- Department of Separation Science, LUT School of Engineering Science, Lappeenranta-Lahti University of Technology LUT, Mikkeli, Finland
In recent years, there has been a considerable rise in the production of novel metabolites derived from fungi compared to the ones originating from bacteria. These organic substances are utilized in various sectors such as farming, healthcare, and pharmaceutical. Since all dividing living cells contain primary metabolites, secondary metabolites are synthesized by utilizing intermediate compounds or by-products generated from the primary metabolic pathways. Secondary metabolites are not critical for the growth and development of an organism; however, they exhibit a variety of distinct biological characteristics. White-rot fungi are the only microorganisms able to decompose all wood components. Hence, they play an important role in both the carbon and nitrogen cycles by decomposing non-living organic substrates. They are ubiquitous in nature, particularly in hardwood (e.g., birch and aspen) forests. White-rot fungi, besides ligninolytic enzymes, produce different bioactive substances during their secondary metabolism including some compounds with antimicrobial and anticancer properties. Such properties could be of potential interest for the pharmaceutical industries. Considering the importance of the untapped biologically active secondary metabolites from white-rot fungi, the present paper reviews the secondary metabolites produced by white-rot fungi with different interesting bioactivities.
1 Introduction
Biologically active compounds are synthesized mostly by fungi, bacteria, archaea, and plants. These compounds possess different properties that make them suitable for various applications including drug development with anti-glycaemic, anticancer, antibiotic, antiviral, anti-inflammatory, enzyme inhibiting, hypercholesteremic, immunomodulator, immunosuppressant, cardiovascular, antithrombotic, antidiabetic, antihypertensive, neuropathic, and anti-infective characteristics for humans (Basit et al., 2021; Conrado et al., 2022; Kijpornyongpan et al., 2022). Starting from the 1940s, microorganisms have played a significant role in uncovering important sources of various natural products used in the agrochemical, cosmetic, pharmaceutical, and food industries (Baltz, 2019; Gakuubi et al., 2022). Because of their exceptional biological activities, these compounds have gained the attention of researchers in various fields, including those investigating natural product-derived medicines as well as chemists, biochemists, and microbiologists. In addition, recently, metabolic engineers seek to enlighten regulations, pathways, and gene clusters to produce bioactive compounds efficiently using suitable host organisms with the assistance of genome sequencing to bioinformatics, transcriptomics, metabolomics, and proteomics (Tsukada et al., 2020; Kijpornyongpan et al., 2022). In this sense, microbial secondary metabolites are beneficial for human wellbeing, owing to their extensive usage in various biological processes spanning across agriculture, medical sciences, food technology, and chemical industry (Yadav, 2021). More specifically, among the different existing microorganisms, fungi have emerged as promising candidates for the discovery of novel biologically active compounds because of their varied pharmacological activities.
The advantageous effects of fungi on human health have been mainly associated with the abundance of various bioactive compounds, including carbohydrates, proteins, amino acids, unsaturated fatty acids, vitamins, and minerals (Gebreyohannes and Sbhatu, 2023) together with bioactive secondary metabolites. The therapeutic use of fungal species can be traced back to as early as 3000 BC. Thus, macrofungi such as Ganoderma lucidum (G. lucidum), Lentinus edodes (L. edodes), Fomes fomentarius, and Fomitopsis officinalis were used as remedies for different diseases in some East countries (Wasser, 2002). Medical professionals have been aware for over five thousand years regarding the presence of immune-enhancing and defensive attributes in fungal species (Ribka et al., 2021). In the literature, reports indicate that a significant number of approved medications are sourced from nature, with approximately 25% of the one million natural compounds examined showing biological activities. Among these compounds, around 60% originated from plants, while the remaining is derived from microbes. Notably, fungi contribute to approximately 42% of microbial resources, underscoring their significance in the exploration and identification of novel molecules (Bharatiya et al., 2021). Furthermore, until 2019, approximately 35% of the naturally derived products approved by the US Food and Drug Administration (FDA) contributed to the development of pharmaceuticals (Shankar and Sharma, 2022). Moreover, fungi are also able to synthesize various biologically active compounds such as pigments, dyes, antioxidants, nutraceuticals, dietary supplements, polysaccharides, and industrial enzymes (Al-Mousa et al., 2022a; Al-Mousa et al., 2022b; Hassane et al., 2022; Mohamed et al., 2022). These fungal products are not only crucial for functional food and nutrition but also serve as important sources of pharmacological/medicinal substances (Keller, et al., 2005; Hoeksma et al., 2019; Fukushima-Sakuno, 2020; Elhusseiny et al., 2021; Bhambri et al., 2022; Krupodorova et al., 2022). These intriguing scientific discoveries have garnered significant interest from researchers who are exploring the potential applications of these metabolites.
Metabolites are small and intermediate metabolism products that serve various purposes in organisms. These products are classified into primary and secondary metabolites. In this sense, primary metabolites are produced for growth, development, and survival and consist of amino acids, sugars, vitamins, lipids, nucleotides, and carbohydrates, which have critical duties in different metabolic processes including respiration and nutrient consumption. On the other hand, secondary metabolites are not integral components of the metabolic pathways; instead, they are synthesized as byproducts in terms of defense mechanisms and derived from primary metabolism. These compounds exhibit diverse biological functions and result from metabolic reactions that are non-essential for growth and reproduction of organisms. The production of secondary metabolites provides a competitive advantage to the organism by increasing the tolerance to environmental stresses and extreme conditions, and thereby indirectly influencing ecological dynamics (Devi and Krishnakumari, 2015; Daley et al., 2017; Thirumurugan et al., 2018; Conrado et al., 2022; Rodríguez-Berríos et al., 2023; Sharma et al., 2023). “Secondary metabolites” term was first introduced by the Nobel Prize laureate Albrecht Kossel in 1891 and the botanist Friedrich Czapek further created the term “secondary modifications” in his work related to plant nitrogen metabolism in the 1920s (Henriksen et al., 2022). Secondary metabolites are a diverse group of organic compounds primarily derived from various sources such as plants, fungi, and bacteria. These bioactive molecules are generally low molecular weight compounds (Molecular weight <1,500 Da) (Zerva et al., 2020a). They present scientific interest due to their multiple applications in industries (e.g., textile, functional food innovation, flavoring, glues, oil). Additionally, they hold promising potential for the development of novel pharmaceuticals, antibiotics, insecticides for pest control, and herbicides targeting unwanted plant growth (Devi and Krishnakumari, 2015; Devi et al., 2022; Shankar and Sharma, 2022).
The biosynthesis of fungal secondary bioactive metabolites is typically based on the mevalonic acid pathway, the acetate pathway, and carbohydrate/polysaccharide synthesis (Kundu, 2021). These fungal-derived bioactive compounds can be divided into high and low molecular weight. The former predominantly comprises polysaccharides and enzymes, while the latter encompasses terpenoids, phenols, and indoles, among others (Ziaja-Sołtys et al., 2022). Conrado et al. (2022) reported that out of the 500,000 secondary metabolites, approximately 70,000 are sourced from microorganisms. Among these compounds, around 33,500 exhibit bioactive properties, and about 47% of these bioactive compounds originate from fungal strains. Up to date, numerous fungal species, particularly filamentous fungi found within the basidiomycetes class, can be considered for an extensive range production of secondary metabolites with significant biological activities (Teoh et al., 2011; Patel and Goyal, 2012). Basidiomycetes are known for their ability to produce numerous secondary metabolites that exhibit antioxidant (Jayakumar et al., 2009), antimicrobial (Bala et al., 2011), anti-inflammatory (Liu et al., 2015), antifungal (Sidorova and Voronina, 2019), and antiviral (Krupodorova et al., 2014) properties. Moreover, they can also produce cytotoxic compounds with the potential use as anticancer agents and immunomodulating polysaccharides. Additionally, some of these metabolites have hallucinogenic effects while others serve as sources for plant growth regulators or flavors (Prasher and Manju, 2019; Halabura et al., 2023).
In recent years, microorganisms belonging to basidiomycetes have become very promising for red (medical) biotechnology (Mizerska-Dudka et al., 2015) and cosmeceuticals (Zerva et al., 2020b). In this sense, white rot fungi are a prominent group within the phylum basidiomycota, which are saprotrophic organisms in the fungal kingdom, encompassing approximately 30%–32% of fungal diversity with an estimated 30,000 distinct species. These fungi can degrade all components of plant cell wall through various mechanisms including extracellular enzymatic processes as well as non-enzymatic ones such as reactive oxygen species (Kijpornyongpan et al., 2022). Due to the distinct characteristics of white rot fungi, these microorganisms and their extracellular enzymes that primarily include lignin peroxidases (LiPs, EC 1.11.1.14), manganese-dependent peroxidases (MnPs, EC 1.11.1.13), and laccases (benzenediol: oxygen oxidoreductases, EC 1.10.3) along with additional enzymes such as peroxidase-generating oxidases and mycelium-associated dehydrogenases (Martínez et al., 2005) have been recognized for their potential in various biotechnological applications. With the aid of these enzymes, white-rot fungi possess the capacity to break down intricate plant cell wall polymers such as cellulose, hemicellulose, and lignin. White-rot fungi represent the only known group of organisms that have evolved to effectively break down lignin into carbon dioxide (CO2) and water (H2O) which contributes significantly to Earth’s ecosystem (Floudas et al., 2012; Kundu, 2021; Llanos-López et al., 2023).
White rot fungi possess a significant capacity to produce numerous enzymes and secondary metabolites, exhibiting potential in the fields of nutrition, medicine, and degradation. These secondary metabolites, including terpenoids, polyphenols, sterols, flavonoids, alkaloids, derivatives of benzoic acid, quinolones, anthraquinones, and lactones possess bioactive characteristics (Jaszek et al., 2013; Jaszek et al., 2014; Fernando et al., 2016; Bogale, 2020; Kundu and Khan, 2021; Mahuri et al., 2023). Moreover, considering the economic significance and the intention of applications based on biologically active secondary metabolites, there has been a rising global interest in these compounds produced by white rot fungi. Thus, recognizing the great potential of biologically active secondary metabolites from white-rot fungi, this review focuses on exploring the secondary metabolites generated by them, emphasizing their fascinating bioactive properties.
2 Biologically active secondary metabolites produced by white-rot fungi
Secondary bioactive metabolites produced by white rot fungi have significant potential of applicability in various sectors such as pharmaceutical production (Zheng et al., 2010), biobleaching processes in pulp and paper industries (Jerusik, 2010), wastewater treatment approaches (Muszyńska et al., 2019), enhancing digestibility of cellulose and lignin in animals (Yilkal, 2015), generation of renewable resources from lignocellulosic materials, and bioremediation technologies (Korcan et al., 2012; Contreras et al., 2023) (Figure 1). In the literature, several works have revealed the production of biologically active secondary metabolites of white-rot fungi (Table 1). Moreover, the chemical structures of some bioactive secondary metabolites from white-rot fungi are illustrated in Table 2. Considering this, the Schizophyllum genus has been an important white-rot fungal genus with the capability of producing bioactive secondary metabolites. The combined treatment of radiotherapy and sizofiran, a polysaccharide extract from the culture broth of Schizophyllum commune (S. commune), resulted in a significantly higher 5-year survival rate with 90 patients compared to 82 patients treated with radiotherapy alone. Sizofiran demonstrated promising potential as an immunotherapeutic agent in the treatment of cervical carcinoma (Miyazaki et al., 1995).
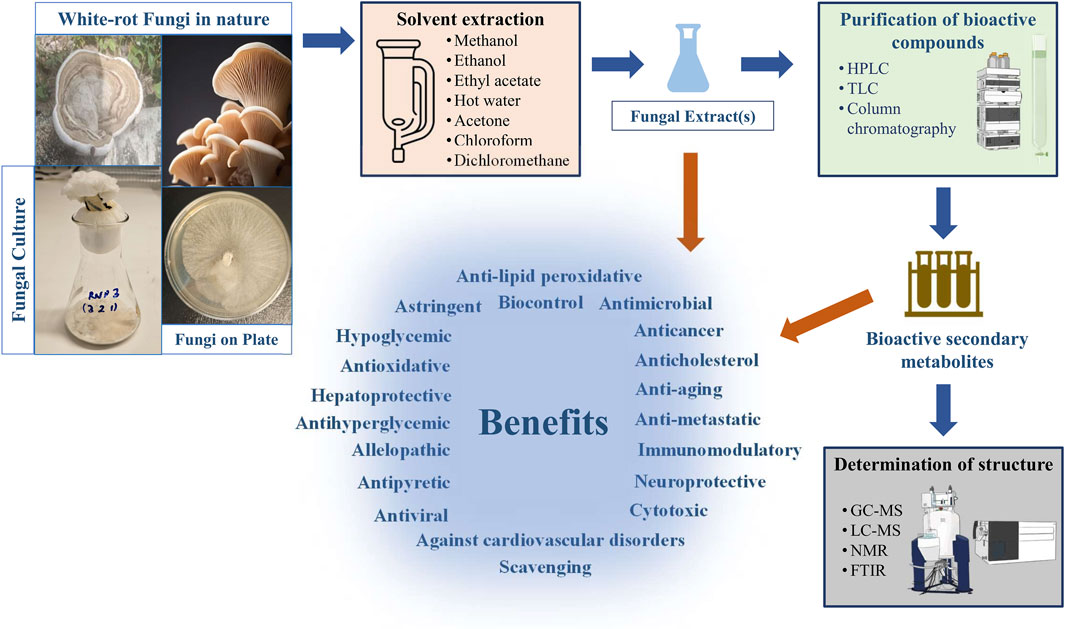
FIGURE 1. General processing steps of biologically active secondary metabolites production from white-rot fungi.
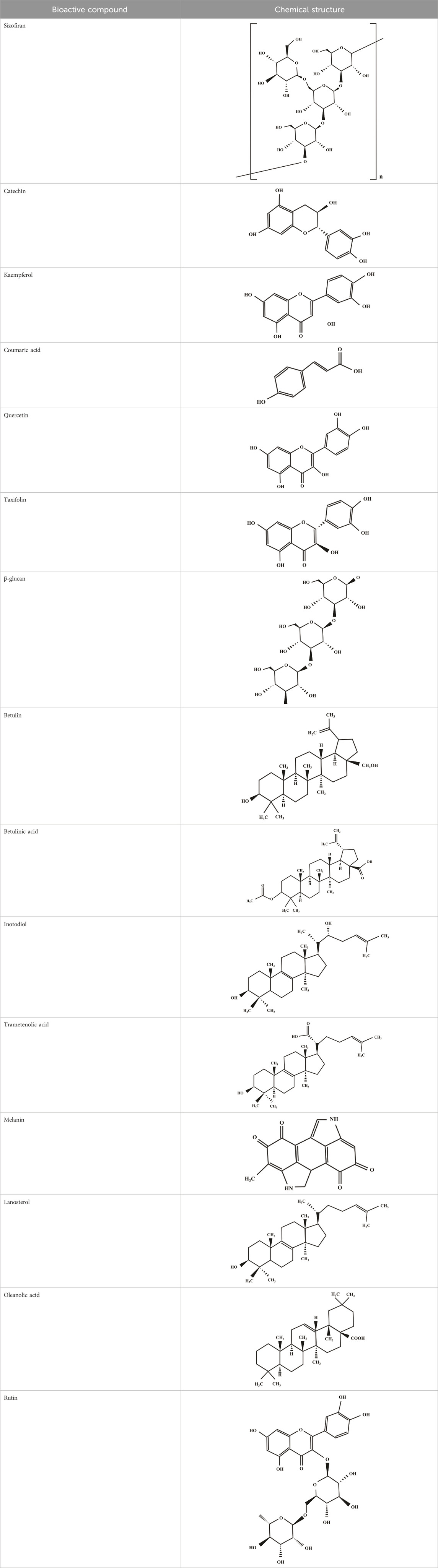
TABLE 2. Illustration of chemical structures of some bioactive secondary metabolites from white-rot fungi.
Tanimoto et al. (1996) extracted and identified a novel metabolite, schizostatin, from S. commune with the ability to inhibit rat liver microsomal squalene synthase to control cholesterol levels. However, in terms of antimicrobial effects, schizostatin showed no antimicrobial activity at a concentration of 1 mg/mL against many microorganisms including Bacillus subtilis (B. subtilis), Candida albicans (C. albicans), Escherichia coli (E. coli), Mycobacterium smegmatis, Mycoplasma mycoides, Proteus vulgaris, Proteus mirabilis, and Staphylococcus aureus (S. aureus).
Tripathi and Tiwary (2013) investigated the production of bioactive compounds from the solvent extracts (methanol, ethanol, acetone, ethyl acetate, and hot water) of S. commune isolated from the Achanakmar-Amarkantak Biosphere Reserve of Central India. In that work, phenolic compounds (i.e., phenyl benzoate (C13H10O2) and 4-(phenyl methoxy) phenol (C13H12O2)) with antioxidant activity and the antibacterial compound pyrrolo (1, 2-a) piperazine-3, 6-dione (C7H10O2N2) were identified from the ethanolic and methanolic extracts, respectively. Moreover, they also detected gallic acid and L-ascorbic acid as antioxidant metabolites of both S. commune ethanol and methanol extracts. Therefore, it was suggested that S. commune could be used for the production of valuable therapeutic agents having antimicrobial and antioxidant activities.
Among several fungal isolates tested for the insecticidal potential against the tobacco cutworm Spodoptera litura (S. litura), ethyl acetate extracts of S. commune, isolated from Aloe vera, showed the strongest insecticidal activity (Kaur et al., 2018). The HPLC analysis of the fungal extract indicated that it contained various phenolic compounds such as gallic acid, catechin, chlorogenic acid, epicatechin, caffeic acid, coumaric acid, rutin quercetin, and kaempferol. Larvae of S. litura treated with that S. commune extract exhibited a notable decrease in the occurrence of living haemocytes with 40.00%–73.33% mortality, as well as an elevated incidence of apoptotic and necrotic cells through the cytotoxic effect of the fungal extract. Moreover, the effect of the fungal extract on tetrazolium dye mammalian viability assay (MTT) on Chinese Hamster Ovary (CHO) cell lines was carried out with a cell viability of 81.82% which was higher than the control (below 60%) consisting of doxorubicin treated cells. Additionally, the evaluation of the genotoxic effect of the S. commune ethyl acetate extract at various exposition times using the comet assay showed that the increasing oxidative stress triggered more DNA damage in haemocytes of S. litura. As a result of all the findings, the S. commune extract was proposed as a potential biocontrol agent.
Water, acetone, and ethanol extracts of S. commune exhibited a free radical scavenging activity of 19.65% at a concentration of 100 μg/mL (Kumar et al., 2018). Moreover, at the same concentration, the superoxide anion scavenging activity and the hydroxyl radical scavenging activity for S. commune extract was determined as 4.84% and 7.50%, respectively, whereas the total antioxidant capacity (TAC) of S. commune extract was found to be 12.15% using ascorbic acid as a reference standard. According to the quantitative analysis of S. commune mycochemicals, phenolics, flavonoids, alkaloids, tannins, and saponins were found to be 10.80 ± 0.76, 4.67 ± 0.23, 4.26 ± 0.54, 1.24 ± 0.16, and 23.83 ± 0.84 (mg/g), respectively. Compared to the edible fungi Tricholoma nudum and Psalliota campestris, a higher number of bioactive compounds (except for the content in phenolics) was obtained from S. commune. Overall, those results indicated that S. commune can serve as a valuable source of antioxidants for human health, and it was proposed that their extracts had the potential to be used for the development of drugs to lower the oxidative stress in the body.
Culture filtrate and bioactive metabolites from chloroform extracts of S. commune were investigated for their antimicrobial properties against different types of plant pathogens (Dutta et al., 2019). In that work, pepper fruits were treated with schizostatin and then infected with Colletotrichum gloeosporioides (C. gloeosporioides) or Botrytis cinerea (B. cinerea). For C. gloeosporioides infection (for anthracnose), significant effect for the control of the disease was observed from the treatment with 10 μg/mL and reached maximum with 97.8% and 100.0% by 100 μg/mL and 150 μg/mL treatment, respectively. Moreover, the control of the disease for B. cinerea (cause of gray mold disease) was 83.2% and 94.6% by treatment with 100 mg/mL and 150 mg/mL, respectively. The incidence of anthracnose in field conditions showed a decrease when treated with a diluted solution (12.5%) of a culture filtrate derived from S. commune. In that paper, the compound responsible for its antifungal and disease-control activity was identified as schizostatin. On the other hand, the growth of fungal pepper plant pathogens was inhibited by S. commune culture filtrate, while bacterial pathogens Ralstonia solanacearum and Pectobacterium carotovorum were unaffected by schizostatin. Thus, it was proposed that schizostatin had the potential to be utilized as a biochemical pesticide for controlling fungal infections, including anthracnose and gray mold, in various types of vegetables.
