- 1Department of Chemistry, Kohat University of Science and Technology, Kohat, Khyber Pakhtunkhwa, Pakistan
- 2Qazi Hussain Ahmad Teaching Hospital, Nowshehra, Khyber Pakhtunkhwa, Pakistan
- 3Department of Chemistry, Bacha Khan University, Charsadda, Khyber Pakhtunkhwa, Pakistan
- 4State Key Laboratory of Chemical Oncogenomics, Guangdong Provincial Key Laboratory of Chemical Genomics, Peking University Shenzhen Graduate School, Shenzhen, Guangdong, China
- 5Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
- 6Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
SARS-CoV-2 infection affects and modulates serum as well as hematological parameters. However, whether it modifies these parameters in the existing disease conditions, which help in the erection of specific treatments for the disease, is under investigation. Here, we aimed to determine whether serum and hematological parameters alteration in various diseases, diabetes mellitus (DM), hypertension (HTN), ischemic heart disease (IHD) and myocardial infarction (MI) conditions correlate and signal SARS-CoV-2 infection, which could be used as a rapid diagnosis tool for SARS-CoV-2 infection in disease conditions. To assess the projected goals, we collected blood samples of 1,113 male and female patients with solo and multiple disease conditions of DM/HTN/IHD/MI with severe COVID-19, followed by biochemical analysis, including COVID-19 virus detection by RT-qPCR. Furthermore, blood was collected from age-matched disease and healthy individuals 502 and 660 and considered as negative control. In our results, we examined higher levels of serum parameters, including D-dimer, ferritin, hs-CRP, and LDH, as well as hematological parameters, including TLC in sole and multiple diseases (DM/HTN/IHD/MI) conditions compared to the control subjects. Besides, the hematological parameters, including Hb, RBC, and platelet levels, decreased in the patients. In addition, we found declined levels of leukocyte count (%), lymphocyte (%), monocyte (%), and eosinophil (%), and elevated level of neutrophil levels (%) in all the disease patients infected with SARS-CoV-2. Besides, NLR and NMR ratios were also statistically significantly (p < 0.05) high in the patients with solo and multiple disease conditions of DM/HTN/IHD/MI infected with the SARS-CoV-2 virus. In conclusion, rapid alteration of sera and hematological parameters are associated with SARS-CoV-2 infections, which could help signal COVID-19 in respective disease patients. Moreover, our results may help to improve the clinical management for the rapid diagnosis of COVID-19 concurrent with respective diseases.
Introduction
The Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic has devastated humanity since its outbreak in 2019 (Chekol Abebe et al., 2022). Great efforts have been made to describe its pathophysiology and develop more effective treatments (Wang and Yang, 2023; Yang and Wang, 2023) In addition to the lungs, the virus has been proven to damage several other systems, including the heart, gastrointestinal tract, neurological system, kidney, coagulation, and endothelium (Iba et al., 2020; Puelles et al., 2020). However, a number of hematological parameters, such as lymphopenia and increased D-dimers, have been observed in COVID-19 patients (Rahi et al., 2021). Several studies have consistently indicated that higher red blood cell distribution width predicts worse outcomes in individuals with COVID-19 infection. However, anemia has been reported to be an independent predictor of disease severity in COVID-19 infection (Bellmann-Weiler et al., 2020; Higgins et al., 2020; Banon et al., 2021).
Red blood cells (RBC) are the carriers of oxygen throughout the body and are known to lyse in cases of infection (Bouchla et al., 2022), especially with the increasing complications of sepsis (Bateman et al., 2017). Previous studies have shown that the virus can invade erythrocytes by interacting its S1 spike protein with erythrocyte CD147 (Al-Salih et al., 2020) and the band-3 protein (Cosic et al., 2020). However, viral spike proteins and complement activation products have been detected on the cell surface of erythrocytes from patients with COVID-19. Therefore, it is thought to affect erythrocyte rheology, leading to intravascular thrombosis and associated lung injury (Lam et al., 2021).
Additionally, the hypercoagulation and inflammatory state lead to more fragility and make elastic the membrane of RBC (Grobler et al., 2020). It has been demonstrated that high serum ferritin levels in COVID-19 patients might result in hyper-activated interactions between erythrocytes and platelets (Youssry et al., 2022), indicating a connection between erythrocyte alterations and thrombosis in COVID-19 disease (Venter et al., 2020).
The understanding about the immune response of SARS-CoV-2 has been studied in detail in different cohort studies. Moreover, due to the significant sequence homology throughout the coronavirus family, several scientists have hypothesized the interaction between SARS-CoV-2 and the host organism may be related to another coronavirus (Vabret et al., 2020). Previously, the immunological dysfunction caused by SARS-CoV-2 confirmed the activation of innate and adaptive immune responses (Yang et al., 2004; Chu et al., 2016). Therefore, viral infection may have damaged the immune system and may be reflected as a change in peripheral blood cells.
Lymphocytes are essential for maintaining immunological homeostasis during infection (Channappanavar et al., 2014). Several studies have shown that lymphopenia can predict longevity in COVID-19 patients (Yang et al., 2004; Channappanavar et al., 2014) Additionally, numerous investigations have revealed that eosinophilia is associated with poor prognosis. (Cortés-Vieyra et al., 2021; Singh et al., 2022). Thus, immunological damage at an early stage of the disease may be indicated by the differentiation of peripheral leukocytes. However, modulation in the levels of eosinophils are the risk factors for changes in peripheral blood cells (Tong et al., 2021).
This study aimed to analyze the effect of COVID-19 on serum and hematological parameters in, DM, HTN, and different cardiovascular diseases before a detailed investigation as a rapid diagnostic tool to reduce the risk of mortality in respective patients.
Material and methods
Data was collected from 1,113 hospitalized patients, including 495 males and 618 females, in the intensive care unit of Qazi Hussain Ahmad Teaching Hospital, Nowshehra, Pakistan, due to severe COVID-19 disease with SpO2 levels less than 75 mmHg, from April 2020 to January 2023. In addition, earliest clinical manifestations such as fever, cough, dyspnea, muscle pain, diarrhea fatigue, and loss of smell and taste recorded in medical history taken upon admission. Furthermore, in the present research work only those patients and normal individuals were considered who were not previously vaccinated. Herein, two control groups were used to better understand the results and their blood was collected from respective departments. Control group 1 was composed of 502 male and female patients with DM, HTN, IHD and MI without COVID-19. However, in control group 2, 660 normal individuals, including 305 males and 355 females, were included without DM, HTN, IHD, MI and COVID-19. Furthermore, patients with renal diseases, hepatic dysfunction, anemia, myeloid tumor, or other diseases were excluded. Whole blood and serum samples were obtained from all participants in EDTA and serum tubes under the supervision of expert and qualified medical physician and standard operating procedures in the hospital. Laboratory investigations, chest CT imaging, and SpO2 levels were performed immediately after the hospitalization of patients. The present study was approved by the ethical committee of Kohat University of Science and Technology, Kohat, Pakistan under Reference No: Ref-No/KUST/Ethical Committee/528. In this study, written informed consent was obtained from all participants. The study was conducted following the guidelines of the Declaration of Helsinki (World Medical Association declaration of Helsinki, 2013: Ethical principles for medical research involving human subjects, 2013).
Hematological and biochemical analysis
An appropriate history and detailed clinical examination were recorded for each patient. Blood samples were collected at the time of admission to the hospital. However, various hematological investigations, including total leukocyte count (TLC), neutrophils, lymphocytes, monocytes, eosinophils, basophils, RBC, hemoglobin (Hb), and platelet count were investigated using Sysmex XP-300 automated analyzer. The standard biochemical analysis of serum components glucose, glycohemoglobin (HbA1c), urea, creatinine, serum glutamyl pyruvate transaminase (SGPT), alkaline phosphatase (ALP) and total bilirubin was performed according to the protocol performed in the routine examination in the hospital. D-dimer (D-Dimer Human ELISA Kit, Cat # EHDDIMER), ferritin (Ferritin Human ELISA Kit, Cat # EHFTL), high-sensitive C reactive protein (hs-CRP) (Boster Bio ELISA KIT, Cat # EK7040), Lactate dehydrogenase (LDH) (L- LDH reagent kit, Cat # TR20015), were performed both in the patients and the control groups according to the instructions provided with the kit.
