
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 05 April 2024
Sec. Medicinal and Pharmaceutical Chemistry
Volume 12 - 2024 | https://doi.org/10.3389/fchem.2024.1372378
This article is part of the Research TopicFive-membered Heterocycles: Synthesis and ApplicationsView all 5 articles
 Sadaf Saeed1
Sadaf Saeed1 Irum Shahzadi1
Irum Shahzadi1 Ameer Fawad Zahoor1*
Ameer Fawad Zahoor1* Aamal A. Al-Mutairi2
Aamal A. Al-Mutairi2 Shagufta Kamal3
Shagufta Kamal3 Shah Faisal4
Shah Faisal4 Ali Irfan1*
Ali Irfan1* Sami A. Al-Hussain2
Sami A. Al-Hussain2 Muhammed Tilahun Muhammed5
Muhammed Tilahun Muhammed5 Magdi E. A. Zaki2*
Magdi E. A. Zaki2*Theophylline, a nitrogen-containing heterocycle, serves as a promising focal point for medicinal researchers aiming to create derivatives with diverse pharmacological applications. In this work, we present an improved synthetic method for a range of theophylline-1,2,4-triazole-S-linked N-phenyl acetamides (4a‒g) utilizing ultrasound-assisted synthetic approach. The objective was to assess the effectiveness of synthesized theophylline-1,2,4-triazoles (4a‒g) as inhibitors of HCV serine protease and as antibacterial agents against B. subtilis QB-928 and E. coli AB-274. Theophylline-1,2,4-triazoles were obtained in good to excellent yields (69%–95%) in a shorter time than conventional approach. 4-Chlorophenyl moiety containing theophylline-1,2,4-triazole 4c displayed significantly higher inhibitory activity against HCV serine protease enzyme (IC50 = 0.015 ± 0.25 mg) in comparison to ribavirin (IC50 = 0.165 ± 0.053 mg), but showed excellent binding affinity (−7.55 kcal/mol) with the active site of serine protease, better than compound 4c (−6.90 kcal/mol) as well as indole-based control compound 5 (−7.42 kcal/mol). In terms of percentage inhibition of serine protease, 2-chlorophenyl compound 4b showed the maximum percentage inhibition (86%), more than that of the 3,4-dichlorophenyl compound 4c (76%) and ribavirin (81%). 3,4-Dimethylphenyl-based theophylline-1,2,4-triazole 4g showed the lowest minimum inhibitory concentration (MIC = 0.28 ± 0.50 μg/mL) against the B. subtilis bacterial strain as compared to the standard drug penicillin (MIC = 1 ± 1.50 μg/mL). The other 4-methylphenyl theophylline-1,2,4-triazole 4e (MIC = 0.20 ± 0.08 μg/mL) displayed the most potent antibacterial potential against E. coli in comparison to the standard drug penicillin (MIC = 2.4 ± 1.00 μg/mL). Molecular docking studies further helped in an extensive understanding of all of the interactions between compounds and the enzyme active site, and DFT studies were also employed to gain insights into the molecular structure of the synthesized compounds. The results indicated that theophylline-linked triazole derivatives 4b and 4c showed promise as leading contenders in the fight against the HCV virus. Moreover, compounds 4e and 4g demonstrated potential as effective chemotherapeutic agents against E. coli and B. subtilis, respectively. To substantiate these findings, additional in vivo studies and clinical trials are imperative, laying the groundwork for their integration into future drug design and development.
Hepatitis C virus (HCV) impacts over 3% of the global population, making it five times more prevalent than HIV (human immunodeficiency virus) infection (De Francesco et al., 2003; Arasappan et al., 2010). If left untreated, HCV infections can progress to cirrhosis, hepatocellular cancer, and liver failure (Bogen et al., 2006). Currently, there is no vaccine available to prevent HCV infection, and there is a lack of broadly effective therapy for individuals with HCV-associated chronic hepatitis (De Francesco and Carfí, 2007). The recommended treatments for HCV involve pegylated interferon-α (PEG IFN-α) injections and oral ribavirin. However, these treatments are only successful in achieving a persistent viral response in 50% of genotype-1 individuals and are associated with significant side effects such as hematological toxicities, flu-like symptoms, and neuropsychiatric events (Koev et al., 2007; Li et al., 2010). Consequently, there is considerable interest in discovering more potent therapeutics for the treatment of HCV infection (Pause et al., 2003; Xue et al., 2012).
Due to their substantial applications across industries, including biological, agricultural, medicinal and pharmacological fields, chemists have drawn their attention to various heterocyclic scaffolds, such as piperazines (Halimehjani et al., 2022), benzoxazole, benzothiazole (Zou et al., 2023), heterocyclic sulfonamides (Apiraksattayakul et al., 2022), benzofurans (Farhat et al., 2022), benzimidazoles (Nardi et al., 2023), thiadiazoles (Janowska et al., 2020), pyarzoles (Nazeer et al., 2023), lamivudines (Henriquez-Camacho et al., 2023), ciprofloxacin-based acetanilides (Zhang et al., 2018), triazoles (Irfan et al., 2024), quinoxalines (Montana et al., 2019), and oxadiazoles (Kerru et al., 2020; Irfan et al., 2022; Irfan et al., 2023). Notably, theophylline (Shahzadi et al., 2020; Shahzadi et al., 2022a), a naturally occurring methylxanthine, has found frequent therapeutic use in treating conditions such as asthma, respiratory deficits in premature infants, and chronic pulmonary obstructive disease (COPD). It also functions as a phosphodiesterase inhibitor (Ito et al., 2002; Saharan and Nantwi, 2006). Acefylline (Shahzadi et al., 2022b), a derivative of theophylline with pharmacological activity, serves various purposes, including bronchodilation, heart stimulation, diuresis, smooth muscle relaxation, antimicrobial and antituberculosis properties, and antiinflammatory effects (Foppoli et al., 2007; Mangasuli et al., 2018; Gopinatha et al., 2020; Shahzadi et al., 2020; Shahzadi et al., 2022a; Montaño et al., 2022), as illustrated in Figure 1.
