
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Chem. , 29 January 2025
Sec. Medicinal and Pharmaceutical Chemistry
Volume 12 - 2024 | https://doi.org/10.3389/fchem.2024.1527946
This article is part of the Research Topic Quinoline as Lead Structures for the Development of Leishmanicidal Agents View all 3 articles
 Angel H. Romero*
Angel H. Romero* Francisco Delgado
Francisco DelgadoLeishmaniasis is one of the most important neglected tropical diseases, with more than two million new cases annually. It is endemic in several regions worldwide, representing a public health problem for more than 88 countries, in particular in the tropical and subtropical regions of developing countries. At the moment, there are neither approved vaccines nor effective drugs for the treatment of human leishmaniasis for any of its three typical clinical manifestations, and, importantly, the drugs of clinical use have several side effects, require complex administration regimens, present high cost, and are ineffective in many populations due to pathogen resistance. Moreover, beyond the pharmacological exigencies, there are other challenges concerning its parasitic nature, such as its great genetic plasticity and adaptability, enabling it to activate a battery of genes to develop resistance quickly. All these aspects demand the identification and development of new, safe, and effective chemical systems, which must not only be focused on medicinal chemistry and pharmacological aspects but also consider key aspects relative to parasite survival.
In this sense, the quinolines and, in particular, 4-aminoquinoline, represent a privileged scaffold for the design of potential leishmanicidal candidates due not only to their versatility to generate highly active and selective compounds but also to their correlation with well-defined biological targets. These facts make it possible to generate safe leishmanicidal agents targeted at key aspects of parasite survival.
The current review summarizes the most current examples of leishmanicidal agents based on 4-aminoquinolines focusing the analysis on two essential aspects: (i) structure–property relationship to identify the key pharmacophores and (ii) mode of action focused on key targets in parasite survival (e.g., depolarization of potential mitochondrial, accumulation into macrophage lysosome, and immunostimulation of host cells). With that information, we seek to give useful guidelines for interested researchers to face the drug discovery and development process for selective and potent leishmanicidal agents based on 4-aminoquinolines.
Leishmaniasis is one of the most important neglected tropical diseases (NTDs). It is caused by obligate intracellular parasites of Leishmania spp. That parasite is transmitted to humans by the bite of dipteran sandflies (Hide et al., 2007; Alvar et al., 2011; Alvar and Arana, 2018; Ryan and Ray, 2004; Torres-Guerrero et al., 2017). The parasite resides within the macrophage, where it differentiates and proliferates. There are approximately 20 species of Leishmania parasites that can infect humans, promoting three types of clinical manifestations of the disease: (i) mucocutaneous leishmaniasis (ML), cutaneous leishmaniasis (CL), and visceral leishmaniasis (VL). Each one of them is caused by a specific species of Leishmania: Leishmania infantum and Leishmania donovani promote VL, Leishmania braziliensis and Leishmania mexicana cause CL in the Americas, and Leishmania major causes CL in the Old world), and L. braziliensis and Leishmania amazonensis are responsible for MC (Hide et al., 2007; Torres-Guerrero et al., 2017). CL is described as lesions in the skin, whereas MC consists of deep lesions with deformation of the mucosa zone as a consequence of a high level of infection (Torres-Guerrero et al., 2017). Meanwhile, the VL is characterized by an over-inflammation or increase of the volume of such organs, including the bazo, the spleen (known as splenomegaly), the liver (hepatomegaly), and lymph nodes (lymphadenopathy) (Hide et al., 2007; Torres-Guerrero et al., 2017). In particular, this last clinical manifestation VL is fatal in more than 95% of untreated cases.
From a demographic point of view, leishmaniasis is prevalent in 98 countries of tropical and subtropical regions, registering between 0.7 and 1.3 million new leishmaniasis cases and between 26,000 and 65,000 deaths annually (World Health Organisation, 2024). The majority of VL cases occur in eight countries: Brazil, Eritrea, Ethiopia, India, Kenya, Somalia, South Sudan, and Sudan; the cases of CL are predominant in Afghanistan, Algeria, Brazil, Colombia, Iraq, Pakistan, and Syria; whereas the cases of MCL are found in South America (Pan American Health Organization, 2023).
Treatment of leishmaniasis represents another challenge for medicinal chemists as a consequence of the high adaptability and genetic plasticity of Leishmania. The parasite is able to elude the defense mechanism of macrophage-inducing changes in macrophage polarization as well as to quickly develop resistance to known drugs. Currently, there are neither vaccines nor effective drugs for leishmaniasis treatment in any of its clinical manifestations. There are a few approved drugs, including glucantime, pentostam, pentamidine, amphotericin B, and miltefosine (Figure 1), but they present multiple disadvantages including diverse toxicity manifestations (affecting heart, liver, and kidneys), high cost, low therapeutic efficacy, prolonged administration treatment times (30–60 days) and parasite resistance (Zulfiqar et al., 2017; Aronson et al., 2017). The use of pentavalent antimonials is currently compromised due to the many resistant cases registered in endemic areas of India and Brazil (Aronson et al., 2017). Alternatively, miltefosine has been used for the treatment of VL and CL as the first oral antileishmanial drug; however, its use is also limited by its high toxicity and effectiveness against different Leishmania species (Aronson et al., 2017).
To minimize side-effects, enhance the therapeutic effect, and reduce the emergence of parasite resistance, binary combinations between two of these approved drugs have been employed in specific regions with epidemic situations in Africa and Asia; however, the use of the combination did not provide a definitive solution. This situation has motivated the scientific community to generate new chemical structural alternatives. Many studies have been done by the academy and non-governmental organizations such as Disease Neglected Innovative (DNDi) and Asian and European consortiums. The DNDi presents a portfolio exhibiting a group of compounds that are currently under investigation in preclinical or clinical stages, with a promising therapeutic profile, including (i) proteasome inhibitors such as GNF8000, GNF6702, LXE408, and DDD01305143/GSK3494245 (preclinical stage); (ii) inhibitors of cyclin-dependent kinase-12 (CDK-12), such as DDD853651/GSK3186899 (preclinical stage); (iii) nitroimidazole compounds such as DNDi-VL2098, DNDi-VL2075, DNDi-VL8219, and DNDi-VL0690 (preclinical stage), (iv) benzoxaboroles, such as DNDi-VL6148 (clinical stage) and DNDi-5421 and DNDi-5640 (preclinical stage), and (v) amino-pyrroles such as DNDi-5561, DNDi-1047, and DNDi-1044 (preclinical stage) (Drugs for Neglected Diseases initiative, 2023). Other heterocyclic derivatives developed by Hilbert´s group are in the preclinical phase (Thomas et al., 2019; Thomas et al., 2021) (Figure 2).
Despite the advances, the development of new leishmanicidal drugs remains a significant challenge for medicinal chemists, not only because of the rigorous pharmacological requirements, which seek to minimize side-effect risks, but also because of the genetic plasticity of the parasite and the challenge of effectively accessing a parasite resident in the host cell. That situation calls for the development of new strategies to identify new effective, selective, and safe leishmanicidal agents, which will not only be focused on classic medicinal chemistry concepts but also on critical aspects of parasite survival (Alvar and Arana, 2018). In this sense, quinoline represents a prominent scaffold for the leishmanicidal drug design due not only to its structural versatility for introducing key pharmacophores but also to the plasticity of the chemical system to be targeted on diverse key aspects of parasite life (Silva et al., 2023; Reynolds et al., 2013; Dorababu, 2021). Details concerning the relationship of the quinoline with critical aspects of Leishmania life are shown in Section 2.
When considering quinolines as leishmanicidal agents, there are many examples that feature a variety of pharmacophores (Silva et al., 2023). In the first studies, antimalarial quinolines such as chloroquine, mefloquine, primaquine, sitamaquine, and tafenoquine (Figure 3) were commonly employed upon repurposing program for the discovery of potential quinoline drugs (Reynolds et al., 2013). From active antimalarial agents, sitamaquine (WR 6026, Figure 3) was identified as the most promising quinoline for the treatment of VL not only by its good antileishmanial activity but also its good aqueous solubility for oral administration, excellent pharmacokinetic (short half-time elimination) and ADME parameters (Loiseau et al., 2011; Coimbra et al., 2010). That quinoline drug has reached clinical trials (phase II) for the treatment of VL caused by Leishmania chagasi (Dietze et al., 2001); however, its investigation has been stopped due to their adverse effects including methemoglobinemia and nephrotoxicity (Dietze et al., 2001). It is important that the potential of sitamaquine as a leishmanicidal agent is restricted to the treatment of VL because poor or discrete results have been found against in vivo models of CL under different topical formulations (Garnier et al., 2006).

Figure 3. Antimalarial drugs with leishmanicidal activity: (A) chloroquine, (B) mefloquine, (C) sitamaquine, (D) quinoline A, (E) tafenoquine, (F) primaquine, and (G) amodiaquine.
Among other antimalarial quinolines used against the Leishmania parasite, a group of 8-substituted quinoline such as the 8-hydroxyquinoline A, tafenoquine and primaquine (see structures in Figure 3) have been identified by their excellent leishmanicidal response against in vitro infective models (Costa Rocha et al., 2013). The 8-hydroxyquinoline A that presents a better leishmanicidal profile than the reference drug miltefosine is in the preclinical stage within the Drugs for Neglected Diseases initiative (DNDi) program (Loiseau et al., 2011). Tafenoquine exhibits a good leishmanicial in vitro profile against a variety of Leishmania species as well as a curative response for the in vivo murine model of L. donovani/BALB/c with a low ED50 dose (1.2–3.5 mg/kg for 5 days) (Manzano et al., 2011). Mechanistic studies revealed that tafenoquine (i) targets respiratory complex III with apoptosis consequences and (ii) is able to increase glycolytic ATP synthesis via a sterol-dependent diffusion process. Meanwhile, good in vitro and in vivo responses for LV models with nontoxic effects were found for primaquine (Shah et al., 2022). However, the potential of the 8-amino/hydroxyquinolines seems to be limited by their tentative side effects (Costa Rocha et al., 2013), making the development of more effective and safer quinolines essential.
In this context, the 4-aminoquinoline emerges as a convenient scaffold, for which a great variety of active compounds against different in vitro and in vivo models of either VL or CL have been identified (see Section 3). Examples of 4-aminoquinolines include chloroquine, hydroxychloroquine, and an analog such as mefloquine (see structures in Figure 3). Chloroquine has shown a better response against in vitro and in vivo models of CL than models of VL (Costa Rocha et al., 2013; Hanif et al., 2016; Mwololo et al., 2015). Against intracellular amastigotes of L. amazonensis in infected macrophages, for example, the chloroquine and other analogs such as mefloquine and hydroxychloroquine exhibited significant antiamastigote response, with IC50 values of 0.78 μM, 1.56 μM, and 0.67 μM, respectively. Interestingly, a more discrete response was found against promastigotes with IC50 values higher than 8 µM (Costa Rocha et al., 2013). Studies at the clinical level have shown the curative properties of chloroquine in infected patients, finding better results upon intralesional administration than upon oral administration (Hanif et al., 2016). Meanwhile, amodiaquine, a traditional 4-aminoquinoline antimalarial drug, has also been demonstrated to possess a remarkable antiproliferative effect against different Leishmania species with low IC50 responses (De Mello et al., 2004). As a consequence of the potential of the 4-aminoquinoline, many active and selective leishmanicidal agents based on 4-aminoquinoline have proved in the last 2 decades against in vitro and in vivo models of CL and VL, making it possible to recognize specific structural requirements and well-defined mechanistic targets for a rational drug design.
This review provides a general perspective on the key issues to consider for the design of safe and selective leishmanicidal agents based on 4-aminoquinolines. The review analyzed the evolution and development of 4-aminoquinolines as leishmanicidal agents focused on two critical aspects: (i) a recompilation of the typical molecular or cellular targets involving 4-aminoquinoline and (ii) a structure–property relationship analysis among key examples. The structure–property relationship seeks to identify the key pharmacophore from biological potency, selectivity, and physicochemical convenience, whereas the mechanistic insight seeks to identify the key targets for the design of a quinoline with a specific biological response. With that information, we seek to offer readers a tool to face the drug design of leishmanicidal agents based on 4-aminoquinolines.
This section presents the main biological targets in which the 4-aminoquinolines are involved as leishmanicidal agents. These therapeutic targets are keys to promoting a specific and selective leishmanicidal response, and they have been identified from the in vitro and in vivo experiments of CL and VL models. Among the most typical targets can be mentioned: (i) accumulation into the mitochondria of the Leishmania parasite leading to depolarization of the mitochondrial membrane potential; (ii) accumulation into the macrophage phagolysosome to favor parasite–drug interaction and (iii) immunostimulation of the host cell.
Before describing how 4-aminoquinoline alters the normal mitochondrial potential in the Leishmania parasite, it is important to describe some key concepts, including a description of the function of the mitochondria in the Leishmania parasite and the role of the mitochondrial potential, its depolarization, and consequences for Leishmania life. Mitochondria, the powerhouses of the cell, are organelles found in most eukaryotic cells. Their main function is the cellular respiration and the production of adenosine triphosphate (ATP) through the enzymatic catalysis reaction between the adenosine diphosphate (ADP) and phosphate ion using mitochondrial ATP synthase (Siekevitz, 1957; McBride et al., 2006; Lackner, 2014). Among other tasks, mitochondria are involved in numerous essential functions such as the production of NADH and GTP in the citric acid cycle, the biosynthesis of amino acids, heme groups, and iron-sulfur clusters or the synthesis of phospholipids for membrane biogenesis, calcium signaling, stress responses, and as cellular signaling hubs (Schmidt et al., 2010; Smith et al., 2011; Liew et al., 2021).
Structurally, a mitochondrion consists of a double membrane structure: the outer membrane and the inner membrane, as depicted in Figure 4. Between the inner and outer membranes of a mitochondrion are three sub-structures: intermembrane space, mitochondrial matrix, and the cristae compartment (Lackner, 2014; Mannella, 2006; Chipuk et al., 2006). The outer membrane is porous, which allows the entrance of ions and small/uncharged molecules through a voltage-dependent ion channel (Lackner, 2014; Mannella, 2006; Chipuk et al., 2006). Other larger molecules, like proteins, tend to enter by special translocases. Meanwhile, the inner membrane is a tight diffusion barrier for diverse types of ions and molecules where oxidative phosphorylation occurs. The mitochondrial matrix, with a pH of 7.9–8 (Lackner, 2014), is the place where the transmembrane electrochemical proton gradient is generated. Also, other processes such as DNA replication, transcription, protein biosynthesis, and numerous enzymatic reactions occur in the mitochondrial matrix (Lackner, 2014). Meanwhile, the intermembrane space, with a pH value of 7.2–7.4, allows the diffusion of chemical components between membranes, whereas the cristae are the location of ATP synthesis (Chipuk et al., 2006).
The Leishmania parasite and other trypanosomatids have a single mitochondrion composed of a compact matrix and inner and outer membranes with many cristae (Jensen and Englund, 2012). The size and functions of the Leishmania mitochondria are substantially different from those size and functions found in higher eukaryotes (Jensen and Englund, 2012; Souza et al., 2009; Braly et al., 1974; Jardim et al., 2018; Coelho et al., 2010). In particular, the Leishmania mitochondria play a role essential in energy production, which is connected with other vital processes in parasite life, such as cellular homeostasis and signaling, biosynthesis of protein and lipid biosynthesis, and beta-oxidation of fatty acids (Jensen and Englund, 2012; Souza et al., 2009; Braly et al., 1974; Jardim et al., 2018). Furthermore, dysfunction of the Leishmania mitochondria is correlated with the production of reactive oxygen species (ROS) as well as the promotion of essential cell processes like apoptosis (Ortiz et al., 2016; Menna-Barreto and de Castro, 2014; Opperdoes and Coombs, 2007; Hart and Coombs, 1982). For example, it is reported that an increase in the levels of ROS and lipid peroxide can depolarize the mitochondrial membrane potential (ΔYm) (Ljubava et al., 2018). The mitochondrial membrane potential (ΔΨm) is a factor derived from redox transformations that occurred during the Krebs cycle, and it serves as a form of energy storage for ATP synthesis (Mitchell, 1966; Glagolev and Skulachev, 1978; Ataullakhanov and Vitvitsky, 2002). Then, the ΔΨm in combination with the proton gradient (ΔpH) (Mitchell, 1966) generates the transmembrane potential of hydrogen ions that is essential for the ATP synthesis (Glagolev and Skulachev, 1978; Ataullakhanov and Vitvitsky, 2002).
Cell stability and normal cell functioning depend on stable levels of ΔΨm and ATP in the cell. A prolonged dysfunction of these key parameters affects the mitochondria function and, consequently, cell viability, promoting a cascade of cell pathologies such as calcium signaling and other key functions (Glagolev and Skulachev, 1978; Ataullakhanov and Vitvitsky, 2002; Zamzami et al., 1995). For example, at a high ΔΨm, the mitochondrial respiratory chain promotes a significant production of reactive oxygen species (ROS), which have an exponential dependence (Yaniv et al., 2010; Yaniv et al., 2014; Izyumov et al., 2004). Whereas sustained low values of ΔΨm are well-documented to minimize the production of ATP as well as to promote “reductive stress” as a consequence of the low ROS production (Jiang et al., 2020).
Leishmania mitochondria represent an attractive target for the drug design, and it is important to understand the rational criteria for a mitochondria-targeted antioxidant (MTAs) (Jiang et al., 2020). The main goal is to seek to alter the normal level of ΔΨm using cationic organic compounds taken in advance on the negative value of the mitochondrial membrane potential inside mitochondria and the lipophilic nature of mitochondrial membranes. First, the negative value of the mitochondrial potential favors the accumulation and transportation of permeating cations inside mitochondria (Ross et al., 2005; Zielonka et al., 2017; Robb et al., 2015). The second key aspect is the permeation of the cationic compounds through the mitochondrial membranes. As mentioned earlier, the mitochondrial membranes are composed of lipid bilayer membranes (BLM) and proteins, incorporating lipophilic chains for the design of permeating cationic compounds. The MTAs possess two essential structural features: the existence of a cationic center and the incorporation of large lipophilic chains or lipophilic chemical functions. For example, among the most relevant MTAs can be mentioned tetraphenylborate, dipicrylamine (DPA), octadecyl rhodamine, triphenylphosphonium, and mitoquinone (Ross et al., 2005; Zielonka et al., 2017; Robb et al., 2015; James et al., 2005; Hoye et al., 2008; Blaikie et al., 2006; Biasutto et al., 2010). This type of compound has been shown to cross the BLM and accumulate inside mitochondria (Ketterer et al., 1971; Andersen and Fuchs, 1975; Melikyan et al., 1996; Zimmermann et al., 2008; Liberman and Topaly, 1969) (Figure 5).
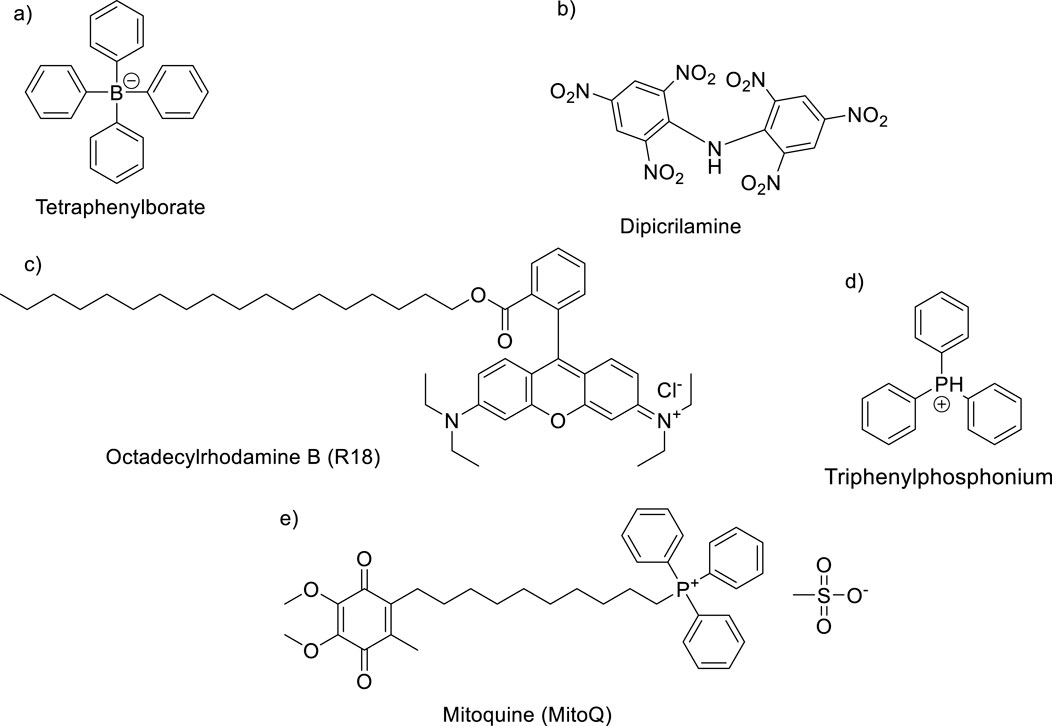
Figure 5. Some typical MTAs: (A) tetraphenylborate, (B) dipicrylamine (DPA), (C) R18, (D) TPP, and (E) mitoquinone (MitoQ).
The known ligands that target mitochondria are characterized by a lipophilic chain that favors the translocation through the BLM of the cell without a complex uptake mechanism and the presence of a cationic region to accumulate inside the negative mitochondria potential. When discussing Leishmania, the miltefosine is the clearest example. It possesses a long lipophilic chain and a cationic region of the terminal ammonium group. More recently, quinolines, including 8-substituted and 4-aminoquinolines, have been correlated with the depolarization of mitochondrial membrane potential into Leishmania parasites. In particular, those quinolines with a terminal tertiary moiety and a lipophilic chain in their molecular structure showed a high ability to alter the ΔΨm with affectation of cell and mitochondrial functions. For example, sitamaquine is a lipophilic weak base that accumulates in the promastigotes through an electrical gradient involving two steps: i) the interaction between the positively charged sitamaquine with the anionic polar head groups of phospholipids in mitochondrial membranes and (ii) the subsequent insertion of quinoline into the parasite plasma membranes through hydrophobic interaction between the acyl chains of phospholipids and the hydrophobic quinoline chains to enter into the lipid monolayer (Duenas-Romero et al., 2007). It is important to mention that the affinity of sitamaquine by mitochondrial membranes is transitory because it can also be located in the cytosol (Coelho et al., 2010). As a consequence of the internalization in the membrane, the sitamaquine affects the normal electrical potential efflux, generating a rapid collapse of the mitochondrial inner-membrane potential (Lopez-Martin et al., 2008; Vercesi et al., 2000).
This evidence has motivated the design of diverse quinoline compounds, including 4-aminoquinolines targeted toward the Leishmania mitochondria. These quinolines are characterized by a weak basic moiety and a lipophilic chain in the quinoline structure, and the leishmanicidal response has shown a consistent correlation with depolarization of mitochondrial potential, promoting a series of biological and biochemical consequences into the parasite, including an increase of ROS production, alteration of parasite morphology and parasite death via apoptosis (Figure 6). In Section 3, we show the effect of a variety of 4-aminoquinolines on the mitochondrial membrane potential of the parasite through the experimental evidence based on the determination of ΔΨm, level of ROS, alteration of parasite morphology, and evidence of apoptosis. In that section, the influence of this depolarization on the in vitro leishmanicidal response is also discussed.
