- 1EaStCHEM School of Chemistry, The University of Edinburgh, Edinburgh, Scotland, United Kingdom
- 2Instituto de Nanociencia y Materiales de Aragón (INMA), CSIC & Universidad de Zaragoza, Zaragoza, Spain
The reaction of MnCl2·4H2O with HL ((1-methyl-1H-imidazol-2-yl)methanol) and pdH2 (1, 3 propanediol) in a basic MeCN solution results in the formation of a mixed-valence [Mn20] cationic cluster and two [MnIICl4] counter anions. The metallic skeleton of the cluster describes two geometrically equivalent mixed-valent, linked [MnIII6MnII4] supertetrahedra in which nearest-neighbor metal ions have a different oxidation state. Magnetic susceptibility, magnetization data and heat capacity measurements support evidence of predominant ferromagnetic correlations, leading to a s = 22 spin ground state for the [MnIII6MnII4] supertetrahedra, which are pair-linked by a weak antiferromagnetic coupling. The properties are discussed in the context of the magnetocaloric effect and the potential application of this compound in cryogenic refrigeration.
1 Introduction
As early as the 1990s, seminal publications promoted the use of ferromagnetic particles for magnetic refrigeration (McMichael et al., 1992; Shull, 1993; Bennett et al., 1994). Clustering spin moments into noninteracting particles results in a net magnetic moment per particle. An applied magnetic field can align the large magnetic moments of ferromagnetic particles more easily than a magnetic domain of similar size in the bulk equivalent paramagnetic material, at least for certain temperatures and particle sizes. In a magnetocaloric material, the change of the applied magnetic field induces a change in the material’s magnetic entropy (ΔSm) and adiabatic temperature (ΔTad). The magnetocaloric effect (MCE) can therefore be substantial, and enhanced, in ferromagnetic particles. However, interparticle interactions, size distributions, and the presence of non-active solvent, are all ingredients that negatively affect their performance in terms of the MCE.
Magnetic molecular clusters inherit the advantages of ferromagnetic particles and are in many ways superior because of ideal monodispersity in size, shape and magnetic moment. In addition, their molecular nature opens avenues for fine tuning properties (Evangelisti et al., 2011; Sharples et al., 2014; Tziotzi et al., 2023; Zhai et al., 2024). This last point is crucial for improving their MCE (Evangelisti and Brechin, 2010). Sought-after molecular clusters are those with a large spin ground state and a small magnetic anisotropy, because of their easier polarization by the applied magnetic field. At first, the archetypal “single-molecule magnets” such as [Mn12] and [Fe8] were proposed for magnetic refrigeration (Torres et al., 2000; Zhang et al., 2001; Spichkin et al., 2001), but their huge anisotropies limit their applicability as refrigerants despite the relatively large s = 10 spin ground state. The search for isotropic molecular clusters led to heterometallic Cr-based wheels, whose limitations are in the small value of their spin ground state (Affronte et al., 2004). The first high-spin and low-anisotropy molecular cluster was the highly symmetric supertetrahedron [Mn10O4Br4(amp)6(ampH2)3(HampH2)]Br3 (ampdH2 = 2-amino-2-methyl-1,3-propanediol), a mixed-valent ([MnIII6MnII4]) ferromagnetic cluster with a remarkable s = 22 ground state displaying negligible anisotropy (Manoli et al., 2007; Manoli et al., 2008). Most of the focus has shifted since then into gadolinium-containing molecular clusters, first in the form of mixed 3d-4f clusters (Karotsis et al., 2009), then as purely gadolinium-based clusters (Evangelisti et al., 2011). Gadolinium is nowadays considered as the standard element for any new molecular cluster for magnetic refrigeration, the advantage residing in its large s = 7/2 moment, zero orbital angular momentum and weak magnetic correlations that, together, facilitate record-high MCE values (Konieczny et al., 2022; Tziotzi et al., 2023). However, for commercial uptake, magnetocaloric materials should be made from safe, inexpensive, and abundant elements (Chaudhary et al., 2019). Since gadolinium is treated as critical because of the concerns surrounding its supply (Zhao et al., 2023), it could be replaced, for instance, by the earth abundant high spin, FeIII and MnII ions without significantly deteriorating the MCE due to a not much smaller s = 5/2. Here, we show that the use of 1,3-propanediol in combination with the N,O-chelate (1-methyl-1H-imidazol-2-yl)methanol (HL) can be used to isolate the cluster [MnIII12MnII8O8(L)16(HL)2(pd)4(pdH2)Cl8][MnIICl4]2·3MeCN·9C2H6O (1·3MeCN·9C2H6O), which is structurally related to [Mn10O4Br4(amp)6(ampH2)3(HampH2)]Br3 (Manoli et al., 2007). Compound 1 contains two analogous [MnIII6MnII4] supertetrahedra carrying a s = 22 ground state, but on this occasion, they are covalently linked into a [Mn10]2 dimer through a single pdH2 bridge. In comparison with [Mn10O4Br4(amp)6(ampH2)3(HampH2)]Br3 (Manoli et al., 2007), the structure of 1 is considerably lighter, which ultimately promotes a larger MCE, favored by the larger weight of magnetic elements with respect to nonmagnetic ones, which act passively (Lorusso et al., 2013; Tziotzi et al., 2023).
