- 1Lady Davis Institute, The Jewish General Hospital, Montreal, QC, Canada
- 2Department of Psychiatry, McGill University, Montreal, QC, Canada
- 3Department of Social and Behavioral Science, Harvard T. H. Chan School of Public Health, Boston, MA, United States
- 4Department of Child and Adolescent Psychiatry and Psychology, Erasmus MC University Medical Center, Rotterdam, Netherlands
- 5Centre for Academic Mental Health, Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, United Kingdom
- 6Department of Psychology, Manchester Metropolitan University, Manchester, United Kingdom
- 7Department of Psychology, University of Oslo, Oslo, Norway
- 8University Institute of Psychological, Social and Life Sciences, Lisbon, Portugal
- 9Psychiatry Monash Health, Monash University, Melbourne, VIC, Australia
- 10Research Department of Clinical, Educational and Health Psychology, UCL, London, United Kingdom
Parenting is a key contributor to child development. The effects of parenting, however, also depend on child characteristics, including genetic factors. A more complete appraisal of the role of parenting thus requires a comprehensive developmental model which explores questions about parenting behavior, child susceptibility to parenting, and child psychopathology. Moving forward, we need to not only be concerned about sample sizes that limit testing of comprehensive models but also the need to replicate findings across multiple settings and samples. A consortium which harmonises key measures offers the opportunity to examine these questions. The Developmental Research in Environmental Adversity, Mental health, BIological susceptibility and Gender (DREAM BIG) consortium includes six international longitudinal prospective birth cohorts to explore the early life origins of major psychiatric disorders in childhood. Here, we will provide a brief overview of parental care research, methodological limitations, and two exciting recent attempts (i.e., the DREAM BIG consortium and the CATS-project), that address key methodological challenges.
The context: the importance of parental care
Humans are among the most helpless of species at birth and they remain dependent on their parents for a long time before being able to navigate the world independently. Parental care has thus direct consequences for children's survival, growth, and psychosocial development (1). The early caregiving environment supplies young children with the necessary experiences and support for achieving their developmental milestones (2), and it plays a key role in shaping children's social-emotional and cognitive development (3). Established models of parenting postulate that the quality of the parent–child relationship is the integrated product of three broad factors: parental characteristics, infant characteristics, and context which may influence parenting in a supportive or stressful way (4, 5). Parent-infant interactions encompass a diverse range of dyadic processes, among which the most heavily investigated construct is maternal sensitivity, or the mother's ability to accurately perceive and interpret their infants' signals and respond to them in a prompt and appropriate manner (6–8). Decades of research on maternal sensitivity have provided evidence on its link with numerous domains of child development, including social adjustment (9), executive functioning (10), cognitive and language outcomes (11, 12), and, not the least, children's attachment relationships (13, 14).
Environmental sensitivity
Children respond differentially to parenting though with genetic and prenatal environmental factors contributing to an increased sensitivity to the environment called postnatal plasticity (15). While Belsky documented that this plasticity (measured as temperament) emerges from genetic factors (differential susceptibility) (16, 17), and Boyce and Ellis posited that this susceptibility would be rather environmentally induced, (16), both show that plasticity factors influence how individuals interact with the environment. Three patterns of environmental sensitivity have been described in the literature: diathesis-stress, differential susceptibility, and vantage sensitivity (17, 18). In diathesis-stress, a biological marker represents a disadvantage in unfavourable environments in that outcomes for carriers of that marker can only be approaching the outcome levels of noncarriers if the individual is exposed to average or advantageous environments. In vantage sensitivity, the opposite is true: the biological marker represents an advantage over noncarriers, such that in unfavorable environments carriers and noncarriers develop similarly, while carriers show increasingly better outcomes as the environment becomes more advantageous. Finally, in differential susceptibility, highly susceptible children are more responsive to both adverse and supportive environments than non-susceptible children for better or worse (15).
Gene-environment interplay
Surprisingly, few studies have examined the associations between genetic risk, prenatal adversity, and maternal care in predicting child psychological functioning, even though the quality of early parental care can be a crucial mitigating factor of the effect of prenatal environmental or genetic risk (19). In a three-way interaction model, we found that maternal looking away behaviour (negatively correlated with maternal sensitivity) moderates the risk associated with prenatal depression and the 5-HTTLPR genotype to predict depressive symptoms at 18 months, but not at 24 months (20). Similarly, we have reported that maternal looking away behaviour moderates the developmental risk from low birthweight and DRD4 to predict disorganized attachment (21). These findings suggest that, in children with genetic and prenatal risk, the risk for psychopathology was attenuated when the mother looked away less frequently. These findings are in line with previous studies reporting that the frequency of self-reported maternal stroking during early infancy moderated the effect of pregnancy anxiety on internalizing problems when the children were 3.5 years of age (22). For mothers who experienced high levels of pregnancy-specific anxiety, high levels of postnatal stroking were related to lower internalizing scores in their children. Similar results of a moderating role of maternal stroking on child internalizing problems have also been reported for prenatal maternal depression (23) and general anxiety (24). Finally, in our recent study in the Maternal Adversity, Vulnerability and Neurodevelopment (MAVAN) sample, we found evidence for the presence of two-way interaction effects on toddler attention function, namely that positive maternal behaviors observed during mother-child interactions at 6 months postpartum mitigated the effects of both prenatal adversity and dopaminergic polygenic risk on toddler attention function (25). However, our sample was limited to find a significant three-way interaction between prenatal adversity, dopaminergic risk, and parenting behavior, and the above two-way interaction effects need to be replicated in independent samples.