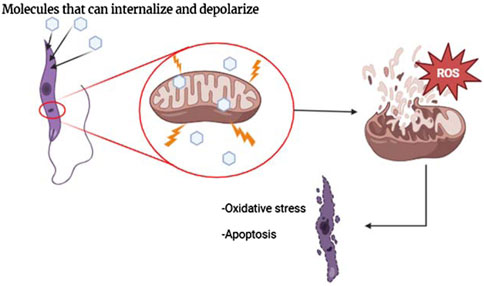
Figure 6. Illustration of the internalization of molecules through the BLM of mitochondria and biological consequences.
The macrophage lysosome is an essential target in the life of the Leishmania parasite. As was mentioned earlier, Leishmania, an intracellular parasite, lives in the phagolysosomes of mammalian macrophages during its infective stage. The location of the parasite inside the phagolysosome is promoted by the presence of lipophosphoglycan (LPG) along the parasite surface (Desjardins and Descoteaux, 1997; Moradin and Descoteaux, 2012; Séguin and Descoteaux, 2016), which are recognized by parasite facilitating its entrance into phagolysosome. The physiological conditions in the phagolysosome consist typically of an acidic pH value of 4–5 and an internal temperature of 37°C. These aspects are critical and play an important role in initiating the phenotypic differentiation of the promastigote form to the amastigote form (Clos et al., 2022). Then, the macrophage lysosome represents an attractive target for the design of novel leishmanicidal drugs, being key to search strategies that favor the accumulation of leishmanicidal drugs into macrophage lysosome. The latter guarantees a direct interaction between the leishmanicidal drug and the parasite, which is key for a direct leishmanicidal effect.
To reach the accumulation of the leishmanicidal drug into the mammalian lysosome (lysosomotropic drug), it is essential to take into account some physicochemical characteristics of the lysosome, such as its internal acidic pH and the lipophilic nature of the outer membrane. These chemical characteristics suggest that the lysosomotropic drugs consist of compounds with weak basic groups and a lipophilic chain, which are essential to favor the internal accumulation inside the lysosome via acid–base ionization and the compound penetration through the lipophilic membrane, respectively. A promising study performed by Trapp et al. (2008) based on a comparison of ten known quinoline antimalarial drugs found a high and selective accumulation in lysosomes for weak mono- and bivalent bases with intermediate to high log Kow. The high lysosome accumulation for quinoline bases showed pKa values between 6 and 10, with an optimum value near 8. The mechanism of this accumulation is the ion trap, involving the uptake of the neutral base into acidic lysosomes with trapping following protonation. The exact maximum concentration ratio of lysosome to outside occurs at pKa = 7.9. In general, Trapp and co-workers found that the optimum association constant for selective accumulation of bivalent bases in lysosomes is at the 3 < log KOW < 6 range, whereas for monovalent bases, it is at the 0 < log KOW < 3 range. For example, chloroquine has a pKa1 value of 9.94 and a pKa2 value of 8.10 (Newton and Kluza, 1978), with a log KOW of 4.38 (Hansch et al., 1995). Quinacrine exhibited pKa values of 10.2 and 8.2, with a log KOW of 4.79 (Hansch et al., 1995). Importantly, the accumulation decreases with an increase in the pH of the lysosomal, finding no ion trapping and accumulation when the pH in lysosomes reaches the pH in the cytosol.
On the other hand, Marceau and co-workers found not only a correlation with the pKa characteristic but also with the lipophilicity (Marceau et al., 2012). They found an optimum lysosome accumulation for organic bases with pKa values between 8 and 10, and the accumulation is more effective if the compound presents a Log P value between 4 and 6. The findings reported by Trapp and Marceu were validated with experimental results and by comparison to the properties of antimalarial drugs in clinical use, for example, Trapp et al. (2008). Further studies have shown that quinoline rapidly accumulates into acidic compartments like acidocalcisomes (Lopez-Martin et al., 2008). Importantly, an NMR study has demonstrated that sitamaquine does not affect lipid trafficking in Leishmania (Coimbra et al., 2010).
In addition to the lysosome-tropic property of quinoline drugs by mammalian cells, several quinolines have received attention for their antitumor effects, which are favored by their accumulation in the lysosomes of tumor cells. Its accumulation in the lysosome blocks autophagy, prevents the degradation of autophagosomal content, activates the ER stress, and induces apoptosis in tumor cells. The accumulation into lysosome is favored by quinoline protonation under acidic pH, decreasing the lysosome function and thus inhibiting autophagy (Solomon and Lee, 2009; McAfee et al., 2012; Degtyarev et al., 2008; Sharma et al., 2012). Recently, the accumulation of quinoline into lysosome in macrophages and mammalian cells has been demonstrated through the use of fluorescence microscopy (Kuo et al., 2016; Fan et al., 2018; Li et al., 2013; Bai and Qian, 2020; Bik et al., 2021). Furthermore, a quinoline compound like Lys05 has demonstrated an effective accumulation into macrophage lysosome via proton trapping, visualized through fluorescence microscopy. Then, the quinoline trapping by parasite phagolysosome not only favors the accumulation into phagolysosome but also facilitates the direct interaction between the quinoline drug and intracellular amastigotes. Although it is still not described, we think that the partial protonation of the basic quinoline into macrophage lysosome generates cationic compounds, which efficiently enter the mitochondria in conjunction with the presence of lipophilic chains, as depicted in Figure 7. Finally, beyond the internalization inside the parasite mitochondria and phagolysosome of leishmanicidal agents based on 4-aminoquinolines, it is reported that sitamaquine can rapidly accumulate into other types of membranous and acidic compartments, mainly into acidocalcisomes (Lopez-Martin et al., 2008; Vercesi et al., 2000). The acidocalcisomes are acidic vacuoles containing most of the cellular calcium, and that type of compartment is present only in trypanosomatids (Leishmania, Trypanosome cruzi, and Trypanosome brucei) and other flagellated parasites such as Plasmodium (causes malaria) and Toxoplasma gondii (causes toxoplasmosis), emerging as another convenient target for the drug design of leishmanicidal agents (Docampo et al., 2005).
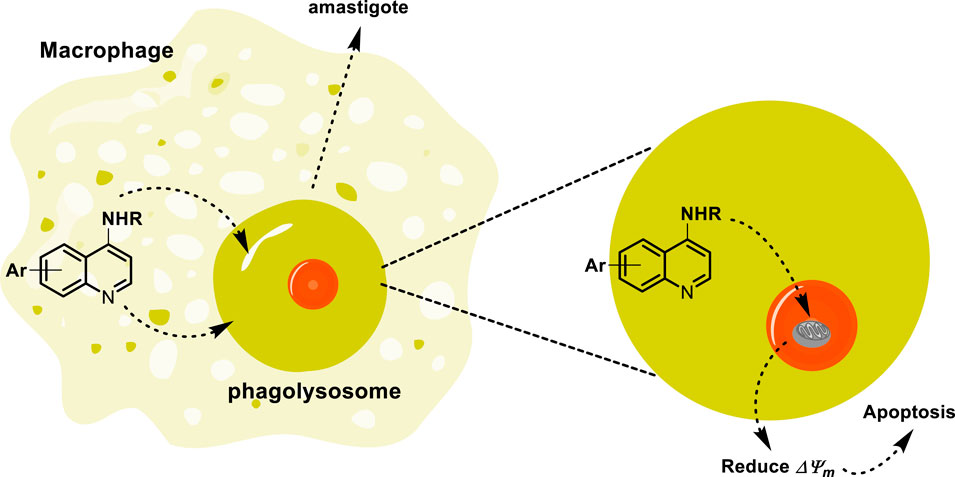
Figure 7. Tentative mechanism of the accumulation of quinoline through the lysosome to reach the mitochondria.
Once the infection within the macrophage is established, the Leishmania parasite is able to elude the immune host defense response through the induction of changes in the macrophage polarization from M1 to M2, resulting in a macrophage more focused on differentiation tasks than on defense actions (Tomiotto-Pellissier et al., 2018; Conceicao and Morgado, 2019). Macrophage polarization occurs through the recognition by the macrophage phagocytes of phosphatidylserine that is placed at the surface of apoptotic parasites (Conceicao and Morgado, 2019). This macrophage stage, M2, is characterized by producing low levels of pro-inflammatory cytokines TNF-α, IL-1, IL-6, and IL-12 (Bogdan, 2020; Tomiotto-Pellissier et al., 2018) and high levels of regulatory cytokines like TFG-1β and IL-10. The latter implies a suppression of the lymphocytes and neutrophils and the production of chemical effectors such as nitric oxide and reactive oxygen species (Smirlis et al., 2019). All these features favor the survival of the parasite within the macrophage and provide a path for its colonization toward other macrophages. The use of immunostimulants like imiquimod and resiquimod (Figure 8A) has been demonstrated to control the parasite proliferation for both in vitro and in vivo infected models. Interestingly, parasite control has shown a good correlation with the increase in the pro-inflammatory cytokine ratio compared with untreated controls (Buates and Matlashewski, 1999; El Hajjldi et al., 2018). These immunostimulants act as agonists of TLR7 and TLR8 receptors. The TLRs are a class of pattern recognition receptors (PRRs), and their signaling pathway represents a primary defense barrier against pathogens to control the activation and progression of adaptive immunity through the production of pro-inflammatory cytokines, interferons, chemokines, and B and T cells (Kawasaki and Kawai, 2014; Tuon et al., 2008; Tuon et al., 2010; He et al., 2013). Macrophages recognize Leishmania parasites through TLR2, TLR4, TLR7, TLR8, TLR9, TLR11, and TLR12 (Polari et al., 2019; Vargas-Inchaustegui et al., 2009; Tuon et al., 2010; Tolouei et al., 2013; Raman et al., 2010; Kaushik et al., 2021; Shukla et al., 2018; Faria et al., 2012).
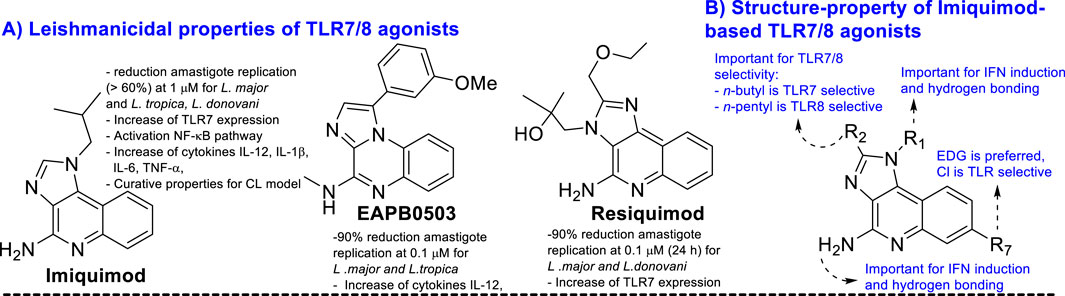
Figure 8. (A) Structure of some key TLR agonists of imiquimod, EAPB0503, and resiquimod. (B) Structure–property relationship of imiquimod based on TLR 7/8 agonist.
Recent studies have demonstrated a significant parasite proliferation in Leishmania-infected Tlr7(-1-) mice compared to normal infected controls (Faria et al., 2012), which highlights the role of the TLR7 receptor in the immune control against Leishmania. Other studies have shown that imiquimod analogs like resiquimod and EAPB0503 (Figures 8A, B) are also able to control parasite proliferation in infected macrophages in combination with stimulation of expression of transcription factors like AP-1 and NF-κB (Smirlis et al., 2019), which are essential for the production of pro-inflammatory cytokines via TLR7 receptor activation. Other types of immunostimulants have controlled parasite proliferation (Regli et al., 2020; Anders et al., 2019; Bernardo et al., 2021; Rodrigues de Santana et al., 2017; Vasilakos and Tomai, 2013; Salyer and David, 2018). It is important to mention that either imiquimod or resiquimod consists of a quinoline structure. In addition, imiquimod and resiquimod are characterized by a weak leishmanicidal response against promastigote and axenic amastigote forms of the parasite, which are the parasite control in the infected model associated with immunostimulant activity (Smirlis et al., 2019; Buates and Matlashewski, 1999). Curiously, most of the proven 4-aminoquinolines have exhibited a significant antiamastigote response against the infected model and a weak leishmanicidal response against axenic forms of the parasite (see Section 3), which suggests a strong association with immunostimulation of the host cell. The ideal situation is the discovery of a leishmanicidal 4-aminoquinoline with a dual leishmanicidal/immunostimulant response. In Section 2.3.2, the use of 4-aminoquinolines as TLR agonists and antagonists is discussed.
A quinoline with a tertiary amine group is a chemical platform involved in immunostimulant response either as a TLR agonist or as a TLR antagonist (Talukdar et al., 2021). In particular, the 2-aminoquinolines have been extensively studied, achieving the discovery of a potent agonist of the TLR8, including some of their analogs such as imiquimod, resiquimod, gardiquimod, which are among the best known (Talukdar et al., 2021). The protonation of the tertiary amino group and the quinolinic nitrogen atom in quinolinic compounds such as chloroquine and quinacrine favors the binding to DNA, emerging as a tool for the inhibition of CpG-DNA (Manzel et al., 1999). Chloroquine was recognized by its CpG-ODN inhibitory effect with an antagonist TLR response. A SAR study of quinoline analogs found that the incorporation of aryl-substituent at the C-2 position, such as phenyl (EC50 = 51 nM) or C-2-naphthyl (EC50 = 9 nM) enhanced the CpG-ODN inhibitory activity by 2-fold and 12-fold, respectively, compared with chloroquine (EC50 = 110 nM) (Figure 9). Interestingly, a correlation between the pKa and leishmanicidal activity was found, where higher pKa values generate greater antagonistic response (Strekowski et al., 1999). Then, either the basicity or the lipophilicity are key features for designing greater antagonistic TLR based on 4-aminoquinolines. Further structures showed that an amodiaquine analog with a phenyl linker between 4-amino and terminal tertiary amine compared with chloroquine was significantly more potent as a TLR antagonist (compounds I-V, Figure 9A). Its prominent compound displayed an EC50 value of 0.24 nM. Subsequently, the naphthyl moiety at the C2 position was replaced by a phenyl-type substituent because the naphthyl moiety is not metabolically stable and is potentially toxic. Other SAR analysis showed that steric terminal dialkylamino at the 4-position compromises the antagonist-receptor interaction, being preferred to a flexible dialkylamino over a cyclic or rigid one like piperazine. Further developments show that compounds VI and VII, which are chloroquine analogs with a 4-(N-methylpiperazine)methyl-phenyl and 4-(N-methylpiperazine)phenyl moieties, exhibited a significant antagonist TLR response with EC50 values of 7.07 nM and 0.76 nM, respectively (Figure 9A) (Strekowski et al., 2003). Finally, quinoline analogs VIII and IX that bear the (4-hydroxyphenyl) substituent at the 4-position showed a potent antagonist response against hTLR and gave IC50 values of 0.5 nM and 0.7 nM, respectively (Figure 9B) (Zhang et al., 2018; Hu et al., 2018).
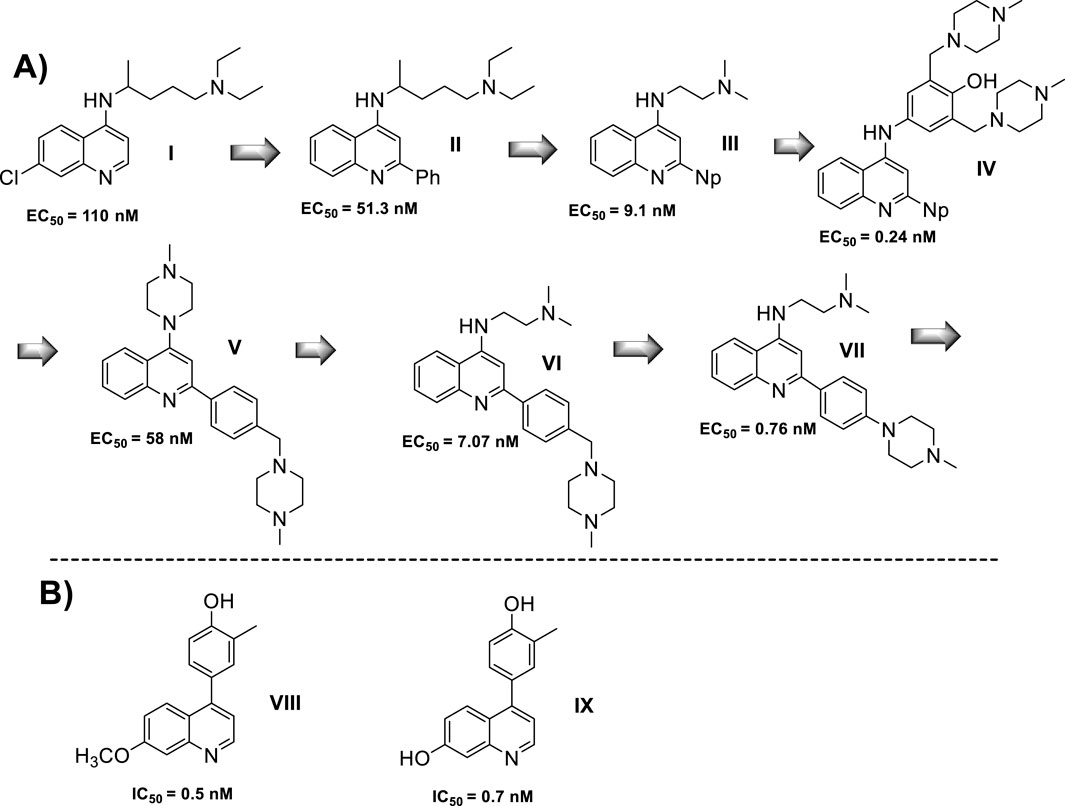
Figure 9. (A) Structure progression of 4-quinolines as antagonists of TLR with their corresponding EC50 values. (B) Derivatives of 4-phenylquinoline as TLR agonists.
In summary, lipophilic quinoline with a strongly basic chain moiety is required for the construction of an antagonist of TLR, which could be of great importance for the immunostimulation of infected macrophages against intracellular amastigotes of Leishmania. In Section 3, the role of the immunostimulation of the host cell is discussed and analyzed for a group of 4-aminoquinolines.
4-Aminoquinoline is the most versatile quinoline scaffold for the construction of active and potent leishmanicidal agents. In particular, the most promising 4-aminoquinolines are characterized by a tertiary amine or/and a lipophilic group (e.g., alkyl or aryl) into the amino chain. In this section, we performed a structure–activity relationship comparison to provide a general perspective about the key structural features for the design of potent leishmanicidal agents based on 4-aminoquinoline and a direct correlation with the three mentioned targets.
Within the 4-aminoquinolines, chloroquine was one of the first antimalarial quinoline compounds to test against Leishmania models, with an excellent in vitro response against intracellular amastigotes of the L. amazonensis parasite (Costa Rocha et al., 2013). In a clinical trial, chloroquine appeared to be as effective as tetracycline for the treatment of CL (Hanif et al., 2016). Its combination with diminazene provided good leishmanicidal responses that merit further development (De Mello et al., 2004), whereas its combination with paromomycin did not yield encouraging results. In summary, chloroquine displayed a good leishmanicidal response against CL models, but its leishmanicidal response is discrete or weak against VL models (Mwololo et al., 2015).
Another antimalarial 4-aminoquinoline that has been proved against Leishmania parasites is amodiaquine. Amodiaquine is a popular antimalarial once used for human treatment, but its use was suppressed by the occurrence of hepatotoxicity due to the metabolization of amodiaquine to form quinone intermediate via hydroxyl oxidation (O’Neill et al., 2003). The amodiaquine has shown an excellent in vitro response against intracellular amastigotes of L. donovani with a selectivity index (SI) higher than 90 (Guglielmo et al., 2009). Beyond the known antimalarial 4-aminoquinolines (chloroquine, amodiaquine, others), the 4-aminoquinoline scaffold has been widely used as a platform for the construction of new leishmanicidal agents.
We performed a chronological (since 2009) summary of current examples of leishmanicidal 4-aminoquinolines, and from them, a structure–property relationship analysis and the correlation of the biological activity with mitochondrial dysfunction and immunostimulation of host cells were made for those cases where mechanistic data were reported. Furthermore, the specificity of 4-aminoquinolines toward intracellular amastigotes in infected in vitro models over promastigote or axenic amastigote in vitro models was discussed for most of the cases.
In 2009, S. Gugliemo and co-workers prepared a series of 22 novel analogs of amodiaquine functionalized with an N-heteroaryl-piperazine (Guglielmo et al., 2009), a 4-(dialkyl)ethylene-diamine, or an N-acyl 4-(dialkyl)ethylene-diamine as the N-amine terminal into the 4-aniline moiety (Figure 10), and its leishmanicidal in vitro effect against L. donovani (responsible of VL) amastigotes was proved. Within the 4-aniline substituted derivatives, the replacement of the terminal N-diethylamino in the AQ by hydroxyl-methyl or chloro-methyl moieties resulted in a significant decrease of the leishmanicidal activity for their amodiaquine analogs by more than 80-fold against the intracellular amastigotes of L. donovani. This implies that the presence of the basic terminal amine, a pharmacophore, is essential for the design of new amodiaquine leishmanicidal quinolines. Derivatives featuring a 4-(dialkyl)ethylene-diamine, group II, showed an antiamastigote response depending on the type of N-substitution at the end of the diamine chain. For example, the incorporation of amine (NH2) or leucine amino acid generates the compounds most active in the family (group II), with IC50 values of 5.65 µM and 4.69 µM and selectivity indexes of 4.5 and 4.8, respectively. These results show the important role of the terminal amino group, but further improvements are needed to reach the potency found for AQ [selectivity index (SI) of about 90].
