
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 28 February 2024
Sec. Organic Chemistry
Volume 12 - 2024 | https://doi.org/10.3389/fchem.2024.1341769
This article is part of the Research Topic Investigation of the Biological Properties of Natural Products Using Experimental Approaches and In Silico Methods View all 10 articles
 Sheila Cristina Almeida Neves Mutran1
Sheila Cristina Almeida Neves Mutran1 Paulo Roberto de Carvalho-Filho2
Paulo Roberto de Carvalho-Filho2 Mara Eliane Soares Ribeiro1
Mara Eliane Soares Ribeiro1 Kelson do Carmo Freitas Faial3
Kelson do Carmo Freitas Faial3 Rafael Rodrigues Lima4
Rafael Rodrigues Lima4 Roberta Souza D’Almeida Couto1*
Roberta Souza D’Almeida Couto1*Introduction: The use of natural products such as essential oils has been suggested due to their promising pharmacological effects and economic viability. This study aimed to determine hydrogenic potential (pH), titratable acidity (TA), and ion concentrations of five solutions containing essential oils (EO), when used as a EO-containing solutions, and evaluate ion concentrations, enamel surface loss, and morphology alterations in enamel.
Materials and methods: The pH, TA, calcium (Ca), potassium (K), and sodium (Na) concentrations of five EO-containing solutions were measured. Bovine enamel specimens were submitted to two daily 30-s immersions in artificial saliva, citric acid, distilled water, BaCloTea (Basil, Clove e Tea Tree), GeLaTeaPep (Geranium, Lavender, Tea Tree and Peppermint), EucaLem (Eucalyptus and Lemon), Cinnamon, or Spearmint solutions for 14 days. Ca, K, Na, and phosphorus (P) were quantified through ions chromatography, enamel surface loss was determined by profilometry, and surface morphology was qualitatively analyzed through scanning electron microscopy. Data were submitted to one-way ANOVA and Tukey (p < 0.05).
Results: The five EO-containing solutions presented significantly lower pH values than distilled water (p < 0.05). The GeLaTeaPep group presented a significantly higher TA value than BaCloTea (p < 0.05), which in turn showed a significantly higher TA value than the other solutions (p < 0.05). The distilled water presented significantly higher Ca, K, and Na concentrations than all EO-containing solutions (p < 0.05). The enamel exposed to EO-containing solutions showed lower Ca and P concentrations than artificial saliva (control) as well as significantly higher surface loss; however, the surface morphology was similar to the artificial saliva.
Conclusion: EO-containing solutions have low pH, TA, and low concentrations of Ca, Na, and K. Moreover, enamel exposed to these solutions showed low Ca and P concentrations and slight surface loss without morphology alteration.
Aromatic plants have been used in cosmetics and folk medicine for many years; however, plant extracts and essential oils (EO) became attractive for the industry in the last decade since consumers seek sustainable therapeutics agents with possible reduced adverse effects (Winska et al., 2019; Ramsey et al., 2020).
The EOs are composed of volatile metabolites secondary extracted from different parts of plants (flowers, buds, seeds, leaves, twigs, bark, herbs, wood, fruits, and roots) and present anticancer, antimicrobial, antiviral, antioxidant, and antibiotic properties (Aziz et al., 2018; Basavegowda et al., 2020; Ramsey et al., 2020). In dentistry, some EO-containing solutions have shown anxiolytic, antimicrobial, antiplaque, antigingivitis, and anticaries effects against oral pathogens (Zero et al., 2004; Dagli et al., 2015; Yadav et al., 2016; Quintas et al., 2017; Ghaderi and Solhjou, 2020).
Current lifestyle and diet habits may lead to tooth wear in young populations (Lussi et al., 2004; Donovan et al., 2021) and the effects of daily-use oral hygiene products on dental tissues need to be addressed. EO-containing mouthrinses do not require professional prescription or application, and are freely sold in supermarkets, drugstores, convenience stores, and on the internet; therefore, these over-the-counter products represent one of the most rapidly growing sectors of the oral care industry (Jardim et al., 2009; Weijden et al., 2015; Favaro et al., 2020). It is expected that products based on natural compounds such EOs may present limited detrimental effects. Some clinical trials have shown a significant reduction in plaque and gingivitis promoted by daily use of an EO-containing mouthrinse in combination with regular brushing and flossing (Sharma et al., 2004; Araujo et al., 2015; Alshehri, 2018). For a better understanding of the present study, it is worth highlighting that the use of the term essential oil-containing solutions mentioned throughout the text refers to mouthwashes.
This study aimed to determine the pH, TA, and mineral concentrations of EO-containing solutions, as well as their effect on enamel surface loss, morphology, and mineral concentrations.
In the present in vitro study, available essential oil mouthwashes commercially (dōTERRA Cosméticos do Brasil Ltda.), BaCloTea (Basil, Clove e Tea Tree), GeLaTeaPep (Geranium, Lavender, Tea Tree and Peppermint), EucaLem (Eucalyptus and Lemon), Cinnamon, and Spearmint solutions were tested according to the chemical characterization of the mouthwashes in relation to pH, TA; and concentrations of Ca, K and Na by induced plasma optical emission spectrometry (ICP OES). In addition, tests of these rinses were carried out on the surface of bovine tooth enamel by: quantifying C, K, Na and P through ion chromatography; analysis of morphology by scanning electron microscopy (SEM); and loss of enamel surface dental by profilometry. Artificial saliva was used as a positive control group, acid citrus 0.3% as a negative control group. Distilled water and other mouthwashes mouthpieces of OEs were the experimental groups. The sample size was determined according to a previously carried out pilot study. As it is an in vitro study, this research did not follow a guideline.
