- 1Eunice Kennedy Shriver Center, University of Massachusetts Chan Medical School, Worcester, MA, United States
- 2Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 3Epidemiology and Prevention, Wake Forest School of Medicine, Winston-Salem, NC, United States
- 4Department of Anatomy & Neurobiology, Boston University Chobanian & Avedisian School of Medicine, Boston, MA, United States
- 5University of Miami School of Nursing & Health Studies, Miami, FL, United States
- 6Department of Radiology, Boston University Chobanian & Avedisian School of Medicine, Boston, MA, United States
- 7Department of Neurology, Boston University Chobanian & Avedisian School of Medicine, Boston, MA, United States
- 8Department of Pediatrics, Section of Developmental and Behavioral Pediatrics and Kennedy Research Center on Intellectual and Neurodevelopmental Disabilities, Comer Children's Hospital, Chicago, IL, United States
Objectives: The prevalence of many psychiatric symptoms, including anxiety and depression, is higher in individuals born extremely preterm (EP) than in term-born individuals during childhood and adolescence. In this prospective study of adolescents born EP, we examined associations between early-life risk factors (prenatal maternal health conditions, socioeconomic and social factors) and anxiety and depression at 15 years of age.
Methods: We included 682 participants (53.2% White, 57.8% male) who were born <28 weeks gestation. Data on demographic factors, maternal health conditions and socioeconomic status (SES) were collected in the first postnatal month, and data on the outcomes (anxiety and depression) were collected at 15 years by a structured clinical diagnostic interview. At the 15-year visit, the mother reported on her own experiences of childhood trauma. Logistic regression models were used to evaluate associations between maternal health indicators, SES factors and mothers' childhood trauma and adolescent outcome variables of anxiety, depression and both anxiety and/or depression, adjusting for potential confounding factors and expressed as adjusted odds ratios (aOR) and 95% confidence intervals (CI).
Results: Maternal pre-pregnancy obesity was associated with anxiety (aOR: 1.84, 95% CI: 1.15, 2.95) and depression (aOR: 1.95, 95% CI: 1.17, 3.23) in adolescents at age 15. Maternal exposure to active or second-hand smoke was associated with depression (aOR: 1.8, 95% CI: 1.08, 3.00) and with anxiety and depression (aOR: 2.83, 95% CI: 1.51, 5.31) at age 15. Other maternal pre-pregnancy health indicators of interest including asthma, hypertension and diabetes mellitus did not demonstrate significant associations with symptoms of anxiety or depression in adolescents at age 15 in univariable and multivariate analyses. Maternal childhood experience of parental upheaval was associated with anxiety and depression (OR: 1.91, 95% CI: 1.01, 3.55) in adolescents, and maternal childhood experience of victim violence was linked with anxiety (OR: 2.4, 95% CI: 1.22, 4.62) and anxiety and depression (OR: 2.49, 95% CI: 1.05, 5.42).
Conclusion: These findings suggest that prenatal maternal health and socioeconomic factors contribute to psychiatric disorders among adolescents born EP. These factors could serve as targets for interventions to improve mental health of individuals born EP.
1 Introduction
Nearly 15 million infants worldwide are born preterm [<37 weeks' gestational age (GA)] every year (1). The subgroup of extremely preterm (EP) births (<28 weeks gestation) comprises approximately 6% of all preterm births and less than 1% of all births. Despite advances in technology and care of these infants leading to increased survival, EP and extremely low birth weight (ELBW; <1,000 grams) infants still remain at high risk for death and disability. There remains a 30%–50% mortality and, in survivors, a 20%–50% risk of morbidity (2, 3). The prevalence of psychiatric symptoms during childhood and adolescence is significantly higher in individuals born EP and/or ELBW than in term-born individuals (2–4 times increased odds) (4–8). We have previously reported that at 15 years of age, 11% of girls and 5% of boys born EP have generalized anxiety and 6% of girls and 2% of boys have depression, and these diagnoses are more prevalent when compared to general population epidemiologic adolescent studies in the U.S. (4).
EP infants may undergo altered development of their stress-regulatory systems since the third trimester of pregnancy represents a sensitive phase of infant brain plasticity (9, 10). Due to their immature neurobiological system and subsequent exposure to intensive medical treatments and extended stay in the Neonatal Intensive Care Unit (NICU), the infant's natural regulatory capacity may be exceeded leading to permanent alterations in their neuroendocrine, autonomic, cardiovascular and neuronal responses (11–13). These early exposures to physical and environmental stressors may increase the brain's vulnerability to stress later in life and subsequent development of psychopathology, especially during adolescence (14). EP infants in the NICU may also experience atypical maternal care due to obstruction of physical and emotional closeness, a critical factor in the early regulation of the infant's stress responses, leading to an increased risk of disruption of the maternal-infant attachment (9, 15). Longer NICU hospitalizations have also been associated with worse neurodevelopmental outcomes at 2–3 years of age (16, 17). Despite the higher prevalence of mental health conditions in individuals born EP, few studies have evaluated their mental health outcomes during adolescence.
Anxiety and depressive disorders are among the most important health challenges faced by adolescents in the general population (18). These disorders are common with an estimated lifetime prevalence of 7.3%–28.8% and are associated with substantial functional impairment, with an estimated cost between $42 and $47 billion to the US economy each year (19, 20). Like many other psychiatric conditions, anxiety and depressive disorders have their onset in childhood. Data from the National Comorbidity Survey Replication (NCS-R), a nationally representative epidemiologic study, reported that exposure to early-life stress and trauma has been associated with the development of depression, panic disorder, and an abnormal stress response (21). Results from nationwide birth cohort studies (22, 23), indicated multifactorial etiologies including psychological, social, familial, and biological factors (eg: puberty, hormones and immune system regulation factors) in increasing vulnerability to internalizing disorders like anxiety in adolescent females. The presence of anxiety and depressive symptoms can have significant long-term impacts including poor academic performance, behavioral problems, poor self-worth, and substance use, which may persist into adulthood (24–27). Despite these detrimental outcomes, anxiety disorders in adolescents are undertreated, with only 18% engaged in treatment (28). Given that children born EP are at an increased risk for anxiety and depression during adolescence and young adulthood (4, 26, 29), it is imperative to investigate and identify factors that may contribute to or mitigate the expression of these disorders.
In individuals exposed to significant early-life stress, such as the Extremely Low Gestational Age Newborn (ELGAN) cohort, social environment has an enormous impact on the individual due to its impact on brain development (30). A maternal childhood history of trauma and maltreatment is associated with increased mental health challenges, social isolation, and altered developmental expectations (31). Children of mothers who have had exposure to adverse childhood experiences have an increased risk for anxiety (30), depressive symptoms, aggression, and hyperactivity (32–34), largely due to resultant lack of access to social support and resources that can potentially address these negative exposures (35, 36). Exposure to trauma in childhood can lead to aggressive response biases in adulthood and can thus influence the immediate family environment and caregiving quality in this already vulnerable population (35–37). Thus, in addition to major life events, abuse, and neglect, the day-to-day experiences in family, neighborhood, school, and work environments may affect neurobiological and behavioral functions. Socioeconomic status (SES), which includes both income and education, is also a strong predictor of brain and body health, including anxiety and depressive symptoms (26, 30).
Our objective was to identify early life risk factors that might be associated with EP birth and anxiety and depression later in life in adolescents born EP. This work can provide targets for intervention to ameliorate or prevent anxiety and depression in this population.
2 Methods
2.1 Study participants
The ELGAN study is a longitudinal, observational study of individuals born EP between 2002 and 2004 in 11 cities in 5 states (38, 39). For the current study, data on the exposure (maternal health conditions and SES) were collected within a few days of the delivery of the ELGAN participant, maternal childhood trauma history and offspring psychiatric outcomes (anxiety and depression) were collected at the age 15-year visit (40). Data about neonatal characteristics were collected from a review of the neonatal medical record at birth. Biological sex was recorded by the neonatologist or pediatrician as biological male, biological female or ambiguous (henceforth referred to as male/female in the manuscript).
During the years 2002–2004, women delivering before 28 weeks' gestation in 11 cities in 5 states were asked to enroll in the first phase of the study. All procedures for this study were approved by the institutional review boards of all participating institutions.
2.2 Perinatal data
Data on maternal demographic factors, pre-pregnancy health, pregnancy complications, and medical treatments were collected by maternal interview within a few days of delivery, and by a review of maternal medical records by research assistants, with oversight from neonatologists at each recruitment site. Data included race, ethnicity, marital status, maternal age, health insurance, pre-pregnancy weight and height, active and passive smoke exposure, maternal medical disorders, and maternal medications. Smoke exposure was measured as described in Venkatesh et al., 2021 (41).
