- 1Department of Materials Chemistry, National Institute of Chemistry, Ljubljana, Slovenia
- 2Department of Atomic Physics, Institute for Nuclear Sciences Vinča, University of Belgrade, Belgrade, Serbia
Electrocatalytic materials are pivotal for clean chemical production and energy conversion in devices like electrolyzers and fuel cells. These materials usually consist of metallic nanoparticles which serve as active reaction sites, and support materials which provide high surface area, conductivity and stability. When designing novel electrocatalytic composites, the focus is often on the metallic sites, however, the significance of the support should not be overlooked. Carbon materials, valued for their conductivity and large surface area, are commonly used as support in benchmark electrocatalysts. However, using alternative support materials instead of carbon can be beneficial in certain cases. In this minireview, we summarize recent advancements and key directions in developing novel supports for electrocatalysis, encompassing both carbon and non-carbon materials.
1 Introduction
Electrocatalysis plays an important role in advancing clean energy and chemical production. Renewable-powered water electrolysis produces green hydrogen (Carmo et al., 2013; Lei et al., 2019), while fuel cells convert different fuels (hydrogen, methanol, ethanol, etc.) into electricity (Carrette et al., 2001). CO2 electrolyzers transform this feedstock into value-added chemicals (Popović et al., 2020; Wang et al., 2023), while ammonia can be produced via nitrogen electro-reduction (Zeng et al., 2020; Lu et al., 2021). The core of these devices are electrocatalysts, typically comprised of metallic nanoparticles (NPs) dispersed on support materials. NPs act as the active sites for reaction enrolling, while supports provide electrical conductivity, a fine distribution of NPs, and stability in (electro)chemical environments.
The focus in the development of novel electrocatalytic materials is primarily on active sites. Strategies for improving intrinsic activity, stability, and selectivity include altering composition (Bing et al., 2010; Mistry et al., 2016; Mukherjee et al., 2022), customizing nanostructures (Erini et al., 2015; L. Pan et al., 2019; Wu et al., 2019) and downsizing catalyst to the level of singe atoms (Li et al., 2019; Speck et al., 2020; Wang et al., 2020). Pt-M alloys (M = Co, Cu, Ni) became benchmark catalysts for fuel cells (Bing et al., 2010; Mistry et al., 2016; Mukherjee et al., 2022). High entropy alloys, formed by mixing five or more metals, create entirely new active sites (Huo et al., 2022). Single-atom electrocatalysts exemplify novel chemistry where isolated atoms yield outstanding performances (Li et al., 2019; Speck et al., 2020; Wang et al., 2020).
Another approach toward novel electrocatalytic composites involves studying the effect of the support on the performance of active sites (Jayabal et al., 2020). Carbon materials are the most common supports in electrocatalysis as they possess unique properties including large surface area and high conductivity (Sharma and Pollet, 2012). Although carbon nanomaterials can serve both as catalysts and catalyst supports (Zhai et al., 2015), they can be enhanced to overcome shortcomings like limited electrochemical stability (Bele et al., 2019; Reier et al., 2014; Pizzutilo et al., 2016). Additionally, the weak interaction between carbon support and NPs may promote certain degradation mechanism such as particle detachment, migration, and coalescence (Campisi et al., 2018). To this end, various directions have been explored, including modification of carbon supports by functionalization, introducing defects and doping with heteroatoms, or developing supports based on transition metal oxides, nitrides, carbides, and organic materials.
This minireview summarizes recent advances in enhancing electrocatalyst performance through support effects. We cover both carbon and non-carbon materials, the two main directions in the field. We explore strategies for improving carbon supports and efforts to design non-carbon materials that combine the benefits of carbon while enhancing active site performance.
2 Carbon-based supports
The diverse range of carbon materials available for catalyst substrates highlights their versatility. In this section, the latest research on the most studied and most promising systems will be presented.
2.1 Graphene
Graphene is a 2D material consisting of a monolayer of sp2 hybridized carbon atoms disposed in a hexagonal packing. Graphene stands out because of large theoretical surface area and easy tenability (Tarcan et al., 2020). Pristine graphene is rarely utilized in electrochemistry, whereas its derivatives like graphene oxide (GO) (Krishnamoorthy et al., 2013) and reduced graphene oxide (rGO) (Erickson et al., 2010) are widely used. The interest in graphene arises from its single-layer structure (Baig et al., 2021) which prevents re-stacking through the formation of a well-organized and interconnected 3D framework. One way to prevent agglomeration is to obtain graphene with more hydroxyl groups at the edges, which can serve also as active sites (Pan et al., 2019).
The interaction between NPs and graphene can be enhanced by heteroatom doping. Xiong et al. (2013) showed that significantly smaller and more homogeneous nanoparticles with enhanced catalytic activity were obtained on N-doped graphene. The introduction of N and B dopants (Y. Sun et al., 2016) can synergistically improve catalyst performance. Pt single-atom catalysts were synthesized using holey N-doped graphene as support and achieved 28 times greater mass activity than Pt/C for hydrogen evolution reaction (HER) (Sun et al., 2022). Our group created advanced PdAu (Rakočević, Srejić, et al., 2021) and PtAu (Rakočević et al., 2021) catalysts on rGO for HER by combining two metals to optimize their electronic structure. Excellent HER activity of PtAu/rGO catalyst can be observed in (Figure 1A). Our recent works revealed that using graphene derivatives as support for Pt-M improves fuel cell catalyst performance. Enhancements were attributed to increased levels of total oxygen, specific oxygen functionalities, sp2 carbon, and reduced structural defects (Pavko et al., 2022; 2023).

FIGURE 1. (A) (a) SEM image of PtAu/rGO; (b) comparison of HER activities in 0.5 M H2SO4; (c) corresponding Tafel slopes (Rakočević et al., 2021). (B) (d) TEM image of Ru@MWCNTs with particle size distribution; (e) and (f) HER activity and stability compared to Pt/C in different media (Kweon et al., 2020). (C) (g) SEM image of Ru1Ni1-NCNFs; (h) HER and (i) OER polarization curves in 1 M KOH (M. Li et al., 2020).
Combining the porous structure of graphene and doping with heteroatoms can significantly improve catalyst performance (Gao et al., 2023). Karaman (2023) synthesized N,P,S triple-doped 3D graphene architectures with interconnected, hierarchical porous structure, which was decorated with Pd NPs. This catalyst showed improved activity and stability for ethanol oxidation reaction (EOR) compared to Pd@C and Pd@3DG. Ma et al. (2022) developed a one-step strategy for preparing N, P co-doped graphene-supported ultrafine Ru2P NPs using GO and deoxyribonucleic acid (DNA) as precursors. In this case, the biomolecule of DNA gives the sites for metal ion adsorption leading to the excellent activity for HER in the whole pH range.
High conductivity of graphene facilitates electron transfer at the interface (Jung et al., 2020) and provides more active sites (Navalon et al., 2017) for electrocatalytic reactions. This is crucial when using poorly conductive materials as catalysts (Li et al., 2017). Bejigo et al. (2023) synthesized Co3O4/rGO catalysts by recycling spent lithium-ion batteries. The activity of Co3O4/rGO for oxygen reduction reaction (ORR) is similar to that of commercial Pt/C but with improved stability. Tang et al. (2022) showed that combining the cobalt metaphosphate with 3D graphene support provides a catalyst with promising HER performance.