Similarly, heterocyclic scaffolds bearing the 1,2,4-triazole ring exhibit a broad range of pharmacological properties such as antihypertensive, antimalarial, antianxiety, antifungal, anticancer, antidiabetic, anticonvulsant and anti-tubercular (Abuo-Rahma et al., 2014; Mohassab et al., 2017; Montana et al., 2019; El-Sayed et al., 2020; Irfan et al., 2022; Irfan et al., 2023). 1,2,4-Triazole ring has been discovered to be essential component of serine protease inhibitor such as carbamoyl triazole 1A and benzothiazole based triazole 1B. Previously, our research group also identified ciprofloxacin-based acetanilide 1C as serine protease inhibitor. In general, compounds containing the 1,2,4-triazole moiety exhibit good inhibitory effect against serine protease (Samanta et al., 2013; McConville et al., 2015; Akhtar et al., 2019; Kerru et al., 2020; Shahzadi et al., 2020; Shahzadi et al., 2022a) as depicted in Figure 2.
In a previous study, our research group outlined a traditional method for synthesizing theophylline-triazoles with moderate to good yields (66%–75%) (Ito et al., 2002). Subsequently, we have shifted our focus to enhancing reaction efficiency and yield by incorporating ultrasound irradiation to expedite the process. Building upon this and considering the importance of theophylline triazoles, our ongoing research involves the synthesis of S-alkylated N-aryl acetamide derivatives with a 1,2,4-triazole core under ultrasonic irradiation. We have undertaken this synthesis with the aim of evaluating the therapeutic potential of the resulting compounds against bacterial strains and as inhibitors of serine proteases.
Ultrasound-assisted irradiation reactions were conducted using an ultrasound cleaner bath operating at a frequency of 47 kHz equipped with a mechanical timer and heater switch. All the chemicals, starting materials, and solvents utilized in these synthetic protocols were of analytical grade and procured from reputable suppliers Merck and Sigma Aldrich. The progression of the reaction and purity of compounds were monitored via thin layer chromatography (TLC) performed at silica gel plates. However, a UV lamp was used to visualize the spots on the TLC plate. Gallenkamp equipment was used to determine the melting points of target analogues. Spectral analysis including proton NMR (1H-NMR) at 400 MHz (δ = ppm) and carbon-13 NMR (13C-NMR) at 100 MHz (δ = ppm) was performed on a Bruker spectrophotometer.
To the mixture of corresponding 1,2,4-triazole-3-thione (0.00037 mol, 1 equiv.) in 10 mL of DMF was added LiH (0.00074, 2 equiv.), and the obtained solution was irradiated by ultrasound over 15 min. Then, the mixture was subjected to 2-bromo-N-phenylacetamide 3a-g (0.00037 mol, 1 equiv.) with further irradiation (Scheme 1). After reaction completion, as indicated by TLC, the reaction content was poured on crushed ice. Precipitates of the desired compounds were formed, which were recrystallized with ethanol. The characterization data of these synthetic molecules 4a–g was in agreement with the previously published data (Batool et al., 2018; Shahzadi et al., 2021).
The protease assay was carried out using a modified Kunitz caseinolytic assay. A test tube was filled with 800 μL of 1% casein dissolved in phosphate buffer at a pH of 7.5 and incubated for 10 min. Following the additive, 100 μL of TCA solution and 100 μL of sample were added. This reaction mixture will be placed in an incubator for 30 min before being filtered. After that, 50 μL of FC reagent and 312 μL of Na2CO3 solution were added to the solution mixture and incubated for 30 min. The optical decimal λmax = 660 nm was measured against a blank (Zhang et al., 2018).
The antibacterial therapeutic efficacy of each targeted compound was assessed using the disc diffusion method (Sarker et al., 2007; Siddiqa et al., 2018). A suspension containing 108 cfu/mL of bacteria per 100 μL was spread on to nutrient agar media using a sterilized loop. The molecules were dissolved in chloroform at a specific concentration (25 μg/100 μL) and infused into the sterilized filter paper discs (5.6 mm in diameter). Penicillin (25 μg/100 mL per disc) was used as a positive control. Following infusion of compound solution, these discs were positioned on agar plates inoculated with selected bacterial strains. After incubating plates at 27°C for 24 h, the inhibition zones (ZI) were measure in millimeters (including 5.6-mm disc diameter) in comparison with reference control drug.
For the computational studies against the NS3/4A protease of HCV, we utilized the protein structure having PDB ID: 6NZT (Meewan et al., 2019), while the structures of the compounds were prepared using the ChemDraw professional Ver-16 software. After structure preparation, the compounds were imported into the MOE (MOE Molecular Operating Environment, 2015) software, where the chemical structures were energy minimized, and then we proceeded with the preparation of the protein molecule, which was prepared by the structure preparation module of the MOE software, whereby all the missing atoms and the necessary hydrogen atoms were added to its structure. Finally, these compounds were docked with the protein, and their interactions with molecules were analyzed using the Biovia Discovery Studio (Mills, 2006) software.
The DFT study was undertaken using the Gaussian program as reported before (Dennington and Keith, 2008; Muhammed and Aki-Yalcin, 2023). The resulting computation results were interpreted based on total energy, the highest occupied molecular orbital (HOMO) energy, and the lowest unoccupied molecular orbital (LUMO) energy obtained from the program, as well as computed parameters with respective formulas.
As depicted in Scheme 1, ultrasound irradiation has been employed to enhance organic reactions in synthetic processes. Noteworthy characteristics of these methods include reduced costs, shorter reaction times, mild conditions, high yields, and an environmentally friendly and convenient methodology compared to traditional approaches. In a previous study (Shahzadi et al., 2021), our research group found that the conventional synthesis of acefylline-triazole resulted in yields ranging from 65% to 80%, requiring several hours to complete the reaction. To improve yield and reduce synthesis time, we utilized ultrasound-assisted techniques to achieve the same acefylline-triazole compounds.
