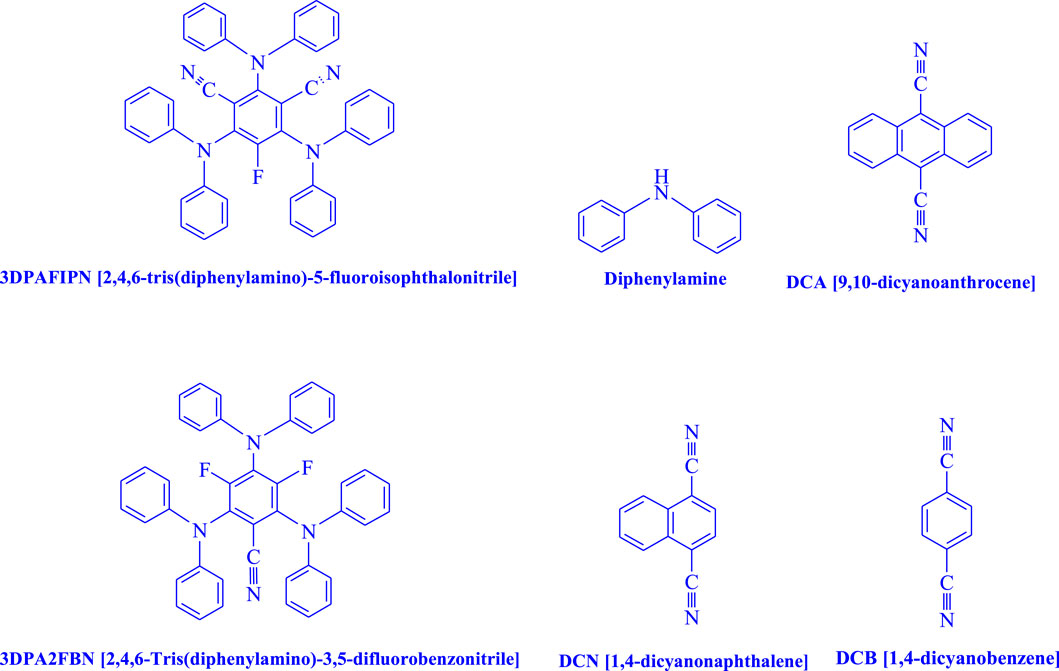
- Department of Medical Nanotechnology, School of Advanced Medical Sciences and Technologies, Shiraz University of Medical Sciences, Shiraz, Iran
Background: Typically, organic dyes show lower excited state lifetimes, a key hindrance in the development of efficient photoredox processes. Due to their distinctive qualities and efficiency, a particular class of organic chromophores has drawn considerable interest from the scientific community. Thermally activated delayed fluorescence (TADF), is only seen in molecules with a minimal energy gap (usually less than 0.2 eV) between their lowest two excited states, i.e., singlet excited state (S1) and triplet excited state (T1), is a distinctive property of the molecules under study. Isophthalonitriles are a promising family of chromophores for use as organic photocatalysts because of the ease with which their redox potentials may be adjusted and the prolonged singlet excited states resulting from TADF.
Methods: A sustainable process for the photosynthesis of polyfunctionalized dihydro-2-oxypyrroles has been developed using the Michael-Mannich cyclocondensation of amines, dialkyl acetylenedicarboxylates, and formaldehyde. The development of a green radical synthesis strategy for this family of chemicals is discussed in detail in the current work. This work used a novel halogenated dicyanobenzene-based photosensitizer was used as a photocatalyst. It was dissolved in ethanol, exposed to air at ambient temperature, and triggered by a blue light-emitting diode as a renewable energy source. This project’s main goal is to use a novel conveniently accessible, reasonably priced donor-acceptor (D-A) based on halogenated cyanoarene.
Findings: When exposed to visible light, the 3DPAFIPN [2,4,6-tris(diphenylamino)-5-fluoroisophthalonitrile] photocatalyst, which is a thermally activated delayed fluorescence (TADF), can induce single-electron transfer (SET), providing a simple and green method that is highly effective, energy-efficient, and environmentally friendly. Also, we calculated the turnover number (TON) and turnover frequency (TOF) for polyfunctionalized dihydro-2-oxypyrroles. Gram-scale cyclization has also been shown to be a practical technique for use in industrial applications.
Introduction
Photoredox catalysis has been used as a starting point for innovative methods in the field of organic chemistry in recent literature (Mohamadpour, 2022a; Mohamadpour, 2022b). Both academia and industries are paying close attention to the topic of photoredox catalysis, which combines metal-promoted reactions with photoredox cycles (Pinosa et al., 2022). To assist the creation of new, potent, and selective metal-promoted reactions, the key area of study involves the use of affordable, easily produced, and effective organic dyes (Gualandi et al., 2021). The widely used inorganic complexes dependent on Ir(III) and Ru(II) must be replaced with organic dyes in this field. These compounds are renowned for their lengthy excited state lifetimes, which may trend toward dynamic quenching when juxtaposed with organic molecules. Typically, organic dyes show lower excited state lifetimes, a key hindrance in the development of efficient photoredox processes. Due to their distinctive qualities and efficiency, a particular class of organic chromophores has drawn considerable interest from the scientific community (Bryden and Zysman-Colman, 2021). Thermally activated delayed fluorescence (TADF), which is only seen in molecules with a minimal energy gap (usually less than 0.2 eV) between their lowest two excited states, i.e., S1 and T1, is a distinctive property of the molecules under study. The molecules under investigation experience reverse intersystem crossing (RISC) under ambient circumstances, which is assisted by a thermally activated route from the triplet excited state (T1) to the singlet excited state (S1). A delayed fluorescence phenomenon is produced as a result, which is frequently seen in systems like this. The current aim is to couple the excellent quantum yield of fluorescence with the outstanding efficiency of reduced instruction set computing (RISC). Through the publication of a fundamental (Uoyama et al., 2012), the year 2012 witnessed a substantial contribution to the area of organic light-emitting diodes (OLEDs). The effective use of dicyanobenzene molecules with desired photophysical characteristics and their proven use in OLEDs are both covered in this procedure. Following these original discoveries, comparable TADF chromophores have been used in other fields, including photocatalysis (Yang et al., 2017; Bryden and Zysman-Colman, 2021). Isophthalonitriles are a promising family of chromophores for use as organic photocatalysts because of the ease with which their redox potentials may be adjusted and the prolonged singlet excited states resulting from TADF (Speckmeier et al., 2018). The compound 2,4,6-tris(diphenylamino)-5-fluoroisophthalonitrile (3DPAFIPN) has been extensively employed in several synthetic techniques that are triggered by visible light. Examples of such procedures include intramolecular cyclizations (Flynn et al., 2020; Wu et al., 2020) as well as the creation of C−C (Cardinale et al., 2020; Donabauer et al., 2020), N−C (Zhou et al., 2020), and P−C (Rothfelder et al., 2021) bonds (Pinosa et al., 2022).
