- 1Department of Conservative Dentistry and Endodontics, Manipal College of Dental Sciences Mangalore, Manipal Academy of Higher Education, Manipal, India
- 2Department of Oral Pathology and Microbiology, Manipal College of Dental Sciences Mangalore, Manipal Academy of Higher Education, Manipal, India
The epoch of Nano-biomaterials and their application in the field of medicine and dentistry has been long-lived. The application of nanotechnology is extensively used in diagnosis and treatment aspects of oral diseases. The nanomaterials and its structures are being widely involved in the production of medicines and drugs used for the treatment of oral diseases like periodontitis, oral carcinoma, etc. and helps in maintaining the longevity of oral health. Chitosan is a naturally occurring biopolymer derived from chitin which is seen commonly in arthropods. Chitosan nanoparticles are the latest in the trend of nanoparticles used in dentistry and are becoming the most wanted biopolymer for use toward therapeutic interventions. Literature search has also shown that chitosan nanoparticles have anti-tumor effects. This review highlights the various aspects of chitosan nanoparticles and their implications in dentistry.
1 Introduction
Over the years, scientific progress in the field of biomedicine has paved the way for the evolution of newer nano-biomaterials which have proved to be progressively efficient and biocompatible. The process of extraction of marine-based nano-biomaterials is at its peak with lot of relevant research being conducted on the same (Bonderer et al., 2008; Ghosh and Urban, 2009). Recent literature search has shown that marine based nano-biomaterials like chitosan is used extensively in medical and dental fields (Ladet et al., 2008; Jones, 2010). As stated in the “European Commission’s Recommendation”, nanomaterial can be elaborated as a natural or synthetic material incorporated with particles, in a bound or unbound state, where 50% or more of the particles are in the range of 1–100 nm (Raura et al., 2020). One of the most commonly used nanoparticle in dentistry is silver (Ag) which has been used in contrasting forms of carbon substrates and ion-oxide species. Nanoparticles are procured with unique physiological and chemical properties like nano-size, better chemical wettability and reactivity and larger surface to volume ratio for better bonding characteristics (Boverhof et al., 2015). These properties of the nanoparticles have been immensely used in treatment of oral—health related problems like treatment of dentinal hypersensitivity, eradication of oral biofilms, diagnosis and treatment of oral cancers.
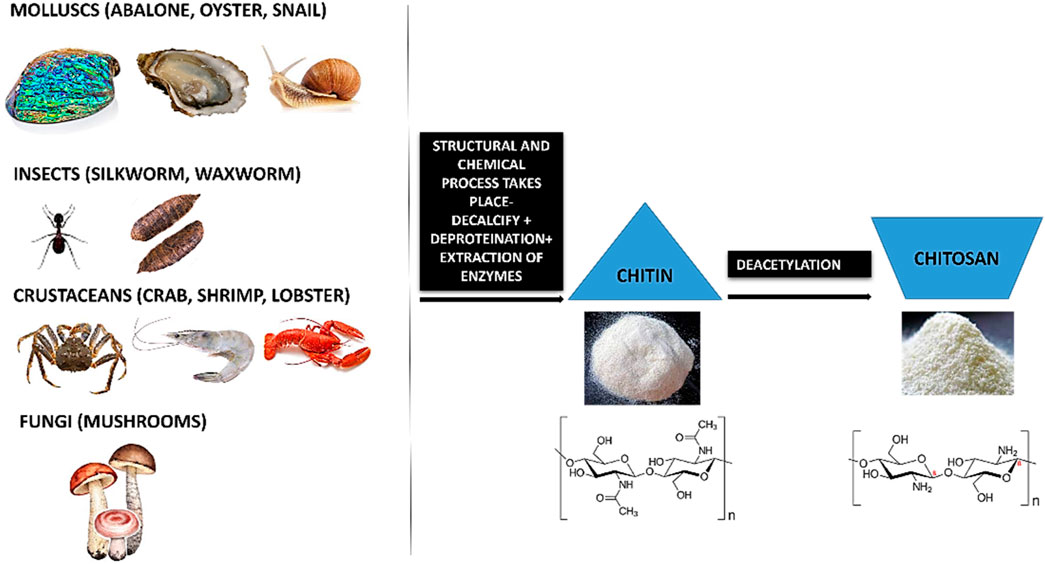
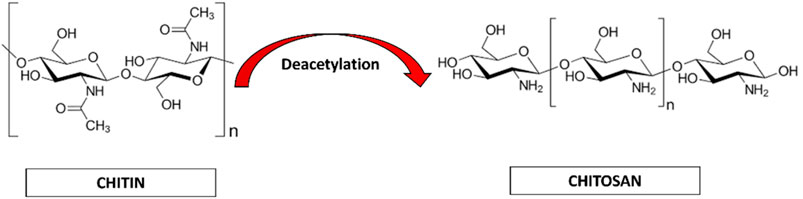
The chemical nature and properties of chitosan like biodegradability, non-toxicity and bioacompatibility lends it to be sustainable. Chitosan is a natural nanopeptide obtained from the purification of chitin which is the main ingredient found in the exoskeletons of marine crustaceans like crabs and prawns (Paul and Sharma, 2004; Younes and Rinaudo, 2015). Other major sources of chitin include fungi (Blumenthal and Roseman, 1957; Merzendorfer, 2011), insects like silkworm, waxworm, etc (Finke, 2007; Mohan et al., 2020), certain spore-bearing plants like mushrooms, fungi (Wu et al., 2004; Vetter, 2007; Ifuku et al., 2011; Nitschke et al., 2011) and molluscs like snail, oysters, etc (Kurita, 2001; Manni et al., 2010; Rasti et al., 2017; Taser et al., 2021). This macromolecule is processed by the repeated formation of D-glucosamine, which is further extracted from de-acetylation of chitin which is a byproduct of marine shells. During the manufacturing process, the shells and the exoskeleton of these marine creatures undergo de-proteinization to form insoluble chitin (CH) which is then converted to CS (Chitosan soluble under acidic conditions) by the removal of acetyl groups (Figure 1).
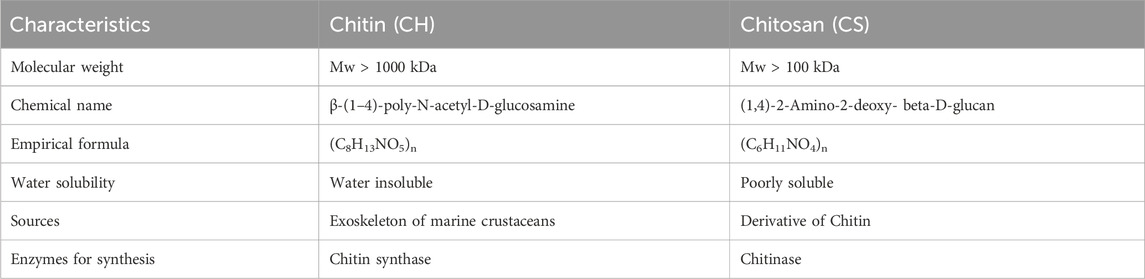
Chitin (CH) is considered as the second most abundant polysaccharide after cellulose (Elieh-Ali-Komi and Hamblin, 2016). This natural amino-polysaccharide copolymer is the building block of the exoskeleton of the marine crustaceans giving them durability and stability against the natural forces. Through enzymatic de-acetylation, chitosan (CS), derivative of chitin is formed. Chitosan is a natural fiber, analogous to cellulose and cannot be digested. This biomaterial is natural, biocompatible, hydrophilic and has a broad antimicrobial and antibacterial spectrum. These natural occurring biopolymers (Chitin and Chitosan) are profusely being used for biomedical applications. Table 1 summarizes the characteristic differences between chitin and chitosan (Imai et al., 2003; Eijsink et al., 2010; Azuma et al., 2015). Chitosan (CS) is composed of N-acetyl glucosamine and glucosamine polymer units (Figure 2) which is derived from Chitin (CH).
Chitosan (CS) has wide range of commendable properties that have been used as a marker for biomedical research. Chitosan has been shown to have positive response for osteo-conductivity when amalgamated with bioactive compounds like Poly-caprolactone (Hayashi et al., 2007; Ignatova et al., 2007; Sarasam et al., 2008; Fakhri et al., 2020). These distinctive characteristics have made a remarkable entry in the field of tissue engineering and biomedical research (Guo et al., 2006; Panahi et al., 2017; Farhadian et al., 2018; Shrestha, 2018). Additionally, Chitosan has also been used as a scaffold substrate for regenerative medicine (Shi et al., 2006; Vázquez et al., 2015) and substratum for growth factor delivery for wound healing (Caetano et al., 2015; Vijayan and Kumar, 2019).
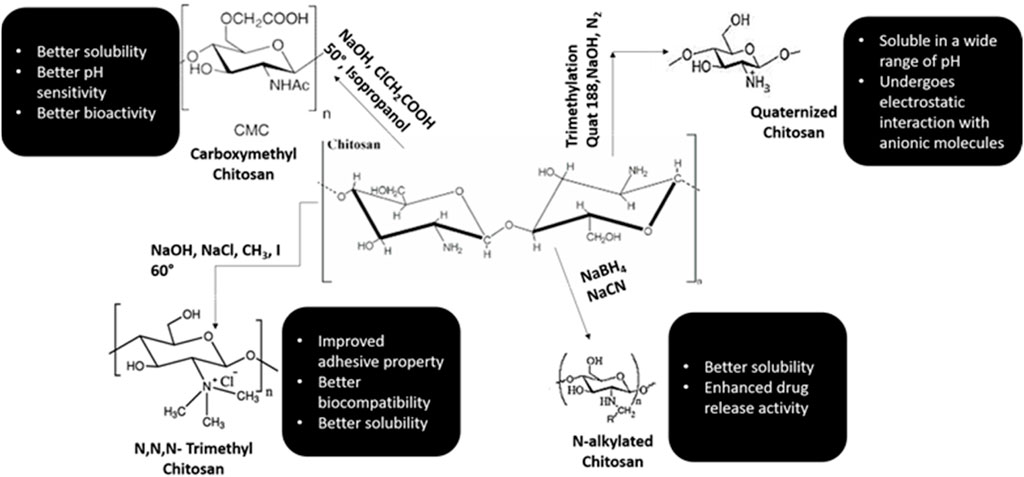
Chitosan is considered to be the only cationic poly-aminosaccharide which can be chemically altered based on the property and function (Fakhri et al., 2020). The degree of deacetylation has a strong influence on its physio-chemical-biological nature. Chitosan and it is by products like chitosan oligosaccharides are similar in nature. These nano-biopolymers can be broken down into simpler compounds through enzymatic process which are biocomapatible and based on required application, they can be modified through chemical or enzymatic reactions to variegated smaller conjugates and various forms like gels, fibers, and sponges (Xia et al., 2011; Qin and Li, 2020). Chitosan and its analogues based on their multitudinous features like cross-linking can be a vital source for the conglomerates of various biomedical materials (Pichayakorn and Boonme, 2013; Li et al., 2014).
Chitosan is well known for its antimicrobial activities, but there are several theories based to this property of chitosan (Rabea et al., 2003; De Carvalho et al., 2011). One theory explains that when chitosan comes in contact with bacterial cell wall, it displaces the calcium ions of the cell membrane resulting in destruction of the membrane (Yadav and Bhise, 2004). Various literature has shown that chitosan is an effective anti-plaque agent and enhances the periodontal health by minimizing the colonies formed by Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Prevotella intermedia (Ikinci et al., 2002; Hanes and Purvis, 2003; Akncbay et al., 2007). In animal studies, chitosan has manifested high potency of biocompatibility and shown to have positive response with implantation of nanomaterials (Levengood and Zhang, 2014; Oryan and Sahvieh, 2017; Li et al., 2021; Sivanesan et al., 2021). Various synthetic monologues of chitosan and their properties are categorized in Figure 3.
The framework of dental materials still holds a place for improvement and lot of research has been carried out for the scope of amelioration. The aim of this review is to highlight the applications of chitosan in dentistry and emphasize its importance in the treatment of various oral diseases. The bioactive properties of chitosan help in synthesis of various drugs and scaffolds for pulpo-dentinal regeneration. Chitosan has shown excellent oseteoconductivity, proliferation of osteoblasts, and mesenchymal cells thereby inducing in vivo neovascularization. (Kim et al., 2008; Costa-Pinto et al., 2011; Saravanan et al., 2013). Chitosan with the above-mentioned properties, makes it the most suitable component for tissue engineering. (Saranya et al., 2011; Sacco et al., 2018; Islam et al., 2020).
Chitosan is the only polycationic nanopolymer and its charge frequency hinges on the degree of acetylation and the pH condition of the media. The solubility of the chitosan depends on molecular weight and acetylation degree. High molecular weight chitosan molecules are easily soluble in acidic media. Hence enormous number of chitosan synthetic derivatives with increased solubility are produced. (Saranya et al., 2011).
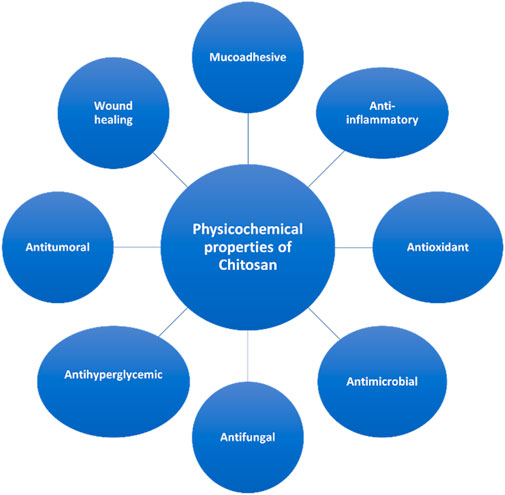
The physiochemical properties of Chitosan are shortlisted in Figure 4.
2 Bio-dental applications of chitosan and its derivatives
Chitosan distinctive properties like biocompatibility (Zhang et al., 2002; Rodrigues et al., 2012; Norowski et al., 2015; Elieh-Ali-Komi and Hamblin, 2016), bioactive nature (Saranya et al., 2011; Prabaharan, 2014; Ainola et al., 2016; Sacco et al., 2018; Islam et al., 2020), antifungal and antimicrobial (Goy et al., 2009; Sahariah and Másson, 2017; Hassan et al., 2018; Kim, 2018; Yilmaz, 2020), anticancer activity (Wimardhani et al., 2014a; Adhikari and Yadav, 2018; Kim, 2018; Alamry et al., 2020) and ability to whisk with other materials.
Chitosan is a natural biopolymer which is easily available and extracted from natural sources. Its biomedical nature makes it one of the most efficient natural nanoparticle which can be used in dentistry. The anti-inflammatory, antifungal, antibacterial, anodyne effect, mucoadhesiveness, osseintegrative property makes it most viable material to be integrated in dentistry. It was also observed that when chitosan was laser coated with chitosan, the osteoblastic activity was enhanced which helps in better remineralization effects. Chitosan, when modulated with apatite coating, enhanced the bioactivity of chitosan which improves the physiological response. (Zhang et al., 2002; Rodrigues et al., 2012; Norowski et al., 2015).
2.1 Classification of nanoparticles in dentistry
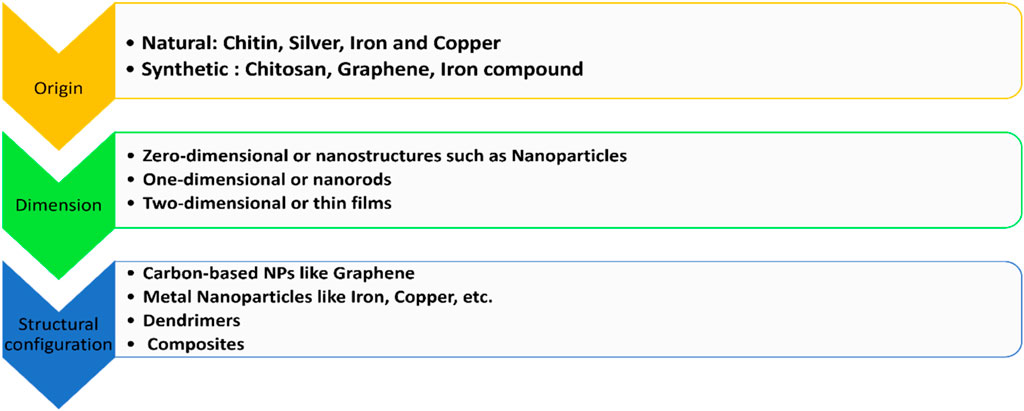
Nanoparticles are classified on the basis of origin, dimension, and structural configuration (Hassan et al., 2018; Raura et al., 2020). The classification is summarized in Figure 5.
2.2 Synthesis of nanoparticles in dentistry
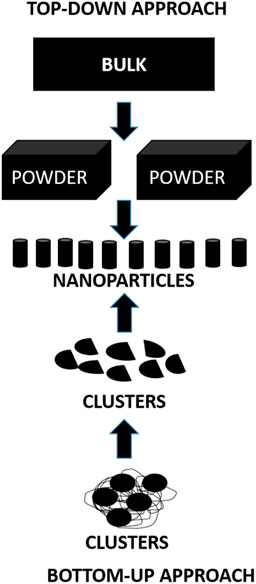
There are two main approaches for the synthesis of nanoparticles (Figure 6):
1) Top-down approach: In this approach the bulk of the material is made to shrink to a nanoscale structure with specialized treatments like grinding, ablation, etching, and sputtering. These techniques are used for manufacturing micron sized particles. This is a simpler technique involving miniaturization of the bulk material to a small sized structure with desired properties. The basic drawback of this technique is the imperfection of the surface architecture. Nanowires made by lithography is an example of top-down approach (Yanat and Schroën, 2021; Abid et al., 2022; Indiarto et al., 2022).
2) Bottom-up approach: In this latter approach the material is made to undergo chemical reactions. This technique is economical, and boasts of reduced wastage of the material. This refers to building-up of the material; i.e., atom-by-atom, molecule-by-molecule, or cluster-by cluster. Some of the well-known bottom-up techniques are organo-metallic chemical route, revere-micelle route, sol-gel synthesis, colloidal precipitation, hydrothermal synthesis, template assisted sol-gel, electrodeposition etc. Luminescent nanoparticles are an example of bottom-up approach (Yanat and Schroën, 2021; Abid et al., 2022; Indiarto et al., 2022).
