- 1Department of Occupational Therapy Sciences, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan
- 2LITALICO Inc., Tokyo, Japan
- 3Minds & Hopes of SeichiAi no Kai Foundation, Saga, Japan
- 4The Porannohiroba Child Development Support Center, Nagasaki, Japan
- 5Department of Rehabilitation for Brain Functions, Research Institute of National Rehabilitation Center for Persons with Disabilities, Saitama, Japan
Background: Restricted and repetitive behavior (RRB) is a core symptom of autism spectrum disorder (ASD). The structure of RRB subcategories and their relationship with atypical sensory processing in Japan are not well understood. This study examined subcategories of the RRB in Japanese children with ASD and explored their relationship with sensory processing.
Methods: A total of 103 children and adolescents with ASD participated in this study, with more than 70% having a co-occurring intellectual disability. First, exploratory factor analysis of the RRB items of the Social Responsiveness Scale second edition (SRS-2) was conducted to identify RRB subcategories. Second, Spearman correlation and multiple regression analysis were run to examine relationships between the RRB subcategories of SRS-2 and subsections of the Short Sensory Profile.
Results: Exploratory factor analysis indicated a two factors solution; repetitive sensory and motor behavior and insistence on sameness. Multiple regression analysis suggested that Movement Sensitivity and Auditory Filtering were associated with insistence on sameness. Furthermore, Underresponsive/Seeks Sensation, Visual/Auditory Sensitivity, and diagnosis of intellectual disabilities were associated with repetitive sensory and motor behavior.
Conclusions: Findings indicate that RRB subcategories are differently related to sensory processing patterns in children with ASD. These results suggested that RRB subcategories are beneficial to consider the relationship between RRB and sensory processing.
1 Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition consisting of differences in social communication styles and a preference for restricted and repetitive behavior (RRB) (1). Both core features are necessary for the diagnosis of ASD, but the impact and clinical symptoms are heterogeneous (2–4).
1.1 RRB subcategories
Several studies have suggested that RRBs can be categorized into two types: repetitive sensory and motor behaviors (RSM) and insistence on sameness (IS) (5, 6). Here, RSM includes repetitive motion, unusual sensory interests, and repetitive use of objects, while IS includes obsessions/rituals, narrow interests, and difficulty with changes in daily life (7). Some studies have pointed out that a three-factor structure including circumscribed interests (CI) is more stable (8–10). Turner proposed that repetitive motor behavior and self-injurious behavior can be classified as lower-order RRB, while IS and CI are higher-order RRB domains (11). Although there are multiple opinions on how to classify RRB, there is a common understanding regarding the existence of the subcategories RSM and IS.
Research findings on how sex, age, and intellectual functioning in the ASD phenotype relate to the expression and severity of different RRB domains have been debated. Sex differences appear to have been reported in relation to RRB (8, 12, 13), while some other studies have not found this relationship (14, 15). Uljarevic et al. reported no sex differences in the IS domain, whereas males were associated with higher severity of RSM (10). In some cases, RSM was reported to be negatively correlated with age and intellectual function (5, 16) while in others indicated no significant association (8, 13). It has been suggested that RSM tends to decrease from later childhood to adolescence, while IS remains stable from early childhood to adolescence (17). It has also been reported that individuals with ASD and intellectual disability exhibited high RMB scores, whereas those without intellectual disability exhibited high scores in IS and CI (18). A large sample study with a wide age range suggested that RRB decreases as age increases and that the relationship between subcategories and age exhibits different patterns (19). Due to the variability across study reports, it is important to examine these factors when investigating RRB. To address these potential age-related differences, we included both children and adolescents in our study.
While it has been consistently suggested that RRB can be classified into several subcategories, the effects of cultural context on the classification of these subcategories are unknown. Some studies have shown differences in ASD symptoms across cultures. A cross-cultural study comparing autistic traits across India, Japan, and the UK revealed that while there was significant overlap in the items most predictive of an autism diagnosis, certain items exhibited cultural differences (20). Cross-cultural research has suggested that cultural aspects may influence RRB items more than other symptoms of ASD (21). It also showed that Autism-Spectrum Quotient (AQ) score variances differed between the UK and Japan (22). Most previous studies on RRB subcategories have been conducted in English-speaking countries; therefore, it is necessary to examine how the RRB subcategories would classify in different cultures, such as Japan, when we consider RRB subcategories.
No study has used factor analysis to identify RRB subcategories in Japanese samples of children with ASD. The Repetitive Behavior Scale-Revised (RBS-R) Japanese version adopted the same six subcategories as the original version (23) without factor analysis (24). In the Japanese version of the Social Responsiveness Scale, second edition (SRS-2), the RRB is treated as one of five subcategories, even though social communication and interaction are divided into four patterns. In this study, we will attempt to identify the RRB subcategories of Japanese children with ASD by examining the exploratory factor structure of the RRB items of SRS-2, which are frequently used to measure ASD symptoms.
1.2 Association between RRB subcategories and sensory processing problems
For understanding ASD symptoms and RRB, sensory processing problems are an important topic. Sensory processing problems in people with ASD have been frequently reported (25, 26). Different aspects of ASD symptoms are associated differently with various patterns of sensory processing. In the correlation analysis of the AQ (27) and the Adult/Adolescent Sensory Profile (AASP) (28), attention to detail and imagination were not significantly correlated with the total score of AASP; social skills, attention switching, and communication were significantly correlated with the total score of AASP (29). Some sensory subtype studies by Lane et al. showed sensory subtypes classified according to modality and responsiveness rather than sensory severity (30–32). Thus, the relationship between sensory processing characteristics and ASD symptoms does not simply increase with severity but rather appears to be heterogeneous in individuals with ASD.
