
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 15 January 2025
Sec. Electrochemistry
Volume 12 - 2024 | https://doi.org/10.3389/fchem.2024.1511600
This article is part of the Research Topic Photoelectrochemistry in Solar Energy Utilization View all articles
An interesting approach of including an upconverter in the MoS2 counter electrode can yield broadband light harvesting Pt-free DSSC assembly. Here different upconverter (UC) nanoparticles (Yb, Er incorporated NaYF4, YF3, CeO2 & Y2O3) were synthesized and loaded in MoS2 thin film by hydrothermal method. The inclusion of UCs in MoS2 films exposed without any secondary formation of upconverters and the uniform deposition of the films are confirmed through XRD and FESEM analysis respectively. The absorption spectrum (UV-Vis-NIR) confirms the increase in absorption intensity in visible and NIR regions due to the incorporation of UCs. Under 980 nm excitation, the UCs-loaded MoS2 films show emission in blue (450–490 nm), green (520–540 nm) and red (600–700 nm) regions due to the f-f inner transition occurring in the Er ions. The oxide and fluoride UCs-based DSSCs were fabricated with an FTO/TiO2/N719/Triiodide electrolyte/UC@MoS2/FTO assembly. Oxide UCs show greater electrocatalytic activity, which might be owed to the films exposed with more catalytic active sites favourable for ion transportation in the I−/I−3 electrolyte. Among them, higher photoconversion efficiency (PCE) of 7.1% was witnessed for (Y2O3: Er, Yb) @MoS2 counter electrode-based DSSC which is due to the good conductivity (RS) of the film, the longer electron lifetime and photon harvesting enhancement by upconversion process. It is notably convinced that the UC-loaded MoS2 films can be used as an effective counter electrode for the possible realization of upconverter DSSC and used for broadband light absorption.
Global energy need and the equivalent sustainable power generation are on the constant rise to deal with the demand for energy for long-term practice. The production of adequate energy employing photovoltaic (PV) technology, i.e., solar cells signifies an assuring way for green and renewable energy generation. However, another prime cause that constrains the photo conversion efficiency (PCE) of solar cells is the spectral disparity between the photon energy of sunlight and the bandgap of the solar cell (Tadge et al., 2020; El Assyry et al., 2022; Tohluebaji et al., 2024). Another constraint of making trade-off between PCE and cost is although met through dye sensitized solar cells (DSSCs), low-priced PV technology is far from reality due to the expensive Pt based counter electrodes (Sarkar and Bera, 2020). Thus, the mutual outcome of having low-cost and high-efficiency Pt-free DSSCs might suggest an evolution in PV manufacturing. Recently, there has been growing interest worldwide in the development of electrocatalyst materials as potential counter electrode materials as it exhibits high electrical conductivity, excellent chemical stability, high mechanical strength and demonstrate superior catalytic activity (Hussain et al., 2015; Kanjana et al., 2023; Pujari et al., 2017). Among them, Platinum (Pt) is the most promising material for counter electrodes in DSSCs as they have outstanding charge carrier mobility and good electrocatalytic activity but is limited by its very expensive nature (Fang et al., 2004; Vikraman et al., 2018; Younas et al., 2020). Thus, cost-effective, environmentally and chemically long-standing Pt free CE such as inorganic compounds (PtAu, PtCo, FeCo2), conducting polymers (PANi, PPy, PEDOT), transition metal compounds including nitrides (VN, TiN, MoN), carbides (TiC, VC, Nb C), phosphides (CoP, Fe2P, Ni12P5), carbon constituents (CNT, carbon black, carbon fibre) to substitute pricy Platinum electrode in DSSCs have been recently explored among different scientific groups (Yu et al., 2018; Jeong et al., 2019). The plentiful and low-cost transition-metal dichalcogenides (TMDCs), a group of two-dimensional atomically thin layered materials which have the form of MX2 where M refers to transition metals and X refers to dichalcogenide elements (S, Se, Te) which validate analogous electrocatalytic behaviour of Platinum that intuitively allow them to be suitable candidates for counter electrodes in DSSC. Amid them, MoS2 (molybdenum disulphide), a typical van der Waals force stacked layered material consists of single layer of Mo packed between two layers of S. (Wei et al., 2016; Vikraman et al., 2019). Existing research on MoS2 proved that sulphur sites are responsible for the electrocatalytic activity of MoS2, which is suitable for photoelectrochemical applications. Recently Durairaj et al., reported that MoS2 nanoplatelets prepared by the hydrothermal method exhibit high electrocatalytic activity and comparable photo conversion efficiency (PCE) with Pt-based DSSC (η = 6.39%) (Sabari Girisun et al., 2023). Thus, MoS2 can be an excellent CE material for the fabrication of Pt-free DSSCs provided the photoconversion efficiency can be improved by certain means.
In this line, PCE of DSSCs is sturdily reliant on the competence of light-harvesting dyes. But commonly used ruthenium-based dyes possess an optical bandgap of 1.8 eV, which limits its photon absorbing capacity to a quite narrow spectral wavelength range between 300 and 700 nm (Shan, 2010). Thus, the prime constraint of improving PCE of DSSC is its inability to utilize solar radiation in the infrared spectrum region, which comprises almost half the energy (49.4%) of the sunlight. One inimitable methodology to subjugate infrared photons is the photon upconversion approach (UC) (Dutta, 2021). Rare-earth doped nanoparticles are guest–host systems actively used as photon upconverters in which trivalent lanthanide ions are incorporated in a suitable host frame. The forbidden quality of 4f–4f transitions in lanthanides produces intermediate energy levels of very long lifetimes (up to tens of a millisecond), thus supporting the successive excitations (low energy photons) in the excited states of one lanthanide ion (sensitizers) as well as granting more eligible ion-ion interactions in the excited states which cause energy transference between two or more lanthanide ions (emitters) that radiate higher energy photons. Among the available lanthanide ions, Ytterbium (Yb) and Erbium (Er) are identified as excellent sensitizer and emitter for UC mechanism (Yao et al., 2016).