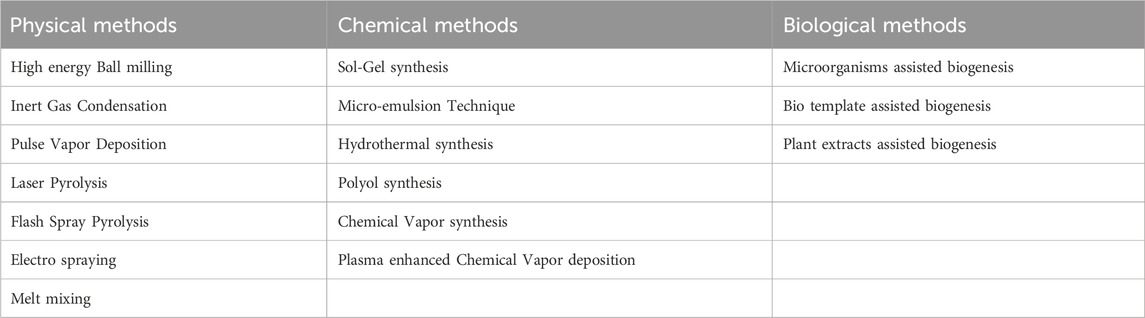
Besides these, there are some physical, chemical and biological methods for synthesis of nanoparticles which are enlisted in Table 2 (Yanat and Schroën, 2021; Abid et al., 2022; Indiarto et al., 2022).
Chitosan is cationic polymer and is efficacious against fungi and bacteria. This microbial action is attributed to the reactive hydroxyl groups at the C-3 and C-6 positions, the structure, physicochemical traits, and environmental factors of chitosan. Chitosan with it is High-MW, potential antimicrobial effects included serving as a chelator of critical metals, inhibiting nutrients from being taken up extracellularly from cells, and changing cell permeability because it is typically unable to permeate the cell wall and cell membrane. Nevertheless, low-MW chitosan affects RNA, protein synthesis, and mitochondrial function in addition to having extracellular and intracellular antibacterial activity. Moreover, the kind of bacteria that chitosan is targeting greatly influences its manner of antimicrobial action. (Ke et al., 2021).
1) Antimicrobial Activity against Bacteria
The cell wall structures of Gram-positive and Gram-negative bacteria differ significantly; Gram-positive bacteria have thicker peptidoglycans, whereas Gram-negative bacteria are more abundant in lipopolysaccharides (LPS). Because LPS is frequently linked to phosphorylated groups, Gram-negative bacteria have a greater negative charge than Gram-positive bacteria. When the pH of the surrounding environment is lower than 6.5, cationic chitosan can attach to phospholipids on more negatively charged cell surfaces. Gram-negative bacteria may be more sensitive to chitosan than Gram-positive bacteria, according to certain theories.
Gram-positive bacteria’s teichoic acids are negatively charged as well because of the phosphate groups that are present in their structure. Nevertheless, Staphylococcus aureus developed a greater resistance to chitosan with loss of the teichoic acid production pathway, suggesting that chitosan’s mechanism of action involves more than just electrostatic interactions. Interestingly, research have shown that DNA transcription can be inhibited by chitosan (≤50 kDa) that can penetrate the cell wall. Therefore, even though chitosan’s molecular size (MW) is crucial for targeting, chitosan’s structure—rather than its MW—determines whether it has extracellular, intracellular, or both extracellular and intracellular antibacterial action.
2) Antimicrobial Activity against Fungi
Chitosan has been demonstrated to have fungicidal effects on a variety of human and plant fungal diseases. The way chitosan interacts with the cell wall or membrane is mostly responsible for its antifungal qualities. However, the MW and level of deacetylation (DDA) of chitosan, the pH of the solvent, and the kind of fungus being targeted are all strongly correlated with the minimum inhibitory concentrations (MICs) of chitosan against fungi. Additionally, it has been suggested that there may be a positive correlation between the amount of unsaturated fatty acids on the cell membrane and chitosan susceptibility. This is because a higher amount of unsaturated fatty acids promotes improved membrane fluidity, which increases the negative charge on the membrane. The contrasting traits between chitosan-resistant and chitosan-sensitive The presence of unsaturated fatty acids in cell membranes is associated with strains of Neurospora crassa. Low-MW chitosan has the ability to pierce both the cell surface and wall, which inhibits the creation of proteins and DNA/RNA.
2.3 Applications of chitosan in dentistry
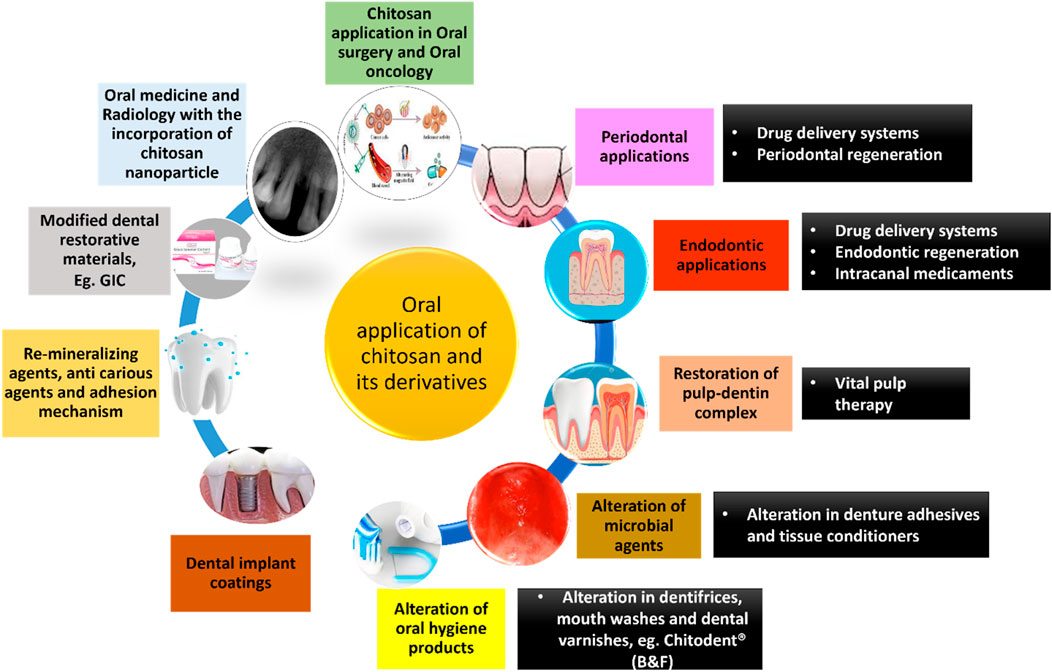
Chitosan has a plethora of applications in the field of dentistry. A schematic diagram summarizing the same has been given below (Figure 7). (Agrawal et al., 2023; Arora et al., 2023; Nava Juárez, 2023; Paradowska-Stolarz et al., 2023; Qu et al., 2023; Minervini et al., 2024)
2.3.1 Oral drug delivery systems
A lot of research has been conducted to confirm the potentiality of chitosan as an oral drug carrier. The basic aim of drug delivery analogues is to produce controlled and sustained release of drugs with prolonged contact time for a specified target with reduced dosage. This results in improving the drug efficacy and reduced side effects of systemic administration (Zivanovic et al., 2007; Priyadarsini et al., 2018). Research has shown that Chitosan-based composites (CBCs) are used to make full-bodied drug delivery carrier systems that have high mechanical strength; maintain good contact time and sustain release of the drug when they are in close contact with the oral mucosa. Chitosan based composites have been used in treatment of oral diseases (Dhand et al., 2015; Huang et al., 2016; Jeyaraj et al., 2019; Sanap et al., 2020).
The Food and Drug Administration (FDA) has approved Chitosan to be used as food supplement. Oral Chitosan nanospheres are non-toxic in nature and help in prolonged activity of drug at the site of pathology (Saboktakin et al., 2010; Stenhagen et al., 2019; Hu et al., 2020). Chitosan has been shown to have promising ingredients for drug delivery of various organic molecules like DNA, RNA, and various growth factors (Zhang et al., 2011; Soran et al., 2012; Abdel Mouez et al., 2014; Ding et al., 2017).
The proton-amino groups on D-glucosamine of the CS microstructure undergoes electrostatic linkage with negatively charged mucus layer and then invades the deepest layers of the epithelium (Kumari and Singh, 2013; Singh et al., 2013). Based on the mucoadhesive property, chitosan can be used as vehicle for drugs which are administered through various routes like nasal, buccal, ocular, and pulmonary (Keegan et al., 2012; Wang et al., 2013). The insoluble nature of chitosan can be modified through chemical alterations like carboxymethylation, acetylation, thiolation, quaternization, etc (Aguilar et al., 2019; Bakshia et al., 2019). Modified chitosan such as Quaternized chitosan with positive ions, thiolated chitosan obtained by chemical alteration of amino groups with thioglycolic acid, carboxymethyl chitosan and N-acylated chitosan are profusely used in pharmaceutical industry due to increased solubility, high pH, high mucoadhesive property and improved drug penetration (Khutoryanskiy, 2011; Bernkop-Schnürch and Dünnhaupt, 2012; Mansuri et al., 2016; Bakshia et al., 2019). CS based drug delivery systems are highly used in the treatment of dental caries, periodontitis, pulp space therapies and prolonged anesthesia.
The adverse effects of systemic administration of drugs have resulted in poor patient compliance; so, to overcome this, local drug deliveries for periodontal pockets has come to the forefront. Local drug delivery systems permit prolonged release of the drug in periodontal pockets with a long contact time resulting in better treatment outcome (Chen et al., 2017; Zhang et al., 2018a; Wang et al., 2020). These systems act as an adjunct to oral prophylaxis and decrease the systemic adverse effects.
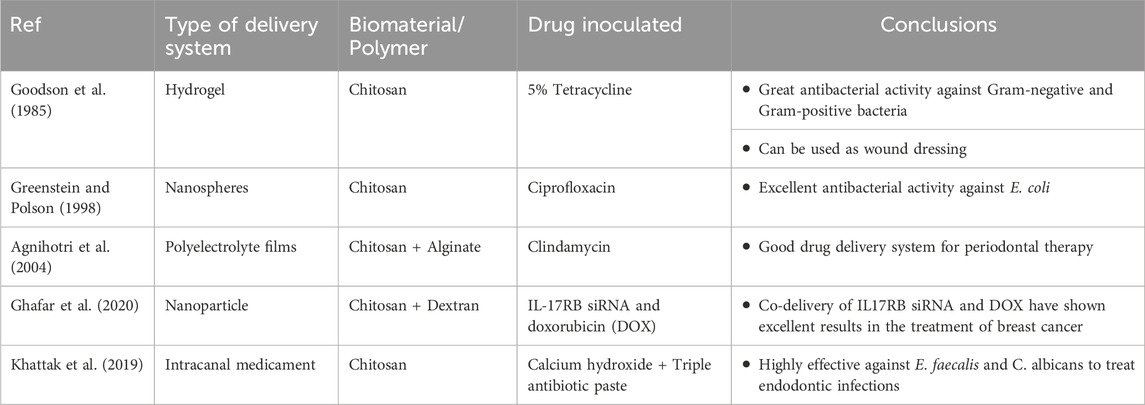
Chitosan nanoparticles have been shown to have anti-inflammatory effects on human gingival fibroblasts by reducing the number of receptors on inflammatory cytokines and chemokines such as IL-1β, TNF-αand CXCL-8. Various studies have shown that human gingival fibroblasts tend to have increased metabolic ability and cellular viability in the presence of chitosan nanoparticles which help in reconstitution of gingival tissue (Goodson et al., 1985; Mahmood et al., 2017; Shen et al., 2017).
Chitosan microspheres are spherical patches ranging from few micrometers to 1000 µm and contain medicinal agents in a polymerized matrix. These patches protect against salivary digestive enzymes and can be applied in mucosal membranes and sub-gingival sites (Greenstein and Polson, 1998; Joshi et al., 2016). Recently, there are micro-formulations of chitosan with anionic bio-particles like alginate, xanthan gum, hyaluronic acid and pectins. These micro-formulations are termed as polyelectrolyte complexes (PEC) which provide sustained release of drugs and are less toxic when compared to cross-linked polymers (Agnihotri et al., 2004; Sinha et al., 2004; Arancibia et al., 2013; Silva et al., 2013; Babrawala et al., 2016). Yadav and co—workers in a study used chitosan, calcium and sodium alginate combination microspheres to contain antibiotics like ornidazole and doxycycline and proved the efficacy, muco-adhesive activity and biodegradability of these microspheres (Yadav et al., 2018). Many attempts to combine microspheres and hydrogel to provide two-layered barrier system has been tried (Zhao et al., 2014).
Chitosan nanoparticles have propitious results when treating oral pathologies. These group drug delivery devices benefit from the small particulate size and the innate characteristics of the polymer. Owing to sizing of the nanoparticles, these particles can penetrate through impervious barriers and also contact the tissues over a larger surface area (Fakhri et al., 2020). These chitosan nanoparticles protect the gastrointestinal tract from enzymatic degradation and acidic environment (Wang et al., 2011; Alcaraz et al., 2016). The beneficial aspects of chitosan nanoparticles encapsulated with antibiotics like doxycycline (Madi et al., 2018; Hu et al., 2019), silver nanocrystals (Xue et al., 2019), tetracycline (Parsa et al., 2019) and ciprofloxacin (Zhao et al., 2013) have been studied and confirmed. A lot of research is conducted on chitosan/PLGA (Polylactic co-glycolic acid) nanoparticles and has shown to have improved stability, enhanced drug release and non-toxic behavior (Lu et al., 2019). Chitosan laden PLGA, Lovastatin and Tetracycline nanoparticles have been explicated and have been revealed to induce osseous formation, alkaline phosphatase activity, biocompatibility, antibacterial activity and controlled and sustained release of these drugs to treat existing pathological condition (Lee et al., 2016).
Chitosan nanofilms are gaining popularity and are effectively used in the interproximal pockets (Salari et al., 2018). The advantages of films as drug delivery devices include biodegradability and ease of placement without intervening with daily activities. The films can be altered and adjusted according to the size of the defect site (Soskolne, 1997). The placement of films in the oral cavity gets disturbed due to saliva and its lubricating efficacy. Muco-adhesiveness is considered to be a pre-requisite property for the manufacturing of nanofilms. In a study by Ghafar et al., thiolated chitosan based nanofilms were introduced for the release of calcium fluoride for dental caries and for the release of lignocaine for diminishing pain. Based on this study, it was found that the thiol groups from the films get released and interact with the oral mucosa for long duration increasing the contact time of the drug, and also regulating the release of fluoride. Hence, thiolated chitosan based nanofilms can be suggested for oral problems (Ghafar et al., 2020). Chitosan–alginate complex films are durable, have improved physical and mucoadhesive properties. These films are said to have higher alginate content with increased concentration which aids in slow drug release (Kilicarslan et al., 2018). CS-alginate films have been used to incorporate natamycin and silver nanoparticles (da Silva et al., 2013). Chitosan laden with risedronate and zinc hydroxyapatite (CRZHF) films are introduced for treatment of periodontitis as anti-resorptive medicament. These CRZHF films are flexible and have good mucoadhesive strength resulting in hard detachment after the placement of the film into the periodontal pockets. Clinical trials with CRZHF have shown increased alkaline phosphatase activity, resulting in bone formation and improved successful treatment outcome (Khajuria et al., 2018). Chitosan-based films with local anesthetics have been implemented to relieve pain and discomfort in patients. Chitosan/collagen films incorporated with lidocaine, tetracaine and benzocaine for effective delivery of local anesthetics have also been tried. These films have good mucoadhesion, and improved flexibility (DiMartino et al., 2019).
Another form of drug delivery systems are chitosan gels. These gels include drug macromolecules incorporated into the polymeric structure and help in controlled and sustained release of the drug. These hydrogels are manufactured by chemical crosslinking to form permanent bonds and physical crosslinking to form provisional bonds. These cross-linked hydrogels have good viscosity, high mucoadhesive properties, better injectability and prolonged release of the drug at the site (Bhattarai et al., 2010). Chitosan gels combined with 15% metronidazole have been used as an adjunct to mechanical debridement for periodontitis to improve the treatment outcome (Akıncıbay et al., 2007). Recently, thermosensitive gels have been introduced into dentistry; these hydrogels reform their gelation nature according to alterations in temperature and display sol-to gel transformation when administered into the body. The first thermosensitive hydrogel composed of chitosan, quarternized chitosan and β-glycerophosphate was introduced against periodontal pathogens like P. gingivalis and P. intermedia (Akncbay et al., 2007; Ji et al., 2010). Chitosan, β-GP hydrogel in injectable form packed with ornidazole and BMP-7 was used for periodontal regeneration of furcation defects (Bansal et al., 2018). Bacterial plaque causes low pH environment in the oral cavity which can alter the release of the drugs from the hydrogel in situ. To overcome this, pH sensitive hydrogels like N-carboxymethyl chitosan-based hydrogel, injectable chitosan-grafted-dihydrocaffeic acid/oxidized pullulan hydrogel, etc. have been introduced. These hydrogels are pH- dependent and exhibit good swelling behavior, drug release and muco-adhesiveness. These hydrogels are well-known for cancer therapy and tumors which create an alteration in pH in the oral environment (Liang et al., 2019). Hydrogels which control the drug release have been found to be very efficient with characteristics like eletro-responsiveness and pH sensitivity and are manufactured by implanting polyaniline onto chitosan and crosslinking with oxidized dextran (CS-P/DO). These hydrogels are cyto-compatible and biodegradable (Alinejad et al., 2016).
Chitosan fibers also form one of the recently introduced drug delivery systems. Chitosan has known to have high fiber-forming property. Chitosan fibers are constructed through electrospinning process producing fibers ranging from nanometers to micrometers. CS fibers are extravagantly used for neural and osseous tissue engineering. These fibers tend to have low molecular weight; hence they are reticulated with epichlorohydrin, hydroxyapatite, PLGA, poly-caprolactone, cellulose, polyvinyl alcohol, etc. (Wei et al., 1992; Tanir et al., 2014). The Electrospun chitosan fibers with drug are potentially used in guided tissue engineering. Chitosan-Polycaprolactone cross-linked with metformin membrane stimulates bone formation, alkaline phospahatase activity and mineralization of bone mesenchymal stem cells (Zhu et al., 2020). Chitosan- Gelatin nano-carriers laden with calcium hydroxide are used for endodontic infections for sustained release of calcium hydroxide for longer duration. The combination of chitosan with calcium hydroxide has shown excellent antibacterial activity against endodontic pathogens like Enterococcus faecalis (Shaik et al., 2014; Malinowska et al., 2017). Table 3 mentions a list of a few of the many studies on nanoparticle drug delivery modes with antibiotics.
