
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Chem., 25 February 2025
Sec. Chemical Biology
Volume 13 - 2025 | https://doi.org/10.3389/fchem.2025.1543455
This article is part of the Research TopicExploration of the Role of Heme Proteins in Biology with Experimental and Computational MethodsView all 7 articles
Oxidative stress is considered as the root-cause of different pathological conditions. Transition metals, because of their redox-active states, are capable of free radical generation contributing oxidative stress. Hemoglobin and myoglobin are two major heme proteins, involved in oxygen transport and oxygen storage, respectively. Heme prosthetic group of heme proteins is a good reservoir of iron, the most abundant transition metal in human body. Although iron is tightly bound in the heme pocket of these proteins, it is liberated under specific circumstances yielding free ferrous iron. This active iron can react with H2O2, a secondary metabolite, forming hydroxyl radical via Fenton reaction. Hydroxyl radical is the most harmful free radical among all the reactive oxygen species. It causes oxidative stress by damaging lipid membranes, proteins and nucleic acids, activating inflammatory pathways and altering membrane channels, resulting disease conditions. In this review, we have discussed how heme-irons of hemoglobin and myoglobin can promote oxidative stress under different pathophysiological conditions including metabolic syndrome, diabetes, cardiovascular, neurodegenerative and renal diseases. Understanding the association of heme proteins to oxidative stress may be important for knowing the complications as well as therapeutic management of different pathological conditions.
Heme proteins, a large class of metallo proteins with heme prosthetic group, play crucial roles in human physiology (Tsiftsoglou et al., 2006), including oxygen transport (hemoglobin), oxygen (O2) storage (myoglobin), antioxidation (peroxidases, catalases), electron transfer (cytochromes), signal transduction (guanylate cyclase), and metabolic processes as enzymes (cyclooxygenase, nitric oxide synthase, etc.) (Table 1).
Heme group consists of a central iron cation bound within a planar ring called protoporphyrin IX (Figure 1). The ring is made up of four pyrrole groups that are joined together by methine bridges. The iron is coordinated by four nitrogen atoms from the protoporphyrin ring. Among the two axial positions of iron, one position is available to bind with amino acid residue from the protein, usually a histidine. The other axial position remains free to bind with molecules like O2. This structure gives heme proteins their abilities to bind oxygen and participate in redox reactions (Smith et al., 2010; Ahmed et al., 2020). The iron in heme moiety typically switches between ferrous (Fe2+) or ferric (Fe3+) state (Kumar and Bandyopadhyay, 2005). Although Fe2+ and Fe3+ states are most common within the heme structure, oxidative states of heme-iron can vary from Fe2+ to Fe5+ (Karpefors et al., 2000; Dey and Ghosh, 2002).
Most of the oxygen, consumed during cellular respiration, is finally reduced to water molecule (H2O) through a four-electron transfer reaction catalyzed by cytochrome oxidase in complex IV of mitochondrial electron transport chain (ETC) (Wilson, 2017). The reaction is coupled with oxidative phosphorylation to produce adenosine triphosphate (ATP). However, a small portion of O2 undergoes partial reduction not only in the respiratory chain, but also during other physiological activities, such as phagocytosis, immune activation and xenobiotics metabolism (Galaris and Pantopoulos, 2008). This leads to formation of potentially harmful intermediates, collectively known as reactive oxygen species (ROS) including superoxide anion (O2.-), hydrogen peroxide (H2O2), hydroxyl radicals (.OH) etc (Table 2). To protect cells from ROS-mediated oxidative damages, several substances called antioxidants are absolutely essential. Under physiological condition, a robust antioxidant mechanism, comprising both enzymatic and non-enzymatic pathways, maintains tight balance between ROS generation and elimination (Kozlov et al., 2024). Oxidative stress, generated due to relative excess of ROS when compared with cellular antioxidants, has been linked to different disease conditions, namely, metabolic syndrome (Martemucci et al., 2023; Masenga et al., 2023), diabetes mellitus (Wronka et al., 2022), cardiovascular disease (Jin and Kang, 2024), neurodegenerative disease (Olufunmilayo et al., 2023), renal dysfunction (Ho and Shirakawa, 2023) and many other pathologies.
Transition metals are able to catalyze the reduction of H2O2, a secondary metabolite, to highly reactive hydroxyl radical (.OH). This is especially prominent with metals like iron (Fe) and copper (Cu), which can readily change the oxidation states (Collin, 2019). Iron is considered as the most biologically relevant transition metal in this regard due to its high concentration in the human body (Song et al., 2022). Because of their diverse biological functions and widespread abundance, heme proteins are among the most studied biomolecules. However, being a major group of proteins in our system, heme proteins, due to their iron-containing porphyrin ring, are also responsible for ROS generation in different pathological conditions (Drvenica et al., 2022; Wilson and Reeder, 2022). In this review, we have discussed pathophysiology of two major heme proteins, hemoglobin and myoglobin, based on their roles in oxidative stress. Transition of hemoglobin between oxy and deoxy form (both in ferrous state) facilitates the transportation of oxygen in different tissues, while methemoglobin or ferrihemoglobin is not capable of oxygen transport. On the other hand, myoglobin, with its higher affinity for oxygen, is involved in oxygen storage in cardiac and skeletal muscle tissues. Besides their important biological functions to maintain cell health, hemoglobin and myoglobin can also contribute to oxidative stress, understanding of which is important to know the disease pathology.
Heme prosthetic group of heme proteins is a major reservoir of iron in human body (Gozzelino et al., 2010). Iron homeostasis is tightly regulated to avoid accumulation of excess free heme (heme group not bound to any protein) (Sawicki et al., 2015). However, the free heme pool can increase under different pathological conditions like sickle cell anemia (Gbotosho et al., 2020), thalassemia (Ali et al., 2021), malaria (Ramos et al., 2024) and paroxysomal nocturnal hemoglobinuria (Gembillo et al., 2020). The underlying causes may be upregulation of heme synthesis, excess hemolysis or myolysis, elevated heme protein degradation, compromised integration of heme into heme proteins, or impaired heme oxygenase activity (Voltarelli et al., 2023). Although all transition metals have the ability to reduce H2O2 to hydroxyl radical (.OH), iron is considered as the most biologically active in this regard, because of its high abundance in the human body (Kontoghiorghe et al., 2015). Free heme acts as a good source of ferrous (Fe2+) ion to generate hydroxyl radical (.OH) through Fenton reaction (Jomova et al., 2023). In this reaction, iron, in its lower oxidation state (Fe2+), reacts with H2O2 to produce hydroxyl radical (.OH), a highly reactive free radical, and itself is oxidized to a higher oxidation state (Fe3+) (Figure 2) (Sadrzadeh et al., 1984; Thomas et al., 2009).

Figure 2. Fenton reaction. Ferrous (Fe2+) ion reacts with hydrogen peroxide (H2O2) to generate Ferric (Fe3+) ion, hydroxyl radical (.OH) and hydroxyl ion (OH−).
Hydroxyl radical (.OH), due to its strong oxidizing property, is capable of severe oxidative damages of biomolecules (Collin, 2019). Heme-driven production of ROS is involved in the pathophysiology of several disorders by damaging lipid membranes (Su et al., 2019), proteins (Pilo et al., 2022) and nucleic acids (Carter et al., 2022), activating inflammatory pathways (Wei et al., 2024), and perturbing membrane channels (Miranda et al., 2023), among other toxic effects.
Free heme is hydrophobic in nature (Tolosano et al., 2010). Because of high degree of lipophilicity, it easily intercalates into phospholipid bilayer of cell membrane and organelles (Higdon et al., 2012). Within oxidizing environment of membrane, H2O2 from various sources (e.g., activated leukocytes) cleaves the heme ring and interacts with the free redox-active iron, leading to enhanced production of hydroxyl radical (Chiabrando et al., 2014). This promotes membrane damage by lipid peroxidation, resulting increased membrane permeability and ultimately leading to cell death (Ryter, 2021).
Further, acting as a potent hemolytic agent, free heme affects stability of red blood cell membrane due to ROS generation and oxidative damage (Deuel et al., 2016), causing release of hemoglobin. Cell-free hemoglobin from high-intensity hemolysis is primarily eliminated from blood by renal clearance (Bolisetty et al., 2017). Post-filtration, the progressive acidification of the urine accelerates hemoglobin oxidation, globin structural destabilization, and heme release (Balla and Zarjou, 2021). Thus, heme-mediated oxidative stress and intravascular hemolysis of red blood cells are related to acute kidney injury (Vallelian et al., 2022).
Hyperglycemia is a condition characterized by elevated circulating blood glucose level. Persistent hyperglycemic state is the primary feature of metabolic syndrome (Cole and Florez, 2020) and diabetes (Minniakhmetov et al., 2024). Glucose and its oxidation by-products slowly but irreversibly react with the amino groups of long-life proteins (Khalid et al., 2022). The reaction is known as non-enzymatic glycosylation or glycation (also called Maillard reaction), leading to the formation of a heterogeneous set of compounds, known as advanced glycation end-products (AGEs) (Uceda et al., 2024). The sequence of non-enzymatic reactions leading to formation of AGEs has been shown schematically (Figure 3A). The reaction occurs when a carbonyl group of a reducing sugar (glucose, fructose, etc.) is exposed to an amino group of protein, leading to Schiff base formation, followed by Amadori rearrangement and formation of Amadori product. The Amadori product undergoes irreversible oxidation, dehydration, enolisation, cyclisation, and fragmentation leading to the formation of reactive intermediate AGE precursors. Reactive AGE precursors interact with lysine or arginine residues of proteins to form AGEs. Several important AGEs are pentosidine, N (6)-carboxymethyl lysine (CML), N (6)-carboxyethyl lysine (CEL), glyoxal-lysine dimer (GOLD), methylglyoxal-lysine dimer (MOLD), pyrraline, etc. Their structures are shown in Figure 3B. A specific cell surface receptor for AGEs (RAGE) has been shown to mediate inflammatory signal transduction via activation of NFκB, and p21 Ras (Deepu et al., 2024; Rojas et al., 2024).
