- 1Department of Pharmacy, University of Salerno, Fisciano, Italy
- 2Department of Agricultural, Food and Forest Sciences, University of Palermo, Palermo, Italy
- 3Department of Soil, Plant, and Food Sciences, University of Bari Aldo Moro, Bari, Italy
- 4Department of Agriculture, Forestry, Food and Environmental Sciences, University of Basilicata, Potenza, Italy
Background: The basidiomycetes Pleurotus eryngii var. ferulae Lanzi and P. eryngii var. elaeoselini Venturella et al. belong to the P. eryngii species complex, acting as facultative biotrophs in association with members of Apiaceae family, i.e., Ferula communis L. and Elaeoselinum asclepium L., respectively. The consumption of these fungi has rapidly increased in recent decades, not only thanks to their nutritional properties and pleasant flavor, but also for their bioactive and medicinal properties.
Methods: A quantitative study of their hydroalcoholic extracts was carried out by liquid chromatography-mass spectrometry. The potential antimicrobial activity of the extracts was also tested against some phytopathogenic bacteria [Clavibacter michiganensis and Bacillus megaterium (Gram-positive), Pseudomonas viridiflava, Xanthomonas campestris, and Escherichia coli (Gram-negative)] and fungi (Aspergillus fumigatus, Penicillium italicum, Monilinia laxa, Botrytis cinerea, Cadophora sp., and Sclerotinia sclerotiorum).
Results: The chemical analysis allowed the identification of secondary metabolites belonging to different classes, as flavonoids, organic acids, amino acids, carbohydrates, vitamins, nucleic acids, fatty acids, and triterpenoids. Both extracts demonstrated antimicrobial activity against of the most tested microorganisms.
Conclusion: The results can broaden the knowledge on the possible use of these fungal species in the agricultural sector.
1 Introduction
The basidiomycetes Pleurotus eryngii var. ferulae Lanzi and P. eryngii var. elaeoselini (Venturella et al., 2015) belong to the P. eryngii species complex, acting as facultative biotrophs in association with Ferula communis L. and Elaeoselinum asclepium L. (Apiaceae), respectively. These edible mushrooms originate from the Mediterranean and are easily cultivated in many parts of Europe for edible purposes (Angeles Flores et al., 2022). The consumption of these fungi has rapidly increased in recent decades, not only thanks to their nutritional properties and pleasant flavor, but also for their bioactive and medicinal and health-enhancing properties (Castronuovo et al., 2019). Recent studies on these two basidiomycetes showed that they exhibited important medicinal properties such as antioxidant, antimicrobial, antidiabetic, anti-inflammatory, immunomodulatory, antihypercholesterolemic, antihypertensive, antimicrobial, hepatoprotective and anti-aging properties and in vitro antitumor effect on the human colon cancer cell lines, HCT116 (Cateni et al., 2020, 2022; Venturella et al., 2021). These activities are attributable to the presence of their mycochemicals with effects depending on their chemical nature; the nature and distribution of these metabolites differs depending on the fungal species (Venturella et al., 2021). Among the most important compounds found in P. eryngii there are certainly polysaccharides, especially α- and β-glucans, but also heteroglycans, peptidoglycans, and polysaccharide-protein complexes (Cateni et al., 2018). They are mainly responsible for the immunomodulatory effects being able to bind to specific membrane receptors, stimulating specific inflammatory responses (Elkhateeb, 2020). Some fungal metabolites (e.g., ergosterol, ergostane-type sterols, etrophasterols E and F, bisabolane-type sesquiterpenes eryngiolide A, pentacyclic triterpenoids) also possess immunomodulatory, as well as anti-inflammatory, antioxidant, and antitumor properties (El Enshasy and Hatti-Kaul, 2013). Pleurotus species are rich in proteins, peptides and lecithins that exhibit cytotoxic, antitumor, immunomodulatory, and antiproliferative properties through various mechanisms, such as binding to specific membrane polysaccharides (Zhao et al., 2020). Moreover, phenolic compounds and medium-long chain fatty acids can exert antioxidant activity (Elkhateeb, 2020).
Today, the massive and ever-increasing use of industrial agrochemicals has become a significant problem for environmental quality and human health. The serious problem of resistance to the most common used pesticides poses a major challenge for the protection of crops most susceptible to bacterial and fungal attack (Devi et al., 2022). For this reason, the scientific research towards is aimed to the discovery of compounds of non-synthetic origin that can contribute to effective control of agricultural pathogens without causing serious problems for the ecosystem and human health. The available literature reports the activity of fungal metabolites against the growth and proliferation of some phytopathogens. Pleurotus eryngii (strain AL142PE) was reported as a potential biological limiter of Phytophthora nicotianae, Fusarium oxysporum f. sp. radicis-lycopersici, F. oxysporum f. sp. lycopersici, F. solani, Sclerotinia minor, S. sclerotiorum, Athelia rolfsii and Verticillium dahliae (D’Ambrosio et al., 2022). Furthermore, an eco-friendly nanomaterial derived from a P. eryngii extract resulted able to inhibit the growth Neoscytalidium dimidiatum, V. dahliae, Bipolaris sorokiniana (Acay et al., 2024). These studies therefore suggest a potential use of P. eryngii extracts as effective and, at the same time, environmentally friendly biocontrol agents.
This research reports data on the chemical composition, achieved by UPLC-HRMSMS, of the hydroalcoholic extracts of both P. eryngii varieties, and on their possible antimicrobial activity against some phytopathogenic bacterial (Clavibacter michiganensis, Bacillus megaterium, Pseudomonas viridiflava, Xanthomonas campestris, and Escherichia coli) and fungal strains (Aspergillus fumigatus, Penicillium italicum, Monilinia laxa, Botrytis cinerea, Cadophora spp., and Sclerotinia sclerotiorum).
2 Materials and methods
2.1 Material and extraction
Basidiomata of P. eryngii var. elaeoselini and P. eryngii var ferulae were collected in autumn 2023 on the Madonie Mts (N. Sicily) in the surroundings of the village of Collesano (province of Palermo), 37°55′40″N, 13°56′51″E, 559 m a.s.l. Whole basidiomes were collected and cleaned of earthy residues with the help of a small knife. Then they were wrapped in aluminum paper and transported to the laboratory for identification. For verification of macro- and microscopic characters, reference was made to the publication by Venturella et al. (2015) and the use of a binocular and Leica light microscope. After identification, the basidiomes were cut into thin slices, dried using a laboratory desiccator and reduced to powder using a Bimby® TM6. The powders were subjected to a solvent extraction with 70% ethanol. The quantities subjected to extraction were 4.00 g for both basidiomata. The extraction was carried out by maceration in glass flasks using 100 mL of solvent for each g of powder. The flasks filled with powder and solvent were stirred using a magnet. Each extraction cycle lasted 5 days and three extraction cycles were carried out to maximize the extraction. Once the extracts were combined, the solvent was removed using a rotary evaporator and the extract was freeze-dried to remove residual water and stored in hermetically sealed falcons away from heat, light and humidity. The freeze-dried extracts were weighed and the extraction yields were calculated: 0.83 g of extract were obtained from P. eryngii var. ferulae and 0.81 g from P. eryngii var. elaeoselini, accounting in both cases for 0.02%. Molecular analysis of Pleurotus eryngii var. ferulae and P. eryngii var. elaeoselini has already been done in a previous paper (Zervakis et al., 2001).
2.2 Chemical analysis
The extracts were analyzed by LC-ESI-HR-MS, by using a Q Exactive: hybrid quadrupole-Orbitrap mass spectrometer (Thermo Fisher, Waltham, MA, United States), operating in negative ion mode following Crescenzi et al. (2023), with some modifications. LC-MS analysis was carried out on a Luna 5 μm C18 100 Å (150 mm × 2 mm) column (Phenomenex, Aschaffenburg, Germany), using a flow rate of 0.2 mL/min. A binary solvent system was utilized [eluent A: H2O with 0.1% HCOOH (99.9:0.1, v/v) and eluent B: H3CN with 0.1% formic acid (99.9:0.1, v/v)]. The HPLC gradient started at 5% B, and after 30 min, percent B was at 95%; this percentage was maintained for another 5 min before coming back to the initial percentage. The autosampler was set to inject 5 μL of each extract (1 mg/mL). The HESI source parameters were the following: capillary voltage −0.2 V; tube lens voltage +50 V; ion source temperature 300.01°C; sheath and auxiliary gas flow (N2), 50.24 and 10.25; and sweep gas 0.00. The full range m/z adapted to the acquisition of MS spectra was 90–1,400. For the fragmentation study, a data-dependent scan was set up, through which the precursor ions corresponding to the most intensive peaks were fragmented in the MS analysis with a collision energy of 30%. Xcalibur software version 2.2 was employed for instrument control, data acquisition, and data analysis.