2.3.2 Alteration of antimicrobial agents
The positively charged amino groups of N-acetyl glucosamine combines with negatively charged ions of the bacterial cell wall containing lipids, phospholipids, carbohydrates and proteins (Kong et al., 2010). This basis remains the same for fungal and viral micro-organisms. The underlying mechanism for the antibacterial action of chitosan is still not clear, but the theories state that the amino groups interact with the negatively charged particles of bacterial cell wall leading to cell wall leakage, increasing the permeability and ultimately leading to cell destruction (Matica et al., 2019). Another theory for the mechanism of action of chitosan believed, is that the low molecular weight of chitosan can penetrate the bacterial cells and impede the bacterial activities like RNA and protein synthesis. High molecular weight molecules of chitosan particles >100 kDa, tend to deposit a polymer material around the cell membrane and cuts down the nutrient supply (Younes et al., 2014). However, the bio-properties of chitosan are magnified by decreasing the deacetylation process and modifying the pH of the environment (Lim and Hudson, 2004; Li et al., 2016).
On the whole, intensifying the positive charges of chitosan molecules increases the electrostatic reciprocity with the cellular contents and thereby increases the antimicrobial efficacy of chitosan. Certain cross-linking methods like carboxymethylation, sulfonation, quaternization, and phosphorylation enhance the solubility of chitosan and increase its antimicrobial efficiency (Lim and Hudson, 2004; Xu et al., 2011). Quaternized chitosan and its derivatives have been extensively studied on the aspects of their antimicrobial efficacy and shown to have the best results (Zhang et al., 2002; Ignatova et al., 2007; De Carvalho et al., 2011; Wang et al., 2016; Xu et al., 2018). Ammonium salts of Chitosan are shown to be highly effective against E. coli and S. aureus (Kim et al., 1997).
Chitosan and its compounds are effective on fungal and bacterial substrates. Congregation of literature has divulged the efficacy of chitosan film on medical instruments against contamination (Ghosh et al., 2011; Islam et al., 2019). Based on the efficacy, chitosan can be cross-linked to tissue conditioners or denture adhesives and help in the prevention of denture stomatitis (Lee et al., 2018; Namangkalakul et al., 2020). Tissue conditioners made of chitosan and chitosan oligosaccharide are excellent alternatives for treatment of denture stomatitis. High anti-microbial property and water solubility of Chitosan oligosaccharide makes it an ideal choice for reducing Candida albicans infections. Tissue conditioners made of quaternized chitosan can be used as provisional lining materials for treating denture stomatitis (Saeed et al., 2019).
Chitosan has played a vital role in the treatment of endodontic infections. Calcium hydroxide cross-linked with chitosan as intracanal medicament has been effective in reducing the periapical infections. Chitosan has anti-biofilm property which aids in controlling the microbial load and effective against E. faecalis, Streptococcus mutans, and various other microbes (Elshinawy et al., 2018; del Carpio-Perochena et al., 2017; Loyola-Rodríguez et al., 2019; Ganss et al., 2011; Young et al., 1997; Ganss et al., 2014). Antibacterial efficacy of endodontic sealers incorporated with chitosan has been seen to be effective for longer periods of time (Pini et al., 2020).
2.3.3 Alteration of oral health products
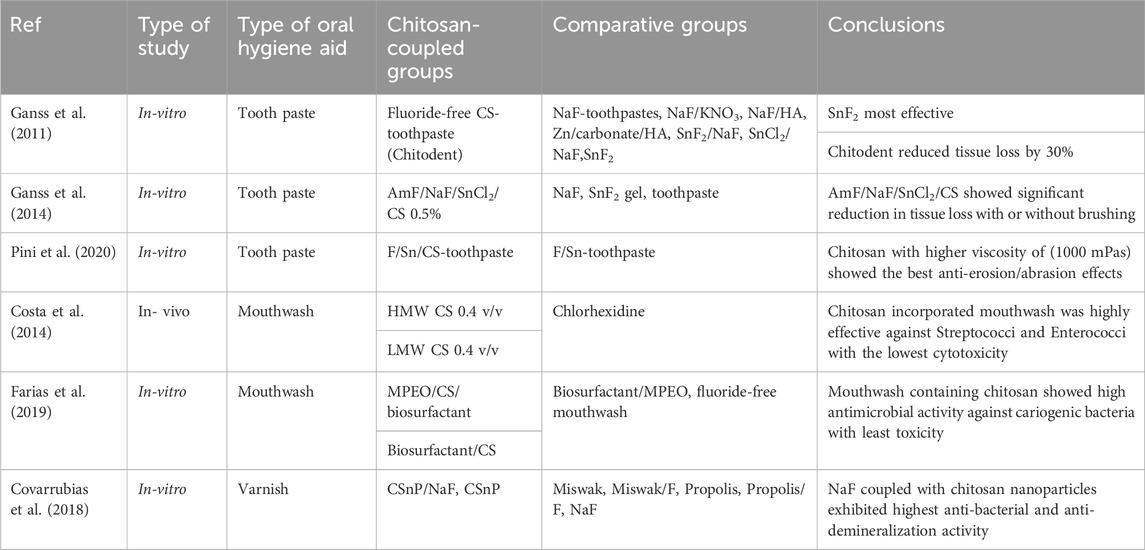
Use of oral hygiene products like toothpastes, brushes and mouth wash plays a very key role in oral hygiene maintenance. Dentifrices are known to fend off the demineralization effects of tooth substrate due to acidic drinks. Numerous dentifrice formulations have been studied and enlisted in table 4. Various toothpaste preparations containing nanoparticles with hydroxyapatite, 5% KNO3, etc., aim at providing fluoride (F) release towards tooth re-mineralization of enamel substrate. In a study by Ganss et al., a non-fluoride, chitosan-based dentifrice (Chitodent® (B&F)) was investigated and showed significant reduction in tooth tissue loss (Costa et al., 2014). This was attributed to the cationic character of chitosan combined with low pH, and affinity to bind to negatively charged structures like enamel and dental biofilm. The presence of nanoparticles like chitosan helps in formation of organic protective layer over the mineralized structures (Costa et al., 2014; Farias et al., 2019; Pini et al., 2020). Certain toothpastes containing Strontium (Sr) and potassium nitrate have shown to diminish the erosion of dentin (Covarrubias et al., 2018). F/Sr containing toothpastes combined with chitosan tend to have anti-erosive and anti-abrasive properties. Pini et al., in their study concluded that increasing the viscosity of chitosan to F/Sr toothpaste, helps in complete inhibition of enamel tissue loss thereby retaining the enamel surface (Costa et al., 2014).
Recently, a lot of research has been involved in the manufacture of chitosan-based oral hygiene maintenance products like mouthwash, varnishes, nanogels (Table 4). Costa and his co-workers, studied the efficacy of chitosan based mouthwashes on biofilm formation and microbial attachment of E. faecalis, C. albicans, S. mutans and P. intermedia and concluded that these mouth washes are effective in controlling dental caries, periodontal problems and fungal infections. Various formulations of mouthwashes like Mentha piperita essential oil (MPEO) with chitosan have shown to have prolonged anti-caries effects. Currently, a new lineage of anti-carious products containing combinations of metallic compounds like silver or copper with chitosan have been experimented and documented that chitosan undergoes electrostatic interaction with tooth structure and bacterial cell wall, thereby escalating anti-biofilm property (Arnaud et al., 2010; Costa et al., 2014; Zafar and Ahmed, 2014; Wassel and Khattab, 2017; Covarrubias et al., 2018; Farias et al., 2019).
2.3.4 Re-mineralization of enamel
Restoration of lost enamel is one of the most challenging tasks in dentistry since enamel is formed only once in the human body and is avascular (Zhang et al., 2018b). Various bioactive dental materials have been developed for regeneration of enamel but till date majority of them are disbelieving. Chitosan structure gets protonated with free amino groups and creates a positive charge around it. This property helps chitosan to bind to negatively charged structures like tooth enamel. In addition, chitosan can invade into the deeper layers of enamel producing mineral content and thereby helping in re-mineralization of carious lesions. Hence, chitosan-based materials help in re-mineralization of lost enamel and prevent the progression of carious lesions (Ren et al., 2019; Zhang et al., 2019). In a study, it was demonstrated that Chitin-bioglass complex helps in deposition of mineral content and refines the eroded or carious enamel surface; improves the microhardness of surface and sub-surface areas. Chitosan nanoparticles coupled with amelogenin in the form of hydrogel help calcium and phosphate ions to reorganize and form enamel like structure. (Ren et al., 2019). The above-mentioned hydrogel has got excellent anti-bacterial and re-mineralization capacities.
2.3.5 Bonding to tooth structure and adhesion mechanism with chitosan
The resin-dentin bond and the longevity of the bond strength has received a lot of attention and is being researched upon. Dental materials like composites are used for bulk filling and dentin replacement which tend to cause polymerization shrinkage and involves technique sensitive steps like acid etching and bonding, and removal of smear layer (Murray et al., 2003). When there is incomplete removal of smear layer, the resin monomer does not flow in properly resulting in polymerization shrinkage and ultimately microleakage (Chen et al., 2003). To overcome this demerit, chitosan hydrogels and polymeric bio adhesives have come into demand. In a study, chitosan hydrogels incorporated with propolis, β carotene and nystatin were evaluated and shown to have increased shear bond strengths over a period of time. The shear bond strengths with respect to chitosan hydrogel have been deemed higher than conventional dentin-bonding systems (Perchyonok et al., 2013).
2.3.6 Chitosan coated dental implants
The extent of osseo-integration of dental implants with alveolar bone marks the clinical outcome of the dental implants (Javed et al., 2014; Alghamdi, 2018). To improve the osseo-integration effects, numerous methods such as chemical surface treatments, and surface coating for implants have been tried and shown favorable results (Najeeb et al., 2016; Guglielmotti et al., 2019). Various bioactive layering on the surface of implants have resulted in improved bone health and osseo-integration in immunocompromised patients (Abtahi et al., 2012; Pang and Huang, 2012; Diz et al., 2013; Javed et al., 2014; Najeeb et al., 2017; Duarte et al., 2021). Multitude of studies have highlighted the beneficial aspects of chitosan coatings for dental implants (Redepenning et al., 2003; El Nady et al., 2017; Li B. et al., 2019; Alnufaiy et al., 2020; Yilmaz, 2020). The chitosan coating on dental implants tends to decrease the surface roughness, hydrophilicity which helps in apaptite formation, cell adhesion and proliferation thus increasing the bioactivity. Chitosan bio-coatings show excellent biocompatibility, no cytotoxicity and better antibacterial activity. It is also stated that increased thickness of chitosan coating on dental implants prolonged the antibacterial activity (Norowski et al., 2011). Literature has also reported that chitosan layering on dental implants reduce stress concentrations by changing the elastic modulus of the bone-implant interface (Levengood and Zhang, 2014). In addition, research has shown that antibiotics can be incorporated on the chitosan coatings for better healing around the implant area (D’Almeida et al., 2017; Cohenca et al., 2013). Antibacterial coatings on implants and medical devices have shown to have good clinical success, but still further investigation is required.
2.3.7 Chitosan modified dental restorative materials
Over the recent years, a lot of noteworthy research has been tried and tested to create opportunities for bioactive dental materials. The level of destruction to the pulpo-dentinal complex dictates the treatment prognosis with the use of biomimetic materials (Drummond, 2008). However, certain flaws have been delineated which include poor adhesion, less mechanical strength when compared to ceramic or resin-based materials (Wimardhani et al., 2014a). Owing to these disparities, poor interfacial bond is created between the tooth and the material resulting in microleakage (Bentley and Drake, 1986; Ho et al., 1999).
Glass ionomers (GI) are tooth colored restorative materials comprising calcium fluor aluminosilicate glass powder and polyacrylic acid liquid. GIs bond chemically to the tooth structure and exhibits anticariogenic property with the release of fluoride (Forsten, 1994). There are numerous applications of Glass ionomers like luting/cementation of crowns and bridges, restorative materials for class V lesions and non-carious lesions like erosions, sandwich technique for class II restorations, core build-ups, atraumatic restorative treatment, lining material under composite restorations and pit and fissure sealants (Sidhu and Nicholson, 2016). Despite all the advantages, GIs have certain limitations like poor mechanical strength, low fracture toughness, low abrasion resistance and poor esthetics. Hence the application of conventional glass ionomers on high stress bearing areas is avoided (Khoroushi and Keshani, 2013). To overcome these discrepancies, experimentation has been done to integrate bioactive polymers into restorative materials and modify their properties.
Currently, nanoparticles like chitosan have been coupled with glass ionomers to modify their mechanical and antibacterial properties. The anionic groups of polyacrylic acid of Glass ionomers co-agglomerate with cationic amine groups of chitosan to form interpenetrating polymerized network. It can be predicted that inclusion of nano-chitosan particles to Glass ionomer materials would enhance the mechanical strength and anti-cariogenic property for high stress bearing area applications (El-Negoly et al., 2014; Senthil et al., 2017). It has also been proved that application of chitosan to liquid component of GIs improves the chemical adhesion and antibacterial activity (Soygun et al., 2021). Chitosan modified GIs have been clinically accepted as a root coverage material for gingival recession which promises minimal to no-genotoxicity and cytotoxicity effects and enhances the proliferation of human gingival fibroblasts (Zhou et al., 2019). It is also noted that chitosan modified GIs helps in sustained release of proteins, growth factors and bioactive polymers which is apt for vital pulp and regenerative therapies. In a study, it was reported that ion release from Chitosan modified Glass ionomers was advantageous to the tooth substrate (Mulder and Anderson-Small, 2019), and also helps in sustained release of proteins without any cytotoxic effects to pulp cells (Limapornvanich et al., 2009). Various bio-molecules like Tumor Necrosis factor, growth factors, peptides, TGFβ-1 can be applied to chitosan modified GICs to stimulate pulp regeneration (Rakkiettiwong et al., 2011).
Enormous amount of research is being carried out to modify the physical properties of various dental materials. Recently, chitosan-based nanocomposites have been developed and are claimed to have superior features like strength, heat stability, electrical conductivity, photoluminescence, antimicrobial, and biomedical features which increase the longevity of dental restorations (Diolosà et al., 2014; Kausar, 2021). In addition, chitosan modified zinc oxide eugenol cements, root canal sealers have also been investigated (Dragland et al., 2019).
2.3.8 Chitosan and pulpal regeneration
Regeneration of dental pulp is one of the most unpredictable situation in dentistry. Tissue engineering approaches like stem-cell based transplantation have been experimented to simulate the dentin-pulp complex. Stem cells are harvested from various sources like dental pulp stem cells (DPSCs), stem cells from human exfoliated deciduous teeth (SHEDs) and stem cells from apical papilla (SCAP) (Huang et al., 2008). These cells have highest potential of differentiating into odontoblast like cells, high proliferation capability, and multi-cell differentiation capacity. DPSCs are easily extracted from human permanent or deciduous teeth. Transplanting human DPSCs onto the chitosan scaffold for pulpo-dentinal complex regeneration is still under research. However, cell homing via DPSCs seem to have promising results in regenerative endodontics. Some of the clinical trials have shown to form connective tissue similar to dental pulp with neovascularization and in some cases, dentin deposition was also noticed (Eramo et al., 2018).
The stimulation of mesenchymal stem cells to form odontoblastic cells forms the basis for endodontic regeneration. It has been reported that porous chitosan scaffolds when coupled with bioactive molecules like growth factors and peptides, help in release of odontoblastic markers like dentin sialo-phosphoprotein, alkaline phosphate, and dentin matrix acidic phosphoprotein which form an extracellular polymerized matrix which acts as niche for the multiplication and proliferation of DPSCs into odontoblastic cells resulting in mineralization process (Soares et al., 2018; Bakopoulou et al., 2019; Raddall et al., 2019; Moreira et al., 2021). In addition, Chitosan scaffolds enriched with signaling molecules like BMP 1and7, vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BNF), fibroblast growth factor (FGF) and drugs like simvastatin and metformin help in inducing reparative dentin formation by promoting cell adhesion and proliferation of DPSCs (Guo et al., 2006; Jingwen et al., 2016; Schmalz et al., 2017; Retana-Lobo, 2018; Soares et al., 2018; Yang et al., 2020). Biomolecules like TGF β-1 when loaded with chitosan are proving to be excellent alternatives to calcium hydroxide as a pulp capping material since it regulates the differentiation of odontoblastic stem cells, alkaline phosphatase, OCN gene/protein expression and bio-mineralization (Farea et al., 2014; Abbass et al., 2020). SCAP/carboxymethyl chitosan/TGF-β1 scaffold is also reported to be favorable for pulp regeneration as it releases CS nanoparticles, dentin matrix protein-1 and dentin sialo-phosphoprotein (Bellamy et al., 2016).
Zhu and his co-workers brought a breakthrough in the field of vital pulp therapy. They introduced injectable Ag-BG/chitosan thermosensitive chitosan hydrogels which promote odontogenic regeneration due to their viscosity, flexibility and thermosensitive nature (Zhu et al., 2019). These hydrogels possess high antibacterial and anti-inflammatory potential and the chitosan release odontogenic markers like PGE2, TNFα, IL-1β, −6 and −8 which are ideal for endodontic regeneration (Silva et al., 2013; Aguilar et al., 2019; Zhu et al., 2019). Another innovation reported with the development of pulp regeneration was chitosan-cellularized fibrin hydrogel which led to proliferation of collagen type I and dental pulp mesenchymal cells producing 3-D collagenous network analogous to innate pulpal tissue (Ducret et al., 2019). Qin et al. in their study inoculated chitosan–metformin with Calcium phosphate cements to enhance the strength, mineralization and osteogenic potential (high alkaline phosphatatse activity and increased proliferation of DPSCs) of biomembranes (Qin et al., 2018). Chitosan along with hDPSCs have shown to have promising results for endodontic regeneration.
2.3.9 Chitosan modified wound dressings
These dressings are highly effectual for controlling hemorrhage and infection after surgical operative procedures. As stated earlier, the positively charged amino groups of chitosan electrostatically interact with negatively charged elements of blood, i.e., RBCs to bring about the hemostatic property (Caetano et al., 2015; Minervini et al., 2024).
Quaternized chitosan-G-polyaniline and benzaldehyde group functionalized poly (ethylene glycol)-co-poly (glycerol sebacate) (PEGS-FA) hydrogels have high antibacterial, antioxidant, antimicrobial property, good self-healing capacity which makes it ideal for the manufacture of wound dressings. These self-healing hydrogels are bestowed with free radical scavenging capacity, adhesiveness, biocompatibility and excellent in vivo blood clotting capacity which stimulate the wound healing mechanism, granulation tissue formation and collagen deposition by upregulating the growth factors like Transforming Growth Factor-β (TGF- β), Epidermal growth factor (EGF) and Vascular endothelial growth factor (VEGF) (Zhao et al., 2017). It is also proved that Chitosan dressings when refined with polyphosphate and silver, escalates the hemostatic and antimicrobial activity. Chitosan-polyphosphate formulation (ChiPP) enhances blood clotting, platelet adhesion, faster thrombin generation and better absorption of blood (Dai et al., 2011).
pH-responsive hydrogel wound dressings containing quaternized chitosan/benzaldehyde-terminated pluronic F127 (PF127) coupled with curcumin have shown enhanced antimicrobial, anti-inflammatory, and improved wound healing activity. These hydrogels enhance the wound healing process by minimizing the inflammatory markers and upregulating the wound healing-related growth factors (Qu et al., 2018). Recently, a major breakthrough, i.e., in the treatment of infected wounds, a photothermal self-healing nanocomposite hydrogels with antibiotics have been introduced. These nanocomposite hydrogels are presented with N-carboxyethyl chitosan/PF127/carbon nanotube and exhibit stable hemostatic, mechanical properties, remarkable photothermal antibacterial property and an increased pH-responsive moxifloxacin release capacity to enhance the healing process (He et al., 2020).