Epigenetic processes
Fresh perspectives in understanding the complexities of the parent-child dynamics are also offered by behavioral epigenetic studies, which posit that the quality of maternal care sets epigenetic processes (e.g., DNA methylation) in motion that may ultimately affect offspring psychological development through modifying expression of genes involved in behavioral and stress regulation (e.g., NR3C1, BDNF, OXTR) (26). For example, harsh parenting contributes to similar epigenetic modifications in the child as early adversity, potentially affecting cognitive and socioemotional development in childhood (27, 28) and attachment style in adulthood (29). Importantly, epigenetic modifications are also affected by positive parent-child interactions (30, 31), translating into “positive” epigenetic mechanisms, which may act as a protective mechanism in the face of adversity-related increased DNA methylation of genes involved in behavioral and stress regulation (32, 33).
The problem: limitations of current parenting research
The replication problem
The replication crisis in science (34) is exemplified in psychological research, where the replication rate of key experiments is just above 10% (35). One reason for the lack of replication is that cohorts do not always assess the same developmental constructs, and, even when they do, they often use different measures to assess them. In addition, without the initial registration of research hypotheses and analytic plans (e.g., in the Open Science Framework) of observational studies, akin to that found with randomized controlled trials (e.g., www.clinicaltrials.gov), it is not often clear which findings (a priori vs. post hoc) are most likely to be replicated. For clinical trials, there are broadly endorsed initiatives of prospective harmonization of outcome measures, such as the COMET initiative (https://www.comet-initiative.org/) and the CROWN initiative (http://www.crown-initiative.org/). For already existing observational studies, the retrospective harmonization of relevant predictor and outcome variables across cohorts with similar measures may prove essential in producing replicable and generalizable research findings. As well, pre-registering planned correlational analyses in intentional initiatives to replicate findings will make for more convincing results.
Measurement error
Gathering detailed observational data on parent-child interaction in large epidemiological cohorts is costly and unfeasible. However, complex developmental models that account for the interplay of genetic and pre- and postnatal environmental influences are incomplete without including precise measures on the quality of parental care. Measurement error can reduce statistical power for detecting true interaction effects in complex developmental models, as it inflates the variance of the estimate of the interaction term, similarly to multi-collinearity and non-normal distribution of the interaction terms (36–38). Observational measures of mother-child interactions are thus strongly preferred to self-report measures of parenting in studying complex developmental models in longitudinal cohorts. Observational measures, due to their complexity and cost, restrict potential sizes of epidemiological developmental cohorts. Thus, the need for larger sample sizes and valid cross-study comparisons, has led to increased interest in co-analyzing already existing data across studies. However, heterogeneity in study design and measures collected limit our capacity to easily compare or integrate data across studies (maelstrom-research.org) (39).
Small sample sizes
To be truly informative, birth cohorts, particularly those with genetic data, require large samples to test complex computational models of developmental trajectories (40, 41). While harmonization of key predictor and outcome variables across multiple birth cohort studies will greatly assist in overcoming the replication problem, a priori or post-hoc harmonization of parenting measures will also help overcome the problem of small sample sizes, that are typical of focus cohorts of large epidemiological samples, where observational measures of parenting are available (42). Combining focus cohorts from multiple large birth cohorts with harmonized parenting data can increase sample size to levels sufficient to conduct tests of complex models.
Aim of the present paper
The present paper discusses the relevance of a key methodological concept (i.e., retrospective data harmonization), which can help mitigate some of the challenges inherent in replicating study findings involving observational parent-child interaction measures. More specifically, this paper offers two valuable approaches to researchers who are interested in retrospective harmonization and integration of parent-child interaction data across independent samples.
The first approach comes from our ongoing initiative, the DREAM BIG consortium, which performs cross-cohort retrospective data harmonization of key constructs relevant to probe complex models of child development (e.g., the prenatal environment, genetic susceptibility, child psychopathology, and early parental care). The second strategy, as used in the CATS-project, focuses on the initial stages of retrospective data harmonization of observed maternal sensitivity. This method first evaluates the theoretical constructs underlying the measures and then the measures themselves prior to the recoding of original values. The DREAM BIG and CATS approaches offer helpful analytical solutions for restructuring parent-child interaction data collected with different instruments across multiple studies to indicate a comparable construct.
A proposed innovative solution: the DREAM BIG consortium as a model of cross-cohort data harmonization of child developmental constructs
The Developmental Research in Environmental Adversity, Mental health, BIological vulnerability, and Gender (DREAM BIG) research consortium was established in 2016 to examine, in a multi-site design, the developmental origins of major mental disorders (www.dreambigresearch.com). DREAM BIG includes six prospective prenatal cohorts: Avon Longitudinal Study of Parents and Children (ALSPAC, UK) (43); Generation R Study (GEN-R, Netherlands) (44); Maternal Adversity, Vulnerability and Neurodevelopment (MAVAN) project (Canada) (45); Mother, Father and Child Cohort (MoBa, Norway) (46); Prediction and prevention of preeclampsia and intrauterine growth restriction (PREDO) study (Finland) (47); and Growing Up in Singapore Towards Healthy Outcomes (GUSTO) cohort (Singapore) (48). These cohorts have comparable measures on prenatal adversity—including prenatal maternal psychopathology and prenatal environmental adversity—genetic data, observed and self-reported early parental care and parent-child interactions, and child psychopathology. Our work thus far supports the hypothesis that prenatal maternal psychopathology, social-environmental adversity, and child genetic susceptibility for multiple psychiatric disorders and psychological traits predict emerging general and internalizing (e.g., depression and anxiety) psychopathology in 4-to-8-year-olds (49, 50).