2.3 Antibacterial activity
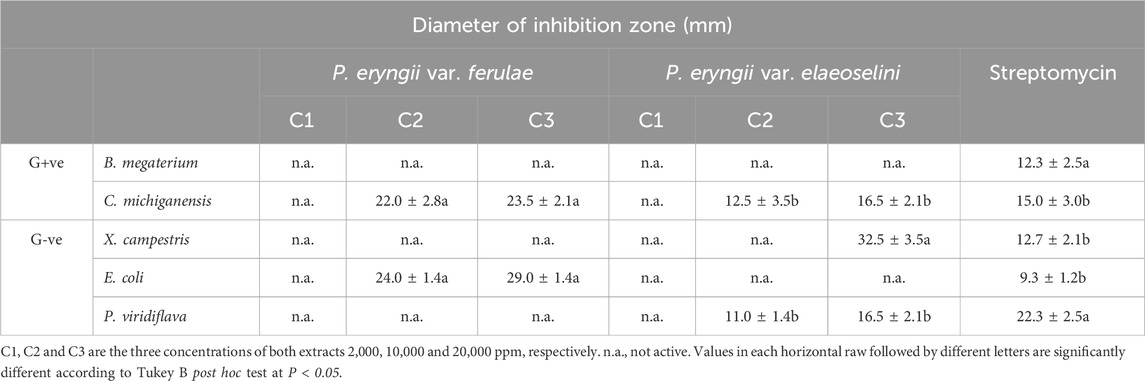
Five bacterial strains were used for this study, two Gram-positive (G+ve) Clavibacter michiganensis Smith and Bacillus megaterium de Bary and three Gram-negative (G-ve) Pseudomonas viridiflava (Burkholder) Dowson, Xanthomonas campestris Pammel and Escherichia coli Migula. All tested bacteria were identified by morphological and molecular methods, stored at 4°C as pure culture in the collection of the Department of Agricultural, Forestry, Food and Environmental Sciences (DAFE), University of Basilicata, Potenza, Italy. All fungal isolated were recultured in King B media (KB). The antibacterial activity was evaluated following the Diffusion Method (Bhunia et al., 1988) using King B (KB) as nutrient media. For the assay, a bacterial suspension (108 CFU/mL) for each strain was prepared by turbidometry in soft agar 0.7%. Four mL of each suspension were poured onto KB petri dishes (Ø 90 mm). Ten µL of three concentrations (C1: 2,000 ppm; C2: 10,000 ppm; C3: 20,000 ppm) of both extracts were applied over agar surface. Streptomycin (100 μg/mL) was used as a positive control. All plates were incubated at 37°C for 24 h. The antibacterial activity was determined by measuring the diameter of the inhibition zone in mm.
2.4 Antifungal activity
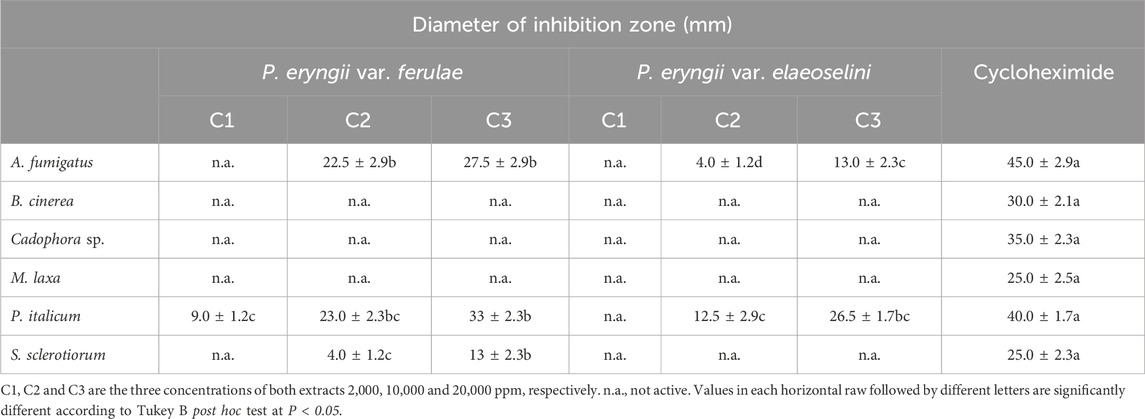
The antifungal activity was tested against some phytopathogenic fungi, Aspergillus fumigatus Fresen, Penicillium italicum Wehmer, Monilinia laxa (Aderh. & Ruhland) Honey, Botrytis cinerea Pers., Cadophora sp. Lagerb. & Melin and Sclerotinia sclerotiorum (Lib.) de Bary. All studied fungi strains were identified by morphological and molecular methods, stored at 4°C as pure culture in the collection of DAFE. All fungal isolated were recultured in Potato Dextrose Agar (PDA). The antifungal activity was evaluated using the agar well diffusion method as reported by Elshafie et al. (2012). Twenty µL of three concentrations (C1: 2,000 ppm; C2: 10,000 ppm; C3: 20,000 ppm) of both extracts were applied to each well: then all plates were inoculated singularly with 0.5 mm agar disk with each fungus and incubated at 22°C ± 2°C for 96 h. Cycloheximide 100 μg/mL was used as a positive control. The antifungal activity was determined by measuring the diameter of eventual inhibition zones (mm).
2.5 Antioxidant activity
2.5.1 DPPH assay
The antioxidant activity was determined using the stable 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical method as reported by Brand-Williams et al. (1995), with some modifications. The analysis was performed in cuvettes by adding 25 μL of a solution of the EOs in MeOH to 975 μL of a DPPH solution (60 μM), which was prepared daily and kept in the dark to have a final volume of 1 mL in a straight-sided cuvette. Methanol alone was used as a blank, and a cuvette with 1 mL of DPPH solution (60 μM) was used as a control. Absorbance at 515 nm was measured in the spectrophotometer Thermo scientific Multiskan GO (Thermo Fischer Scientific, Vantaa, Finland) after 45 min. The absorbance of DPPH without the antioxidant (control sample) was used for a baseline measurement. The percent inhibition of free radical formation by DPPH (I%) was calculated as follows:
where Ablank is the absorbance of the control reaction (containing all reagents except the test compound) and Asample is the absorbance of the test compound read at 515 nm after 45 min. The scavenging activity was expressed as the 50% effective concentration (IC50), which is defined as the sample concentration (mg mL−1) necessary to inhibit DPPH radical activity by 50% after 45 min of incubation. Experiments were performed in triplicate and the results are expressed as the mean ± standard deviation. Trolox was used as the standard reference.
2.5.2 FRAP assay
The FRAP assay (FRAP is an acronym for “Ferric Ion Reducing Antioxidant Power”) was performed following the protocol of Benzie and Strain (1996). A FRAP reagent is a solution consisting of 23 mM acetate buffer (pH 3.6), 10 mM of tripyridyl triazine (TPTZ) in 40 mM of HCl, and 20 mM of FeCl3 (in a 10:1:1 ratio). Different concentrations of ferrous sulfate heptahydrate, FeSO4 7H2O, in a range from 1 mM to 0.1 mM were prepared to obtain the calibration curve. The reaction was carried out for each sample in a final volume of 272 µL in wells. The reaction mixture was incubated at 37°C for 30 min in dark conditions. The absorbance of the blank, consisting of FRAP alone and monitored spectrophotometrically at the wavelength of 593 nm, was subtracted from the absorbance of the FRAP with the sample to determine the FRAP value for each sample. The FRAP values were determined using the FeSO4 7H2O calibration curve (Amamcharla and Metzger, 2014) and expressed as μmol Fe2+/g of hydroalcoholic extract. Trolox was used as the standard reference.
2.5.3 ABTS•+ assay
The 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) test was carried out following the method of Ud-Daula et al. (2016). In triplicate, 10 μL of the different concentrations of EOs dissolved previously in methanol (final concentrations, ranging from 0.1 to 40 mg/mL) and 190 μL ABTS• were added to the wells for analysis. Amounts of 10 μL of PBS and 190 μL of ultrapure water were added to the wells for the control. The results are presented as Trolox equivalent antioxidant capacity (TEAC μmol/g). Ascorbic acid (vitamin C) was used as the standard reference.
2.6 Statistical analysis
For the statistical analysis, the data were analyzed via a one-way ANOVA using Statistical Package for the Social Sciences (SPSS) version 13.0, 2004 (Chicago, IL, United States). The Tukey’s B post hoc multiple comparison test was applied to determine the significance level with a probability of p ≤ 0.05.
Moreover, the tested bacteria and fungi strains were considered as original variables and subjected, after normalization, for doing Principal Component Analysis (PCA). Hierarchical Cluster Heatmap analysis of the same strains, was also conducted. The statistical analyses were performed using Matlab software with three principal components (PC) and the number of clusters was determined using scaled distances in the Hierarchical Cluster Heatmap. PCA and Hierarchical Cluster Heatmap were used to understand the similarity between the tested samples (Pleurotus eryngii var elaeoselini and Pleurotus eryngii var. ferulae at three different concentrations) and the two standard reference antibiotics (streptomycin and cycloheximide), in relation to the variables considered above.
3 Results and discussion
3.1 Chemical composition
The LC-HRESIMS/MS analyses of hydroalcoholic extracts led to the separation and annotation of the most constituents (Figure 1).
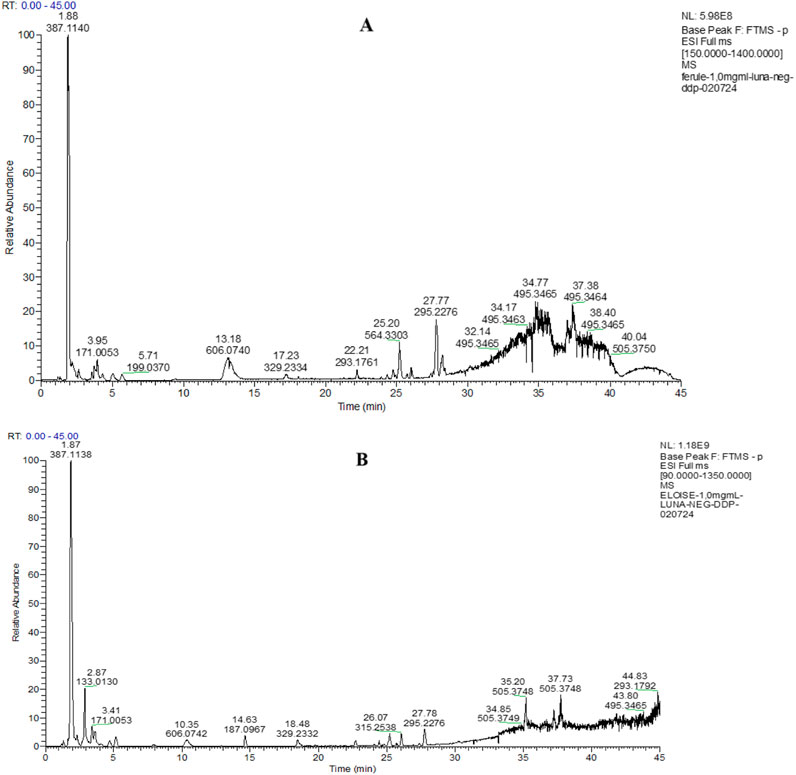
Figure 1. Full scan LC-MS chromatograms (negative ion HRESIMS) of hydroalcoholic extracts of P. eryngii var. ferulae (A) and P. eryngii var. elaeoselini (B).