Five essential oil mouthwashes (EOs) were handled individually or associated according to the manufacturer’s recommendations. The description of the groups, plants and main components of each EO, according to information from gas chromatography and mass spectrometry (GC/MS) made available by the company, are described in Table 1. The EO mouthwashes were prepared with the same distilled water of the control group.
Five EO-containing solutions were manipulated according to the manufacturer’s recommendations; in addition, artificial saliva and 0.3% citric acid were respectively used as positive and negative controls (Table 1).
A total of 10 mL of each solution was added to a glass beaker and the pH value at 25°C was measured by a benchtop electrode previously calibrated with standard solutions (pH 4.01, 7.00, and 10.00). The electrode was washed with distilled water between each one of the three measurements.
Aliquots of 0.1 M sodium hydroxide were added to 10 mL of each solution (eight samples per group), and the total base volume (µL) of base needed to raise the initial pH to 7 was recorded. The TA was not determined for solutions with initial pH above 7 or neutral.
A total of 0.5 mL of each EO-containing solution was mixed with 0.5 mL of 10% (v/v) nitric acid (six samples per group), sieved with filter paper, diluted in 1:10 deionized water, and placed into polyethylene bottles (Medeiros et al., 2020). Ca2+, K+, and Na+ ions were quantified by using inductively coupled plasma optical emission spectrometry (ICP-OES) under axial configuration, equipped with an automatic sampling system (Vista-MPX CCD Simultaneous; Varian, Mulgrave, Australia), and operating conditions determined by specific software (ICP Expert Vista; Varian).
Bovine incisors (Bos taurus indicus) were disinfected into 0.1% thymol solution for 1 week, scraped with periodontal curettes, brushed with pumice paste, and ultrasonically cleaned with distilled water for 10 min. The teeth with enamel cracks or defects were excluded after observation under a stereomicroscope with ×80 magnification, while sound teeth were stored for up to 30 days in distilled water at 4°C (ISO/TR 11405:1994 standards).
A total of 158 enamel specimens (4 × 4 × 2 mm) were obtained from the buccal surface of the teeth by using a water-cooled low-speed diamond saw (Isomet; Buehler, Lake Bluff, IL, United States of America) and ultrasonically cleaned with distilled water for 2 min (Machado et al., 2019). Seventy-two specimens were ground flat and wet-polished at 300 rpm for 1 min with aluminum oxide papers (400-, 600-, 1200-, and 4000-grit) and 1-μm-grit diamond paste) and ultrasonically cleaned with distilled water for 3 min in between every polishing step. Two areas of 72 specimens with curvature <0.3 µm were covered with an adhesive unplasticized polyvinyl chloride (UPVC) tape and an approximately 4 × 1 mm window was left exposed for further analysis of enamel surface loss.
Eight groups (BaCloTea, GeLaTeaPep, EucaLem, Cinnamon, Spearmint, Artificial saliva, Citric acid and Distilled water) were randomly assigned in accordance to different solutions in which the specimens were twice a day immersed under agitation for 30 s in solutions and then washed with distilled water. The specimens were stored in the artificial saliva in between exposure cycles, except for the distilled water group. The exposure cycles were repeated for 14 days to simulate the complete consumption of a mouthrinse bottle (Zero et al., 2004; Vieira-Junior et al., 2019).
The chemical quantification of the specimens was carried out after removing the dentin layer from 72 specimens that were divided into 9 experimental groups, in which 3 crushed specimens formed 1 sample (n = 3), using a tapered round-edge diamond bur mounted in a water-cooled high-speed air turbine, the enamel was ground, autoclaved, and placed in polytetrafluoroethylene vials previously decontaminated in nitric (1%, v/v) acid and washed with deionized water. Approximately 0.1 g of ground enamel, 3 mL of nitric acid, 1 mL of hydrochloric acid, and 1 mL of hydrogen peroxide were mixed and subjected to acidic digestion by microwave radiation (MARSXpress; CEM, Matthews, NC, United States). The samples were transferred to polypropylene bottles with a final volume of 50 mL and diluted (303x approximately) to quantify Ca, K, Na, and P through ions chromatography (ICS-2000 Dual; Dionex, Sunnyvale, CA, United States) (Petta et al., 2017).
The enamel surface loss was determined through profilometric measurements after 14-day exposure to the solutions (n = 9). In each specimen, the UPVC tapes were removed and these two areas were used as references. A 2-mm long (X) and 1-mm wide (Y) scanned that included both exposed and reference areas were performed by using an optical profilometer (Proscan 2000; Scantron, Taunton, United Kingdom) (Figure 1). The profilometer scanned two hundred 0.01-mm steps and ten 0.1-mm steps in the X- and Y-axis, respectively. The depth of the exposed area was determined by subtracting the average height of the two reference areas (3-point height tool) (Proscan Application Software 2.0.17) (Lopes et al., 2020).