Maternal health characteristics included pre-pregnancy body mass index (BMI) categories (underweight: <18.5, healthy weight: 18.5–25, overweight: >25–30, and obese: >30), pre-pregnancy diagnosis of asthma and diabetes, and pre-pregnancy/pregnancy/delivery hypertension symptoms. Potentially life-threatening maternal pregnancy complications and indications for premature delivery, as well as Hemolysis, Elevated Liver enzymes and Low Platelets (HELLP) syndrome and preeclampsia, were included. A composite variable was defined as having displayed any of the aforementioned hypertensive disorders, HELLP, or preeclampsia. Whether mother smoked or was exposed to second-hand smoke during pregnancy was ascertained by maternal interview; and a composite exposure was defined for mothers' who either smoked or were exposed to second-hand smoke during pregnancy.
The following socioeconomic variables were included: education (less than high school), marital status (not married or not living together), insurance (public insurance or no insurance), and Supplemental Nutrition Assistance Program (SNAP) eligibility.
Newborn characteristics included sex, birth weight, gestational age, and medical conditions known to commonly occur in EP infants such as, intraventricular hemorrhage, white matter damage, chronic lung disease, sepsis, severe retinopathy of prematurity, and necrotizing enterocolitis. Since the focus of the current manuscript was on maternal health and psychosocial antecedents of anxiety and depression, these variables were not included and reported in the present analysis.
The Childhood Trauma Questionnaire [CTQ; (42); ECHO-wide Cohort version 01.20] was completed by 438 mothers during the age 15 visit. It provided a description of maternal exposure to death of a family member/very close friend, parent upheaval such as divorce, separation, etc., sexual trauma, victim violence (other than sexual violence), being extremely ill/injured, or any other upheaval. It also obtained data on a 7-point Likert scale on how traumatic the experience was for the mother. Internal consistency in a community sample was acceptable for the entire measure (Cronbach's alpha = 0.91) and four of the five subscales (ranging from 0.58 for Physical Neglect to 0.94 for Sexual Abuse (43). In clinical samples, the CTQ has demonstrated test-retest reliability coefficients from 0.79 to 0.86, internal consistency reliability coefficients ranging from 0.66 to 0.92, convergent validity with clinician ratings of abuse, and a consistent five-factor structure (42).
2.3 Neuropsychiatric assessments
The Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID 7.0.2) (44) is a structured clinical diagnostic interview tool designed to assess the presence of current DSM-5 and ICD-10 psychiatric disorders in children and adolescents aged 6–17 years. It is used for psychiatric evaluation and outcome monitoring in clinical psychopharmacology trials and epidemiological studies in more than 100 countries. The interview is administered to the child/adolescent together with the parent(s), although it can be administered to adolescents without a parent present. The MINI-KID follows the structure and format of the adult version of the interview (MINI), which has been validated against the Structured Clinical Interview for DSM-III-R and against the World Health Organization–designed Composite International Diagnostic Interview. Like its adult counterpart, the MINI-KID is organized in diagnostic sections or modules, and is administered using branching tree logic (e.g., 2–4 screening questions for each disorder, with additional questions being asked only if the screen questions are positively endorsed). The instrument screens for 24 DSM-5 and ICD-10 psychiatric disorders and suicidality and takes approximately 30 min to complete. The MINI-KID has substantial to excellent concordance with the gold standard K-SADS-PL (area under the curve = 0.81–0.96, ≥0.56–0.87). Sensitivity was 0.61–1.00 for 15/20 individual disorders, and specificity was 0.81–1.00 for 18 disorders and >0.73 for the remaining two. Interrater and test-retest kappas were 0.64–1.00 for all individual disorders except dysthymia. It has recently been updated to map onto DSM-5 (MINI 7.0.2) diagnostic criteria.
The primary outcomes for our analyses were presence of any form of anxiety (i.e., generalized anxiety disorder, separation anxiety disorder, social anxiety disorder, panic disorder lifetime/current, specific phobia, and agoraphobia), depression (major depressive disorder current/recurrent/past), as assessed by the MINI-KID, anxiety-or-depression, in which participants met the cut-offs for either disorder, and anxiety-and-depression, indicating that they met cut-offs for the comorbidity of both disorders, thereby indicating greater illness severity.
2.4 Statistical analysis
We evaluated the association between the following sets of exposures: maternal health conditions, SES indicators during pregnancy, history of maternal childhood trauma with four outcomes: anxiety, depression, and anxiety-or-depression, and anxiety-and-depression at 15 years of age. Based on sex differences in the prevalence of psychiatric disorders, we also evaluated sex as a modifier of associations between perinatal factors and adolescent psychiatric disorder (45, 46). Regression models were used to evaluate associations between psychiatric outcomes of anxiety, depression, anxiety-or-depression, and anxiety-and-depression, and antecedents, including prenatal maternal health factors as well as SES factors. For maternal education and marital status variables, both of which consisted of five categories, a secondary set of binary variables- college education and married- were also defined to explore these factors' associations without the small sample size effects of some categories. In the same regard, the very small number of mothers whose marital status were “widowed” were analyzed along with mothers “separated or divorced”, under the “separated or divorced or widowed” category (Table 2). Statistical significance was defined as p < 0.05. After conducting univariate analyses, multivariate regression analyses were conducted. The variables for adjustment were determined using directed acyclic graphs, DAGs, to determine a minimally sufficient set of adjustment variables for inclusion in the models (see Supplementary Figures). Adjustment variables are listed in Supplementary Table 1. Based on the finding that pre-pregnancy exposure to adverse experiences has been associated with increased risk of maternal health conditions like hypertension, preterm birth and detrimental maternal mental health outcomes (31), severity of trauma as measured by the CTQ was included as a confounding variable.
3 Results
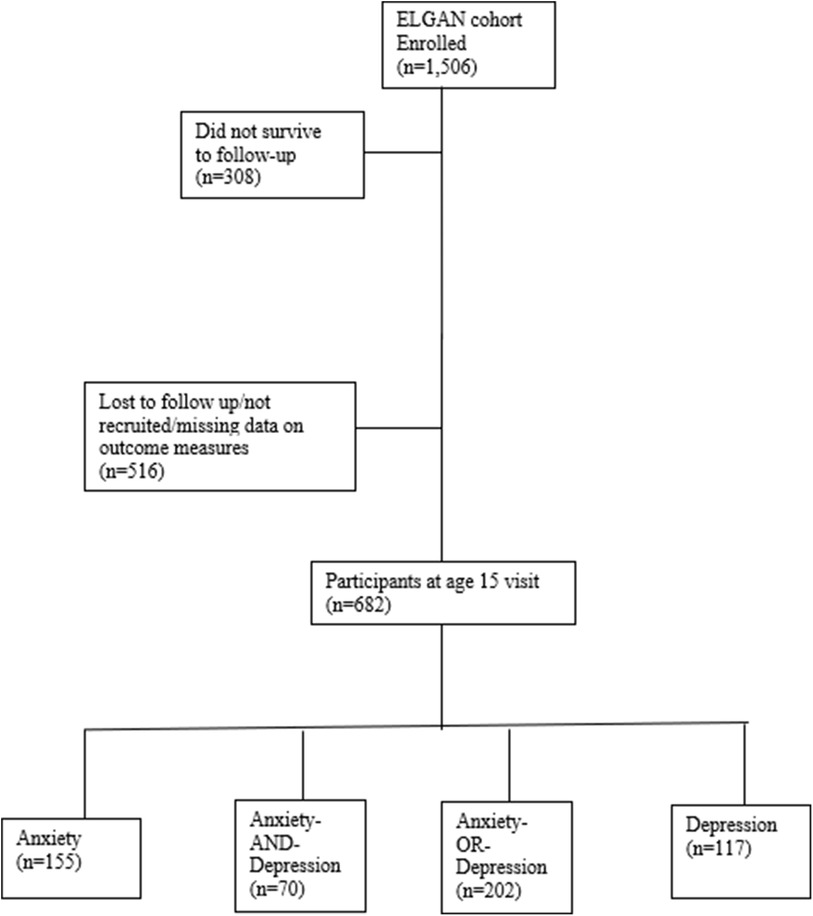
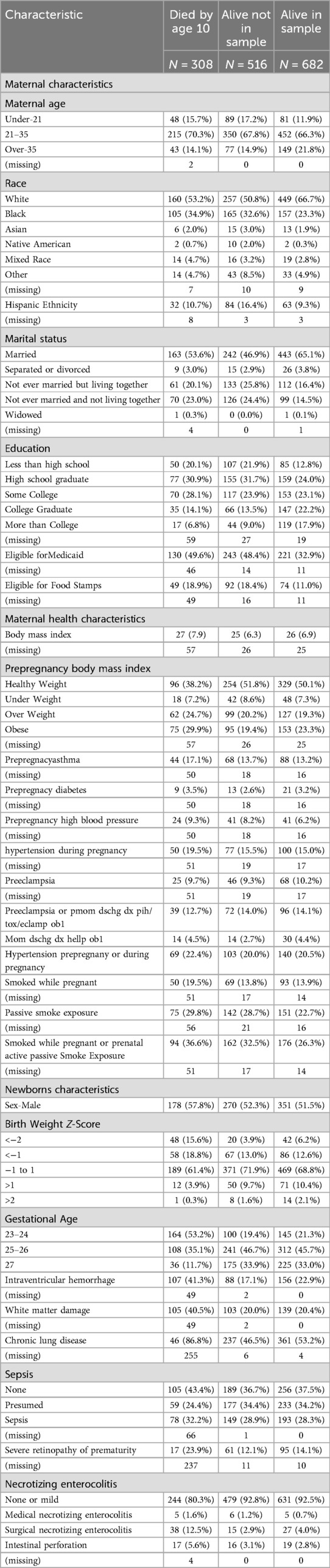
A total of 1,506 infants, born to 1,249 mothers, were enrolled in the ELGAN study. Of the 1,198 children assessed in this longitudinal study at age ten, 516 participants were lost to follow-up or not recruited in this age 15 analysis. Four participants did not have any measures of depression or anxiety completed and were excluded from this current report. Youth with anxiety symptoms (N = 155) included those with anxiety alone (N = 85) and those with anxiety and depression comorbid (N = 70). Youth under the depression category (N = 117) included those with depression only (N = 47) and those with both, depression and anxiety symptoms (N = 70; see Figure 1). No significant differences were noted in demographic characteristics, maternal health and socioeconomic variables among those assessed and not assessed at age 15 (see Table 1). Given that anxiety and depressive disorders are highly comorbid with a significant overlap in symptoms, this combination can contribute to an increased risk of comorbid general medical illnesses, and worse treatment outcomes than with either condition alone (47–50), the combined variable, anxiety-and-depression, was an indicator of greater illness burden in our analyses at age 15 in the ELGAN cohort.
