- Centre for Cellular and Molecular Biology-CSIR, Hyderabad, India
Pharmacological drugs targeting specific pathways involved in various diseases have seen recent advancement with newer and more efficient emerging drug targets, but these drugs are limited in terms of their side effects and patient adherence. The potential of plant-based diets in the form of functional foods is increasingly being realized as an option to treat and/or prevent several diseases. In this work, we have selected flaxseed (Linum usitatissimum), also known as linseed, to study its pharmacological efficacy and proposed mechanisms of action for medicinal purposes. The target genes of linseed with Disease Specificity Index (DSI >0.6) are compared to the associated genes of diabetes mellitus, decrease in appetite, addictive behavior, cardiovascular diseases (CVDs), inflammatory bowel diseases (IBDs), and Polycystic Ovary Syndrome (PCOS), and the selected genes are further evaluated using in silico methods. The binding affinity of flaxseed to three common target proteins (CCDC28b, PDCD6IP, and USP34) is assessed by docking and molecular dynamics (MD) simulations. The results show that linseed is safe to use for mutagenic toxicity and other cardiotoxicity measures, but linseed is unsafe for embryotoxicity, hERG toxicity, and cardiac failure. The analysis of the protein–protein interaction (PPI) network, Gene Ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways indicates that flaxseed can be used as a medicinal herb for treatment of diabetes mellitus, cardiovascular diseases, IBDs, and PCOS.
Introduction
Flaxseed or linseed (Linum usitatissimum L.) belongs to the Linaceae family, which is used as an edible medicinal herb due to the large amount of high-quality protein and soluble fiber. Flaxseed oil has many health benefits as it has a proper balance of essential fatty acids such as α-linolenic acid (ALA); an omega-3 biological precursor, linoleic acid; an omega-6 fatty acid; and omega-9 fatty acids. Potassium, lecithin, magnesium, 6% mucilage, traces of cyanogenic glycoside linamarin (El-galil and Mohammed, 2021), and vitamins (A, B, D, and E) are present in flaxseed. Flaxseed also includes phytoactive chemicals such as phenolic compounds, terpenoids, pigments, and other naturally occurring antioxidants. Cyclic peptides (cyclopeptides and cyclotides) isolated from plants and animals show various biological functions such as antidiabetic, cardioprotective, and immunosuppressive properties, which can be used to develop many therapeutic agents (Górski et al., 2001; Drygała et al., 2009; Thevenard et al., 2010; Yu et al., 2010; Hu and Xu, 2012; Chang et al., 2013; Fang et al., 2013; Goyal et al., 2014; Boivin et al., 2015). High contents of ALA in flaxseed oil, lignans, dietary fibers, and flaxseed proteins have drawn the attention of the scientific community in exploiting the maximum benefits of flaxseed for medicinal purposes (Marambe and Wanasundara, 2017; Zou et al., 2017; Wu et al., 2019; Yang et al., 2021). Dietary supplementation with flaxseed is beneficial for cardiovascular diseases (CVDs) as use of flaxseed supplement shows antihypertensive action, antiatherogenic effects, lowering of cholesterol, anti-inflammatory action, and inhibition of arrhythmias. Although not well-known, few biological actions of flaxseed are attributed to potential bioactive compounds such as proteins, cyclolinopeptides, and cyanogenic glycosides. The cardioprotective effects of polyunsaturated fatty acids (PUFAs) has been observed in several clinical trials such as DART (Burr et al., 1989), the GISSI-Prevenzione trial (Bang et al., 1971), GISSI-HF (Bang et al., 1976), and JELIS (Gonzalez et al., 2011). Previous studies have shown that the consumption of flaxseed (for 8 to 12 weeks) in the long term reduces blood glucose (Moreira et al., 2022), glycated hemoglobin (Pan et al., 2007; Hasaniani et al., 2019), triglycerides (Torkan et al., 2015), total cholesterol (Toulabi et al., 2022), and blood pressure (Khalesi et al., 2015) in patients with T2DM (Hasaniani et al., 2019) and insulinemia (Rhee and Brunt, 2011). Consumption of flaxseed improves insulin sensitivity in individuals with prediabetes (Javidi et al., 2016) and obesity-related issues (Bongartz et al., 2022). Several studies indicate that the consumption of flaxseed improves glycemic control (Mohammadi-Sartang et al., 2018; Hajiahmadi et al., 2020; Villarreal-Renteria et al., 2022). The acute effects of flaxseed on postprandial hyperglycemia in individuals with T2DM have not been investigated until now (Moreira et al., 2022). Flaxseed is also used as a therapeutic agent in inflammatory bowel disease (IBD), which is a heterogeneous disease in which multiple triggers act simultaneously (Palla et al., 2020). The main targets of IBD are immune dysregulation, polyendocrinopathy, and microbial defects, which is accompanied with symptoms such as abdominal spasms and colic (Xavier and Podolsky, 2007). Even with advance IBD therapeutics, the rate of failure remains high, possibly due to multiple causes of the disease (Duke et al., 2002; Rahimi et al., 2010). The aqueous-methanolic crude extract of Flaxseed (Fs.Cr) has improved the severity in the mouse model of colitis by reducing mediators of inflammation (myeloperoxidase and cytokines) and inducers of oxidative stress (Palla et al., 2016). Flaxseed has been reported to have antihyperglycemic properties without any reported severe side effects (Mani et al., 2011; Parikh et al., 2019). The functional compounds of flaxseed provide several health benefits related to improvement in disease in individuals with metabolic syndrome (Hutchins et al., 2013; Shayan et al., 2020; Yari et al., 2020). Consumption of 30 g of flaxseed added to a bread recipe or in a 50-g glucose challenge reduces the blood glucose area under the curve (AUC) for more than 2 h in healthy young adults (Dahl et al., 2005; Vuksan et al., 2017). Lignan in flaxseed reduces androgen levels in men with prostate cancer (Demark-Wahnefried et al., 2008). Polycystic Ovary Syndrome (PCOS) is a chronic endocrinopathy which affects few women of reproductive age (Nowak et al., 2007, Yumiceba et al., 2020). Persistent anovulation and hyperandrogenism are the characteristics of PCOS, which results in high blood sugar and type 2 diabetes (T2D) at an early stage, dyslipidemia, cardiovascular disease, and infertility (Franks and Hardy, 2020). The most important PCOS candidate genes are those that encode for molecules involved in androgen synthesis, transportation, and control the secretion and activity of insulin receptors, signaling cascade proteins, and growth factors (Wang et al., 2020).
The main genomic investigations are focused on the genes involved in metabolism and biosynthetic pathways, reproductive function, signaling pathways, transportation and development, cellular senescence, and other biological processes (Deswal et al., 2019). Drugs such as metformin, clomiphene citrate, and glucocorticoids and aromatase inhibitors like anastrozole are used for the treatment of PCOS (Badawy and Elnashar, 2011), which cause numerous side effects such as nausea, discomfort in the abdomen, and vaginal bleeding (Ndefo et al., 2013). So herbal drugs as phytoestrogen (isoflavonoids, flavonoids, stilbenes, and lignans), which are abundant in soy isoflavones and flaxseed lignans (Mehraban et al., 2020), are recommended as an alternative.
Materials and methods
Conventional methods to discover and characterize potential bioactive peptides from food proteins are time-consuming and costly, so in silico approaches are used to predict the potential bioactive peptides from various food proteins (Udenigwe et al, 2012; Poustforoosh et al., 2023, Ji et al., 2020). Computational resources are cost-effective and provide a significant amount of information in a limited time span for herbal complexes. Computational tools can predict the physicochemical and biological properties of herbal medicine in less time and accurately predict the possible target–drug interactions. As flaxseed has multifactorial activities and no proper detailed information related to the pharmacodynamics, mechanism of action, absorption, and toxicity on flaxseed is reported, we carried out a detailed computational work to study the multifarious activities of flaxseed. At first, the physicochemical and absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties of the main constituent (α-linolenic acid) of flaxseed are studied. Then, the potent targets for linseed are identified. After determining the disease-associated genes, the protein–protein interaction (PPI) network, Gene ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway were analyzed. Docking studies are carried out for the three target–flaxseed complexes, and then the molecular dynamics (MD) simulations for the three target–flaxseed complexes are carried out with a Galaxy platform using open-source GROMACS tools (Galaxy, 2023). The results were analyzed by Root Mean Square Deviation, Root Mean Square Fluctuation, and principal component analysis (PCA) plots.
Physicochemical and ADMET properties of flaxseed (α-linolenic acid)
Flaxseed consists of chemical compounds with specific biological activity and functional properties: PUFAs, omega-3 family, soluble dietary fibers, lignans, proteins, and carbohydrates. The major composition of flaxseed includes fatty acids (α-linolenic acid, linoleic acid, oleic acid, stearic acid, and palmitic acid) (Bernacchia et al., 2014). ALA comprises approximately 55% of the total fatty acid content of flaxseed fatty acids (McCullough et al., 2011). Experimental trials showed that ALA-rich diets reduced the occurrence of both fatal and non-fatal myocardial infarction (de Lorgeril et al., 1994; Albert et al., 2005), cardiac arrhythmias (Albert et al., 2005), and atherosclerotic lesions (Albert et al., 2005; Tziomalos et al., 2008). The SMILES notation for the main constituent of linseed: ligand (DB00132, α-linolenic acid) is obtained from the DrugBank (https://go.drugbank.com/drugs/DB00132) for predicting the physicochemical properties and toxicity (mutagenic effect, irritant effect, tumorigenicity, and effect on the reproductive system) effects. Various conformational structures of α-linolenic acid are generated from the PDB file (DB00132), and then all the structures are optimized by the wB97XD (Chai and Head-Gordon, 2008)/6–31G (d,p) method with G09 software suites (Frisch et al., 2009). The integral equation formalism-polarized continuum model (IEF-PCM) is utilized with water as a solvent (Marenich et al., 2009). Finally, the lower minima structure with no negative frequency is considered for further studies. The SMILES notation of α-linolenic acid is used as the input to study the physicochemical properties and toxicity measures by Molinspiration software (www.molinspiration.com) (Molinspiration Chemoinformatics software, 2023) and Osiris software program (Osiris property explorer (www.organicchemistry.org/prog/peo/)), (Osiris property explorer, 2023) respectively. Molinspiration software is used to predict the physicochemical properties including logP, molecular polar surface area (PSA), and the descriptors from Lipinski “Rule of 5.” The Osiris software program is used to predict various drug-relevant properties. The mutagenic toxicity-related results are color-coded. Properties with high risks of undesired effects like mutagenicity or poor intestinal absorption are shown in red. The green color indicates drug-conformation behavior, whereas yellow color indicates mild risk.