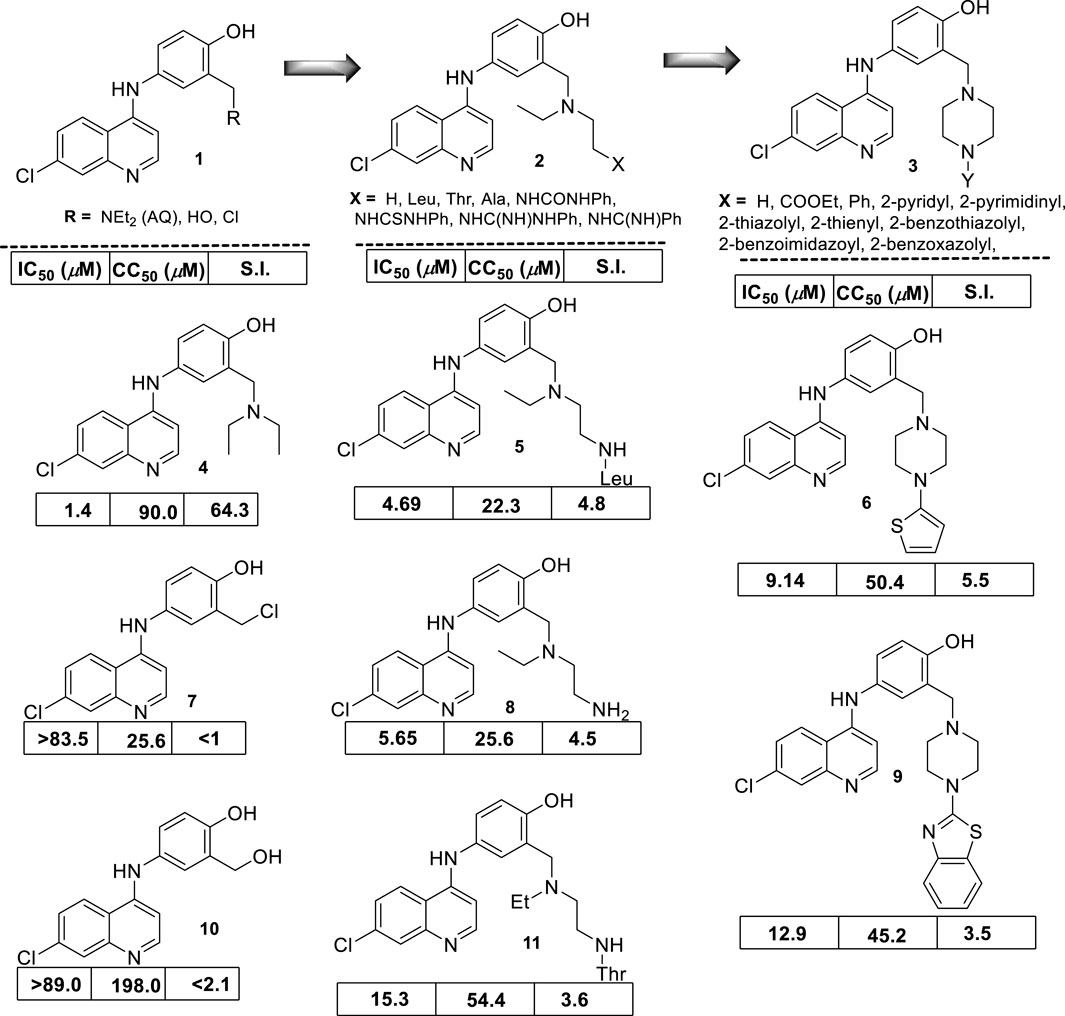
Figure 10. Structure of a series of modified amodiaquine derivatives reported by Bertinaria´s group (Guglielmo et al., 2009). Leishmanicidal response (expressed as IC50) against intracellular amastigote L. donovani cytotoxicity (expressed as CC50) determined on KB cells and selectivity index (SI).
The remaining compounds with acyl-amine moieties as terminal substituents showed a similar leishmanicidal activity but higher toxicity, which compromises the selectivity of their analogs. Finally, from group III, the incorporation of heteroaromatic basic moieties was not convenient because their analogs displayed a comparable IC50 lower than AQ, but their derivatives were highly toxic, with an SI lower than 3. Only a derivative with a thienyl, a not basic moiety, displayed an acceptable selectivity (SI = 5.5) derived from an IC50 of 9.14 µM and a CC50 of 50.4 µM, whereas other analogs with a benzothiazolyl moiety showed a selectivity index of 3.5 and an IC50 of 12.9 µM and a CC50 of 45.2 µM.
From the results, it is clear that the amodiaquine represents a lead platform for the leishmanicidal drug design, and the elongation of the amino chain by the incorporation of an extra group (e.g., amino, acyl-amino, or basic heteroaryl) did not improve the leishmanicidal response compared with the amodiaquine standard. No mechanism of action assays were performed.
In 2010, E.S. Coimbra and co-workers prepared a series of three N-(2-(indol-3-yl)ethyl)-7-chloroquinolin-4-amines (compound 11, 12 and 13, Figure 11A) (Coimbra et al., 2010), and they were proved against promastigotes of L. braziliensis, L. amazonensis, L. chagasi, and L. major parasites. In general, these derivatives showed a weak or no leishmanicidal response (IC50 > 200 µM) against the different promastigote Leishmania spp. In particular, the derivative with the ester moiety as an R substituent (compound 13) was the most active of the compounds, whereas the compounds with carboxylic moiety (compound 14) proved to be highly toxic and less selective with an IC50 higher than 200 µM and a CC50 of approximately 10 µM on Vero and kB cells. These results indicate that a minimal lipophilicity is an essential requirement for the design of a leishmanicidal 7-chloroquinoline featuring a 4-(dialkyl)ethylene-diamine chain. As was described in Section 3, most of the 4-aminoquinolines are usually more active against intracellular amastigotes than promastigotes or axenic amastigotes, which suggests that further experiments of these compounds, in particular, for compound 13, are required to evaluate the real potential of the hybridized N-(2-(indol-3-yl)ethyl)-7-chloroquinolin-4-amines.
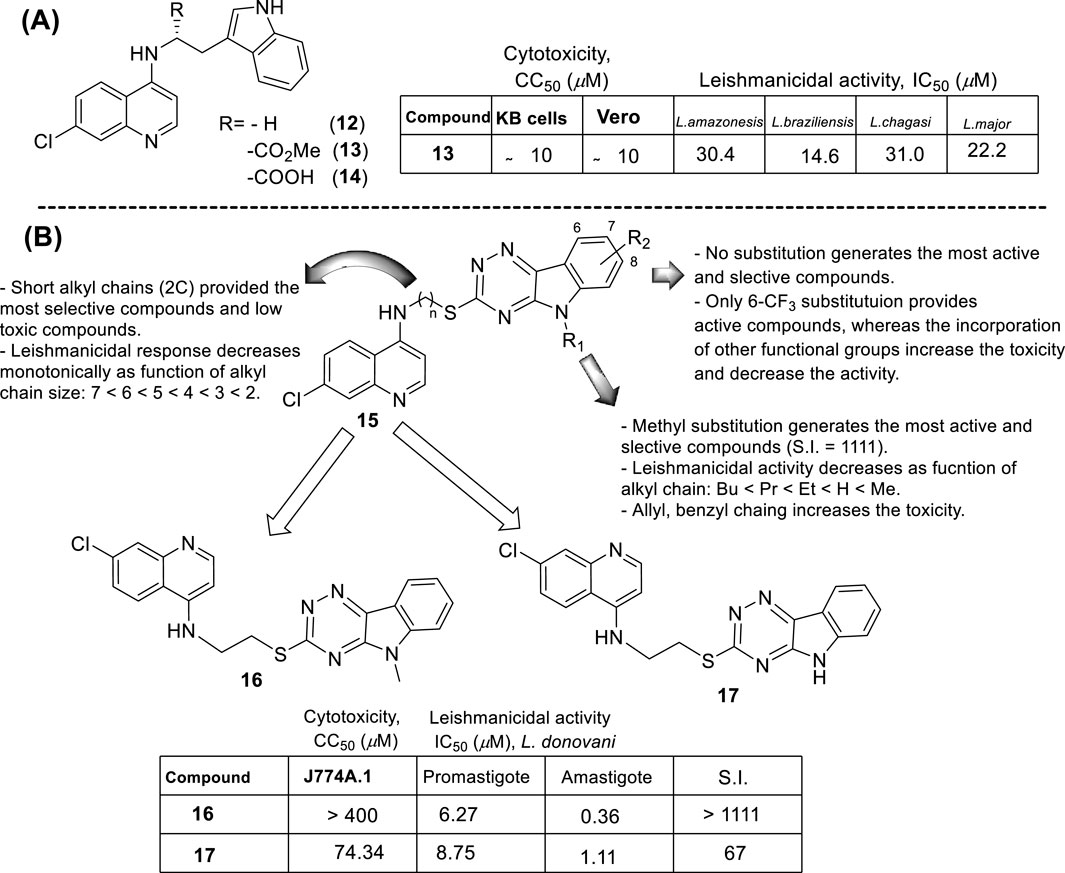
Figure 11. (A) Structure of a series of N-(2-(indol-3-yl)ethyl)-7-chloroquinolin-4-amines by Coimbra et al. (2010); (B) Structure of a series of 7-chloroquinolin-4-amines with a 1,2,4-triazine-benzomidazol fragment reported by P.M.S. Chauhan´s group (Sharma et al., 2014).
In 2013, Chauhan´s group prepared a series of 19 novel quinolines with a triazine indole-thiol alkyl amine at the 4-position (structure 15 in Figure 11B) to be proved against L. donovani promastigotes and amastigotes (Sharma et al., 2014). The nature of the hybridization of the triazino indole-thiol-ethylene amino focused on three structural features: (i) the length of the linker (sulfur-alkyl-amino) between the quinoline and the triazino indole; (ii) the N-alkyl group incorporated at the indole nitrogen; and (iii) the substitution pattern on the indole’s aromatic ring (C-6 and C-8 position).
From a SAR analysis, the influence of the chain length of the linker, the N-indole alkylation, and indole pattern substitution are clear. In general, the short alkyl chain (two carbons, ethylene, compound 16) provided the most active and selective compounds, finding a monotonic increase of potency and selectivity as a function of the number of carbon atoms as follows: 2C > 3C > 4C > 5C > 6C > 7C > 8C. Interestingly, increasing this carbon chain increases the toxicity of the compounds on J-774A.1 macrophage cells, which compromises the selectivity. Regarding the N-alkyl indole substitution, it was found that N-methyl substitution provided the most active compound, finding a monotonic increase of leishmanicidal response as a function of N-alkyl substitution as follows: N-methyl > N-ethyl > N-propyl > N-butyl > N-pentyl. N-methyl derivative showed an IC50 value of 0.36 µM, and the no N-substituted hybrid displayed an IC50 value of 1.11 µM (compound 17, Figure 11B), whereas a monotonic increase of IC50 was found with an increase of the N-alkyl chain length higher than 1 µM. Other nonlinear N-alkyl chains, like isopropyl, sec-butyl, allyl, and benzyl, were found to have lower activity levels with IC50 values as high as 29.48 µM. Finally, regarding the substitution pattern on the indole’s aromatic ring (C-6 and C-8), except for the introduction of a 6-CF3 group (IC50 = 6.46 µM), the introduction of any other functional group led to toxic derivatives or derivatives with no leishmanicidal activity (Figure 11B). In summary, Chauhan´s group identified a high selective compound, compound 16, which presented a high level of activity (IC50 = 0.36–7.10 µM) and considerable selectivity indexes (SI from 7 to >1111). Interestingly, the compounds were significantly more selective toward the amastigote form than the promastigote strain.
In 2014, S. Adhikari and co-workers prepared a series of 7-chloro-N-[2-(1H-5-ferrocenyl-1,2,3-triazol-1-yl)ethyl]quinolin-4-amines (compound 18, Figure 12A) (Yousuf et al., 2015), and they were proved against in vitro models of L. major and L. donovani. The designed chemical system presents two important structural characteristics: (i) a heteroaryl moiety with basic characteristics at the end of the amino-ethylene chain and (ii) a ferrocenyl moiety that can be interesting due to the role of iron in Leishmania recognition. The compound displayed IC50 values of 21.86 µM and 15.26 µM against the promastigotes of L. major and L. donovani, respectively, which was in same range as the leishmanicidal derived from reference miltefosine (IC50 = 21 µM) and to other quinoline references such as chloroquine (IC50 = 30.00 µM) and ferroquine (IC50 = 19.62 µM). The compound exhibited an apparently more significant leishmanicidal response against intracellular amastigotes of L. donovani in peritoneal macrophages without cytotoxicity toward murine splenocytes, achieving an inhibition of 83.62% upon treatment of 15 µM (value of IC50 determined from the anti-promastigote response against L. donovani). The latter revealed that compound 18 was more active against the amastigote form than against promastigotes, which implies that specific targets concerning macrophage-amastigote biological systems could be involved. Further studies showed that compounds promoted changes in the mitochondrial depolarization potential with further biological consequences in promastigote parasites. Scanning electron microscopy (SEM) studies revealed changes in the shape of promastigotes under compound 18 treatment, including loss of flagella and the appearance of porosity in cell membranes with respect to the flagellated and slender non-treated promastigote controls. Flow cytometric analysis showed an increase of the sub-G0/G1 phase from 2.98% (at 6 h) to 9.43% (at 24 h) in comparison with 3.49% in control cells at 24 h. Further assays based externalization of phosphatidylserine residues showed that the percentages of early and late apoptotic cells were increased significantly with respect to untreated cells, finding values of 1.60% and 1.94% (early apoptotic) and of 0.58% and 0.24% (late apoptotic) at 24 h and 48 h, respectively, for untreated promastigotes, and a significant increase under treatment to 12.74% and 23.45% (early apoptotic) and to 18.67% and 24.69% (late apoptotic) at 24 h and 48 h, respectively. Further assays showed that (i) compound 18 promoted a depletion of GSH in a time-dependent manner after 3 h with the highest MFI with respect to the untreated cells; (ii) DNA fragmentation in promastigotes; (iii) increase of ROS level in a time-dependent manner after 3 h of treatment with respect to control culture, (iv) a substantial increase of NO production in comparison with an untreated model of infected macrophages with intracellular amastigotes, and (v) an increase of the levels of lipid peroxides in a time-dependent manner after 1 h and, in particular, after 3 h. With this evidence, the authors proposed that compound 18 acts through an oxidative mechanistic pathway, which is promoted by the presence of the ferrocene moiety in compound 18. That chemical function induces peroxidation of lipids in the treated promastigotes, which subsequently promotes a decrease in the protein content and changes in the nature of lipidic membranes. This latter induces a loss of mitochondrial membrane potential, leading to apoptosis in L. donovani promastigotes. In addition, the release of NO in the infected macrophage model suggests that the compound can also upregulate the innate immune response.
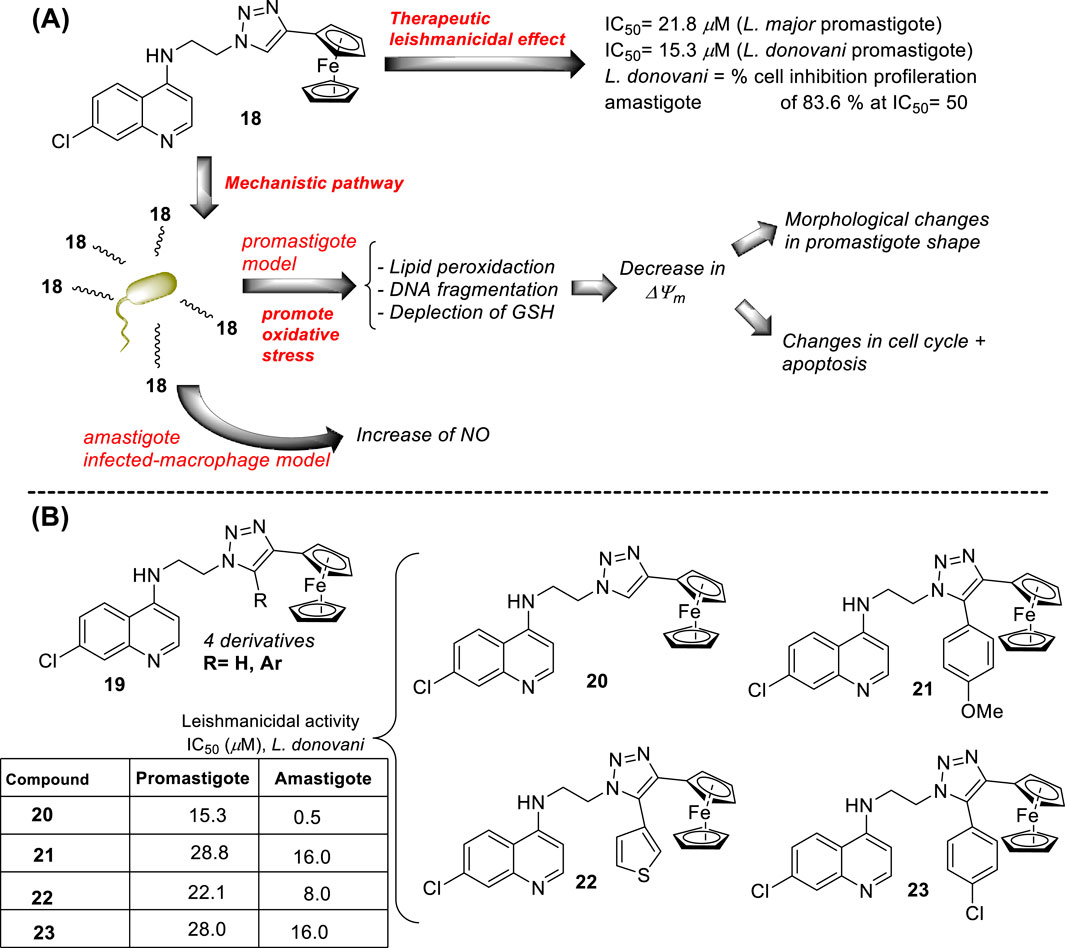
Figure 12. (A) Structure of ferrocenylquinoline 18 as a potential antileishmanial agent reported by E.S. Adhikari´s group (Yousuf et al., 2015). (B) Structure of ferrocenylquinolines 20–23 as a promising antileishmanial agent reported by Yousuf et al. (2016).
Next, in 2015, following the Adhikari research, Yousuf and co-workers prepared a series of 13 derivatives of ferrocenylquinolines to be proved against L. donovani and L. major (Yousuf et al., 2016). Four compounds were identified as promising agents against promastigote strains of L. donovani AG83 (compounds 19–23, Figure 12B). Compound 20, which bears a hydrogen atom as the R substituent (structure 19 in Figure 12B), showed the highest antipromastigote activity, with an IC50 value of 15.26 µM, which was more potent than miltefosine (IC50 = 21 µM). Compound 20 is the same as compound 18 (Figure 12A), which was studied by Adhikari (Yousuf et al., 2015). Lower antipromastigote responses with IC50 values between 22 µM and 28 µM were found for those ferrocenylquinoline derivatives with a heteroaryl/aryl as the R substituent. It revealed that the incorporation of an extra aryl/heteroaryl ring did not favor the leishmanicidal response with respect to the prior compound 18. Further assays showed that the ferrocenylquinoline 20 was also able to inhibit the growth and proliferation of L. donovani LV9 (44.28%) and L. major LV39 (52.74%) at 21.8 µM.
Regarding the amastigote stage of L. donovani AG83, compound 20 was also able to considerably affect the amastigotes in 50% at 0.5 µM, which supported the specificity of this type of quinolinic compound toward the amastigote form over the promastigote form. Further mechanistic experiments showed, as was described for compound 18, that this type of compound could arrest cell cycle progression from the increase of sub-G0/G1 cells to 32.88%, 27.31%, and 25.14%, respectively, compared to 2.07% of the control cells after 48 h of treatment. The parasite death was induced by triggering apoptosis. Furthermore, an increase in NO production was recognized from the infected macrophage model under treatment with compound 20, which opens the door to an immunostimulation role.
In 2015, E.S. Coimbra and co-workers prepared a series of novel ten derivatives of 7-dichloroquinoline functionalized at the 4-position by sulfonamides- (24), hydrazide (25) or hydrazine (26) substitutions (Figure 13A), and the compounds were proved against the amastigote and promastigote forms of L. amazonensis (Antinarelli et al., 2015). From sulfonamide derivatives 24, only those functionalized with pyridine (27) displayed good leishmanicidal activity against the promastigote and amastigote forms of L. amazonensis, giving IC50 values of 10.9 µM and 26.6 µM, respectively. However, its discrete cytotoxicity on macrophages (CC50 > 30 µM) compromises its potential by low SI values close to 1.1. Meanwhile, within the hydrazide compounds 25, only derivative 28 featuring a 3-pyridin heteroaryl exhibited a good antiamastigote response (IC50 = 1.9 µM) with a selectivity index of 15.8. Other derivatives consisting of a phenyl or 4-pyridin as the R group in compound 9 generated a discrete anti-amastigote response (IC50 between 16.5 µM and 20.7 µM). Finally, quinolin-4-hydrazine and compound 29 exhibited the best antileishmanial activity with IC50 of 0.8 µM and 2.4 µM and SI of 37.5 and 12.5, respectively.
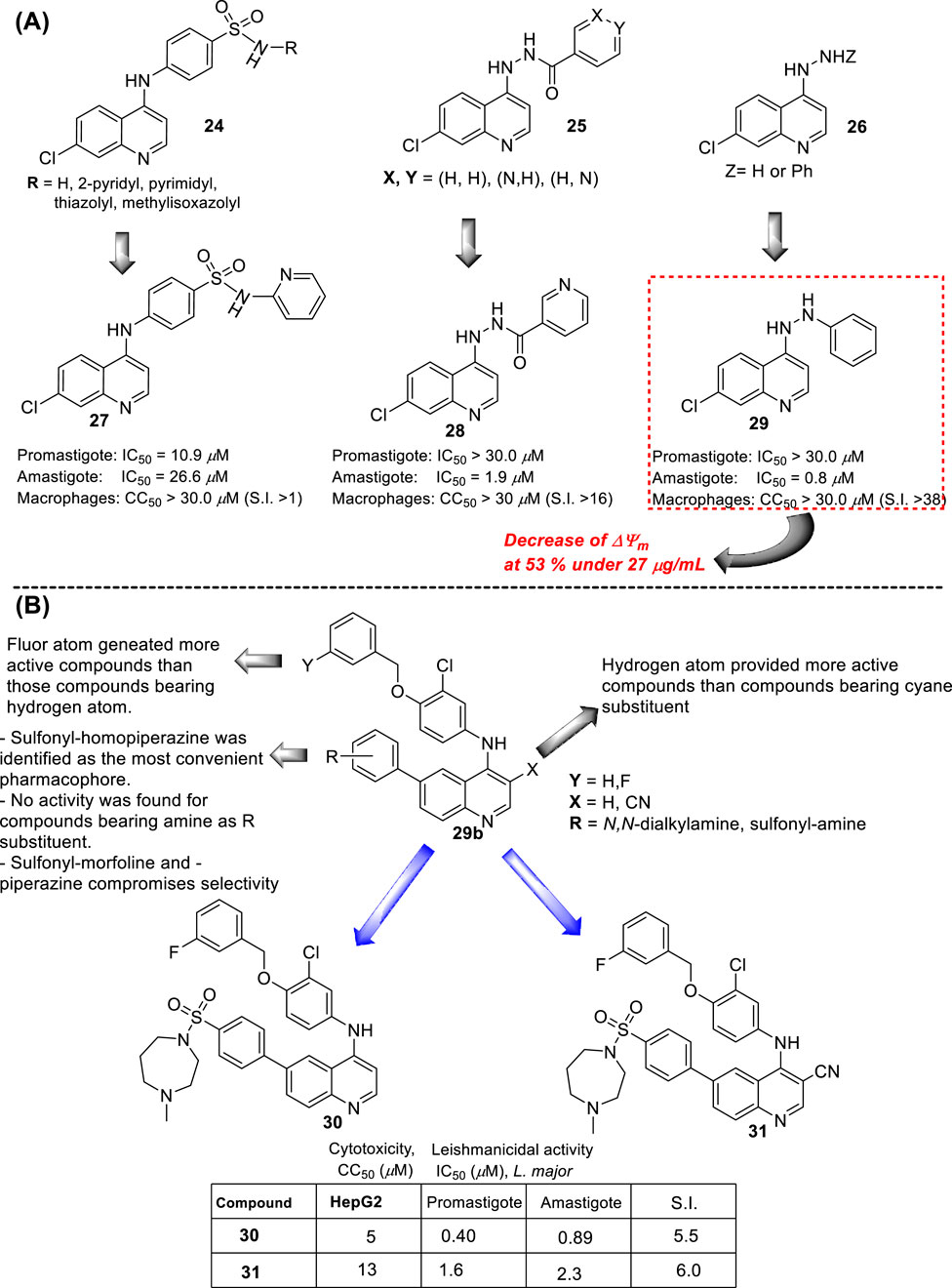
Figure 13. (A) Structure of 4-aminoquinolines reported by E.S. Coimbra´s group (Antinarelli et al., 2015). (B) Structure of 4-aminoquinolines reported by M. Pollastri´s group (Devine et al., 2015).
