- 1Department of Pharmacy, General Hospital of Ningxia Medical University, Yinchuan, China
- 2Department of Pharmacy, People's Hospital of Ningxia Hui Autonomous Region, Ningxia Medical University, Yinchuan, China
- 3College of Pharmacy, Ningxia Medical University, Yinchuan, China
- 4School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China
Jujube (Ziziphus Jujuba Mill.) is an excellent medicinal and edible plant owing to its high nutritional and health-promoting properties. As an important bioactive component, Z. Jujuba polysaccharides have aroused wide attention due to their various pharmacological activities, including anti-inflammatory, immunomodulatory, anti-oxidant, anti-tumor, anti-viral, regulating gut microbiota, hepatoprotective effects and prebiotic activity, and so on. This review highlights the advancements in the extraction methods, structural characteristics, structural elucidation, and functional analysis of polysaccharides derived from Jujube fruits over the past decade, aiming to provide valuable insights for future development and commercialization of Jujube fruits polysaccharides.
1 Introduction
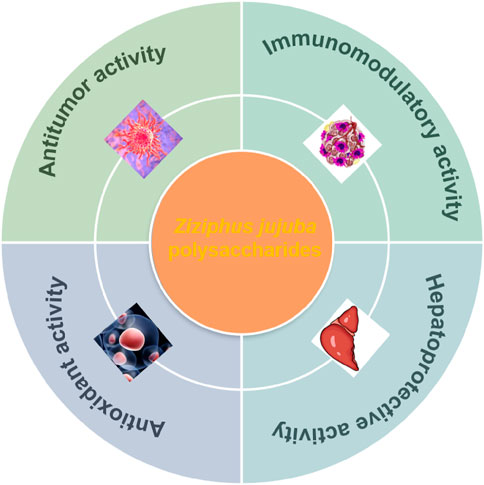
Jujube (Ziziphus Jujuba Mill.) is a small deciduous tree that belongs to the genus Ziziphus (Rhamnaceae family), is native to China with over 7,000 years of cultivation history, and is extensively distributed in Asia, Europe, and Australia (Figure 1A), mostly in Xinjiang, Hebei, Shandong, Shaanxi, Henan, Ningxia, Shanxi, and other places in China (Gao et al., 2013; LIU et al., 2015b; Liu et al., 2024). There are different jujube trees around the world, most of which have been extensively cultivating in China, including Ziziphus jujuba cv Jinsixiaozao, Ziziphus Jujuba cv. Muzao, Ziziphus Jujuba cv. Huizao, Ziziphus Jujuba cv. Shaanbeitanzao, Ziziphus Jujuba var. spinosa (Bunge) Hu ex H. F. Chou, Ziziphus Jujuba cv. Ruoqiangzao, Ziziphus Jujuba. var. inermis (Bunge) Rehd, Ziziphus Jujuba cv. Junzao, Ziziphus Jujuba cv. Hamidazao, Ziziphus Jujuba cv. Huanghetanzao, etc., (Figure 1B). It is worth mentioning that the edible part of the plant is generally the fruits of Ziziphus Jujuba Mill. has been extensively used as a medicinal and functional fruit. The medicinal history of Ziziphus Jujuba Mill. first appeared in Huangdi Neijing (475–221 BCE) to treat various diseases two thousand years ago. The historical documentation of jujube application can be traced back to these monumental works such as Shanghanzabinglun, Mingyibielu, Compendium of Materia Medica, Chinese Medicine Dictionary, Chinese Herbal Medicine, and Chinese Pharmacopoeia (Chen et al., 2015; Chen et al., 2014). In traditional Chinese medicines, Z. Jujuba has the favorable functions of strengthening sleep quality, improving food digestion, delaying the life-span, and nourishing the body. In recent years, a large number of attentions have paid to the chemical components, biological activities and application of Z. Jujuba. Notably, as a homology of medicine and food, Ziziphus Jujuba Mill. comprises abundant nutrients: polysaccharides, proteins, fatty acids, flavonoids, dietary fiber, triterpenoids, and vitamins, and other active constituents (Liu M. et al., 2020; Wojdyło et al., 2016), which demonstrates heterogeneous pharmacological activities, containing neuroprotective, anti-tumor, anti-inflammatory and antioxidant, immune-stimulation, liver protection (Bahrami et al., 2024; Șumălan et al., 2025; Xiao et al., 2022; Zou et al., 2024). Polysaccharides, among these components, are deemed as one of the main bioactive compounds of Z. Jujuba (total content 21.9 g/100 g), their biological activities have attracted increasing attention in decades (Ji et al., 2023). For decades, numerous investigations have been conducted to explore the pharmacological properties and prospective applications of Z. Jujuba polysaccharides. It is noteworthy that Jujuba polysaccharide exhibited satisfactory physicochemical properties and an abundance of biological activities, involving anti-inflammatory, immunomodulatory, anti-oxidant, anti-cancer, anti-viral, gut microbiota regulation, hepatoprotective effects and prebiotic activity, demonstrating multifarious benefits to human health (Han et al., 2024; Hua et al., 2022; Ji et al., 2019a). Polysaccharides from distinct natural products have different biological activities due to their unique structures, and this activity varies with glycoside bond, repeat unit, monosaccharide content, space conformation, linking group, and molecular weight. Different extraction and purification methods of Z. Jujuba have an impact on the structures and biological activities of its polysaccharides. As a result, the Z. Jujuba polysaccharides extracted by various procedures must be recognized for their specific structures, and their corresponding structures need to be thoroughly investigated in terms of biological efficacy and molecular mechanisms. At the moment, investigation into Z. Jujuba polysaccharides remains in its early stages. Although various biological effects and beginning mechanisms have been discovered, numerous issues remain, including the impact that extraction on the structure of polysaccharides and the structure-activity correlation of polysaccharide. This paper reviews the separation, chemical structures, and pharmacological properties of Jujuba polysaccharides, with the objective of offering references for subsequent investigation and application.
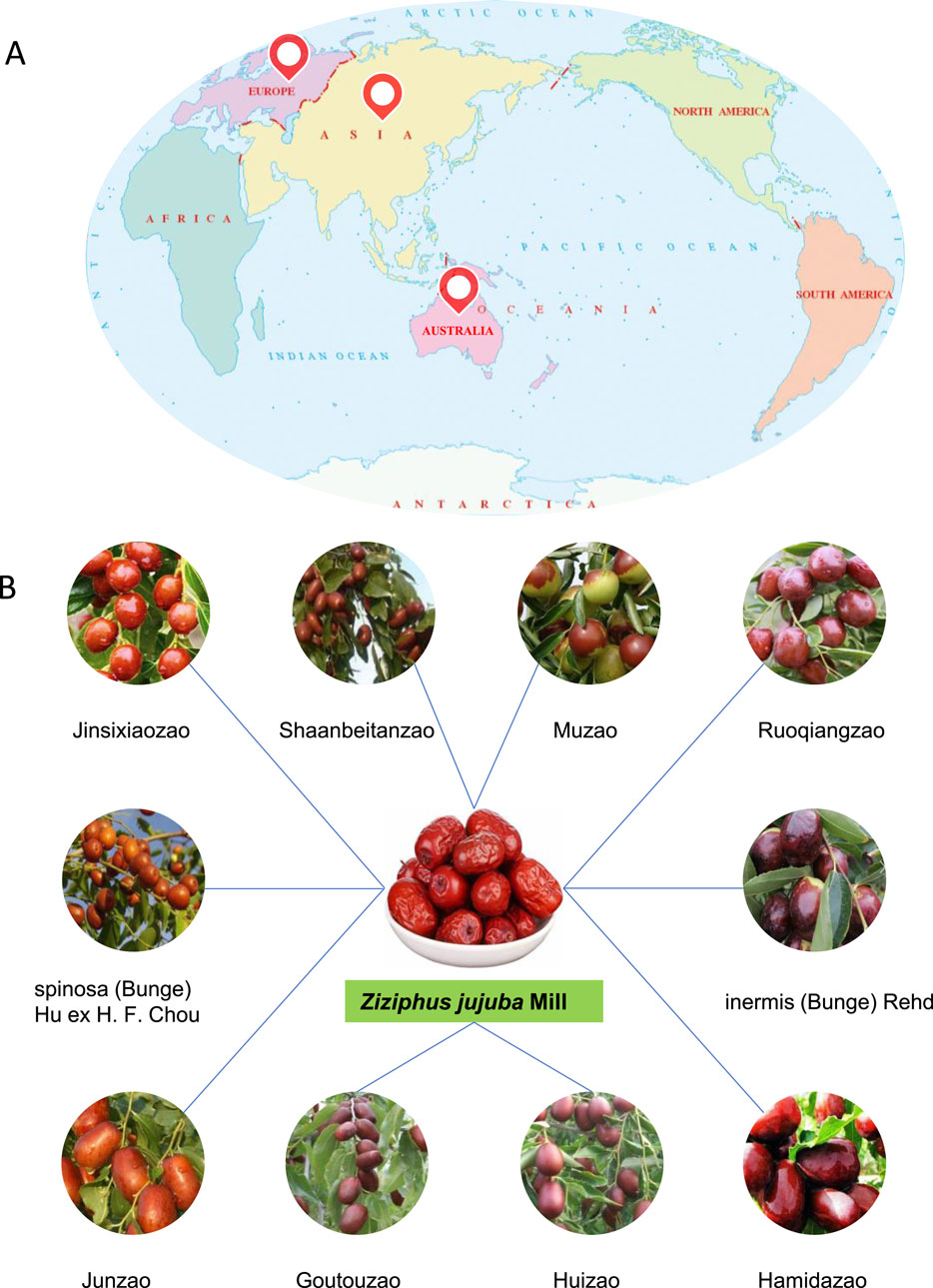
Figure 1. The distribution of Ziziphus jujuba Mill (A) (https://www.gbif.org/species/2536891), and the source of Ziziphus jujuba Mill (B).
2 Extraction, isolation and purification of jujuba polysaccharides
Polysaccharides are both polar and hydrophilic macromolecules made up of the same or various monosaccharide units connected by glycosidic linkages. They are often soluble in water but insoluble in organic solvents. Polysaccharides are considered a substantial component of the Z. jujuba fruit, and their extraction and purification have received increasing attention. A schematic diagram is shown in Figure 2.