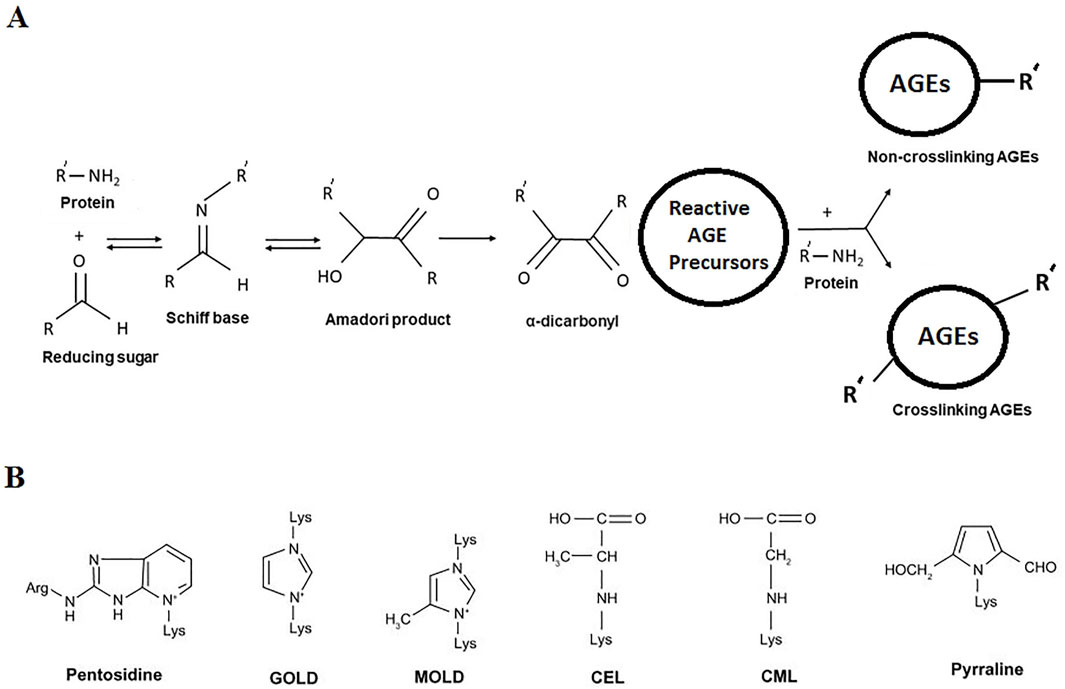
Figure 3. (A) Overview of non-enzymatic glycation (Maillard reaction) leading to formation of AGEs. (B) Structures of common AGEs.
Chronic hyperglycemia increases non-enzymatic glycation of proteins in metabolic syndrome and diabetes (Rabbani and Thornalley, 2021). Concentration of major glycosylated hemoglobin HbA1c, in which glucose is linked to N-terminal valine residues of β-chains increases proportionately with progression of hyperglycemia (Woleffenbuttel et al., 1996) and is used to monitor the extent or control of the disease condition. Several in vitro studies (Roy et al., 2004; Sen et al., 2005; Sen et al., 2007; Roy et al., 2010) have reported that, free iron release increases from the glycated form of major heme proteins, hemoglobin and myoglobin, resulting iron-mediated ROS generation by Fenton reaction. These findings are well supported by experimental studies in diabetic animals (Roy et al., 2008; Sen et al., 2011). Clinical studies on diabetic patients have reported a positive correlation between the serum free iron, glycated hemoglobin and fasting blood glucose (Kar and Chakraborti, 1999; Thomas et al., 2004; Zafar et al., 2011; Senghor et al., 2012; Achuthan and Mageswari, 2017). The sequence of hyperglycemia-associated protein glycation and free radical reactions has been shown in Figure 4.
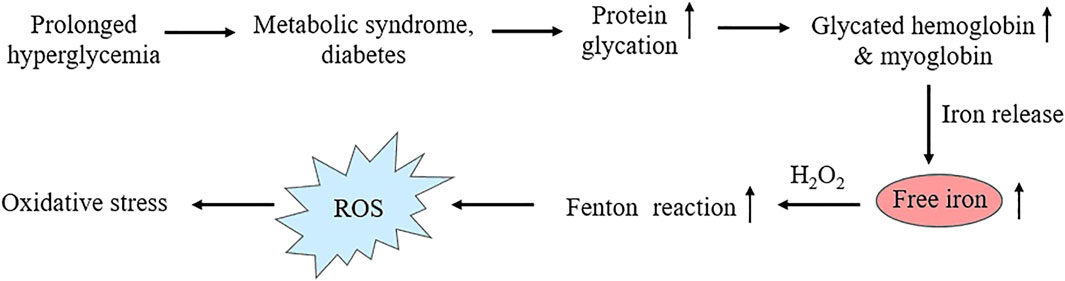
Figure 4. Schematic representation showing heme-protein glycation under hyperglycemic conditions, resulting enhanced glycated hemoglobin and myoglobin level followed by free iron release and Fenton reaction leading to oxidative stress.
In addition to glucose, several other reducing monosaccharides may initiate Maillard type reaction. For example, fructose is almost eight times more reactive than glucose (Bunn and Higgins, 1981). Like glucose, fructose (fructation) also induces structural and functional modification of hemoglobin leading to release of iron and iron-mediated oxidative reactions (Bose and Chakraborti, 2008). High concentration of fructose induces metabolic syndrome, which is characterized by insulin resistance, hyperglycemia, dyslipidemia and obesity (Taskinen et al., 2019). Animal model of this metabolic disorder also exhibits enhanced hemoglobin glycation, leading to increased iron release and oxidative reactions (Sil et al., 2013; Sil et al., 2015). Metabolic syndrome and diabetes are associated with elevated oxidative damage and inflammation (Darenskaya et al., 2021; Masenga et al., 2023). Free iron released from glycated hemoglobin leads to oxidative stress in these pathological conditions (Shetty et al., 2008).
In addition, a variety of highly reactive α-oxoaldehydes such as, 3-deoxy-glucosone, glyoxal and methylglyoxal are formed by auto-oxidation of glucose, Schiff base or Amadori products. The concentration of α-oxoaldehydes increases significantly in diabetic patients (Lapolla et al., 2003). These carbonyl intermediates can react again with free amino groups of proteins to form different AGEs (Thornally et al., 1999). Methylglyoxal has been reported to cause structural and functional modifications of heme proteins hemoglobin and myoglobin (Bose et al., 2013; Banerjee and Chakraborti, 2013; Banerjee et al., 2016).
If hyperglycemia is therapeutically controlled, heme protein glycation and free iron-mediated oxidative reactions are significantly reduced, as shown by using different phytoconstituents in experimental diabetes (Roy et al., 2008; Sen et al., 2011) and metabolic syndrome (Sil et al., 2013; 2015). Compared to chemical drugs, herbal therapeutic agents exhibit less or almost no side-effects during long-term uses (Jun et al., 2021; Kushwah et al., 2023; Guan et al., 2024). However, the limited solubility of most of the herbal components in aqueous media causing poor bioavailability restrict their therapeutic applications. To make them bioavailable, different techniques of nanonization have been developed (Hillaireau and Couvreur, 2009; Singh et al., 2017; Tran and Tran, 2019). Nanoformulations enhance the therapeutic potential of herbal agents in treatment of different diseases. Phytoconstituents in different nanocarriers exhibit better management of hyperglycemia and associated complications (Mukhopadhyay and Prajapati, 2015; Samaddar et al., 2017; Sun et al., 2020; Hou et al., 2021; Maity et al., 2022). Moreover, compared to free herbal agents, herbal agent-nanoparticle conjugates (entrapped, enveloped or tagged) appear to be more effective in preventing heme protein glycation and free iron-mediated oxidative reactions in experimental models (Bhattacherjee et al., 2016; Bhattacherjee et al., 2017; Roy et al., 2017; Mukhopadhyay et al., 2018; Maity and Chakraborti, 2020). These findings further confirm the role of heme proteins in hyperglycemia-associated oxidative stress.
Cardiovascular disease indicates problems in heart or blood vessels, including narrowing of the blood vessels in heart, other organs or throughout the body (Kim et al., 2023a). The condition is frequently associated in patients with chronic hyperglycemia (Rosengren and Dikaiou, 2023). Atherosclerosis is considered as the main underlying cause of cardiovascular disease (Poznyak et al., 2022). Oxidative stress, inflammation, endothelial dysfunction, and altered lipid metabolism are potential mechanisms leading to atherosclerosis (Jebari-Benslaiman et al., 2022). Oxidation of low-density lipoprotein (LDL) particles in the vascular endothelium has been reported to be an initial event in the atherosclerotic plaque formation (Khatana et al., 2020). The atherosclerotic plaques, especially the necrotic core of unstable plaques, contain apoptotic macrophages, erythrocytes and its metabolites - heme and hemoglobin (Li et al., 2006), and cytotoxic substances (cholesterol crystals, cholesterol esters, oxidized lipids, fibrin, inorganic minerals like hydroxyapatite, iron, and calcium) (Tong et al., 2023).
Several epidemiologic and experimental studies have shown an association between iron and atherosclerosis (Ma et al., 2022; Naito et al., 2022). Iron accumulates in the plaque either as inorganic or hemoglobin-bound iron (Vinchi et al., 2014; Vinchi et al., 2020). Heme-derived iron from hemoglobin can access the plaque upon intravascular hemolysis and intraplaque hemorrhage, affecting endothelial cells and macrophages (Turpin et al., 2021). In various types of cardiovascular diseases, impaired metabolism and exposure to heme occur in pathological processes, including neovascularization, internal hemorrhage, ischemia, and reperfusion (Guo et al., 2021). Free iron, heme and hemoglobin increase LDL oxidation, resulting enhanced sub-endothelial LDL retention favoring plaque progression (Vinchi et al., 2014; Michel and Martin-Ventura, 2020; Meegan et al., 2021).
The underlying mechanism is based on heme-iron accumulation, causing activation of multiple signaling pathways and impacting cell interactions within the atherosclerotic lesion (Choi et al., 2021; Gusev and Sarapultsev, 2023). Catalytically active iron is involved in producing ROS and promoting lipid peroxidation, which is crucial in the development of atherosclerosis. ROS generated by iron overload can damage DNA, proteins, and lipid structures in cell membranes, ultimately accelerating cardiomyocyte death (Drvenica et al., 2022). Thus, heme iron-mediated ROS generation plays an important role in physiological signaling pathways related to cardiovascular tissue injury and disease.