2 Experimental methods
2.1 Synthesis
MnCl2·4H2O (198 mg, 1 mmol), HL (112 mg, 1 mmol), pdH2 (72 μL, 1 mmol) and NEt3 (420 μL, 3 mmol) were dissolved in MeCN (15 mL) and stirred for 1 h. The solution was then filtered and diffused with acetone. Brown crystals of 1 were obtained after 2 days. Elemental analysis (%) calculated for Mn22O45N56C133H216Cl16: C, 31.35; H, 4.27; N, 15.39. Found: C, 31.42; H, 4.38; N, 15.27. Yield ≤40%.
2.2 Single-crystal X-ray diffraction
A suitable brown, blade-shaped crystal of 1·3MeCN·9C2H6O with dimensions 0.18 × 0.07 × 0.04 mm3 was selected and mounted on a MITIGEN holder in perfluoroether oil on a Rigaku Oxford Diffraction SuperNova diffractometer. The crystal was kept at a steady T = 100.00 K during data collection. The structure was solved with the ShelXT (Sheldrick, 2015a) solution program using dual methods and by using Olex2 1.5-beta (Dolomanov et al., 2009) as the graphical interface. The model was refined with ShelXL 2018/3 (Sheldrick, 2015b) using full matrix least squares minimization on F2. Crystal Data. C133.4H216.41Cl16Mn22N36.39O45.32, Mr = 4,831.09, triclinic, P-1 (No. 2), a = 14.7918(17) Å, b = 18.444(2) Å, c = 19.275(2) Å, α = 82.653(5)°, β = 69.696(5)°, γ = 80.063(5)°, V = 4,844.7(10) Å3, T = 100.00 K, Z = 1, Z' = 0.5, m(MoKa) = 1.675, 279539 reflections measured, 26124 unique (Rint = 0.0580) which were used in all calculations. The final wR2 was 0.1620 (all data) and R1 was 0.0558 (I ≥ 2σ(I)). CCDC = 2342033.
2.3 Physical properties measurements
Magnetization and magnetic susceptibility data were collected on a freshly prepared polycrystalline powder of 1 on a Quantum Design MPMS3 SQUID magnetometer, equipped with a 7 T magnet in the temperature range 2–300 K. Diamagnetic corrections were applied to the observed paramagnetic susceptibilities using Pascal’s constants. Heat capacity measurements were carried out using a Quantum Design PPMS, equipped with a 3He option and a 9 T magnet in the temperature range 0.3–30 K. The polycrystalline sample of 1 was in the form of a thin pressed pellet (ca. 1 mg), thermalized by ca. 0.2 mg of Apiezon N grease, whose contribution was subtracted by using a phenomenological expression.
3 Results and discussion
The reaction of MnCl2·4H2O with HL ((1-methyl-1H-imidazol-2-yl)methanol) and pdH2 (1,3-propanediol) in a basic MeCN solution results in the formation of the mixed-valence compound [MnIII12MnII8O8(L)16(HL)2(pd)4(pdH2)Cl8][MnIICl4]2·3MeCN·9C2H6O in (1), upon diffusion of acetone into the mother liquor (Figure 1). Compound 1 crystallizes in the triclinic space group P-1 with the asymmetric unit comprising half of the formula.
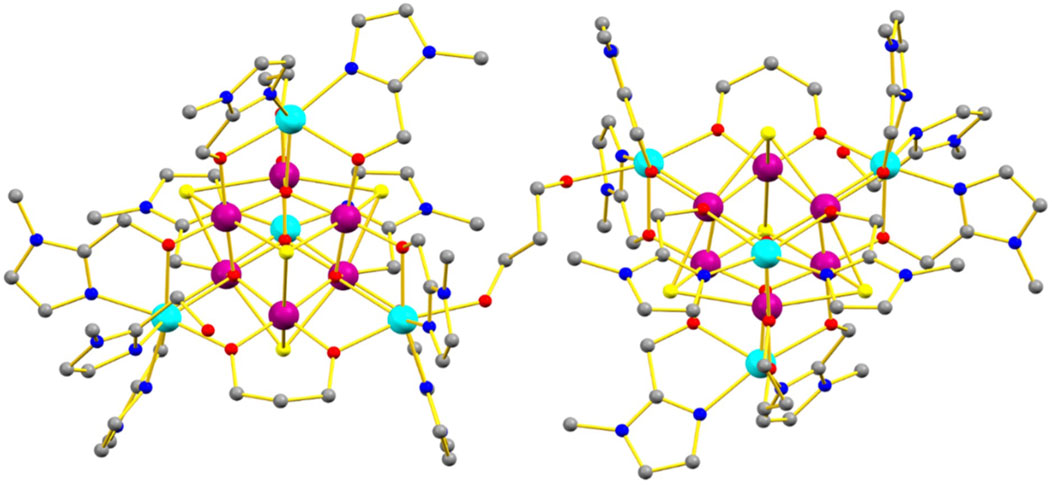
Figure 1. Molecular structure of 1. Color code: MnIII = purple, MnII = cyan, O = red, N = blue, C = grey, Cl = yellow. Solvent molecules, anions and H atoms omitted for clarity.