3.1 Maternal health characteristics at birth
3.1.1 Univariate analyses
Maternal hypertension was significantly associated with symptoms of anxiety and depression in youth at age 15. Specifically, hypertensive disorder during pregnancy was linked with depression (OR: 1.59, 95% CI: 1.00, 2.50). A discharge diagnosis of hypertensive disorder in the mother was associated with anxiety (OR: 1.68, 95% CI: 1.03, 2.67), which was noted in females (OR: 1.81, 95% CI: 1.03, 3.15) but not males. Another maternal health characteristic of significance was pre-pregnancy BMI. In female participants, maternal obesity displayed significant relationships with anxiety (OR: 1.86, 95% 95% CI: 1.03, 3.38), depression (OR: 2.57, 95% CI: 1.33, 5.02), anxiety-or-depression (OR 1.95, 95% CI 1.10, 3.46), and anxiety-and-depression variables (OR 2.9, 95% CI 1.36, 3.61). Maternal smoking during pregnancy was associated with depression (OR 1.73, 95% CI 1.01, 2.87), and anxiety-and-depression (OR: 2.00, 95% CI: 1.06, 3.61). Maternal exposure to second-hand smoke was also associated with depression (OR: 1.73, 95% CI: 1.10, 2.68) and anxiety-and-depression (OR: 1.79, 95% CI: 1.03, 3.03) in the overall sample. This was also noted in females whose mothers were exposed to secondhand smoke with presence of depression (OR: 2.26, 95% CI: 1.26, 4.00) and anxiety-and-depression (OR: 2.76, 95% CI: 1.45, 5.24; see Table 2 and Supplementary Table 2A and 2B for sex differences in outcome variables).
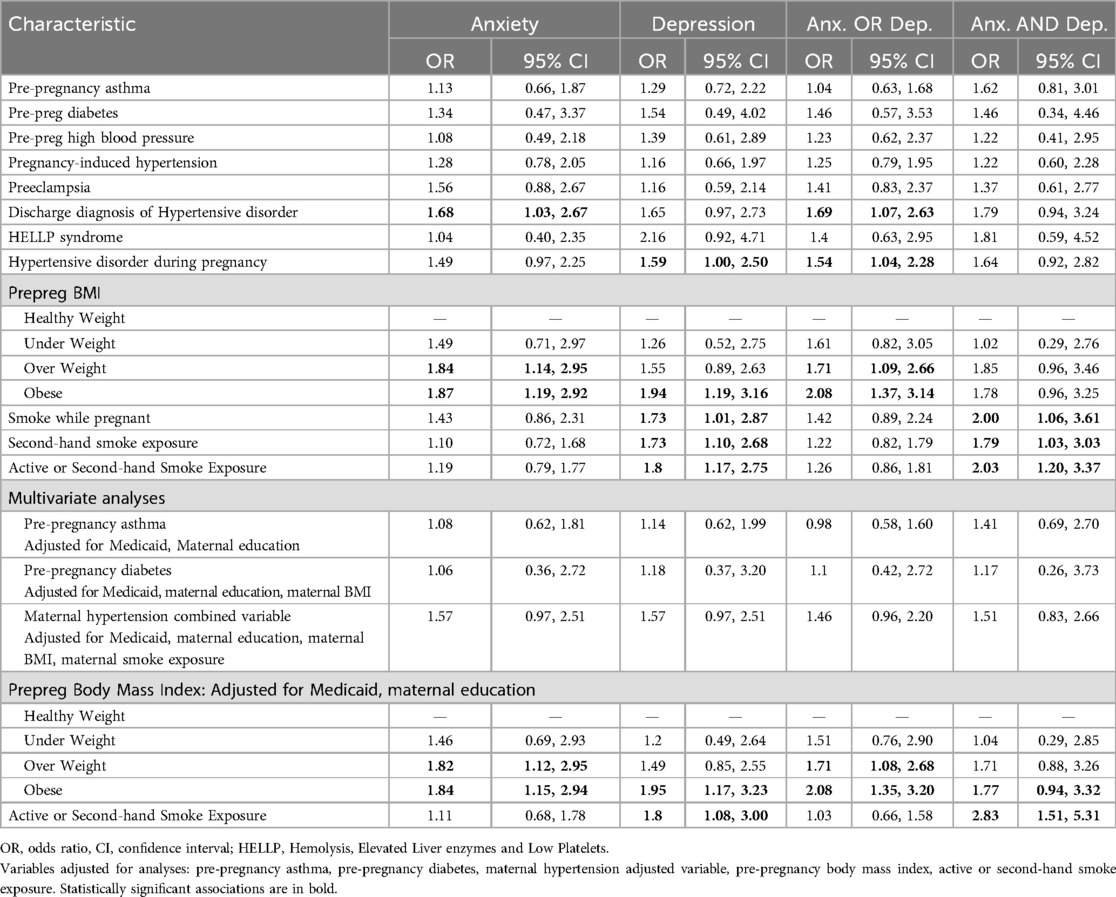
Table 2. Maternal health characteristics at birth: univariate and multivariate associations with adolescent anxiety, depression, anxiety-or-depression, and anxiety-and-depression.
3.1.2 Multivariable analyses
Mothers who were overweight (BMI: 25.0–29.9) had youth with higher odds of anxiety (aOR: 1.82, 95% CI: 1.12, 2.95) when adjusted for insurance/Medicaid and maternal education. Mothers who were classified as being obese (BMI > 30) had youth with higher odds of anxiety (aOR: 1.84, 95% CI: 1.15, 2.94), depression (aOR: 1.95, 95% CI: 1.17, 3.23), and anxiety-and-depression (aOR: 1.77, 95% CI: 0.94, 3.32). Female youth who had mothers with BMI > 30 were more likely to have depression (OR: 2.44, 95% CI: 1.23, 4.88) and anxiety-and-depression (aOR: 2.58, 95% CI: 1.17, 5.82) when adjusted for Medicaid and maternal education. In male youth, anxiety symptoms were associated with overweight status in mothers (aOR: 2.27, 95% CI: 1.07, 4.75) when adjusted for Medicaid and maternal education. When adjusted for Medicaid status, maternal education and marital status of the mother, smoke exposure (active and second-hand smoke exposure combined) was associated with depression (aOR: 1.8, 95% CI: 1.08, 3.00) and anxiety-and-depression (aOR: 2.83, 95% CI: 1.51, 5.31).
3.2 Maternal socioeconomic characteristics
3.2.1 Univariate analyses
Youth whose mother had a high school education were more likely to have anxiety-and-depression (OR: 2.46, 95% CI: 1.03, 6.84) than those with less than college education or higher with stronger associations noted in females (OR: 6.27, 95% CI: 1.70, 40.6). Mothers who did not have a college degree were also more likely to have female children with depression (OR: 1.86, 95% CI: 1.04, 3.45) and anxiety-and-depression (OR: 2.26, 95% CI: 1.13,4.82). Female youth whose mothers were on Medicaid displayed greater likelihood for the development of anxiety (OR: 1.71, 95% CI: 1.04, 2.81) while males had decreased likelihood for the presence of anxiety-and-depression (OR: 0.19, 95% CI: 0.03, 0.67; see Table 3 and Supplementary Table 3A and 3B for sex differences in outcome variables).