2.2 Carbon nanotubes (CNTs)
CNTs are nanometer-sized cylindrical structures, which can be either single-walled (SW) or multi-walled (MW), depending on the number of concentric layers. Due to their chemical stability, they can be used in different pH environments. High electrical conductivity and large surface area of CNTs enable efficient electron transfer and more active sites (Ortiz-Herrera et al., 2022) making them interesting supports for electrocatalysis.
Wang et al. (2023) developed electrocatalyst for HER by depositing Pt nanoparticles onto activated CNTs. Outstanding HER performance was ascribed to the improved electron conductivity of CNTs and the increased number of exposed active sites. Liu et al. (2023) synthesized three-metallic PtPdRh nanoparticles with CNTs and obtained a highly active catalyst for EOR. Multicomponent catalyst, denoted as Co@CNTs|Ru, showed excellent results for HER in the whole pH range (Chen et al., 2022). The arrangement of Co NPs constrained within nanotubes and a small amount of Ru uniformly deposited on their outer walls led to a redistribution of charges and electron coupling, resulting in excellent HER activity. Wang et al. (2020) reported an HER catalyst containing Co nanoparticles, along with Pt and CNTs. They used dealloying of PtCo/CNT catalyst to reach excellent HER performance. Kweon et al. (2020) uniformly deposited small Ru nanoparticles on MWCNTs modified with–COOH groups, which showed exceptional HER performance in both acidic and alkaline media (Figure 1B). DFT calculations suggest that Ru-C bonds likely serve as the primary active sites for HER. MWCNTs were also utilized for developing low-cost catalysts with MnSe nanostructures for both alkaline oxygen evolution reaction (OER) and ORR (Singh et al., 2022).
Regarding heteroatom doping, nitrogen-doped CNTs (N-CNTs) are the most widely studied. For example, excellent catalysis was obtained on N-CNTs supporting Pt-Ru for methanol oxidation reaction (MOR) (Forootan Fard et al., 2020). Similarly, Cu-Fe oxide alloy nanoparticles supported on N-CNTs exhibited promising activities for HER and ORR (Liu et al., 2022). The B,N co-doped CNTs with highly dispersed Ru nanoclusters showed high activity for water splitting. Simultaneous doping with B and N significantly reduces the adsorption energy of the hydrogen intermediate at Ru sites, which enhances the HER kinetics (Fan et al., 2022). S-doped (Tavakol et al., 2020) or P-doped (Song et al., 2022) CNTs have also shown potential in improving HER kinetics.
2.3 Other carbon-based supports
In addition to graphene and CNTs, various other carbon-based materials have potential as catalyst substrates. Carbon nanofibers (CNFs) are similar to CNTs in structure but have a more disordered arrangement. Wang et al. (2017) synthesized well-dispersed Pt-Cu nanoparticles enclosed in CNFs as a highly effective catalyst for HER. The outstanding performance originated from synergistic interaction between Pt and Cu, the uniform distribution of the alloy nanoparticles, and the use of CNFs with 3D architectures. Hodnik et al. reported a facile and scalable synthesis of 3D-structured electrodes composed of Pt NPs deposited on graphitized CNFs which were used as catalysts for ORR and MOR (Hodnik et al., 2020). RuNi nanoparticles grafted in nitrogen-doped CNFs also showed very good activity for water-splitting reactions (Figure 1C) (Li et al., 2020).
Carbon aerogels (CA) are 3D structures that can be organic-based, graphitic materials-based, or biomass-based (Lee and Park, 2020). The aerogel’s porosity affects the distribution and size of the NPs and inhibits their aggregation (Peles-Strahl et al., 2023). Li et al. synthesized a highly stable non-precious metal catalyst with template-assisted few-layer graphene aerogel as support with high activities for OER and ORR (Li et al., 2023). Hou et al. (2023) developed a Ni-WC/CA anode for MOR, using the highly conductive bacterial cellulose-derived carbon as the substrate.
Another strategy to create catalysts with excellent corrosion resistance involves the combination of fullerene C60 and platinum. The attachment of Pt clusters onto two-dimensional fullerene nanosheets (Chen et al., 2023), as well as confining single Pt atoms using two fullerene molecules were achieved (Zhang et al., 2023). In both cases, the enhanced catalytic effect originated from the varied bonding characteristics of Pt-sites at the Pt/C60 interface. Ruthenium nanoparticles and singe atoms were grafted into a 3D crystalline fullerene network, providing an outstanding catalyst for alkaline HER (T. Luo et al., 2023).
3 Non-carbon-based supports
Despite the mentioned advantages and feasibility of carbon supports, there are cases where alternative materials are necessary. In this section, we will explore recent advancements in the design of non-carbon supports for electrocatalysis.
3.1 Transition metal oxides-based supports
Transition metal oxides (TMOs) are attractive in electrocatalysis due to their (electro)chemical stability and ability to trigger metal-support interaction (MSI) with active sites (Tauster et al., 1978; Pan et al., 2017; Luo et al., 2022). However, TMOs often suffer from limited conductivity. For example, TiO2, a cost-effective, low-toxicity semiconductor with excellent corrosion resistance. To address insufficient conductivity, Kwon et al. developed a TiO2/C composite by covering carbon with a sub-nanometer-thick TiO2 layer, which enhanced the durability of carbon support and the performance of supported Pt for ORR through MSI (Shi et al., 2021). MSI weakens the interaction of Pt with intermediate species, which results in more active sites available for O2 adsorption. Gasteiger’s group achieved a different effect by partially reducing a TiO2-y layer on carbon and decorating it with Pt nanoparticles using atomic layer deposition (Geppert et al., 2020). This led to the encapsulation of Pt particles with a TiO2-y coating, enabling selective catalysis of hydrogen reactions while inhibiting ORR, CO oxidation, and Pt oxidation. Such selectivity is crucial for fuel cells’ lifetime, as it prevents catalyst deactivation due to the enrolling of the undesired ORR at Pt-based anodes (Jung et al., 2020). A similar encapsulation effect was demonstrated for Ru/TiOx/C composites (Stühmeier et al., 2022). In summary, blending TiO2 with carbon is a viable strategy to enhance its conductivity.
Another way to increase TiO2 conductivity is transition metal doping. For instance, incorporating W into TiO2 generates a highly conductive composite with a high surface area (Pham et al., 2021). Pt supported on W-doped TiO2 (Pt/W-TiO2) exhibited significantly improved ORR activity compared to undoped Pt/TiO2 and surpassed the durability of Pt/C (T. M. Pham et al., 2023). A similar effect was shown for Pt supported on Ta-doped TiO2 (Liu et al., 2014; Anwar et al., 2017) and Nb-doped TiO2 (Liu et al., 2014). Analogously, IrO2 and IrRuOx nanoparticles loaded on Nb-doped TiO2 nanotubes exhibited high activity for OER (Genova-Koleva et al., 2019).