Figure 2. Extraction, purification, and structural characterization of polysaccharides from Z. jujuba Mill.
2.1 Extraction methods
Extraction is an important stage in polysaccharide investigation since it allows for the isolation of polysaccharides for further structural and pharmacological analysis, establishing the framework for the creation of new medicinal agents and health supplements. Developments in science and technology have resulted in considerable advances to extraction and purification processes. Given the increased knowledge of the nutritional and therapeutic benefits of traditional Chinese medicine polysaccharides (TCMPs), there has been an abundance of interest in researching these molecules. Over the last few decades, multiple techniques have been used to extract polysaccharides from Z. jujuba fruit, highlighting the significant potential of jujube polysaccharides as valuable natural compounds and advocating for their further exploration and application in industrial and therapeutic settings. Table 1 summarizes the different extraction, purification methods, and the corresponding Z. jujuba fruit yield. Prior to the extraction of polysaccharides, Z. jujuba fruit undergo a series of pretreatment steps are generally required, mainly including milling the fruits into a fine powder (Liu et al., 2020b), followed by removing the lipids and pigments either by soaking in 85% ethanol or H2O2 at 50°C for 1.5 h (Guo et al., 2019; Zhao et al., 2008). Moreover, the fruits could also be defatted with petroleum ether or other organic solvents (Lin et al., 2018). These pre-treatment methods effectively remove pigments of the fruits and the solid residue is used for subsequent reflux extraction.
Traditionally, the primary extraction technique for Z. jujuba polysaccharides involves solvent extraction, utilizing solvents such as hot water, alkaline solutions, which yield varying amounts of Z. jujuba polysaccharides depending on the solvent used. However, various eco-friendly and green technologies, such as microwave-assisted extraction (MAE), ultrasonic-assisted extraction (UAE), ultrasonic-enzyme assisted extraction (UEAE), ultrahigh pressure-assisted DES extraction (UPADESE), ultrahigh pressure extraction (UPE), deep eutectic solvent (DES), ultrasound-assisted aqueous two-phase extraction (UAATPE) have been increasingly reported for the efficient and selective extraction of Z. jujuba polysaccharides. Currently, hot water extraction (HWE) is a widely employed and accessible extraction method, offers advantages such as low cost and simplicity, which is generally employed for obtaining polysaccharides from natural resources in laboratory and industrial applications. Nevertheless, it has disadvantages like low extraction purity, high temperatures, and prolonged processing times. To increase extraction amount, fruits are usually crushed into powder beforehand, necessitating proper particle size selection; extremely fine powder forms colloids in extraction, delaying transfer, and filtration, perhaps resulting in polysaccharide loss. Zhan et al. demonstrated that polysaccharide ZJP extracted from Zizyphus jujuba cv. Junzao using water extraction, ethanol precipitation, savage protein removal, and activated carbon decolorization yielded 0.17% (Zhan et al., 2018).
Furthermore, dilute alkali techniques, another conventional extraction method, can improve the extraction efficiency and decrease the separation time of jujuba polysaccharides to a particular degree. Nevertheless, alkaline conditions must be rigorously managed to prevent the structural damage of jujube polysaccharides. Yue et al. demonstrated that polysaccharide WJPs extracted from Z. jujuba cv. Muzao using alkali extraction, ethanol precipitation, savage protein removal, and hydrogen peroxide decolorization yielded 3.3% (Lin et al., 2019).
In addition, another study demonstrated that UAE and HWE were employed to extract polysaccharide from Zizyphus jujuba cv. Junzao. They compared the yield, physicochemical properties, structural properties, and biological activities of these extracts. It was found that UAE altered several characteristics of CZP compared to HWE. Specifically, the yield of polysaccharides via UAE increased from 6.23% to 7.95%, which represents an increase of about 27.6%. Conversely, the carbohydrate content decreased by 82.3% and 78.7%. (Li et al., 2014).
MAE has been employed as a supplementary extraction method to improve the solid-liquid extraction process since the past few years. Research indicates that MAE is effective in extracting polysaccharides from Z. jujube Mill fruits. An orthogonal design confirmed that a material-to-liquid ratio of 1:30, microwave power of 400 W, and an extraction time of 60 min yielded a 9.02% extraction rate (Rostami and Gharibzahedi, 2016).
Subcritical water extraction has recently been recognized as a beneficial polysaccharide extraction technology due to its high yield, inexpensive price, simplicity of usage, and friendly to the environment. Liu et al. selected the optimal extraction process for supercritical water extraction through single factor experiments, which was: extraction time of 60 min, 140°C, liquid-solid ratio of 20 mL/g, polysaccharide extraction rate of 7.9%, significantly higher than the traditional HWE extraction of 6.76% and MAE extraction of 7.2% (Liu et al., 2020c). Thus, subcritical water extraction is a rapid and efficient methods.
The ultrahigh pressure extraction (UPE) is a complementary technological advances that has appeared in recent decades, which understands rapid, cost-effective, highly precise, and little solvent consumption, and solves the shortcomings of traditional methods of extraction like soaking, maceration, water percolation, and Soxhlet extraction, etc., (Xi, 2017). Zou et al. reported that the comparison among UPE, HWE and deep eutectic solvent extract (DESE) of Polysaccharide from Zizyphus jujube cv. Hui-zao was carried out. Among these four extraction methods, the extraction yield of UPE (7.98% ± 0.13) was significantly higher than HWE (3.12% ± 0.24) and DESE (6.36% ± 0.43). While the extraction time of UPE (8.25 min) was much shorter than the extraction time of DESE (120 min) and HWE (120 min) as well. In addition to using one method alone, there are also ways to combine the two methods. Zou et al. combined UPE with DESE, achieving an extraction rate of 10.42% ± 0.28% for JPs. Through characterization using scanning electron microscopy (SEM), High performance liquid chromatography (HPLC), Fourier transform infrared spectroscopy (FT-IR), and Nuclear magnetic resonance (NMR), they confirmed that the UPE/DESE method does not degrade polysaccharides, offering a high extraction rate and short extraction time. This method shows potential to replace traditional hot water extraction as a simple and efficient industrial extraction method (Zou et al., 2022).
With developments in extraction technology, various combinations of extraction methods, including ultrasound-assisted aqueous two-phase extraction (UAATPE), have emerged as viable alternatives to conventional polysaccharide extraction methods from Ziziphus jujuba Mill. Ji et al. (2018b) extracted Z. jujube polysaccharides by using UAATPE and optimized the extraction process via RSM based on single factor experimental results. The results show that the optimal combination of process parameters was as follows: extraction temperature of 48°C, and extraction time of 38 min, microwave power of 70 W, a solid-to-liquid ratio of 1 g–30 mL, and the yield of polysaccharides was 8.18% under the optimal extraction conditions.
2.2 Isolation and purification methods
After extraction, jujuba polysaccharides usually remain with many impurities, including inorganic salts, oligosaccharides, proteins, and lignin. The presence of these impurities will directly impact on the following purification, structural characterization, physicochemical properties, and biological function of jujuba polysaccharides, rendering it challenging to assess the link between the structure and function of polysaccharides. As a result, prior to separating and purifying polysaccharides, certain steps are required to eliminate non polysaccharide impurities, such as proteins and pigments, from the crude polysaccharides.
The elimination of proteins in crude jujube polysaccharides is frequently evaluated by Sevag, a repeated freeze-thaw method, trichloroacetic acid, and chloroform–butyl alcohol methods. Pigments can oxidize jujuba polysaccharides, thus affecting the chromato-graphic analysis and compromising an accurate identification of the polymer; in view of this, the removal of pigments is an essential step during the separation process. Activated carbon, microporous adsorption resin, and hydrogen peroxide solution are used to remove pigments.
To obtain homogeneous polysaccharides, crude jujuba polysaccharides should be further separated and purified. Column chromatography is an effective method for purifying natural components. In general, two types of column chromatography methods, ion exchange chromatography and the gel filtration chromatography are frequently employed to purify crude polysaccharides. Ion-exchange chromatography can separate neutral polysaccharides and acidic polysaccharides via gradient salt elution, whereas gel filtration can separate polysaccharides of different molecular weights (Mw). Ji et al. (2020a) using DEAE-Sepharose Fast Flow column (2.6 × 100 cm), neutral polysaccharides together with acidic polysaccharides were obtained by eluting with distilled water and different concentrations of NaCl gradient. Three main fraction ZMP1, ZMP2, ZMP3 was further separated by a Sephacryl S-300 column (2.6 cm × 100 cm) with deionized water at a flow rate of 0.8 mL/min, to obtain three homogeneous polysaccharide PZMP1, PZMP2 and PZMP3. At present, the commonly used anion exchange chromatographic columns for jujube polysaccharides are the DEAE-cellulose 52 column (Liu et al., 2020c; Wu et al., 2021), DEAE-Sepharose CL-6B (Li et al., 2013; Li et al., 2011) and DEAE-Sepharose fast flow column (Ji et al., 2019b; Zhang et al., 2017). The commonly used gel chromatographic columns are mainly the Sephacry (Ji et al., 2020a; Ji et al., 2019c), Sepharose (Ji et al., 2021a) and Sephadex (Rostami and Gharibzahedi, 2016; Wang et al., 2018). Dialysis, concentration and lyophilize treatment of jujube polysaccharides can be used for purification after column chromatography for better storage. The differences in purification processes result in significant variations in the monosaccharide composition, Mw, and main chain structure of jujube polysaccharides (Ji et al., 2020a; Wang et al., 2023; Zou et al., 2018). Therefore, the separation and purification of polysaccharides is very complex, and researchers must carefully choose appropriate separation and purification methods based on the characteristics of the polysaccharides being studied.