Intravascular hemolysis, i.e., destruction of red blood cells is a fundamental feature of chronic hereditary and acquired hemolytic anemias (Kato et al., 2017), including those associated with hemoglobinopathies, complement disorders and vector-borne disease such as malaria (Schaer et al., 2013). Hemolysis results in the presence of excess amount of cell-free hemoglobin and heme in blood circulation, compared to the levels of their scavengers haptoglobin and hemopexin, respectively (Kumar and Bandyopadhyay, 2005). Free hemoglobin and heme present in plasma are filtered by the kidney, exposing the kidney to the injurious effects of heme and iron (Van-Avondt et al., 2019). Rhabdomyolysis can be induced by hereditary as well as acquired factors (Barbano et al., 2015; Stahl et al., 2020). The hereditary factors include metabolic myopathies occurring due to disorders of fatty acid oxidation, glycogen metabolism, purine nucleotide cycle, muscular dystrophies, calcium influx and caveolinopathy, etc. On the other hand, excess physical activity, influence of extreme temperatures, crush injury and trauma, vascular ischemia, drug toxicity, infections and sepsis, endocrine disorders, hyperthermia, electric current, toxins and alcohol, etc., may contribute as acquired factors leading to rhabdomyolysis. It is a clinical syndrome caused by skeletal muscle damage and release of its breakdown products including myoglobin into the circulation, followed by myoglobinuria and acute kidney injury (Gupta et al., 2021). Thus, both hemolysis and myolysis cause exposure of kidney to free heme proteins - hemoglobin and myoglobin (Van-Avondt et al., 2019; Nath et al., 2022).
High renal oxygen demand is associated with tubular oxygen consumption which is necessary for solute reabsorption (Bullen et al., 2017). In this oxygen-rich environment, H2O2, present in urine, promotes oxidation of heme proteins resulting conversion of ferrous (Fe2+) iron to ferric (Fe3+) state, accompanied by the generation of superoxide radical. Further oxidation of heme proteins causes redox cycling between ferric (Fe3+) and ferryl (Fe4+) forms, finally leading to heme degradation and iron release (Nath et al., 2022). Free iron then increases hydroxyl radical generation by Fenton reaction (Andrianova et al., 2020; Rashid et al., 2023). Moreover, urinary acidification heightens lipid peroxidation caused by ferryl (Fe4+) -form of heme proteins and the accompanying generation of the potent renal vasoconstrictor, isoprostanes (Karamouzis et al., 2008). Isoprostanes are prostaglandin-like compounds that are generated by free radical-induced oxidation of membrane arachidonic acids (Milne et al., 2011). Excessive plasma and urinary isoprostanes are established biomarkers of oxidative stress in humans with chronic kidney disease (Granick et al., 2021). The findings suggest that heme protein-induced oxidative stress may act as a key mediator in acute renal injury as well as chronic kidney dysfunction.
Neurodegeneration is a complex process resulting in progressive and selective loss of neuronal functions (Katsnelson et al., 2016). Oxidative stress (Kim et al., 2015), protein aggregation (Sweeney et al., 2017), mitochondrial dysfunction (Bustamante-Barrientos et al., 2023) and endoplasmic reticulum stress (Ghemrawi and Khair, 2020) are well established pathways driving neurodegenerative processes.
Several findings suggest that dysfunction in iron and heme metabolism plays a crucial role in Parkinson’s disease and other neurodegenerative disorders (Carocci et al., 2018; Chiabrando et al., 2018; Ndayisaba et al., 2019). Neurodegeneration is often triggered by intracerebral hemorrhage (Schrag and Kirshner, 2020; Watson et al., 2022). The toxic properties of heme in the brain have been observed in intracerebral hemorrhage (Bulters et al., 2018; Pandya et al., 2021; Vasconcellos and Pimentel-Coelho, 2022). The intra-cerebral hemorrhage occurs with a bleeding event and the extravasation of blood components into brain parenchyma (Wu et al., 2002). With time, extravasated erythrocytes are lysed, releasing cytosolic components in the brain, including huge amounts of hemoglobin (Wagner et al., 2003). In the highly oxygen-rich environment of the brain, free hemoglobin in extracellular spaces undergoes oxidation and releases heme as well as iron in their free form. Iron has an inflammatory and pro-oxidative potential with the ability to activate the inflammasome, promoting oxidative stress, lipid peroxidation, inflammatory response and finally cell death (Righy et al., 2018; Sun et al., 2022). The involvement of iron in neurodegeneration has been well documented (Dusek et al., 2016; Ashraf et al., 2018), and iron chelation has been proposed as a therapeutic option in this disorder (Kupershmidt and Youdim, 2023; Marupudi and Xiong, 2024). It has been reported that intracellular iron-overload contributes to neuronal cell death via apoptosis and ferroptosis pathways (Zeng et al., 2021), while desferrioxamine (DFO), a well-known iron chelator, inhibits ferroptosis in Parkinson’s disease cell model improving expression levels of glutathione peroxidase 4 (GPX4) and ferritin heavy chain. Deferasirox (DFX), a trivalent iron chelator, exerts ameliorative effect in animal models of Alzheimer’s disease and tauopathy (Kwan et al., 2022). Another iron chelating drug, deferiprone, effectively improves patient’s condition in Parkinson’s disease (Martin-Bastida et al., 2017; Negida et al., 2024).
Ferroptosis is a relatively new form of programmed cell death (Dixon et al., 2012). It is characterized by iron-dependent accumulation of ROS and peroxidation of polyunsaturated fatty acids of membrane phospholipids. It is different from other cell death modalities in many aspects. Cells that undergo ferroptosis have morphological, biochemical, genetic, and metabolic features distinct from those of previously identified programmed cell deaths, such as apoptosis, pyroptosis, entosis, mitoptosis, necroptosis, and autophagy (Lin et al., 2022). Ferroptosis is triggered by excessive peroxidative damage of membrane lipid bilayer due to labile iron overload causing Fenton reaction-mediated hydroxyl radical generation and lipid peroxidation, and compromised antioxidant defense systems, including reduced glutathione (GSH)/GPX4-dependent and independent pathways (Dixon and Pratt, 2023; Xu et al., 2024). Recent findings suggest that ferroptosis plays a key role in pathogenesis of diabetes (Sha et al., 2021; Liu et al., 2024a), cardiovascular disease (Wang et al., 2021a; Zhang et al., 2022), kidney disease (Martin-Sanchez et al., 2020; Wang et al., 2023), non-alcoholic fatty liver disease (Wang et al., 2022; Zhao et al., 2023), neurodegenerative disorders (Lane et al., 2021; Ryan et al., 2023) and tumor progression (Gong et al., 2022; Kim et al., 2023b). Release of free iron from heme proteins, especially hemoglobin (Cao and Dixon, 2016; Liu et al., 2024b) and myoglobin (Luan et al., 2023; Qiao et al., 2023) under different pathophysiological conditions may promote Fenton reaction and oxidative stress leading to ferroptosis. Iron chelators effectively prevent the occurrence of ferroptosis, which may be an effective approach for the treatment of iron-related disorders (Chen et al., 2020; Pei et al., 2022; Liu et al., 2024a). FerroTerminator1, a novel iron chelator, has been reported to ameliorate liver damage by inhibiting hepatic iron accumulation and ferroptosis in various metabolic dysfunction-associated steatohepatitis (MASH) (Tao et al., 2024).
Iron is an essential component of heme proteins regulating several biochemical functions. However, free redox-active iron, can be harmful for cells by promoting oxidative stress. Iron metabolism is, therefore, tightly regulated to fulfill the demand for heme protein biosynthesis as well as by avoiding detrimental effect of the redox-active iron (Gozzelino and Arosio, 2016; Muckenthaler et al., 2017). Under normal physiological condition, iron balance is finely controlled via binding to proteins, namely, transferrin (involved in iron transport) and ferritin (responsible for iron storage) (Kawabata, 2022). However, in various pathological conditions, as discussed in earlier sections, generation of free redox-active iron exerts adverse effects.
In diabetes, iron release increases from glycated hemoglobin resulting ROS production. Increased generation of ROS induces lipid peroxidation. Cell membranes and organelle membranes are especially sensitive to ROS damage due to high content of polyunsaturated fatty acids. Lipid peroxidation and accumulation of peroxidation products are the main risk factors of diabetes-induced vascular dysfunction (Negre-Salvayre et al., 2010). The markers of lipid peroxidation include malonaldehyde (MDA), hydroxynonenal (HNE), and 8-isoprostaglandin F2⍺ (Gopaul et al., 1995; Gaweł et al., 2004). 8-isoprostaglandin F2⍺ exhibits multiple activities to induce vascular dysfunction, including plateles adhesion and aggregation as well as vasoconstriction. Growing evidence has suggested that oxysterols (lipid peroxidation products of cholesterol) are involved in the pathology of diabetes mellitus (Samadi et al., 2021). Oxysterols are also found elevated in the brains of diabetic rodent models and in the blood of diabetic patients (Weigel et al., 2019). Increased levels of oxysterols were also found in the plasma and vascular walls of patients with cardiovascular diseases, particularly in atherosclerotic lesions (Vejux and Lizard, 2009). Macrophages absorb excessive oxysterols in the presence of high level of peripheral cholesterol. Accumulation of these cholesterol-rich immune cells on blood vessel walls contributes to vascular dysfunction and atherosclerosis (Chistiakov et al., 2016). Under diabetic condition, excess ROS, thus, plays a critical role in the occurrence and development of cardiovascular diseases (Wang et al., 2021b).
Protein carbonylation is one of the most detrimental oxidative protein modifications, which are not easily reversed (Wong et al., 2013). It is also regarded as a crucial biomarker of oxidative stress-related diseases (Cattaruzza, and Hecker, 2008). ROS can oxidize amino acid side groups of protein to introduce carbonyl group at specific sites, which leads to loss of catalytic or structural function of the modified proteins (Hecker and Wagner, 2018). For example, carbonylation of actin leads to changes in cytoskeleton dynamics and damage of barrier function of blood vessels (Dalle-Donne et al., 2001). Moderately carbonylated proteins are susceptible to degradation by the proteasomal system. However, heavily carbonylated proteins tend to form aggregates that are resistant to proteolytic degradation, and accumulate as damaged or unfolded proteins (Dalle-Donne et al., 2006). A large number of neurodegenerative diseases are directly associated with the accumulation of proteolysis-resistant aggregates of carbonylated proteins in tissues (Gonos et al., 2018).
Besides induction of tissue oxidative stress damage, ROS can also trigger the aggregation of inflammatory cells, and formation of inflammatory cytokines related to various pathological processes. ROS-mediated activation of various inflammatory signaling pathways including nuclear factor kappa B (NF-κB) signaling, Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling, and mitogen-activated protein kinase (MAPK) signaling, have been reported in diabetes and associated complications (An et al., 2019; Chen et al., 2021; Lei et al., 2021). ROS can directly attack the free sulfhydryl (-SH) groups, which are necessary to maintain protein folding. It thus induces oxidative modification of proteins, and triggers endoplasmic reticulum (ER) stress due to the prolonged accumulation of unfolded or mis-folded proteins in the ER lumen (Lenna et al., 2014; Zeeshan et al., 2016). Studies have shown that elevated ROS and ER stress can lead to endothelial dysfunction in hyperglycemic condition (Kapadia et al., 2021; Chen et al., 2022).