Our group has developed a way to increase the conductivity of TiO2 using partial nitridation to form titanium-oxynitride (TiON). Initial work by Bele et al. showed that TiON is a conductive material capable of anchoring small-sized Ir nanoparticles (Bele et al., 2019). The OER activity and stability of the Ir/TiON composite surpassed the performance of Ir-benchmarks. Further developments included mixing TiON with carbon to increase its surface area. Ir/TiON/C composite with nearly three times higher OER mass activity than Ir-black was reported by Loncar et al. (2020). Ir nanoparticles immobilized on nanotubular TiON exhibited exceptional OER performance due to MSI. The presence of nitrogen was shown to be crucial for Ir anchoring and nanoparticle stability (Bele et al., 2020). Another work reported that the presence of Ir stabilized TiON support against oxidation during electrochemical treatments (Koderman Podborsek et al., 2022). We have also explored the effect of TiON on other catalysts and reactions beyond Ir and OER. Graphene covered with TiON was decorated with PtCu, Ir and Ru nanoparticles (Moriau et al., 2019). This composite (scheme given in Figure 2A) exhibited high catalytic performance for various reactions occurring in fuel cells and electrolyzers (Figures 2B, C). More recently, we have shown that Pt/TiON outperforms Pt/C as a HER catalyst due to the dual effect of MSI (Figures 2D–F): i) adjustment of H adsorption energetics; ii) more effective anchoring of Pt particles with respect to carbon (Smiljanić et al., 2022).
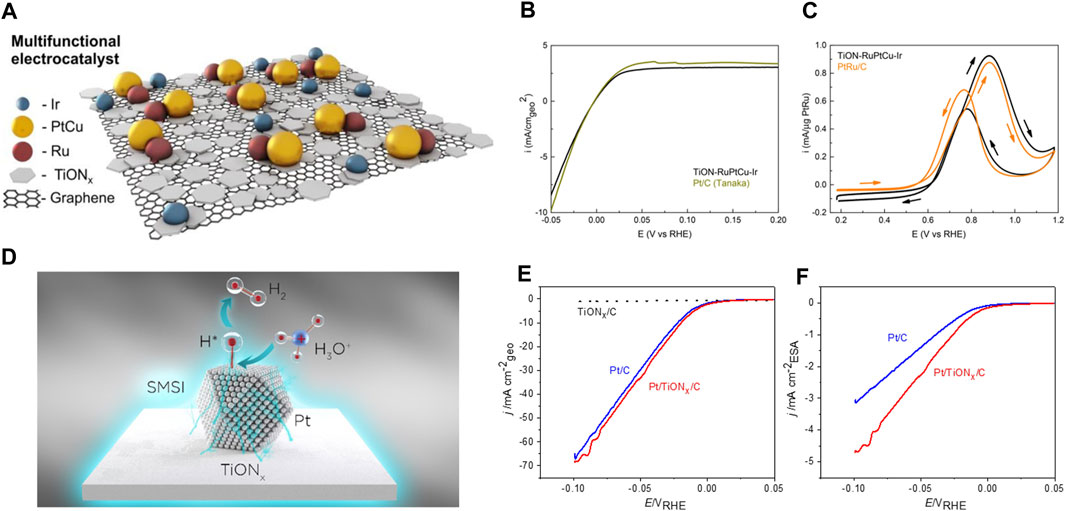
FIGURE 2. (A) Scheme of multifunctional electrocatalyst comprised of Ir, PtCu and Ru nanoparticles dispersed over TiON-graphene support; (B) comparison of HER/HOR activity of TiON-RuPtCu-Ir catalyst with Pt/C benchmark; (C) comparison of MOR performance of TiON-RuPtCu-Ir catalyst with PtRu/C bechmark; (D) scheme of MSI between Pt and TiON; (E) comparison of HER activities of TiON/C, Pt/TiON/C and Pt/C; (F) intrinsic HER activities of Pt/C and Pt/TiON/C obtained by taking into account Pt electrochemical surface area. Reprinted with permission from Moriau et al. (2019); Smiljanić et al. (2022). Further requests regarding reproduction of the used materials should be directed to the ACS.
Tin-oxide (SnO2) is another interesting material in this context. Sasaki et al. demonstrated that Pt/SnO2 composite is more stable than Pt/C in fuel cell operations and that doping of SnO2 with hypervalent anions (Nb5+, Sb5+, Al3+) can increase its conductivity (Takasaki et al., 2011). The approach of doping antimony into tin-oxide (ATO) and decoration with Pt was adopted for obtaining better-performing catalysts for ORR (Hussain et al., 2019; He et al., 2020), and electro-oxidation of methanol and ethanol (Lee et al., 2008). In terms of OER, ATO is considered state of the art support for iridium nanocatalysts (Puthiyapura et al., 2014). Many works reported impressive OER catalysts prepared by supporting IrO2 on various types of ATO (Tong et al., 2017; Han et al., 2020; Moriau et al., 2022; Khan et al., 2023). The advantages of SnO2 over carbon include higher durability, an oxophilic effect beneficial for CO tolerance of supported nanoparticles, and the ability to induce MSI.
Tungsten-oxide (WO3) also found application in electrocatalysis research. Recent findings reveal that Pt/WO3 has remarkable HER activity, attributed to electron transfer from the support to Pt sites, leading to the adjustment of the free energy of H adsorption (Fan et al., 2023). Conversely, Kim et al. reported a unique effect of WO3 support, known as a metal-insulator transition (Jung et al., 2020). When exposed to hydrogen, WO3 transforms into a metallic conductor (HxWO3-x), enabling supported Pt to catalyze HOR. In contrast, HxWO3-x reverts to insulating WO3 in an oxygen atmosphere and suppresses ORR. Such selective catalysis of Pt-materials is beneficial for the fuel cell lifespan.
3.2 Other non-carbon-based supports
Many other materials gained attention in electrocatalysis, including nitrides, carbides, 2D-materials, organic matrices, etc. Transition metal nitrides are known for high conductivity which makes them interesting for electrocatalysis. For instance, Ir nanoparticles loaded onto TiN exhibited improved OER performance due to electronic interactions between TiN and Ir (G. Li et al., 2018). A recent study also reported electron transfer between Ru nanorods anchored on TiN, contributing to the excellent HER activity of Ru/TiN composite (Yang et al., 2022). However, the instability of TiN is a concern for its electrocatalytic applications (Avasarala and Haldar, 2011). To address this, DiSalvo et al. prepared a ternary Ti0.5Nb0.5N nitride as a support for Pt, which exhibited better ORR performance than Pt/C (Cui et al., 2013). Similar benefits were observed with various transition metal carbides. Sun and others synthesized a RuO2-WC composite with exceptional activity for water-splitting, attributed to the regulation of Ru electronic structures via MSI (Sun et al., 2022). Ru singe atoms supported on nitrogen-doped molybdenum carbide displayed excellent HER performance due to the synergistic effect between support and active Ru sites (Yu et al., 2020).
Recently, a group of 2D layered materials called MXenes gained prominence in electrocatalysis (Liu et al., 2020; Bai and Guan, 2022). For instance, Pt nanoclusters deposited on Ti3C2Tx MXene exhibited higher HER activity than Pt/C (Jian et al., 2021). Sun’s team reported MXene catalysts with low Pt loading and impressive HER performance, which was linked with the influence of surface terminals (OH and O) in MXene on the electronic state of Pt (Yuan et al., 2019).