In 2017, Wenwu Liu et al., synthesized Nb2O5-covered TiO2 nanowires doped with Er3+/Yb3+ as photoanode and attained a high efficiency of 8.2% as an effect of making fermi levels in the TiO2 (Lu et al., 2019). Dutta et al. (2019) reported an enhanced PCE that proves the add-on of the Y2O3: Ho3+/Yb3+ UC nanophosphors into the TiO2 film can effectively outdo the photovoltaic performance. Although several attempts so far have been made to incorporate UC in photoanode, it is limited by the restriction in reducing the conductivity, transparency and dye adsorption on the semiconductor metal oxide layer. Another way to improve the PCE of DSSC without altering electrode work functions is to incorporate UC in the counter electrode. The legitimate selection of the host material also plays an important role in obtaining high UC luminescence, as it influences the UC process through either the phonon dynamics or the local crystal field occurrence around the rare-earth ions (Ambapuram et al., 2020a). So far examined host materials for UC are oxides, fluorides, vanadates, oxysulphides and oxyfluorides. In this series, Ceria (CeO2) and Yttria (Y2O3) as oxide host materials and Sodium Yttrium Fluoride (NaYF4) and Yttrium Fluoride (YF3) as fluoride host materials can be an interesting upconversion process in DSSCs since they have been well explored in upconverter loaded photoanode based DSSCs (Yao et al., 2016; Durairaj and Sabari Girisun, 2023). Thus, exploring the effect of the incorporation of UCs in the counter electrode of DSSC, will provide a deep understanding of the role and suitable position of UCs in DSSCs. The present work UC-loaded MoS2 counter electrode based DSSC can pave the way towards the realization of broadband Pt-free DSSCs. A straightforward hydrothermal technique has opted to produce four UC-incorporated MoS2 counter electrodes namely CeO2/NaYF4/YF3/Y2O3: Yb, Er and analyse the performance of different broadband Pt free DSSCs (Durairaj and Sabari Girisun, 2023).
High purity (Sigma-Aldrich, United States) Thiourea (CH4N2S), Ammonium molybdate tetrahydrate (NH4)6Mo7O24.4H2O), Cerium nitrate [Ce (NO3)2], Sodium fluoride (NaF), Erbium chloride hexahydrate (ErCl3), Ytterbium chloride hexahydrate (YbCl3), Yttrium chloride hexahydrate (YCl3), Ammonium fluoride (NH4F), Poly Vinyl Alcohol [PVA- (C2H4O)n] and Fluoride Tin Oxide substrate (FTO) were purchased and used without any prior purification.
The simple sol-gel procedure is employed to synthesise the oxide (CeO2: Yb, Er & Y2O3: Yb, Er) UCs. The precursor solution for CeO2: Yb, Er was prepared by dissolving 1 g of Cerium nitrate, 0.04 g of Erbium chloride, and 0.36 g of Ytterbium chloride in 10 mL of deionized water through constant stirring for 1 h. Then 1 g of PVA was added into the stirring solution and allowed for an extra 1 h stirring. Next, the precursor solution was transferred into the crucible and placed in the oven at 120°C for 12 h. Thus, the obtained pale-yellow colour powder was further annealed (600°C, 3 h). The same procedure was followed for Y2O3: Yb, Er by using yttrium chloride hexahydrate as the host precursor and yellow colour powder was obtained.
The hydrothermal method is used to synthesise the fluoride (NaYF4: Yb, Er & YF3: Yb, Er) UCs. The precursor solution for YF3: Yb, Er was prepared by taking 0.8 g of ammonium fluoride, 2.6 g of yttrium chloride, 0.36 g of ytterbium chloride and 0.04 g of erbium chloride in 50 mL DI water and after 1 h continuous stirring the solution was poured into a 75 mL Teflon-lined autoclave. The solution taken in 75 mL Teflon-lined autoclave was heated (180°C, 24 h) and the collected UC powder was further washed using DI water. Finally, the obtained UCs were dried (120°C, 12 h). The same procedure was followed for NaYF4: Yb, Er by using sodium fluoride as the host precursor and white colour powder was obtained.
Initially the FTO substrates were cleaned by ultrasonication using a solution containing acetone, DD water and isopropanol taken in a 1:1:1 ratio. The MoS2 precursor solution was prepared by dissolving 1 g of ammonium molybdate tetrahydrate and 2 g of thiourea in 50 mL of DI water through constant stirring for 1 h at 70°C. As-prepared CeO2: Yb, Er weighing 0.02 g were separately sonicated in 10 mL DI water for 30 min. Then, the sonicated UC solution was poured into MoS2 precursor solution and the stirring was continued for another 1 h without heat treatment. The pre-cleaned FTO plates (2 × 2 cm2) were placed at 45° angle against the wall of the Teflon container. Next, the solution was heated treated (180°C, 36 h) in a steel autoclave to obtain UC-loaded MoS2 thin films. Finally the DI water washed films were dried at 80°C for 12 h to achieve crystalline CEs (see in Figure 1). The same procedure was followed for other UCs and samples were labelled as CM (CeO2: Yb, Er), YOM (Y2O3: Yb, Er), NM (NaYF4: Yb, Er), and YM (YF3: Yb, Er).