We have discussed, in brief, the potential mechanism leading to hemoglobin and myoglobin-mediated oxidative stress in various disease conditions. Under pathophysiological stress, heme and free iron release from major heme proteins, hemoglobin and myoglobin, leads to harmful hydroxyl radical (.OH) generation via Fenton reaction. Hydroxyl radicals (.OH) cause oxidative damage of different cellular components by lipid peroxidation, protein carbonylation and DNA damage. Such events may be associated with ferroptosis and inflammation causing atherosclerosis, renal injury, neuronal cell damage and hyperglycemia-related complications (Figure 5).
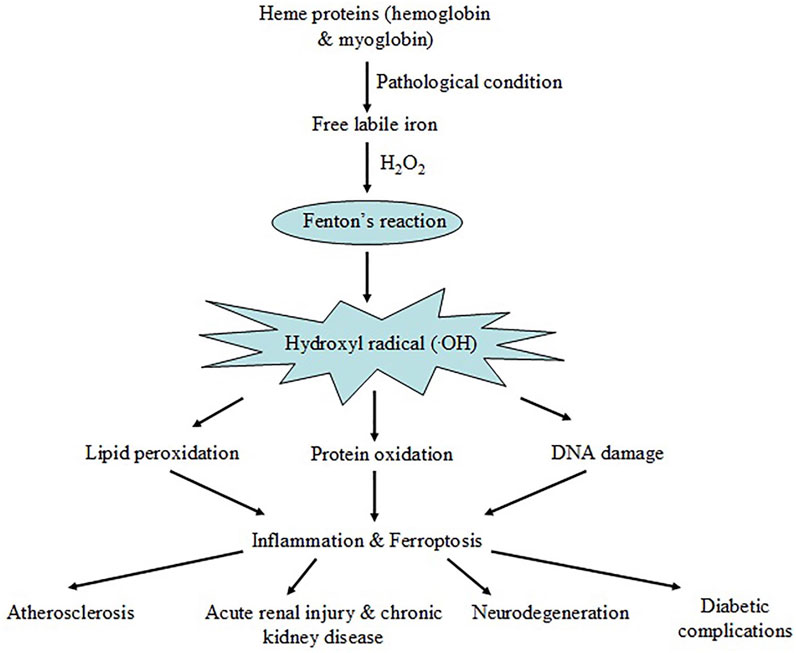
Figure 5. Schematic representation showing iron release from major heme-proteins, hemoglobin and myoglobin, under pathological conditions resulting Fenton reaction and ROS generation. The event leads to oxidative damage of cellular macromolecules triggering ferroptosis and inflammation causing disease complications.
Iron is not only an essential cofactor for vital biochemical activities in human physiology, but also a potential biohazard. Cells have evolved stringent mechanism to control iron metabolism and to satisfy metabolic needs, minimizing the risk of iron toxicity. However, in pathophysiological condition, iron can contribute oxidative stress worsening the situation. An increasing number of experimental studies has provided evidence regarding involvement of heme iron in several disease progression. Hence a comprehensive understanding of the mechanism linking heme proteins to oxidative stress is potentially beneficial for future therapeutic intervention.
RS: Writing–original draft, Writing–review and editing. AC: Conceptualization, Supervision, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The senior author (ASC) acknowledges the receipt of funds from University Grants Commission and Council of Scientific and Industrial Research, New Delhi to undertake different earlier studies, some of which have been cited as the published reports.
Author Rajarshi Sil was employed by Allied Scientific Products.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Achuthan, A., and Mageswari, U. (2017). Correlation between serum free iron, glycated hemoglobin and insulin resistance in uncontrolled type-2 diabetic patients. Natl. J. Physiol. Pharm. Pharmacol. 7 (6), 642. doi:10.5455/njppp.2017.7.1234002032017
Ahmed, M. H., Ghatge, M. S., and Safo, M. K. (2020). Hemoglobin: structure, function and allostery. Subcell. Biochem. 94, 345–382. doi:10.1007/978-3-030-41769-7_14
Ali, S., Mumtaz, S., Shakir, H. A., Khan, M., Tahir, H. M., Mumtaz, S., et al. (2021). Current status of beta-thalassemia and its treatment strategies. Mol. Genet. Genomic Med. 9 (12), e1788. doi:10.1002/mgg3.1788
An, Y., Zhang, H., Wang, C., Jiao, F., Xu, H., Wang, X., et al. (2019). Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 33 (11), 12515–12527. doi:10.1096/fj.201802805RR
Andrianova, N. V., Zorov, D. B., and Plotnikov, E. Y. (2020). Targeting inflammation and oxidative stress as a therapy for ischemic kidney injury. Biochem. (Mosc) 85 (12), 1591–1602. doi:10.1134/S0006297920120111
Ashraf, A., Clark, M., and So, P. W. (2018). The aging of iron man. Front. Aging Neurosci. 10, 65. doi:10.3389/fnagi.2018.00065
Balla, J., and Zarjou, A. (2021). Heme burden and ensuing mechanisms that protect the kidney: insights from bench and bedside. Int. J. Mol. Sci. 22 (15), 8174. doi:10.3390/ijms22158174
Banerjee, S., and Chakraborti, A. S. (2013). In vitro study on structural alteration of myoglobin by methylglyoxal. Protein J. 32 (3), 216–222. doi:10.1007/s10930-013-9480-7
Banerjee, S., Maity, S., and Chakraborti, A. S. (2016). Methylglyoxal-induced modification causes aggregation of myoglobin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 155, 1–10. doi:10.1016/j.saa.2015.10.022
Barbano, B., Sardo, L., Gasperini, M. L., Gigante, A., Liberatori, M., Di Lazzaro, G. G., et al. (2015). Drugs and rhabdomyolysis: from liver to kidney. Curr. Vasc. Pharmacol. 13 (6), 725–737. doi:10.2174/1570161113666150130151839
Bhattacherjee, A., Dhara, K., and Chakraborti, A. S. (2016). Argpyrimidine-tagged rutin-encapsulated biocompatible (ethylene glycol dimers) nanoparticles: synthesis, characterization and evaluation for targeted drug delivery. Int. J. Pharm. 509 (1-2), 507–517. doi:10.1016/j.ijpharm.2016.05.042
Bhattacherjee, A., Dhara, K., and Chakraborti, A. S. (2017). Bimolecular interaction of argpyrimidine (a Maillard reaction product) in in vitro non-enzymatic protein glycation model and its potential role as an antiglycating agent. Int. J. Biol. Macromol. 102, 1274–1285. doi:10.1016/j.ijbiomac.2017.04.108
Bolisetty, S., Zarjou, A., and Agarwal, A. (2017). Heme oxygenase 1 as a therapeutic target in acute kidney injury. Am. J. Kidney Dis. 69 (4), 531–545. doi:10.1053/j.ajkd.2016.10.037
Bose, T., Bhattacherjee, A., Banerjee, S., and Chakraborti, A. S. (2013). Methylglyoxal-induced modifications of hemoglobin: structural and functional characteristics. Arch. Biochem. Biophys. 529 (2), 99–104. doi:10.1016/j.abb.2012.12.001
Bose, T., and Chakraborti, A. S. (2008). Fructose-induced structural and functional modifications of hemoglobin: implication for oxidative stress in diabetes mellitus. Biochim. Biophys. Acta. 1780 (5), 800–808. doi:10.1016/j.bbagen.2008.02.001
Bullen, A., Liu, Z. Z., Hepokoski, M., Li, Y., and Singh, P. (2017). Renal oxygenation and hemodynamics in kidney injury. Nephron 137 (4), 260–263. doi:10.1159/000477830
Bulters, D., Gaastra, B., Zolnourian, A., Alexander, S., Ren, D., Blackburn, S. L., et al. (2018). Haemoglobin scavenging in intracranial bleeding: biology and clinical implications. Nat. Rev. Neurol. 14 (7), 416–432. doi:10.1038/s41582-018-0020-0
Bunn, H. F., and Higgins, P. J. (1981). Reaction of monosaccharides with proteins: possible evolutionary significance. Science 213 (4504), 222–224. doi:10.1126/science.12192669
Bustamante-Barrientos, F. A., Luque-Campos, N., Araya, M. J., Lara-Barba, E., Solminiac, J. D., Pradenas, C., et al. (2023). Mitochondrial dysfunction in neurodegenerative disorders: potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. J. Transl. Med. 21 (1), 613. doi:10.1186/s12967-023-04493-w
Cao, J. Y., and Dixon, S. J. (2016). Mechanisms of ferroptosis. Cell. Mol. Life Sci. 73, 2195–2209. doi:10.1007/s00018-016-2194-1
Carocci, A., Catalano, A., Sinicropi, M. S., and Genchi, G. (2018). Oxidative stress and neurodegeneration: the involvement of iron. Biometals 31 (5), 715–735. doi:10.1007/s10534-018-0126-2
Carter, A., Racey, S., and Veuger, S. (2022). The Role of iron in DNA and genomic instability in cancer, a target for iron chelators that can induce ROS. Appl. Sci. 12 (19), 10161. doi:10.3390/app121910161
Cattaruzza, M., and Hecker, M. (2008). Protein carbonylation and decarboylation: a new twist to the complex response of vascular cells to oxidative stress. Circ. Res. 102 (3), 273–274. doi:10.1161/CIRCRESAHA.108.172148
Chen, C., Zhang, B., Xue, J., Li, Z., Dou, S., Chen, H., et al. (2022). Pathogenic role of endoplasmic reticulum stress in diabetic corneal endothelial dysfunction. Invest. Ophthalmol. Vis. Sci. 63 (3), 4. doi:10.1167/iovs.63.3.4
Chen, D., Liu, Y., Chen, J., Lin, H., Guo, H., Wu, Y., et al. (2021). JAK/STAT pathway promotes the progression of diabetic kidney disease via autophagy in podocytes. Eur. J. Pharmacol. 902, 174121. doi:10.1016/j.ejphar.2021.174121
Chen, X., Yu, C., Kang, R., and Tang, D. (2020). Iron metabolism in ferroptosis. Front. Cell Dev. Biol. 8, 590226. doi:10.3389/fcell.2020.590226
Chiabrando, D., Fiorito, V., Petrillo, S., and Tolosano, E. (2018). Unraveling the role of heme in neurodegeneration. Front. Neurosci. 12, 712. doi:10.3389/fnins.2018.00712
Chiabrando, D., Vinchi, F., Fiorito, V., Mercurio, S., and Tolosano, E. (2014). Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharmacol. 5, 61. doi:10.3389/fphar.2014.00061