RT-qPCR analysis
All the patients and control groups were investigated for C0VID-19 infection through RT-qPCR. In addition, only those patients who showed positive results were considered for further investigation while in the control groups who showed negative results were selected for further study. The RNA was collected through the MagMAX™ Viral/Pathogen Nucleic Acid Isolation Kit, Cat # A42352, Applied Biosystems, using the instructions provided with the kit. cDNA was synthesized from the RNA of the COVID-19 virus with the High-Capacity RNA-to-cDNA™ Kit, Cat #: 4387406, Applied Biosystems, using the instructions provided with the kit. cDNA was amplified through RT-qPCR with SYBR™ Select Master Mix, Cat #: 4472913, Applied Biosystems, using the instructions provided with the kit.
Statistical analysis
The SPSS 21 service (IBM Corp., Armonk, NY, United States) was used to analyze statistics among different groups using ANOVA followed by Tukey’s multiple comparison test. In addition, Student’s t-test was performed for comparison between the two groups. However, qualitative analysis was performed using Mann-Whitney t-test. All the data is presented as the mean ± SD. *p < 0.05 was considered to be statistically significant.
Results
In this study, hospitalized SARS-CoV-2 patients with different diseases, including COVID-19 only, DM, HTN, and other cardiovascular diseases, were included. In addition, an age and gender-matched healthy control group for each disease group was also included. The demographic characteristics showed that the ratio of COVID-19 was high in female patients of COVID-19 only, DM, MI, MI with HTN, MI with DM, and MI with DM and HTN patients. In addition, the ratio of COVID-19 was high in male patients in the HTN, IHD, IHD with DM, and DM with HTN groups given in Figure 1.
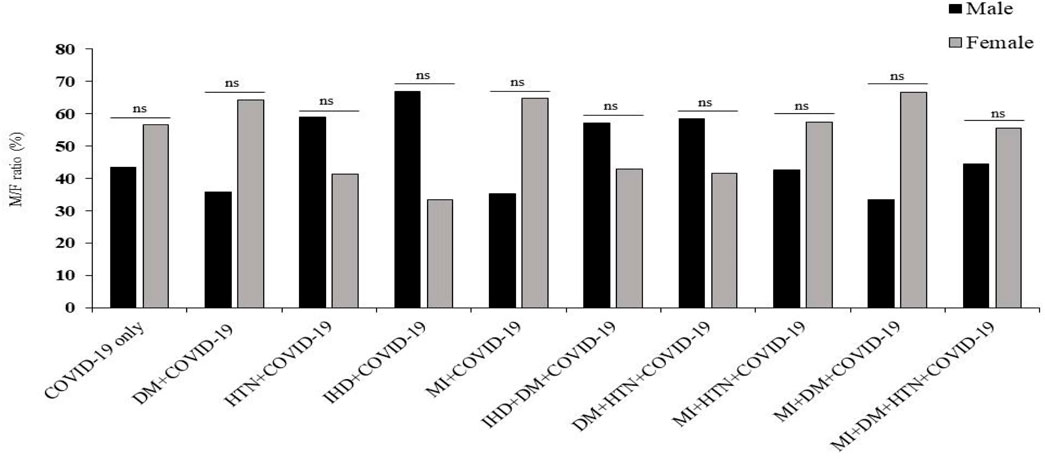
Figure 1. Prevalence of COVID-19 infection in male and female patients with DM, HTN, IHD and MI. δ Mann-Whitney t-test. *p-value was significant when less than 0.05. ns: non-significant.
Serum markers
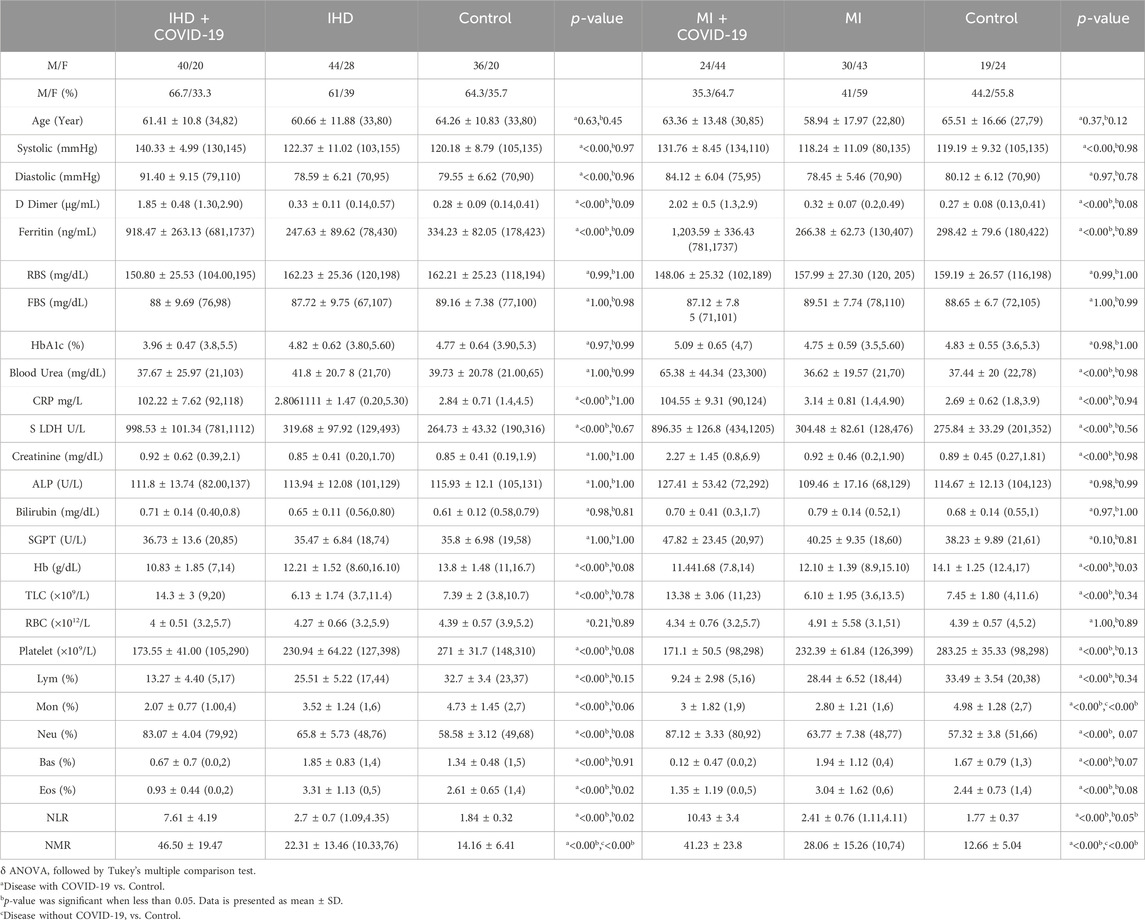
In this cohort study, we have thoroughly investigated and compared patient characteristics among the patients with severe COVID-19 disease. D-dimer is one of the investigations performed in COVID-19 patients to detect thrombosis. However, previous studies showed that a 3 to 4-fold increase in D-dimer levels is associated with a better prognosis in COVID-19 patients (Yao et al., 2020). In the present finding, the levels of D-dimer were significantly increased in patients with COVID-19 only, and solo disease condition of DM, and HTN infected with COVID-19 (2 ± 0.55, 1.80 ± 0.34 and 1.50 ± 0.12 μg/mL, respectively) compared to control. Ferritin is recognized as a critical mediator of immune system irregularities, particularly in cases of severe hyperferritinemia, through direct immune suppression and pro-inflammatory activities. It helps create cytokine storms that damage many organs. However, serum ferritin is also a unique severity risk factor in COVID-19 patients (Vargas-Vargas and Cortés-Rojo, 2020). In the present finding, the serum ferritin level was significantly high in the respective COVID-19 patients (1,191.39 ± 332.15, 1,142.21 ± 272.73, 812.82 ± 142.81 μg/L respectively). The significantly elevated level of hs-CRP was also observed in COVID-19 patients with the values 107.29 ± 10.26, 109.5 ± 9.27 and 102.96 ± 7.96 mg/L, respectively. Different clinical disorders, including hemolysis, cancer, severe infections, sepsis, and liver illnesses, have high serum LDH levels. The progression of COVID-19 may also be associated with LDH elevation. The severity and status of the disease may therefore be correlated with LDH levels (Wu et al., 2020). Herein, it has also been shown that the LDH levels were significantly higher in patients who were infected with COVID-19 only and solo disease condition of DM and HTN infected with COVID-19 (896.2 ± 167.23, 860.07 ± 167.63 and 1,009.65 ± 99.68 U/L) when compared with controls (Table 1 and Figures 2A–L).