The cardioToxCSM (Iftkhar et al., 2022) webserver was used to study six types of cardiotoxicity outcomes in flaxseed: arrhythmia, cardiac failure, heart block, hERG toxicity, hypertension, and myocardial infarction. The cardioToxCSM is a machine learning (ML)-based webserver which is developed by using the concept of graph-based signatures, molecular descriptors, toxicophore matchings, and molecular fingerprints. The datasets are internally and externally validated via different cross-validation schemes and low-redundancy blind sets, respectively. The embryoTox (Aljarf et al., 2023) webserver is used to predict and classify molecules which are likely to be safe to use during pregnancy in women. The webserver is trained and validated using in vitro bioactivity data of over 700 small molecules. embryoTox utilizes a graph-based signature representation of the chemical structure with teratogenicity effects.
Identification of flaxseed potential targets
The ChEMBL database (https://www.ebi.ac.uk/chembl) is utilized to identify the potential targets of linseed. “Flaxseed” or linseed or “Linum usitatissimum” or SMILES notation of α-linolenic acid are used as the keywords. A total of 150 potential targets for Homo sapiens were obtained from the target summary in the compound’s report card with similar structures and extensive search.
Determination of disease-associated genes
The associated genes for various diseases are determined using the DisGeNET database (https://www.disgenet.org). As flaxseed is used as therapeutic agents for cardiovascular diseases (Rodriguez-Leyva et al., 2010), DM (Javidi et al., 2016), addictive behavior (Prior et al., 2015), IBD (Palla et al., 2020), and PCOS (Nowak et al., 2007), the associated genes with Disease Specificity Index (DSI) greater than 0.6 for Homo sapiens are compared. Ten potential target genes were identified, out of which three potential target proteins which are common to cardiovascular diseases, DM, IBD, and PCOS, were selected for further studies. Therefore, no common target genes are identified for addictive behavior.
Protein–protein interaction network, enrichment of Gene Ontology, and Kyoto Encyclopedia of Genes and Genomes pathway
The STRING database v11.5 (https://stringdb.org/) (String database V11.5, 2023) was used to find the PPI network of the potential targets. The suggested pathways are based on the known interactions (previously reported studies), predicted interactions (e.g., gene co-occurrence), or various other reports (e.g., text mining) to investigate the molecular functions and biological processes. The GO and KEGG (https://www.genome.jp/kegg/) analyses were carried out to find the pathways modulated by flaxseed targets. GO and KEGG enrichment analyses are commonly used to analyze genes (Chen et al., 2017). GO, a database from the Gene Ontology Consortium, with a set of dynamic administered vocabularies was used to understand the roles of genes and proteins in cells, thereby extensively characterizing genes and gene products by biological process (BP), cellular component (CC), and molecular function (Ashburner et al., 2000; The Gene Ontology Consortium, 2023) in living organisms. KEGG, a manually curated database, is used to integrate various biological objects that are divided into systems, genomes, and other health-related resources (Kanehisa et al., 2023). The core pathways revealed by KEGG enrichment analysis show the core pathways, and the connections between these basic genes can further help understand the key roles of these genes.
Docking and MD simulations
Once the key roles of target genes are identified, these common target genes are selected for docking and MD simulations with flaxseed (α-linolenic acid). Molecular docking in drug discovery plays a vital role as it includes structure–activity relationship (SAR), lead optimization, finds potential leads through virtual screening, and provides binding measures to facilitate predictions for inhibitors (Schleinkofer et al., 2006). The 3D structures of the selected three target proteins are obtained from the EBI AlphaFold2 database (Jumper et al., 2021) (EMBL-EBI alphafold, 2023). These three target proteins are CCDC28b (Q9BUN5), PDCD61P (UniProt ID: Q8WUM4), and USP34 (UniProt ID: Q9P2H5) with source species as Homo sapiens. The lower energy minima structure with no negative frequency of flaxseed (α-linolenic acid) is selected for the target–ligand interaction. The binding energies of target–flaxseed complexes are calculated with AutoDock tools (Morris et al., 2009). The output structures of these docked structures are used as input for energy minimization and MD simulations with the Galaxy webserver platform (Galaxy webserver; usegalaxy.org). The Galaxy platform is used for high-throughput molecular dynamics simulation to study protein–ligand interactions using the open-source GROMACS tools. For protein topology, the TIP3P model with an AMBER99SB force field is used. Ligand topology is generated by the GAFF force field with default BCC charge method (0 charge, multiplicity 1). The simulation box is created with box dimensions of 1.0 nm and triclinic shape for energy minimization. The system is charged (depending on the pH) with water as a solvent, and it also adds sodium or chloride ions (replacing existing water molecules) as per requirement for neutralization. The EM tolerance = 1,000, and 50,000 steps are considered for energy minimization. Energy minimization (EM) is used to relax the structure, and any steric clashes or unusual geometry are removed. The equilibration of the solvent around the solute (i.e., the protein) is performed in two steps; the equilibration under an NVT (or isothermal–isochoric) ensemble, followed by an NPT (or isothermal–isobaric) ensemble. For NVT and NPT calculations, the following parameters are used: bond constraints (constraints)—all bonds, temperature/K—300, step length in ps—0.001, and number of steps that elapse between saving data points (velocities, forces, and energies)—1,000 and number of simulation steps—50000. For production simulation, the parameter settings are as follows: ensemble—NPT, temperature /K—300, step length in ps—0.001, number of steps that elapse between saving data points (velocities, forces, and energies)—1,000, and number of simulation steps—100000. In this manner, the simulation will run for 1 million steps, with the total length of 1 ns. This post-study is repeated 20 times for 20-ns MD simulations, and then the final plots for Root Mean Square Deviation, Root Mean Square Fluctuation, and PCA are reported independently for target–flaxseed interactions. The Root Mean Square Deviation is used to calculate the difference between a protein’s backbone Cα atoms (at final position) compared to its original conformational structure. The deviation during protein simulation is used to determine the stability of its conformational structure (Jiang et al., 2019). Root Mean Square Deviation is used to examine the free-energy landscape (FEL) and display the compactness and conformational stability of proteins during the dynamic period. Root Mean Square Fluctuation is a crucial measure that is used to assess how much an atom group deviates from its distinct place in a constituted system during an MD simulation (Jiang et al., 2019). The obtained results from docking and MD simulations are visualized with PyMOL software (Schrödinger DeLano, 2020).
Results
The chemical structures, SMILES notation, physicochemical parameters, and various toxicity measurements of flaxseed (α-linolenic acid) are given in Table 1. The oral active nature of a drug complex is predicted by Lipinski’s ‘‘rule of 5’’ (Lipinski et al., 1997) which follows i) the molecular weight (MW) < 500, ii) the calculated octanol/water partition coefficient (log P) < 5, iii) fewer than five hydrogen bond donors (HBDs) (OH and NH groups), and iv) less than 10 hydrogen bond acceptors (HBAs) (notably, N and O). During drug discovery, lipophilicity and molecular weight are often increased in order to improve the affinity and selectivity of the drug candidate. Hence, sometimes it is difficult to maintain drug-likeness (i.e., RO5 compliance) during hit and lead optimization. In flaxseed, it is seen that one parameter violates the Lipinski’s ‘‘rule (log P) > 5.” If the calculated logP (logarithm of partition coefficient) values for the LOBs are between 2 and 5 (from ALOGpS, http://www.vcclab.org), it is suitable for both oral and topical administrations. The topological polar surface area (TPSA) is interconnected to the hydrogen-bonding potential of the complexes (Deconinck et al., 2007). The complexes with TPSA values >140 Å2 or more showed poor intestinal absorption (Daina et al., 2017), and TPSA <90 Å2 is required to penetrate the blood−brain barrier, and thus, act on receptors in the CNS. The TPSA of flaxseed is 37.30 Å2, which is suitable to penetrate the BBB. The bioactivity score of more than 0.00 for flaxseed indicates considerable biological activities. Since the inappropriate ADMET properties of drugs prohibit their usage at the clinical level, the toxicity risks (mutagenicity, tumorigenicity, irritation, and reproductive effect) are also predicted for flaxseed (α-linolenic acid). The results show that flaxseed is safe to use for mutagenic toxicity (green tick), which is also validated by drug-score prediction. As toxicity is the main issue related to the drugs, computational approaches with quantitative structure–activity relationship (QSAR) models and ML methods are used to identify six types of toxicity as cardiac toxicity outcomes: arrhythmia, cardiac failure, heart block, hERG toxicity, hypertension, and myocardial infarction efficiently and accurately. The cardiotoxicity results show that it is harmful to use flaxseed in hERG toxicity and cardiac failure, while it is safe to use in arrhythmia, heart block, hypertension, and myocardial infarction. The embryoTox results indicate that it is unsafe to use flaxseed during pregnancy in women (Table 1).