Two random specimens per group (except for distilled water) were ultrasonically cleaned, dried, mounted on aluminum stubs, sputter-coated with gold-palladium (JFC 1100E Ion Sputter, Jeol), and the enamel surface morphology was qualitatively analyzed under a scanning electron microscope (SEM; VEGA3 Tescan, Brno, Czech Republic) at ×6,000 and ×10,000 magnifications.
The Shapiro-Wilk and Levene tests were used to respectively assess the normal distribution and homoscedasticity of pH, TA, ions concentration, and enamel surface loss data. Groups were compared using one-way analysis of variance (ANOVA) and post hoc Tukey multiple comparisons at a significance level of p < 0.05 (SPSS 17.0, IBM Corp., Chicago, IL, United States). The chromatography data were descriptively analyzed through means and standard deviations.
The five EO-containing solutions presented significantly lower pH than distilled water (p < 0.05) and below the critical value (5.5) for enamel dissolution. BaCloTea (Basil, Clove e Tea Tree), GeLaTeaPep (Geranium, Lavender, Tea Tree and Peppermint), EucaLem (Eucalyptus and Lemon), and Cinnamon groups showed the lowest pH values. The pH and TA mean values for each group are shown in Table 2. The GeLaTeaPep group presented a significantly higher TA value than BaCloTea (p < 0.05), which in turn showed a significantly higher TA value in comparison to the other EO-containing solutions (p < 0.05). The distilled water presented significantly higher Ca, K, and Na concentrations than all EO-containing solutions (p < 0.05) (Figure 2).
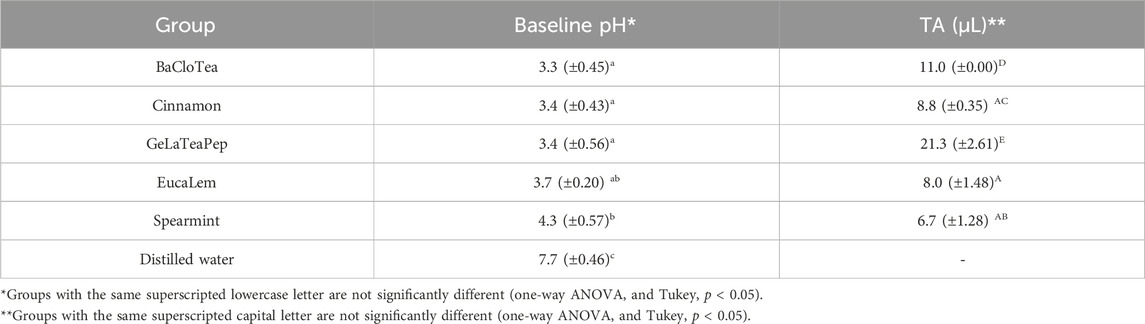
TABLE 2. pH and AT mean values (± standard deviations) for EO-containing solutions and distilled water.
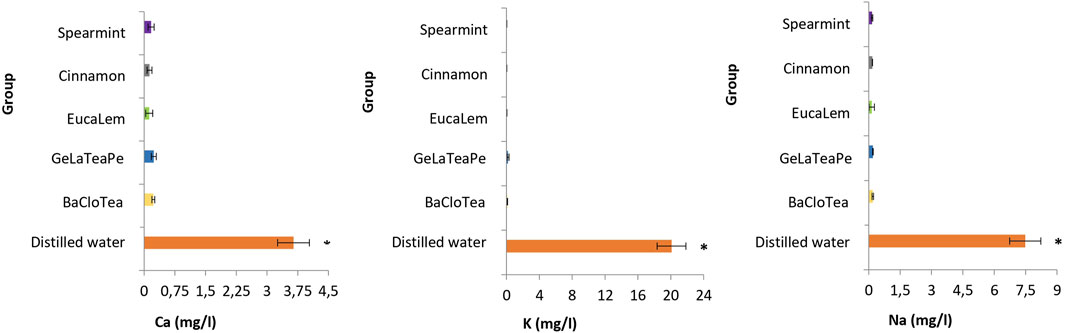
FIGURE 2. Ca, K, and Na concentrations (mg/L) in EO-containing solutions and distilled water. Groups followed by an asterisk (*) are significantly different from the other groups (ANOVA and Tukey, p < 0.05).
The Ca, K, Na, and P concentrations in enamel after 14-day exposure to EO-containing solutions are described in Table 3. Artificial saliva (positive control) and citric acid (negative control) presented the highest and the lowest Ca, K, Na, and P concentrations, respectively. GeLaTeaPep and EucaLem were the EO-containing solutions with the lowest Ca, Na, and P concentrations. All EO-containing solutions showed lower Ca and P concentrations than artificial saliva. Except for GeLaTeaPep and EucaLem groups, the other EO-containing solutions showed higher Ca and P concentrations than citric acid.