Although several studies have suggested that RRB is associated with sensory processing, there is no consensus on whether these behavioral categories are conceptually distinct (33, 34). Unusual sensory responses and RRB are included in the same group of ASD as different behavioral types in the DSM-5 (1). Furthermore, RRB was associated with sensory characteristics even when adjusted for age and IQ (35, 36). An exploratory model has proposed that atypical sensory function is a significant mediator of RRB through intolerance of uncertainty (37). Schulz et al. showed that sensory hypersensitivity predicted repetitive behaviors in both typically developing (TD) groups and ASD groups (38). In a study analyzing video recordings, hyperresponsive behaviors were more associated with everyday sensory stimuli and family-initiated stimuli (e.g., TV sound, sunlight) and everyday actions (e.g., brushing teeth, washing face), while sensory seeking was more associated with free play and child-initiated stimuli (39). Sensory over-responsivity and high RRB were significantly associated with internalizing symptoms (40). These results suggest that a unidirectional or reciprocal relationship between RRB symptoms and sensory processing exists.
It has been suggested that each of the RRB subcategories has a different relationship with sensory processing characteristics. In a study using the Repetitive Behavior Questionnaire-2 and ASSP, IS was significantly correlated with all types of responsiveness, and RSM was significantly correlated with Avoidance/Seeking (6). Another study showed that sensory sensitivity predicts RRB, regardless of the ASD diagnosis (38). In this study, repetitive motor movements or rigidity and adherence to routine had a small association with RRB, and preoccupation with restricted patterns of interest and unusual sensory interests were predictors. However, the associations between RRB subcategories and each sensory modality are unclear.
1.3 Research questions
There are two research questions in the present study. The first research question was to determine what structure was appropriate for RRB subcategories in children and adolescents with ASD in Japan. We hypothesized that RRB would be divided into two subcategories (RSM, IS) as in previous studies (5, 6, 41, 42). The second research question was to investigate how the RRB subcategories relate to sensory processing in children and adolescents with ASD. Our hypothesis was that the RRB subcategories might be associated with sensory processing properties in different patterns.
2 Methods
2.1 Participants
Since all participants were minors, we obtained written informed consent from all the parents/caregivers of the participants, in accordance with the Declaration of Helsinki. The present study obtained approval from the Ethics Committee of the Graduate School of Biomedical Sciences at Nagasaki University (17110906).
Our study included 103 children with ASD aged 6–18 years (mean ± SD = 12.8 ± 4.0). Among them, eighty-one participants (78.6%) were male. They were recruited from autism parent associations across various prefectures and from day-service centers for children with developmental disorders in Japan. Caregivers provided reports on their children's neurodevelopmental disorder diagnoses from medical institutions. All participants had received a diagnosis of ASD (including autism and Asperger syndrome). The average total T-score on the Social Responsiveness Scale, Second Edition, which indicates the severity of autism symptoms, was 84.7 (SD = 14.8). Additionally, 71.8% of participants were diagnosed with intellectual disabilities. Children with genetic disorders (e.g., Fragile X, Down syndrome) or known medical conditions such as cerebral palsy, epilepsy, or brain injury were excluded from the study.
2.2 Materials
2.2.1 Short Sensory Profile (SSP)
The SSP is a 38-item parent-reported questionnaire for assessing sensory processing characteristics (43). The SSP is organized into seven subsections: Tactile Sensitivity, Taste/Smell Sensitivity, Movement Sensitivity, Underresponsive/Seeks Sensation, Auditory Filtering, Low Energy/Weak, and Visual/Auditory Sensitivity. Parents were asked to rate the frequency with which their child engages in behaviors related to sensory processing in each subsection. Possible scores ranged from 1 to 5, from “never” (1 point) to “always” (5 points). In the Japanese version of SSP, a lower score indicated typical sensory responses, while a higher score indicated atypical sensory responses, which is opposite to the original version of SSP (44). The Japanese version of the SSP also calculated standard scores of children ages 11–18 years, unlike the English version. In this study, the SSP total score and the raw score of each subsection are included in the analysis.
2.2.2 Social Responsiveness Scale, second edition (SRS-2)
The SRS-2 is a standardized, parent-reported questionnaire used for screening ASD risk and assessing ASD symptoms (45). The Japanese version of the SRS-2 School-Age Form, which was standardized for ages 4–18 years, was utilized in this research (46). It includes 65 items about children's behavior over the past six months. In the Japanese version of SRS-2, caregivers were required to respond 0 (Not True) to 3 (Almost Always True). Higher scores indicate more severe autism symptoms in each subdomain. The total raw score and each subdomain score of the SRS-2 can be converted to T-scores based on chronological age and sex norms. The symptomatology scores are classified into three categories: “Mild” (60–65), “Moderate” (66–75), and “Severe” (76 and above). In the present study, participants with a total T-score of less than 60 on the SRS-2 were excluded; however, no samples were excluded because all the collected data had T-scores of 60 or above. All items are clustered into a two-factor structure based on DSM-5 diagnostic criteria, which includes five subdomains corresponding to these two factors; social communication and interaction (social awareness, social cognition, social communication, and social motivation) and restricted, repetitive behaviors and interests (12 items).
