
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 17 March 2025
Sec. Organic Chemistry
Volume 13 - 2025 | https://doi.org/10.3389/fchem.2025.1558075
This article is part of the Research TopicIsolation, Structural Elucidation, and Biological Evaluation of Bioactive Products from Traditional Medicine-Volume IIView all 5 articles
A bioassay-guided phytochemical study of the fruits of Cornus officinalis led to the isolation of six new iridoid glycoside dimers, named corndiridoside A-F (1–6), along with 11 analogs (7–17). The structure of these dimers was elucidated using HRESIMS, 1D and 2D NMR, IR, and UV spectra, as well as literature comparisons. The anti-inflammatory activity of all compounds was evaluated, revealing a significant inhibitory effect on all dimers on the production of NO in LPS-stimulated RAW 264.7 cells at concentrations of 25 and 50 μM. Of the six, compounds 2 and 3 showed the strongest anti-inflammatory activity.
Inflammation is a defensive response of host tissues to injuries, external pathogens, and foreign bodies; long-term inflammatory responses and persistent chronic inflammation can damage the body’s homeostasis (Kotas and Medzhitov, 2015). Inflammatory responses include both acute and chronic inflammation. Chronic inflammation is associated with diseases such as diabetes, obesity, cancer, atherosclerosis, neurological diseases, and atopic dermatitis (Headland and Norling, 2015; Germolec et al., 2018). Natural products with anti-inflammatory activity and extracted from plants play an important role in the development of anti-inflammatory drugs or functional foods.
Cornus officinalis plant belongs to the Cornaceae family and is mainly found in East Asia, including China, Korea, and Japan. Its dried mature fruits are widely used both as traditional Chinese medicine as well as healthy edible food and have anti-inflammatory, antioxidation, neuroprotective, and hypoglycemic effects (Huang et al., 2018; Gao et al., 2021). The extract of C. officinalis has shown significant anti-inflammatory activity in several studies (Sung et al., 2009; Quah et al., 2020; Yang et al., 2024). For example, Quanh et al. (2020) reported that the ethanol extract of C. officinalis had a potential therapeutic effect on atopic dermatitis by inhibiting iNOS mRNA expression, and pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and LPS-induced nitric oxide (NO) production in RWA 264.7 cells (Quah et al., 2020). Iridoid glycosides, gallate derivatives, and triterpenoids are considered to be anti-inflammatory components of C. officinalis (Jang et al., 2014; Yuan et al., 2020; Li et al., 2021). Of these components, iridoid glycosides, including monomers and dimers, are the main anti-inflammatory active ingredients in C. officinalis. Total cornel iridoid glycoside and some iridoid glycosides including morroniside, loganin, cornuside, and iridoid dimers have been reported to show significant anti-inflammatory activity by regulating different inflammatory factors (Ye et al., 2017; Park et al., 2021; Peng et al., 2022; Wang et al., 2022; Zheng et al., 2022; Tong et al., 2023; Yan et al., 2024). Although some iridoid glycosides with anti-inflammatory activity have been extracted from C. officinalis, their anti-inflammatory components remain poorly understood. To further elucidate the anti-inflammatory activity of iridoid glycosides, based on an in vitro anti-inflammatory activity test, the 50% ethanol aqueous extract of C. officinalis was chromatographically isolated to obtain the iridoid glycoside enrichment site with the strongest anti-inflammatory activity. Herein, six new iridoid glycoside dimers (1–6) and 11 (7–17) analogs were isolated from the strongest anti-inflammatory fraction, and their isolated procedures, and structural elucidation were reported in the present study. Additionally, the in vitro anti-inflammatory activity of the isolated compounds was measured on the LPS-stimulated RAW 264.7 cells model.
Nuclear magnetic resonance (NMR) spectroscopy of isolated compounds (CD3OD) was acquired on a Bruker AV-III-500 (500 MHz for 1H and 125 MHz for 13C) spectrometers (Bruker, Billerica, MA, United States). High-resolution electrospray ionization mass spectroscopy (HRESIMS) data were obtained using a Thermo QE Plus spectrometer (Thermo Scientific, Waltham, MA, United States). Optical rotation data were recorded using a Rudolph Research Autopol III automatic polar spectrometer (Rudolph Research Analytical, Zurich, Switzerland). Infrared (IR) spectra were recorded on a Nicolet Impact 400 FT-IR spectrophotometer (Nicolet, Madison, WI, United States). Furthermore, column chromatographical separation was performed on Diaion HP-20 macroporous resin (Mitsubishi Chemical Corporation, Tokyo, Japan), Sephadex LH-20 gel (Pharmacia Biotech AB, Uppsala, Sweden), silica gel (Qingdao Marin Chemical Inc., Qingdao, China). A Waters HPLC (Waters 2,545 controller with a Waters 2,998 dual-wavelength absorbance detector) with a Waters preparative Rp C18 chromatographic column (X-bridge, 250 mm × 19 mm, 5 μm) was employed for HPLC preparative (Waters, Milford, MA, United States). The chemical reagents, including analytical grade and chromatographic grade, were purchased from Tianjin Fuyu (Tianjin, China). The RAW 264.7 cells (No. 1101MOU-PUMC000146) used in this study were obtained from the National Infrastructure of Cell Line Resource (Peking Union Medical College, Beijing, China).