Our group recently explored tris(aza)pentacene (TAP) as a support for Pt nanoparticles (Vélez Santa et al., 2021; Smiljanić, Bele, et al., 2022). TAP belongs to the family of polyheterocycles, which are organic compounds interesting in various fields of chemistry and material science due to their electronic, optical, and conductive properties. In aqueous media, TAP features tunable conductivity due to reversible protonation/deprotonation. When protonated at lower potentials (<0.5 VRHE), the conductivity of TAP increases, allowing supported Pt to run HOR/HER, while at higher potentials limited TAP conductivity prevents Pt/TAP from running ORR (Vélez Santa et al., 2021). Additionally, this feature of TAP reduces Pt dissolution during rapid potential spikes encountered during device switching on/off, which is otherwise very harmful for Pt/C benchmarks (Smiljanić, Bele, et al., 2022).
4 Summary and outlook
This mini-review provides a concise overview of recent advancements in designing support materials for electrocatalysis. High surface area carbon-based materials have shown their versatility in electrocatalysis but also have room for further improvements. The most important challenge is in optimizing the activity of supported nanoparticles and enhancing the carbon corrosion resistance to prevent degradation of the catalytic composites. High-temperature treatments that lead to graphitization offer a path to more stable materials, but this comes with the trade-off of potentially reducing catalytic activity by removing defects and heteroatoms. Strategies for manufacturing advanced carbon-based materials will be the focus of future research. Despite all advantages, in some cases, however, it is necessary to replace carbon with some alternative supports. After addressing their low conductivity, TMOs offer several key advantages over carbon in tackling specific challenges in energy conversion devices. Their corrosion resistance enables the design of supported nanocatalysts for OER, which significantly improves the utilization of precious and scarce metals such as Ir and Ru. The ability to induce MSI and to improve the activity and durability of supported active sites can be used to create advanced electrocatalysts for a range of reactions. Materials such as ATO and TiON are certainly TMO derivates with interesting features as electrocatalytic supports for different reactions. Further work is needed to additionally understand the MSI provided by these supports for each particular case (i.e., each combination of metal and reaction), which will allow more straightforward tailoring of the advanced electrocatalytic composites. Similar is valid for nitrides, carbides, MXenes, and organic matrices, which can be used for reactions in both fuel cells and electrolyzers.
Overall, we anticipate that carbon materials will remain a key focus in electrocatalysis, particularly in cases when they provide sufficient durability for stable catalyst operation. Non-carbon supports are foreseen to play a role when a specific issue is to be addressed (such as carbon corrosion) and when additional enhancement of the performance of active sites via MSI is desired. While TMOs, nitrides, and carbides are already well-known as supports for electrocatalysis, the research on MXenes and a few other types of 2D materials (phosphorenes, boridenes, etc.) is in its early stages, offering ample opportunities for the synthesis of advanced electrocatalytic composites. In the case of non-carbon-based materials, the choice of support can be guided by the specific demand in front of the catalyst.
Author contributions
MS: Conceptualization, Project administration, Writing–original draft, writing—review & editing. IS: Conceptualization, Formal Analysis, Writing–original draft, writing—review & editing. JG: Writing–original draft. AM: Writing–original draft. MB: Supervision, Visualization, Writing–review and editing. NH: Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. IS, JG, and AM acknowledge the funding from the Ministry of Education, Science and Technological Development of the Republic of Serbia. MS, MB, and NH acknowledge funding from the Slovenian Research and Innovation Agency (projects N2-0248, N2-0155, N1-0224 and programs P2-0393 and IO-0003), the European Research Council (ERC) Starting Grant 123STABLE (Grant agreement ID: 852208) and NATO Science for Peace and Security Program under grant G5729.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anwar, M. T., Yan, X., Shen, S., Husnain, N., Zhu, F., Luo, L., et al. (2017). Enhanced durability of Pt electrocatalyst with tantalum doped titania as catalyst support. Int. J. Hydrogen Energy 42 (52), 30750–30759. doi:10.1016/j.ijhydene.2017.10.152
Avasarala, B., and Haldar, P. (2011). On the stability of TiN-based electrocatalysts for fuel cell applications. Int. J. Hydrogen Energy 36 (6), 3965–3974. doi:10.1016/j.ijhydene.2010.12.107
Bai, X., and Guan, J. (2022). MXenes for electrocatalysis applications: modification and hybridization. Chin. J. Catal. 43 (8), 2057–2090. doi:10.1016/S1872-2067(21)64030-5
Baig, N., Waheed, A., Sajid, M., Khan, I., Kawde, A.-N., and Sohail, M. (2021). Porous graphene-based electrodes: advances in electrochemical sensing of environmental contaminants. Trends Environ. Anal. Chem. 30, e00120. doi:10.1016/j.teac.2021.e00120
Bejigo, K. S., Natarajan, S., Bhunia, K., Elumalai, V., and Kim, S.-J. (2023). Recycling of value-added products from spent lithium-ion batteries for oxygen reduction and methanol oxidation reactions. J. Clean. Prod. 384, 135520. doi:10.1016/j.jclepro.2022.135520
Bele, M., Jovanovič, P., Marinko, Ž., Drev, S., Šelih, V. S., Kovač, J., et al. (2020). Increasing the oxygen-evolution reaction performance of nanotubular titanium oxynitride-supported Ir nanoparticles by a strong metal–support interaction. ACS Catal. 10 (22), 13688–13700. doi:10.1021/acscatal.0c03688
Bele, M., Stojanovski, K., Jovanovič, P., Moriau, L., Koderman Podboršek, G., Moškon, J., et al. (2019). Towards stable and conductive titanium oxynitride high-surface-area support for iridium nanoparticles as oxygen evolution reaction electrocatalyst. ChemCatChem 11 (20), 5038–5044. doi:10.1002/cctc.201901487