The metallic skeleton of 1 (Figure 2) describes two geometrically equivalent mixed-valent [MnIII6MnII4] supertetrahedra, bridged via a protonated pdH2 ligand through Mn8 (and symmetry equivalent (s. e.), MnII-MnII). Nearest neighbors within each supertetrahedron have a different oxidation state (MnII = Mn1, Mn6, Mn8, Mn10; MnIII = Mn2, Mn3, Mn4, Mn5, Mn7, Mn9).
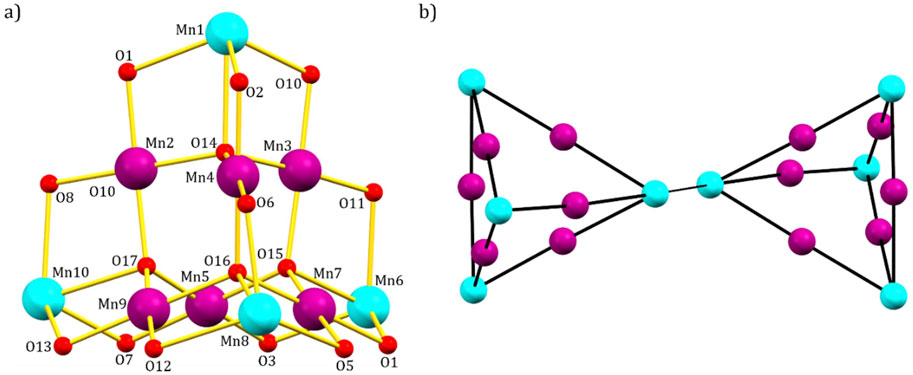
Figure 2. (A) Metal-oxygen core; (B) metallic skeleton of 1. Color code: MnIII = purple, MnII = cyan, O = red.
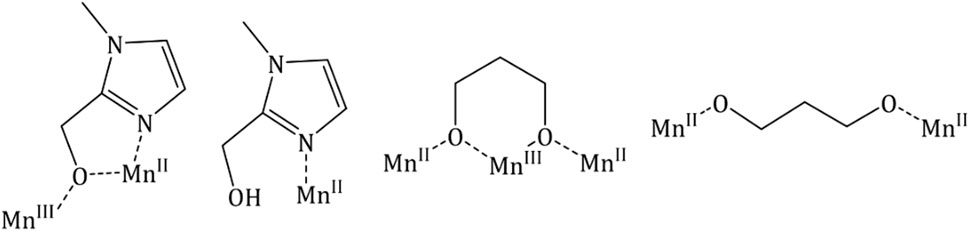
The MnII ions define the four apices of the tetrahedron while the six MnIII ions lie along each edge, therefore describing a trigonal antiprism. The metal ions are connected via four μ4-O2− ions to give a [MnIII6MnII4O4]18+ core such that the supertetrahedron can be thought of as being built from four vertex-sharing [MnIII3MnIIO]9+ tetrahedra. Each chloride ion caps one face of the tetrahedron acting as a μ3-bridge for the MnIII ions (Mn-Br, ∼2.7 Å), while all L1− ligands display the same coordination mode, chelating to each MnII ion and bridging between two different MnIII ions (Scheme 1).
The two protonated HL ligands complete the coordination sphere of Mn10 (and s. e.), by terminally bonding to it through the N-atom. The metal ions are all six-coordinate and in distorted {MnO4N2} and {MnO4Cl2} octahedral geometries for the MnII and MnIII ions, respectively. The only exceptions are Mn8 and Mn10, that are hepta-coordinated and in distorted {MnO5N2} and {MnO4N3} pentagonal bipyramidal geometries, respectively.