Harmonization of major constructs within DREAM BIG
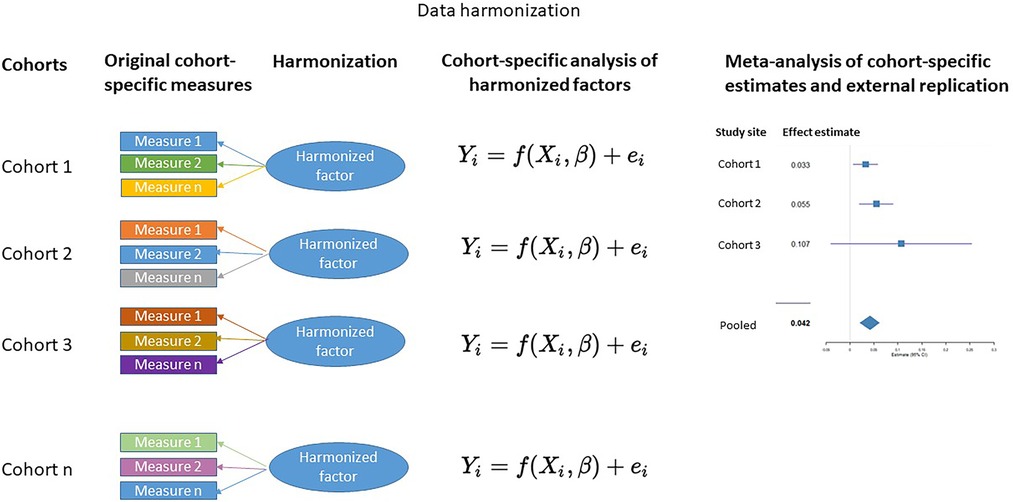
To date, DREAM BIG has harmonized measures of prenatal adversity and genetic susceptibility, and created cross-diagnostic and hierarchical harmonized measures of child psychopathology by integrating information across multiple informants at multiple time points (Figure 1). A brief description of these measures are provided below and a summary of the main findings to date are presented in the Supplementary Material Table S1.
Prenatal social-environmental adversity
This is a harmonized prenatal cumulative risk index derived from four major areas: stressful life events (i.e., death in family, accident, illness), contextual risks (i.e., poor housing conditions, financial problems), parental risks (i.e., alcohol and substance abuse, criminal involvement), and interpersonal risks (i.e., family conflict, domestic violence) (51, 52) using confirmatory factor analysis with a second-order hierarchical model.
Prenatal maternal affective symptoms
This is a set of harmonized prenatal maternal psychological symptoms constructed using confirmatory factor analyses that identified a general prenatal affective symptoms factor and three specific factors: anxiety/depression; somatic symptoms; and pregnancy-specific anxieties across cohorts (50). These prenatal maternal affective symptoms factors predicted offspring psychopathology at age 4–8 years in a meta-analysis of three cohorts (50).
Both measures of adversity (i.e., prenatal social- environmental and maternal affective) build on previous successful harmonization initiatives between ALSPAC and Generation R (51, 52). DREAM BIG innovated by separating these two measures.
Childhood psychopathology
This includes a harmonized, age-adjusted, general psychopathology factor (P-factor), and specific uncorrelated internalizing and externalizing factors consistent with the Hierarchical Taxonomy of Psychopathology (HiTOP) model (53), constructed using psychopathology measures rated by different informants (parent, child, teacher) at multiple time points between the ages of 4 and 8 years (54). A HiTOP approach to harmonizing psychopathology addresses important methodological concerns about diagnostic co-morbidity, homotypic and heterotypic discontinuities, and rater differences (53, 55).
Genetic susceptibility
Polygenic scores (PGS) for internalizing (anxiety, depression), neurodevelopmental (ADHD, ASD), psychotic (schizophrenia, bipolar), and compulsive problems (anorexia nervosa, obsessive-compulsive, Tourette syndrome) will be computed in each cohort based on results of a Genomic Structural Equation Model (GenomicSEM) of 11 common psychiatric disorders using publicly available GWAS summary statistics (56). This approach models the structure of psychopathology at the genomic level and exploits genetic correlations between multiple psychiatric disorders modeled simultaneously. Further details about the GenomicSEM approach are available elsewhere (56).
Maternal care
Four of the six DREAM BIG cohorts have observational measures available on maternal sensitivity and parent-child interactions. In MAVAN, maternal care and mother-child interactions were observed during free play using the Parent-Child Early Relational Assessment (PCERA) (57) at 6, 18, 36 and 60 months, the Ainsworth Maternal Sensitivity Scales (6) at 6 and 18 months, and the Behavioral Evaluation Strategies and Taxonomies (BEST) (Educational Consulting, Inc. Florida, US; S & K NorPark Computer Design, Toronto) at 6 months. In Generation R, maternal sensitivity was observed during free play using two subscales of the Ainsworth Maternal Sensitivity Scales at 14 months (sensitivity and cooperation), and during two structured mother-child interaction tasks using the revised Erickson 7-point rating scales for supportive presence and intrusiveness (58) at 36 and 48 months in a subsample (n = 1,079). In GUSTO, maternal sensitivity was observed during free play using the Revised Mini-A short form of the Maternal Behavioral Q-Sort-V (Mini-MBQS-V) (59) at 6 months, and during a structured mother-child interaction using the Erickson 7-point rating scale at 54 months. Finally, in a subsample (N = 1,240) of the ALSPAC cohort, the Mellow Parenting Observational System (60) was used to code mother-child interactions during the Thorpe Interaction Measure (61) at 12 months.