3 Chemical composition and structures of jujube polysaccharides
According to studies, the biological activity of jujube polysaccharides is significantly affected by their complex physicochemical properties, such as Mw, chain length, type and quantity of functional groups, type of glycosidic bonds, monosaccharide composition, molar ratio, and branching degree, which can be assessed using a combination of chemical and physical analysis methods. To date, literature data provide diverse results, nearly 71 polysaccharides have been obtained and purified from Ziziphus jujuba Mill. by exploring different technology technologies of extraction, isolation, and purification methods, which have determined the different properties and structural features of the jujuba polysaccharides. As the main foundation for the quality evaluation of jujuba polysaccharides, the total sugar contents are commonly determined by phenol sulfuric acid, anthrone-sulfuric acid methods. Homogeneity is the premise of jujuba polysaccharide structure characterization, which is usually represented by using nuclear magnetic resonance (NMR) spectroscopy, high performance liquid chromatography (HPLC), high performance gel permeation chromatography (HPGPC), high-performance size-exclusion chromatography (HPSEC), fourier transform infrared spectroscopy (FT-IR), ultraviolet (UV)-visible spectroscopy, gas chromatography (GC), gas chromatography-mass spectrometry (GC-MS), liquid chromatography-tandem mass spectrometry (LC-ESI-MS), GPC-multi-angle laser light scattering (MALLS), high-performance anion-exchange pulsed amperometric detection chromatography (HPAEC-PAD), X-ray diffraction (XRD), atomic force microscopy (AFM), scanning electron microscopy (SEM), capillary zone electrophoresis (CZE), methylation analysis, periodate oxidation-Smith degradation, and partial acid-hydrolysis, etc. The biological activity of jujuba polysaccharides can be greatly influenced by various factors such as different Mw, composition of monosaccharides, type of linkage, and modification. Herein, the reported jujuba polysaccharide in the past few years are listed and integrated information on their source, Mw, monosaccharide composition, Structural characteristics and bioactivities are comprehensively introduced in Table 2.
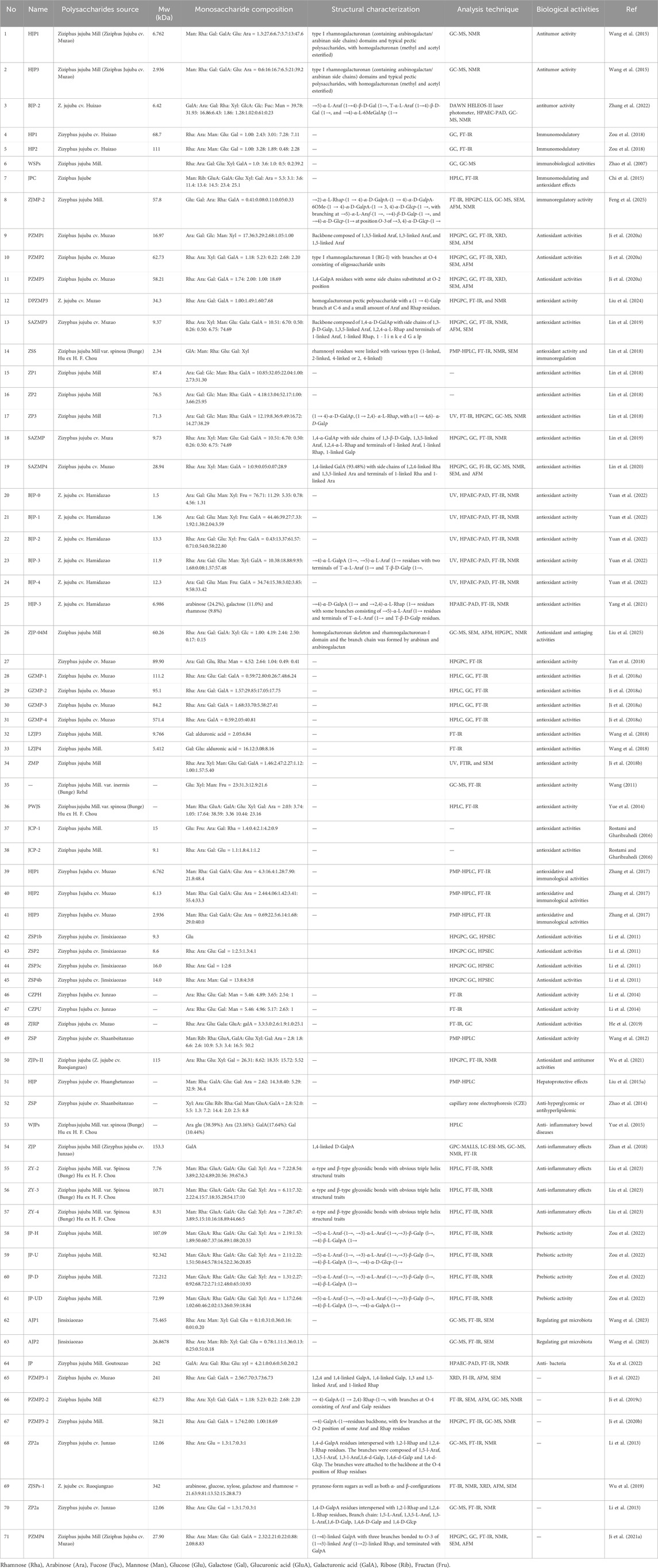
Table 2. Monosaccharide compositions, molecular weights, and structural characteristics of jujuba polysaccharide.
3.1 Relative molecular mass
Mw of jujuba polysaccharide is a key parameter that needs to be considered in understanding their biological activities and functions, thus determination of the Mw of polysaccharides can help determine the optimal range of biological activities and provide guidance for further research and application. The Mw of jujuba polysaccharide is currently determined using HPGPC, HPSEC, HPLC. Differentiation of average Mw of jujuba polysaccharides vary across studies due to variations in plant source, extraction techniques, purification methods, and analytical approaches. For instance, the JP-H, JP-U, JP-UD and JP-D reported come from Zizyphus jujube cv. Hui-zao using different extraction methods, with high and low Mw in the order of JP-H (107.09 kDa) (HWE) > JP-U (92.342 kDa) (UPE) > JP-UD (72.99 kDa) (UPADESE) > JP-D (72.212 kDa) (DESE) (Zou et al., 2022). In addition, Mw fraction of Z. jujuba cv. Junzao polysaccharide obtained by HWE (ZJP) (153.3 kDa), was higher than that of Z. jujuba cv. Junzao polysaccharide obtained by HWE (ZP2a) (12.06 kDa) (Li et al., 2013; Zhan et al., 2018). As shown in Table 2, different experimental conditions observed that the Mw of jujuba polysaccharides ranged from 1 to 100 kDa.
3.2 Monosaccharide composition
The polysaccharides found in Ziziphus jujuba Mill plants exhibit significant variations in monosaccharide composition. These differences can be attributed to the variations in extraction, separation, purification methods, and detection techniques, leading to inconsistencies in the composition and proportion of monosaccharides. However, it can be seen from Table 2 that the monosaccharide composition of various jujuba polysaccharides was different, but most of them were composed of mannose (Man), rhamnose (Rha), glucose (Glu), galactose (Gal), xylose (Xyl), arabinose (Ara), glucuronic acid (GluA), and galacturonic acid (GalA), and the proportion of each component was different. It is worth noting that fructose (Fuc) main in Z. jujuba cv. Huizao polysaccharides were found, which is mainly composed of GalA, Ara, Gal, Rha, Xyl, GlcA, Glc, Fuc, Man with a molar ratio of 39.78: 31.93: 16.86:6.43: 1.86: 1.28:1.02:0.61:0.23 (Zhang et al., 2022). In addition, ribose (Rib) main in Ziziphus. jujuba cv. Jinsixiaozao and Shaanbeitanzao were found. It is worth noting that some jujuba polysaccharides are homogeneous. Moreover, significant differences in the monosaccharide composition and molar ratio of jujuba polysaccharides obtained from same extraction method and different purification processes have been observed. For instance, a crude jujuba polysaccharides CZSP were extracted from Zizyphus jujuba cv. Jinsixiaozao (Li et al., 2011). Four kinds of jujuba polysaccharides ZSP1b, ZSP2, ZSP3c and ZSP4b were obtained by DEAE-SepharoseCL-6B and SepharoseCL-6B column chromatography methods. The results showed that the composition of four water-soluble polysaccharide components ZSP1b, ZSP2, ZSP3c, and ZSP4b was different.
3.3 Chemical structures
The biological activity of polysaccharides is intimately related to their structure, which includes the branch chain, configuration, and mechanism of connection of the glycosidic bond. Nevertheless, according to the existing literatures, the chemical structure of jujuba polysaccharides is rarely mentioned in literature reports. Researchers have attempted to categorize and examine the chemical structure of the current jujuba polysaccharides in an effort to close this knowledge gap and gather more useful data. By using this categorization analysis method, we intend to uncover some possible traits and attributes of jujuba polysaccharides and offer more detailed recommendations for subsequent research. Liu et al. (2020b) extracted a water-soluble ZP3 from Ziziphus jujuba Mill. fruit. The structures of the compounds were determined by UV, IR-RT, 1H NMR and 13C NMR spectra. It was found that the purified ZP3 were mainly composed a main chain of (1→4)-α-D-GalAp, (1→2,4)-α-L-Rhap, with a (1→4,6)-α-D-Galp branches. A homogeneous acidic polysaccharide (PZMP4) was isolated from Ziziphus jujuba Mill. by Ji et al. (2021b) in their study. AFM and SEM analysis results showed that PZMP4 was a netted structure of molecular aggregates. Using a combination of HPGPC, GC, FT-IR, GC-MS, NMR, a comprehensive analysis of PZMP4 was conducted. The results revealed that the main chain of PZMP4 mainly composed of (1→4)-linked GalpA, with three branches boned to O-3 of (1→3)-linked Araf, (1→2)-linked Rhap, and terminated with GalpA. In addition, Zhan et al. (2018) extracted a pectin polysaccharide with anti-inflammatory activity and found that they had 1,4-linked D-GalpA. Wang et al. (2015) deciphered the chemical structures of two pectin like HJP1 and HJP3, revealing that both of them have a type I rhamnolactouronic acid (containing arabinogalactan/arabinan side chain) domain and a typical pectin polysaccharide. Pectin is classified into Xylogalacturonan (XGA), Homogalacturonan (HG), and Rhamnogalacturonan-I (RG-I) regions. Similarly, Zou et al. (2022) isolated with four different extraction techniques, and purified four polysaccharides (JP-H, JP-U, JP-D and JP-UD) from Ziziphus jujuba Mill. Through modern instrumental analysis methods, including HPLC, FT-IR and NMR, each refined JP-H, JP-U, JP-D and JP-UD was analyzed, and its primary structure was inferred. NMR results showed that four polysaccharides contained similar glycosidic linkage of →5)-α-L-Araf-(1→, →3)-α-L-Araf-(1→, →3)-β-Galp-(1→ and →4)-β-L-GalpA-(l→, while the specific glycosidic linkage of →4)-α-D-Glcp-(l→ appeared in JP-U and JP-UD, the esterified units of galacturonic acid and the CONH2 group appeared in JP-D and JP-UD, as well the Terminal β-D-Galp and →4)-α-GalpA-(1→ appeared in JP-UD. The structural characterization of jujuba polysaccharide were summarized as presented in Table. 2, and the potential structures of polysaccharide were depicted in Figure 3.