The layout of the constructed DSSC and real-time image of the assembled DSSC are shown in Figure 2. The doctor-blade method was used to prepare mesoporous TiO2 photoanodes on an FTO substrate and the film was sintered at 450°C for 1 h. The prepared photoanodes were immersed for 24 h in 0.5 mM of N719 dye solution containing acetonitrile and tert-butanol taken in a 1:1 ratio. The DSSCs were fabricated by coupling the dye-sensitized TiO2 photoanodes and the UC incorporated MoS2 counter electrodes concurrently and the iodine electrolyte (containing Iodine, Lithium Iodide, 4-tert-butylpyridine and acetonitrile) was inoculated between the photoanode and counter electrode.
The X-ray diffraction analysis was done by an Empyrean instrument with Cu-Kα (λ = 1.54 Å) for the prepared UC-loaded MoS2 counter electrodes for crystallization and structural evaluation. Field emission scanning electron microscopy (FESEM) imaging by ZEISS Sigma instrument was used to examine the surface morphology of the films. The absorption properties of the prepared thin films were studied by UV-Vis-NIR spectrometer of wavelength from 175 nm to 3,300 nm (Shimadzu 3600). The emission features of the samples were analysed by a photoluminescence spectrometer. The fabricated DSSCs were illuminated by the solar simulator (150 W, Oriel) and their corresponding I-V characteristics were recorded. Enlitech’s IPCE spectral instrument was utilized to study the PCE of the DSSCs. Electrochemical impedance spectra in 1–106 MHz frequency range were obtained using a solar simulator and a potentiostat with an impedance analyser (AUTO-LAB12/FRA2).
XRD was used to analyse the structure and phase of UC-loaded MoS2 thin films. As shown in Figure 3, the indexed XRD peaks signify the existence of both MoS2 and upconverter nanoparticles validating the formation and deposition of nanocomposites in the films. Here in the nanocomposite thin films, MoS2 exist in 1T-2H mixed-phase in oxide UCs and 2H phase in fluoride UCs [JCPDS Card No. (65-1951)]. Further the presence of upconverter nanoparticles NaYF4: (Yb, Er) in hexagonal phase [JCPDS Card No. 16-0334], YF3: (Yb, Er) in orthorhombic phase [JCPDS Card No. 74-0911], CeO2: (Yb, Er) in cubic phase [JCPDS Card No. 04-0593] and Y2O3: (Yb, Er) in cubic phase [JCPDS Card No. 25-1200] is also well accorded. The Yb and Er lanthanide ions are completely incorporated into the host lattices of upconverters (NaYF4, YF3, CeO2, Y2O3) in the lattice site of Y and Ce appropriately, which justifies the non-emergence of any impurity segments of upconverters which proved the phase purity of nanocomposites (Ambapuram et al., 2020a; Durairaj and Sabari Girisun, 2023). In Figure 3, the (002), (004), (100), (102), (103), (106), (107), (200) and (205) planes refer to the 2H-hexagonal phase which is a semiconductor phase of MoS2. The (002) plane of 2H MoS2 located at 14.2° is identified as the basal plane, which exists in NM and YM films. Also, the CM and YOM films display a 1T-2H mixed phase of MoS2 which is identified by a lower peak shift at 11.2° that corresponds to the (002) basal plane of the 1T phase which is a metallic phase of MoS2 (Sabari Girisun et al., 2023). The (002) plane of MoS2 specified as the basal plane arises due to interlayer scattering of Mo–Mo and this plane appears with low intensity in all the films which shows that the MoS2 nanograins and upconverter nanoparticles were grown upon the MoS2 nanosheets during the hydrothermal process, consequently resulting in the poor crystallinity due to the lacking of the layered structure of MoS2 (Vikraman et al., 2018; Ghaleghafi et al., 2021). The active centres present in the basal plane of the 2H phase (14.2°) show low conductivity when compared to the edge plane active centres whereas the basal plane of the 1T phase (11.2°) is as catalytically active as edge planes (Das and Parida, 2021). Thus, the mixed 1T-2H phase MoS2 in CM and YOM films facilitate the fast electron transportation during the electrocatalytic reaction which can enable high current density in oxide UCs-loaded MoS2 CE-based DSSCs.

Figure 3. X-Ray Diffraction Pattern for UC-loaded MoS2 Thin films (


The surface morphology of UCs-loaded MoS2 films is shown in Figure 4 and the hydrothermal method of producing films reveals aggregated morphology with the uniform and continuous distribution of dissimilar small grains in all the films with an average particle size of 40–50 nm. The existence of UCs such as CeO2: (Yb, Er), Y2O3: (Yb, Er), NaYF4: (Yb, Er) and YF3: (Yb, Er) has a less significant impact on the nanograin morphology of MoS2 which is mainly due to the incorporation of low concentration of UCs but greatly induce the formation of porosity in the films. These MoS2 nanograins are linked to form bonded clusters throughout the films. These fused clusters commence the porosity and voids during the growth of the MoS2 films on the FTO substrate (Pujari et al., 2017). This porous grouping is observed in all the films and the distribution of MoS2 nanograins is associated with each other which enables good electron transportation in electrochemical performance (Gopakumar et al., 2019). MoS2 nanograins congregated all over the MoS2 nanosheets which is confirmed by the presence of lattice orientation for the layered structure of MoS2 indicated by the observed conservative plane of (002). Furtherly, the porous appearance of UCs-loaded MoS2 films would provide a large specific area having large reduction sites to subjugate high electrocatalytic activity that promotes the I−/I3− redox reaction which constitutes a better electrolyte-counter electrode interface and hence can improve the electrocatalytic activity (Kanjana et al., 2023).