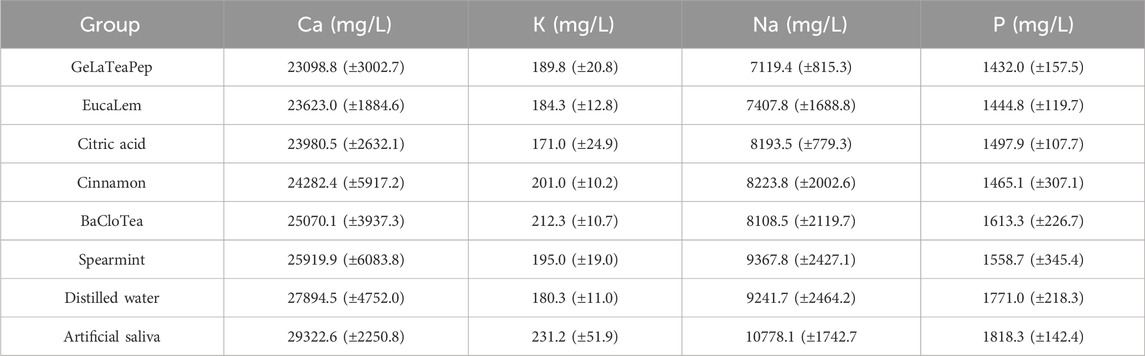
TABLE 3. Ca, K, Na, and P mean concentrations (± standard deviations) in enamel after 14-day exposure to EO-containing solutions, citric acid, distiller water, and artificial saliva.
All EO-containing solutions induced significantly higher enamel surface loss than artificial saliva and were not different from distilled water (except for the BaCloTea group) (Table 4). BaCloTea and Cinnamon groups showed the highest enamel surface loss and were not different from citric acid. EucaLem, GeLaTeaPep, and Spearmint groups present the lowest enamel surface loss.
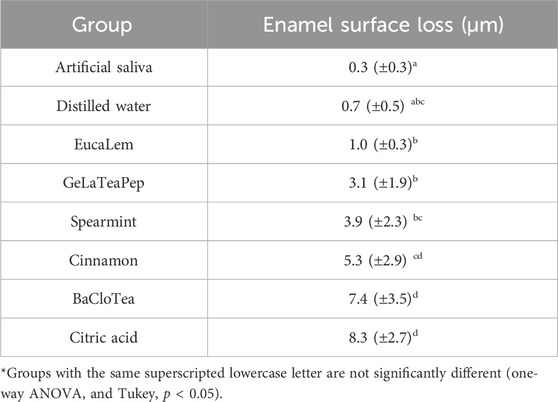
TABLE 4. Means (± standard deviations) of enamel surface loss after 14-day exposure to EO containing solutions, citric acid, distiller water, and artificial saliva.
The morphology of the enamel surface only exposed to artificial saliva was found predominantly smooth, regular, and uniform. Although slight marks of specimen preparation can be observed, the surface still presented aprismatic enamel without prisms exposure (Figures 3A, B). Conversely, the exposure to 0.3% citric acid resulted in the exposure of enamel prisms, interprismatic regions, and grooves that indicate accentuated demineralization (Figures 3C, D). The enamel surface of the specimens exposed to EO-containing solutions showed similar morphology of the positive control group (artificial saliva) (Figures 3E–N).
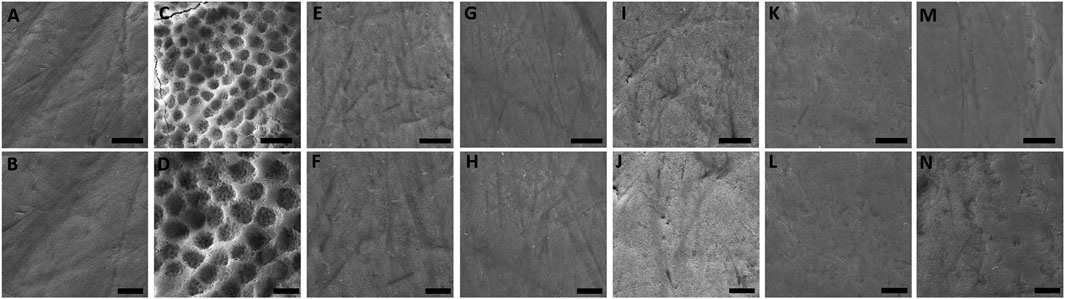
FIGURE 3. SEM photomicrographs (×6,000 and ×10,000 magnifications) of enamel after 14-day exposure to (A, B) artificial saliva, (C, D) citric acid, (E, F) BaCloTea, (G, H) GeLaTeaPep, (I, J) EucaLem, (K, L) Cinnamon, and (M, N) Spearmint.
The EO-containing solutions investigated in this study have low pH, TA, and Ca, K, and Na concentrations. In addition, these solutions released low Ca and P concentrations into the enamel and induced different levels of surface loss without affecting remarkable morphology alterations.
The EO-containing solutions presented pH values below 5.5, which is widely considered critical for enamel dissolution; however, this process also depends on the buffer capacity, TA, and Ca, P, and fluoride (F) concentrations (Dawes, 2003; Saads Carvalho and Lussi, 2020; Harper et al., 2021). Several oral care products such as F-containing mouthrinses have a low pH (Hellwig and Lussi, 2006). The results of this study are in agreement with other investigations on EO- and F-containing mouthrinses (Zero et al., 2004; Delgado et al., 2018; Valdivia-Tapia et al., 2021) and pH values similar to sports drinks (Sato et al., 2021). The EO-containing solutions investigated in this study showed low pH; however, they needed a small amount of base to be neutralized (low TA) (Saads Carvalho and Lussi, 2020), which represents a low erosive potential for tooth tissues (Harper et al., 2021). Delgado et al. (2018) observed some commercially available mouthrinses (Listerine Total Care, Listerine Ultraclean, Listerine Original, and Scope Classic) with pH below 5.5 and high TA; thus, both parameters were significantly correlated to dentin loss and erosive potential. Similar findings were also reported by Cavalcanti et al. (2010) regarding other branded mouthrinses.
