- Pharmacy Department, Jiang Men Maternity and Child Healthcare Hospital, Jiangmen, China
Tyrosinase is one important rate limiting enzyme in melanin synthesis, directly affecting the melanin synthesis. Quercetagetin is one active ingredient from marigold. Thence, the inhibition effects of quercetagetin against tyrosinase were investigated. The results showed quercetagetin could inhibit tyrosinase activity with IC50 value of 0.19 ± 0.01 mM and the inhibition type was a reversible mixed-type. Results of fluorescence quenching showed quercetagetin could quench tyrosinase fluorescence in static process. CD and 3D fluorescence results showed the interaction of quercetagetin to tyrosinase could change tyrosinase conformation to inhibit activity. Moreover, docking revealed details of quercetagetin’s interactions with tyrosinase.
1 Introduction
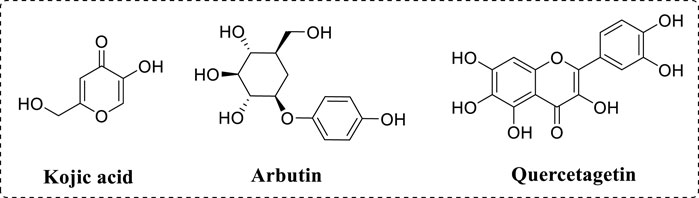
As we all know, various diseases affect people’s health (Zhang et al., 2020; Zhang X. et al., 2021; Shi et al., 2023; Zhou S. et al., 2022). As a therapeutic target, tyrosinase is one important metal enzyme containing two copper, which widely presents in the organism (Min et al., 2023; Fan et al., 2017). Tyrosinase has been confirmed to be involved in the synthesis of melanin (Li et al., 2023; Hassan et al., 2023; Li J. et al., 2021). The first reaction process is monophenolase activity, in which L-tyrosine is hydroxylated into L-dopa and the second reaction process is diphenolase activity, in which L-dopa is subsequently oxidized into dopaquinone (Djafarou et al., 2023; Lee et al., 2023; Zargaham et al., 2023). In the organism, melanin acts crucial roles to protect the skin from UV radiation (Lu et al., 2023a; Znajafi et al., 2023). But, excessive elevated melanin content leads to lots of pigmentation disorders, including age spots and melanoma (Broulier et al., 2023; De Barros et al., 2023; Xue et al., 2023). Inhibition of tyrosinase activity would reduce melanin generation, thence, the finding on novel tyrosinase inhibitors is attracting more attention due to their potential application in medicine and cosmetics fields (Wang and Mu, 2021). Although kojic acid and arbutin (Figure 1) are applied as tyrosinase inhibitors in the medical and industrial fields, they still have been found to lots of adverse side effects (Wang et al., 2021; Esma et al., 2023). Now, to find novel tyrosinase inhibitors is essential for treatment of melanin synthesis (Romagnoli et al., 2022; Nasab et al., 2023).
Natural products have been the vital sources for clinical drugs (Li Y. et al., 2021; Wu et al., 2021; Chen et al., 2022; Zang et al., 2022). Many natural products display widely biological activities, such as antioxidant (Zhang Y. et al., 2021; Tao et al., 2022; Tang et al., 2023), anti-tumor (Liu et al., 2022; Chen et al., 2023; Song et al., 2023), anti-inflammatory (Wang et al., 2020; Wang X. et al., 2022; Zhou Y. et al., 2022), and so on (Wang Y. et al., 2022; Qi et al., 2022; Ma et al., 2023). In particular, natural products show low toxicity (Shao et al., 2020; Jiang et al., 2021; Pei et al., 2021). Thence the development of natural products as tyrosinase inhibitors attracts much attention.
Quercetagetin (Figure 1) is one active ingredient from marigold and has a chemical structure of 3,3′,4′,5,6,7-hexahydroxyflavone (Bulut and Yilmaz, 2021; Wang X. et al., 2022). As one polyhydroxyphenol molecule with multiple hydrogen donor substituents, quercetagetin represents rich biological activities (Wu F. et al., 2023). For example, quercetagetin shows strong antioxidant activity and can effectively scavenge DPPH and ABTS (Fuentes et al., 2021). Quercetagetin also enhances the antioxidant enzymatic activities in broilers tissue by Nrf2/ARE signal pathway (Wu et al., 2022). In addition, quercetagetin displays immunomodulatory and anti-inflammatory to inhibit the release of macrophage-derived chemokine (Rufino et al., 2021). Besides, quercetagetin display other beneficial functions, including anti-virus and anti-diabetes (Seyedi et al., 2016). However, to our knowledge, the inhibition effects of quercetagetin against tyrosinase have not been reported yet.
Hence, in this study, we investigated the inhibition effects of quercetagetin against tyrosinase by the multispectral method, followed by the molecular docking.
2 Results and discussion
2.1 Inhibitory activity
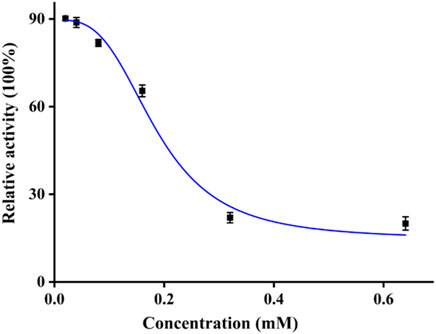
The tyrosinase inhibitory activity of quercetagetin was examined on mushroom tyrosinase. The tyrosinase activity was measured under different concentrations of quercetagetin (Figure 2). It could be observed that tyrosinase relative activity gradually reduced with quercetagetin concentration (0–0.64 mM), meaning that quercetagetin could inhibit the tyrosinase activity with quercetagetin concentration. Its IC50 value was calculated to be 0.19 ± 0.01 mM, which was lower than that of kojic acid. This result showed that quercetagetin could be used as a natural tyrosinase inhibitor.