The pd2− ligands chelate Mn3 and Mn9 (and s. e.) and bridge between two different MnII ions (Mn1/Mn6 and Mn8/Mn10, respectively), completing their coordination sphere. The MnIII ions are Jahn-Teller distorted with the JT axes being defined by the {Cl-Mn-Cl} vectors. Charge balance is maintained by the presence of the two [MnIICl4]2− counter anions. The protonated pdH2 ligands form intramolecular H-bonds to the acetone molecules of crystallization (O(H)⋯O, ∼2.9 Å), and the clusters pack in a brickwork like fashion in the extended structure (Figure 3).

Figure 3. Packing of the clusters of 1 in the extended structure viewed down the b-axis of the unit cell. H-bonds are highlighted with thin black dotted lines. Color code: MnIII = purple, MnII = cyan, O = red, N = blue, C = grey. Solvent molecules and H atoms omitted for clarity.
Direct current (DC) magnetic susceptibility (χ) data on a sample of 1 were collected in the 2–300 K temperature range in an applied magnetic field of B = 0.1 T, and are plotted as χT versus T in Figure 4 (inset). The experimental χT value at room temperature (113.1 cm3 K mol−1) is higher than the value expected for 10 MnII and 12 MnIII noninteracting ions per formula unit (79.75 cm3 K mol−1). More importantly, χT steadily increases on lowering the temperature, reaching a maximum of 476.4 cm3 K mol−1 at 12.5 K, before dropping down to 356.8 cm3 K mol−1 at 2.0 K. The magnetic behavior of 1 denotes predominant ferromagnetic interactions, likely associated with a s = 22 ground state for each [MnIII6MnII4] supertetrahedron in close analogy with previous studies (Manoli et al., 2007). Indeed, assuming that all interactions within each super supertetrahedron are ferromagnetic, the effective spin at low temperatures is the sum of 4 s = 5/2 (MnII) and 6 s = 2 (MnIII) spins, leading to a net s = 22. Weaker antiferromagnetic correlations between the supertetrahedra and/or Zeeman effects can account for the low-temperature χT decrease. Alternating current (AC) magnetic susceptibility measurements show no out-of-phase signal, hence no single-molecule magnet behavior for 1, as expected from the high symmetry of the supertetrahedron (Manoli et al., 2007). Isothermal magnetization (M) measurements were collected in the ranges 0–7 T and 2–10 K. The M data plotted versus B/T merge nicely into a single curve, except for the slight deviation of the data at the lowest temperature of 2 K (Figure 4). The relatively fast variation at low fields further confirms the predominant ferromagnetic interactions in 1. The saturation reached at low temperatures corresponds precisely to 98.0 NμB, which is consistent with the value expected from 2 s = 22 [MnIII6MnII4] supertetrahedra and 2 s = 5/2 [MnIICl4] anions, all for g = 2.0 and negligible anisotropies. For T > 2 K, the experimental data are well modeled by the sum of the corresponding Brillouin functions (solid line in Figure 4).
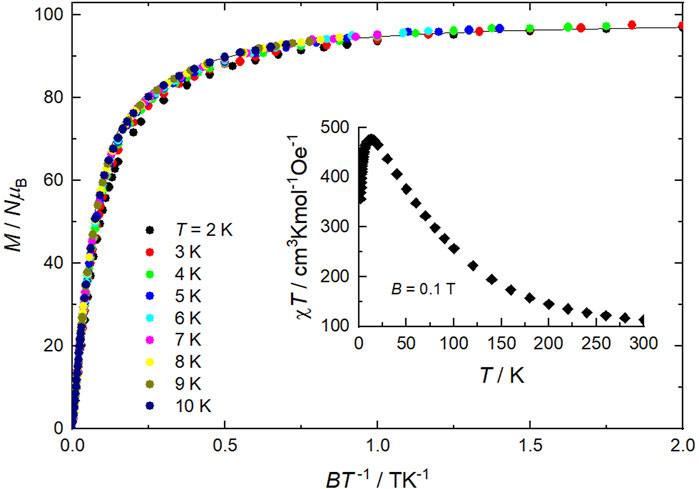
Figure 4. Isothermal low-temperature magnetization (M) curves versus B/T in the ranges T = 2–10 K and B = 0–7 T, and temperature dependence of the DC magnetic susceptibility at B = 0.1 T (as χΤ, inset) for 1. The solid line is the fit of the M data, see main text.