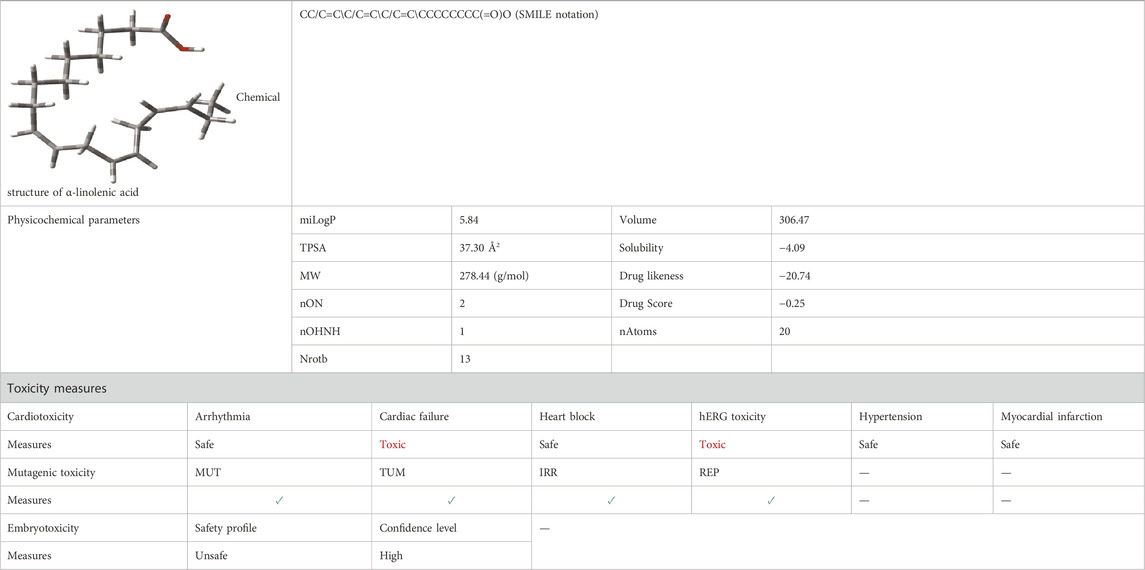
TABLE 1. Chemical structure of α-linolenic acid, SMILES notation, physicochemical parameters, and toxicity measures for flaxseed. Abbreviations: mutagenic effect (MUT), irritant effect (IRRI), tumorigenicity (TUM), and effects on the reproductive system (REP). Green tick indicates that the complex is safe to use. SMILES notation for α-linolenic acid is taken from DB00132, and the chemical structure is optimized by the wB97XD/6–31G (d,p) method with G09 software suites. Physicochemical parameters are taken from Molinspiration software, and the Osiris software is used to estimate MUT, IRRI, TUM, and REP. The cardiotoxicity and embryotoxicity is predicted by CardioToxCSM and EmbryoTox webserver respectively.
The genes associated with the cardiovascular diseases (C0007222)-517 genes, diabetes mellitus (C0011849)-516 genes, inflammatory bowel disease (C0021390)-500 targets, addiction behavior (C0085281)-332 genes, and PCOS-297 are obtained from the DisGeNET database, and then these genes are compared to the flaxseed genes with the help of the DisGeNET database, with DSI >0.6 (Homo sapiens). Ten potential target genes were identified, out of which three common potential target genes (CCDC28b, PDCD6IP, and USP34) were selected, which are most effective for cardiovascular diseases, diabetes mellitus, IBD, and PCOS (Table 2).
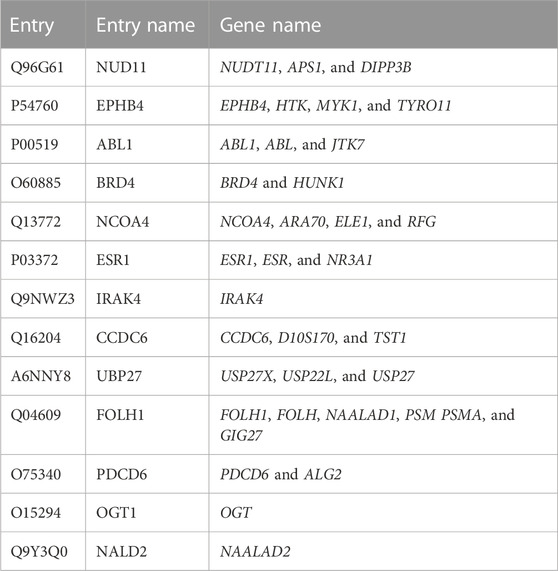
TABLE 2. Target proteins of flaxseed associated with cardiovascular diseases (C0007222), diabetes mellitus (C0011849), inflammatory bowel disease (C0021390), and polycystic ovary syndrome for Homo sapiens (organism). The selected target genes with disease specificity index > 0.6 (Homo sapiens) are obtained from the DisGeNET database.
After determining the potential targets of flaxseed, they are assessed for its association in various diseases. One of the targets CCDC28b with a PPI network ID PMID:29445114: kinesin 1 regulates cilia length through an interaction with the Bardet–Biedl syndrome, associated to the Bardet–Biedl syndrome disease which is a new player in hypertension and other cardiovascular risk factors such as obesity and renal abnormalities (Zhao and Rahmouni, 2022). Complications of obesity can include type 2 diabetes, high blood pressure (Caligiuri et al., 2016), (hypertension), and abnormally high cholesterol levels (hypercholesterolemia) (Jiamset and Hanprasertpong, 2016). The PPI network analysis for CCDC28b is given in Figure 1. Other targets, PCDC6 and PCDC6IP, are associated with PMID:35396512:MAT2A, which facilitates PDCD6 methylation and promotes cell growth under glucose deprivation in cervical cancer. PMID:25644331; programmed cell death 6-interacting protein (PDCD6IP) and Rabenosyn-5 (ZFYVE20) are potential urinary biomarkers for upper gastrointestinal cancer (Sarosiek et al., 2016), PMID:23777424; a functional insertion–deletion polymorphism in the promoter of PDCD6IP is associated with the susceptibility of hepatocellular carcinoma in a Chinese population and PMID:22369209; PDCD6 is an independent predictor of progression-free survival in epithelial ovarian cancer (Figure 2). Previous studies during the last decade indicate significant epidemiological evidence to firmly connect certain cancers, especially breast, colorectal, endometrial, hepatic, pancreatic, and kidney, with type 2 DM (Zhan et al., 2010; Larsson and Wolk, 2011; Szablewski, 2014), though the mechanisms of cancer development related with DM remain unclear. Recent studies showed that there is an association between potential urinary biomarkers for upper gastrointestinal cancer and CVD, but the pathophysiological mechanisms underlying these associations are unclear (Jovani et al., 2022). There is also a link stating that diabetes may increase the risk of gastric cancer through shared risk factors including obesity, insulin resistance, hyperinsulinemia, and smoking. Hyperglycemia, even before the clinical diagnosis of diabetes, may predict gastric cancer in some epidemiological in vitro and in vivo studies (Tseng and Tseng, 2014). IBD, GI cancers, and celiac disease have shown variations in the urinary metabolomics which are associated with possible GI dysbiosis, but there is no study which has systematically assessed the GI microbiota profile simultaneously. The third target gene, USP34 PMID:25975428, has an association with polycystic ovary syndrome. (Zhao et al., 2015) PCOS is a kind of reproductive and metabolic disorder that is characterized by hyperandrogenism and insulin resistance and has affected mostly reproductive-aged women in Caucasia and China (Azziz et al., 2004; Goodarzi and Azziz, 2006; Li et al., 2013) (Figure 3). As PCOS is a heterogeneous endocrine disorder which is characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovaries, recent epidemiological findings showed that women with PCOS have high chances to develop certain cancer types due to their shared metabolic and endocrine abnormalities.
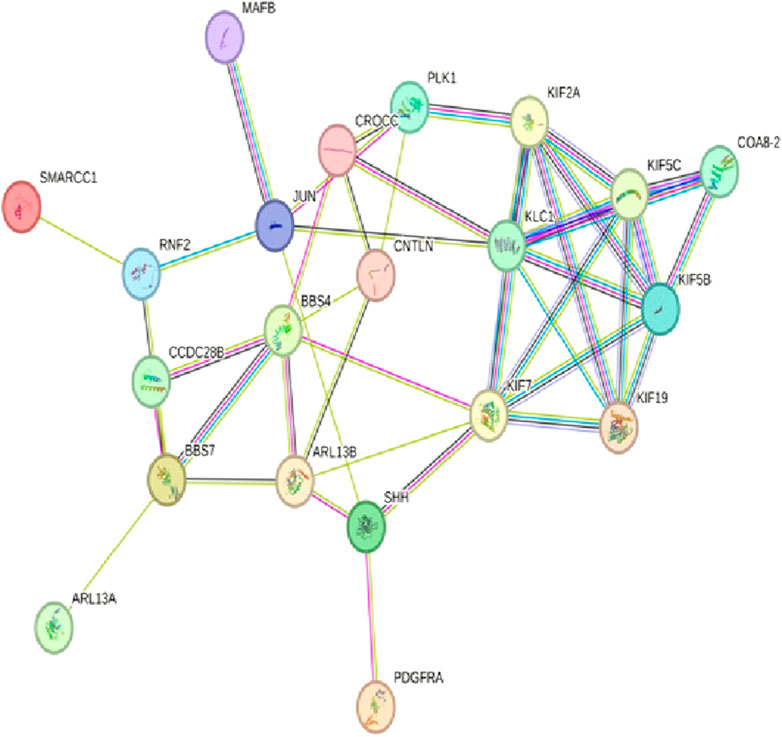
FIGURE 1. The PPI network of CCDC28b pathways engaged in PMID:29445114: kinesin 1 regulates cilia length through an interaction with the Bardet–Biedl syndrome using the STRING database v11.5 (https://stringdb.org/).
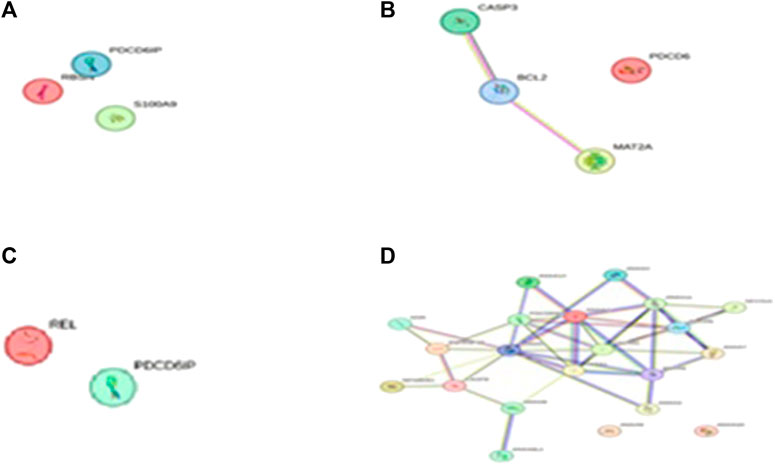
FIGURE 2. The PPI network of PDCD6IP pathways engaged in (A) (D) PMID:25644331 Programmed cell death 6 interacting protein (PDCD6IP) and Rabenosyn-5 (ZFYVE20) are potential urinary biomarkers for upper gastrointestinal cancer. String database V11.5 (https://stringdb.org/) is used to predict the PPI network pathways, (B) PMID:23777424 A functional insertion–deletion polymorphism in the promoter of PDCD6IP is associated with the susceptibility of hepatocellular carcinoma in a Chinese population, and (C) PMID:22369209: PDCD6 is an independent predictor of progression free survival in epithelial ovarian cancer.