2.3.10 Chitosan based tissue engineering aspects
Periodontitis is a chronic inflammatory disease affecting the periodontium and the tooth supporting structures resulting in loss of alveolar bone and mobility of teeth. To overcome this situation, surgical approaches like guided tissue regeneration (GTR) and guided bone regeneration (GBR) can be considered. In these procedures, a scaffold barrier is placed to allow osteloblastic proliferation and osseous formation around the bone defect. The barrier obstructs the epithelial cell migration resulting in formation of long junctional epithelium in lieu of bone formation (Newman et al., 2011).
The basic aim of tissue engineering is to construct a 3-D scaffold which bears a close similitude to the structure of bone. Tissue engineering aims to create a regenerated tissue on polymerized decomposable scaffold as a layering for stem cell attachment, adhesion, and proliferation. The manufacture of bio-membrane should be biocompatible, non-toxic, biodegradable and should resemble the extracellular matrix (ECM) containing glycosaminoglycans, glycolipids and glycolipids for neo-regeneration of the tissue (Baranwal et al., 2018).
Natural and synthetic biopolymers are present to provide scaffolding. Chitosan and various subordinates meet the fundamental requirements of tissue engineering. It has been advocated that chitosan implantation does not provoke any immune response and the breakdown of chitosan by lysozyme upon the formation of new tissue does not produce any toxic effects. In dentistry, chitosan scaffolds are extensively used for pulpo-periodontal-bone regeneration (Li et al., 2014; Vázquez et al., 2015; Sultankulov et al., 2019). Chitosan is amenable to the characteristics of scaffold; however it lacks superior mechanical property and bioactivity which are the essential basis for osseous tissue engineering (Azevedo et al., 2014). To achieve this point, chitosan is coupled with synthetic biopolymers, growth factors and proteins to enhance the osseous regeneration with improved mechanical strength. Chitosan membrane laden with bioactive nanoparticles like hydroxyapaptite (HA), silica and tricalcium phosphate (TCP) stimulates bone formation and improves mechanical properties (Faqhiri et al., 2019). Additionally, nano-ceramic particles like Bioactive glass can be applied to chitosan to promote osteogenesis in load bearing areas (Denuziere et al., 1998). Chitosan/chondroitin sulfate/Bioactive glass nanoparticles facilitate osteogenesis in-vivo (Nie et al., 2019). Nanocomposite scaffolds containing chitosan-gelatin- nanobioactive glass are proven to create a sterile environment for cell attachment to promote protein adsorption and mineral deposition to promote osseous formation (Januariyasa et al., 2020). Combination of nanomaterials with bioactive growth factors on a 3-D scaffold directs the mesenchymal stem cells to differentiate to osseous formation (Guo et al., 2006; Oryan and Sahvieh, 2017).
Various studies have reported that chitosan-hydroxyapaptite combination coated scaffolds provide excellent environment for cell differentiation and proliferation promoting osseous formation similar to the original bone structure. The incorporation of nanohydroxyapatite in the scaffold increases the apatite content in the defective area which provides the basis for bone tissue engineering which led to the development of chitosan double faced membranes (Gümüşderelioğlu et al., 2020). These membranes were used for periodontal regeneration. The porous side of the membrane (in contact with bone) was loaded with nanohydroxyapatite and BMP-6, and the opposite side was laden with poly-caprolactone nanofibers to mitigate the epithelial cell migration. The porous surface induced multiplication of MC3T3-E1 preosteoblasts, while the other side of the membrane acted as a guard against epithelial cell migration (Marrazzo et al., 2016; Suneetha et al., 2020).
Injectable hydrogels have always been reliable and used as a part of tissue engineering process. Chitosan membranes in the form of injectable hydrogels do not require surgical intervention. These hydrogels are highly used for the treatment of periodontal pockets. It has been observed that PEC hydrogels coupled with chitosan and sodium alginate in a polymerized network have improved mechanical properties and biocompatibility which are requisites for osseous tissue engineering. These hydrogels tend to form porous ladder like interconnected mesh with fibrous structure on which osteoblasts proliferation is enhanced (Tatullo et al., 2017). Drug–based chitosan scaffolds have gained lot of popularity and are extensively used for bone and periodontal healing. (Agrawal et al., 2023; Arora et al., 2023; Nava Juárez, 2023).
Cell-based approaches are becoming a trend in regenerative medicine to treat health related conditions. However, safety concerns with respect to the adverse effects of the cell-based approach is always controversial (Sukpaita et al., 2019). These cell-based approaches are gaining attention in the field of dentistry. The human pulp stem cells, Dental pulp Stem cells (PDSCs), Stem cells from Apical Papilla (SCAP), Stem cells from exfoliated deciduous teeth (SHED), and human periodontal ligament cells (HPLCs) have high multi-differential potential and are giving promising results in regenerative dentistry. Besides these, another cell fraction has been identified from periapical granulation tissues and are termed as “human periapical cyst-mesenchymal stem cells (hPCy-MSCs)” and suffice with high proliferation and multi-differentiation capacity (Tatullo et al., 2017; Liao et al., 2020). Periodontal regeneration with human periodontal ligament cells (HPLCs) has resulted in excellent clinical success. Clinical trials have reported that HPLCs when seeded on chitosan scaffolds enhance osteogenesis without any toxic effects. It is said that the above-mentioned cells along with chitosan tend to increase the gene expression of osteoblastic cells (RUNX2, ALP, OPN) resulting in enhanced osteogenic potential (Li Y. et al., 2019). Recently in a study by Liao et al., Mesoporous hydroxyapatite/chitosan scaffold recombined with human amelogenenin promoted the proliferation of HPLCs leading to formation of cementum and bone (Liao et al., 2020). Studies have revealed that Stem cells from human exfoliated teeth (SHED) with TGF-β1 when inoculated on chitosan scaffolds show osteogenic potential (Li Y. et al., 2019). Chitosan laden scaffolds and sponges are considered as good delivery vehicles for periodontal regeneration (Su et al., 2014; Vining and Mooney, 2017).
Understanding the mechano-biology of stem cells, a sterile artificial environment which fulfills all the requisites of tissue engineering including the biochemical and mechanical forces should be designed. Stem cells respond to intracellular and extracellular forces. The physiological environment can be altered to regulate the stem cell behavior to achieve their beneficial effects. Mechanical cues like stiffness and viscoelasticity of the scaffold on which the cells are seeded, can regulate the organogenesis and the cell fate ex-vivo (Thomas et al., 2017). Modifying the viscoelasticity and stiffness of the chitosan-hyaluronic acid hydrogel as connective tissue tends to promote the proliferation of chondrocytes and the gene expression of ECM markers (Jagodzinski et al., 2004; Brindley et al., 2011).
Mechano-regulation of stem cells through gene expression, proliferation, differentiation forms the basis for osseous regeneration (Lovecchio et al., 2019). It is believed that shear and compressive forces enhance the proliferation of human mesenchymal cells into osteoblasts and produce extracellular matrix. Human bone marrow stromal cells (hBMSCs) due to cyclic loading tend to enhance collagen–I fiber, AL and OC levels leading to osteogenesis (Choi et al., 2018). Tissue engineering models are conducted on 3-D scaffolds to create a physiochemical environment for stem cells. It has been evaluated that cell proliferation, and differentiation and ECM matrix deposition occurs when hBMSCs cultured on chitosan-graphene 3-D scaffold undergo mechano-stimulation (Choi et al., 2018).
2.3.11 Chitosan in medical imaging
Targeted tumor therapy using chitosan nanoparticles permit exceptional prospects beyond standard cancer therapies. Combining such nanoparticles with diagnostic test such as computed tomography, magnetic resonance imaging, and ultrasound imaging, or multi-modal imaging compounds aids easy cancer detection (Sun et al., 2014). Chitosan nanoparticles with multiple contrast agents have been tested for multimodal imaging procedures to counteract disadvantages of single imaging modality. A multimodal approach provides both in-vitro and in-vivo results effectively. Sun et al. reported that solubility of glycol chitosan when treated with hydrophilic ethylene glycol or PEGylation made it suitable for tumor imaging (Zhang et al., 2013). Besides, chitosan nanoparticles show passive targeting due to higher permeability and retention in cancerous lesions for prolonged duration (Min et al., 2015). This property is also known as enhanced permeability and retention effect.
The traditional method for synthesizing gold nanoparticles lacks stability due to presence of sodium citrate salt. Hence, Sun et al., modified the surface of the gold nanoparticles by eliminating the salt and including a reducing agent, glycol chitosan, which acts as a stabilizer biologically. Also, glycol chitosan is already known as an effective tumor targeting agent in animal models. Coating success was established after observing the change in the refractive index around the coated nanoparticles, using a UV-visible spectrometer, transmission electron microscopy, and Fourier transform infrared spectroscopy. The targeted accumulation in the tumor and its bio-distribution was assessed in-vivo using computed tomography (CT) in mice induced with colon cancer. The study results showed enhanced stability of the nanoparticles biologically due to a stabilizing surface coat of chitosan. Moreover, high contrast images of tumor were acquired from the mice by using the coated nanoparticles as a CT contrast agent. The images matched the results of cellular uptake and were highly sensitive to metastatic zones. A selective uptake of the coated nanoparticles was observed in the colon cancer cells compared to macrophages. Thus, the characteristics accumulation of the glycol chitosan coated nanoparticles in cancerous cells demonstrates its efficient tumor targeting capacity which promotes imaging. The authors observed that the ample amine groups of glycol chitosan coated gold nanoparticles serves as zones for chemical conjugation for chemotherapeutic agents and enhances stability to support cancer imaging (Sun et al., 2014).
Zhang et al., studied the efficacy of gadolinium loaded chitosan nanoparticles as magnetic resonance imaging (MRI) contrast agents to target cancerous tissues by enhanced permeability and retention effect. To counteract the shortcomings of gadolinium based chelates such as easy renal filtration and poor contrast, gadolinium was chemically conjugated using ionic gelation with chitosan to prevent its early release and achieve improved retention for extended imaging time. In-vitro MRI revealed comparatively high relaxation time of gadolinium loaded chitosan nanoparticles because of surface modification demonstrating its capability as an effective contrast agent. Compared to another commercial contrast agent Magnevist® (same amount of gadonilium without chitosan coating), gadolinium loaded chitosan nanoparticles presented advanced imaging capacity and a high sensitivity aiding early diagnosis. During in-vivo MRI, gadolinium loaded chitosan nanoparticles showed higher brightness and retention time compared to Magnevist®, prolonging the imaging time considerably. This improvement was attributed to chitosan conjugation with gadolinium. The authors concluded that chemically conjugated gadolinium loaded chitosan nanoparticles holds vast possibility as MRI contrast agent (Zhang et al., 2013).
Min et al., developed an echogenic glycol chitosan-based nanoparticles for ultrasound-based imaging of malignant lesions. The authors used a chemotherapeutic bio-inert agent named perfluoropentane (PFP) which served as an ultrasound gas precursor. The components were turned into glycol chitosan nanoparticles using an oil-water emulsion approach, bearing an anti-cancer drug (docetaxel or doxorubicin)/PFP inner core coated with hydrophilic glycol chitosan. The authors demonstrated that the hydrophobic inner core was essential to stabilize the glycol chitosan coating. The ultrasound imaging capacity of echogenic particles were confirmed by injecting them intravenously in cancer induced mice. Within a minute of injection strong bright images were detected via ultrasound imaging due to effective accumulation of the particles in the cancerous cells. PFP gas production within the nanoparticles helped in retaining the ultrasound signals for an hour. The accumulation was noticed until 2 days after the injection because of due to the enhanced permeability and retention effect produced by promising size of the coated nanoparticles. The ultrasound treated samples displayed 4–7 times increased accumulation and wider distribution of chitosan coated nanoparticles in excised growths. This represents the thorough penetration of chitosan coated nanoparticles into the major vessels and its effective spread to the cancerous tissues. Based on their experimental observations the authors concluded that the echogenic glycol chitosan coated nanoparticles may be tried as ultrasound contrast enhancers in cancer imaging (Min et al., 2015).
Choi et al., developed iodine based echogenic diatrizoic acid-conjugated glycol chitosan nanoparticles as multimodal contrast agents for computed tomography-ultrasound dual imaging. Glycol chitosan-diatrizoic acid compound was formulated chemical conjugating with the amine groups on chitosan. Oil-water emulsion technique was used to introduced iodinated nanoparticles. The resultant contrast agents were directly inserted into cancerous tissues. Notably, the iodide-based echogenic glycol chitosan nanoparticles showed significant accumulation in cancerous tissues. Clear signals were obtained on injecting the contrast agents into tumor sites using both computed tomography and ultrasound imaging modalities. The authors considered the developed agent to perform effectively during a multi-modal imaging approach (Choi et al., 2018).
With all the clinical trial data challenge remains to replicate the same responses in human subjects with tumors and other cancer drug interactions. It is necessary to observe the immune responses and related toxicities. Genetic mutations and varied vascularity of cancerous tissues may generate dissimilar response compared to lab animals. If all such limitations are taken care of then chitosan would have a promising future in targeted cancer imaging and drug therapy.
2.3.12 Chitosan in oral pathology and oncology
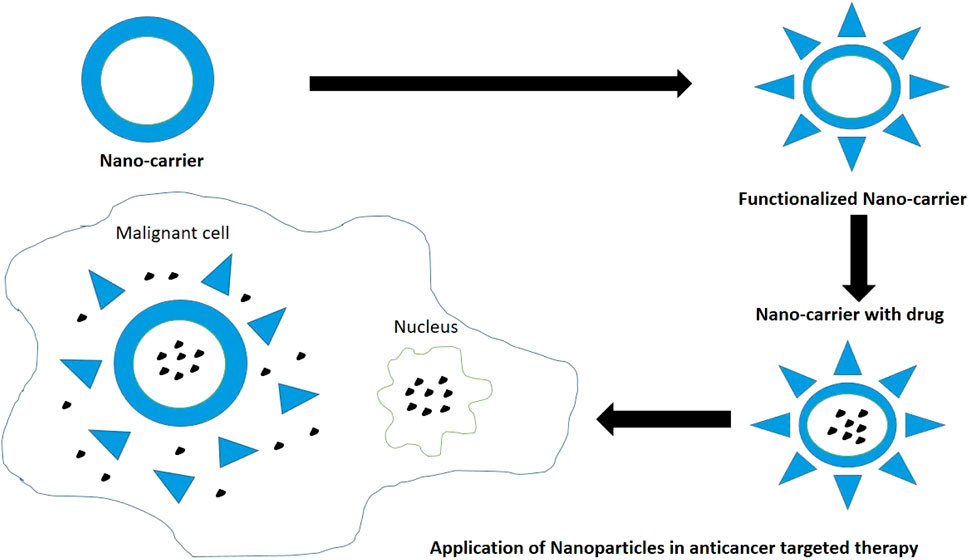
Chitosan modified nanocarrier systems can be used as a potential vehicle to target anti-cancer drugs to the various tumors and cause tumor apoptosis as per the individual efficacy of loaded drugs (Figure 8). Surface modifications of chitosan nanoparticles have been postulated which aim to enhance the tumor targeting ability via different mechanisms like receptor or carrier mediated transcytosis. Chitosan presents significant biocompatibility and promotes wound healing at molecular and cell level. Chitosan also acts as a bio-adhesive or mucoadhesive and hydrates the underlying tissues to effectively heal ulcers and relieve pain. It repairs tissues, contracts wound by acting as hemostat, secretes inflammatory mediators, and induces macrophage actions by providing a non-proteinaceous matrix for 3D-tissue growth. Chitosan serves as collagen depositor by releasing N-acetyl-D-glucosamine on depolymerization to promote fibroblast formation. Chitosan modifies bacterial surface morphology, improves cell permeability, causes intracellular constituents’ seepage, and prevents nutrient transport. Hence prevents erythema and secondary infections related to cancer therapy. Chitosan also presents fungistatic property by improving permeability of yeast cells (Mahima et al., 2015).
Min et al., developed an echogenic glycol chitosan-based nanoparticles for ultrasound-based drug delivery to areas of induced squamous cell carcinoma (SCC7 cell lines). An echogenic approach is known to enhance the drug release because of external ultrasound and result in a targeted burst release of the therapeutic agents to tumor sites. In the study the inner core behaved as an efficient reservoir for both the anti-cancer agents and PFPs. The gas precursor successfully retarded gas expansion and maintained stability. The authors demonstrated that the chitosan coated nanosized echogenic particles persisted in the bloodstream for longer periods promoting targeted delivery. Moreover, the outer glycol chitosan coating improved the overall physiochemical characteristics and targeted delivery (Min et al., 2015).
Wimardhani et al., studied the influence of low-molecular-weight chitosan on Ca9-22 cells derived from gingival carcinoma. The study was based on the premise that the low-molecular-weight chitosan showed anticancer effects and were relatively less toxic to non-cancerous cells. Cytotoxic effects were observed with low-molecular-weight chitosan on Ca9-22 cells leading to cell cycle arrest, upsurge in apoptotic DNA fragmentation, and subtle elevation in caspase expressions. The author concluded that the short-term exposure to low-molecular-weight chitosan has encouraging application as an anti-cancer agent (Wimardhani et al., 2014b).
Muthukrishnan et al., studied the effect of chitosan with zinc sulphate for treating and preventing oral mucositis as a side effect of radiotherapy. A WHO mucositis scale was used to grade oral mucositis three times a week. Although during early treatment days no significant changes were observed, around the sixth week a reduced severity was noticed. Pain levels and dysphagia were also reduced. The authors concluded that chitosan-zinc sulphate combination lowered the mucositis severity and aids healing (Muthukrishnan and Shanmughapriya, 2017).