Chistiakov, D. A., Bobryshev, Y. V., and Orekhov, A. N. (2016). Macrophage-mediated cholesterol handling in atherosclerosis. J. Cell. Mol. Med. 20 (1), 17–28. doi:10.1111/jcmm.12689
Choi, Y., Won, Ki., Kang, H. H., and Change, H. (2021). Association of serum hemoglobin level with the risk of carotid plaque beyond metabolic abnormalities among asymptomatic adults without major adverse clinical events: a cross-sectional cohort study. BMC Cardiovasc. Disord. 21 (1), 35. doi:10.1186/s12872-021-01852-7
Cole, J. B., and Florez, J. C. (2020). Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 16 (7), 377–390. doi:10.1038/s41581-020-0278-5
Collin, F. (2019). Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 20 (10), 2407. doi:10.3390/ijms20102407
Dalle-Donne, I., Aldini, G., Carini, M., Colombo, R., Rossi, R., and Milzani, A. (2006). Protein carbonylation, cellular dysfunction, and disease progression. J. Cell Mol. Med. 10 (2), 389–406. doi:10.1111/j.1582-4934.2006.tb00407.x
Dalle-Donne, I., Rossi, R., Giustarini, D., Gagliano, N., Lusini, L., Milzani, A., et al. (2001). Actin carbonylation: from a simple marker of protein oxidation to relevant signs of severe functional impairment. Free Radic. Biol. Med. 31 (9), 1075–1083. doi:10.1016/s0891-5849(01)00690-6
Darenskaya, M. A., Kolesnikova, L. I., and Kolesnikov, S. I. (2021). Oxidative stress: pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull. Exp. Biol. Med. 171 (2), 179–189. doi:10.1007/s10517-021-05191-7
Deepu, V., Rai, V., and Agrawal, D. K. (2024). Quantitative assessment of intracellular effectors and cellular response in RAGE activation. Arch. Intern. Med. Res. 7 (2), 80–103. doi:10.26502/aimr.0168
Deuel, J. W., Schaer, C. A., Boretti, F. S., Opitz, L., Garcia-Rubio, I., Baek, J. H., et al. (2016). Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis. 7 (1), e2064. doi:10.1038/cddis.2015.392
Dey, A., and Ghosh, A. (2002). “True” iron(V) and iron (VI) porphyrins: a first theoretical exploration. J. Am. Chem. Soc. 124 (13), 3206–3207. doi:10.1021/ja012402s
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
Dixon, S. J., and Pratt, D. A. (2023). Ferroptosis: a flexible constellation of related biochemical mechanisms. Mol. Cell 83 (7), 1030–1042. doi:10.1016/j.molcel.2023.03.005
Drvenica, I. T., Stančić, A. Z., Maslovarić, I. S., Trivanović, D. I., and Ilić, V. L. (2022). Extracellular hemoglobin: modulation of cellular functions and pathophysiologicaleffects. Biomolecules 12 (11), 1708. doi:10.3390/biom12111708
Dusek, P., Schneider, S. A., and Aaseth, J. (2016). Iron chelation in the treatment of neurodegenerative diseases. J. Trace Elem. Med. Biol. 38, 81–92. doi:10.1016/j.jtemb.2016.03.010
Galaris, D., and Pantopoulos, K. (2008). Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit. Rev. Clin. Lab. Sci. 45 (1), 1–23. doi:10.1080/10408360701713104
Gaweł, S., Wardas, M., Niedworok, E., and Wardas, P. (2004). Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 57 (9-10), 453–455.
Gbotosho, O. T., Kapetanaki, M. G., and Kato, G. J. (2020). The worst things in life are free: the role of free heme in sickle cell disease. Front. Immunol. 11, 561917. doi:10.3389/fimmu.2020.561917
Gembillo, G., Siligato, R., Cernaro, V., and Santoro, D. (2020). Complement inhibition therapy and dialytic strategies in paroxysmal nocturnal hemoglobinuria: the Nephrologist’s opinion. J. Clin. Med. 9 (5), 1261. doi:10.3390/jcm9051261
Ghemrawi, R., and Khair, M. (2020). Endoplasmic reticulum stress and unfolded protein response in neurodegenerative diseases. Int. J. Mol. Sci. 21 (17), 6127. doi:10.3390/ijms21176127
Gong, C., Ji, Q., Wu, M., Tu, Z., Lei, K., Luo, M., et al. (2022). Ferroptosis in tumor immunity and therapy. J. Cell Mol. Med. 26 (22), 5565–5579. doi:10.1111/jcmm.17529
Gonos, E. S., Kapetanou, M., Sereikaite, J., Bartosz, G., Naparlo, K., Grzesik, M., et al. (2018). Origin and pathophysiology of protein carbonylation, nitration and chlorination in age-related brain diseases and aging. Aging 10 (5), 868–901. doi:10.18632/aging.101450
Gopaul, N. K., Änggård, E. E., Mallet, A. I., Betteridge, D. J., Wolff, S. P., and Nourooz-Zadeh, J. (1995). Plasma 8-epi-PGF2 α levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 368, 225–229. doi:10.1016/0014-5793(95)00649-t
Gozzelino, R., and Arosio, P. (2016). Iron homeostasis in health and disease. Int. J. Mol. Sci. 17 (1), 130. doi:10.3390/ijms17010130
Gozzelino, R., Jeney, V., and Soares, M. P. (2010). Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 50, 323–354. doi:10.1146/annurev.pharmtox.010909.105600
Granick, M., Leuin, A. S., and Trepanier, L. A. (2021). Plasma and urinary F2-isoprostane markers of oxidative stress are increased in cats with early (stage 1) chronic kidney disease. J. Feline Med. Surg. 23 (8), 692–699. doi:10.1177/1098612X20969358
Guan, Y., Tang, G., Li, L., Shu, J., Zhao, Y., Huang, L., et al. (2024). Herbal medicine and gut microbiota: exploring untapped therapeutic potential in neurodegenerative disease management. Arch. Pharm. Res. 47 (2), 146–164. doi:10.1007/s12272-023-01484-9
Guo, Y., Zhao, H., Lin, Z., Ye, T., Xu, D., and Zeng, Q. (2021). Heme in cardiovascular diseases: aubiquitous dangerous molecule worthy of vigilance. Front. Cell Dev. Biol. 9, 781839. doi:10.3389/fcell.2021.781839
Gupta, A., Thorson, P., Penmatsa, K. R., and Gupta, P. (2021). Rhabdomyolysis: revisited. Ulst. Med. J. 90 (2), 61–69.
Gusev, E., and Sarapultsev, A. (2023). Atherosclerosis and inflammation: insights from the theory of general pathological processes. Int. J. Mol. Sci. 24 (9), 7910. doi:10.3390/ijms24097910
Hecker, M., and Wagner, A. H. (2018). Role of protein carbonylation in diabetes. J. Inherit. Metab. Dis. 41 (1), 29–38. doi:10.1007/s10545-017-0104-9
Higdon, A. N., Benavides, G. A., Chacko, B. K., Ouyang, X., Johnson, M. S., Landar, A., et al. (2012). Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy. Am. J. Physiol. Heart Circ. Physiol. 302 (7), H1394–H1409. doi:10.1152/ajpheart.00584.2011
Hillaireau, H., and Couvreur, P. (2009). Nanocarriers' entry into the cell: relevance to drug delivery. Cell Mol. Life Sci. 66 (17), 2873–2896. doi:10.1007/s00018-009-0053-z
Ho, H. J., and Shirakawa, H. (2023). Oxidative stress and mitochondrial dysfunction in chronic kidney disease. Cells 12 (1), 88. doi:10.3390/cells12010088
Hou, Y., Xin, Q., Li, Q., and Wu, X. (2021). Glycyrrhizin micelle as a genistein nanocarrier: synergistically promoting corneal epithelial wound healing through blockage of the HMGB1 signaling pathway in diabetic mice. Exptl. Eye Res. 204, 108454–108464. doi:10.1016/j.exer.2021.108454
Jebari-Benslaiman, S., Galicia-García, U., Larrea-Sebal, A., Olaetxea, J. R., Alloza, I., Vandenbroeck, K., et al. (2022). Pathophysiology of atherosclerosis. Int. J. Mol. Sci. 23 (6), 3346. doi:10.3390/ijms23063346
Jin, S., and Kang, P. M. (2024). A systematic review on advances in management of oxidative stress-associated cardiovascular diseases. Antioxidants (Basel) 13 (8), 923. doi:10.3390/antiox13080923
Jomova, K., Raptova, R., Alomar, S. Y., Alwasel, S. H., Nepovimova, E., Kuca, K., et al. (2023). Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 97 (10), 2499–2574. doi:10.1007/s00204-023-03562-9
Jun, J. H., Choi, T. Y., Lee, H. W., Ang, L., and Lee, M. S. (2021). Herbal medicine for behçet’s disease: a systematic review and meta-analysis. Nutrients 13 (1), 46. doi:10.3390/nu13010046
Kapadia, P., Bikkina, P., Landicho, M. A., Parekh, S., Haas, M. J., and Mooradian, A. D. (2021). Effect of anti-hyperglycemic drugs on endoplasmic reticulum (ER) stress in human coronary artery endothelial cells. Eur. J. Pharmacol. 907, 174249. doi:10.1016/j.ejphar.2021.174249
Kar, M., and Chakraborti, A. S. (1999). Release of iron from haemoglobin - a possible source of free radicals in diabetes mellitus. Indian J. Exp. Biol. 37 (2), 190–192.