The matured and dried fruits of C. officinalis were obtained in June 2023 from the Beijing Hospital of Traditional Chinese Medicine (Beijing, China), and authenticated by Pro. Sheng Lin (Beijing University of Traditional Chinese Medicine). A voucher specimen (No. 20230101) has been deposited at the Department of Dermatology, Beijing Hospital of Traditional Chinese Medicine, Beijing, China.
The air-dried fruits (10 kg) of C. officinalis were powdered and extracted with 50% EtOH aqueous (100 L × 3) by ultrasound at room temperature. After filtration, the extract was evaporated under reduced pressure to obtain crude extract. After suspending into H2O, a Diaion HP-20 Macroporous Resin column was used to separate the extract with EtOH-H2O (0:100, 20:80, 40:60, 70:30, and 95:5, v/v), yielding five fractions (Fr.A –Fr. E). The EtOH-H2O (40:60) fraction (Fr. C) was further subjected to a silica gel chromatography eluting with CHCl3-MeOH (15:1 – 8:1, v/v) to afford six major fractions (Fr.C1 – Fr.C6). The anti-inflammatory activity test exhibited that Fr.C4 had the strongest inhibitory activity; therefore, it was selected as the target fraction for further separation. Fraction Fr.C4 was subjected to a reversed-phase C18 silica gel with MeOH-H2O (10:90 – 100:0, v/v) to obtain seven faction Fr.C4-1 ∼ Fr.C4-7. Subfraction Fr.C4-2 was further separated by a reversed-phase C18 silica gel eluting with MeOH-H2O (20:80 ∼ 100:0, v/v) to yield five fractions Fr.C4-2-1∼ Fr.C4-2-5. Subfraction Fr.C4-2-2 was separated by a Sephadex LH-20 gel column (CHCl3-MeOH 2:1, v/v) and further purified by preparative HPLC using MeCN-H2O (20:80, 18 mL/min) to yield compounds 7, 9, and 12. Subfraction Fr.C4-2-3 was subjected to a Sephadex LH-20 gel column eluting with CHCl3-MeOH (2:1, v/v) and further purified by preparative HPLC eluting with MeOH-H2O (40:60, 18 mL/min) to yield 4, 14 and 8. Fr.C4-2-4 was purified using preparative HPLC eluting with MeCN-H2O (20:80, 18 mL/min) to yield compounds 5 and 6. Subfraction Fr.C4-2-5 was given to a Sephadex LH-20 gel column eluting with CHCl3-MeOH (2:1, v/v) and further purified by using preparative HPLC (20% MeCN/H2O, 18 mL/min) to yield 1, 10 and 11. Fr.C4-3 was purified on a Sephadex LH-20 gel column with CHCl3-MeOH (2:1, v/v) elution and further prepared by HPLC (20% MeCN/H2O, 18 mL/min) to yield compounds 13 and 15. Fr.C4-4 was given to HPLC using 40% MeOH/H2O (18 mL/min) to yield 16 and 17. Fr.C4-6 was separated on a Sephadex LH-20 gel column with CHCl3-MeOH (2:1, v/v) and further purified by using preparative HPLC (25% MeCN/H2O, 18 mL/min) to yield compounds 2 and 3.
Corndiridoside A (1): White amorphous powder; [α] −45.6 (0.03, MeOH); UV (MeOH) λmax (log ε) 239 (3.95) nm; IR vmax 3,401, 2,916, 1703, 1,636, 1,077 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD) ( see Table 1); HRESIMS m/z 775.26660 [M−H]− (calculated for C34H47O20, 775.26662).
Corndiridoside B (2) White amorphous powder; [α] −65.6 (0.02, MeOH); UV (MeOH) λmax (log ε) 238 (4.00), 282 (3.24) nm; IR vmax 3,392, 2,939, 1,682, 1,639, 1,078 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD) see Table 1; HRESIMS m/z 901.29828 [M−H]− (calculated for C40H53O23, 901.29831).
