- 1Graduate Program for Biomedical and Materials Science, Tunghai University, Taichung, Taiwan
- 2Department of Chemistry, Tunghai University, Taichung, Taiwan
A library of β-enamino diketones was prepared via base-mediated, three-component reaction of 4-hydroxycoumarins with various aromatic/aliphatic amines and β-nitrostyrenes under microwave irradiation conditions to investigate their photochemical properties. Among the prepared compounds, a thiophene derived β-enamino diketone was found to be light-sensitive and to exhibit unique photochromic behavior, that is, positive photochromism in solution and negative photochromism in crystalline phase. In addition, this prepared photochromic compound was further covalently linked to a structure-related, piezochromic β-enamino diketone moiety to explore its potential multi-stimuli responsive properties.
1 Introduction
The β-enamino diketones represent important and versatile synthons for the synthesis of natural products and other heterocycles (Michael et al., 2001; Valduga et al., 1998; Ferraz et al., 1995; White and Lhle, 2006; Li et al., 2007). Compounds bearing β-enamino diketone moiety, especially six-membered cyclic ones, were found to possess many biological properties. For instance, as shown in Figure 1, β-enamino diketone 1 is an antioxidant agent (Mladenovic et al., 2009). Compound 2 possesses strong inhibitory activity against fungus C. albicans (Vukovic et al., 2010). Compound 3 exhibits potent cytotoxicity against A546, HeLa, and K562 cells (Budzisz et al., 2004). Owing to their diverse biological activities, a plethora of multi-component reaction methodologies for the synthesis of β-enamino diketones have been reported in the literature (Kuo et al., 2009; Ghabraie et al., 2011; Ye et al., 2015). While most efforts were focused on the biological activities of the prepared compounds, their potential photochemical and functional properties were much less explored. Recently, we have reported that coumarin-based N-aryl-β-enamino diketone 4 (Figure 1) exhibits piezochromic properties (Hsieh et al., 2019); that is, upon grinding, compound 4 changes from yellow to red and can be swiftly reverted to the original color when exposed to methylene chloride vapor. Further, the furan-derived enaminones 5 serve as amine-protected compounds for primary alkyl amines protection. These acid/base stable amine-protected 5 can be readily deprotected by treating with ethylene diamine under reflux conditions (Chithanna and Yang, 2019). Similarly, the phenyl-derived enaminones 6 function as amine-protected products for aryl amines and amino acids in both solution and solid phase peptide synthesis. The free aryl amines and amino acids can be regenerated by treating with hydrazine hydrate in ethanol under mild conditions (Chithanna et al., 2020). These successful examples prompted us to speculate that new or unique functional behavior of β-enamino diketones via structural tuning of their three major molecular scaffolds, namely, cyclic diketone, aryl/alkyl amine, and aryl group. Further, we envisioned that the β-enamino diketones are prone to undergo the excited state intramolecular proton transfer (ESIPT) process upon photoirradiation by means of proton transfer from amine to nearby keto-group of coumarin. Thus, in our continuous efforts to unearth novel functional properties from the heterocycles synthesized via multi-component reaction, herein we reported the preparation of a library of β-enamino diketones from a microwave-assisted, three-component reaction of 4-hydroxycoumarins with various aromatic/aliphatic amines and β-nitrostyrenes in toluene under basic conditions. Their stimuli responses towards UV and visible light in solution, thin film, and crystalline state were examined. Further, the photochromic compound prepared from 7-N,N-dimethylamino-4-hydroxycoumarin, benzylamine, and 5-chlorothiophene-2-carbaldehyde was further linked to a structure-related, pressure-sensitive β-enamino diketone scaffold to investigate its potential multi-stimuli responsive properties.
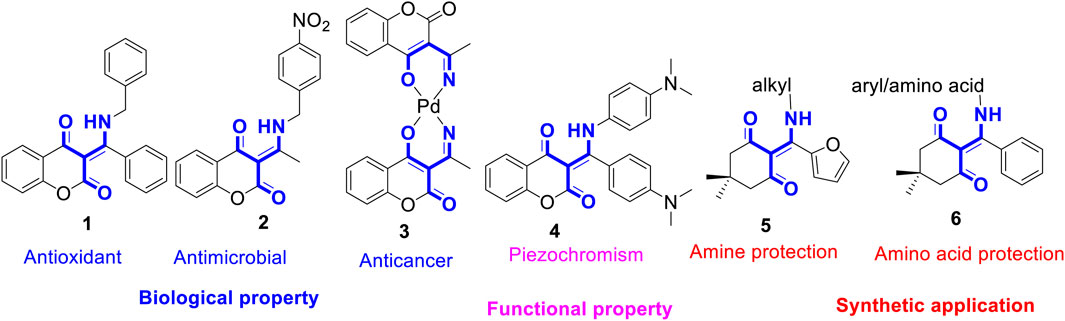
FIGURE 1. Structures of representative β-enamino diketones with biological/functional properties and synthetic applications.
2 Results and discussion
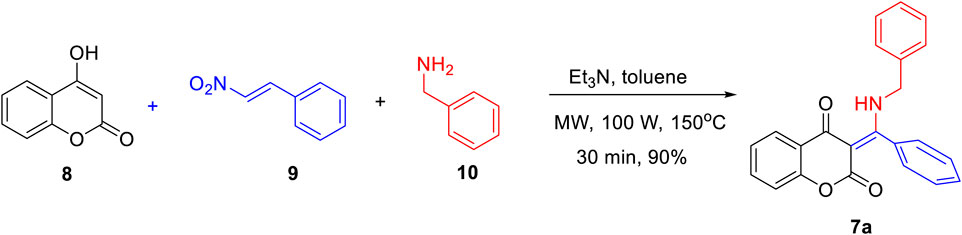
Scheme 1 outlines the microwave-assisted, three-component preparation of β-enamino diketone 7a. With slight modifications from the literature reported procedure (Manjappa et al., 2018; Hsieh et al., 2019), it could be easily obtained via triethylamine-mediated coupling of 4-hydroxycoumarin (8), β-nitrostyrene (9), and benzylamine (10) in toluene under microwave conditions for 30 min in 90% yield.