Visible light irradiation is regarded as a trustworthy approach for synthesizing organic molecules due to its abundant energy reserves, affordable cost, and the possibility of accessing sustainable energy sources (Mohamadpour, 2021a; Mohamadpour, 2021b; Mohamadpour, 2022c).
Oxypyrrole rings are anticipated to have strong pharmacological and biological properties (Figure 1). HCMV, a protease from the human cytomegalovirus, is one example of pyrrole rings (Borthwick et al., 2002), in addition, human cytosolic carbonic anhydrase isozymes (Alp et al., 2010), PI-091 (Shiraki et al., 1995), Oteromycin (Singh et al., 1995), cardiac cAMP phosphodiestrase (Lampe et al., 1993), and most alkaloids (Chen et al., 2005) are other examples of pyrrole rings.
Several catalysts are used for producing polyfunctionalized dihydro-2-oxypyrroles such as methylene blue (Mohamadpour, 2022d), I2 (Khan et al., 2012), glycine (Mohamadpour, 2020), AcOH (Zhu et al., 2009), Cu(OAc)2.H2O (Lv et al., 2013), Fe3O4@nano-cellulose–OPO3H (Salehi and Mirjalili, 2017), tartaric acid (Mohamadpour et al., 2017), nano-Fe3O4@SiO2/SnCl4 (Mirjalili et al., 2019), glutamic acid (Mohamadpour, 2019a), graphene oxide (Bavadi and Niknam, 2018), caffeine (Mohamadpour, 2019b), 2,6-pyridinedicarboxylic acid (Khan et al., 2016), saccharin (Mohamadpour et al., 2016), BiFeO3 nanoparticles (Singh and Rajput, 2018), and CoFe2O4@SiO2@IRMOF-3 (Zhang et al., 2017). Intricate processes, long reaction times, the use of expensive reagents, and decreased yields are just a few of the factors that have a big influence on how waste is handled and disposed of. Furthermore, separating homogeneous catalysts from reaction mixtures can be a difficult process. The use of photocatalysts in the creation of organic compounds is discussed in the current studies (Srivastava et al., 2022), with a focus on the adoption of environmentally friendly procedures. According to the inquiry, it is also economically viable to use halogenated organic dye photo-redox catalysts. Using the aforementioned method, a powerful donor-acceptor (D-A) cyanoarene is used as an effective organo-photocatalyst.
Due to its remarkable photophysical and photochemical capabilities, 2,4,6-tris(diphenylamino)-5-fluoroisophthalonitrile (3DPAFIPN) was the main study focus. The variety of photocatalysts available to organic chemists has increased with the introduction of dicyanobenzene-based photosensitizers, which exhibit outstanding photoelectric activity and thermally activated delayed fluorescence (TADF).
A novel halogenated cyanoarene-based donor-acceptor (D-A) photocatalyst; 3DPAFIPN that functions through a series of visible-light-induced electron transfers has been studied in the current work. The process described here uses tandem Michael-Mannich cyclocondensation amines with formaldehyde, dialkyl acetylenedicarboxylates. Additionally, this reaction uses blue LED, a renewable and eco-friendly energy source in an ethanol media as a green solvent, and at room temperature. Regardless of the efficient and prompt fulfillment of all responsibilities and compliance with the agreed financial plan.
Experimental
General
A 9100 electrothermal instrument was used to determine the melting points of the different compounds. The DRX-300 Avance equipment from Bruker were used to collect 1HNMR spectra with CDCl3. Reagents and materials were purchased from Fluka, Merck, and Acros and used immediately.
The environmentally friendly approach for manufacturing polyfunctionalized dihydro-2-oxypyrroles (5a-s)
Amine 1 (1.0 mmol) and dialkyl acetylenedicarboxylate 2 (1.0 mmol) were agitated for 15 min at room temperature in EtOH (3 mL) with 3DPAFIPN (1 mol%) and blue light (5 W). Then, the reaction mixture was agitated in the presence of amine 3 (1.0 mmol) and formaldehyde 4 (1.5 mmol). Through the use of thin-layer chromatography (TLC), we monitored the progress of the reaction. The unrefined solid was subjected to screening after the chemical reaction, followed by washing with ethanol, eliminating the need for additional purification methods. Current research focuses on whether it is possible to produce the above compounds on a gram-scale for use in pharmaceutical research and development (R&D) processes. 25 mmol of aniline, 25 mmol of n-butylamine, 37.5 mmol of formaldehyde, and 25 mmol of dimethyl acetylenedicarboxylate (DMAD) were used in a single experiment. The 15-min reaction time was followed by the application of a typical filtering technique to recover the end product. A spectroscopic data is included in the Supplementary Material file.
Results and discussion
In the current investigation, a 3 mL ethanol medium containing formaldehyde (1.5 mmol), aniline (2 mmol), and dimethyl acetylenedicarboxylate (DMAD) (1.0 mmol) was used to evaluate the reaction. A trace amount of 5c was generated at room temperature in the presence of 3 mL of EtOH for 45 min without the use of a photocatalyst. Table 1, entry 2, gives a thorough summary of this observation. The addition of photocatalysts accelerated the reaction rate. According to the data shown in Figure 2, the chemicals include 3DPAFIPN, diphenylamine, DCA, 3DPA2FBN, DCN, and DCB. With different yields, 5c can be produced with the current process. The results presented above indicated an increase in the operational effectiveness of 3DPAFIPN. According to the information in Table 1, entry 1, a reaction with 1 mol% 3DPAFIPN produced a 95% yield. Table 2 shows results for toluene, CH3CN, MeOH, EtOH, THF, EtOAc, H2O, DCM, CHCl3, DMSO, and solvent-free conditions. The reaction was shown to have a noticeably elevated rate and subsequent yield in the presence of EtOH. A yield of 95% was attained based on the information shown in Table 2, notably entry 9. Various light sources have been utilized in research aiming at evaluating the influence of blue light on output, as shown in Table 2. During the evaluation that was carried out without the implementation of an illuminating device, the 5c was found in a minuscule quantity. The current study shows that the presence of 3DPAFIPN and visible light is a crucial need for the efficient synthesis of product 5c. Blue light-emitting diode (LED) intensities of 3 W, 5 W, and 7 W were used to find the best designs. According to the study’s findings, it was found that using blue light-emitting diodes (LEDs) with a 5-W power output produced the best outcomes. Table 3 and Scheme 1 show the results of experiments carried out under idealized circumstances on a variety of substrates. The outcome of the reaction was not significantly affected by the addition of an aniline substituent. The substitution of halide functionality in the current reaction was approved. Both reactions involving functional groups that may donate electrons and those involving functional groups that display electron acceptance are permissible in the reaction’s present state. It was discovered that the reactivity shown in aliphatic and benzylic amines was similar. Dimethyl acetylenedicarboxylate (DMAD) and diethyl acetylenedicarboxylate (DEAD) react similarly.
