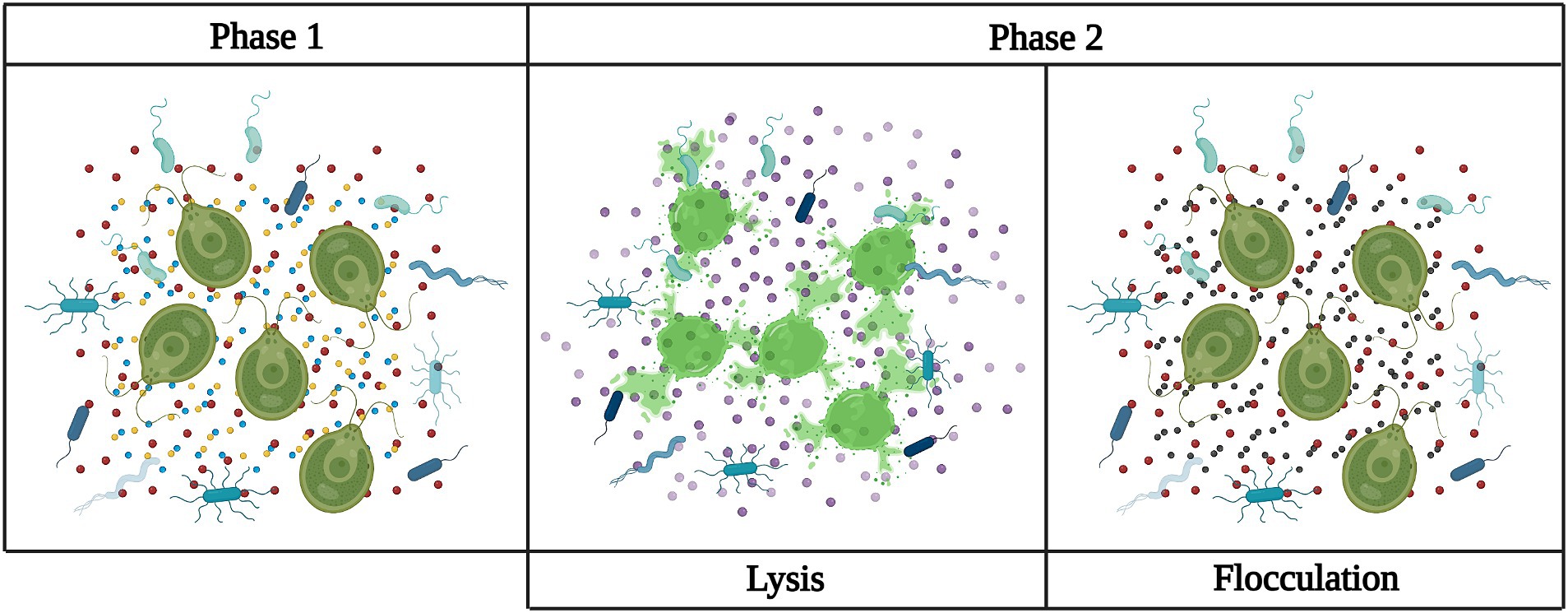
Figure 2. Examples of how engineering the phycosphere can aid in algal pond growth and harvesting. Phase 1: Algae and bacteria are inoculated into pond as a co-culture. The bacteria in this phase is producing phytohormones (yellow dots) to upregulate the alga’s growth and lipid production, QS compounds (red dots) to regulate its own growth, and QQ compounds (blue dots) to prevent invasion. Meanwhile, the algae is being focused solely on growth and lipid production. Phase 2: The bacteria has detected compounds indicating the algae has reached the desired cell density and will initiate the second phase of the culture. In the lysis panel, the bacteria were given the means to produce a lysing compound (yellow dots) that will rupture the cells and aid in lipid harvesting. In the flocculation panel, the bacteria were instead given the means to produce a compound to induce bio-flocculation (gray dots) in the algae resulting in easier to harvest algal clumps.