2.2 Kinetic study
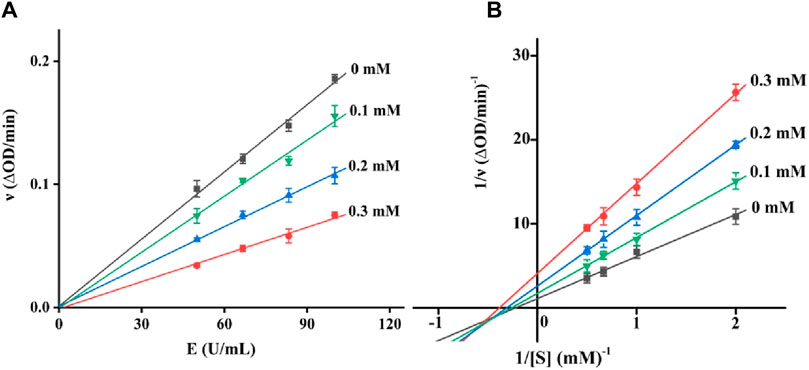
There are two kinds of inhibitors, reversible and non-reversible inhibitors. For a reversible inhibitor, it can reduce the enzyme activity by the binding to enzyme, which can restore the enzyme activity through the remove of inhibitor. There are three reversible inhibitors, including competitive, non-competitive and mixed-type inhibitors. The inhibition type of quercetagetin on tyrosinase was subsequently investigated. With different concentration of quercetagetin and tyrosinase, the absorbance changes were measured (Figure 3A) and found that the lines of quercetagetin with different concentration passed origin and slopes reduced with quercetagetin concentration. The results suggested quercetagetin as a reversible inhibitor. With different concentration of quercetagetin and substrate, the absorbance changes were analyzed using Lineweaver-Burk plots (Figure 3B). The lines of quercetagetin intersected in the third quadrant and their slops increased with quercetagetin concentrations. The results indicated that quercetagetin inhibited tyrosinase in a mixed-type, meaning that quercetagetin bound to tyrosinase and tyrosinase-substrate complex to inhibit its activity. Similar phenomena were obtained in inhibition type of indole-carbohydrazides (Iraji et al., 2022).
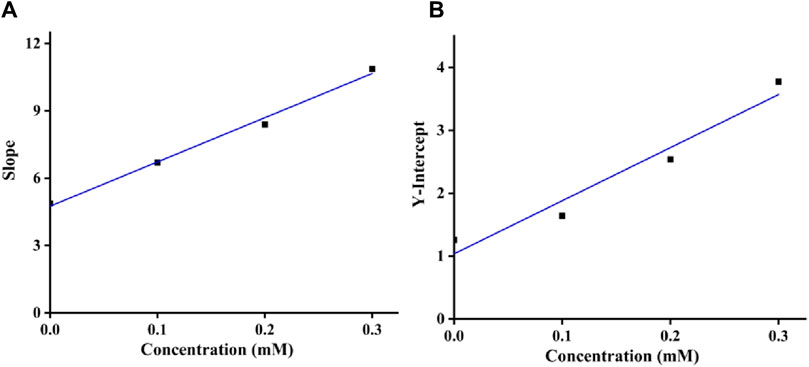
Moreover, as shown in Figures 4A, B, the inhibition constants Ki and Kis were obtained from the secondary curves of inhibitory kinetics to be 0.24 and 0.12 mM. Smaller Kis value than Ki meant that binding force of quercetagetin with tyrosinase-substrate complex was stronger than with tyrosinase. That was to say that quercetagetin preferred to bind with tyrosinase-substrate complex.
2.3 Fluorescence quenching
The fluorescence quenching process of tyrosinase by quercetagetin was investigated. For fluorescence spectra of tyrosinase, they all showed characteristic peaks at 344 nm at 295, 298, and 305 K, respectively (Figures 5A–C). But, quercetagetin did not show effective fluorescence spectra. Moreover, when treated by quercetagetin, tyrosinase presented the gradually decreasing peak intensity (Figures 5A–C), which indicated that quercetagetin bound to tyrosinase.
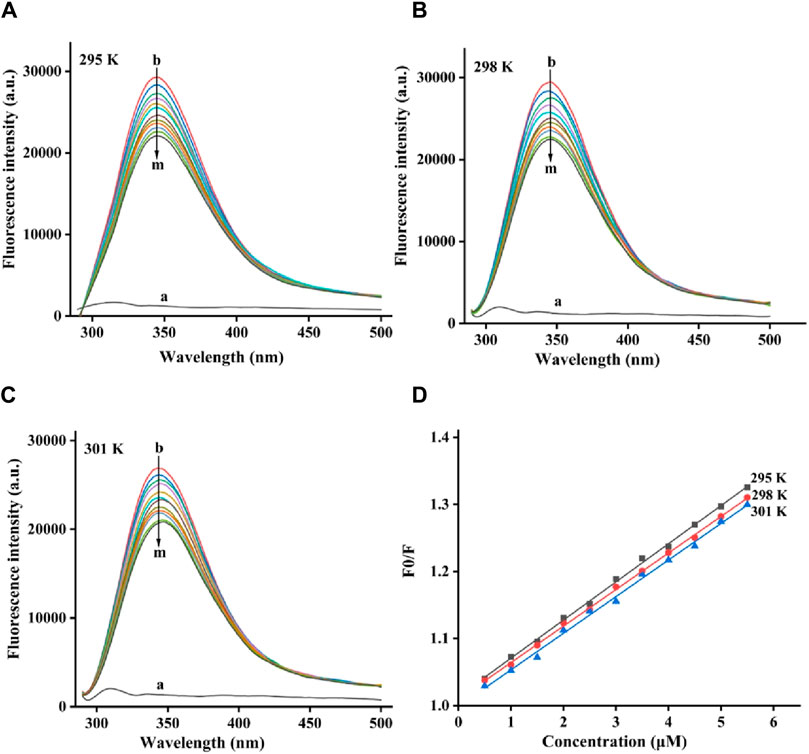
Figure 5. (A–C) Fluorescence quenching spectra of quercetagetin on tyrosinase at 295, 298, and 305 K, respectively; (D) Stern-Volmer plots of quercetagetin.