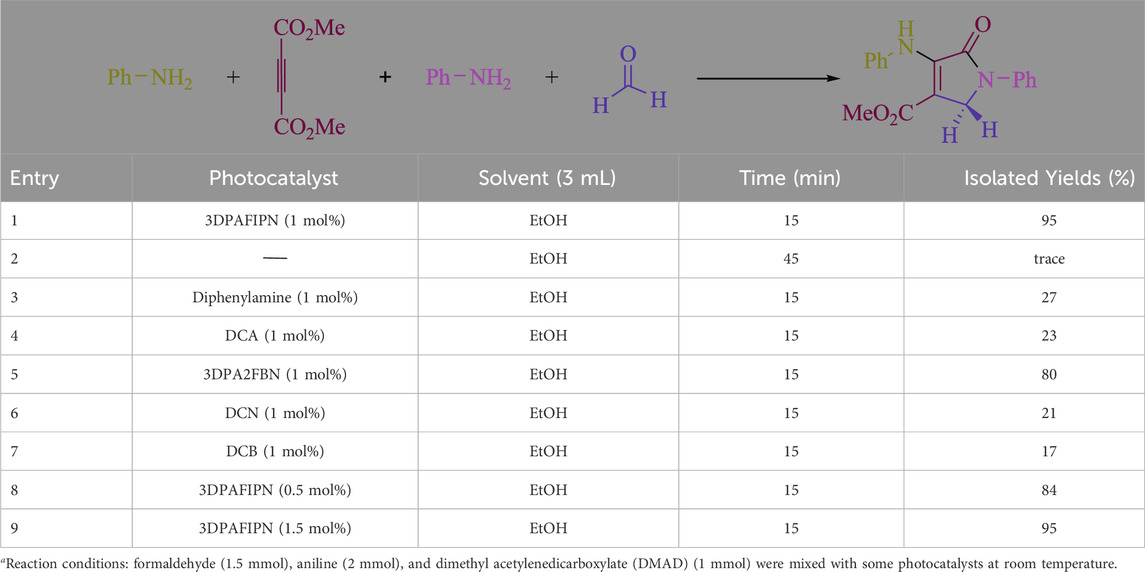
Table 1. Here is a photocatalyst optimization table for the synthesis of 5ca.
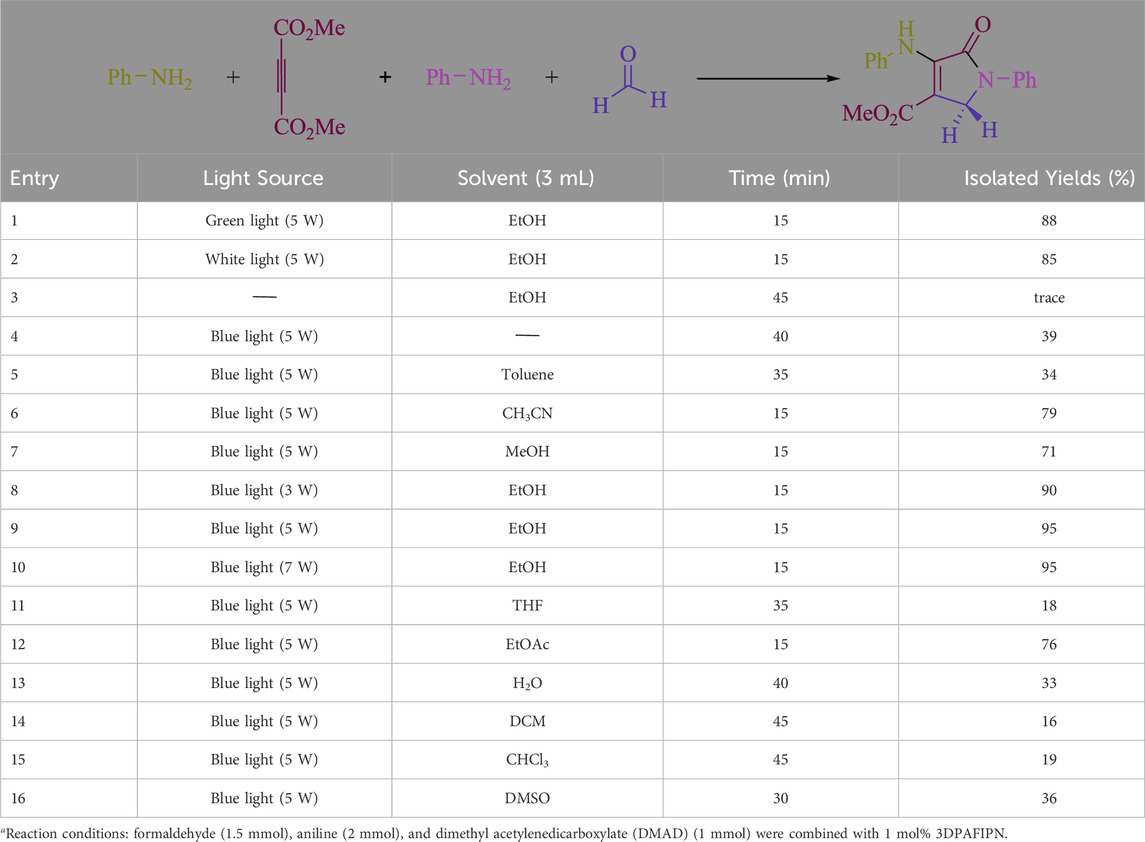
Table 2. The solvent and visible light optimization table for the synthesis of 5ca.