Our current work in DREAM BIG will also harmonize the measures of observed maternal sensitivity across cohorts to test for the presence of replicable two-way and three-way interactions between prenatal adversity, child genetic susceptibility, and early maternal sensitivity on the development of child mental health problems. Integrating both harmonized parenting and child measures allows for the inclusion of complex questions assessing a wide range of well-defined observable parental care measures.
The CATS-project as a solution for the initial stages of retrospective harmonization of observed maternal sensitivity
Assessing the nature of dyadic dynamic processes, such as maternal sensitivity, is challenging, and brings critical attention to the core issue of assessment (26, 62, 63). The Collaboration on Attachment Synthesis CATS-project is a multi-site meta-analytic study focusing on synthesizing the literature regarding the association between parental cognitive representation of attachment and the child-parent attachment relationship (64). The strategy pertains to the early stages of data harmonization and can be applied to observational measures of maternal sensitivity. This three-step method includes a top-down approach to evaluating the theoretical constructs underlying the measures and a bottom-up approach to evaluating the measures prior to recoding the values (Figure 2) (64).
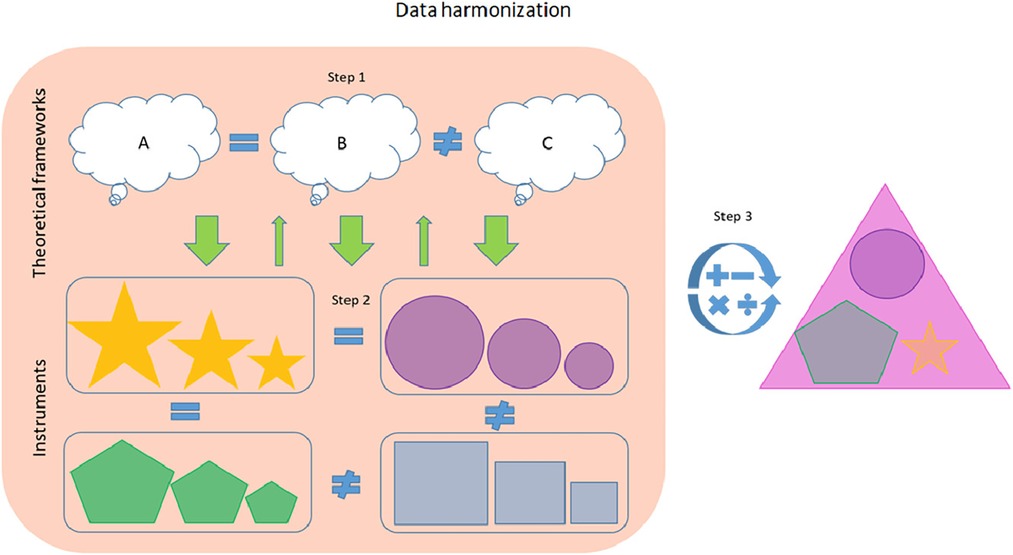
Figure 2. Initial stages of retrospective data harmonization using the CATS-project. Source: Graphical abstract from Verhage et al. (65).
The first step represents a top-down strategy of defining a unitary construct by reviewing the existing literature screening for one or more dominant developmental framework(s). The second step is a bottom-up strategy whereby each instrument is evaluated against the theoretical frameworks identified in the first step. Here the researchers assess which theoretical subdimensions are measured by which subscale or item(s) and decide which (sub)scales or items to retain. The final step entails the recoding of scores to an identical metric based on the existing literature. So, in the case of parental sensitivity, the authors' search of the literature (step 1) indicated that the construct of parental sensitivity is derived from the attachment theory framework. The individual studies in the CATS database included eight different measures of parental sensitivity in total, which then needed to be evaluated (step 2) against the construct of maternal sensitivity derived in step 1. Finally, the authors recalculated the scores (step 3) of all the instruments to match the reference scale of one of the available instruments, the Ainsworth sensitivity scale, which is considered “gold standard” for measuring parental sensitivity (64). The authors recommend their method to be used in conjunction with the existing literature on the restructuring of measurements from different instruments into the same format (i.e., the later stages of the harmonization process) before analysis.
This exciting new strategy is filling a gap in the literature on data harmonization by providing researchers with a tool for pooling, amongst others, observational data on parental behaviors. However, this approach, which could be expanded to other predictor and/or outcome measures, is facilitated greatly by the development of new large scale epidemiological cohort studies in which predictor and outcome measures are harmonized before study onsets.