Bing, Y., Liu, H., Zhang, L., Ghosh, D., and Zhang, J. (2010). Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction. Chem. Soc. Rev. 39 (6), 2184–2202. doi:10.1039/b912552c
Campisi, S., Chan-Thaw, C. E., and Villa, A. (2018). Understanding heteroatom-mediated metal-support interactions in functionalized carbons: a perspective review. Appl. Sci. Switz. 8 (7), 1159. doi:10.3390/app8071159
Carmo, M., Fritz, D. L., Mergel, J., and Stolten, D. (2013). A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 38 (12), 4901–4934. doi:10.1016/j.ijhydene.2013.01.151
Carrette, L., Friedrich, K. A., and Stimming, U. (2001). Fuel cells - fundamentals and applications. Fuel Cells 1 (1), 5–39. doi:10.1002/1615-6854(200105)1:1<5::aid-fuce5>3.0.co;2-g
Chen, J., Aliasgar, M., Zamudio, F. B., Zhang, T., Zhao, Y., Lian, X., et al. (2023). Diversity of platinum-sites at platinum/fullerene interface accelerates alkaline hydrogen evolution. Nat. Commun. 14 (1), 1711. doi:10.1038/s41467-023-37404-0
Chen, J., Ha, Y., Wang, R., Liu, Y., Xu, H., Shang, B., et al. (2022). Inner Co synergizing outer Ru supported on carbon nanotubes for efficient pH-universal hydrogen evolution catalysis. Nano-Micro Lett. 14 (1), 186. doi:10.1007/s40820-022-00933-2
Cui, Z., Burns, R. G., and Disalvo, F. J. (2013). Mesoporous Ti0.5Nb0.5N ternary nitride as a novel noncarbon support for oxygen reduction reaction in acid and alkaline electrolytes. Chem. Mater. 25 (19), 3782–3784. doi:10.1021/cm4027545
Erickson, K., Erni, R., Lee, Z., Alem, N., Gannett, W., and Zettl, A. (2010). Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv. Mater. 22 (40), 4467–4472. doi:10.1002/adma.201000732
Erini, N., Rudi, S., Beermann, V., Krause, P., Yang, R., Huang, Y., et al. (2015). Exceptional activity of a Pt-Rh-Ni ternary nanostructured catalyst for the electrochemical oxidation of ethanol. ChemElectroChem 2 (6), 903–908. doi:10.1002/celc.201402390
Fan, M., Chen, X., Zhang, M., Cui, L., He, X., and Zou, X. (2022). Highly dispersed Ru nanoclusters anchored on B,N co-doped carbon nanotubes for water splitting. Inorg. Chem. Front. 9 (5), 968–976. doi:10.1039/D1QI01672E
Fan, X., Liu, C., Gao, B., Li, H., Zhang, Y., Zhang, H., et al. (2023). Electronic structure engineering of Pt species over Pt/WO3 toward highly efficient electrocatalytic hydrogen evolution. Small 19, e2301178. doi:10.1002/smll.202301178
Forootan Fard, H., Khodaverdi, M., Pourfayaz, F., and Ahmadi, M. H. (2020). Application of N-doped carbon nanotube-supported Pt-Ru as electrocatalyst layer in passive direct methanol fuel cell. Int. J. Hydrogen Energy 45 (46), 25307–25316. doi:10.1016/j.ijhydene.2020.06.254
Gao, Z., Chen, Z., Zhan, X., Zhou, L., Xie, Y., Yang, X., et al. (2023). Pt nanoparticles supported on iron and nitrogen-doped holey graphene for boosting ORR performance. ACS Appl. Nano Mater. 6 (12), 10521–10530. doi:10.1021/acsanm.3c01502
Genova-Koleva, R. V., Alcaide, F., Álvarez, G., Cabot, P. L., Grande, H. J., Martínez-Huerta, M. V., et al. (2019). Supporting IrO2 and IrRuO nanoparticles on TiO2 and Nb-doped TiO2 nanotubes as electrocatalysts for the oxygen evolution reaction. J. Energy Chem. 34, 227–239. doi:10.1016/j.jechem.2019.03.008
Geppert, T. N., Bosund, M., Putkonen, M., Stühmeier, B. M., Pasanen, A. T., Heikkilä, P., et al. (2020). HOR activity of Pt-TiO 2-Y at unconventionally high potentials explained: the influence of SMSI on the electrochemical behavior of Pt. J. Electrochem. Soc. 167 (8), 084517. doi:10.1149/1945-7111/ab90ae
Han, S. B., Mo, Y. H., Lee, Y. S., Lee, S. G., Park, D. H., and Park, K. W. (2020). Mesoporous iridium oxide/Sb-doped SnO2 nanostructured electrodes for polymer electrolyte membrane water electrolysis. Int. J. Hydrogen Energy 45 (3), 1409–1416. doi:10.1016/j.ijhydene.2019.11.109
He, C., Wang, X., Sankarasubramanian, S., Yadav, A., Bhattacharyya, K., Liang, X., et al. (2020). Highly durable and active Pt/Sb-doped SnO2Oxygen reduction reaction electrocatalysts produced by atomic layer deposition. ACS Appl. Energy Mater. 3 (6), 5774–5783. doi:10.1021/acsaem.0c00717
Hodnik, N., Romano, L., Jovanovic, P., Ruiz-Zepeda, F., Bele, M., Fabbri, F., et al. (2020). Assembly of Pt nanoparticles on graphitized carbon nanofibers as hierarchically structured electrodes. ACS Appl. Nano Mater. 3 (10), 9880–9888. doi:10.1021/acsanm.0c01945
Hou, G., Shen, Z., Tang, Y., Chen, Q., Cao, H., Zhang, H., et al. (2023). Ni-WC nanoparticles/carbon aerogel electrocatalytic electrode for methanol and urea electrooxidation. Int. J. Hydrogen Energy 48 (3), 991–1000. doi:10.1016/j.ijhydene.2022.09.311
Huo, X., Yu, H., Xing, B., Zuo, X., and Zhang, N. (2022). Review of high entropy alloys electrocatalysts for hydrogen evolution, oxygen evolution, and oxygen reduction reaction. Chem. Rec. 22 (12), e202200175. doi:10.1002/tcr.202200175
Hussain, S., Kongi, N., Erikson, H., Rähn, M., Merisalu, M., Matisen, L., et al. (2019). Platinum nanoparticles photo-deposited on SnO2-C composites: an active and durable electrocatalyst for the oxygen reduction reaction. Electrochimica Acta 316, 162–172. doi:10.1016/j.electacta.2019.05.104
Jayabal, S., Saranya, G., Geng, D., Lin, L. Y., and Meng, X. (2020). Insight into the correlation of Pt-support interactions with electrocatalytic activity and durability in fuel cells. J. Mater. Chem. A 8 (19), 9420–9446. doi:10.1039/d0ta01530j
Jian, X., Wei, T.-T., Zhang, M.-M., Li, R., Liu, J.-X., and Liang, Z.-H. (2021). Construction of Ti 3 C 2 T x MXene supported low-platinum electrocatalyst for hydrogen evolution reaction by direct electrochemical strategy. J. Electrochem. Soc. 168 (9), 096504. doi:10.1149/1945-7111/ac2103
Jung, S. M., Yun, S. W., Kim, J. H., You, S. H., Park, J., Lee, S., et al. (2020). Selective electrocatalysis imparted by metal–insulator transition for durability enhancement of automotive fuel cells. Nat. Catal. 3 (8), 639–648. doi:10.1038/s41929-020-0475-4
Karaman, C. (2023). Engineering of N,P,S-Triple doped 3-dimensional graphene architecture: catalyst-support for “surface-clean” Pd nanoparticles to boost the electrocatalysis of ethanol oxidation reaction. Int. J. Hydrogen Energy 48 (17), 6691–6701. doi:10.1016/j.ijhydene.2022.02.093