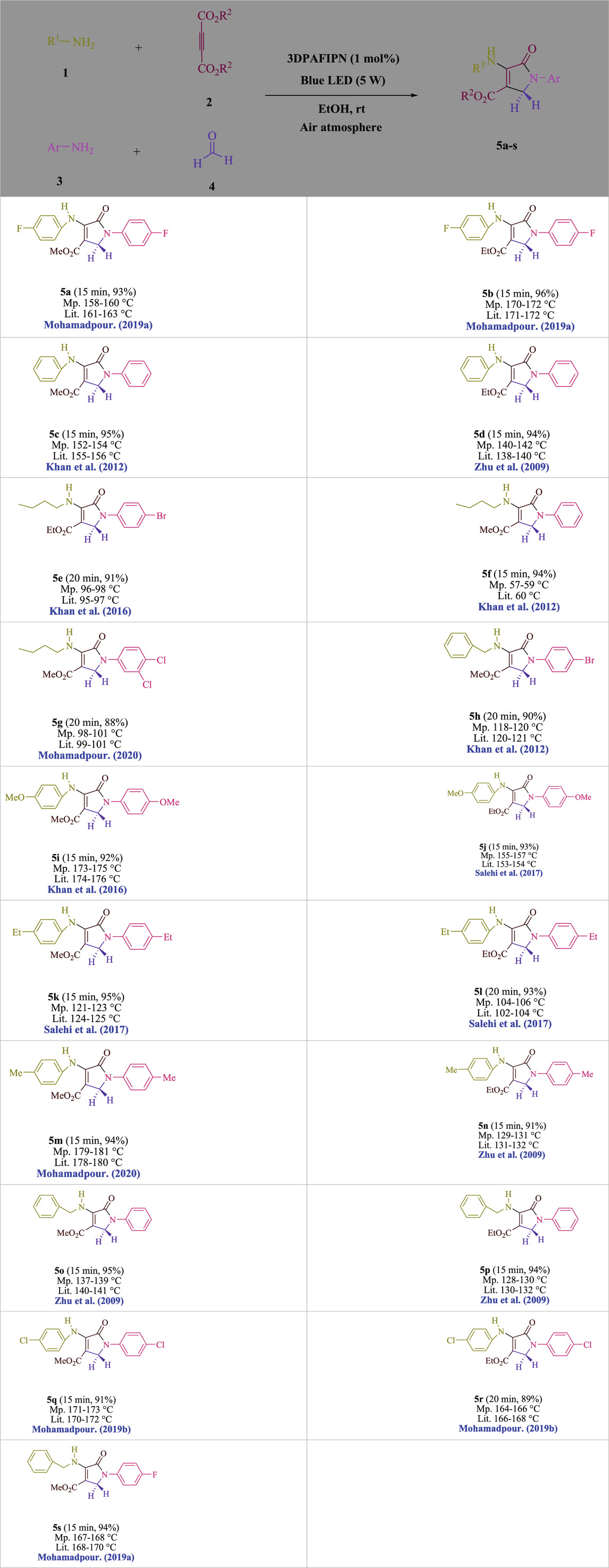
Table 3. Utilizing the halogenated dicyanobenzene-based photosensitizer; 3DPAFIPN allows for the creation of polyfunctionalized dihydro-2-oxypyrroles.

Scheme 1. This process presents a green technique for producing polyfunctionalized dihydro-2-oxypyrroles by radical synthesis.
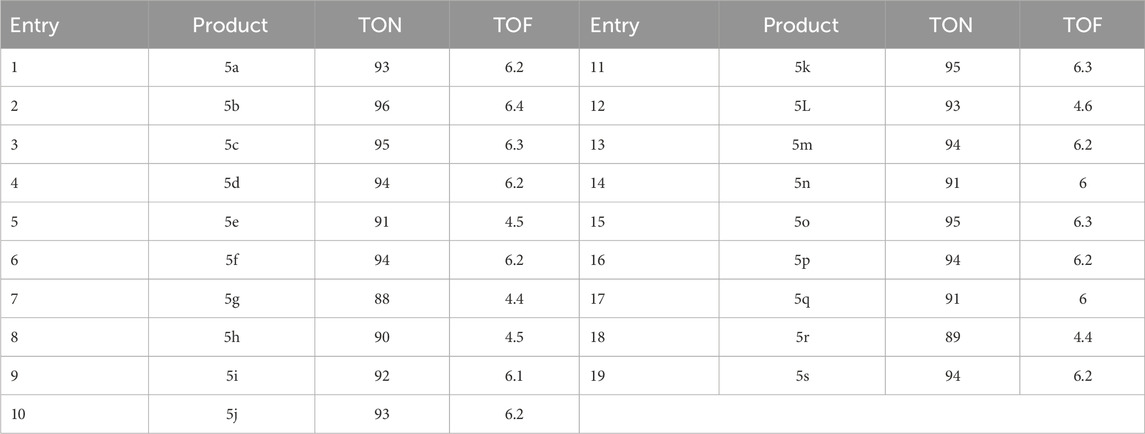
The turnover number (TON) and turnover frequency (TOF) are presented as objective value measurements in Table 4. In academic literature, the two different forms of yield—Yield/Amount of Catalyst (mol) and Yield/Time/Amount of Catalyst (mol)—are frequently written as TON and TOF, respectively. Increased turnover number (TON) and turnover frequency (TOF) values have the potential to improve catalyst performance since they need less catalyst to provide desired yields. When it comes to 5c, a TOF of 6.3 and a TON of 95 are both regarded as high. A TOF of 6.2 is considered high concerning 5d, although a TON of 94 is also considered high. The investigation’s goal was to maximize production, cut down on reaction times, and use the least amount of catalysts possible.
Control experiments
The results of the control tests carried out to clarify the mechanism using the visible-light-induced are shown in Scheme 2. As shown in Scheme 2, aniline 3) and formaldehyde 4) were condensed to produce the related imine I) under conventional conditions (3DPAFIPN in EtOH under blue LED). No product was produced when formaldehyde 4) was mixed with dimethyl acetylenedicarboxylate (DMAD) 2). The yield for 5c was 95% for the condensation of imine I) and enamine radical II). When the reaction was conducted in the dark, a very small amount of the equivalent product 5c was produced. According to the findings of this experiment, Scheme 3 depicts a logical and cogent chemical progression.