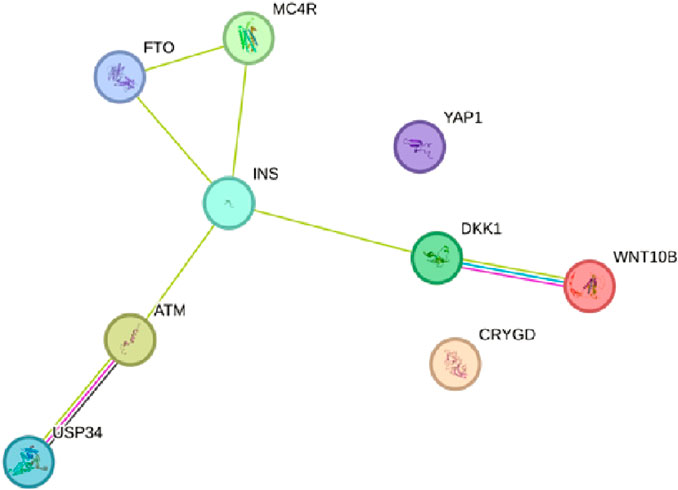
FIGURE 3. PPI network of the USP34 pathways engaged in polycystic ovary syndrome using the STRING database v11.5 (https://stringdb.org/).
The GO analysis for CCDC28b show heart looping, heart morphogenesis, and heart development related to cardiovascular diseases (Table 3). Another PPI network GO analysis for PDCD6 and PDCDIP showed the vascular endothelial growth factor receptor-2 signaling pathway, negative regulation of the biological process, and negative regulation of phospholipase A2 activity which is related to cancer and other related diseases (Table 4). The GO terms for USP34 indicate a response to oxygen-containing compound and regulation of fat cell differentiation, which are closely related to diabetes mellitus and cardiovascular diseases (Table 5). The KEGG pathway analysis predicts hsa:79140 KEGG genes for CCDC28b, hsa:10016 and hsa:10015 for PDCD6, and hsa:9736 KEGG genes for USP34.
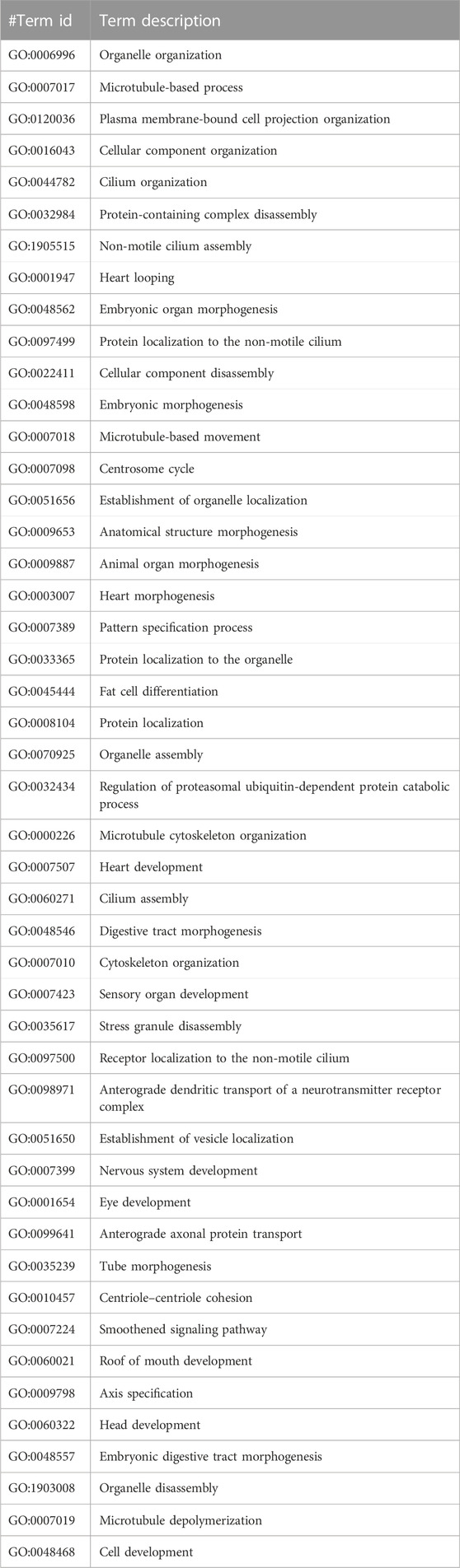
TABLE 3. The Gene Ontology (GO) terms of biological processes with a minimum false discovery rate for the CCDC28b PPI network using the STRING database v11.5 (https://stringdb.org/).
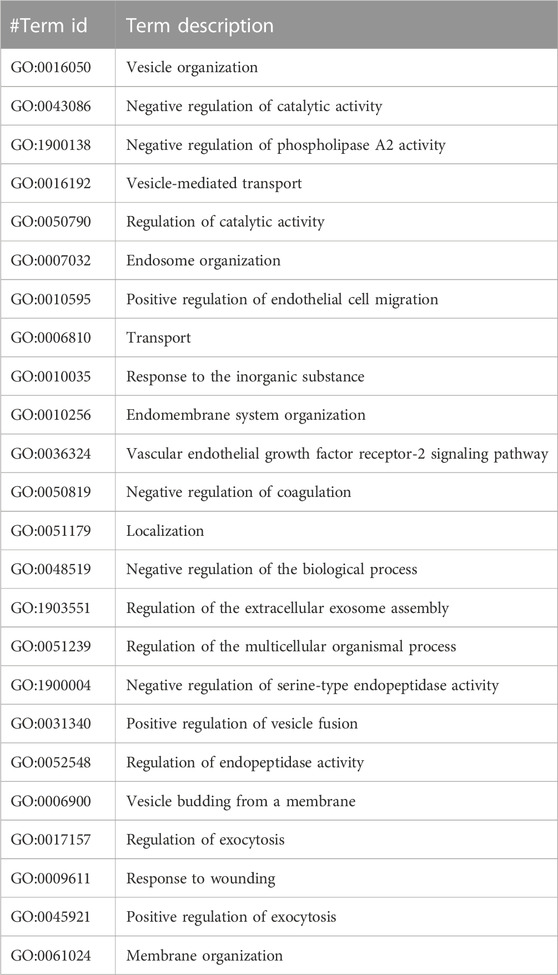
TABLE 4. The Gene Ontology (GO) terms of biological processes with a minimum false discovery rate for the PDCD6IP PPI network using the STRING database v11.5 (https://stringdb.org/).
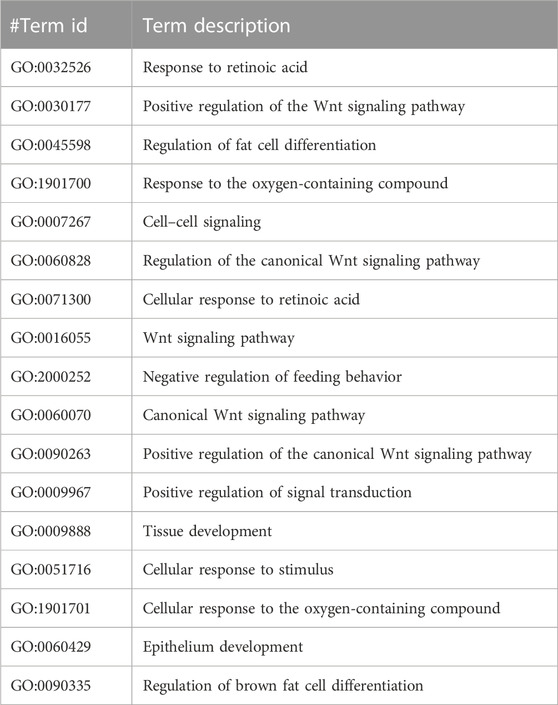
TABLE 5. The Gene Ontology (GO) terms of biological processes with a minimum false discovery rate for the USP34 PPI network using the STRING database v11.5 (https://stringdb.org/).
The calculated binding energies of these three protein–ligand complexes CCDC28b–flaxseed, PDCD6IP–flaxseed, and USP34–flaxseed, are −2.30 kcal/mol, −2.05 kcal/mol, and −4.52 kcal/mol, respectively. The binding energy is lower for the USP34–linseed complex (Figure 4). These output structures are used as input for MD simulation studies for CCDC28b–flaxseed, PDCD6IP–flaxseed, and USP34–flaxseed complexes. The parameters used for MD simulations in the Galaxy webserver are set with the step length of 0.001 ps and 100,000 steps with 300 K temperature. The Root Mean Square Deviation plot, Root Mean Square Fluctuation, and PCA cluster plot for CCDC28b–linseed, PDCD6IP–linseed, and USP34–linseed complexes are shown in Figure 5, Figure 6, and Figure 7, respectively. Root Mean Square Deviation and Root Mean Square Fluctuation are calculated to check the stability and conformation of the ligand during the simulations. Root Mean Square Deviation is a quantitative measurement used to check the stability of protein–ligand complexes (Kulkarni et al., 2022). Root Mean Square Deviation indicates any changes in the atomic position from the initial structure. The initial increase in the Root Mean Square Deviation for all the three protein–ligand complexes shows system adaptability. However, the flat Root Mean Square Deviation after that shows that the protein conformation has not changed much, and finally, the small increase in Root Mean Square Deviation shows that there is not much deviation of the protein from its original conformation, which is seen in CCDC28b–linseed and USP34–linseed complexes. Root Mean Square Fluctuation measures the average deviation of a protein residue over time from a reference position which is basically the time-averaged position of the protein. Root Mean Square Fluctuation is used to analyze how (much or least) the portions of protein structures are fluctuating. The higher Root Mean Square Fluctuation values at the other end of the graph are most likely the loop regions with more conformational flexibility, where no well-defined structure was observed. All the three (target–linseed) complexes showed the higher Root Mean Square Fluctuation values with time. This will allow a connection with experimental spectroscopic techniques to detect the secondary structure of a protein. The ligand-binding-induced correlated motions are assessed by PCA (Kulkarni et al., 2022; Kumar et al., 2023). The first few eigenvectors control the overall important motion of the protein (Parate et al., 2021). It is anticipated that the resulting plot will indicate higher eigenvalues for the first few vectors, and the most significant motions are contained in the first, second, and third eigenvectors. The PCA results obtained from the Galaxy server indicate the first three eigenvectors which is 43.29%, 27.79%, and 22.39% for the CCDC28b–linseed complex; 40.5%, 27.87%, and 17.59% for PDCD6IP-linseed; and 35.7%, 30.86%, and 7.9% for USP34–linseed complexes, indicating the better integrity of the three target–ligand complexes.