Calorimetry experiments were conducted on a pressed pellet sample of 1 for B up to 7 T and temperatures between 0.3 and 30 K. At high temperatures, the heat capacity (cp, Figure 5) is dominated by the nonmagnetic contribution associated with lattice phonon vibrations, which follows Debye’s law (dashed line) that, below ca. 5 K, simplifies to cp/R = aT 3, where a = 2.5 × 10−2 K−3 and R is the gas constant. At low temperatures, cp depends strongly on B, as expected in the case of ferromagnetic correlations. Note that the heat capacity of an equivalent system with ten MnII and twelve MnIII ions per formula unit, but without any interaction, would be drastically different from the experimental data. We tentatively modeled the magnetic contribution to cp for B
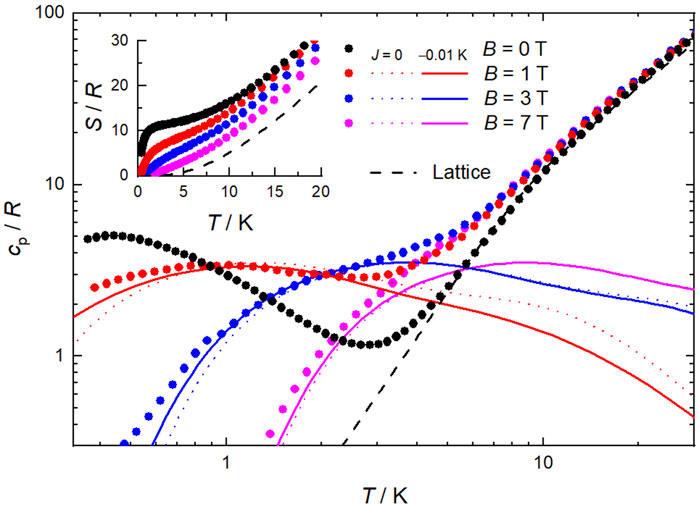
Figure 5. Temperature dependence of the heat capacity (cp) and entropy (S, inset), both normalized to the gas constant, for selected applied field values for 1. Experimental data = symbols, modelling = lines, including the lattice contribution (dashed line).
The magnetic contribution to S at zero-applied field is seen to level off at temperatures between ca. 2–4 K to the value corresponding to 2 s = 22 [MnIII6MnII4] supertetrahedra and 2 s = 5/2 [MnIICl4] anions, i.e., S = 2×[ln(2 × 22 + 1)+ln(2 × 5/2 + 1)]R = 11.2R = 19.3 Jkg−1 K−1. At higher temperatures, S increases steadily with temperature, mainly because of the nonmagnetic lattice entropy.
Next, we evaluate the magnetocaloric effect, as differences between the entropy curves in Figure 5. The temperature dependencies of ΔSm and ΔTad are depicted in Figure 6 for several applied-field changes ΔB = (B−0), i.e., full demagnetization from B = 1, 3 or 7 T. The entropy changes are also computed by applying the Maxwell relation to the magnetization data, namely
whose results are plotted in Figure 6. As can be seen, both sets of ΔSm data, complementarily evaluated from S (hence cp) and M data, respectively, agree very well with one another, therefore proving the validity of our approach. The strongest MCE takes place at low temperatures near 2 K. Specifically, the maximal −ΔSm = 19.2 Jkg−1K−1 (which corresponds to the full entropy content for s = 22 spins) occurs at T = 2.1 K, while the maximal ΔTad = 9.6 K at T ≡ Te = 1.4 K, both for ΔB = 7 T (Figure 6), where Te is the ending temperature of the demagnetization process. The observed maximum of −ΔSm is relatively large and compares positively with most Mn-based molecular clusters reported for magnetic refrigeration (Table 1), but less favorably with respect to selected Gd-containing molecular compounds (Evangelisti et al., 2011; Sharples et al., 2014; Konieczny et al., 2022; Tziotzi et al., 2023; Zhai et al., 2024). Where 1 excels is in the temperature range over which the entropy change remains fairly large, e.g., −ΔSm = 10.5 Jkg−1K−1 at T = 20 K and −ΔSm = 8.7 Jkg−1 K−1 at T = 30 K, which are equivalent to 54% and 45% of the observed maximal value, respectively. That is, the strength of the MCE decreases just by half after increasing the temperature by an order of magnitude. This behavior originates from the broad Schottky-like anomaly (Figure 5), which in turn results from the ferromagnetic interactions that are robust to the applied fields. Clusters with a high-spin ground state generated from strong ferromagnetic correlations, such as 1, are uncommon (Konieczny et al., 2022). These characteristics can be an advantage to MCE, if exploited properly. Clearly, the entropy of a hypothetical molecule having n spins s is smaller when the spins couple ferromagnetically, yielding a net spin ns at low temperature, than when they do not, i.e., ln(ns) < n×ln(s). Therefore, if one targets the largest MCE value, interactions should be avoided. However, the release of the entropy with temperature/field depends on the strength of the ferromagnetic interactions, or their lack thereof, being more abrupt when such interactions are important and hence facilitating a relatively large MCE with small changes of temperature/field (Evangelisti and Brechin, 2010). This behavior is clearly seen in 1, specifically in the temperature and field dependence of its −ΔSm and ΔTad figures of merit that we plot in Figure 6 together with those calculated for a system of ten MnII and twelve MnIII noninteracting ions per formula unit, for comparison. For any given ΔB, larger −ΔSm and ΔTad can be produced at the lowest temperatures in the absence of interactions, while 1 outperforms the noninteracting system over a broad temperature/field range. For instance, to reach −ΔSm = 4.5 Jkg−1K−1 at T = 8.4 K, or similarly ΔTad = 3.3 K at T = 5.0 K, an applied-field change of ΔB = 2 T would be needed with the noninteracting system, while just ΔB = 1 T would suffice with 1 (Figure 6).