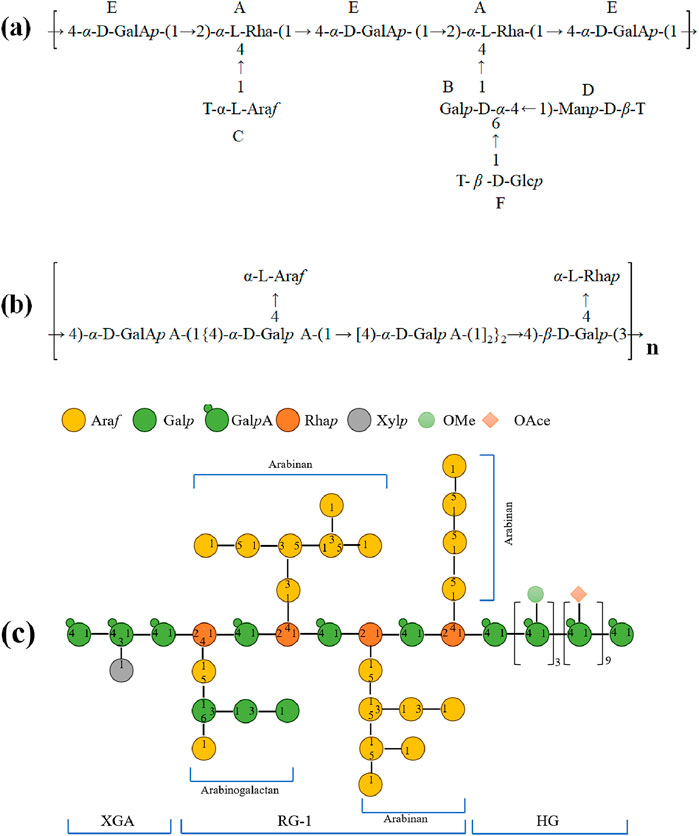
Figure 3. Summary of the typical structural features of diverse polysaccharides from Ziziphus jujuba Mill. (a) ZP3; (b) JP; (c) ZJP-04M.
3.4 Molecular morphology
At present, SEM, AFM and XRD have been widely used to determine the molecular morphological properties of jujuba polysaccharides. Among them, SEM is the most commonly used method for determining the morphology of polysaccharide molecules, which can perform continuous slicing and imaging of the sample, and then use computer software packages for segmentation and three-dimensional (3D) reconstruction to achieve visualization of the three-dimensional structure. Wang et al. (2023) used SEM to observe the microstructure of AJP1 and AJP2, and found that AJP2 has a smooth surface and dense sheet-like interior, while the surface of AJP1 is relatively rough, loose, and porous. The results showed that the loose pores between AJP1 polysaccharides were more likely to bind with water, increasing the surface area of polysaccharides and improving their solubility. Another study used SEM and AFM to image PZMP1, PZMP2 and PZMP3 obtained by HWE extraction methods. At ×10,000 magnification, SEM found that PZMP1, PZMP2, and PZMP3 have different surface morphologies. Among them, PZMP1 is in an aggregated state, appearing as thin flakes with a smooth surface, while PZMP2 has a spherical network structure. PZMP3 has a rough surface with flocculent fibers and different branch chains. They inferred that the different microstructural features are mainly due to the different monosaccharide compositions of the three PZMPs, especially the different content of uronic acid and glycosidic bonds links. In addition, at a magnification of 5,000 times, AFM observation revealed a clear spherical mass in PZPP1, indicating molecular aggregation. PZMP2 constituted of random linear chains with a small number of spherical aggregates, forming a spherical structure as a whole, while PZMP3 dispersed in extremely dilute solutions could be exhibit monodisperse, spherical, and irregular particles. They inferred that the different microstructural characteristics can be attributed to differences in hydrogen bonding and galacturonic acid content (Ji et al., 2020a).
4 Biological activities of jujuba polysaccharides
The bioactivities of jujuba polysaccharide from their unique structural characteristics, which promote interactions with biological systems and mechanisms of action that contribute to the potential health benefits. Jujuba polysaccharide exhibit anti-cancer, hepatoprotective effects, anti-oxidant, anti-inflammatory, anti-bacterial, anti-hyperglycemic, regulating gut microbiota and immunomodulatory effects which have attracted significant interest from researchers worldwide. The diverse bioactivity of jujuba polysaccharides highlights their potential as natural agents for the management and prevention of a range of illnesses as well as their role in the creation of novel medical goods and treatments in the future. Figure 4 shows the combined health benefits.
4.1 Antitumor activity
Cancer is a major global public health issue and the third leading cause of death globally (Li et al., 2024). The latest data reported that approximately 2,001,140 new cancer cases and 611,720 cancer deaths the United States in 2024 (Siegel et al., 2024). The medical community still seeks the causes of cancer and effective treatments, as current chemical drugs therapy methods often cause harm and approved drugs have significant side effects, emphasizing the urgent need for safer anti-tumor therapies. Medicinal plant polysaccharides, as natural active ingredients, are used to inhibit tumor cell growth through various molecular mechanisms, including: 1) preventing tumor invasion and metastasis by lowering tumor cell adhesion or trophic factor levels; 2) stimulating T and NK cells to play an immune role in preventing cancer growth; 3) decreasing tumor cell viability or displaying cytotoxic effects; 4) preventing cancer cell division and proliferation by modifying the expression levels of genes and proteins involved in the cell cycle; 5) inducing mitochondrial dysfunction and antioxidant system imbalance, and increasing cell apoptosis mediated by cysteine protease (Xue et al., 2024). So far, compared to the researches provided for other polysaccharides, there are few findings presented on the anti-tumor activity of jujuba polysaccharides. Wang et al. (2015) prepared the water-soluble polysaccharides (HJP1 and HJP3) by UAE and found that HJP1 and HJP3 exhibited a significant inhibitory effect on human HepG2 cells in vitro. Additionally, the significantly stronger cytotoxicity of HJP3 against HepG2 cells compared to HJP1 suggests that both HJP1 and HJP3 have potential as natural anti-tumor candidates. Wu et al. (2021) extracted Ziziphus jujuba polysaccharides (ZJPs) using UAE, and then purified the crude ZJPs to obtain homogeneous polysaccharides fractions (ZJPs-II). The results observed that ZJPs-II could effectively inhibit the proliferation of SW620 cells, arresting them in the G2/M phase, reducing the cell colony-formation, and inducing apoptosis and arrested. Zhang et al. (2022) found that BJP-2 could induce apoptosis in a dose-dependent manner, leading to the inhibition of cell proliferation. In addition, cell scratching and Trans well assays demonstrated that BJP-2 could effectively inhibit the invasion and metastasis of tumor cells. Moreover, BJP-2 could upregulate the expression of Bax, Cleaved Caspase-3/Caspase-3 and Cleaved Caspase-9/Caspase-9 and downregulation of Bcl-2, suggesting that the anti-tumor activity of BJP-2 is mediated through mitochondrial dependent pathways. Most importantly, HJP3 with lower molecular weight (2.94 kDa) exhibited greater anti-tumor activity than HJP1 (6.76 kDa). The structure-function relationship indicated that type I rhamnogalacturonan (containing arabinogalactan/arabinan side chains) domains and typical pectic polysaccharides, with homogalacturonan (methyl and acetyl esteried), thereby exerting remarkable anti-tumor activity. Additionally, the special structure with the backbone of the main chain of →5)-α-L-Araf (1→4)-β-D-Gal (1→, T-α-L-Araf (1→4)-β-D-Gal (1→, and →4)-α-L-6MeGalAp (1→, is also of great importance for promoting the anti-tumor effect.
4.2 Immunomodulatory activity
Natural polysaccharides are well known to have better immunomodulatory properties. Han et al. (2020) found that JP could improve the immune response and restore intestinal barrier function in immunosuppressed mice (induced by cyclophosphamide). In comparison with the model group, JP (150, 300, and 600 mg/kg BW per day) significantly increased the lymphocyte proliferation in the spleen and decrease the proportion of CD3+ and CD4+ and the ratio of CD4+/CD8+ in cyclophosphamide-induced mice in a dose-dependent manner after 28 continuous days of treatment. In addition, JP treatment in immunosuppressed mice also increased the levels of IL-2, IL-4, IL-10, IFN-γ, and TNF-α in serum and the intestine, and the improvement effects were proportional to the dose of JP. JP at a dose of 600 mg/kg BW demonstrated better immune activation. In vitro, JP significantly enhanced the proliferation of T lymphoma cells, and they triggered significant changes in immune cells at concentrations as low as 150 μg/mL. Zou et al. (2018) found that both medium and high concentrations of HP1 and HP2 (100 and 200 mg/kg BW per day) could significantly increase spleen and thymus indices, promote serum hemolysin formation, enhance the phagocytic activity of macrophages and inhibit footpad edema of mice after seven consecutive days of treatment. HP2 at a dose of 200 mg/kg BW demonstrated better immune activation. It is worth noting that JPC can also promote NK cell activities, T cell proliferation, CD4+/CD8+ ratio and CD4+ counts in CFS rats, thereby improving the body and helping restore immune function (Chi et al., 2015). Extensive researches have displayed the anti-inflammatory and immunomodulatory effects of polysaccharides were closely related to their structures, especially the Mw, monosaccharide composition. HP-2 with a relatively higher molecular weight (111 kDa) than HP-1 (68.7 kDa) and higher ratio of galacturonic acid (35.9%) than HP-1 (7.32%) significantly exert immunomodulatory activity.