The optical responses and Tauc’s plots of the pure MoS2 and UC-loaded MoS2 films are shown in Figures 5A, B. From the absorption spectra, UC-loaded MoS2 films disclose enhanced absorption behaviour in the visible region than pure MoS2. The thin films show considerable absorbance featuring in the NIR spectral range up to 1,200 nm due to the incorporation of UCs, whereas the absorbance is intensified in the UV and visible regions, which is effectively heightened by the upconversion process facilitated by Er and Yb ions in the CEs during the illumination (Gopakumar et al., 2019). The absorption spectrum displays absorption bands around UV (350–400 nm) and visible regions (450–550 nm) due to the electron-transfer activity coupled with extrinsic states of MoS2 such as the presence of defect states and oxygen vacancies developed because of the inclusion of UCs which created more sub-band gaps between them (Ghaleghafi et al., 2021). The absorption over the 600–800 nm region corresponds to the transition from the k-point of the Brillouin zone of MoS2 and the edge observed at 390 nm and 420 nm can be consigned to the MoS2 charge transition from the deep valence band to the conduction band (Hussain et al., 2015). The optical bandgap of the UCs-loaded MoS2 thin films was determined to be 3.80 eV (NM), 3.84 eV (YM), 3.79 eV (CM) and 3.75 eV (YOM) respectively by Tauc plot shown in Figure 5B. The incorporation of UCs altered the band configuration of MoS2 and the oxide UCs loaded MoS2 thin films show lower bandgap compared to the fluoride UCs loaded MoS2 thin films due to the presence of oxygen vacancies which facilitates electron conductivity in the film (Sabari Girisun et al., 2023). The outcomes confirm the insertion of upconverter nanoparticles in DSSCs can stimulate the large visible light absorption ability of N719 dye (400–700 nm), which guarantees an opening for the harvesting of NIR light, a major component of sunlight. Here the oxide UCs, CM, and YOM exhibit higher absorption intensity than fluoride UCs due to the high chemical and optical stability of oxide UCs which helps in improving the photovoltaic performance whereas the fluorides are often hygroscopic, which have a limitation towards stability.
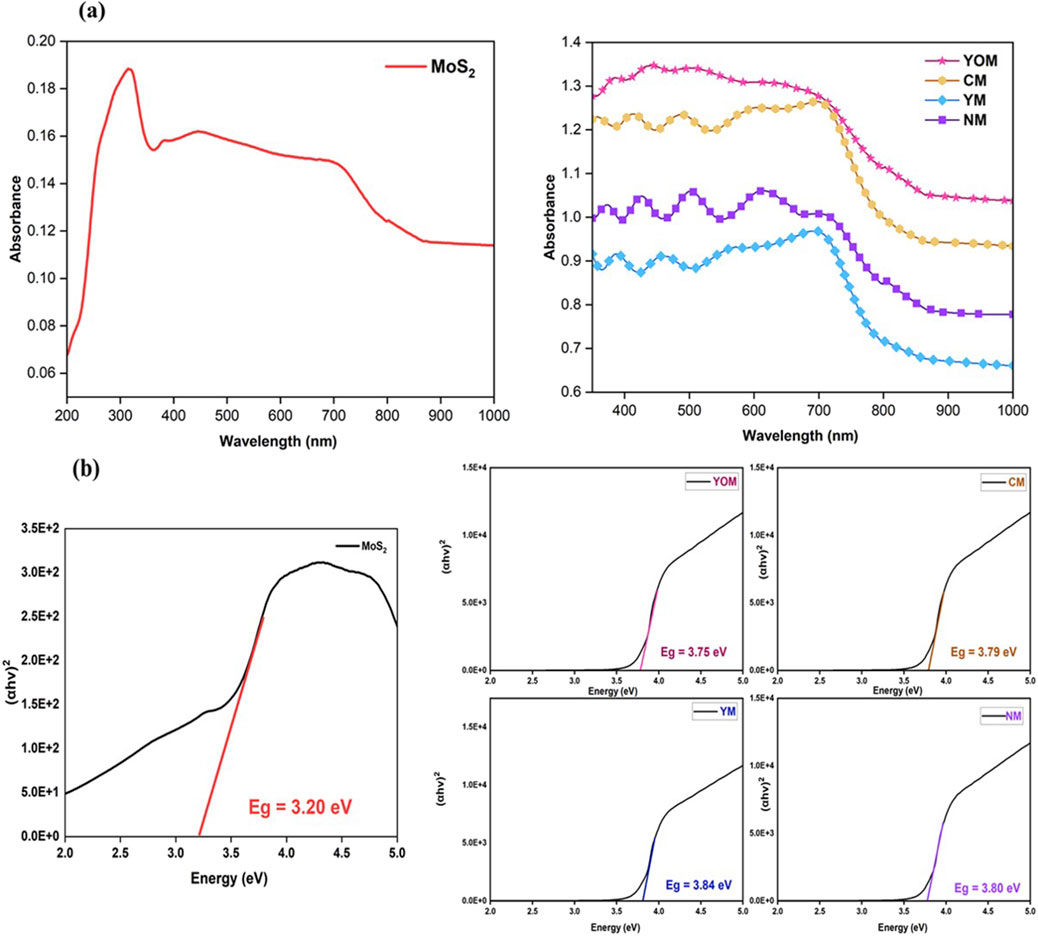
Figure 5. (A) Absorption spectra of MoS2 and UC-loaded MoS2 CEs 5. (B) Tauc plot of MoS2 and UC-loaded MoS2 CEs.