Alam et al. (2009) discovered that feeding hypercholesterolemic rats with a 5% of fruiting body powders of Pleurotus ostreatus (P. ostreatus), Pleurotus sajor-caju (P. sajor-caju), and Pleurotus florida (P. florida) resulted in substantial reductions in total cholesterol levels (by 37.0%, 21.0%, and 16.0%, respectively) and triglyceride levels (by 45.0%, 24.0%, and 14.0%, respectively) in plasma which were attributed to the content of lovastatin in the fungal powders. Also, they compared the effect of P. sajor-caju on plasma and fecal lipid profiles as well as liver and kidney function in rats with high and normal cholesterol levels. The low-density lipoproteins (LDL)/high-density lipoproteins (HDL) ratio also exhibited significant decreases of 64.0%, 45.0%, and 41.0% for P. sajor-caju, P. ostreatus, and P. florida-fed rats, respectively. These findings based on mice studies suggested that consumption of the aforementioned Pleurotus species could bring notable health advantages by modulating physiological functions, particularly in addressing various atherogenic lipid profiles in cases of hypercholesterolemia, potentially serving as a nutritious source and a preventative measure against related complications and known risk factors for atherosclerosis.
Fagade and Oyelade (2009) identified and assessed 12 different species including Auricularia auricula, Coriolus versicolor (C. versicolor), Daedalea elegans, Fomes lignosus, G. lucidum, Lentinus subnudus, Leptoporus sp., S. commune, Panus fulvus (P. fulvus), P. florida, Trametes saepiara, and Trametes betulina for antibacterial activity. Among them, the ethanol extracts of P. florida and P. fulvus exhibited the strongest antibacterial activity against a range of bacteria including S. aureus, Streptococcus sp., Streptococcus pyogenes (S. pyogenes), E. coli, Klebsiella pneumoniae (K. pneumoniae), Flavobacterium sp., and the yeast C. albicans at a concentration of 1 g/mL for each microorganism. Additionally, P. florida displayed the lowest minimum inhibitory concentrations (MIC) value (0.01 g/mL) when tested against the yeast C. albicans, whereas the highest MIC was observed for P. florida (1 g/mL) against Flavobacterium sp. However, ethanolic extracts of S. commune and C. versicolor showed no inhibition against any of the tested bacteria.
The predominant bioactive component, total phenols, of the methanolic extract of Pleurotus pulmonarius (P. pulmonarius) was found to be 5.79 ± 0.03 mg/mL expressed as milligrams of gallic acid equivalent (GAE) per Gram of fruiting body (Ramesh and Pattar, 2010). The extract also contained flavonoids at a concentration of 1.76 ± 0.06 mg/mL and a minimal amount of ascorbic acid at 0.13 ± 0.00 mg/mL. The radical scavenging activity (RAS) on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was measured at 1.62 ± 0.2 mg/mL for P. pulmonarius. Antimicrobial activity against a range of standard pathogenic Gram-positive and Gram-negative bacteria, along with yeast, was assessed demonstrating a notable antibacterial effect. MIC values indicated antimicrobial activity even at low concentrations (from 1 to 5 mg/mL). The methanolic extract of P. pulmonarius showed promising biopharmaceutical potential with its antioxidant and antimicrobial properties. However, additional research is essential to assess its effectiveness as a therapeutic compound. It is also crucial to identify the bioactive compounds and understand their mechanisms of action before considering practical applications.
Ghaly et al. (2011) evaluated the antihyperglycemic properties of an ethanol extract from P. ostreatus and its influence on potential DNA damage, chromosome aberrations, and sperm abnormalities in diabetic rats induced by streptozotocin. The research involved five groups of adult male albino rats, with the control group consisting of normal animals and the remaining groups comprising hyperglycemic animals. These hyperglycemic groups were orally administered with the antidiabetic drug Amaryl and different levels of mushroom extract doses: low (100 mg/kg.body weight/dL), or high (200 mg/kg.body weight/dL) for 30 days. According to their findings, the application of a higher dosage of P. ostreatus extract exhibited superior therapeutic effects compared to the treatment with a lower dosage. Notably, P. ostreatus extract, especially at high concentration, effectively lowered blood glucose levels in hyperglycemic rats, though to a lesser extent than Amaryl treatment. Significantly, mushroom treatments exhibited greater efficacy in reducing genetic alterations and sperm abnormalities in diabetic conditions compared to Amaryl treatment. In conclusion, this study highlighted the potential of P. ostreatus extract, particularly at higher concentration, to alleviate elevated blood glucose levels and mitigate genetic and reproductive abnormalities associated with diabetes, offering a promising alternative to conventional treatments.
Two polysaccharide fractions, namely PSPO-1a (composed of mannose, glucose, galactose, xylose, and rhamnose) and PSPO-4a (composed of rhamnose, mannose, and galactose), both containing protein and uronic acid, were isolated from ethanol extracts of P. ostreatus (Zhang et al., 2012). These fractions demonstrated stronger DPPH and superoxide anion radical scavenging activities, which increased with concentrations up to 2.1 mg/mL and 3.0 mg/mL, respectively. However, their efficacy in scavenging hydroxyl radicals was comparatively lower compared to DPPH and superoxide anion radical scavenging activities. Consequently, PSPO-1a proved to be a more potent free-radical scavenger than PSPO-4a which could be resulted from their different polysaccharide compositions and their varied molar ratios.
A study conducted by Mitra et al. (2013) focused on exploring the antioxidant potential and nitric oxide synthase (NOS) activation properties of water-soluble crude polysaccharides derived from P. ostreatus. Their results indicated that the polysaccharides, primarily composed of carbohydrates, notably β-glucan, displayed good antioxidant activity, demonstrating superiority in free radical scavenging and NOS activation compared to other components including low levels of protein and phenolic compounds. The yield from hot water extraction of dried fruit bodies revealed a total polysaccharide content of 62.67 ± 7.67 mg/100 mg, with the total glucan component as 43.9 ± 1.2 mg/100 mg. Furthermore, the polysaccharides exhibited significant NOS activation properties. Moreover, considering the antioxidant activity, the EC50 (the concentration required to obtain a 50% antioxidant effect) values for scavenging hydroxyl radicals, superoxide radicals, and chelating ferrous ions were 665, 390, and 370 μg/mL, respectively. That study highlighted the potential of P. ostreatus as a valuable source of bioactive compounds, suggesting that its crude polysaccharides, rich in β-glucan, could serve as an effective antioxidant food additive or find applications in the pharmaceutical industry.
Assis et al. (2013) investigated the antitumor activity of the exopolysaccharides (EPSs) and the mycelial biomass (intracellular polysaccharides, IPSs) of P. sajor-caju against Sarcoma 180 (S180) cells. According to that test, the antitumor efficacy of the produced EPS was 86%, and two IPSs from the mycelial biomass showed 80% and 82%. Since the chemical characterization of these bioactive compounds was not determined within that study, the main concern surrounding these exo- and intracellular polysaccharides lies in the lack of identification of bioactive secondary metabolites presented in EPS and IPS produced by P. sajor-caju. Therefore, after further investigation, that findings could aid in the exploration of new bioactive substances, introducing innovative perspectives to the medical and pharmaceutical fields.
Corrêa et al. (2015) focused on the chemical characterization and bioactivity of ethanol extracts of fruiting bodies and submerged mycelia from Pleurotus ostreatoroseus (P. ostreatoroseus). The fruiting body and mycelia extracts contained a minimum of five free sugars, four organic acids, four phenolic compounds, and two tocopherols. The culture filtrates from submerged cultivation exhibited superior reducing activity only for fruiting body (1.79 ± 0.01 mg/mL). Furthermore, DPPH scavenging activity (4.78 ± 0.02 mg/mL for fruiting body and 15.62 ± 0.13 mg/mL for mycelia), β-carotene bleaching inhibition (0.40 ± 0.01 mg/mL for fruiting body and 7.62 ± 0.25 for mycelia), and lipid peroxidation inhibition (0.29 ± 0.00 mg/mL for fruiting body and 2.34 ± 0.08 mg/mL for mycelia) in porcine (Sus scrofa) brain homogenates were detected in terms of bioactivity. Additionally, P. ostreatoroseus demonstrated higher anti-inflammatory and antimicrobial activities, while showing no hepatotoxicity in porcine liver primary cells. These functional responses could be associated with varying levels of bioactive metabolites in the fruiting body extract and the submerged culture filtrate, including phenolic acids, organic acids, and tocopherols. These bioactive compounds can be utilized to create dietary supplements for nutraceutical purposes.
P. sajor caju, a medicinal fungus, was reported as a notable source of secondary metabolites such as phenols (3.35 ± 0.20 mg/g), flavonoids (5.36 ± 0.31 mg/g), tannins (6.84 ± 0.12 mg/g), and alkaloids (2.81 ± 0.61 mg/g), in addition to carbohydrates, protein, amino acids, and vitamins (A, C, and E) (Devi and Krishnakumari, 2015). These secondary metabolites could have significant potential for exhibiting antimicrobial, anticancer, antipyretic, astringent, and antiviral properties. The findings in that work strongly indicated the commercial and pharmaceutical significance of the secondary bioactive compounds found in P. sajor caju.
Chowdhury et al. (2015) studied the antimicrobial and antioxidant properties of methanolic extracts from three edible mushrooms (P. ostreatus, Lentinula edodes (formerly Lentinus edodes) (L. edodes), and Hypsizigus tessulatus (H. tessulatus)) isolated from Bangladesh. Antimicrobial activity against 8 microbial strains was evaluated, revealing substantial effectiveness with diameters of inhibition zone (DIZ) ranging from 7 ± 0.2 to 20 ± 0.1 mm. MIC values exhibited notable activity at concentrations ranging from 1 mg/mL to 9 mg/mL, with L. edodes exhibiting the most potent antimicrobial activity. Pseudomonas aeruginosa (P. aeruginosa) showed the maximum resistance, while Saccharomyces cerevisiae (S. cerevisiae) was more sensitive for the three fungal tested extracts. To reveal the antioxidant efficiency, free radical scavenging activity (EC50, μg/ml) on DPPH was determined as 100 ± 1.20, 105.0 ± 1.23, and 110.0 ± 1.24 μg/mL respectively for P. ostreatus, H. tessulatus, and L. edodes (with ascorbic acid as a control, 5.25 ± 0.21 μg/mL). The total phenols, a major bioactive component, ranged from 3.20 ± 0.05 to 10.66 ± 0.52 mg/mL expressed as mg of GAE per Gram of fruiting bodies. Furthermore, the flavonoid concentration detected spectrophotometrically in all isolates ranged from 2.50 ± 0.008 mg/mL to 4.76 ± 0.11 mg/mL. The potential of these extracts to serve as effective therapeutic agents requires further investigation and a detailed study of their mechanisms of action prior to application.
Koutrotsios et al. (2018) cultivated P. ostreatus, Pleurotus eryngii (P. eryngii), and Pleurotus nebrodensis (P. nebrodensis) on unconventional substrates such as grape marc (GMC) and olive mill byproducts (OMB), with wheat straw (WHS) serving as the control. GMC-based media demonstrated comparable or superior mushroom productivity compared to WHS for P. eryngii and P. nebrodensis, while P. eryngii exhibited enhanced cultivation performance in OMB-based media. Both GMC and OMB substrates led to a substantial increase in the content of fruiting bodies in phenolic acids, resveratrol, triterpenic compounds, and ergosterol. Specifically, P. eryngii methanol extract displayed significantly high total phenolics, showing a substantial 2- to 8-fold increase in antioxidant activity based on DPPH and ferric reducing/antioxidant power assays. Moreover, substrates containing GMC or OMB resulted in up to a 27.0% increase in mushroom β-glucans. Pleurotus species responded differentially and mostly in a substrate-specific manner, selectively absorbing organic compounds. The phenolics and squalene content in substrates showed a strong correlation with the antioxidant activity of fungi and ergosterol, respectively. Similarly, a comparable correlation was noted between the triterpene content in substrates and fungi.
In a study by Beltrán Delgado et al. (2021), the aqueous extract of mature fruiting bodies of P. ostreatus exhibited higher levels of proteins, reducing sugars, and flavonoids than those in the extract of early-stage fungus. However, carbohydrates and total phenols were higher in the extract from the early stage of fungal development than in mature fruiting body extract. According to that work, the antioxidant characteristics of P. ostreatus aqueous extractions (earliest stage of fungal development and mature fruiting bodies) were influenced by changes in the levels of bioactive compounds, considering the physiological attributes during different growth phases. These findings could be valuable for developing protocols to obtain bioproducts from P. ostreatus with potential applications as antioxidants in food and medical-pharmaceutical industries and for the design and formulation of new related therapeutic products.
Ogidi et al. (2020) focused on the production of EPSsby submerged cultures of P. pulmonarius, containing diverse agricultural wastes. The highest EPS yield (5.60 g/L) was achieved by P. pulmonarius submerged cultures supplemented with groundnut shells (20.0 g/L) (EPS-B). The observed zones of inhibition by EPS-A (without agro-waste), EPS-B (groundnut shell), EPS-C (coconut husk), and EPS-D (pineapple peel) against Shigella dysenteriae and E. coli did not show significant differences. All the obtained EPS variants inhibited the growth not only of Gram-positive bacteria, including B. subtilis and S. aureus, but also against C. albicans and Gram-negative bacteria. All the obtained EPS exhibited DIZs (5.00–14.00 mm) against different tested microorganisms. The MIC also ranged from 0.25 to 1.00 mg/mL against the tested microorganisms. The EPS-A to D demonstrated scavenging activity within the ranges of 67.80%–81.80%, 60.60%–81.20%, 70.40%–84.70%, and 78.40%–88.50% against DPPH, OH, Fe2+, and NO radicals, respectively. The potential applications of the EPS, obtained from submerged cultures of P. pulmonarius supplemented with different agro-wastes, make it a promising natural product with the possibility of being utilized as a preservative in the food industry. Additionally, the method of generating natural bioactive compounds through fungal submerged culture using agricultural waste offers a potential solution to the unregulated disposal of agricultural waste into the environment.
Aqueous extracts from P. ostreatus and L. edodes (shiitake mushroom) exhibited the expression of 753 and 432 proteins, respectively (Elhusseiny et al., 2021). Common bioactive peptides such as Rab GDP dissociation inhibitor, superoxide dismutase, thioredoxin reductase, serine proteinase, and lectin were identified in both white rot fungal extracts Additionally, P. ostreatus extract contained phenolics and flavonoids, such as catechin, kaempferol, and apigenin, whereas catechin and quercetin were detected in the extract of L. edodes. Vitamins, including ascorbic acid, nicotinic acid, nicotinamide, and pyridoxine, together with various amino acids were also detected in both extracts. The antioxidant impact of both fungi can be ascribed to the existence of numerous bioactive elements, such as flavonoids, phenolics, bioactive peptides, and vitamin C. Notably, both extracts demonstrated significant antiviral activities, particularly P. ostreatus extract exhibited a selectivity index (SI) of 4.5 and 2.0 against adenovirus (Ad7) and herpes simplex virus-II, respectively, while L. edodes extract showed values of 2.7 and 2.5 for the respective viruses. The aqueous extracts from L. edodes and P. ostreatus demonstrated an approximately 20.0% reduction in viability among the tested cancer cell lines LS-513 (cecum carcinoma), HepG2 (hepatocellular carcinoma), DU-145 (prostate cancer), and PC-3 (prostate cancer). Cytotoxicity analysis was conducted on aqueous fungal extracts against leukemia (CCR-CEM, NB-4, THP-1) and lymphoma (U937) cells. The L. edodes extract exhibited a viability decrease of 66.02% in THP1 cells, while the P. ostreatus extract reduced the viability of CCRF-CEM cells to 70.64%. Additionally, minimal cytotoxic effects on normal human peripheral blood mononuclear cells (PBMC) from the extracts with untreated cells and doxorubicin treated cells as negative and positive controls, respectively, was observed. Considering the effects of a wide range of bioactive compounds in the aqueous extracts of the white rot fungi P. ostreatus and L. edodes, the study suggested the potential pharmacological application of these fungal strains. It underscored their minimal cytotoxicity on normal PBMCs, while also emphasizing their beneficial properties in terms of antiviral, antitumor, and antioxidant properties.
Oba et al. (2009) assessed the impact of immunochemotherapy using lentinan derived from L. edodes in comparison to chemotherapy alone in individuals with advanced gastric cancer through a meta-analysis of 650 individual patient data. Based on their findings, lentinan demonstrated a potentially higher efficacy in patients with lymph node metastasis in contrast to those without such metastasis. Moreover, the proportion of hepatic metastasis in the group receiving chemotherapy plus lentinan was smaller than that in the group receiving chemotherapy alone, with percentages of 34.5% and 43.1%, respectively. It was indicated that lentinan extended the overall survival period of the patients. In summary, the inclusion of lentinan alongside standard chemotherapy showed a notable and significant advantage over chemotherapy alone in terms of survival for individuals having advanced gastric cancer.
Resurreccion et al. (2016) reported the isolation of ergosterol and trilinolein from dichloromethane extracts of L. edodes, obtained from the Mushroom Burger in Tagaytay City, Philippines. Their structures were identified by comparing their NMR data with those of the existing literature. Ergosterol from the water extract of Polyporus showed significant protective properties against bladder tumor promotion in Wistar rats (Yazawa et al., 2000). Previous research also indicated that ergosterol in P. ostreatus extracts had the potential to inhibit lipid peroxidation (Dissanayake et al., 2009). On the other hand, trilinolein exhibited protective effects against cardiovascular disorders, including its ability to inhibit ischemia-induced ventricular arrhythmias and display antioxidant properties (Chan et al., 2002; Chan et al., 2005). In addition, trilinolein from the water extract of Polyporus inhibited the growth of human non-small cell lung carcinoma A549 and induce programmed cell death, with the effects being contingent on both the dosage and duration of exposure (Chou et al., 2011).
Sevindik (2018a) evaluated the antioxidant capacity of the Lentinus tigrinus (L. tigrinus) by determining the total antioxidant status (TAS), the total oxidant status (TOS), and the oxidative stress index (OSI) as 1.748 ± 0.071 mmol/L, 19.294 ± 0.237 μmol/L, and 1.106 ± 0.031, respectively. Additionally, the antimicrobial properties of ethanol, methanol, and dichloromethane extracts of L. tigrinus were investigated against several bacterial and yeast strains, including S. aureus, Enterococcus faecalis (E. faecalis), E. coli, P. aeruginosa, C. albicans, Candida krusei (C. krusei) and Candida glabrata (C. glabrata) in a range from 800 to 100 MIC (µg/mL) values with highest anticandidal activity. In that paper, it was proposed that L. tigrinus could serve as a natural antioxidant and antimicrobial source. On the other hand, since L. tigrinus is an edible mushroom (Mohammadnejad et al., 2019), the restriction of over-consumption of this white-rot fungus could be necessary because of its high level of antioxidants. Moreover, the fungal extracts should be analyzed to determine the responsible bioactive secondary metabolites for its antimicrobial and antioxidant activities.
The antioxidant and antidiabetic properties of mycelium and fruiting body ethanol extracts of Lentinus swartzii (L. swartzii) was examined by Austria et al. (2021). The inhibition of α-amylase, which is an enzyme responsible for breaking down carbohydrates during digestion, has the potential to cause a decrease in blood sugar levels (Tundis et al., 2010). Considering this, the ethanolic extract of L. swartzii mycelium demonstrated a notable α-amylase inhibitory activity of 81.98%, while the fruiting body ethanolic extract exhibited an α-amylase inhibitory activity of 71.08%. The mycelial extract contained essential oils, triterpenes, sugars, tannins, flavonoids, fatty acids, and phenols, while the fruiting body extract presented the same components except for fatty acids and sugars. At a concentration of 1,000 μg/mL, the mycelial ethanolic extract showed scavenging effects against DPPH (35.29%) and NO (36.04%), contained 20.25 mg GAE/g sample, and demonstrated high inhibitory activity against α-amylase (81.98%). Similarly, the fruiting body ethanolic extract, at the same concentration, scavenged 43.69% of DPPH, 31.75% of nitric oxide, contained 16.92 mg GAE/g sample, and exhibited high inhibitory activity against α-amylase (71.08%). Consequently, both L. swartzii mycelia and fruiting body ethanolic extracts held promise as valuable sources of bioactive compounds with antioxidant and antidiabetic activities. A notable observation in that paper was that mycelia grown in coconut water exhibited superior activities compared to the fruiting body cultivated in sawdust and rice straw substrate. This signifies that the chemical properties and biological effects are impacted not just by elements like species, strain type, growth media, and solvents for extraction but also by the specific medium composition used for fungal cultivation. Further steps, including the isolation and characterization of the compounds responsible for these significant bioactivities, are crucial for a comprehensive understanding of the extracts’ potential in different applications.
Muslihin et al. (2022) reported that the wild mushroom Lentinus squarrosulus (L. squarrosulus) possessed notable characteristics such as rapid mycelium growth, having potential to be a food source, and various other benefits. Notably, it serves as a source of bioactive compounds. The ethyl acetate extract from L. squarrosulus was analyzed at 516.8 nm using a UV-Vis spectrophotometer and it revealed a strong antioxidant activity with an EC50 of 54.93 mg/L. This highlights its possible utility in various applications.