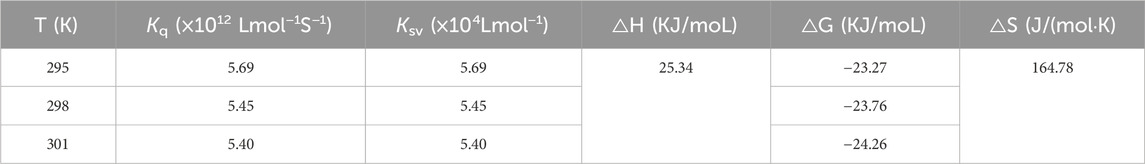
The fluorescence quenching data at 295, 298, and 305 K were future analyzed by Stern-Volmer plots. As is shown in Figure 5D the Stern-Volmer plots of quercetagetin, lines with different temperature presented good linearity, meaning that there was only one quenching type of static or dynamic in quenching process. Then the quenching constant (Ksv) the bimolecular quench rate constant (Kq) were obtained (Table 1). The Ksv results found that Ksv values decreased with the temperature. And Kq values at 295, 298, and 305 K were higher than 2 × 1010 L mol−1 S−1. Above results suggested the quenching process of quercetagetin against tyrosinase was a static process. This quenching type presented in the quenching of indole derivatives on α-glucosidase (Hu et al., 2024). The quenching process also was the process of thermodynamic changes. Thence, the thermodynamic parameters were calculated (Table 1). The negative ΔG value unlocked one spontaneous process of quercetagetin binding to tyrosinase. The positive ΔH and ΔS values suggested hydrophobic interactions as the important forces between quercetagetin and tyrosinase.
2.4 CD spectra
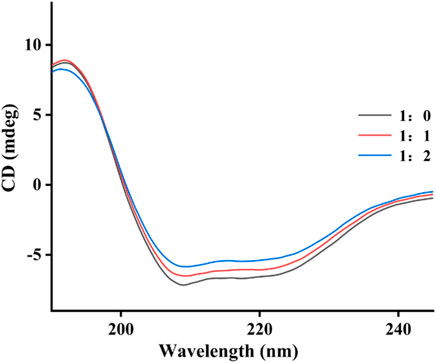
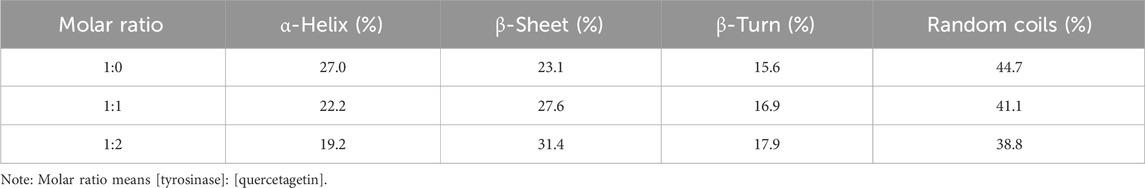
The effect of quercetagetin on conformation change of tyrosinase was investigated using CD spectra. Figure 6 showed CD spectra of tyrosinase, the characteristic peaks around 210–220 nm stood for the peptide chains of tyrosinase. When treated with quercetagetin, the CD spectra of tyrosinase appeared some changes, meaning its conformation change. Then its secondary structure contents were calculated (Table 2) and found that quercetagetin treatment (molar ratio: 2:1) caused reduction of α-helix and random coils and increase of β-sheet and β-turn. These results indicated that quercetagetin treatment could lead to conformation changes of tyrosinase.
2.5 3D fluorescence spectra
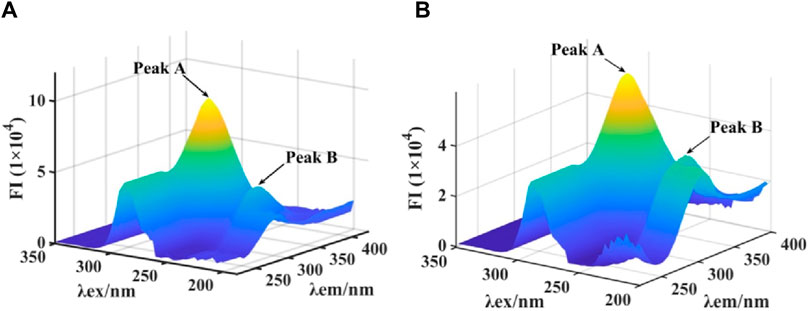
3D fluorescence spectra were monitored to further investigate the effect on conformation of tyrosinase by quercetagetin. As shown in Figure 7A the 3D fluorescence spectra tyrosinase, two characteristic peaks appeared, including Peak A for Tyr and Trp residues and Peak B for peptide backbone. When treated with quercetagetin, Peak A and Peak B were reduced the intensity by 35.4% and 13.3%, respectively (Figure 7B), which suggested that quercetagetin treatment would cause the changes of Tyr and Trp residues, and peptide backbone. That was to say that quercetagetin treatment would cause the conformation change of tyrosinase.