Corndiridoside C (3) White amorphous powder; [α] −63.1 (0.02, MeOH); UV (MeOH) λmax (log ε) 238 (4.01), 282 (3.13) nm; IR vmax 3,424, 2,906, 1,681, 1,639, 1,076 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD) see Table 1; HRESIMS m/z 901.29816 [M−H]− (calculated for C40H53O23, 901.29831).
Corndiridoside D (4) White amorphous powder; [α] −35.6 (0.03, MeOH); UV (MeOH) λmax (log ε) 243 (4.00) nm; IR vmax 3,418, 2,922, 1,693, 1,616, 1,077 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD) see Table 2; HRESIMS m/z 745.25659 [M−H]− (calculated for C33H45O19, 745.25605).
Corndiridoside E (5) White amorphous powder; [α] −27.4 (0.02, MeOH); UV (MeOH) λmax (log ε) 244 (4.20) nm; IR vmax 3,393, 2,912, 1,696, 1,617, 1,074 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD) see Table 2; HRESIMS m/z 745.25513 [M−H]− (calculated for C33H45O19, 745.25605).
Corndiridoside F (6) White amorphous powder [α] −31.6 (0.02, MeOH); UV (MeOH) λmax (log ε) 239 (4.20) nm; IR vmax 3,403, 2,927, 1,697, 1,618, 1,074 cm−1; 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD) see Table 2; HRESIMS m/z 745.25549 [M−H]− (calculated for C33H45O19, 745.25605).
The acid hydrolysis method of each compound has previously been reported (Wang et al., 2018). Each compound (1–6, 1.0 mg) was dissolved in 2 M HCl (2.0 mL) and reacted at 80°C for 1.5 h. EtOAc was used to extract the reaction mixture and obtain an H2O layer. After evaporation and dilution by H2O, a neutral residue was obtained. Then, anhydrous pyridine (1.0 mL) and 1 mg of L-cysteine methyl ester hydrochloride was added. After stirring at 60°C for 2 h, the mixture was evaporated to dryness, and 0.1 mL of N-trimethylsilyl imidazole was added. After stirring 2 h at 60°C, the reaction mixture was extracted with n-hexane. The n-hexane extract was subjected to GC analysis with an HP-5 capillary column (30 m, 0.25 mm, Dikma), FID detection, 280°C detector temperature, and a stepwise heating from 160°C to 280°C at 5°C/min, using N2 as the carrier gas. Compared with the retention time of D-glucose (20.55 min), the sugar of all compounds was found to be D-glucose.
The anti-inflammatory activity of the isolated compounds was determined by a previously reported method (Xu et al., 2024). The cell viability assay was measured using CCK-8 assay and NO production in LPS-stimulated RAW264.7 cells was measured using Griess assay. All experiments were performed in triplicate. Data were processed using GraphPad Prism 9.0.
Guided by the anti-inflammatory activity test, six new iridoid glycoside dimers (1–6) and eleven known iridoid glycoside dimers (11–17) were isolated from the fraction with strong anti-inflammatory activity using chromatography techniques (Figure 1).
Compound 1 was obtained as an amorphous white powder. HRESIMS data exhibited an ion at m/z 775.26660 [M−H]−, confirming the molecular formula to be C34H48O20 with an unsaturation of 11. The IR spectra had absorption bands at 3,401 cm−1 (hydroxyl group), 1737 and 1703 cm−1 (carbonyl groups). The 1H NMR data (Table 1) of compound 1 displayed two olefinic protons at δH 7.52 (1H, s, H-3″) and 7.51 (1H, d, J = 1.1 Hz, H-3), four oxygenated methine protons at δH 5.66 (1H, d, J = 2.8 Hz, H-1), 5.88 (1H, d, J = 9.3 Hz, H-1″), 4.79 (1H, dd, J = 9.6, 2.3 Hz, H-6″), and 4.01 (1H, m, H-8″), two methoxy protons at δH 3.71 (3H, s, H-12) and 3.70 (3H, s, H-12″), and a series of glycosyl protons. The molecular formula and 13C NMR data suggested that structure 1 could be an iridoid dimer. 13C NMR data combined with HSQC spectrum suggested the presence of one ketone carbonyl carbon signal (δC 220.8), two ester carbonyl carbon signals (168.6 and 168.9), four olefin carbon signals (δC 111.1, 153.2, 110.8, 154.7), two methoxy carbon signals (δC 51.8 and 51.7), two secondary methyl carbon signals (δC 13.5 and 19.7), five acetal carbon signals (δC 94.9, 96.4, 99.7, 100.7, 103.3) and one oxygenated methine carbon signal (δC 74.4). A series of signals for two glycosyl compounds were displayed at δC 86.0, 78.7, 78.2, 78.0, 74.9, 73.2, 71.8, 70.2, 62.9, and 62.5. A comparison of the data for 1 with those of 7α-morroniside (Han et al., 2004) and 7-dehydrologanin (Chen et al., 2017) suggested that 1 might be a condensation product of the above two compounds.