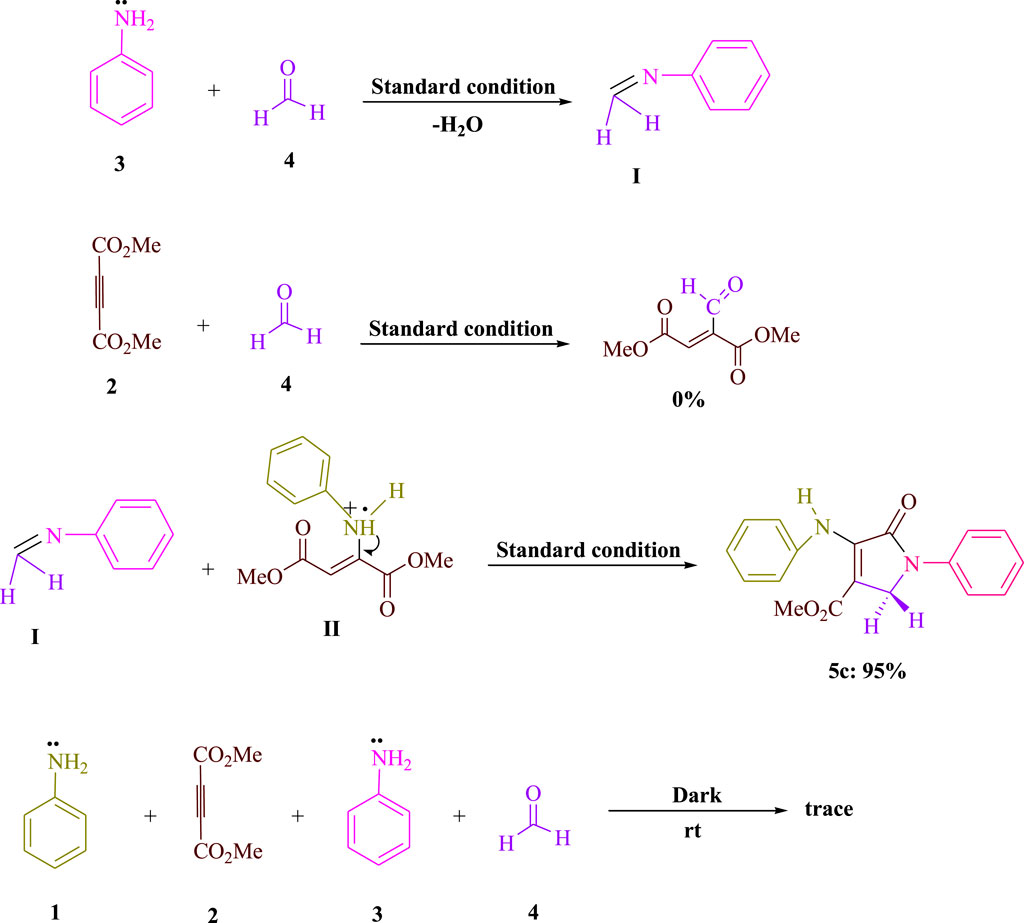
Scheme 2. Significant control experiments are done to understand the condensations of formaldehyde (4, 1.5 mmol), dimethyl acetylenedicarboxylate (DMAD) (2, 1 mmol), and aniline (1 and 3, 2 mmol).
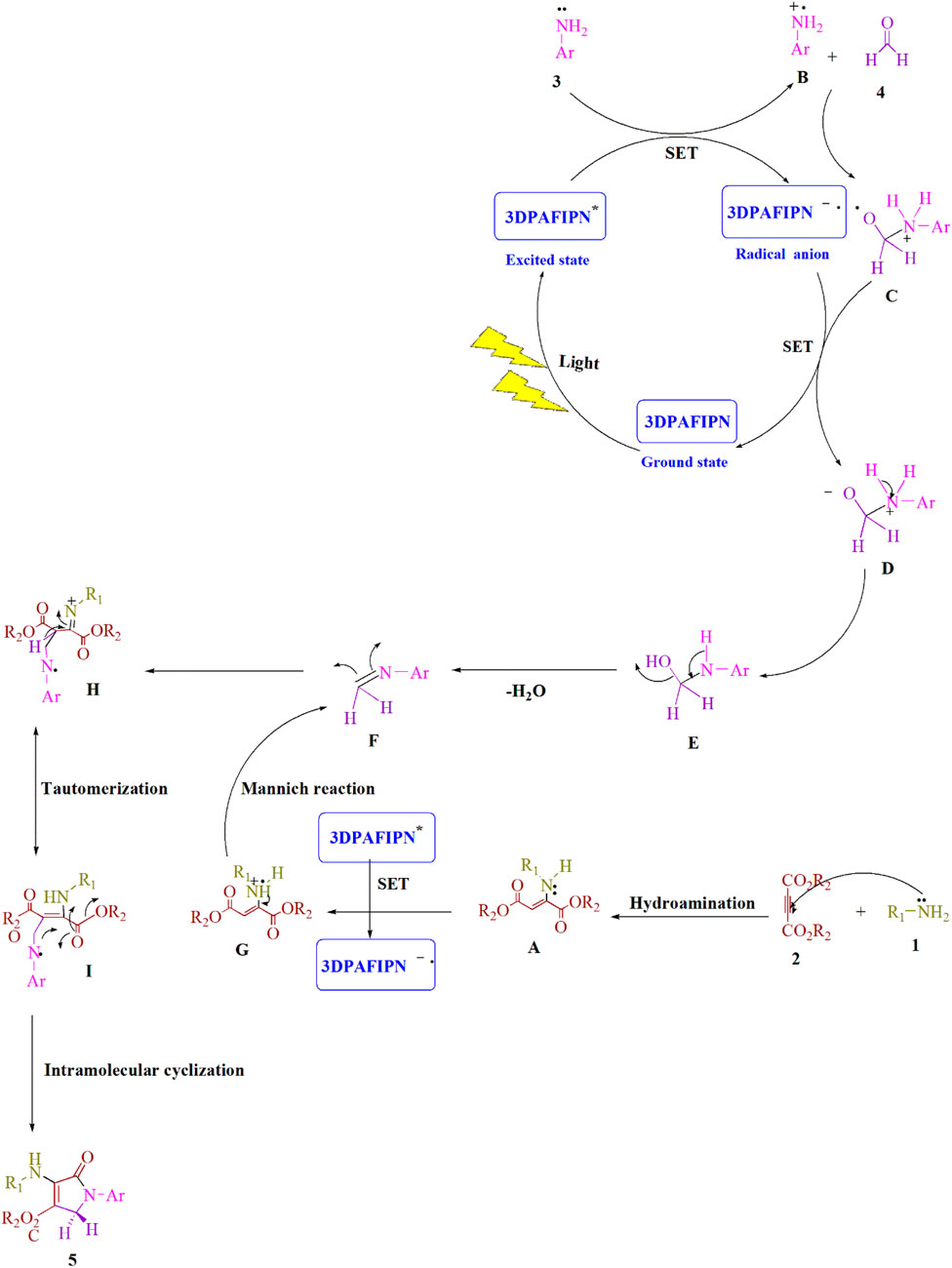
Scheme 3. Here, a thorough representation of the synthetic process for producing polyfunctionalized dihydro-2-oxypyrroles is provided.