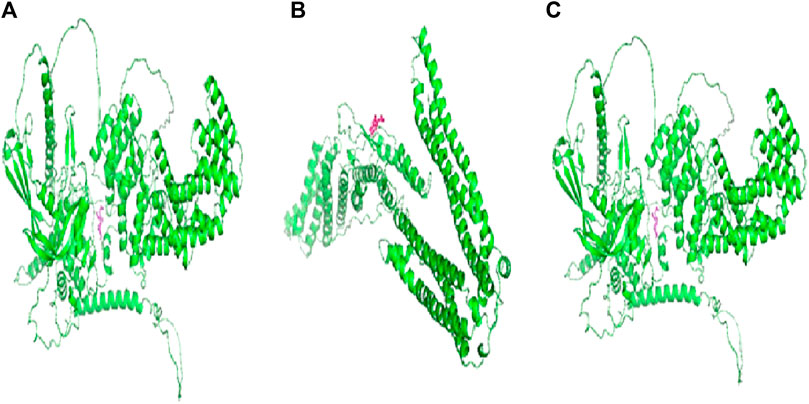
FIGURE 4. Docking structures and binding energies of (A) CCDC28b–flaxseed (BE –2.30 kcal/mol), (B) PDCD6IP–flaxseed (BE –2.05 kcal/mol), and (C) USP34–flaxseed (BE –4.52 kcal/mol) complexes. AutoDock software tools are used for calculating docking and binding energies for the studied target–flaxseed complexes. The flaxseed (α-linolenic acid) is highlighted with a magenta color.
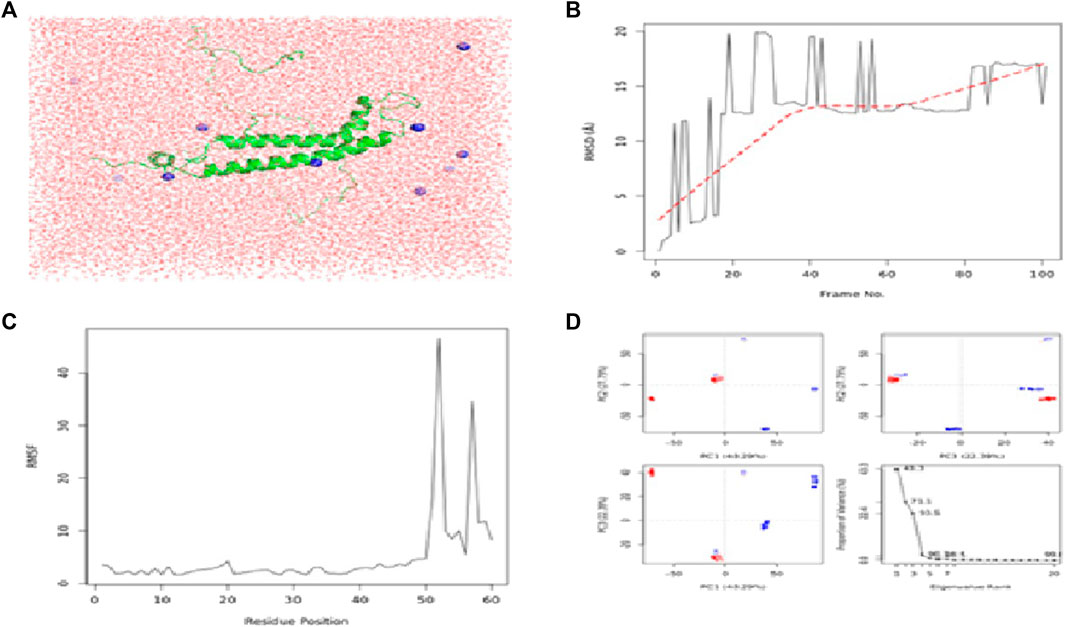
FIGURE 5. Results of MD simulations for CCDC28b–flaxseed interactions. (A) MD simulation structure; (B) Root Mean Square Deviation plot; (C) Root Mean Square Fluctuation plot; and (D) PCA plot. MD simulations for the CCDC28b–flaxseed complex are carried out by the Galaxy platform using open-source GROMACS tools.
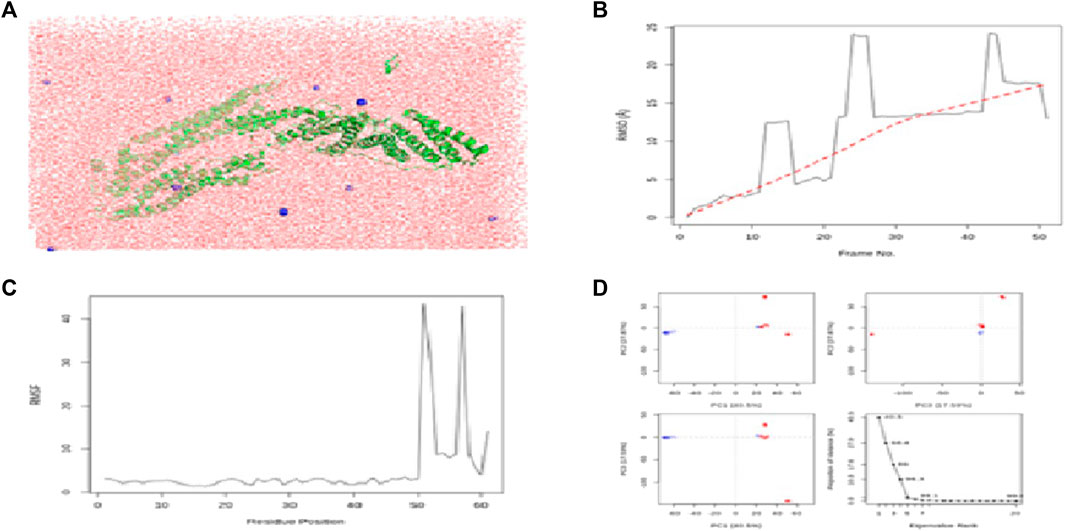
FIGURE 6. Results of MD simulations for PDCD6IP–flaxseed interactions. (A) MD simulation structure; (B) Root Mean Square Deviation plot; (C) Root Mean Square Fluctuation plot; and (D) PCA plot. MD simulations for the PDCD6IP–flaxseed complex are carried out by the Galaxy platform using open-source GROMACS tools.
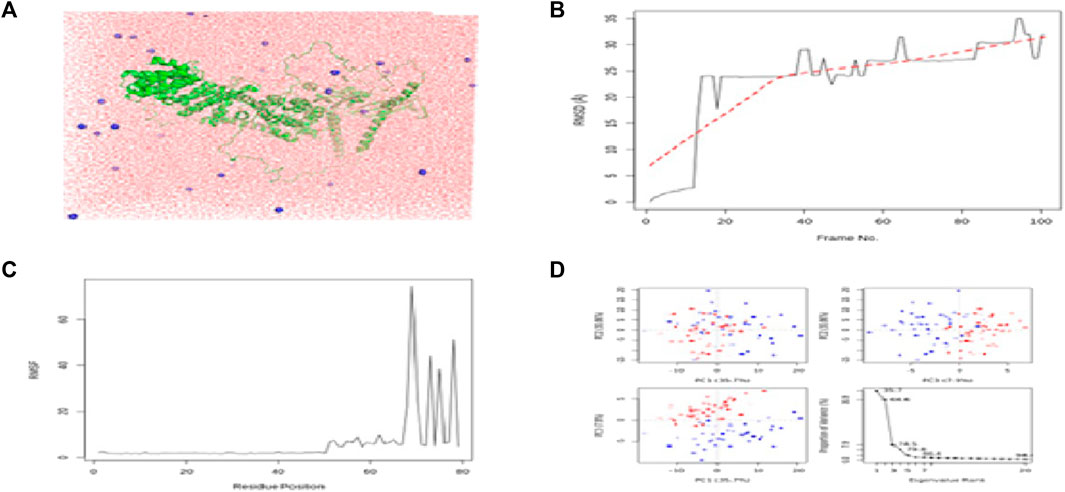
FIGURE 7. Results of MD simulations for USP34–flaxseed interactions. (A) MD simulation structure; (B) Root Mean Square Deviation plot; (C) Root Mean Square Fluctuation plot; and (D) PCA plot. MD simulations for the USP34-flaxseed complex are carried out by the Galaxy platform using open-source GROMACS tools.
The computational investigations of the potential targets using bioinformatics databases predict that flaxseed may inhibit development of cardiovascular diseases, diabetes mellitus, inflammatory bowel disease, and polycystic ovary syndrome. These targets have an association with various stages of the diseases. The ingestion of 15 g of raw ground golden flaxseed containing complex carbohydrates before breakfast decreases the 2-h postprandial glycemic response in men with T2DM (Moreira et al., 2022). Flaxseeds have antihyperglycemic properties without severe reported side effects. Many health benefits of flaxseed protein hydrolysates, such as anti-hypertension ability, antibacterial activity, antioxidant capacity, anti-diabetic ability, and the inhibition ability of calmodulin-dependent neuronal nitric oxide synthase, have been reported (Franck et al., 2019; Logarušić et al., 2020; Nwachukwu and Aluko, 2018a; Nwachukwu and Aluko, 2018b; Perreault et al., 2017; Wu et al., 2019) previously. Flaxseed yogurt is useful not only in women with PCOS but also for women during menopause (Tang, 2019a; Tang, 2019b).