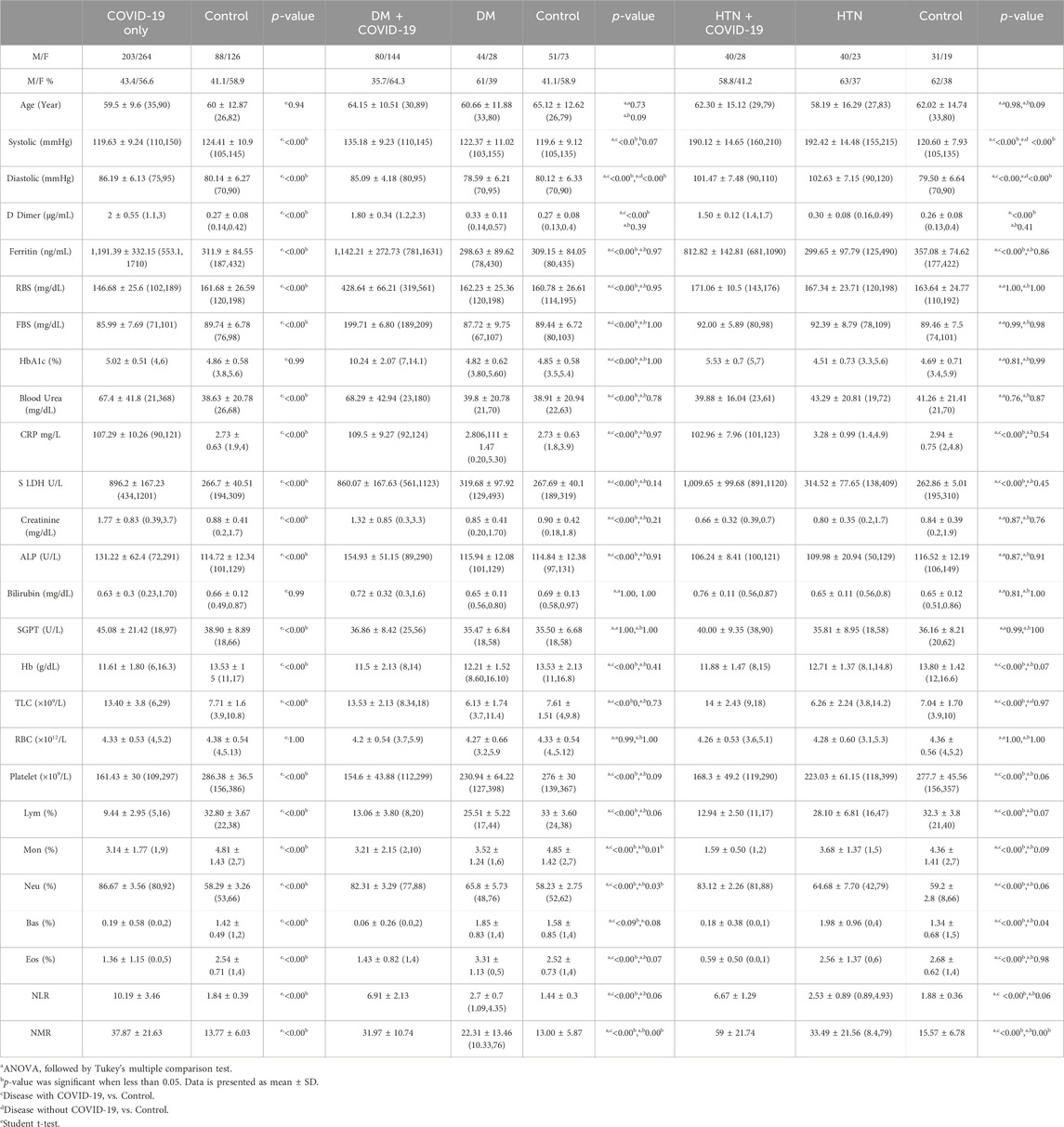
Table 1. Clinical characteristics of patients infected with COVID-19 only and patients of DM and HTN infected with COVID-19.
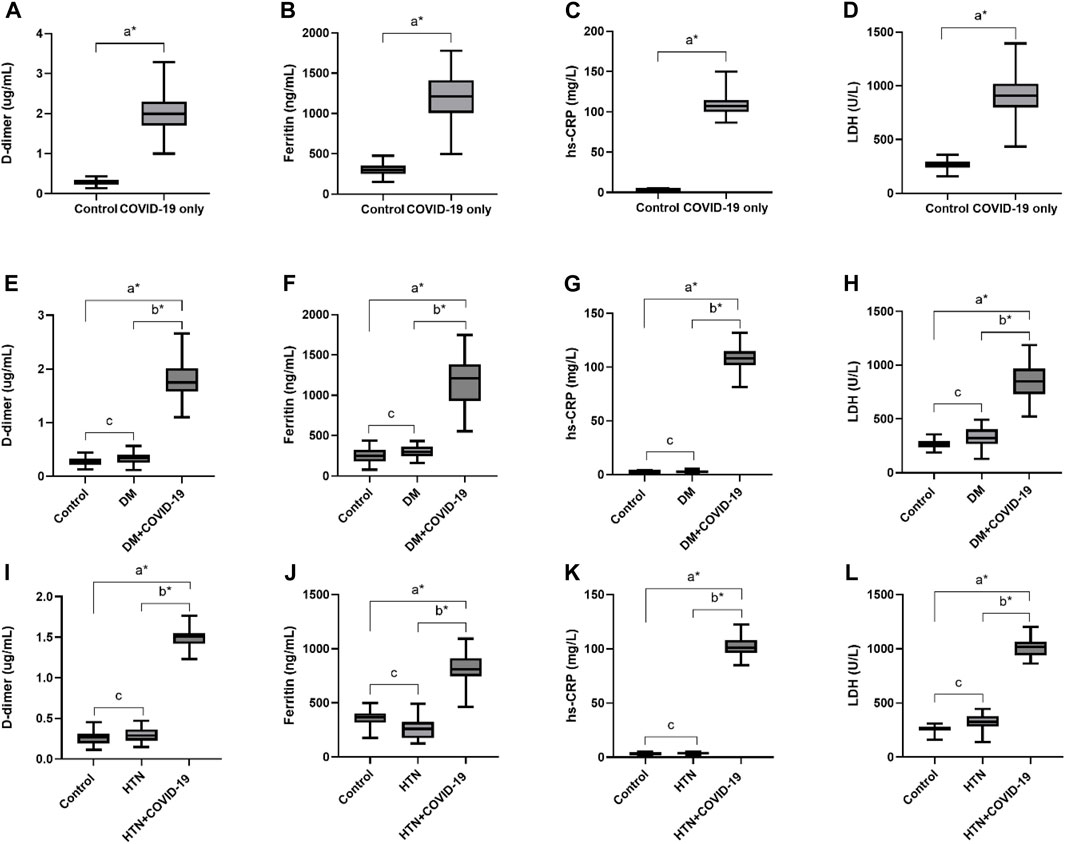
Figure 2. Increased levels of D-dimer, Ferritin, hs-CRP and LDH in COVID-19 only and COVID-19 patients with DM and HTN. (A), D-dimer. (B), Ferritin, (C), hs-CRP and (D), LDH levels in COVID-19 patients only. (E), D-dimer. (F), Ferritin, (G), hs-CRP and (H), LDH levels in diabetes mellitus patients infected with COVID-19. (I), D-dimer. (J), Ferritin, (K), hs-CRP and (L), LDH levels in hypertensive patients suffered from COVID-19 infection. δ *p value was significant when less than 0.05 a Disease with COVID-19 vs healthy control b Disease with COVID-19 vs disease control c Disease without COVID-19 vs healthy control.