Overall, 23 components (Table 1) were identified, belonging to several representative classes of constituents, mainly organic acids (peaks 5,6,9–13) and carboxylic acids (peaks 19–22).
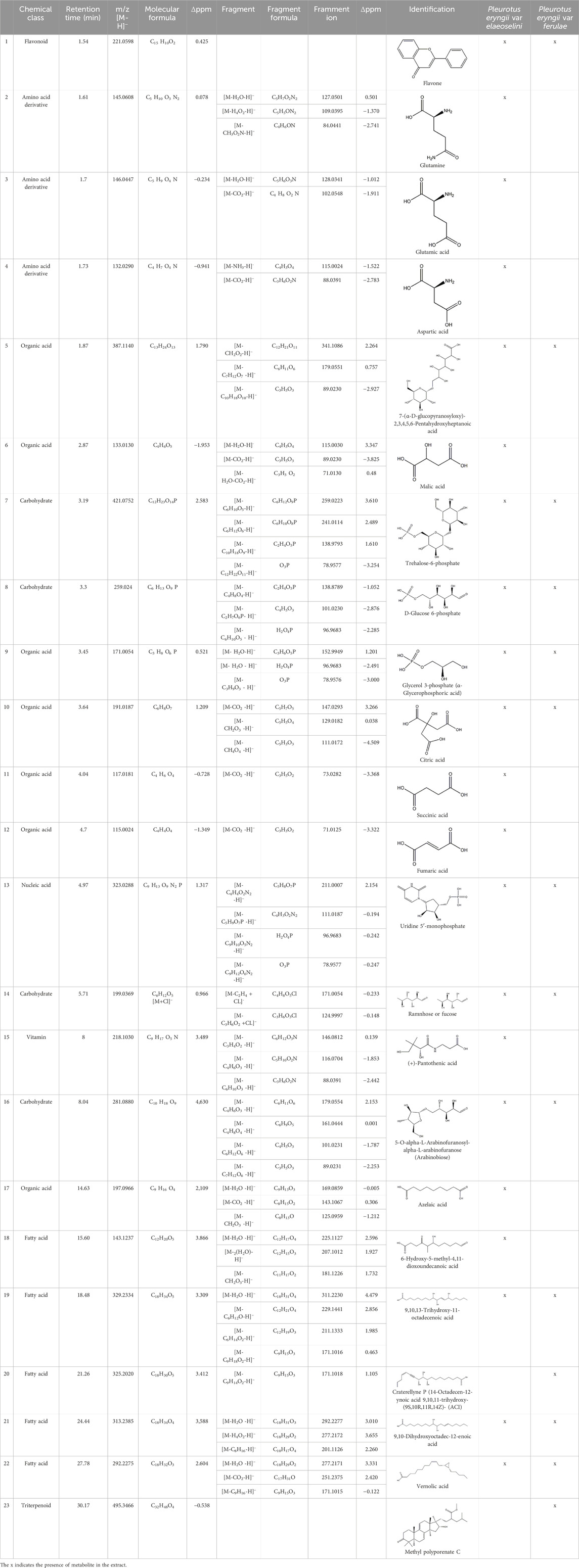
Table 1. Composition of the hydroalcoholic extracts of Pleurotus eyngii var. elaesolini and Pleutorus eryngii var. ferulae.
Compound (1) appeared at tR = 1.54 min and yielded a precursor ion [M-H]− at m/z 221.0598, attributed to flavone (2-phenyl-4H-1-benzopyran-4-one), previously reported by Smith et al. (2023) in some Pleurotus species.
The compounds 2, 3 and 4, identified only in P. eryngii var. elaeoselini, belonged to the amino acid derivatives: in particular, the compound 2 gave a [M-H]− ion at m/z 145.0608, corresponding to the deprotonated molecular form of glutamine. The substance, already reported in P. eryngii by Tagkouli et al. (2020), gave MS2 fragments at m/z 127.0501, 109.0395, 84.0441, attributable to the amino acid. Amino acids such as glutamine, leucine and alanine seem to dominate in a strain of P. ostreatus (Tagkouli et al., 2020). The compounds 3 and 4 were respectively identified as glutamic and aspartic acid, previously reported in several edible mushroom including Pleurotus (Chanvorleak et al., 2016). Also in these case, MS2 fragmentations revealed characteristic fragments at m/z 128.0341, 102.0548, 84.0441 for glutamic acid, and 115.0024 and 88.0391 for aspartic acid, respectively. According to Yamaguchi (1991), among all free amino acids, only aspartic acid and glutamic acid contribute to the characteristic umami taste.
The compound 5 (C13H24O13) was identified in both varieties and characterized as 7-(α-D-glucopyranosyloxy)-2,3,4,5,6-pentahydroxyheptanoic acid, a compound previously reported in Vitex negundo L. (Lamiaceae) (Nadeem et al., 2020). The major fragments in MS2 spectrum for this compound appeared at m/z 341.1086, resulting from neutral loss of HCOOH, and m/z 179.0551 resulting from neutral loss of C7H12O7.
Malic acid (compound 6), present only in the extract of P. eryngii var. elaeoselini, presented a deprotonated [M-H]− ion at m/z 133.0130 and a diagnostic fragment at 115.0030 [M-H-18]−, that was appeared to due to a loss of H2O, while the fragment at 89.0230 was due to the elimination of CO2 from the precursor ion. The compound was previously reported in P. eryngii (Li et al., 2014; Li et al., 2015; Wang et al., 2016).
The peaks 9, 10, 11 and 12 were also attributed to organic acids: in particular, compound 9 gave a [M-H]− ion at m/z 171.0054 and was attributed to α-glycerophosphoric acid. Xiao et al. (2019) reported the presence of this compound in the metabolism in P. ostreatus. The peaks 10, 11 and 12 were attributed respectively to citric, succinic and fumaric acids, that presented deprotonated [M-H]− ions at m/z 191.0187, 117.0181 and 115.0024: the major fragments in MS/MS spectrum of citric acid appeared at m/z 111.0172, due to the loss of H2O and CO2 molecules [M-2H2O-CO2-H]−. The MS2 analysis of succinic and fumaric acid revealed major fragments at m/z 73.0282, correspond to a loss of CO2 [M-44-H]−, as previously reported (Ibrahim et al., 2024; Thakur et al., 2024). The three substances were non-volatile taste components present in several edible mushrooms, including P. eryngii, as reported in literature (Li et al., 2014; Li et al., 2015; Wang et al., 2016). Furthermore, malic and succinic acids were found in many plants and animals, probably because they were both intermediates in the tricarboxylic acid cycle (Wang et al., 2016). Previously, it has been noted that organic acids, as malic, citric or succinic acids, also play a beneficial role in combating various illnesses due to their antioxidant properties. Additionally, they are crucial flavor elements in beverages such as wine and sake, suggesting their potential as safer additives for food flavoring (Wang et al., 2016).
The peaks 7 and 8 were attributed to substances involved in carbohydrate metabolism but linked to two different sub pathways: the first one was identified as trehalose-6-phosphate, which showed a deprotonated [M-H]− ion at m/z 421.0752, mainly involved in sucrose, glucose, and fructose metabolism of Pleurotus ostreatus, as reported by Luo et al. (2017). Later, a detailed MS fragmentation pathway of this substance characterized by peaks at m/z 241, 139 and 79.1, also present in MS2 analysis here conducted, was reported (Luo et al., 2019). Trehalose-6-phosphate can be a key regulator of fungal cell wall biosynthesis; moreover, it was an active component that regulated the trehalose metabolic pathway (Paul, 2007) and, considering the trehalose antioxidant activity, the substance can play a meaningful function in protecting cells from oxidative damages, mainly in cell membranes (Herdeir et al., 2006). The peak 8 was attributed to glucose-6-phosphate, which gave a deprotonated [M-H]− ion at m/z 259.0224; this compound is involved in the glycolysis sub pathway (Luo et al., 2017). As reported by Benvenuti et al. (2023), the fragmentation pathway of molecule, with the characteristic ion at m/z 138.8789 and 96.9683, confirmed the identification.
Also, the peak 14 was recognized as belonging to carbohydrate metabolism: it showed a precursor ion [M+Cl]− at m/z 199.0369, having a molecular formula C6H12O5. The MS/MS spectrum formed fragment ions at m/z 171.0054 [C4H8O5+Cl]− and 124.9997 [C3H6O3+Cl]−, compatible with rhamnose or fucose, monosaccharides both reported in P. eryngii (Cui et al., 2014; Li et al., 2014).