Figure 2 lists the structures and yields of the prepared 7a–o. Most of the reactions gave good to excellent yields, except for compound 7k in which 4-bromofuran-2-carbaldehyde was used as the aldehyde source. This observation could be attributed to the furan’s high propensity to be attacked by a nucleophilic amine (Helmy et al., 2014; Chithanna and Yang, 2019). In the present investigation, we focused mainly on 7-N,N-dimethylamino-4-hydroxycoumarin, benzylamine, and thiophene aldehydes derived β-enaminone diketones. The molecular structures of diketones 7a–o were elucidated by spectroscopic data along with X-ray crystal analysis (7b, 7g, 7i, and 7m) (CCDC No, 2023). A broad signal appearing around 13–14 ppm in the proton NMR spectrum was detected for all prepared compounds, indicating the presence of an intramolecular hydrogen bond in the molecular scaffold. Indeed, an intramolecular hydrogen bonding between the amine hydrogen (N-H) and carbonyl oxygen (C=O) of coumarin was distinctly observed in the ORTEP diagrams shown in Figure 2. Generally, changes of amines (aryl or aliphatic) or aromatic/heterocyclic aldehydes had little effect on product yields.
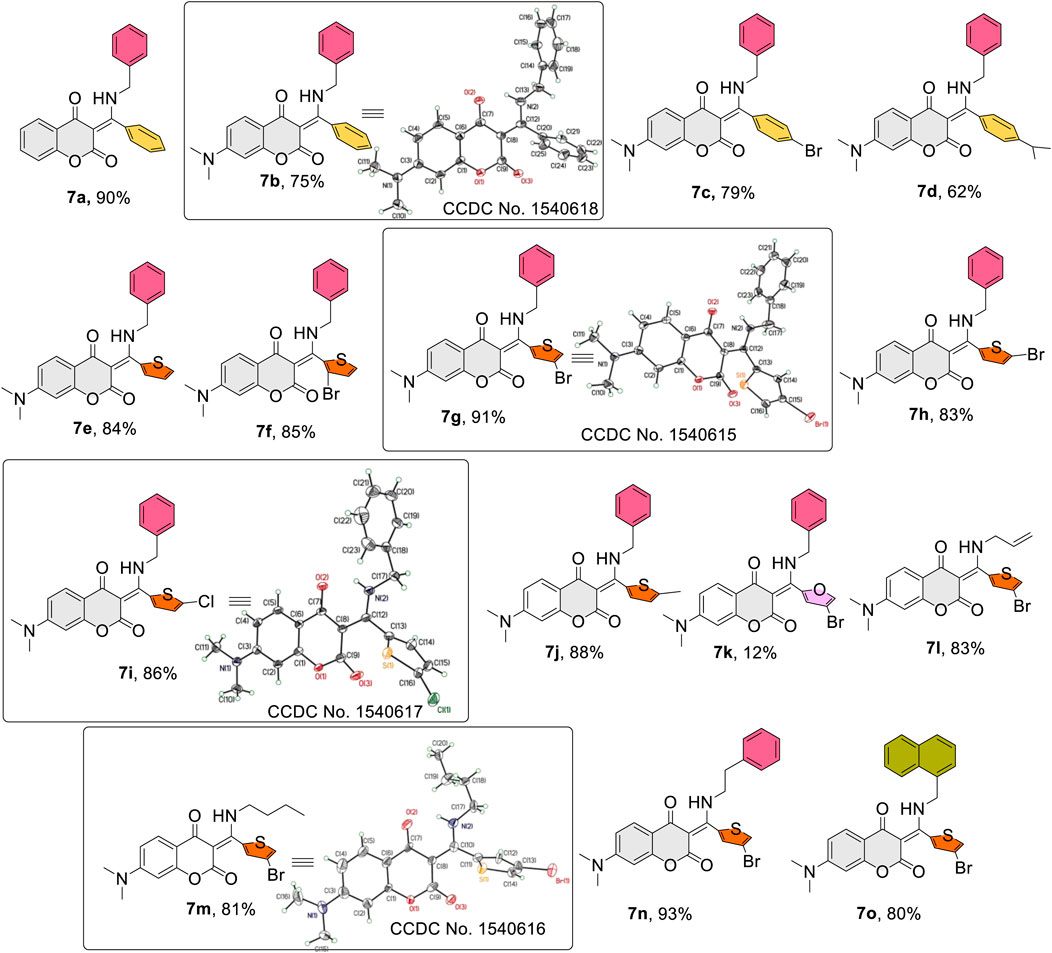
FIGURE 2. Structures and yields of the prepared β-enamino diketones (7a–o) and ORTEP crystal structures of (7b, 7g, 7i, 7m) with atomic displacement shown at 50% probability.
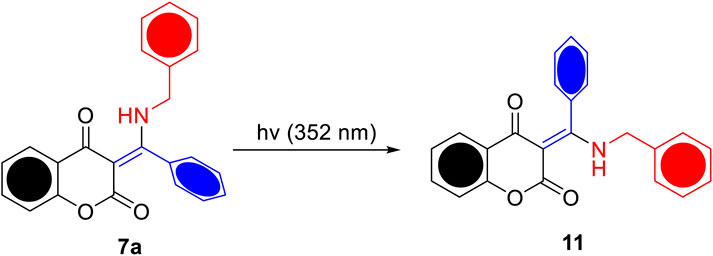
To investigate the potential ESIPT behavior, the prepared β-enaminone diketones 7a–o were subjected to photoirradiation and their photochemical properties were explored. Compound 7a, in which benzaldehyde was used as the aldehyde substrate, was found to be sensitive to UV light. Figure 3 shows the absorption spectra of 7a in acetonitrile when exposed to UV irradiation (352 nm) for 60 s. With the increase of exposure time, the absorbance at wavelength of 330 and 342 nm gradually decreased, along with the emergence of broad shoulder at around 380 nm. Also, two isosbestic points located at 305 and 355 nm were clearly observed. Presumably, upon UV irradiation, compound 7a undergoes E-Z isomerization of the central carbon-carbon double bond to generate the isomer 11, as shown in Scheme 2. To further support this hypothesis, a time-dependent proton NMR study was carried out. Prior to irradiation, a small doublet appeared at 4.42 ppm and a broad singlet appeared at 14.34 ppm which correspond to the respective benzylic -CH2 and the intramolecular hydrogen bonding absorptions were observed in the proton NMR spectrum of 7a. During the process of UV irradiation (up to 60 min), these peaks gradually diminished and the concomitant emergence of two new peaks at 4.83 and 9.03 ppm which correspond to the benzylic -CH2 and intramolecular hydrogen bonding absorptions of 11 was witnessed (see the Supporting Information). These observations suggest that indeed compound 7a undergoes E-Z isomerization to give the compound 11 upon UV irradiation. On the other hand, when an N,N-dimethylamino group was introduced onto the 7-position of the coumarin moiety, the resulting compound 7b became light-insensitive. Prolonged irradiation of 7b did not exhibit considerable changes in the UV-vis absorption profiles (Figure 3B). This observation implies that the photochemical property of the β-enaminone diketones can be influenced by the substituents on the coumarin moiety.