Limitations
As inherent in all retrospective data harmonization techniques, the two approaches presented here are also subject to limitations including the complexity and necessity of expert domain knowledge to pool data, and the possibility that, despite best efforts, some data may not be comparable across cohorts due to, for instance, gross heterogeneity in measures. When the available data are not comparable, there is risk of data loss (66). Moreover, and also inherent to data harmonization, details with regard to the observational context can get lost. For example, parental sensitivity can be observed during free play, unstructured home observations, or stress-inducing tasks in a laboratory. Such observational contextual information can be included in analyses as potential moderating factors, but type of context and measure may be confounded when specific measures are only used in specific contexts.
Implications
Harmonization and replication of complex models improve our ability to detect and understand methodologically robust and key nodes of environmental influences in children's social-emotional development. Given the rich primary and secondary intervention literature aimed at the modification of early parental care and parent-child interactions, more precise identification of susceptibility to and effects of these interactions, will additionally inform public health and primary care practices. For example, harmonized indicators of observed parent-child interaction may be used in the assessment, selection of target behaviors, intervention, and monitoring the effect of the intervention in the treatment of families with mental health problems (67).
Conclusions
The CATS approach contributes to an important avenue of the initial stages of retrospective harmonization, while the DREAM BIG approach focuses on the later stages of retrospective data harmonization of developmental and parenting research to overcome the replication problem. Both approaches can be applied, in a complementary manner, to the cross-cohort harmonization of observed mother-child interaction data. In conclusion, the above strategies offer helpful analytical solutions for restructuring data collected with different instruments across multiple studies to the same format. Further, the DREAM BIG model of harmonization and replication permits the following questions about the impact of parenting on development across the lifespan to be addressed: (i) What aspects of parental care are most important?; (ii) During what phase of development?; (iii) Which children are at highest risk?; and (iv) For what kind of outcomes?
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval has been granted by each respective DREAM BIG cohort institution for the studies conducted within the DREAM BIG consortium. For this publication, no direct access to research data was required.
Author contributions
ES, DL, HT, JE, RP, MB, MB-K, MI and AW: have made substantial contributions to the conception or design of the work, in the creation of the consortium, acquisition of data and harmonization of constructs, have drafted the work or revised it critically for important intellectual content, provided approval for publication of the content, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Funding
This research was made possible by the Canadian Institutes of Health Research (CIHR; grants MWG 146 330, MOP 111251, PJT-148721, UIP-179221, WI2-179938, PJT-185872, EDC-186603), the Fonds de la recherche en sante du Quebec (FRSQ; grant 22418 and 331904), and the March of Dimes Foundation (grant 12- FY12-198).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors RP, HT and ES declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frcha.2023.1206922/full#supplementary-material
References
1. Rogers FD, Bales KL. Mothers, fathers, and others: neural substrates of parental care. Trends Neurosci. (2019) 42(8):552–62. doi: 10.1016/j.tins.2019.05.008
2. Brinker RP, Seifer R, Sameroff AJ. Relations among maternal stress, cognitive development, and early intervention in middle- and low-SES infants with developmental disabilities. Am J Ment Retard. (1994) 98(4):463–80. Available at: https://europepmc.org/article/med/8148123 (Cited Apr 13, 2023).8148123
3. Landry SH, Smith KE, Swank PR. Responsive parenting: establishing early foundations for social, communication, and independent problem-solving skills. Dev Psychol. (2006) 42(4):627–42. doi: 10.1037/0012-1649.42.4.627
4. Taraban L, Shaw DS. Parenting in context: revisiting belsky’s classic process of parenting model in early childhood. Dev Rev. (2018) 48:55–81. doi: 10.1016/j.dr.2018.03.006
5. Belsky J. The determinants of parenting: a process model. Child Dev. (1984) 55(1):83–96. doi: 10.2307/1129836
7. Deans CL. Maternal sensitivity, its relationship with child outcomes, and interventions that address it: a systematic literature review. Early Child Dev Care. (2020) 190(2):252–75. doi: 10.1080/03004430.2018.1465415
8. Bilgin A, Wolke D. Maternal sensitivity in parenting preterm children: a meta-analysis. Pediatrics. (2015) 136(1):e177–93. doi: 10.1542/peds.2014-3570
9. Stams GJJM, Juffer F, van IJzendoorn MH. Maternal sensitivity, infant attachment, and temperament in early childhood predict adjustment in middle childhood: the case of adopted children and their biologically unrelated parents. Dev Psychol. (2002) 38(5):806–21. doi: 10.1037/0012-1649.38.5.806