Author contributions
JY: Writing – review & editing, Writing – original draft, Visualization, Investigation, Conceptualization. EK: Writing – review & editing, Data curation, Formal analysis, Validation, Visualization. NB: Writing – review & editing, Visualization, Supervision, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the DOE Office of Science, Office of Biological and Environmental Research (BER), grant no. DE-SC0019171.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arnolds, K. L., Dahlin, L. R., Ding, L., Wu, C., Yu, J., Xiong, W., et al. (2021). Biotechnology for secure biocontainment designs in an emerging bioeconomy. Curr. Opin. Biotechnol. 71, 25–31. doi: 10.1016/j.copbio.2021.05.004
Arora, S., and Mishra, G. (2021). Effect of gibberellin, methyl jasmonate and myoinositol on biomass and eicosapentaenoic acid productivities in the eustigmatophyte Monodopsis subterranea CCALA 830. J. Appl. Phycol. 33, 287–299. doi: 10.1007/s10811-020-02317-8
Chen, X., Hu, Z., Qi, Y., Song, C., and Chen, G. (2019). The interactions of algae-activated sludge symbiotic system and its effects on wastewater treatment and lipid accumulation. Bioresour. Technol. 292:122017. doi: 10.1016/j.biortech.2019.122017
Cheng, S.-H., Aoki, S., Maeda, M., and Hino, A. (2004). Competition between the rotifer Brachionus rotundiformis and the ciliate Euplotes vannus fed on two different algae. Aquaculture 241, 331–343. doi: 10.1016/j.aquaculture.2004.08.006
Choudhary, S., and Schmidt-Dannert, C. (2010). Applications of quorum sensing in biotechnology. Appl. Microbiol. Biotechnol. 86, 1267–1279. doi: 10.1007/s00253-010-2521-7
Contreras-Pool, P. Y., Peraza-Echeverria, S., Ku-González, Á. F., and Herrera-Valencia, V. A. (2016). The phytohormone abscisic acid increases triacylglycerol content in the green microalga Chlorella saccharophila (Chlorophyta). Algae 31, 267–276. doi: 10.4490/algae.2016.31.9.3
Dao, G. H., Wu, G. X., Wang, X. X., Zhuang, L. L., Zhang, T. Y., and Hu, H. Y. (2018). Enhanced growth and fatty acid accumulation of microalgae Scenedesmus sp. LX1 by two types of auxin. Bioresour. Technol. 247, 561–567. doi: 10.1016/j.biortech.2017.09.079
Davis, R. E., Markham, J. N., Kinchin, C. M., Canter, C., Han, J., Li, Q., et al. (2017). “Algae harmonization study: evaluating the potential for future algal biofuel costs, sustainability, and resource assessment from harmonized modeling.” National Renewable Energy Lab. (NREL), Golden, CO (United States), 2018.
Dow, L. (2021). How do quorum-sensing signals mediate algae–bacteria interactions? Microorganisms 9:1391. doi: 10.3390/microorganisms9071391
EIA (2023). Biofuels explained—U.S. Energy Information Administration (EIA). Available at: https://www.eia.gov/energyexplained/biofuels/
Fuentes, J. L., Garbayo, I., Cuaresma, M., Montero, Z., González-Del-Valle, M., and Vílchez, C. (2016). Impact of microalgae-Bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs 14:100. doi: 10.3390/md14050100
González-González, L. M., and De-Bashan, L. E. (2021). Toward the enhancement of microalgal metabolite production through microalgae-Bacteria consortia. Biology 10:282. doi: 10.3390/biology10040282
Gupta, A., Brockman Reizman, I. M., Reisch, C. R., and Prather, K. L. J. (2017). Dynamic regulation of metabolic flux in engineered bacteria using a pathway-independent quorum-sensing circuit. Nat. Biotechnol. 35, 273–279. doi: 10.1038/nbt.3796