D-dimer levels were also significantly high in the IHD and MI group infected with COVID-19 (1.85 ± 0.48 and 2.02 ± 0.5 μg/mL, respectively). In addition, serum ferritin levels were also significantly high in the present disease group (918.47 ± 263.13 and 1,203.59 ± 336.43 μg/L). The overproduction of inflammatory cytokines in COVID-19 patients with severe disease may cause high CRP values. Herein, the hs-CRP level was also significantly high in the severe disease group of COVID-19, with 102.22 ± 7.62 and 104.55 ± 9.31 mg/L. However, the level of LDH was also high in the respective disease group of COVID-19 patients (998.53 ± 101.34, 896.35 ± 126.8 U/L) shown in Table 2 and Figures 3A–H.
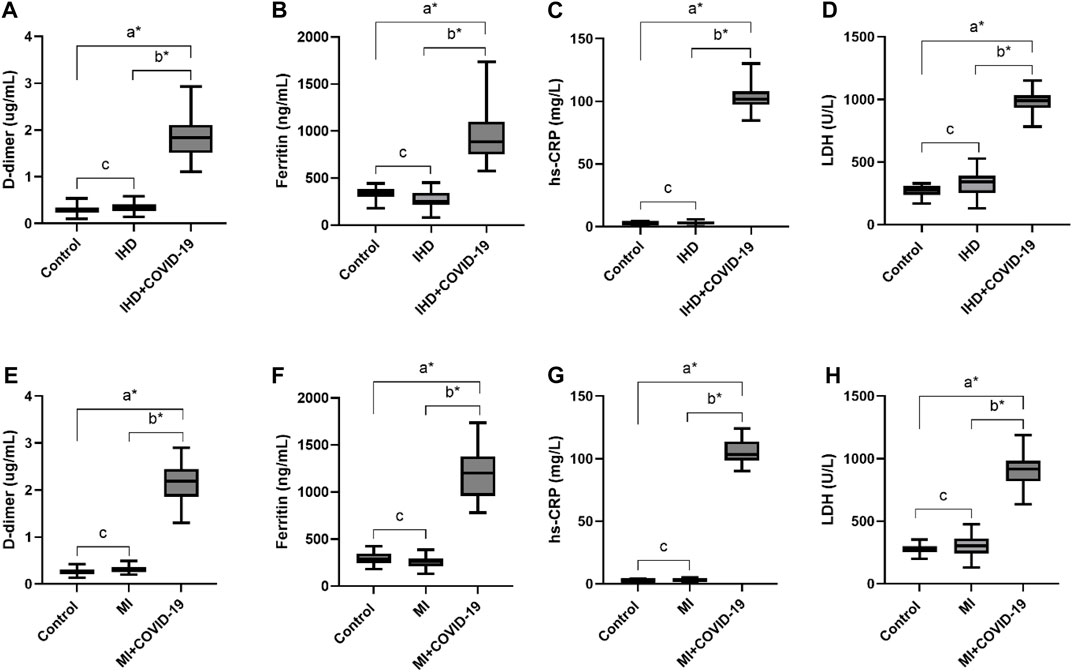
Figure 3. Elevated levels of D-dimer, Ferritin, hs-CRP and LDH in IHD and MI patients infected with COVID-19. (A), D-dimer. (B), Ferritin, (C), hs-CRP and (D), LDH levels in IHD patients suffered from COVID-19 infection. (E), D-dimer. (F), Ferritin, (G), hs-CRP and (H), LDH levels in MI patients infected with COVID-19.δ *p value was significant when less than 0.05 a Disease with COVID-19 vs healthy control b Disease with COVID-19 vs disease control c Disease without COVID-19 vs healthy control.
Previous studies have shown that D-dimer and fibrinogen concentrations increase in the early stages of COVID-19 disease. In the present cohort, patients of the multiple disease groups IHD with DM, DM with HTN, MI with HTN, and MI with DM infected with COVID-19 also showed significantly elevated levels of D-dimers (2.13 ± 0.50, 1.56 ± 0.21, 1.58 ± 0.20 and 1.77 ± 0.19 μg/mL). Furthermore, serum ferritin levels were also significantly high in disease present disease group of COVID-19 with high values of 1,177.7 ± 284.95, 848.58 ± 116.54, 913.50 ± 110.61, and 1,046.08 ± 181.30 μg/L, respectively. The hs-CRP level was also significantly high in the disease group of COVID-19 (112.14 ± 5.88, 98.29 ± 4.48, 102.33 ± 4.45, 105.00 ± 8.87 mg/L, respectively). In the cells of the majority of body tissues, lactate is converted to pyruvate by an enzyme called lactate dehydrogenase (LDH), and its activity is elevated after tissue breakdown. However, the LDH concentration was also significantly high in the group of COVID-19 patients with respective disease conditions (858.64 ± 155.87, 982.25 ± 103.27, 934.75 ± 54.73, 817.50 ± 131.05 U/L respectively) given in Table 3 and Figures 4A–P.

Table 3. Clinical characteristics of IHD + DM, DM + HTN, MI + HTN and MI + DM patients infected with COVID-19.

Figure 4. Increased levels of D-dimer, Ferritin, hs-CRP and LDH in different disease conditions of IHD with DM, DM with HTN, MI with HTN and MI with DM patients infected with COVID-19. (A), D-dimer. (B), Ferritin, (C), hs-CRP and (D), LDH levels in IHD with DM patients suffered from COVID-19 infection. (E), D-dimer. (F), Ferritin, (G), hs-CRP and (H), LDH levels in DM with HTN patients infected with COVID-19. (I), D-dimer. (J), Ferritin, (K), hs-CRP and (L), LDH levels in MI with HTN patients suffered from COVID-19 infection. (M), D-dimer. (N), Ferritin, (O), hs-CRP and (P), LDH levels in MI with DM patients suffered from COVID-19 infection.δ *p value was significant when less than 0.05 a Disease with COVID-19 vs healthy control b Disease with COVID-19 vs disease control c Disease without COVID-19 vs healthy control.