Under ultrasound irradiation, compound 2 was reacted with 2-bromo-N-phenylacetamides 3a-h, leading to the formation of S-alkylated analogues of triazole 4a-h with good to excellent yields (69%–95%), as detailed in Table 1 (Batool et al., 2018). The ultrasound-assisted approach proved significantly more efficient, yielding substituted 1,2,4-triazole products at 69%–95% in a much shorter timeframe of 50–60 min as compared to the previously reported method, which required 24–48 h to furnish products at 65%–80% yield.
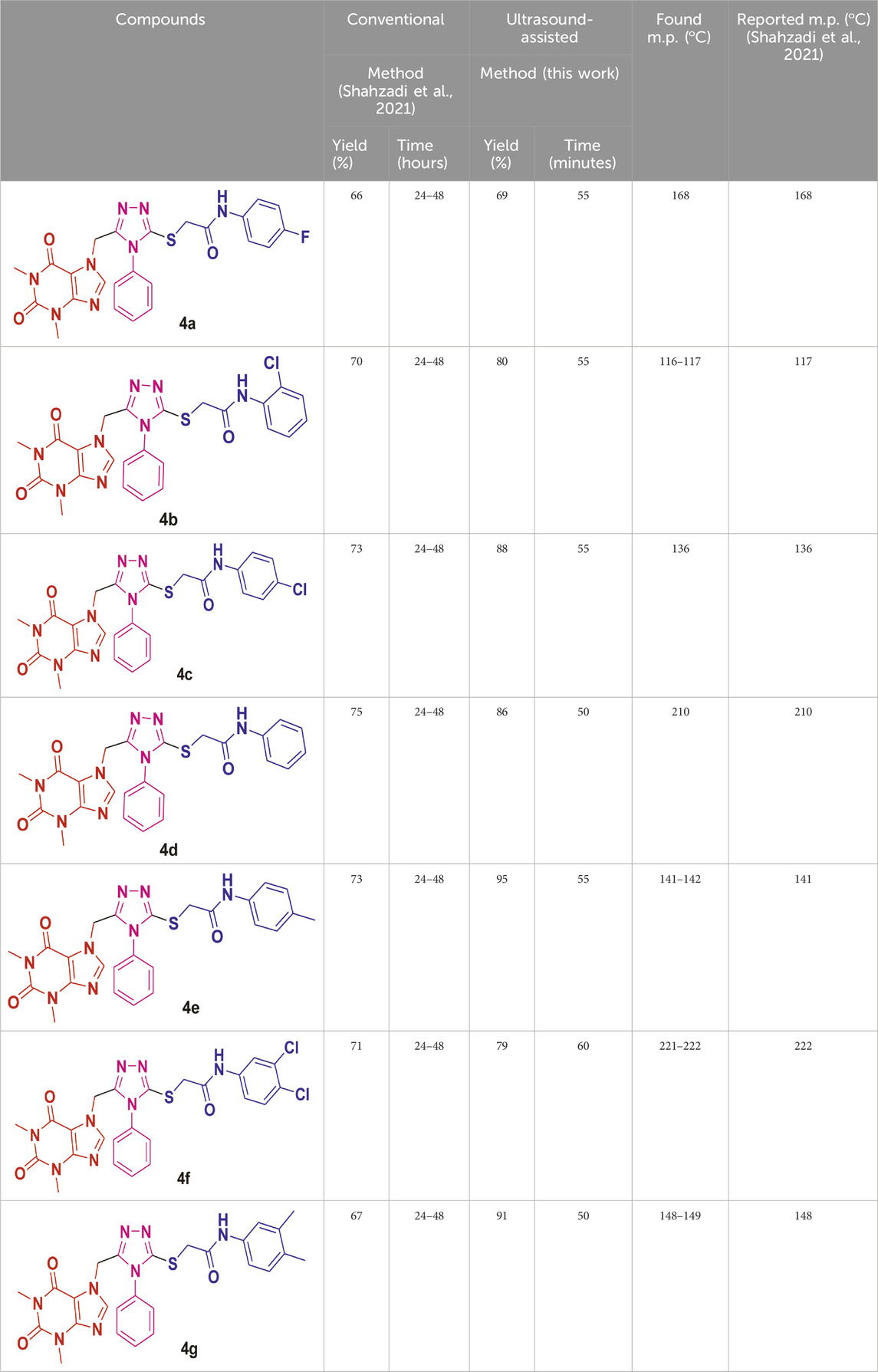
Table 1. Yields of theophylline–1,2,4-triazole analogues via ultrasound irradiation synthetic pathway.
The inhibitory potential of a series of synthesized triazole derivatives (4a–g) against serine protease was investigated. The results of their serine protease inhibition activity are summarized in Table 2. The IC50 values, indicative of the concentration required for 50% inhibition, revealed that all the designed triazole analogues exhibited noteworthy inhibitory potential (IC50 values = 0.015 ± 0.25 mg to 1.314 ± 0.00 mg) against serine protease in comparison to the reference standard drug ribavirin, with an IC50 value of 0.165 ± 0.053 mg.
Among the synthesized derivatives, compound 4c, featuring an electron-withdrawing chloro group on the acetanilide aryl ring, demonstrated the most robust inhibitory activity against serine protease, boasting an IC50 value of 0.015 ± 0.25 mg. The second-most active molecule, 4b, displayed an IC50 value of 0.197 ± 0.00 mg. Compounds 4a, 4e, and 4f also exhibited promising inhibitory activity against serine protease, with IC50 values ranging from 0.203 ± 0.00 to 0.940 ± 0.50 mg. In contrast, compound 4d was considered relatively less active, showing an IC50 value of 1.314 ± 0.00 mg. These findings underscore the potential of the synthesized triazole derivatives as effective inhibitors of serine protease, with compound 4c standing out as particularly potent in this regard.