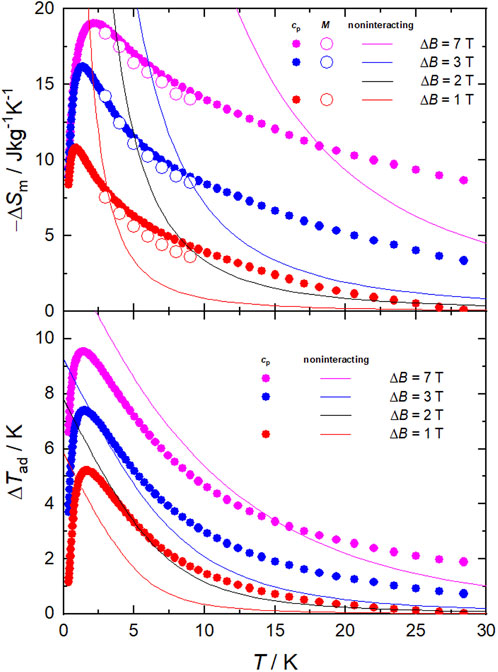
Figure 6. Temperature dependence of the magnetic entropy change (−ΔSm, top) and adiabatic temperature change (ΔTad, bottom) for 1, obtained from the experimental magnetization (empty symbols) and heat capacity (filled symbols) data. Depicted for comparison are the calculated −ΔSm and ΔTad (solid lines), at the indicated ΔB, for a hypothetical compound equivalent to 1 but with no magnetic interactions.
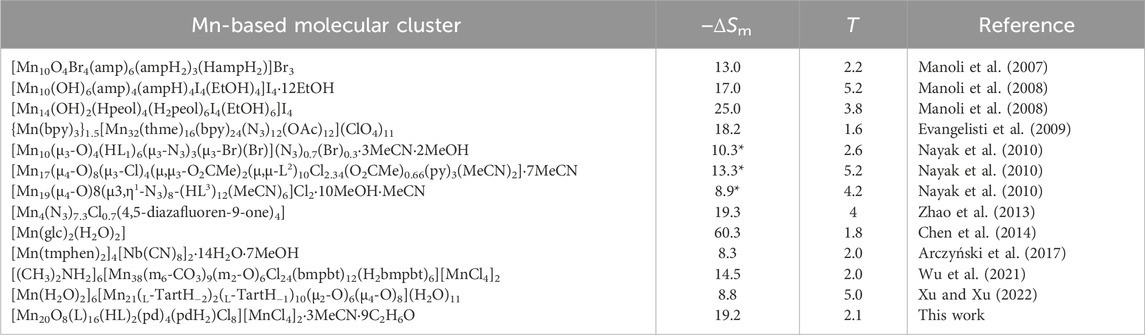
Table 1. Mn-based molecular clusters proposed for magnetic refrigeration; maximal magnetic entropy changes, −ΔSm, in units of Jkg−1K−1 and for ΔB = 7 T (or 9 T, where indicated by *); temperatures of maximal −ΔSm, in K; references.