Comprehensive analysis of the two dimensional NMR (2D NMR) data displayed the planar structure of 1 (Figure 1). 1H-1H COSY spectrum demonstrated the spin system involving C6-C5-C9-C1 and C8-C9, combined with the HMBC correlations from H-5 to C-7, from H-10 to C-9 and C-7, from H-1 to C-3, C-5 and C-1″, from H-6 to C-4, from H-3 to C-11, and from H-12 to C-11 indicated the presence of a 7-dehydrologanin unit (Figure 2). Additionally, 1H-1H COSY correlations of H-1′′/H-9″, H-9′′/H-8″, H-9′′/H-5″, H-5′′/H-6″ combined with HMBC correlations from H-8″ to C-5″ and C-7″, from H-7″ to C-5″, H-3″ to C-5″ and C-1‴ established the 7α-morroniside unit. The key HMBC correlations of H-7″ with C-3′ suggested the linkage of C-7″-O-C-3′. The ROESY spectra exhibited correlations of H-1/H-8, H-1′′/H-10″, suggesting H-1, H-8, H-1″, and H-10″ were co-facial and defined as α-orientation (Figure 2). Consequently, ROESY correlations of H-10/H-5, H-5/H-9, H-8′′/H-7′′/H-5″, H-5′′/H-9″ indicated the H-5, H-9, H-10, H-5″, H-7″, H-8″ and H-9″ were in β-oriented. Based on the fact that iridoid compounds in C. officinalis are all 5β and 9β configurations, according to the coupling constants and chemical shifts of H-1, H-1″, H-7″, the absolute configurations of C-1, C-1″, and C-7 were determined to be 1R, 1″R and 7″S. Furthermore, the configurations of C-1, C-5, C-8, C-9, C-1″, C-5″, C-7″, C-8″ and C-9″ were identified as 1R, 5S, 8R, 9S, 1″R, 5″S, 7″S, 8″S and 9″S, which were consistent with 7α-morroniside (Han et al., 2004) and 7-dehydrologanin (Chen et al., 2017). After acid hydroxylation and derivatization, 1 was confirmed as D-glucose by GC analysis. The coupling constants of the anomeric proton signal at δH 4.75 (1H, d, J = 7.8 Hz, H-1′) and 4.76 (1H, d, J = 7.8 Hz, H-1‴) confirmed the β-configuration of D-glucose. Compound 1 was assigned as corndiridoside A.
Compound 2 was obtained as an amorphous powder with the molecular formula C40H54O23 by HRESIMS m/z 901.29828 [M-H]- analysis. Its 1H NMR displayed four olefin protons at δH 6.67 (1H, d, J = 3.5 Hz, H-3′′′′), 7.38 (1H, d, J = 3.5 Hz, H-4′′′′), 7.51 (1H, s, H-3″), and 7.52 (1H, s, H-3), six acetal protons at δH 4.80 (1H, d, J = 7.9 Hz, H-1‴), 4.81 (1H, J = 7.9 Hz, H-1′), 4.87 (1H, d, J = 3.2 Hz, H-7″), 4.92 (1H, d, J = 3.2 Hz, H-7), 5.84 (1H, d, J = 9.3 Hz, H-1″), and 5.90 (1H, d, J = 9.3 Hz, H-1). There are two oxygenated methine protons at δH 4.30 (1H, m, H-8″) and 4.41 (1H, m, H-8), and oxygenated methylene protons at δH 4.59 (1H, d, J = 13.7 Hz, H-6a′′′′) and 4.63 (1H, d, J = 13.7 Hz, H-6b′′′′); a series of protons displayed between δH 3.24 ∼ 3.97 correspond to the sugar moieties. The 13C NMR data showed 40 carbon signals, including two carbonyl carbon signals at δC 168.6 and 168.6, one ketone carbonyl carbon signals at δC 179.5, eight olefin carbon signals at δC 111.7, 111.7, 112.9, 124.9, 154.2, 154.5, 154.6, and 160.0, six acetal carbon signals at δC 95.7, 95.8, 97.9, 99.1, 100.0, and 100.4, two oxygenated methine carbon signals at δC 62.8 and 66.3, one oxygenated methylene carbon signal at δC 61.9, two methoxy carbon signals at δC 51.7. These NMR data showed close similarity to those of 7β-morroniside (Han et al., 2004). Combined with the molecular formula, 2 was speculated as a 7β-morroniside dimer with a 5-hydroxymethylfurfuraly moiety. The planar structure of 2 (Figure 1) was confirmed by the comprehensive analysis of the 2D NMR data (Figure 2). The key HMBC correlations of H-7″ with C-6′ indicated linkage of C-6′-O-C-7″ between two 7β-morroniside moieties. The structure of 5-hydroxymethylfurfuraly moiety was determined by the key HMBC correlations of H-3′′′′ with C-1′′′′ and C-5′′′′, H-4′′′′ with C-2′′′′ and C-6′′′′, which was connected to 7β-morroniside through 7-O-C-6′′′′ according to the HMBC correlations of H-7 with C-6′′′′. The configuration was found similar to that of the 7β-morroniside moieties by the NOESY correlations analysis as shown in Figure 2 and consistent with 7β-morroniside. Thus, compound 2 was determined as corndiridoside B.