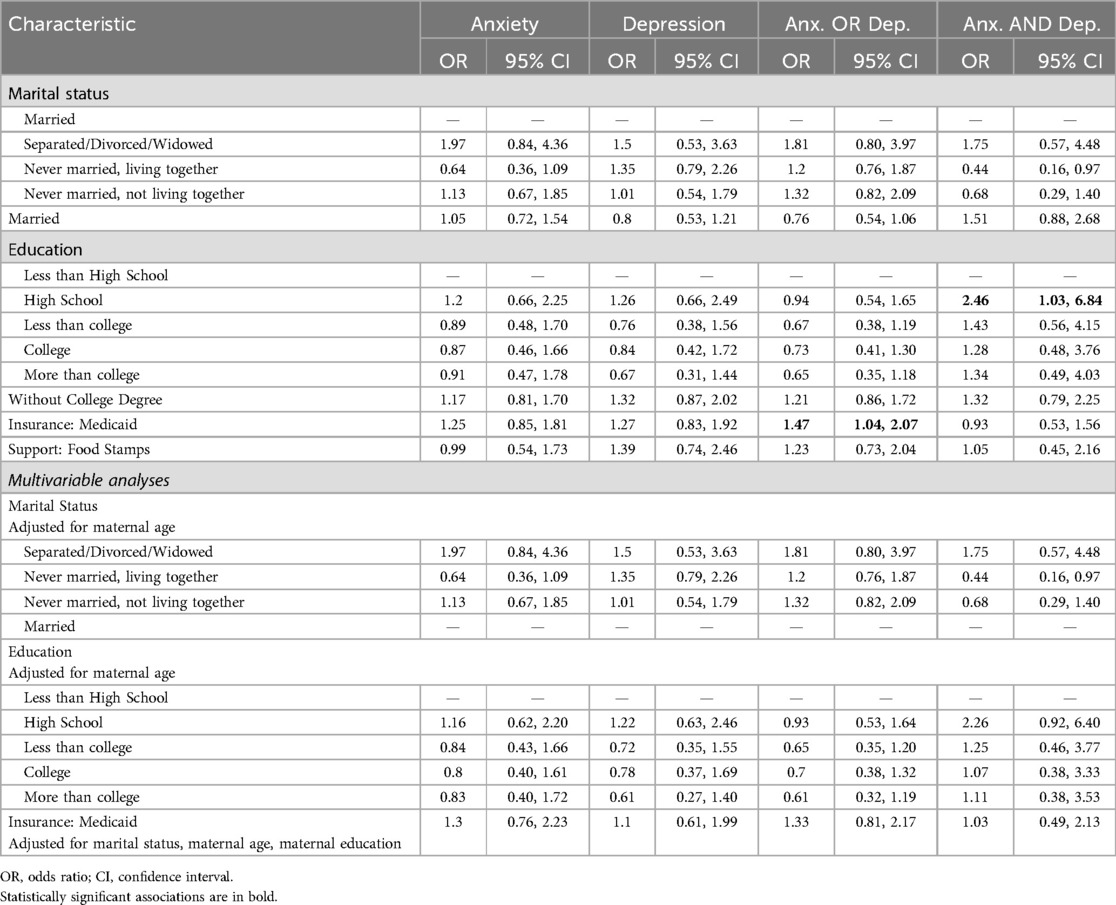
Table 3. Maternal socioeconomic characteristics at birth: univariate and multivariable associations with adolescent anxiety, depression, anxiety-or-depression, and anxiety-and-depression.
3.2.2 Multivariable analyses
When adjusted for maternal age, no statistically significant associations were noted between maternal marital status, maternal education and outcomes of anxiety and/or depression in the overall sample as well as in females and males. When adjusted for marital status, maternal age and maternal education, there were no significant associations noted with Medicaid/insurance status.
3.3 Maternal childhood trauma history
3.3.1 Univariable analyses
Mother's childhood experience of parental upheaval, defined as experiencing parental separation or divorce, was associated with anxiety-and-depression (OR: 1.91, 95% CI: 1.01, 3.55) in both male and female adolescents, and was associated with anxiety among male adolescents (OR: 2.26, 95% CI: 1.08, 4.65). Maternal childhood exposure to sexual trauma was associated with anxiety-and-depression in female adolescents (OR: 2.55, 95% CI: 1.12, 5.67). Mothers who were victims of violence in childhood were more likely to have adolescents with anxiety (OR: 2.4, 95% CI: 1.22, 4.62), and anxiety-and-depression (OR: 2.49, 95% CI: 1.05, 5.42). In the analysis by sex, this association was only seen in the male offspring. Other upheaval, defined as any other experience or upheaval that may have shaped the reporter's life or personality significantly, was associated with anxiety-and-depression in the overall sample (OR: 2.21, 95% CI: 1.04, 4.44), and anxiety (OR: 3.21, 95% CI: 1.30, 7.68) and anxiety-and-depression (OR: 5.83, 95% CI: 1.80, 18.0) in males. See Table 4 describing maternal childhood trauma characteristics with univariate associations with adolescent anxiety or depression with overall sample, female and male participants.
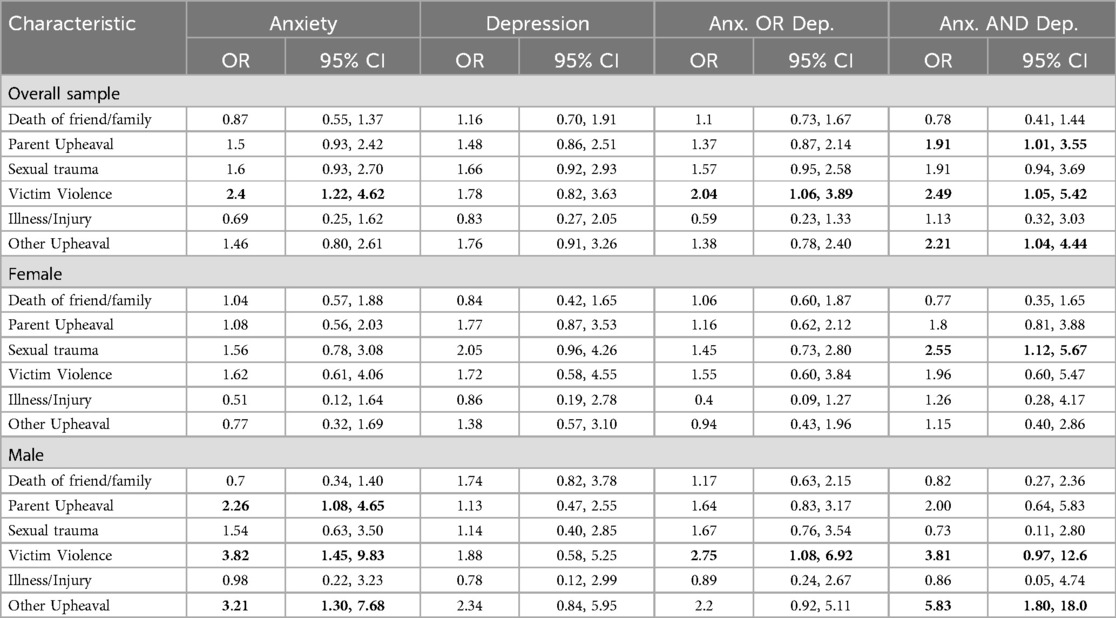
Table 4. Maternal childhood trauma characteristics: univariate associations with adolescent anxiety or depression with overall sample, female and male participants.
3.3.2 Multivariable analyses with severity of traumatic experiences
When adjusted for maternal age and CTQ severity rating, mothers who were exposed to smoke (active and secondhand smoke exposure) had a greater likelihood of having children with anxiety-and-depression (OR: 3.26, 95% CI: 1.39, 7.63). When adjusting for CTQ severity, mothers whose marital status was classified as not ever married, living together, had a greater likelihood of having children with anxiety-and-depression at age 15 years (aOR: 029, 95% CI: 0.07, 0.85).
4 Discussion
Children born extremely preterm are at a higher risk of psychiatric disorders in adolescence, notably anxiety and depression (4, 26, 51). We sought to identify modifiable prenatal and early-life risk factors of these highly prevalent conditions in this already vulnerable population by examining associations between youth's psychiatric diagnoses of anxiety and depression and measures of maternal health, socioeconomic factors, and maternal report of childhood trauma in this study. Our study assessed for sex differences for anxiety and depression in the youth born extremely preterm and noted the findings are consistent with sex differences in the prevalence of psychiatric disorders reported in adolescents in epidemiologic studies in the general population (4, 52, 53).