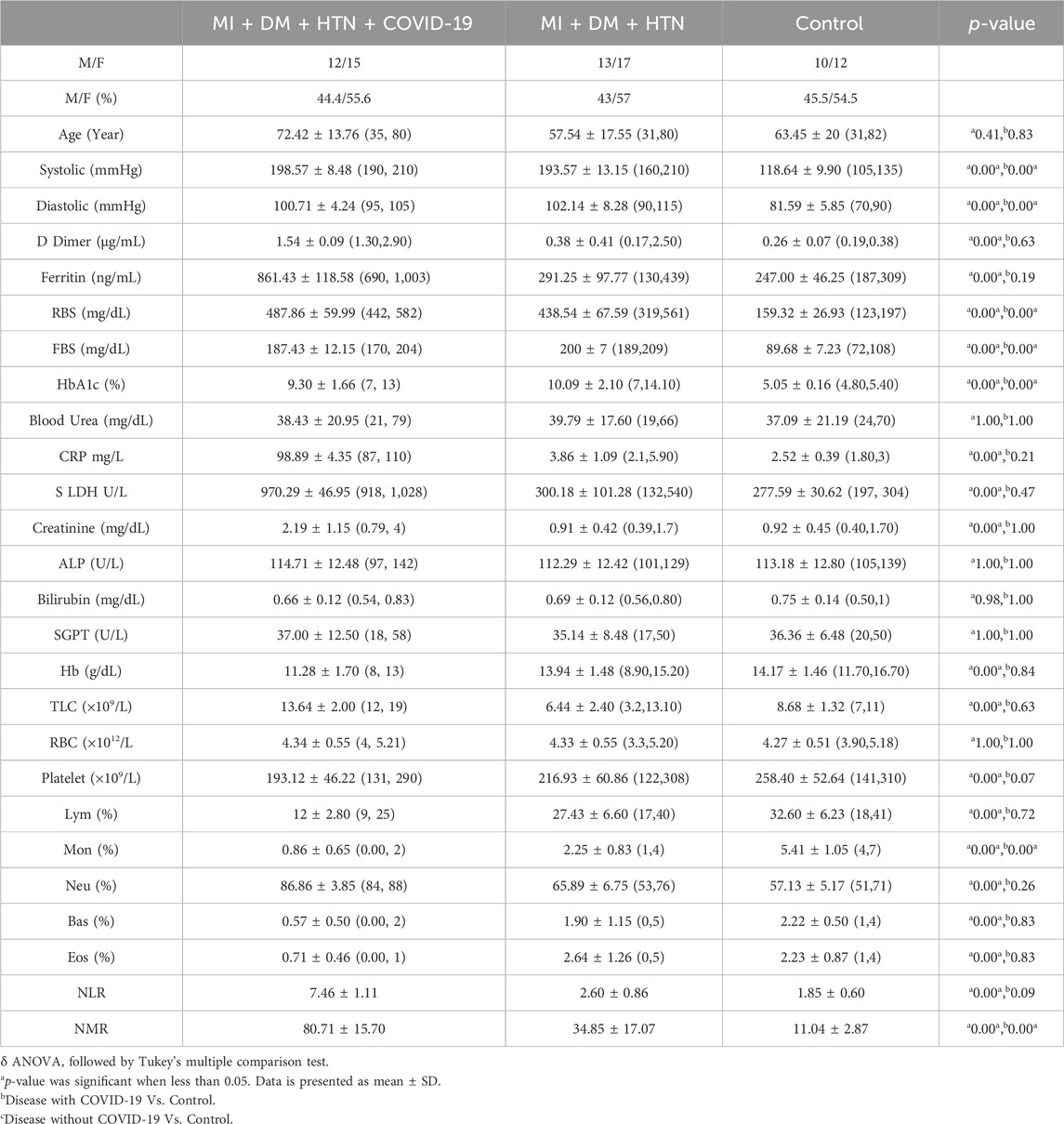
The D-dimer level was also significantly high (1.54 ± 0.09 μg/mL) in the group of COVID-19 patients with multiple disease condition of MI, DM, and HTN. Furthermore, serum ferritin levels were also significantly high in the present disease group (861.43 ± 118.58) infected with COVID-19. The severe COVID-19 disease groups of MI, DM, and HTN also have significantly high levels of hs-CRP (87.00, 110.00 mg/L). In addition, the LDH level (970.29 ± 46.95 U/L) was also significantly raised in the present disease group of COVID-19 patients with MI, DM, and HTN (Table 4 and Figures 5A–D).
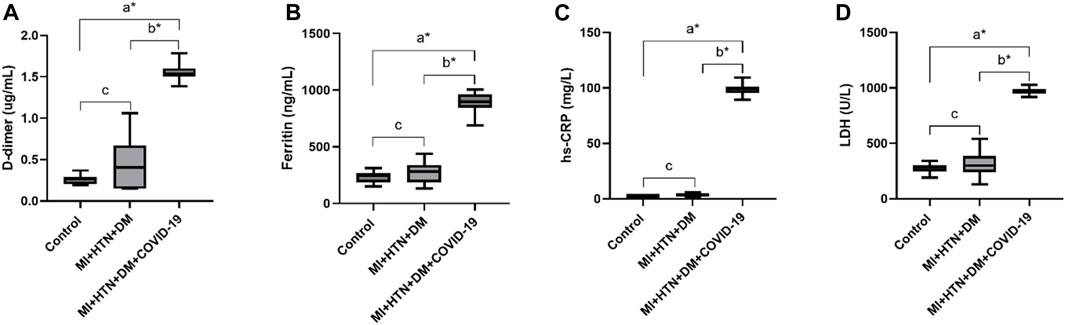
Figure 5. Increased levels of D-dimer, Ferritin, hs-CRP and LDH in disease condition of combine MI, HTN and DM patients infected with COVID-19. (A), D-dimer. (B), Ferritin, (C), hs-CRP and (D), LDH.δ *p value was significant when less than 0.05 a Disease with COVID-19 vs healthy control b Disease with COVID-19 vs disease control c Disease without COVID-19 vs healthy control.
Hematological changes
Hemoglobinopathy may seriously compromise the capacity of erythrocytes to transport oxygen under hypoxia. Therefore, low levels of oxygen were observed in different patients with COVID-19. The Hb level was observed low in patients with COVID-19 only, and solo disease conditions of DM and HTN with COVID-19 infection (11.61 ± 1.80, 11.5 ± 2.13, and (11.88 ± 1.47 g/dL). In addition, different viral infections, including COVID-19, also cause thrombocytopenia. However, thrombocytopenia was observed in the present disease group of patients with COVID-19 (161.43 ± 30, 154.6 ± 43.88, 168.3 ± 49.2/uL) compared with the control. Hemophagocytic lymphohistiocytosis disorder is widely associated with different species of viral infections, including COVID-19. Lymphopenia was noted in the disease groups of COVID-19 patients with respective disease conditions (9.44 ± 2.95, 13.06 ± 3.80, and 12.94% ± 2.50%). The level of monocytes was significantly low in the present group of patients with COVID-19 (3.14 ± 1.77, 3.21 ± 2.15, and 1.59% ± 0.50%). The levels of basophils and eosinophils were also significantly low in the COVID-19 patients in the respective disease group. Neutrophils play an essential role in clearing pathogens and debris through phagocytosis. However, its level also increases during COVID-19 infection. The elevated levels of neutrophils were observed in different patients with COVID-19 only and solo disease conditions of DM and HTN (86.67 ± 3.56, 82.31 ± 3.29, and 83.12% ± 2.26%). The NLR data showed significant increased by 10.19 ± 3.46, 6.91 ± 2.13, and 6.67 ± 1.29. In addition, the NMR was also significantly high 37.87 ± 21.63, 31.97 ± 10.74, and 59 ± 21.74 in the present disease group of COVID-19 (Table 1).
The Hb level was also found to be low in the group of COVID-19 patients with solo disease condition of IHD and MI (10.83 ± 1.85 and 11.441.68 g/dL). Platelets play an essential role in inflammatory signaling and the infectious response. However, the level of platelets was also low within the normal limit, at 173.55 ± 41.00 and 171.1 ± 50.5/ul. In addition, lymphopenia was also observed in the present solo disease conditions of IHD and MI infected with SARS-CoV-2 (13.27% ± 4.40% and 9.24% ± 2.98%). The level of monocytes was also significantly low in the COVID-19 disease group of IHD and MI (2.07% ± 0.77% and 3% ± 1.82%). The SARS-CoV-2 patients with respective disease conditions also showed low levels of basophils and eosinophils. However, the neutrophil level was also significantly high in the IHD and MI groups of COVID-19 patients, with 83.07% ± 4.04% and 87.12% ± 3.33%. The NLR was significantly high in the present population of patients with disease (7.61 ± 4.19 and 10.43 ± 3.4. In addition, the NMR was significantly high 46.50 ± 19.47 and 41.23 ± 23.8 shown in Table 2.