The peak 13, with a precursor deprotonated ion [M-H]− at m/z 323.0288, was attributed to uridine 5′-monophosphate, a nucleic acid already reported in Pleurotus genus (Benvenuti et al., 2023): the same Authors reported also the m/z of the respective fragment ions, which corroborate the attribution here reported: the fragment at m/z 211.0007 is due to the loss of uracil molecule (C4H4O2N2); the fragment at m/z 96.9683 is due to phosphoric acid (H3O4P-H)− (Fan et al., 2022).
Compound 15, present only in P. eryngii var. elaeoselini extract, was attributed to a vitaminic substance, (+)-pantothenic acid, which gave a precursor ion deprotonated [M-H]− at m/z 218.1030: the MS2 analysis, with the presence of fragment at m/z 88.0391, was in agreement to Benvenuti et al. (2023). Pantothenic acid, along with mineral salts and vitamins such as B1, B2, B6, B12, D, H, and niacin, has been found in fungi in greater amounts compared to vascular plants (La Guardia et al., 2005).
Compound 16, another carbohydrate, gave a deprotonated ion [M-H]− at m/z 281.0880, attributed to arabinobiose, previously reported in P. ostreatus (Lee et al., 2023): its MS/MS spectrum is characterized by the presence of ions at m/z 161.0444, 101.0231 and 89.0231, attributable, respectively, to cross-ring cleavage with loss of C4H8O4 (−120 Da), to the neutral loss of CH2O from the ion [M-C5H10O5]− arising from the cleavage of glycosidic bonds and to the neutral loss of C2H4O2 from the ion [M-C7H12O6], also arising from the cleavage of glycosidic bonds. This fragmentation pathway agrees with literature (da Costa et al., 2012).
The peak 17 was attributed to azelaic acid, a dicarboxylic acid, also known as 1,9-nonanedioic acid. The compound, with a precursor deprotonated ions [M-H]− at m/z 187.0969, was found only in P. eryngii var. elaeoselini extract and was previously reported in P. ostreatus (Fogarasi et al., 2018). The MS2 analysis of azelaic acid showed the presence of fragment ions [M-H]- at m/z 169.0859, attributed to the loss of H2O, and at m/z 143.1067, due to the loss of CO2; moreover, the presence of a fragment at m/z 125.0959 was also reported by Okomo Aloo et al. (2024). The same authors reported that natural compounds as azelaic acid can be changed significantly after fermentation, a process in which bacteria break down carbohydrates as sucrose to produce a variety of organic acids, which could explain these changes; so, these metabolites could substantially contribute to sensory properties of fermented foods.
Also compound 18, with a precursor deprotonated ion [M-H]− at m/z 243.1237, was only found in P. eryngii var. elaeoselini extract and was identified as 6-hydroxy-5-methyl-4,11-dioxoundecanoic acid. In the MS/MS spectrum, the predominant fragment ions compared at m/z 225.1127 and 207.1012, compatible with [M-H2O-H]- and [M-2(H2O)-H]−, which correspond to the elemental compositions of C12H17O4 and C12H15O3, respectively. The compound was already reported in P. ostreatus (Lee et al., 2023).
The peaks 19–22 were attributed to carboxylic acids, three of which (peaks 19, 21 and 22) were found in both extracts: the compound 19 was identified as 9,10,13-trihydroxy-11-octadecenoic acid, which gave a precursor deprotonated ion [M-H]− at m/z 329.2334. Lee et al. (2023) reported this compound in P. ostreatus. It is a monounsaturated fatty acid, also reported in other edible fungi, as Morchella sp. (Zhao et al., 2022). Compound 21 was attributed to 9,10-dihydroxyoctadec-12-enoic acid, which gave a precursor ion [M-H]− at m/z 313.2385. The MS/MS fragmentation pathway, with the ions at m/z 295.227, 277.2172 and 201.1126, corresponding respectively to a losses of H2O, H4O2 and C8H16, was previously reported in P. ostreatus (Benvenuti et al., 2023). The compound 22 was identified as vernolic acid by a precursor ion [M-H]− at m/z 295.2275 and was previously reported in P. ostreatus (Ferraz et al., 2023): product ion scan of the deprotonated molecule formed characteristic fragment ions at m/z 277.2171, 251.2375 and 171.1015, compatible with losses of H2O, CO2 and C9H16, respectively. Recently, the compound was reported as one of the lowering cholesterol agents, found in the seeds of Caesalpinia bonducella L. (Caesalpiniaceae) (Musa et al., 2023).
Compound 20, with formula C18H30O5, was identified as craterellyne P, a fatty acid only present in P. eryngii var. ferulae: ectract in the MS/MS spectrum, a characteristic fragment ion of [M-C9H14O2-H]− at m/z 171.1018 was observed and confirmed by literature (Huang et al., 2017).
Compound 23 displayed a deprotonated molecule [M–H]− at m/z 495.3466, corresponding to the molecular formula C32H48O4 and was identified as methyl polyporenate C. The compound was previously reported in the Polyporaceae and Pleurotaceae families (Yokoyama and Natori, 1974).
3.2 Antibacterial activity
Table 2 reports the antibacterial activity of the extracts. In the case of the extract of P. eryngii var. ferulae, some concentrations inhibited the growth of the majority of the tested bacterial strains, except for X. campestris, while both extracts did not show any activity against B. megaterium. P. eryngii var. ferulae extract showed higher activity against C. michiganensis and P. viridiflava, at the two higher concentrations tested compared to positive control; however, this extract showed activity against E. coli only at the highest concentration tested compared to positive control.
P. eryngii var. elaeoselini extract exerted the highest activity against X. campestris only at the higher concentration, compared to positive control. This sample showed also moderate activity against C. michiganensis and P. viridiflava only at the higher tested concentration compared to positive control.
3.3 Antifungal activity
Table 3 reports the antifungal activity of the extracts. In the case of P. eryngii var. ferulae extract, it showed the highest antifungal activity against P. italicum and A. fumigatus at the two higher tested concentrations. The same sample showed low activity against S. sclerotiorum. No activity was observed against the other tested fungi.
P. eryngii var. elaeoselini extract showed moderate and low antifungal effect against P. italicum and A. fumigatus, only at the highest tested concentration. No activity was observed against the other tested fungi.
Pleurotus eryngii complex show pronounced host/substrate specificity, growing as saprotrophs (or facultative biotrophs) on various plants of the Apiaceae family (Zervakis et al., 2014).
P. eryngii has gained significant attention due to its potential antimicrobial properties. Several studies have shown that P. eryngii produces a range of bioactive compounds, including polysaccharides, proteins, and secondary metabolites, many of which exhibit notable antimicrobial activity (Akyuz and Kirbag, 2009; Yuan et al., 2017; Yin et al., 2016; Murgia et al. 2014).
Yu et al. (2018) reported that ethanolic extracts of P. eryngii demonstrated antimicrobial effects against various microorganisms, including Enterobacter cloacae and E. coli. In addition, proteins produced by P. eryngii can directly target microbial cell walls or interfere with microbial enzyme functions. Phenolic compounds also contribute to its antimicrobial action by disrupting microbial cell membranes or functioning as antioxidants (Yu et al., 2018).
Akyuz and Kirbag (2009) further investigated the antimicrobial activity of extracts from P. eryngii var. ferulae against several bacterial species, including B. megaterium, Staphylococcus aureus, and E. coli. They found that methyl alcohol extracts of P. eryngii var. ferulae inhibited the growth of tested microorganisms to varying degrees.
On the other hand, beside the notable antimicrobial activity of P. eryngii, other species within the Pleurotus genus, such as P. ostreatus, have also demonstrated promising antimicrobial properties. For instance, Prastiyanto et al. (2016) reported that P. ostreatus extracts exhibited antimicrobial activity against Enterobacter aerogenes, S. aureus, and Candida albicans. Similarly, Iwalokun et al. (2007) identified bioactive compounds, including terpenoids, alkaloids, saponins, and tannins, commonly present in P. ostreatus and other Pleurotus species, as key contributors to their antibacterial effects.
Recently, Jiang et al. (2022) reported that glutamine, one of the amino acid found in P. eryngii var elaeoselini played critical roles in host immunity against M. tuberculosis infection. Moreover, also the other two amino acid derivatives found in the same extract, glutamic and aspartic acids, were previously reported in literature as major amino acids in New Zealand honeydew honey, already known for its several biological properties, including antimicrobial one (Chessum et al., 2022).
Trehalose-6-phosphate, a carbohydrate found in both extracts, was involved in the most widely distributed trehalose biosynthetic pathway (Magalhães et al., 2017), who produced trehalose throught the glucose-6-phosphate formation. Since this pathway was totally absent in mammalian cells and employed very specific enzymes, trehalose-6-phosphate could be considered an interesting target for the fight some pathogens whose virulence depends on trehalose, essential for stress tolerance and virulence (Magalhães et al., 2017). Fosfomycin was one of the most important antibiotic because of its effciacy against common drug-resistant bacteria: the compound acted by blocking the first step in bacterial cell wall synthesis, as unique mechanism of action: glucose 6-phosphate, the second carbohydrate found in the extract, was reported in literature for the its capability to let enter the antibitoic fosfomycin in drug-resistant Klebsiella pneumoniae isolate (Aydemir et al., 2022).
The organic acids (malic acid, fumaric acid, succinic acid, citric acid) were previously investigated as potential candidate replacements for in-feed antibiotics (Skřivanová et al., 2006) and also for their capability to act as antimicrobial agents for controlling E coli in beef trimmings (Mohan and Pohlman, 2016): malic and fumaric acids were also considered as a substitute for monensin to prevent subacute acidosis in feedlots (Castillo et al., 2004); succinic acid, when added to lactating cows, was decarboxylated by rumen microbes to propionate, of which the increase production is a major effect of antibiotic feed additives in the rumen (Skřivanová et al., 2006).