Lin et al. (2008) investigated the impact of Lycium barbarum (L. barbarum) fruit extract on the growth and extracellular polysaccharopeptide (ePSP) production by C. versicolor (now Trametes versicolor (T. versicolor)) in a 20-L fermenter under submerged fermentation conditions. The addition of L. barbarum extract (LBE) into the culture medium led to a notable increase in ePSP production as from 0.61 g/L to 1.66 g/L. Significantly, ePSP from C. versicolor cultured with supplemental L. barbarum extract demonstrated noteworthy immunomodulatory activity, influencing the production of nitric oxide and various cytokines by murine RAW264.7 cells. The approach in that work can open up new possibilities for the future advancement of dietary supplements centered around C. versicolor LH1 polysaccharopeptides.
From a submerged culture of Panus strigellus (P. strigellus), Llanos-López et al. (2023) isolated three metabolites, including a new bioactive compound called panapophenanthrin and two known compounds identified as panepophenanthrin and dihydrohypnophilin which are defined in an uncommon group of oligocyclic terpenoidal metabolites, exclusively identified in the Panus genus. While panapophenanthrin and dihydrohypnophilin exhibited not a very strong antimicrobial effect with MIC ranging from 33.3 to 66.6 g/mL on various fungal strains together with Gram-positive and Gram-negative bacteria, panepophenanthrin showed no activity against any of the tested microorganisms. Moreover, panapophenanthrin showed strong cytotoxic effects on mammalian cell lines including mouse fibroblast (L929) and human endocervical adenocarcinoma (KB3.1) with 13.2 and 17.9 EC50 (µM) values, respectively. Panus species are predominantly found in tropical and subtropical areas. Hence, this discovery emphasized the significance of examining tropical species to uncover new bioactive compounds, but additional research is necessary to thoroughly understand the bioactivity of these compounds and explored their potential uses in different applications.
Cyclocybe cylindracea (C. cylindracea) ethanol extract was investigated to determine its phenolic content, heavy metal content, and antioxidant activity to evaluate possible medical benefits (Sevindik et al., 2018). In that work, TAS, TOS, and OSI values were determined as 4.325 mmol/L, 21.109 μmol/L, and 0.488. Phenolic compounds including gallic acid, hesperidin, catechin, syringic acid, and hydroxybenzoic acid, were detected in the ethanolic extracts of C. cylindracea. These bioactive compounds presented diverse health advantages, encompassing antioxidant properties, anti-inflammatory effects, and potential anti-cancer properties (Sevindik et al., 2018). Despite possible benefits, since C. cylindracea is edible (Landingin et al., 2020), its levels of Pb (16.54 ± 0.93 mg/kg) and TOS values should be considered.
Masuda et al. (2008) explored the antimetastatic properties of Grifola frondosa (G. frondosa) (maitake mushroom) extracts, using an experimental mice model of lung metastasis. The observed inhibition of lung metastasis by G. frondosa extract was attributed to the activation of NK cells. Additionally, the G. frondosa extract demonstrated inhibition of ICAM-1 (Intercellular Adhesion Molecule 1) expression in vascular endothelial cells, suggesting that its mechanism of action involved blocking the adhesion of tumor cells to lung tissue, thereby inhibiting metastasis. Their findings suggested that G. frondosa extract was effective for cancer prevention and the inhibition of tumor metastasis when consumed regularly.
Masuda et al. (2009) explored the ability of an extract from G. frondosa to enhance the immune system by antitumor and antimetastatic activities together with cisplatin, a well-known anticancer drug. Based on their findings, the increased antitumor and antimetastatic effectiveness observed in the combination of cisplatin with G. frondosa extract was attributed to a synergistic interaction. This synergy was sourced from the dual action of cisplatin’s cytotoxic impact on tumor cells and the simultaneous activation of the immune response in antigen-presenting cells (APC) and natural killer (NK) cells by G. frondosa extract. Moreover, this combination not only exhibited antitumor and antimetastatic activity but also caused a decrease in cisplatin-induced myelotoxicity and nephrotoxicity. Consequently, the joint administration of G. frondosa extract with cisplatin holds promise as a beneficial approach to cancer treatment.
The clinical evaluation of the immunological effects of hot water and alcohol extract from the fruit body of G. frondosa at different oral dosage levels was first investigated for a group of 34 eligible study subjects in the work performed by Deng et al. (2009). Based on their results, it was shown that the administration of G. frondosa extract was linked to notable alterations in specific immunologic parameters within the peripheral blood. According to their findings, this extract was recognized for its role as an immunomodulator rather than simply an immune enhancer. Moreover, cancer patients should be aware of that G. frondosa extracts may have complex effects on immune function, and while the clinical impact on cancer prevention or treatment remains uncertain, it is crucial to conduct experimental investigations to clarify its potential anticancer effects.
Su et al. (2020) prepared a G. frondosa extract through a process involving hot water extraction from the fruiting body, followed by enzymatic digestion and dialysis, resulting in high and low molecular weight fractions. They examined the water-soluble polysaccharides of G. frondosa to understand their impact on inflammation and receptor interactions using parental RAW264.7 macrophages and Dectin-1-expressing RAW264.7 macrophages. The results of cell-based assays indicated that the high molecular weight fraction (1,260 kDa) as the major bioactive fraction demonstrated inhibitory effects on tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) production, while also reducing NF-κB (important transcription factor regulating inflammatory responses in eukaryotes) activation in lipopolysaccharide-induced macrophages. That research suggested that the nondigestible β-(1→6)-branched (1→4)-β-D-glucan found in high molecular weight fraction might be responsible for its anti-inflammatory properties by interacting with TLR2 receptors rather than Dectin-1 or CR3 receptors. The discovered polysaccharide was identified as a non-digestible glucan with a β-linked core and side groups. Moreover, the receptor-binding properties and anti-inflammatory activity of G. frondosa polysaccharides may be influenced by their molecular weight and arrangement of linkages.
Eckhardt et al. (2000) reported the impact of a bioactive fungal compound on pancreatic cancer in humans. This involved a phase I trial and a pharmacokinetic examination of irofulven (a fungal cytotoxin) (doses ranging from 1.0 to 17.69 mg/m2) and a novel cytotoxin derived from the white-rot fungus Omphalotus olearius (O. olearius), which was conducted on 46 patients (given daily for 5 consecutive days every 4 weeks) with advanced solid malignancies. According to the trial, evidence of antitumor activity was observed in an individual with advanced pancreatic cancer, coupled with the remarkable preclinical antitumor effects demonstrated by irofulven.
Chandra et al. (2019) assessed the antioxidant activity of the white-rot fungi Phanerochaete chrysosporium (P. chrysosporium), Phlebia brevispora (P. brevispora), and Phlebia floridensis (P. floridensis) against various free radicals, including DPPH, nitric oxide, ferrous ion, and ferric ion, in addition to their total phenolic content. All the studied fungal strains produced phenolics ranging from 5.2 to 16.7 mg/mL and exhibited diverse free radical and metal ion scavenging activities. The growth medium significantly influenced these activities. Thus, all the studied fungi presented similar antioxidant activity (approximately 72.0% DPPH scavenging) in yeast extract glucose medium, while it was lower in Czapek dox’s medium (ranging from 60.0% to 45.0%). The fungal extracts showed no mutagenic or cytotoxic effects, highlighting the fungi’s potential as a new source for the rapid production of extracellular antioxidants. These white rot fungi displayed strong antioxidant potential and could serve as a valuable source of natural antioxidant compounds. Further studies are recommended to isolate and characterize the bioactive compounds for potential use in new therapeutic approaches.
Hericium erinaceus (H. erinaceus) is a well-known traditional medicinal fungus acclaimed for its anti-dementia properties, Alzheimer’s disease, depression, Parkinson’s disease, and spinal cord injuries together with the production of cyathane diterpenoids (erinacines). Numerous structurally diverse bioactive compounds, exceeding 80 types, have been extracted from both the fruiting bodies and mycelia of H. erinaceus. These compounds, including terpenoids (erinacines), phenols (hericenone AE), pyrones (erinapyrones AC), sterols (erinarol, hericerins, hericenes), fatty acids, and non-ribosomal peptides, exhibit various therapeutic effects such as anti-tumor, antibacterial, hypoglycemic, and neuroprotective activities. Around 20 of the 25 isolated diterpenoids extracted from H. erinaceus share a characteristic 5-6-7 tricarbocyclic fused core structure (Kawagishi et al., 1992; Kawagishi et al., 1996; Lee et al., 2000; Li et al., 2001; Kenmoku et al., 2002; Kawagishi et al., 2006; Kobayashi et al., 2014; Lu et al., 2014; Friedman, 2015; Li et al., 2015; Wang et al., 2015; Brandalise et al., 2023).
Lee et al. (2014) reported that erinacine A from the ethanolic extract of H. erinaceus was able to inhibit inflammatory cytokine expression as a neuroprotective effect in adult male Sprague–Dawley rats having ischemia injury. In that work, it was also shown that the brain tissue trauma effectors of nitrotyrosine (RNS) via inducible nitric oxide synthase (iNOS) (Alderton et al., 2001)/p38 mitogen-activated protein kinase (MAPK) (Darling and Cook, 2014; Han et al., 2020)/a transcription factor (i.e., CHOP) (Bruhat et al., 1997) contributed to the neuroprotective effect. Furthermore, in a stroke animal model, erinacine A led to the suppression of reactive nitrogen species and the downregulation of iNOS, p38 MAPK, and CHOP, which are factors involved in ischemia injury.
H. ernaceus methanolic extracts inhibited the inflammatory activity induced by lipopolysaccharide/interferon-γ in murine RAW264.7 cells, with a maximum decrease in nitric oxide production of 39.6% (Lee et al., 2016). The bioactive metabolites in these methanol extracts were identified as three different hericenones, C, D, and F. Consequently, they suggested that the anti-inflammatory effect of the H. ernaceus extract was likely based on the hericenones F. Also, ethanolic extracts of H. erinaceus myceliaeffectively inhibited glutamate-induced apoptosis in PC12 cells against 20 mM glutamate-induced damage (Chang et al., 2016). The following biochemical parameters glutathione at 2.5 ± 0.7 nmol/mg protein, glutathione peroxidase at 28.2 ± 3.2 mU/mg protein, glutathione reductase at 2.3 ± 0.4 mU/mg protein, calcium influx at 360 ± 23 nmol/L, reactive oxygen species at 140.0% ± 7.0%, superoxide dismutase at 23.2 ± 4.2 U/mg protein, H2O2 at 20.4 ± 3.5 nmol/mg protein, and thiobarbituric acid reactive substances (malondialdehyde) at 13.3 ± 2.5 mmol/mg protein, were affected by glutamate insult. Overall, their findings underlined the potential neuroprotective effect of erinacine A from H. erinaceus ethanolic extracts.
Despite the elucidation of chemical synthesis, the biosynthetic pathway and gene regulation remain unknown. A comparative genome analysis of 42 basidiomycota fungal species, including H. erinaceus, revealed abundant gene clusters related to terpenoid and polyketide biosynthesis (Chen et al., 2017). The genome analysis of H. erinaceus will provide important understanding into the biosynthetic pathways of bioactive secondary compounds, which is crucial for improving the production of these compounds.
Zhang et al. (2017) explored the neuroprotective and neuritogenic properties of several secondary metabolites, including 4-chloro-3,5-dimethoxybenzoic methyl ester, 3-(hydroxymethyl)-2-furaldehyde, erinacine A, erinacerin G, herierin III, and herierin IV from the methanol extract of H. erinaceus mycelium. Among them, 4-chloro-3,5-dimethoxybenzoic methyl ester and erinacine A metabolites not only enhanced nerve growth factor-induced neurite outgrowth but also protected neuronally differentiated cells against lack of nerve growth factor in PC12 pheochromocytoma cells. Erinacine A additionally stimulated neuritogenesis in primary rat cortex neurons. Their findings suggest that H. erinaceus holds promise as a potential therapeutic agent for reducing the risk of various neurodegenerative diseases.
Erinacine A and S, isolated from of H. erinaceus mycelia, displayed anti-neurodegenerative and neuroprotective effects in the cerebrum of transgenic mice (Tzeng et al., 2018). Thus, 30-days application of erinacine A and S attenuated cerebral plaque loading by inhibiting plaque growth, diminishing glial cell activation, and promoting hippocampal neurogenesis in transgenic mice as Alzheimer’s disease model. Additionally, it was showed that erinacine A recovered behavioral deficits in transgenic mice. These findings suggested the possibility that erinacine A may have therapeutic potential for treating Alzheimer’s disease.
Ratto et al. (2019) reported that ethanol extract of H. erinaceus, containing erinacine A, hericenone C, and hericenone D bioactive metabolites, was able to partially revert the cognitive and locomotor frailty index during physiological aging in a mice model. They observed an increase in proliferating cell nuclear antigen (PCNA) and doublecortin (DCX) levels in the hippocampus and cerebellum of mice supplemented (2 months) with H. erinaceus extract orally, indicating the occurrence of neurogenesis in elderly frail mice. Therefore, it was demonstrated that the supplementation of H. erinaceus extract reversed the age-related decline in recognition memory.
Roda et al. (2021) demonstrated that a two-month oral supplementation of ethanol extract from H. erinaceus, which contained erinacine A, hericenone C, hericenone D, and ergothioneine, could reverse age-induced cerebellar alterations in C57BL-6J wild-type male mice. These alterations included volume reduction, molecular layer thickness decrease, and dwindled neurons. Additionally, the supplementation led to a decrease in inflammation, oxidative stress, and reactive gliosis. In another study, they investigated the preventive effects of H. erinaceus ethanol extract, which contained a high amount of ergothioneine, on cognitive and locomotor decline during physiological aging in C57BL-6J mice. The ergothioneine-rich extract exhibited neuroprotective and preventive actions, mitigating age-dependent deficiencies (Roda et al., 2022). Moreover, same extract was shown to reduce oxidative stress and inflammation in the hippocampus, prevent recognition memory decline, and increase the expression of specific receptors crucially involved in glutamatergic neurotransmission in the same mice (Roda et al., 2023).
A tremulane sesquiterpene, named irpexlacte A (yellowish needle crystals), along with three novel furan derivatives, identified as irpexlacte B (yellowish oil), irpexlacte C (yellowish powder), irpexlacte D (brown flaky solid), were obtained from the fungus Irpex lacteus (I. lacteus) isolated from waterlogging tolerant plant Distylium chinense by Duan et al. (2019). Furthermore, they also isolated two known metabolites, irlactin E and 3β-hydroxycinnamolide. Irpexlacte A and D demonstrated robust antioxidant activity, with EC50 values of 2.50 and 5.75 μM, respectively. Moreover, in contrast to gentamicin (0.18 μM) as the positive control, four new compounds, irpexlacte A, B, C and D, demonstrated moderate activity, displaying MIC values of 24.1, 32.3, 35.5, and 23.8 μM, respectively, against P. aeruginosa. On the other hand, the isolated compounds showed no activity against tested cancer cell lines. However, irpexlacte A-D displayed significant antioxidant activity, underscoring the need for further investigations to evaluate their importance and clarify underlying mechanisms.
Porodaedalea pini (P. pini) has been an esteemed traditional mushroom known for its therapeutic properties against various diseases. In this context, Devi et al. (2022) determined the antioxidant potential of hexane, chloroform, ethyl acetate, and methanol extracts of P. pini using DPPH assay (EC50, 253.98 μg/mL, maximum with hexane extraction), total antioxidant capacity (231.04 ± 1.75 μg ascorbic acid equivalents/g of dried extract, maximum with methanol extraction), total phenolic content (277.67 ± 9.46 μg GAE/g of the sample, maximum with methanol extraction), and total flavonoid content (4.95 ± .013 μg rutin equivalent/g of dried extract, maximum with methanol extraction). The presence of 12 polyphenolic metabolites, including gallic acid, catechin, chlorogenic acid, epicatechin, caffeic acid, umbelliferone, coumaric acid, tert-butyl-hydroquinone, and quercetin was revealed. However, rutin, elagic acid, and kaempferol were not detected. The identified polyphenols of P. pini. could potentially contribute to its antioxidant activity, Moreover, further exploration of P. pini extracts is necessary to unveil its nutraceutical and pharmacological potential.
Ildız et al. (2022) employed molecular techniques to analyze the total phenolic compound content, antioxidant activity using the DPPH scavenging method, and antimicrobial activity for Bjerkandera adusta (B. adusta). Ethanol and methanol were used for extraction and the methanolic extract of B. adusta exhibited a total phenolic content of 772.28 μg GAE/mL. The ethanol extract demonstrated a substantial 79.66% scavenging activity against a 0.1 mM DPPH solution. For antimicrobial activity, the ethanolic extract exhibited significant antimicrobial activity, showing the largest DIZ of 28 ± 1 mm against P. aeruginosa. In contrast, the methanol extract displayed the lowest antimicrobial efficacy, with a DIZ of 8.7 ± 1.2 mm against Salmonella typhimurium (S. typhimurium). These findings suggested that both ethanolic and methanolic extracts of B. adusta possess antioxidant and antibacterial properties. More comprehensive investigations into wild-collected fungal strains should be done for an extensive exploration of the bioactive constituents present in fungi, drawing attention to their potential applications in the development of functional foods and other potential uses.
The antioxidant and oxidant potentials of the ethanolic extracts of Hohenbuehelia myxotricha (H. myxotricha) was determined for the first time by Krupodorova et al. (2022). The highest recorded TAS, TOS, and OSI values for H. myxotricha were 5.416 ± 0.150 mmol/L, 1.320 ± 0.156 μmol/L, and 0.024 ± 0.003, respectively. The ethanolic extracts of H. myxotricha exhibited antimicrobial activities with concentrations ranging from 25 to 200 μg/mL against various bacteria and yeasts. The extract demonstrated a better antifungal activity compared to its antibacterial activity. The antioxidant, oxidant, and antimicrobial potentials of H. myxotricha mycelia exhibited variations based on the culture media employed. According to their findings, glucose peptone yeast (GPY) medium was found more suitable for the synthesis of antibacterial bioactive metabolites against E. coli, while Sabouraud dextrose broth (SDB) medium was more proper for the production of antioxidant and antifungal bioactive metabolites. Thus, their findings underscored the importance of identifying an optimal cultivation medium to maximize antimicrobial and antioxidant activities. Overall, the ethanolic extract of H. myxotricha mycelia presented significant pharmacological potential, serving as a natural source of antioxidants and antimicrobials with potential health benefits. Like various studies in the literature, further research is required to isolate and identify the bioactive secondary metabolites responsible for the observed antioxidant and antimicrobial effects, offering potential sources for pharmacological drug designs.
Jaszek et al. (2013) isolated bioactive compounds (crude endopolysaccharides - c-EPL, and low molecular secondary metabolites - ex-LMS) extracted from Cerrena unicolor (C. unicolor) submerged cultures that exhibited antioxidant and antibacterial properties., Ex-LMS demonstrated the highest antioxidant capability (39.0%–90.0% for chemiluminometric measurement, 20.0%–90.0% for ABTS, and 10.0%–59.0% for DPPH reduction at 6.25–800 μg/mL). Moreover, c-EPL scavenging abilities ranged from 36.0% to 70.0% for chemiluminometric measurement, 2%–60% for ABTS, and 28.0%–32.0% for DPPH reduction at 6.25–800 μg/mL. Preliminary data for the toxic effect against Vibrio fischeri (V. fischeri) were found to be 85.37% for c-EPL, and 99.8% for ex-LMS. In this sense, c-EPL showed antibacterial activity against S. aureus with an 18.96 ± 0.4 mm DIZ while ex-LMS displayed activity with 11.83 ± 0.2 and 25.86 ± 0.2 mm DIZs, respectively, against E. coli and S. aureus. These compounds have the potential to serve as a novel and easily producible source of effective antioxidants within laboratory-scale conditions. Additionally, further investigation of the aforementioned bioactive secondary metabolites is crucial in terms of applications, as they may play a critical role in new therapies and serve as a natural source of antioxidative molecules.
Mizerska-Dudka et al. (2015) explored the antiviral, immunostimulatory, cytotoxic, and antitumor effects of bioactive compounds from C. unicolor, specifically endopolysaccharides (c-EPL) and an extracellular low molecular weight compound (ex-LMS) obtained from the culture filtrate below 10 kDa. The study employed THP-1-derived macrophages to assess immunomodulatory activity, revealing that the fungal c-EPL stimulated the production and secretion of TNF-α and IL-6. Antitumor activity was evaluated using cervical carcinoma cell lines SiHa and CaSki, with SiHa showing cytotoxic EC50 (µg/mL) value of 1.2 for ex-LM, and CaSki values of 2.3 for ex-LMS. The research highlighted the promising immunomodulatory effect of c-EPL samples and the need for further investigations into these multifaceted bioactive compounds.