The Hb level was low in different patients with SARS-CoV-2 with multiple disease conditions of IHD with DM, DM with HTN, MI with HTN, and MI and DM 11.00 ± 1.34, 11.69 ± 1.35, 11.16 ± 1.40, and 11.83 ± 1.53 g/dL, respectively. In addition, low platelet levels were also noted in the present group of COVID-19 patients (160.10 ± 49.00, 177.80 ± 49.00, 161.70 ± 33.70, 175.10 ± 58.60/µL). The SARS-CoV-2 patients in the present disease group also showed lymphopenia (13.50 ± 2.37, 18.42 ± 19.48, 12.92 ± 1.96, 13.25% ± 2.45%). However, a low level of monocytes was also observed in SARS-CoV-2 patients with the respective disease conditions. In addition, basophil and eosinophil levels were also low in different COVID-19 patients in the present disease group. A significantly high level of neutrophils was also noted in COVID-19 patients with the respective multiple disease conditions (83.50 ± 2.04, 85.08 ± 2.42, and 85.25% ± 2.15% and 84.25% ± 2.65%). In addition, NLR was significantly elevated at 6.40 ± 1.27, 6.77 ± 1.43, 6.76 ± 1.09 and 6.59 ± 1.30. However, NMR was also significantly elevated at 59.50 ± 20.44, 70.96 ± 20.54, 78.08 ± 16.00 and 80.67 ± 11.77 (Table 3).
The patients with SARS-CoV-2 with multiple disease condition of MI, DM, and HTN also showed low levels of Hb. The present group of COVID-19 patients with respective diseases also showed low levels of platelets (193.12 ± 46.22). In addition, the COVID-19 patients in this group also showed lymphopenia (12.00% ± 2.80%). Moreover, the monocytes and basophils levels were also low in the present COVID-19-infected group, with respective disease 0.86% ± 0.65% and 0.57 ± 0.50. Low levels of basophils and eosinophils were also observed. However, a significantly elevated neutrophil count (86.86% ± 3.85%) was also observed. The NLR was significantly high in the present group of patients with 7.46 ± 1.11, while the NMR was also significantly increased by 80.71 ± 15.70, as shown in Table 4.
Discussion
SARS-CoV-2 can affect various physiological systems, including the blood and immune system. The SARS-CoV-2 primarily targets the respiratory system (Rahi et al., 2021). However, the infection can have systemic effects. The impact on serum and hematological parameters can vary among individuals and is influenced by factors such as age, overall health, and the severity of the infection (Bellmann-Weiler et al., 2020; Higgins et al., 2020). This statement agrees with our results, which demonstrate that SARS-CoV-2 infection significantly modulates serum and hematological parameters in the existing health condition.
The elevated levels of liver enzymes are frequently observed by different physicians during their clinical practice. Therefore, it can be difficult to evaluate such problems in people with no symptoms. In addition, liver enzymes are also elevated during different hepatitis infections, fatty liver disease, obesity and hemochromatosis, etc (Malakouti et al., 2017). Kidney failure may also occur during chronic DM and may elevate the levels of urea and creatinine in blood (Dabla, 2010). Furthermore, high levels of urea and creatinine are also found during glomerulonephritis, use of certain drugs and consuming large amounts of protein diets, etc. It indicates that it may be difficult to identify the cause of elevated levels of liver enzymes, urea and creatinine. Therefore, in the present cohort study we selected precise markers that modulate during SARS-CoV-2 infection. The abnormal coagulation during COVID-19 results in the elevated level of D-dimer (Han et al., 2020). The clinical investigations to diagnose the deep vein thrombosis or pulmonary embolism suggested that the elevated level of D-dimer directly linked with the risk of irregular blood clotting (Querol-Ribelles et al., 2004; Yao et al., 2020). However, several studies depicted 3 to 4-folds increase in D-dimer levels to be associated with the better prognosis in COVID-19 patients. However, in the present study elevated D-dimer levels have also been identified in solo and multiple disease conditions of DM, HTN, IHD and MI infected with COVID-19. Therefore, the early stages of the disease’s coagulation parameters can also help to prevent and treat COVID-19 infection. The detailed investigation of serum ferritin as a marker of iron metabolism demonstrated that the changes in its concentrations is one of the systemic symptoms of the acute phase reaction (de Lucena et al., 2020). Serum ferritin has been investigated for a long time as a marker of iron metabolism (Wang et al., 2010; Henry et al., 2020b; Vargas-Vargas and Cortés-Rojo, 2020). Moreover, it has been demonstrated that the high ferritin levels result due to acute inflammatory reactions. Likewise in the current investigation, the recorded serum ferritin level was significantly increased both in solo as well as multiple disease conditions of the patients infected with COVID-19. CRP level is the key indicator associated with the degree of inflammation irrespective of the age and gender (Bilgir et al., 2015). The high CRP levels can be utilized to the early diagnosis of the severe lung infections in the patients with severe pneumonia (Warusevitane et al., 2016). In the present study, the significantly increased levels of inflammatory marker such as hs-CRP were observed in all the disease conditions of COVID-19 infection that can also be used as rapid diagnostic markers for disease severity at early stages of infection. The inflammatory responses showed the association between infectious cells, the immune system, and the inflammatory response, mimicking nonspecific responses to hypoxia, tissue injury, and necrosis. Moreover, previous studies also explored that several conditions such as cancer, heart failure, hypothyroidism, and viral diseases, including COVID-19, can increase LDH levels (Henry et al., 2020a; Wu et al., 2020). In the present cohort, the LDH level was significantly increased in patients of solo and multiple disease conditions of DM, HTN, IHD and MI infected with COVID-19.
Previous studies showed that specific laboratory parameters at the time of hospitalization were linked to mortality and the severity of the COVID-19 infection (Allouche et al., 2021; Anai et al., 2021; Al-Saadi and Abdulnabi, 2022; Gajendra, 2022). However, the present findings showed a low level of Hb in COVID-19 patients at the time of admission to the ICU and were associated with disease severity (Freeman and Morando, 2018). Therefore, a decline in Hb level upon diagnosis of COVID-19 may be a sign of acute respiratory failure. Moreover, the low level of Hb was also observed in all the patients infected with COVID-19 and hence could be assigned to acute respiratory failure in COVID-19 infection. The state of leukocytosis could be substantially associated to the death of individual which can be occurred in response to the external stimuli including infection, inflammation, drugs, malignancies, poisoning and ketoacidosis. Furthermore, it is also associated with acute leukemias, chronic leukemias and myeloproliferative disorders (Asadollahi et al., 2011). Therefore, in COVID-19 infection, a higher TLC count should receive more significant consideration, which would help practitioners to take an even more effective treatment (Zhu et al., 2021). However, in the present study, elevated levels of TLC were observed in all the patients with different disease conditions infected with SARS-CoV-2. In addition, during the onset of COVID-19 infection, viral spike proteins and complement activation products have been detected on the cell surface of erythrocytes and reduce the level of RBC. However, the RBC levels were not significantly reduced in the present investigation. In the severe stage of SARS-CoV-2 infection, the increase risk of mortality is also associated with thrombocytopenia. The first cellular response to vascular injuries and thrombosis is the elevation in the levels of platelets. Hence by releasing numerous potent cytokines and chemokines, the platelets can link the immune system to thrombotic events (Franco et al., 2015). In the present finding, thrombocytopenia has been observed in all the patients infected with COVID-19. The findings of the current investigation observed thrombocytopenia in all the studied patients infected with SARS-CoV-2. Moreover, the present findings also showed significant differences for some parameters including Hb, TLC, RBC, and platelets as compared to control, although the index was in the normal range.
Hemophagocytic lymphohistiocytosis disorder is considerable associated with the several species of viral infections (Mostaza-Fernández et al., 2014). However, differentiation in the peripheral white blood cell composition could indicate immunologic dysfunction during COVID-19 infection (Higgins et al., 2020). Likewise, neutrophilia is another sign of cytokine storm and hyper-inflammation state and hence considered as a predictor of disease severity in COVID-19 infection. (Chan et al., 2021). Therefore, neutrophilia is also considered a predictor of disease severity in COVID-19 infection. On the other side, as a poor prognostic indicator several studies also demonstrated the association of eosinophilia with COVID-19 disease severity. Severe SARS-CoV-2 patients have lower lymphocyte counts and higher leukocyte counts, identified as a prognostic indicator (Tong et al., 2021). However, elevated levels of neutrophil-to-lymphocyte ratio (NLR) and neutrophil-to-monocyte (NMR) ratio have emerged as independent hallmarks of severe COVID-19 infection (Rizo-Téllez et al., 2020; Reusch et al., 2021). In the present cohort, lymphopenia, monocytopenia, neutrophilia, eosinopenia, and basopenia have been observed in all the patients with solo and multiple disease conditions of DM, HTN, IHD and MI. In addition, high NLR and NMR were also observed in all the respective disease conditions infected with Severe SARS-CoV-2.