A rapid SAR analysis reveals that the incorporation of basic moieties, such as can be seen in structures of groups 24 and 25, can be an essential pharmacophore for generating active and selective leishmanicidal compounds. Furthermore, the inclusion of a lipophilic moiety into a hydrazine compound (group 26) could be essential. Interestingly, as seems to be typical in quinoline derivatives, this type of quinoline showed higher activity against the amastigote form than against the promastigote. Mechanistic experiments showed that the compounds can inhibit mitochondrial depolarization potential either in promastigotes or in infected macrophages. For example, a significant reduction in the potential of the mitochondrial membrane in the infected macrophage model was reported with values of 43.6%, 36.7%, and 53.0% under 7.0 μg/mL, 13.0 μg/mL, and 27 μg/mL doses of compound 29, respectively. The latter was comparable with the depolarization induced by miltefosine (25 μg/mL) of 58%, which is known as a disrupter of membrane potential. The authors found a linear correlation between the anti-amastigote response and the decrease in the ΔΨm magnitude.
In the same year, M. Pollastri and co-workers prepared a series of 16 quinolines 29b with a 4-(benzyloxyl)-aniline and another with a dialkylamine-sulfoxylfenil moieties at the 4- and 6-position (Figure 13B) (Devine et al., 2015). The compounds were evaluated against a diverse type of neglected tropical disease parasite strains, including T. brucei, T. cruzi, Plasmodium falciparum, and L. major. The quinolinic compounds were divided into two parts: (i) 4- and 6-substituted quinolines and (ii) 4-, 6-substituted 3-cyanequinoline. A consistent SAR analysis was extracted against the L. major amastigote parasite. The authors focused their study on the pattern of benzyloxy moiety, quinoline substitution at the 3-position, and the nature of the aryl-substitution at the 6-position. First, the incorporation of benzyloxy-aniline moiety generated the most active compound of the 4-aminoquinolines 29b. Regarding the benzyloxy moiety, it was found that the fluorine substitution at the 3-position of the benzyloxy ring provides a more active and selective compound than compounds featuring hydrogen atoms. Meanwhile, at the 6-substitution, the sulfonyl-homopiperazine was identified as the most convenient aryl-substitution for the development of the most active and selective compounds. A direct amine (e.g., morpholine) implied a decrease in leishmanicidal activity. Meanwhile, the incorporation of sulfonyl-morpholine and -piperazine aryl-substitution compromises the selectivity in compound 29b. The inclusion of a cyane moiety at the 3-position of quinoline decreases the activity and selectivity, suggesting that unsubstituted quinolines maintain a significant leishmanicidal response. From the study, two compounds, 30 and 31, were identified as the most promising compounds, giving IC50 values of 0.4 µM and 1.6 µM against promastigotes, respectively, and IC50 values of 0.89 µM and 2.3 µM against the amastigote strain, respectively. It implied SI values of 5.5 and 6.0 for compounds 30 and 31, respectively. No mechanistic exploration was explored for the 29b type of quinolines.
In 2016, E.S. Coimbra prepared a series of 7-chloroquinolin-4-arylhydrazones 32 and evaluated them against promastigote and amastigote forms of L. amazonensis (Antinarelli et al., 2016; Antinarelli et al., 2020) (Figure 14A). Although it is not exactly a 4-aminoquinoline type compound, the existence of the NH-moiety at the 4-position is the key point for our selection. Only two of the ten synthesized compounds displayed an acceptable leishmanicidal response against intracellular amastigotes and low toxicity on murine macrophages (CC50 > 150 µM). In particular, compounds 33 and 34 with a 3-hydroxy-phenyl and a 3-formyl moiety, respectively, displayed IC50 values of 8.1 µM and 15.6 µM, respectively, which implied selectivity indexes higher than 18 and 10, respectively. Compound 33 was identified as a promising candidate with a better SI (higher than 18) than miltefosine (SI 15.4). Interestingly, those compounds displaying a good antiamastigote activity showed a weak response against promastigotes, which confirmed the high specificity of the quinoline scaffold toward the amastigote stage over the promastigote stage.
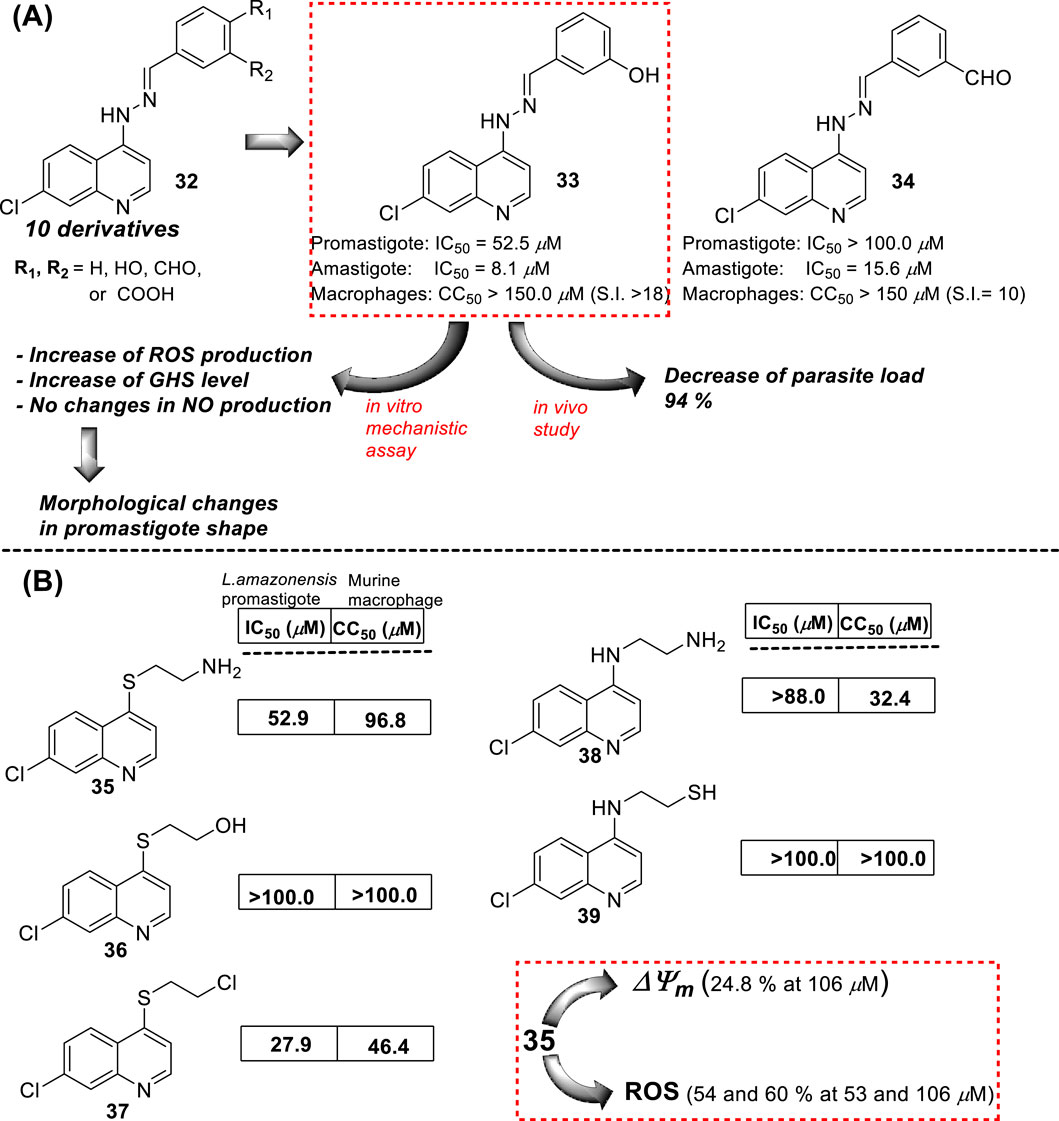
Figure 14. (A) Structure of 4-hydrazinylquinoline as an antileishmanial agent reported by E.S. Coimbra´s group (Antinarelli et al., 2016; Antinarelli et al., 2020). (B) Structure of 4-amino/sulfur-ethylene-aminequinoline as an antileishmanicidal agent reported by Coimbra et al. (2016).
Mechanistic studies showed that compounds using compound 33 induced mitochondrial protozoan dysfunction with a dose-dependence, which was in good correlation with a reduction of parasite proliferation in intracellular parasites with intracellular parasite death. Further mechanistic studies reflected that active compound 33 promoted an increase in the ROS levels in vitro models of promastigote- and L. amazonensis-infected macrophages. In addition, the authors found changes in the level of key factors such as GSH in promastigote models. Through SEM, morphological changes were seen in the parasite shape with loss of flagella compared with flagellated untreated control promastigotes. Further experiments in the amastigote model showed no significant increase in NO production under different concentrations compared with untreated L. amazonensis-infected macrophages. This fact suggested that the leishmanicidal activity against the intracellular parasite promoted by compound 33 is possibly more associated with ROS production than immunological activation. In vivo studies in the CL model showed that compound 33 led to a significant reduction (48.3%) of cutaneous lesions compared with the control group, finding results comparable to amphotericin reference. Interestingly, the parasitic load was found to be significantly reduced by 93.8% compared to the control group, which was comparable to the parasitic load reduction derived from the AmpB group.
In the same year, E.S. Coimbra presented a series of five 7-chloroquinolines 35–39 featuring an ethylene chain with thiol, amine, or hydroxyl at the beginning or end of the 4-functionalized chain (Coimbra et al., 2016) (Figure 14B), and they were proved against in vitro models of L. amazonensis (intracellular amastigotes and promastigotes). Only the derivatives 35 and 37, which bear a 2-aminoethyl-thionyl or a 2-chloroethyl-thionyl chain, respectively, showed a leishmanicidal response with a discrete IC50 of 52.9 µM and 27.9 µM against promastigotes of L. amazonensis, respectively. Compound 35 exhibited a strong inhibition of the proliferation of L. amazonensis amastigotes, giving an IC50 value of 0.0911 µM and a selectivity index of 1063. This compound was 139-fold more potent than miltefosine (IC50 of 12.7 µM) against amastigotes of the L. amazonensis parasite. The high activity against amastigotes and low toxicity on murine macrophages and human erythrocytes were interesting for further assay. Further experiments using compound 35 demonstrated that the compound could promote a decrease in mitochondrial membrane potential and an increase in the ROS level. No substantial NO production in infected macrophages treated with this compound was detected. Compound 35 showed a decrease in the mitochondrial membrane potential (24.8%) only under a high compound concentration (106.0 µM, two times of IC50 against promastigotes). Further experiments showed that compound 35 promoted ROS production with percentages of 60.8% and 54.2% under 53.0 µM or 106.0 µM, respectively.
In 2017, B. Insuasty and co-workers prepared a series of 37 new derivatives of 7-chloroquinolines functionalized at the 4-position by substituted 4,5-dihydro-1H-pyrazole- or chalcone-anilines (40 and 41) (Ramírez-Prada et al., 2017) (Figure 15A), and they were proved against in vitro models of Leishmania panamensis amastigotes. In general, the quinoline-chalcone derivatives 40 displayed better antileishmanial activity against Leishmania panamensis intracellular amastigotes than those quinolines based on 4,5-dihydro-1H-pyrazoles 41. Most of the studied quinoline-chalcones 40 showed good IC50 values ranging between 0.79 μg/mL and 2.54 μg/mL. The most active compounds had a 4-chloro, 4-bromo, or 4-methyl substitution in the aryl moiety. However, they were highly cytotoxic with low CC50 values of approximately 0.7–2.0 μg/mL on promonocytic human cell U-937 cells. Meanwhile, among the quinoline-based 4,5-dihydro-1H-pyrazoles 41, although their derivatives displayed lower leishmanicidal response than quinoline-chalcones, their derivatives displayed acceptable SI values of about 17.1, 14.9, 16.6, and 72.2 for some derivatives including (R2 = Ph, R1 = 4Cl), (R2 = Ph, R1 = 3,4,5-(OMe)3), (R2 = 3,4-DiClPh, R1 = 4Cl), and (R2 = 3,4-DiClPh, R1 = 4Br) as consequence of their low cytotoxicity. Then, the leishmanicidal activity of the quinoline-based 4,5-dihydro-1H-pyrazoles was dependent on the nature of the R1 and R2. The R1-substitution was essential to generate active compounds, where the phenyl moiety provided more active compounds than those derivatives with formyl or acetyl moieties, which implies the importance of the incorporation of a highly lipophilic moiety like phenyl. Meanwhile, the R2 substitution allowed modulating the level of biological activity, finding the most active and selective response for the R1-substitution of 4-bromo-, 4-chloro-, or, eventually, 3,4,5-trimethoxy-. Importantly, none of the compounds presented an IC50 lower than amphotericin B (0.05 μg/mL). From both chemical platforms 40 and 41, compounds 42 and 43 were identified as the most active and selective of the compounds, respectively, although they were sufficiently active for further assays. No mechanistic assays were reported by the authors.
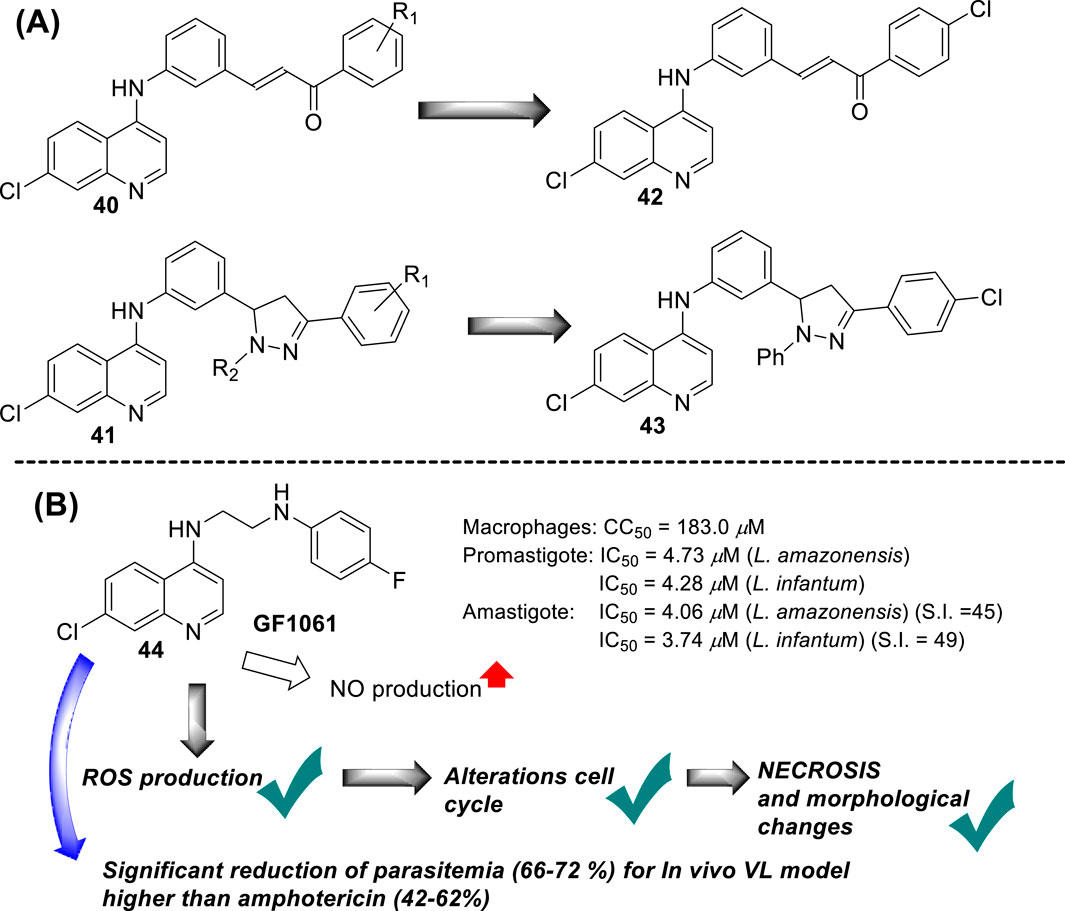
Figure 15. (A) Structure of 7-chloroquinolines functionalized at the 4-position by substituted 4,5-dihydro-1H-pyrazole- or chalcone-anilines (Ramírez-Prada et al., 2017). (B) Structure of 4-aminoquinoline 44 reported by E.A.F Coelho´s group (Tavares et al., 2019).
In 2018, E.A.F Coelho’s group prepared compound GF1061 (compound 44) from the coupling between the N-(2-bromoethyl)-7-chloroquinolin-4-amine with an excess of 4-fluoroaniline in the presence of potassium carbonate in DMF under reflux. The compound was proved against in vitro models of L. amazonensis and L. infantum. GF1061 showed a good leishmanicidal response with EC50 values of 4.73 μM and 4.28 μM against promastigotes of L. amazonensis and L. infantum parasites, respectively (Tavares et al., 2019) (Figure 15B). Against axenic amastigotes, GF1061 displayed EC50 values of 4.06 μM and 3.74 μM against L. amazonensis and L. infantum, respectively. AmpB presented IC50 values of 0.13 μM and 0.09 μM against L. amazonensis and L. infantum promastigotes, respectively, and of 0.39 μM and 0.30 μM against their amastigote strains, respectively. Despite the higher biological response of amphotericin to compound 44, the quinoline compound displayed a lower toxicity (CC50 of 183.0 μg/mL) than amphotericin (57.64 μg/mL), which implied SI values of 45.0 and 48.9 against the amastigotes, respectively, which was superior to SI values of 2.2 and 2.7 found for amphotericin. Regarding the study against intracellular amastigotes, a significant reduction in infection was found for infected macrophages treated with GF1061, finding % infection lower than 25% for a GF1061 concentration of approximately 2.5 μM. That higher leishmanicidal response against intracellular than axenic forms of the parasite confirms the high preference of the 4-aminoquinoline scaffold toward clinically relevant amastigotes in infected models. Further assay showed that GF1061 promoted a significant reduction in the mitochondrial membrane potential (Δψm) in the in vitro promastigote model of L. infantum, giving values of 34.3% and 34.5% upon compound concentrations of 2.98 μg/mL and 5.96 μg/mL, respectively. Further assay showed that compound 44 induced an increase in the levels of intracellular ROS in promastigote parasites upon the action of the compound at 6 h or 24 h. Analysis of parasite membrane integrity showed that the treatment with GF1061 induced significant morphological alterations in Leishmania promastigotes. Further evaluations showed that alterations in the parasite cell cycle were found in the G0/G1 phase for the treated parasites, which suggests the ability of the compound to promote DNA fragmentation. The cell integrity alterations were interpreted from the significant reductions in the G1 and G2 phases in treated parasites.
Further assays based on phosphatidylserine exposure in the cell surface showed the absence of an apoptotic process in the GF1061-treated parasites with strong evidence of death via necrosis. No substantial NO production in infected macrophages treated with this compound was detected. The leishmanicidal response of compound 44 could be associated with the depolarization of mitochondrial potential, which promoted ROS production that subsequently induced alteration in the cell cycle by death via necrosis with morphological consequences. Finally, compound 44 showed a significant in vivo efficacy for a VL model, finding an appreciable reduction in parasitemia in the infected tissue, liver, spleen, and dLN, giving a percentage of parasitemia reduction of 66%, 69%, 71%, and 72%, respectively, which was comparable with results found under the amphotericin drug of 62%, 44%, 38%, and 48%, respectively. Compound 44 emerged as a potential candidate as a leishmanicidal agent with an appreciable effect of depolarization on mitochondrial potential as the main mechanism of action.
In 2019, Coelho’s group prepared a new type of 4-aminoquinoline called AM1009, compound 45. That compound was synthesized through a direct reaction between the N-(3-bromopropyl)-7-chloroquinolin-4-amine and an excess of cyclohexanamine in the presence of potassium carbonate under reflux (Sousa et al., 2019) (Figure 16). AM1009 was evaluated in vitro against L. amazonensis and L. infantum promastigotes and their axenic amastigotes. AM1009 showed a good antileishmanial response with EC50 values of 2.41 μM and 0.38 μM against the promastigotes of L. amazonensis and L. infantum, respectively. Against axenic amastigotes, AM1009 displayed EC50 values of 1.03 μM and 0.98 μM against L. amazonensis and L. infantum, respectively. AM 1009 showed lower leishmanicidal activity than amphotericin drug (0.13 μM and 0.09 μM against L. amazonensis and L. infantum promastigotes, respectively, and of 0.34 μM and 0.18 μM, respectively), but interestingly, AM1009 was less toxic on murine macrophages than amphotericin (CC50 value of 148.94 μM vs. 0.85 μM), which also implies better SI values of 144.6 and 392.0 against axenic amastigotes of L. amazonensis and L. infantum, respectively. Amphotericin displayed SI values of 2.5 and 4.7. Importantly, AM1009 showed lower toxicity against red blood cells with an RBC50 value of 1739.69 μM. Against the infected model of macrophages, AM1009 displayed a reduction of infection higher than 50% for concentrations of 0.18 and 0.34 μM against models of L. infantum and L. amazonensis, respectively, which are in same range to IC50 values found for the amphotericin drug against these infected models. Mechanistic assays revealed that AM1009 induced a significant decrease of mitochondrial membrane potential at 2 μM (24.6%) against promastigotes of L. amazonensis, which was comparable to that found for the FCCF reference (35.9%). These results were in good concordance with a higher production of ROS and significant alterations in their cell cycle by the occurrence of cells in the sub-G0/G1 phase and the formation of autophagic vacuoles promoted on L. amazonensis promastigote parasites treated with AM1009. All these results indicate cell apoptosis promoted by AM1009. A further assay based on immunological studies showed high levels of the cytokines IFN-γ, IL-12, and GM-CSF, a significant increase of nitrite ions, and an increase in levels of the parasite-specific IgG2a isotype antibody. These results suggested that AM1009 could be acting as an immunostimulating agent, favoring the development of polarized Th1 response, which increases the levels of essential citoquines and NO. In addition, in the parasite, the compound promoted depolarization of mitochondrial potential, which induces the increase of ROS, and, subsequently, changes in the cell cycle. Therefore, AM1009 could have a double action, an immunostimulant activity and a leishmanicidal action derived from alteration in mitochondria functions. Finally, for a murine model of CL infected with L. amazonensis, AM1009 achieved a significant decrease in the size of the lesion and parasite load in infected mice, with a better efficacy than amphotericin.