10. Bernier A, Carlson SM, Whipple N. From external regulation to self-regulation: early parenting precursors of young children’s executive functioning. Child Dev. (2010) 81(1):326–39. doi: 10.1111/j.1467-8624.2009.01397.x
11. Lemelin JP, Tarabulsy GM, Provost MA. Predicting preschool cognitive development from infant temperament, maternal sensitivity, and psychosocial risk. Merrill-Palmer quarterly. J Dev Psychol. (2006) 52(4):779–806. doi: 10.1353/mpq.2006.0038
12. Hirsh-Pasek K, Burchinal M. Mother and caregiver sensitivity over time: predicting language and academic outcomes with variable- and person-centered approaches. Merrill Palmer Q. (2006) 52(3):449–85 doi: 10.1353/mpq.2006.0027
13. Verhage ML, Schuengel C, Madigan S, Pasco Fearon RM, Oosterman M, Cassibba R, et al. Narrowing the transmission gap: a synthesis of three decades of research on intergenerational transmission of attachment. Psychol Bull. (2016) 142(4):337–66. doi: 10.1037/bul0000038
14. Madigan S, Deneault AA, Duschinsky R, Bakermans-Kranenburg MJ, Schuengel C, van IJzendoorn Marinus H, et al. Parent sensitivity and child attachment: a meta-analysis revisited. Psychol Bull. (2023) 149(1–2):99–132. doi: 10.1037/bul0000388
15. Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary–neurodevelopmental theory. Dev Psychopathol. (2011) 23(1):7–28. doi: 10.1017/S0954579410000611
16. Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. (2005) 17(2):271–301. doi: 10.1017/S0954579405050145
17. Pluess M, Belsky J. Differential susceptibility to rearing experience: the case of childcare. J Child Psychol Psychiatry. (2009) 50(4):396–404. doi: 10.1111/j.1469-7610.2008.01992.x
18. Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary–developmental theory. Dev Psychopathol. (2005) 17(2):303–28. doi: 10.1017/S0954579405050157
19. Cost K, McGowan P, Pawluski J. Gestational stress and parenting: A review of human and animal literature. In: Wazana A, Székely E, Oberlander TF, editors. Prenatal stress and child development. Cham: Springer (2021) p. 317–49. doi: 10.1007/978-3-030-60159-1_12
20. Green CG, Babineau V, Jolicoeur-Martineau A, Bouvette-Turcot AA, Minde K, Sassi R, et al. Prenatal maternal depression and child 5-HTTLPR and DRD4 genotype predict negative emotionality from 3 to 36 months. Dev Psychopathol. (2017) 29(3):901. doi: 10.1017/S0954579416000560
21. Wazana A, Moss E, Jolicoeur-Martineau A, Graffi J, Tsabari G, Lecompte V, et al. The interplay of birth weight, dopamine receptor D4 gene (DRD4), and early maternal care in the prediction of disorganized attachment at 36 months of age. Dev Psychopathol. (2015) 27(4pt1):1145–61. doi: 10.1017/S0954579415000735
22. Pickles A, Sharp H, Hellier J, Hill J. Prenatal anxiety, maternal stroking in infancy, and symptoms of emotional and behavioral disorders at 3.5 years. Eur Child Adolesc Psychiatry. (2017) 26(3):325–34. doi: 10.1007/s00787-016-0886-6
23. Sharp H, Pickles A, Meaney M, Marshall K, Tibu F, Hill J. Frequency of infant stroking reported by mothers moderates the effect of prenatal depression on infant behavioural and physiological outcomes. PLoS One. (2012) 7(10):e45446. doi: 10.1371/journal.pone.0045446
24. Sharp H, Hill J, Hellier J, Pickles A. Maternal antenatal anxiety, postnatal stroking and emotional problems in children: outcomes predicted from pre- and postnatal programming hypotheses. Psychol Med. (2015) 45(2):269–83. doi: 10.1017/S0033291714001342
25. Szekely E, Jolicoeur-Martineau A, Atkinson L, Levitan RD, Steiner M, Lydon JE, et al. The interplay between prenatal adversity, offspring dopaminergic genes, and early parenting on toddler attentional function. Front Behav Neurosci. (2021) 15:701971. doi: 10.3389/fnbeh.2021.701971
26. Provenzi L, di Minico GS, Giusti L, Guida E, Müller M. Disentangling the dyadic dance: theoretical, methodological and outcomes systematic review of mother-infant dyadic processes. Front Psychol. (2018) 9(MAR):348. doi: 10.3389/fpsyg.2018.00348
27. Bueno D. Epigenetics and learning: how the environment shapes gene expression, and the possible consequences for learning and behaviour. (2021). Available at: https://solportal.ibe-unesco.org/articles/epigenetics-and-learning-how-the-environment-shapes-gene-expression-and-the-possible-consequences-for-learning-and-behaviour/ (Cited Jun 8 2023)
28. Lewis CR, Breitenstein RS, Henderson A, Sowards HA, Piras IS, Huentelman MJ, et al. Harsh parenting predicts novel HPA receptor gene methylation and NR3C1 methylation predicts cortisol daily slope in middle childhood. Cell Mol Neurobiol. (2021) 41(4):783–93. doi: 10.1007/s10571-020-00885-4
29. Ein-Dor T, Verbeke WJMI, Mokry M, Vrtička P. Epigenetic modification of the oxytocin and glucocorticoid receptor genes is linked to attachment avoidance in young adults. Attach Hum Dev. (2018) 20(4):439–54. doi: 10.1080/14616734.2018.1446451
30. Jensen Peña CL, Champagne FA. Epigenetic and neurodevelopmental perspectives on variation in parenting behavior. Parent Sci Pract. (2012) 12(2–3):202–11. doi: 10.1080/15295192.2012.683358
31. Barreto-Zarza F, Arranz-Freijo EB. Family context, parenting and child development: an epigenetic approach. Soc Sci (2022). 11(3):113. doi: 10.3390/socsci11030113
32. Naumova OY, Hein S, Suderman M, Barbot B, Lee M, Raefski A, et al. Epigenetic patterns modulate the connection between developmental dynamics of parenting and offspring psychosocial adjustment. Child Dev. (2016) 87(1):98–110. doi: 10.1111/cdev.12485
33. Kundakovic M, Champagne FA. Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology. (2015) 40(1):141–53. doi: 10.1038/npp.2014.140