Mahima V.G., examined the influence of 1% chitosan mouthwash on oral mucositis on 20 patients developed after radio-chemotherapy. The study showed significant results in lowering symptoms of oral mucositis such as pain, erythema, ulcerations, and other associated side effects. Chitosan not only demonstrated enhanced healing but also acted as a promising occlusive dressing to alleviate pain and ulcer discomfort due to bio-adhesive properties. No secondary infections and adverse biocompatibility issues were reported during the tests reinforcing chitosan’s ability to work effectively as solution to side effects of radio-chemotherapy. The authors concluded that chitosan is far more efficient than chlorhexidine in alleviating symptoms of oral mucositis (Mahima et al., 2015).
Pornpitchanarong et al., investigated a muco-adhesive based on catechol-modified chitosan/hyaluronic acid nanoparticles as a carrier of doxorubicin for oral cancer. Chitosan and catechol functionalized drug delivery were already reported to boost muco-adhesion individually. Muco-adhesion provides prolonged retention with sustained release of the agents. The authors demonstrated that a significant portion of drug could be loaded into the nanoparticles and sustained release was attainable. A better muco-adhesive property was observed. The combination also induced effective apoptosis of HN22 human oral cancer cells. The authors concluded the said combination as a potential drug carrier for doxorubicin to prevent localized oral cancers (Pornpitchanarong et al., 2020). Figure 7 depicts the usage of nanoparticles as drug carriers in targeted therapy for treatment of various cancers (Sharifi-Rad et al., 2021).
Zhu et al. also reported that a fluorinated chitosan-chlorin e6 (FC-Ce6) nanocarrier for intracellular delivery catalase enhanced photodynamic therapy of oral cancer. They established that fluorine conjugated chitosan nanoparticles exhibited superior anti-cancer activity in contrast to free Ce6 and non-fluorinated CS-Ce6/catalase nanoparticles. Chen et al., tested chitosan nanoparticles encapsulated with 5-aminolevulinic acid (a photo-sensitizer), and IR780 (a near-infrared fluorescence dye used as a photo-thermal agent). However, a single approach using 5-aminolevulinic acid and IR780 had many drawbacks such as tumor recurrence, hydrophilicity of 5-aminolevulinic acid, and low specificity. Chitosan served as a nano-carrier with high biocompatibility and cell membrane permeability. Hence, a combination of photo-thermal and photodynamic agents for a non-invasive oral cancer treatment was developed in the study. The author demonstrated that the said combination displayed improved accumulation in cancerous tissues and showed fluorescence during imaging. Improved photo-thermally augmented photodynamic result for tumor excision was reported with no apparent toxicity. The authors concluded that the combination was safe for use as non-invasive oral cancer therapy (Zhu et al., 2021). Chen et al. formulated a new photothermally enhanced photodynamic therapy platform based on orally administered Chitosan Nanoparticles. Improved photodynamic cytotoxicity to cancer cells was seen with a combination of PTT (photothermal therapy) and PDT (photodyanamic therapy) when compared with photodynamic therapy alone. Additionally, 5-ALA (5-aminolevulinic acid) &IR780 coated Chitosan Nanoparticles exhibited high tumor accumulation and greater ability to fluorescently image tumor tissue (Chen et al., 2020).
Takeuchi et al., designed a sustained release film loaded with rebamipide and chitosan for treating side effects of oral mucositis after chemotherapy. Rebamipide was enforced as a gargle to counter side effects of oral mucositis but its effects were short lived. Chitosan was reinforced into rebamipide for its muco-adhesive and antibacterial characteristics. Along with chitosan, pluronic was added as adhesion enhancer and hydroxyl-propyl methylcellulose served as film former. The release behavior was studied. The authors reported that chitosan caused suppression in the release of rebamipide for nearly half an hour. Whereas, hydroxyl-propyl methylcellulose helped in sustained release and maintained the films shape. The authors concluded that the combination can be effectively used as base for sustained drug delivery. However, additional clinical trials are warranted (Takeuchi et al., 2019).
3 Conclusion
Chitosan shows a notable range of properties which makes it valuable for sustainable development due to it being plentiful, decomposable, eco-friendly, and adaptable. Chitosan production has improved in terms of green chemistry due to the harmful chemicals being replaced by solvents with minimum melting points (Eutectic). This has also lead to an overall decrease in energy consumption. An important reason for using chitosan is the presence of a large number of organic groups (hydroxyl and amino groups) in its structure which makes it amenable to chemical modifications (Maliki et al., 2022). This versatility of chitosan makes it especially remarkable for the preparation of suspensions, composites, functionalized materials, or (nano)hybrids for diverse environment-friendly usage and in industrial and health related applications. Chitosan-based nanocomposites, hydrogels, and membranes are being used in regenerative medicine and dentistry. In field of dentistry, it has gained enormous popularity due to its natural existence and biocompatibility and decreased cytotoxicity. However, it still presents certain limitations with regard to its structure and molecular weight. Nonetheless, this natural nanomaterial is being extensively used and has an excellent potential to stretch out its biotic properties in the near future. There is hardly any clinical evidence of chitosan-based derivatives in the field of dentistry and more clinical data should be added on. Being the second abundant biopolymer in nature after cellulose, the potential of chitosan as sustainable future material in dentistry and medical needs further exploration. Continuous investigation into nanobiotechnology related to computer science ought to be carried out to enhance the present state of medicine and create pharmaceuticals with strong therapeutic efficacy to reduce patients’ discomfort while optimizing the effectiveness of therapeutic agents.
Author contributions
RM: Conceptualization, Project administration, Validation, Writing–original draft, Writing–review and editing. SH: Data curation, Supervision, Writing–original draft, Writing–review and editing. NM: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbass, M. M. S., El-Rashidy, A. A., Sadek, K. M., El Moshy, S., Radwan, I. A., Rady, D., et al. (2020). Hydrogels and dentin–pulp complex regeneration: from the benchtop to clinical translation. Polymers 12, 2935. doi:10.3390/polym12122935
Abdel Mouez, M., Zaki, N. M., Mansour, S., and Geneidi, A. S. (2014). Bioavailability enhancement of verapamil HCl via intranasal chitosan microspheres. Eur. J. Pharm. Sci. 51, 59–66. doi:10.1016/j.ejps.2013.08.029
Abid, N., Khan, A. M., Shujait, S., Chaudhary, K., Ikram, M., Imran, M., et al. (2022). Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: a review. Adv. Colloid Interface Sci. 300, 102597. doi:10.1016/j.cis.2021.102597
Abtahi, J., Tengvall, P., and Aspenberg, P. (2012). A bisphosphonate-coating improves the fixation of metal implants in human bone. A randomized trial of dental implants. Bone 50, 1148–1151. doi:10.1016/j.bone.2012.02.001
Adhikari, H. S., and Yadav, P. N. (2018). Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. Int. J. Biomaterials 2018, 1–29. doi:10.1155/2018/2952085
Agnihotri, A. S., Mallikarjuna, N. N., and Aminabhavi, T. M. (2004). Recent advances on chitosan based micro-and nanoparticles in drug delivery. J. Control. Release 100 (1), 5–28. doi:10.1016/j.jconrel.2004.08.010
Agrawal, A., Reche, A., Agrawal, S., and Paul, P. (2023). Applications of chitosan nanoparticles in dentistry: a review. Cureus 15 (12), e49934. doi:10.7759/cureus.49934
Aguilar, A., Zein, N., Harmouch, E., Hafdi, B., Bornert, F., Offner, D., et al. (2019). Application of chitosan in bone and dental engineering. Molecules 24 (16), 3009. doi:10.3390/molecules24163009
Ainola, M., Tomaszewski, W., Ostrowska, B., Wesolowska, E., Wagner, H. D., Swieszkowski, W., et al. (2016). A bioactive hybrid three-dimensional tissue-engineering construct for cartilage repair. J. Biomater. Appl. 30, 873–885. doi:10.1177/0885328215604069
Akıncıbay, H., Şenel, S., and Yetkin, Z. A. (2007). Application of chitosan gel in the treatment of chronic periodontitis. J. Biomed.Mater. Res. B Appl. Biomater. 80 (2), 290–296. doi:10.1002/jbm.b.30596
Akncbay, H., Senel, S., and Ay, Z. Y. (2007). Application of chitosan gel in the treatment of chronic periodontitis. J. Biomed. Mater Res. B Appl. Biomater. 80 (2), 290–296. doi:10.1002/jbm.b.30596
Alamry, K. A., Almehmadi, S. J., Elfaky, M. A., Al-Shareef, H. F., Samah, J. A., and Hussein, M. A. (2020). Enhanced antimicrobial activity of new arylidene-based polyketone nanocomposite materials. Polymer-Plastics Technol. Mater. 59 (18), 1973–1986. doi:10.1080/25740881.2020.1784213
Alcaraz, A. L., Mendoza, J. L., Goycoolea, F., Ciapara, L. H., and Monal, W. A. (2016). Preparation of chitosan nanoparticles by nanoprecipitation and their ability as a drug nanocarrier. RSC Adv. 6 (64), 59250–59256. doi:10.1039/c6ra06563e
Alghamdi, H. S. (2018). Methods to improve osseointegration of dental implants in low quality (Type-IV) bone: an overview. J. Funct. Biomater. 9 (1), 7. doi:10.3390/jfb9010007
Alinejad, V., Somi, M. H., Baradaran, B., Akbarzadeh, P., Atyabi, F., Kazerooni, H., et al. (2016). Co-delivery of IL17RB siRNA and doxorubicin by chitosan-based nanoparticles for enhanced anticancer efficacy in breast cancer cells. Biomed. Pharmacother. 83, 229–240. doi:10.1016/j.biopha.2016.06.037
Alnufaiy, B. M., Lambarte, R. N. A., and Al-Hamdan, K. S. (2020). The osteogenetic potential of chitosan coated implant: an in vitro study. J. Stem Cells Regen. Med. 16 (2), 44–49. doi:10.46582/jsrm.1602008
Arancibia, R., Maturana, C., Silva, D., Tobar, N., Tapia, C., Salazar, J., et al. (2013). Effects of chitosan particles in periodontal pathogens and gingival fibroblasts. J.Dent. Res. 92 (8), 740–745. doi:10.1177/0022034513494816
Arnaud, T. M. S., de Barros, B. N., and Diniz, F. B. (2010). Chitosan effect on dental enamel deremineralization: an in vitro evaluation. J. Dent. 38 (11), 848–852. doi:10.1016/j.jdent.2010.06.004
Arora, S., Das, G., Alqarni, M., Grover, V., Manzoor Baba, S., Saluja, P., et al. (2023). Role of chitosan hydrogels in clinical dentistry. Gels 9 (9), 698. doi:10.3390/gels9090698
Azevedo, A. S., Sá, M. J., Fook, M. V., Neto, P. I. N., Sousa, O. B., Azevedo, S. S., et al. (2014). Use of chitosan and β-tricalcium phosphate, alone and in combination, for bone healing in rabbits. J. Mater Sci. Mater Med. 25 (2), 481–486. doi:10.1007/s10856-013-5091-2
Azuma, K., Izumi, R., Osaki, T., Ifuku, S., Morimoto, M., Saimoto, H., et al. (2015). Chitin, chitosan, and its derivatives for wound healing: old and new materials. J. Funct. biomaterials 6 (1), 104–142. doi:10.3390/jfb6010104
Babrawala, I. S., Prabhuji, M. L. V., Karthikeyan, B. V., and Divya, K. (2016). A novel approach using natural 1% (W/W) chitosan as a local drug delivery system in the management of non-surgical periodontal treatment: a pilot study. J. Int. Acad. Periodontology 18 (4), 129–133.
Bakopoulou, A., Georgopoulou, A., Grivas, I., Bekiari, C., Prymak, O., Loza, K., et al. (2019). Dental pulp stem cells in chitosan/gelatin scaffolds for enhanced orofacial bone regeneration. Dent. Mater. 35 (2), 310–327. doi:10.1016/j.dental.2018.11.025
Bakshia, P. S., Selvakumara, D., Kadirvelub, K., and Kumara, N. (2019). Chitosan as an environment friendly biomaterial–a review on recent modifications and applications. Int. J. Biol. Macromol. 150, 1072–1083. doi:10.1016/j.ijbiomac.2019.10.113
Bansal, M., Mittal, N., Yadav, S. K., Khan, G., Gupta, P., Mishra, B., et al. (2018). Periodontal thermoresponsive, mucoadhesive dual antimicrobial loaded in-situ gel for the treatment of periodontal disease: preparation, in-vitro characterization and antimicrobial study. J. Oral Biol. Craniofac. Res. 8 (2), 126–133. doi:10.1016/j.jobcr.2017.12.005
Baranwal, A., Kumar, A., Priyadharshini, A., Oggu, G. S., Bhatnagar, I., Srivastava, A., et al. (2018). Chitosan: an undisputed bio-fabrication material for tissue engineering and bio-sensing applications. Int. J. Biol. Macromol. 110, 110–123. doi:10.1016/j.ijbiomac.2018.01.006
Bellamy, C., Shrestha, S., Torneck, C., and Kishen, A. (2016). Effects of a bioactive scaffold containing a Sustained transforming growth factor-β1–releasing nanoparticle system on the Migration and differentiation of stem cells from the apical papilla. J. Endod. 42 (9), 1385–1392. doi:10.1016/j.joen.2016.06.017
Bentley, C., and Drake, C. (1986). Longevity of restorations in a dental school clinic. J. Dent. Educ. 50, 594–600. doi:10.1002/j.0022-0337.1986.50.10.tb02046.x
Bernkop-Schnürch, A., and Dünnhaupt, S. (2012). Chitosan-based drug delivery systems. Eur. J.Pharm. Biopharm. 81 (3), 463–469. doi:10.1016/j.ejpb.2012.04.007
Bhattarai, N., Gunn, J., and Zhang, M. (2010). Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 62 (1), 83–99. doi:10.1016/j.addr.2009.07.019
Blumenthal, H. J., and Roseman, S. (1957). Quantitative estimation of chitin in fungi. J. Bacteriol. 74, 222–224. doi:10.1128/jb.74.2.222-224.1957
Bonderer, L. J., Studart, A. R., and Gauckler, L. J. (2008). Bioinspired design and assembly of platelet reinforced polymer films. Science 319, 1069–1073. doi:10.1126/science.1148726
Boverhof, D. R., Bramante, C. M., Butala, J. H., Clancy, S. F., Lafranconi, M., West, J., et al. (2015). Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 73 (1), 137–150. doi:10.1016/j.yrtph.2015.06.001
Brindley, D., Moorthy, K., Lee, J. H., Mason, C., Kim, H. W., and Wall, I. (2011). Bioprocess forces and their impact on cell behavior: implications for bone regeneration therapy. J. Tissue Eng. 2011, 620247. doi:10.4061/2011/620247
Caetano, G. F., Frade, M. A. C., Andrade, T. A. M., Leite, M. N., Bueno, C. Z., Moraes, A. M., et al. (2015). Chitosan-alginate membranes accelerate wound healing. J. Biomed. Mater. Res. Part B. Appl. Biomaterials 103 (5), 1013–1022. doi:10.1002/jbm.b.33277
Chen, G., Zhao, Y., Xu, Y., Zhu, C., Liu, T., and Wang, K. (2020). Chitosan nanoparticles for oral photothermally enhanced photodynamic therapy of colon cancer. Int. J. Pharm. 589, 119763. doi:10.1016/j.ijpharm.2020.119763
Chen, K., Guo, B., and Luo, J. (2017). Quaternized carboxymethyl chitosan/organic montmorillonite nanocomposite as a novel cosmetic ingredient against skin aging. Carbohydr. Polym. 173, 100–106. doi:10.1016/j.carbpol.2017.05.088
Chen, R., Liu, C., Tseng, W., Jeng, J., and Lin, C. (2003). Cytotoxicity of three dentin bonding agents on human dental pulp cells. J. Dent. 31, 223–229. doi:10.1016/s0300-5712(02)00088-x
Choi, D., Jeon, S., You, D. G., Um, W., Kim, J. Y., Yoon, H. Y., et al. (2018). Iodinated echogenic glycol chitosan nanoparticles for X-ray CT/US dual imaging of tumor. Nanotheranostics 2 (2), 117–127. doi:10.7150/ntno.18643
Cohenca, N., Paranjpe, A., and Berg, J. (2013). Vital pulp therapy. Dent. Clin. N. Am. 57, 59–73. doi:10.1016/j.cden.2012.09.004
Costa, E., Silva, S., Madureira, A., Cardelle-Cobas, A., Tavaria, F., and Pintado, M. (2014). A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism's biofilm formation in vitro. Carbohydr. Polym. 101, 1081–1086. doi:10.1016/j.carbpol.2013.09.041
Costa-Pinto, A. R., Reis, R. L., and Neves, N. M. (2011). Scaffolds based bone tissue engineering: the role of chitosan. Tissue Eng. Part B Rev. 17 (5), 331–347. doi:10.1089/ten.teb.2010.0704
Covarrubias, C., Trepiana, D., and Corral, C. (2018). Synthesis of hybrid copper-chitosan nanoparticles with antibacterial activity against cariogenic Streptococcus mutans. Dent. Mat. J. 37 (3), 379–384. doi:10.4012/dmj.2017-195
Dai, T., Tanaka, M., Huang, Y. Y., and Hamblin, M. R. (2011). Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev. Anti Infect. Ther. 9 (7), 857–879. doi:10.1586/eri.11.59
D’Almeida, M., Attik, N., Amalric, J., Brunon, C., Renaud, F., Abouelleil, H., et al. (2017). Chitosan coating as an antibacterial surface for biomedical applications. PLoS ONE 12 (12), e0189537. doi:10.1371/journal.pone.0189537
da Silva, M. A., Iamanaka, B. T., Taniwaki, M. H., and Kieckbusch, T. G. (2013). Evaluation of the antimicrobial potential of alginate and alginate/chitosan films containing potassium sorbate and natamycin. Packag. Technol. Sci. 26 (8), 479–492. doi:10.1002/pts.2000
De Carvalho, M., Stamford, T., Pereira, E., Dos Santos, P., and Sampaio, F. (2011). Chitosan as an oral antimicrobial agent. Formatex 2012, 13.
del Carpio-Perochena, A., Kishen, A., Felitti, R., Bhagirath, A. Y., Medapati, M. R., Lai, C., et al. (2017). Antibacterial properties of chitosan nanoparticles and propolis associated with calcium hydroxide against single-and multispecies biofilms: an in vitro and in situ study. J. Endod. 43 (8), 1332–1336. doi:10.1016/j.joen.2017.03.017
Denuziere, A., Ferrier, D., Damour, O., and Domard, A. (1998). Chitosan-chondroitin sulfate and chitosan-hyaluronate polyelectrolyte complexes: biological properties. Biomaterials 19 (14), 1275–1285. doi:10.1016/s0142-9612(98)00036-2
Dhand, C., Dwivedi, N., Loh, X. J., Ying, A. N. J., Verma, N. K., Beuerman, R. W., et al. (2015). Methods and strategies for the synthesis of diverse nanoparticles and their applications: a comprehensive overview. RSC Adv. 5, 105003–105037. doi:10.1039/c5ra19388e
DiMartino, A., Drannikov, A., Surgutskaia, N. S., Ozaltin, K., Postnikov, P. S., Marina, T. E., et al. (2019). Chitosan-collagen based film for controlled delivery of a combination of short life anesthetics. Int. J. Biol. Macromol. 140, 1183–1193. doi:10.1016/j.ijbiomac.2019.08.228
Ding, C. M., Chen, Z. X., and Li, J. S. (2017). From molecules to macrostructures: recent development of bioinspired hard tissue repair. Biomater. Sci. 5, 1435–1449. doi:10.1039/c7bm00247e
Diolosà, M., Donati, I., Turco, G., Cadenaro, M., Di Lenarda, R., Breschi, L., et al. (2014). Use of methacrylate-modified chitosan to increase the durability of dentine bonding systems. Biomacromolecules 15 (12), 4606–4613. doi:10.1021/bm5014124
Diz, P., Scully, C., and Sanz, M. (2013). Dental implants in the medically compromised patient. J. Dent. 41 (3), 195–206. doi:10.1016/j.jdent.2012.12.008
Dragland, I. S., Wellendorf, H., Kopperud, H., Stenhagen, I., and Valen, H. (2019). Investigation on the antimicrobial activity of chitosan-modified zinc oxide-eugenol cement. Biomaterial Investigations Dent. 6 (1), 99–106. doi:10.1080/26415275.2019.1697621
Drummond, J. L. (2008). Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 87, 710–719. doi:10.1177/154405910808700802
Duarte, F., Pinheiro, L., Ramos, C., and Silva, J. N. (2021). Rehabilitation of down syndrome with zygomatic implants-case report. SVOA Dent., 15–19.