In this study, the EO-containing solutions presented ions that are important for the oral environment. Ca is essential for remineralization, while P and Na play a major role in membrane potential and hypersensitivity regulation (Cummins, 2009; González-Cabezas and Fernández, 2018; Li et al., 2020). Moreover, Ca and P are the main components of hydroxyapatite [Ca10(PO4)6(OH)2]. The Spearmint showed the highest pH value, lowest TA, and Ca concentration similar to the other EO-containing solutions. This EO has 58% of carvone (C10H14O), which is a monoterpene ketone with promising antimicrobial, antispasmodic, anti-inflammatory, antioxidant, antinociceptive, and anticonvulsant activities (Dehsheikh et al., 2020; Pina et al., 2022); in addition, Mentha spicata has demonstrated to retard biofilm formation and can be considered an effective, cheap and safe alternative to improve oral health (Rasooli et al., 2009; Wiwattanarattanabut et al., 2017).
Among the scarce evidence on the effect of EO-containing mouthrinses, their use against cariogenic bacteria (Almeida Freires et al., 2015; Braga et al., 2019; Ma et al., 2020) and to reduce plaque and gingivitis have been suggested (Haas et al., 2016; Richards, 2017; Lynch et al., 2018). Some vegetable oils have been demonstrated to form a lipid-rich pellicle that minimizes enamel and dentin demineralization (Buchalla et al., 2003; Kensche et al., 2013; Ionta et al., 2017). The preventive potential of vegetable oils has been widely studied since they are natural, edible, cheap, and worldwide accessible (Ionta et al., 2017).
In this study, the 14-day protocol of exposure of enamel specimens to EO-containing solutions simulated two daily mouthrinses (morning and afternoon) usually performed by individuals (Vieira-Junior et al., 2019) and evaluated the sole effect of five EO-containing solutions on enamel without other variables such as erosion, wear, or toothbrushing (Zero et al., 2004; Delgado et al., 2018).
Most commercially available EO-containing mouthrinses usually contain 5%–27% of alcohol, which acts as a preservative, germicide, and solubilizer to maintain EO bioavailability (Silverman and Wilder, 2006; Gandini et al., 2012; Lynch et al., 2018; Pelino et al., 2018). The five EO-containing solutions evaluated in this study were prepared without the addition of alcohol by following the manufacturer’s recommendations for at-home use. Some studies have shown reliable antibacterial and antiplaque effects of alcohol-free EO-containing mouthrinses (Quintas et al., 2017; Lynch et al., 2018).
The highest Ca, K, Na, and P concentrations were observed in the enamel specimens exposed to artificial saliva (positive control). Despite the variety of artificial saliva formulations used for in vitro research, this product usually induces greater enamel remineralization than water (Ionta et al., 2014). The chromatography revealed that the EO-containing solutions released smaller amounts of Ca2+ and P ions when compared to the distilled water group.
Except for the negative control, the BaCloTea group showed the greatest enamel surface loss, which is in agreement with its lowest pH among the investigated EO-containing solutions. EucaLem, GeLaTeaPep, and Spearmint showed the lowest enamel surface loss mean values and were not significantly different from the distilled water group. However, in the SEM photomicrographs, the enamel surfaces of the specimens exposed to the EO-containing solutions were found similar to the positive control and distilled water groups. Loss of enamel surface was only clearly observed in the negative control group. While SEM is a method for qualitative analysis of the enamel surface microanatomy (Zhang et al., 2000), profilometry is a quantitative method that can accurately determine surface losses greater than 0.4 µm (Attin and Wegehaupt, 2014). Therefore, it can be assumed that profilometry can detect clinically imperceptible losses on the enamel surface that are not visualized through SEM.
Under slightly acidic pH, the Ca2+ ions from the tooth surface react with F ions to form a layer of calcium fluoride (CaF2) or CaF2-like molecule, which is more acid-resistant (Lussi et al., 2012; Schiffner, 2021). Interestingly, the EO-containing solutions investigated in this study showed chemical parameters that could benefit from the addition of F ions to remineralize the abovementioned enamel surface losses.
The effects of oral hygiene products on tooth structure must be well known, since they are widely used on a daily basis to maintain oral health. Thus, EO-containing solutions can be a viable and non-aggressive alternative to preserve tooth structure. Since this in vitro study did not fully simulate the oral environment, the effect of human saliva on acquired pellicle formation and enamel remineralization was not addressed (Nahsan et al., 2018; Lopes et al., 2020). Although the specimens were stored in artificial saliva, the effect of the EO-containing solutions on the enamel surface may have been more pronounced. Therefore, further in situ studies and clinical trials are encouraged to overcome these limitations.
The EO-containing solutions have low pH, TA, and low concentrations of Ca, Na, and K. Moreover, enamel exposed to these solutions showed low Ca and P concentrations and slight surface loss without morphology alteration.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The animal study was approved by the Ethic Committee on Animal Use of the Federal University of Para (CEUA/UFPA protocol number 6179220321 - ID 00164). The study was conducted in accordance with the local legislation and institutional requirements.
SM: Writing–original draft. PC-F: Writing–review and editing. MR: Writing–review and editing. KF: Writing–review and editing. RL: Writing–review and editing. RD’A: Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was developed with support from Brazilian National Council for Scientific and Technological Development (CNPq) in awarding a scholarship. The APC was paid by Federal University of Pará, by the Pró-reitoria de Pesquisa e Pós-graduação.