Compound 3 had the same molecular formula as 2 according to HREISMS (m/z 901.29816 [M-H]-) and NMR data (Table 1). The NMR spectrum of compound 3 was highly similar to that of 2, except for the chemical downshift of C-7″ and C-8′′ (from δC 99.1, 66.5 to δC 102.4, 74.1), which indicated that one of the 7β-morroniside in 2 was replaced by 7α-morroniside in 3. HMBC correlation of H-7″ with C-6′ confirmed that 7α-morroniside and 7β-morroniside were connected through C-6′-O-C-7′′ bond (Figure 2). The HMBC correlation of H-7 with C-6′′′′ indicated that the 5-hydroxymethylfurfural moiety was linked to 7α-morroniside via an ester bond. Furthermore, the NOESY correlation between H-7″ and H-8β confirmed that H-7 was the β-oriented, and the absolute configuration was the same as 7α-morroniside and 7β-morroniside; hydrolysis and GC analysis proved that the sugar groups were D-glucose, so compound 3 was identified as corndiridoside C.
Compound 4 was a white amorphous powder. The molecular formula of C33H46O19 was determined by the HRESIMS ion at m/z: 745.25659 [M−H]−. The 1H NMR data of 4 (Table 2) showed two four olefin protons, including two terminal olefinic protons at δH 5.53 (1H, m, H-8) and 5.29 (2H, m, H-10) and two olefin protons at δH 7.52 (1H, s, H-3″), 7.61 (1H, d, J = 2.4 Hz, H-3), five acetal protons at δH 5.87 (1H, d, J = 9.4 Hz, H-1″), 4.74 (1H, d, J = 7.8 Hz, H-1‴), 4.77 (1H, d, J = 7.9 Hz, H-1′), 5.56 (1H, d, J = 1.7 Hz, H-1), 4.80 (1H, d, J = 2.3 Hz, H-7″), one oxygenated methylene proton at δH 4.44 (1H, m, H-7a) and 4.46 (1H, m, H-7b), one oxygenated methine proton at δH 4.01 (1H, m, H-8″), one methoxy protons at δH 3.70 (3H, s, H-12″), and a series of protons at δH 3.19 ∼ 4.78 were assigned to the glycosyl groups. 13C NMR and HSQC data were assigned to 33 carbon signals. Among them, there was one methyl carbon (δC 19.7), one methoxy carbon signal (δC 51.7), two oxygenated olefin carbon signals (δC 154.7, 153.8), four olefin carbon signals (δC 106.2, 110.8, 133.3, 120.8), five acetal carbon signals (δC 103.0, 96.5, 100.8, 97.6, 99.1), one oxygenated methylene carbon signal (δC 69.8), one oxygenated methine carbon signal (δC 74.4), two methylene carbon signals (δC 35.5 and 25.9), four methine carbon signals (δC 28.4, 43.7, 32.1, 39.7), two carbonyl carbon signals (δC 168.7 and 168.5), and two sets of glycosyl carbons (δC 62.4 ∼ 100.8). The above data of 4 were very similar to those of 7α-morroniside (Han et al., 2004) and sweroside (Chen et al., 2017), indicating that 4 was an iridoid glycoside dimer. Detailed 2D NMR analysis confirmed the structures of the two moieties (Figure 2). In the 13C NMR data of sweroside, the chemical shift of C-2′ was significantly shifted up (from δC 74.9 to 73.2) and the chemical shift of C-3′ was significantly shifted down (from δC 78.9 to 85.7), indicating that sweroside and 7α-morroniside were connected via C-3′-O-C-7″, which was also confirmed by the HMBC correlation from H-7″ to C-3′. The NOESY spectral correlations of H-8/H-1, H-8/H-6a, H-6b/H-5, H-6b/H-9, H-1′′/H-10″, H-8′′/H-5″, H-8′′/H-7″, H-5′′/H-7″ and H-5′′/H-9″, combined with chemical shift and coupling constants, thereby confirming the structure of compound 4, which was named corndiridoside D.