We noted that maternal health attributes of hypertension, elevated BMI (overweight or obese status), and smoke exposure (active or secondhand smoke) were associated with the presence of anxiety and depression in the youth. After adjusting for maternal education and Medicaid status, elevated maternal BMI continued to be significantly associated with anxiety and depression in adolescents. In contrast to earlier findings from our group that did not find an association between smoke exposure and cognitive outcomes at age 10 (41), we noted that smoke exposure even after statistical adjustment for Medicaid, maternal education and marital status remained associated with psychiatric outcomes at age 15. Exposure to maternal metabolic disorders during pregnancy, including hypertension and obesity, has been linked to adverse outcomes in the behavior and physiology of their offspring, and the development of neuropsychiatric disorders such as anxiety and depression (54–57). High maternal BMI increases the risk of adverse childhood outcomes including preterm and extreme preterm birth as well as likelihood of high or low birth weight (58, 59), which in turn increases the risk of the development of anxiety and depression in adolescence (4). A comprehensive meta-analysis by Zhang and colleagues also demonstrated associations between maternal pre-pregnancy overweight status/obesity and adverse neurodevelopmental outcomes in their offspring including anxiety, depression, emotional symptoms, autism spectrum disorder among others. Obesity during pregnancy can have an impact on neuroendocrine, metabolic and inflammatory systems leading to altered neuronal plasticity, impaired reward circuitry, and dysregulated brain metabolism (60–62). Additionally, due to disturbances in metabolic and endocrine factors in adipose tissue in obesity, there is an increased risk of insulin resistance which can increase the risk for the development of type 2 diabetes (63, 64). A nationwide cohort study in Finland (65) found that maternal moderate and severe obesity along with type 2 diabetes and pregestational diabetes were associated with development of mood disorders in their children. The association of severe obesity in combination with diabetes had a stronger link with psychiatric and neurodevelopmental outcomes in this study, suggesting a stronger neural exposure to inflammation, oxidative stress, lipotoxicity and insulin resistance. In the Avon Longitudinal Study for Parents and Children (ALSPAC), hypertensive pregnancy disorders in mothers predicted a significantly greater risk of anxiety and depression in children at age 7 and age 15 respectively (66). In another large cohort study, maternal smoking in early pregnancy was associated with childhood internalizing symptoms of anxiety and depression (67). It has been hypothesized based on animal models, that exposure to nicotine and other components of cigarette smoke may interfere with neurodevelopmental processes in utero, thus leading to this increased risk (68, 69); however, no direct causal relationship between these factors has been demonstrated in human studies (67).
The link between SES and child mental health and wellbeing is well established in multiple population-based studies (70, 71). We also noted an association between lower maternal education and greater prevalence of anxiety at age 15, but this did not persist when adjusted for maternal age. Studies have examined the psychophysiological pathways underlying this association which have suggested that exposure to chronic stressful events secondary to low SES and long-term alterations in physiological systems make individuals more vulnerable to anxiety, depression and aberrant hypothalamic-pituitary-adrenal (HPA) axis functioning (McEwen, 2000). Receipt of public health insurance was additionally used as an estimate of socioeconomic disadvantage in the ELGAN cohort. Similar to our results, other longitudinal studies involving youth exposed to poverty and socioeconomic disadvantage were noted to have increased prevalence of symptoms of anxiety and depression in adolescence (72, 73).
Parental mental health plays a critical role in the child's physiological as well as psychological response to stress (71). Exposure to childhood trauma can lead to altered emotional and behavioral response patterns that often persist into adulthood and thus negatively impact caregiving (36, 74). Maternal report of a history of childhood trauma has been linked to mood and anxiety disorders, posttraumatic stress disorder, and other significant mental health conditions in their children (75–78). This critical public health implication was noted in our studied sample as well, as mothers who were exposed to traumatic events in their childhood were more likely to have children diagnosed with anxiety and depression in adolescence. Specifically, the association between mothers exposed to parental upheaval, sexual trauma or being victims of any other traumatic event, and the presence of anxiety and depressive symptoms in the adolescent was significant in both biological sexes. Maternal social isolation, dysfunction in intimate relationships, academic and social challenges may occur as downstream effects of a traumatic exposure in childhood and can impact access to healthcare and other resources for the child and family (35, 79–81). Surprisingly, the severity of childhood trauma experienced by the mother did not significantly alter the associations between perinatal factors and socioeconomic status and anxiety and/or depression outcomes. In our sample, it is important to consider the role of extreme preterm birth, the associated stressors related to medical investigations, interventions and monitoring, the child's co-occurring medical diagnoses and any signs of distress and helplessness that they may display, which could serve as traumatic reminders for mothers who report having experienced childhood trauma (82). This could lead to an added increased risk of insensitive caregiving, child maltreatment and long-term impact on mother-child relationships and maladaptive interaction styles, in part due to the mother's own impaired self-regulation abilities (34, 51, 82, 83).
5 Strengths and limitations
In addition to its prospective, longitudinal design with recruitment of a large number of children who were born extremely preterm, our study is unique in the use of a structured diagnostic interview to evaluate symptoms of anxiety and depression in adolescents at age 15. Information about maternal health was obtained by self-report leading to concerns about inaccurate reporting and the resultant misclassification bias could lead to an underestimation of the strength of associations with those health disorders (84). We have previously reported that in the ELGAN cohort some adolescents who had anxiety and depression symptoms on dimensional measures did not meet the clinical threshold for the diagnosis on the MINI-KID. This suggests that the interview does not fully capture subclinical symptoms in our study population (4). Another limitation of our study is the relatively high attrition rate at age 15 years, similar to that noted in other longitudinal studies (85). It is also important to consider the impact of our selected study population of extreme preterm infants where effects of maternal factors on the development of anxiety and depression may be underestimated since the study design controls for gestational age.
In addition, we do not have information on parental attachment styles in our analysis, making it difficult to assess the extent of emotional dysregulation's impact on anxiety and depression compared to other presented factors. A methodologic concern with including parental psychopathology as a confounder in our analyses is that ascertainment of parent psychiatric diagnoses was completed simultaneous with ascertainment of child's psychiatric diagnoses. It is possible that any symptoms of parental psychopathology reported at age 15 may not have been present at birth for all participants and may have arisen due to associated stressors related to EP birth, associated medical and psychiatric comorbidities in the child and accurate date regarding the timeline of the onset of symptoms in the parent was not available. Only data on maternal psychiatric diagnoses was available for a subset of the sample and we did not have paternal psychiatric diagnostic information on all youth. Similarly, our inclusion of maternal report of early life trauma obtained when adolescents were 15 years of age is not in temporal alignment with our other early life indicators. While we believe this variable is an important consideration in examining the psychiatric status of our adolescent sample, the ascertainment of this information in a retrospective manner may have biased our data in an unknown fashion, and follow-up of the influence of this variable for this population of adolescents remains a future area of scientific inquiry into modifiable maternal factors.
6 Conclusions
The current study underscores the importance of monitoring for psychiatric disorders, especially anxiety and depression, in children born extremely preterm. Maternal health conditions including exposure to smoke and elevated BMI were noted to be associated with the development of anxiety and depression in their child in adolescence. Additionally, socioeconomic stressors and maternal childhood trauma were independently associated with symptoms of anxiety and depression in these youth born extremely preterm. Identification of these modifiable risk factors serves to inform future research efforts and the design of interventions to effectively address these challenging and prevalent conditions in preterm children. These findings set the stage for further work aimed at identifying mediating pathways between maternal childhood traumatic exposures and social determinants of health and child mental health outcomes in adolescents who are vulnerable.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by IRB of all participating institutions. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
IJ: Writing – original draft, Writing – review & editing. AO: Formal Analysis, Writing – review & editing. JF: Conceptualization, Writing – review & editing. DC: Writing – review & editing. SK: Writing – review & editing. EJ: Writing – review & editing. RJ: Writing – review & editing. SH: Writing – review & editing. HS: Writing – review & editing. HJ: Writing – review & editing. KK: Writing – review & editing. MM: Writing – review & editing. RS: Writing – review & editing. LW: Writing – review & editing. SG: Writing – review & editing. SH: Writing – review & editing. LV: Writing – review & editing. RF: Writing – review & editing, Conceptualization, Funding acquisition, Investigation. MO: Funding acquisition, Investigation, Writing – review & editing, Project administration.
Project lead for ELGAN-2 & ELGAN ECHO: Julie V. Rollins, MA
Site Principal Investigators
Baystate Medical Center, Springfield, MA: Bhavesh Shah, MD; Rachana Singh, MD, MS, Ruben Vaidya, MD.
Boston Children’s Hospital, Boston, MA: Linda Van Marter, MD, MPH and Camilla Martin, MD, MPH; Janice Ware, PhD, Caitlin Rollins, MD.
Tufts Medical Center, Boston, MA: Cynthia Cole, MD; Ellen Perrin, MD, Christina Sakai, MD.
University of Massachusetts Medical School, Worcester, MA: Frank Bednarek, MD (deceased); Jean Frazier, MD.
Yale University School of Medicine, New Haven, CT: Richard Ehrenkranz, MD (deceased); Jennifer Benjamin, MD, Angela Montgomery, MD.
Wake Forest University, Winston-Salem, NC: T Michael O’Shea, MD, MPH, Lisa Washburn, MD, Semsa Gogcu, MD, MPH.
University of North Carolina, Chapel Hill, NC: Carl Bose, MD; Diane Warner, MD, MPH, T Michael O’Shea, MD, MPH.