2.3 Statistical analyses
2.3.1 Research question 1
Exploratory factor analysis (EFA) was conducted on RRB items of SRS-2. The EFA was carried out using the minimum residual solution with Promax rotation. Parallel analysis, which is based on the Monte Carlo simulation (47), and minimum average partial (MAP) were used to determine the number of factors, which have been proposed as suggested criteria (48). Factor loadings for items were set at .30. EFA was based on the raw scores of RRB items of SRS-2, and was performed using the GPA rotation (49) and psych (50) packages in R (v4.3.3, Vienna, Austria).
2.3.2 Research question 2
Spearman correlation coefficients were calculated for the raw scores of the subscales of the SRS-2 (including IS and RSM), SRS-2-total T-score, and the SSP, to examine the relationship between detailed sensory processing features and ASD symptoms. To account for multiple comparisons, correlation results are presented with Bonferroni adjusted alpha levels. Next, we conducted a two-step hierarchical linear regression to investigate SSP subscales that explained the unique variances of RRB subscales. These analyses were performed using the corrplot (51) and stats (52) in R (v4.3.3, Vienna, Austria).
3 Results
3.1 Research question 1
The Kaiser–Meyer–Olkin measure of sampling adequacy was .80, and Bartlett's test of sphericity was significant (p < .001, approximate χ2 = 429.23).
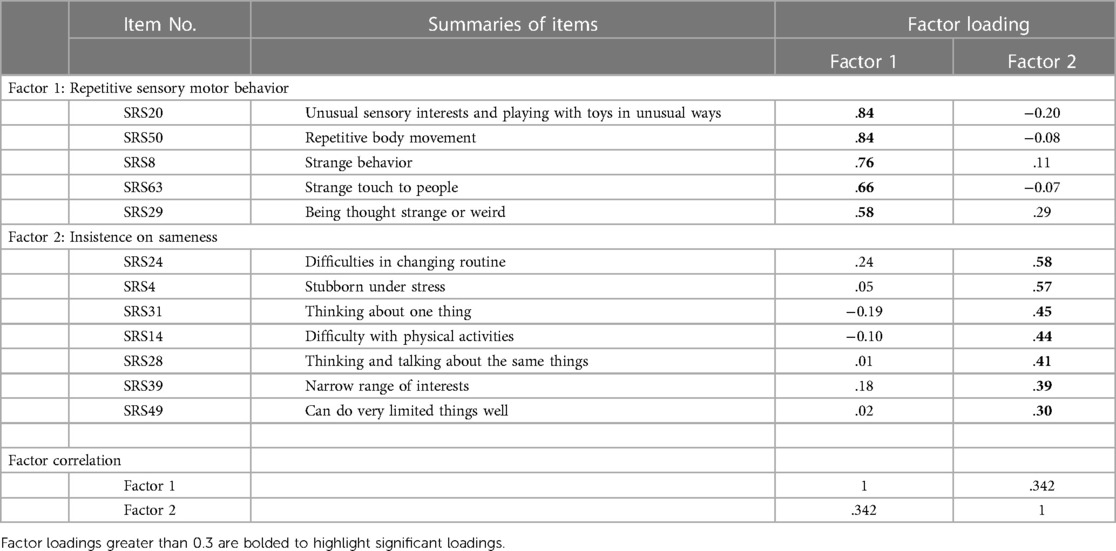
The results of the parallel analysis showed that the eigenvalues after the second factor were smaller than those from the simulation, indicating that a two-factor solution should be retained in the final analysis. Detailed results of the parallel analysis are shown in Supplementary Table S1. The MAP also indicated the two-factor structure was appropriate, therefore the two-factor structure was selected for the EFA.
Table 1 shows the final two-factor solution, which accounted for 38.0% of the variance (RSM 24.4%, IS 13.6%). All 12 items in the RRB factor of SRS-2 were included in the factor analysis, and no items were loaded on two factors.
3.2 Research question 2
3.2.1 Correlation
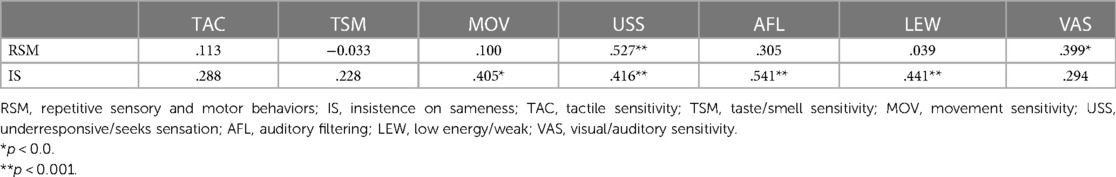
Spearman's correlation analyses were conducted to examine the relationships between RRB subcategories and SSP subsections. Significant correlations were shown between RSM and Underresponsive/Seeks Sensation (r = . 527, p < .001), Visual/Auditory Sensitivity (r = .399, p < .01); IS and Movement sensitivity (r = .405, p > .01), Underresponsive/Seeks Sensation (r = .416, p < .001), Auditory Filtering (r = .399, p > .001), and Low Energy/Weak (r = .441, p < .001), respectively (Table 2).