Compound 5 has the molecular formula C33H46O19 by the HRESIMS ion at m/z: 745.25513 [M-H]-. Its NMR data (Table 2) was consistent with compound 4, except for the obvious chemical upshift of C-7′′ (4, δC 103.0; 5, δC 99.0 and C-8′′ (4, δC 74.4; 5, δC 66.5) indicated the presence of 7β-morroniside unit in 5 instead of 7α-morroniside in 4. The linkage of C-3′-O-C-7″ between two units was confirmed by the HMBC correlations of H-7″ and C-3′ (Figure 2). Compound 5 was determined as corndiridoside E.
Compound 6 has the same molecular formula of C33H46O19 as compounds 4 and 5 based on the HREISMS (m/z: 745.25549 [M-H]-) and NMR data (Table 2). The NMR data of 6 was very similar to those of 4, except for the chemical shifts of C-3′, C-4′, and C-5′. The upshift of C-3′ (from δC 85.7 in 4 to δC 77.4 in 6), C-5′ (from δC 78.1 in 4 to δC 76.9 in 6) and downshift of C-4′ (from δC 70.1 in 4 to δC 76.6 in 6) indicated that the 7α-morroniside moiety was linked via C-4′. HMBC correlation analysis of H-7″ at δH 4.93 and C-4′ at δC 76.6 confirmed that the C-7″ and C-4″ were lined via an ether bond (Figure 2). Thus, compound 6 was determined as corndiridoside F.
Comparing the NMR and HRESIMS data with literatures, 11 known compounds were identified as: cornuofficinaliside L (7) (Hao et al., 2023), cornuside C (8) (Ye et al., 2017), cornuside B (9) (Ye et al., 2017), cornuside E (10) (Ye et al., 2017), cornuside J (11) (Ye et al., 2017), cornuside A (12) (Ye et al., 2017), cornuside G (13) (Ye et al., 2017), cornuside K (14) (Ye et al., 2017), cornuofficinali side D (15) (Hao et al., 2023), cornuside L (16) (Ye et al., 2017), cornuside M (17) (Ye et al., 2017).
The inhibitory effects of the isolated compounds on NO production in LPS-stimulated RAW264.7 cells were evaluated. First, the cell viability assays exhibited that compounds 1–17 had no cytotoxic effect on RAW264.7 cells at a concentration below 50 μM (p > 0.05) (Supplementary Figure S1). Therefore, the inhibitory activity of compounds 1–17 on NO production in LPS-stimulated RAW264.7 cells was measured at concentrations of 12.5, 25, and 50 μM. As the results showed (Figure 3; Supplementary Table S1), compounds 1–17 showed significant anti-inflammatory activity at concentrations of 25 and 50 μM. Among them, compounds 2 and 3 exhibited the strongest anti-inflammatory activity in a dose-dependent manner, thereby suggesting that the 5-hydroxymethylfurfural group might enhance the activity. In addition, compound 14 showed the weakest anti-inflammatory activity compared with the other compounds, suggesting that the ethoxy group at the C-7 position might reduce its anti-inflammatory activity.
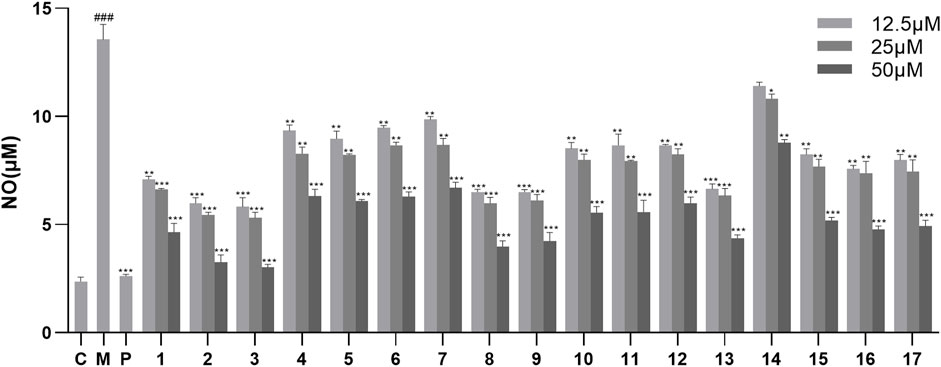
Figure 3. Effects of NO production in LPS-stimulated RAW246.7 cells treated with compounds 1–17. Data are expressed as mean ± S. D (n = 3). C = control, M = model, P = hydrocortisone. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. LPS-simulated group, ###p < 0.001 vs. control group.