East Carolina University, Greenville, NC: Steve Engelke, MD, Amanda Higginson, MD, Jason Higginson, MD, Kelly Bear, MD.
Helen DeVos Children’s Hospital, Grand Rapids, MI: Mariel Poortenga, MD; Steve Pastyrnak, PhD.
Sparrow Hospital, Lansing, MI and Michigan State University, East Lansing, MI; Padu Karna, MD; Nigel Paneth, MD, MPH; Madeleine Lenski, MSPH.
University of Chicago Medical Center, Chicago, IL: Michael Schreiber, MD; Scott Hunter, PhD; Michael Msall, MD.
William Beaumont Hospital, Royal Oak, MI: Danny Batton, MD; Judith Klarr, MD, Young Ah Lee, MD, Rawad Obeid, MD.
Site Study Coordinators
Baystate Medical Center, Springfield, MA: Karen Christianson, RN; Deborah Klein, BSN, RN, Katie Wagner, MS, Victoria Cobb, Shaula Paula, Andres Santana.
Boston Children’s Hospital, Boston MA: Maureen Pimental, BA; Collen Hallisey, BA; Taryn Coster, BA, Maddie Dolins, Maggie Mittleman, Hannah Haile, Julia Rohde, Kaysi Herrera Pujols, Susie Rodriquez, Kyla Waring.
Tufts Medical Center, Boston, MA: Ellen Nylen, RN; Emily Neger, MA; Kathryn Mattern, BA, Catherine Ma, Deanna Toner, Elizabeth Vitaro, Allison Nolan.
University of Massachusetts Medical School, Worcester, MA: Lauren Venuti, BA; Beth Powers, RN; Ann Foley, EdM, Taylor Merk, BA.
Yale University School of Medicine, New Haven, CT: Joanne Williams, RN; Elaine Romano, APRN, Christine Henry.
Wake Forest University, Winston-Salem, NC: Debbie Hiatt, BSN (deceased); Nancy Peters, RN; Patricia Brown, RN; Emily Ansusinha, BA, Jazmyne James, MS, Nou Yang, MS, Nicole Froelich, Kristi Lanier.
University of North Carolina, Chapel Hill, NC: Gennie Bose, RN; Janice Wereszczak, MSN; Janice Bernhardt, MS, RN.
East Carolina University, Greenville, NC: Joan Adams (deceased); Donna Wilson, BA, BSW, Nancy Darden-Saad, BS, RN, Bree Williams, Emily Jones, Hannah Morris, Taiara Williams, Isabella Carter, Emily Jones.
Helen DeVos Children’s Hospital, Grand Rapids, MI: Dinah Sutton, RN; Julie Rathbun, BSW, BSN, Stephanie Fagerman, William Boshoven, Jalen Johnson, Brandon James; Cynthia Gile, BS, CCRC, Megan Maynard, Emina Nakic, Cynthia Gile, Duvonna Haynes.
Sparrow Hospital, Lansing, MI and Michigan State University, East Lansing, MI: Karen Miras, RN, BSN; Carolyn Solomon, RN, Deborah Weiland, MSN, Chloe Caltrider.
University of Chicago Medical Center, Chicago, IL: Grace Yoon, RN; Rugile Ramoskaite, BA; Suzanne Wiggins, MA; Krissy Washington, MA; Ryan Martin, MA; Barbara Prendergast, BSN, RN, Emma Lynch, MPH, Sabina Hajdarovic.
William Beaumont Hospital, Royal Oak, MI: Beth Kring, RN.
Other Study Personnel
Baystate Medical Center, Springfield, MA: Anne Smith, PhD; Susan McQuiston, PhD.
Boston Children’s Hospital: Samantha Butler, PhD; Rachel Wilson, PhD; Kirsten McGhee, PhD; Patricia Lee, PhD; Aimee Asgarian, PhD; Anjali Sadhwani, PhD; Brandi Henson, PsyD.
Tufts Medical Center, Boston MA: Cecelia Keller, PT, MHA; Jenifer Walkowiak, PhD; Susan Barron, PhD.
University of Massachusetts Medical School, Worcester MA: Alice Miller, PT, MS; Brian Dessureau, PhD; Molly Wood, PhD; Jill Damon-Minow, PhD.
Yale University School of Medicine, New Haven, CT: Elaine Romano, MSN; Linda Mayes, MD; Kathy Tsatsanis, PhD; Katarzyna Chawarska, PhD; Sophy Kim, PhD; Susan Dieterich, PhD; Karen Bearrs, PhD.
Wake Forest University Baptist Medical Center, Winston-Salem NC: Ellen Waldrep, MA; Jackie Friedman, PhD; Gail Hounshell, PhD; Debbie Allred, PhD.
University Health Systems of Eastern Carolina, Greenville, NC: Rebecca Helms, PhD; Lynn Whitley, PhD Gary Stainback, PhD.
University of North Carolina at Chapel Hill, NC: Lisa Bostic, OTR/L; Amanda Jacobson, PT; Joni McKeeman, PhD; Echo Meyer, PhD.
Helen DeVos Children’s Hospital, Grand Rapids, MI: Steve Pastyrnak, PhD.
Sparrow Hospital, Lansing, MI and Michigan State University, East Lansing, MI: Joan Price, EdS; Megan Lloyd, MA, EdS.
University of Chicago Medical Center, Chicago, IL: Susan Plesha-Troyke, OT; Megan Scott, PhD.
William Beaumont Hospital, Royal Oak, MI: Katherine M Solomon, PhD; Kara Brooklier, PhD; Kelly Vogt, PhD.
We would like to thank David Sheehan of the University of South Florida College of Medicine, for his consultation regarding the use of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID).
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Institute of Neurological Disorders and Stroke (5U01NS040069-05 to Alan Leviton) and the Office of the NIH Director (1UG3OD023348-01 to TO) The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Acknowledgments
The authors gratefully acknowledge the contributions of the ELGAN Study participants and their families, as well as those of their colleagues listed separately.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frcha.2024.1334316/full#supplementary-material
References
2. Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. (2015) 120(6):1337–51. doi: 10.1213/ANE.0000000000000705
3. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. Jama. (2015) 314(10):1039–51. doi: 10.1001/jama.2015.10244
4. Frazier JA, Cochran D, Kim S, Jalnapurkar I, Joseph RM, Hooper SR, et al. Psychiatric outcomes, functioning, and participation in extremely low gestational age newborns at age 15 years. J Am Acad Child Adolesc Psychiatry. (2022) 61(7):892–904.e2. doi: 10.1016/j.jaac.2021.12.008
5. Frazier JA, Wood ME, Ware J, Joseph RM, Kuban KC, O’Shea M, et al. Antecedents of the child behavior checklist–dysregulation profile in children born extremely preterm. J Am Acad Child Adolesc Psychiatry. (2015) 54(10):816–23. doi: 10.1016/j.jaac.2015.07.008
6. Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. (2011) 69(8):11–8. doi: 10.1203/PDR.0b013e318212faa0
7. Johnson S, Marlow N. Growing up after extremely preterm birth: lifespan mental health outcomes. Semin Fetal Neonat Med. (2014) 19.
8. Johnson S, Wolke D. Behavioural outcomes and psychopathology during adolescence. Early Hum Dev. (2013) 89(4):199–207. doi: 10.1016/j.earlhumdev.2013.01.014
9. Lammertink F, Vinkers CH, Tataranno ML, Benders MJ. Premature birth and developmental programming: mechanisms of resilience and vulnerability. Front Psychiatry. (2021) 11:531571. doi: 10.3389/fpsyt.2020.531571
10. Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. (2010) 35(1):147–68. doi: 10.1038/npp.2009.115
11. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. (2007) 87(3):873–904. doi: 10.1152/physrev.00041.2006
12. Puchalski M, Hummel P. The reality of neonatal pain. Adv Neonatal Care. (2002) 2(5):233–47.12881937
13. Valeri BO, Holsti L, Linhares MB. Neonatal pain and developmental outcomes in children born preterm: a systematic review. Clin J Pain. (2015) 31(4):355–62. doi: 10.1097/AJP.0000000000000114
14. Doom JR, Gunnar MR. Stress physiology and developmental psychopathology: past, present, and future. Dev Psychopathol. (2013) 25(4pt2):1359–73. doi: 10.1017/S0954579413000667
15. Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. (2005) 28(9):456–63. doi: 10.1016/j.tins.2005.07.006
16. Faramarzi R, Darabi A, Emadzadeh M, Maamouri G, Rezvani R. Predicting neurodevelopmental outcomes in preterm infants: a comprehensive evaluation of neonatal and maternal risk factors. Early Hum Dev. (2023) 184:105834. doi: 10.1016/j.earlhumdev.2023.105834
17. Vohr B, McGowan E, McKinley L, Tucker R, Keszler L, Alksninis B. Differential effects of the single-family room neonatal intensive care unit on 18-to 24-month bayley scores of preterm infants. J Pediatr. (2017) 185:42–8.e1. doi: 10.1016/j.jpeds.2017.01.056
18. Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in US adolescents: results from the national comorbidity survey replication–adolescent supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. (2010) 49(10):980–9. doi: 10.1016/j.jaac.2010.05.017
19. Andlin-Sobocki P, Wittchen H-U. Cost of affective disorders in Europe. Eur J Neurol. (2005) 12(Suppl 1):34–8. doi: 10.1111/j.1468-1331.2005.01195.x
20. Baxter AJ, Scott KM, Vos T, Whiteford HA. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol Med. (2013) 43(5):897–910. doi: 10.1017/S003329171200147X
21. Kessler RC, Merikangas KR. The national comorbidity survey replication (NCS-R): background and aims. Int J Methods Psychiatr Res. (2004) 13(2):60–8. doi: 10.1002/mpr.166
22. Hyland P, Shevlin M, Elklit A, Christoffersen M, Murphy J. Social, familial and psychological risk factors for mood and anxiety disorders in childhood and early adulthood: a birth cohort study using the Danish registry system. Soc Psychiatry Psychiatr Epidemiol. (2016) 51:331–8. doi: 10.1007/s00127-016-1171-1
23. Khanal P, Ståhlberg T, Luntamo T, Gyllenberg D, Kronström K, Suominen A, et al. Time trends in treated incidence, sociodemographic risk factors and comorbidities: a Finnish nationwide study on anxiety disorders. BMC psychiatry. (2022) 22(1):144. doi: 10.1186/s12888-022-03743-3
24. Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr Clin. (2009) 32(3):483–524.
25. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. (2005) 62(6):593–602. doi: 10.1001/archpsyc.62.6.593
26. Moore PS, Mokrova I, Frazier JA, Joseph RM, Santos HP Jr, Dvir Y, et al. Anxiety and depression correlates at age 10 in children born extremely preterm. J Pediatr Psychol. (2021) 46(4):422–32. doi: 10.1093/jpepsy/jsaa118
27. Wright MF. Cyber victimization and perceived stress: linkages to late adolescents’ cyber aggression and psychological functioning. Youth Soc. (2015) 47(6):789–810. doi: 10.1177/0044118X14537088
28. Merikangas KR, He J-P, Burstein M, Swendsen J, Avenevoli S, Case B, et al. Service utilization for lifetime mental disorders in US adolescents: results of the national comorbidity survey–adolescent supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. (2011) 50(1):32–45. doi: 10.1016/j.jaac.2010.10.006
29. Pyhälä R, Wolford E, Kautiainen H, Andersson S, Bartmann P, Baumann N, et al. Self-reported mental health problems among adults born preterm: a meta-analysis. Pediatrics. (2017) 139(4). doi: 10.1542/peds.2016-2690
30. McEwen BS, Gianaros PJ. Stress-and allostasis-induced brain plasticity. Annu Rev Med. (2011) 62:431–45. doi: 10.1146/annurev-med-052209-100430
31. Collender P, Bozack AK, Veazie S, Nwanaji-Enwerem JC, Van Der Laan L, Kogut K, et al. Maternal adverse childhood experiences (ACEs) and DNA methylation of newborns in cord blood. Clin Epigenetics. (2023) 15(1):162. doi: 10.1186/s13148-023-01581-y
32. Madigan S, Wade M, Plamondon A, Maguire JL, Jenkins JM. Maternal adverse childhood experience and infant health: biomedical and psychosocial risks as intermediary mechanisms. J Pediatr. (2017) 187:282–9.e1. doi: 10.1016/j.jpeds.2017.04.052
33. McDonald S, Madigan S, Racine N, Benzies K, Tomfohr L, Tough S. Maternal adverse childhood experiences, mental health, and child behaviour at age 3: the all our families community cohort study. Prev Med. (2019) 118:286–94. doi: 10.1016/j.ypmed.2018.11.013
34. Sroufe LA, Egeland B, Carlson EA, Collins WA. The Development of the Person: The Minnesota Study of Risk and Adaptation from Birth to Adulthood. Guilford Press (2009).
35. Berlin LJ, Appleyard K, Dodge KA. Intergenerational continuity in child maltreatment: mediating mechanisms and implications for prevention. Child Dev. (2011) 82(1):162–76. doi: 10.1111/j.1467-8624.2010.01547.x
36. Englund Enlow M, Englund MM, Egeland B. Maternal childhood maltreatment history and child mental health: mechanisms in intergenerational effects. J Clin Child Adolesc Psychol. (2018) 47(Suppl 1):S47–62. doi: 10.1080/15374416.2016.1144189
37. Egeland B, Bosquet M, Chung AL. Continuities and discontinuities in the intergenerational transmission of child maltreatment: implications for breaking the cycle of abuse. In: Early Prediction and Prevention of Child Abuse: A Handbook (2002). p. 217232.
38. O’shea T, Allred E, Dammann O, Hirtz D, Kuban K, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. (2009) 85(11):719–25. doi: 10.1016/j.earlhumdev.2009.08.060
39. Taylor GL, O’Shea TM. Extreme prematurity: risk and resiliency. Curr Probl Pediatr Adolesc Health Care. (2022) 52(2):101132. doi: 10.1016/j.cppeds.2022.101132
40. O’Shea TM, Register HM, Joe XY, Jensen ET, Joseph RM, Kuban KC, et al. Growth during infancy after extremely preterm birth: associations with later neurodevelopmental and health outcomes. J Pediatr. (2023) 252:40–7.e5. doi: 10.1016/j.jpeds.2022.08.015
41. Venkatesh K, Leviton A, Fichorova R, Joseph R, Douglass L, Frazier J, et al. Prenatal tobacco smoke exposure and neurological impairment at 10 years of age among children born extremely preterm: a prospective cohort. BJOG: Int J Obstet Gynaecol. (2021) 128(10):1586–97. doi: 10.1111/1471-0528.16690
42. Bernstein DP, Fink L, Handelsman L, Foote J. Childhood trauma questionnaire. In: Assessment of Family Violence: A Handbook for Researchers and Practitioners (1998).
43. Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. J Trauma Stress. (2001) 14:843–57. doi: 10.1023/A:1013058625719
44. Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. Reliability and validity of the mini international neuropsychiatric interview for children and adolescents (MINI-KID). J Clin Psychiatry. (2010) 71(3):17393. doi: 10.4088/JCP.09m05305whi
45. Sundrani DP, Roy SS, Jadhav AT, Joshi SR. Sex-specific differences and developmental programming for diseases in later life. Reproduction. Fertil Dev. (2017) 29(11):2085–99. doi: 10.1071/RD16265
46. Sutherland S, Brunwasser SM. Sex differences in vulnerability to prenatal stress: a review of the recent literature. Curr Psychiatry Rep. (2018) 20:1–12. doi: 10.1007/s11920-018-0961-4
47. Bobo WV, Yawn BP, St. Sauver JL, Grossardt BR, Boyd CM, Rocca WA. Prevalence of combined somatic and mental health multimorbidity: patterns by age, sex, and race/ethnicity. J Gerontol Ser A. (2016) 71(11):1483–91.
48. Fava M, Rush AJ, Alpert JE, Balasubramani G, Wisniewski SR, Carmin CN, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR* D report. Am J Psychiatry. (2008) 165(3):342–51. doi: 10.1176/appi.ajp.2007.06111868
49. Kessler RC, Ormel J, Petukhova M, McLaughlin KA, Green JG, Russo LJ, et al. Development of lifetime comorbidity in the world health organization world mental health surveys. Arch Gen Psychiatry. (2011) 68(1):90–100. doi: 10.1001/archgenpsychiatry.2010.180
50. Volirath M, Angst J. Outcome of panic and depression in a seven-year follow-up: results of the Zurich study. Acta Psychiatr Scand. (1989) 80(6):591–6. doi: 10.1111/j.1600-0447.1989.tb03031.x
51. Dvir Y, Ford JD, Hill M, Frazier JA. Childhood maltreatment, emotional dysregulation, and psychiatric comorbidities. Harv Rev Psychiatry. (2014) 22(3):149. doi: 10.1097/HRP.0000000000000014
52. Copeland WE, Angold A, Shanahan L, Costello EJ. Longitudinal patterns of anxiety from childhood to adulthood: the great smoky mountains study. J Am Acad Child Adolesc Psychiatry. (2014) 53(1):21–33. doi: 10.1016/j.jaac.2013.09.017
53. Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. (2012) 21(3):169–84. doi: 10.1002/mpr.1359
54. Brekke HK, van Odijk J, Ludvigsson J. Predictors and dietary consequences of frequent intake of high-sugar, low-nutrient foods in 1-year-old children participating in the ABIS study. Br J Nutr. (2007) 97(1):176–81. doi: 10.1017/S0007114507244460
55. Maftei O, Whitrow M, Davies M, Giles L, Owens J, Moore V. Maternal body size prior to pregnancy, gestational diabetes and weight gain: associations with insulin resistance in children at 9–10 years. Diabetic Med. (2015) 32(2):174–80. doi: 10.1111/dme.12637
56. Olson CM, Strawderman MS, Dennison BA. Maternal weight gain during pregnancy and child weight at age 3 years. Matern Child Health J. (2009) 13:839–46. doi: 10.1007/s10995-008-0413-6