34. Schooler JW. Metascience could rescue the “replication crisis”. Nature. (2014) 515(7525):9. doi: 10.1038/515009a
35. Aarts AA, Anderson JE, Anderson CJ, Attridge PR, Attwood A, Axt J, et al. Estimating the reproducibility of psychological science. Science. (1979) 349(6251):253–67. doi: 10.1126/science.aac4716
36. Jaccard J, Turrisi R. Interaction effects in multiple regression. 2nd edn. Framework (2003). p. 1–92. Available at: https://books.google.com/books/about/Interaction_Effects_in_Multiple_Regressi.html?id=n0pIZTQqvmIC (Cited Jun 18 2023)
37. Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. (2013) 36(1):27–46. doi: 10.1111/j.1600-0587.2012.07348.x
38. Wong MY, Day NE, Luan JA, Chan KP, Wareham NJ. The detection of gene-environment interaction for continuous traits: should we deal with measurement error by bigger studies or better measurement? Int J Epidemiol. (2003) 32(1):51–7. doi: 10.1093/ije/dyg002
39. Maelstrom Research. Maelstrom research. Data processing methods (n.d.). Available at: https://www.maelstrom-research.org/page/data-processing-methods
40. Burton PR, Hansell AL, Fortier I, Manolio TA, Khoury MJ, Little J, et al. Size matters: just how big is BIG? Quantifying realistic sample size requirements for human genome epidemiology. Int J Epidemiol. (2009) 38(1):263–73. doi: 10.1093/ije/dyn147
41. Fortier I, Doiron D, Little J, Ferretti V, L'Heureux F, Stolk RP, et al. Is rigorous retrospective harmonization possible? Application of the DataSHaPER approach across 53 large studies. Int J Epidemiol. (2011) 40(5):1314–28. doi: 10.1093/ije/dyr106
42. Schmidt RA, Wey TW, Harding KD, Fortier I, Atkinson S, Tough S, et al. A harmonized analysis of five Canadian pregnancy cohort studies: exploring the characteristics and pregnancy outcomes associated with prenatal alcohol exposure. BMC Pregnancy Childbirth. (2023) 23(1):128. doi: 10.1186/s12884-023-05447-2
43. Golding G, Pembrey P, Jones J. ALSPAC–the avon longitudinal study of parents and children. I. Study methodology. Paediatr Perinat Epidemiol. (2001) 15(1):74–87. doi: 10.1046/j.1365-3016.2001.00325.x
44. Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IJzendoorn MH, et al. The generation R study: design and cohort update 2017. Eur J Epidemiol. (2016) 31(12):1243–64. doi: 10.1007/s10654-016-0224-9
45. O’Donnell KA, Gaudreau H, Colalillo S, Steiner M, Atkinson L, Moss E, et al. The maternal adversity, vulnerability and neurodevelopment project: theory and methodology. Can J Psychiatry. (2014) 59(9):497–508. doi: 10.1177/070674371405900906
46. Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, et al. Cohort profile update: the Norwegian mother and child cohort study (MoBa). Int J Epidemiol. (2016) 45(2):382–8. doi: 10.1093/ije/dyw029
47. Girchenko P, Lahti M, Tuovinen S, Savolainen K, Lahti J, Binder EB, et al. Cohort profile: prediction and prevention of preeclampsia and intrauterine growth restriction (PREDO) study. Int J Epidemiol. (2017) 46(5):1380–1g. doi: 10.1093/ije/dyw154
48. Soh SE, Saw SM, Tint MT, Chong YS, Gluckman PD, Rifkin-Graboi A, et al. Cohort profile: growing up in Singapore towards healthy outcomes (GUSTO) birth cohort study. Int J Epidemiol. (2014) 43(5):1401–9. doi: 10.1093/ije/dyt125
49. Neumann A, Jolicoeur-Martineau A, Szekely E, Sallis HM, O’Donnel K, Greenwood CMT, et al. Combined polygenic risk scores of different psychiatric traits predict general and specific psychopathology in childhood. J Child Psychol Psychiatry. (2022) 63(6):636–45. doi: 10.1111/jcpp.13501
50. Szekely E, Neumann A, Sallis H, Jolicoeur-Martineau A, Verhulst FC, Meaney MJ, et al. Maternal prenatal mood, pregnancy-specific worries, and early child psychopathology: findings from the DREAM BIG consortium. J Am Acad Child Adolesc Psychiatry. (2021) 60(1):186–97. doi: 10.1016/j.jaac.2020.02.017
51. Cecil CAM, Lysenko LJ, Jaffee SR, Pingault JB, Smith RG, Relton CL, et al. Environmental risk, oxytocin receptor gene (OXTR) methylation and youth callous-unemotional traits: a 13-year longitudinal study. Mol Psychiatry. (2014) 19(10):1071–7. doi: 10.1038/mp.2014.95
52. Rijlaarsdam J, Pappa I, Walton E, Bakermans-Kranenburg MJ, Mileva-Seitz VR, Rippe RCA, et al. An epigenome-wide association meta-analysis of prenatal maternal stress in neonates: a model approach for replication. Epigenetics. (2016) 11(2):140–9. doi: 10.1080/15592294.2016.1145329
53. Kotov R, Waszczuk MA, Krueger RF, Forbes MK, Watson D, Clark LA, et al. The hierarchical taxonomy of psychopathology (HiTOP): a dimensional alternative to traditional nosologies. J Abnorm Psychol. (2017) 126(4):454–77. doi: 10.1037/abn0000258
54. Sallis H, Szekely E, Neumann A, Jolicoeur-Martineau A, van IJzendoorn M, Hillegers M, et al. General psychopathology, internalising and externalising in children and functional outcomes in late adolescence. J Child Psychol Psychiatry. (2019) 60(11):1183–90. doi: 10.1111/jcpp.13067
55. Ruggero CJ, Kotov R, Hopwood CJ, First M, Clark LA, Skodol AE, et al. Integrating the hierarchical taxonomy of psychopathology (HiTOP) into clinical practice. J Consult Clin Psychol. (2019) 87(12):1069–84. doi: 10.1037/ccp0000452
56. Grotzinger AD, Mallard TT, Akingbuwa WA, Ip HF, Adams MJ, Lewis CM, et al. Genetic architecture of 11 major psychiatric disorders at biobehavioral, functional genomic and molecular genetic levels of analysis. Nat Genet. (2022) 54(5):548–59. doi: 10.1038/s41588-022-01057-4
57. Clark R. The parent-child early relational assessment: Instrument and manual. Madison: University of Wisconsin Medical School, Department of Psychiatry (1985).