The emission spectrum of the prepared UC-loaded MoS2 thin films is shown in Figure 6. Under 980 nm excitation, the photoluminescence spectrum demonstrates strong blue (440–490 nm), green (520–540 nm) and weak red emissions (600–700 nm) which are attributed to 2H11/2, 4S3/2 → 4I15/2, 4F9/2 → 4I15/2 and 4I13/2 → 4I15/2 f-f transitions of emitter (Er3+) ions respectively (Li et al., 2014; Zhao et al., 2014). The emission peaks that occur at the visible region (570–580 nm) correspond to the surface states of MoS2 and also the peaks around 740 nm correspond to the valence band splitting caused by the strong spin-orbit coupling of MoS2 (Vikraman et al., 2018; Wilcoxon and Samara, 1996). Notably, the emission peaks are just falling in the absorption range of N719 dye, which has strong absorption in the visible range (400–700 nm). Here, the oxide UCs (CM, and YOM) deliver better emission intensity in the dye-absorbing region. The suppressed emission noticed for fluoride UCs is due to the happening of multiphonon relaxation in the meta-stable states as they have lower phonon energies. To understand the upconversion luminescence, the energy transfer mechanism is shown in Figure 7. The Yb3+ ions present in the UCs are excited from 2F7/2 energy (ground) state to the 2F5/2 energy (higher) state which has a large absorption coefficient in that state and they go back to the ground state by radiating the energy. The radiated energy from the sensitizer Yb3+ ions is transferred to various energy states of emitter Er3+ ions, which have ladder-like energy levels, resulting in the 4F9/2 and 4G11/2 levels. After undergoing a multiphonon relaxation, a fraction of the 4G11/2 level decays to the 4S3/2 level. Meantime, another photon is absorbed by Yb3+ ions and the energy is transferred one more time to the emitter Er3+ ions. Thus, the 4F9/2 and 4G11/2 energy states are populated in Er3+. Afterwards, the excited electrons undertake non-radiative processes and decay to the ground state of Er (4I15/2) (Dutta, 2021; Yao et al., 2016). Therefore, visible emissions are observed owing to this f-f transition of UCs. Thus, the emission studies clearly emphasize that the IR photons from the sunlight can be reabsorbed by the dye N719 in the form of visible photons by upconversion phenomenon and the absorption potential of N719 dye can be prolonged, consequently the performance of the DSSC can be improved. To witness this, the UC-loaded TiO2 film (Y2O3:Yb, Er) was prepared and sensitized with N719 dye and both the films with and without N719 dye sensitization were subjected to 980 nm excitation and the upconversion emission intensity was recorded. The emission intensity of N719-sensitized UC-loaded TiO2 film reduces as the dye has high absorbance in the visible region as shown in Figure 8. This evidences the visible emission from the upconverter nanophosphors are reabsorbed by the dye, hence the DSSC’s efficiency is enhanced.
The dynamics of the reactions and the internal resistance of DSSCs were analyzed by Electrochemical Impedance Spectroscopy (EIS). By fitting the data to an equivalent electrical circuit, the electrochemical parameters such as electron (τe) lifetime, series (RS) and charge transfer (RCT) resistance were measured. The obtained Nyquist plot and Bode-Phase plot are shown in Figures 9, 10 respectively. The equivalent circuit represented in Figure 9 determines the internal resistances of the films by fitting the EIS spectrum data in the ZSimpWin software.
The semicircles observed at low and high-frequency ranges in the Nyquist plot correspond to the redox reaction happening in the CE/electrolyte interface and the electron transportation occurring at the photoanode/electrolyte interface (RCT). Table 1 displays the calculated RS and RCT values for the UC-loaded MoS2 CE-based DSSCs. Here in the Nyquist plot, the third semicircle is absent which is usually observed and this is because of the negligible separation of the TiO2 photoanode and UC-loaded MoS2 counter electrode and also the used iodine liquid electrolyte is a low viscous electrolyte. MoS2-based CEs generally have higher RS values, which could be attributed to the minimal conductive value of the FTO substrate. Here, the Rs values of the UCs-loaded MoS2 counter electrodes are identical since Rs is correlated to the film on the substrate surface and the properties of each coated film obtained from the hydrothermal method on the FTO substrates are the same (Pujari et al., 2017). The YOM-based DSSC shows a lower RS (30.31 Ω) value than other UCs (NM, YM& CM) loaded DSSCs, which suggests the higher conductivity of YOM-MoS2 CE. It is an indicator to assess the electronic conductivity and the low RCT (1.76 Ω) value exhibited by YOM-based DSSC than other DSSC devices signifies a low electron recombination rate between CE and the electrolyte and also indicates the quicker mobility of electrons in the DSSC at the photoanode/electrolyte interface. This is mainly due to the in-depth exchange of hydrophilic act between the oxide UCs-loaded MoS2 (1T-2H phase) films and the FTO substrates (Yun et al., 2015). The hydrophilic nature of the mixed-phase of MoS2 shows good adhesion of the film onto the substrate. Also, the small RCT value of oxide UCs might be owed to the films exposed with more catalytic active sites (1T-2H phase) favourable for the ion transportation in the I−/I−3 electrolyte. The bode phase plots of UC-loaded MoS2-based DSSCs are shown in Figure 10. The characteristic peak frequency (fmax) for UC-loaded CEs was measured individually and the charge carrier lifetime (τe) was determined by using the relation, τe = 1/(2πfmax) and it was found to be 2.6 ms, 0.56 ms, 4.6 ms and 21.8 ms for NM, YM, CM, and YOM respectively. Here, the obtained results show that YOM-based DSSC has a longer electron lifetime which specifies the lower chance of recombination reactions to occur and thus exhibits excellent electrical conductivity and electrocatalytic activity than other DSSCs. Also, the existence of a large surface area with the porous nature of the YOM film prevents the electron-hole recombination reactions and possesses higher charge carrier concentration by having a large flat band potential and offers a prolonged lively pathway for faster electron transportation in the DSSC which necessarily enhance the JSC of the DSSC (Sabari Girisun et al., 2023).