The authors would like to thank the funding of this research carried out by the National Council for Scientific and Technological Development (CNPq), Biomaterials Lab (UFPA), Evandro Chagas Institute (IEC/SVSA/MS), and University of São Paulo.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Almeida Freires, N., Denny, C., Benso, B., Matias De Alencar, S., and Rosalen, P. L. (2015). Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: a systematic review. Molecules 20, 7329–7358. doi:10.3390/molecules20047329
Alshehri, F. A. (2018). The use of mouthwash containing essential oils (LISTERINE®) to improve oral health: a systematic review. Saudi Dent. J. 30, 2–6. doi:10.1016/j.sdentj.2017.12.004
Araujo, M. W. B., Charles, C. A., Weinstein, R. B., McGuire, J. A., Parikh-Das, A. M., Du, Q., et al. (2015). Meta-analysis of the effect of an essential oil–containing mouthrinse on gingivitis and plaque. J. Am. Dent. Assoc. 146, 610–622. doi:10.1016/J.ADAJ.2015.02.011
Attin, T. S., and Wegehaupt, F. J. (2014). Methods for assessment of dental erosion. Monogr. Oral Sci. 25, 123–142. doi:10.1159/000360355
Aziz, Z. A. A., Ahmad, A., Setapar, S. H. M., Karakucuk, A., Azim, M. M., Lokhat, D., et al. (2018). Essential oils: extraction techniques, pharmaceutical and therapeutic potential - a review. Curr. Drug Metab. 19, 1100–1110. doi:10.2174/1389200219666180723144850
Basavegowda, N., Patra, J., and Baek, K.-H. (2020). Essential oils and mono/bi/tri-metallic nanocomposites as alternative sources of antimicrobial agents to combat multidrug-resistant pathogenic microorganisms: an overview. Molecules 27, 1058. doi:10.3390/molecules25051058
Braga, A. S., Girotti, L. D., Melo Simas, L. L., Pires, J. G., Pelá, V. T., Buzalaf, M. A. R., et al. (2019). Effect of commercial herbal toothpastes and mouth rinses on the prevention of enamel demineralization using a microcosm biofilm model. Biofouling 35, 796–804. doi:10.1080/08927014.2019.1662897
Buchalla, W., Attin, T., Roth, P., and Hellwig, E. (2003). Influence of olive oil emulsions on dentin demineralization in vitro. Caries Res. 37, 100–107. doi:10.1159/000069017
Cavalcanti, A. L., Ramos, I. A., Leite, R. B., Oliveira, M. C., Menezes, K. M., Fernandes, L. V., et al. (2010). Endogenous pH, titratable acidity and total soluble solid content of mouthwashes available in the Brazilian market. Eur. J. Dent. 4, 156–159. doi:10.1055/s-0039-1697823
Cummins, D. (2009). Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief. J. Clin. Dent. 20, 1–9.
Dagli, N., Dagli, R., Mahmoud, R. S., and Baroudi, K. (2015). Essential oils, their therapeutic properties, and implication in dentistry: a review. J. Int. Soc. Prev. Community Dent. 5, 335–340. doi:10.4103/2231-0762.165933
Dawes, C. (2003). What is the critical pH and why does a tooth dissolve in acid? J. Can. Dent. Assoc. 69, 722–724.
Dehsheikh, A. B., Sourestani, M. M., Dehsheikh, P. B., Mottaghipisheh, J., Vitalini, S., and Iriti, M. (2020). Monoterpenes: essential oil components with valuable features. Mini Rev. Med. Chem. 20, 958–974. doi:10.2174/1389557520666200122144703
Delgado, A., Ribeiro, A., Quesada, A., Rodríguez, L. E., Hernández, R., Wynkoop, B., et al. (2018). Potential erosive effect of mouthrinses on enamel and dentin. Gen. Dent. 66, 75–79.
Donovan, T., Nguyen-Ngoc, C., Abd Alraheam, I., and Irusa, K. (2021). Contemporary diagnosis and management of dental erosion. J. Esthet. Restor. Dent. 33, 78–87. doi:10.1111/jerd.12706
Favaro, J. C., Ribeiro, E., Guiraldo, R. D., Lopes, M. B., Aranha, A. M. F., and Berger, S. B. (2020). Effect of mouth rinses on tooth enamel surface. J. Oral Sci. 62, 103–106. doi:10.2334/josnusd.18-0370
Gandini, S., Negri, E., Boffetta, P., La Vecchia, C., and Boyle, P. (2012). Mouthwash and oral cancer risk quantitative meta-analysis of epidemiologic studies. Ann. Agric. Environ. Med. 19, 173–180.