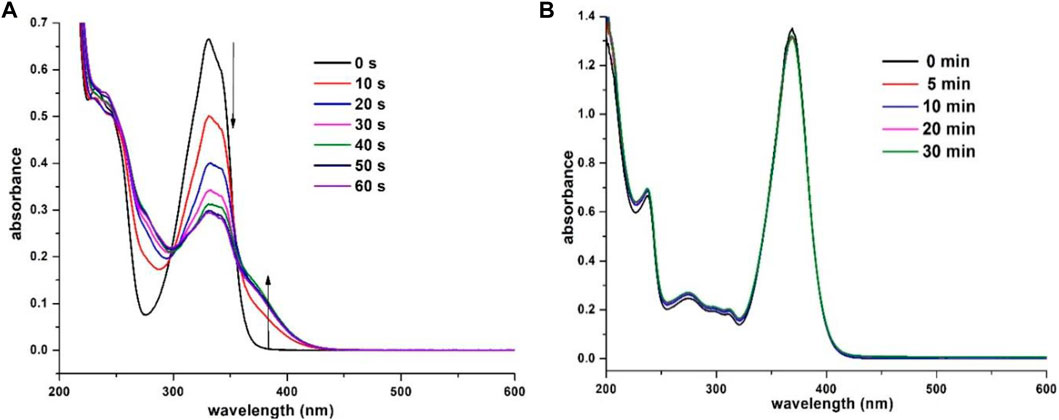
FIGURE 3. Absorption spectra of (A) 7a (3.0 × 10−5 M in CH3CN) and (B) 7b (3.0 × 10−5 M in CH3CN) obtained with different exposure times (352 nm).
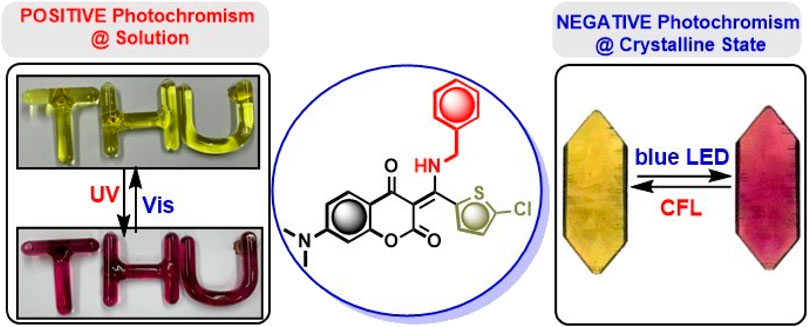
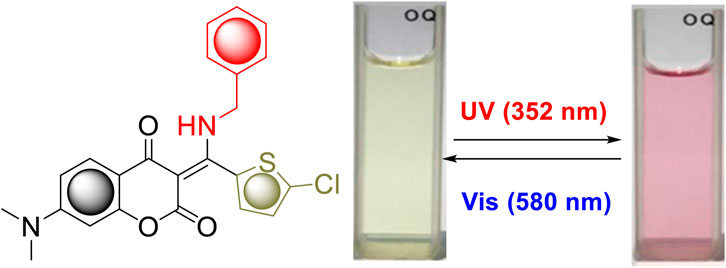
Interestingly, compound 7i bearing a 5-chlorothiophene moiety was found to be highly sensitive to UV light and exhibit unique photochemical properties. As shown in Figure 4, it turned from light yellow to red within seconds when exposed to UV irradiation in solution. Figure 5A displays the time course of the UV–vis absorption spectra of 7i in acetonitrile under continuous irradiation (352 nm) for 5 min. With the increase of exposure time, two broad absorbance peaks which centered at 527 and 551 nm exhibited smooth continuous growth. This process could be reverted by visible light irradiation (580 nm), indicating that compound 7i possesses photochromic property (Figure 5B).

FIGURE 5. Absorption spectra of (A) 7i (3.0 × 10−5 M in CH3CN) obtained with different exposure times (352 nm), 0–5 min, in increments of 1 min (B) the photogenerated product obtained with different exposure times (580 nm), 0–7 min, in increments of 2 min.
Isolation of the photogenerated product and subsequent characterization of its molecular structure proved to be difficult since prolonged UV irradiation of 7i resulted in the E-Z isomerization to be the dominant process. Figure 6 depicts the evolution of absorbance profile of 7i during the prolonged UV irradiation. Starting from the initial 5 min of UV irradiation, there was a surge in a broad absorption band around 520–560 nm, indicating the formation of photogenerated products. However, as the irradiation time was further extended (up to 120 min), the decrease in absorption at 372 nm and the appearance of a broad shoulder near 455 nm were also recorded. This observation suggests that prolonged irradiation of compound 7i renders the E-Z isomerization to be a dominant process over the formation of photogenerated product. Therefore, current efforts to characterize the photogenerated product resulting from this photochromic behavior were futile.