2.6 Molecular docking
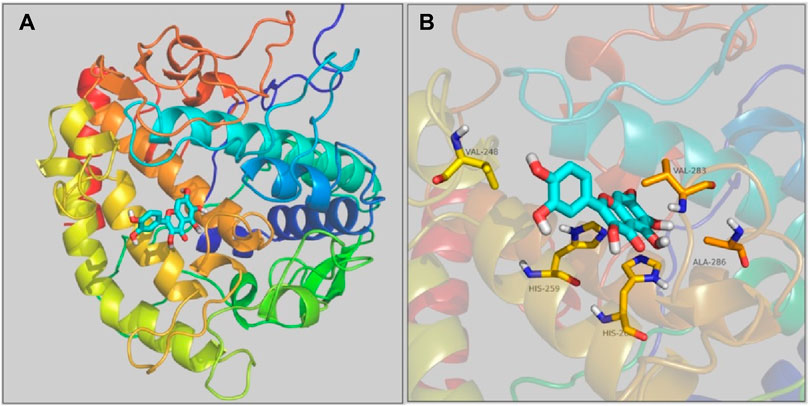
The interaction of quercetagetin to tyrosinase was simulated by molecular docking method. As shown in Figure 8A the docking results, quercetagetin bound to the active pocket of tyrosinase with trihydroxychromone section in the active catalytic zone of active pocket. From Figure 8B the docking results in detail, it could be observed that quercetagetin made one hydrogen bond with His259 and hydrophobic interaction with Val248, His263, Val283, and Ala286. The results indicated that the interactions between quercetagetin and tyrosinase resulted in the reduction of tyrosinase activity.
2.7 Copper-chelating activity
The copper-chelating activity of quercetagetin was finally assayed using copper sulfate. Figure 9A showed the quercetagetin-copper sulfate mixture. Quercetagetin showed its UV spectra with characteristic peak at 370 nm. While, after added copper sulfate, the UV characteristic peak of quercetagetin was gradually decreased. These results indicated that copper might complex with quercetagetin to change its UV characteristic peak. The peak intensity at different molar ratios of quercetagetin to copper sulfate was analyzed (Figure 9B) and found that the decreasing trend of quercetagetin peak intensity become equilibrium, meaning that the binding molar ratio of quercetagetin with copper sulfate was 1. The copper-chelating activity of quercetagetin also might be one reason of the reduction of tyrosinase activity.
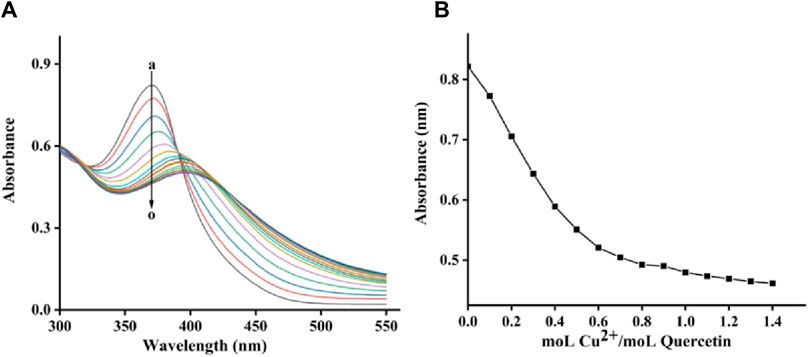
Figure 9. (A) UV spectra of quercetagetin-copper sulfate mixture; (B) UV peak of quercetagetin versus molar ratios of quercetagetin to copper sulfate.
3 Conclusion
Tyrosinase is one important rate limiting enzyme in melanin synthesis, directly affecting the melanin synthesis. Quercetagetin is one active ingredient from marigold. In this study, the inhibition effects of quercetagetin against tyrosinase were investigated. The results showed that quercetagetin could inhibit the tyrosinase activity with IC50 value of 0.19 ± 0.01 mM and the inhibition type was a reversible mixed-type. Results of fluorescence quenching showed that quercetagetin could quench tyrosinase fluorescence in static process. CD and 3D fluorescence results showed interaction of quercetagetin to tyrosinase could change tyrosinase conformation to inhibit activity. Moreover, docking revealed details of quercetagetin’s interactions with tyrosinase.
4 Materials and methods
4.1 Tyrosinase activity assay
Tyrosinase inhibitory activity of quercetagetin was measured (Lu et al., 2023b). 10 μL of quercetagetin in DMSO solutions was added into 140 μL of tyrosinase in PBS solution and incubated for 10 min. 50 μL of L-dopa (in PBS) was added as the substrates. Then, the absorbance was measured at 475 nm on a microplate reader. Kojic acid was selected as the positive control. All experiments were tested in triplicate. Inhibition rate (%) = [(OD1-OD0)/OD0]×100%. OD0 and OD1 were the absorbance of tyrosinase and tyrosinase-quercetagetin mixture.
4.2 Inhibitory kinetics
The reversibility of quercetagetin against tyrosinase was analyzed at different concentration of quercetagetin with tyrosinase or L-dopa. And the inhibitory kinetics of quercetagetin against tyrosinase was analyzed at different concentration of quercetagetin with tyrosinase or L-dopa. The inhibition constants Ki and Kis were obtained from the secondary curves of inhibitory kinetics (Xu et al., 2020; Deng et al., 2022; Lin et al., 2023).