Ghaderi, F., and Solhjou, N. (2020). The effects of lavender aromatherapy on stress and pain perception in children during dental treatment: a randomized clinical trial. Complement. Ther. Clin. Pract. 40, 101182. doi:10.1016/j.ctcp.2020.101182
González-Cabezas, C., and Fernández, C. E. (2018). Recent advances in remineralization therapies for caries lesions. Adv. Dent. Res. 29, 55–59. doi:10.1177/0022034517740124
Haas, A., Wagner, T., Muniz, F., Fiorini, T. F., Cavagni, J., and Celeste, R. K. (2016). Essential oils-containing mouthwashes for gingivitis and plaque: meta-analyses and meta-regression. J. Dent. 55, 7–15. doi:10.1016/j.jdent.2016.09.001
Harper, R., Shelton, R., James, J., Salvati, E., Besnard, C., Korsunsky, A. M., et al. (2021). Acid-induced demineralisation of human enamel as a function of time and pH observed using X-ray and polarised light imaging. Acta Biomater. 15, 240–248. doi:10.1016/j.actbio.2020.04.045
Hellwig, E., and Lussi, A. (2006). Oral hygiene products and acidic medicines. Monogr. Oral Sci. 20, 112–118. doi:10.1159/000093358
Ionta, F. Q., Alencar, C. R. B., Val, P. P., Boteon, A. P., Jordão, M. C., Honório, H. M., et al. (2017). Effect of vegetable oils applied over acquired enamel pellicle on initial erosion. J. Appl. Oral Sci. 25, 420–426. doi:10.1590/1678-7757-2016-0436
Ionta, F. Q., Mendonça, F. L., De Oliveira, G. C., De Alencar, C. R. B., Honório, H. M., Magalhães, A. C., et al. (2014). In vitro assessment of artificial saliva formulations on initial enamel erosion remineralization. J. Dent. 42, 175–179. doi:10.1016/J.JDENT.2013.11.009
Jardim, J. J., Alves, L. S., and Maltz, M. (2009). The history and global market of oral home-care products. Braz Oral Res. 23, 17–22. doi:10.1590/s1806-83242009000500004
Kensche, A., Reich, M., Kümmerer, K., Hannig, M., and Hannig, C. (2013). Lipids in preventive dentistry. Clin. Oral Investig. 17, 669–685. doi:10.1007/S00784-012-0835-9
Li, H., Liu, W., Zhou, H., Sun, Y., Zhang, M., Wang, J., et al. (2020). In vitro dentine tubule occlusion by a novel toothpaste containing calcium silicate and sodium phosphate. J. Dent. 103, 100024. doi:10.1016/j.jjodo.2020.100024
Lopes, R., Silva, J., João-Souza, S. H., Maximiano, V., Machado, A. C., Scaramucci, T., et al. (2020). Enamel surface loss after erosive and abrasive cycling with different periods of immersion in human saliva. Arc Oral Biol. 109, 104549. doi:10.1016/j.archoralbio.2019.104549
Lussi, A., Hellwig, E., and Klimek, J. (2012). Fluorides -mode of action and recommendations for use. Schweiz Monatsschr Zahnmed. 122, 1030–1042.
Lussi, A., Jaeggi, T., and Zero, D. (2004). The role of diet in the aetiology of dental erosion. Caries Res. 38, 34–44. doi:10.1159/000074360
Lynch, M. C., Cortelli, S. C., McGuire, J. A., Zhang, J., Ricci-Nittel, D., Mordas, C. J., et al. (2018). The effects of essential oil mouthrinses with or without alcohol on plaque and gingivitis: a randomized controlled clinical study. BMC Oral Health 18, 6–10. doi:10.1186/s12903-017-0454-6
Ma, L., Chen, J., Han, H., Liu, P., Wang, H., Lin, S., et al. (2020). Effects of lemon essential oil and limonene on the progress of early caries: an in vitro study. Arc Oral Biol. 111, 104638. doi:10.1016/j.archoralbio.2019.104638
Machado, A., Bezerra, S., João-Souza, S. H., Caetano, T. M., Russo, L. C., Carvalho, T. S., et al. (2019). Using fluoride mouthrinses before or after toothbrushing: effect on erosive tooth wear. Arc Oral Biol. 108, 104520. doi:10.1016/j.archoralbio.2019.104520
Medeiros, T. L. M., Mutran, S. C. A. N., Espinosa, D. G., Do Carmo Freitas Faial, K., Pinheiro, H. H. C., and D’Almeida Couto, R. S. (2020). Prevalence and risk indicators of non-carious cervical lesions in male footballers. BMC Oral Health 20, 215. doi:10.1186/S12903-020-01200-9
Nahsan, F., Reis, M., Francisconi-Dos-Rios, L. F., Leão, L. V., and Paranhos, L. R. (2018). Effectiveness of whitening mouthwashes on tooth color: an in vitro study. Gen. Dent. 66, e7–e10.
Pelino, J., Passero, A., Martin, A. A., and Charles, C. A. (2018). In vitro effects of alcohol-containing mouthwashes on human enamel and restorative materials. Braz Oral Res. 32, e25. doi:10.1590/1807-3107bor-2018.vol32.0025
Petta, T. M., Gomes, Y. S. B. L., Esteves, R. A., Faial, K. C. F., Couto, R. S. D., and Silva, C. M. (2017). Chemical composition and microhardness of human enamel treated with fluoridated whintening agents. A study in situ. Open Dent. J. 11, 34–40. doi:10.2174/1874210601711010034
Pina, L., Serafini, M., Oliveira, M., Sampaio, L. A., Guimarães, J. O., and Guimarães, A. G. (2022). Carvone and its pharmacological activities: a systematic review. Phytochemistry 196, 113080. doi:10.1016/j.phytochem.2021.113080
Quintas, V., Prada-López, I., Carreira, M. J., Suárez-Quintanilla, D., Balsa-Castro, C., and Tomás, I. (2017). In situ antibacterial activity of essential oils with and without alcohol on oral biofilm: a randomized clinical trial. Front. Microbiol. 8, 2162. doi:10.3389/fmicb.2017.02162
Ramsey, J. T., Shropshire, B. C., Nagy, T. R., Chambers, K. D., Li, Y., and Korach, K. S. (2020). Essential oils and health. Yale J. Biol. Med. 93, 291–305.