In summary, six new iridoid glycoside dimers, named corndiridoside A-F (1–6), and eleven known analogs (7–17) were isolated from the anti-inflammatory active fraction of C. officinalis fruits in the current study. The constituent units in these dimers are composed of morrnoiside, 7-dehydrologanin, and sweroside analogs. Among them, compound 1, a dimer containing 7-dehydrologanin unit, was discovered for the first time from C. officinalis. In addition, their anti-inflammatory activities were assayed on the LPS-stimulated 264.7 RAW cell model. All compounds showed no cytotoxic effect on the cell viability of 264.7 RAW cells at 50 μM, and a majority of compounds exhibited significant anti-inflammatory activity at concentrations of 12.5, 25, and 50 μM. Compounds 2 and 3 containing 5-hydroxymethylfurfural group showed the strongest anti-inflammatory, indicating that the 5-hydroxymethylfurfural group might play an important role in enhancing anti-inflammatory activity. These compounds with anti-inflammatory activity may represent promising natural anti-inflammatory compounds that can be used in the development of drugs and functional foods. Moreover, this study also provides a basic scientific basis for the clinical anti-inflammatory application of C. officinalis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Y-CS: investigation, visualization, and writing–original draft. Y-XY: investigation, methodology, visualization, and writing–original draft. J-XG: formal analysis, methodology, and writing–original draft. XW: investigation, methodology, visualization, and writing–original draft. X-YS: methodology and writing–review and editing. JX: conceptualization, funding acquisition, project administration, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by Capital’s Funds for Health Improvement and Research (CFH) (No. 2024-3-7141).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2025.1558075/full#supplementary-material
Chen, Y. J., Wang, Z. B., Yu, Y., Gao, Y., Yang, C. J., Bi, X. Y., et al. (2017). Structural identification of iridoids from leaves of Hydrangea macrophylla. Chin. Tradit. Herb. Drugs 48, 232–235. doi:10.7501/j.issn.0253-2670.2017.02.002
Gao, X., Liu, Y., An, Z., and Ni, J. (2021). Active components and pharmacological effects of Cornus officinalis: literature review. Front. Pharmacol. 12, 633447. doi:10.3389/fphar.2021.633447
Germolec, D. R., Shipkowski, K. A., Frawley, R. P., and Evans, E. (2018). Markers of inflammation. Methods Mol. Biol. 1803, 57–79. doi:10.1007/978-1-4939-8549-4_5
Han, S. Y., Pan, Y., Ding, G., and Cai, B. C. (2004). The 1H-NMR and 13C-NMR application in structural identification of iridoid compounds in Cornus officinalis. Chin. Arch. Tradit. Chin. Med. 24, 56. doi:10.13193/j.archtcm.2004.01.55.hanshy.028
Hao, Z. Y., Wang, X. L., Yang, M., Cao, B., Zeng, M. N., Zhou, S. Q., et al. (2023). Minor iridoid glycosides from the fruits of Cornus officinalis Sieb. et Zucc. and their anti-diabetic bioactivities. Phytochemistry 205, 113505. doi:10.1016/j.phytochem.2022.113505
Headland, S. E., and Norling, L. V. (2015). The resolution of inflammation: principles and challenges. Semin. Immunol. 27 (3), 149–160. doi:10.1016/j.smim.2015.03.014
Huang, J., Zhang, Y., Dong, L., Gao, Q., Yin, L., Quan, H., et al. (2018). Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J. Ethnopharmacol. 213, 280–301. doi:10.1016/j.jep.2017.11.010
Jang, S. E., Jeong, J. J., Hyam, S. R., Han, M. J., and Kim, D. H. (2014). Ursolic acid isolated from the seed of Cornus officinalis ameliorates colitis in mice by inhibiting the binding of lipopolysaccharide to Toll-like receptor 4 on macrophages. J. Agric. Food Chem. 62 (40), 9711–9721. doi:10.1021/jf501487v
Kotas, M. E., and Medzhitov, R. (2015). Homeostasis, inflammation, and disease Susceptibility. Cell 160, 816–827. doi:10.1016/j.cell.2015.02.010