Ducret, M., Montembault, A., Josse, J., Pasdeloup, M., Celle, A., Benchrih, R., et al. (2019). Design and characterization of a chitosan-enriched fibrin hydrogel for human dental pulp regeneration. Dent. Mat. 35 (4), 523–533. doi:10.1016/j.dental.2019.01.018
Eijsink, V., Hoell, I., and Vaaje-Kolstada, G. (2010). Structure and function of enzymes acting on chitin and chitosan. Biotechnol. Genet. Eng. Rev. 27, 331–366. doi:10.1080/02648725.2010.10648156
Elieh-Ali-Komi, D., and Hamblin, M. R. (2016). Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 4 (3), 411–427.
El Nady, D. G., Hashem, A. M., Shoeib, M. A., Shalaby, H. A., and Abdelraouf, R. M. (2017). Electro-deposition of biological active chitosan/composite coating on titanium dental implant. Egypt. Dent. J. 63 (4), 1417–1428.
El-Negoly, S. A., El-Fallal, A. A., and El-Sherbiny, I. M. (2014). A new modification for improving shear bond strength and other mechanical properties of conventional glass-ionomer restorative materials. J. Adhes. Dent. 16 (1), 41–47. doi:10.3290/j.jad.a30541
Elshinawy, M. I., Al-Madboly, L. A., Ghoneim, W. M., and El-Deeb, N. M. (2018). Synergistic effect of newly introduced root canal medicaments; ozonated olive oil and chitosan nanoparticles, against persistent endodontic pathogens. Front. Microbiol. 9, 1371. doi:10.3389/fmicb.2018.01371
Eramo, S., Natali, A., Pinna, R., and Milia, E. (2018). Dental pulp regeneration via cell homing. Int. Endod.J 51 (4), 405–419. doi:10.1111/iej.12868
Fakhri, E., Eslami, H., Maroufi, P., Pakdel, F., Taghizadeh, S., Ganbarov, K., et al. (2020). Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 162, 956–974. doi:10.1016/j.ijbiomac.2020.06.211
Faqhiri, H., Hannula, M., Kellomäki, M., Calejo, M. T., and Massera, J. (2019). Effect of melt-derived bioactive glass particles on the properties of chitosan scaffolds. J. Funct. Biomater. 10 (3), 38. doi:10.3390/jfb10030038
Farea, M., Husein, A., Halim, A. S., Abdullah, N. A., Mokhtar, K. I., Lim, C. K., et al. (2014). Synergistic effects of chitosan scaffold and TGFβ1 on the proliferation and osteogenic differentiation of dental pulp stem cells derived from human exfoliated deciduous teeth. Archives Oral Biol. 59 (12), 1400–1411. doi:10.1016/j.archoralbio.2014.08.015
Farhadian, N., Godiny, M., Moradi, S., Azandaryani, A. H., and Shahlaei, M. (2018). Chitosan/gelatin as a new nano-carrier system for calcium hydroxide delivery in endodontic applications: development, characterization and process optimization. Mater. Sci. Eng. C 92, 540–546. doi:10.1016/j.msec.2018.07.002
Farias, J. M., Stamford, T. C. M., Resende, A. H. M., Aguiar, J. S., Rufino, R. D., Luna, J. M., et al. (2019). Mouthwash containing a biosurfactant and chitosan: an eco-sustainable option for the control of cariogenic microorganisms. Int. J. Biol. Macromol. 129, 853–860. doi:10.1016/j.ijbiomac.2019.02.090
Finke, M. D. (2007). Estimate of chitin in raw whole insects. Zoo. Biol. 26, 105–115. doi:10.1002/zoo.20123
Forsten, L. (1994). Flouride release of glass ionomers. J. Esthet. Dent. 6 (5), 216–222. doi:10.1111/j.1708-8240.1994.tb00862.x
Ganss, C., Klimek, J., and Schlueter, N. (2014). Erosion/abrasion-preventing potential of NaF and F/Sn/chitosan toothpastes in dentine and impact of the organic matrix. Caries Res. 4, 163–169. doi:10.1159/000354679
Ganss, C., Lussi, A., Grunau, O., Klimek, J., and Schlueter, N. (2011). Conventional and anti-erosion fluoride toothpastes: effect on enamel erosion and erosion-abrasion. Caries Res. 45, 581–589. doi:10.1159/000334318
Ghafar, H., Khan, M. I., Sarwar, H. S., Yaqoob, S., Hussain, S. Z., Tariq, I., et al. (2020). Development and characterization of bioadhesive film embedded with lignocaine and calcium fluoride nanoparticles. AAPS PharmSciTech 21 (2), 60–12. doi:10.1208/s12249-019-1615-5
Ghosh, B., and Urban, M. W. (2009). Self-repairing oxetane-substituted chitosan polyurethane networks. Science 323, 1458–1460. doi:10.1126/science.1167391
Ghosh, S., Ranebennur, T. S., and Vasan, H. N. (2011). Study of antibacterial efficacy of hybrid chitosan-silver nanoparticles for prevention of specific biofilm and water purification. Int. J. Carbohydr. Chem. 2011, 1–11. doi:10.1155/2011/693759
Goodson, J., Offenbacher, S., Farr, D., and Hogan, P. (1985). Periodontal disease treatment by local drug delivery. J. Periodontol. 56 (5), 265–272. doi:10.1902/jop.1985.56.5.265
Goy, R. C., de Britto, D., and Assis, O. B. G. (2009). A review of the antimicrobial activity of chitosan. Polímeros Ciência Tecnol. 19 (3), 241–247. doi:10.1590/s0104-14282009000300013
Greenstein, G., and Polson, A. (1998). The role of local drug delivery in the management of periodontal diseases: a comprehensive review. J. Periodontol. 69 (5), 507–520. doi:10.1902/jop.1998.69.5.507
Guglielmotti, M. B., Olmedo, D. G., and Cabrini, R. L. (2019). Research on implants and osseointegration. Periodontol. 2000 79 (1), 178–189. doi:10.1111/prd.12254
Gümüşderelioğlu, M., Sunal, E., Demirtaş, T. T., and Kiremitçi, A. S. (2020). Chitosan-based double faced barrier membrane coated with functional nanostructures and loaded with BMP-6. J. Mat. Sci. Mat. Med. 31 (1), 4. doi:10.1007/s10856-019-6331-x
Guo, T., Zhao, J. N., Chang, J. B., Ding, Z., Hong, H., Chen, J. N., et al. (2006). Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-β1 for chondrocytes proliferation. Biomaterials 27, 1095–1103. doi:10.1016/j.biomaterials.2005.08.015
Hanes, P. J., and Purvis, J. P. (2003). Local anti-infective therapy: pharmacological agents. A systematic review. Ann. Periodontol. 8 (1), 79–98. doi:10.1902/annals.2003.8.1.79
Hassan, M. A., Omer, A. M., Abbas, E., Baset, W. M. A., and Tamer, T. M. (2018). Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci. Rep. 8, 11416. doi:10.1038/s41598-018-29650-w
Hayashi, Y., Ohara, N., Ganno, T., Yamaguchi, K., Ishizaki, T., Nakamura, T., et al. (2007). Chewing chitosan-containing gum effectively inhibits the growth of cariogenic bacteria. Arch. Oral Biol. 52, 290–294. doi:10.1016/j.archoralbio.2006.10.004
He, J., Shi, M., Liang, Y., and Guo, B. (2020). Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 394, 124888. doi:10.1016/j.cej.2020.124888
Ho, T. F. T., Smales, R. J., and Fang, D. T. S. (1999). A 2-year clinical study of two glass ionomer cements used in the atraumatic restorative treatment (ART) technique. Community Dent. Oral Epidemiol. 27, 195–201. doi:10.1111/j.1600-0528.1999.tb02010.x
Hu, D., Ren, Q., Li, Z., and Zhang, L. (2020). Chitosan-based biomimetically mineralized composite materials in human hard tissue repair. Molecules 25, 4785. doi:10.3390/molecules25204785
Hu, F., Zhou, Z., Xu, Q., Fan, C., Wang, L., Ren, H., et al. (2019). A novel pH responsive quaternary ammonium chitosan-liposome nanoparticles for periodontal treatment. Int. J. Biol. Macromol. 129, 1113–1119. doi:10.1016/j.ijbiomac.2018.09.057
Huang, G., Zhai, J., Cheng, S., Wang, Y., Yang, L., Liu, H., et al. (2016). The application of chitosan and its derivatives as nanosized carriers for the delivery of chemical drugs and genes or proteins. Curr. Drug Targets 17, 811–816. doi:10.2174/1389450116666151019100106
Huang, G. T., Sonoyama, W., Liu, Y., Liu, H., Wang, S., and Shi, S. (2008). The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J. Endod. 34 (6), 645–651. doi:10.1016/j.joen.2008.03.001
Ifuku, S., Nomura, R., Morimoto, M., and Saimoto, H. (2011). Preparation of chitin nanofibers from mushrooms. Materials 4, 1417–1425. doi:10.3390/ma4081417
Ignatova, M., Manolova, N., and Rashkov, I. (2007). Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) prepared by electrospinning. Eur. Polym. J. 43, 1112–1122. doi:10.1016/j.eurpolymj.2007.01.012
Ikinci, G., Senel, S., Akincibay, H., Kaş, S., Erciş, S., Wilson, C. G., et al. (2002). Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int. J. Pharm. 235 (1-2), 121–127. doi:10.1016/s0378-5173(01)00974-7
Imai, T., Watanabe, T., Yui, T., and Sugiyama, J. (2003). The directionality of chitin biosynthesis: a revisit. Biochem. J. 374 (Pt 3), 755–760. doi:10.1042/bj20030145
Indiarto, R., Indriana, L. P. A., Andoyo, R., Subroto, E., and Nurhadi, B. (2022). Bottom–up nanoparticle synthesis: a review of techniques, polyphenol-based core materials, and their properties. Eur. Food Res. Technol. 248, 1–24. doi:10.1007/s00217-021-03867-y
Islam, M. M., Shahruzzaman, M., Biswas, S., Nurus Sakib, M., and Rashid, T. U. (2020). Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater 5 (1), 164–183. doi:10.1016/j.bioactmat.2020.01.012
Islam, N., Dmour, I., and Taha, M. O. (2019). Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon 5 (5), e01684. doi:10.1016/j.heliyon.2019.e01684
Jagodzinski, M., Drescher, M., Zeichen, J., Hankemeier, S., Krettek, C., Bosch, U., et al. (2004). Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur. Cell Mater 7, 35–41. doi:10.22203/ecm.v007a04
Januariyasa, I. K., Ana, I. D., and Yusuf, Y. (2020). Nanofibrous poly (vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mat. Sci. Eng. C 107, 110347. doi:10.1016/j.msec.2019.110347
Javed, F., Vohra, F., Zafar, S., and Almas, K. (2014). Significance of osteogenic surface coatings on implants to enhance osseointegration under osteoporotic-like conditions. Implant Dent. 23, 679–686. doi:10.1097/id.0000000000000161
Jeyaraj, M., Gurunathan, S., Qasim, M., Kang, M. H., and Kim, J. H. (2019). A comprehensive review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials 9 (12), 1719. doi:10.3390/nano9121719
Ji, Q. X., Zhao, Q. S., Deng, J., and Lü, R. (2010). A novel injectable chlorhexidine thermosensitive hydrogel for periodontal application: preparation, antibacterial activity and toxicity evaluation. J. Mat. Sci. Mat. Med. 21 (8), 2435–2442. doi:10.1007/s10856-010-4098-1
Jingwen, Y., Guohua, Y., and Zhi, C. (2016). Pulp regeneration: current approaches and future challenges. Front. Physiology 7, 58. doi:10.3389/fphys.2016.00058
Joshi, D., Garg, T., Goyal, A. K., and Rath, A. (2016). Advanced drug delivery approaches against periodontitis. Drug Deliv. 23 (2), 363–377. doi:10.3109/10717544.2014.935531
Kausar, A. (2021). Polymer and modified chitosan-based nanocomposite: impending material for technical application. Polymer-Plastics Technol. Mater. 58 (9), 934–947. doi:10.1080/25740881.2019.1587771
Ke, C.-L., Deng, F.-S., Chuang, C.-Y., and Lin, C.-H. (2021). Antimicrobial actions and applications of chitosan. Polymers 13, 904. doi:10.3390/polym13060904
Keegan, G. M., Smart, J. D., Ingram, M. J., Barnes, L., Burnett, G. R., and Rees, G. D. (2012). Chitosan microparticles for the controlled delivery of fluoride. J. Dent. 40, 229–240. doi:10.1016/j.jdent.2011.12.012
Khajuria, D. K., Zahra, S. F., and Razdan, R. (2018). Effect of locally administered novel biodegradable chitosan based risedronate/zinc-hydroxyapatite intra-pocket dental film on alveolar bone density in rat model of periodontitis. J. Biomater. Sci. Polym. Ed. 29 (1), 74–91. doi:10.1080/09205063.2017.1400145
Khattak, S., Wahid, F., Liu, L. P., Jia, S. R., Chu, L. Q., Xie, X. Y., et al. (2019). Applications of cellulose and chitin/chitosan derivatives and composites as antibacterial materials: current state and perspectives. Appl. Microbiol. Biotechnol. 103 (5), 1989–2006. doi:10.1007/s00253-018-09602-0
Khoroushi, M., and Keshani, F. (2013). A review of glass-ionomers: from conventional glass-ionomer to bioactive glass-ionomer. Dent. Res. J. (Isfahan) 10 (4), 411–420.