Higgins, B. T., Gennity, I., Samra, S., Kind, T., Fiehn, O., and Vander Gheynst, J. S. (2016). Cofactor symbiosis for enhanced algal growth, biofuel production, and wastewater treatment. Algal Res. 17, 308–315. doi: 10.1016/j.algal.2016.05.024
Jiang, L., Li, Y., and Pei, H. (2021). Algal–bacterial consortia for bioproduct generation and wastewater treatment. Renew. Sust. Energ. Rev. 149:111395. doi: 10.1016/j.rser.2021.111395
Kazamia, E., Czesnick, H., Nguyen, T. T. V., Croft, M. T., Sherwood, E., Sasso, S., et al. (2012). Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ. Microbiol. 14, 1466–1476. doi: 10.1111/j.1462-2920.2012.02733.x
Kim, S., Park, J., Kim, J. H., Lee, J., Bang, B., Hwang, I., et al. (2013). RNAseq-based transcriptome analysis of Burkholderia glumae quorum sensing. Plant Pathol. J. 29, 249–259. doi: 10.5423/PPJ.OA.04.2013.0044
Kizhakkekalam, V. K., and Chakraborty, K. (2020). Marine macroalgae-associated heterotrophic Firmicutes and gamma-proteobacteria: prospective anti-infective agents against multidrug resistant pathogens. Arch. Microbiol. 202, 905–920. doi: 10.1007/s00203-019-01800-2
Kozlova, T. A., Hardy, B. P., Krishna, P., and Levin, D. B. (2017). Effect of phytohormones on growth and accumulation of pigments and fatty acids in the microalgae Scenedesmus quadricauda. Algal Res. 27, 325–334. doi: 10.1016/j.algal.2017.09.020
Le Henry, M., Charton, M., Alignan, M., Maury, P., Luniov, A., Pelletier, I., et al. (2017). Ethylene stimulates growth and affects fatty acid content of Synechocystis sp. PCC 6803. Algal Res. 26, 234–239. doi: 10.1016/j.algal.2017.07.032
Li, Y., Horsman, M., Wu, N., Lan, C. Q., and Dubois-Calero, N. (2008). Biofuels from microalgae. Biotechnol. Prog. 24, 815–820. doi: 10.1021/bp070371k
Li, D., Liu, R., Cui, X., He, M., Zheng, S., du, W., et al. (2021). Co-culture of bacteria and microalgae for treatment of high concentration biogas slurry. J. Water Process Eng. 41:102014. doi: 10.1016/j.jwpe.2021.102014
Lin, H., Li, Y., and Hill, R. T. (2022). Microalgal and bacterial auxin biosynthesis: implications for algal biotechnology. Curr. Opin. Biotechnol. 73, 300–307. doi: 10.1016/j.copbio.2021.09.006
Lu, Y., and Xu, J. (2015). Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends Plant Sci. 20, 273–282. doi: 10.1016/j.tplants.2015.01.006
Lutzu, G. A., and Turgut Dunford, N. (2018). Interactions of microalgae and other microorganisms for enhanced production of high-value compounds. Front. Biosci. 23, 1487–1504. doi: 10.2741/4656
Miller, M. B., and Bassler, B. L. (2001). Quorum sensing in bacteria. Ann. Rev. Microbiol. 55, 165–199. doi: 10.1146/annurev.micro.55.1.165
Miller, S. R., and Bergmann, D. (1993). Biocontainment design considerations for biopharmaceutical facilities. J. Ind. Microbiol. 11, 223–234. doi: 10.1007/BF01569595
Mugnai, S., Derossi, N., and Hendlin, Y. (2023). Algae communication, conspecific and interspecific: the concepts of phycosphere and algal-bacteria consortia in a photobioreactor (PBR). Plant Signal. Behav. 18:2148371. doi: 10.1080/15592324.2022.2148371
Nagarajan, D., Lee, D. J., Varjani, S., Lam, S. S., Allakhverdiev, S. I., and Chang, J. S. (2022). Microalgae-based wastewater treatment–microalgae-bacteria consortia, multi-omics approaches and algal stress response. Sci. Total Environ. 845:157110. doi: 10.1016/j.scitotenv.2022.157110
Patidar, S. K., Kim, S. H., Kim, J. H., Park, J., Park, B. S., and Han, M. S. (2018). Pelagibaca bermudensis promotes biofuel competence of Tetraselmis striata in a broad range of abiotic stressors: dynamics of quorum-sensing precursors and strategic improvement in lipid productivity. Biotechnol. Biofuels 11, –16. doi: 10.1186/s13068-018-1185-x