Li, H. B., Feng, Q. M., Zhang, L. X., Wang, J., Chi, J., Chen, S. Q., et al. (2021). Four new gallate derivatives from wine-processed Corni fructus and their anti-inflammatory activities. Molecules 26 (7), 1851. doi:10.3390/molecules26071851
Park, C., Lee, H., Kwon, C. Y., Kim, G. Y., Jeong, J. W., Kim, S. O., et al. (2021). Loganin inhibits lipopolysaccharide-induced inflammation and oxidative response through the activation of the Nrf2/HO-1 signaling pathway in RAW264.7 macrophages. Biol. Pharm. Bull. 44 (6), 875–883. doi:10.1248/bpb.b21-00176
Peng, Z., Wang, Y., He, J., Zhang, J., Pan, X., Ye, X., et al. (2022). Chemical constituents and their antioxidant and anti-inflammatory activities from edible Cornus officinalis fruits. Eur. Food Res. Technol. 248, 1003–1010. doi:10.1007/s00217-021-03940-6
Quah, Y., Lee, S. J., Lee, E. B., Birhanu, B. T., Ali, M. S., Abbas, M. A., et al. (2020). Cornus officinalis ethanolic extract with potential anti-allergic, anti-Inflammatory, and antioxidant activities. Nutrients 12 (11), 3317. doi:10.3390/nu12113317
Sung, Y. H., Chang, H. K., Kim, S. E., Kim, Y. M., Seo, J. H., Shin, M. C., et al. (2009). Anti-inflammatory and analgesic effects of the aqueous extract of Corni fructus in murine RAW 264.7 macrophage cells. J. Med. Food 12 (4), 788–795. doi:10.1089/jmf.2008.1011
Tong, Q., Xi, J., Cao, Y., He, R., Zhao, Y., Shao, Y., et al. (2023). Morroniside delays NAFLD progression in fructose-fed mice by normalizing lipid metabolism and inhibiting the inflammatory response. J. Food Biochem. 2023, 1–13. doi:10.1155/2023/9952583
Wang, X., Liu, C. H., Li, J. J., Zhang, B., Ji, L. L., and Shang, X. Y. (2018). Iridoid glycosides from the fruits of Cornus officinalis. J. Asian. Nat. Prod. Res. 20, 934–942. doi:10.1080/10286020.2018.1497609
Wang, X., Wu, Y., Wang, Z. Y., Li, J. J., Li, W., Li, Q., et al. (2022). Anti-inflammatory iridoid glycosides from fruits of Cornus officinalis. Phytochem. Lett. 52, 122–125. doi:10.1016/j.phytol.2022.10.006
Xu, Q. J., Liu, J. C., Huang, C. J., Wang, X., and Shang, X. Y. (2024). Seco-nortriterpenoids from Cirsium setosum and their anti-inflammatory activity. Fitoterapia 175, 105879. doi:10.1016/j.fitote.2024.105879
Yan, F., Wang, L., Zhang, J., Liu, Z., Yu, B., Li, W., et al. (2024). Cornuside alleviates psoriasis-like skin lesions in mice by relieving inflammatory effects. Int. Immunopharmacol. 134, 112183. doi:10.1016/j.intimp.2024.112183
Yang, H., Liu, H., Zheng, Y., Li, B., Wang, S., Zhang, J., et al. (2024). Cornus Officinalis total glycosides alleviate granulomatous lobular mastitis via the B7-CD28/CTLA-4 costimulatory pathway. Chem. Biodivers. 22, e202401539. doi:10.1002/cbdv.202401539
Ye, X. S., He, J., Cheng, Y. C., Zhang, L., Qiao, H. Y., Pan, X. G., et al. (2017). Cornusides A-O, bioactive iridoid glucoside dimers from the fruit of Cornus officinalis. J. Nat. Prod. 80 (12), 3103–3111. doi:10.1021/acs.jnatprod.6b01127
Yuan, J., Cheng, W., Zhang, G., Ma, Q., Li, X., Zhang, B., et al. (2020). Protective effects of iridoid glycosides on acute colitis via inhibition of the inflammatory response mediated by the STAT3/NF-кB pathway. Int. Immunopharmacol. 81, 106240. doi:10.1016/j.intimp.2020.106240
Keywords: Cornus officinalis, iridoid glycoside dimers, isolation, structure identification, anti-inflammatory activity
Citation: Shi Y-C, Yu Y-X, Gao J-X, Wang X, Shang X-Y and Xu J (2025) Iridoid glycoside dimers from fruits of Cornus officinalis and their anti-inflammatory activity. Front. Chem. 13:1558075. doi: 10.3389/fchem.2025.1558075
Received: 09 January 2025; Accepted: 19 February 2025;
Published: 17 March 2025.
Edited by:
Wei Li, Toho University, JapanReviewed by:
Bingyang Zhang, Tianjin University of Traditional Chinese Medicine, ChinaCopyright © 2025 Shi, Yu, Gao, Wang, Shang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Xu, ZG9jdG9yeHVqaWFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.