The antioxidant and antimicrobial properties of ethanol, methanol, and dichloromethane extracts of C. unicolor were studied by Sevindik (2018b). Considering antioxidant effects, TSS, TOS, and OSI were measured as 6.706 ± 0.059 mmol/L, 19.308 ± 0.114 μmol/L, and 0.288 ± 0.003. Additionally, all the extracts presented antimicrobial efficacy within the concentration range of 25–400 μg/mL, spanning a spectrum of MIC values from 400 to 50 μg/mL against S. aureus, E. faecalis, E. coli, P. aeruginosa, Acinetobacter baumannii, C. albicans, C. glabrata, and C. krusei with higher anticandidal activity. The primary issue about these extracts is their unidentified contents regarding the bioactive secondary metabolites.
Matuszewska et al. (2019) explored the anticancer and antioxidant properties of low molecular weight secondary metabolites produced by C. unicolor. These secondary metabolites consisted of protein, sugars, and phenolic compounds. The findings revealed that the low molecular weight compounds displayed inhibitory effects on human colon cancer cells HT-29 within the concentration range of 25–200 μg/mL and demonstrated dose-dependent inhibition of cell proliferation, ranging from 47.5% to 9.2% at the highest concentrations. Microscopic observations indicated that all compounds induced programmed cell death, specifically apoptosis (up to 44.4% for a compound in HT-29 and less than 20.0% for most compounds in CCD 841 CoTr), with minimal or significantly low levels of necrosis observed in both cell lines simultaneously.
Romorosa et al. (2017) found that distilled water, aqueous, and acetonitrile extracts of Auricularia fuscosuccinea (A. fuscosuccinea) fruiting bodies contained alkaloids and tannins glycosides, while saponins and flavonoids were absent. The antibacterial properties of the aqueous and acetonitrile extracts were evaluated against S. aureus and E. coli. The results indicated low antibacterial activity against S. aureus for all the fungal extracts with DIZs of 5.0 mm, 13.5 mm, and 5.0 mm, respectively, compared to cotrimoxazole (control) with a 33.54 mm DIZ. For E. coli, the corresponding DIZs were 5.0 mm, 22.98 mm, and 22.41 mm, which were lower than the control with a 32.00 mm zone.
Kalaw and Albinto (2014) evaluated the antibacterial properties, phytochemical composition, and antioxidant activity of ethanol and acetone extracts of Coprinus comatus (C. comatus) and Pleurotus cystidiosus (P. cystidiosus). Both ethanol and acetone extracts exhibited antibacterial activity against S. aureus. The ethanol extract of C. comatus displayed a slightly larger DIZ (14.09 ± 4.65 mm) compared to the acetone extract (13.16 ± 3.39 mm). Conversely, in P. cystidiosus, the acetone extract exhibited a larger DIZ (15.25 ± 2.76 mm) than the ethanol extract (13.43 ± 0.15 mm). There was no inhibition against E. coli for both fungal extracts. Moreover, phytochemical screening of the extracts revealed the presence of alkaloids, flavonoids, saponins, and terpenoids in both fungal species. Steroids and cardiac glycosides were absent in P. cystidiosus while tannins were not detected in any of the studied species. P. cystidiosus registered higher DPPH radical scavenging activity (72.97% ± 0.68% to 66.59% ± 0.83%) indicating its potential antioxidant capacity, and lower total phenolic content (3.41 ± 0.12 mg GAE/g) than C. comatus (17.82 ± 0.51 mg GAE/g).
In a research conducted by Stilinović et al. (2020), it was reported that C. comatus methanol extract contained significant amounts of proteins (23.07 ± 0.28 g/100 g dry matter), carbohydrates (40.42 ± 0.48 g/100 g dry matter), dietary fibers (21.13 ± 0.34 g/100 g dry matter) and fats (2.04 ± 0.03 g/100 g dry matter). Furthermore, methanol extract of C. comatus served as a valuable flavonoid content (0.39 ± 0.08 (mg quercetin equivalents (QE)/g dry weight extract) and a total phenolic source (107.02 ± 2.42 mg GAE/g dry weight extract) including 4-hydroxybenzoic acid, protocatechuic acid, cinnamic acid, p-coumaric acid, caffeic acid, and quinic acid with concentrations of 11.41 ± 1.17, 0.13 ± 0.03, 4.34 ± 0.27, 10.48 ± 0.94, 0.15 ± 0.02, and 9.10 ± 1.39 μg/g, respectively, based on spectrometric analysis. According to the findings of experiments involving rats having liver damage induced by carbon tetrachloride, the administration of C. comatus orally for 42 days exhibited hepatoprotective effects in oxidative stress-induced liver damage by triggering repair mechanisms. Based on the results indicated in that paper, C. comatus had the potential to be utilized as a readily available food source with high levels of natural antioxidants. It also can be used as an additive or component for producing nutraceuticals and functional foods. Considering the reported work, an important issue arises regarding the complex composition of C. comatus extracts. Additional investigation is needed to ascertain whether the positive effects result from a singular active compound, or the synergistic activities of various metabolites present in the extract.
Phylloporia ribis (P. ribis), traditionally used in China for natural medicine, is recognized for its functional ingredients beneficial in treating conditions like pharyngitis, laryngitis, tonsillitis, and hyperglycemia. Ribka et al. (2021) reported bioactive compounds, and antifungal activity of methanolic (from 5% to 25%) extracts of P. ribis. Such extracts contained a diverse array of bioactive compounds, including carbohydrates, proteins, amino acids, lipids, alkaloids, glycosides, cardiac glycerides, flavonoids, phenols, terpenoids, steroids, sterols, saponins, tannins, and phosphate. The methanolic extract of P. ribis presented superior antifungal activity, particularly against Aspergillus niger (A. niger), causing soft rot in carrots, with 100% inhibition observed in all methanolic extracts except at 5% concentration. The diverse components present in P. ribis hold promise for applications as immunity boosters, food supplements, and in the field of drug discovery. Further investigations are also required to isolate the bioactive compounds responsible for immunity-boosting, drug development, antioxidant, anti-inflammatory, antibiotic, and antimicrobial activities in their pure form.
Polyporus grammocephalus (P. grammocephalus) ethanol extract was studied for its nutraceutical potential considering its bioactive metabolites. Aquino et al. (2018) identified sugars, alkaloids, flavonoids, triterpenes, essential oils, phenols, fatty acids, anthraquinones, coumarins, anthrones, tannins, and steroids, while terpenoids, cardiac glycosides, whereas saponins were not present in the P. grammocephalus ethanol extract. The ethanol extract of P. grammocephalus displayed DPPH radical scavenging activity (26.37%) and total phenolic content of 38.58 mg GAE/g. Brine shrimp toxicity assay indicated high toxicity with an LC50 value of 73.78 μg/mL These findings suggested that P. grammocephalus extract was rich in bioactive compounds with significant pharmacological activities, including antioxidant properties and cytotoxic effects.
The antioxidant and antimicrobial properties of ethyl acetate extracts from Alternaria alternata (A. alternata) were investigated by Chatterjee et al. (2019). The ethyl acetate extracts showed MIC ranging from 300 to 400 μg/mL against both Gram-positive and Gram-negative bacteria. Moreover, the ethyl acetate extract of A. alternata displayed antibacterial inhibition on B. subtilis, Listeria monocytogenes, S. aureus, E. coli, and S. typhimurium with up to 14 ± 1.5 mm DIZ. Furthermore, a reduction in the activity of key metabolic pathways, including the EMP pathway, TCA cycle, and gluconeogenic enzymes, suggested interference with the central carbohydrate metabolism. Additionally, A. alternata extract demonstrated strong antioxidant potential through DPPH and superoxide radical scavenging assays, with EC50 values of 38.0 ± 1.7 μg/mL and 11.38 ± 1.2 μg/mL, respectively. Within this analysis, ascorbic acid, used as a positive control, had an EC50 value of 20.23 ± 2.3 μg/mL. These results suggest that A. alternata showed potential as a source of bioactive compounds with medicinal importance, demonstrating strong antibacterial effects.
Phenylpropanoid (PPPN) compounds are widely utilized in various industries due to their diverse bioactivities, including applications in agriculture, medicine, food, and cosmetics. In this sense, Alternaria sp., which is, a novel natural source of PPPNs, was isolated from grapes by Lu J. et al. (2020). However, starvation is known to stimulate the PPPN pathway in plants, its impact on fungi remains underexplored. In that study, metabolomics analysis revealed that starvation treatment significantly increased the accumulation of shikimate and PPPN compounds in Alternaria sp. Notably, the study also identified additional PPPNs, such as sinapate, 4-hydroxystyrene, piceatannol, and taxifolin, under starvation conditions. These findings indicated that starvation treatment offers an effective strategy to enhance PPPN production and unveil compounds undetectable under non-starvation conditions. Overall, subjecting Alternaria sp. to starvation treatment during cultivation resulted in the robust activation of both the shikimate and PPPN pathways. These findings can shed light on the potential for optimizing the production of PPPN compounds by fungi, offering insights into the genetic resources and secondary metabolite pathways of Alternaria sp. for future functional studies.
Inonotus obliquus (I. obliquus), naturally grows on the trunks of birch wood trees in colder northern climates and is a medicinal fungus that has been used for therapeutic purposes since the 16th century (Ern et al., 2023). To investigate the antihyperglycemic and anti-lipid peroxidative effects of the dry matter of the culture broth (DMCB) of I. obliquus, Sun et al. (2008) utilized normal, glucose-induced hyperglycemic, and alloxan-induced diabetic mice. The DMCB exhibited a mild hypoglycemic effect in normal mice and achieved euglycemia in glucose-loaded mice after 2 h at a higher dose (1,000 mg/kg compared to 500 mg/kg). In alloxan-induced diabetic mice, the DMCB significantly reduced blood glucose levels, with a notable reduction observed for 21 days. The treatment also decreased serum levels of free fatty acids, total cholesterol, triglycerides, and LDL-cholesterol, while increasing HDL-cholesterol, insulin levels, and hepatic glycogen contents. Additionally, the DMCB enhanced antioxidant enzyme activities and histologically restored pancreas tissues in diabetic mice. Overall, the DMCB of I. obliquus demonstrated significant antihyperglycemic, anti-lipid peroxidative, and antioxidant effects in alloxan-induced diabetic mice.
Ma et al. (2013) identified the anti-inflammatory and anticancer compounds present in ethanol, petroleum ether, ethyl acetate, n-butyl alcohol, and water extracts of I. obliquus. Among all extracts, the petroleum ether extract was the most active one against human prostatic carcinoma cells and breast carcinoma cell lines with 64.66% and 63.26% inhibitory percentages, respectively. They also isolated lanosterol, 3β-hydroxy-8,24-dien-21-al, ergosterol, inotodiol, ergosterol peroxide, and trametenolic acid from both petroleum ether and ethyl acetate extracts. Among these metabolites, ergosterol, ergosterol peroxide, and trametenolic acid exhibited anti-inflammatory properties, while ergosterol peroxide and trametenolic acid demonstrated cytotoxic effects on human prostatic carcinoma cells and breast carcinoma cell lines. Additionally, these metabolites significantly inhibited nitric oxide production and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) luciferase activity in murine macrophage RAW 264.7 cells.
Xu et al. (2016) discovered flavonoids from ethanol, chloroform, ethyl acetate and n-buthanol extracts of I. obliquus. In these extracts epicatechin-3-gallate, epigallocatechin-3-gallate, and naringin, as well as phenolic acids, such as ferulic acid and gallic acid were identified. DPPH radical-scavenging abilities were significantly higher in Tween 20 medium (minimum EC50; 40.63 ± 0.89 mg/L) and linoleic acid medium (minimum EC50; 43.54 ± 0.92 mg/L) than the control medium (minimum EC50; 95.80 ± 1.99).
Baek et al. (2018) isolated triterpenoids from the methanol extract of I. obliquus to assess its cytotoxic effects on four human lung adenocarcinoma cell lines, each one with a different p53 tumor protein conditions (human lung adenocarcinoma cell lines A549, H1264, H1299, and Calu-6). They identified several metabolites including 3β-hydroxylanosta-8,24-dien-21, (+)-fuscoporianol C, inonotsutriol E, inotodiol, inonotsutriol A, trametenolic acid, saponaceoic acid I, and a novel lanostane-type triterpenoid, chagabusone A from methanol extracts from I. obliquus. Among these, 3β-hydroxylanosta-8,24-dien-21, trametenolic acid, and chagabusone A exhibited the most potent cytotoxicity against all human lung cancer cell lines tested, with EC50 values ranging from 75.1 to 227.4 µM. Notably, these compounds reduced the viability of human adenocarcinoma cell lines regardless of p53 mutations or null phenotype. This suggests that the cytotoxic effects observed against human lung cancer cells were independent of p53-related pathways, but rather mediated by apoptosis with caspase-3 activation.
Wold et al. (2020) identified melanin and six triterpenoids from dichloromethane and ethanol extracts of I. obliquus, chemically characterized, and evaluated for their anti-inflammatory, immunological, antimicrobial, anticancer, and cytotoxic effects. Among these compounds, melanin, and the triterpenoids 3β-hydroxy-8,24-dien-21-al and inotodiol significantly inhibited the hemolysis of antibody-sensitized sheep red blood cells by human sera. Specifically, 3β-hydroxy-8,24-dien-21-al and inotodiol activated the complement cascade, while the melanin fraction inhibited it. Inotodiol, betulinic acid, and betulin demonstrated anti-proliferative effects against the methotrexate-resistant human adenocarcinoma cell line HT29-MTX (viability: 3.8 ± 0.8 µM, 0.8 ± 0.3 µM, and 1.6 ± 0.4 µM, respectively) and the human lung carcinoma cell line NCI-H460 (viability: 4.7 ± 1 μM, 2.1 ± 0.5 µM, and 2.8 ± 0.4 µM, respectively). Additionally, the melanin fraction and betulinic acid-3-O-caffeate reduced the nitric oxide production in primary murine macrophages. However, these metabolites showed no antimicrobial activity against the tested four bacterial strains (E. coli, S. aureus, B. subtilis, and P. aeruginosa) and the yeast C. albicans.
Betulin, betulinic acid, inotodiol, and trametenolic acid, extracted with methanol from inner and outer parts (the sclerotium) of I. obliquus fruiting bodies, were tested against cancer cell lines (HT-29, AGS, MCF-7, and PC3) by Kim et al. (2020). The MTT assay was conducted to test the effect of triterpenoids on cancer cell lines. They found that the triterpenoids from the outer part extract showed significantly higher anti-proliferative activity against AGS, MCF-7, and PC3 cells compared to the inner part extract.
The hypouricemic properties of triterpenoid acids isolated from I. obliquus in mice with hyperuricemia were investigated by Luo et al. (2021). They identified various triterpenoid acids, including 3β,22,24-trihydroxy-lanosterol-8,25-diene, oleanolic acid, 3β-hydroxy-lanoster-8,24-dien-21-acid, 3β,21-dihydroxy-lanosterol-8,24 diene, betulin, inotodiol, 3β-Hydroxy-lanoster-8,24 dien-21-aldehyde, and lanosterol. Their research demonstrated that triterpenoid acids extracted from ethanol extracts of I. obliquus effectively inhibited xanthine oxidase activity (with an EC50 of 0.065 ± 0.01 mg/mL), displaying a mixed and reversible inhibition pattern. These triterpenoid acids also significantly reduced uric acid levels, hepatic xanthine oxidase, and serum blood urea nitrogen activities in mice with hyperuricemia. This suggests that triterpenoid acids from I. obliquus may help suppress kidney damage, lower inflammation in hyperuricemic mice, and exhibit inhibitory effects on xanthine oxidase activity. These findings underscore the potential of triterpenoids derived from I. obliquus as a promising dietary or medicinal supplement for managing hyperuricemia.
Zhao et al. (2021) identified flavonoids, including gallic acid, ferulic acid, flavonoids epicatechin-3-gallate, epigallocatechin-3-gallate, naringin, rutin, naringenin, phelligridin G, inoscavin B, and davallialectone, from the ethanol extract of I. obliquus. Moreover, they showed that cultivating I. obliquus on wheat straw led to increased levels of inoscavin B and davallialectone. In that work, the degradation of lignocellulose boosted the synthesis of flavonoids such as epicatechin-3-gallate, epigallocatechin-3-gallate, rutin, and naringin, thereby enhancing the antioxidative capabilities of I. obliquus. The highest antioxidant potential was observed in the extract obtained on day 9 (EC50 of 30.96 mg/L) against DPPH radicals.
In a study by Kou et al. (2021), seven newly discovered lanostane-type triterpenoids, named inonotusols H to N, were identified from the ethanol extract of I. obliquus. These metabolites exhibited significant inhibition of nitric oxide production in lipopolysaccharide-stimulated BV-2 microglial cells, with EC50 values ranging from 2.32 to 23.83 µM. At the concentration of 25.0 µM of these metabolites, no cytotoxicity was observed towards lipopolysaccharide-stimulated BV2 cells. Through molecular docking and Western blotting studies, two of the inonotusols showed the most potent inhibitory effects on iNOS and nitric oxide production. These findings suggest that these bioactive metabolites hold promise for development into therapeutic agents for neurodegenerative disorders, including Alzheimer’s disease.
Abu-Reidah et al. (2021) identified phenolics and flavonoids, including gallic acid, protocatechuic acid, salicylic acid, vanillic acid, 2,3-dihydroxybenzaldehyde, 2,5-dihydroxyterephthalic acid, coumaric acid, caffeic acid, 4-methoxycinnamic acid, hispidin, ferrulic acid, isorhamnetin, myricetin, quercetin, syringic acid, ellagic acid, hispolon, 3,4-dihydroxybenzalacetone, and 3-O-methylellagic acid, from I. obliquus extract using the modified Swiss water method. These metabolites showed hydrophilic, lipophilic, and total antioxidant activities.
Li et al. (2021) reported that phelligridin D, extracted from I. obliquus using both petroleum ether and ethyl acetate, presented good antioxidant properties. This metabolite reduced reactive oxygen species and malondialdehyde levels while increasing the activity of superoxide dismutase and catalase in human glomerular mesangial cells under high glucose concentration (30 mM). Additionally, it enhanced the capacity of the nuclear factor erythroid 2-related factor 2 (Nrf2), a master transcription factor that upregulates antioxidant response elements (ARE) (Zhao et al., 2017), to promote the transcription of ARE. It was also shown that phelligridin D activated Nrf2 in mesangial cells exposed to high glucose concentration, contributing to its protective effects. Their findings indicated the potential discovery of novel therapies targeting diabetic nephropathy and the applications of I. obliquus metabolites in clinical practices.
Wang et al. (2021) identified polyphenol compounds from ethanol extracts of I. obliquus such as procyanidin, caffeic acid, p-coumaric acid, isorhamnetin-3-O-glucoside, astilbin, tangeretin, gallic acid, kaempferol, quercetin, and catechin. According to the antioxidant activity of these polyphenols, their DPPH radical scavenging activity increased from 45.12% to 85.64% as the concentration increased from 1.0 to 5.0 mg/mL, respectively. As for their hydroxyl radical scavenging activity, it was found to be 38.76% at 1.0 mg/mL. When the concentration of polyphenols increased from 1.0 to 5.0 mg/mL, its stronger ferric-reducing antioxidant power increased from 0.11 to 0.39 mmol/mL. Their findings suggested that polyphenols from I. obliquus possessed promising potential as natural antioxidants.
Chen et al. (2021) extracted forty-six triterpenoids, twelve of which were newly discovered, from I. obliquus using ethanol and ethyl acetate stepwise. Among these 46 triterpenoids, thirteen of them showed strong α-glucosidase inhibition, with EC50 values from 11.5 to 81.8 µM. This study highlighted the significance of triterpenoids in clarifying the hypoglycemic effects associated with I. obliquus.
Peng et al. (2022) showed that ethanol extracts from I. obliquus, containing inotodiol, lanosterol, and trametenolic acid, significantly improved lipid accumulation in mouse livers induced by a methionine-choline deficient diet or in human LO2 hepatocyte cells lines induced by oleic acid. These metabolites exhibited protective properties against non-alcoholic fatty liver disease (NAFLD) by mitigating lipid deposition effects, reversing liver weight loss, and reducing liver triglyceride content together with restoring lower levels of alanine transaminase (ALT) and aspartate aminotransferase (AST). Inotodiol specifically demonstrated its anti-NAFLD properties by regulating the lipid metabolism pathway, farnesoid X receptor (FXR)/small heterodimer partner (SHP)/sterol regulatory element-binding protein-1c (SREBP-1c) (Liu et al., 2016). Their findings suggested that these bioactive compounds hold promise as potential drugs for NAFLD treatment.