The structure-activity relationship (SAR) was estimated for all the screen triazole molecules on the basis of nature of substituents as well as position of substituents on the aryl ring of N-phenylacetamide. It was shown that electron-donating and electron-withdrawing groups on the aryl ring of N-phenylacetamide increased the inhibitory potential as compared to the unsubstituted phenyl ring. The most potent hybrid in the series, compound 4c, with the chloro group at the para position on the acetanilide aryl ring, was found to have an IC50 value of 0.015 ± 0.25 mg.
However, changing the position of the chlorine atom from para to ortho in the acetanilide aryl ring decreased the activity as compared to 4c such as compound 4b (0.197 ± 0.00 mg). Furthermore, the placement of two chloro groups at meta as well as para positions further decreased the inhibitory potential of compound 4f (0.940 ± 0.50 mg). Compound 4e (0.45 ± 0.00 mg) bearing a methyl group at the para position exhibited significant activity, while the incorporation of another methyl group at the meta position slightly decreased the activity such as compound 4g (0.855 ± 0.25 mg). Compound 4a (0.658 ± 0.00 mg) having a mono-substituted para fluoro phenyl ring showed considerable activity. The least active compounds in the series were 4d (1.314 ± 0.00 mg) which have an unsubstituted phenyl ring (Figure 3).
Triazole-based compounds were analyzed for their in vitro efficacy against Gram-positive B. subtilis QB-928 and Gram-negative E. coli AB-274 bacterial strains via the disc diffusion method. Results predicted in Table 3 demonstrated that synthesized hybrids 4a–h displayed moderate antibacterial potential. In particular, compounds 4e and 4g exhibited the most promising antibacterial potential values (0.20 ± 0.08 μg/mL and 0.28 ± 0.50 μg/mL) against E. coli and B. subtilis, respectively. The antibacterial effect of compound 4c was observed to be similar in activity against E. coli (0.5 ± 0.00 μg/mL) and B. subtilis (0.5 ± 1.75 μg/mL), respectively. Compounds 4a, 4d, and 4f showed lower antibacterial activity values, that is, 20 ± 2.25 μg/mL, 20 ± 1.25 μg/mL, and 18.48 ± 0.95, whereas compounds 4a, and 4g did not possess any antibacterial activity against E. coli. Compounds 4c (0.5 ± 1.75 μg/mL) and 4e (2.94 ± 0.06 μg/mL) when evaluated against B. subtilis showed moderate antibacterial activity, while compounds 4b, 4d, and 4f did not provide any detectable results as shown in Table 3.
SAR (structure-activity relationship) of all synthesized compounds (4a–h) demonstrated that only compound 4e having an electron-donating (CH3) substituent displayed the highest activity against E. coli (0.20 ± 0.08 μg/mL), whereas this hybrid possessed little activity against B. subtilis (2.94 ± 0.06 μg/mL). The compound 4g, having a methyl group at the meta as well as para positions of the anilide ring, was the second-most potent hybrid of the series against B. subtilis (0.28 ± 0.50 μg/mL), but it was inactive against E. coli. The existence of a chloro substituent at the ortho position of the anilide ring as in compound 4b (3.54 ± 1.34 μg/mL) and at the meta as well as para positions in compound 4f (18.48 ± 0.95 μg/mL) decreased the antibacterial activity against E. coli and produced no detectable results towards B. subtilis, while compound 4d (20 ± 1.25 μg/mL) having an unsubstitued anilide ring showed a similar effect. Placing a fluoro substituent at the para position of N-phenyl acetamide (4a) exhibited the least activity against B. subtilis (20 ± 2.25 μg/mL) and was found ineffective towards E. coli.
Molecular modeling studies were conducted for the acefylline derivatives, which showed good anti-HCV serine protease inhibition activities in the in vitro experimental assays. We employed molecular docking techniques to identify the binding affinities and conformational bindings of these two acefylline derivatives (4b and 4c) to understand the inhibitory mechanism of the HCV viral serine protease, also known as the NS3/4A protease enzyme.
In these investigations, it was found that compounds 4b and 4c possess binding affinities of −7.55 and −6.90 kcal/mol with the active site of the HCV serine protease enzyme. The control drug, which is compound 5 (Meewan et al., 2019), is a small-molecule inhibitor of this serine protease enzyme. This compound 5 was able to show a binding affinity of −7.42 kcal/mol with this target enzyme, which, in comparison to our compounds, has a relatively similar binding affinity. Furthermore, analysis of the molecular interactions and binding conformations of compounds 4b and 4c revealed that these compounds show diverse types of molecular interactions with the active site of the target enzyme. It was found in the binding conformation analysis of these two compounds inside the serine protease active pocket that these compounds occupy the active site pocket and block access to the catalytic amino acids of this enzyme, which are key amino acid residues involved in the processing of the HCV viral proteins after infection with the HCV virus.
It can be seen in Figure 4 that compound 4b is in contact with the important ASP1081 and HIS1057 catalytic residues, engaging it via multiple types of molecular interactions. The acefylline moiety as well as the triazole scaffold of this compound can be seen interacting via diverse types of interactions, e.g., hydrogen bonds (both conventional as well as C-H hydrogen bonds along with Pi-Donor H-Bond). Other than these multiple types of hydrophobic interactions, which further stabilize a compound inside the active site of an enzyme like Alkyl, Pi-Alkyl Pi-Cation and Sigma, Pi-Sulfur, Amide-Pi Stacked, etc., other moieties of the 4b compounds and the amino acid residues of the active site pocket of HCV serine protease can also be seen in Figure 4.
Similarly, the other compound 4c was also able to show a similar type of conformation and molecular interaction with the target enzyme active site amino acid residues. The acefylline moiety of the 4c was able to engage the important catalytic amino acid residues multiple times via conventional and carbon-hydrogen-type hydrogen bonding. The important SER1139 and HIS1057, which are the main catalytic amino acid residues, can be seen in Figure 4, making multiple strong interactions with the acefylline moiety of the 4c compound. Apart from the acefylline moiety, other important scaffolds like the triazole and the other phenyl moieties can also be seen engaging in different types of molecular interactions with the important active site residues of this enzyme. Table 4 contains the binding energies and chemical structures of the investigated compounds with the target enzyme.