Gheita et al. (2020) reported the potential role of pantothenic acid (vitamin B5), highlighting its antimicrobial activity and generally its capability to improve the immune function, thus providing potentially important therapeutic implications. Recently Choi et al. (2024) reported that the biosynthetic pathway of Co-enzyme A (CoA) and Acetyl-CoA (AcCoA) from pantothenic acid has been considered as an excellent target for the development of new antimicrobials against fungi and protozoa.
Azelaic acid, a 9-carbon straight-chain saturated dicarboxylic acid, naturally and abundantly available in wheat was also previously reported for its antimicrobial effect (Khojali et al., 2023). Between identified carboxylic acids, 9,10-dihydroxyoctadec-12-enoic acid was reported as plant defensive metabolite against rice blast disease and also was a precurosor to the hydroxylated and/or unsaturated fatty acids which possess several biological activites including antifungal and nematidicidal (Pang et al., 1994).
Craterellyne P, a derivative acetylenic acid, was isolated from the fruiting bodies of edible mushroom, Craterellus lutescens, and was previously tested for a poitential anitfungal activitiy against Candida albicans (Huang et al., 2017).
Vernolic acid was a plant long-chain monounsaturated epoxy fatty acid: fatty acids were known to have antimicrobial activity, throught destabilizing bacterial membranes and interfering with bacterial metabolic processes (Mohammed et al., 2023).
3.4 Antioxidant activity
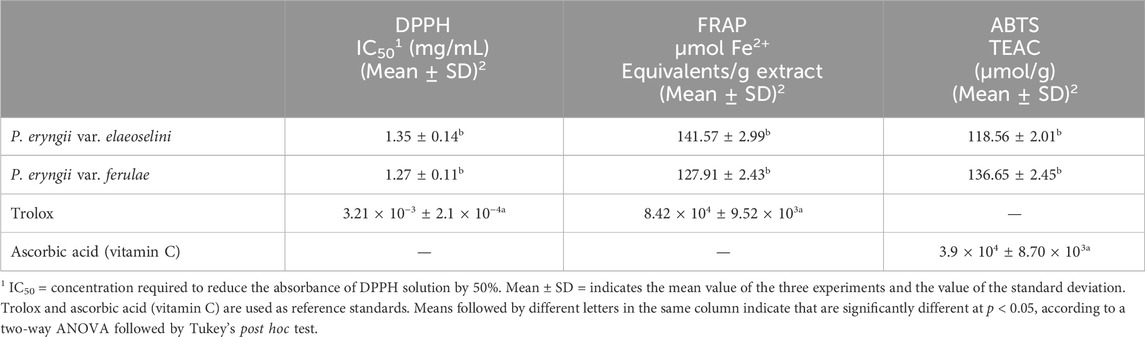
The results of the tests for the evaluation of the antioxidant activity, reported in Table 4, show that both extracts possess some antioxidant activity in all three tests conducted. However, in all three tests the activity was lower than that shown for the standards. The extract obtained from P. eryngii var. eleoselini showed a slightly higher activity than the other extract both in the FRAP test, with a value of Fe2+ equivalents/g extract equal to 141.57 ± 2.99 μmol against a value of 127.91 ± 2.43 μmol for the extract obtained from the var. ferulae, and in the ABTS test with a TEAC value of 118.56 ± 2.01 μmol/g against the 136.65 ± 2.45 μmol/g of the extract obtained from var. ferulae. In the DPPH test, however, the extract obtained from the var. ferulae was found to be more active, with an IC50 value of 1.27 ± 0.11 mg/mL against 1.35 ± 0.14 mg/mL of the extract obtained from the var. elaeoselini. The antioxidant activity of extracts obtained from Pleurotus eryngii is reported in few works. Akyüz et al. (2012) through DPPH test established an IC50 value equal to 24.67 ± 0.72 for a methanolic extract of P. eryngii var ferulae, a value that demonstrates a higher activity than that highlighted in this work. Higher activity was also reported by Choi et al. (2017) who reported an IC50 value of 139.46 ± 3.2 μg for an aqueous extract of the fruiting bodies of P. eryngii var ferulae. There are no contributions investigating the antioxidant activity on var. elaeoselini. Several works report the antioxidant activity of various extracts obtained from the P. eryngii biotype, typically using the DPPH test for the evaluation and sometimes exposing the results differently than what was done in this work. Yldirim et al. (2012) measured the antioxidant activity by DPPH test of methanolic extracts obtained from P. eryngii collected in different areas of Turkey, the results showed a good antioxidant activity with an inhibition ranging from 25.08% to 39.13%. Lin et al. (2014) highlighted, through DPPH tests, IC50 values ranging from 1.08 ± 0.06 to 1.30 ± 0.10 mg/mL for ethanolic extracts of P. eryngii obtained at different times, showing an activity similar to that found in this work. Gąsecka et al. (2016) instead finds a lower activity than that highlighted in this work, with IC50 values equal to 7.34 ± 0.11 and 3.35 ± 0.11 mg/mL for methanolic extracts of P. eryngii enriched with selenium and zinc. Finally, in 2018 a comparison was made between the antioxidant activity, measured by DPPH assay, of various extracts of P. eryngii. Results reported a general strong activity for all extracts. The EtAC extract showed the highest activity with an inhibition of 81.0% ± 0.95%, while the activity of acetone extract and EtOH extract was found to be 79.1% ± 0.56% and 77.4% ± 0.33%, respectively (Yu et al., 2018).
3.5 PCA and Hierarchical Cluster Heatmap
The PCA biplot (Figure 2) visualizes the relationships among different microbial species and their respective observations in a three-dimensional space defined by the first three principal components (PC1, PC2, and PC3).
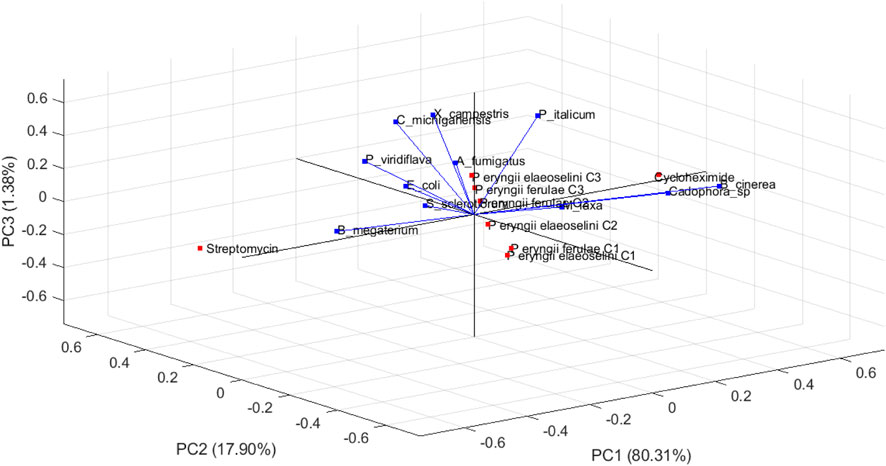
Figure 2. Biplot (loading and scores plots) obtained by principal component analysis (PCA) of two extracts (at three different concentrations), and two standard compounds (streptomycin and cycloheximide) based on the eleven different variables (the bacterial and fungal tested strains) in the three dimensional space. The vectors shown are the eigenvectors of the covariance matrix.
This representation allows for an intuitive understanding of how these species relate to each other based on the data provided. The first principal component explained 80.31% of the total variance in the dataset, indicating that it is the most significant dimension for distinguishing between the microbial species. The direction of the PC1 axis suggests that it differentiates species based on a specific set of characteristics or measurements that are predominant in this dimension.
The second principal component accounts for 17.90% of the total variance, adding further differentiation among the species. The orientation of PC2 relative to PC1 provides insights into how different species cluster or separate based on their measurements. The third principal component contributes 1.38% to the variance, allowing for minimal additional separation among observations.
Generally, the arrows represent the original variables (microbial species, B. megaterium, C. michiganensis, X. campestris, E. coli, P. viridiflava, A. fumigatus, B. cinerea, Cadophora sp., M. laxa, P. italicum, and S. sclerotiorum) in relation to the principal components: the length and direction of each arrow indicate how much each variable contributes to the respective principal components. The direction of these arrows indicates how each variable contributes to different principal components. Longer arrows, such as B. cinerea and B. megaterium, indicate that these variables have a strong contribution to the variance in the principal components being visualized. Conversely, A. fumigatus has a shorter arrow, so indicating a lesser contribution to the variance captured by PC1, PC2 and PC3. E. coli points towards the upper right quadrant, suggesting it is positively associated with observations in that area. The variables that point in the same direction are positively correlated: C michiganensis and X campestris arrows are oriented similarly; it means they share a similar relationship to the data structure.
The observations, represented as points, include: P eryngii ferulae C1, P eryngii ferulae C2, P eryngii ferulae C3, P eryngii elaeoselini C1, P eryngii elaeoselini C2, P eryngii elaeoselini C3, Streptomycin, and Cycloheximide.
The observations may cluster together, suggesting shared traits or responses among those microbes: if certain strains were located close to each other in the biplot, it implies they had similar measurement profiles across the evaluated conditions. The observations P eryngii ferulae C1, C2, and C3 cluster closely together in the lower left quadrant, indicating similar responses across the measured variables. This means that they are influenced in similar ways by the principal components.
The observations Cycloheximide and Streptomycin are positioned far from other observations, indicating it may have unique characteristics compared to others.
The direction of the points relative to the arrows also provides insights: samples that lie along the direction of an arrow are highly influenced by the corresponding variable. Om the contrary, there is a clear negative correlation between Streptomycin and B cinerea, as indicated by their positions on opposite sides of the plot.