3.2.2 Multiple regression analysis
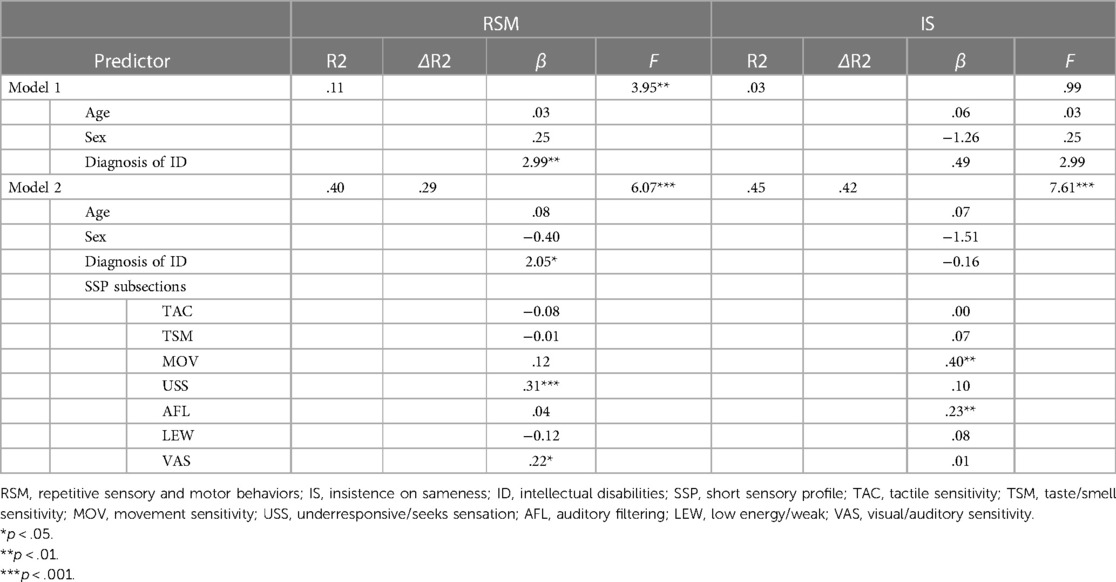
In the hierarchical regression predicting IS, the base model with demographic variables was not a significant predictor, but intellectual disability was a significant predictor of RSM (Table 3). In Model 2, SSP subsections were added as a predictor. Movement Sensitivity and Auditory Filtering were significant predictors of IS [R2 = .4525, p < .001, F (10, 92) = 7.605]. In the model of RSM, Underresponsive/Seeks Sensation (p < .001), Visual/Auditory Sensitivity (p < .01), and Intellectual disabilities (p < .01) were significant predictors [R2 = 0.3974, p < .001, F (10, 92) = 6.067].
4 Discussion
The purpose of our study was to identify RRB subcategories and examine their relationship to sensory processing patterns. RRB items of the SRS-2 were structured into a two-factor solution similar to previous studies: RSM and IS, which were strongly associated with different sensory processing characteristics.
4.1 Research question 1
The result of the EFA showed that RRB items of the SRS-2 were explained by the two-factor structure; RSM and IS. This two-factor structure is similar to past research (5, 7). Although there are studies suggesting that RRB items may be affected by culture (21), the present study may imply that easily noticeable symptoms may vary by culture but may not significantly affect the structure of the RRB subgroups.
Some studies have suggested that a three-factor solution that includes CI is appropriate (8, 9) and some others proposed more factors (18, 23). In this regard, the bias of the concepts measured by the RRB items of the SRS-2 and the small number of items may have influenced the results. However, some studies that support more than three factors also agree on the existence of the RSM and IS factors (8, 9, 18). Although the number of subcategories is a subject of debate in previous studies (5, 8, 10), our analysis showed a two-factor structure was appropriate for the RRB items of the SRS-2. Therefore, the present data suggest that the RRB of SRS-2 can be classified into two subgroups (RSM and IS) at least in Japanese children and adolescents with ASD.
4.2 Research question 2
Research Question 2 examined what sensory processing characteristics are strongly associated with each RRB subcategory. The results showed that significant predictors differed between RSM and IS, providing new evidence for the existing literature reporting the association between RRBs and sensory processing (6, 38, 53).
For Underresponsive/Seeks Sensation and Visual/Auditory Sensitivity, RSM exhibited significant correlations and was a significant predictor in the hierarchical regression analysis. As a clinical implication, RSM may function as a compensatory behavior. An association between vestibular stimulation and low-order RRB has been reported; Gal and Dyck (54) found that blind children were more likely than low-vision children to engage in rocking and repetitive head movements, which are proprioceptive and vestibular-seeking behavior. This suggested that they may function as compensatory behaviors in situations lacking sensory input, and it supports our finding that Underresponsive/Seeks Sensation is associated with RSM. On the other hand, there are several studies supporting a relationship between RSM and hypersensitivity in the visual or auditory modality (38, 55), which is consistent with our findings. These results suggest that some IS behaviors may be compensatory behaviors triggered by discomfort due to atypical visual and auditory functions.
On the other hand, it is debatable whether it was appropriate to treat sensory reactivity collectively as the Underresponsive/Seeks Sensation subsection in the SSP. Boyd et al. suggested a significant association between sensory seeking and ritualistic/sameness (36), which is similar to the present study. Lidstone et al. suggested RSM was significantly correlated with seeking, but not with low registration (6). While Underresponsive/Seeks Sensation was higher in the ASD group than the TD group (26), it also showed a negative correlation between AQ and low registration and a positive correlation with seeking (29). Given the variability in research findings regarding the relationship between ASD symptoms and response characteristics, it may be a more appropriate way to separate Underresponsive/Seeks Sensation as hyposensitivity and seeking. This is a limitation caused by the factor construction of SSP.