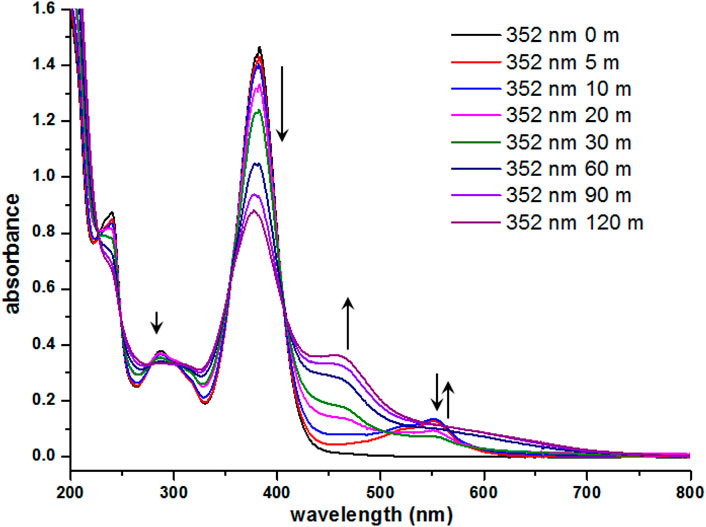
FIGURE 6. UV-vis spectra of 7i (3.0 × 10−5 M in CH3CN) after continuous irradiation at 352 nm for 120 min.
Inspired by the photochromic behavior in solution, we chose to investigate the photosensitivity of 7i in solid phase. The thin film of 7i was prepared by spin coating on a quartz plate and then irradiated with light. When exposed to blue LED for 60 min, no apparent color change of 7i was observed. Conversely, compound 7i (thin film) changed from colorless to red in 5 min upon UV (352 nm) irradiation. Figure 7 shows the color change and absorption profile of 7i (thin film) prior to and after irradiation. As UV exposure increased, the absorbance centered at 388 nm decreased along with a smooth enhancement of the shoulders near 450 nm and 530 nm. This evolution of the absorbance resulted in the formation of two isosbestic points at 366 and 412 nm, which indicates the presence of two distinguishable species in the film. The fact that UV-vis spectra of 7i in thin film (solid state) exhibited strong resemblance to that of 7i in solution phase (Figure 6) implies that similar photochromic mechanisms may be involved for 7i in solution and solid state.
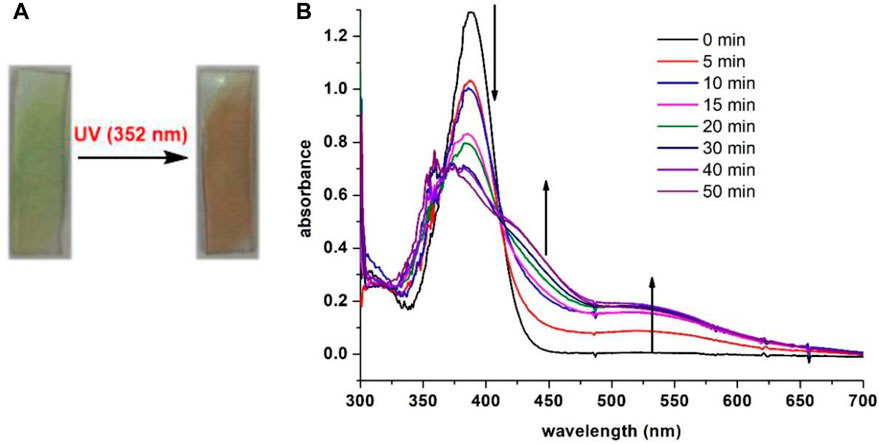
FIGURE 7. (A) Color change prior to and after UV irradiation of 7i (thin film). (B) UV-vis spectra of 7i (spin-coated on quartz plate) after continuous irradiation at 352 nm for 50 min.
After discovering the photochromism of 7i in both solution and solid state, we embarked upon our investigations on its photochromic behavior in crystalline state (Avadanei et al., 2014; Irie et al., 2014; Funasako et al., 2019; Zhang et al., 2021; Sun et al., 2022; Loan et al., 2023). A fine, moderate-sized crystal of 7i was grown and subjected to photoirradiation. To our surprise, 7i was found to be highly sensitive to visible light in crystalline state. When exposed to blue LED (465 nm), the crystal of 7i changed from yellow to violet within 15 s. Gratifyingly, this process was reversible upon exposure to the compact fluorescent lamp (CFL) for 2 h. Figure 8 shows the color transition of 7i at crystalline state as well as UV-vis absorption profiles of 7i prior to and after the blue LED irradiation. Before the exposure, 7i displayed a maximum absorbance (λmax) around 416 nm. After blue LED exposure (40 min), the λmax was red-shifted to 425 nm along with the broad absorbance increase from 425 to 550 nm. This change in the absorbance was visible to the naked eye as blue LED imparted dark violet color to the 7i crystal. The violet crystal of 7i gradually returned to yellow upon exposure to CFL light (315–400 nm for UVA), suggesting possible reversibility. In the case of color change of 7i in solution photochromism, the number of cycles is limited to 3–4 times only. On the other hand, the number of photochromic cycles for 7i in crystalline state is up to 25 times (see the Supplementary Material for details), which is comparatively higher than that of in solution state.
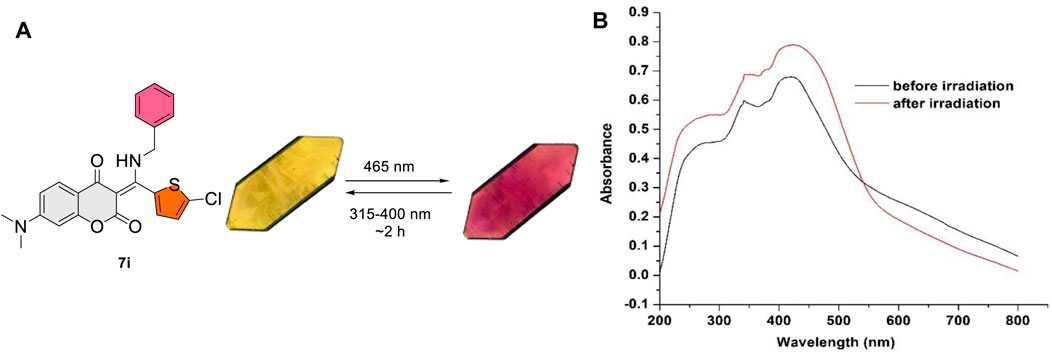
FIGURE 8. (A) Reversible color change in crystalline state for 7i. (B) UV-vis absorption profiles of 7i prior to and after blue LED irradiation (40 min).