The Hierarchical Cluster Heatmap (Figure 3) represents the relationships between different observations of Pleurotus eryngii strains (along the Y-axis) and antibiotic treatments (cycloheximide and streptomycin) and different strain types (along the X-axis). The heatmap uses color intensity to convey the degree of similarity or difference between observations and variables.
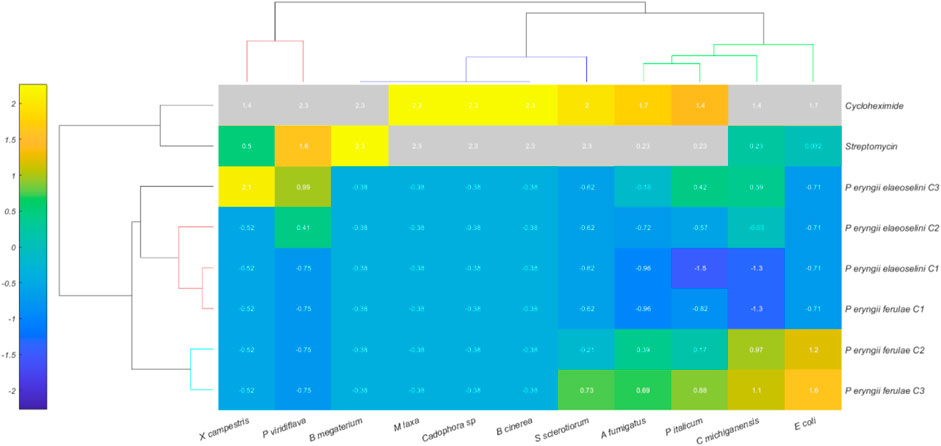
Figure 3. Hierarchical Cluster Heatmap of 8 treatments (rows) and 11 variables (columns), with normalized data values represented by a color scale ranging from blue (low) to yellow (high). The rows represent the two extracts (at three different concentrations), and two standard compounds (streptomycin and cycloheximide). The columns represent the tested bacterial and fungal strains.
The observations along the Y-axis include the different concentrations of the extracts obtained from Pleurotus eryngii var ferulae and var elaeoselini, specifically:
P. eryngii ferulae C1, C2, C3
P. eryngii elaeoselini C1, C2, C3
The Y-axis also includes antibiotic treatments such as cycloheximide and streptomycin.
The X-axis includes the bacterial and fungal strains studied.
The heatmap displays how each treatment responds to these variables, with different color intensities indicating the strength of the response or similarity between observations.
The Euclidean distance between different treatments, based on their response to the variables, reveals that: P. eryngii ferulae group (C1, C2, C3) appears to be more similar internally, indicated by the consistent color pattern across their interactions with the variables.
Similarly, P. eryngii elaeoselini (C1, C2, C3) also exhibits clustering with each other, suggesting consistent responses within this subgroup.
Cycloheximide and Streptomycin exhibit distinct clustering patterns compared to the other treatments, indicating that their effects are clearly differentiated from the biological properties of the Pleurotus strains.
The similarity or variability in color intensity between different bacterial and fungal strains when exposed to these treatments may indicate differing levels of resistance or susceptibility, which could be pivotal in understanding how these microbes respond to antimicrobial agents.
The heatmap clearly highlights the relationships between the microbial strains and treatments, providing a visual summary of which strains respond in a similar manner to specific conditions. The use of Euclidean distance as the metric for clustering helps in quantifying these relationships, showing both high similarity within groups and significant divergence between different clusters.
This clustering can help identify potential leads for further analysis, such as which Pleurotus strain might have resistance to a specific antibiotic or which strains are biologically similar in their growth and response under various experimental conditions.
4 Conclusion
This study provides a comprehensive analysis of the composition, antimicrobial activity, and antioxidant properties of hydroalcoholic extracts derived from Pleurotus eryngii var. ferulae and P. eryngii var. elaeoselini. The findings demonstrate that both varieties possess significant antimicrobial properties, which can be attributed to their unique phytochemical profiles rich in bioactive compounds.
Chemical analyses revealed a diverse range of constituents, including organic acids, fatty acids, amino acid derivatives, known for their health benefits and antimicrobial effects. The extracts exhibited varying degrees of effectiveness against a spectrum of microbial strains, indicating their potential application in food preservation and as natural antimicrobial agents.
In addition to their antimicrobial properties, the hydroalcoholic extracts displayed notable antioxidant activity. The presence of high levels of organic acids correlates with their ability to scavenge free radicals and mitigate oxidative stress. This dual action not only enhances the nutritional value of these mushrooms but also suggests their potential role in reducing oxidative damage associated with various chronic diseases. The antioxidant properties observed may also contribute to enhancing the antimicrobial efficacy of these extracts, providing a multifaceted approach to treatment strategies.
In conclusion, the hydroalcoholic extracts from P. eryngii var. ferulae and P. eryngii var. elaeoselini represent a valuable source of natural antimicrobial and antioxidant agents with significant potential for various applications in health and food industries. Their rich composition underscores their role as functional foods that can contribute to improved public health outcomes while offering promising avenues for future research.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://doi.org/10.7910/DVN/QZMGMV.
Author contributions
FP: Conceptualization, Validation, Formal Analysis, Methodology, Writing–original draft. LD: Formal Analysis, Data curation, Writing–original draft. GM: Data curation, Formal Analysis, Writing–original draft. GV: Conceptualization, Resources, Supervision, Validation, Writing–review and editing. MG: Conceptualization, Writing–review and editing, Formal Analysis. VD: Conceptualization, Writing–review and editing, Resources, Supervision, Validation. HE: Data curation, Formal Analysis, Validation, Writing–original draft. IC: Methodology, Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acay, H., Güney, I. G., Yildirim, A., Derviş, S., and Dereli, E. (2024). Green synthesis of Pleurotus eryngii-derived nanomaterials for phytopathogen control. Chem. Biodivers. 21, e202401972. doi:10.1002/cbdv.202401972
Akyuz, M., and Kirbag, S. (2009). Antimicrobial activity of Pleurotus eryngii var. ferulae grown on various agro-wastes. EurAsian J. Biosci. 3, 58–63. doi:10.5053/ejobios.2009.3.0.8
Akyüz, M., Onganer, A. N., Erecevit, P., and Kirbag, S. (2012). Flavonoid contents and 2, 2-diphenyl-1-picrylhydrazyl radical scavenging activity of some edible mushrooms from Turkey: A. bisporus and Pleurotus spp. Cur. Top. Nutraceut. R. 10, 133.
Amamcharla, J. K., and Metzger, L. E. (2014). Modification of the ferric reducing antioxidant power (FRAP) assay to determine the susceptibility of raw milk to oxidation. Int. Dairy J. 34, 177–179. doi:10.1016/j.idairyj.2013.09.004
Angeles Flores, G., Girometta, C. E., Cusumano, G., Angelini, P., Tirillini, B., Ianni, F., et al. (2022). Untargeted metabolomics used to describe the chemical composition, antioxidant and antimicrobial effects of extracts from Pleurotus spp. mycelium grown in different culture media. Antibiotics 11, 1468. doi:10.3390/antibiotics11111468
Aydemir, Ö., Şahin, E. Ö., Ayhancı, T., Ormanoğlu, G., Aydemir, Y., Köroğlu, M., et al. (2022). Investigation of in-vitro efficacy of intravenous fosfomycin in extensively drug-resistant Klebsiella pneumoniae isolates and effect of glucose 6-phosphate on sensitivity results. Int. J. Antimicrob. Agents 59 (1), 106489. doi:10.1016/j.ijantimicag.2021.106489
Benvenuti, M., Di Piazza, S., Salis, A., Cecchi, G., Zotti, M., Scarfi, S., et al. (2023). A novel method for the extraction and characterization of metabolites from Basidiomycota: Pleurotus ostreatus (Jacq.) P. Kumm., 1871 as a case study. Sep. Sci. Plus 6 6, 2300116. doi:10.1002/sscp.202300116
Benzie, I. F., and Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239, 70–76. doi:10.1006/abio.1996.0292
Bhunia, A. K., Johnson, M. C., and Ray, B. (1988). Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J. Appl. Bacteriol. 65, 261–268. PMID: 2906056. doi:10.1111/j.1365-2672.1988.tb01893.x
Brand-Williams, W., Cuvelier, M. E., and Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 28, 25–30. doi:10.1016/S0023-6438(95)80008-5
Castillo, C., Benedito, J. L., Mendez, J., Pereira, V., LopezAlonso, M., Miranda, M., et al. (2004). Organic acids as a substitute for monensin in diets for beef cattle. Anim. Feed Sci. Technol. 115, 101–116. doi:10.1016/j.anifeedsci.2004.02.001
Castronuovo, D., Mang, S. M., Becce, A., Candido, V., Cardone, L., and Camele, I. (2019). Morphological and productivity comparison between commercial and wild isolates of Pleurotus eryngii (D.C.: Fr.) Quél. Ital. J. Agron. 14, 170–175. doi:10.4081/ija.2019.1458