Ogidi et al. (2018) analyzed raw and fermented ethyl acetate and ethanol extracts of Lenzites quercina (L. quercina) for their total phenol and flavonoid contents, alongside assessments of their antioxidant properties. The scavenging efficacy of fungal extracts was found against various free radicals, including DPPH, OH−, nitric oxide, and Fe2+ ranging from 0.12 to 1.80 mg/mL EC50 values. Furthermore, petroleum ether, ethyl acetate, and ethanol extracts exhibited EC50 lower than the positive controls butylated hydroxytoluene (BHT) and ethylenediaminetetraacetic acid (EDTA). The ethyl acetate extract from fermented L. quercina exhibited a higher phenolic content of 67.6 mg GAE/g extract, while the ethyl acetate extract from raw L. quercina displayed the highest flavonoid content of 51.4 mg QE/g extract. The antioxidant property, measured by FeCl3 reducing power, ranged from 18.1 (fermented L. quercina extracted with petroleum ether) to 127.6 mg (raw L. quercina extracted with petroleum ether) Ascorbic Acid Equivalent (AAE)/g extract for extracts obtained from both raw and fermented L. quercina. Fermented L. quercina demonstrated pronounced scavenging properties against nitric oxide and ferrous ion radicals, and it also exhibited superior inhibition of thiobarbituric acid reactive species (TBARS) with the highest inhibitory effect of 109.3%. The study suggested that the high total phenol and flavonoid content in L. quercina extracts positioned them as effective antioxidant agents, potentially serving as alternative therapy in healthcare.
A study by Prasher and Manju (2019) analyzed the active constituents present in ethyl acetate, methanol, and hexane extracts of Peniophora nuda (P. nuda) isolated from mango twigs. GC–MS chromatograms revealed 60, 9, and 60 major peaks in the ethyl acetate, methanol, and hexane crude extracts, respectively. The ethyl acetate extract exhibited 29 peaks with area percentages greater than one, with 13-docosenamide, (Z)- occupying the highest at 12.88%. In the methanolic extract, all 9 peaks had area percentages exceeding 1%, with tricaproin being the highest at 49.82%. The hexane extract displayed 28 peaks with area percentages greater than 1, and 13-docosenamide, (Z)- was the highest at 14.46%. According to their GC-MS findings, P. nuda was found to contain significant bioactive compounds with known antioxidant, anti-tumor, antibacterial, immunostimulant, lipoxygenase-inhibitor, anti-aging, analgesic, antidiabetic, anti-inflammatory, antidermatitic, antileukemic, anticancer, hepatoprotective, hypocholesterolemic, antiulcerogenic, vasodilator, antispasmodic, and antibronchitic properties (Prasher and Manju, 2019). These results could serve for identifying and understanding the nature of various bioactive components, with potential applications in biotechnological processes. Further isolation of individual phytochemicals may lead to the discovery of novel drugs. An extensive study of the pharmacological importance, diversity, and chemical composition can provide valuable insights and advance knowledge in this area. Further isolation of bioactive compounds has the potential to reveal new drugs.
The antioxidant properties of terrestrial Flavodon flavus (F. flavus) and Xylaria feejeensis (X. feejeensis), harvested from the dry zone forest from Sri Lanka were investigated by Fernando et al. (2016). The study also aimed to determine the contribution of phenolic and flavonoid substances to the antioxidant capabilities of these white rot fungi. Both species exhibited strong antioxidant capacity, indicating the presence of an effective antioxidative system. F. flavus demonstrated potent antioxidant activity with an EC50 of 77.00 ± 0.18 μg/mL based on DPPH radical scavenging capacity, while X. feejeensis exhibited promising antioxidant capacity with an EC50 value of 98.4 ± 0.28 μg/mL. Additionally, both analyzed species contained high levels of phenolic and flavonoid substances, suggesting their contribution to the prominent antioxidant activity. F. flavus and X. feejeensis showed higher total phenol contents of 55.7 ± 10.89 μg gallic acid/mg and 31.33 ± 8.87 μg gallic acid/mg, respectively. They also exhibited elevated levels of total flavonoids, with values of 82.4 ± 4.0 μg epicatechin/mg and 23.35 ± 7.0 μg epicatechin/mg, respectively. Notably, F. flavus exhibited a higher amount of total phenolics and flavonoids compared to X. feejeensis.
Fuscoporia torulosa (F. torulosa) is a fungus that develops woody fruiting bodies on both living and deceased trees. From methanol extract of F. torulosa fruiting bodies, two distinctive pentacyclic triterpenoids, namely fuscotorunones A and B, were isolated using ethyl acetate and purification by Noji et al. (2021). In vitro antimicrobial testing against B. subtilis, S. aureus, and C. albicans was conducted for fuscotorunones A and B. However, the ethyl acetate extract of F. torulosa demonstrated antimicrobial activity, with a MIC of 25 μg/mL against S. aureus and MIC of 100 μg/mL against B. subtilis, fuscotorunones A and B exhibited no activity against all tested microorganisms.
The Ganoderma genus belongs to the basidiomycota division, agaricomycetes class, polyporales order and ganodermataceae familiy. Among them, the species G. lucidum (Ling-Zhi in Chinese, Reishi in Japanese and Yeongji in Korean) is an outstanding medicinal mushroom having different therapeutical properties. Thus, this fungus is being cultivated worldwide, especially in Southeast Asian countries and many health products are being produced and sold (Bijalwan et al., 2020). Even in Europe there is a biotechnology company named Hifas da Terra (https://hifasdaterra.com/en/) that grow some white-rot fungi, G. lucidum among them, to extract active biomolecules that are commercialized in different products with diverse benefits for human health (e.g., immune system, oncology, mental health).
Several research works have reported different secondary metabolites from the Ganoderma genus, particularly from the G. lucidum species (Zhou et al., 2012; Sharma et al., 2019; Lu Y. et al., 2020; Wu et al., 2024), with interesting bioactivities. In this context, Baby et al. (2015) reviewed the biologically active secondary metabolites produced by different species belonging to the Ganoderma genus. They stated that phytochemical studies resulted in the isolation of 431 secondary metabolites, from which 240 were isolated from G. lucidum. Most of the isolated biologically active secondary metabolites were triterpenes, steroids, and polysaccharides (Seo et al., 2009; Cör et al., 2018). The latter showed to diminish the levels of serum glucose in normal fasted mice after 3 and 6 h of administration (Zhang and Lin, 2004). Likewise, Yang et al. (2007) observed that the administration of a Ganoderma applanatum (G. applanatum) exopolymer to induced diabetic rats reduced the glucose levels in plasma by 22%. Additionally, it decreased the total levels of cholesterol and triglycerides in plasma by 20.3% and 22.5%, respectively. Also, the activity of alanine transaminase and aspartate transaminase was reduced by 23.2% and 20.7%. Therefore, these compounds could find application to treat diabetes in animals.
Other researchers found that Ganoderma extracts presented interesting anti-cancer activities. Thus, for example, lucidenic acids, isolated from triterpenoids of ethanolic extracts of a new G. lucidum strain, exhibited anti-invasive activity on human hepatoma carcinoma cells (Weng et al., 2007). Similarly, Li et al. (2013) identified a new triterpenoid, named ethyl lucidenate (ethyl 7β-hydroxy-4,4,14α-trimethyl-3,11,15-trioxo-5α-chol-8-en-24-oate) from ethyl acetate extracts of G. lucidum with cytotoxicity against the cancer cell lines HL-60 and CA46. Also, exopolysaccharides obtained from G. applanatum presented antitumor activity against carcinoma cell lines (Osińska -Jaroszuk et al., 2014). In addition, G. lucidum ganodermic acid was able to inhibit the proliferation of HeLa and U87 human glioma cells, indicating its potential utilization as an anticancer drug (Upadhyay et al., 2014). Li et al. (2018) found that the polysaccharides from G. lucidum and Ganoderma sinense (G. sinense) presented similar chemical characteristics and tumor suppressive activity in mice, which indicated that polysaccharides from Ganoderma are therapeutic agents. Also, Wang K. et al. (2019) isolated from ethanolic extracts of G. lucidum fruiting bodies 1 new lanostane triterpene and 2 known aromatic meroterpenoids showing high antioxidant and neuroprotective activities. Zhang et al. (2019) showed that ganoderic acids from chloroform extracts of three Ganoderma species presented high antiproliferative activity (inhibition percentages from 70.8% to 80.7%) against three cancer cell lines (i.e., gastric carcinoma, liver carcinoma, and colon carcinoma). Bhat et al. (2021) reviewed the bioactivities of polysaccharides produced by different Ganoderma genera, mainly by G. lucidum, and stated that they presented antitumor, antioxidant, immunomodulatory, antibacterial, neuroprotective, hypoglycemic, and hepatoprotective activities. Therefore, they hold promise for further research to formulate natural efficient drugs to prevent and treat several diseases. More recently, Milhorini et al. (2022) isolated a fucoxylomannan from G. lucidum fruting bodies by alkaline extraction with important antimelanomic properties.
On the other hand, there are many research papers reporting antimicrobial activities of Ganoderma strains. Thus, Hassan et al. (2019) reported the high antibiotic activity of water extracts from G. applanatum against P. aeruginosa, Pseudomonas fluorescens (P. fluorescens), B. subtilis, Staphylococcus epidermidis (S. epidermidis), and Micrococcus luteus (M. luteus) strains. In addition, chloroform extracts of G. lucidum basidiocarp displayed high antibacterial activity against Salmonella typhi (S. typhi) (18 ± 2.1 mm DIZ for 100 µL extract) and B. subtilis (17 ± 1.9 mm DIZ for 100 µL extract) and high antifungal activity against the yeast C. albicans (17 ± 1.7 mm DIZ for 100 µL extract) which was related to their content in polysaccharides and triterpenoids. In addition, G. lucidum chloroform extracts also presented high antioxidant activity (Uma Gowrie et al., 2014). Also, methanolic extracts of G. lucidum exhibited strong antimicrobial activity against the yeast S. cerevisiae (MIC50 value 3 μg/mL) but low antimicrobial activities against Gram-positive bacteria (S. epidermidis and Enterococcus raffinosus (E. raffinosus)) and no activity against Gram-negative bacteria (E. coli and P. aeruginosa) and the yeast C. albicans (Hleba et al., 2014). Also, Ismail et al. (2014) studied the antimicrobial activities of methanol, chloroform, dichloromethane, and hexane extracts of Ganoderma boninense (G. boninense). The methanol and chloroform extracts showed significant antibacterial activities against different food-borne and skin disease bacterial pathogens (i.e., E. coli, B. subtilis Bacillus cereus (B. cereus), P. aeruginosa, S. pyogenes, Streptococcus pneumoniae, S. aureus, and Klebsiella spp.). Further, GC-MS results confirmed that G. boninense contained bioactive compounds such as dodecanoic acid, cyclododecane, octadecanoic acid, 9-octadecenoic acid, hexadecanoic acid, methyl tetradecanoate, 9, 12-octadecadienoic acid, dodecyl acrylate and hexadecanoic acid. In addition, exopolysaccharides from G. applanatum presented antibacterial activity against S. aureus (17.98 ± 0.4 mm DIZ and MIC value 1 mg/mL) and toxicity against V. fischeri (82.8% cell damage) (Osińska-Jaroszuk et al., 2014). Moreover, ganodermic acid from G. lucidum presented antibacterial properties against the Gram-negative bacteria E. coli and P. aeruginosa (MIC 1 mg/mL) and the Gram-positive bacteria S. aureus and S. epidermidis (MIC 0.25 mg/mL), pointing out its potential use as a broad-spectrum antibiotic (Upadhyay et al., 2014). Hoque et al. (2015) investigated the antioxidant, antimicrobial and cytotoxic potential of pet ether, chloroform, and methanol extracts of a G. lucidum strain collected from Bangladesh. Their results revealed that all the extracts presented high antioxidant activity, low to moderate antibacterial activity (DIZs ranging from 7 mm to 21 mm) against different strains of both Gram-positive (Sarcina lutea, Bacillus megaterium, B. subtilis, S. aureus, and B. cereus) and Gram-negative bacteria (P. aeruginosa, S. typhi, E. coli, Vibrio parahemolyticus, Vibrio mimicus, Shigella boydii, and Shigella disenteriae) and weak cytotoxic activity (brine shrimp nauplii bioassay). However, Romorosa et al. (2017) showed that aqueous extracts of G. lucidum, isolated from decaying logs in Isabela State University, Philippines, contained alkaloids, tannins, glycosides and in less extent saponins but not flavonoids. Nevertheless, these extracts had low antimicrobial activity against E. coli and especially against S. aureus. This was likely because they were devoid in flavonoids. According to the review by Ahmad et al. (2021), G. lucidum has a wide range of pharmacological activities, including antiviral activities, due to its content in triterpenoids and polysaccharides. Nonetheless, further studies on the clinical application of the biologically active compounds of this strain are needed. More recently, Chan and Chong (2022) reported the strong antibacterial activity against methicillin-resistant S. aureus (MRSA) (DIZ 41.08 ± 0.04 mm and MIC 0.078 mg/mL) of ethyl acetate extracts of a G. boninense strain collected from Malaysia. This strong antibacterial activity against MRSA was attributed to its content in aristolochic acid and tamoxifen, which are known to be effective against MRSA (Flores et al., 2016; Bartha et al., 2019), as well as its content in other metabolites with reported antimicrobial properties (i.e., aminoimidazole ribotide, lysine sulfonamide, carbocyclic puromycin, fenbendazole, acetylcaranine, and tigecycline) (Vince et al., 1986; Livermore, 2005; Stranix et al., 2006; Kim et al., 2015; Ločárek et al., 2015; Qadir et al., 2016; Miro-Canturri et al., 2019; de Oliveira et al., 2020). Hence, it could be a promising solution to develop drugs able to fight against multi-antibiotic resistant bacteria. In another recent work, it was shown that hot water extracts of Ganoderma neo-japonicum (G. neo-japonicum) exhibited 2-fold higher antioxidant and antimicrobial activities (against S. typhimurium, Salmonella enteritidis, and E. coli) than those of G. lucidum ones. This was presumably related to the higher content in flavonoids of the G. neo-japonicum extracts (Ayimbila et al., 2023).
Additionally, Wang et al. (2017) reported anti-aging activities of G. lucidum extracts which were mainly exerted through anti-oxidation, immunomodulation, and anti-neurodegeneration. The bioactive compounds responsible for these antiaging effects consisted of polysaccharides, triterpenes, peptides, and polysaccharide peptides. More studies are needed to clarify the mechanisms involved in these antiaging properties.
The Trametes genus belongs to the basidiomycota division, agaricomycetes class, polyporales order and family polyporaceae. Different research studies have reported the production of secondary metabolites with various biological activities by several strains of the Trametes genus. Among them, T. versicolor (also known as C. versicolor) is the most studied species. Thus, methanolic extracts of T. versicolor exhibited strong antimicrobial activity against the yeast S. cerevisiae (MIC50 value 24 μg/mL), low against Gram-positive bacteria (S. epidermidis and E. raffinosus) and no activity against Gram-negative bacteria (E. coli and P. aeruginosa) and the yeast C. albicans (Hleba et al., 2014). Also, acetonitrile and aqueous extracts of Trametes hirsuta (T. hirsuta), isolated from decaying logs in Isabela State University, Philippines, presented strong antimicrobial activity against E. coli (DIZ 26.36 mm) and S. aureus (DIZ 13.87 mm). This could be related to the flavonoid content in the extracts (Romorosa et al., 2017). Furthermore, isolated cerevisterol (ergosta-7, 22E-diene-3β5α, 6β -triol) from methanol extracts of Trametes gibbosa (T. gibbosa) and Trametes elegans (T. elegans), collected from farms and forests in Ghana, exhibited a broad-spectrum antibiotic activity. Thus, the isolated cerevisterol from T. gibbosa and T. elegans inhibited the growth of S. typhi (MIC25 and MIC 50, µg/mL, respectively), S. aureus (MIC25 and 100 μg/mL, respectively), A. niger (MIC25 and 100 μg/mL, respectively) and E. faecalis (MIC50 and 200 μg/mL, respectively) (Appiah et al., 2020). Nanglihan et al. (2018) showed that the ethanol extracts of T. elegans, collected from the Lingap Kalikasan Park of Central Luzon State University, Philippines, contained flavonoids, tannins, phenols, steroids, alkaloids, anthraquinones, anthrones, coumarins, essential oils, and fatty acids. Also, T. elegans extracts presented significant scavenging activity, antibacterial activities against S. aureus (DIZ 8.30 mm) and E. coli (DIZ 8.07 mm) and high cytotoxicity (brine shrimp nauplii bioassay). Gebreyohannes et al. (2019) found that chloroform, ethanol and hot extracts of two wild fungi, collected from National Reserve Forests, in Kenya, and further identified as Trametes spp. showed interesting antimicrobial activities against different test strains (E. coli, K. pneumoniae, P. aeruginosa, S. aureus, MRSA, C. albicans, and Candida parapsilosis), the highest one being obtained for S. aureus (MIC values 0.83 ± 0.29, 0.67 ± 0.29, and 0.67 ± 0.29 for chloroform, ethanol and hot water extracts, respectively). In addition, Hassan et al. (2019) reported the high antibiotic activity of water extracts from T. versicolor against P. aeruginosa, P. fluorescens, B. subtilis, S. epidermidis, and M. luteus strains. Bains and Chawla (2020) reported that the methanolic extracts from T. versicolor, collected from the forest of Chail in India, contained phenolics as the main compounds followed by flavonoids, ascorbic acid, β-carotene, and lycopene and presented significant antimicrobial activities against S. aureus, P. aeruginosa, K. pneumonia, and E. coli (DIZs ranging from 24.14 to 30.18 mm). It also showed anti-inflammatory activities presumably due to its content in glycopeptides. Furthermore, Oyetayo and Akingbesote (2022) tested the antimicrobial properties of acetone and methanolic extracts from raw and submerged and solid-state fermented Trametes polyzona (T. polyzona), collected from dead wood in Nigeria, against S. aureus isolated from blood, soil, water, and urine. The methanolic extract from submerged fermented T. polyzona showed the highest antimicrobial activity against blood isolated S. aureus (DIZ 28 mm), probably due to its ability to dissolve the endogenous compounds of the fungus. However, the acetonic extracts presented low antimicrobial activity. GC-MS analysis of T. polyzona methanolic extracts showed the following 14 bioactive compounds: caprylic acid methyl ester, tridecanoic acid methyl ester, myristoleic acid methyl ester, cis-10 pentadecanoic acid methyl ester, palmitoleic acid methyl ester, heptadecanoic acid methyl ester, stearic acid methyl ester, elaidic acid methyl ester, oleic acid methyl ester, linolelaidic acid methyl ester, g-linoleic acid methyl ester, x-linolenic acid methyl ester, heneicosanoic acid methyl ester, and cis-11-14-eicosadienoic acid methyl ester. Recently, Begum et al. (2023) reported that the aqueous extracts of T. hirsuta exhibited antimicrobial activity against S. aureus (DIZ 16.00 ± 0.66 mm for 20 mg/mL extract), K. pneumonia (DIZ 14.66 ± 0.88 mm for 20 mg/mL extract) and Salmonella enterica (DIZ 13.00 ± 0.88 mm for 20 mg/mL extract). They also related that ethanolic extracts of T. hirsuta had significant analgesic, anti-inflammatory and antispasmodic activities. Therefore, T. hirsuta could be a valuable source of bioactive compounds to develop new drugs to treat pain, fever and anti-inflammatory disorders, bacterial infections, and gastrointestinal problems. Moreover. Wei et al. (2023) characterized four new sesquiterpenes (three bisabolane sesquiterpenes and one drimane sequisterpene) from T. versicolor, one of them (drimarene sequisterpene) showing antimicrobial activity against S. aureus (MIC50 value 22.2 µM).
On the other hand, Leliebre-Lara et al. (2015) found that n-hexane, dichloromethane, ethyl acetate, and ethanol extracts of T. versicolor, collected from a dead and dry trunk in Cuba, presented anti-leishmanial activity against the parasite Leishmania amazonensis, being higher in ethyl acetate and ethanol extracts. Also, a partially purified exoproteome of T. versicolor culture filtrates highly inhibited the growth and the T2 toxin production of the cereal pathogen Fusarium langsethiae (Parroni et al., 2019). Wang K. et al. (2019) reported that the bioactive macromolecule polysaccharopeptide from T. versicolor (TPSP), purchased from Fujian Fuzhou Green Valley Biopharmaceutical Technology Research, inhibited the development of morphine addiction in rats. They pointed out that TPSP could be used as an adjunctive therapy approach for the alleviation of morphine resistance in the clinic.
Additionally, a polysaccharide from Trametes orientalis (T. orientalis) presented chemoprotective effects against cyclophosphamide-induced immunosuppression and oxidative stress in mice (Zheng et al., 2017). Furthermore, Roca-Lema et al. (2019) assessed the anticancer effects of polysaccharide-rich extracts from T. versicolor on LoVo and HT-29 human colon cancer cells. Their studies showed that T. versicolor extracts inhibited human colon proliferation and cause cytotoxicity. Moreover, blending the extracts with the known anticancer drug 5-fluoruoracil boosted cell cytotoxicity. More recently, He et al. (2021) purified a protein named musarin from T. versicolor extract which strongly inhibited the growth of human colorectal cancer cell lines in vitro. Therefore, musarin protein holds promise to develop drugs against colorectal cancers, especially against the chemo-resistant ones.