4.3 Fluorescence quenching
To the 3.0 mL of tyrosinase solution, 1 μL of quercetagetin was added by titration method (Li et al., 2024a; Min et al., 2024). The mixture was measured fluorescence spectra at excitation of 280 nm. The experiments were conducted at temperatures of 295, 298, and 305 K, respectively. The Stern-Volmer equation and the vanʼt Hoff equation were employed to obtained parameters.
4.4 CD spectra
To the 100 μL of tyrosinase solution, 1 μL of quercetagetin was added. CD spectra were measured at room temperature (Xiao et al., 2023; Li et al., 2024b). Tyrosinase solution without quercetagetin was also measured its CD spectra.
4.5 3D fluorescence spectra
To the 3.0 mL of tyrosinase solution, 1 μL of quercetagetin was added. 3D fluorescence spectra were measured (Wu X. et al., 2023).
4.6 Copper-chelating activity
To the 2 mL of quercetagetin solution, 5 μL of copper sulfate solution was gradually added. UV absorption spectra were detected. The molar ratios of quercetagetin to copper sulfate ranged from 10: 0 to 10: 14.
4.7 Molecular docking
Docking of quercetagetin with tyrosinase was simulated using the SYBYL software (Zhang et al., 2022; Feng et al., 2024). The tyrosinase crystal structure (PDB: 2Y9X) was optimized by removing water, adding hydrogen, and generation of active pocket. Quercetagetin structure was performed energy minimization. Thence, docking procedure was run in the default format.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
FL: Conceptualization, Investigation, Methodology, Project administration, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Broulier, R. I., Lazinski, L. M., Pérès, B., Olleik, H., Royal, G., Fishman, A., et al. (2023). Resorcinol-based hemiindigoid derivatives as human tyrosinase inhibitors and melanogenesis suppressors in human melanoma cells. Eur. J. Med. Chem. 246, 114972. doi:10.1016/j.ejmech.2022.114972
Bulut, O., and Yilmaz, M. D. (2021). Concise synthesis of quercetagetin (3,3’,4’,5,6,7-hexahydroxyflavone) with antioxidant and antibacterial activities. Results Chem. 3, 100255. doi:10.1016/j.rechem.2021.100255
Chen, J., Cao, D., Jiang, S., Liu, X., Pan, W., Cui, H., et al. (2022). Triterpenoid saponins from Ilex pubescens promote blood circulation in blood stasis syndrome by regulating sphingolipid metabolism and the PI3K/AKT/eNOS signaling pathway. Phytomedicine 104, 154242. doi:10.1016/j.phymed.2022.154242
Chen, Y., Yin, S., Liu, R., Yang, Y., Wu, Q., Lin, W., et al. (2023). β-Sitosterol activates autophagy to inhibit the development of hepatocellular carcinoma by regulating the complement C5a receptor 1/alpha fetoprotein axis. Eur. J. Pharmacol. 957, 175983. doi:10.1016/j.ejphar.2023.175983
De Barros, M. R., Menezes, T. M., Garcia, Y. S., and Neves, J. L. (2023). Inhibitory effects of iron-based carbonaceous nanocomposites on mushroom tyrosinase activity: molecular aspects and mechanistic insights. New J. Chem. 47, 9134–9142. doi:10.1039/d3nj00882g
Deng, X., Ke, J., Zheng, Y., Li, D., Zhang, K., Zheng, X., et al. (2022). Synthesis and bioactivities evaluation of oleanolic acid oxime ester derivatives as α-glucosidase and a-amylase inhibitors. J. Enzyme Inhib. 37, 451–461. doi:10.1080/14756366.2021.2018682
Djafarou, S., Mermer, A., Barut, B., Yılmaz, G. T., Khodja, I. A., and Boulebd, H. (2023). Synthesis and evaluation of the antioxidant and anti-tyrosinase activities of thiazolyl hydrazone derivatives and their application in the anti-browning of fresh-cut potato. Food Chem. 414, 135745. doi:10.1016/j.foodchem.2023.135745
Esma, A. T. K., Abd, E. H. K., Rabah, A., Djamila, B., Mohamed, S. B., Chawki, B., et al. (2023). In vitro assessment of antioxidant, neuroprotective, anti-urease and anti-tyrosinase capacities of Tamarix africana leaves extracts. J. Tradit. Chin. Med. 43, 252–264. doi:10.19852/j.cnki.jtcm.20230105.003
Fan, M., Zhang, G. W., Pan, J., and Gong, D. (2017). An inhibition mechanism of dihydromyricetin on tyrosinase and the joint effects of vitamins B6, D3 or E. Food Funct. 8, 2601–2610. doi:10.1039/c7fo00236j
Feng, M., Liang, B., Sun, J., Min, X., Wang, S., Lu, Y., et al. (2024). Synthesis, anti-α-glucosidase activity, inhibition interaction, and anti-diabetic activity of novel cryptolepine derivatives. J. Mol. Struct. 1310, 138311. doi:10.1016/j.molstruc.2024.138311
Fuentes, J., de Camargo, A. C., Atala, E., Gotteland, M., Olea-Azar, C., and Speisky, H. (2021). Quercetin oxidation metabolite present in onion peel protects caco-2 cells against the oxidative stress, NF-kB activation, and loss of epithelial barrier function induced by NSAIDs. J. Agric. Food Chem. 69, 2157–2167. doi:10.1021/acs.jafc.0c07085