The HOMO, LUMO and total energies of the relatively active synthesized compounds were obtained from the DFT computation. Then, the other related parameters were calculated with the respective formulas (Table 5) (Zeyrek et al., 2021; Arslan et al., 2023).
The DFT analysis showed that all the investigated compounds had similar electrochemical values with slim differences. As HOMO represents electron donors and LUMO represents electron acceptors of a molecule, they are utilized to predict electron exchange capacity (Miar et al., 2021). According to predictions, 4g had the highest HOMO energy value, while 4b had the lowest. The HOMO energy value of 4g was predicted to be the highest whereas 4b had the lowest value. Similarly, 4e had the highest LUMO value whereas 4b had the lowest value (Table 5). Hence, 4g is expected to give its electrons relatively easy whereas 4e is expected to accept electrons with relatively higher affinity. HOMO-LUMO energy gaps of molecules give clue about the relative stability of them. In general, higher energy gap manifests higher chemical stability (Ruiz-Morales, 2002). In this study, the greatest energy gap among the tested compounds was produced by compound 4b (Table 5). Hence, 4b is anticipated to display the highest chemical stability. Global hardness displays resistance of atoms to electron transfer. The DFT computation revealed that 4b had the highest global hardness value as expected. This study implicated that 4b might be the most stable as well the least reactive (Han et al., 2022).
HOMO-LUMO orbital orientations were similar for some derivatives and different for some others. In this regard, orbital orientations of 4e and 4g were similar with each other. HOMO orbitals were predominantly observed around the p-tolyl acetamide group and sulfur next to it (Figure 5). Similarly, LUMO orbitals were mainly observed around the purine heterocyclic group and its substituents for both of them. In addition to this, some LUMO orbitals were observed around the triazole ring (Figure 5). However, HOMO and LUMO orbitals of compound 4b were mainly concentrated around the purine heterocyclic group and also sparsely located around the triazole ring. Furthermore, LUMO orbitals were observed around the sulfur of compound 4b. Orbital distribution of 4c was different from the other compounds. The LUMO orbitals were concentrated mainly around the triazole ring and its phenyl substituent. On the other hand, the HOMO orbital distribution was concentrated around the purine ring and some more orbitals were also observed around 4-chlorophenyl group next to the acetamide functional group (Figure 5).
The synthesis of theophylline-linked 1,2,4-triazole compounds (4a‒g) was achieved with excellent yield under ultrasonic irradiation, surpassing the conventional approach’s reported yield of 66%–75%. The ultrasonic-assisted method demonstrated advantages such as shorter reaction times and higher yields of 1,2,4-triazole products (69%–95%). Notably, among the synthesized structural hybrids, the 1,2,4-triazole compound 4c (IC50 = 0.015 ± 0.25 mg), featuring a 4-chlorophenyl ring, exhibited superior serine protease inhibitory activity compared to the standard drug ribavirin (IC50 = 0.165 ± 0.053 mg). Molecular docking studies revealed that triazole compound 4b exhibited a stronger binding affinity score than 4c and the control drug 5 with the active site of serine protease enzyme. DFT study results were consistent with in vitro and molecular docking findings. In terms of inhibition, compound 4b demonstrated higher inhibition (86%) of serine protease compared to 3,4-dichlorophenyl compound 4c (76%) and the standard drug ribavirin (81%). Thus, compounds 4b and 4c emerged as more promising serine protease inhibitors than the standard drug, ribavirin.
Structure-activity relationship (SAR) analysis revealed that nature of substituents as well as position of substituents on the aryl ring of N-phenylacetamide played a crucial role in protease inhibition. Additionally, the synthesized compounds exhibited significant antibacterial potential against Bacillus subtilis and Escherichia coli. Theophylline-1,2,4-triazole 4g, based on 3,4-dimethylphenyl, showed the lowest minimum inhibitory concentration (MIC = 0.28 ± 0.50 μg/mL) against B. subtilis compared to the standard drug penicillin (MIC = 1 ± 1.50 μg/mL). Another compound, 4e, featuring 4-methylphenyl theophylline-1,2,4-triazole, displayed the most potent antibacterial potential against E. coli (MIC = 0.20 ± 0.08 μg/mL) compared to penicillin (MIC = 2.4 ± 1.00 μg/mL). In conclusion, this study suggests that modifications in triazole hybrids could lead to the development of more effective antibacterial and serine protease inhibitor compounds.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
SS: Data curation, Investigation, Methodology, Writing–original draft. IS: Validation, Visualization, Writing–review and editing. AZ: Conceptualization, Project administration, Resources, Supervision, Writing–review and editing. AA-M: Data curation, Formal Analysis, Writing–review and editing. SK: Formal Analysis, Investigation, Writing–review and editing, Formal Analysis. SF: Software, Writing–review and editing. AI: Conceptualization, Funding acquisition, Visualization, Writing–review and editing. SA-H: Data curation, Funding acquisition, Resources, Writing–review and editing. MM: Software, Writing–original draft. MZ: Funding acquisition, Project administration, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research through the project number IFP-IMSIU-2023131. The authors also appreciate the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for supporting and supervising this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abuo-Rahma, G. E. D. A., Abdel-Aziz, M., Beshr, E. A., and Ali, T. F. (2014). 1, 2, 4-Triazole/oxime hybrids as new strategy for nitric oxide donors: synthesis, anti-inflammatory, ulceroginicity and antiproliferative activities. Eur. J. Med. Chem. 71, 185–198. doi:10.1016/j.ejmech.2013.11.006
Akhtar, R., Zahoor, A. F., Rasul, A., Ahmad, M., Anjum, M. N., Ajmal, M., et al. (2019). Design, synthesis, in-silico study and anticancer potential of novel n-4-piperazinyl-ciprofloxacin-aniline hybrids. Pak. J. Pharm. Sci. 32 (5), 2215–2222.