In order to gain more insights into the photosensitivity of 7i towards blue LED, the X-ray crystallography was sought to examine the possible structural changes at crystalline state during irradiation. A crystal of 7i was irradiated with blue LED for 24 h and its X-ray ORTEP diagram was obtained (CCDC No 2300435). Figure 9 depicts the superimposition of the ORTEP diagrams of 7i before and after blue LED irradiation. Some conformational differences in the crystals were noticed, that is, the benzylamine and thiophene moieties were found to be tilted to certain degrees after irradiation. For instance, prior to the blue LED irradiation, the dihedral angle between coumarin and thiophene moieties in 7i was found to be −81.82o. After irradiation, however, it changed to −70.79o. Similarly, the torsion angle between coumarin and benzylamine moieties switched from 34.35o to 56.09o after irradiation. These subtle yet significant conformational variations during blue LED irradiation suggested that the compound 7i is indeed sensitive to visible light and is capable of undergoing reversible changes upon irradiation with suitable wavelength. Although the mechanistic details for the photochromic switch of 7i in solution, thin film, and crystalline state remain to be investigated, compound 7i represents one of the rare examples that exhibit opposite photochromic property under different states (Funasako et al., 2020), that is, positive photochromism in solution and thin film as well as negative photochromism in crystalline state.
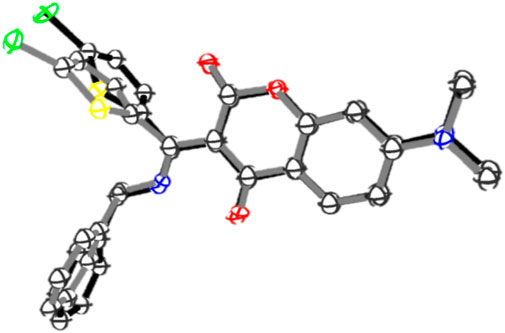
FIGURE 9. Superimposition of the ORTEP diagrams of 7i before (black) and after (grey) blue LED irradiation. Hydrogens are omitted for clarity.
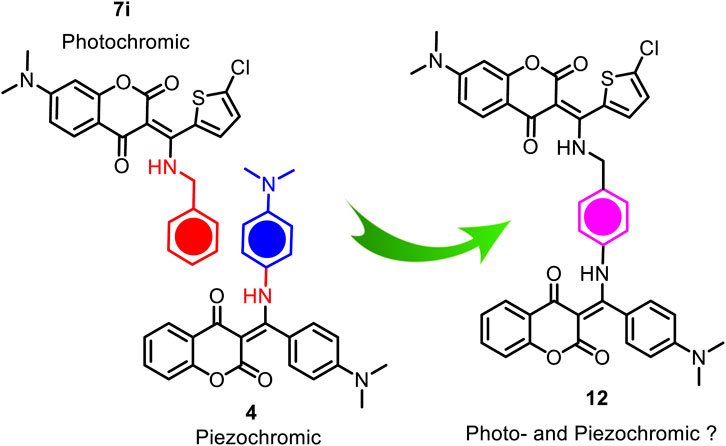
As illustrated in Figure 1, our previous study has demonstrated that coumarin-based N-aryl-β-enamino diketone 4 exhibits piezochromic behavior (Figure 1) (Hsieh et al., 2019). We speculate that by linking this pressure-sensitive molecular scaffold (Sagara et al., 2007; Sagara and Kato, 2009; Ma et al., 2015; Naumov et al., 2015; Wang et al., 2015; Li et al., 2021; Wei et al., 2023) of 4 with the present light-sensitive moiety of 7i into one molecule, the resulting hybrid compound may exhibit multi-stimuli responsive properties in solid state (Zhang et al., 2011; Chiu and Yang, 2020; Vaidya et al., 2021; Martinez-Junquera et al., 2022; Wang et al., 2022). Scheme 3 depicts the conceptual design of the potential dual responsive N-aryl-β-enamino diketone 12. Since both piezochromic 4 and photochromic 7i share a phenyl group on the N-substituent, this common phenyl group was then used to connect the two molecular scaffolds to form the hybrid 12.
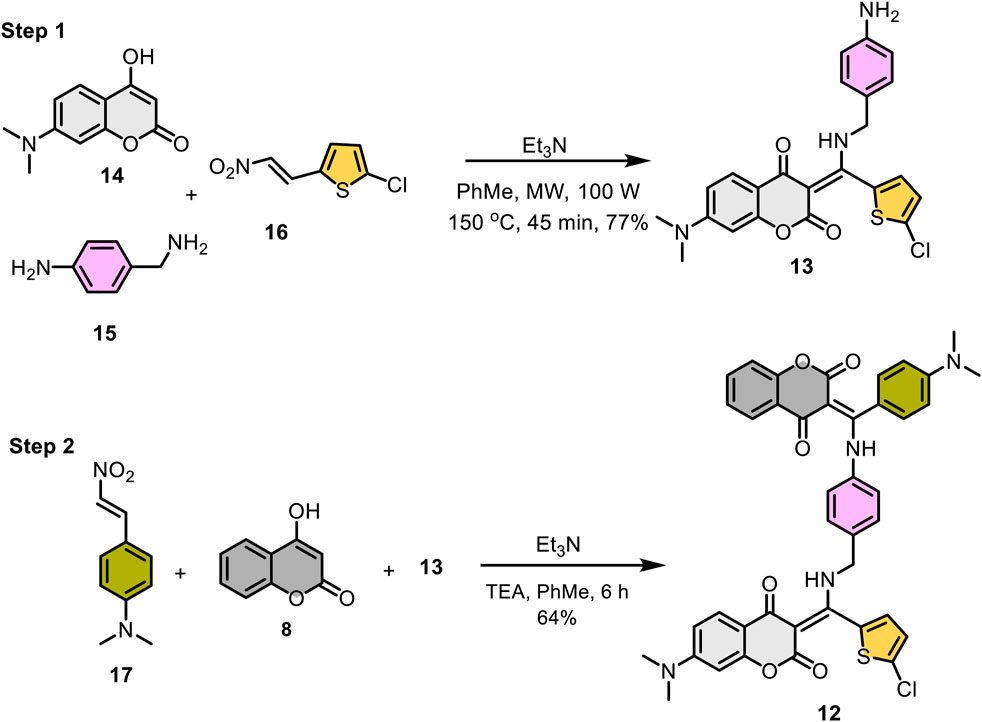
Scheme 4 outlines the two-step synthesis of the target compound 12. The photochromic unit 13 was synthesized via base-promoted, three-component reaction of 7-N,N-dimethylamino-4-hydroxycoumarin (14), 4-aminobenzylamine (15), and β-nitrostyrene 16 under microwave conditions. As expected, the less nucleophilic aniline nitrogen on 4-aminobenzylamine (15) did not participate in the reaction, and the desired β-enamino diketone 13 was isolated as an exclusive product in 77% yield. The subsequent three-component reaction of 13 with 4-hydroxycoumarin (8) and p-N,N-dimethylamino-β-nitrostyrene (17) under basic conditions yielded the hybrid β-enaminone 12 in 64% yield.