Hassan, M., Shahzadi, S., and Kloczkowski, A. (2023). Tyrosinase inhibitors naturally present in plants and synthetic modifications of these natural products as anti-melanogenic agents: a review. Molecules 28, 378. doi:10.3390/molecules28010378
Hu, C., Liang, B., Sun, J., Li, J., Xiong, Z., Wang, S., et al. (2024). Synthesis and biological evaluation of indole derivatives containing thiazolidine-2,4-dione as α-glucosidase inhibitors with antidiabetic activity. Eur. J. Med. Chem. 264, 115957. doi:10.1016/j.ejmech.2023.115957
Iraji, A., Sheikhi, N., Attarroshan, M., Ardani, G. R. S., Kabiri, M., Bafghi, A. N., et al. (2022). Design, synthesis, spectroscopic characterization, in vitro tyrosinase inhibition, antioxidant evaluation, in silico and kinetic studies of substituted indole-carbohydrazides. Bioorg. Chem. 129, 106140–140. doi:10.1016/j.bioorg.2022.106140
Jiang, T., Ji, C., Cheng, X., Gu, S., Wang, R., Li, Y., et al. (2021). α-Mangostin alleviated HIF-1α-mediated angiogenesis in rats with adjuvant-induced arthritis by suppressing aerobic glycolysis. Front. Pharmacol. 12, 785586. doi:10.3389/fphar.2021.785586
Lee, J., Park, Y. J., Jung, H. J., Ullah, S., Yoon, D., Jeong, Y., et al. (2023). Design and synthesis of (Z)-2-(benzylamino)-5-benzylidenethiazol-4(5H)-one derivatives as tyrosinase inhibitors and their anti-melanogenic and antioxidant effects. Molecules 28, 848. doi:10.3390/molecules28020848
Li, J., Feng, L., Liu, L., Wang, F., Ouyang, L., Zhang, L., et al. (2021a). Recent advances in the design and discovery of synthetic tyrosinase inhibitors. Eur. J. Med. Chem. 224, 113744. doi:10.1016/j.ejmech.2021.113744
Li, J., Min, X., Zheng, X., Wang, S., Xu, X., and Peng, J. (2023). Synthesis, anti-tyrosinase activity, and spectroscopic inhibition mechanism of cinnamic acid-eugenol esters. Molecules 28, 5969. doi:10.3390/molecules28165969
Li, M., Li, H., Min, X., Sun, J., Liang, B., Xu, L., et al. (2024a). Identification of 1,3,4-thiadiazolyl-containing thiazolidine-2,4-dione derivatives as novel PTP1B inhibitors with anti-diabetic activity. J. Med. Chem. doi:10.1021/acs.jmedchem.4c00676
Li, M., Sun, J., Liang, B., Min, X., Hu, J., Wu, R., et al. (2024b). Thiazolidine-2,4-dione derivatives as potential α-glucosidase inhibitors: synthesis, inhibitory activity, binding interaction and hypoglycemic activity. Bioorg. Chem. 144, 107177. doi:10.1016/j.bioorg.2024.107177
Li, Y., Dai, M., Wang, L., and Wang, G. (2021b). Polysaccharides and glycosides from Aralia echinocaulis protect rats from arthritis by modulating the gut microbiota composition. J. Ethnopharmacol. 269, 113749. doi:10.1016/j.jep.2020.113749
Lin, J., Xiao, D., Lu, L., Liang, B., Xiong, Z., and Xu, X. (2023). New β-carboline derivatives as potential α-glucosidase inhibitor: synthesis and biological activity evaluation. J. Mol. Struct. 1283, 135279. doi:10.1016/j.molstruc.2023.135279
Liu, J., Zhao, X., Qin, F., Zhou, J., Ding, F., Zhou, G., et al. (2022). Isoliquiritigenin mitigates oxidative damage after subarachnoid hemorrhage in vivo and in vitro by regulating Nrf2-dependent Signaling Pathway via Targeting of SIRT1. Phytomedicine 105, 154262. doi:10.1016/j.phymed.2022.154262
Lu, L., Hu, C., Min, X., Liu, Z., Xu, X., and Gan, L. (2023a). In vitro and in vivo biological evaluation of indole-thiazolidine-2,4-dione derivatives as tyrosinase inhibitors. Molecules 28, 7470. doi:10.3390/molecules28227470
Lu, L., Zhang, X., Kang, Y., Xiong, Z., Zhang, K., Xu, X., et al. (2023b). Novel coumarin derivatives as potential tyrosinase inhibitors: synthesis, binding analysis and biological evaluation. Arab. J. Chem. 16, 104724. doi:10.1016/j.arabjc.2023.104724
Ma, M., Wei, N., Yang, J., Ding, T., Song, A., Chen, L., et al. (2023). Schisandrin B promotes senescence of activated hepatic stellate cell via NCOA4-mediated ferritinophagy. Pharm. Biol. 61, 621–629. doi:10.1080/13880209.2023.2189908
Min, X., Guo, S., Lu, Y., and Xu, X. (2024). Investigation on the inhibition mechanism and binding behavior of cryptolepine to α-glucosidase and its hypoglycemic activity by multi-spectroscopic method. J. Lumin. 269, 120437. doi:10.1016/j.jlumin.2024.120437
Min, X., Lu, L., Xu, X. T., Wen, Y., and Zheng, X. (2023). Investigation on the inhibition mechanism and binding behavior of paeonol to tyrosinase and its anti-browning property by multi-spectroscopic and molecular docking methods. Int. J. Biol. Macromol. 253, 126962. doi:10.1016/j.ijbiomac.2023.126962