Apiraksattayakul, S., Pingaew, R., Prachayasittikul, V., Ruankham, W., Jongwachirachai, P., Songtawee, N., et al. (2022). Neuroprotective properties of bis-sulfonamide derivatives against 6-OHDA-induced Parkinson's model via sirtuin 1 activity and in silico pharmacokinetic properties. Front. Mol. Neurosci. 15, 890838. doi:10.3389/fnmol.2022.890838
Arasappan, A., Bennett, F., Bogen, S. L., Venkatraman, S., Blackman, M., Chen, K. X., et al. (2010). Discovery of narlaprevir (SCH 900518): a potent, second generation HCV NS3 serine protease inhibitor. Acs. Med. Chem. Lett. 1, 64–69. doi:10.1021/ml9000276
Arslan, G., Gökçe, B., Muhammed, M. T., Albayrak, Ö., Önkol, T., and Özçelik, A. B. (2023). Synthesis, DFT calculations, and molecular docking study of acetohydrazide-based sulfonamide derivatives as paraoxonase 1 inhibitors. Chem. Sel. 8, e202204630. doi:10.1002/slct.202204630
Batool, M., Tajammal, A., Farhat, F., Verpoort, F., Khattak, Z. A., Shahid, M., et al. (2018). Molecular docking, computational, and antithrombotic studies of novel 1, 3, 4-oxadiazole derivatives. Int. J. Mol. Sci. 19, 3606. doi:10.3390/ijms19113606
Bogen, S. L., Arasappan, A., Bennett, F., Chen, K., Jao, E., Liu, Y. T., et al. (2006). Discovery of SCH446211 (SCH6): a new ketoamide inhibitor of the HCV NS3 serine protease and HCV subgenomic RNA replication. J. Med. Chem. 49, 2750–2757. doi:10.1021/jm060077j
Chattaraj, P. K., Sarkar, U., and Roy, D. R. (2006). Electrophilicity index. Chem. Rev. 106, 2065–2091. doi:10.1021/cr040109f
De Francesco, R., and Carfí, A. (2007). Advances in the development of new therapeutic agents targeting the NS3-4A serine protease or the NS5B RNA-dependent RNA polymerase of the hepatitis C virus. Adv. Drug Deliv. Rev. 59, 1242–1262. doi:10.1016/j.addr.2007.04.016
De Francesco, R., Tomei, L., Altamura, S., Summa, V., and Migliaccio, G. (2003). Approaching a new era for hepatitis C virus therapy: inhibitors of the NS3-4A serine protease and the NS5B RNA-dependent RNA polymerase. Antivir. Res. 58, 1–16. doi:10.1016/s0166-3542(03)00028-7
El-Sayed, H. A., Assy, M. G., and Mohamed, A, S. (2020). An efficient synthesis and antimicrobial activity of N-bridged triazolo [3, 4-b] thiadiazine and triazolo [3,4-b] thiadiazole derivatives under microwave irradiation. Synth. Commun. 50, 997–1007. doi:10.1080/00397911.2020.1726397
Farhat, J., Alzyoud, L., Alwahsh, M., and Al-Omari, B. (2022). Structure–activity relationship of benzofuran derivatives with potential anticancer activity. Cancers 14 (9), 2196. doi:10.3390/cancers14092196
Foppoli, A., Zema, L., Gazzaniga, A., Caira, M. R., Nassimbeni, L. R., Borkum, E., et al. (2007). Solid-state chemistry of ambroxol theophylline-7-acetate. J. Pharm. Sci. 96, 1139–1146. doi:10.1002/jps.20951
Gopinatha, V. K., Mantelingu, K., and Rangappa, K. S. (2020). Synthesis and biological evaluation of theophylline acetohydrazide hydrazone derivatives as antituberculosis agents. J. Chin. Chem. Soc. 67, 1453–1461. doi:10.1002/jccs.201900558
Halimehjani, A. Z., Dehghan, F., Tafakori, V., Amini, E., Hooshmand, S. E., and Nossod, Y. L. (2022). Synthesis of novel antibacterial and antifungal dithiocarbamate-containing piperazine derivatives via re-engineering multicomponent approach. Heliyon 8, e09564. doi:10.1016/j.heliyon.2022.e09564
Han, M. İ., Dengiz, C., Doğan, Ş. D., Gündüz, M. G., Köprü, S., and Özkul, C. (2022). Isoquinolinedione-urea hybrids: synthesis, antibacterial evaluation, drug-likeness, molecular docking and DFT studies. J. Mol. Struct. 1252, 132007. doi:10.1016/j.molstruc.2021.132007
Henriquez-Camacho, C., Hijas-Gomez, A. I., Risco Risco, C., Ruiz Lapuente, M. A., Escudero-Sanchez, R., and Cuerda, V. M. (2023). Lamivudine and entecavir for acute hepatitis B: a systematic review and meta-analysis. Viruses 15, 2241. doi:10.3390/v15112241
Irfan, A., Zahoor, A. F., Kamal, S., Hassan, M., and Kloczkowski, A. (2022). Ultrasonic-assisted synthesis of benzofuran appended oxadiazole molecules as tyrosinase inhibitors: mechanistic approach through enzyme inhibition, molecular docking, chemoinformatics, ADMET and drug-likeness studies. Int. J. Mol. Sci. 23, 10979. doi:10.3390/ijms231810979
Irfan, A., Zahoor, A. F., Rasul, A., Al-Hussain, S. A., Faisal, S., Ahmad, S., et al. (2023). BTEAC catalyzed ultrasonic-assisted synthesis of bromobenzofuran-oxadiazoles: unravelling anti-HepG-2 cancer therapeutic potential through in vitro and in silico studies. Int. J. Mol. Sci. 24, 3008. doi:10.3390/ijms24033008
Irfan, M., Khan, H. A., Bibi, S., Wu, G., Ali, A., Khan, S. G., et al. (2024). Exploration of nonlinear optical properties of 4-methyl-4H-1,2,4-triazol-3-y lthio-N-phenylpropanamide based derivatives: experimental and DFT approach. Sci. Rep. 14, 2732. doi:10.1038/s41598-024-51788-z