3 Concluding remarks
In the evolving landscape of natural bioactive metabolite discovery, white-rot fungi have emerged as prolific sources of novel metabolites, offering versatile applications, including agriculture, healthcare, and pharmaceuticals. These compounds constitute a rich reservoir of bioactive substances, synthesized during secondary metabolism by utilizing intermediate compounds or by-products from primary metabolic pathways. While secondary metabolites are non-essential for an organism’s growth, they show diverse biological characteristics, underscoring their potential significance. White-rot fungi exhibit a unique ability to decompose all wood components, contributing to carbon and nitrogen cycles and producing bioactive substances with several effects, such as antioxidant, antimicrobial, and anticancer properties. In light of explanations, this article reviews the potential application of biologically active secondary metabolites from white-rot fungi in different fields like nutrition, medicine, and degradation. As shown, the diversity of these compounds highlights their importance in forthcoming research, advancements, and practical applications across various industries. This underscores the crucial contribution that white-rot fungi can make in influencing the field of biotechnology and sustainable development. Nevertheless, scaling up production on a large scale is necessary to assess the feasibility of commercial applications.
Author contributions
OP: Conceptualization, Visualization, Writing–original draft, Writing–review and editing. SR-C: Conceptualization, Funding acquisition, Project administration, Supervision, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Mikkeli University Consortium (MUC) with the project number 23B350E6YT10.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-Reidah, I. M., Critch, A. L., Manful, C. F., Rajakaruna, A., Vidal, N. P., Pham, T. H., et al. (2021). Effects of pH and temperature on water under pressurized conditions in the extraction of nutraceuticals from chaga (Inonotus obliquus) mushroom. Antioxidants 10 (8), 1322. doi:10.3390/antiox10081322
Ahmad, M. F., Ahmad, F. A., Khan, M. I., Alsayegh, A. A., Wahab, S., Alam, M. I., et al. (2021). Ganoderma lucidum: a potential source to surmount viral infections through β-glucans immunomodulatory and triterpenoids antiviral properties. Int. J. Biol. Macromol. 187, 769–779. doi:10.1016/j.ijbiomac.2021.06.122
Alam, N., Amin, R., Khan, A., Ara, I., Shim, M. J., Lee, M. W., et al. (2009). Comparative effects of oyster mushrooms on lipid profile, liver, and kidney function in hypercholesterolemic rats. Mycobiology 37 (1), 37–42. doi:10.4489/MYCO.2009.37.1.037
Alderton, W. K., Cooper, C. E., and Knowles, R. G. (2001). Nitric oxide synthases: structure, function and inhibition. Biochem. J. 357 (3), 593–615. doi:10.1042/bj3570593
Al-Mousa, A., Abo-Dahab, N. F., Hassane, A. M. A., Gomaa, A. E. F., Aljuriss, J. A., and Dahmash, N. D. (2022b). Harnessing Mucor spp. for xylanase production: statistical optimization in submerged fermentation using agro-industrial wastes. Biomed. Res. Int. 2022, 1–17. doi:10.1155/2022/3816010
Al-Mousa, A., Hassane, A. M. A., Gomaa, A. E. F., Aljuriss, J. A., Dahmash, N. D., and Abo-Dahab, N. F. (2022a). Response-surface statistical optimization of submerged fermentation for pectinase and cellulase production by Mucor circinelloides and M. hiemalis. Fermentation 8, 205. doi:10.3390/fermentation8050205
Appiah, T., Agyare, C., Luo, Y., Boamah, V. E., and Boakye, Y. D. (2020). Antimicrobial and resistance modifying activities of cerevisterol isolated from Trametes species. Curr. Bioact. Compd. 16 (2), 115–123. doi:10.2174/1573407214666180813101146
Aquino, Y. K. D. C., Vega, L. D. P., Medrano, N. R. M., and Dulay, R. M. R. (2018). Mycochemicals, antioxidant and cytotoxic activities of Polyporus grammocephalus Berk (BIL7749). Int. J. Biol. Pharm. Allied. Sci. 7 (6), 966–975. doi:10.31032/IJBPAS/2018/7.6.4455
Assis, I. S., Chaves, M. B., Silveira, M. L. L., Gern, R. M. M., Wisbeck, E., Júnior, A. F., et al. (2013). Production of bioactive compounds with antitumor activity against sarcoma 180 by Pleurotus sajor-caju. J. Med. Food. 16 (11), 1004–1012. doi:10.1089/jmf.2012.0267
Austria, A. B., Dulay, R. M. R., and Pambid, R. C. (2021). Mycochemicals, antioxidant and anti-diabetic properties of Philippine sawgill mushroom Lentinus swartzii (Higher Basidiomycetes). Asian J. Agric. Biol. 2, 1–8. doi:10.35495/ajab.2020.06.365
Ayimbila, F., Siriwong, S., Chaiyama, V., Srihanant, N., and Keawsompong, S. (2023). Comparative study of bio-functional profile and bioactivities of polysaccharides from Ganoderma lucidum and Ganoderma neo-japonicum. Biocatal. Agric. Biotechnol. 53, 102875. doi:10.1016/j.bcab.2023.102875
Baby, S., Johnson, A. J., and Govindan, B. (2015). Secondary metabolites from Ganoderma. Phytochemistry 114, 66–101. doi:10.1016/j.phytochem.2015.03.010
Baek, J., Roh, H. S., Baek, K. H., Lee, S., Lee, S., Song, S. S., et al. (2018). Bioactivity-based analysis and chemical characterization of cytotoxic constituents from Chaga mushroom (Inonotus obliquus) that induce apoptosis in human lung adenocarcinoma cells. J. Ethnopharmacol. 224, 63–75. doi:10.1016/j.jep.2018.05.025
Bains, A., and Chawla, P. (2020). In vitro bioactivity, antimicrobial and anti-inflammatory efficacy of modified solvent evaporation assisted Trametes versicolor extract. 3 Biotech. 10 (9), 404. doi:10.1007/s13205-020-02397-w
Bala, N., Aitken, E. A., Fechner, N., Cusack, A., and Steadman, K. J. (2011). Evaluation of antibacterial activity of Australian basidiomycetous macrofungi using a high-throughput 96-well plate assay. Pharm. Biol. 49, 492–500. doi:10.3109/13880209.2010.526616
Baltz, R. H. (2019). Natural product drug discovery in the genomic era: realities, conjectures, misconceptions, and opportunities. J. Ind. Microbiol. Biotechnol. 46, 281–299. doi:10.1007/s10295-018-2115-4
Bartha, G. S., Tóth, G., Horváth, P., Kiss, E., Papp, N., and Kerényi, M. (2019). Analysis of aristolochlic acids and evaluation of antibacterial activity of Aristolochia clematitis L. Biol. Futur. 70 (4), 323–329. doi:10.1556/019.70.2019.36
Basit, A., Shah, S. T., Ullah, I., Ullah, I., and Mohamed, H. I. (2021). “Microbial bioactive compounds produced by endophytes (bacteria and fungi) and their uses in plant health,” in Plant growth-promoting microbes for sustainable biotic and abiotic stress management. Editors S. T. Shah,, and M. H. I. Mohamed (Berlin, Germany: Springer Cham), 285–318.
Begum, H. A., Ahmad, W., Rafiq, N., Ali, H., Hussain, S., Ali, B., et al. (2023). Exploring the pharmacological potential of Trametes hirsuta (White Rot Fungi): analgesic, anti-Inflammatory, antispasmodic and antimicrobial activities. Pure Appl. Biol. 12 (2), 1183–1193. doi:10.19045/bspab.2023.120121
Beltrán Delgado, Y., Morris Quevedo, H., Domínguez, O. D., Batista Corbal, P., and Llauradó Maury, G. (2021). Composición micoquímica y actividad antioxidante de la seta Pleurotos ostreatus en diferentes estados de crecimiento. Acta Biológica Colomb. 26 (1), 89–98. doi:10.15446/abc.v26n1.84519
Bhambri, A., Srivastava, M., Mahale, V. G., Mahale, S., and Karn, S. K. (2022). Mushrooms as potential sources of active metabolites and medicines. Front. Microbiol. 13, 837266. doi:10.3389/fmicb.2022.837266
Bharatiya, P., Rathod, P., Hiray, A., and Kate, A. S. (2021). Multifarious elicitors: invoking biosynthesis of various bioactive secondary metabolite in fungi. Appl. Biochem. Biotechnol. 193 (3), 668–686. doi:10.1007/s12010-020-03423-6
Bhat, Z. A., Wani, A. H., War, J. M., and Bhat, M. Y. (2021). Major bioactive properties of Ganoderma polysaccharides: a review. Asian J. Pharm. Clin. Res. 14 (3), 11–24. doi:10.22159/ajpcr.2021.v14i3.40390
Bijalwan, A., Bahuguna, K., Vasishth, A., Singh, A., Chaudhary, S., Tyagi, A., et al. (2020). Insights of medicinal mushroom (Ganoderma lucidum): prospects and potential in India. Biodivers. Int. J. 4 (5), 202–209.
Bogale, T. T. (2020). Biotechnological applications of white rot fungi: a review. GSC Adv. Res. Rev. 5 (2), 97–103. doi:10.30574/gscarr.2020.5.2.0043
Brandalise, F., Roda, E., Ratto, D., Goppa, L., Gargano, M. L., Cirlincione, F., et al. (2023). Hericium erinaceus in neurodegenerative diseases: from bench to bedside and beyond, how far from the shoreline? J. Fungus 9 (5), 551. doi:10.3390/jof9050551
Bruhat, A., Jousse, C., Wang, X. Z., Ron, D., Ferrara, M., and Fafournoux, P. (1997). Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both transcriptional and post-transcriptional levels. J. Biol. Chem. 272 (28), 17588–17593. doi:10.1074/jbc.272.28.17588
Chan, P., Kao, P. F., and Tomlinson, B. (2005). Cardiovascular effects of trilinolein, a natural triglyceride isolated from the herb sanchi (Panax notoginseng). Acta Cardiol. Sin. 21 (2), 71–76.
Chan, P., Thomas, G. N., and Tomlinson, B. (2002). Protective effects of trilinolein extrated from Panax notoginseng against cardiovascular disease. Acta Pharmacol. Sin. 23 (12), 1157–1162.
Chan, Y. S., and Chong, K. P. (2022). Bioactive compounds of Ganoderma boninense inhibited methicillin-resistant Staphylococcus aureus growth by affecting their cell membrane permeability and integrity. Molecules 27 (3), 838. doi:10.3390/molecules27030838
Chandra, P., Arora, D. S., Pal, M., and Sharma, R. K. (2019). Antioxidant potential and extracellular auxin production by white rot fungi. Appl. Biochem. Biotechnol. 187, 531–539. doi:10.1007/s12010-018-2842-z
Chang, C. H., Chen, Y., Yew, X. X., Chen, H. X., Kim, J. X., Chang, C. C., et al. (2016). Improvement of erinacine A productivity in Hericium erinaceus mycelia and its neuroprotective bioactivity against the glutamate-insulted apoptosis. LWT - Food Sci. Technol. 65, 1100–1108. doi:10.1016/j.lwt.2015.08.014
Chatterjee, S., Ghosh, R., and Mandal, N. C. (2019). Production of bioactive compounds with bactericidal and antioxidant potential by endophytic fungus Alternaria alternata AE1 isolated from Azadirachta indica A. Juss. PLoS One 14 (4), e0214744. doi:10.1371/journal.pone.0214744
Chen, J., Zeng, X., Yang, Y. L., Xing, Y. M., Zhang, Q., Li, J. M., et al. (2017). Genomic and transcriptomic analyses reveal differential regulation of diverse terpenoid and polyketides secondary metabolites in Hericium erinaceus. Sci. Rep. 7 (1), 10151. doi:10.1038/s41598-017-10376-0
Chen, S. D., Yong, T. Q., Xiao, C., Gao, X., Xie, Y. Z., Hu, H. P., et al. (2021). Inhibitory effect of triterpenoids from the mushroom Inonotus obliquus against α-glucosidase and their interaction: inhibition kinetics and molecular stimulations. Bioorg. Chem. 115, 105276. doi:10.1016/j.bioorg.2021.105276
Chou, P. Y., Huang, G. J., Pan, C. H., Chien, Y. C., Chen, Y. Y., Wu, C. H., et al. (2011). Trilinolein inhibits proliferation of human non-small cell lung carcinoma A549 through the modulation of PI3K/Akt pathway. Am. J. Chin. Med. 39 (4), 803–815. doi:10.1142/S0192415X11009214
Chowdhury, M. M. H., Kubra, K., and Ahmed, S. R. (2015). Screening of antimicrobial, antioxidant properties and bioactive compounds of some edible mushrooms cultivated in Bangladesh. Ann. Clin. Microbiol. Antimicrob. 14 (1), 8–6. doi:10.1186/s12941-015-0067-3
Conrado, R., Gomes, T. C., Roque, G. S. C., and De Souza, A. O. (2022). Overview of bioactive fungal secondary metabolites: cytotoxic and antimicrobial compounds. Antibiotics 11 (11), 1604. doi:10.3390/antibiotics11111604
Contreras, E., Flores, R., Gutiérrez, A., Cerro, D., and Sepúlveda, L. A. (2023). Agro-industrial wastes revalorization as feedstock: production of lignin-modifying enzymes extracts by solid-state fermentation using white rot fungi. Prep. Biochem. Biotechnol. 53 (5), 488–499. doi:10.1080/10826068.2022.2109048
Cör, D., Knez, Ž., and Knez Hrncic, M. (2018). Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: a review. Molecules 23, 649. doi:10.3390/molecules23030649
Corrêa, R. C. G., de Souza, A. H. P., Calhelha, R. C., Barros, L., Glamoclija, J., Sokovic, M., et al. (2015). Bioactive formulations prepared from fruiting bodies and submerged culture mycelia of the Brazilian edible mushroom Pleurotus ostreatoroseus Singer. Food Funct. 6 (7), 2155–2164. doi:10.1039/c5fo00465a
Daley, D. K., Brown, K. J., and Badal, S. (2017). “Fungal metabolites,” in Pharmacognosy. Editors S. Badal,, and R. Delgoda (United States: Academic Press), 413–421.
Darling, N. J., and Cook, S. J. (2014). The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta, Mol. Cell Res. 1843 (10), 2150–2163. doi:10.1016/j.bbamcr.2014.01.009
Deng, G., Lin, H., Seidman, A., Fornier, M., D’Andrea, G., Wesa, K., et al. (2009). A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: immunological effects. J. Cancer Res. Clin. Oncol. 135, 1215–1221. doi:10.1007/s00432-009-0562-z
de Oliveira, H. C., Joffe, L. S., Simon, K. S., Castelli, R. F., Reis, F. C., Bryan, A. M., et al. (2020). Fenbendazole controls in vitro growth, virulence potential, and animal infection in the Cryptococcus model. Antimicrob. Agents Chemother. 64, 002866–e320. doi:10.1128/AAC.00286-20
Devi, M. R., and Krishnakumari, S. (2015). Quantitative estimation of primary and secondary metabolites in hot aqueous extract of Pleurotus sajor caju. J. Pharmacogn. Phytochem. 4 (3), 198–202.
Devi, R., Sharma, P., Rajput, A., Kaur, J., and Arora, S. (2022). Evaluation of taxonomic, phytochemical and antioxidant characteristics of wild mushroom Porodaedalea pini. Pharmacol. Res. - Mod. Chin. 5, 100189. doi:10.1016/j.prmcm.2022.100189
Dissanayake, D. P., Abeytunga, D. T. U., Vasudewa, N. S., and Ratnasooriya, W. D. (2009). Inhibition of lipid peroxidation by extracts of Pleurotus ostreatus. Pharmacogn. Mag. 5 (19), 266–271.
Duan, X. X., Qin, D., Song, H. C., Gao, T. C., Zuo, S. H., Yan, X., et al. (2019). Irpexlacte AD, four new bioactive metabolites of endophytic fungus Irpex lacteus DR10-1 from the waterlogging tolerant plant Distylium chinense. Phytochem. Lett. 32, 151–156. doi:10.1016/j.phytol.2019.06.001
Dutta, S., Woo, E. E., Yu, S. M., Nagendran, R., Yun, B. S., and Lee, Y. H. (2019). Control of anthracnose and gray mold in pepper plants using culture extract of white-rot fungus and active compound schizostatin. Mycobiology 47 (1), 87–96. doi:10.1080/12298093.2018.1551833
Eckhardt, S. G., Baker, S. D., Britten, C. D., Hidalgo, M., Siu, L., Hammond, L. A., et al. (2000). Phase I and pharmacokinetic study of irofulven, a novel mushroom-derived cytotoxin, administered for five consecutive days every four weeks in patients with advanced solid malignancies. J. Clin. Oncol. 18 (24), 4086–4097. doi:10.1200/JCO.2000.18.24.4086
Elhusseiny, S. M., El-Mahdy, T. S., Awad, M. F., Elleboudy, N. S., Farag, M. M., Yassein, M. A., et al. (2021). Proteome analysis and in vitro antiviral, anticancer and antioxidant capacities of the aqueous extracts of Lentinula edodes and Pleurotus ostreatus edible mushrooms. Molecules 26 (15), 4623. doi:10.3390/molecules26154623
Ern, P. T. Y., Quan, T. Y., Yee, F. S., and Yin, A. C. Y. (2023). Therapeutic properties of Inonotus obliquus (Chaga mushroom): a review. Mycology 2023, 1–18. doi:10.1080/21501203.2023.2260408
Fagade, O. E., and Oyelade, A. A. (2009). A comparative study of the antibacterial activities of some wood-decay fungi to synthetic antibiotic discs. Elec. J. Env. Agric. Food Chem. 8, 184–188.
Fernando, M. D. M., Wijesundera, R. L. C., Soysa, S. S. B. D. P., de Silva, E. D., and Nanayakkara, C. M. (2016). Antioxidant potential and content of the polyphenolic secondary metabolites of white rot macrofungi; Flavodon flavus (Klotzsch.) and Xylaria feejeensis (Berk.). SDRP J. Plant Sci. 1 (1), 1–6. doi:10.25177/jps.1.1.2
Flores, R., Insel, P. A., Nizet, V., and Corriden, R. (2016). Enhancement of neutrophil antimicrobial activity by the breast cancer drug tamoxifen. FASEB J. 30, 969–1014. doi:10.1096/fasebj.30.1_supplement.969.14
Floudas, D., Binder, M., Riley, R., Barry, K., Blanchette, R. A., Henrissat, B., et al. (2012). The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336 (6089), 1715–1719. doi:10.1126/science.1221748
Friedman, M. (2015). Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (Lion’s Mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 63 (32), 7108–7123. doi:10.1021/acs.jafc.5b02914
Fukushima-Sakuno, E. (2020). Bioactive small secondary metabolites from the mushrooms Lentinula edodes and Flammulina velutipes. J. Antibiot. 73 (10), 687–696. doi:10.1038/s41429-020-0354-x
Gakuubi, M. M., Ching, K. C., Munusamy, M., Wibowo, M., Liang, Z. X., Kanagasundaram, Y., et al. (2022). Enhancing the discovery of bioactive secondary metabolites from fungal endophytes using chemical elicitation and variation of fermentation media. Front. Microbiol. 13, 898976. doi:10.3389/fmicb.2022.898976
Gebreyohannes, G., Nyerere, A., Bii, C., and Berhe Sbhatu, D. (2019). Determination of antimicrobial activity of extracts of indigenous wild mushrooms against pathogenic organisms. Evid. Based Complement. Altern. Med. 2019, 1–7. doi:10.1155/2019/6212673
Gebreyohannes, G., and Sbhatu, D. B. (2023). Wild mushrooms: a hidden treasure of novel bioactive compounds. Int. J. Anal. Chem. 2023, 6694961–6695020. doi:10.1155/2023/6694961
Ghaly, I. S., Ahmed, E. S., Booles, H. F., Farag, I. M., and Nada, S. A. (2011). Evaluation of antihyperglycemic action of oyster mushroom (Pleurotus ostreatus) and its effect on DNA damage, chromosome aberrations and sperm abnormalities in streptozotocin-induced diabetic rats. Glob. Vet. 7 (6), 532–544.