Khan, I. A., Morgen, P., Gyergyek, S., Sharma, R., and Andersen, S. M. (2023). Selection on antimony-doped tin oxide (ATO) as an efficient support for iridium-based oxygen evolution reaction (OER) catalyst in acidic media. Mater. Chem. Phys. 308, 128192. doi:10.1016/j.matchemphys.2023.128192
Koderman Podborsek, G., Suhadolnik, L., Loncar, A., Bele, M., Hrnjic, A., Marinko, Z., et al. (2022). Iridium stabilizes ceramic titanium oxynitride support for oxygen evolution reaction. ACS Catal. 12 (24), 15135–15145. doi:10.1021/acscatal.2c04160
Krishnamoorthy, K., Veerapandian, M., Yun, K., and Kim, S.-J. (2013). The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 53, 38–49. doi:10.1016/j.carbon.2012.10.013
Kweon, D. H., Okyay, M. S., Kim, S.-J., Jeon, J.-P., Noh, H.-J., Park, N., et al. (2020). Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency. Nat. Commun. 11 (1), 1278. doi:10.1038/s41467-020-15069-3
Lee, J.-H., and Park, S.-J. (2020). Recent advances in preparations and applications of carbon aerogels: a review. Carbon 163, 1–18. doi:10.1016/j.carbon.2020.02.073
Lee, K. S., Park, I. S., Cho, Y. H., Jung, D. S., Jung, N., Park, H. Y., et al. (2008). Electrocatalytic activity and stability of Pt supported on Sb-doped SnO2 nanoparticles for direct alcohol fuel cells. J. Catal. 258 (1), 143–152. doi:10.1016/j.jcat.2008.06.007
Lei, Q., Wang, B., Wang, P., and Liu, S. (2019). Hydrogen generation with acid/alkaline amphoteric water electrolysis. J. Energy Chem. 38, 162–169. doi:10.1016/j.jechem.2018.12.022
Li, G., Li, K., Yang, L., Chang, J., Ma, R., Wu, Z., et al. (2018). Boosted performance of Ir species by employing TiN as the support toward oxygen evolution reaction. ACS Appl. Mater. Interfaces 10 (44), 38117–38124. doi:10.1021/acsami.8b14172
Li, M., Wang, H., Zhu, W., Li, W., Wang, C., and Lu, X. (2020). RuNi nanoparticles embedded in N-doped carbon nanofibers as a robust bifunctional catalyst for efficient overall water splitting. Adv. Sci. 7 (2), 1901833. doi:10.1002/advs.201901833
Li, Q., Sun, Z., Yin, C., Chen, Y., Pan, D., Yu, B., et al. (2023). Template-assisted synthesis of ultrathin graphene aerogels as bifunctional oxygen electrocatalysts for water splitting and alkaline/neutral zinc-air batteries. Chem. Eng. J. 458, 141492. doi:10.1016/j.cej.2023.141492
Li, T., Lv, Y., Su, J., Wang, Y., Yang, Q., Zhang, Y., et al. (2017). Anchoring CoFe 2 O 4 nanoparticles on N-doped carbon nanofibers for high-performance oxygen evolution reaction. Adv. Sci. 4 (11), 1700226. doi:10.1002/advs.201700226
Li, X., Yang, X., Huang, Y., Zhang, T., and Liu, B. (2019). Supported noble-metal single atoms for heterogeneous catalysis. Adv. Mater. 31 (50), e1902031. doi:10.1002/adma.201902031
Liu, A., Liang, X., Ren, X., Guan, W., Gao, M., Yang, Y., et al. (2020). Recent progress in MXene-based materials: potential high-performance electrocatalysts. Wiley-VCH Verl. 30 (38). doi:10.1002/adfm.202003437
Liu, C., Wu, T., Zeng, F., Pan, X., Li, G., Teng, K., et al. (2023). High-selective and effective carbon nanotubes supported ultrasmall PtPdRh electrocatalysts for ethanol oxidation. Electrochimica Acta 437, 141531. doi:10.1016/j.electacta.2022.141531
Liu, J., Fan, X., Ning, G., Zheng, M., Shi, K., Sun, Y., et al. (2022). The high-efficiency electrochemical catalysis of nitrogen-doped carbon nanotubes materials modified with Cu–Fe oxide alloy nanoparticles for HER and ORR. Int. J. Hydrogen Energy 47 (80), 34090–34101. doi:10.1016/j.ijhydene.2022.08.011
Liu, X., Wu, X., and Scott, K. (2014). Study of niobium and tantalum doped titania-supported Pt electrocatalysts for methanol oxidation and oxygen reduction reactions. Catal. Sci. Technol. 4 (11), 3891–3898. doi:10.1039/c4cy00393d
Loncar, A., Moriau, L., Stojanovski, K., Ruiz-Zepeda, F., Jovanovic, P., Bele, M., et al. (2020). Ir/TiONx/C high-performance oxygen evolution reaction nanocomposite electrocatalysts in acidic media: synthesis, characterization and electrochemical benchmarking protocol. JPhys Energy 2 (2), 02LT01. doi:10.1088/2515-7655/ab69a2
Lu, X., Song, H., Cai, J., and Lu, S. (2021). Recent development of electrochemical nitrate reduction to ammonia: a mini review. Electrochem. Commun. 129, 107094. doi:10.1016/j.elecom.2021.107094
Luo, T., Huang, J., Hu, Y., Yuan, C., Chen, J., Cao, L., et al. (2023). Fullerene lattice-confined Ru nanoparticles and single atoms synergistically boost electrocatalytic hydrogen evolution reaction. Adv. Funct. Mater. 33 (12). doi:10.1002/adfm.202213058
Luo, Z., Zhao, G., Pan, H., and Sun, W. (2022). Strong metal–support interaction in heterogeneous catalysts. John Wiley Sons Inc 12 (37). doi:10.1002/aenm.202201395
Ma, J., Li, X., Lei, G., Wang, J., Wang, J., Liu, J., et al. (2022). A general synthetic strategy for N, P co-doped graphene supported metal-rich noble metal phosphides for hydrogen generation. Green Energy & Environ. 2022. doi:10.1016/j.gee.2022.04.009
Mistry, H., Varela, A. S., Kühl, S., Strasser, P., and Cuenya, B. R. (2016). Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 1, 16009. doi:10.1038/natrevmats.2016.9
Moriau, L., Smiljanić, M., Lončar, A., and Hodnik, N. (2022). Supported iridium-based oxygen evolution reaction electrocatalysts - recent developments. ChemCatChem 14 (20), e202200586. doi:10.1002/cctc.202200586
Moriau, L. J., Bele, M., Vižintin, A., Ruiz-Zepeda, F., Petek, U., Jovanovič, P., et al. (2019). Synthesis and advanced electrochemical characterization of multifunctional electrocatalytic composite for unitized regenerative fuel cell. ACS Catal. 9 (12), 11468–11483. doi:10.1021/acscatal.9b03385
Mukherjee, P., Kakade, B., and Swami, A. (2022). Current trends in platinum-based ternary alloys as promising electrocatalysts for the oxygen reduction reaction: a mini review. Energy Fuels 36 (5), 2306–2322. doi:10.1021/acs.energyfuels.1c03667
Navalon, S., Dhakshinamoorthy, A., Alvaro, M., Antonietti, M., and García, H. (2017). Active sites on graphene-based materials as metal-free catalysts. Chem. Soc. Rev. 46 (15), 4501–4529. doi:10.1039/C7CS00156H