After realizing compound 12, we then explored its functional properties by applying external stimuli such as mechanical force and UV irradiation to examine its potential piezochromic and photochromic responses. Upon grinding, compound 12 turned slowly from yellow to dark brown (Figure 10), indicating that the hybrid 12 remained pressure-sensitive. Nevertheless, the ground 12 failed to return to its original color when exposed to various solvent vapors such as methylene chloride, dichloroethane, acetone, chloroform, and THF, etc. This irreversible response of the hybrid 12 towards mechanical force suggests that it is not piezochromic anymore.
Compound 12 was also subjected to UV (352 nm) irradiation in solid state as it consisted of a light-sensitive β-enamino diketone moiety. Regrettably, the hybrid 12 failed to respond to UV light in solid state. As shown in Figure 11A compound 12 did not show noticeable color change even after exposed to UV light (352 nm) for 30 min. Further, no major change was observed in the solid-state absorbance spectra of 12 prior to and after UV irradiation (Figure 11B). Our studies suggest that a multi-stimuli responsive molecule cannot be constructed simply through combination of two different chromic moieties into one, even though structures of the two chromic molecular scaffolds are closely related.
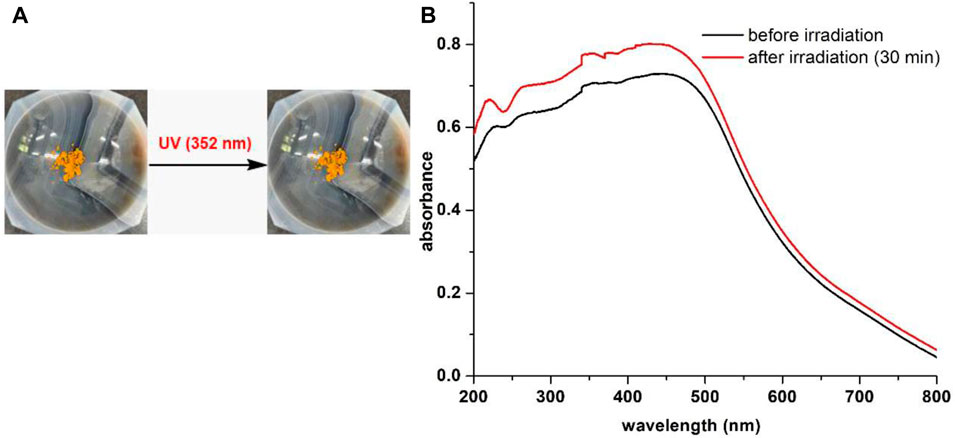
FIGURE 11. (A) No color change of 12 prior to and after UV (352 nm) irradiation. (B) Solid state absorption profiles of 12 prior to and after UV (352 nm) irradiation.
3 Methods and materials
3.1 General information
Microwave reactions were performed using a CEM Discover unit (operating at 110 V, microwave irradiation of 2.45 GHz, maximum microwave output of 300 W) in 50 mL capacity open round-bottom flasks. Visualization was accomplished by using portable UV light and an iodine chamber. Flash chromatography was performed in columns of various diameters with Merck silica gel (230–400 mesh ASTM 9385 kieselgel 60H) by elution with the solvent systems. Solvents, unless otherwise specified, were reagent grade and distilled once before use. All new compounds exhibited satisfactory spectroscopic and analytical data. 1H NMR (400 MHz) and 13C NMR (100) spectra were recorded on a Bruker 400 spectrometer. Chemical shifts were reported in parts per million on the scale relative to an internal standard (tetramethylsilane, or appropriate solvent peaks) with coupling constants given in hertz. 1H NMR multiplicity data are denoted by s (singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). Analytical thin-layer chromatography (TLC) was carried out on Merck silica gel 60G-254 plates (25 mm) and developed with the solvents mentioned. Melting points were determined on a Mel-Temp melting point apparatus in open capillaries and are uncorrected. High-resolution mass spectra (HRMS) were obtained on a Thermo Fisher Scientific Finnigan MAT95XL spectrometer using a magnetic sector analyzer. Infrared (IR) spectra were recorded using 1725XFT-IR spectrophotometer. Single-crystal structures were determined with a Bruker AXS SMART-1000 X-ray single-crystal diffractometer. The absorption spectra were obtained using a UV/vis/NIR spectrophotometer (Jasco V-770) with a deuterium lamp (190–350 nm) and halogen lamp (300–2,700 nm) light sources and the detector was a photomultiplier tube.
3.2 Synthesis of compounds 7a–o, 12, and 13
Mixtures of appropriately substituted 4-hydroxycoumarin (1 equiv.), β-nitrostyrene (1.2 equiv.), substituted amine (1.2 equiv.), and a few drops of triethylamine in toluene (∼20 mL) were irradiated under microwave (100 W, 150 C) for 25–30 min (unless otherwise specified). The cooled reaction mixture was concentrated and then re-dissolved in DCM. The solution was washed with water, brine, dried over MgSO4, and evaporated in vacuo. The crude product was purified by column chromatography and was further recrystallized from DCM/hexanes.