The suggested mechanism
A detailed explanation of the recommended technique is shown in Scheme 3. The cyanoarene organic dye 3DPAFIPN has been used to create photocatalytic reactions that use visible light energy as a sustainable resource by employing single-electron transfer (SET) processes. Visible light is used to speed up the process. Amine 1) and dialkylacetylenedicarboxylate 2) are combined in the Michael reaction to produce enamine A). The aniline radical B) is created using the SET procedure and visible light irradiation to enhance the visible-light-induced 3DPAFIPN*. The result of the reaction between the radical cation B) and formaldehyde 4) is the radical cation C). The radical adduct C) and the 3DPAFIPN radical produce the intermediate D) and the ground state of 3DPAFIPN by a single-electron transfer (SET) mechanism. The intermediate F) is created when one H2O molecule from E) is removed. The SET method is used to create the enamine radical G) to improve visible-light-induced 3DPAFIPN*. A more stable tautomeric form I) is created via the Mannich reaction between an activated imine F) and an enamine radical G). Finally, a polyfunctionalized dihydro-2-oxypyrrole 5) is produced by intramolecular cyclization in intermediate I).
A comparison of the effectiveness of several catalysts in promoting the production of polyfunctionalized dihydro-2-oxypyrroles is shown in Table 5. The method in issue uses minuscule amounts of photocatalyst and precipitates quick chemical changes without producing any leftover materials. This method can be applied in situations where there are measurable light wavelengths. Atom-economical procedures are very effective and have a significant impact on the industrial domain at multigram scales.
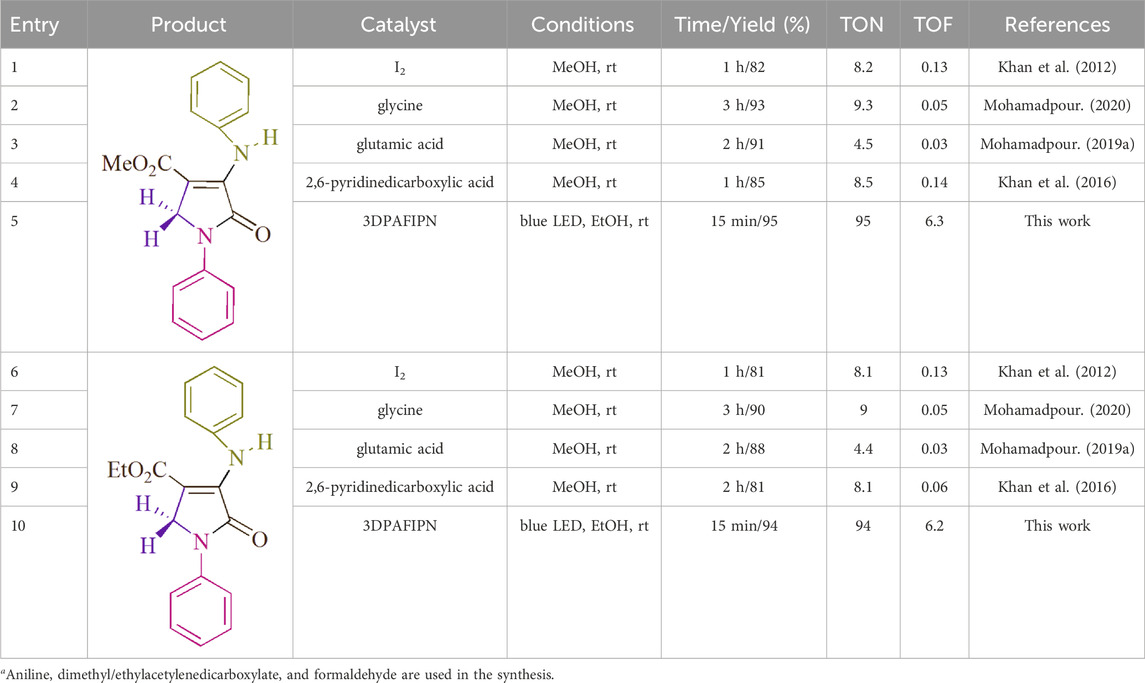
Table 5. The outcome of evaluating the catalytic effectiveness of the numerous catalysts for the production of 5c and 5daysa.