IS showed that significant positive correlations were found with Movement Sensitivity, Underresponsive/Seeks Sensation, Auditory Filtering, and Low Energy/Weak. Furthermore, hierarchical regression analysis showed that IS was significantly predicted by Movement Sensitivity and Auditory Filtering. Movement Sensitivity was an SSP subsection with a small effect size compared to ASD and TD (26) and not a significant difference compared to developmental delays (56), but the results of the present study suggested that it significantly predicts IS. Both IS and RSM have been shown to correlate with the center of pressure sway area during a quiet stance (57). However, the Movement Sensitivity of the SSP consists of items that measure mainly vestibular anxiety or sensitivity and not simple postural control. Therefore, it may be the vestibular anxiety part that is more closely related to IS rather than the ability to maintain posture. It has been suggested that IS, not RSM, is associated with anxiety. The relationship between IS and anxiety is mediated by sensory avoidance/hypersensitivity (6) and is partially mediated by social motivation, not RSM (58). Given the notion that repetitive behaviors may work as actions to gain reassurance through sensory input (33), Movement Sensitivity may be more likely to increase heightened anxiety in daily life, and consequently IS behaviors also increase to reassure themselves. Tomchek et al. demonstrated in their SSP study that Auditory Filtering is significantly higher in ASD than TD (26). This subsection includes difficulty noticing social information and distractibility of sounds, indicating a tendency of disruption by an auditory stimulus. An examination of auditory modalities showed a significant correlation (r = −0.37 in the ASD group) between overall RRB and auditory sensitivity, whereas auditory sensitivity was not a predictor (6, 38, 53). In the present study, Visual/Auditory Sensitivity was one of the significant predictors of RSM. Although the association between auditory modality and RRB cannot be ruled out, differences in reactivity, such as distractibility and hypersensitivity within the modality, may each be associated with different aspects of RRB.
It was also shown that the diagnosis of intellectual disability did not predict IS but significantly predicted RSM. Although the relationship between RRB and intellectual disability or cognitive functioning is debated, some studies support our findings. It has been reported that RSM was correlated with IQ and age, whereas IS was unrelated to these factors (5, 59). Individuals with ASD and intellectual disability have been reported to exhibit high scores in RSM (18). IQ score was a significant predictor of the total score on the RBS-R (38). Although the variability in previous research findings should be considered, the results of the present study suggest that RSM may be more strongly associated with intellectual aspects compared to IS.
4.3 Limitations
This study has several limitations. First, our results are based on parental responses. It is difficult to distinguish between hypo-sensitivity and hypo-reactivity based on observational scaling studies alone. The present study would be enhanced by incorporating directly observed data, such as ASD symptoms or experimental data on the physiology of sensory processing. Second, the reliability of the SSP scale: As Williams et al. point out, there are concerns about measuring sensory processing characteristics of individuals with ASD using the SSP factor (60). While similar concerns exist regarding constructs, the distributional bias in the ASD group due to the change to the Japanese version remains untested. In the future, it may be necessary to examine the reliability of the Japanese version of the SSP scale. Third, there may be sample bias. Although we were not able to measure the IQ of everyone in our sample, the proportion of children with intellectual disability was relatively high compared with prevalence studies (61, 62). The study results might be captured based on a sample with a relatively lower IQ. Therefore, it is desirable to verify these findings with a larger sample study. Fourth, the wide age range of the participants, from 6 to 18 years, is a limitation. This broad age range may obscure distinct characteristics of RRB that exist across narrower age bands. If distinct characteristics of RRB exist across narrower age bands, these differences might not be clear in the present study. Fifth, this study conducted EFA, not CFA. While it is desirable to perform the CFA following the EFA to statistically validate the factor structure, this study was unable to prepare a separate dataset for the CFA. Future research should report the fit indices from the CFA to confirm construct validity in more detail.
4.4 Implications and future direction
Although there are limitations, the findings from this study provide insights into the relationship between RRB and sensory processing patterns in Japanese children and adolescents with ASD. The finding that RSM and IS are related to sensory processing in different patterns suggests that dividing RRB into subcategories rather than treating it as a single entity may be more effective in identifying the underlying causes and developing better support strategies. These findings may help develop approaches for children with ASD. Our results may provide evidence that intervention strategies can be tailored based on whether clients exhibit different patterns of RSM or IS, and on the specific sensory processing characteristics they struggle with.
Future research should investigate two directions. First, it should explore the developmental trajectories of RRB subcategories and their relationship with sensory processing over time. Longitudinal studies can provide deeper insights into how these behaviors evolve and the long-term effectiveness of targeted interventions. Second, CFA should be applied to other datasets of the Japanese sample of with ASD. This analysis will provide more robust evidence for the validity of the two-factor structure examined in this study.