4 Conclusion
In summary, a total of 15 structurally diverse β-enamino diketone derivatives were synthesized in good to excellent yields via microwave-assisted, base-mediated, three-component reaction between 4-hydroxycoumarins, substituted β-nitrostyrene, and primary amines. Among prepared compounds, compound 7i was found to exhibit positive photochromism in solution/thin film and negative photochromism in crystalline state. Further, compound 12 bearing pressure- and light-sensitive molecular scaffolds was designed and synthesized in two steps as a potential dual-responsive material. Unfortunately, the prepared 12 failed to show any expected functional behavior. The in-depth investigation of the photochromic mechanism of this intriguing thiophene-derived β-enamino diketone 7i at the molecular level is currently underway and will be reported in due course.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
KBM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing–original draft, Writing–review and editing. S-CF: Formal Analysis, Investigation, Methodology, Writing–original draft, DYY: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. National Science and Technology Council of Taiwan Contract No. NSTC 109-2113-M-029-010-MY3 (DYY) and 108-2113-M-029-004 (KBM).
Acknowledgments
We thank the National Science and Technology Council of Taiwan for financially supporting this research. Also, the support for the X-ray, mass spectroscopy, and NMR measurements from the Instrument Center of National Chung Hsing University (NCHU) is greatly acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2023.1295347/full#supplementary-material
References
Avadanei, M., Cozan, V., Shova, S., and Paixão, J. A. (2014). Solid state photochromism and thermochromism of two related N-salicylidene anilines. Chem. Phy. 444, 43–51. doi:10.1016/j.chemphys.2014.10.007
Budzisz, E., Keppler, B. K., Giester, G., Wozniczka, M., Kufelnicki, A., and Nawrot, B. (2004). Synthesis, crystal structure and biological characterization of a novel palladium(II) complex with a coumarin-derived ligand. Eur. J. Inorg. Chem. 22, 4412–4419. doi:10.1002/ejic.200400483
CCDC No (2023). Crystallographic data (excluding structure factors) for 7b, 7g, 7i (prior Blue LED irradiation), 7i (after blue LED irradiation), and 7m have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC-1540618, -1540615, -1540617, 2300435 and -1540616, respectively. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, by emailingZGF0YV9yZXF1ZXN0QGNjZGMuY2FtLmFjLnVrLA== or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Chithanna, S., and Yang, D. Y. (2019). Multicomponent synthesis of 1,3-diketone-linked N-substituted pyrroles, pyrrolo[1,2-a]pyrazines, pyrrolo[1,4]diazepines, and pyrrolo[1,4]diazocines. J. Org. Chem. 84, 1339–1347. doi:10.1021/acs.joc.8b02819
Chithanna, S., Vyasamudri, S., and Yang, D. Y. (2020). Application of dimedone enamines as protecting groups for amines and peptides. Org. Lett. 22, 2391–2395. doi:10.1021/acs.orglett.0c00586
Chiu, C.-W., and Yang, J.-S. (2020). Photoluminescent and photoresponsive iptycene-incorporated π-conjugated systems: fundamentals and applications. ChemPhotoChem 4, 538–563. doi:10.1002/cptc.201900300
Ferraz, H. M. C., de Oliveira, E. O., Payret-Arrua, M. E., and Brandt, C. A. (1995). A new and efficient approach to cyclic.beta.-Enamino esters and.beta.-Enamino ketones by iodine-promoted cyclization. J. Org. Chem. 60, 7357–7359. doi:10.1021/jo00127a051
Funasako, Y., Ason, M., Takebayashi, J., and Inokuchi, M. (2019). Solid-state photochromism of salts of cationic spiropyran with various anions: a correlation between reaction cavity volumes and reactivity. Cryst. Growth Des. 19, 7308–7314. doi:10.1021/acs.cgd.9b01185
Funasako, Y., Miyazaki, H., Sasaki, T., Goshima, K., and Inokuchi, M. (2020). Synthesis, photochromic properties, and crystal structures of salts containing a pyridinium-fused spiropyran: positive and negative photochromism in the solution and solid state. J. Phys. Chem. B 124, 7251–7257. doi:10.1021/acs.jpcb.0c04994
Ghabraie, E., Bararjanian, M., Balalaie, S., Rominger, F., and Bijanzadeh, H. R. (2011). Efficient synthesis of (3e)-3-[amino(aryl)methylidene]chromane-2,4-diones (=(3E)-3-[Amino(aryl)methylene]-2H-1-benzopyran-2,4(3H)-diones) via a three-component reaction. Helv. Chim. Acta 94, 1440–1447. doi:10.1002/hlca.201100001
Helmy, S., Oh, S., Leibfarth, F. A., Hawker, C. J., and Read deAlaniz, J. (2014). Design and synthesis of donor–acceptor stenhouse adducts: a visible light photoswitch derived from furfural. J. Org. Chem. 79, 11316–11329. doi:10.1021/jo502206g
Hsieh, W. C., Manjappa, K. B., and Yang, D. Y. (2019). Structural tuning enables piezochromic and photochemical properties in N-aryl-β-enaminonesN-aryl-β-enaminones. RSC Adv. 9, 34088–34094. doi:10.1039/C9RA07598D
Irie, M., Fukaminato, T., Matsuda, K., and Kobatake, S. (2014). Photochromism of diarylethene molecules and crystals: memories, switches, and actuators. Chem. Rev. 114, 12174–12277. doi:10.1021/cr500249p
Kuo, P. Y., Chuang, R. R., and Yang, D. Y. (2009). Reactions of 3-benzoyl-7-dimethylamino-4-hydroxycoumarin and their potential applications in solution- and solid-phase synthesis. Mol. Divers. 13, 253–260. doi:10.1007/s11030-009-9107-2
Li, G., Watson, K., Buckheit, R. W., Zhang, Y., and Zhang, Y. (2007). Total synthesis of anibamine, a novel natural product as a chemokine receptor CCR5 antagonist. Org. Lett. 9, 2043–2046. doi:10.1021/ol070748n