Cateni, F., Gargano, M. L., Procida, G., Venturella, G., Cirlincione, F., and Ferraro, V. (2022). Mycochemicals in wild and cultivated mushrooms: nutrition and health. Phytochem. Rev. 21, 339–383. doi:10.1007/s11101-021-09748-2
Cateni, F., Zacchigna, M., Caruso Bavisotto, C., Procida, G., Bonaventura, G., Saporita, P., et al. (2018). Structural characterization of polysaccharides of a productive strain of the culinary-medicinal king oyster mushroom, Pleurotus eryngii (agaricomycetes), from Italy. Int. J. Med. Mushrooms 20, 717–726. doi:10.1615/IntJMedMushrooms.2018027011
Cateni, F., Zacchigna, M., Procida, G., Venturella, G., Procida, V., and Gargano, M. L. (2020). Polysaccharides from Pleurotus eryngii var. elaeoselini (agaricomycetes), a new potential Culinary−Medicinal oyster mushroom from Italy. Int. J. Med. Mushrooms 22, 431–444. doi:10.1615/IntJMedMushrooms.2020034539
Chanvorleak, P., Moon, B., and Chan, L. (2016). Evaluation of umami taste in mushroom extracts by chemical analysis, sensory evaluation, and an electronic tongue system. Food Chem. 192, 1068–1077. doi:10.1016/j.foodchem.2015.07.113
Chessum, K. J., Chen, T., Hamid, N., and Kam, R. (2022). A comprehensive chemical analysis of New Zealand honeydew honey. Food Res. Int. 157, 111436. doi:10.1016/j.foodres.2022.111436
Choi, J. H., Kim, D. W., Kim, S., and Kim, S. J. (2017). In vitro antioxidant and in vivo hypolipidemic effects of the king oyster culinary-medicinal mushroom, Pleurotus eryngii var. Ferulae DDL01 (agaricomycetes), in rats with high-fat diet–induced fatty liver and hyperlipidemia. Int. J. Med. Mushrooms 19, 107–119. doi:10.1615/IntJMedMushrooms.v19.i2.20
Choi, J. Y., Gihaz, S., Munshi, M., Singh, P., Vydyam, P., Hamel, P., et al. (2024). Vitamin B5 metabolism is essential for vacuolar and mitochondrial functions and drug detoxification in fungi. Commun. Biol. 7, 894. doi:10.1038/s42003-024-06595-7
Crescenzi, M. A., Cerulli, A., Montoro, P., and Piacente, S. (2023). Metabolite Profiling for Typization of “Rucola della Piana del Sele” (PGI), Eruca sativa, through UHPLC-Q-Exactive-Orbitrap-MS/MS Analysis. Foods 12, 3384. doi:10.3390/foods12183384
Cui, F., Li, Y., Yang, Y., Sun, W., Wu, D., and Ping, L. (2014). Changes in chemical components and cytotoxicity at different maturity stages of Pleurotus eryngii fruiting body. J. Agric. Food Chem. 62, 12631–12640. doi:10.1021/jf5048354
da Costa, E. V., Moreira, A. S. P., Nunes, F. M., Coimbra, M. A., Evtuguin, D. V., and Domingues, M. R. M. (2012). Differentiation of isomeric pentose disaccharides by electrospray ionization tandem mass spectrometry and discriminant analysis. Rapid Commun. Mass Spectrom. 26, 2897–2904. doi:10.1002/rcm.6415
D’Ambrosio, G., Cariddi, C., Mannerucci, F., and Bruno, G. L. (2022). In vitro screening of new biological limiters against some of the main soil-borne phytopathogens. Sustainability 14, 2693. doi:10.3390/su14052693
Devi, P. I., Manjula, M., and Bhavani, R. V. (2022). Agrochemicals, environment, and human health. Annu. Rev. Environ. Resour. 47, 399–421. doi:10.1146/annurev-environ-120920-111015
El Enshasy, H. A., and Hatti-Kaul, R. (2013). Mushroom immunomodulators: unique molecules with unlimited applications. Trends Biotechnol. 31, 668–677. doi:10.1016/j.tibtech.2013.09.003
Elshafie, H. S., Camele, I., Racioppi, R., Scrano, L., Iacobellis, N. S., and Bufo, S. A. (2012). In vitro antifungal activity of Burkholderia gladioli pv. agaricicola against some phytopathogenic fungi. Int. J. Mol. Sci. 13, 16291–16302. doi:10.3390/ijms131216291
Fan, Y., Sun, G., Kaw, H. Y., Zhu, L., and Wang, W. (2022). Analytical characterization of nucleotides and their concentration variation in drinking water treatment process. Sci. Total Environ. 817, 152510. doi:10.1016/j.scitotenv.2021.152510
Ferraz, C. G., Ribeiro, P. R., Verde, B. V., Silva, R. S., Silva, M. C., Do Carmo, C. O., et al. (2023). Metabolite profiling of Pleurotus ostreatus grown on sisal agro-industrial waste supplemented with cocoa almond tegument and wheat bran. Chem. Biodivers. 20, e202300346. doi:10.1002/cbdv.202300346
Fogarasi, M., Socaci, S. A., Dulf, F. V., Diaconeasa, Z. M., Fărcaș, A. C., Tofană, M., et al. (2018). Bioactive compounds and volatile profiles of five transylvanian wild edible mushrooms. Molecules 23, 3272. doi:10.3390/molecules23123272
Gąsecka, M., Mleczek, M., Siwulski, M., and Niedzielski, P. (2016). Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 242, 723–732. doi:10.1007/s00217-015-2580-1
Gheita, A. A., Gheita, T. A., and Kenawy, S. A. (2020). The potential role of B5: a stitch in time and switch in cytokine. Phytother. Res. 34 (2), 306–314. doi:10.1002/ptr.6537
Herdeir, R. S., Pereira, M. D., Panek, A. D., and Eleutherio, E. C. (2006). Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochim. Biophys. Acta 1760, 340–346. doi:10.1016/j.bbagen.2006.01.010
Huang, Y., Zhang, S. B., Chen, H. P., Zhao, Z. Z., Zhou, Z. Y., Li, Z. H., et al. (2017). New acetylenic acids and derivatives from the edible mushroom Craterellus lutescens (cantharellaceae). J. Agric. Food Chem. 65, 3835–3841. doi:10.1021/acs.jafc.7b00899
Ibrahim, R. M., Abdel-Baki, P. M., El-Rashedy, A. A., and Mahdy, N. E. (2024). LC-MS/MS profiling of Tipuana tipu flower, HPLC-DAD quantification of its bioactive components, and interrelationships with antioxidant, and anti-inflammatory activity: in vitro and in silico approaches. BMC Complement. Med. Ther. 24, 176. doi:10.1186/s12906-024-04467-5
Iwalokun, B. A., Usen, U. A., Otunba, A. A., Olukoya, D. K., and Otunba, (2007). Comparative phytochemical evaluation, antimicrobial and antioxidant properties of Pleurotus ostreatus. Afr. J. Biotechnol. 6, 1732–1739. doi:10.5897/AJB2007.000-2254
Jiang, Q., Qiu, Y., Kurland, I. J., Drlica, K., Subbian, S., Tyagi, S., et al. (2022). Glutamine is required for M1-like polarization of macrophages in response to Mycobacterium tuberculosis infection. mBio 13, e0127422. doi:10.1128/mbio.01274-22
Khojali, W. M. A., Hussein, W., Bin Break, M. K., Alafnan, A., Huwaimel, B., Khalifa, N. E., et al. (2023). Chemical composition, antibacterial activity and in vitro anticancer evaluation of Ochradenus baccatus methanolic extract. Medicina 59, 546. doi:10.3390/medicina59030546
La Guardia, M., Venturella, G., and Venturella, F. (2005). On the chemical composition and nutritional value of Pleurotus taxa growing on umbelliferous plants (Apiaceae). J. Agric. Food Chem. 53, 5997–6002. doi:10.1021/jf0307696
Lee, A. M. L., Chin, C. F. S., Seelan, J. S. S., Chye, F. Y., Lee, H. H., and Rakib, M. R. M. (2023). Metabolites profiling of protein enriched oyster mushroom (Pleurotus ostreatus (Jacq.) P. Kumm.) grown on oil palm empty fruit bunch substrate. LWT-Food Sci. Technol. 181, 114731. doi:10.1016/j.lwt.2023.114731
Li, W., Gu, Z., Yang, Y., Zhou, S., Liu, Y., and Zhang, J. S. (2014). Non-volatile taste components of several cultivated mushrooms. Food Chem. 143, 427–431. doi:10.1016/j.foodchem.2013.08.006
Li, X., Feng, T., Zhou, F., Zhou, S., Liu, Y., Li, W., et al. (2015). Effects of drying methods on the tasty compounds of Pleurotus eryngii. Food Chem. 166, 358–364. doi:10.1016/j.foodchem.2014.06.049
Lin, J. T., Liu, C. W., Chen, Y. C., Hu, C. C., Juang, L. D., Shiesh, C. C., et al. (2014). Chemical composition, antioxidant and anti-inflammatory properties for ethanolic extracts from Pleurotus eryngii fruiting bodies harvested at different time. LWT-Food Sci. Technol. 55, 374–382. doi:10.1016/j.lwt.2013.08.023
Luo, F., Zhong, Z., Liu, L., Igarashi, Y., Xie, D., and Li, N. (2017). Metabolomic differential analysis of interspecific interactions among white rot fungi Trametes versicolor, Dichomitus squalens and Pleurotus ostreatus. Sci. Rep. 7, 5265. doi:10.1038/s41598-017-05669-3
Luo, X. T., Cai, B. D., Jiang, H. P., Xiao, H. M., Yuan, B. F., and Feng, Y. Q. (2019). Sensitive analysis of trehalose-6-phosphate and related sugar phosphates in plant tissues by chemical derivatization combined with hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1592, 82–90. doi:10.1016/j.chroma.2019.01.040