Karamouzis, I., Sarafidis, P. A., Karamouzis, M., Iliadis, S., Haidich, A. B., Sioulis, A., et al. (2008). Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am. J. Nephrol. 28 (3), 397–404. doi:10.1159/000112413
Karpefors, M., Adelroth, P., Namslauer, A., Zhen, Y., and Brzezinski, P. (2000). Formation of the “peroxy” intermediate in cytochrome c oxidase is associated with internal proton/hydrogen transfer. Biochemistry 39 (47), 14664–14669. doi:10.1021/bi0013748
Kato, G. J., Steinberg, M. H., and Gladwin, M. T. (2017). Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Invest. 127 (3), 750–760. doi:10.1172/JCI89741
Katsnelson, A., De Strooper, B., and Zoghbi, H. Y. (2016). Neurodegeneration: from cellular concepts to clinical applications. Sci. Transl. Med. 8 (364), 364ps18. doi:10.1126/scitranslmed.aal2074
Kawabata, T. (2022). Iron-induced oxidative stress in human diseases. Cells 11 (14), 2152. doi:10.3390/cells11142152
Khalid, M., Petroianu, G., and Adem, A. (2022). Advanced glycation end products and diabetes mellitus: mechanisms and perspectives. Biomolecules 12 (4), 542. doi:10.3390/biom12040542
Khatana, C., Saini, N. K., Chakrabarti, S., Saini, V., Sharma, A., Saini, R. V., et al. (2020). Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid. Med. Cell Longev. 2020, 1–14. doi:10.1155/2020/5245308
Kim, G. H., Kim, J. E., Rhie, S. J., and Yoon, S. (2015). The Role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 24 (4), 325–340. doi:10.5607/en.2015.24.4.325
Kim, R., Taylor, D., Vonderheide, R. H., and Gabrilovich, D. I. (2023a). Ferroptosis of immune cells in the tumor microenvironment. Trends Pharmacol. Sci. 44 (8), 542–552. doi:10.1016/j.tips.2023.06.005
Kim, S. J., Mesquita, F. C. P., and Hochman-Mendez, C. (2023b). New biomarkers for cardiovascular disease. Tex. Heart. Inst. J. 50 (5), e238178. doi:10.14503/THIJ-23-8178
Kontoghiorghe, C. N., Kolnagou, A., and Kontoghiorghes, G. J. (2015). Phytochelators intended for clinical use in iron overload, other diseases of iron imbalance and free radical pathology. Molecules 20 (11), 20841–20872. doi:10.3390/molecules201119725
Kozlov, A. V., Javadov, S., and Sommer, N. (2024). Cellular ROS and antioxidants: physiological and pathological role. Antioxidants (Basel) 13 (5), 602. doi:10.3390/antiox13050602
Kumar, S., and Bandyopadhyay, U. (2005). Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 157 (3), 175–188. doi:10.1016/j.toxlet.2005.03.004
Kupershmidt, L., and Youdim, M. B. H. (2023). The Neuroprotective activities of the novel multi-target iron-chelators in models of Alzheimer’sdisease, amyotrophic lateral sclerosis and aging. Cells 12 (5), 763. doi:10.3390/cells12050763
Kushwah, S., Maurya, N. S., Kushwaha, S., Scotti, L., Chawade, A., and Mani, A. (2023). Herbal therapeutics for alzheimer's disease: ancient indian medicine system from the modern viewpoint. Curr. Neuropharmacol. 21 (4), 764–776. doi:10.2174/1570159X21666230216094353
Kwan, P., Ho, A., and Baum, L. (2022). Effects of deferasirox in Alzheimer’s disease and tauopathy animal models. Biomolecules 12 (3), 365. doi:10.3390/biom12030365
Lane, D. J. R., Metselaar, B., Greenough, M., Bush, A. I., and Ayton, S. J. (2021). Ferroptosis and NRF2: an emerging battlefield in the neurodegeneration of Alzheimer's disease. Essays Biochem. 65 (7), 925–940. doi:10.1042/EBC20210017
Lapolla, A., Flamini, R., Vedova, A. D., Senesi, A., Reitano, R., Fedele, D., et al. (2003). Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method. Clin. Chem. Lab. Med. 41 (9), 1166–1173. doi:10.1515/CCLM.2003.180
Lei, L., Zhao, J., Liu, X. Q., Chen, J., Qi, X. M., Xia, L. L., et al. (2021). Wogonin alleviates kidney tubular epithelial injury in diabetic nephropathy by inhibiting PI3K/akt/NF-κB signaling pathways. Drug Des. Devel Ther. 15, 3131–3150. doi:10.2147/dddt.S310882
Lenna, S., Han, R., and Trojanowska, M. (2014). Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life 66 (8), 530–537. doi:10.1002/iub.1292
Li, W., Östblom, M., Xu, L. H., Hellsten, A., Leanderson, P., Liedberg, B., et al. (2006). Cytocidal effects of atheromatous plaque components: the death zone revisited. FASEB J. 20 (13), 2281–2290. doi:10.1096/fj.06-6114com
Lin, L., Zhang, M. X., Zhang, L., Zhang, D., Li, C., and Li, Y. L. (2022). Autophagy, pyroptosis, and ferroptosis: new regulatory mechanisms for atherosclerosis. Front.Cell Dev. Biol. 9, 809955. doi:10.3389/fcell.2021.809955
Liu, C., Wang, G., Han, W., Tian, Q., and Li, M. (2024a). Ferroptosis: a potential therapeutic target for stroke. Neural. Regen. Res. 19 (5), 988–997. doi:10.4103/1673-5374.385284
Liu, P., Zhang, Z., Cai, Y., Li, Z., Zhou, Q., and Chen, Q. (2024b). Ferroptosis: mechanisms and role in diabetes mellitus and its complications. Ageing Res. Rev. 94, 102201. doi:10.1016/j.arr.2024.102201
Luan, Y., Huang, E., Huang, J., Yang, Z., Zhou, Z., Liu, Y., et al. (2023). Serum myoglobin modulates kidney injury via inducing ferroptosis after exertional heatstroke. J. Transl. Int. Med. 11 (2), 178–188. doi:10.2478/jtim-2023-0092
Ma, J., Zhang, H., Chen, Y., Liu, X., Tian, J., and Shen, W. (2022). The role of macrophage iron overload and ferroptosis in atherosclerosis. Biomolecules 12 (11), 1702. doi:10.3390/biom12111702
Maity, S., Acharyya, A., and Chakraborti, A. S. (2022). Flavonoid-based polymeric nanoparticles: a promising approach for cancer and diabetes treatment. Eur. Polym. J. 177, 455–465. doi:10.1016/j.eurpolymj.2022.111455
Maity, S., and Chakraborti, A. S. (2020). Formulation, physico-chemical characterization and antidiabetic potential of naringenin-loaded poly D, L-lactide-co-glycolide (N-PLGA) nanoparticles. Eur. Polym. J. 134, 109818–109910. doi:10.1016/j.eurpolymj.2020.109818
Martemucci, G., Fracchiolla, G., Muraglia, M., Tardugno, R., Dibenedetto, R. S., and D’Alessandro, A. G. (2023). Metabolic syndrome: anarrative review from the oxidative stress to the management of related diseases. Antioxidants (Basel) 12 (12), 2091. doi:10.3390/antiox12122091
Martin-Bastida, A., Ward, R. J., Newbould, R., Piccini, P., Sharp, D., Kabba, C., et al. (2017). Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci. Rep. 7, 1398. doi:10.1038/s41598-017-01402-2
Martin-Sanchez, D., Fontecha-Barriuso, M., Martinez-Moreno, J. M., Ramos, A. M., Sanchez-Niño, M. D., Guerrero-Hue, M., et al. (2020). Ferroptosis and kidney disease. Nefrologia 40 (4), 384–394. doi:10.1016/j.nefro.2020.03.005
Marupudi, N., and Xiong, M. P. (2024). Genetic targets and applications of iron chelators for neurodegeneration with brain iron accumulation. ACS Bio. Med. Chem. Au. 4 (3), 119–130. doi:10.1021/acsbiomedchemau.3c00066
Masenga, S. K., Kabwe, L. S., Chakulya, M., and Kirabo, A. (2023). Mechanisms of oxidative stress in metabolic syndrome. Int. J. Mol. Sci. 24 (9), 7898. doi:10.3390/ijms24097898
Meegan, J. E., Bastarache, J. A., and Ware, L. B. (2021). Toxic effects of cell-free hemoglobin on the microvascular endothelium: implications for pulmonary and nonpulmonary organ dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 321 (2), L429–L439. doi:10.1152/ajplung.00018.2021
Michel, J. B., and Martin-Ventura, J. L. (2020). Red blood cells and hemoglobin in human atherosclerosis and related arterial diseases. Int. J. Mol. Sci. 21 (18), 6756. doi:10.3390/ijms21186756
Milne, G. L., Yin, H., Hardy, K. D., Davies, S. S., and Roberts, L. J. (2011). Isoprostane generation and function. Chem. Rev. 111 (10), 5973–5996. doi:10.1021/cr200160h
Minniakhmetov, I., Yalaev, B., Khusainova, R., Bondarenko, E., Melnichenko, G., Dedov, I., et al. (2024). Genetic and epigenetic aspects of type 1 diabetes mellitus: modern view on the problem. Biomedicines 12 (2), 399. doi:10.3390/biomedicines12020399
Miranda, M. R., Vestuto, V., Moltedo, O., Campiglia, P., Manfra, M., and Pepe, G. (2023). The ion channels involved in oxidative stress-related gastrointestinal diseases. Oxygen 3 (3), 336–365. doi:10.3390/oxygen3030022
Muckenthaler, M. U., Rivella, S., Hentze, M. W., and Galy, B. (2017). A red carpet for iron metabolism. Cell 168 (3), 344–361. doi:10.1016/j.cell.2016.12.034