Peng, H., de-Bashan, L. E., and Higgins, B. T. (2021). Comparison of algae growth and symbiotic mechanisms in the presence of plant growth promoting bacteria and non-plant growth promoting bacteria. Algal Res. 53:102156. doi: 10.1016/j.algal.2020.102156
Philmus, B., Shaffer, B. T., Kidarsa, T. A., Yan, Q., Raaijmakers, J. M., Begley, T. P., et al. (2015). Investigations into the biosynthesis, regulation, and self-resistance of toxoflavin in Pseudomonas protegens Pf-5. ChemBioChem 16, 1782–1790. doi: 10.1002/cbic.201500247
Renuka, N., Guldhe, A., Singh, P., Ansari, F. A., Rawat, I., and Bux, F. (2017). Evaluating the potential of cytokinins for biomass and lipid enhancement in microalga Acutodesmus obliquus under nitrogen stress. Energy Convers. Manag. 140, 14–23. doi: 10.1016/j.enconman.2017.02.065
Rolland, J. L., Stien, D., Sanchez-Ferandin, S., and Lami, R. (2016). Quorum sensing and quorum quenching in the phycosphere of phytoplankton: a case of chemical interactions in ecology. J. Chem. Ecol. 42, 1201–1211. doi: 10.1007/s10886-016-0791-y
Saad, M. G., Dosoky, N. S., Zoromba, M. S., and Shafik, H. M. (2019). Algal biofuels: current status and key challenges. Energies 12:1920. doi: 10.3390/en12101920
Salama, E. S., Kabra, A. N., Ji, M. K., Kim, J. R., Min, B., and Jeon, B. H. (2014). Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour. Technol. 172, 97–103. doi: 10.1016/j.biortech.2014.09.002
Samo, T. J., Kimbrel, J. A., Nilson, D. J., Pett-Ridge, J., Weber, P. K., and Mayali, X. (2018). Attachment between heterotrophic bacteria and microalgae influences symbiotic microscale interactions. Environ. Microbiol. 20, 4385–4400. doi: 10.1111/1462-2920.14357
Seymour, J. R., Amin, S. A., Raina, J.-B., and Stocker, R. (2017). Zooming in on the phycosphere: the ecological interface for phytoplankton–bacteria relationships. Nat. Microbiol. 2:17065. doi: 10.1038/nmicrobiol.2017.65
Smith, V. H., Sturm, B. S., Denoyelles, F. J., and Billings, S. A. (2010). The ecology of algal biodiesel production. Trends Ecol. Evol. 25, 301–309. doi: 10.1016/j.tree.2009.11.007
Stirling, F., Bitzan, L., O’Keefe, S., Redfield, E., Oliver, J. W. K., Way, J., et al. (2017). Rational design of evolutionarily stable microbial kill switches. Mol. Cell 68, 686–697.e3. doi: 10.1016/j.molcel.2017.10.033
Stock, F., Bilcke, G., de Decker, S., Osuna-Cruz, C. M., van den Berge, K., Vancaester, E., et al. (2020). Distinctive growth and transcriptional changes of the diatom Seminavis robusta in response to quorum sensing related compounds. Front. Microbiol. 11:1240. doi: 10.3389/fmicb.2020.01240
Stock, F., Syrpas, M., Graff van Creveld, S., Backx, S., Blommaert, L., Dow, L., et al. (2019). N-acyl homoserine lactone derived tetramic acids impair photosynthesis in Phaeodactylum tricornutum. ACS Chem. Biol. 14, 198–203. doi: 10.1021/acschembio.8b01101
Tandon, P., Jin, Q., and Huang, L. (2017). A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microb. Cell Factories 16:219. doi: 10.1186/s12934-017-0834-2
Tarakhovskaya, E. R., Maslov, Y. I., and Shishova, M. F. (2007). Phytohormones in algae. Russ. J. Plant Physiol. 54, 163–170. doi: 10.1134/S1021443707020021
Teplitski, M., Chen, H., Rajamani, S., Gao, M., Merighi, M., Sayre, R. T., et al. (2004). Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiol. 134, 137–146. doi: 10.1104/pp.103.029918