Magalhães, R. S. S., De Lima, K. C., de Almeida, D. S. G., De Mesquita, J. F., and Eleutherio, E. C. A. (2017). Trehalose-6-Phosphate as a potential lead candidate for the development of Tps1 inhibitors: insights from the trehalose biosynthesis pathway in diverse yeast species. Appl. Biochem. Biotechnol. 181, 914–924. doi:10.1007/s12010-016-2258-6
Mohammed, A. E., Alghamdi, S. S., Shami, A., Suliman, R. S., Aabed, K., Alotaibi, M. O., et al. (2023). In silico prediction of Malvaviscus arboreus metabolites and green synthesis of silver nanoparticles – opportunities for safer anti-bacterial and anti-cancer precision medicine. Int. J. Nanomedicine18 Vol. 18, 2141–2162. doi:10.2147/IJN.S400195
Mohan, A., and Pohlman, F. W. (2016). Role of organic acids and peroxyacetic acid as antimicrobial intervention for controlling Escherichia coli O157:H7 on beef trimmings. LWT--Food Sci. Technol. 65, 868–873. doi:10.1016/j.lwt.2015.08.077
Murgia, M., Pani, S. M., Sanna, A., Marras, L., Manis, C., Banchiero, A., et al. (2024). Antimicrobial activity of grapefruit seed extract on edible mushrooms contaminations: efficacy in preventing Pseudomonas spp. in Pleurotus eryngii. Foods 13, 1161. doi:10.3390/foods13081161
Musa, W. J. A., Bialangi, N., Kilo, A. K., Situmeang, B., Susparini, N. T., and Rusydi, I. D. (2023). Antioxidant, cholesterol lowering activity, and analysis of Caesalpinia bonducella seeds extract. Pharmacia 70, 97–103. doi:10.3897/pharmacia.70.e96817
Nadeem, M., Mumtaz, M. W., Danish, M., Rashid, U., Mukhtar, H., and Irfan, A. (2020). Antidiabetic functionality of Vitex negundo L. leaves based on UHPLC-QTOF-MS/MS based bioactives profiling and molecular docking insights. Ind. Crops Prod. 152, 112445. doi:10.1016/j.indcrop.2020.112445
Okomo Aloo, S., Park, S., Martins Oyinloye, T., and Oh, D. H. (2024). Rheological properties, biochemical changes, and potential health benefits of dehulled and defatted industrial hempseeds after fermentation. Food Chem. 439, 138086. doi:10.1016/j.foodchem.2023.138086
Pang, Z., Anke, H., Sterner, O., Bergson, G., Ayllón, J. A., Paulsen, G. B., et al. (1994). A chemical investigation of the fruit bodies of Lepista nebularis. Acta Chem. Scand. 48 (5), 408–410. doi:10.3891/acta.chem.scand.48-0408
Paul, M. (2007). Trehalose 6-phosphate. Curr. Opin. Plant Biol. 10, 303–309. doi:10.1016/j.pbi.2007.04.001
Prastiyanto, M., Darmawati, S., Setyaningtyas, A., Trisnawati, L., and Syafira, A. (2016). Antimicrobial activity and identification of the compounds of methanol extract from the Pleurotus ostreatus fruiting body. El-Hayah J. Biologi6, 29–34. doi:10.18860/elha.v6i1.4082
Skřivanová, E., Benda, V., Březina, P., and Brezina, P. (2006). Susceptibility of Escherichia coli, Salmonella sp and Clostridium perfringens to organic acids and monolaurin. Vet. Med. (Praha) 51, 81–88. doi:10.17221/5524-VETMED
Smith, H., Doyle, S., and Murphy, R. (2023). Target directed identification of natural bioactive compounds from filamentous fungi. Food Chem. 405, 134743. doi:10.1016/j.foodchem.2022.134743
Tagkouli, D., Kaliora, A., Bekiaris, G., Koutrotsios, G., Christea, M., Zervakis, G. I., et al. (2020). Free amino acids in three Pleurotus species cultivated on agricultural and agro-industrial by-products. Molecules 25, 4015. doi:10.3390/molecules25174015
Thakur, N., Murali, K., Bhadoriya, T. K. Y. C., Varshneyet, V. K., and Varshney, V. K. (2024). Phytochemical exploration of Neolitsea pallens leaves using UPLC-Q-TOF-MS/MS approach. Sci. Rep. 14, 7770. doi:10.1038/s41598-024-58282-6
Ud-Daula, A. S., Demirci, F., Salim, K. A., Demirci, B., Lim, L. B., Baser, K. H. C., et al. (2016). Chemical composition, antioxidant and antimicrobial activities of essential oils from leaves, aerial stems, basal stems, and rhizomes of Etlingera fimbriobracteata (K. Schum.). Rm. Sm.’ Ind. Crops Prod. 84, 189–198. doi:10.1016/j.apjtm.2017.08.006
Venturella, G., Ferraro, V., Cirlincione, F., and Gargano, M. L. (2021). Medicinal mushrooms: bioactive compounds, use, and clinical trials. Int. J. Mol. Sci. 22, 634. doi:10.3390/ijms22020634
Venturella, G., Gargano, M. L., and Compagno, R. (2015). The genus Pleurotus in Italy. Flora Medit. 25, 143–156. doi:10.7320/FlMedit25SI.143
Wang, W.-K., Zhu, Y., Tang, Y., Lu, N., Song, J. L., Yuan, W.-D., et al. (2016). Non-volatile taste components of different cultivated mushrooms at mycelia, primordium, and fruit body cultivation stages. Int. J. Food Prop. 19, 1938–1948. doi:10.1080/10942912.2015.1089891
Xiao, Q., Yu, H., Zhang, J., Li, F., Li, C., Zhang, X., et al. (2019). The potential of cottonseed hull as biorefinery substrate after biopretreatment by Pleurotus ostreatus and the mechanism analysis based on comparative proteomics. Ind. Crops Prod. 130, 151–161. doi:10.1016/j.indcrop.2018.12.057
Yamaguchi, S. (1991). Fundamental properties of umami taste. J. Agric. Chem. Soc. Jpn. 65, 903–906. doi:10.1016/0031-9384(91)90192-q
Yildirim, N. C., Turkoglu, S., Yildirim, N., and Kaplan Ince, O. (2012). Antioxidant properties of wild edible mushroom Pleurotus eryngii collected from Tunceli province of Turkey. Dig. J. Nanomater. Biostruct (DJNB) 7, 1647–1654.
Yin, H. H., Cho, B. O., Lee, H. S., Chu, J. I., and Jang, S. I. (2016). Synergistic effects of grape branch and Pleurotus eryngii extract combination against inflammation on activated mast cells and atopic dermatitis-like skin lesions in mice. J. Korean Food Sci. Technol. 48 (6), 582–589. doi:10.9721/KJFST.2016.48.6.582
Yokoyama, A., and Natori, S. (1974). Triterpenoids of lanostane group from fruit bodies of nine basidiomycetous species. Chem. Pharm. Bull. 22, 877–883. doi:10.1248/cpb.22.877
Yu, E.-J., Han, S.-R., Kim, K.-H., Park, B.-R., Lim, K.-O., and Oh, T.-J. (2018). Antibacterial and antioxidant activity of Pleurotus eryngii extracts. Indian J. Public Health Res. Dev. 9, 2206. doi:10.5958/0976-5506.2018.02191.5
Yuan, B., Zhao, L., Rakariyatham, K., Han, Y., Gao, Z., Muinde Kimatu, B., et al. (2017). Isolation of a novel bioactive protein from an edible mushroom Pleurotus eryngii and its anti-inflammatory potential. Food Funct. 8, 2175–2183. doi:10.1039/C7FO00244K
Zervakis, G. I., Ntougias, S., Gargano, M. L., Besi, M. I., Polemis, E., Typas, M. A., et al. (2014). A reappraisal of the Pleurotus eryngii complex. New species and taxonomic combinations based on the application of a polyphasic approach, and an identification key to Pleurotus taxa associated with Apiaceae plants. Fungal Biol. 118, 814–834. doi:10.1016/j.funbio.2014.07.001
Zervakis, G. I., Venturella, G., and Papadopoulou, K. (2001). Genetic polymorphism and taxonomic infrastructure of the Pleurotus eryngii species complex as determined by RAPD analysis, isozyme profiles and ecomorphological characters. Microbiology 147, 3183–3194. doi:10.1099/00221287-147-11-3183
Zhao, S., Gao, Q., Rong, C., Wang, S., Zhao, Z., Liu, Y., et al. (2020). Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. J. Fungi 6, 269–306. doi:10.3390/jof6040269
Keywords: Pleurotus eryngii var. ferulae, Pleurotus eryngii var. elaeoselini, LC-MS, antibacterial activity, antifungal activity
Citation: Polito F, De Martino L, Mirabile G, Venturella G, Gargano ML, De Feo V, Elshafie HS and Camele I (2024) Composition and antimicrobial activity of hydroalcoholic extracts of Pleurotus eryngii var. ferulae and P. eryngii var. elaeoselini. Front. Chem. 12:1498787. doi: 10.3389/fchem.2024.1498787
Received: 20 September 2024; Accepted: 11 November 2024;
Published: 04 December 2024.
Edited by:
Tara Louise Pukala, University of Adelaide, AustraliaReviewed by:
Abdallah M. A. Hassane, Al-Azhar University, EgyptYusufjon Gafforov, New Uzbekistan University, Uzbekistan
Maura Téllez Téllez, Autonomous University of the State of Morelos, Mexico
Copyright © 2024 Polito, De Martino, Mirabile, Venturella, Gargano, De Feo, Elshafie and Camele. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincenzo De Feo, ZGVmZW9AdW5pc2EuaXQ=
 Flavio Polito
Flavio Polito Laura De Martino
Laura De Martino Giulia Mirabile2
Giulia Mirabile2 Giuseppe Venturella
Giuseppe Venturella Vincenzo De Feo
Vincenzo De Feo Ippolito Camele
Ippolito Camele