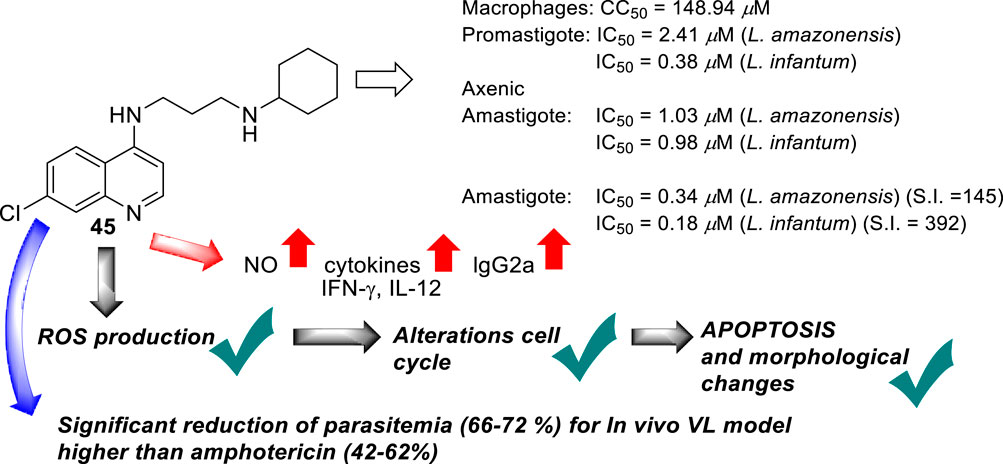
Figure 16. Structure of 4-ethylenediamine quinoline as a potential antileishmanicidal agent reported by E.S. Coimbra´s group (Sousa et al., 2019).
In 2018, Bogdan A. Solaja’s group designed and synthesized a series of 30 7-chloroquinolines featuring alkyl-diamino chains at the 4-position with different structural characteristics involving the incorporation of aryl- or rigid alkyl groups at the end of the alkyl-diamino chain (Konstantinović et al., 2018) (Figure 17). Furthermore, other chemical functions at the 3- and 7-positions were incorporated into synthesized compounds. The compounds were divided into seven groups: (i) group I consisting of chloroquine analogs functionalized at 3-position by nitro and amino moieties, (ii) group II consisting of chloroquine analogs with an adamantino moiety at the end of the alkyl-diamine chain, (iii) group III consisting of chloroquine analogs with a benzothiazole moiety at the end of the alkyl-diamine chain, (iv) group IV consisting of chloroquine analogs with a phenyl-3-benzothiazole moiety at the end of the alkyl-diamine chain, (v) group V consisting of tetrahydroquinoline with a diethylamino moiety at the end of the alkyl-diamine chain like chloroquine, (vi) group VI consisting of tetrahydroquinoline with a (4-cyanephenyl)-2-thiophenyl-4-fenil moiety at the end of the alkyl-diamine chain, and (vii) group VII consisting of chloroquine with a (4-cyanophenyl)-2-thiophenyl-4-fenil moiety at the end of the alkyl-diamine chain. A structure–property relationship analysis showed compounds from group II to group VII showed a sub-micromolar response against L. infantum and Leishmania tropica promastigotes (0.3–4.0 µM), whereas the compounds of group I showed a weaker response (4.0–10.0 µM). From the antipromastigote response, no significant differences in biological activity were found between compounds featuring an ethylene-diamine chain and compounds with a propylamine chain. No significant changes in antipromastigote response were found by the suppression of the 7-chloro substitution by hydrogen. Meanwhile, the functionalization at the end of the alkyl-diamine chain leads to appreciable changes in the leishmanicidal response. From this QSAR relationship, eight compounds, 46–53, from group II through group VII were chosen as the most promising compounds for an in vitro evaluation against L. infantum and L. tropica promastigotes (Figure 17), and further in vitro evaluations against intracellular amastigotes of L. infantum parasite were performed. A comparison between selected candidates revealed that the compound with the (4-cyanophenyl)-2-thiophenyl-4-fenil 53 moiety (0.6–0.8 µM) was the most active, followed by those with a benzothiazole (1.0–1.4 µM), whereas a derivative of adamatino showed a significant IC50 of 0.31 µM. Results showed that lipophilicity must be considered, but it seems important that elongated alkyl-diamine chains are not convenient because although active compounds are generated, they promote more side effects on the host cell.
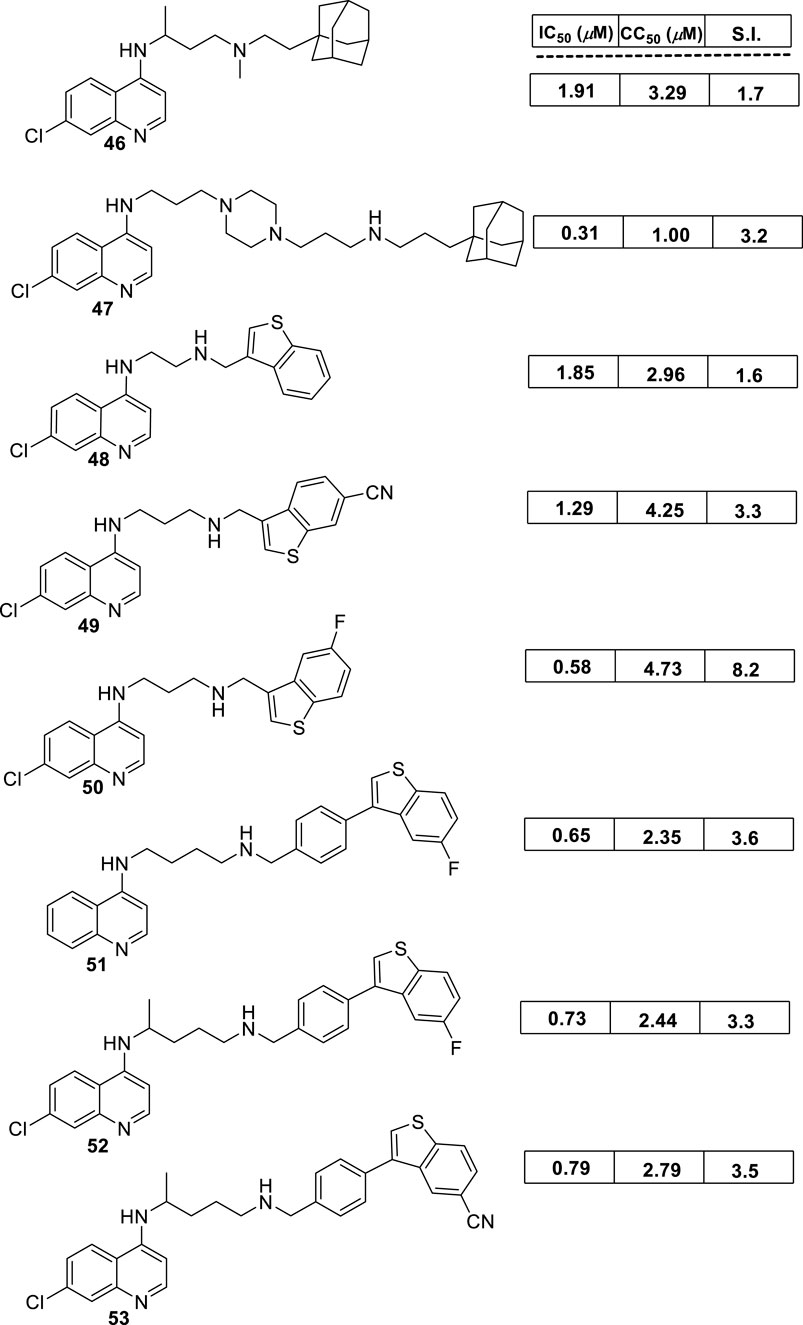
Figure 17. Structure of complex 4-aminoquinolines functionalized through diamine-alkyl chain reported by B.A. Solaja´s group (Konstantinović et al., 2018).
Solaja selected compounds 47 and 50 with selectivity indexes of 3.2 and 8.2, respectively, for further in vivo evaluation for a model of VL. Interestingly, the authors found a significant in vivo response, finding a significant reduction of liver parasitemia by about 99%, using a low compound dose of 5 mg/kg. Despite the results, further toxicity assays are needed to confirm the potential of the compounds as lead candidates. No mechanistic experiments were reported. This report put in evidence the structural relevance of the incorporation of a weak basic moiety like an alkyl-diamine chain with a tertiary amine as well as the incorporation of a lipophilic moiety composed by an aryl-thiophenyl or an adamant moiety.
In 2018, Calixto and co-workers synthesized a series of organic salts from an N1-(7-chloroquinolin-4-yl)-N2,N2-di(prop-2-yn-1-yl)ethane-1,2-diamine 54a-b, 55, and 56 (Calixto et al., 2018) (Figure 18A), and they were evaluated against in vitro models of L. amazonensis, and L. braziliensis. The 4-aminoquinoline 54a previously showed a discrete activity against different Leishmania parasites for in vitro models (Carmo et al., 2011). The new organic salts were based on the N-alkyl protonation of 4-aminoquinoline 54a, the N1-methylation, and the double action, N-alkyl protonation, and N1-methylation, to give the corresponding water-soluble compounds 54b, 55, and 56, respectively. This cationization seeks to improve the leishmanicidal response through an improvement of the physical-chemical properties. The three cationic compounds were evaluated for their leishmanicidal response against L. amazonensis and L. braziliensis promastigotes and amastigotes. The results of the in vitro evaluation against promastigotes demonstrated that only the derivative 54b was effective against both species of Leishmania (IC50 [L. amazonensis] = 43.25 µM and IC50 [L. braziliensis] = 39.19 µM). Furthermore, only compound 54b was active against L. amazonensis-GFP intracellular amastigotes (IC50 = 5.48 µM), and that antiamastigote activity was very similar to that observed against L. amazonensis-Wild type amastigotes (IC50 = 5.62 µM). Importantly, compound 54b presented a low level of toxicity against murine macrophages (IC50 = 226.70 µM), making compound 54b a promising candidate by its higher SI (by about 40 units) against L. amazonensis amastigote strains. Further experiments confirmed the ability of compound 54b to induce oxidative stress via depolarization of mitochondrial membrane potential. Compound 54b induced a reduction of ∆Ψm of 28% at a compound concentration of 86 µM. Interestingly, the compound displayed a reduction of mitochondrial potential comparable with that displayed under miltefosine, and no effect on the mitochondrial potential was observed for uninfected macrophages under treatment with compound 54b. On the other hand, compound 54b at 86 µM in culture promoted an increase of ROS production by about 62%, which was similar to miltefosine (68%) (Figure 18B). In addition, TEM revealed that compound 54b promoted some morphological modifications such as rounded bodies and reduction of cell volume. To analyze the reason for death, TEM showed that promastigotes, upon treatment with compound 54b, induced exposure of phosphatidylserine on the parasite surface, reflecting an increase of the cell population in the sub-G0/G1 phase with condensed and marginalized chromatin, suggesting death via apoptosis.

Figure 18. (A) Leishmanicidal in vitro activity of 4-aminoquinolinic salts with terminal 3-propinyl reported by Calixto et al. (2018). (B) Mechanistic data derived from compound 54b. (C) Structure of 6-methoxy-quinalidine 57 reported by Stevanovic et al. (2018).
In addition, in 2018, S. Stevanovic’s group identified a substituted 6-methoxy-quinalidine 57 (Figure 18C) from a virtual screening of the L. infantum type 2 NADH dehydrogenase (NDH2) for a group of ubiquinone analogs including a 4-aminoquinoline, phthalazine, quinolone, and other N-heteroarenes (Stevanovic et al., 2018). The quinolinic compound 57, as well as other N-heteroarenes ubiquinone analogs, were evaluated in vitro against L. infantum axenic amastigotes and promastigotes. Compound 57 exhibited an excellent leishmanicidal response against the wild type of L. infantum, giving IC50 values of 0.03 and 0.2 µM against promastigotes and amastigotes, respectively. Later, Rivas’s group reported a good response against L. donovani promastigotes and Leishmania pifanoi amastigotes, giving IC50 values of 4.4 µM and 6.2 µM, respectively. Rivas´s group also reported a moderate cytotoxicity with a CC50 value higher than 50 µM using J774A.1 macrophages, which implies SI indexes higher than 250 and 8.1 for in vitro amastigote models of L. infantum and L. pifanoi, respectively. This report is an interesting finding because it showed that the incorporation of an extra basic alkyl chain is needed to generate a potent and selective compound, although it cannot be discarded if the N-alkyl chain could increase the leishmanicidal effect even more.
Based on the widely known potential of quinoline derivatives as antileishmanial agents, another research group designed a new class of 4-amino-2-styryl quinolines 58 and evaluated them against L. donovani promastigotes and L. pifanoi amastigotes (Staderini et al., 2019) (Figure 19). Furthermore, some other derivatives were also synthesized and evaluated to serve as a control group, allowing a deeper understanding of the structural influences of the 4-amino-2-styryl quinolines. Considering their effects against L. donovani promastigotes, the results demonstrated that, except for 2-styrylquinoline (not bear 4-substitution), all the evaluated molecules with a 4-N-alkyl chain present considerable leishmanicidal properties, giving IC50 values ranging from 0.2 µM to 35.1 µM. Interestingly, most of the evaluated compounds were more potent, making it possible to verify the importance of a 2-styryl group, whose remotion led to a considerable loss of leishmanicidal activity. Four compounds 59–62 were chosen as promising candidates for their good leishmanicidal profile (Figure 19). From the leishmanicidal response, compounds displayed a higher activity against the amastigote strain than against the promastigote strain. Against Leishmania amastigotes, compounds presented low IC50 values from 0.9 µM to 1.6 µM for compounds 59–62. In particular, compound 61 exhibited the highest SI value, giving a magnitude higher than 42. The compound is characterized by an aniline as terminal moiety on the N,N-dialkyldiamine chain, which seems to be more convenient to increase the selectivity than dialkyl amine or methoxy as terminal moieties on the N,N-dialkyldiamine chain.
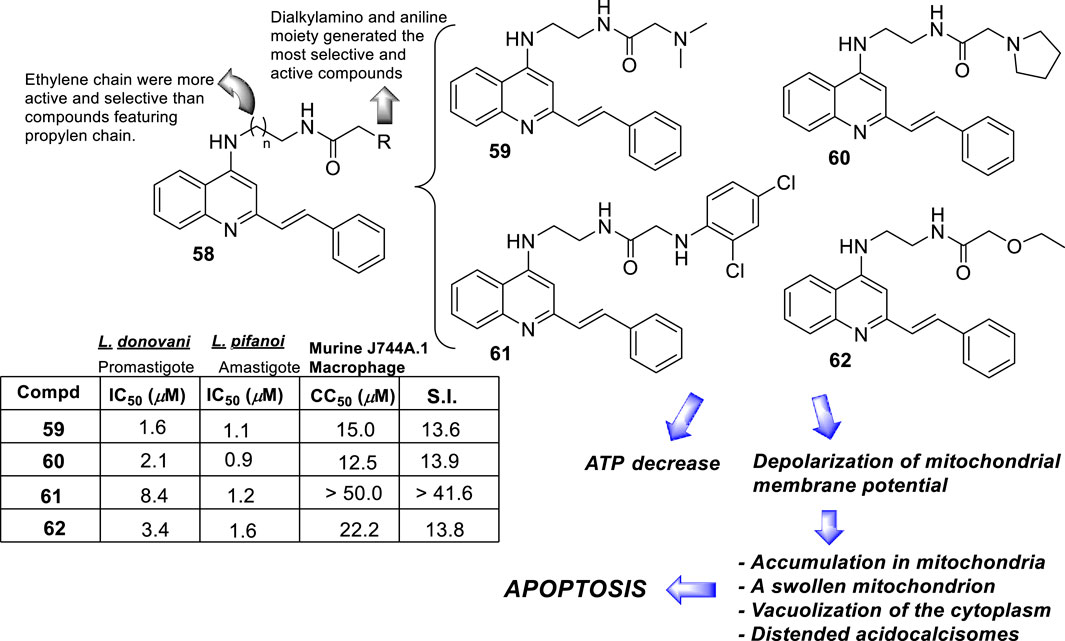
Figure 19. Structure of 4-amino-2-styrylquinolines 58 and their most active derivatives 59–62 reported by Rivas´s group (Staderini et al., 2019).
Beyond the leishmanicidal effect, the authors explored the mechanism of action based on mitochondrial dysfunction. First, the author studied the levels of intracellular ATP upon compound treatment using L. donovani promastigotes from the 3-Luc strain. This parasite expresses a cytoplasmic form of firefly luciferase that is episomally encoded. At 50 mM compound concentrations, most of the studied compounds 58 induced a decrease in luminescence, which supports the decrease in ATP synthesis. To demonstrate that the drop in ATP production is associated with permeabilization, the authors explored the permeabilization by the addition of triton X-100, finding no alteration of the plasma membrane permeability. Alternatively, the authors studied the effect of these compounds on membrane mitochondrial potential, finding a decay of ΔΨm compared to negative controls. Accumulation into mitochondria using mitotracker supports the role of the parasite mitochondria as a target for compounds 58, which was demonstrated through confocal microscopy. A TEM study on L. donovani promastigotes showed three key morphological characteristics: (i) a swollen mitochondria, (ii) strong vacuolization of the cytoplasm, and (iii) the appearance of distended acidocalcisomes. This evidence reflects the role of the mitochondria as key targets for promoting apoptosis and also provides evidence for the role of other organelles like acidocalcisome as targets to store divalent Ca2+ and heavy cations, Fe2+ and Zn2+, which could be able to capture quinoline compounds via coordination binding.
In 2019, we reported the activity of a series of dehydroxylated derivatives of isoquine and isotebuquine 63 against in vitro models of L. braziliensis and L. Mexicana (Figure 20) (Romero et al., 2019). These derivatives previously displayed a good antimalarial response with curative properties for an in vivo model of Plasmodium berghei (Romero et al., 2015; Valverde et al., 2017). Regarding Leishmania parasites, interestingly, these derivatives, dehydroxilated from isotebuquine, showed a higher leishmanicidal response than their isoquine analogs either against L. braziliensis promastigotes and L. mexicana parasites (Romero et al., 2019). The latter revealed that the incorporation of the extra phenyl ring on the aniline moiety was essential for promoting a significant leishmanicidal response. Three of the 12 evaluated compounds (64, 65, and 66) showed a promising response for further studies. From cytotoxic assay, derivatives 64, 65, and 66 showed good SI values against L. braziliensis of 11.4, 14.1, and 52.1 and of 17.9, 18.3, and 24.1 against L. mexicana, respectively. Against amastigote strains, compound 64 showed better antiamastigote response (IC50 = 13.88 µM) than compound 65 (IC50 = 22.56 µM) and compound 66 (IC50 = 19.34 µM), which implied SI values of 14.3, 6.1, and 5.2, respectively. The antiamastigote response of compound 65 was barely higher than glucantime (IC50 = 15.12 µM). Interestingly, compound 66 showed the best antiamastigote response against a resistant strain of L. braziliensis, giving an IC50 value of 25.23 µM (SI 7.8), higher than that found by using glucantime (IC50 > 50 µM). From a mechanistic point of view, a discrete increase of NO was found from an intracellular amastigote model with a concentration dependence, which suggested a specific effect against infected macrophages that could be associated with an immunostimulation response. Currently, we are studying the potential of these derivatives against in vitro models of L. infantum, finding excellent responses against infected models in sub-micromolar ranges.
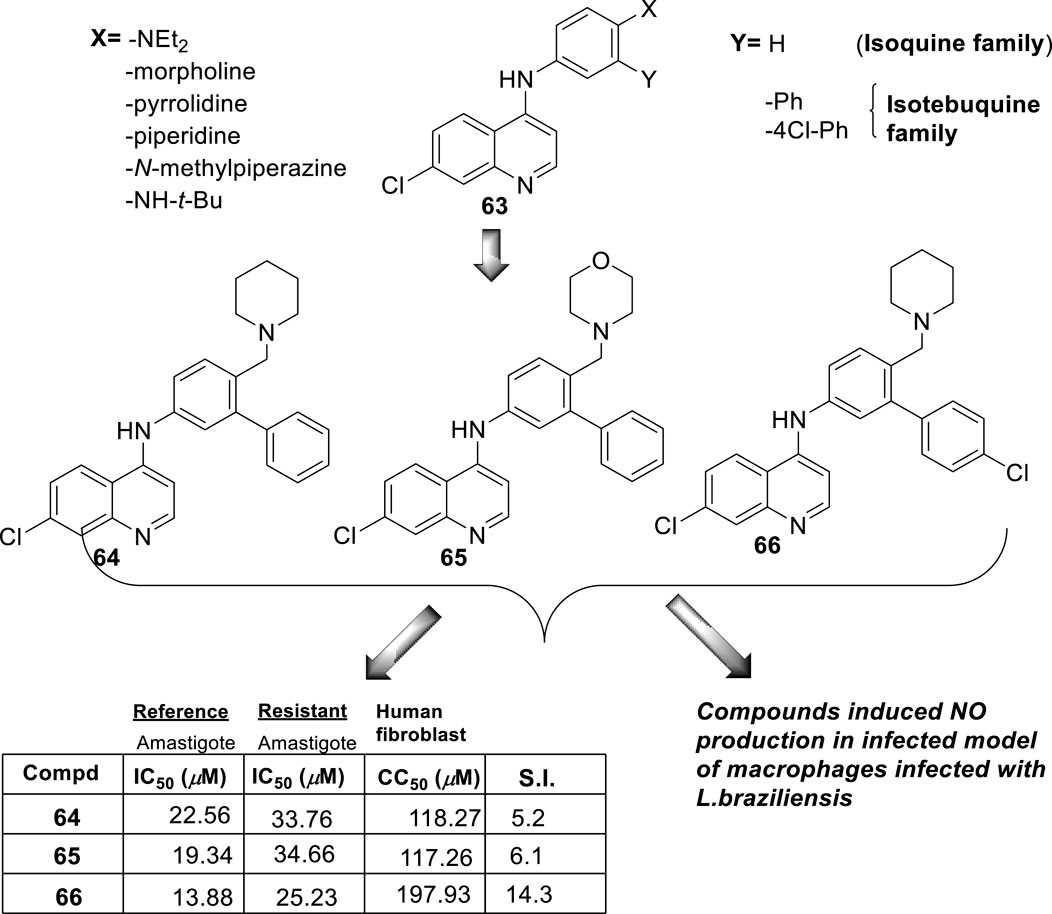
Figure 20. Structure of isoquine and isotebuquine derivatives 63–66 reported by Romero et al. (2019).
In 2019, N. Basilico, F. Gamarro, and co-workers development a series of five quinoline hybrids 67–71 as depicted in Figure 21A (Manzano et al., 2019). This research is a continuation of a previous report (Konstantinović et al., 2018). All compounds exhibited significant antipromastigote and antiamastigote responses, giving IC50 values lower than 1 µM (Figure 21A), finding similar leishmanicidal responses between promastigote and amastigote strains. In particular, among the five compounds, compound 67 showed the best SI profile with an SI value of 8.2, whereas the remaining compounds displayed SI values between 2 and 4. A small superficial structure–activity analysis reflects that the location of the 2-benzothiazol at the 3-position of the benzylic-amine is preferred over the location at the 4-position in order to decrease the cytotoxic effect and achieve a higher SI. It is important to mention that the leishmanicidal effect is similar among all studied compounds. On the other hand, mechanistic experiments demonstrated that compound 67 promoted (i) an alteration in the energetic metabolism with a significant loss of ATP that is associated with the depolarization of mitochondrial potential (ΔΨm), (ii) a decrease of intracellular ATP levels with an alteration of plasma membrane permeability, and (iii) a significant ROS production, which is the typical mode of action found for 4-aminoquinolines. This type of mechanism initiates a depolarization of mitochondrial membrane potential, which subsequently promotes ROS production that promotes alterations in parasite morphology with apoptotic consequences as the mode of cell death.