Nasab, N., Raza, H., Eom, Y., Hassan, M., Kloczkowski, A., and Kim, S. (2023). Synthesis and discovery of potential tyrosinase inhibitor of new coumarin-based thiophenyl-pyrazolylthiazole nuclei: in-vitro evaluation, cytotoxicity, kinetic and computational studies. Chem. Biol. Drug. Des. 101, 13–88. doi:10.1111/cbdd.14209
Pei, Y. Q., Zheng, Y. Q., Ding, Y. D., Xu, Q. X., Cao, D., Wu, Y. N., et al. (2021). Triptolide attenuates vascular calcification by upregulating expression of miRNA-204. Front. Pharmacol. 11, 581230. doi:10.3389/fphar.2020.581230
Qi, X., Zheng, S., Ma, M., Lian, N., Wang, H., Chen, L., et al. (2022). Curcumol suppresses CCF-mediated hepatocyte senescence through blocking LC3B-Lamin B1 interaction in alcoholic fatty liver disease. Front. Pharmacol. 13, 912825. doi:10.3389/fphar.2022.912825
Romagnoli, R., Oliva, P., Prencipe, F., Manfredini, S., Germanò, M., De Luca, L., et al. (2022). Cinnamic acid derivatives linked to arylpiperazines as novel potent inhibitors of tyrosinase activity and melanin synthesis. Eur. J. Med. Chem. 231, 114147. doi:10.1016/j.ejmech.2022.114147
Rufino, A. T., Ramalho, A., Sousa, A., de Oliveira, J. M. P. F., Freitas, P., Gómez, M. A. G., et al. (2021). Protective role of flavonoids against intestinal pro-inflammatory effects of silver nanoparticles. Molecules 26, 6610. doi:10.3390/molecules26216610
Seyedi, S. S., Shukri, M., Hassandarvish, P., Oo, A., Shankar, E. M., Abubakar, S., et al. (2016). Computational approach towards exploring potential anti-chikungunya activity of selected flavonoids. Sci. Rep. 6, 24027. doi:10.1038/srep24027
Shao, X., Li, B., Shen, J., Wang, Q., Chen, S., Jiang, X., et al. (2020). Ghrelin alleviates traumatic brain injury-induced acute lung injury through pyroptosis/NF-κB pathway. Int. Immunopharmacol. 79, 106175. doi:10.1016/j.intimp.2019.106175
Shi, L., Jiang, C., Xu, H., Wu, J., Lu, J., He, Y., et al. (2023). Hyperoside ameliorates cerebral ischaemic–reperfusion injury by opening the TRPV4 channel in vivo through the IP 3-PKC signalling pathway. Pharm. Biol. 61, 1000–1012. doi:10.1080/13880209.2023.2228379
Song, A., Ding, T., Wei, N., Yang, J., Ma, M., Zheng, S., et al. (2023). Schisandrin B induces HepG2 cells pyroptosis by activating NK cells mediated anti-tumor immunity. Toxicol. Appl. Pharmacol. 472, 116574. doi:10.1016/j.taap.2023.116574
Tang, Z., Zhang, M., Gao, L., Bao, Y., Li, P., Wang, M., et al. (2023). Optimal extraction of polysaccharides from Stevia rebaudiana roots for protection against hydrogen peroxide-induced oxidative damage in RAW264.7 cells. Nat. Prod. Res. 2023, 2263905. doi:10.1080/14786419.2023.2263905
Tao, Z. S., Li, T. L., and Wei, S. (2022). Silymarin prevents iron overload induced bone loss by inhibiting oxidative stress in an ovariectomized animal model. Chem. Biol. Interact. 366, 110168. doi:10.1016/j.cbi.2022.110168
Wang, D., Li, Y., Wu, Y., Wu, Y., Han, J., Olatunji, O., et al. (2021). Xanthones from Securidaca inappendiculata antagonized the antirheumatic effects of methotrexate in vivo by promoting its secretion into urine. Expert Opin. Drug Metab. toxico. 17, 241–250. doi:10.1080/17425255.2021.1843634
Wang, L., Wang, P., Wang, D., Tao, M., Xu, W., and Olatunji, O. J. (2020). Anti-inflammatory activities of kukoamine A from the root bark of lycium chinense miller. Nat. Prod. Commun. 15, 1934578X2091208. doi:10.1177/1934578x20912088
Wang, R., and Mu, J. (2021). Arbutin attenuates ethanol-induced acute hepatic injury by the modulation of oxidative stress and Nrf-2/HO-1 signaling pathway. J. Biochem. Mol. Toxicol. 35, 22872. doi:10.1002/jbt.22872
Wang, X., Zhou, D., Zhou, W., Liu, J., Xue, Q., Huang, Y., et al. (2022a). Clematichinenoside AR inhibits the pathology of rheumatoid arthritis by blocking the circPTN/miR-145-5p/FZD4 signal axis. Int. Immunopharm. 113, 109376. doi:10.1016/j.intimp.2022.109376
Wang, Y., Wu, H., Han, Z., Sheng, H., Wu, Y., Wang, Y., et al. (2022b). Guhong injection promotes post-stroke functional recovery via attenuating cortical inflammation and apoptosis in subacute stage of ischemic stroke. Phytomedicine 99, 154034. doi:10.1016/j.phymed.2022.154034
Wu, F., Wang, F., Tang, Z., Yang, X., Liu, Y., Zhao, M., et al. (2023a). Quercetagetin alleviates zearalenone-induced liver injury in rabbits through Keap1/Nrf2/ARE signaling pathway. Front. Pharmacol. 14, 1271384. doi:10.3389/fphar.2023.1271384
Wu, F., Wang, H., Li, S., Wei, Z., Han, S., and Chen, B. (2022). Effects of dietary supplementation with quercetagetin on nutrient digestibility, intestinal morphology, immunity, and antioxidant capacity of broilers. Front. Vet. Sci. 9, 1060140. doi:10.3389/fvets.2022.1060140