5 Conclusion
The present study suggests that the RRB subcategories are classified as RSM and IS in the Japanese sample, similar to previous studies. RSM and IS were associated with different sensory processing characteristics. This information is valuable for examining the common neural basis and reasons for the appearance of sensory processing characteristics and RRB subcategories.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Graduate School of Biomedical Sciences at Nagasaki University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
HN: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. NY: Data curation, Investigation, Writing – review & editing. KK: Data curation, Investigation, Writing – review & editing. GT: Supervision, Writing – review & editing. MI: Funding acquisition, Supervision, Writing – review & editing. RI: Conceptualization, Data curation, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was funded by the Japanese Academy of Sensory Integration Research Grant (2017), Grant-in-Aid from the Japan Society for the Promotion of Science (JSPS) Fellows (21J11906), and JSPS KAKENHI Grant Number (18H03140).
Acknowledgments
We would like to thank all the participants in this study.
Conflict of interest
HN was employed by LITALICO Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frcha.2024.1411445/full#supplementary-material
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association (2013).
2. Lai M-C, Lombardo MV, Chakrabarti B, Baron-Cohen S. Subgrouping the autism “spectrum”: reflections on DSM-5. PLoS Biol. (2013) 11(4):e1001544. doi: 10.1371/journal.pbio.1001544
3. Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. (2007) 17(1):103–11. doi: 10.1016/j.conb.2007.01.009
4. Happé F. Criteria, categories, and continua: autism and related disorders in DSM-5. J Am Acad Child Adolesc Psychiatry. (2011) 50(6):540–2. doi: 10.1016/j.jaac.2011.03.015
5. Bishop SL, Hus V, Duncan A, Huerta M, Gotham K, Pickles A, et al. Subcategories of restricted and repetitive behaviors in children with autism spectrum disorders. J Autism Dev Disord. (2013) 43(6):1287–97. doi: 10.1007/s10803-012-1671-0
6. Lidstone J, Uljarević M, Sullivan J, Rodgers J, McConachie H, Freeston M, et al. Relations among restricted and repetitive behaviors, anxiety and sensory features in children with autism spectrum disorders. Res Autism Spectr Disord. (2014) 8(2):82–92. doi: 10.1016/j.rasd.2013.10.001
7. Cuccaro ML, Shao Y, Grubber J, Slifer M, Wolpert CM, Donnelly SL, et al. Factor analysis of restricted and repetitive behaviors in autism using the autism diagnostic interview-R. Child Psychiatry Hum Dev. (2003) 34(1):3–17. doi: 10.1023/A:1025321707947
8. Lam KSL, Bodfish JW, Piven J. Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. J Child Psychol Psychiatry. (2008) 49(11):1193–200. doi: 10.1111/j.1469-7610.2008.01944.x
9. Turner-Brown LM, Lam KSL, Holtzclaw TN, Dichter GS, Bodfish JW. Phenomenology and measurement of circumscribed interests in autism spectrum disorders. Autism. (2011) 15(4):437–56. doi: 10.1177/1362361310386507
10. Uljarević M, Frazier TW, Jo B, Billingham WD, Cooper MN, Youngstrom EA, et al. Big data approach to characterize restricted and repetitive behaviors in autism. J Am Acad Child Adolesc Psychiatry. (2022) 61(3):446–57. doi: 10.1016/j.jaac.2021.08.006
11. Turner M. Annotation: repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. (1999) 40(6):839–49. doi: 10.1111/1469-7610.00502
12. Sutherland R, Hodge A, Bruck S, Costley D, Klieve H. Parent-reported differences between school-aged girls and boys on the autism spectrum. Autism. (2017) 21(6):785–94. doi: 10.1177/1362361316668653
13. Hus V, Pickles A, Cook EH Jr, Risi S, Lord C. Using the autism diagnostic interview–revised to increase phenotypic homogeneity in genetic studies of autism. Biol Psychiatry. (2007) 61(4):438–48. doi: 10.1016/j.biopsych.2006.08.044
14. Antezana L, Factor RS, Condy EE, Strege MV, Scarpa A, Richey JA. Gender differences in restricted and repetitive behaviors and interests in youth with autism. Autism Res. (2019) 12(2):274–83. doi: 10.1002/aur.2049
15. Frazier TW, Hardan AY. Equivalence of symptom dimensions in females and males with autism. Autism. (2017) 21(6):749–59. doi: 10.1177/1362361316660066
16. Bishop SL, Richler J, Lord C. Association between restricted and repetitive behaviors and nonverbal IQ in children with autism spectrum disorders. Child Neuropsychol. (2006) 12(4–5):247–67. doi: 10.1080/09297040600630288
17. Richler J, Huerta M, Bishop SL, Lord C. Developmental trajectories of restricted and repetitive behaviors and interests in children with autism spectrum disorders. Dev Psychopathol. (2010) 22(1):55–69. doi: 10.1017/S0954579409990265
18. Uljarević M, Carrington SJ, Hardan AY, Leekam SR. Subdomains of restricted and repetitive behaviors within autism: exploratory structural equation modeling using the diagnostic interview for social and communication disorders. Autism Res. (2022) 15(5):861–9. doi: 10.1002/aur.2687
19. Esbensen AJ, Seltzer MM, Lam KSL, Bodfish JW. Age-related differences in restricted repetitive behaviors in autism spectrum disorders. J Autism Dev Disord. (2009) 39(1):57–66. doi: 10.1007/s10803-008-0599-x
20. Carruthers S, Kinnaird E, Rudra A, Smith P, Allison C, Auyeung B, et al. A cross-cultural study of autistic traits across India, Japan and the UK. Mol Autism. (2018) 9(1):52. doi: 10.1186/s13229-018-0235-3
21. Matson JL, Matheis M, Burns CO, Esposito G, Venuti P, Pisula E, et al. Examining cross-cultural differences in autism spectrum disorder: a multinational comparison from Greece, Italy, Japan, Poland, and the United States. Eur Psychiatry. (2017) 42:70–6. doi: 10.1016/j.eurpsy.2016.10.007
22. Kurita H, Koyama T, Osada H. Autism-spectrum quotient-Japanese version and its short forms for screening normally intelligent persons with pervasive developmental disorders. Psychiatry Clin Neurosci. (2005) 59(4):490–6. doi: 10.1111/j.1440-1819.2005.01403.x
23. Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. (2000) 30(3):237–43. doi: 10.1023/A:1005596502855
24. Inada N, Ito H, Yasunaga K, Kuroda M, Iwanaga R, Hagiwara T, et al. Psychometric properties of the repetitive behavior scale-revised for individuals with autism spectrum disorder in Japan. Res Autism Spectr Disord. (2015) 15–16:60–8. doi: 10.1016/j.rasd.2015.01.002
25. Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory experiences questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. J Child Psychol Psychiatry. (2006) 47(6):591–601. doi: 10.1111/j.1469-7610.2005.01546.x
26. Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am J Occup Ther. (2007) 61(2):190–200. doi: 10.5014/ajot.61.2.190
27. Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. (2001) 31(1):5–17. doi: 10.1023/A:1005653411471