Tong, C. Y., Honda, K., and Derek, C. J. C. (2023). A review on microalgal-bacterial co-culture: the multifaceted role of beneficial bacteria towards enhancement of microalgal metabolite production. Environ. Res. 228:115872. doi: 10.1016/j.envres.2023.115872
Turnau, K., Fiałkowska, E., Ważny, R., Rozpądek, P., Tylko, G., Bloch, S., et al. (2021). Extraordinary multi-organismal interactions involving bacteriophages, Bacteria, Fungi, and rotifers: quadruple microbial trophic network in water droplets. Int. J. Mol. Sci. 22:2178. doi: 10.3390/ijms22042178
Ubeda, B., Galvez, J. A., Michel, M., and Bartual, A. (2017). Microalgae cultivation in urban wastewater: Coelastrum cf. pseudomicroporum as a novel carotenoid source and a potential microalgae harvesting tool. Bioresour. Technol. 228, 210–217. doi: 10.1016/j.biortech.2016.12.095
Voß, U., Bishopp, A., Farcot, E., and Bennett, M. J. (2014). Modelling hormonal response and development. Trends Plant Sci. 19, 311–319. doi: 10.1016/j.tplants.2014.02.004
Wang, C., Qi, M., Guo, J., Zhou, C., Yan, X., Ruan, R., et al. (2021). The active phytohormone in microalgae: the characteristics, efficient detection, and their adversity resistance applications. Molecules 27:46. doi: 10.3390/molecules27010046
Wellington, S., and Greenberg, E. P. (2019). Quorum sensing signal selectivity and the potential for interspecies cross talk. MBio 10:e01128. doi: 10.1128/mBio.00146-19
Xu, J., Fan, X., Li, X., Liu, G., Zhang, Z., Zhu, Y., et al. (2017). Effect of salicylic acid on fatty acid accumulation in Phaeodactylum tricornutum during stationary growth phase. J. Appl. Phycol. 29, 2801–2810. doi: 10.1007/s10811-017-1191-6
Yao, S., Lyu, S., An, Y., Lu, J., Gjermansen, C., and Schramm, A. (2019). Microalgae–bacteria symbiosis in microalgal growth and biofuel production: a review. J. Appl. Microbiol. 126, 359–368. doi: 10.1111/jam.14095
Yu, Z., Song, M., Pei, H., Jiang, L., Hou, Q., Nie, C., et al. (2017). The effects of combined agricultural phytohormones on the growth, carbon partitioning and cell morphology of two screened algae. Bioresour. Technol. 239, 87–96. doi: 10.1016/j.biortech.2017.04.120
Zhou, J., Lyu, Y., Richlen, M. L., Anderson, D. M., and Cai, Z. (2016). Quorum sensing is a language of chemical signals and plays an ecological role in algal-bacterial interactions. Crit. Rev. Plant Sci. 35, 81–105. doi: 10.1080/07352689.2016.1172461
Zhu, J., Chen, G., Zhou, J., Zeng, Y., Cheng, K., and Cai, Z. (2022). Dynamic patterns of quorum sensing signals in phycospheric microbes during a marine algal bloom. Environ. Res. 212:113443. doi: 10.1016/j.envres.2022.113443
Zhu, J., Tang, S., Cheng, K., Cai, Z., Chen, G., and Zhou, J. (2023). Microbial community composition and metabolic potential during a succession of algal blooms from Skeletonema sp. to Phaeocystis sp. Front. Microbiol. 14:1147187. doi: 10.3389/fmicb.2023.1147187
Glossary
Keywords: biofuels, microalgae, consortia, phytohormones, quorum sensing
Citation: Yarbro J, Khorunzhy E and Boyle N (2024) The phycosphere and its role in algal biofuel production. Front. Clim. 6:1277475. doi: 10.3389/fclim.2024.1277475
Edited by:
Melissa Paola Mezzari, Independent Researcher, Houston, TX, United StatesReviewed by:
Imran Pancha, Gujarat Biotechnology University, IndiaKimberly Wright, Ginkgo BioWorks (United States), United States
Copyright © 2024 Yarbro, Khorunzhy and Boyle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nanette Boyle, bmJveWxlQG1pbmVzLmVkdQ==
†These authors have contributed equally to this work
 Jake Yarbro
Jake Yarbro Emma Khorunzhy2
†
Emma Khorunzhy2
†
 Nanette Boyle
Nanette Boyle