Conversely, ZJMP-2, a relatively ratio of galacturonic acid (0.33%), with a very small Mw of 57.8 kDa and the backbone comprised →2)-α-L-Rhap-(1 → 4)-α-D-GalpA-(1 → 4)-α-D-GalpA-6OMe-(1 → 4)-α-D-GalpA-(1 → 3, 4)-α-D-Glcp-(1 →, with branching at →5)-α-L-Araf-(1 →, →4)-β-D-Galp-(1 →, and →4)-α-D-Glcp-(1→ at position O-3 of →3, 4)-α-DGlcp-(1 →, significantly promoted the expression levels of TLR4, NF-κB, and TRAF6 proteins, increasing RAW264.7 cell activity, index of splenic lymphocytes, and the production of cytokines and NO, thereby activating macrophages and elevating lymphocyte proliferation. So far, the relationship of jujuba polysaccharides structure with the immunomodulatory function is still unclear. Further research on the structure-activity relationship of jujuba polysaccharides will be beneficial for the development and application of Z. jujuba polysaccharides as a immunomodulatory drug.
4.3 Antioxidant activity
Reactive oxygen species (ROS) have been implicated in the development of multiple diseases, including hypertension, diabetes, aging and cancer (Li et al., 2021). The antioxidant properties of Jujuba polysaccharides are closely linked to their ability to neutralize ROS and inhibit lipid peroxidation. This action helps protect cells and tissues from oxidative damage, maintains cellular redox balance, and may enhance overall cellular health while mitigating conditions related to oxidative stress. Research on the antioxidant activity of Jujuba polysaccharides has shown promising results and potential therapeutic benefits.
In vitro antioxidant activity is typically assessed through straightforward methods, including the measurement of free radical and reduce Fe2+ (Yu-Hao et al., 2021). He et al. (2019) optimized the alkali extraction process to obtain a water-soluble ZJRP from the Ziziphus jujuba cv. Muzao residue. Their study shown that ZJRP have a strong antioxidant effect at a lower concentration (1 mg/mL) and can effectively scavenge DPPH free radicals, with scavenging rate of 70.67%. However, their antioxidant activities were not surprising when compared to the corresponding concentration of ascorbic acid. They speculated that the antioxidant activity of ZJRP may be related to high levels of galacturonic acid and extraction methods. Guo et al. (2024) found that the JPS obtained by HWE, UAE, EAE and UAEE had the ability to scavenge hydroxyl radicals, ABTS radical, reducing power and DPPH free radical and exhibited a reducing capacity in a dose-dependent manner. The results of DPPH, ABTS and hydroxyl free radicals scavenging experiments showed that JPS-UAEE had the strongest scavenging ability on three kinds of free radicals, and the scavenging effects were 77.98%, 91.82% and 66.85%, respectively. Additionally, absorbance represents reducibility, and the larger the reduction capacity, the higher the anti-oxidant capacity. The reducing capacities and ABTS radical scavenging ability of JPS obtained from UAEE higher than that of EAE, UAE and HWE. However, the extraction of JPS using UAEE method significantly reduced the IC50 value compared to other samples, which may be due to the fact that UAEE in these frequency modes induced more severe degradation of JPS during the extraction process, changed the Mw, thus enhancing hydrogen ion donor ability and DPPH scavenging efficiency. Research indicates that polysaccharides with lower Mw exhibit higher antioxidant activity. Liu et al. (2024) investigated the antioxidant activity of three jujuba polysaccharide fractions (DPZMP3, DZMP, and ZMP) extracts (i.e., purified polysaccharides, degraded polysaccharides, and crude polysaccharides) obtained from Ziziphus Jujuba cv. Muzao. The oxidation ability of different polysaccharide fractions was found to be concentration-dependent. When the concentration of DPZMP3, DZMP, and ZMP used was 2.0 mg/mL, the scavenging rates of DPPH radical reached 92.89%, 89.64%, and 81.55%, respectively. In a specific concentration range, the DPPH radical scavenging ability of ZP1, ZP2 and ZP3 (extracted using subcritical water and purified using DEAE-52 anion-exchange chromatography) were 74.8%, 64.9%, and 88.3%, respectively. Further structure-activity relationship indicated DPZMP3 had a smaller Mw of 34.3 kDa than ZP1 (874 kDa), ZP2 (765 kDa), and ZP3 (713 kDa), and was mainly composed of Rha, Ara, Gal, and GalA, which may be related to the fact that after the treatment, the molecular chain of polysaccharide is broken, and more active groups are exposed to the solution, making them easier to create contact between active sites and free radicals, thus exerting the anti-oxidant activities.
The most commonly utilized in vivo models for investigating antioxidant activity are normal mice and C. elegans. The activities of antioxidant enzymes and the content of ROS are used to measure the ability of polysaccharides to reduce oxidative damage in C. elegans models. Han et al. (2024) isolated and purified a polysaccharide (CPJE) with a molecular weight of 98 kDa from Ziziphus jujuba Mill cv. Jinsixiaozao. CPJE can significantly reduce the levels of ROS, and increased the activities of SOD, CAT, and the content of GSH. The research indicates that CPJE can regulate the transcription factors DAF-16 and SKN-1 to modulate oxidative stress in Caenorhabditis elegans, and can also exert an antiaging role by mediating DAF-16 and SKN-1 pathways and inhibiting IIS pathways in nematode model. In addition, the homogeneous polysaccharide ZJP-04M, isolated and purified from Ziziphus jujuba fruit, exhibits significant anti-aging and antioxidant effects. It also exerts an anti-aging role by alleviating oxidative stress in nematodes and modulating the downstream target of the conserved insulin/IGF-1 signaling pathway (IIS), FOXO homolog DAF-16. Further structure-activity relationship indicated ZJP-04M had Mw of 60.26 kDa, and was mainly composed of Rha, Ara, Gal, and GalA, with small amounts of Xyl and Glu, which may be related to a fairly long linear HG skeleton was alternately connected with RG-I to form the trunk chain, and multibranched arabinan and AG domains coexist in the C4 site of Rhap on the trunk chain, thus exerting the anti-oxidant and antiaging activities (Liu et al., 2025).
Further investigation reveals that the anti-oxidant effect of Z. Jujuba polysaccharides is mainly associated with the Mw, monosaccharide content, glycosidic bond type, and uronic acid content. Based to the published literature (Table 2), Z. Jujuba polysaccharides with molecular weights ranging from 1.36 kDa to 571.4 kDa exhibited remarkable anti-oxidant action. Notably, the antioxidant activity of polysaccharides is proportional to their galacturonic acid, glucose, galactose, arabinose, fucose, mannose, xylose content, plays an important role in the anti-oxidant impact of Z. Jujuba polysaccharides. In general, the antioxidant activity of polysaccharides is connected to their uronic acid content due to more functional groups including -COOH and -OH in these monosaccharides, which can offer more hydrogen ions to neutralize unpaired electrons in free radicals. For instance, ZSP3c and ZSP4b comprising higher galacturonic acid content had the stronger free radical scavenging activities than ZSP1b including no uronic acid. The antioxidant impact of PZMP3 higher than PZMP2 and PZMP1 by neutralizing unpaired electrons in free radical activity due to their functional groups, including higher galacturonic acid groups. Additionally, the structure-function relationship indicated BJP-1 with the highest content of arabinose (44.46%) and lowest molecular weight (1.36 kDa) exhibited great anti-oxidant activity than other compounds by scavenging DPPH and ABTS radicals. The polysaccharides with a low-molecular weight tend to possess strong antioxidant activity due to more reducing hydroxyl groups at their terminals.
Other monosaccharides, in addition to uronic acid, also play an important role in the antioxidant activity of polysaccharides. The content of glucose affected the in vitro antioxidant activity of jujuba polysaccharide fractions against hydroxyl radical and ferric-reducing.
Furthermore, various factors influence the antioxidant activity of polysaccharides. Polysaccharide biological activities are thus determined by interactions between various structural variables, most notably monosaccharide content and molecular weight.
The structure-activity relationship revealed that the antioxidant effect of polysaccharides was closely related to its structure. structural characteristics demonstrated Galacturonic acid occupied the terminal region of the branched chains and attached to the main chain, connecting by α-(1→4) bonds at O-4 and O-6 position and accompanying by arabinose, xylose, rhamnose, galactose and glucose residues, thereby exerting remarkable anti-oxidant activity. So far, despite the abundance of literature on the anti-oxidant effect of jujuba polysaccharides, research on the structure-activity relationship of Z. jujuba polysaccharides is still obscure, and it requires immediate attention to elaborate comprehensively, which provides a strong basis to further investigate the functional food or anti-oxidant medicine of Z. jujuba in the coming years.
4.4 Hepatoprotective activity
Carbon tetrachloride (CCl4) administration catalyzes the formation of reactive trichloromethyl radicals (CCl3) via cytochrome P450, which subsequently convert to trichloromethyl peroxide radicals (CCl3OO), precursors of lipid peroxidation. This lipid peroxidation leads to liver damage, resulting in the release of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (McCay et al., 1984; Yang et al., 2010). Consequently, serum levels of ALT and AST are important indicators of liver injury. The polysaccharides from jujuba have been reported to have protective effect on liver injury (Gao et al., 2013; Liu et al., 2015a). For example, Sun et al. (2022) investigated the potential health benefits of WJP-F80, particularly in vivo assessment of protective effects against CCl4-induced liver injury in mice. The results indicate that the prophylactic treatment with WJP-F80 before CCl4 administration showed the ability to decrease the levels of both ALT and AST in a dose-dependent manner by comparing with the CCl4-model group. At a dose of 100 mg/kgBW, the ALT level decreased sharply, while the AST levels had no significant statistical difference. At a dose of 200 body weight of WJP-F80, the activities of ALT and AST were close to those of the normal control group, and that 400 mg/kg·WJP-F80 significantly reduced the CCl4-elevated levels of ALT and AST. Histopathological analysis further demonstrated that WJP-F80 treatment improved liver histological integrity in a dose-dependent manner. Mice receiving low-dose WJP-F80 exhibited only mild inflammatory cell infiltration, whereas high-dose treatment preserved normal liver architecture, effectively preventing cell necrosis and inflammatory infiltration.