Conclusion
This study provides evidence of a relationship between COVID-19 infection and modulation in serum and hematological parameters. However, severe SARS-CoV-2 infection induces changes in the serum and hematological parameters which could be used as a marker for the rapid diagnosis of SARS-CoV-2 infection in patients with solo and multiple disease conditions of DM, HTN, IHD and MI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The present study was approved by the ethical committee of Kohat University of Science and Technology, Kohat, Pakistan under Reference No: Ref-No/KUST/Ethical Committee/528. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MIJ: Conceptualization, Investigation, Supervision, Writing–original draft. RAK: Conceptualization, Writing–review and editing. NK: Data curation, Methodology, Writing–review and editing. SMI: Investigation, Writing–original draft. SA: Formal Analysis, Writing–original draft. MIK: Data curation, Writing–review and editing. SG: Validation, Writing–review and editing. UN: Formal Analysis, Investigation, Writing–review and editing. TA: Investigation, Writing–original draft. RU: Conceptualization, Funding acquisition, Writing–original draft. AB: Writing–review and editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research work is supported by researchers supporting project number (RSP2024R346) at King Saud University Riyadh Saudi Arabia.
Acknowledgments
The authors wish to thank the Researchers Supporting Project number (RSP 2024R346) at King Saud University Riyadh Saudi Arabia for financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allouche, A., Ben Amar, J., Echi, K., Hemissi, K., Zaibi, H., Ben Jemia, E., et al. (2021). Hematological changes in COVID-19 and their association with the severity of the disease., PA3881. doi:10.1183/13993003.congress-2021.pa3881
Al-Saadi, E. A. K. D., and Abdulnabi, M. A. (2022). Hematological changes associated with COVID-19 infection. J. Clin. Lab. Anal. 36, e24064. doi:10.1002/jcla.24064
Al-Salih, M., Samsudin, S., Alsalih, S. W., Arshad, S. S., Warid, F., Sfoog, A. A., et al. (2020). Identify human cluster of differentiation 147 (CD147), a new target of SARS-CoV-2 invasion. Int. J. Pharm. Res. 12, 2654–2667. doi:10.31838/ijpr/2020.12.02.359
Anai, M., Akaike, K., Iwagoe, H., Akasaka, T., Higuchi, T., Miyazaki, A., et al. (2021). Decrease in hemoglobin level predicts increased risk for severe respiratory failure in COVID-19 patients with pneumonia. Respir. Investig. 59, 187–193. doi:10.1016/j.resinv.2020.10.009
Asadollahi, K., Hastings, I. M., Beeching, N. J., Gill, G. V., and Asadollahi, P. (2011). Leukocytosis as an alarming sign for mortality in patients hospitalized in general wards. Iran. J. Med. Sci. 36, 45–49.
Banon, T., Wortsman, J., Ben Moshe, S., Gazit, S., Peretz, A., Ben Tov, A., et al. (2021). Evaluating red blood cell distribution width from community blood tests as a predictor of hospitalization and mortality in adults with SARS-CoV-2: a cohort study. Ann. Med. 53, 1410–1418. doi:10.1080/07853890.2021.1968484
Bateman, R. M., Sharpe, M. D., Singer, M., and Ellis, C. G. (2017). The effect of sepsis on the erythrocyte. Int. J. Mol. Sci. 18, 1932. doi:10.3390/ijms18091932
Bellmann-Weiler, R., Lanser, L., Barket, R., Rangger, L., Schapfl, A., Schaber, M., et al. (2020). Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J. Clin. Med. 9, 2429–2511. doi:10.3390/jcm9082429
Bilgir, O., Bilgir, F., Calan, M., Calan, O. G., and Yuksel, A. (2015). Comparison of pre- and post-levothyroxine highsensitivity c-reactive protein and fetuin-a levels in subclinical hypothyroidism. Clinics 70, 97–101. doi:10.6061/clinics/2015(02)05
Bouchla, A., Kriebardis, A. G., Georgatzakou, H. T., Fortis, S. P., Thomopoulos, T. P., Lekkakou, L., et al. (2022). Red blood cell abnormalities as the mirror of SARS-CoV-2 disease severity: a pilot study. Front. Physiol. 12, 825055. doi:10.3389/fphys.2021.825055
Chan, L., Karimi, N., Morovati, S., Alizadeh, K., Kakish, J. E., Vanderkamp, S., et al. (2021). The roles of neutrophils in cytokine storms. Viruses 13, 2318. doi:10.3390/v13112318
Channappanavar, R., Zhao, J., and Perlman, S. (2014). T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 59, 118–128. doi:10.1007/s12026-014-8534-z
Chekol Abebe, E., Medhin, M. T. G., Mariam, A. B. T., Dejenie, T. A., Ayele, T. M., Admasu, F. T., et al. (2022). Mutational pattern, impacts and potential preventive strategies of omicron SARS-CoV-2 variant infection. Infect. Drug Resist. 15, 1871–1887. doi:10.2147/IDR.S360103
Chu, H., Zhou, J., Wong, B. H. Y., Li, C., Chan, J. F. W., Cheng, Z. S., et al. (2016). Middle East respiratory Syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 213, 904–914. doi:10.1093/infdis/jiv380
Cortés-Vieyra, R., Gutiérrez-Castellanos, S., Álvarez-Aguilar, C., Baizabal-Aguirre, V. M., Nuñez-Anita, R. E., Rocha-López, A. G., et al. (2021). Behavior of eosinophil counts in recovered and deceased covid-19 patients over the course of the disease. Viruses 13, 1675. doi:10.3390/v13091675
Cosic, I., Cosic, D., and Loncarevic, I. (2020). RRM prediction of erythrocyte band3 protein as alternative receptor for SARS-CoV-2 virus. Appl. Sci. 10, 4053. doi:10.3390/app10114053
Dabla, P. K. (2010). Renal function in diabetic nephropathy. World J. Diabetes 1, 48. doi:10.4239/wjd.v1.i2.48
de Lucena, T. M. C., da Silva Santos, A. F., de Lima, B. R., de Albuquerque Borborema, M. E., and de Azevêdo Silva, J. (2020). Mechanism of inflammatory response in associated comorbidities in COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 14, 597–600. doi:10.1016/j.dsx.2020.05.025
Franco, A. T., Corken, A., and Ware, J. (2015). Platelets at the interface of thrombosis, inflammation, and cancer. Blood 126, 582–588. doi:10.1182/blood-2014-08-531582
Gajendra, S. (2022). Spectrum of hematological changes in COVID-19. Am. J. Blood Res. 12, 43–53. Available at: http://www.ncbi.nlm.nih.gov/pubmed/35291254%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC8918700.