Khutoryanskiy, V. V. (2011). Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 11 (6), 748–764. doi:10.1002/mabi.201000388
Kilicarslan, M., Ilhan, M., Inal, O., and Orhan, K. (2018). Preparation and evaluation of clindamycin phosphate loaded chitosan/alginate polyelectrolyte complex film as mucoadhesive drug delivery system for periodontal therapy. Eur. J. Pharm. Sci. 123, 441–451. doi:10.1016/j.ejps.2018.08.007
Kim, C. H., Choi, J. W., Chun, H. J., and Choi, K. S. (1997). Synthesis of chitosan derivatives with quaternary ammonium salt and their antibacterial activity. Polym. Bull. 38 (4), 387–393. doi:10.1007/s002890050064
Kim, I. Y., Seo, S. J., Moon, H. S., Yoo, M. K., Park, I. Y., Kim, B. C., et al. (2008). Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 26 (1), 1–21. doi:10.1016/j.biotechadv.2007.07.009
Kim, S. (2018). Competitive biological activities of chitosan and its derivatives: antimicrobial, antioxidant, anticancer, and anti-inflammatory activities. Polysaccharides Biomed. Appl. 2018, 1–13. doi:10.1155/2018/1708172
Kong, M., Chen, X. G., Xing, K., and Park, H. J. (2010). Antimicrobial properties of chitosan and mode of action: a state of the art review. Int. J. Food Microbiol. 144 (1), 51–63. doi:10.1016/j.ijfoodmicro.2010.09.012
Kumari, S., and Singh, R. P. (2013). Glycolic acid-functionalized chitosan-Co3O4-Fe3O4 hybrid magnetic nanoparticles-based nanohybrid scaffolds for drug-delivery and tissue engineering. J. Mat. Sci. 48, 1524–1532. doi:10.1007/s10853-012-6907-z
Kurita, K. (2001). Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 26, 1921–1971. doi:10.1016/s0079-6700(01)00007-7
Ladet, S., David, L., and Domard, A. (2008). Multi-membrane hydrogels. Nature 452, 76–79. doi:10.1038/nature06619
Lee, B. S., Lee, C. C., Wang, Y. P., Chen, H. J., Lai, C. H., Hsieh, W. L., et al. (2016). Controlled-release of tetracycline and lovastatin by poly (d, l-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. Int. J. Nanomedicine 11, 285–297. doi:10.2147/ijn.s94270
Lee, H. L., Wang, R. S., Hsu, Y. C., Chuang, C. C., Chan, H. R., Chiu, H. C., et al. (2018). Antifungal effect of tissue conditioners containing poly (acryloyloxyethyltrimethyl ammonium chloride)-grafted chitosan on Candida albicans growth in vitro. J. Dent. Sci. 13 (2), 160–166. doi:10.1016/j.jds.2017.06.004
Levengood, S. L., and Zhang, M. (2014). Chitosan-based scaffolds for bone tissue engineering. J. Mater Chem. B 2 (21), 3161–3184. doi:10.1039/c4tb00027g
Li, B., Xia, X., Guo, M., Jiang, Y., Zhang, Z., Liu, S., et al. (2019a). Biological and antibacterial properties of the micro-nanostructured hydroxyapatite/chitosan coating on titanium. Sci. Rep. 9, 14052. doi:10.1038/s41598-019-49941-0
Li, H., Li, P., Yang, Z., Gao, C., Fu, L., Liao, Z., et al. (2021). Meniscal regenerative scaffolds based on biopolymers and polymers: recent status and applications. Front. Cell Dev. Biol. 9, 661802. doi:10.3389/fcell.2021.661802
Li, J., Wu, Y., and Zhao, L. (2016). Antibacterial activity and mechanism of chitosan with ultra-high molecular weight. Carbohydr. Polym. 148, 200–205. doi:10.1016/j.carbpol.2016.04.025
Li, W., Long, Y., Liu, Y., Long, K., Liu, S., Wang, Z., et al. (2014). Fabrication and characterization of chitosan–collagen crosslinked membranes for corneal tissue engineering. J. Biomater. Sci. Polym. Ed. 25, 1962–1972. doi:10.1080/09205063.2014.965996
Li, Y., Qiao, Z., Yu, F., Hu, H., Huang, Y., Xiang, Q., et al. (2019b). Transforming growth factor-β3/chitosan sponge (TGF-β3/CS) facilitates osteogenic differentiation of human periodontal ligament stem cells. Int. J. Mol. Sci. 20 (20), 4982. doi:10.3390/ijms20204982
Liang, Y., Zhao, X., Ma, P. X., Guo, B., Du, Y., and Han, X. (2019). pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 536, 224–234. doi:10.1016/j.jcis.2018.10.056
Liao, Y., Li, H., Shu, R., Chen, H., Zhao, L., Song, Z., et al. (2020). Mesoporous hydroxyapatite/chitosan loaded with recombinant-human amelogenin could enhance antibacterial effect and promote periodontal regeneration. Front. Cell. Infect. Microbiol. 10, 180. doi:10.3389/fcimb.2020.00180
Lim, S. H., and Hudson, S. M. (2004). Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 339 (2), 313–319. doi:10.1016/j.carres.2003.10.024
Limapornvanich, A., Jitpukdeebodintra, S., Hengtrakool, C., and Kedjarune-Leggat, U. (2009). Bovine serum albumin release from novel chitosan-fluoro-aluminosilicate glass ionomer cement: stability and cytotoxicity studies. J. Dent. 37 (9), 686–690. doi:10.1016/j.jdent.2009.05.007
Lovecchio, J., Gargiulo, P., Vargas Luna, J. L., Giordano, E., and Sigurjónsson, Ó. E. (2019). A standalone bioreactor system to deliver compressive load under perfusion flow to hBMSCseeded 3D chitosan-graphene templates. Sci. Rep. 9 (1), 16854. doi:10.1038/s41598-019-53319-7
Loyola-Rodríguez, J. P., Torres-Méndez, F., Espinosa-Cristobal, L. F., García-Cortes, J. O., Loyola-Leyva, A., González, F. J., et al. (2019). Antimicrobial activity of endodontic sealers and medications containing chitosan and silver nanoparticles against Enterococcus faecalis. J. Appl. Biomater. Func. 17 (3), 228080001985177. doi:10.1177/2280800019851771
Lu, B., Lv, X., and Le, Y. (2019). Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers 11 (2), 304. doi:10.3390/polym11020304
Madi, M., Pavlic, V., Samy, W., and Alagl, A. (2018). The anti-inflammatory effect of locally delivered nano-doxycycline gel in therapy of chronic periodontitis. Acta Odontol. Scand. 76 (1), 71–76. doi:10.1080/00016357.2017.1385096
Mahima, V. G., Patil, K., Kulkarni, P. K., Tayal, S., and Keshari, D. (2015). Use of chitosan mouth-wash in radio-chemotherapy induced oral mucositis: a case-control study. J. Adv. Clin. Res. Insights 2, 248–252. doi:10.15713/ins.jcri.88
Mahmood, A., Lanthaler, M., Laffleur, F., Huck, C. W., and Bernkop-Schnürch, A. (2017). Thiolated chitosan micelles: highly mucoadhesive drug carriers. Carbohydr. Polym. 167, 250–258. doi:10.1016/j.carbpol.2017.03.019
Maliki, S., Sharma, G., Kumar, A., Moral-Zamorano, M., Moradi, O., Baselga, J., et al. (2022). Chitosan as a tool for sustainable development: a mini review. Polym. (Basel) 14 (7), 1475. doi:10.3390/polym14071475
Malinowska, S. K., Kaczmarek, U., Malicka, B., Walczak, K., and Zietek, M. (2017). Application of chitosan and propolis in endodontic treatment: a review. Mini Rev. Med. Chem. 17 (5), 410–434. doi:10.2174/1389557516666160418122510
Manni, L., Ghorbel-Bellaaj, O., Jellouli, K., Younes, I., and Nasri, M. (2010). Extraction and characterization of chitin, chitosan, and protein hydrolysates prepared from shrimp waste by treatment with crude protease from Bacillus cereus SV1. Appl. Biochem. Biotechnol. 162 (2), 345–357. doi:10.1007/s12010-009-8846-y
Mansuri, S., Kesharwani, P., Jain, K., Tekade, R. K., and Jain, N. (2016). Mucoadhesion: a promising approach in drug delivery system. React. Funct. Polym. 100, 151–172. doi:10.1016/j.reactfunctpolym.2016.01.011
Marrazzo, P., Paduano, F., Palmieri, F., Marrelli, M., and Tatullo, M. (2016). Highly efficient in vitro reparative behaviour of dental pulp stem cells cultured with standardised platelet lysate supplementation. Stem Cells Int. 2016, 1–16. doi:10.1155/2016/7230987
Matica, M. A., Aachmann, F. L., Tøndervik, A., Sletta, H., and Ostafe, V. (2019). Chitosan as a wound dressing starting material: antimicrobial properties and mode of action. Int. J. Mol. Sci. 20 (23), 5889. doi:10.3390/ijms20235889
Merzendorfer, H. (2011). The cellular basis of chitin synthesis in fungi and insects: common principles and differences. Eur. J. Cell Biol. 90, 759–769. doi:10.1016/j.ejcb.2011.04.014
Min, H. S., You, D. G., Son, S., Jeon, S., Park, J. H., Lee, S., et al. (2015). Echogenic glycol chitosan nanoparticles for ultrasound-triggered cancer theranostics. Theranostics 5 (12), 1402–1418. doi:10.7150/thno.13099
Minervini, G., Franco, R., Marrapodi, M. M., Di Blasio, M., Cicciù, M., and Ronsivalle, V. (2024). The effectiveness of chitosan as a hemostatic in dentistry in patients with antiplatelet/anticoagulant therapy: systematic review with meta-analysis. BMC Oral Health 24 (1), 70. doi:10.1186/s12903-023-03568-w
Mohan, K., Ganesan, A. R., Muralisankar, T., Jayakumar, R., Sathishkumar, P., Uthayakumar, V., et al. (2020). Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 105, 17–42. doi:10.1016/j.tifs.2020.08.016
Moreira, M. S., Sarra, G., Carvalho, G. L., Gonçalves, F., Caballero-Flores, H. V., Pedroni, A. C. F., et al. (2021). Physical and biological properties of a chitosan hydrogel scaffold associated to photobiomodulation therapy for dental pulp regeneration: an in vitro and in vivo study. BioMed Res. Int. 2021, 1–10. doi:10.1155/2021/6684667
Mulder, R., and Anderson-Small, C. (2019). Ion release of chitosan and nanodiamond modified glass ionomer restorative cements. Clin. Cosmet. Investig. Dent. 11, 313–320. doi:10.2147/ccide.s220089
Murray, P. E., Windsor, L. J., Hafez, A. A., Stevenson, R. G., and Cox, C. F. (2003). Comparison of pulp responses to resin composites. Oper. Dent. 28 (3), 242–250.
Muthukrishnan, A., and Shanmughapriya, G. (2017). Chitosan in the treatment of radiotherapy induced oral mucositis in head and neck cancer patients: a randomisd clinical trial. Oral Surg. Oral Med. Oral Pathology Oral Radiology 124, E198–E199. doi:10.1016/J.Oooo.2017.05.502
Najeeb, S., Khurshid, Z., Siddiqui, F., Zohaib, S., and Zafar, M. S. (2017). Outcomes of dental implant therapy in patients with down syndrome: a systematic review. J. Evid. Based Dent. Pract. 17 (4), 317–323. doi:10.1016/j.jebdp.2017.05.003
Najeeb, S., Khurshid, Z., Zohaib, S., and Zafar, M. S. (2016). Bioactivity and osseointegration of PEEK are inferior to those of titanium-A systematic review. J. Oral Implantol. 42, 512–516. doi:10.1563/aaid-joi-d-16-00072
Namangkalakul, W., Benjavongkulchai, S., Pochana, T., Promchai, A., Satitviboon, W., Howattanapanich, S., et al. (2020). Activity of chitosan antifungal denture adhesive against common Candida species and Candida albicans adherence on denture base acrylic resin. J. Prosthet. Dent. 123 (1), 181. e1–e181.e7. doi:10.1016/j.prosdent.2019.09.026
Nava Juárez, E. (2023). Biological effects of chitosan in Dentistry. Mexican J. Med. Res. ICSA 11 (22), 36–40. doi:10.29057/mjmr.v11i22.10635
Newman, M. G., Takei, H., Klokkevold, P. R., and Carranza, F. A. (2011). Carranza's clinical periodontology. Elsevier health sciences.
Nie, L., Wu, Q., Long, H., Hu, K., Li, P., Wang, C., et al. (2019). Development of chitosan/gelatin hydrogels incorporation of biphasic calcium phosphate nanoparticles for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 30 (17), 1636–1657. doi:10.1080/09205063.2019.1654210
Nitschke, J., Altenbach, H., Malolepszy, T., and Mölleken, H. (2011). A new method for the quantification of chitin and chitosan in edible mushrooms. Carbohydr. Res. 346, 1307–1310. doi:10.1016/j.carres.2011.03.040
Norowski, P. A., Courtney, H. S., Babu, J., Haggard, W. O., and Bumgardner, J. D. (2011). Chitosan coatings deliver antimicrobials from titanium implants: a preliminary study. Implant Dent. 20 (1), 56–67. doi:10.1097/id.0b013e3182087ac4
Norowski, P. A., Fujiwara, T., Clem, W. C., Adatrow, P. C., Eckstein, E. C., Haggard, W. O., et al. (2015). Novel naturally crosslinked electrospun nanofibrous chitosan mats for guided bone regeneration membranes: material characterization and cytocompatibility. J. Tissue Eng. Regen. Med. 9, 577–583. doi:10.1002/term.1648
Oryan, A., and Sahvieh, S. (2017). Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 104, 1003–1011. doi:10.1016/j.ijbiomac.2017.06.124
Panahi, F., Rabiee, S. M., and Shidpour, R. (2017). Synergic effect of chitosan and dicalcium phosphate on tricalcium silicate-based nanocomposite for root-end dental application. Mater. Sci. Eng. C 80, 631–641. doi:10.1016/j.msec.2017.07.012
Pang, X., and Huang, Y. (2012). Physical properties of nano-HAs/ZrO2 coating on surface of titanium materials used in dental-implants and its biological compatibility. J. Nanosci. Nanotechnol. 12, 902–910. doi:10.1166/jnn.2012.5666
Paradowska-Stolarz, A., Mikulewicz, M., Laskowska, J., Karolewicz, B., and Owczarek, A. (2023). The importance of chitosan coatings in dentistry. Mar. Drugs 21 (12), 613. doi:10.3390/md21120613
Parsa, P., Paydayesh, A., and Davachi, S. M. (2019). Investigating the effect of tetracycline addition on nanocomposite hydrogels based on polyvinyl alcohol and chitosan nanoparticles for specific medical applications. Int. J. Biol. Macromol. 121, 1061–1069. doi:10.1016/j.ijbiomac.2018.10.074
Paul, W., and Sharma, C. P. (2004). Chitosan and alginate wound dressings: a short review. Trends Biomater. Artif. Organs 18, 18–23.
Perchyonok, V. T., Zhang, S., Grobler, S. R., and Oberholzer, T. G. (2013). Insights into and relative effect of chitosan-H, chitosan-H-propolis, chitosan-H-propolis-nystatin and chitosan-H-nystatin on dentine bond strength. Eur. J. Dent. 7, 412–418. doi:10.4103/1305-7456.120666
Pichayakorn, W., and Boonme, P. (2013). Evaluation of cross-linked chitosan microparticles containing metronidazole for periodontitis treatment. Mat. Sci. Eng. C 33, 1197–1202. doi:10.1016/j.msec.2012.12.010
Pini, N. I. P., Lima, DANL, Luka, B., Ganss, C., and Schlueter, N. (2020). Viscosity of chitosan impacts the efficacy of F/Sn containing toothpastes against erosive/abrasive wear in enamel. J. Dent. 92, 103247. doi:10.1016/j.jdent.2019.103247
Pornpitchanarong, C., Rojanarata, T., Opanasopit, P., Ngawhirunpat, T., and Patrojanasophon, P. (2020). Catechol-modified chitosan/hyaluronic acid nanoparticles as a new avenue for local delivery of doxorubicin to oral cancer cells. Colloids Surf. B Biointerfaces 196, 111279. doi:10.1016/j.colsurfb.2020.111279
Prabaharan, M. (2014). Bioactivity of chitosan derivative. Polysaccharides, 1–14. doi:10.1007/978-3-319-03751-6_17-1
Priyadarsini, S., Mukherjee, S., and Mishra, M. (2018). Nanoparticles used in dentistry: a review. J. oral Biol. craniofacial Res. 8 (1), 58–67. doi:10.1016/j.jobcr.2017.12.004
Qin, W., Chen, J. Y., Guo, J., Ma, T., Weir, M. D., Guo, D., et al. (2018). Novel calcium phosphate cement with metformin-loaded chitosan for odontogenic differentiation of human dental pulp cells. Stem Cells Int. 2018, 1–10. doi:10.1155/2018/7173481
Qin, Y., and Li, P. (2020). Antimicrobial chitosan conjugates: current synthetic strategies and potential applications. Int. J. Mol. Sci. 21 (2), 499. doi:10.3390/ijms21020499
Qu, J., Zhao, X., Liang, Y., Zhang, T., Ma, P. X., and Guo, B. (2018). Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 183, 185–199. doi:10.1016/j.biomaterials.2018.08.044
Qu, S., Ma, X., Yu, S., and Wang, R. (2023). Chitosan as a biomaterial for the prevention and treatment of dental caries: antibacterial effect, biomimetic mineralization, and drug delivery. Front. Bioeng. Biotechnol. 11, 1234758. doi:10.3389/fbioe.2023.1234758
Rabea, E. I., Badawy, M. E., Stevens, C. V., Smagghe, G., and Steurbaut, W. (2003). Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4 (6), 1457–1465. doi:10.1021/bm034130m
Raddall, G., Mello, I., and Leung, B. M. (2019). Biomaterials and scaffold design strategies for regenerative endodontic therapy. Front. Bioeng. Biotechnol. 7, 317. doi:10.3389/fbioe.2019.00317
Rakkiettiwong, N., Hengtrakool, C., Thammasitboon, K., and Kedjarune-Leggat, U. (2011). Effect of novel chitosan-fluoroaluminosilicate glass ionomer cement with added transforming growth factor beta-1 on pulp cells. J. Endod. 37 (3), 367–371. doi:10.1016/j.joen.2010.11.031
Rasti, H., Parivar, K., Baharara, J., Iranshahi, M., and Namvar, F. (2017). Chitin from the mollusc Chiton: extraction, characterization and chitosan preparation. Iran. J. Pharm. Res. 16 (1), 366–379.