At present, comprehensive researches on the hepatoprotective effects of jujuba polysaccharides are lacking. Existing theories suggest that their liver-protective functions may involve scavenging free radicals and enhancing antioxidant enzyme activity. Therefore, further detailed investigations are needed to elucidate the hepatoprotective activities of jujuba polysaccharides.
4.5 Other bioactivities
Except for the aforementioned pharmacological effects, jujuba polysaccharides also exhibit various physiological activities, including antihyperglycemic, anti-hyperlipidemic, prebiotic activity, anti-bacteria, and regulating gut microbiota. An earlier study revealed that jujuba polysaccharides can significantly reduce the serum levels of glucose, insulin, TC, TG, LDL-C, and VLDLC, improve the HDL-C level, homeostasis model assessment for insulin resistance (HOMA-IR), b-cell function (HOMA-b), and decrease the atherogenic index (AI) of the mice exposed to high-fructose water, and address hyperglycemic or hyperlipidemic (Zhao et al., 2014). Furthermore, Xu et al. (2022) investigated the mechanism of jujuba polysaccharides in preventing and treating on oral biofilm pathogens by human saliva collection. Their findings indicate that jujuba polysaccharides can significantly inhibit the activity of oral biofilm pathogens Streptococcus mutans, MRSA, Porphyromonas gingivalis and prevent and treat oral infectious diseases.
Jujuba polysaccharides demonstrate significant potential for functional food applications due to their diverse bioactive properties. In the formulation of functional foods that incorporate jujuba polysaccharides, it is crucial to address several key factors, including formulation stability, bioavailability, and potential interactions with other ingredients. For example, due to its dietary fiber content and prebiotic properties, Jujuba polysaccharides has the potential to promote digestive health. Furthermore, their prebiotic effects can promote the growth of beneficial gut bacteria, thereby enhancing gut microbiota composition. Given these attributes, jujuba polysaccharides represent a promising ingredient for functional foods and dietary supplements aimed at promoting human health and preventing diseases (Wang et al., 2023; Zou et al., 2022).
Furthermore, the prebiotic actions of Jujuba polysaccharides may indirectly contribute to immune support by encouraging the growth of beneficial gut bacteria involved in immune system regulation and function. Jujuba polysaccharides have the potential to have prebiotic effects, which encourage the development and reproduction of beneficial gut bacteria. As a prebiotic, Jujuba polysaccharides act as a substrate for some beneficial microbes in the gut, promoting their growth and potentially improving gut microbiota composition. Jujuba polysaccharides may help to improve the health and diversity of the gut microbiota by supporting these beneficial gut bacteria. Jujuba polysaccharides prebiotic actions suggest that they could be used in functional foods, nutritional supplements, or probiotic formulae to promote gut health.
5 Conclusion and perspectives
As a homolog of medicinal and edible food, Z. jujuba fruit has been widely exploited for functional food, dietary supplement and therapeutic medicines owing to its excellent nutritional and medicinal benefits, as well as its range of therapeutic effects and health-promoting properties. This review summarized the extraction, separation, and purification, structure identification, and biological functions research on polysaccharides from Ziziphus Jujuba Mill. It highlights the crucial potential of jujuba polysaccharides as precious natural compounds and advocates for their further exploration and application in both industry and therapeutics. Previous researches have addressed that the content of jujuba polysaccharides varies with different extraction techniques and purification techniques. jujuba polysaccharides have been shown to have a variety of potential health benefits, including antioxidant activity, immunomodulatory function, antitumor activity, hypoglycemic and hypolipidemic effects, modulation of gut microbiota, prebiotic activity, and antiaging activities, among others, making them beneficial for the development of novel therapeutics.
Despite these potential characteristics, present research identifies several areas requiring further exploration: (1) The yield, purity, stability, reproducibility and massive production of jujuba polysaccharides is difficult to guarantee due to the limitations of current extraction methods, and the interaction of polysaccharides with other components of the food matrix is not fully understood during the extraction process. Therefore, the researchers may confront with challenges in improving these processes to acquire high-purity jujuba polysaccharides for their investigations; (2) Numerous investigations prioritize extraction yield optimization over detailed characterization of the resulting jujuba polysaccharides, especially its structure and functionality. The specific chemical structure and composition of jujuba polysaccharides remain difficult to determine. Jujuba polysaccharides is a complex plant polysaccharide, which extraction process is affected by varieties of differences and extraction methods, resulting in the significant differences in polysaccharide structure. All of these variables contribute to the difficulty of the development of jujuba polysaccharides-based drugs. (3) Though multiple pharmacological activities of jujuba polysaccharides have been confirmed by diverse experiments in vivo and in vitro in animals, the mechanisms underlying the biological functions have not been completely clarified. Compared with other polysaccharides, investigation on the structural characterization and structure-activity relationship of jujuba polysaccharides is still in the early stages. (4) The research on jujuba polysaccharides demonstrating pharmacological activities primarily focuses on cell models and animal experiments. However, the safety evaluation, toxicity, efficacy, pharmacokinetics, and clinical trials of jujuba polysaccharides have not been thoroughly elucidated, which are essential prerequisites for the development of functional foods, nutritional supplements, and pharmaceuticals.
Future research on jujuba polysaccharides should focus on the following objectives: including the development of the innovative extraction and purification techniques; the optimization of production processes for commercialization; the continued efforts to elucidate structural features for the better understanding of bioactivities and potential mechanisms of jujuba polysaccharides; the conduction of the in vitro and in vivo experiments in animals and clinical studies with regards to antioxidant activity, immunomodulatory function, antitumor activity, hypoglycemic and hypolipidemic effects, modulation of gut microbiota, prebiotic activity of jujuba polysaccharides.
In conclusion, t this review systematically summarizes the research progress on the preparation, structural characteristics, and biological activities of jujuba polysaccharides. A more detailed understanding of the structure, activity and structure-activity relationship of jujuba polysaccharides will offer valuable insights and references to facilitate their development and application in functional foods, nutritional supplements, and pharmaceuticals.
Author contributions
FJ: Writing – original draft, Writing – review and editing, Data curation. BW: Data curation, Software, Writing – original draft, Writing – review and editing. HM: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Writing – review and editing. CB: Formal Analysis, Funding acquisition, Writing – original draft, Writing – review and editing. YZ: Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (No. 81860755); Class A, “Western Light” and “Western Young Scholars” of the Chinese Academy of Sciences in 2019 (2009A-6).; Ningxia Natural Science Foundation (grant number. 2020 A0564); Ningxia Natural Science Foundation (grant number. 2020 A0450).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bahrami, S., Babaei, N., Esmaeili Gouvarchin Ghaleh, H., Mohajeri Borazjani, J., and Farzanehpour, M. (2024). Anti-inflammatory activity of Ziziphus jujuba hydroalcoholic extract in acetic acid-induced ulcerative colitis model. J. Complement. Integr. Med. 21, 481–489. doi:10.1515/jcim-2024-0178
Chen, J., Chan, P. H., Lam, C. T., Li, Z., Lam, K. Y., Yao, P., et al. (2015). Fruit of Ziziphus jujuba (Jujube) at two stages of maturity: distinction by metabolic profiling and biological assessment. J. Agric. Food Chem. 63, 739–744. doi:10.1021/jf5041564
Chen, J., Maiwulanjiang, M., Lam, K. Y., Zhang, W. L., Zhan, J. Y., Lam, C. T., et al. (2014). A standardized extract of the fruit of Ziziphus jujuba (Jujube) induces neuronal differentiation of cultured PC12 cells: a signaling mediated by protein kinase A. J. Agric. Food Chem. 62, 1890–1897. doi:10.1021/jf405093f