Li, A., Xu, S., Bi, C., Geng, Y., Cuib, H., and Xu, w. (2021). Piezochromic mechanism of organic crystals under hydrostatic pressure. Mater. Chem. Front. 5, 2588–2606. doi:10.1039/D0QM00975J
Loan, T., Santra, M., and Bradley, M. (2023). Novel class of photochromic molecules exhibiting photo-switching in the solid state. Front. Chem. 11, 1205452. doi:10.3389/fchem.2023.1205452
Ma, Z., Wang, Z., Teng, M., Xu, Z., and Jia, Ma (2015). Mechanically induced multicolor change of luminescent materials. ChemPhysChem 16, 1811–1828. doi:10.1002/cphc.201500181
Manjappa, K. B., Yang, Y.-A., Santhosh, M. S., and Yang, D. Y. (2018). Nitroalkane-mediated multicomponent synthesis of β-enaminones. ChemistrySelect 3, 10701–10705. doi:10.1002/slct.201802742
Martinez-Junquera, M., Lalinde, E., and Moreno, M. T. (2022). Multistimuli-responsive properties of aggregated isocyanide cycloplatinated(II) complexes. Inorg. Chem. 61, 10898–10914. doi:10.1021/acs.inorgchem.2c01400
Michael, J. P., Koning, C. B., Hosken, G. D., and Stanbury, T. V. (2001). Reformatsky reactions with N-arylpyrrolidine-2-thiones: synthesis of tricyclic analogues of quinolone antibacterial agents. Tetrahedron 57, 9635–9648. doi:10.1016/S0040-4020(01)00964-4
Mladenovic, M., Vukovic, N., Niciforovic, N., Sukdolak, S., and Solujic, S. (2009). Synthesis and molecular descriptor characterization of novel 4-Hydroxy-chromene-2-one derivatives as antimicrobial agents. Molecules 14, 1495–1512. doi:10.3390/molecules14041495
Naumov, P., Chizhik, S., Panda, M. K., Nath, N. K., and Boldyreva, E. (2015). Mechanically responsive molecular crystals. Chem. Rev. 115, 12440–12490. doi:10.1021/acs.chemrev.5b00398
Sagara, Y., and Kato, T. (2009). Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 1, 605–610. doi:10.1038/nchem.411
Sagara, Y., Mutai, T., Yoshikawa, I., and Araki, K. (2007). Material design for piezochromic luminescence: hydrogen-bond-directed assemblies of a pyrene derivative. J. Am. Chem. Soc. 6, 1520–1521. doi:10.1021/ja0677362
Sun, F., Xiong, X., Gao, A., Duan, Y., Mao, L., Gu, L., et al. (2022). Fast photochromism in solid: microenvironment in metal-organic frameworks promotes the isomerization of donor-acceptor Stenhouse adducts. J. Chem. Eng. 427, 132037. doi:10.1016/j.cej.2021.132037
Vaidya, S., Sharma, M., Bruckner, C., and Kasi, R. M. (2021). Rhodamine-installed polynorbornenes: molecular design, structure, and stimuli-responsive properties. ACS Omega 6, 15017–15028. doi:10.1021/acsomega.1c01160
Valduga, C. J., Braibante, H. S., and Braibante, M. E. F. (1998). Reactivity of p-phenyl substituted β-enamino compounds using k-10/ultrasound. I. synthesis of pyrazoles and pyrazolinones. J. Heterocycl. Chem. 35, 189–192. doi:10.1002/jhet.5570350136
Vukovic, N., Sukdolak, S., Solujic, S., and Niciforovic, N. (2010). Substituted imino and amino derivatives of 4-hydroxycoumarins as novel antioxidant, antibacterial and antifungal agents: synthesis and in vitro assessments. Food Chem. 120, 1011–1018. doi:10.1016/j.foodchem.2009.11.040
Wang, Y., Tan, X., Zhang, Y.-M., Zhu, S., Zhang, I., Yu, B., et al. (2015). Dynamic behavior of molecular switches in crystal under pressure and its reflection on tactile sensing. J. Am. Chem. Soc. 137, 931–939. doi:10.1021/ja511499p
Wang, J., Zhang, M., Han, S., Zhu, L., and Jia, X. (2022). Multiple-stimuli-responsive multicolor luminescent self-healing hydrogel and application in information encryption and bioinspired camouflage. Journal of Materials Chemistry 10, 15565–15572. doi:10.1039/D2TC03072A
Wei, Y., Yang, R., Cui, G., Dai, S., Pan, G., Wang, J., et al. (2023). Low-pressure sensitive piezochromic fluorescence switching of tetraphenylethylene-anthraquinone. Chem. Eur. J. 29, e202301070. doi:10.1002/chem.202301070
White, J. D., and Lhle, D. C. (2006). Tandem Photocycloaddition−Retro-mannich fragmentation of enaminones. A route to spiropyrrolines and the tetracyclic core of koumine. Org. Lett. 8, 1081–1084. doi:10.1021/ol052955y
Ye, W. J., Li, Y., Zhou, L. X., Liu, J. J., and Wang, C. D. (2015). Three-component reaction between substituted β-nitrostyrenes, β-dicarbonyl compounds and amines: diversity-oriented synthesis of novel β-enaminones. Green Chem. 17, 188–192. doi:10.1039/C4GC01234H
Zhang, Z., Yao, D., Zhou, T., Zhang, H., and Wang, Y. (2011). Reversible piezo- and photochromic behaviors accompanied by emission color switching of two anthracene-containing organic molecules. Chem. Commun. 47, 7782–7784. doi:10.1039/C1CC11882J
Keywords: β-enamino diketones, thiophene, photochromism, piezochromism, multi-responsive, photosensitive, microwave, functional property
Citation: Manjappa KB, Fan S-C and Yang D-Y (2023) Structural tuning of β-enamino diketones: exploration of solution and crystalline state photochromism. Front. Chem. 11:1295347. doi: 10.3389/fchem.2023.1295347
Received: 16 September 2023; Accepted: 24 October 2023;
Published: 07 November 2023.
Edited by:
Kamaldeep Paul, Thapar Institute of Engineering and Technology, IndiaReviewed by:
Borys Ośmiałowski, Nicolaus Copernicus University in Toruń, PolandArindam Mukhopadhyay, Idaho National Laboratory (DOE), United States
Copyright © 2023 Manjappa, Fan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kiran B. Manjappa, a2lyYW5AdGh1LmVkdS50dw==; Ding-Yah Yang, eWFuZ0B0aHUuZWR1LnR3
 Kiran B. Manjappa
Kiran B. Manjappa Sheng-Chieh Fan2
Sheng-Chieh Fan2