Ito, K., Lim, S., Caramori, G., Cosio, B., Chung, K. F., Adcock, I. M., et al. (2002). A molecular mechanism of action of theophylline: induction of histone deacetylase activity to decrease inflammatory gene expression. P. Natl. A. Sci. 99, 8921–8926. doi:10.1073/pnas.132556899
Janowska, S., Paneth, A., and Wujec, M. (2020). Cytotoxic properties of 1,3,4-thiadiazole derivatives—a review. Molecules 25, 4309. doi:10.3390/molecules25184309
Kerru, N., Gummidi, L., Maddila, S., Gangu, K. K., and Jonnalagadda, S. B. (2020). A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 25 (8), 1909. doi:10.3390/molecules25081909
Koev, G., Dekhtyar, T., Han, L., Yan, P., Ng, T. I., Lin, C. T., et al. (2007). Antiviral interactions of an HCV polymerase inhibitor with an HCV protease inhibitor or interferon in vitro. Antivir. Res. 73, 78–83. doi:10.1016/j.antiviral.2006.07.009
Koopmans, T. (1934). About the assignment of wave functions and eigenvalues to the individual electrons of an atom. Physica 1, 104–113. doi:10.1016/s0031-8914(34)90011-2
Li, X., Zhang, Y. K., Liu, Y., Ding, C. Z., Li, Q., Zhou, Y., et al. (2010). Synthesis and evaluation of novel α-amino cyclic boronates as inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 20, 3550–3556. doi:10.1016/j.bmcl.2010.04.129
Mangasuli, S. N., Hosamani, K. M., Devarajegowda, H. C., Kurjogi, M. M., and Joshi, S. D. (2018). Synthesis of coumarin-theophylline hybrids as a new class of anti-tubercular and anti-microbial agents. Eur. J. Med. Chem. 146, 747–756. doi:10.1016/j.ejmech.2018.01.025
McConville, M., Fernández, J., Angulo-Barturen, Í., Bahamontes-Rosa, N., Ballell-Pages, L., Castañeda, P., et al. (2015). Carbamoyl triazoles, known serine protease inhibitors, are a potent new class of antimalarials. J. Med. Chem. 58, 6448–6455. doi:10.1021/acs.jmedchem.5b00434
Meewan, I., Zhang, X., Roy, S., Ballatore, C., O’Donoghue, A. J., Schooley, R. T., et al. (2019). Discovery of new inhibitors of hepatitis C virus NS3/4A protease and its D168A mutant. ACS Omega 4, 16999–17008. doi:10.1021/acsomega.9b02491
Miar, M., Shiroudi, A., Pourshamsian, K., Oliaey, A. R., and Hatamjafari, F. (2021). Theoretical investigations on the HOMO–LUMO gap and global reactivity descriptor studies, natural bond orbital, and nucleus-independent chemical shifts analyses of 3-phenylbenzo [d] thiazole-2 (3 H)-imine and its para-substituted derivatives: solvent and substituent effects. J. Chem. Res. 45, 147–158. doi:10.1177/1747519820932091
Mills, N. (2006). ChemDraw ultra 10.0 CambridgeSoft, 100 CambridgePark drive, cambridge, MA 02140. Www.Cambridgesoft.Com. Commercial price: $1910 for download, $2150 for CD-ROM; academic price: $710 for download, $800 for CD-ROM. J. Am. Chem. Soc. 128, 13649–13650. doi:10.1021/ja0697875
MOE (Molecular Operating Environment) (2015). Integrated computer-aided molecular design platform. Sci. Comput. Instrum. 32.
Mohassab, A. M., Hassan, H. A., Abdelhamid, D., Abdel-Aziz, M., Dalby, K. N., and Kaoud, T. S. (2017). Novel quinoline incorporating 1, 2, 4-triazole/oxime hybrids: synthesis, molecular docking, anti-inflammatory, COX inhibition, ulceroginicity and histopathological investigations. Bioorg. Chem. 75, 242–259. doi:10.1016/j.bioorg.2017.09.018
Montana, M., Mathias, F., Terme, T., and Vanelle, P. (2019). Antitumoral activity of quinoxaline derivatives: a systematic review. Eur. J. Med. Chem. 163, 136–147. doi:10.1016/j.ejmech.2018.11.059
Montaño, L. M., Sommer, B., Gomez-Verjan, J. C., Morales-Paoli, G. S., Ramírez-Salinas, G. L., Solís-Chagoyán, H., et al. (2022). Theophylline: old drug in a new light, application in COVID-19 through computational studies. Int. J. Mol. Sci. 23, 4167. doi:10.3390/ijms23084167
Muhammed, M. T., and Aki-Yalcin, E. (2023). Computational Insight into the mechanism of action of DNA gyrase inhibitors; revealing a new mechanism. Curr. Comput-Aid Drug Des. 20 (3), 224–235. doi:10.2174/1573409919666230419094700
Nardi, M., Cano, N. C. H., Simeonov, S., Bence, R., Kurutos, A., Scarpelli, R., et al. (2023). A review on the green synthesis of benzimidazole derivatives and their pharmacological activities. Catalysts 13, 392. doi:10.3390/catal13020392
Nazeer, U., Mushtaq, A., Zahoor, A. F., Hafeez, F., Shahzadi, I., and Akhtar, R. (2023). Cloke-Wilson rearrangement: a unique gateway to access five-membered heterocycles. RSC Adv. 13 (50), 35695–35732. doi:10.1039/D3RA07410B
Parr, R. G., Donnelly, R. A., Levy, M., and Palke, W. E. (1978). Electronegativity: the density functional viewpoint. J. Chem. Phys. 68, 3801–3807. doi:10.1063/1.436185