Raura, N., Garg, A., Arora, A., and Roma, M. (2020). Nanoparticle technology and its implications in endodontics: a review. Biomater. Res. 24 (21), 21–28. doi:10.1186/s40824-020-00198-z
Redepenning, J., Venkataraman, G., Chen, J., and Stafford, N. (2003). Electrochemical preparation of chitosan/hydroxyapatite composite coatings on titanium substrates. J. Biomed. Mater Res. A 66 (2), 411–416. doi:10.1002/jbm.a.10571
Ren, Q., Ding, L., Li, Z., Wang, X., Wang, K., Han, S., et al. (2019). Chitosan hydrogel containing amelogenin-derived peptide: inhibition of cariogenic bacteria and promotion of remineralization of initial caries lesions. Arch. Oral Biol. 100, 42–48. doi:10.1016/j.archoralbio.2019.02.004
Retana-Lobo, C. (2018). Dental pulp regeneration: insights from biological processes. Odovtos 20 (1), 10–16. doi:10.15517/ijds.v0i0.31269
Rodrigues, S., Dionísio, M., López, C. R., and Grenha, A. (2012). Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 3 (3), 615–641. doi:10.3390/jfb3030615
Saboktakin, M. R., Tabatabaie, R., Maharramov, A., and Ramazanov, M. A. (2010). Synthesis and characterization of superparamagnetic chitosan—dextran sulfate hydrogels as nano carriers for colon-specific drug delivery. Carbohydr. Polym. 81, 372–376. doi:10.1016/j.carbpol.2010.02.034
Sacco, P., Furlani, F., De Marzo, G., Marsich, E., Paoletti, S., and Donati, I. (2018). Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 4 (3), 67. doi:10.3390/gels4030067
Saeed, A., Haider, A., Zahid, S., Khan, S. A., Faryal, R., and Kaleem, M. (2019). In-vitro antifungal efficacy of tissue conditioner-chitosan composites as potential treatment therapy for denture stomatitis. Int. J. Biol. Macromol. 125, 761–766. doi:10.1016/j.ijbiomac.2018.12.091
Sahariah, P., and Másson, M. (2017). Antimicrobial chitosan and chitosan derivatives: a review of the structure–activity relationship. Biomacromolecules 18 (11), 3846–3868. doi:10.1021/acs.biomac.7b01058
Salari, M., Khiabani, M. S., Mokarram, R. R., Ghanbarzadeh, B., and Kafil, H. S. (2018). Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 84, 414–423. doi:10.1016/j.foodhyd.2018.05.037
Sanap, P., Hegde, V., Ghunawat, D., Patil, M., Nagaonkar, N., and Jagtap, V. (2020). Current applications of chitosan nanoparticles in dentistry: a review. Int. J. Appl. Dent. Sci. 6 (4), 81–84. doi:10.22271/oral.2020.v6.i4b.1050
Saranya, N., Saravanan, S., Moorthi, A., Ramyakrishna, B., and Selvamurugan, N. (2011). Enhanced osteoblast adhesion on polymeric nano-scaffolds for bone tissue engineering. J. Biomed. Nanotechnol. 7 (2), 238–244. doi:10.1166/jbn.2011.1283
Sarasam, A. R., Brown, P., Khajotia, S. S., Dmytryk, J. J., and Madihally, S. V. (2008). Antibacterial activity of chitosan-based matrices on oral pathogens. J. Mat. Sci. Mat. Med. 19, 1083–1090. doi:10.1007/s10856-007-3072-z
Saravanan, S., Sameera, D. K., Moorthi, A., and Selvamurugan, N. (2013). Chitosan scaffolds containing chicken feather keratin nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 62, 481–486. doi:10.1016/j.ijbiomac.2013.09.034
Schmalz, G., Widbiller, M., and Galler, K. M. (2017). Signaling molecules and pulp regeneration. J. Endod. 43 (9S), S7–S11. doi:10.1016/j.joen.2017.06.003
Senthil, K. R., Ravikumar, N., Kavitha, S., Mahalaxmi, S., Jayasree, R., Sampath, K. T. S., et al. (2017). Nanochitosan modified glass ionomer cement with enhanced mechanical properties and fluoride release. Int. J. Biol. Macromol. 104, 1860–1865. doi:10.1016/j.ijbiomac.2017.05.120
Shaik, J., Garlapati, R., Nagesh, B., Sujana, V., Jayaprakash, T., and Naidu, S. (2014). Comparative evaluation of antimicrobial efficacy of triple antibiotic paste and calcium hydroxide using chitosan as carrier against Candida albicans and Enterococcus faecalis: an in vitro study. J. Conserv. Dent. 17 (4), 335–339. doi:10.4103/0972-0707.136444
Sharifi-Rad, J., Quispe, C., Butnariu, M., Rotariu, L. S., Sytar, O., Sestito, S., et al. (2021). Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 21 (1), 318. doi:10.1186/s12935-021-02025-4
Shen, J., Jin, B., Qi, Y. C., Jiang, Q. Y., and Gao, X. F. (2017). Carboxylated chitosan/silverhydroxyapatite hybrid microspheres with improved antibacterial activity and cytocompatibility. Mat. Sci. Eng. C 78, 589–597. doi:10.1016/j.msec.2017.03.100
Shi, C., Zhu, Y., Ran, X., Wang, M., Su, Y., and Cheng, T. (2006). Therapeutic potential of chitosan and its derivatives in regenerativeMedicine. J. Surg. Res. 133 (2), 185–192. doi:10.1016/j.jss.2005.12.013
Shrestha, A., and Kishen, A. (2016). Antibacterial nanoparticles in endodontics: a review. J. Endod. 42 (10), 1417–1426. doi:10.1016/j.joen.2016.05.021
Sidhu, S. K., and Nicholson, J. W. (2016). A review of glass-ionomer cements for clinical dentistry. J. Funct. Biomater. 7 (3), 16. doi:10.3390/jfb7030016
Silva, D., Arancibia, R., Tapia, C., Acuña-Rougier, C., Diaz-Dosque, M., Cáceres, M., et al. (2013). Chitosan and platelet-derived growth factor synergistically stimulate cell proliferation in gingival fibroblasts. J. Periodontal Res. 48 (6), 677–686. doi:10.1111/jre.12053
Singh, K., Tiwary, A. K., and Rana, V. (2013). Spray dried chitosan-EDTA superior microparticles as solid substrate for the oral delivery of amphotericin B. Int. J. Biol. Macromol. 58, 310–319. doi:10.1016/j.ijbiomac.2013.04.053
Sinha, V., Singla, A. K., Wadhawan, S., Kaushik, R., Kumria, R., Bansal, K., et al. (2004). Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 274 (1–2), 1–33. doi:10.1016/j.ijpharm.2003.12.026
Sivanesan, I., Gopal, J., Muthu, M., Shin, J., and Oh, J. W. (2021). Reviewing chitin/chitosan nanofibers and associated nanocomposites and their attained medical milestones. Polymers 13 (14), 2330. doi:10.3390/polym13142330
Soares, D. G., Anovazzi, G., Bordini, E. A. F., Zuta, U. O., Silva Leite, M. L. A., Basso, F. G., et al. (2018). Biological analysis of simvastatin-releasing chitosan scaffold as a cell-free system for pulp-dentin regeneration. J. Endod. 44 (6), 971–976.e1. doi:10.1016/j.joen.2018.02.014
Soran, Z., Aydin, R. S., and Gumusderelioglu, M. (2012). Chitosan scaffolds with BMP-6 loaded alginate microspheres for periodontal tissue engineering. J. Microencapsul. 29, 770–780. doi:10.3109/02652048.2012.686531
Soskolne, W. A. (1997). Subgingival delivery of therapeutic agents in the treatment of periodontal diseases. Crit. Rev. Oral Biol. Med. 8 (2), 164–174. doi:10.1177/10454411970080020501
Soygun, K., Soygun, A., and Dogan, M. C. (2021). The effects of chitosan addition to glass ionomer cement on microhardness and surface roughness. J. Appl. Biomater. Funct. Mater 19, 228080002198970. doi:10.1177/2280800021989706
Stenhagen, I. S. R., Rukke, H. V., Dragland, I. S., and Kopperud, H. M. (2019). Effect of methacrylated chitosan incorporated in experimental composite and adhesive on mechanical properties and biofilm formation. Eur. J. Oral Sci. 127 (1), 81–88. doi:10.1111/eos.12584
Su, W. T., Wu, P. S., Ko, C. S., and Huang, T. Y. (2014). Osteogenic differentiation and mineralization of human exfoliated deciduous teeth stem cells on modified chitosan scaffold. Mat. Sci. Eng. C 41, 152–160. doi:10.1016/j.msec.2014.04.048
Sukpaita, T., Chirachanchai, S., Suwattanachai, P., Everts, V., Pimkhaokham, A., and Ampornaramveth, R. S. (2019). In vivo bone regeneration induced by a scaffold of chitosan/dicarboxylic acid seeded with human periodontal ligament cells. Int. J. Mol. Sci. 19, 4883. doi:10.3390/ijms20194883
Sultankulov, B., Berillo, D., Sultankulova, K., Tokay, T., and Saparov, A. (2019). Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 9 (9), 470. doi:10.3390/biom9090470
Sun, I. C., Na, J. H., Jeong, S. Y., Kim, D. E., Kwon, I. C., Choi, K., et al. (2014). Biocompatible glycol chitosan-coated gold nanoparticles for tumor-targeting CT imaging. Pharm. Res. 31 (6), 1418–1425. doi:10.1007/s11095-013-1142-0
Suneetha, M., Rao, K. M., and Han, S. S. (2020). Mechanically improved porous hydrogels with polysaccharides via polyelectrolyte complexation for bone tissue engineering. Int.J. Biol. Macromol. 144, 160–169. doi:10.1016/j.ijbiomac.2019.12.096
Takeuchi, I., Togo, C., and Makino, K. (2019). Rebamipide-containing film using chitosan and HPMC for oral mucositis induced by cancer chemotherapy. Anticancer Res. 39 (12), 6531–6536. doi:10.21873/anticanres.13868
Tanir, T. E., Hasirci, V., and Hasirci, N. (2014). Electrospinning of chitosan/poly (lactic acid-coglycolic acid)/hydroxyapatite composite nanofibrous mats for tissue engineering applications. Polym. Bull. 71 (11), 2999–3016. doi:10.1007/s00289-014-1234-y
Taser, B., Ozkan, H., Adiguzel, A., Orak, T., Baltaci, M. O., and Taskin, M. (2021). Preparation of chitosan from waste shrimp shells fermented with Paenibacillus jamilae BAT1. Int. J. Biol. Macromol. 183, 1191–1199. doi:10.1016/j.ijbiomac.2021.05.062
Tatullo, M., Codispoti, B., Pacifici, A., Palmieri, F., Marrelli, M., Pacifici, L., et al. (2017). Potential use of human periapical cyst-mesenchymal stem cells (hPCy-MSCs) as a novel stem cell source for regenerative medicine applications. Front. Cell Dev. Biol. 5, 103. doi:10.3389/fcell.2017.00103
Thomas, L. V., Rahul, V., and Nair, P. D. (2017). Effect of stiffness of chitosan-hyaluronic acid dialdehyde hydrogels on the viability and growth of encapsulated chondrocytes. Int. J. Biol. Macromol. 104, 1925–1935. doi:10.1016/j.ijbiomac.2017.05.116
Vázquez, M. R., Vega-Ruiz, B., Zúñiga, R. R., Koppel, D. A. S., and Olvera, L. F. Q. (2015). Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed Res. Int. 2015, 1–15. doi:10.1155/2015/821279
Vetter, J. (2007). Chitin content of cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. Food Chem. 102, 6–9. doi:10.1016/j.foodchem.2006.01.037
Vijayan, A. A. S., and Kumar, G. S. V. (2019). PEG grafted chitosan scaffold for dual growth factor delivery for enhanced wound healing. Sci. Rep. 9, 19165. doi:10.1038/s41598-019-55214-7
Vining, K., and Mooney, D. (2017). Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 18, 728–742. doi:10.1038/nrm.2017.108
Wang, C. H., Liu, W. S., Sun, J. F., Hou, G. G., Chen, Q., Cong, W., et al. (2016). Non-toxic Oquaternized chitosan materials with better water solubility and antimicrobial function. Int. J. Biol. Macromol. 84, 418–427. doi:10.1016/j.ijbiomac.2015.12.047
Wang, J. J., Zeng, Z. W., Xiao, R. Z., Xie, T., Zhou, G. L., Zhan, X. R., et al. (2011). Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomedicine 6, 765–774. doi:10.2147/ijn.s17296
Wang, W., Meng, Q., Li, Q., Liu, J., Zhou, M., Jin, Z., et al. (2020). Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 21 (2), 487. doi:10.3390/ijms21020487
Wang, Y., Liu, P., Du, J., Sun, Y., Li, F., and Duan, Y. (2013). Targeted siRNA delivery by anti-HER2 antibody-modified nanoparticles of mPEG-chitosan diblock copolymer. J. Biomater. Sci. Polym. Ed. 24, 1219–1232. doi:10.1080/09205063.2012.745716
Wassel, M. O., and Khattab, M. A. (2017). Antibacterial activity against Streptococcus mutans and inhibition of bacterial induced enamel demineralization of propolis, miswak, and chitosan nanoparticles based dental varnishes. J. Adv. Res. 8 (4), 387–392. doi:10.1016/j.jare.2017.05.006
Wei, Y., Hudson, S., Mayer, J., and Kaplan, D. (1992). The crosslinking of chitosan fibers. J. Polym. Sci. A Polym. Chem. 30 (10), 2187–2193. doi:10.1002/pola.1992.080301013
Wimardhani, Y. S., Suniarti, D. F., Freisleben, H. J., Wanandi, S. I., Siregar, N. C., and Ikeda, M. A. (2014a). Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J. Oral Sci. 56 (2), 119–126. doi:10.2334/josnusd.56.119
Wimardhani, Y. S., Suniarti, D. F., Freisleben, H. J., Wanandi, S. I., Siregar, N. C., and Ikeda, M. A. (2014b). Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J. Oral Sci. 56 (2), 119–126. doi:10.2334/josnusd.56.119
Wu, T., Zivanovic, S., Draughon, F. A., and Sams, C. E. (2004). Chitin and chitosan--value-added products from mushroom waste. J. Agric. Food Chem. 52 (26), 7905–7910. doi:10.1021/jf0492565
Xia, W., Liu, P., Zhang, J., and Chen, J. (2011). Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 25 (2), 170–179. doi:10.1016/j.foodhyd.2010.03.003
Xu, H., Fang, Z., Tian, W., Wang, Y., Ye, Q., Zhang, L., et al. (2018). Green fabrication of amphiphilic quaternized β-chitin derivatives with excellent biocompatibility and antibacterial activities for wound healing. Adv. Mat. 30 (29), 1801100. doi:10.1002/adma.201801100
Xu, T., Xin, M., Li, M., Huang, H., Zhou, S., and Liu, J. (2011). Synthesis, characterization, and antibacterial activity of N, O-quaternary ammonium chitosan. Carbohydr. Res. 346 (15), 2445–2450. doi:10.1016/j.carres.2011.08.002
Xue, Y., Hong, X., Gao, J., Shen, R., and Ye, Z. (2019). Preparation and biological characterization of the mixture of poly (lactic-co-glycolic acid)/chitosan/Ag nanoparticles for periodontal tissue engineering. Int. J. Nanomedicine 14, 483–498. doi:10.2147/ijn.s184396
Yadav, A., and Bhise, S. (2004). Chitosan: a potential biomaterial effective against typhoid. Curr. Sci. 87, 1176–1178.
Yadav, S. K., Khan, G., Bonde, G. V., Bansal, M., and Mishra, B. (2018). Design, optimization and characterizations of chitosan fortified calcium alginate microspheres for the controlled delivery of dual drugs. Artif. Cells Nanomed. Biotechnol. 46 (6), 1180–1193. doi:10.1080/21691401.2017.1366331
Yanat, M., and Schroën, K. (2021). Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 161, 104849. doi:10.1016/j.reactfunctpolym.2021.104849
Yang, X., Han, G., Pang, X., and Fan, M. (2020). Chitosan/collagen scaffold containing bone morphogenetic protein-7 DNA supports dental pulp stem cell differentiation in vitro and in vivo. J. Biomed. Mater Res. A 108 (12), 2519–2526. doi:10.1002/jbm.a.34064
Yilmaz, A. H. (2020). Antibacterial activity of chitosan-based systems. Funct. Chitosan, 457–489. doi:10.1007/978-981-15-0263-7_15
Younes, I., and Rinaudo, M. (2015). Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 13, 1133–1174. doi:10.3390/md13031133
Younes, L., Sellimi, S., Rinaudo, M., Jellouli, K., and Nasri, M. (2014). Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 185, 57–63. doi:10.1016/j.ijfoodmicro.2014.04.029
Young, A., Smistad, G., Karlsen, J., Rolla, G., and Rykke, M. (1997). Zeta potentials of human enamel and hydroxyapatite as measured by the Coulter® DELSA 440. Adv. Dent. Res. 11, 560–565. doi:10.1177/08959374970110042501
Zafar, M. S., and Ahmed, N. (2014). Nanomechanical characterization of exfoliated and retained deciduous incisors. Technol. Health Care 22, 785–793. doi:10.3233/thc-140852
Zhang, J., Boyes, V., Festy, F., Lynch, R. J., Watson, T. F., and Banerjee, A. (2018b). In-vitro subsurface remineralisation of artificial enamel white spot lesions pre-treated with chitosan. Dent. Mat. 34 (8), 1154–1167. doi:10.1016/j.dental.2018.04.010
Zhang, J., Lynch, R. J., Watson, T. F., and Banerjee, A. (2019). Chitosan-bioglass complexes promote subsurface remineralisation of incipient human carious enamel lesions. J. Dent. 84, 67–75. doi:10.1016/j.jdent.2019.03.006
Zhang, J., Tan, W., Wang, G., Yin, X., Li, Q., Dong, F., et al. (2018a). Synthesis, characterization, and the antioxidant activity of N, N, N-trimethyl chitosan salts. Int. J. Biol. Macromol. 118, 9–14. doi:10.1016/j.ijbiomac.2018.06.018
Zhang, L., Liu, Y., Yu, D., and Zhangl, N. (2013). Gadolinium-loaded chitosan nanoparticles as magnetic resonance imaging contrast agents for the diagnosis of tumor. J. Biomed. Nanotechnol. 9 (5), 863–869. doi:10.1166/jbn.2013.1584
Zhang, M., Li, X. H., Gong, Y. D., Zhao, N. M., and Zhang, X. F. (2002). Properties and biocompatibility of chitosan films modified by blending with PEG. Biomaterials 23 (13), 2641–2648. doi:10.1016/s0142-9612(01)00403-3
Zhang, Y., Wei, W., Lv, P., Wang, L., and Ma, G. (2011). Preparation and evaluation of alginate—chitosan microspheres for oral delivery of insulin. Eur. J. Pharm. Biopharm. 77, 11–19. doi:10.1016/j.ejpb.2010.09.016
Zhao, J., Guo, B., and Ma, P. X. (2014). Injectable alginate microsphere/PLGA–PEG–PLGA composite hydrogels for sustained drug release. RSC Adv. 4 (34), 17736–17742. doi:10.1039/c4ra00788c
Zhao, L., Zhu, B., Jia, Y., Hou, W., and Su, C. (2013). Preparation of biocompatible carboxymethyl chitosan nanoparticles for delivery of antibiotic drug. Biomed. Res. Int. 2013, 1–7. doi:10.1155/2013/236469
Zhao, X., Wu, H., Guo, B., Dong, R., Qiu, Y., and Ma, P. X. (2017). Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 122, 34–47. doi:10.1016/j.biomaterials.2017.01.011
Zhou, J., Xu, Q., Fan, C., Ren, H., Xu, S., Hu, F., et al. (2019). Characteristics of chitosan-modified glass ionomer cement and their effects on the adhesion and proliferation of human gingival fibroblasts: an in vitro study. J. Mat. Sci. Mat. Med. 30 (3), 39. doi:10.1007/s10856-019-6240-z
Zhu, J., Ye, H., Deng, D., Li, J., and Wu, Y. (2020). Electrospun metformin-loaded polycaprolactone/chitosan nanofibrous membranes as promoting guided bone regeneration membranes: preparation and characterization of fibers, drug release, and osteogenic activity in vitro. J. Biomater. Appl. 34 (9), 1282–1293. doi:10.1177/0885328220901807
Zhu, N., Chatzistavrou, X., Ge, L., Qin, M., Papagerakis, P., and Wang, Y. (2019). Biological properties of modified bioactive glass on dental pulp cells. J. Dent. 83, 18–26. doi:10.1016/j.jdent.2019.01.017
Zhu, T., Shi, L., Ma, C., Xu, L., Yang, J., Zhou, G., et al. (2021). Fluorinated chitosan-mediated intracellular catalase delivery for enhanced photodynamic therapy of oral cancer. Biomater. Sci. 9 (3), 658–662. doi:10.1039/d0bm01898h
Keywords: chitosan, sustainable nanomaterials, organic-inorganic hybrid nanomaterial, bio-medical applications, dentistry
Citation: Mascarenhas R, Hegde S and Manaktala N (2024) Chitosan nanoparticle applications in dentistry: a sustainable biopolymer. Front. Chem. 12:1362482. doi: 10.3389/fchem.2024.1362482
Received: 28 December 2023; Accepted: 26 March 2024;
Published: 10 April 2024.
Edited by:
Qingli Hao, Nanjing University of Science and Technology, ChinaReviewed by:
Ceylan Hepokur, Cumhuriyet University, TürkiyeZiqing Wang, The University of Texas at Austin, United States
Copyright © 2024 Mascarenhas, Hegde and Manaktala. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nidhi Manaktala, bWFuYWt0YWxhLm5pZGhpQG1hbmlwYWwuZWR1
†ORCID: Roma Mascarenhas, orcid.org/0000-0003-3407-8685; Shreya Hegde, orcid.org/0000-0001-9084-3383; Nidhi Manaktala, orcid.org/0000-0003-0730-0914
 Roma Mascarenhas
Roma Mascarenhas Shreya Hegde
Shreya Hegde Nidhi Manaktala
Nidhi Manaktala