Ortiz-Herrera, J. C., Cruz-Martínez, H., Solorza-Feria, O., and Medina, D. I. (2022). Recent progress in carbon nanotubes support materials for Pt-based cathode catalysts in PEM fuel cells. Int. J. Hydrogen Energy 47 (70), 30213–30224. doi:10.1016/j.ijhydene.2022.03.218
Pan, C. J., Tsai, M. C., Su, W. N., Rick, J., Akalework, N. G., Agegnehu, A. K., et al. (2017). Tuning/exploiting strong metal-support interaction (SMSI) in heterogeneous catalysis. J. Taiwan Inst. Chem. Eng. 74, 154–186. doi:10.1016/j.jtice.2017.02.012
Pan, L., Ott, S., Dionigi, F., and Strasser, P. (2019). Current challenges related to the deployment of shape-controlled Pt alloy oxygen reduction reaction nanocatalysts into low Pt-loaded cathode layers of proton exchange membrane fuel cells. Curr. Opin. Electrochem. 18, 61–71. doi:10.1016/j.coelec.2019.10.011
Pan, M., Wang, J., Hua, M., Gao, G., Wang, X., and Chew, J. W. (2019). Augmentation of hydroxyl groups as electrocatalytic active sites in porous graphene. Carbon 154, 384–390. doi:10.1016/j.carbon.2019.08.033
Pavko, L., Gatalo, M., Đukić, T., Ruiz-Zepeda, F., Surca, A. K., Šala, M., et al. (2023). Correlating oxygen functionalities and electrochemical durability of carbon supports for electrocatalysts. Carbon 215, 118458. doi:10.1016/j.carbon.2023.118458
Pavko, L., Gatalo, M., Finšgar, M., Ruiz-Zepeda, F., Ehelebe, K., Kaiser, P., et al. (2022). Graphene-derived carbon support boosts proton exchange membrane fuel cell catalyst stability. ACS Catal. 12 (15), 9540–9548. doi:10.1021/acscatal.2c01753
Peles-Strahl, L., Persky, Y., and Elbaz, L. (2023). Design of advanced aerogel structures for oxygen reduction reaction electrocatalysis. SusMat 3 (1), 44–57. doi:10.1002/sus2.104
Pham, H. Q., Huynh, T. T., Bach, L. G., and Ho, V. T. T. (2021). Synthesis and characterization the multifunctional nanostructures TixW1-xO2 (x = 0.5; 0.6; 0.7; 0.8) supports as robust non-carbon support for Pt nanoparticles for direct ethanol fuel cells. Int. J. Hydrogen Energy 46 (48), 24877–24890. doi:10.1016/j.ijhydene.2020.03.066
Pham, T. M., Im, K., and Kim, J. (2023). A highly stable Tungsten-Doped TiO2-Supported platinum electrocatalyst for oxygen reduction reaction in acidic media. Appl. Surf. Sci. 611, 155740. doi:10.1016/j.apsusc.2022.155740
Pizzutilo, E., Geiger, S., Grote, J.-P., Mingers, A., Mayrhofer, K. J. J., Arenz, M., et al. (2016). On the need of improved accelerated degradation protocols (ADPs): examination of platinum dissolution and carbon corrosion in half-cell tests. J. Electrochem. Soc. 163 (14), F1510–F1514. doi:10.1149/2.0731614jes
Popović, S., Smiljanić, M., Jovanovič, P., Vavra, J., Buonsanti, R., and Hodnik, N. (2020). Stability and degradation mechanisms of copper-based catalysts for electrochemical CO2 reduction. Angew. Chem. - Int. Ed. 59 (35), 14736–14746. doi:10.1002/anie.202000617
Puthiyapura, V. K., Mamlouk, M., Pasupathi, S., Pollet, B. G., and Scott, K. (2014). Physical and electrochemical evaluation of ATO supported IrO2 catalyst for proton exchange membrane water electrolyser. J. Power Sources 269, 451–460. doi:10.1016/j.jpowsour.2014.06.078
Rakočević, L., Simatović, I. S., Maksić, A., Rajić, V., Štrbac, S., and Srejić, I. (2021). PtAu nanoparticles supported by reduced graphene oxide as a highly active catalyst for hydrogen evolution. Catalysts 12 (1), 43. doi:10.3390/catal12010043
Rakočević, L., Srejić, I., Maksić, A., Golubović, J., and Štrbac, S. (2021). Hydrogen evolution on reduced graphene oxide-supported PdAu nanoparticles. Catalysts 11 (4), 481. doi:10.3390/catal11040481
Reier, T., Teschner, D., Lunkenbein, T., Bergmann, A., Selve, S., Kraehnert, R., et al. (2014). Electrocatalytic oxygen evolution on iridium oxide: uncovering catalyst-substrate interactions and active iridium oxide species. J. Electrochem. Soc. 161 (9), F876–F882. doi:10.1149/2.0411409jes
Sharma, S., and Pollet, B. G. (2012). Support materials for PEMFC and DMFC electrocatalysts - a review. J. Power Sources 208, 96–119. doi:10.1016/j.jpowsour.2012.02.011
Shi, W., Park, A. H., Li, Z., Xu, S., Kim, J. M., Yoo, P. J., et al. (2021). Sub-nanometer thin TiO2-coating on carbon support for boosting oxygen reduction activity and durability of Pt nanoparticles. Electrochimica Acta 394, 139127. doi:10.1016/j.electacta.2021.139127
Singh, H., Marley-Hines, M., Chakravarty, S., and Nath, M. (2022). Multi-walled carbon nanotube supported manganese selenide as a highly active bifunctional OER and ORR electrocatalyst. J. Mater. Chem. A 10 (12), 6772–6784. doi:10.1039/D1TA09864K
Smiljanić, M., Bele, M., Moriau, L. J., Vélez Santa, J. F., Menart, S., Šala, M., et al. (2022). Suppressing platinum electrocatalyst degradation via a high-surface-area organic matrix support. ACS Omega 7 (4), 3540–3548. doi:10.1021/acsomega.1c06028
Smiljanić, M., Panić, S., Bele, M., Ruiz-Zepeda, F., Pavko, L., Gašparič, L., et al. (2022). Improving the HER activity and stability of Pt nanoparticles by titanium oxynitride support. ACS Catal. 12 (20), 13021–13033. doi:10.1021/acscatal.2c03214
Song, L., Fan, H., Fan, X., Gong, H., Wang, T., and He, J. (2022). A simultaneous phosphorization and carbonization strategy to synthesize a defective Co2P/doped-CNTs composite for bifunctional oxygen electrocatalysis. Chem. Eng. J. 435, 134612. doi:10.1016/j.cej.2022.134612
Speck, F. D., Paul, M. T. Y., Ruiz-Zepeda, F., Gatalo, M., Kim, H., Kwon, H. C., et al. (2020). Atomistic insights into the stability of Pt single-atom electrocatalysts. J. Am. Chem. Soc. 142 (36), 15496–15504. doi:10.1021/jacs.0c07138
Stühmeier, B. M., Schuster, R. J., Hartmann, L., Selve, S., El-Sayed, H. A., and Gasteiger, H. A. (2022). Modification of the electrochemical surface oxide formation and the hydrogen oxidation activity of ruthenium by strong metal support interactions. J. Electrochem. Soc. 169 (3), 034519. doi:10.1149/1945-7111/ac58c9
Sun, S. C., Jiang, H., Chen, Z. Y., Chen, Q., Ma, M. Y., Zhen, L., et al. (2022). Bifunctional WC-supported RuO2 nanoparticles for robust water splitting in acidic media. Angew. Chem. - Int. Ed. 61 (21), e202202519. doi:10.1002/anie.202202519