Conclusion
We have used the radical-induced Michael-Mannich cyclocondensation reaction to green photosynthesize polyfunctionalized dihydro-2-oxypyrroles from amines, dialkyl acetylenedicarboxylates, and formaldehyde. The novel halogenated dicyanobenzene-based photosensitizer; 3DPAFIPN was used in current work as a donor-acceptor (D-A) photocatalyst that functions through a series of visible-light-induced electron transfers. The use of blue-light-emitting diode (LED) technology has been shown to produce a sustainable energy-generating mechanism within an ethanol medium at room temperature and in an air environment. The field of chemical synthesis benefits greatly from the proposed technique. These advantages include quick reaction times, elimination of dangerous solvents, increased product yields, simplified reaction mechanisms, durable conditions, and use of a sustainable energy source. Chromatography is not required for the separation technique. It is conceivable to speed up a multigram-scale reaction of model substrates by maintaining the outcome. Thus, the technique may be adopted within a setting that supports long-term ecological and economic viability.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
FM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. AMA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This work was carried out with the cooperation of Iran National Science Foundation (INSF) (no. 4015618), Iran's National Elites Foundation (no. 4015618), and Shiraz University of Medical Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2024.1407071/full#supplementary-material
References
Alp, C., Ekinci, D., Gültekin, M. S., Şentürk, M., Şahin, E., and Küfrevioğlu, Ö. İ. (2010). A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis of carbonic anhydrase inhibitory potencies. Bioorg. Med. Chem. 18, 4468–4474. doi:10.1016/j.bmc.2010.04.072
Bavadi, M., and Niknam, K. (2018). Synthesis of functionalized dihydro-2-oxopyrroles using graphene oxide as heterogeneous catalyst. Mol. Divers. 22, 561–573. doi:10.1007/s11030-017-9809-9
Borthwick, A. D., Crame, A. J., Ertl, P. F., Exall, A. M., Haley, T. M., Hart, G. J., et al. (2002). Design and synthesis of pyrrolidine-5, 5-trans-lactams (5-oxohexahydropyrrolo [3, 2-b] pyrroles) as novel mechanism-based inhibitors of human cytomegalovirus protease. 2. Potency and chirality. J. Med. Chem. 45, 1–18. doi:10.1021/jm0102203
Bryden, M. A., and Zysman-Colman, E. (2021). Organic thermally activated delayed fluorescence (TADF) compounds used in photocatalysis. Chem. Soc. Rev. 50, 7587–7680. doi:10.1039/D1CS00198A
Cardinale, L., Konev, M. O., and Jacobi von Wangelin, A. (2020). Photoredox-catalyzed addition of carbamoyl radicals to olefins: a 1, 4-dihydropyridine approach. Chem. Eur. J. 26, 8239–8243. doi:10.1002/chem.202002410
Chen, Y., Zeng, D. X., Xie, N., and Dang, Y. Z. (2005). Study on photochromism of diarylethenes with a 2, 5-dihydropyrrole bridging unit: a convenient preparation of 3, 4-diarylpyrroles from 3, 4-diaryl-2, 5-dihydropyrroles. J. Org. Chem. 70, 5001–5005. doi:10.1021/jo050236r
Donabauer, K., Murugesan, K., Rozman, U., Crespi, S., and König, B. (2020). Photocatalytic reductive radical-polar crossover for a base-free corey–seebach reaction. Chem. Eur. J. 26, 12945–12950. doi:10.1002/chem.202003000
Flynn, A. R., McDaniel, K. A., Hughes, M. E., Vogt, D. B., and Jui, N. T. (2020). Hydroarylation of arenes via reductive radical-polar crossover. J. Am. Chem. Soc. 142, 9163–9168. doi:10.1021/jacs.0c03926
Gualandi, A., Anselmi, M., Calogero, F., Potenti, S., Bassan, E., Ceroni, P., et al. (2021). Metallaphotoredox catalysis with organic dyes. Org. Biomol. Chem. 19, 3527–3550. doi:10.1039/D1OB00196E
Khan, A. T., Ghosh, A., and Khan, M. M. (2012). One-pot four-component domino reaction for the synthesis of substituted dihydro-2-oxypyrrole catalyzed by molecular iodine. Tetrahedron Lett. 53, 2622–2626. doi:10.1016/j.tetlet.2012.03.046
Khan, M. M., Khan, S., Iqbal, S., and Yousuf, R. (2016). Synthesis of functionalized dihydro-2-oxypyrroles and tetrahydropyridines using 2, 6-pyridinedicarboxylic acid as an efficient and mild organocatalyst. New J. Chem. 40, 7504–7512. doi:10.1039/C6NJ01170E
Lampe, J. W., Chou, Y. L., Hanna, R. G., Di Meo, S. V., Erhardt, P. W., Hagedorn III, A. A., et al. (1993). (Imidazolylphenyl) pyrrol-2-one inhibitors of cardiac cAMP phosphodiesterase. J. Med. Chem. 36, 1041–1047. doi:10.1021/jm00060a012
Lv, L., Zheng, S., Cai, X., Chen, Z., Zhu, Q., and Liu, S. (2013). Development of four-component synthesis of tetra-and pentasubstituted polyfunctional dihydropyrroles: free permutation and combination of aromatic and aliphatic amines. ACS Comb. Sci. 15, 183–192. doi:10.1021/co300148c
Mirjalili, B. B. F., Araqi, R., and Mohajeri, S. A. (2019). A simple and green approach for the synthesis of substituted dihydro-2-oxypyrroles catalyzed by nano-Fe3O4@SiO2/SnCl4 superparamagnetic nanoparticles. Iran. J. Catal. 9, 11–19.
Mohamadpour, F. (2019a). Glutamic acid as green and bio-based α-amino acid catalyst promoted one-pot access to polyfunctionalized dihydro-2-oxypyrroles. J. Serb. Chem. Soc. 84, 1083–1092. doi:10.2298/JSC180720006M
Mohamadpour, F. (2019b). Caffeine as a naturally green and biodegradable catalyst promoted convenient and expedient synthetic route for the synthesis of polysubstituted dihydro-2 oxypyrroles. Bull. Chem. Soc. Ethiop. 33, 149–158. doi:10.4314/bcse.v33i1.15
Mohamadpour, F. (2020). Imin-based synthesis of polyfunctionalized dihydro-2-oxypyrroles catalyzed by glycine amino acid via tandem Michael–Mannich cyclocondensation reaction under ambient temperature. Res. Chem. Intermed. 46, 1931–1940. doi:10.1007/s11164-019-04072-z
Mohamadpour, F. (2021a). Catalyst-free, visible light irradiation promoted synthesis of spiroacenaphthylenes and 1H-pyrazolo[1,2-b]phthalazine-5,10-diones in aqueous ethyl lactate. J. Photochem. Photobiol. A Chem. 407, 113041. doi:10.1016/j.jphotochem.2020.113041