57. Rivera HM, Christiansen KJ, Sullivan EL. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci. (2015) 194.
58. Djelantik A, Kunst A, Van Der Wal M, Smit H, Vrijkotte T. Contribution of overweight and obesity to the occurrence of adverse pregnancy outcomes in a multi-ethnic cohort: population attributive fractions for Amsterdam. BJOG: Int J Obstet Gynaecol. (2012) 119(3):283–90. doi: 10.1111/j.1471-0528.2011.03205.x
59. Gavard JA, Artal R. The association of gestational weight gain with birth weight in obese pregnant women by obesity class and diabetic status: a population-based historical cohort study. Matern Child Health J. (2014) 18:1038–47. doi: 10.1007/s10995-013-1356-0
60. Cirulli F, Musillo C, Berry A. Maternal obesity as a risk factor for brain development and mental health in the offspring. Neuroscience. (2020) 447:122–35. doi: 10.1016/j.neuroscience.2020.01.023
61. Evsyukova II. The impact of maternal obesity and diabetes on fetal brain development (mechanisms and prevention). J Obstet Women’s Dis. (2020) 69(3):33–8. doi: 10.17816/JOWD69333-38
62. Shook LL, Kislal S, Edlow AG. Fetal brain and placental programming in maternal obesity: a review of human and animal model studies. Prenat Diagn. (2020) 40(9):1126–37. doi: 10.1002/pd.5724
63. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. (2006) 444(7121):840–6. doi: 10.1038/nature05482
64. Wenkeová J, Páv J. Release of non-esterified fatty acids from adipose tissue in normal and diabetic rats. Nature. (1959) 184(4693):1147. doi: 10.1038/1841147a0
65. Kong L, Nilsson IA, Brismar K, Gissler M, Lavebratt C. Associations of different types of maternal diabetes and body mass index with offspring psychiatric disorders. JAMA Netw Open. (2020) 3(2):e1920787. doi: 10.1001/jamanetworkopen.2019.20787
66. Dachew BA, Scott JG, Betts K, Mamun A, Alati R. Hypertensive disorders of pregnancy and the risk of offspring depression in childhood: findings from the avon longitudinal study of parents and children. Dev Psychopathol. (2020) 32(3):845–51. doi: 10.1017/S0954579419000944
67. Moylan S, Gustavson K, Øverland S, Karevold EB, Jacka FN, Pasco JA, et al. The impact of maternal smoking during pregnancy on depressive and anxiety behaviors in children: the Norwegian mother and child cohort study. BMC Med. (2015) 13(1):1–12. doi: 10.1186/s12916-014-0257-4
68. DeBry SC, Tiffany ST. Tobacco-induced neurotoxicity of adolescent cognitive development (TINACD): a proposed model for the development of impulsivity in nicotine dependence. Nicotine Tob Res. (2008) 10(1):11–25. doi: 10.1080/14622200701767811
69. Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. (2004) 198(2):132–51. doi: 10.1016/j.taap.2003.06.001
70. Reiss F, Meyrose A-K, Otto C, Lampert T, Klasen F, Ravens-Sieberer U. Socioeconomic status, stressful life situations and mental health problems in children and adolescents: results of the German BELLA cohort-study. PLoS One. (2019) 14(3):e0213700. doi: 10.1371/journal.pone.0213700
71. Zhu Y, Chen X, Zhao H, Chen M, Tian Y, Liu C, et al. Socioeconomic status disparities affect children’s anxiety and stress-sensitive cortisol awakening response through parental anxiety. Psychoneuroendocrinology. (2019) 103:96–103. doi: 10.1016/j.psyneuen.2019.01.008
72. Bitsko RH, Holbrook JR, Ghandour RM, Blumberg SJ, Visser SN, Perou R, et al. Epidemiology and impact of health care provider–diagnosed anxiety and depression among US children. J Dev Behav Pediatr. (2018) 39(5):395. doi: 10.1097/DBP.0000000000000571
73. Spence SH, Najman JM, Bor W, O'Callaghan MJ, Williams GM. Maternal anxiety and depression, poverty and marital relationship factors during early childhood as predictors of anxiety and depressive symptoms in adolescence. J Child Psychol Psychiatry. (2002) 43(4):457–69. doi: 10.1111/1469-7610.00037
74. De Bellis MD. Developmental traumatology: the psychobiological development of maltreated children and its implications for research, treatment, and policy. Dev Psychopathol. (2001) 13(3):539–64. doi: 10.1017/S0954579401003078
75. Brent DA, Oquendo M, Birmaher B, Greenhill L, Kolko D, Stanley B, et al. Familial transmission of mood disorders: convergence and divergence with transmission of suicidal behavior. J Am Acad Child Adolesc Psychiatry. (2004) 43(10):1259–66. doi: 10.1097/01.chi.0000135619.38392.78
76. Collishaw S, Dunn J, O'Connor TG, Golding J, Parents ALSo, Team CS. Maternal childhood abuse and offspring adjustment over time. Dev Psychopathol. (2007) 19(2):367–83. doi: 10.1017/S0954579407070186
77. Plant D, Barker ED, Waters C, Pawlby S, Pariante C. Intergenerational transmission of maltreatment and psychopathology: the role of antenatal depression. Psychol Med. (2013) 43(3):519–28. doi: 10.1017/S0033291712001298
78. Roberts R, O’Connor T, Dunn J, Golding J, Team AS. The effects of child sexual abuse in later family life; mental health, parenting and adjustment of offspring. Child Abuse Negl. (2004) 28(5):525–45. doi: 10.1016/j.chiabu.2003.07.006
79. Colman RA, Widom CS. Childhood abuse and neglect and adult intimate relationships: a prospective study. Child Abuse Negl. (2004) 28(11):1133–51. doi: 10.1016/j.chiabu.2004.02.005
80. Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychol Rev. (1999) 106(3):458. doi: 10.1037/0033-295X.106.3.458
81. Yates TM, Obradović J, Egeland B. Transactional relations across contextual strain, parenting quality, and early childhood regulation and adaptation in a high-risk sample. Dev Psychopathol. (2010) 22(3):539–55. doi: 10.1017/S095457941000026X
82. Enlow MB, Egeland B, Carlson E, Blood E, Wright RJ. Mother–infant attachment and the intergenerational transmission of posttraumatic stress disorder. Dev Psychopathol. (2014) 26(1):41–65. doi: 10.1017/S0954579413000515
83. Liotti G. Trauma, dissociation, and disorganized attachment: three strands of a single braid. Psychother: Theory Res Pract, Train. (2004) 41(4):472. doi: 10.1037/0033-3204.41.4.472
84. Demetriou C, Ozer BU, Essau C. Self-report questionnaires. In: Cautin RL, Lilienfeld SO, editors. The Encyclopedia of Clinical Psychology, 5 Volume Set. John Wiley & Sons (2015).
Keywords: adolescents, preterm, anxiety, depression, socioeconomic status, maternal health
Citation: Jalnapurkar I, Oran A, Frazier JA, Cochran D, Kim S, Jensen E, Joseph R, Hooper SR, Santos H Jr, Jara H, Kuban KCK, Msall ME, Singh R, Washburn L, Gogcu S, Hanson S, Venuti L, Fry RC and O’Shea TM (2024) Maternal and psychosocial antecedents of anxiety and depression in extremely low gestational age newborns at age 15 years. Front. Child Adolesc. Psychiatry 3:1334316. doi: 10.3389/frcha.2024.1334316
Received: 6 November 2023; Accepted: 26 August 2024;
Published: 13 September 2024.
Edited by:
Ujjwal Ramtekkar, University of Missouri, United StatesReviewed by:
Vito Giordano, Medical University of Vienna, AustriaTiia Ståhlberg, University of Turku, Finland
Copyright: © 2024 Jalnapurkar, Oran, Frazier, Cochran, Kim, Jensen, Joseph, Hooper, Santos, Jara, Kuban, Msall, Singh, Washburn, Gogcu, Hanson, Venuti, Fry and O'Shea. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isha Jalnapurkar, aXNoYS5qYWxuYXB1cmthckB1bWFzc21lbW9yaWFsLm9yZw==
 Isha Jalnapurkar
Isha Jalnapurkar Ali Oran2
Ali Oran2 Jean A. Frazier
Jean A. Frazier David Cochran
David Cochran Sohye Kim
Sohye Kim Elizabeth Jensen
Elizabeth Jensen Robert Joseph
Robert Joseph Stephen R. Hooper
Stephen R. Hooper Hudson Santos Jr
Hudson Santos Jr Hernan Jara
Hernan Jara Michael E. Msall
Michael E. Msall Rachana Singh
Rachana Singh Shannon Hanson
Shannon Hanson Rebecca C. Fry
Rebecca C. Fry