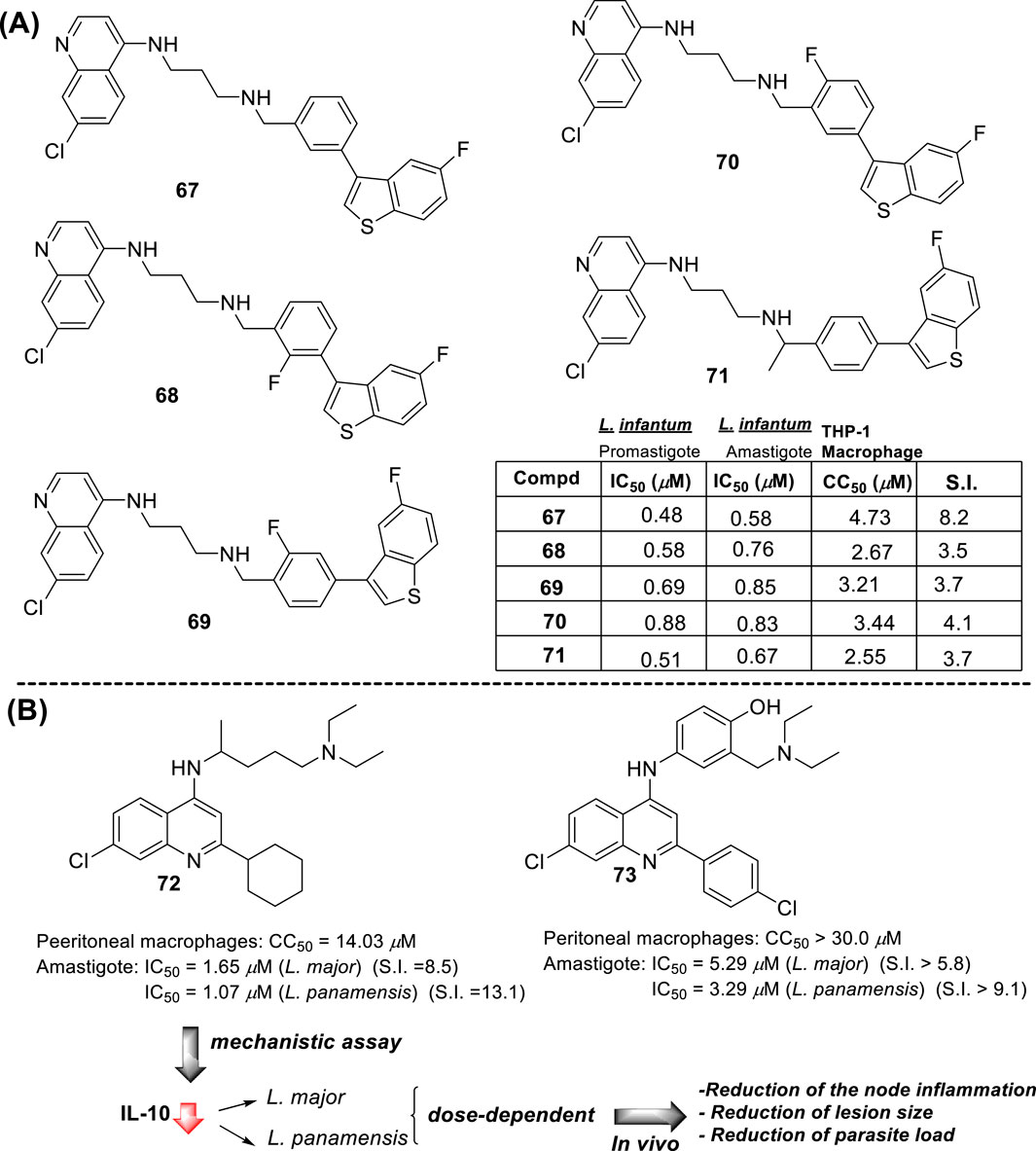
Figure 21. (A) Structure of complete 4-aminoquinoline hybrid functionalized through diamine-alkyl with 2-benzothiazol-benzyl-amine moiety reported by Manzano et al. (2019). (B) Structure of a chloroquine 72 and an amodiaquine 73 analog with a lipophilic moiety at the 2-position of quinoline core, reported by P. Fernandez (Herrera et al., 2016; Herrera et al., 2020).
In 2020, P. Fernandez synthesized and evaluated the leishmanicidal activity of two 7-chloroquinoline derivatives 72 and 73. Compound 72 displayed IC50 values of 1.65 and 1.07 against intracellular amastigotes of L. major and L. panamensis, respectively, whereas compound 73 displayed IC50 values of 5.29 and 3.29 µM against L. major and L. panamensis, respectively (Herrera et al., 2016; Herrera et al., 2020) (Figure 21B). Compounds showed CC50 values of 14.03 μM and higher than 30 μM, respectively, which implied SI values of 8.5 and 13.1 for compound 72 and 5.8 and 9.1 for compound 73, against L. major and L. panamensis, respectively. Interestingly, neither compound showed a response against promastigotes at 50 µM (IC50 > 50 µM), which supports the high specificity of this type of quinolines toward the amastigote form over the promastigote form. Further assays showed the tentative immunostimulating response of these compounds. In particular, compound 72 inhibited the production of the pro-inflammatory cytokine of IL-10 by macrophages infected with L. panamensis and L. major in a dose-dependent manner. These results suggest that the compound induced a parasite-killing mechanism through regulation of the macrophage activation, as was explained in Section 2.3.
Further studies demonstrated the efficacy of compound 72 on a murine model of L. panamensis (Herrera et al., 2020). The compound promoted an appreciable reduction of the node inflammation, lesion size, and parasitemia index at the lesion region in a dose-dependent manner. In addition, the authors found a significant reduction in the IL-10 production from the lymph node cells of infected mice. Then, it seems that the incorporation of either a basic moiety or a lipophilic moiety is essential for the development of selective and potent leishmanicidal agents based on 4-aminoquinoline.
At the beginning of 2020, L. Ferrins and M. Pollastri followed a target repurposing and parasite-hopping approach and developed a series of quinoline derivatives originating from the reoptimization of lapatinib to NEU-1953 and further optimizations of this (Ferrins et al., 2018; Mehta et al., 2018; Bachovchin et al., 2019). This entire series of quinoline derivatives was then evaluated for its leishmanicidal potential against L. major and L. donovani intracellular amastigotes (Singh et al., 2020). Focusing on the in vitro leishmanicidal effect against intracellular amastigotes of L. major parasite, many of the designed quinoline hybrid 74 compounds displayed a remarkable leishmanicidal activity (Figure 22A). A SAR analysis showed that the nature of the R1 and R2 substituents is pivotal for leading an active and selective compound. Talking about the R2 substitution, the incorporation of dialkyl amine chains decreased the leishmanicidal activity, whereas the inclusion of amino-1,4-pyrazine generated active compounds. More improvement was found when the pyrazine moiety at the 4-position was replaced by composed phenyl rings. In particular, the substituted anilines such as 3′-chloro-4′-methoxyaniline 75 and 4-(trifluoromethoxy)aniline 76 generated the most active compounds (Figure 22A).
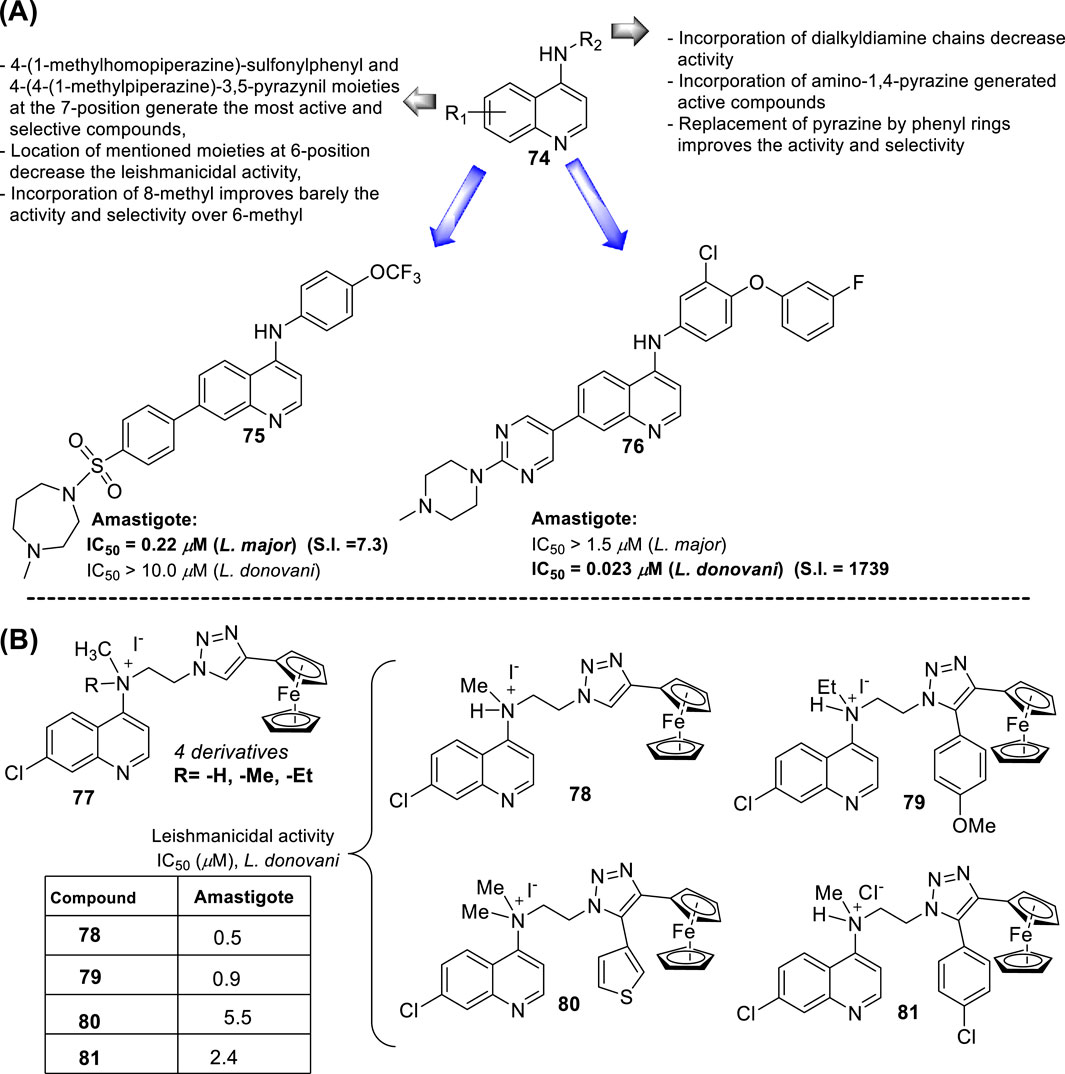
Figure 22. (A) Structure of 4-aminoquinolines 74–76 reported by L. Ferrins-M. Pollastri (Singh et al., 2020). (B) Structure of flexible and water-soluble ferrocenylquinoline N-quaternized compounds 78–81 (Mukherjee et al., 2020).
Meanwhile, regarding the R1 substitution, the 4-(1-methyl homopiperazine)-sulfamoylphenyl and 4-(4-(1-methylpiperazine)-3,5-pyrazinyl moieties at the 7-position generate the most active and selective compounds, whereas its location at the 6-position decrease the leishmanicidal activity. Interestingly, the introduction of methyl-groups at different positions of the quinoline core provides different effects. 6-Methylation improves the leishmanicidal effect, whereas an 8-methylation promotes a loss of activity. From the proved compounds, compounds 75 and 76 were chosen as promising candidates for their high antiamastigote response against infected models of L. major and L. donovani macrophages. Compound 75 was more active against L. major (IC50 = 0.22 µM, SI = 7.3) than against L. donovani (IC50 > 10.0 µM). In contrast, compound 76 was more active against L. donovani (IC50 = 0.023 µM, S.I = 1739 using HepG2 cell as reference) than against L. major (IC50 > 1.5 µM). Compound 76, with an SI of 1739, represents a potential candidate for further evaluation of either mechanistic or in vivo efficacy.
In 2020, S. Adhikari-C.Pal and co-workers prepared a series of novel flexible and water-soluble ferrocenyl quinoline N-quaternized compounds 77 (compounds 78–81, Figure 22B) (Mukherjee et al., 2020). From an in vitro evaluation against intracellular amastigotes of L. donovani, compound 78 displayed the best leishmanicidal response, giving an IC50 value of 0.50 μM, whereas compounds 79, 80, and 81 displayed IC50 values of 0.99 µM, 5.05 µM, and 2.44 μM, respectively. Their activities were comparable with amphotericin B (IC50 = 0.26 μM) and higher than sodium antimony gluconate (IC50 = 170 μM), miltefosine (IC50 = 13.60 μM), and paromomycin (IC50 = 8 μM). Compound 77 was evaluated for in vivo efficacy experiments and mechanistic assays. The ferrocenyl quinoline demonstrated curative properties for mice under L. donovani infection with a dose-dependent response. The in vivo therapeutic effect was achieved either upon noninvasive oral or invasive intramuscular administration. Then, several experiments focused on immunostimulation were performed for compound 77. The water-soluble ferrocenyl quinoline 77 stimulated the secretion of Th1 either under oral or intramuscular administration. Furthermore, a significant induction in the expressions of key pro-inflammatory cytokines, IL-6, IL-12, TNF-α, and IL-1β, was observed at the mRNA level in the treated groups, either oral or intramuscular, in comparison to the infected group. By contrast, the mRNA expressions of the anti-inflammatory cytokines like IL-10 and TGF β were reduced for the in vivo model. Additionally, a significant increase in the level of NO in vivo models was found. The expression of pro-inflammatory cytokines, secretion of Th1, and reduction of expression of anti-inflammatory cytokines suggest that this type of compound must be inducing an immunostimulating response in the infected host. Further mechanistic experiments showed that compound 77 reduced the expression of some enzymes, such as γ-glutamylcysteine synthetase, glutathione synthetase, ornithine decarboxylase, and trypanothione reductase. To verify the induction of apoptosis, downregulation in the expression of Sir2 and enzymes NAD + biosynthetic pathway enzymes, nicotinamidase, Na phosphoribosyltransferase (NAPRT), NAMN adenyltransferase (NAMNAT), and ammonia-dependent NAD + synthetase were found under treatment of compound 77 against in vitro or in vivo model when compared with the untreated models. Finally, from the ADME-properties point of view, compound 77 showed a good pharmacokinetic and pharmacodynamics profile. In summary, compound 77 showed an effective leishmanicidal effect against in vitro and in vivo models of LV with curative properties, convenient ADME properties, and an immunostimulating response.
In 2021, Glanzmann and co-workers prepared a novel series of quinoline-triazole hybrids 82–84 (Figure 23A), and they were evaluated against L. amazonensis promastigotes and intracellular amastigotes (Glanzmann et al., 2021). Considering both promastigotes and amastigotes, the results demonstrated that only derivative 84 displayed a promising leishmanicidal response, with IC50 values of 5.7 µM and 1.1 µM against promastigote and amastigote strains, respectively. That compound was the most selective, displaying an SI value of 16.5, which was higher than typical 1 or 3 of the remaining compounds. On the other hand, mechanistic experiments based on compound 84 showed that it induces a significant reduction of the mitochondrial membrane potential, leading to the disruption of its function. Furthermore, a pronounced generation of ROS was noted under treatment with compound 84. Apoptotic evidence was observed through cytometry. In conclusion, the introduction of a triazole ring induces an improvement in leishmanicidal response, leading to a specific mode of action through interference in the bioenergetic system and plasma membrane permeabilization, with subsequent activation of apoptosis-like and necrosis processes, culminating in cell death.
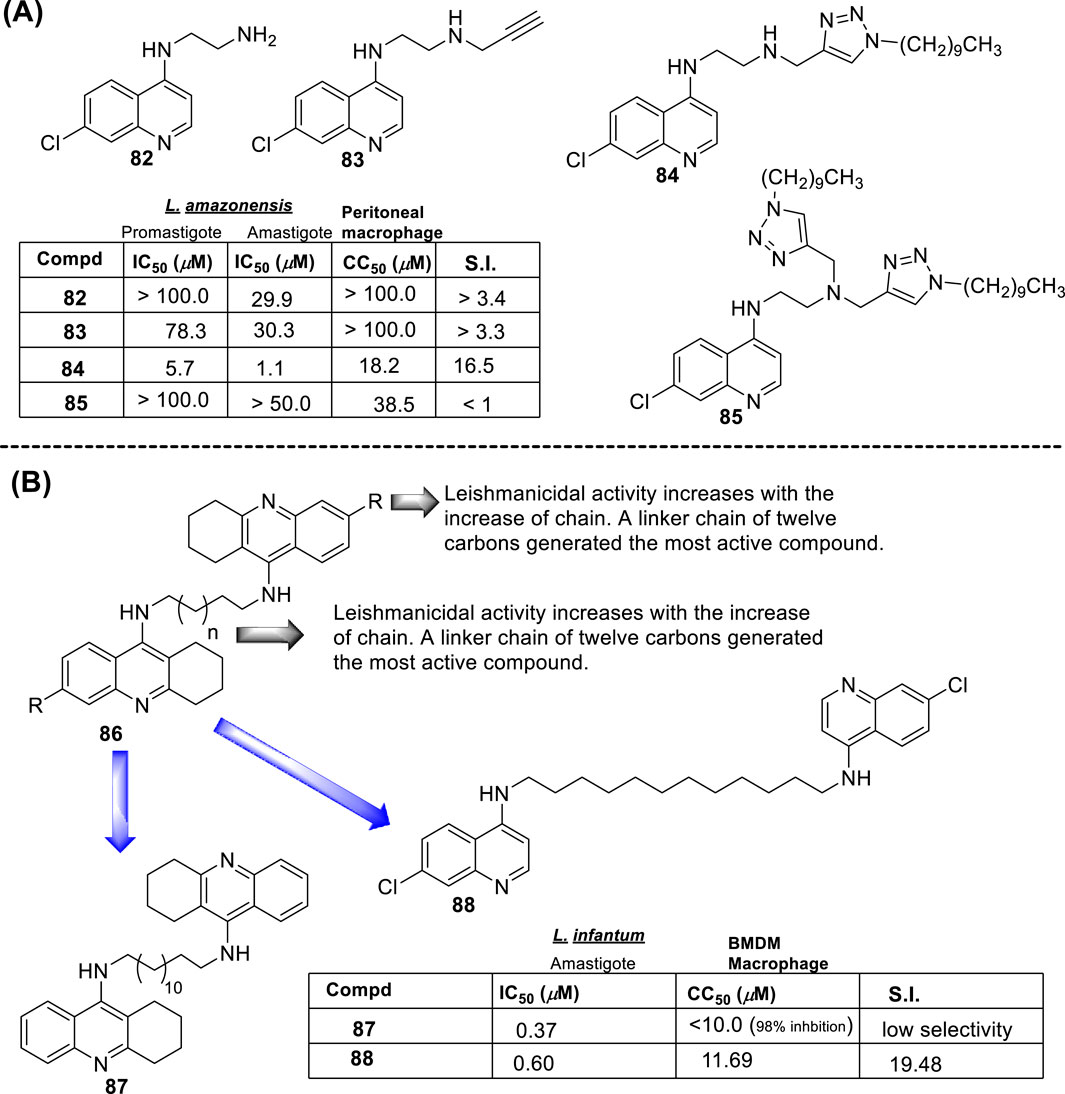
Figure 23. (A) Structure of 4-aminoquinolines with triazole rings at 4-diamine chain 82–85 reported by Glanzmann et al. (2021). (B) Structure of a quinoline dimer 86–88 with promising antileishmanial properties against Leishmania infantum promastigotes reported by Silva et al. (2023).
Finally, Tavares and co-workers developed a series of 1,2,3,4-tetrahydroacridines 86 based on a virtual screening performed against the enzyme S-adenosylmethionine decarboxylase and evaluated them against L. infantum promastigotes (Figure 23B) (Silva et al., 2023). Forty-two compounds were assayed. A SAR study was structured based on their results, and it was possible to verify that the length of the alkyl chain had a significant effect on the leishmanicidal activity of the compound, especially in the case of dimers. Most of the 1,2,3,4-tetrahydroacridines presented high levels of toxicity, with a percentage of macrophage death of about 90% at 10 µM. From the SAR analysis, only compound 87 was a promising candidate with an IC50 value of 0.37 µM, although high toxicity derived from an inhibition percentage of 98% in macrophage proliferation at 10 µM. Based on the relevance of a long alkyl chain as a linker between the quinoline core and the high toxicity of the acridine core, this research group decided to replace the tetrahydroacridine scaffold with a 7-chloroquinoline core to prepare compound 88, which consists of two 7-chloroquinolines connected through an N,N-decan-1,10-diamine chain. The new compound showed a good leishmanicidal response with an IC50 value of 0.60 µM and a CC50 value of 11.69 µM, which implies a good SI value near 20. This replacement allowed the retention of the leishmanicidal response with decreased toxicity.
In summary, the following remarks about generating potent and selective compounds from the different 4-aminoquinolines can be extracted from these reports:
i) The inclusion of a chlorine atom at the 7-position has a detrimental influence;
ii) The incorporation of tertiary amine at the end of the alkyl-diamine chain at the 4-position is essential;
iii) The incorporation of lipophilic moieties like aryl- or extended alkyl chains either on the diamine chain or on the quinoline core improved potency and selectivity;
iv) An extensive alkyl-diamine chain can compromise the selectivity and increase toxicity;
v) A higher specificity toward the amastigote than toward the promastigote stage was found for most of the analyzed 4-aminoquinolines;
vi) The 4-aminoquinolines target the reduction of mitochondrial membrane potential, which promotes the reduction of ATP synthesis and ROS production that induces morphological alterations and death via apoptosis.