Conclusion
As flaxseed is widely used as a medicinal herb in many countries and is an affordable dietary supplement which is used by individuals with T2DM to improve glycemic control, we studied the physicochemical parameters and toxicity measures of flaxseed (α-linolenic acid) by computational methods and bioinformatics tools. The results predicted that flaxseed is a biologically active medicinal plant. Flaxseed is safe to use in mutagenicity, tumorigenicity, irritation, and reproductive effect, while it is unsafe for use in hERG toxicity and cardiac failure. The use of flaxseed is not recommended for pregnant women. The docking results showed good binding affinities for the three potential (CCDC28b–flaxseed), (PDCD6IP–flaxseed), and (USP34–flaxseed) interactions. The GO analysis for the three target–flaxseed complexes shows heart looping, heart morphogenesis and heart development, vascular endothelial growth factor receptor-2 signaling pathway, negative regulation of biological process, negative regulation of phospholipase A2 activity, oxygen-containing compound, and regulation of fat cell differentiation, which is related to cardiovascular, cancer, diabetes mellitus, and other related diseases. The PPI analysis shows that flaxseed can be used for anti-cholesterol, antioxidant, anti-tumor, and antihyperglycemic activities. Flaxseed is also useful to serve the basic pathophysiological theory of IBD, which involves immune dysregulation, barrier defects, and microbial dysregulation. The results indicate that flaxseed can be used as an option to treat PCOS, which may be influenced by environmental factors, prenatal hormone imbalance, lifestyle, and genetic abnormalities. Therefore, more preclinical trials and clinical trials of a longer duration are needed to identify extensive bioactive components from flaxseed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
RS: conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing–original draft, and writing–review and editing. SJ: formal analysis, methodology, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. RS acknowledges the financial assistance by DST WOSA project (SR/WOS-A/CS-69/2018).
Acknowledgments
RS is thankful to her Mentor Shrish Tiwari, Bioinformatics, CSIR-Centre for Cellular and Molecular Biology, and G. Narahari Sastry, Director, CSIR-NEIST for the technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albert, C. M., Oh, K., Whang, W., Manson, J. E., Chae, C. U., Stampfer, M. J., et al. (2005). Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation 112, 3232–3238. doi:10.1161/CIRCULATIONAHA.105.572008
Aljarf, R., Tang, S., Pires, D. E. V., and Ascher, D. B. (2023). embryoTox: using graph-based signatures to predict the teratogenicity of small molecules. J. Chem. Inf. Model 63 (2), 432–441. doi:10.1021/acs.jcim.2c00824
Ashburner, M., Ball, C. A., Blake, J. A., Botstein, D., Butler, H., Cherry, J. M., et al. (2000). Gene ontology: tool for the unification of biology. Nat. Genet. 25 (1), 25–29. doi:10.1038/75556
Azziz, R., Woods, K. S., Reyna, R., Key, T. J., Knochenhauer, E. S., and Yildiz, B. O. (2004). The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 89 (6), 2745–2749. doi:10.1210/jc.2003-032046
Badawy, A., and Elnashar, A. (2011). Treatment options for polycystic ovary syndrome. Int. J. Womens Health 3, 25–35. doi:10.2147/IJWH.S11304
Bang, H. O., Dyerberg, J., and Hjørne, N. (1976). The composition of food consumed by Greenland Eskimos. Acta Med. Scand. 200, 69–73. doi:10.1111/j.0954-6820.1976.tb08198.x
Bang, H. O., Dyerberg, J., and Nielsen, A. (1971). Plasma lipid and lipoprotein pattern in Greenlandic West-Coast Eskimos. Lancet 297, 1143–1146. doi:10.1016/S0140-6736(71)91658-8
Bernacchia, R., Preti, R., and Vinci, G. (2014). Chemical composition and health benefits of flaxseed. Austin J. Nutri Food Sci. 2 (8), 1045. ISSN: 2381-8980.
Boivin, V., Beyersdorf, N., Palm, D., Nikolaev, V. O., Schlipp, A., Müller, J., et al. (2015). Novel receptorderived cyclopeptides to treat heart failure caused by anti-1-adrenoceptor antibodies in a human-analogous rat model. PLOS ONE 10, e0117589. doi:10.1371/journal.pone.0117589
Bongartz, U., Hochmann, U., Grube, B., Uebelhack, R., Alt, F., Erlenbeck, C., et al. (2022). Flaxseed mucilage (IQP-LU-104) reduces body weight in overweight and moderately obese individuals in a 12-week, three-arm, double-blind, randomized, and placebo-controlled clinical study. Obes. Facts 15 (3), 395–404. doi:10.1159/000522082
Burr, M. L., Gilbert, J. F., Holliday, R. M., Elwood, P. C., Fehily, A. M., Rogers, S., et al. (1989). Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 334, 757–761. doi:10.1016/S0140-6736(89)90828-3
Caligiuri, S. P., Rodriguez-Leyva, D., Aukema, H. M., Ravandi, A., Weighell, W., Guzman, R., et al. (2016). Dietary flaxseed reduces central aortic blood pressure without cardiac involvement but through changes in plasma oxylipins. Hypertension 68, 1031–1038. doi:10.1161/HYPERTENSIONAHA.116.07834
Chai, J.-D., and Head-Gordon, M. (2008). Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620. doi:10.1039/B810189B
Chang, M., Li, X., Sun, Y., Cheng, F., Li, Y., Zhao, W., et al. (2013). A potential mechanism of a cationic cyclopeptide for enhancing insulin delivery across Caco-2 cell monolayers. Biol. Pharm. Bull. 36, 1602–1607. doi:10.1248/bpb.b13-00487
Chen, L., Zhang, Y. H., Wang, S., Zhang, Y., Huang, T., and Cai, Y. D. (2017). Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways. PLoS One 12 (9), e0184129. doi:10.1371/journal.pone.0184129
Dahl, W. J., Lockert, E. A., Cammer, A. L., and Whiting, S. J. (2005). Effects of flax fiber on laxation and glycemic response in healthy volunteers. J. Med. Food 8, 508–511. doi:10.1089/jmf.2005.8.508
Daina, A., Michielin, O., and Zoete, V. (2017). SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7, 42717–42730. doi:10.1038/srep42717
Deconinck, E., Ates, H., Callebaut, N., Van Gyseghem, E., and Vander Heyden, Y. (2007). Evaluation of chromatographic descriptors for the prediction of gastro-intestinal absorption of drugs. J. Chromatogr. A 1138, 190–202. doi:10.1016/j.chroma.2006.10.068
de Lorgeril, M., Renaud, S., Mamelle, N., Salen, P., Martin, J. L., Monjaud, I., et al. (1994). Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 343, 1454–1459. doi:10.1016/S0140-6736(94)92580-1
Demark-Wahnefried, W., Polascik, T. J., George, S. L., Switzer, B. R., Madden, J. F., Ruffin, M. T., et al. (2008). Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol. Biomarkers Prev. 17 (12), 3577–3587. doi:10.1158/1055-9965.EPI-08-0008
Deswal, R., Nanda, S., and Dang, A. S. (2019). Association of Luteinizing hormone and LH receptor gene polymorphism with susceptibility of Polycystic ovary syndrome. Syst. Biol. Reprod. Med. 65 (5), 400–408. doi:10.1080/19396368.2019.1595217
Drygała, P., Olejnik, J., Mazur, A., Kierus, K., Jankowski, S., Zimecki, M., et al. (2009). Synthesis and immunosuppressive activity of cyclolinopeptide A analogues containing homophenylalanine. Eur. J. Med. Chem. 44, 3731–3738. doi:10.1016/j.ejmech.2009.03.037
Duke, J. A., Bogenschutz-Godwin, M. J., du Cellier, M. J., and Duke, P. A. (2002). Catalog of herbs (Aˆa€“Z): flax, handbook of medicinal herbs. Boca Raton, FL, USA: CRC Press.
El-galil, M. M. A., and Mohammed, S. F. (2021). The possible effect of flaxseed extract on letrozole-induced polycystic ovary rat model. Correl. Histological Funct. Study 50 (4)–2021. doi:10.21608/amj.2021.196448
EMBL-EBI alphafold (2023). EMBL-EBI alphafold. https://www.alphafold.ebi.ac.uk/search/text/Pdcd6ip?suggested=true.
Fang, X. Y., Chen, W., Fan, J. T., Song, R., Wang, L., Gu, Y. H., et al. (2013). Plant cyclopeptide RA-V kills human breast cancer cells by inducing mitochondria-mediated apoptosis through blocking PDK1- AKT interaction. Toxicol. Appl. Pharmacol. 267, 95–103. doi:10.1016/j.taap.2012.12.010
Franck, M., Perreault, V., Suwal, S., Marciniak, A., Bazinet, L., and Doyen, A. (2019). High hydrostatic pressure-assisted enzymatic hydrolysis improved protein digestion of flaxseed protein isolate and generation of peptides with antioxidant activity. Food Res. Int. 115, 467–473. doi:10.1016/j.foodres.2018.10.034
Franks, S., and Hardy, K. (2020). What causes anovulation in polycystic ovary syndrome? Curr. Opin. Endocr. Metab. Res. 12, 59–65. doi:10.1016/j.coemr.2020.03.001
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2009). Gaussian 16 software suite. Wallingford CT: Gaussian, Inc.
Galaxy (2023). Galaxy | europe | Australia accessible history | protein-ligand HTMD simulation. https://cheminformatics.usegalaxy.eu/u/sbray/w/protein-ligand-htmd-sim.