Rasooli, I., Shayegh, S., and Astaneh, S. D. A. (2009). The effect of mentha spicata and eucalyptus camaldulensis essential oils on dental biofilm. Int. J. Dent. Hyg. 7, 196–203. doi:10.1111/J.1601-5037.2009.00389.x
Richards, D. (2017). Effect of essential oil mouthrinses on plaque and gingivitis. Evid. Based Dent. 18, 39–40. doi:10.1038/sj.ebd.6401233
Saads Carvalho, T., and Lussi, A. (2020). Chapter 9: acidic beverages and foods associated with dental erosion and erosive tooth wear. Monogr. Oral Sci. 28, 91–98. doi:10.1159/000455376
Sato, T., Fukuzawa, Y., Kawakami, S., Suzuki, M., Tanaka, Y., Terayama, H., et al. (2021). The onset of dental erosion caused by food and drinks and the preventive effect of alkaline ionized water. Nutrients 13, 3440. doi:10.3390/nu13103440
Schiffner, U. (2021). Use of fluorides for caries prevention. Bundesgesundheitsblatt - Gesundheitsforsch. Gesundheitsschutz. 64, 830–837. doi:10.1007/S00103-021-03347-4
Sharma, N., Charles, C., Lynch, M., Qaqish, J., McGuire, J. A., Galustians, J. G., et al. (2004). Adjunctive benefit of an essential oil–containing mouthrinse in reducing plaque and gingivitis in patients who brush and floss regularly: a six-month study. J. Am. Dent. Assoc. 135, 496–504. doi:10.14219/jada.archive.2004.0217
Silverman, S., and Wilder, R. (2006). Antimicrobial mouthrinse as part of a comprehensive oral care regimen: safety and compliance factors. J. Am. Dent. Assoc. 137, 22S–S26. doi:10.14219/jada.archive.2006.0406
Valdivia-Tapia, A. C., Botelho, J. N., Tabchoury, C. P. M., Ricomini-Filho, A. P., Giacaman, R. A., and Cury, J. A. (2021). Fluoride bioavailability on demineralized enamel by commercial mouth rinses. Braz Dent. J. 32, 45–54. doi:10.1590/0103-6440202104475
Vieira-Junior, W. F., Ferraz, L. N., Giorgi, M. C. C., Ambrosano, G. M. B., Aguiar, F. H. B., and Lima, D. A. N. L. (2019). Effect of mouth rinse treatments on bleached enamel properties, surface morphology, and tooth color. Oper. Dent. 44, 178–187. doi:10.2341/17-250-L
Weijden, F. V. der., Sluijs, E. V. der., Ciancio, S. G., and Slot, D. E. (2015). Can chemical mouthrinse agents achieve plaque/gingivitis control? Dent. Clin. North Am. 59, 799–829. doi:10.1016/j.cden.2015.06.002
Winska, K., Maczka, W., Łyczko, J., Grabarczyk, M., Czubaszek, A., and Szumny, A. (2019). Essential oils as antimicrobial agents - myth or real alternative? Molecules 24, 2130. doi:10.3390/molecules24112130
Wiwattanarattanabut, K., Choonharuangdej, S., and Srithavaj, T. (2017). In vitro anti-cariogenic plaque effects of essential oils extracted from culinary herbs. J. Clin. Diagn Res. 11, DC30–DC35. doi:10.7860/JCDR/2017/28327.10668
Yadav, H. K., Yadav, R. K., Chandra, A. R., and Thakkar, R. (2016). The effectiveness of eucalyptus oil, orange oil, and xylene in dissolving different endodontic sealers. J. Conserv. Dent. 19, 332–337. doi:10.4103/0972-0707.186447
Zero, D., Zhang, J., Harper, D., Wu, M., Kelly, S., Waskow, J., et al. (2004). The remineralizing effect of an essential oil fluoride mouthrinse in an intraoral caries test. J. Am. Dent. Assoc. 135, 231–237. doi:10.14219/jada.archive.2004.0157
Keywords: oils, volatile, dental enamel, calcium, chromatography, scanning electron microscopy
Citation: Mutran SCAN, Carvalho-Filho PRd, Ribeiro MES, Faial KdCF, Lima RR and D’Almeida Couto RS (2024) Essential oil-containing solutions (mouthwashes) preserve dental enamel with releasing low Ca and P concentrations without morphology alterations: an in vitro study. Front. Chem. 12:1341769. doi: 10.3389/fchem.2024.1341769
Received: 20 November 2023; Accepted: 31 January 2024;
Published: 28 February 2024.
Edited by:
Joaquín Campos Rosa, University of Granada, SpainReviewed by:
Luiz Renato Paranhos, Federal University of Uberlandia, BrazilCopyright © 2024 Mutran, Carvalho-Filho, Ribeiro, Faial, Lima and D’Almeida Couto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberta Souza D’Almeida Couto, cmRhbG1laWRhY291dG9AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.