Additionally, Mott–Schottky (M–S) analysis was recorded with a 1 kHz frequency for all the UC-loaded MoS2 films to study the band potentials and the curves are shown in Figure 11. All the films exhibited a positive slope structure, which specifies that NM, YM, CM, and YOM films are n-type semiconductor electrodes in which electrons are the majority charge carriers. The flat band potentials (Vfb) were calculated by taking slopes in the linear portion of the mott-Schottky curves. The estimated flat band potentials (Vfb) for NM, YM, CM and YOM are −0.35 V, −0.22 V, −0.59 V and −0.72 V respectively. Generally, the flat band potential is directly associated to the charge carrier density in the semiconductor material. Here, it is evident from the plot, that YOM and CM oxide UCs show higher Vfb values than fluoride UCs which confirms the presence of a higher concentration of charge carriers in oxide UC films that arise mainly due to the presence of oxygen vacancies created by the oxide UCs in the MoS2 films. Among the chosen films, YOM reveals a larger flat band potential of −0.72 V which aids in improved electron conductivity and electrocatalytic activity and enhances the overall efficiency of the DSSC (Kumari and Kumar, 2023).
The I-V characteristics shown in Figure 12 for UC-loaded MoS2 CE-based DSSC under dark conditions are performed to study the process of current leakage, i.e., dark current due to the electron recombination in the DSSCs. The dark current for YOM (0.011 mA cm−2) and CM (0.012 mA cm−2) is smaller than for fluoride UCs-loaded DSSCs (NM & YM), as shown in the insert image in Figure 12. This indicates that the YOM and CM oxide UCs based DSSC can capably overturn the electron recombination process between the FTO substrate and the triiodide electrolyte and suggest a better photovoltaic performance under light condition by constraining the dark current and improving the short-circuit current. To analyze the impact of upconversion on photovoltaic performance of DSSCs, the UC-loaded MoS2 CE-based DSSCs fabricated with 0.25 cm2 active area were placed under Air Mass 1.5 illumination with input power of 85 mW cm−2. To facilitate upconversion-induced broadband absorption, an aluminium reflector beneath the CE of the DSSC to minimize the photon loss was placed. Figure 13 shows the light J-V curves of the DSSCs with UC-loaded MoS2 CE. The corresponding device characteristics (i.e., open-circuit (Voc) voltage, short-circuit current (Jsc) density, fill factor (
In summary, the fluoride and oxide upconverter nanoparticles were successfully synthesized and introduced into the MoS2 counter electrodes, and the DSSCs were fabricated. Among the DSSCs, YOM-based DSSC Y2O3: (Yb, Er) shows high photovoltaic performance with 7.19% efficiency. The higher photocurrent generation of YOM-based DSSC is mainly due to the high electrocatalytic activity showed by low RCT and RS and the longer electron lifetime, which suggests the low electron recombination rate inside the DSSC. The absorption spectra demonstrate the wide-ranging absorption to NIR, i.e., up to 1,200 nm due to the inclusion of sensitizer (Yb3+ ions) in UCs and the PL spectra show that light emission is observed in the visible region by the non-radiative process of the emitter (Er3+ ions) where the N719 dye can captivate them for more electron excitation. These outcomes can deliver an effective understanding of the upconverter nanoparticles with the NIR light upconverting into visible light for high-performance dye-sensitized photovoltaic devices. It is intriguingly assured the UC-loaded MoS2 thin films can be used as an effective counter electrode for the possible realization of upconverter DSSC and this study provides an aspect for expanding the research to incorporating the UCs in DSSC for broadband absorption.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
JK: Data curation, Investigation, Visualization, Writing–original draft, Writing–review and editing. MD: Conceptualization, Methodology, Writing–review and editing. SA: Software, Validation, Writing–review and editing. TS: Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors declare that financial support was received for research from the project grant MHRD RUSA 2.0 and DST SERB SURE (SUR/2022/000885).