58. Egeland B. Revised erickson scales: 24 month tools coding manual. Project STEEP-revised 1990. From mother-child project scales 1978. Minneapolis, MN: University of Minnesota, Department of Psychology (1990).
59. Tarabulsy GM, Provost MA, Bordeleau S, Trudel-Fitzgerald C, Moran G, Pederson DR, et al. Validation of a short version of the maternal behavior Q-set applied to a brief video record of mother–infant interaction. Infant Behav Dev. (2009) 32(1):132–6. doi: 10.1016/j.infbeh.2008.09.006
60. Puckering C, Allely CS, Doolin O, Purves D, McConnachie A, Johnson PCD, et al. Association between parent-infant interactions in infancy and disruptive behaviour disorders at age seven: a nested, case-control ALSPAC study. BMC Pediatr. (2014) 14(1):223. doi: 10.1186/1471-2431-14-223
61. Thorpe K, Rutter M, Greenwood R. Twins as a natural experiment to study the causes of mild language delay: II: family interaction risk factors. J Child Psychol Psychiatry. (2003) 44(3):342–55. doi: 10.1111/1469-7610.00126
62. Cerezo MA, Abdelmaseh M, Trenado RM, Pons-Salvador G, Bohr Y. The temporal dimension in the understanding of maternal sensitivity in caregiver-infant interactions: the “early mother-child interaction coding system.” Infant Behav Dev. (2021) 63:101563. doi: 10.1016/j.infbeh.2021.101563
63. Bohr Y, Putnick DL, Lee Y, Bornstein MH. Evaluating caregiver sensitivity to infants: measures matter. Infancy. (2018) 23(5):730–47. doi: 10.1111/infa.12248
64. Verhage ML, Schuengel C, Holopainen A, Bakermans-Kranenburg MJ, Bernier A, Brown GL, et al. Conceptual comparison of constructs as first step in data harmonization: parental sensitivity, child temperament, and social support as illustrations. MethodsX. (2022) 9:101889. doi: 10.1016/j.mex.2022.101889
65. Verhage ML, Schuengel C, Duschinsky R, van IJzendoorn MH, Fearon RMP, Madigan S, et al. The collaboration on attachment transmission synthesis (CATS): A move to the level of individual-participant-data meta-analysis. Curr Dir Psychol Sci. (2020) 29(2):199–206. doi: 10.1177/0963721420904967
66. Gurugubelli VS, Fang H, Shikany JM, Balkus S V, Rumbut J, Ngo H, et al. A review of harmonization methods for studying dietary patterns. Smart Health. (2022) 23:100263. doi: 10.1016/j.smhl.2021.100263
Keywords: maternal sensitivity, mother-child interactions, replication, developmental model, prenatal, genetic risk, data harmonization
Citation: Szekely E, Laplante DP, Tiemeier H, Evans J, Pearson RM, Bekkhus M, Bakermans-Kranenburg M, van IJzendoorn MH and Wazana A (2023) The DREAM BIG project as a model for harmonizing early measures of parental care and parent-child interactions across epidemiological cohorts. Front. Child Adolesc. Psychiatry 2:1206922. doi: 10.3389/frcha.2023.1206922
Received: 16 April 2023; Accepted: 28 September 2023;
Published: 20 October 2023.
Edited by:
Jonathan Hill, University of Reading, United KingdomReviewed by:
Federico Amianto, University of Turin, ItalySilvia Cimino, Sapienza University of Rome, Italy
Luca Cerniglia, Università Telematica Internazionale Uninettuno, Italy
© 2023 Szekely, Laplante, Tiemeier, Evans, Pearson, Bekkhus, Bakermans-Kranenburg, van IJzendoorn and Wazana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashley Wazana YXNobGV5LndhemFuYUBtY2dpbGwuY2E=
 Eszter Szekely
Eszter Szekely David P. Laplante
David P. Laplante Henning Tiemeier
Henning Tiemeier Jonathan Evans5
Jonathan Evans5 Rebecca M. Pearson
Rebecca M. Pearson Mona Bekkhus
Mona Bekkhus Marian Bakermans-Kranenburg
Marian Bakermans-Kranenburg Marinus H. van IJzendoorn
Marinus H. van IJzendoorn Ashley Wazana
Ashley Wazana