Grobler, C., Maphumulo, S. C., Grobbelaar, L. M., Bredenkamp, J. C., Laubscher, G. J., Lourens, P. J., et al. (2020). Covid-19: The rollercoaster of fibrin(ogen), d-dimer, von willebrand factor, p-selectin and their interactions with endothelial cells, platelets and erythrocytes. Int. J. Mol. Sci. 21, 5168–5225. doi:10.3390/ijms21145168
Han, H., Yang, L., Liu, R., Liu, F., Liu, F., Wu, K. L., et al. (2020). Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 58, 1116–1120. doi:10.1515/cclm-2020-0188
Henry, B. M., Aggarwal, G., Wong, J., Benoit, S., Vikse, J., Plebani, M., et al. (2020a). Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am. J. Emerg. Med. 38, 1722–1726. doi:10.1016/j.ajem.2020.05.073
Henry, B. M., De Oliveira, M. H. S., Benoit, S., Plebani, M., and Lippi, G. (2020b). Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 58, 1021–1028. doi:10.1515/cclm-2020-0369
Higgins, J. M., Foy, B. H., Carlson, J. C. T., Reinertsen, E., Padros, I., Valls, R., et al. (2020). Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS-CoV-2 infection. JAMA Netw. Open 3, e2022058. doi:10.1001/jamanetworkopen.2020.22058
Iba, T., Levy, J. H., Levi, M., and Thachil, J. (2020). Coagulopathy in COVID-19. J. Thromb. Haemost. 18, 2103–2109. doi:10.1111/jth.14975
Lam, L. K. M., Reilly, J. P., Rux, A. H., Murphy, S. J., Kuri-Cervantes, L., Weisman, A. R., et al. (2021). Erythrocytes identify complement activation in patients with COVID-19. Am. J. Physiol. - Lung Cell. Mol. Physiol. 321, L485–L489. doi:10.1152/AJPLUNG.00231.2021
Malakouti, M., Kataria, A., Ali, S. K., and Schenker, S. (2017). Elevated liver enzymes in asymptomatic patients – what should i do? J. Clin. Transl. Hepatol. 5, 1–10. doi:10.14218/JCTH.2017.00027
Mostaza-Fernández, J. L., Guerra Laso, J., Carriedo Ule, D., and Ruiz de Morales, J. M. G. (2014). Hemophagocytic lymphohistiocytosis associated with viral infections: diagnostic challenge and therapeutic dilemma. Rev. Clínica Española 214, 320–327. doi:10.1016/j.rceng.2014.03.004
Puelles, V. G., Lütgehetmann, M., Lindenmeyer, M. T., Sperhake, J. P., Wong, M. N., Allweiss, L., et al. (2020). Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 383, 590–592. doi:10.1056/nejmc2011400
Querol-Ribelles, J. M., Tenias, J. M., Grau, E., Querol-Borras, J. M., Climent, J. L., Gomez, E., et al. (2004). Plasma d-dimer levels correlate with outcomes in patients with community-acquired pneumonia. Chest 126, 1087–1092. doi:10.1378/chest.126.4.1087
Rahi, M. S., Jindal, V., Reyes, S. P., Gunasekaran, K., Gupta, R., and Jaiyesimi, I. (2021). Hematologic disorders associated with COVID-19: a review. Ann. Hematol. 100, 309–320. doi:10.1007/s00277-020-04366-y
Reusch, N., De Domenico, E., Bonaguro, L., Schulte-Schrepping, J., Baßler, K., Schultze, J. L., et al. (2021). Neutrophils in COVID-19. Front. Immunol. 12, 652470. doi:10.3389/fimmu.2021.652470
Rizo-Téllez, S. A., Méndez-García, L. A., Flores-Rebollo, C., Alba-Flores, F., Alcántara-Suárez, R., Manjarrez-Reyna, A. N., et al. (2020). The neutrophil-to-monocyte ratio and lymphocyte-to-neutrophil ratio at admission predict in-hospital mortality in mexican patients with severe sars-cov-2 infection (Covid-19). Microorganisms 8, 1560. doi:10.3390/microorganisms8101560
Singh, A., Verma, S. P., Kushwaha, R., Ali, W., Reddy, H. D., and Singh, U. S. (2022). Hematological changes in the second wave of SARS-CoV-2 in north India. Cureus 14, e23495. doi:10.7759/cureus.23495
Tong, X., Cheng, A., Yuan, X., Zhong, X., Wang, H., Zhou, W., et al. (2021). Characteristics of peripheral white blood cells in COVID-19 patients revealed by a retrospective cohort study. BMC Infect. Dis. 21, 1236. doi:10.1186/s12879-021-06899-7
Vabret, N., Britton, G. J., Gruber, C., Hegde, S., Kim, J., Kuksin, M., et al. (2020). Immunology of COVID-19: current state of the science. Immunity 52, 910–941. doi:10.1016/j.immuni.2020.05.002
Vargas-Vargas, M., and Cortés-Rojo, C. (2020). Ferritin levels and COVID-19. Rev. Panam. Salud Publica/Pan Am. J. Public Heal. 44, 1. doi:10.26633/RPSP.2020.72
Venter, C., Bezuidenhout, J. A., Laubscher, G. J., Lourens, P. J., Steenkamp, J., Kell, D. B., et al. (2020). Erythrocyte, platelet, serum ferritin, and p-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int. J. Mol. Sci. 21, 8234–8314. doi:10.3390/ijms21218234
Wang, W., Knovich, M. A., Coffman, L. G., Torti, F. M., and Torti, S. V. (2010). Serum ferritin: past, present and future. Biochim. Biophys. Acta - Gen. Subj. 1800, 760–769. doi:10.1016/j.bbagen.2010.03.011
Wang, Z., and Yang, L. (2023). The therapeutic potential of natural dietary flavonoids against SARS-CoV-2 infection. Nutrients 15, 3443. doi:10.3390/nu15153443
Warusevitane, A., Karunatilake, D., Sim, J., Smith, C., and Roffe, C. (2016). Early diagnosis of pneumonia in severe stroke: clinical features and the diagnostic role of C-Reactive protein. PLoS One 11, e0150269. doi:10.1371/journal.pone.0150269
World Medical Association declaration of Helsinki (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama 310, 2191–2194. doi:10.1001/jama.2013.281053
Wu, M. Y., Yao, L., Wang, Y., Zhu, X. Y., Wang, X. F., Tang, P. J., et al. (2020). Clinical evaluation of potential usefulness of serum lactate dehydrogenase (LDH) in 2019 novel coronavirus (COVID-19) pneumonia. Respir. Res. 21, 171. doi:10.1186/s12931-020-01427-8
Yang, L., and Wang, Z. (2023). Bench-to-bedside: innovation of small molecule anti-SARS-CoV-2 drugs in China. Eur. J. Med. Chem. 257, 115503. doi:10.1016/j.ejmech.2023.115503
Yang, M., Li, C. K., Li, K., Hon, K. L., Ng, M. H., Chan, P. K. S., et al. (2004). Hematological findings in SARS patients and possible mechanisms (review). Int. J. Mol. Med. 14, 311–315. doi:10.3892/ijmm.14.2.311
Yao, Y., Cao, J., Wang, Q., Shi, Q., Liu, K., Luo, Z., et al. (2020). D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J. Intensive Care 8, 49. doi:10.1186/s40560-020-00466-z
Youssry, I., Abd Elaziz, D., Ayad, N., and Eyada, I. (2022). The cause–effect dilemma of hematologic changes in COVID-19: one year after the start of the pandemic. Hematol. Rep. 14, 95–102. doi:10.3390/hematolrep14020014
Keywords: COVID-19, D-dimer, ferritin, hs-CRP, and LDH
Citation: Jan MI, Anwar Khan R, Khan N, Iftikhar SM, Ali S, Khan MI, Gul S, Nishan U, Ali T, Ullah R and Bari A (2024) Modulation in serum and hematological parameters as a prognostic indicator of COVID-19 infection in hypertension, diabetes mellitus, and different cardiovascular diseases. Front. Chem. 12:1361082. doi: 10.3389/fchem.2024.1361082
Received: 24 December 2023; Accepted: 10 April 2024;
Published: 29 April 2024.
Edited by:
Diego Brancaccio, University of Naples Federico II, ItalyReviewed by:
Liyan Yang, Qufu Normal University, ChinaAjmir Khan, Michigan State University, United States
Uroos Akber, Gwangju Institute of Science and Technology, Republic of Korea
Copyright © 2024 Jan, Anwar Khan, Khan, Iftikhar, Ali, Khan, Gul, Nishan, Ali, Ullah and Bari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Ishtiaq Jan, bWlzaHRpYXFqYW5Aa3VzdC5lZHUucGs=
 Muhammad Ishtiaq Jan
Muhammad Ishtiaq Jan Riaz Anwar Khan2
Riaz Anwar Khan2 M. I. Khan
M. I. Khan Umar Nishan
Umar Nishan Riaz Ullah
Riaz Ullah Ahmed Bari
Ahmed Bari