Pause, A., Kukolj, G., Bailey, M., Brault, M., Dô, F., Halmos, T., et al. (2003). An NS3 serine protease inhibitor abrogates replication of subgenomic hepatitis C virus RNA. J. Biol. Chem. 278, 20374–20380. doi:10.1074/jbc.m210785200
Ruiz-Morales, Y. (2002). HOMO− LUMO gap as an index of molecular size and structure for polycyclic aromatic hydrocarbons (PAHs) and asphaltenes: a theoretical study. I. J. Phys. Chem. A 106, 11283–11308. doi:10.1021/jp021152e
Saharan, R. S., and Nantwi, K. D. (2006). Changes in the biochemical profiles of mid-cervically located adenosine A1 receptors after repeated theophylline administration in adult rats. J. Spinal Cord. Med. 29, 520–526. doi:10.1080/10790268.2006.11753902
Samanta, S., Lim, T. L., and Lam, Y. (2013). Synthesis and in vitro evaluation of west nile virus protease inhibitors based on the 2-{6-[2-(5-phenyl-4H-{1, 2, 4] triazol-3-ylsulfanyl) acetylamino] benzothiazol-2-ylsulfanyl} acetamide Scaffold. Chem. Med. Chem. 8, 994–1001. doi:10.1002/cmdc.201300114
Sarker, S. D., Nahar, L., and Kumarasamy, Y. (2007). Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth and its application in the in vitro antibacterial screening of phytochemicals. Methods 42, 321–324. doi:10.1016/j.ymeth.2007.01.006
Shahzadi, I., Zahoor, A. F., Parveen, B., Rasul, A., Raza, Z., Ahmad, S., et al. (2022b). Acefylline derivatives as a new class of anticancer agents: synthesis, molecular docking, and anticancer, hemolytic, and thrombolytic activities of acefylline-triazole hybrids. J. Chem. 2022, 1–8. doi:10.1155/2022/3502872
Shahzadi, I., Zahoor, A. F., Rasul, A., Mansha, A., Ahmad, S., and Raza, Z. (2021). Synthesis, hemolytic studies, and in silico modeling of novel acefylline–1,2,4-triazole hybrids as potential anti-cancer agents against MCF-7 and A549. ACS Omega 6, 11943–11953. doi:10.1021/acsomega.1c00424
Shahzadi, I., Zahoor, A. F., Rasul, A., Rasool, N., Raza, Z., Faisal, S., et al. (2020). Synthesis, anticancer, and computational studies of 1, 3, 4-oxadiazole-purine derivatives. J. Heterocycl. Chem. 57, 2782–2794. doi:10.1002/jhet.3987
Shahzadi, I., Zahoor, A. F., Tüzün, B., Mansha, A., Anjum, M. N., Rasul, A., et al. (2022a). Repositioning of acefylline as anti-cancer drug: synthesis, anticancer and computational studies of azomethines derived from acefylline tethered 4-amino-3-mercapto-1,2,4-triazole. PLoS One 17 (12), e0278027. doi:10.1371/journal.pone.0278027
Siddiqa, A., Abbasi, M. A., Siddiqui, S. Z., Khan, S. G., Rasool, S., Ali Shah, S. A., et al. (2018). Synthesis, spectral analysis and antibacterial activity of some novel 5-substituted-2-((6-bromo-3,4-methylenedioxybenzyl)thio)-1,3,4-oxadiazole derivatives. Pak. J. Pharm. Sci. 31 (5), 1783–1790.
Xue, W., Pan, D., Yang, Y., Liu, H., and Yao, X. (2012). Molecular modeling study on the resistance mechanism of HCV NS3/4A serine protease mutants R155K, A156V and D168A to TMC435. Antivir. Res. 93, 126–137. doi:10.1016/j.antiviral.2011.11.007
Zeyrek, C. T., Arpacı, Ö. T., Arısoy, M., and Onurdağ, F. K. (2021). Synthesis, antimicrobial activity, density functional modelling and molecular docking with COVID-19 main protease studies of benzoxazole derivative: 2-(p-chloro-benzyl)-5-[3-(4-ethly-1-piperazynl) propionamido]-benzoxazole. J. Mol. Struct. 1237, 130413. doi:10.1016/j.molstruc.2021.130413
Zhang, G. F., Liu, X., Zhang, S., Pan, B., and Liu, M. L. (2018). Ciprofloxacin derivatives and their antibacterial activities. Eur. J. Med. Chem. 146, 599–612. doi:10.1016/j.ejmech.2018.01.078
Keywords: ultrasonic assisted approach, high yields, theophylline-1,2,4-triazoles, serine protease inhibitors, antibacterial agents, molecular docking, DFT study, SAR
Citation: Saeed S, Shahzadi I, Zahoor AF, Al-Mutairi AA, Kamal S, Faisal S, Irfan A, Al-Hussain SA, Muhammed MT and Zaki MEA (2024) Exploring theophylline-1,2,4-triazole tethered N-phenylacetamide derivatives as antimicrobial agents: unraveling mechanisms via structure-activity relationship, in vitro validation, and in silico insights. Front. Chem. 12:1372378. doi: 10.3389/fchem.2024.1372378
Received: 17 January 2024; Accepted: 20 March 2024;
Published: 05 April 2024.
Edited by:
Md. Musawwer Khan, Aligarh Muslim University, IndiaReviewed by:
Radhika Amaradhi, University of Texas at San Antonio, United StatesCopyright © 2024 Saeed, Shahzadi, Zahoor, Al-Mutairi, Kamal, Faisal, Irfan, Al-Hussain, Muhammed and Zaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ameer Fawad Zahoor, ZmF3YWQuemFob29yQGdjdWYuZWR1LnBr; Ali Irfan, Mi1yYWlhbGlpcmZhbkBnbWFpbC5jb20=; Magdi E. A. Zaki, bWV6YWtpQGltYW11LmVkdS5zYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.