Table 1 summarizes the most highlighted compounds based on in vitro evaluation against intracellular amastigotes, cytotoxicity on macrophages, and a selectivity index higher than 10. From Table 1, compounds 16 (SI > 1111), 45 (SI = 145 vs. L. amazonensis and 392 vs. L. infantum), 54b (SI = 41.3), 57 (SI > 250), 61 (SI = 41.6), and 76 (SI = 1739) are highlighted as the most promising candidate for further assays and as lead structures to inspire new design. These compounds are within the strictest parameters for the drug discovery of leishmanicidal agents (Don and Ioset, 2014). From the structural point of view, the most selective compounds (16, 45, 57, and 76) are better adjusted to the strict parameters for the design of leishmanicidal agents (Don and Ioset, 2014). The inclusion of a lipophilic moiety (aryl or cyclohexyl) at the end of the 4-diamine chain, as well as the incorporation of a composed N-aniline moiety, such as 4′-phenoxy-aniline, into the structure of compounds 57 and 76 at the 4-position of the quinoline core is highly convenient for generating selective compounds as a consequence of the decrease of the cytotoxicity, conserving the leishmanicidal response. In particular, a comparison of compounds 57 and 76, which bear an N-anilino moiety at the 4-position of quinoline core, showed that the incorporation of an extra basic moiety like phenyl-piperazine into compound 76 significantly enhances the selectivity compared with compound 57. A comparison between 4-aminoquinolines featuring an alkyl-amine chain at the 4-position revealed that the inclusion either of a basic moiety or of a lipophilic moiety at the end of the amine chain improves the activity and selectivity, such as can be seen in compounds 16 and 45. These features are also of great relevance for the accumulation in the host lysosome (Trapp et al., 2008) and parasite mitochondria (Duenas-Romero et al., 2007; Coimbra et al., 2010) as well as for promoting a consistent immunostimulant response acting as a TLR agonist/antagonist (Talukdar et al., 2021). Interestingly, when the size of the alkyl-amine chain at the 4-position is increased, the selectivity is compromised as a consequence of an increase of the cytotoxicity on macrophages, as can be seen in compounds 67 (SI = 8.2), 72 (SI = 13.1), 84 (SI = 16.5) and 88 (SI = 19.5). That tendency was appreciated in the quinolinic compound prepared by Bertinaria´s group (Guglielmo et al., 2009) (Figure 10), B.A. Solaja´s group (Konstantinović et al., 2018) (Figure 22), and Manzano et al. (2019) (Figure 21A). On the other hand, the ferrocenyl quinoline also proved to be a convenient scaffold for generating potent and active compounds against in vitro and in vivo models of VL. In addition, it is important to mention that the presence of an extra basic moiety is highly convenient for generating water-soluble compounds by the generation of protonated salts, such as can be seen for compounds 54b and 78, which is a physicochemical property that is highly desired for in vivo models using oral administration.
A few of the compounds mentioned in Table 1 have been proved against in vivo models of VL. In particular, compound 45 was proved against an in vivo model of VL of L. infantum, promoting a significant reduction of parasitemia (66%–72%) larger than that promoted by amphotericin (42%–62%). Other groups of compounds, including compounds 20, 33 (SI > 18), 72 (SI = 13.1), and 78, displayed a good in vivo efficacy with parasitemia reduction. In particular, for compounds 20 and 78, beyond the in vivo efficacy, an immunological activation was proved through the increase of pro-inflammatory cytokines. Compound 78 is an attractive alternative to favor the administration and pharmacokinetics due to its solubility in water.
From the synthetic point of view and based on promising compounds shown in Table 1, compounds 20, 29, 33, 45, 54b, 57, 78, 84, and 88 can be prepared from simple and short synthetic routes involving a simple or three-step simple route, requiring only the extra functionalization of 4-alkyl amine substitution and the final step of nucleophilic aromatic substitution at the 4-position in 4,7-dichloroquinoline substrate. Meanwhile, the synthesis of compounds 16, 61, 67, 72, and 76 require complex synthetic routes involving several steps as a consequence of specific functionalization at the quinoline core as well as the incorporation of complex functionalization at the 4-amine chain. In summary, compounds 45 and 78 are attractive scaffolds due to their significant leishmanicidal activity against in vitro and in vivo models of VL and also due to their synthetic feasibility and physicochemical properties for oral administration. Meanwhile, compounds 16 and 76 emerge as promising candidates for further studies to evaluate their potential as leishmanicidal agents.
In the last decades, compounds based on 4-aminoquinoline have shown great potential for the drug discovery of leishmanicidal agents with a well-defined mechanism of action. The present review showed how the 4-aminoquinolines possess a high specificity toward the amastigote strain over the promastigote, which is highly relevant because the first is the stage in clinical manifestations. Furthermore, a SAR analysis comparison based on diverse examples showed that two factors, basicity and lipophilicity, are key parameters leading to a significant leishmanicidal response and are selective. In particular, a tertiary terminal amine and long hydrocarbon chains are required. From the mechanistic point of view, it was found that 4-aminoquinoline targets two key organelles, the lysosome macrophage and parasite mitochondria as well as playing a role in immunostimulation. The incorporation of lipophilic chains is essential when targeting macrophage lysosomes, and fundamental reports indicate that Log P values between 4 and 6 are required. Once within the lysosome, the 4-aminoquinoline enters the parasite, and under the protonated form (under an acidic lysosome pH), the compounds target the mitochondria, where they promote depolarization of mitochondrial potential, which is key to conserving energy production. This latter favors ROS production, decreases ATP production, and promotes parasite apoptosis. Additionally, 4-aminoquinolines could act as TLR agonists or antagonists, which could favor macrophage activation and activate the innate immune macrophage system, which emerges as an extra defense mechanism to attack parasites within the macrophage. Interestingly, a more selective TLR response is found for 4-aminoquinolines with terminal tertiary amine and lipophilic groups.
The present review provides a considerable update on development, taking into account two key aspects: (i) SAR analysis and (ii) identification of key targets and their modulation based on SAR analysis. The information seeks to give the scientific community a more in-depth understanding of the most suitable functionalizations to perform in this particular family of compounds for the construction of potent and selective leishmanicidal agents and how it is possible to modulate the mentioned targets. Our report is a starting point to inspire future investigations directed to the design of leishmanicidal agents based on 4-aminoquinolines.
AR: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing–original draft, and writing–review and editing. FD: formal analysis, investigation, validation, visualization, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by PEDECIBA (Programa de Desarrollo de las Ciencias Básicas) of the Universidad de la República (Uruguay) under Despegue-Cientifico 2023 funds. Angel H. Romero thanks the Sistema Nacional de Investigadores (SNI) under grant code SNI_2020_1-1010584.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alvar, J., and Arana, B. (2018). “Leishmaniasis-impact and therapeutic needs, p 3-23,” in Drug discovery for leishmaniasis. Editors L. Rivas,, and C. Gil (Croydon, United Kingdom: The Royal Society of Chemistry).
Alvar, J., Vélez, I. D., Bern, C., Herrero, M., Desjeux, P., Cano, J., et al. (2011). Leishmaniasis worldwide and global estimates of its incidence. Plos One 7, e35671. doi:10.1371/journal.pone.0035671
Anders, C. B., Lawton, T. M. W., and Ammons, M. C. B. (2019). Metabolic immunomodulation of macrophage functional plasticity in nonhealing wounds. Curr. Opin. Infect. Dis. 32, 204–209. doi:10.1097/qco.0000000000000550
Andersen, O. S., and Fuchs, M. (1975). Potential energy barriers to ion transport within lipid bilayers. Studies with tetraphenylborate. Biophys. J. 15, 795–830. doi:10.1016/s0006-3495(75)85856-5
Antinarelli, L. M. R., de Souza, I. O., Glanzmann, N., Almeida, A. C., Porcino, G. N., Vasconcelos, E. G., et al. (2016). Aminoquinoline compounds: effect of 7-chloro-4-quinolinylhydrazone derivatives against Leishmania amazonensis. Exp. Parasitol. 171, 10–16. doi:10.1016/j.exppara.2016.10.009
Antinarelli, L. M. R., Dias, R. M. P., Souza, I. O., Lima, W. P., Gameiro, J., da Silva, A. D., et al. (2015). 4-Aminoquinoline derivatives as potential antileishmanial agents. Chem. Biol. Drug Des. 86, 704–714. doi:10.1111/cbdd.12540
Antinarelli, L. M. R., Souza, M. V. N., Coelho, E. A. F., Lima, W. P., and Coimbra, E. S. (2020). Efficacy of the 7-chloro-4-(3-hydroxy-benzilidenehydrazo) quinoline derivative against infection caused by Leishmania amazonensis. Rev. Soc. Bras. Med. Trop. 53, e20200091. doi:10.1590/0037-8682-0091-2020
Aronson, N., Herwaldt, B. L., Libman, M., Pearson, R., Lopez-Velez, R., Weina, P., et al. (2017). Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the infectious diseases society of America (IDSA) and the American society of tropical medicine and hygiene (ASTMH). Am. J. Trop. Med. Hyg. 96, 24–45. doi:10.4269/ajtmh.16-84256
Ataullakhanov, F. I., and Vitvitsky, V. M. (2002). What determines the intracellular ATP concentration. Biosci. Rep. 22, 501–511. doi:10.1023/a:1022069718709
Bachovchin, K. A., Sharma, A., Bag, S., Klug, D. M., Schneider, K. M., Singh, B., et al. (2019). Improvement of aqueous solubility of lapatinib-derived analogues: identification of a quinolinimine lead for human african trypanosomiasis drug development. J. Med. Chem. 62, 665–687. doi:10.1021/acs.jmedchem.8b01365
Bai, J., and Qian, Y. (2020). Construction of an NIR and lysosome-targeted quinoline-BODIPY photosensitizer and its application in photodynamic therapy for human gastric carcinoma cells. Dyes Pigments 181, 108615. doi:10.1016/j.dyepig.2020.108615
Bernardo, L., Solana, J. C., Romero-Kauss, A., Sánchez, C., Carrillo, E., and Moreno, J. (2021). Effect of immunosuppressants on the parasite load developed in, and immune response to, visceral leishmaniasis: a comparative study in a mouse model. PLoS Negl. Trop. Dis. 15, e0009126. doi:10.1371/journal.pntd.0009126
Biasutto, L., Sassi, N., Mattarei, A., Marotta, E., Cattelan, P., Toninello, A., et al. (2010). Impact of mitochondriotropic quercetin derivatives on mitochondria. Biochim. Biophys. Acta 1797, 189–196. doi:10.1016/j.bbabio.2009.10.001
Bik, E., Mateuszuk, L., Orleanska, J., Baranska, M., Chlopicki, S., and Majzner, K. (2021). Chloroquine-induced accumulation of autophagosomes and lipids in the endothelium. Int. J. Mol. Sci. 22, 2401. doi:10.3390/ijms22052401
Blaikie, F. H., Brown, S. E., Samuelsson, L. M., Brand, M. D., Smith, R. A., and Murphy, M. P. (2006). Targeting dinitrophenol to mitochondria: limitations to the development of a self-limiting mitochondrial protonophore. Biosci. Rep. 26, 231–243. doi:10.1007/s10540-006-9018-8
Bogdan, C. (2020). Macrophages as host, effector and immunoregulatory cells in leishmaniasis: impact of tissue micro-environment and metabolism. Cytokine x. 2, 100041. doi:10.1016/j.cytox.2020.100041
Braly, P., Simpson, L., and Kretzer, F. (1974). Isolation of kinetoplast-mitochondrial complexes from Leishmania tarentolae. J. Eukaryot. Microbiol. 21, 782–790. doi:10.1111/j.1550-7408.1974.tb03752.x
Buates, S., and Matlashewski, G. (1999). Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. J. Infect. Dis. 179, 1485–1494. doi:10.1086/314782
Calixto, S. L., Glanzmann, N., Xavier Silveira, M. M., da Trindade Granato, J., Gorza Scopel, K. K., Torres de Aguiar, T., et al. (2018). Novel organic salts based on quinoline derivatives: the in vitro activity trigger apoptosis inhibiting autophagy in Leishmania spp. Chem. Biol. Interact. 293, 141–151. doi:10.1016/j.cbi.2018.08.003
Carmo, A. M. L., Silva, F., Machado, P., Fontes, A., Pavan, F., Leite, S., et al. (2011). Synthesis of 4-aminoquinoline analogues and their platinum(II) complexes as new antileishmanial and antitubercular agents. Biomed. Pharmacother. 65, 204–209. doi:10.1016/j.biopha.2011.01.003
Chipuk, J. E., Bouchier-Hayes, L., and Green, D. R. (2006). Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 13, 1396–1402. doi:10.1038/sj.cdd.4401963
Clos, J., Grünebast, J., and Holm, M. (2022). Promastigote-to-Amastigote conversion in Leishmania spp.- A molecular view. Pathogens 11, 1052. doi:10.3390/pathogens11091052
Coelho, E. A. F., Tavares, G. S. V., Mendonça, D. V. C., Ribeiro, P. A. F., Dias, D. S., Oliveira, M., et al. (2010). Mechanism of interaction of sitamaquine with Leishmania donovani. J. Antimicrob. Chemother. 65, 2548–2555. doi:10.1093/jac/dkq371
Coimbra, E. S., Antinarelli, L. M. R., Silva, N. P., Souza, I. O., Meinel, R. S., Rocha, M. N., et al. (2016). Quinoline derivatives: synthesis, leishmanicidal activity and involvement of mitochondrial oxidative stress as mechanism of action. Chem. Biol. Int. 260, 50–57. doi:10.1016/j.cbi.2016.10.017
Coimbra, E. S., Carvalhaes, R., Grazul, R. M., Machado, P. A., De Souza, M. V. N., and Da Silva, A. D. (2010). Synthesis, cytotoxicity and antileishmanial activity of some N-(2-(indol-3-yl)ethyl)-7-chloroquinolin-4-amines. Chem. Biol. Drug Des. 75, 628–631. doi:10.1111/j.1747-0285.2010.00962.x
Conceicao, F., and Morgado, F. N. (2019). Leishmania spp.-host interaction: there is always an onset, but is there an end? Front. Cell Infect. Microbiol. 9, 330. doi:10.3389/fcimb.2019.00330
Costa Rocha, V. P., Nonato, F. R., Teixeira Guimaraes, E., Rodrigues de Freitas, L. A., and Pereira Soares, M. B. (2013). Activity of antimalarial drugs in vitro and in a murine model of cutaneous leishmaniasis. J. Med. Microbiol. 62, 1001–1010. doi:10.1099/jmm.0.058115-0
Degtyarev, M., De Mazière, A., Orr, C., Lin, J., Lee, B. B., Tien, J. Y., et al. (2008). Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J. Cell Biol. 183, 101–116. doi:10.1083/jcb.200801099
De Mello, H., Echevarría, A., Bernardino, A. M., Cavalheiro, M., and Leon, L. L. (2004). Antileishmanial pyrazolopyridine derivatives: synthesis and structure-activity relationship analysis. J. Med. Chem. 47, 5427–5432. doi:10.1021/jm0401006
Desjardins, M., and Descoteaux, A. (1997). Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J. Exp. Med. 185, 2061–2068. doi:10.1084/jem.185.12.2061
Devine, W., Woodring, J. L., Swaminathan, U., Amata, E., Patel, G., Erath, J., et al. (2015). Protozoan parasite growth inhibitors discovered by cross-screening yield potent scaffolds for lead discovery. J. Med. Chem. 58, 5522–5537. doi:10.1021/acs.jmedchem.5b00515
Dietze, R., Carvalho, S. F., Valli, L. C., Berman, J., Brewer, T., Milhous, W., et al. (2001). Phase 2 trial of WR6026, an orally administered 8-aminoquinoline, in the treatment of visceral leishmaniasis caused by Leishmania chagasi. Am. J. Trop. Med. Hyg. 65, 685–689. doi:10.4269/ajtmh.2001.65.685
Docampo, R., De Souza, W., Miranda, K., Rohloff, P., and Moreno, S. N. (2005). Acidocalcisomes- Conserved from bacteria to man. Nat. Rev. Microbiol. 3, 251–261. doi:10.1038/nrmicro1097
Don, R., and Ioset, J. R. (2014). Screening strategies to identify new chemical diversity for drug development to treat kinetoplastid infections. Parasitol. 141, 140–146. doi:10.1017/s003118201300142x
Dorababu, A. (2021). Quinoline: a promising scaffold in recent antiprotozoal drug discovery. Chem. Sel. 6, 2164–2177. doi:10.1002/slct.202100115
Drugs for Neglected Diseases initiative (2023). Research-Development and portfolio. Available at: https://www.dndi.org/research-development/portfolio (Accessed April 28, 2023).
Duenas-Romero, A. M., Loiseau, P. M., and Saint-Pierre-Chazalet, M. (2007). Interaction of sitamaquine with membrane lipids of Leishmania donovani promastigotes. Biochim. Biophys. Acta 1768, 246–252. doi:10.1016/j.bbamem.2006.07.003
El Hajjldi, R., Youness, H. B., Lachaud, L., Bastien, P., Masquefa, C., Bonnet, P. A., et al. (2018). EAPB0503: an imiquimod analog with potent in vitro activity against cutaneous leishmaniasis caused by Leishmania major and Leishmania tropica. PLoS Negl. Trop. Dis. 12, e0006854. doi:10.1371/journal.pntd.0006854
Fan, L., Nan, M., Ge, J., Wang, X., Lin, B., Zhang, W., et al. (2018). Imaging of lysosomal pH changes with a novel quinoline/benzothiazole probe. New J. Chem. 42, 13479–13485. doi:10.1039/c8nj02455c
Faria, M. S., Reis, F. C. G., and Lima, A. P. C. A. (2012). Toll-like receptors in Leishmania infections: guardians or promoters? J. Parasitol. Res. 2012, 1–12. doi:10.1155/2012/930257
Ferrins, L., Sharma, A., Thomas, S. M., Mehta, N., Erath, J., Tanghe, S., et al. (2018). Anilinoquinoline-based inhibitors of trypanosomatid proliferation. PLoS Negl. Trop. Dis. 12, e0006834. doi:10.1371/journal.pntd.0006834
Garnier, T., Brown, M. B., Lawrence, M. J., and Croft, S. L. (2006). In vitro and in vivo studies on a topical formulation of sitamaquine dihydrochloride for cutaneous leishmaniasis. J. Pharm. Pharmacol. 58, 1043–1054. doi:10.1211/jpp.58.8.0004
Glagolev, A. N., and Skulachev, V. P. (1978). The proton pump is a molecular engine of motile bacteria. Nature 272, 280–282. doi:10.1038/272280a0
Glanzmann, N., Antinarelli, L. M. R., Nunes, I. K. C., Pereira, H. M. G., Coelho, E. A. F., Coimbra, E. S., et al. (2021). Synthesis and biological activity of novel 4-aminoquinoline/1,2,3-triazole hybrids against Leishmania amazonensis. Biomed. Pharmacother. 141, 111857. doi:10.1016/j.biopha.2021.111857
Guglielmo, S., Bertinaria, M., Rolando, B., Crosetti, M., Fruttero, R., Yardley, V., et al. (2009). A new series of amodiaquine analogues modified in the basic side chain with in vitro antileishmanial and antiplasmodial activity. Eur. J. Med. Chem. 44, 5071–5079. doi:10.1016/j.ejmech.2009.09.012
Hanif, M. M., Akram, K., and Mustafa, G. (2016). Intralesional versus oral chloroquine in cutaneous leishmaniasis: comparison of outcome, duration of treatment and total dose of drug. J. Coll. Physicians Surg. Pak 26, 260–262.
Hansch, C., Leo, A., and Hoekman, D. (1995). Exploring QSAR: fundamentals and applications in chemistry and biology. Washington, DC. Am. Chem. Soc.
Hart, D., and Coombs, G. H. (1982). Leishmania mexicana: energy metabolism of amastigotes and promastigotes. Exp. Parasitol. 54, 397–409. doi:10.1016/0014-4894(82)90049-2
He, X., Jia, H., Jing, Z., and Liu, D. (2013). Recognition of pathogen-associated nucleic acids by endosomal nucleic acid-sensing toll-like receptors. Acta Biochim. Biophys. Sin. 45, 241–258. doi:10.1093/abbs/gms122
Herrera, L., Llanes, A., Alvarez, J., Degracia, K., Restrepo, C. M., Rivera, R., et al. (2020). Antileishmanial activity of a new chloroquine analog in an animal model of Leishmania panamensis infection. Int. J. Parasitol. Drug Drug Res. 14, 56–61. doi:10.1016/j.ijpddr.2020.08.002
Herrera, L., Stephens, D., D'Avila, A., George, K. G., Arman, H., Zhang, Y., et al. (2016). Insights into the structural patterns of the antileishmanial activity of bi- and tricyclic N-heterocycles. Org. Biomol. Chem. 14, 7053–7060. doi:10.1039/c6ob01149g
Hide, M., Bucheton, B., Kamhawi, S., Bras-Gonçalves, R., Sundar, S., Lemesre, J., et al. (2007). Understanding human leishmaniasis: the need for an integrated approach. Encycl. Infect. Dis. 6, 87–123. Chapter. doi:10.1002/9780470114209.ch6
Hoye, A. T., Davoren, J. E., Wipf, P., Fink, M. P., and Kagan, V. E. (2008). Targeting mitochondria. Acc. Chem. Res. 41, 87–97. doi:10.1021/ar700135m
Hu, Z., Tanji, H., Jiang, S., Zhang, S., Koo, K., Chan, J., et al. (2018). Small molecule TLR8 antagonists via structure-based rational design. Cell Chem. Biol. 25, 1286–1291.e3. doi:10.1016/j.chembiol.2018.07.004
Izyumov, D. S., Avetisyan, A. V., Pletjushkina, O. Y., Sakharov, D. V., Wirtz, K. W., Chernyak, B. V., et al. (2004). “Wages of fear”: transient threefold decrease in intracellular ATP level imposes apoptosis. Biochim. Biophys. Acta 1658, 141–147. doi:10.1016/j.bbabio.2004.05.007
James, A. M., Cocheme, H. M., and Murphy, M. P. (2005). Mitochondria-targeted redox probes as tools in the study of oxidative damage and ageing. Mech. Ageing Dev. 126, 982–986. doi:10.1016/j.mad.2005.03.026
Jardim, A., Hardie, D. B., Boitz, J., and Borchers, C. H. (2018). Proteomic profiling of Leishmania donovani promastigote subcellular organelles. J. Proteome Res. 17, 1194–1215. doi:10.1021/acs.jproteome.7b00817
Jensen, R. E., and Englund, P. T. (2012). Network news: the replication of kinetoplast DNA. Annu. Rev. Microbiol. 66, 473–491. doi:10.1146/annurev-micro-092611-150057
Jiang, Q., Yin, J., Chen, J., Ma, X., Wu, M., Liu, G., et al. (2020). Mitochondria-targeted antioxidants: a step towards disease treatment. Oxid. Med. Cell Longev. 2020, 1–18. doi:10.1155/2020/8837893
Kaushik, D., Granato, J. T., Macedo, G. C., Dib, P. R. B., Piplani, S., Fung, J., et al. (2021). Toll like receptor-7/8 agonist kill Leishmania amazonensis by acting as pro-oxidant and pro-inflammatory agent. J. Pharm. Pharmacol. 73, 1180–1190. doi:10.1093/jpp/rgab063
Kawasaki, T., and Kawai, T. (2014). Toll-like receptor signaling pathways. Front. Immunol. 5, 461. (section Cancer Immunity and Immunotherapy). doi:10.3389/fimmu.2014.00461
Ketterer, B., Neumcke, B., and Lauger, P. (1971). Transport mechanism of hydrophobic ions through lipid bilayer membranes. J. Membr. Biol. 5, 225–245. doi:10.1007/bf01870551
Konstantinović, J., Videnović, M., Orsini, S., Bogojevic, K., D'Alessandro, S., Scaccabarozzi, D., et al. (2018). Novel aminoquinoline derivatives significantly reduce parasite load in Leishmania infantum infected mice. ACS Med. Chem. Lett. 9, 629–634. doi:10.1021/acsmedchemlett.8b00053
Kuo, H.-H., Kakadiya, R., Wu, Y.-C., Su, T.-L., Lee, T.-C., Lin, Y.-W., et al. (2016). Derivatives of 6-cinnamamido-quinoline-4-carboxamide impair lysosome function and induce apoptosis. Oncotarget 7, 38078–38090. doi:10.18632/oncotarget.9348
Lackner, L. L. (2014). Shaping the dynamic mitochondrial network. BMC Biol. 12, 35. doi:10.1186/1741-7007-12-35
Li, G., Zhu, D., Xue, L., and Jiang, H. (2013). Quinoline-based fluorescent probe for ratiometric detection of lysosomal pH. Org. Lett. 15, 5020–5023. doi:10.1021/ol4023547
Liberman, E. A., and Topaly, V. P. (1969). Permeability of biomolecular phospholipid membranes for fat-soluble ions. Biophysics 14, 477.