Finally, we compare the magnetic and magnetocaloric properties of 1 with those of the structurally related cluster [Mn10O4Br4(amp)6(ampH2)3(HampH2)]Br3 (Manoli et al., 2007). Magnetically, both compounds behave similarly owing to the s=22 ground state of the ferromagnetic and isotropic [MnIII6MnII4] supertetrahedra. The “dimer” coupling in 1 is relatively weak (
4 Concluding remarks
We have synthesized a molecular magnetic refrigerant, characterized by a mixed-valence [Mn20] cationic cluster and two [MnIICl4] counter anions. Each [Mn20] unit can be magnetically described as formed by two ferromagnetic and isotropic [MnIII6MnII4] supertetrahedra with s=22, coupled mutually by a weak antiferromagnetic interaction. Such a large spin ground state promotes an enhanced magnetocaloric response to the applied magnetic field and a MCE that remains relatively large over a broad temperature range, e.g., from −ΔSm = 19.2 Jkg−1K−1 at 2.1 K to −ΔSm = 8.7 Jkg−1K−1 at T = 30 K, for ΔB = 7 T, that is roughly a decrease by a factor of 2 upon increasing the temperature by an order of magnitude. Complex 1 differs from the closely related and previously reported cluster [Mn10O4Br4(amp)6(ampH2)3(HampH2)]Br3 (Manoli et al., 2007) because 1 is significant lighter, that is, its larger magnetic density makes it a better magnetic refrigerant.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
EA: Writing–review and editing, Investigation, Formal Analysis, Data curation. EC: Writing–review and editing, Investigation, Formal Analysis, Data curation. GN: Data curation, Formal Analysis, Investigation, Writing–review and editing. DG: Writing–review and editing, Investigation, Formal Analysis, Data curation. ME: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Supervision, Writing–original draft, Writing–review and editing. EB: Funding acquisition, Conceptualization, Data curation, Formal Analysis, Investigation, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by MICINN (PID 2021-124734OB-C21) and Diputación General de Aragón (E11-23R, E12-23R). EKB thanks the EPSRC for funding grant EP/V010573/1. EKC and DG acknowledge financial supports from MICINN and the Gobierno de Aragón, respectively, through their doctoral fellowships.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Affronte, M., Ghirri, A., Carretta, S., Amoretti, G., Piligkos, S., Timco, G. A., et al. (2004). Engineering molecular rings for magnetocaloric effect. Appl. Phys. Lett. 84, 3468–3470. doi:10.1063/1.1737468
Arczyński, M., Rams, M., Stanek, J., Fitta, M., Sieklucka, B., Dunbar, K. R., et al. (2017). A family of octahedral magnetic molecules based on [NbIV(CN)8]4–. Inorg. Chem. 56, 4021–4027. doi:10.1021/acs.inorgchem.6b03134
Bennett, L. H., McMichael, R. D., Tang, H. C., and Watson, R. E. (1994). Monte Carlo simulations of the magnetocaloric effect in superferromagnetic clusters having uniaxial magnetic anisotropy. J. Appl. Phys. 75, 5493–5495. doi:10.1063/1.355667
Chaudhary, V., Chen, X., and Ramanujan, R. V. (2019). Iron and manganese based magnetocaloric materials for near room temperature thermal management. Prog. Mater. Sci. 100, 64–98. doi:10.1016/j.pmatsci.2018.09.005
Chen, Y.-C., Guo, F.-S., Liu, J.-L., Leng, J.-D., Vrábel, P., Orendáč, M., et al. (2014). Switching of the magnetocaloric effect of MnII glycolate by water molecules. Chem. Eur. J. 20, 3029–3035. doi:10.1002/chem.201304423
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., and Puschmann, H. (2009). OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341. doi:10.1107/S0021889808042726
Evangelisti, M., and Brechin, E. K. (2010). Recipes for enhanced molecular cooling. Dalton Trans. 39, 4672–4676. doi:10.1039/b926030g
Evangelisti, M., Candini, A., Affronte, M., Pasca, E., de Jongh, L. J., Scott, R. T. W., et al. (2009). Magnetocaloric effect in spin-degenerated molecular nanomagnets. Phys. Rev. B 79, 104414. doi:10.1103/PhysRevB.79.104414
Evangelisti, M., Roubeau, O., Palacios, E., Camón, A., Hooper, T. N., Brechin, E. K., et al. (2011). Cryogenic magnetocaloric effect in a ferromagnetic molecular dimer. Angew. Chem. Int. Ed. 50, 6606–6609. doi:10.1002/anie.201102640
Karotsis, G., Evangelisti, M., Dalgarno, S. J., and Brechin, E. K. (2009). A calix[4]arene 3d/4f magnetic cooler. Angew. Chem. Int. Ed. 48, 9928–9931. doi:10.1002/anie.200905012
Konieczny, P., Sas, W., Czernia, D., Pacanowska, A., Fitta, M., and Pełka, R. (2022). Magnetic cooling: a molecular perspective. Dalton Trans. 51, 12762–12780. doi:10.1039/d2dt01565j