Mukhopadhyay, P., Maity, S., Mandal, S., Chakraborti, A. S., Prajapati, A. K., and Kundu, P. P. (2018). Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr. Polym. 182, 42–51. doi:10.1016/j.carbpol.2017.10.098
Mukhopadhyay, P., and Prajapati, A. K. (2015). Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers – a review. RSC Adv. 5, 97547–97562. doi:10.1039/c5ra18896b
Naito, Y., Tsujino, T., Masuyama, T., and Ishihara, M. (2022). Crosstalk between iron and arteriosclerosis. J. Atheroscler. Thromb. 29 (3), 308–314. doi:10.5551/jat.RV17060
Nath, K. A., Singh, R. D., Croatt, A. J., and Adams, C. M. (2022). Heme proteins and kidney injury: beyond rhabdomyolysis. Kidney 3 (11), 1969–1979. doi:10.34067/KID.0005442022
Ndayisaba, A., Kaindlstorfer, C., and Wenning, G. K. (2019). Iron in neurodegeneration - cause or consequence? Front. Neurosci. 13, 180. doi:10.3389/fnins.2019.00180
Negida, A., Hassan, N. M., Aboeldahab, H., Zain, Y. E., Negida, Y., Cadri, S., et al. (2024). Efficacy of the iron-chelating agent, deferiprone, in patients with Parkinson's disease: a systematic review and meta-analysis. CNS Neurosci. Ther. 30, e14607. doi:10.1111/cns.14607
Negre-Salvayre, A., Auge, N., Ayala, V., Basaga, H., Boada, J., Brenke, R., et al. (2010). Pathological aspects of lipid peroxidation. Free Radic. Res. 44 (10), 1125–1171. doi:10.3109/10715762.2010.498478
Olufunmilayo, E. O., Gerke-Duncan, M. B., and Damian Holsinger, R. M. (2023). Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants (Basel) 12 (2), 517. doi:10.3390/antiox12020517
Pandya, C. D., Vekaria, H., Joseph, B., Slone, S. A., Gensel, J. C., Sullivan, P. G., et al. (2021). Hemoglobin induces oxidative stress and mitochondrial dysfunction in oligodendrocyte progenitor cells. Transl. Res. 231, 13–23. doi:10.1016/j.trsl.2021.01.005
Pei, Z., Qin, Y., Fu, X., Yang, F., Huo, F., Liang, X., et al. (2022). Inhibition of ferroptosis and iron accumulation alleviates pulmonary fibrosis in a bleomycin model. Redox Biol. 57, 102509. doi:10.1016/j.redox.2022.102509
Pilo, F., Cilloni, D., Giovanni Della Porta, M., Forni, G. L., Piperno, A., Santini, V., et al. (2022). Iron-mediated tissue damage in acquired ineffective erythropoiesis disease: it's more a matter of burden or more of exposure to toxic iron form? Leuk. Res. 114, 106792. doi:10.1016/j.leukres.2022.106792
Poznyak, A. V., Litvinova, L., Poggio, P., Moschetta, D., Sukhorukov, V. N., and Orekhov, A. N. (2022). From diabetes to atherosclerosis: potential of metformin for management of cardiovascular disease. Int. J. Mol. Sci. 23 (17), 9738. doi:10.3390/ijms23179738
Qiao, O., Wang, X., Wang, Y., Li, N., and Gong, Y. (2023). Ferroptosis in acute kidney injury following crush syndrome: a novel target for treatment. J. Adv. Res. 54, 211–222. doi:10.1016/j.jare.2023.01.016
Rabbani, N., and Thornalley, P. J. (2021). Protein glycation - biomarkers of metabolic dysfunction and early-stage decline in health in the era of precision medicine. Redox Biol. 42, 101920. doi:10.1016/j.redox.2021.101920
Ramos, S., Jeney, V., Figueiredo, A., Paixão, T., Sambo, M. R., Quinhentos, V., et al. (2024). Targeting circulating labile heme as a defense strategy against malaria. Life Sci. Alliance 7 (4), e202302276. doi:10.26508/lsa.202302276
Rashid, H., Jali, A., Akhter, M. S., and Abdi, S. A. H. (2023). Molecular mechanisms of oxidative stress in acute kidney injury: targeting the loci by resveratrol. Int. J. Mol. Sci. 25 (1), 3. doi:10.3390/ijms25010003
Righy, C., Turon, R., Freitas, G., Japiassú, A. M., Faria Neto, H. C. C., Bozza, M., et al. (2018). Hemoglobin metabolism by-products are associated with an inflammatory response in patients with hemorrhagic stroke. Rev. Bras. Ter. Intensiva. 30 (1), 21–27. doi:10.5935/0103-507x.20180003
Rojas, A., Lindner, C., Schneider, I., Gonzalez, I., and Uribarri, J. (2024). The RAGE axis: a relevant inflammatory hub in human diseases. Biomolecules 14 (4), 412. doi:10.3390/biom14040412
Rosengren, A., and Dikaiou, P. (2023). Cardiovascular outcomes in type 1 and type 2 diabetes. Diabetologia 66 (3), 425–437. doi:10.1007/s00125-022-05857-5
Roy, A., Sen, S., and Chakraborti, A. S. (2004). In vitro nonenzymatic glycation enhances the role of myoglobin as a source of oxidative stress. Free Radic. Res. 38 (2), 139–146. doi:10.1080/10715160310001638038
Roy, A., Sil, R., and Chakraborti, A. S. (2010). Non-enzymatic glycation induces structural modifications of myoglobin. Mol. Cell Biochem. 338 (1-2), 105–114. doi:10.1007/s11010-009-0343-7
Roy, M., Pal, R., and Chakraborti, A. S. (2017). Pelargonidin-PLGA nanoparticles: fabrication, characterization, and their effect on streptozotocin induced diabetic rats. Ind. J. Exptl. Biol. 55, 819–830.
Roy, M., Sen, S., and Chakraborti, A. S. (2008). Action of pelargonidin on hyperglycemia and oxidative damage in diabetic rats: implication for glycation-induced hemoglobin modification. Life Sci. 82 (21-22), 1102–1110. doi:10.1016/j.lfs.2008.03.011
Ryan, S. K., Zelic, M., Han, Y., Teeple, E., Chen, L., Sadeghi, M., et al. (2023). Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 26 (1), 12–26. doi:10.1038/s41593-022-01221-3
Ryter, S. W. (2021). Significance of heme and heme degradation in the pathogenesis of acute lung and inflammatory disorders. Int. J. Mol. Sci. 22 (11), 5509. doi:10.3390/ijms22115509
Sadrzadeh, S. M., Graf, E., Panter, S. S., Hallaway, P. E., and Eaton, J. W. (1984). Hemoglobin. A biologic fenton reagent. J. Biol. Chem. 259 (23), 14354–14356. doi:10.1016/s0021-9258(17)42604-4
Samaddar, A., Tarafdar, D., Abraham, S. K., Ghosh, K., and Khuda-Bukhsh, A. R. (2017). Nanopelargonidin protects hyperglycemic-induced L6 cells against mitochondrial dysfunction. Planta Medica 83 (5), 468–475. doi:10.1055/s-0043-100017
Samadi, A., Sabuncuoglu, S., Samadi, M., Isikhan, S. Y., Chirumbolo, S., Peana, M., et al. (2021). A comprehensive review on oxysterols and related diseases. Curr. Med. Chem. 28 (1), 110–136. doi:10.2174/0929867327666200316142659
Sawicki, K. T., Chang, H. C., and Ardehali, H. (2015). Role of heme in cardiovascular physiology and disease. J. Am. Heart Assoc. 4 (1), e001138. doi:10.1161/JAHA.114.001138
Schaer, D. J., Buehler, P. W., Alayash, A. I., Belcher, J. D., and Vercellotti, G. M. (2013). Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 121 (8), 1276–1284. doi:10.1182/blood-2012-11-451229
Schrag, M., and Kirshner, H. (2020). Management of intracerebral hemorrhage: JACC focus seminar. J. Am. Coll. Cardiol. 75 (15), 1819–1831. doi:10.1016/j.jacc.2019.10.066
Sen, S., Bose, T., Roy, A., and Chakraborti, A. S. (2007). Effect of non-enzymatic glycation on esterase activities of hemoglobin and myoglobin. Mol. Cell Biochem. 301 (1-2), 251–257. doi:10.1007/s11010-007-9418-5
Sen, S., Kar, M., Roy, A., and Chakraborti, A. S. (2005). Effect of nonenzymatic glycation on functional and structural properties of hemoglobin. Biophys. Chem. 113 (3), 289–298. doi:10.1016/j.bpc.2004.05.005
Sen, S., Roy, M., and Chakraborti, A. S. (2011). Ameliorative effects of glycyrrhizin on streptozotocin-induced diabetes in rats. J. Pharm. Pharmacol. 63 (2), 287–296. doi:10.1111/j.2042-7158.2010.01217.x
Senghor, A., Bhrathya, N., Kumar, J. S., William, E., and Balasubramaniam, S. (2012). Serum ferritin, iron, TIBC, hemoglobin in male patients with dysglycemia. Int. J. Biol. Med. Res. 3 (2), 1609–1611.
Sha, W., Hu, F., Xi, Y., Chu, Y., and Bu, S. (2021). Mechanism of ferroptosis and its role in type 2 diabetes mellitus. J. Diabetes Res. 28, 1–10. doi:10.1155/2021/9999612
Shetty, J. K., Prakash, M., and Ibrahim, M. S. (2008). Relationship between free iron and glycated hemoglobin in uncontrolled type 2 diabetes patients associated with complications. Indian J. Clin. biochem. 23 (1), 67–70. doi:10.1007/s12291-008-0016-4
Sil, R., Ray, D., and Chakraborti, A. S. (2013). Glycyrrhizin ameliorates insulin resistance, hyperglycemia, dyslipidemia and oxidative stress in fructose-induced metabolic syndrome-X in rat model. Ind. J. Exp. Biol. 51 (2), 129–138.