Chi, A., Kang, C., Zhang, Y., Tang, L., Guo, H., Li, H., et al. (2015). Immunomodulating and antioxidant effects of polysaccharide conjugates from the fruits of Ziziphus Jujube on Chronic Fatigue Syndrome rats. Carbohydr. Polym. 122, 189–196. doi:10.1016/j.carbpol.2014.12.082
Feng, L., Ju, M., Ma, C., Li, K., and Cai, S. (2025). Immunomodulatory acidic polysaccharide from jujube fruit (Zizyphus jujuba Mill.): insight into their chemical characteristics and modes of action. J. Agric. Food Chem. 73, 450–463. doi:10.1021/acs.jafc.4c06905
Gao, Q. H., Wu, C. S., and Wang, M. (2013). The jujube (Ziziphus jujuba Mill.) fruit: a review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 61, 3351–3363. doi:10.1021/jf4007032
Guo, X., Zhang, J., Cui, Y., Chen, S., Sun, H., Yang, Q., et al. (2019). Highly biocompatible jujube polysaccharide-stabilized palladium nanoparticles with excellent catalytic performance. New J. Chem. 43, 7646–7652. doi:10.1039/c9nj00950g
Guo, Y., Nan, S., Qiu, C., Song, C., Wu, B., Tang, Y., et al. (2024). Ultrasound-assisted enzymatic extraction of jujube (Ziziphus jujuba Mill.) polysaccharides: extraction efficiency, antioxidant activity, and structure features. Ultrason. Sonochem 111, 107088. doi:10.1016/j.ultsonch.2024.107088
Han, S., Hu, F., Ji, X., Liu, Y., Zhang, S., Wang, Z., et al. (2024). Polysaccharides from Ziziphus jujuba prolong lifespan and attenuate oxidative stress in Caenorhabditis elegans via DAF-16 and SKN-1. Int. J. Biol. Macromol. 282, 137482. doi:10.1016/j.ijbiomac.2024.137482
Han, X., Bai, B., Zhou, Q., Niu, J., Yuan, J., Zhang, H., et al. (2020). Dietary supplementation with polysaccharides from Ziziphus Jujuba cv. Pozao intervenes in immune response via regulating peripheral immunity and intestinal barrier function in cyclophosphamide-induced mice. Food Funct. 11, 5992–6006. doi:10.1039/d0fo00008f
He, Z., Zhu, Y., Bao, X., Zhang, L., Li, N., Jiang, G., et al. (2019). Optimization of alkali extraction and properties of polysaccharides from Ziziphus jujuba cv. Residue. Molecules 24, 2221. doi:10.3390/molecules24122221
Hua, Y., Xu, X. X., Guo, S., Xie, H., Yan, H., Ma, X. F., et al. (2022). Wild jujube (Ziziphus jujuba var. spinosa): a review of its Phytonutrients, health benefits, metabolism, and applications. J. Agric. Food Chem. 70, 7871–7886. doi:10.1021/acs.jafc.2c01905
Ji, X., Cheng, Y., Tian, J., Zhang, S., Jing, Y., and Shi, M. (2021a). Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem. Biol. Technol. Agric. 8, 54–57. doi:10.1186/s40538-021-00255-2
Ji, X., Cheng, Y., Tian, J., Zhang, S., Jing, Y., and Shi, M. (2021b). Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem. Biol. Technol. Agric. 8, 54. doi:10.1186/s40538-021-00255-2
Ji, X., Hou, C., Yan, Y., Shi, M., and Liu, Y. (2020a). Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 149, 1008–1018. doi:10.1016/j.ijbiomac.2020.02.018
Ji, X., Hou, C., Zhang, X., Han, L., Yin, S., Peng, Q., et al. (2019a). Microbiome-metabolomic analysis of the impact of Zizyphus jujuba cv. Muzao polysaccharides consumption on colorectal cancer mice fecal microbiota and metabolites. Int. J. Biol. Macromol. 131, 1067–1076. doi:10.1016/j.ijbiomac.2019.03.175
Ji, X., Liu, F., Ullah, N., and Wang, M. (2018a). Isolation, purification, and antioxidant activities of polysaccharides from Ziziphus Jujuba cv. Muzao. Int. J. Food Prop. 21, 1–11. doi:10.1080/10942912.2018.1425702
Ji, X., Peng, Q., Li, H., Liu, F., and Wang, M. (2017). Chemical characterization and anti-inflammatory activity of polysaccharides from Zizyphus jujube cv. Muzao. Int. J. Food Eng. 13, 20160382. doi:10.1515/ijfe-2016-0382
Ji, X., Peng, Q., Yuan, Y., Liu, F., and Wang, M. (2018b). Extraction and physicochemical properties of polysaccharides from Ziziphus Jujuba cv. Muzao by ultrasound-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 108, 541–549. doi:10.1016/j.ijbiomac.2017.12.042
Ji, X., Wang, Z., Hao, X., Zhu, Y., Lin, Y., Li, G., et al. (2022). Structural characterization of a new high molecular weight polysaccharide from jujube fruit. Front. Nutr. 9, 1012348. doi:10.3389/fnut.2022.1012348
Ji, X., Yan, Y., Hou, C., Shi, M., and Liu, Y. (2020b). Structural characterization of a galacturonic acid-rich polysaccharide from Ziziphus Jujuba cv. Muzao. Int. J. Biol. Macromol. 147, 844–852. doi:10.1016/j.ijbiomac.2019.09.244
Ji, X., Zhang, F., Zhang, R., Liu, F., Peng, Q., and Wang, M. (2019b). An acidic polysaccharide from Ziziphus Jujuba cv. Muzao: purification and structural characterization. Food Chem. 274, 494–499. doi:10.1016/j.foodchem.2018.09.037
Ji, X., Zhang, F., Zhang, R., Liu, F., Peng, Q., and Wang, M. (2019c). An acidic polysaccharide from Ziziphus Jujuba cv. Muzao: purification and structural characterization. Food Chem. 274, 494–499. doi:10.1016/j.foodchem.2018.09.037
Ji, X., Zhang, S., Jin, X., Yin, C., Zhang, Y., Guo, X., et al. (2023). Systematic comparison of structural characterization of polysaccharides from Ziziphus jujuba cv. Muzao. Molecules 28, 562. doi:10.3390/molecules28020562
Li, J., Ai, L., Hang, F., Ding, S., and Liu, Y. (2014). Composition and antioxidant activity of polysaccharides from jujuba by classical and ultrasound extraction. Int. J. Biol. Macromol. 63, 150–153. doi:10.1016/j.ijbiomac.2013.10.043
Li, J., Ai, L., Yang, Q., Liu, Y., and Shan, L. (2013). Isolation and structural characterization of a polysaccharide from fruits of Zizyphus jujuba cv. Junzao. Int. J. Biol. Macromol. 55, 83–87. doi:10.1016/j.ijbiomac.2012.12.017
Li, J., Liu, Y., Fan, L., Ai, L., and Shan, L. (2011). Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus Jujuba cv. Jinsixiaozao. Carbohydr. Polym. 84, 390–394. doi:10.1016/j.carbpol.2010.11.051
Li, L., Qiu, Z., Dong, H., Ma, C., Qiao, Y., and Zheng, Z. (2021). Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from the roots of Arctium lappa L.: a comparison. Int. J. Biol. Macromol. 182, 187–196. doi:10.1016/j.ijbiomac.2021.03.177
Li, W., Liang, H., Wang, W., Liu, J., Liu, X., Lao, S., et al. (2024). Global cancer statistics for adolescents and young adults: population based study. J. Hematol. Oncol. 17, 99. doi:10.1186/s13045-024-01623-9
Lin, T., Liu, Y., Lai, C., Yang, T., Xie, J., and Zhang, Y. (2018). The effect of ultrasound assisted extraction on structural composition, antioxidant activity and immunoregulation of polysaccharides from Ziziphus jujuba Mill var. spinosa seeds. Industrial Crops Prod. 125, 150–159. doi:10.1016/j.indcrop.2018.08.078
Lin, X., Ji, X., Wang, M., Yin, S., and Peng, Q. (2019). An alkali-extracted polysaccharide from Zizyphus jujuba cv. Muzao: structural characterizations and antioxidant activities. Int. J. Biol. Macromol. 136, 607–615. doi:10.1016/j.ijbiomac.2019.06.117
Lin, X., Liu, K., Yin, S., Qin, Y., Shen, P., and Peng, Q. (2020). A novel pectic polysaccharide of jujube Pomace: structural analysis and Intracellular antioxidant activities. Antioxidants (Basel) 9, 127. doi:10.3390/antiox9020127
Liu, G., Liu, X., Zhang, Y., Zhang, F., Wei, T., Yang, M., et al. (2015a). Hepatoprotective effects of polysaccharides extracted from Zizyphus jujube cv. Huanghetanzao. Int. J. Biol. Macromol. 76, 169–175. doi:10.1016/j.ijbiomac.2015.01.061
Liu, M., Wang, J., Wang, L., Liu, P., Zhao, J., Zhao, Z., et al. (2020a). The historical and current research progress on jujube-a superfruit for the future. Hortic. Res. 7, 119. doi:10.1038/s41438-020-00346-5
Liu, M.-j., Wang, J.-r., Liu, P., Zhao, J., Zhao, Z.-h., Dai, L., et al. (2015b). Historical achievements and frontier advances in the production and research of Chinese jujube (Ziziphus jujuba) in China. Acta Hortic. Sin. 42, 1683. doi:10.16420/j.issn.0513-353x.2015-0538
Liu, Q., Yi, Y. L., Liang, X. F., Wu, M. T., Li, J., Chen, X., et al. (2025). Antioxidant and antiaging activities of the polysaccharide ZJP-04M from Ziziphus jujuba in Caenorhabditis elegans. Int. J. Biol. Macromol. 284, 138208. doi:10.1016/j.ijbiomac.2024.138208
Liu, X.-X., Liu, H.-M., Yan, Y.-Y., Fan, L.-Y., Yang, J.-N., Wang, X.-D., et al. (2020b). Structural characterization and antioxidant activity of polysaccharides extracted from jujube using subcritical water. Lwt 117, 108645. doi:10.1016/j.lwt.2019.108645
Liu, X.-X., Liu, H.-M., Yan, Y.-Y., Fan, L.-Y., Yang, J.-N., Wang, X.-D., et al. (2020c). Structural characterization and antioxidant activity of polysaccharides extracted from jujube using subcritical water. Lwt 117, 108645. doi:10.1016/j.lwt.2019.108645
Liu, Y., Meng, Y., Ji, H., Guo, J., Shi, M., Lai, F., et al. (2024). Structural characteristics and antioxidant activity of a low-molecular-weight jujube polysaccharide by ultrasound assisted metal-free Fenton reaction. Food Chem. X 24, 101908. doi:10.1016/j.fochx.2024.101908
Liu, Y., Zhang, Y., Mei, N., Li, W., Yang, T., and Xie, J. (2023). Three acidic polysaccharides derived from sour jujube seeds protect intestinal epithelial barrier function in LPS induced Caco-2 cell inflammation model. Int. J. Biol. Macromol. 240, 124435. doi:10.1016/j.ijbiomac.2023.124435