Lorusso, G., Sharples, J. W., Palacios, E., Roubeau, O., Brechin, E. K., Sessoli, R., et al. (2013). A dense metal-organic framework for enhanced magnetic refrigeration. Adv. Mater. 25, 4653–4656. doi:10.1002/adma.201301997
Manoli, M., Collins, A., Parsons, S., Candini, A., Evangelisti, M., and Brechin, E. K. (2008). Mixed-valent Mn supertetrahedra and planar discs as enhanced magnetic coolers. J. Am. Chem. Soc. 130, 11129–11139. doi:10.1021/ja802829d
Manoli, M., Johnstone, R. D. L., Parsons, S., Murrie, M., Affronte, M., Evangelisti, M., et al. (2007). A ferromagnetic mixed-valent Mn supertetrahedron: towards low-temperature magnetic refrigeration with molecular clusters. Angew. Chem. Int. Ed. 46, 4456–4460. doi:10.1002/anie.200701027
McMichael, R. D., Shull, R. D., Swartzendruber, L. J., Bennett, L. H., and Watson, R. E. (1992). Magnetocaloric effect in superparamagnets. J. Magn. Magn. Mater. 111, 29–33. doi:10.1016/0304-8853(92)91049-Y
Nayak, S., Evangelisti, M., Powell, A. K., and Reedijk, J. (2010). Magnetothermal studies of a series of coordination clusters built from ferromagnetically coupled {MnII4MnIII6} supertetrahedral units. Chem. Eur. J. 16, 12865–12872. doi:10.1002/chem.201001988
Sharples, J. W., Collison, D., McInnes, E. J. L., Schnack, J., Palacios, E., and Evangelisti, M. (2014). Quantum signatures of a molecular nanomagnet in direct magnetocaloric measurements. Nat. Commun. 5, 5321. doi:10.1038/ncomms6321
Sheldrick, G. M. (2015a). SHELXT-integrated space-group and crystal-structure determination. Acta Cryst. A71, 3–8. doi:10.1107/S2053273314026370
Sheldrick, G. M. (2015b). Crystal structure refinement with SHELXL. Acta Cryst. C71, 3–8. doi:10.1107/S2053229614024218
Shull, R. D. (1993). Magnetocaloric effect of ferromagnetic particles. IEEE Trans. Magn. 29, 2614–2615. doi:10.1109/20.280849
Spichkin, Yu. I., Zvezdin, A. K., Gubin, S. P., Mischenko, A. S., and Tishin, A. M. (2001). Magnetic molecular clusters as promising materials for refrigeration in low-temperature regions. J. Phys. D. Appl. Phys. 34, 1162–1166. doi:10.1088/0022-3727/34/8/306
Torres, F., Hernández, J. M., Bohigas, X., and Tejada, J. (2000). Giant and time-dependent magnetocaloric effect in high-spin molecular magnets. Appl. Phys. Lett. 77, 3248–3250. doi:10.1063/1.1325393
Tziotzi, T. G., Gracia, D., Dalgarno, S. J., Schnack, J., Evangelisti, M., Brechin, E. K., et al. (2023). A {Gd12Na6} molecular quadruple-wheel with a record magnetocaloric effect at low magnetic fields and temperatures. J. Am. Chem. Soc. 145, 7743–7747. doi:10.1021/jacs.3c01610
Wu, T.-X., Tao, Y., He, Q.-J., Li, H.-Y., Bian, H.-D., and Huang, F.-P. (2021). Constructing an unprecedented {MnII38} matryoshka doll with a [Mn18(CO3)9] inorganic core and magnetocaloric effect. Chem. Commun. 57, 2732–2735. doi:10.1039/d0cc07884k
Xu, X., and Xu, F. (2022). A heniconuclear {Mn21} cluster-based coordination polymer with Manganese(II) linkers showing high proton conductivity. Inorg. Chem. 61, 16038–16044. doi:10.1021/acs.inorgchem.2c02441
Zhai, Y.-Q., Chen, W.-P., Evangelisti, M., Fu, Z., and Zheng, Y.-Z. (2024). Gd-based molecular coolants: aggregating for better magnetocaloric effect. Aggregate 5, e520. doi:10.1002/agt2.520
Zhang, X. X., Wei, H. L., Zhang, Z. Q., and Zhang, L. (2001). Anisotropic magnetocaloric effect in nanostructured magnetic clusters. Phys. Rev. Lett. 87, 157203. doi:10.1103/PhysRevLett.87.157203
Zhao, J. P., Zhao, R., Yang, Q., Hu, B. W., Liu, F. C., and Bu, X. H. (2013). Magnetocaloric effect and slow magnetic relaxation in two only azido bridged ferromagnetic tetranuclear metal clusters. Dalton Trans. 42, 14509–14515. doi:10.1039/C3DT51655E
Keywords: cluster compounds, magnetic properties, ferromagnetism, magnetocaloric effect, manganese, manganese
Citation: Agapaki E, Charkiolakis EK, Nichol GS, Gracia D, Evangelisti M and Brechin EK (2024) Magnetocaloric effect in a high-spin ferromagnetic molecular cluster. Front. Chem. 12:1494609. doi: 10.3389/fchem.2024.1494609
Received: 11 September 2024; Accepted: 22 October 2024;
Published: 31 October 2024.
Edited by:
Ming Wu, Xi’an Jiaotong University, ChinaReviewed by:
Cai-Ming Liu, Chinese Academy of Sciences (CAS), ChinaSilvia Gómez-Coca, University of Barcelona, Spain
Copyright © 2024 Agapaki, Charkiolakis, Nichol, Gracia, Evangelisti and Brechin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Evangelisti, ZXZhbmdlQHVuaXphci5lcw==; Euan K. Brechin, ZS5icmVjaGluQGVkLmFjLnVr
 Eleftheria Agapaki1
Eleftheria Agapaki1 Marco Evangelisti
Marco Evangelisti