The authors JK and TS acknowledge MHRD RUSA 2.0 and DST SERB SURE (SUR/2022/000885) for the project grant.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ambapuram, M., Maddala, G., Simhachalam, N. B., Sripada, S., Kalvapalli, S., Pedda, V. S. Y., et al. (2020a). Highly effective SnS composite counter electrode sandwiched bi-function CeO2: Er3+/Yb3+ assisted surface modified photoelectroded dye sensitized solar cell exceeds 9.5% efficiency. J. Sol. Energy. 207, 1158–1164. doi:10.1016/j.solener.2020.07.030
Ambapuram, R. R., Maddala, G., Godugunuru, S., Yerva, P. V. S., and Mitty, R. (2020b). Effective upconverter and light scattering dual function LiYF4: Er3+/Yb3+ assisted photoelectrode for high performance cosensitized dye sensitized solar cells. ACS Appl. Electron. Mater. 2 (4), 962–970. doi:10.1021/acsaelm.0c00014
Das, G. S., and Parida, K. (2021). One step towards the 1T/2H-MoS2 mixed phase: a journey from synthesis to application. Mater. Chem. Front. 5 (5), 2143–2172. doi:10.1039/D0QM00802H
Dubey, R. S., Jadkar, S. R., and Bhorde, A. B. (2021). Synthesis and characterization of various doped TiO2 nanocrystals for dye-sensitized solar cells. ACS omega 5, 3470–3482. doi:10.1021/acsomega.0c01614
Durairaj, T. C. S. G., and Sabari Girisun, T. C. (2023). Demonstration of enhanced saturable absorption in upconverter integrated MoS2 heterostructure thin film using nanopulsed green laser. Phys. Status Solidi B Basic Res. 260 (11), 2300216. doi:10.1002/pssb.202300216
Dutta, J., Rai, V. K., Durai, M. M., and Thangavel, R. (2019). Development of Y2O3: Ho3+/Yb3+ upconverting nanophosphors for enhancing solar cell efficiency of dye-sensitized solar cells. IEEE J. Photovolt. 9 (4), 1040–1045. doi:10.1109/JPHOTOV.2019.2912719
Dutta, V. K. R. (2021). Upconverting BiYO3 nanophosphors in DSSCs applications. Opt. Laser Technol. 140, 107087. doi:10.1016/j.optlastec.2021.107087
El Assyry, A., Rafiq, I., Rbaa, M., Derouiche, A., and Lakhrissi, B. (2022). Optical and photovoltaic properties of new synthesized quinoxaline-2, 3 dione derivatives for efficient dye sensitized solar cell application. Trends Sci. 19 (19), 6173. doi:10.48048/tis.2022.6173
Fang, X., Ma, T., Guan, G., Akiyama, M., Kida, T., and Abe, E. (2004). Effect of the thickness of the Pt film coated on a counter electrode on the performance of a dye-sensitized solar cell. J. Electroanal. Chem. 570 (2), 257–263. doi:10.1016/j.jelechem.2004.04.004
Ghaleghafi, E., Rahmani, M. B., and Wei, Z. H. (2021). Photoluminescence and UV photosensitivity of few-layered MoS2 nanosheets synthesized under different hydrothermal growth times. J. Mater. Sci. 56, 11749–11768. doi:10.1007/s10853-021-06083-x
Gopakumar, G., Nair, S. V., and Shanmugam, M. (2019). Hydrothermal processed heterogeneous MoS2 assisted charge transport in dye sensitized solar cells. Appl. Phys. 125 (12), 822. doi:10.1007/s00339-019-3126-3
Gurulakshmi, A. M., Siddeswaramma, G., Susmitha, K., Subbaiah, Y. P. V., Narayana, T., and Raghavender, M. (2020). Electrodeposited MoS2 counter electrode for flexible dye sensitized solar cell module with ionic liquid assisted photoelectrode. J. Sol. Energy. 199, 447–452. doi:10.1016/j.solener.2020.02.047
Hussain, S. F. S., Vikraman, D., Mane, R. S., Joo, O. S., Naushad, M., Jung, J., et al. (2015). High-performance platinum-free dye-sensitized solar cells with molybdenum disulfide films as counter electrodes. ChemPhysChem 16 (18), 3959–3965. doi:10.1002/cphc.201500644
Jeong, H., Kim, J. Y., Koo, B., Son, H. J., Kim, D., and Ko, M. J. (2016). Rapid sintering of MoS2 counter electrode using near-infrared pulsed laser for use in highly efficient dye-sensitized solar cells. J. Power Sources. 330, 104–110. doi:10.1016/j.jpowsour.2016.09.002
Jeong, T., Ham, S. Y., Koo, B., Lee, P., Min, Y. S., Kim, J. Y., et al. (2019). Transparent 3 nm-thick MoS2 counter electrodes for bifacial dye-sensitized solar cells. J. Ind. Eng. Chem. 80, 106–111. doi:10.1016/j.jiec.2019.07.037
Kanjana, W. M., Lunnoo, T., Laokul, P., Chaiya, I., Ruammaitree, A., Wongjom, P., et al. (2023). One-step hydrothermal synthesis and electrocatalytic properties of MoS2/activated carbon composite derived from shallots. J. Appl. Electrochem. 53 (12), 2311–2320. doi:10.1007/s10800-023-01921-z
Kumari, R., and Kumar, R. (2023). Exploring the influence of temperature and time on the formation and properties of 3D flower-like MoS2 nanostructures synthesized via hydrothermal method. ECS J. Solid State Sci. Technol. 12 (9), 097004. doi:10.1149/2162-8777/acf8f1
Li, L., Yang, Y., Fan, R., Chen, S., Wang, P., Yang, B., et al. (2014). Conductive upconversion Er, Yb-FTO nanoparticle coating to replace Pt as a low-cost and high-performance counter electrode for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 6 (11), 8223–8229. doi:10.1021/am5009776