28. Brown C, Dunn W. Adult/Adolescent Sensory Profile. San Antonio, Tex: Psychological Corporation (2002).
29. Mayer JL. The relationship between autistic traits and atypical sensory functioning in neurotypical and ASD adults: a Spectrum approach. J Autism Dev Disord. (2017) 47(2):316–27. doi: 10.1007/s10803-016-2948-5
30. Lane AE, Young RL, Baker AEZ, Angley MT. Sensory processing subtypes in autism: association with adaptive behavior. J Autism Dev Disord. (2010) 40(1):112–22. doi: 10.1007/s10803-009-0840-2
31. Lane AE, Dennis SJ, Geraghty ME. Brief report: further evidence of sensory subtypes in autism. J Autism Dev Disord. (2011) 41(6):826–31. doi: 10.1007/s10803-010-1103-y
32. Lane AE, Molloy CA, Bishop SL. Classification of children with autism spectrum disorder by sensory subtype: a case for sensory-based phenotypes. Autism Res. (2014) 7(3):322–33. doi: 10.1002/aur.1368
33. Leekam SR, Prior MR, Uljarevic M. Restricted and repetitive behaviors in autism spectrum disorders: a review of research in the last decade. Psychol Bull. (2011) 137(4):562–93. doi: 10.1037/a0023341
34. Rogers SJ, Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry. (2005) 46(12):1255–68. doi: 10.1111/j.1469-7610.2005.01431.x
35. Gabriels RL, Agnew JA, Miller LJ, Gralla J, Pan Z, Goldson E, et al. Is there a relationship between restricted, repetitive, stereotyped behaviors and interests and abnormal sensory response in children with autism spectrum disorders? Res Autism Spectr Disord. (2008) 2:660–70. doi: 10.1016/j.rasd.2008.02.002
36. Boyd BA, McBee M, Holtzclaw T, Baranek GT, Bodfish JW. Relationships among repetitive behaviors, sensory features, and executive functions in high functioning autism. Res Autism Spectr Disord. (2009) 3:959–66. doi: 10.1016/j.rasd.2009.05.003
37. South M, Rodgers J. Sensory, emotional and cognitive contributions to anxiety in autism Spectrum disorders. Front Hum Neurosci. (2017) 11(January):1–7. doi: 10.3389/fnhum.2017.00020
38. Schulz SE, Stevenson RA. Sensory hypersensitivity predicts repetitive behaviours in autistic and typically-developing children. Autism. (2019) 23(4):1028–41. doi: 10.1177/1362361318774559
39. Kirby AV, Boyd BA, Williams KL, Faldowski RA, Baranek GT. Sensory and repetitive behaviors among children with autism spectrum disorder at home. Autism. (2017) 21(2):142–54. doi: 10.1177/1362361316632710
40. Istvan EM, Nevill RE, Mazurek MO. Sensory over-responsivity, repetitive behavior, and emotional functioning in boys with and without autism spectrum disorder. Res Autism Spectr Disord. (2020) 75:101573. doi: 10.1016/j.rasd.2020.101573
41. McDermott CR, Farmer C, Gotham KO, Bal VH. Measurement of subcategories of repetitive behaviors in autistic adolescents and adults. Autism Adulthood. (2020) 2(1):48–60. doi: 10.1089/aut.2019.0056
42. Barrett SL, Uljarević M, Baker EK, Richdale AL, Jones CRG, Leekam SR. The adult repetitive behaviours questionnaire-2 (RBQ-2A): a self-report measure of restricted and repetitive behaviours. J Autism Dev Disord. (2015) 45(11):3680–92. doi: 10.1007/s10803-015-2514-6
43. Tani I, Ito H, Hirashima T, Iwanaga R, Hagiwara T, Yukihiro R, et al. Standardization of the Japanese version of the short sensory profile: reliability and validity. Seishin Igaku. (2015) 57(6):419–29. doi: 10.11477/mf.1405204926
44. McIntosh DN, Miller LJ, Shyu V, Dunn W. Overview of the short sensory profile (SSP). In: Dunn W, Dunn W, editors. The Sensory Profile: Examiners Manual, Vol. 38. San Antonio, TX: The Psychological Corporation (1999). p. 59–73.