Sun, Y., Du, C., Han, G., Qu, Y., Du, L., Wang, Y., et al. (2016). Boron, nitrogen co-doped graphene: a superior electrocatalyst support and enhancing mechanism for methanol electrooxidation. Electrochimica Acta 212, 313–321. doi:10.1016/j.electacta.2016.06.168
Sun, Z., Yang, Y., Fang, C., Yao, Y., Qin, F., Gu, H., et al. (2022). Atomic-level Pt electrocatalyst synthesized via iced photochemical method for hydrogen evolution reaction with high efficiency. Small 18 (33), e2203422. doi:10.1002/smll.202203422
Takasaki, F., Matsuie, S., Takabatake, Y., Noda, Z., Hayashi, A., Shiratori, Y., et al. (2011). Carbon-free Pt electrocatalysts supported on SnO2 for polymer electrolyte fuel cells: electrocatalytic activity and durability. J. Electrochem. Soc. 158 (10), B1270. doi:10.1149/1.3625918
Tang, Z., Wei, S., Wang, Y., and Dai, L. (2022). Three-dimensional reduced graphene oxide decorated with cobalt metaphosphate as high cost-efficiency electrocatalysts for the hydrogen evolution reaction. RSC Adv. 12 (17), 10522–10533. doi:10.1039/D2RA01271E
Tarcan, R., Todor-Boer, O., Petrovai, I., Leordean, C., Astilean, S., and Botiz, I. (2020). Reduced graphene oxide today. J. Mater. Chem. C 8 (4), 1198–1224. doi:10.1039/C9TC04916A
Tauster, S. J., Fung, S. C., and Garten, R. L. (1978). Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 100, 170–175. doi:10.1021/ja00469a029
Tavakol, H., Zhiani, M., and Shareifyan-ghahfarokhi, F. (2020). Gold-decorated sulfur-doped carbon nanotubes as electrocatalyst in hydrogen evolution reaction. Gold Bull. 53 (2), 63–76. doi:10.1007/s13404-020-00275-0
Tong, J., Liu, Y., Peng, Q., Hu, W., and Wu, Q. (2017). An efficient Sb-SnO2-supported IrO2 electrocatalyst for the oxygen evolution reaction in acidic medium. J. Mater. Sci. 52 (23), 13427–13443. doi:10.1007/s10853-017-1447-1
Vélez Santa, J. F., Menart, S., Bele, M., Ruiz-Zepeda, F., Jovanovič, P., Jovanovski, V., et al. (2021). High-surface-area organic matrix tris(aza)pentacene supported platinum nanostructures as selective electrocatalyst for hydrogen oxidation/evolution reaction and suppressive for oxygen reduction reaction. Int. J. Hydrogen Energy 46 (49), 25039–25049. doi:10.1016/J.IJHYDENE.2021.05.041
Wang, F., Yu, H., Feng, T., Zhao, D., Piao, J., and Lei, J. (2020). Surface roughed and Pt-rich bimetallic electrocatalysts for hydrogen evolution reaction. Front. Chem. 8, 422. doi:10.3389/fchem.2020.00422
Wang, H., Xue, J., Liu, C., Chen, Z., Li, C., Li, X., et al. (2023). CO2 electrolysis toward acetate: a review. Curr. Opin. Electrochem. 39, 101253. doi:10.1016/j.coelec.2023.101253
Wang, J., Chen, J. W., Chen, J. D., Zhu, H., Zhang, M., and Du, M. L. (2017). Designed synthesis of size-controlled Pt-Cu alloy nanoparticles encapsulated in carbon nanofibers and their high efficient electrocatalytic activity toward hydrogen evolution reaction-Cu alloy nanoparticles encapsulated in carbon nanofibers and their high efficient electrocatalytic activity toward hydrogen evolution reaction. Adv. Mater. Interfaces 4(12), 1700005. doi:10.1002/admi.201700005
Wang, K., Lan, X., Jing, Y., Liu, X., Liu, B., Zhang, P., et al. (2023). Ultrafine Pt nanoparticles decorated carbon nanotubes for enhanced electrocatalytic hydrogen evolution reaction. Mater. Lett. 336, 133871. doi:10.1016/j.matlet.2023.133871
Wang, Y., Su, H., He, Y., Li, L., Zhu, S., Shen, H., et al. (2020). Advanced electrocatalysts with single-metal-atom active sites. Chem. Rev. 120 (21), 12217–12314. doi:10.1021/acs.chemrev.0c00594
Wu, Z., Su, Y. Q., Hensen, E. J. M., Tian, X., You, C., and Xu, Q. (2019). Highly stable Pt3Ni nanowires tailored with trace Au for the oxygen reduction reaction. J. Mater. Chem. A 7 (46), 26402–26409. doi:10.1039/c9ta08682j
Xiong, B., Zhou, Y., Zhao, Y., Wang, J., Chen, X., O’Hayre, R., et al. (2013). The use of nitrogen-doped graphene supporting Pt nanoparticles as a catalyst for methanol electrocatalytic oxidation. Carbon 52, 181–192. doi:10.1016/j.carbon.2012.09.019
Yang, Y., Wu, D., Li, R., Rao, P., Li, J., Deng, P., et al. (2022). Engineering the strong metal support interaction of titanium nitride and ruthenium nanorods for effective hydrogen evolution reaction. Appl. Catal. B Environ. 317, 121796. doi:10.1016/j.apcatb.2022.121796
Yu, J., Wang, A., Yu, W., Liu, X., Li, X., Liu, H., et al. (2020). Tailoring the ruthenium reactive sites on N doped molybdenum carbide nanosheets via the anti-Ostwald ripening as efficient electrocatalyst for hydrogen evolution reaction in alkaline media. Appl. Catal. B Environ. 277, 119236. doi:10.1016/j.apcatb.2020.119236
Yuan, Y., Li, H., Wang, L., Zhang, L., Shi, D., Hong, Y., et al. (2019). Achieving highly efficient catalysts for hydrogen evolution reaction by electronic state modification of platinum on versatile Ti3C2Tx (MXene). ACS Sustain. Chem. Eng. 7 (4), 4266–4273. doi:10.1021/acssuschemeng.8b06045
Zeng, Y., Priest, C., Wang, G., and Wu, G. (2020). Restoring the nitrogen cycle by electrochemical reduction of nitrate: progress and prospects. John Wiley Sons Inc 4 (12). doi:10.1002/smtd.202000672
Zhai, Y., Zhu, Z., and Dong, S. (2015). Carbon-based nanostructures for advanced catalysis. ChemCatChem 7 (18), 2806–2815. doi:10.1002/cctc.201500323
Keywords: electrocatalysis, fuel cells, electrolyzers, support, metal-support interaction
Citation: Smiljanić M, Srejić I, Georgijević JP, Maksić A, Bele M and Hodnik N (2023) Recent progress in the development of advanced support materials for electrocatalysis. Front. Chem. 11:1304063. doi: 10.3389/fchem.2023.1304063
Received: 28 September 2023; Accepted: 27 October 2023;
Published: 09 November 2023.
Edited by:
Jean-Paul Chopart, Université de Reims Champagne-Ardenne, FranceReviewed by:
Subhasis Shit, Free University of Berlin, GermanyCopyright © 2023 Smiljanić, Srejić, Georgijević, Maksić, Bele and Hodnik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Smiljanić, bWlsdXRpbi5zbWlsamFuaWNAa2kuc2k=; I. Srejić, aXJpbmFAdmluLmJnLmFjLnJz
†These authors have contributed equally to this work
 M. Smiljanić
M. Smiljanić I. Srejić
I. Srejić J. P. Georgijević
J. P. Georgijević A. Maksić2
A. Maksić2