Lu, X. Q., Zhang, H. U., Li, R., Zhang, M., and Guo, M. (2019). Nb2O5 coating on the performance of flexible dye sensitized solar cell based on TiO2 nanoarrays/upconversion luminescence composite structure. Inorg. Mater. 34 (6), 590–598. doi:10.15541/jim20180406
Pujari, R. B., Lokhande, A. C., Shelke, A. R., Kim, J. H., and Lokhande, C. D. (2017). Chemically deposited nano grain composed MoS2 thin films for supercapacitor application. J. Colloid Interface Sci. 496, 1–7. doi:10.1016/j.jcis.2016.11.026
Sabari Girisun, T. C., Durairaj, M., Vijaya, S., and Anandan, S. (2023). 1T and 2H phase molybdenum disulfide as a counter electrode for Pt free dye-sensitized solar cells. Mater. Sci. Eng. B 287, 116123. doi:10.1016/j.mseb.2022.116123
Sarkar, A., and Bera, S. (2020). Chakraborty, CoNi2S4-reduced graphene oxide nanohybrid: an excellent counter electrode for Pt-free DSSC. J. Sol. Energy. 208, 139–149. doi:10.1016/j.solener.2020.07.075
Shan, G. P. D. (2010). Near-infrared sunlight harvesting in dye-sensitized solar cells via the insertion of an upconverter-TiO2 nanocomposite layer. J. Adv. Mater. 22 (39), 4373–4377. doi:10.1002/adma.201001816
Tadge, R. S. Y., Vishwakarma, P. K., Rai, S. B., Chen, T. M., Sapra, S., Ray, S., et al. (2020). Enhanced photovoltaic performance of Y2O3: Ho3+/Yb3+ upconversion nanophosphor based DSSC and investigation of color tunability in Ho3+/Tm3+/Yb3+ tridoped Y2O3. J. Alloys Compd. 821, 153230. doi:10.1016/j.jallcom.2019.153230
Tohluebaji, N., Siri, R., Muensit, N., Putson, C., Channuie, P., Porrawatkul, P., et al. (2024). Hydrophobic and optical properties of P (VDF-HFP) nanofiber filled with nickel (II) chloride hexahydrate for dye-sensitized solar cells application. Trends Sci. 21 (9), 8762. doi:10.48048/tis.2024.8762
Vikraman, A. A. A., Hussain, S., Shrestha, N. K., Jeong, S. H., Jung, J., Patil, S. A., et al. (2019). Design of WSe2/MoS2 heterostructures as the counter electrode to replace Pt for dye-sensitized solar cell. ACS Sustain. Chem. Eng. 7 (15), 13195–13205. doi:10.1021/acssuschemeng.9b02430
Vikraman, S. A. P., Hussain, S., Mengal, N., Kim, H. S., Jeong, S. H., Jung, J., et al. (2018). Facile and cost-effective methodology to fabricate MoS2 counter electrode for efficient dye-sensitized solar cells. Dyes Pigm 151, 7–14. doi:10.1016/j.dyepig.2017.12.037
Wei, W., Sun, K., and Hu, Y. H. (2016). An efficient counter electrode material for dye-sensitized solar cells—flower-structured 1T metallic phase MoS2. J. Mater. Chem. 4 (32), 12398–12401. doi:10.1039/C6TA04743B
Wilcoxon, P. P. N., and Samara, G. A. (1996). Synthesis and optical properties of MoS2 nanoclusters. Mater Res. Soc. Symp. Proc. 452, 371. doi:10.1557/PROC-452-371
Yang, Q., Duan, J., Yang, P., and Tang, Q. (2016). Counter electrodes from platinum alloy nanotube arrays with ZnO nanorod templates for dye-sensitized solar cells. Electrochim. Acta. 190, 648–654. doi:10.1016/j.electacta.2015.12.206
Yao, N., Huang, J., Fu, K., Deng, X., Ding, M., and Xu, X. (2016). Rare earth ion doped phosphors for dye-sensitized solar cells applications. RSC Adv. 6 (21), 17546–17559. doi:10.1039/C5RA27033B
Yoon, C., Vittal, R., Lee, J., Chae, W.-S., and Kim, K.-J. (2008). Enhanced performance of a dye-sensitized solar cell with an electrodeposited-platinum counter electrode. Electrochim. Acta. 53 (6), 2890–2896. doi:10.1016/j.electacta.2007.10.074
Younas, M., Baroud, T. N., Gondal, M. A., Dastageer, M. A., and Giannelis, E. P. (2020). Highly efficient, cost-effective counter electrodes for dye-sensitized solar cells (DSSCs) augmented by highly mesoporous carbons. J. Power Sources. 468, 228359. doi:10.1016/j.jpowsour.2020.228359
Yu, F., Shi, Y., Shen, X., Yao, W., Han, S., and Ma, J. (2018). Three-dimensional MoS2-nanosheet-based graphene/carbon nanotube aerogel as a Pt-free counter electrode for high-efficiency dye-sensitized solar cells. ACS Sustain. Chem. Eng. 6 (12), 17427–17434. doi:10.1021/acssuschemeng.8b03143
Yun, S., Liu, Y., Zhang, T., and Ahmad, S. (2015). Recent advances in alternative counter electrode materials for Co-mediated dye-sensitized solar cells. Nanoscale 7 (28), 11877–11893. doi:10.1039/C5NR02433A
Keywords: MoS2 counter electrode, upconverter nanoparticles, DSSC, broadband absorption, upconversion (UC) materials
Citation: Kawya J, Durairaj M, Anandan S and Sabari Girisun TC (2025) Upconverter loaded MoS2 counter electrode for broadband dye-sensitized solar cell applications. Front. Chem. 12:1511600. doi: 10.3389/fchem.2024.1511600
Received: 17 October 2024; Accepted: 23 December 2024;
Published: 15 January 2025.
Edited by:
Jianhua Han, Civil Aviation University of China, ChinaReviewed by:
Auttasit Tubtimtae, Kasetsart University, ThailandCopyright © 2025 Kawya, Durairaj, Anandan and Sabari Girisun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: T. C. Sabari Girisun, c2FiYXJpZ2lyaXN1bkBiZHUuYWMuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.