McCay, P. B., Lai, E. K., Poyer, J. L., DuBose, C. M., and Janzen, E. G. (1984). Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism. Observation of lipid radicals in vivo and in vitro. J. Biol. Chem. 259, 2135–2143. doi:10.1016/s0021-9258(17)43327-8
Qu, C., Yu, S., Jin, H., Wang, J., and Luo, L. (2013). The pretreatment effects on the antioxidant activity of jujube polysaccharides. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 114, 339–343. doi:10.1016/j.saa.2013.05.084
Rostami, H., and Gharibzahedi, S. M. (2016). Microwave-assisted extraction of jujube polysaccharide: optimization, purification and functional characterization. Carbohydr. Polym. 143, 100–107. doi:10.1016/j.carbpol.2016.01.075
Siegel, R. L., Giaquinto, A. N., and Jemal, A. (2024). Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49. doi:10.3322/caac.21820
Șumălan, R. L., Copolovici, D. M., Crișan, M., Stănică, F., Șumălan, R. M., Lupitu, A., et al. (2025). Assessment of fruit traits and antioxidant capacity in Wild and cultivated Genotypes of Ziziphus sp. Plants (Basel) 14, 134. doi:10.3390/plants14010134
Sun, S., Lan, W., Ji, L., Ai, L., Wu, Y., and Zhang, H. (2022). A homogalacturonan from Peel of Winter jujube (Zizyphus jujuba Mill. Cv. Dongzao): characterization and protective effects against CCl(4)-induced liver injury. Foods 11, 4087. doi:10.3390/foods11244087
Wang, B. (2011). Chemical characterization and Ameliorating effect of polysaccharide from Chinese jujube on intestine oxidative injury by ischemia and reperfusion. Int. J. Biol. Macromol. 48, 386–391. doi:10.1016/j.ijbiomac.2010.12.005
Wang, D., Zhao, Y., Jiao, Y., Yu, L., Yang, S., and Yang, X. (2012). Antioxidative and hepatoprotective effects of the polysaccharides from Zizyphus jujube cv. Shaanbeitanzao. Carbohydr. Polym. 88, 1453–1459. doi:10.1016/j.carbpol.2012.02.046
Wang, N., Li, Q., Liu, M., Liu, M., and Zhao, Z. (2023). Structural characterization of alkali-extracted jujube polysaccharides and their effects on the fecal microbiota in vitro. Lwt 184, 115087. doi:10.1016/j.lwt.2023.115087
Wang, Y., Liu, X., Zhang, J., Liu, G., Liu, Y., Wang, K., et al. (2015). Structural characterization and in vitro antitumor activity of polysaccharides from Zizyphus jujuba cv. Muzao. RSC Adv. 5, 7860–7867. doi:10.1039/c4ra13350a
Wang, Y., Xu, Y., Ma, X., Liu, X., Yang, M., Fan, W., et al. (2018). Extraction, purification, characterization and antioxidant activities of polysaccharides from Zizyphus jujuba cv. Linzexiaozao. Int. J. Biol. Macromol. 118, 2138–2148. doi:10.1016/j.ijbiomac.2018.07.059
Wojdyło, A., Carbonell-Barrachina Á, A., Legua, P., and Hernández, F. (2016). Phenolic composition, ascorbic acid content, and antioxidant capacity of Spanish jujube (Ziziphus jujube Mill.) fruits. Food Chem. 201, 307–314. doi:10.1016/j.foodchem.2016.01.090
Wu, Z., Gao, R., Li, H., Wang, Y., Luo, Y., Zou, J., et al. (2021). New insight into the joint significance of dietary jujube polysaccharides and 6-gingerol in antioxidant and antitumor activities. RSC Adv. 11, 33219–33234. doi:10.1039/d1ra03640h
Wu, Z., Li, H., Wang, Y., Yang, D., Tan, H., Zhan, Y., et al. (2019). Optimization extraction, structural features and antitumor activity of polysaccharides from Z. jujuba cv. Ruoqiangzao seeds. Int. J. Biol. Macromol. 135, 1151–1161. doi:10.1016/j.ijbiomac.2019.06.020
Xi, J. (2017). Ultrahigh pressure extraction of bioactive compounds from plants-A review. Crit. Rev. Food Sci. Nutr. 57, 1097–1106. doi:10.1080/10408398.2013.874327
Xiao, F., Shao, S., Zhang, H., Li, G., Piao, S., Zhao, D., et al. (2022). Neuroprotective effect of Ziziphi Spinosae Semen on rats with p-chlorophenylalanine-induced insomnia via activation of GABA(A) receptor. Front. Pharmacol. 13, 965308. doi:10.3389/fphar.2022.965308
Xu, D., Xiao, J., Jiang, D., Liu, Y., Gou, Z., Li, J., et al. (2022). Inhibitory effects of a water-soluble jujube polysaccharide against biofilm-forming oral pathogenic bacteria. Int. J. Biol. Macromol. 208, 1046–1062. doi:10.1016/j.ijbiomac.2022.03.196
Xue, H., Zhang, P., Zhang, C., Gao, Y., and Tan, J. (2024). Research progress in the preparation, structural characterization, and biological activities of polysaccharides from traditional Chinese medicine. Int. J. Biol. Macromol. 262, 129923. doi:10.1016/j.ijbiomac.2024.129923
Yan, X., Wang, W., Liu, M., and Zhao, Z. (2018). Preparation of oligosaccharides by degradation of polysaccharides from Chinese jujube and its biological activity. Int. J. Polym. Sci. 2018, 6464051–6464058. doi:10.1155/2018/6464051
Yang, J., Li, Y., Wang, F., and Wu, C. (2010). Hepatoprotective effects of apple polyphenols on CCl4-induced acute liver damage in mice. J. Agric. Food Chem. 58, 6525–6531. doi:10.1021/jf903070a
Yang, Y., Qiu, Z., Li, L., Vidyarthi, S. K., Zheng, Z., and Zhang, R. (2021). Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao: a comparison. Carbohydr. Polym. 261, 117879. doi:10.1016/j.carbpol.2021.117879
Yuan, L., Qiu, Z., Yang, Y., Liu, C., and Zhang, R. (2022). Preparation, structural characterization and antioxidant activity of water-soluble polysaccharides and purified fractions from blackened jujube by an activity-oriented approach. Food Chem. 385, 132637. doi:10.1016/j.foodchem.2022.132637
Yue, Y., Wu, S., Li, Z., Li, J., Li, X., Xiang, J., et al. (2015). Wild jujube polysaccharides protect against experimental inflammatory bowel disease by enabling enhanced intestinal barrier function. Food Funct. 6, 2568–2577. doi:10.1039/c5fo00378d
Yue, Y., Wu, S., Zhang, H., Zhang, X., Niu, Y., Cao, X., et al. (2014). Characterization and hepatoprotective effect of polysaccharides from Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex HF Chou sarcocarp. Food Chem. Toxicol. 74, 76–84. doi:10.1016/j.fct.2014.09.006
Yu-Hao, D., Chun, C., Qiang, H., and Xiong, F. (2021). Study on a novel spherical polysaccharide from Fructus Mori with good antioxidant activity. Carbohydr. Polym. 256, 117516. doi:10.1016/j.carbpol.2020.117516
Zhan, R., Xia, L., Shao, J., Wang, C., and Chen, D. (2018). Polysaccharide isolated from Chinese jujube fruit (Zizyphus jujuba cv. Junzao) exerts anti-inflammatory effects through MAPK signaling. J. Funct. Foods 40, 461–470. doi:10.1016/j.jff.2017.11.026
Zhang, G., Liu, C., and Zhang, R. (2022). A novel acidic polysaccharide from blackened jujube: structural features and antitumor activity in vitro. Front. Nutr. 9, 1001334. doi:10.3389/fnut.2022.1001334
Zhang, L., Liu, X., Wang, Y., Liu, G., Zhang, Z., Zhao, Z., et al. (2017). In vitro antioxidative and immunological activities of polysaccharides from Zizyphus Jujuba cv. Muzao. Int. J. Biol. Macromol. 95, 1119–1125. doi:10.1016/j.ijbiomac.2016.10.102
Zhao, Y., Yang, X., Ren, D., Wang, D., and Xuan, Y. (2014). Preventive effects of jujube polysaccharides on fructose-induced insulin resistance and dyslipidemia in mice. Food Funct. 5, 1771–1778. doi:10.1039/c3fo60707k
Zhao, Z., Li, J., Wu, X., Dai, H., Gao, X., Liu, M., et al. (2006). Structures and immunological activities of two pectic polysaccharides from the fruits of Ziziphus jujuba Mill. cv. jinsixiaozao Hort. Food Res. Int. 39, 917–923. doi:10.1016/j.foodres.2006.05.006
Zhao, Z., Liu, M., and Tu, P. (2007). Characterization of water soluble polysaccharides from organs of Chinese Jujube (Ziziphus jujuba Mill. cv. Dongzao). Eur. Food Res. Technol. 226, 985–989. doi:10.1007/s00217-007-0620-1
Zhao, Z., Liu, M., and Tu, P. (2008). Characterization of water soluble polysaccharides from organs of Chinese Jujube (Ziziphus jujuba Mill. cv. Dongzao). Eur. Food Res. Technol. 226, 985–989. doi:10.1007/s00217-007-0620-1
Zou, M., Chen, Y., Sun-Waterhouse, D., Zhang, Y., and Li, F. (2018). Immunomodulatory acidic polysaccharides from Zizyphus jujuba cv. Huizao: insights into their chemical characteristics and modes of action. Food Chem. 258, 35–42. doi:10.1016/j.foodchem.2018.03.052
Zou, Q., Zhang, Y., Niu, X., Yang, H., Chu, M., Wang, N., et al. (2024). Antifungal activity of Rhizosphere Bacillus isolated from Ziziphus jujuba against Alternaria alternata. Microorganisms 12, 2189. doi:10.3390/microorganisms12112189
Keywords: Ziziphus jujuba polysaccharides, pharmacological activities, structure-activity relationship, structural characteristics, Ziziphus jujuba Mill
Citation: Jia F, Wang B, Ma H, Bai C and Zhang Y (2025) Research progress on extraction, separation, structure, and biological activities of polysaccharides from jujube fruit (Ziziphus jujuba Mill.): a review. Front. Chem. 13:1581947. doi: 10.3389/fchem.2025.1581947
Received: 23 February 2025; Accepted: 07 April 2025;
Published: 16 April 2025.
Edited by:
Wei Li, Toho University, JapanReviewed by:
Liqin Ding, Tianjin University of Traditional Chinese Medicine, ChinaChandra Has, GSFC University, India
Copyright © 2025 Jia, Wang, Ma, Bai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changcai Bai, Y2hhbmdjYWliYWlAMTYzLmNvbQ==; Yuanyuan Zhang, enl5MTAxMDFAMTI2LmNvbQ==; Hui Ma, MTczOTUxNjg4MzVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Feilong Jia1†
Feilong Jia1† Bo Wang
Bo Wang Changcai Bai
Changcai Bai Yuanyuan Zhang
Yuanyuan Zhang