Halabura, M. I. W., Avelino, K. V., Araújo, N. L., Kassem, A. S. S., Seixas, F. A. V., Barros, L., et al. (2023). Light conditions affect the growth, chemical composition, antioxidant and antimicrobial activities of the white-rot fungus Lentinus crinitus mycelial biomass. Photochem. Photobiol. Sci. 22 (3), 669–686. doi:10.1007/s43630-022-00344-7
Han, J., Wu, J., and Silke, J. (2020). An overview of mammalian p38 mitogen-activated protein kinases, central regulators of cell stress and receptor signaling. F1000Research 9, 653. doi:10.12688/f1000research.22092.1
Hassan, F., Ni, S., Becker, T. L., Kinstedt, C. M., Abdul-Samad, J. L., Actis, L. A., et al. (2019). Evaluation of the antibacterial activity of 75 mushrooms collected in the vicinity of Oxford, Ohio (USA). Int. J. Med. Mushrooms 21 (2), 131–141. doi:10.1615/IntJMedMushrooms.2018029710
Hassane, A. M. A., Hussien, S. M., Abouelela, M. E., Taha, T. M., Awad, M. F., Mohamed, H., et al. (2022). In vitro and in silico antioxidant efficiency of bio-potent secondary metabolites from different taxa of black seed-producing plants and their derived mycoendophytes. Front. Bioeng. Biotechnol. 10, 930161. doi:10.3389/fbioe.2022.930161
He, Y., Liu, S., and Newburg, D. S. (2021). Musarin, a novel protein with tyrosine kinase inhibitory activity from Trametes versicolor, inhibits colorectal cancer stem cell growth. Biomed. Pharmacother. 144, 112339. doi:10.1016/j.biopha.2021.112339
Henriksen, N. N., Lindqvist, L. L., Wibowo, M., Sonnenschein, E. C., Bentzon-Tilia, M., and Gram, L. (2022). Role is in the eye of the beholder—the multiple functions of the antibacterial compound tropodithietic acid produced by marine Rhodobacteraceae. FEMS Microbiol. Rev. 46 (3), fuac007. doi:10.1093/femsre/fuac007
Hleba, L., Vuković, N., Petrová, J., and Kačániová, M. (2014). Antimicrobial activity of crude methanolic extracts from Ganoderma lucidum and Trametes versicolor. Anim. Sci. Biotechnol. 47, 89–93.
Hoeksma, J., Misset, T., Wever, C., Kemmink, J., Kruijtzer, J., Versluis, K., et al. (2019). A new perspective on fungal metabolites: identification of bioactive compounds from fungi using zebrafish embryogenesis as read-out. Sci. Rep. 9 (1), 17546. doi:10.1038/s41598-019-54127-9
Hoque, N., Ahmed, I., Akanda, M. R. U. Z., and Chowdhury, N. S. (2015). In vitro antioxidant, antimicrobial and cytotoxic activities of the various extracts of Ganoderma lucidum available in Bangladesh. J. Pharmacogn. Phytochem. 4 (3), 42–46.
Ildız, E., Canpolat, Ş., İşlek, C., Canpolat, E. Y., İşlek, Y., and Akata, I. (2022). Bjerkandera adusta collected from niğde: analysis of total phenolic compound, antioxidant, vnd antimicrobial properties. TURJAF 10, 2996–3000. doi:10.24925/turjaf.v10isp2.2996-3000.5750
Ismail, K., Abdullah, S., and Chong, K. P. (2014). Screening for potential antimicrobial compounds from Ganoderma boninense against selected foodborne and skin disease pathogens. Int. J. Pharm. Pharm. Sci. 6 (2), 771–774.
Jaszek, M., Kos, K., Matuszewska, A., Grąz, M., Stefaniuk, D., Osińska-Jaroszuk, M., et al. (2014). Effective stimulation of the biotechnological potential of the medicinal white rot fungus: Phellinus pini by menadione-mediated oxidative stress. Appl. Biochem. Biotechnol. 174, 644–656. doi:10.1007/s12010-014-1064-2
Jaszek, M., Osińska-Jaroszuk, M., Janusz, G., Matuszewska, A., Stefaniuk, D., Sulej, J., et al. (2013). New bioactive fungal molecules with high antioxidant and antimicrobial capacity isolated from Cerrena unicolor idiophasic cultures. Biomed. Res. Int. 2013, 1–11. doi:10.1155/2013/497492
Jayakumar, T., Thomas, P. A., and Geraldine, P. (2009). In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Innov. Food Sci. Emerg. Technol. 10 (2), 228–234. doi:10.1016/j.ifset.2008.07.002
Jerusik, R. J. (2010). Fungi and paper manufacture. Fungal Biol. Rev. 24 (1-2), 68–72. doi:10.1016/j.fbr.2010.04.003
Kalaw, S. P., and Albinto, R. F. (2014). Functional activities of Philippine wild strain of Coprinus comatus (OF Müll.: Fr.) Pers and Pleurotus cystidiosus OK Miller grown on rice straw based substrate formulation. Mycosphere 5 (5), 646–655. doi:10.5943/mycosphere/5/5/5
Kaur, M., Chadha, P., Kaur, S., Kaur, A., Kaur, R., Yadav, A. K., et al. (2018). Schizophyllum commune induced genotoxic and cytotoxic effects in Spodoptera litura. Sci. Rep. 8 (1), 4693. doi:10.1038/s41598-018-22919-0
Kawagishi, H., Masui, A., Tokuyama, S., and Nakamura, T. (2006). Erinacines J and K from the mycelia of Hericium erinaceum. Tetrahedron 62 (36), 8463–8466. doi:10.1016/j.tet.2006.06.091
Kawagishi, H., Shimada, A., Hosokawa, S., Mori, H., Sakamoto, H., Ishiguro, Y., et al. (1996). Erinacines E, F, and G, stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 37 (41), 7399–7402. doi:10.1016/0040-4039(96)01687-5
Kawagishi, H., Shirai, R., Sakamoto, H., Yoshida, S., Ojima, F., and Ishiguro, Y. (1992). Erinapyrones A and B from the cultured mycelia of Hericium erinaceum. Chem. Lett. 21 (12), 2475–2476. doi:10.1246/cl.1992.2475
Keller, N. P., Turner, G., and Bennett, J. W. (2005). Fungal secondary metabolism from biochemistry to genomics. Nat. Rev. Microbiol. 3, 937–947. doi:10.1038/nrmicro1286
Kenmoku, H., Shimai, T., Toyomasu, T., Kato, N., and Sassa, T. (2002). Erinacine Q, a new erinacine from Hericium erinaceum, and its biosynthetic route to erinacine C in the basidiomycete. Biosci. Biotechnol. Biochem. 66 (3), 571–575. doi:10.1271/bbb.66.571
Kijpornyongpan, T., Schwartz, A., Yaguchi, A., and Salvachúa, D. (2022). Systems biology-guided understanding of white-rot fungi for biotechnological applications: a review. iScience 25 (7), 104640. doi:10.1016/j.isci.2022.104640
Kim, A., Wolf, N. M., Zhu, T., Johnson, M. E., Deng, J., Cook, J. L., et al. (2015). Identification of Bacillus anthracis PurE inhibitors with antimicrobial activity. Bioorg. Med. Chem. 23 (7), 1492–1499. doi:10.1016/j.bmc.2015.02.016
Kim, J., Yang, S. C., Hwang, A. Y., Cho, H., and Hwang, K. T. (2020). Composition of triterpenoids in Inonotus obliquus and their anti-proliferative activity on cancer cell lines. Molecules 25 (18), 4066. doi:10.3390/molecules25184066
Kobayashi, S., Tamanoi, H., Hasegawa, Y., Segawa, Y., and Masuyama, A. (2014). Divergent synthesis of bioactive resorcinols isolated from the fruiting bodies of Hericium erinaceum: total syntheses of hericenones A, B, and I, hericenols B–D, and erinacerins A and B. J. Org. Chem. 79 (11), 5227–5238. doi:10.1021/jo500795z
Korcan, S. E., Ciğerci, İ. H., and Konuk, M. (2012). “White-rot fungi in bioremediation,” in Fungi as bioremediators. Editors E. M. Goltapeh, Y. R. Danesh, and A. Varma (Heidelberg: Springer-Verlag Berlin Heidelberg), 371–390.
Kou, R. W., Han, R., Gao, Y. Q., Li, D., Yin, X., and Gao, J. M. (2021). Anti-neuroinflammatory polyoxygenated lanostanoids from Chaga mushroom Inonotus obliquus. Phytochemistry 184, 112647. doi:10.1016/j.phytochem.2020.112647
Koutrotsios, G., Kalogeropoulos, N., Kaliora, A. C., and Zervakis, G. I. (2018). Toward an increased functionality in oyster (Pleurotus) mushrooms produced on grape marc or olive mill wastes serving as sources of bioactive compounds. J. Agric. Food Chem. 66 (24), 5971–5983. doi:10.1021/acs.jafc.8b01532
Krupodorova, T., Barshteyn, V., and Sevindik, M. (2022). Antioxidant and antimicrobial potentials of mycelial extracts of Hohenbuehelia myxotricha grown in different liquid culture media. BioTechnologia 103 (1), 19–28. doi:10.5114/bta.2022.113912
Krupodorova, T., Rybalko, S., and Barshteyn, V. (2014). Antiviral activity of basidiomycete mycelia against influenza type A (serotype H1N1) and herpes simplex virus type 2 in cell culture. Virol. Sin. 29 (5), 284–290. doi:10.1007/s12250-014-3486-y
Kumar, A., Ali, S., Lal, S. B., and Sinha, M. P. (2018). Mycochemical screening and determination of nutritive potency and antioxidant activity of edible macrofungi Dacryopinax spathularia (Schwein) and Schizophyllum commune (Fries). World J. Pharm. Res. 7 (16), 1311–1321. doi:10.20959/wjpr201816-13240
Kundu, S. (2021). Study of Secondary Metabolites Produced by White rot fungi for knowing their antimicrobial properties. J. Emerg. Technol. Innov. Res. 8 (2), 1–12.
Kundu, S., and Khan, M. A. (2021). Diversity of metabolites produced by White rot fungi possess different biological properties: a review. Eco. Env. Cons. 27 (2021), S165–S177.
Landingin, H. R. R., Francisco, B. E., Dulay, R. M. R., Kalaw, S., and Reyes, R. (2020). Optimization of culture conditions for mycelial growth and basidiocarp production of Cyclocybe cylindracea (Maire). CLSU-IJST 4 (1), 1–17. doi:10.22137/ijst.2020.v4n1.01
Lee, D. G., Kang, H. W., Park, C. G., Ahn, Y. S., and Shin, Y. (2016). Isolation and identification of phytochemicals and biological activities of Hericium ernaceus and their contents in Hericium strains using HPLC/UV analysis. J. Ethnopharmacol. 184, 219–225. doi:10.1016/j.jep.2016.02.038
Lee, E. W., Shizuki, K., Hosokawa, S., Suzuki, M., Suganuma, H., Inakuma, T., et al. (2000). Two novel diterpenoids, erinacines H and I from the mycelia of Hericium erinaceum. Biosci. Biotechnol. Biochem. 64 (11), 2402–2405. doi:10.1271/bbb.64.2402
Lee, K. F., Chen, J. H., Teng, C. C., Shen, C. H., Hsieh, M. C., Lu, C. C., et al. (2014). Protective effects of Hericium erinaceus mycelium and its isolated erinacine A against ischemia-injury-induced neuronal cell death via the inhibition of iNOS/p38 MAPK and nitrotyrosine. Int. J. Mol. Sci. 15 (9), 15073–15089. doi:10.3390/ijms150915073
Leliebre-Lara, V., García, M., Nogueiras, C., and Monzote, L. (2015). Qualitative analysis of an ethanolic extract from Trametes versicolor and biological screening against Leishmania amazonensis. Emir. J. Food Agric. 592, 592–595. doi:10.9755/ejfa.2015.05.194
Li, J. L., Lu, L., Dai, C. C., Chen, K., and Qiu, J. Y. (2001). A comparative study on sterols of ethanol extract and water extract from Hericium erinaceus. Zhongguo Zhong Yao Za Zhi= Zhongguo Zhongyao Zazhi= China J. Chin. Materia Medica 26 (12), 831–834.
Li, L. F., Liu, H. B., Zhang, Q. W., Li, Z. P., Wong, T. L., Fung, H. Y., et al. (2018). Comprehensive comparison of polysaccharides from Ganoderma lucidum and G. sinense: chemical, antitumor, immunomodulating and gut-microbiota modulatory properties. Sci. Rep. 8 (1), 6172. doi:10.1038/s41598-018-22885-7
Li, P., Deng, Y. P., Wei, X. X., and Xu, J. H. (2013). Triterpenoids from Ganoderma lucidum and their cytotoxic activities. Nat. Prod. Res. 27 (1), 17–22. doi:10.1080/14786419.2011.652961
Li, W., Zhou, W., Kim, E. J., Shim, S. H., Kang, H. K., and Kim, Y. H. (2015). Isolation and identification of aromatic compounds in Lion’s Mane Mushroom and their anticancer activities. Food Chem. 170, 336–342. doi:10.1016/j.foodchem.2014.08.078
Li, Y., Zhou, Y., Wu, J., Li, J., and Yao, H. (2021). Phelligridin D from Inonotus obliquus attenuates oxidative stress and accumulation of ECM in mesangial cells under high glucose via activating Nrf2. J. Nat. Med. 75 (4), 1021–1029. doi:10.1007/s11418-021-01534-w
Lin, F. Y., Lai, Y. K., Yu, H. C., Chen, N. Y., Chang, C. Y., Lo, H. C., et al. (2008). Effects of Lycium barbarum extract on production and immunomodulatory activity of the extracellular polysaccharopeptides from submerged fermentation culture of Coriolus versicolor. Food Chem. 110 (2), 446–453. doi:10.1016/j.foodchem.2008.02.023
Liu, C., Dunkin, D., Lai, J., Song, Y., Ceballos, C., Benkov, K., et al. (2015). Anti-inflammatory effects of Ganoderma lucidum triterpenoid in human Crohn’s disease associated with downregulation of NF-κB signaling. Inflamm. Bowel. Dis. 21 (8), 1918–1925. doi:10.1097/MIB.0000000000000439
Liu, N., Zhao, J., Wang, J., Teng, H., Fu, Y., and Yuan, H. (2016). Farnesoid X receptor ligand CDCA suppresses human prostate cancer cells growth by inhibiting lipid metabolism via targeting sterol response element binding protein 1. Am. J. Transl. Res. 8 (11), 5118–5124.
Livermore, D. M. (2005). Tigecycline: what is it, and where should it be used? J. Antimicrob. Chemother. 56, 611–614. doi:10.1093/jac/dki291
Llanos-López, N. A., Ebada, S. S., Vasco-Palacios, A. M., Sánchez-Giraldo, L. M., López, L., Rojas, L. F., et al. (2023). Panapophenanthrin, a rare oligocyclic diterpene from Panus strigellus. Metabolites 13 (7), 848. doi:10.3390/metabo13070848
Ločárek, M., Nováková, J., Klouček, P., Hošt’álková, A., Kokoška, L., Gábrlová, L., et al. (2015). Antifungal and antibacterial activity of extracts and alkaloids of selected Amaryllidaceae species. Nat. Prod. Commun. 10, 1934578X1501000–1540. doi:10.1177/1934578X1501000912
Lu, J., He, R., Sun, P., Zhang, F., Linhardt, R. J., and Zhang, A. (2020a). Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 150, 765–774. doi:10.1016/j.ijbiomac.2020.02.035
Lu, Q. Q., Tian, J. M., Wei, J., and Gao, J. M. (2014). Bioactive metabolites from the mycelia of the basidiomycete Hericium erinaceum. Nat. Prod. Res. 28 (16), 1288–1292. doi:10.1080/14786419.2014.898145
Lu, Y., Che, J., Xu, X., Pang, B., Zhao, X., Liu, Y., et al. (2020b). Metabolomics reveals the response of the phenylpropanoid biosynthesis pathway to starvation treatment in the grape endophyte Alternaria sp MG1. J. Agric. Food Chem. 68 (4), 1126–1135. doi:10.1021/acs.jafc.9b05302
Luo, L. S., Wang, Y., Dai, L. J., He, F. X., Zhang, J. L., and Zhou, Q. (2021). Triterpenoid acids from medicinal mushroom Inonotus obliquus(Chaga) alleviate hyperuricemia and inflammation in hyperuricemic mice: possible inhibitory effects on xanthine oxidase activity. J. Food Biochem. 46 (3), 13932. doi:10.1111/jfbc.13932
Ma, L., Chen, H., Dong, P., and Lu, X. (2013). Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 139 (1-4), 503–508. doi:10.1016/j.foodchem.2013.01.030
Mahuri, M., Paul, M., and Thatoi, H. (2023). A review of microbial laccase production and activity toward different biotechnological applications. Syst. Appl. Microbiol. 3, 533–551. doi:10.1007/s43393-023-00163-6
Martínez, A. T., Speranza, M., Ruiz-Dueñas, F. J., Ferreira, P., Camarero, S., Guillén, F., et al. (2005). Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 8 (3), 195–204.
Masuda, Y., Inoue, M., Miyata, A., Mizuno, S., and Nanba, H. (2009). Maitake β-glucan enhances therapeutic effect and reduces myelosupression and nephrotoxicity of cisplatin in mice. Int. Immunopharmacol. 9 (5), 620–626. doi:10.1016/j.intimp.2009.02.005
Masuda, Y., Murata, Y., Hayashi, M., and Nanba, H. (2008). Inhibitory effect of MD-Fraction on tumor metastasis: involvement of NK cell activation and suppression of intercellular adhesion molecule (ICAM)-1 expression in lung vascular endothelial cells. Biol. Pharm. Bull. 31 (6), 1104–1108. doi:10.1248/bpb.31.1104
Matuszewska, A., Stefaniuk, D., Jaszek, M., Pięt, M., Zając, A., Matuszewski, Ł., et al. (2019). Antitumor potential of new low molecular weight antioxidative preparations from the white rot fungus Cerrena unicolor against human colon cancer cells. Sci. Rep. 9 (1), 1975. doi:10.1038/s41598-018-37947-z
Milhorini, S. D., Bellan, D. D., Zavadinack, M., Simas, F. F., Smiderle, F. R., de Santana-Filho, A. P., et al. (2022). Antimelanoma effect of a fucoxylomannan isolated from Ganoderma lucidum fruiting bodies. Carbohydr. Polym. 294, 119823. doi:10.1016/j.carbpol.2022.119823
Miro-Canturri, A., Ayerbe-Algaba, R., and Smani, Y. (2019). Drug repurposing for the treatment of bacterial and fungal infections. Front. Microb. 10, 41–12. doi:10.3389/fmicb.2019.00041
Mitra, P., Khatua, S., and Acharya, K. (2013). Free radical scavenging and NOS activation properties of water-soluble crude polysaccharide from Pleurotus ostreatus. Asian J. Pharm. Clin. Res. 6 (3), 67–70.
Miyazaki, K., Mizutani, H., Katabuchi, H., Fukuma, K., Fujisaka, S., and Okamura, H. (1995). Activated (HLA-DR+) T-lymphocyte subsets in cervical carcinoma and effects of radiotherapy and immunotherapy with sizofiran on cell-mediated immunity and survival. Gynecol. Oncol. 56 (3), 412–420. doi:10.1006/gyno.1995.1073
Mizerska-Dudka, M., Jaszek, M., Błachowicz, A., Rejczak, T. P., Matuszewska, A., Osińska-Jaroszuk, M., et al. (2015). Fungus Cerrena unicolor as an effective source of new antiviral, immunomodulatory, and anticancer compounds. Int. J. Biol. Macromol. 79, 459–468. doi:10.1016/j.ijbiomac.2015.05.015
Mohamed, H., Awad, M. F., Shah, A. M., Nazir, Y., Naz, T., Hassane, A., et al. (2022). Evaluation of different standard amino acids to enhance the biomass, lipid, fatty acid, and ɣ-linolenic acid production in Rhizomucor pusillus and Mucor circinelloides. Front. Nutr. 9, 876817. doi:10.3389/fnut.2022.876817
Mohammadnejad, S., Pourianfar, H. R., Drakhshan, A., Jabaleh, I., and Rezayi, M. (2019). Potent antiproliferative and pro-apoptotic effects of a soluble protein fraction from culinary-medicinal mushroom Lentinus tigrinus on cancer cells. J. Food Meas. Charact. 13 (4), 3015–3024. doi:10.1007/s11694-019-00222-4
Muslihin, A. M., Rifai, Y., and Rante, H. (2022). Isolation and identification of endophytic fungi producing antioxidant compound from Azadirachta indica A. juss based on gen 18S rRNA. Teikyo Med. J. 45 (1), 3635–3644.
Muszyńska, B., Dąbrowska, M., Starek, M., Żmudzki, P., Lazur, J., Pytko-Polończyk, J., et al. (2019). Lentinula edodes mycelium as effective agent for piroxicam mycoremediation. Front. Microbiol. 10, 313. doi:10.3389/fmicb.2019.00313
Nanglihan, K. E. M. V., Dulay, R. M. R., and Kalaw, S. P. (2018). Myko-actives and functional activities of Philippine wild mushroom Trametes elegans. Int. J. Biosci. 13 (5), 402–408. doi:10.12692/ijb/13.5.402-408
Noji, M., Yoneyama, T., Nishihama, K., Elshamy, A. I., Hashimoto, T., and Umeyama, A. (2021). Pentacyclic triterpenoids, fuscotorunones A and B, with ε-caprolactone in ring E from Fuscoporia torulosa. Phytochem 187, 112748. doi:10.1016/j.phytochem.2021.112748
Oba, K., Kobayashi, M., Matsui, T., Kodera, Y., and Sakamoto, J. (2009). Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res. 29 (7), 2739–2745.