45. Constantino JN, Gruber CP. Social Responsiveness Scale-Second Edition: SRS-2. Torrance, CA: Western Psychological Services (2012).
46. Kamio Y, Inada N, Moriwaki A, Kuroda M, Koyama T, Tsujii H, et al. Quantitative autistic traits ascertained in a national survey of 22 529 Japanese schoolchildren. Acta Psychiatr Scand. (2013) 128(1):45–53. doi: 10.1111/acps.12034
48. Velicer WF, Eaton CA, Fava JL. Construct explication through factor or component analysis: a review and evaluation of alternative procedures for determining the number of factors or components. In: Goffin RD, Helmes E, editors. Problems and Solutions in Human Assessment: Honoring Douglas N Jackson at Seventy. Boston, MA: Springer US (2000). p. 41–71.
49. Bernaards CA, Jennrich RI. Gradient projection algorithms and software for arbitrary rotation criteria in factor analysis. Educ Psychol Meas. (2005) 65(5):676–96. doi: 10.1177/0013164404272507
50. Revelle W. psych: Procedures for Psychological, Psychometric, and Personality Research. Evanston, Illinois: Northwestern University (2024). Available online at: https://CRAN.R-project.org/package=psych
51. Wei T, Simko V. R package “corrplot”: Visualization of a Correlation Matrix (2021). Available online at: https://github.com/taiyun/corrplot (Accessed June 8, 2024).
52. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2024). Available online at: https://www.R-project.org/
53. Chen Y-H, Rodgers J, McConachie H. Restricted and repetitive behaviours, sensory processing and cognitive style in children with autism spectrum disorders. J Autism Dev Disord. (2009) 39(4):635–42. doi: 10.1007/s10803-008-0663-6
54. Gal E, Dyck MJ. Stereotyped movements among children who are visually impaired. J Vis Impair Blind. (2009) 103(11):754–65. doi: 10.1177/0145482X0910301105
55. Zetler NK, Cermak SA, Engel-Yeger B, Baranek G, Gal E. Association between sensory features and high-order repetitive and restricted behaviors and interests among children with autism spectrum disorder. Am J Occup Ther. (2022) 76(3). doi: 10.5014/ajot.2022.048082
56. Wiggins LD, Robins DL, Bakeman R, Adamson LB. Breif report: sensory abnormalities as distinguishing symptoms of autism spectrum disorders in young children. J Autism Dev Disord. (2009) 39(7):1087–91. doi: 10.1007/s10803-009-0711-x
57. Radonovich KJ, Fournier KA, Hass CJ. Relationship between postural control and restricted, repetitive behaviors in autism spectrum disorders. Front Integr Neurosci. (2013) 7:28. doi: 10.3389/fnint.2013.00028
58. Factor RS, Condy EE, Farley JP, Scarpa A. Brief report: insistence on sameness, anxiety, and social motivation in children with autism Spectrum disorder. J Autism Dev Disord. (2016) 46(7):2548–54. doi: 10.1007/s10803-016-2781-x
59. Chowdhury M, Benson BA, Hillier A. Changes in restricted repetitive behaviors with age: a study of high-functioning adults with autism Spectrum disorders. Res Autism Spectr Disord. (2010) 4(2):210–6. doi: 10.1016/j.rasd.2009.09.006
60. Williams ZJ, Failla MD, Gotham KO, Woynaroski TG, Cascio C. Psychometric evaluation of the short sensory profile in youth with autism Spectrum disorder. J Autism Dev Disord. (2018) 48(12):4231–49. doi: 10.1007/s10803-018-3678-7
61. Elsabbagh M, Divan G, Koh Y-J, Kim YS, Kauchali S, Marcín C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. (2012) 5(3):160–79. doi: 10.1002/aur.239
62. Saito M, Hirota T, Sakamoto Y, Adachi M, Takahashi M, Osato-Kaneda A, et al. Prevalence and cumulative incidence of autism spectrum disorders and the patterns of co-occurring neurodevelopmental disorders in a total population sample of 5-year-old children. Mol Autism. (2020) 11(1):35. doi: 10.1186/s13229-020-00342-5
Keywords: autism spectrum disorder, sensory, restricted and repetitive behavior, repetitive sensory and motor behavior, insistence on sameness
Citation: Noda H, Yoneda N, Kamogawa K, Tanaka G, Ide M and Iwanaga R (2024) Sensory processing associated with subcategories of restricted and repetitive behaviors in Japanese children and adolescents with autism spectrum disorder. Front. Child Adolesc. Psychiatry 3: 1411445. doi: 10.3389/frcha.2024.1411445
Received: 3 April 2024; Accepted: 10 July 2024;
Published: 1 August 2024.
Edited by:
Marija Colic, University of Hawaii at Manoa, United StatesReviewed by:
Mikle South, Emory Autism Center, United StatesAnnalisa Levante, University of Salento, Italy
© 2024 Noda, Yoneda, Kamogawa, Tanaka, Ide and Iwanaga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haruka Noda, aGFydWthbm9kYTFAZ21haWwuY29t
 Haruka Noda
Haruka Noda Naoto Yoneda
Naoto Yoneda Ryoichiro Iwanaga
Ryoichiro Iwanaga