Liew, S. S., Qin, X., Zhou, J., Li, L., Huang, W., and Yao, S. Q. (2021). Smart design of nanomaterials for mitochondria-targeted nanotherapeutics. Angew. Chem. Int. Ed. 60, 2232–2256. doi:10.1002/anie.201915826
Ljubava, D., Zorova, S., Popkov, V. A., Plotnikov, E. Y., Silachev, D. N., Pevzner, I. B., et al. (2018). Mitochondrial membrane potential. Anal. Biochem. 552, 50–59. doi:10.1016/j.ab.2017.07.009
Loiseau, P. M., Cojean, S., and Schrével, J. (2011). Sitamaquine as a putative antileishmanial drug candidate: from the mechanism of action to the risk of drug resistance. Parasite 18 (2), 115–119. doi:10.1051/parasite/2011182115
Lopez-Martin, C., Perez-Victoria, J. M., Carvalho, L., Castanys, S., and Gamarro, F. (2008). Sitamaquine sensitivity in Leishmania species is not mediated by drug accumulation in acidocalcisomes. Antimicrob. Agents Chemother. 52, 4030–4036. doi:10.1128/aac.00964-08
Mannella, C. A. (2006). Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1763, 542–548. doi:10.1016/j.bbamcr.2006.04.006
Manzano, J. I., Carvalho, L., Pérez-Victoria, J. M., Castanys, S., and Gamarro, F. (2011). Increased glycolytic ATP synthesis is associated with tafenoquine resistance in Leishmania major. Antimicrob. Agent. Chemother. 55, 1045–1052. doi:10.1128/AAC.01545-10
Manzano, J. I., Konstantinović, J., Scaccabarozzi, D., Perea, A., Pavić, A., Cavicchini, L., et al. (2019). 4-Aminoquinoline-based compounds as antileishmanial agents that inhibit the energy metabolism of Leishmania. Eur. J. Med. Chem. 180, 28–40. doi:10.1016/j.ejmech.2019.07.010
Manzel, L., Strekowski, L., Ismail, F. M. D., Smith, J. C., and Macfarlane, D. E. (1999). Antagonism of immunostimulatory CpG-oligodeox ynucleotides by 4-aminoquinolines and other weak bases: mecha nistic studies. J. Pharmacol. Exp. Ther. 291, 1337–1347. doi:10.1016/s0022-3565(24)35244-9
Marceau, F., Bawolak, M. T., Lodge, R., Bouthillier, J., Gagné-Henley, A., Gaudreault, R. C., et al. (2012). Cation trapping by cellular acidic compartments: beyond the concept of lysosomotropic drugs. Tox. Appl. Pharm. 259, 1–12. doi:10.1016/j.taap.2011.12.004
McAfee, Q., Zhang, Z., Samanta, A., Levi, S. M., Ma, X. H., Piao, S., et al. (2012). Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc. Natl. Acad. Sci. U. S. A. 109, 8253–8258. doi:10.1073/pnas.1118193109
McBride, H. M., Neuspiel, M., and Wasiak, S. (2006). Mitochondria: more than just a powerhouse. Curr. Biol. 16, R551–R560. doi:10.1016/j.cub.2006.06.054
Mehta, N., Ferrins, L., Leed, S. E., Sciotti, R. J., and Pollastri, M. P. (2018). Optimization of physicochemical properties for 4-anilinoquinoline inhibitors of Plasmodium falciparum proliferation. ACS Infect. Dis. 4, 577–591. doi:10.1021/acsinfecdis.7b00212
Melikyan, G. B., Deriy, B. N., Ok, D. C., and Cohen, F. S. (1996). Voltage-dependent translocation of R18 and DiI across lipid bilayers leads to fluorescence changes. Biophys. J. 71, 2680–2691. doi:10.1016/s0006-3495(96)79459-6
Menna-Barreto, R. F. S., and de Castro, S. L. (2014). The double-edged sword in pathogenic trypanosomatids: the pivotal role of mitochondria in oxidative stress and bioenergetics. Biomed. Res. Int. 2014, 1–14. doi:10.1155/2014/614014
Mitchell, P. (1966). Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol. Rev. Camb. Philos. Soc. 41, 445–501. doi:10.1111/j.1469-185x.1966.tb01501.x
Moradin, N., and Descoteaux, A. (2012). Leishmania promastigotes: building a safe niche within macrophages. Front. Cell. Inf. Microbiol. 2, 121. doi:10.3389/fcimb.2012.00121
Mukherjee, D., Yousuf, M., Dey, S., Chakraborty, S., Chaudhuri, A., Kumar, V., et al. (2020). Targeting the trypanothione reductase of tissue-residing Leishmania in hosts’ reticulo endothelial system: a flexible water soluble ferrocenylquinoline-based preclinical drug candidate. J. Med. Chem. 63, 15621–15638. doi:10.1021/acs.jmedchem.0c00690
Mwololo, S. W., Mutiso, J. M., Macharia, J. C., Bourdichon, A. J., and Gicheru, M. M. (2015). In vitro activity and in vivo efficacy of a combination therapy of diminazene and chloroquine against murine visceral leishmaniasis. J. Biomed. Res. 29, 214. doi:10.7555/jbr.29.20140072
Newton, D. W., and Kluza, R. B. (1978). pKa values of medicinal compounds in pharmacy practice. Drug Intell. Clin. Pharm. 12, 546–554. doi:10.1177/106002807801200906
O’Neill, P. M., Mukhtar, A., Stocks, P. A., Randle, L. E., Hindley, S., Ward, S. A., et al. (2003). Isoquine and related amodiaquine analogues: a new generation of improved 4-aminoquinoline antimalarials. J. Med. Chem. 46 (23), 4933–4945. doi:10.1021/jm030796n
Opperdoes, F. R., and Coombs, G. H. (2007). Metabolism of Leishmania: proven and predicted. Trends Parasitol. 23, 149–158. doi:10.1016/j.pt.2007.02.004
Ortiz, D., Forquer, I., Boitz, J., Soysa, R., Elya, C., Fulwiler, A., et al. (2016). Targeting the cytochrome bc1 complex of Leishmania parasites for discovery of novel drugs. Antimicrob. Agents Chemother. 60, 4972–4982. doi:10.1128/aac.00850-16
Pan American Health Organization (2023). Leishmaniasis: epidemiological report in the americas. Number 12, december 2023. Washington, D.C.: PAHO. Available at: https://iris.paho.org/handle/10665.2/53090.
Polari, L. P., Carneiro, P. P., Macedo, M., Machado, P. R. L., Scott, P., Carvalho, E. M., et al. (2019). Leishmania braziliensis infection enhances toll-like receptors 2 and 4 expression and triggers TNF-α and IL-10 production in human cutaneous leishmaniasis. Front. Cell Infect. Microbiol. 9, 120. doi:10.3389/fcimb.2019.00120
Raman, V. S., Bhatia, A., Picone, A., Whittle, J., Bailor, H. R., O'Donnell, J., et al. (2010). Applying TLR synergy in immunotherapy: implications in cutaneous leishmaniasis. J. Immunol. 185, 1701–1710. doi:10.4049/jimmunol.1000238
Ramírez-Prada, J., Robledo, S. M., Vélez, I. D., Crespo, M. P., Quiroga, J., Abonia, R., et al. (2017). Synthesis of novel quinoline–based 4,5–dihydro–1H–pyrazoles as potential anticancer, antifungal, antibacterial and antiprotozoal agents. Eur. J. Med. Chem. 131, 237–254. doi:10.1016/j.ejmech.2017.03.016
Regli, I. B., Passelli, K., Martínez-Salazar, B., Amore, J., Hurrell, B. P., Müller, A. J., et al. (2020). TLR7 sensing by neutrophils is critical for the control of cutaneous leishmaniasis. Cell Rep. 31, 107746. doi:10.1016/j.celrep.2020.107746
Reynolds, K. A., Loughlin, W. A., and Young, D. J. (2013). Quinolines as chemotherapeutic agents for leishmaniasis. Mini.-Rev. Med. Chem. 13, 730–743. doi:10.2174/1389557511313050010
Robb, E. L., Gawel, J. M., Aksentijevic, D., Cocheme, H. M., Stewart, T. S., Shchepinova, M. M., et al. (2015). Selective superoxide generation within mitochondria by the targeted redox cycler MitoParaquat. Free Radic. Biol. Med. 89, 883–894. doi:10.1016/j.freeradbiomed.2015.08.021
Rodrigues de Santana, F., Dalboni, L. C., Nascimento, K. F., Konno, F. T., Alvares-Saraiva, A. M., Correia, M. S. F., et al. (2017). High dilutions of antimony modulate cytokines production and macrophage-Leishmania (L.) amazonensis interaction in vitro. Cytokine 92, 33–47. doi:10.1016/j.cyto.2017.01.004
Romero, A. H., Acosta, M. E., Gamboa, N., Charris, J., Salazar, J., and López, S. E. (2015). Synthesis β-hematin inhibition studies and antimalarial evaluation of dehydroxy Isotebuquine derivatives against Plasmodium berghei. Bioorg. Med. Chem. 23, 4755–4762. doi:10.1016/j.bmc.2015.05.040
Romero, A. H., Rodríguez, N., López, S. E., and Oviedo, H. (2019). Identification of dehydroxy isoquine and isotebuquine as promising antileishmanial agents. Arch. Pharm. 352 (5), 1800281. doi:10.1002/ardp.201800281
Ross, M. F., Kelso, G. F., Blaikie, F. H., James, A. M., Cocheme, H. M., Filipovska, A., et al. (2005). Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochem. 70, 222–230. doi:10.1007/s10541-005-0104-5
Salyer, A. C. D., and David, S. A. (2018). Transcriptomal signatures of vaccine adjuvants and accessory immunostimulation of sentinel cells by toll-like receptor 2/6 agonists. Hum. Vacc. Immunother. 14, 1686–1696. doi:10.1080/21645515.2018.1480284
Schmidt, O., Pfanner, N., and Meisinger, C. (2010). Mitochondrial protein import: from proteomics to functional mechanisms. Nat. Rev. Mol. Cell Biol. 11, 655–667. doi:10.1038/nrm2959
Séguin, O., and Descoteaux, A. (2016). Leishmania, the phagosome, and host responses: the journey of a parasite. Cell Immunol. 309, 1–6. doi:10.1016/j.cellimm.2016.08.004
Shah, S. I., Nasir, F., Malik, N. S., Alamzeb, M., Abbas, M., Rehman, I. U., et al. (2022). Efficacy evaluation of 10-hydroxy chondrofoline and tafenoquine against Leishmania tropica (HTD7). Pharmaceuticals 15, 1005. doi:10.3390/ph15081005
Sharma, N., Thomas, S., Golden, E. B., Hofman, F. M., Chen, T. C., Petasis, N. A., et al. (2012). Inhibition of autophagy and induction of breast cancer cell death by mefloquine, an antimalarial agent. Cancer Lett. 326, 143–154. doi:10.1016/j.canlet.2012.07.029
Sharma, R., Pandey, A. K., Shivahare, R., Srivastava, K., Gupta, S., and Chauhan, P. M. S. (2014). Triazino indole-quinoline hybrid: a novel approach to antileishmanial agents. Bioorg. Med. Chem. Lett. 24, 298–301. doi:10.1016/j.bmcl.2013.11.018
Shukla, D., Singh-Chandel, H., Srivastava, S., Chauhan, P., Pandey, S. P., Patidar, A., et al. (2018). TLR11 or TLR12 silencing reduces Leishmania major infection. Cytokine 104, 110–113. doi:10.1016/j.cyto.2017.10.005
Siekevitz, P. (1957). Powerhouse of the cell. Sci. Am. 197, 131–144. doi:10.1038/scientificamerican0757-131
Silva, C. F. M., Leão, T., Dias, F., Tomás, A. M., Pinto, D. C. G. A., Oliveira, E. F. T., et al. (2023). Structure-activity relationship studies of 9-alkylamino-1,2,3,4-tetrahydroacridines against Leishmania (Leishmania) infantum promastigotes. Pharmaceutics 15, 669. doi:10.3390/pharmaceutics15020669
Singh, B., Bernatchez, J. A., McCall, L. I., Calvet, C. M., Ackermann, J., Souza, J. M., et al. (2020). Scaffold and parasite hopping: discovery of new Protozoal proliferation inhibitors. ACS Med. Chem. Lett. 11, 249–257. doi:10.1021/acsmedchemlett.9b00453
Smirlis, D., Dingli, F., Pescher, P., Prina, E., Loew, D., Rachidi, N., et al. (2019). SILAC-based quantitative proteomics reveals pleiotropic, phenotypic modulation in primary murine macrophages infected with the protozoan pathogen Leishmania donovani. J. Proteomics 213, 103617. doi:10.1016/j.jprot.2019.103617
Smith, R. A., Hartley, R. C., and Murphy, M. P. (2011). Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox Signal. 15, 3021–3038. doi:10.1089/ars.2011.3969
Solomon, V. R., and Lee, H. (2009). Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur. J. Pharmacol. 625, 220–233. doi:10.1016/j.ejphar.2009.06.063
Sousa, J. K. T., Antinarelli, L. M. R., Mendonça, D. V. C., Lage, D. P., Tavares, G. S. V., Dias, D. S., et al. (2019). A chloroquinoline derivate presents effective in vitro and in vivo antileishmanial activity against Leishmania species that cause tegumentary and visceral leishmaniasis. Parasitol. Int. 73, 101966. doi:10.1016/j.parint.2019.101966
Souza, W. D., Attias, M., and Rodrigues, J. (2009). Particularities of mitochondrial structure in parasitic protists (Apicomplexa and Kinetoplastida). Int. J. Biochem. Cell Biol. 41, 2069–2080. doi:10.1016/j.biocel.2009.04.007
Staderini, M., Piquero, M., Abengózar, M. Á., Nachér-Vázquez, M., Romanelli, G., López-Alvarado, P., et al. (2019). Structure-activity relationships and mechanistic studies of novel mitochondria-targeted, leishmanicidal derivatives of the 4-aminostyrylquinoline scaffold. Eur. J. Med. Chem. 171, 38–53. doi:10.1016/j.ejmech.2019.03.007
Stevanovic, S., Perdih, A., Sencanski, M., Glisic, S., Duarte, M., Tomas, A. M., et al. (2018). In silico discovery of a substituted 6-methoxy-quinalidine with leishmanicidal activity in Leishmania infantum. Molecules 23, 772. doi:10.3390/molecules23040772
Strekowski, L., Say, M., Henary, M., Ruiz, P., Manzel, L., Macfarlane, D. E., et al. (2003). Synthesis and activity of substituted 2 phenylquinolin-4-amines, antagonists of immunostimulatory CpG oligodeoxynucleotides. J. Med. Chem. 46, 1242–1249. doi:10.1021/jm020374y
Strekowski, L., Zegrocka, O., Henary, M., Say, M., Mokrosz, M. J., Kotecka, B. M., et al. (1999). Structure-activity relationship analysis of substituted 4-quinolinamines, antagonists of immunostimulatory CpG-oligodeoxynucleotides. Bioorg. Med. Chem. Lett. 9, 1819–1824. doi:10.1016/s0960-894x(99)00291-7
Talukdar, A., Ganguly, D., Roy, S., Das, N., and Sarkar, D. (2021). Structural evolution and translational potential for agonists and antagonists of endosomal toll-like receptors. J. Med. Chem. 64, 8010–8041. doi:10.1021/acs.jmedchem.1c00300
Tavares, G. S. V., Mendonca, D. V. C., Lage, D. P., Antinarelli, L. M. R., Soyer, T. G., Senna, A. J. S., et al. (2019). In vitro and in vivo antileishmanial activity of a fluoroquinoline derivate against Leishmania infantum and Leishmania amazonensis species. Acta Trop. 191, 29–37. doi:10.1016/j.actatropica.2018.12.036
Thomas, M., Brand, S., De Rycker, M., Zuccotto, F., Lukac, I., Dodd, P. G., et al. (2021). Scaffold-hopping strategy on a series of proteasome inhibitors led to a preclinical candidate for the treatment of visceral leishmaniasis. J. Med. Chem. 64, 5905–5930. doi:10.1021/acs.jmedchem.1c00047
Thomas, M. G., De Rycker, M., Ajakane, M., Albrecht, S., Álvarez-Pedraglio, A. I., Boesche, M., et al. (2019). Identification of gsk3186899/ddd853651 as a preclinical development candidate for the treatment of visceral leishmaniasis. J. Med. Chem. 62, 1180–1202. doi:10.1021/acs.jmedchem.8b01218
Tolouei, S., Hejazi, S., Ghaedi, K., Khamesipour, A., and Hasheminia, S. J. (2013). TLR2 and TLR4 in cutaneous leishmaniasis caused by Leishmania major. Scand. J. Immunol. 78, 478–484. doi:10.1111/sji.12105
Tomiotto-Pellissier, F., Da Silva-Bortoleti, B. T., Assolini, J. P., Gonçalves, M. D., Machado-Carloto, A. C., Miranda-Sapla, M., et al. (2018). Macrophage polarization in leishmaniasis: broadening horizons. Front. Immunol. 9, 2529. doi:10.3389/fimmu.2018.02529
Torres-Guerrero, E., Quintanilla-Cedillo, M. R., Ruiz-Esmenjaud, J., and Arenas, R. (2017). Leishmaniasis: a review. F1000Research 6, 750. doi:10.12688/f1000research.11120.1
Trapp, S., Rosania, G. R., Horobin, R. W., and Kornhuber, J. (2008). Quantitative modeling of selective lysosomal targeting for drug design. Eur. Biophys. J. 37, 1317–1328. doi:10.1007/s00249-008-0338-4
Tuon, F. F., Fernandes, E. R., Duarte, M. I., and Amato, V. S. (2010). The expression of TLR2, TLR4 and TLR9 in the epidermis of patients with cutaneous leishmaniasis. J. Dermatol. Sci. 59, 55–57. doi:10.1016/j.jdermsci.2010.04.009
Tuon, F. F., Gomes-Silva, A., Da-Cruz, A. M., Duarte, M. I., Neto, V. A., and Amato, V. S. (2008). Local immunological factors associated with recurrence of mucosal leishmaniasis. Clin. Immunol. 128, 442–446. doi:10.1016/j.clim.2008.05.007
Valverde, E. A., Romero, A. H., Acosta, M. E., Gamboa, N., Henriques, G., Rodrigues, J. R., et al. (2017). Synthesis, β-hematin inhibition studies and antimalarial evaluation of new dehydroxy isoquine derivatives against Plasmodium berghei: a promising antimalarial agent. Eur. J. Med. Chem. 148, 498–506. doi:10.1016/j.ejmech.2017.10.051
Vargas-Inchaustegui, D. A., Tai, W., Xin, L., Hogg, A. E., Corry, D. B., and Soong, L. (2009). Distinct roles for MyD88 and toll-like receptor 2 during Leishmania braziliensis infection in mice. Infect. Immun. 77, 2948–2956. doi:10.1128/iai.00154-09
Vasilakos, J. P., and Tomai, M. A. (2013). The use of Toll-like receptor 7/8 agonists as vaccine adjuvants. Expert Rev. Vaccines 12, 809–819. doi:10.1586/14760584.2013.811208
Vercesi, A., Rodrigues, C., Castisti, R., and Docampo, R. (2000). Presence of a Na(+)/H(+) exchanger in acidocalcisomes of Leishmania donovani and their alkalization by anti-leishmanial drugs. FEBS Lett. 473, 203–206. doi:10.1016/s0014-5793(00)01531-3
World Health Organisation (2024). Leishmaniasis fact sheet. Available at: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (Accessed September 28, 2024).
Yaniv, Y., Juhaszova, M., Nuss, H. B., Wang, S., Zorov, D. B., Lakatta, E. G., et al. (2010). Matching ATP supply and demand in mammalian heart: in vivo, in vitro, and in silico perspectives. Ann. NY. Acad. Sci. 1188, 133–142. doi:10.1111/j.1749-6632.2009.05093.x
Yaniv, Y., Juhaszova, M., Sollott, S. J., and Zorov, D. B. (2014). Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94, 909–950. doi:10.1152/physrev.00026.2013
Yousuf, M., Mukherjee, D., Dey, S., Pal, C., and Adhikari, S. (2016). Antileishmanial ferrocenylquinoline derivatives: synthesis and biological evaluation against Leishmania donovani. Eur. J. Med. Chem. 124, 468–479. doi:10.1016/j.ejmech.2016.08.049
Yousuf, M., Mukherjee, D., Pal, A., Dey, S., Mandal, S., Pal, C., et al. (2015). Synthesis and biological evaluation of ferrocenylquinoline as a potential antileishmanial agent. ChemMedChem 10, 546–554. doi:10.1002/cmdc.201402537
Zamzami, N., Marchetti, P., Castedo, M., Decaudin, D., Macho, A., Hirsch, T., et al. (1995). Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 182, 367–377. doi:10.1084/jem.182.2.367
Zhang, S., Hu, Z., Tanji, H., Jiang, S., Das, N., Li, J., et al. (2018). Small-molecule inhibition of TLR8 through stabilization of its resting state. Nat. Chem. Biol. 14, 58–64. doi:10.1038/nchembio.2518
Zielonka, J., Joseph, J., Sikora, A., Hardy, M., Ouari, O., Vasquez-Vivar, J., et al. (2017). Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem. Rev. 117, 10043–10120. doi:10.1021/acs.chemrev.7b00042
Zimmermann, D., Kiesel, M., Terpitz, U., Zhou, A., Reuss, R., Kraus, J., et al. (2008). A combined patch-clamp and electrorotation study of the voltage- and frequency-dependent membrane capacitance caused by structurally dissimilar lipophilic anions. J. Membr. Biol. 221, 107–121. doi:10.1007/s00232-007-9090-4
Keywords: 4-aminoquinoline, Leishmania, mitochondria, phagolysosome, immunostimulation
Citation: Romero AH and Delgado F (2025) 4-Aminoquinoline as a privileged scaffold for the design of leishmanicidal agents: structure–property relationships and key biological targets. Front. Chem. 12:1527946. doi: 10.3389/fchem.2024.1527946
Received: 14 November 2024; Accepted: 26 December 2024;
Published: 29 January 2025.
Edited by:
Kaushik Chanda, VIT University, IndiaReviewed by:
Esther Del Olmo, University of Salamanca, SpainCopyright © 2025 Romero and Delgado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angel H. Romero, YW5nZWwudWN2LnVzYkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.