Ogidi, C. O., Oyetayo, V. O., and Akinyele, B. J. (2018). Estimation of total phenolic, flavonoid contents and free radical scavenging activity of a wild macrofungus, Lenzites quercina (L.) P. Karsten. Curr. Res. Environ. Appl. 8 (4), 425–437. doi:10.5943/cream/8/4/2
Ogidi, C. O., Ubaru, A. M., Ladi-Lawal, T., Thonda, O. A., Aladejana, O. M., and Malomo, O. (2020). Bioactivity assessment of exopolysaccharides produced by Pleurotus pulmonarius in submerged culture with different agro-waste residues. Heliyon 6 (12), e05685. doi:10.1016/j.heliyon.2020.e05685
Osińska-Jaroszuk, M., Jaszek, M., Mizerska-Dudka, M., Błachowicz, A., Rejczak, T. P., Janusz, G., et al. (2014). Exopolysaccharide from Ganoderma applanatum as a promising bioactive compound with cytostatic and antibacterial properties. Biomed. Res. Int. 2014, 1–10. doi:10.1155/2014/743812
Oyetayo, V. O., and Akingbesote, E. T. (2022). Assessment of the antistaphylococcal properties and bioactive compounds of raw and fermented Trametes polyzona (Pers.) Justo extracts. Microb. Biosyst. 7 (1), 1–7. doi:10.21608/mb.2022.129214.1054
Parroni, A., Bellabarba, A., Beccaccioli, M., Scarpari, M., Reverberi, M., and Infantino, A. (2019). Use of the secreted proteome of Trametes versicolor for controlling the cereal pathogen Fusarium langsethiae. Int. J. Mol. Sci. 20 (17), 4167. doi:10.3390/ijms20174167
Patel, S., and Goyal, A. (2012). Recent developments in mushrooms as anti-cancer therapeutics: a review. 3 Biotech. 2 (1), 1–15. doi:10.1007/s13205-011-0036-2
Peng, A., Liu, S., Fang, L., Zhu, Z., Zhou, Y., Yue, S., et al. (2022). Inonotus obliquus and its bioactive compounds alleviate non-alcoholic fatty liver disease via regulating FXR/SHP/SREBP-1c axis. Eur. J. Pharmacol. 921, 174841. doi:10.1016/j.ejphar.2022.174841
Prasher, I. B., and Manju, (2019). Screening of Peniophora nuda (a white rot fungus) for the presence of commercially important bioactive metabolites. Vegetos 32 (3), 307–315. doi:10.1007/s42535-019-00038-z
Qadir, M. A., Ahmed, M., and Khaleeq, A. (2016). Synthesis, antibacterial and antifungal possession of amino acids containing sulfonamide moieties. Pak. J. Pharm. Sci. 29, 1609–1613.
Ramesh, C. H., and Pattar, M. G. (2010). Antimicrobial properties, antioxidant activity and bioactive compounds from six wild edible mushrooms of western ghats of Karnataka, India. Pharmacogn. Res. 2 (2), 107. doi:10.4103/0974-8490.62953
Ratto, D., Corana, F., Mannucci, B., Priori, E. C., Cobelli, F., Roda, E., et al. (2019). Hericium erinaceus improves recognition memory and induces hippocampal and cerebellar neurogenesis in frail mice during aging. Nutrients 11 (4), 715. doi:10.3390/nu11040715
Resurreccion, N. G. U., Shen, C. C., and Ragasa, C. Y. (2016). Chemical constituents of Lentinus edodes. Der Pharm. Lett. 8 (4), 117–120.
Ribka, T., Nagadesi, P. K., Ponnuru, V., Thatha, V., and Pratyusha, A. V. (2021). Phenotypical, mycochemicals, proximate composition and antifungal activity of Phylloporia ribis (schumach.) ryvarden from India. J. Chem. Pharm. Res. 13 (2), 20–28.
Roca-Lema, D., Martinez-Iglesias, O., de Ana Portela, C. F., Rodríguez-Blanco, A., Valladares-Ayerbes, M., Díaz-Díaz, A., et al. (2019). In vitro anti-proliferative and anti-invasive effect of polysaccharide-rich extracts from Trametes versicolor and Grifola frondosa in colon cancer cells. Int. J. Med. Sci. 16 (2), 231–240. doi:10.7150/ijms.28811
Roda, E., De Luca, F., Ratto, D., Priori, E. C., Savino, E., Bottone, M. G., et al. (2023). Cognitive healthy aging in mice: boosting memory by an ergothioneine-rich Hericium erinaceus primordium extract. Biology 12 (2), 196. doi:10.3390/biology12020196
Roda, E., Priori, E. C., Ratto, D., De Luca, F., Di Iorio, C., Angelone, P., et al. (2021). Neuroprotective metabolites of Hericium erinaceus promote neuro-healthy aging. Int. J. Mol. Sci. 22 (12), 6379. doi:10.3390/ijms22126379
Roda, E., Ratto, D., De Luca, F., Desiderio, A., Ramieri, M., Goppa, L., et al. (2022). Searching for a longevity food, we bump into Hericium erinaceus primordium rich in ergothioneine: the “longevity vitamin” improves locomotor performances during aging. Nutrients 14 (6), 1177. doi:10.3390/nu14061177
Rodríguez-Berríos, R. R., Ríos-Delgado, A. M., Perdomo-Lizardo, A. P., Cardona-Rivera, A. E., Vidal-Rosado, Á. G., Narváez-Lozano, G. A., et al. (2023). Extraction, Isolation, characterization, and bioactivity of polypropionates and related Polyketide metabolites from the Caribbean region. Antibiotics 12 (7), 1087. doi:10.3390/antibiotics12071087
Romorosa, E. S., De Guzman, C. T., Martin, J. R. G., and Jacob, J. K. S. (2017). Preliminary investigation on the pharmacological properties of wood-rotting mushrooms collected from Isabela State University, Echague, Isabela, Philippines. Int. J. Agric. Technol. 13 (7), 2591–2596.
Seo, H. W., Hung, T. M., Na, M., Jung, H. J., Kim, J. C., Choi, J. S., et al. (2009). Steroids and triterpenes from the fruit bodies of Ganoderma lucidum and their anti-complement activity. Arch. Pharm. Res. 32, 1573–1579. doi:10.1007/s12272-009-2109-x
Sevindik, M. (2018a). Investigation of antioxidant/oxidant status and antimicrobial activities of Lentinus tigrinus. Adv. Pharmacol. Pharm. Sci. 2018, 1–4. doi:10.1155/2018/1718025
Sevindik, M. (2018b). Antioxidant and antimicrobial activity of Cerrena unicolor. Mycopath 16 (1), 11–14.
Sevindik, M., Akgul, H., Bal, C., and Selamoglu, Z. (2018). Phenolic contents, oxidant/antioxidant potential and heavy metal levels in Cyclocybe cylindracea. Indian J. Pharm. Educ. Res. 52 (3), 437–441. doi:10.5530/ijper.52.3.50
Shankar, A., and Sharma, K. K. (2022). Fungal secondary metabolites in food and pharmaceuticals in the era of multi-omics. Appl. Microbiol. Biotechnol. 106 (9-10), 3465–3488. doi:10.1007/s00253-022-11945-8
Sharma, C., Bhardwaj, N., Sharma, A., Tuli, H. S., Batra, P., Beniwal, V., et al. (2019). Bioactive metabolites of Ganoderma lucidum: factors, mechanism and broad spectrum therapeutic potential. J. Herb. Med. 17, 100268. doi:10.1016/j.hermed.2019.100268
Sharma, N., Takkar, S., Bhatia, K., Vardhan, H., Tripathi, M., Iqbal, K., et al. (2023). “Recent advances in fungal secondary metabolites and their applications,” in Fungal resources for sustainable economy: current status and future perspectives. Editors I. Singh, V. R. Rajpal, and S. S. Navi (Singapore: Springer), 411–432.
Sidorova, I., and Voronina, E. (2019). “Bioactive secondary metabolites of basidiomycetes and its potential for agricultural plant growth promotion,” in Secondary metabolites of plant growth promoting rhizomicroorganisms. Editors H. Singh, C. Keswani, M. Reddy, E. Sansinenea, and C. García-Estrada (Singapore: Springer), 3–26.
Stilinović, N., Čapo, I., Vukmirović, S., Rašković, A., Tomas, A., Popović, M., et al. (2020). Chemical composition, nutritional profile and in vivo antioxidant properties of the cultivated mushroom Coprinus comatus. R. Soc. Open Sci. 7 (9), 200900. doi:10.1098/rsos.200900
Stranix, B. R., Lavallée, J. F., Sévigny, G., Yelle, J., Perron, V., LeBerre, N., et al. (2006). Lysine sulfonamides as novel HIV-protease inhibitors: Nε-acyl aromatic α-amino acids. Bioorg. Med. Chem. Lett. 16 (13), 3459–3462. doi:10.1016/j.bmcl.2006.04.011
Su, C. H., Lu, M. K., Lu, T. J., Lai, M. N., and Ng, L. T. (2020). A (1→ 6)-branched (1→ 4)-β-D-glucan from Grifola frondosa inhibits lipopolysaccharide-induced cytokine production in RAW264. 7 macrophages by binding to TLR2 rather than dectin-1 or CR3 receptors. J. Nat. Prod. 83 (2), 231–242. doi:10.1021/acs.jnatprod.9b00584
Sun, J. E., Ao, Z. H., Lu, Z. M., Xu, H. Y., Zhang, X. M., Dou, W. F., et al. (2008). Antihyperglycemic and antilipidperoxidative effects of dry matter of culture broth of Inonotus obliquus in submerged culture on normal and alloxan-diabetes mice. J. Ethnopharmacol. 118 (1), 7–13. doi:10.1016/j.jep.2008.02.030
Tanimoto, T., Onodera, K., Hosoya, T., Takamatsu, Y., Kinoshita, T., Tago, K., et al. (1996). Schizostatin, a novel squalene synthase inhibitor produced by the mushroom, Schizophyllum commune. I. Taxonomy, fermentation, isolation, physico-chemical properties and biological activities. J. Antibiot. 49 (7), 617–623. doi:10.7164/antibiotics.49.617
Teoh, Y. P., Don, M. M., and Ujang, S. (2011). Media selection for mycelia growth, antifungal activity against wood-degrading fungi, and GC-MS study by Pycnoporus sanguineus. BioResources 6 (3), 2719–2731. doi:10.15376/biores.6.3.2719-2731
Thirumurugan, D., Cholarajan, A., Raja, S. S., and Vijayakumar, R. (2018). “An introductory chapter: secondary metabolites,” in Secondary metabolites-sources and applications. Editors R. Vijayakumar,, and S. S. S. Raja. London, United Kingdom: (IntechOpen), 3–21.
Tripathi, A. M., and Tiwary, B. N. (2013). Biochemical constituents of a wild strain of Schizophyllum commune isolated from Achanakmar-Amarkantak Biosphere Reserve (ABR), India. World J. Microbiol. Biotechnol. 29, 1431–1442. doi:10.1007/s11274-013-1306-4
Tsukada, K., Shinki, S., Kaneko, A., Murakami, K., Irie, K., Murai, M., et al. (2020). Synthetic biology based construction of biological activity-related library of fungal decalin-containing diterpenoid pyrones. Nat. Commun. 11, 1830. doi:10.1038/s41467-020-15664-4
Tundis, R., Loizzo, M. R., and Menichini, F. (2010). Natural products as α-Amylase and α-Glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini-Rev. Med. Chem. 10 (4), 315–331. doi:10.2174/138955710791331007
Tzeng, T. T., Chen, C. C., Chen, C. C., Tsay, H. J., Lee, L. Y., Chen, W. P., et al. (2018). The cyanthin diterpenoid and sesterterpene constituents of Hericium erinaceus mycelium ameliorate Alzheimer’s disease-related pathologies in APP/PS1 transgenic mice. Int. J. Mol. Sci. 19 (2), 598. doi:10.3390/ijms19020598
Uma Gowrie, S., Chathurdevi, G., and Rani, K. (2014). Evaluation of bioactive potential of basidiocarp extracts of Ganoderma lucidum. Int. J. Pharm. Res. Allied Sci. 3, 36–46.
Upadhyay, M., Shrivastava, B., Jain, A., Kidwai, M., Kumar, S., Gomes, J., et al. (2014). Production of ganoderic acid by Ganoderma lucidum RCKB-2010 and its therapeutic potential. Ann. Microbiol. 64 (2), 839–846. doi:10.1007/s13213-013-0723-9
Vince, R., Daluge, S., and Brownell, J. (1986). Carbocyclic purmocyin: synthesis and inhibition of protein biosynthesis. J. Med. Chem. 29, 2400–2403. doi:10.1021/jm00161a044
Wang, C., Liu, X., Lian, C., Ke, J., and Liu, J. (2019b). Triterpenes and aromatic meroterpenoids with antioxidant and neuroprotective activity from Ganoderma lucidum. Molecules 24 (23), 4353. doi:10.3390/molecules24234353
Wang, J., Cao, B., Zhao, H., and Feng, J. (2017). Emerging roles of Ganoderma lucidum in anti-aging. Aging Dis. 8 (6), 691. doi:10.14336/AD.2017.0410
Wang, K., Bao, L., Qi, Q., Zhao, F., Ma, K., Pei, Y., et al. (2015). Erinacerins C–L, isoindolin-1-ones with α-glucosidase inhibitory activity from cultures of the medicinal mushroom Hericium erinaceus. J. Nat. Prod. 78 (1), 146–154. doi:10.1021/np5004388
Wang, K., Wang, Z., Cui, R., and Chu, H. (2019a). Polysaccharopeptide from Trametes versicolor blocks inflammatory osteoarthritis pain-morphine tolerance effects via activating cannabinoid type 2 receptor. Int. J. Biol. Macromol. 126, 805–810. doi:10.1016/j.ijbiomac.2018.12.212
Wang, Y., Ouyang, F., Teng, C., and Qu, J. (2021). Optimization for the extraction of polyphenols from Inonotus obliquus and its antioxidation activity. Prep. Biochem. Biotechnol. 51 (9), 852–859. doi:10.1080/10826068.2020.1864642
Wasser, S. P. (2002). Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 60, 258–274. doi:10.1007/s00253-002-1076-7
Wei, W. K., Chi, M. J., Yang, Y. L., and Feng, T. (2023). Bisabolane and drimane sesquiterpenes from the fungus Trametes versicolor. Phytochem. Lett. 57, 73–77. doi:10.1016/j.phytol.2023.07.015
Weng, C. J., Chau, C. F., Chen, K. D., Chen, D. H., and Yen, G. C. (2007). The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain. Mol. Nutr. Food Res. 51 (12), 1472–1477. doi:10.1002/mnfr.200700155
Wold, C. W., Gerwick, W. H., Wangensteen, H., and Inngjerdingen, K. T. (2020). Bioactive triterpenoids and water-soluble melanin from Inonotus obliquus (Chaga) with immunomodulatory activity. J. Funct. Foods. 71, 104025. doi:10.1016/j.jff.2020.104025
Wu, S., Zhang, S., Peng, B., Tan, D., Wu, M., Wei, J., et al. (2024). Ganoderma lucidum: a comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci. Hum. Wellness. 13 (2), 568–596. doi:10.26599/FSHW.2022.9250051
Xu, X., Zhao, W., and Shen, M. (2016). Antioxidant activity of liquid cultured Inonotus obliquus polyphenols using tween-20 as a stimulatory agent: correlation of the activity and the phenolic profiles. J. Taiwan Inst. Chem. Eng. 69, 41–47. doi:10.1016/j.jtice.2016.10.011
Yadav, A. N. (2021). Microbial biotechnology for bio-prospecting of microbial bioactive compounds and secondary metabolites. J. App. Biol. Biotech. 9 (2), 1–6. doi:10.7324/JABB.2021.92ed
Yang, B. K., Jung, Y. S., and Song, C. H. (2007). Hypoglycemic effects of Ganoderma applanatum and Collybia confluens exo-polymers in streptozotocin-induced diabetic rats. Phytother. Res. 21 (11), 1066–1069. doi:10.1002/ptr.2214
Yazawa, Y., Yokota, M., and Sugiyama, K. (2000). Antitumor promoting effect of an active component of Polyporus, ergosterol and related compounds on rat urinary bladder carcinogenesis in a short-term test with concanavalin A. Biol. Pharm. Bull. 23 (11), 1298–1302. doi:10.1248/bpb.23.1298
Yilkal, T. (2015). Role of white rot fungi as a biological treatment of low-quality animal feeds. Sch. J. Agric. Sci. 5 (7), 247–255.
Zerva, A., Tsafantakis, N., and Topakas, E. (2020a). Production of biocatalysts and bioactive compounds from Greek basidiomycete wild strains grown in different induction media. Proceedings 2020, 1–7.
Zerva, A., Tsafantakis, N., and Topakas, E. (2020b). Greek basidiomycete wild strains for the production of bioactive compounds and enzymes with applications in cosmetic and biocatalysis industries. Chem. Proc. 1, 1–7.
Zhang, C., Fu, D., and Guo, M. (2019). Comparative and chemometric analysis of correlations between the chemical fingerprints and anti-proliferative activities of ganoderic acids from three Ganoderma species. Phytochem. Anal. 30 (4), 474–480. doi:10.1002/pca.2830
Zhang, C. C., Cao, C. Y., Kubo, M., Harada, K., Yan, X. T., Fukuyama, Y., et al. (2017). Chemical constituents from Hericium erinaceus promote neuronal survival and potentiate neurite outgrowth via the TrkA/Erk1/2 pathway. Int. J. Mol. Sci. 18 (8), 1659. doi:10.3390/ijms18081659
Zhang, H. N., and Lin, Z. B. (2004). Hypoglycemic effect of Ganoderma lucidum polysaccharides. Acta Pharmacol. Sin. 25 (2), 191–195.
Zhang, Y., Dai, L., Kong, X., and Chen, L. (2012). Characterization and in vitro antioxidant activities of polysaccharides from Pleurotus ostreatus. Int. J. Biol. Macromol. 51 (3), 259–265. doi:10.1016/j.ijbiomac.2012.05.003
Zhao, H., Eguchi, S., Alam, A., and Ma, D. (2017). The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. Am. J. Physiol. Lung Cell Mol. 312 (2), L155–L162. doi:10.1152/ajplung.00449.2016
Zhao, W., Huang, P., Zhu, Z., Chen, C., and Xu, X. (2021). Production of phenolic compounds and antioxidant activity via bioconversion of wheat straw by Inonotus obliquus under submerged fermentation with the aid of a surfactant. J. Sci. Food Agric. 101 (3), 1021–1029. doi:10.1002/jsfa.10710
Zheng, W., Miao, K., Liu, Y., Zhao, Y., Zhang, M., Pan, S., et al. (2010). Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl. Microbiol. Biotechnol. 87, 1237–1254. doi:10.1007/s00253-010-2682-4
Zheng, Y., Zong, Z. M., Chen, S. L., Chen, A. H., and Wei, X. Y. (2017). Ameliorative effect of Trametes orientalis polysaccharide against immunosuppression and oxidative stress in cyclophosphamide-treated mice. Int. J. Biol. Macromol. 95, 1216–1222. doi:10.1016/j.ijbiomac.2016.11.013
Zhou, X. W., Su, K. Q., and Zhang, Y. M. (2012). Applied modern biotechnology for cultivation of Ganoderma and development of their products. Appl. Microbiol. Biotechnol. 93, 941–963. doi:10.1007/s00253-011-3780-7
Ziaja-Sołtys, M., Kołodziej, P., Stefaniuk, D., Matuszewska, A., Jaszek, M., and Bogucka-Kocka, A. (2022). Low-molecular-weight secondary metabolites from fungi: Cerrena unicolor as a new proposal of an effective preparation against rhabditis nematodes. Molecules 27 (5), 1660. doi:10.3390/molecules27051660
Keywords: white-rot fungi, secondary metabolites, biologically active compounds, bioactive properties, therapeutic substances
Citation: Pinar O and Rodríguez-Couto S (2024) Biologically active secondary metabolites from white-rot fungi. Front. Chem. 12:1363354. doi: 10.3389/fchem.2024.1363354
Received: 30 December 2023; Accepted: 04 March 2024;
Published: 13 March 2024.
Edited by:
Raha Orfali, King Saud University, Saudi ArabiaReviewed by:
Abdallah M. A. Hassane, Al-Azhar University, EgyptIshtiaq Jeelani, University of California, San Diego, United States
Copyright © 2024 Pinar and Rodríguez-Couto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susana Rodríguez-Couto, c3VzYW5hLnJvZHJpZ3Vlei5jb3V0b0BsdXQuZmk=
 Orkun Pinar
Orkun Pinar Susana Rodríguez-Couto
Susana Rodríguez-Couto