Wu, X., Zhu, W., Lu, L., Hu, C., Zheng, Y., Zhang, X., et al. (2023b). Synthesis and anti-α-glucosidase activity evaluation of betulinic acid derivatives. Arab. J. Chem. 16, 104659. doi:10.1016/j.arabjc.2023.104659
Wu, Z., Liang, D., Xu, M., Liu, Y., and Xie, H. (2021). A comparative pharmacokinetic study of schisandrol B after oral administration of schisandrol b monomer and schisandra chinensis extract. Curr. Pharm. Anal. 17, 273–284. doi:10.2174/1573412916666191114122101
Xiao, D., Lu, L., Liang, B., Xiong, Z., Xu, X., and Chen, W. (2023). Identification of 1,3,4-oxadiazolyl-containing β-carboline derivatives as novel α-glucosidase inhibitors with antidiabetic activity. Eur. J. Med. Chem. 261, 115795. doi:10.1016/j.ejmech.2023.115795
Xu, X., Deng, X., Chen, J., Liang, Q., Zhang, K., Li, D., et al. (2020). Synthesis and biological evaluation of coumarin derivatives as aglucosidase Inhibitors. Eur. J. Med. Chem. 112, 112013. doi:10.1016/j.ejmech.2019.112013
Xue, S., Li, Z., Ze, X., Wu, X., He, C., Shuai, W., et al. (2023). Design, synthesis, and biological evaluation of novel hybrids containing dihydrochalcone as tyrosinase inhibitors to treat skin hyperpigmentation. J. Med. Chem. 66, 5099–5117. doi:10.1021/acs.jmedchem.3c00012
Zang, L., Xu, H., Huanh, C., Wang, C., Wang, R., Chen, R., et al. (2022). A link between chemical structure and biological activity in triterpenoids. Recent Pat. Anti-Canc 17, 145–161. doi:10.2174/1574892816666210512031635
Zargaham, M. K., Ahmed, M., Akhtar, N., Ashraf, Z., Abdel-Maksoud, M. A., Aufy, M., et al. (2023). Synthesis, in silico studies, and antioxidant and tyrosinase inhibitory potential of 2-(substituted phenyl) thiazolidine-4-carboxamide derivatives. Pharmaceuticals 16, 835. doi:10.3390/ph16060835
Zhang, X., Lu, Y., Li, W., Tao, T., Peng, L., Wang, W., et al. (2021a). Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Brit. J. Pharmacol. 178, 1114–1132. doi:10.1111/bph.15346
Zhang, X., Peng, L., Zhang, J., Dong, Y. P., Wang, C. J., Liu, C., et al. (2020). Berberine ameliorates subarachnoid hemorrhage injury via induction of sirtuin 1 and inhibiting HMGB1/Nf-κB pathway. Front. Pharmacol. 11, 1073. doi:10.3389/fphar.2020.01073
Zhang, X., Zheng, Y., Hu, C., Wu, X., Lin, J., Xiong, Z., et al. (2022). Synthesis and biological evaluation of coumarin derivatives containing oxime ester as α-glucosidase inhibitors. Arab. J. Chem. 15, 104072. doi:10.1016/j.arabjc.2022.104072
Zhang, Y., Zhang, X., Li, H. J., Zhou, T. F., Zhou, A. C., Zhong, Z. L., et al. (2021b). Antidepressant-like effects of helicid on a chronic unpredictable mild stress-induced depression rat model: inhibiting the IKK/IκBα/NF-κB pathway through NCALD to reduce inflammation. Int. Immunopharm. 93, 107165. doi:10.1016/j.intimp.2020.107165
Zhou, S., Sun, Y., Xing, Y., Wang, Z., Wan, S., Yao, X., et al. (2022a). Exenatide ameliorates hydrogen peroxide-induced pancreatic β-cell apoptosis through regulation of METTL3-mediated m6A methylation. Eur. J. Pharmacol. 924, 174960. doi:10.1016/j.ejphar.2022.174960
Zhou, Y., Xiang, R., Qin, G., Ji, B., Yang, S., Wang, G., et al. (2022b). Xanthones from Securidaca inappendiculata Hassk. attenuate collagen-induced arthritis in rats by inhibiting the nicotinamide phosphoribosyltransferase/glycolysis pathway and macrophage polarization. Int. Immunopharm. 111, 109137. doi:10.1016/j.intimp.2022.109137
Znajafi, E. A., Chehardoli, G., Ziaei, M., Akbarzadeh, T., Saeedi, M., Gholamhoseini, P., et al. (2023). Design, synthesis, in vitro, and in silico studies of novel benzylidene 6-methoxy-1-tetralone linked to benzyloxy and benzyl-1, 2, 3-triazole rings as potential tyrosinase inhibitors. J. Mol. Struct. 1271, 134018. doi:10.1016/j.molstruc.2022.134018
Keywords: nature product, quercetagetin, tyrosinase, inhibition effects, inhibitor
Citation: Liang F (2024) Inhibition mechanism investigation of quercetagetin as a potential tyrosinase inhibitor. Front. Chem. 12:1411801. doi: 10.3389/fchem.2024.1411801
Received: 03 April 2024; Accepted: 16 May 2024;
Published: 03 June 2024.
Edited by:
Xuetao Xu, Wuyi University, ChinaReviewed by:
Guanghao Zhu, Shanghai University of Traditional Chinese Medicine, ChinaDanying Huang, Guangdong University of Petrochemical Technology, China
Haoxing Zhang, Shenzhen University, China
Copyright © 2024 Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faliang Liang, ZmFsaWFuZzIwMjRAMTYzLmNvbQ==
 Faliang Liang
Faliang Liang