Mohamadpour, F. (2021b). Catalyst-free and solvent-free visible light irradiation-assisted Knoevenagel–Michael cyclocondensation of aryl aldehydes, malononitrile, and resorcinol at room temperature. Monatsh. Chem. 152, 507–512. doi:10.1007/s00706-021-02763-1
Mohamadpour, F. (2022a). The development of Friedländer heteroannulation through a single electron transfer and energy transfer pathway using methylene blue (MB+). Sci. Rep. 12, 7253. doi:10.1038/s41598-022-11349-8
Mohamadpour, F. (2022b). Visible-light-induced radical condensation cyclization to synthesize 3, 4-dihydropyrimidin-2-(1H)-ones/thiones using photoexcited Na2 eosin Y as a direct hydrogen atom transfer (HAT) catalyst. ACS Omega 7, 8429–8436. doi:10.1021/acsomega.1c05808
Mohamadpour, F. (2022c). Catalyst-free and solvent-free visible light assisted synthesis of tetrahydrobenzo[b]Pyran scaffolds at room temperature. Polycycl. Aromat. Compd. 42, 7607–7615. doi:10.1080/10406638.2021.2006244
Mohamadpour, F. (2022d). The development of imin-based tandem Michael–Mannich cyclocondensation through a single-electron transfer (SET)/energy transfer (EnT) pathway in the use of methylene blue (MB+) as a photo-redox catalyst. RSC Adv. 12, 10701–10710. doi:10.1039/D2RA01190E
Mohamadpour, F., Maghsoodlou, M. T., Heydari, R., and Lashkari, M. (2016). Saccharin: a green, economical and efficient catalyst for the one-pot, multi-component synthesis of 3,4-dihydropyrimidin-2-(1H)-one derivatives and 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives and substituted dihydro-2-oxypyrrole. J. Iran. Chem. Soc. 13, 1549–1560. doi:10.1007/s13738-016-0871-5
Mohamadpour, F., Maghsoodlou, M. T., Heydari, R., and Lashkari, M. (2017). Tartaric acid: a naturally green and efficient di-functional Brønsted acid catalyst for the one-pot four-component synthesis of polysubstituted dihydropyrrol-2-ones at ambient temperature. Iran. J. Sci. Technol. Trans. A Sci. 41, 843–849. doi:10.1007/s40995-016-0049-0
Pinosa, E., Bassan, E., Cetin, S., Villa, M., Potenti, S., Calogero, F., et al. (2022). Light-induced access to carbazole-1, 3-dicarbonitrile: a thermally activated delayed fluorescent (TADF) photocatalyst for cobalt-mediated allylations. J. Org. Chem. 88, 6390–6400. doi:10.1021/acs.joc.2c01825
Rothfelder, R., Streitferdt, V., Lennert, U., Cammarata, J., Scott, D. J., Zeitler, K., et al. (2021). Photocatalytic arylation of P4 and PH3: reaction development through mechanistic insight. Angew. Chem. 133, 24855–24863. doi:10.1002/ange.202110619
Salehi, N., and Mirjalili, B. B. F. (2017). Synthesis of highly substituted dihydro-2-oxopyrroles using Fe3O4@ nano-cellulose–OPO3H as a novel bio-based magnetic nanocatalyst. RSC Adv. 7, 30303–30309. doi:10.1039/C7RA04101B
Shiraki, R., Sumino, A., Tadano, K. I., and Ogawa, S. (1995). Total synthesis of PI-091. Tetrahedron Lett. 36, 5551–5554. doi:10.1016/0040-4039(95)01049-N
Singh, H., and Rajput, J. K. (2018). Chelation and calcination promoted preparation of perovskite-structured BiFeO3 nanoparticles: a novel magnetic catalyst for the synthesis of dihydro-2-oxypyrroles. J. Mater. Sci. 53, 3163–3188. doi:10.1007/s10853-017-1790-2
Singh, S. B., Goetz, M. A., Jones, E. T., Bills, G. F., Giacobbe, R. A., Herranz, L., et al. (1995). Oteromycin: a novel antagonist of endothelin receptor. J. Org. Chem. 60, 7040–7042. doi:10.1021/jo00126a071
Speckmeier, E., Fischer, T. G., and Zeitler, K. (2018). A toolbox approach to construct broadly applicable metal-free catalysts for photoredox chemistry: deliberate tuning of redox potentials and importance of halogens in donor–acceptor cyanoarenes. J. Am. Chem. Soc. 140, 15353–15365. doi:10.1021/jacs.8b08933
Srivastava, V., Singh, P. K., and Singh, P. P. (2022). Recent advances of visible-light photocatalysis in the functionalization of organic compounds. J. Photochem. Photobiol. C. Photochem. Rev. 50, 100488. doi:10.1016/j.jphotochemrev.2022.100488
Uoyama, H., Goushi, K., Shizu, K., Nomura, H., and Adachi, C. (2012). Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238. doi:10.1038/nature11687
Wu, Q. A., Chen, F., Ren, C. C., Liu, X. F., Chen, H., Xu, L. X., et al. (2020). Donor–acceptor fluorophores as efficient energy transfer photocatalysts for [2+ 2] photodimerization. Org. Biomol. Chem. 18, 3707–3716. doi:10.1039/C9OB02735A
Yang, Z., Mao, Z., Xie, Z., Zhang, Y., Liu, S., Zhao, J., et al. (2017). Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 46, 915–1016. doi:10.1039/C6CS00368K
Zhang, J. N., Yang, X. H., Guo, W. J., Wang, B., and Zhang, Z. H. (2017). Magnetic metal–organic framework CoFe2O4@SiO2@IRMOF-3 as an efficient catalyst for one-pot synthesis of functionalized dihydro-2-oxopyrroles. Synlett 28, 734–740. doi:10.1055/s-0036-1588924
Zhou, C., Lei, T., Wei, X. Z., Ye, C., Liu, Z., Chen, B., et al. (2020). Metal-free, redox-neutral, site-selective access to heteroarylamine via direct radical–radical cross-coupling powered by visible light photocatalysis. J. Am. Chem. Soc. 142, 16805–16813. doi:10.1021/jacs.0c07600
Keywords: dicyanobenzene-based photosensitizer (3DPAFIPN), polyfunctionalized dihydro-2oxypyrroles, visible-light-induced electron transfer, photosynthesis, renewable energy source
Citation: Mohamadpour F and Amani AM (2024) Gram-scale photosynthesis of polyfunctionalized dihydro-2-oxypyrroles using 3DPAFIPN as a halogenated dicyanobenzene-based photosensitizer via a consecutive visible-light-induced electron transfer process. Front. Chem. 12:1407071. doi: 10.3389/fchem.2024.1407071
Received: 26 March 2024; Accepted: 10 July 2024;
Published: 08 August 2024.
Edited by:
Ravi Kumar, J C Bose University of Science and Technology, IndiaReviewed by:
Vishal Srivastava, University of Allahabad, IndiaArindam Mukhopadhyay, Idaho National Laboratory (DOE), United States
Copyright © 2024 Mohamadpour and Amani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farzaneh Mohamadpour, Zl9tb2hhbWFkcG91ckBzdW1zLmFjLmly, bW9oYW1hZHBvdXIuZi43QGdtYWlsLmNvbQ==; Ali Mohammad Amani, YW1hbmlfYUBzdW1zLmFjLmly, YWxpYW1hbmlAc3Vtcy5hYy5pcg==
 Farzaneh Mohamadpour
Farzaneh Mohamadpour Ali Mohammad Amani*
Ali Mohammad Amani*