Sil, R., Ray, D., and Chakraborti, A. S. (2015). Glycyrrhizin ameliorates metabolic syndrome-induced liver damage in experimental rat model. Mol. Cell Biochem. 409 (1-2), 177–189. doi:10.1007/s11010-015-2523-y
Singh, N. A., Mandal, A. K. A., and Khan, Z. A. (2017). Fabrication of PLA-PEG nanoparticles as delivery systems for improved stability and controlled release of catechin. Intl. J. Nanomed. 1, 1–9. doi:10.1155/2017/6907149
Smith, L. J., Kahraman, A., and Thornton, J. M. (2010). Heme proteins–diversity in structural characteristics, function, and folding. Proteins 78 (10), 2349–2368. doi:10.1002/prot.22747
Song, C. C., Pantopoulos, K., Chen, G. H., Zhong, C. C., Zhao, T., Zhang, D. G., et al. (2022). Iron increases lipid deposition via oxidative stress-mediated mitochondrial dysfunction and the HIF1α-PPARγ pathway. Cell Mol. Life Sci. 79 (7), 394. doi:10.1007/s00018-022-04423-x
Stahl, K., Rastelli, E., and Schoser, B. (2020). A systematic review on the definition of rhabdomyolysis. J. Neurol. 26 (4), 877–882. doi:10.1007/s00415-019-09185-4
Su, L. J., Zhang, J. H., Gomez, H., Murugan, R., Hong, X., Xu, D., et al. (2019). Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell Longev. 2019, 1–13. doi:10.1155/2019/5080843
Sun, M., Xie, Q., Cai, X., Liu, Z., Wang, Y., Dong, X., et al. (2020). Preparation and characterization of epigallocatechin gallate, ascorbic acid, gelatin, chitosan nanoparticles and their beneficial effect on wound healing of diabetic mice. Int. J. Biol. Macromol. 148, 777–784. doi:10.1016/j.ijbiomac.2020.01.198
Sun, Y., Li, Q., Guo, H., and He, Q. (2022). Ferroptosis and iron metabolism after intracerebral hemorrhage. Cells 12 (1), 90. doi:10.3390/cells12010090
Sweeney, P., Park, H., Baumann, M., Dunlop, J., Frydman, J., Kopito, R., et al. (2017). Protein misfolding in neurodegenerative diseases: implications and strategies. Transl. Neurodegener. 6, 6. doi:10.1186/s40035-017-0077-5
Tao, L., Yang, X., Ge, C., Zhang, P., He, W., Xu, X., et al. (2024). Integrative clinical and preclinical studies identify FerroTerminator1 as a potent therapeutic drug for MASH. Cell Metab. 36 (10), 2190–2206.e5. doi:10.1016/j.cmet.2024.07.013
Taskinen, M. R., Packard, C. J., and Borén, J. (2019). Dietary fructose and the metabolic syndrome. Nutrients 11 (9), 1987. doi:10.3390/nu11091987
Thomas, C., Mackey, M. M., Diaz, A. A., and Cox, D. P. (2009). Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: implications for diseases associated with iron accumulation. Redox Rep. 14 (3), 102–108. doi:10.1179/135100009X392566
Thomas, M. C., MacIsaac, R. J., Tsalamandris, C., and Jerums, G. (2004). Elevated iron indices in patients with diabetes. Diabet. Med. 21 (7), 798–802. doi:10.1111/j.1464-5491.2004.01196.x
Thornally, P. J., Langborg, H. S., and Minhas, H. S. (1999). Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 344, 109–116. doi:10.1042/0264-6021:3440109
Tolosano, E., Fagoonee, S., Morello, N., Vinchi, F., and Fiorito, V. (2010). Heme scavenging and the other facets of hemopexin. Antioxid. Redox Signal. 12 (2), 305–320. doi:10.1089/ars.2009.2787
Tong, W., Zhang, Y., Hui, H., Feng, X., Ning, B., Yu, T., et al. (2023). Sensitive magnetic particle imaging of haemoglobin degradation for the detection and monitoring of intraplaque haemorrhage in atherosclerosis. eBioMedicine 90, 104509. doi:10.1016/j.ebiom.2023.104509
Tran, T., and Tran, P. (2019). Nanoconjugation and encapsulation strategies for improving drug delivery and therapeutic efficacy of poorly water-soluble drugs. Pharmaceutics 11, 325–335. doi:10.3390/pharmaceutics11070325
Tsiftsoglou, A. S., Tsamadou, A. I., and Papadopoulou, L. C. (2006). Heme as key regulator of major mammalian cellular functions: molecular, cellular, and pharmacological aspects. Pharmacol. Ther. 111 (2), 327–345. doi:10.1016/j.pharmthera.2005.10.017
Turpin, C., Catan, A., Meilhac, O., Bourdon, E., Canonne-Hergaux, F., and Rondeau, P. (2021). Erythrocytes: central actors in multiple scenes of atherosclerosis. Int. J. Mol. Sci. 22 (11), 5843. doi:10.3390/ijms22115843
Uceda, A. B., Mariño, L., Casasnovas, R., and Adrover, M. (2024). An overview on glycation: molecular mechanisms, impact on proteins, pathogenesis, and inhibition. Biophys. Rev. 16 (2), 189–218. doi:10.1007/s12551-024-01188-4
Vallelian, F., Buehler, P. W., and Schaer, D. J. (2022). Hemolysis, free hemoglobin toxicity, and scavenger protein therapeutics. Blood 140 (17), 1837–1844. doi:10.1182/blood.2022015596
Van-Avondt, K., Nur, E., and Zeerleder, S. (2019). Mechanisms of haemolysis-induced kidney injury. Nat. Rev. Nephrol. 15 (11), 671–692. doi:10.1038/s41581-019-0181-0
Vasconcellos, L. R., and Pimentel-Coelho, P. M. (2022). Heme as an inducer of cerebral damage in hemorrhagic stroke: potential therapeutic implications. Neural Regen. Res. 17 (9), 1961–1962. doi:10.4103/1673-5374.335148
Vejux, A., and Lizard, G. (2009). Cytotoxic effects of oxysterols associated with human diseases: induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Asp. Med. 30 (3), 153–170. doi:10.1016/j.mam.2009.02.006
Vinchi, F., Muckenthaler, M. U., Da Silva, M. C., Balla, G., Balla, J., and Jeney, V. (2014). Atherogenesis and iron: from epidemiology to cellular level. Front. Pharmacol. 5, 94. doi:10.3389/fphar.2014.00094
Vinchi, F., Porto, G., Simmelbauer, A., Altamura, S., Passos, S. T., Garbowski, M., et al. (2020). Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur. Heart J. 41 (28), 2681–2695. doi:10.1093/eurheartj/ehz112
Voltarelli, V. A., Alves de Souza, R. W., Miyauchi, K., Hauser, C. J., and Otterbein, L. E. (2023). Heme: the lord of the iron ring. Antioxidants (Basel) 12 (5), 1074. doi:10.3390/antiox12051074
Wagner, K. R., Sharp, F. R., Ardizzone, T. D., Lu, A., and Clark, J. F. (2003). Heme and iron metabolism: role in cerebral hemorrhage. J. Cereb. Blood Flow. Metab. 23 (6), 629–652. doi:10.1097/01.WCB.0000073905.87928.6D
Wang, H., Liu, D., Zheng, B., Yang, Y., Qiao, Y., Li, S., et al. (2023). Emerging role of ferroptosis in diabetic kidney disease: molecular mechanisms and therapeutic opportunities. Int. J. Biol. Sci. 19 (9), 2678–2694. doi:10.7150/ijbs.81892
Wang, M., Liu, Y., Liang, Y., Naruse, K., and Takahashi, K. (2021a). Systematic understanding of pathophysiological mechanisms of oxidative stress-related Conditions—diabetes mellitus, cardiovascular diseases, and ischemia-reperfusion Injury. Front. Cardiovasc. Med. 8, 649785. doi:10.3389/fcvm.2021.649785
Wang, S., Liu, Z., Geng, J., Li, L., and Feng, X. (2022). An overview of ferroptosis in non-alcoholic fatty liver disease. Biomed. Pharmacother. 153, 113374. doi:10.1016/j.biopha.2022.113374
Wang, Y., Zhao, Y., Ye, T., Yang, L., Shen, Y., and Li, H. (2021b). Ferroptosis signaling and regulators in atherosclerosis. Front. Cell Dev. Biol. 9, 809457. doi:10.3389/fcell.2021.809457
Watson, N., Bonsack, F., and Sukumari-Ramesh, S. (2022). Intracerebral hemorrhage: the effects of aging on brain injury. Front. Aging Neurosci. 14, 859067. doi:10.3389/fnagi.2022.859067
Wei, X., Zhang, F., Cheng, D., Wang, Z., Xing, N., Yuan, J., et al. (2024). Free heme induces neuroinflammation and cognitive impairment by microglial activation via the TLR4/MyD88/NF-κB signaling pathway. Cell Commun. Signal. 22 (1), 16. doi:10.1186/s12964-023-01387-8
Weigel, T. K., Kulas, J. A., and Ferris, H. A. (2019). Oxidized cholesterol species as signaling molecules in the brain: diabetes and Alzheimer’s disease. Neuronal Signal 3, NS20190068. doi:10.1042/NS20190068
Wilson, D. F. (2017). Oxidative phosphorylation: regulation and role in cellular and tissue metabolism. J. Physiol. 595 (23), 7023–7038. doi:10.1113/JP273839
Wilson, M. T., and Reeder, B. J. (2022). The peroxidatic activities of myoglobin and hemoglobin, their pathological consequences and possible medical interventions. Mol. Asp. Med. 84, 101045. doi:10.1016/j.mam.2021.101045
Woleffenbuttel, B. H. R., Giordano, D., Founds, H. W., and Bucala, R. (1996). Long-term assessment of glucose control by haemoglobin-AGE measurement. Lancet 347 (9000), 513–515. doi:10.1016/s0140-6736(96)91141-1
Wong, C. M., Marcocci, L., Das, D., Wang, X., Luo, H., Zungu-Edmondson, M., et al. (2013). Mechanism of protein decarbonylation. Free Radic. Biol. Med. 65, 1126–1133. doi:10.1016/j.freeradbiomed.2013.09.005
Wronka, M., Krzemińska, J., Młynarska, E., Rysz, J., and Franczyk, B. (2022). The influence of lifestyle and treatment on oxidative stress and inflammation in diabetes. Int. J. Mol. Sci. 23 (24), 15743. doi:10.3390/ijms232415743
Wu, J., Hua, Y., Keep, R. F., Schallert, T., Hoff, J. T., and Xi, G. (2002). Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Res. 953 (1-2), 45–52. doi:10.1016/s0006-8993(02)03268-7
Xu, X., Xu, X. D., Ma, M. Q., Liang, Y., Cai, Y. B., Zhu, Z. X., et al. (2024). The mechanisms of ferroptosis and its role in atherosclerosis. Biomed. Pharmacother. 171, 116112. doi:10.1016/j.biopha.2023.116112
Zafar, U., Qureshi, H. J., and Karim, A. (2011). Insulin resistance and serum parameters of iron status in type 2 diabetics. Pak. J. Physiol. 7 (2), 1–31.
Zeeshan, H. M. A., Lee, G. H., Kim, H. R., and Chae, H. J. (2016). Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 17 (3), 327. doi:10.3390/ijms17030327
Zeng, X., An, H., Yu, F., Wang, K., Zheng, L., Zhou, W., et al. (2021). Benefits of iron chelators in the treatment of Parkinson’s disease. Neurochem. Res. 46 (5), 1239–1251. doi:10.1007/s11064-021-03262-9
Zhang, Y., Xin, L., Xiang, M., Shang, C., Wang, Y., Wang, Y., et al. (2022). The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed. Pharmacother. 145, 112423. doi:10.1016/j.biopha.2021.112423
Keywords: hemoglobin, myoglobin, oxidative stress, free iron, fenton reaction
Citation: Sil R and Chakraborti AS (2025) Major heme proteins hemoglobin and myoglobin with respect to their roles in oxidative stress – a brief review. Front. Chem. 13:1543455. doi: 10.3389/fchem.2025.1543455
Received: 11 December 2024; Accepted: 05 February 2025;
Published: 25 February 2025.
Edited by:
Dragan M. Popovic, University of Belgrade, SerbiaReviewed by:
Changlin Liu, Central China Normal University, ChinaCopyright © 2025 Sil and Chakraborti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abhay Sankar Chakraborti, YWJoYXlfY2hha3JhYm9ydGlAeWFob28uY28uaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.