Gonzalez, M., Sealls, W., Jesch, E. D., Brosnan, M. J., Ladunga, I., Ding, X., et al. (2011). Defining a relationship between dietary fatty acids and the cytochrome P450 system in a mouse model of fatty liver disease. Physiol. Genomics 43, 121–135. doi:10.1152/physiolgenomics.00209.2010
Goodarzi, M. O., and Azziz, R. (2006). Diagnosis, epidemiology, and genetics of the polycystic ovary syndrome. Best. Pract. Res. Clin. Endocrinol. Metab. 20 (2), 193–205. doi:10.1016/j.beem.2006.02.005
Górski, A., Kasprzycka, M., Nowaczyk, M., Wieczoreck, Z., Siemion, I. Z., Szelejewski, W., et al. (2001). Cyclolinopeptide: a novel immunosuppressive agent with potential anti-lipemic activity. Transpl. Proc. 33, 553. doi:10.1016/s0041-1345(00)02139-4
Goyal, A., Sharma, V., Upadhyay, N., Gill, S., Sihag, M., Upadhyay, N., et al. (2014). Flax and flaxseed oil: an ancient medicine & modern functional food. J. Food Sci. Technol. 51, 1633–1653. doi:10.1007/s13197-013-1247-9
Hajiahmadi, S., Nadjarzadeh, A., Gharipour, M., Hosseinzadeh, M., Fallahzadeh, H., Mohsenpour, M. A., et al. (2020). Effect of flaxseed oil on glycemic control and inflammatory markers in overweight adults with pre-diabetes: a double-blind randomized controlled clinical trial. J. Herb. Med. 24, 100387. doi:10.1016/j.hermed.2020.100387
Hasaniani, N., Rahimlou, M., Ahmadi, A. R., Khalifani, A. M., and Alizadeh, M. (2019). The effect of flaxseed enriched yogurt on the glycemic status and cardiovascular risk factors in patients with type 2 diabetes mellitus: randomized, open-labeled, controlled study. Clin. Nutr. Res. 8, 284–295. doi:10.7762/cnr.2019.8.4.284
Hu, C. Q., and Xu, J. C. (2012). “Amino acids and peptides,” in Introduction to natural products Chemistry. Editors R. Xu, Y. Ye, and W. Zhao (Boca Roton: CRC Press), 147–167.
Hutchins, A. M., Brown, B. D., Cunnane, S. C., Domitrovich, S. G., Adams, E. R., and Bobowiec, C. E. (2013). Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: a randomized study. Nutr. Res. 33, 367–375. doi:10.1016/j.nutres.2013.02.012
Iftkhar, S. de Sá, Velloso, A. G. C., Aljarf, J. P. L., Pires, R., Ascher, D. E. V., and cardioToxCSM, D. B. (2022). cardioToxCSM: a web server for predicting cardiotoxicity of small molecules. J. Chem. Inf. Model 62 (20), 4827–4836. doi:10.1021/acs.jcim.2c00822
Javidi, A., Mozaffari-Khosravi, H., Nadjarzadeh, A., Dehghani, A., and Eftekhari, M. H. (2016). The effect of flaxseed powder on insulin resistance indices and blood pressure in prediabetic individuals: a randomized controlled clinical trial. J. Res. Med. Sci. 21, 70. doi:10.4103/1735-1995.189660
Ji, D., Xu, M., Udenigwe, C. C., and Agyei, D. (2020). Physicochemical characterisation, molecular docking, and drug-likeness evaluation of hypotensive peptides encrypted in flaxseed proteome. Curr Res Food Sci. 3, 41–50. doi:10.1016/j.crfs.2020.03.001
Jiamset, I., and Hanprasertpong, J. (2016). Impact of diabetes mellitus on oncological outcomes after radical hysterectomy for early stage cervical cancer. J. Gynecol. Oncol. 27 (3), e28. doi:10.3802/jgo.2016.27.e28
Jiang, Z., You, L., Dou, W., Sun, T., and Xu, P. (2019). Effects of an electric field on the conformational transition of the protein: a molecular dynamics simulation study. Polym. (Basel) 11 (2), 282. doi:10.3390/polym11020282
Jovani, M., Liu, E. E., Paniagua, S. M., Lau, E. S., Li, S. X., Takvorian, K. S., et al. (2022). Cardiovascular disease related circulating biomarkers and cancer incidence and mortality: is there an association? Cardiovasc Res. 27 (10), 2317–2328. doi:10.1093/cvr/cvab282
Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. doi:10.1038/s41586-021-03819-2
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M., and Ishiguro-Watanabe, M. (2023). KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51 (D1), D587–D592. doi:10.1093/nar/gkac963
Khalesi, S., Irwin, C., and Schubert, M. (2015). Flaxseed consumption may reduce blood pressure: a systematic review and meta-analysis of controlled trials. J. Nutr. 145 (4), 758–765. doi:10.3945/jn.114.205302
Kulkarni, A. M., Kumar, V., Parate, S., Lee, G., Yoon, S., and Lee, K. W. (2022). Identification of new KRAS G12D inhibitors through computer-aided drug discovery methods. Int. J. Mol. Sci. 23, 1309. doi:10.3390/ijms23031309
Kumar, V., Kumar, R., Parate, S., Danishuddin, , Lee, G., Kwon, M., et al. (2023). Identification of activated cdc42-associated kinase inhibitors as potential anticancer agents using pharmacoinformatic approaches. Biomolecules 13, 217. doi:10.3390/biom13020217
Larsson, S. C., and Wolk, A. (2011). Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia 54, 1013–1018. doi:10.1007/s00125-011-2051-6
Li, R., Zhang, Q., Yang, D., Li, S., Lu, S., Wu, X., et al. (2013). Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum. Reprod. 28 (9), 2562–2569. doi:10.1093/humrep/det262
Lipinski, C. A., Lombardo, F., Dominy, B. W., Feeney, P. J., and Dominy, B. W. (1997). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25. doi:10.1016/s0169-409x(96)00423-1
Logarušić, M., Radošević, K., Bis, A., Panić, M., Slivac, I., and Srček, V. G. (2020). Biological potential of flaxseed protein hydrolysates obtained by different proteases. Plant Foods Hum. Nutr. 75 (4), 518–524. doi:10.1007/s11130-020-00841-z
Mani, U. V., Mani, I., Biswas, M., and Kumar, S. N. (2011). An open-label study on the effect of flax seed powder (Linum usitatissimum) supplementation in the management of diabetes mellitus. J. Diet. Suppl. 8, 257–265. doi:10.3109/19390211.2011.593615
Marambe, H. K., and Wanasundara, J. P. D. (2017). “Protein from flaxseed (Linum usitatissimum L.),” in Sustainable protein sources. Editors S. R. Nadathur, J. P. D. Wanasundara, and L. Scanlin (UK: Academics Press), 133–144. doi:10.1016/B978-0-12-802778-3.00008-1
Marenich, A. V., Cramer, C. J., and Truhlar, D. G. (2009). Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396. doi:10.1021/jp810292n
McCullough, R. S., Edel, A. L., Bassett, C. M., Lavallée, R. K., Dibrov, E., Blackwood, D. P., et al. (2011). The alpha linolenic acid content of flaxseed is associated with an induction of adipose leptin expression. Lipids 46 (11), 1043–1052. doi:10.1007/s11745-011-3619-0
Mehraban, M., Jelodar, G., and Rahmanifar, F. (2020). A combination of spearmint and flaxseed extract improved endocrine and histomorphology of ovary in experimental PCOS. J. Ovarian Res. 13 (1), 32–38. doi:10.1186/s13048-020-00633-8
Mohammadi-Sartang, M., Sohrabi, Z., Barati-Boldaji, R., Raeisi-Dehkordi, H., and Mazloom, Z. (2018). Flaxseed supplementation on glucose control and insulin sensitivity: a systematic review and meta-analysis of 25 randomized, placebo-controlled trials. Nutr. Rev. 76 (2), 125–139. doi:10.1093/nutrit/nux052
Molinspiration Chemoinformatics software (2023). Molinspiration chemoinformatics software. https://www.molinspiration.com (Accessed date July 15, 2023).
Moreira, F. D., Reis, C. E. G., Welker, A. F., and Gallassi, A. D. (2022). Acute flaxseed intake reduces postprandial glycemia in subjects with type 2 diabetes: a randomized crossover clinical trial. Nutrients 14 (18), 3736. doi:10.3390/nu14183736
Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., et al. (2009). AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 3016, 2785–2791. doi:10.1002/jcc.21256
Ndefo, U. A., Eaton, A., and Green, M. R. (2013). Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. P T 38 (6), 336–355. PMID: 23946629.
Nowak, D. A., Snyder, D. C., Brown, A. J., and Demark-Wahnefried, W. (2007). The effect of flaxseed supplementation on hormonal levels associated with Polycystic Ovary Syndrome: a case study. Curr. Top. Nutraceutical Res. 5 (4), 177–181. PMID: 19789727.
Nwachukwu, I. D., and Aluko, R. E. (2018a). Antioxidant properties of flaxseed protein hydrolysates: influence of hydrolytic enzyme concentration and peptide size. J. Am. Oil Chemists Soc. 95 (8), 1105–1118. doi:10.1002/aocs.12042
Nwachukwu, I. D., and Aluko, R. E. (2018b). Physicochemical and emulsification properties of flaxseed (Linum usitatissimum) albumin and globulin fractions. Food Chem. 255, 216–225. doi:10.1016/j.foodchem.2018.02.068
Osiris property explorer (2023). Osiris property explorer. www.organicchemistry.org/prog/peo/ (Accessed date- July 17, 2023).
Palla, A. H., Gilani, A. U., Bashir, S., and Ur Rehman, N. (2020). Multiple mechanisms of flaxseed: effectiveness in inflammatory bowel disease. Evid. Based Complement. Altern. Med. 2020, 1–16. doi:10.1155/2020/7974835
Palla, A. H., Iqbal, N. T., Minhas, K., and Gilani, A. H. (2016). Flaxseed extract exhibits mucosal protective effect in acetic acid induced colitis in mice by modulating cytokines, antioxidant and antiinflammatory mechanisms. Int. Immunopharmacol. 38, 153–166. doi:10.1016/j.intimp.2016.04.043
Pan, A., Sun, J., Chen, Y., Ye, X., Li, H., Yu, Z., et al. (2007). Effects of a flaxseed-derived lignan supplement in type 2 diabetic patients: a randomized, double-blind, cross-over trial. PLoS ONE 2, e1148. doi:10.1371/journal.pone.0001148
Parate, S., Kumar, V., Hong, J. C., and Lee, K. W. (2021). Putative dual inhibitors of mTOR and RET kinase from natural products: pharmacophore-based hierarchical virtual screening. J. Mol. Liq. 350 (2022), 118562. doi:10.1016/j.molliq.2022.118562
Parikh, M., Maddaford, T. G., Austria, J. A., Aliani, M., Netticadan, T., and Pierce, G. N. (2019). Dietary flaxseed as a strategy for improving human health. Nutrients 11, 1171. doi:10.3390/nu11051171
Perreault, V., Henaux, L., Bazinet, L., and Doyen, A. (2017). Pretreatment of flaxseed protein isolate by high hydrostatic pressure: impacts on protein structure, enzymatic hydrolysis and final hydrolysate antioxidant capacities. Food Chem. 221, 1805–1812. doi:10.1016/j.foodchem.2016.10.100
Poustforoosh, A., Faramarz, S., Negahdaripour, M., Tüzün, B., and Hashemipour, H. (2023). Tracing the pathways and mechanisms involved in the anti-breast cancer activity of glycyrrhizin using bioinformatics tools and computational methods. J. Biomol. Struct. Dyn., 1–15. doi:10.1080/07391102.2023.2196347
Prior, P. L., Ramos, A. C., Eserian, J. K., Zaparoli, J., and Galdurózc, J. C. F. (2015). Flaxseed oil decreases craving for chocolate: preliminary results. Int. Arch. Addict. Res. Med. 1, 010. doi:10.23937/2474-3631/1510010
Rahimi, R., Shams-Ardekani, M. R., and Abdollahi, M. (2010). A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J. Gastroenterology 16 (36), 4504. doi:10.3748/wjg.v16.i36.4504
Rhee, Y., and Brunt, A. (2011). Flaxseed supplementation improved insulin resistance in obese glucose intolerant people: a randomized crossover design. Nutr. J. 10, 44. doi:10.1186/1475-2891-10-44
Rodriguez-Leyva, D., Dupasquier, C. M., McCullough, R., and Pierce, G. N. (2010). The cardiovascular effects of flaxseed and its omega-3 fatty acid, alpha-linolenic acid. Can. J. Cardiol. 26 (9), 489–496. doi:10.1016/s0828-282x(10)70455-4
Sarosiek, I., Schicho, R., Blandon, P., and Bashashati, M. (2016). Urinary metabolites as noninvasive biomarkers of gastrointestinal diseases: a clinical review. World J. Gastrointest. Oncol. 8 (5), 459–465. doi:10.4251/wjgo.v8.i5.459
Schleinkofer, K., Wang, T., and Wade, R. C. (2006). Molecular docking. Encycl. Ref. Genomics Proteomics Mol. Med. 443, 1149–1153. doi:10.1007/3-540-29623-9-3820
Schrödinger, L., and DeLano, W. (2020). PyMOL. Accessed http://www.pymol.org/pymol.
Shayan, M., Kamalian, S., Sahebkar, A., and Tayarani-Najaran, Z. (2020). Flaxseed for health and disease: review of clinical trials. Comb. Chem. High. Throughput Screen 23, 699–722. doi:10.2174/1386207323666200521121708
String database V11.5 (2023). String database V11.5. https://stringdb.org/.
Szablewski, L. (2014). Diabetes mellitus: influences on cancer risk. Diabetes Metab. Res. Rev. 30, 543–553. doi:10.1002/dmrr.2573
Tang, Z. X. (2019a). Flaxseed fat-reducing yogurt nutrition bar and a preparation method thereof. CN 201910603833.X. doi:10.1590/fst.22021
Tang, Z. X. (2019b). Flaxseed yogurt for women and a making method thereof. CN 201910814791. doi:10.1590/fst.22021
The Gene Ontology Consortium Aleksander, S. A., Balhoff, J., Carbon, S., Cherry, J. M., Drabkin, H. J., et al. (2023). The gene ontology knowledgebase in 2023. Genetics 224 (1), iyad031. doi:10.1093/genetics/iyad031
Thevenard, J., Ramont, L., Devy, J., Brassart, B., Dupont-Deshorgue, A., Floquet, N., et al. (2010). The YSNSG cyclopeptide derived from tumstatin inhibits tumor angiogenesis by down-regulating endothelial cell migration. Int. J. Cancer 126, 1055–1066. doi:10.1002/ijc.24688
Torkan, M., Entezari, M. H., and Siavash, M. (2015). Effect of flaxseed on blood lipid level in hyperlipidemic patients. Rev. Recent Clin. Trials 10 (1), 61–67. doi:10.2174/1574887110666150121154334
Toulabi, T., Yarahmadi, M., Goudarzi, F., Ebrahimzadeh, F., Momenizadeh, A., and Yarahmadi, S. (2022). Effects of flaxseed on blood pressure, body mass index, and total cholesterol in hypertensive patients: a randomized clinical trial. Explore (NY) 18 (4), 438–445. doi:10.1016/j.explore.2021.05.003
Tseng, C. H., and Tseng, F. H. (2014). Diabetes and gastric cancer: the potential links. World J. Gastroenterol. 20 (7), 1701–1711. doi:10.3748/wjg.v20.i7.1701
Tziomalos, K., Athyros, V. G., Karagiannis, A., and Mikhailidis, D. P. (2008). Omega-3 fatty acids: how can they be used in secondary prevention? Curr. Atheroscler. Rep. 10, 510–517. doi:10.1007/s11883-008-0079-y
Udenigwe, C. C., Adebiyi, A. P., Doyen, A., Li, H., Bazinet, L., and Aluko, R. E. (2012). Low molecular weight flaxseed protein-derived arginine-containing peptides reduced blood pressure of spontaneously hypertensive rats faster than amino acid form of arginine and native flaxseed protein. Food Chem. 132 (1), 468–75. doi:10.1016/j.foodchem.2011.11.024
Villarreal-Renteria, A. I., Herrera-Echauri, D. D., Rodríguez-Rocha, N. P., Zuñiga, L. Y., Muñoz-Valle, J. F., García-Arellano, S., et al. (2022). Effect of flaxseed (Linum usitatissimum) supplementation on glycemic control and insulin resistance in prediabetes and type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 70, 102852. doi:10.1016/j.ctim.2022.102852
Vuksan, V., Choleva, L., Jovanovski, E., Jenkins, A. L., Au-Yeung, F., Dias, A. G., et al. (2017). Comparison of flax (Linum usitatissimum) and Salba-chia (Salvia hispanica L.) seeds on postprandial glycemia and satiety in healthy individuals: a randomized, controlled, crossover study. Eur. J. Clin. Nutr. 71, 234–238. doi:10.1038/ejcn.2016.148
Wang, T., Sha, L., Li, Y., Zhu, L., Wang, Z., Li, K., et al. (2020). Dietary α-linolenic acid-rich flaxseed oil exerts beneficial effects on polycystic ovary syndrome through sex steroid hormones—microbiota—inflammation Axis in rats. Front. Endocrinol. 11, 284. doi:10.3389/fendo.2020.00284
Wu, S., Wang, X., Qi, W., and Guo, Q. (2019). Bioactive protein/peptides of flaxseed: a review. Trends Food Sci. Technol. 92, 184–193. doi:10.1016/j.tifs.2019.08.017
Xavier, R. J., and Podolsky, D. K. (2007). Unravelling the pathogenesis of inflammatory bowel disease. Nature 448 (7152), 427–434. doi:10.1038/nature06005
Yang, J., Wen, C., Duan, Y., Deng, Q., Peng, D., Zhang, H., et al. (2021). The composition, extraction, analysis, bioactivities, bioavailability and applications in food system of flaxseed (Linum usitatissimum L.) oil: a review. Trends Food Sci. Technol. 118, 252–260. doi:10.1016/j.tifs.2021.09.025
Yari, Z., Cheraghpour, M., and Hekmatdoost, A. (2020). Flaxseed and/or hesperidin supplementation in metabolic syndrome: an openlabeled randomized controlled trial. Eur. J. Nutr. 60, 287–298. doi:10.1007/s00394-020-02246-9
Yu, R., Wang, J., Li, J., Wang, Y., Zhang, H., Chen, J., et al. (2010). A novel cyclopeptide from the cyclization of PACAP(1-5) with potent activity towards PAC1 attenuates STZ-induced diabetes. Peptides 31, 1062–1067. doi:10.1016/j.peptides.2010.03.008
Yumiceba, V., Lo´ pez-Corte´, s A., Pe´rez-Villa, A., Yumiseba, I., Guerrero, S., Garc´ıa-Ca´rdenas, J. M., et al. (2020). Oncology and pharmacogenomics insights in polycystic ovary syndrome: an integrative analysis. Front. Endocrinol. 11, 585130. doi:10.3389/fendo.2020.585130
Zhan, Y. S., Feng, L., Tang, S. H., Li, W. G., Xu, M., Liu, T. F., et al. (2010). Glucose metabolism disorders in cancer patients in a Chinese population. Med. Oncol. 27, 177–184. doi:10.1007/s12032-009-9189-9
Zhao, S., Tian, Y., Zhang, W., Xing, X., Li, T., Liu, H., et al. (2015). An association study between USP34 and polycystic ovary syndrome. J. Ovarian Res. 8, 30. doi:10.1186/s13048-015-0158-y
Zhao, Y., and Rahmouni, K. (2022). BBSome: a new player in hypertension and other cardiovascular risks. Hypertension 79 (2), 303–313. doi:10.1161/HYPERTENSIONAHA.121.17946
Keywords: flaxseed, hypertension, addictions, Docking, side effects
Citation: Joshi S and Srivastava R (2024) Tracing the pathways and mechanisms involved in medicinal uses of flaxseed with computational methods and bioinformatics tools. Front. Chem. 11:1276052. doi: 10.3389/fchem.2023.1276052
Received: 11 August 2023; Accepted: 12 December 2023;
Published: 12 January 2024.
Edited by:
Konda Reddy Karnati, Bowie State University, United StatesReviewed by:
Malay Kumar Rana, Indian Institute of Science Education and Research Berhampur (IISER), IndiaChandrabose Selvaraj, Alagappa University, India
Copyright © 2024 Joshi and Srivastava. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruby Srivastava, YW1pdHJ1YnkxQGdtYWlsLmNvbQ==
†ORCID: Ruby Srivastava, orcid.org/0000-0002-2367-0176
 Sravani Joshi
Sravani Joshi Ruby Srivastava
Ruby Srivastava