
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 20 July 2023
Sec. Organic Chemistry
Volume 11 - 2023 | https://doi.org/10.3389/fchem.2023.1233443
A series of 1,4-benzoxazin-3-one derivatives containing an acylhydrazone moiety were designed, synthesized and evaluated for their in vitro antifungal activities against Gibberella zeae, Pellicularia sasakii, Phytophthora infestans, Capsicum wilt, and Phytophthora capsica. The structures of target compounds were characterized by 1H NMR, 13H NMR, 19F NMR and HRMS. The preliminary antifungal evaluation of all target compounds showed that some target compounds possessed moderate to good activities against G. zeae, P. sasakii, P. infestans and C. wilt. Among them, compounds 5L and 5o exhibited noticeable inhibition effects against G. zeae with the EC50 values (effective concentration for 50% activity) of 20.06 and 23.17 μg/ml, respectively, which were even nearly double effective than that of hymexazol (40.51 μg/ml). Meanwhile, compound 5q displayed a notable inhibitory effect toward P. sasakii, with the EC50 value of 26.66 μg/ml, which was better than that of hymexazol (32.77 μg/ml). In addition, compound 5r yielded the EC50 value of 15.37 μg/ml against P. infestans, which was less than those of hymexazol (18.35 μg/ml) and carbendazim (34.41 μg/ml). Eventually, compound 5p showed higher inhibitory effect against C. wilt, with EC50 value of 26.76 μg/ml, which was better than that of hymexazol (>50 μg/ml).
Phytopathogenic fungi can invade plants and cause plant diseases, which not only bring about dramatic financial lose, but also can cause food safety problem because mycotoxins produced by some plant pathogenic fungi can threaten the health of humans and animals (Fisher et al., 2018). At present, one of the most efficient and immediate strategies to prevent plant diseases caused by phytopathogenic fungi is to use chemical fungicides. Meanwhile, the serious pesticide resistance, pesticide interaction and environmental pollution had been dramatically increased with the use of long-term and frequently traditional chemical fungicides (Yang et al., 2020). Therefore, there is a pressing need for the development of green fungicides with a new type of molecular scaffolds or new action mechanisms appears urgent necessary.
1,4-Benzoxazin-3-one, firstly reported in rye in the 1960 s (Hietala et al., 1960), possesses a pivotal scaffold structure in a large amount of nature products and pharmaceutical molecules as well as some useful building fragments in organic synthetic chemistry (Ylijoki and Kündig, 2011). Thus, 1,4-benzoxazin-3-one derivatives have attracted many interesting attentions of chemists and pharmacologist, which proved that the introduction of this bioactive framework may give rise to high potential biological activities such as herbicidal (Wang et al., 2021), antitumor (Yang T. et al., 2019), anticonvulsant (Piao et al., 2008), antioxidants (Sonia et al., 2014) and antibacterial (Konda et al., 2015) properties, and so on. Quinolone analogue is one of the classical antimicrobial agents containing the 1,4-benzoxazine ring in its structure. Recently, this pharmacophore core has been introduced in antifungal agents (Figure 1). For example, compound A1 is efficiently synthesized and strongly inhibited the growth of many different strains of phytopathogenic fungi (for example, F. culmorum, R. solani, P. betae, P. cactorum, B. cinerea and F. oxysporum) (Śmist et al., 2016). Compound A2 has been found good inhibitory activities against some strains of reported fungi, such as Saccharomyces cerevisiae (Gleńsk et al., 2016). Compound A3 possesses strong inhibitory activities against Candida albicans MTCC 3017, Candida albicans ATCC 90028 and Candida glabrata ATCC 90030 (Bollu et al., 2017). Compound A4 exhibits promising antifungal activities toward a wide spectrum of fungi, especially the synthetic compounds with benzyl groups on the nitrogen atom exhibited prominent antifungal activity (Zamani et al., 2021). Thus, the development of efficient and convenient synthetic strategy for bioactive backbone of 1,4-benzoxazin-3-one derived antifungal agents is of great interest.
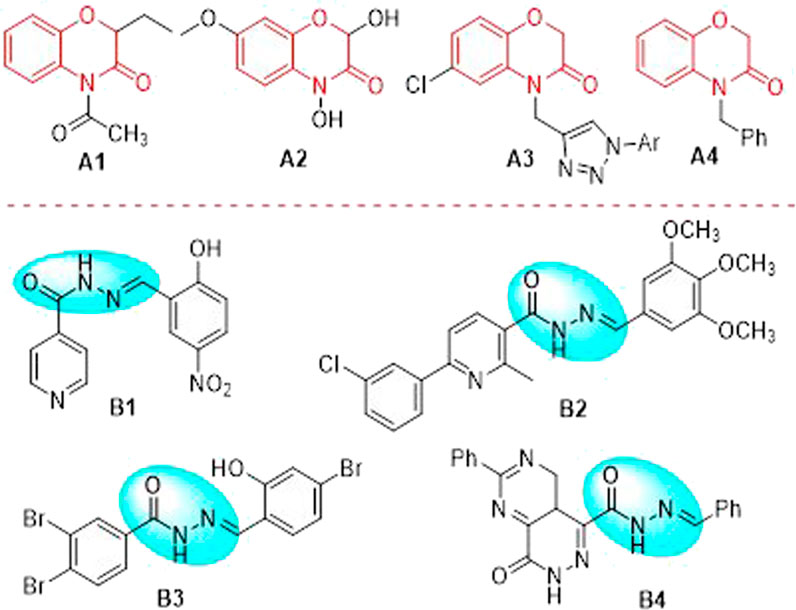
FIGURE 1. Some 1,4-benzoxazin-3-one derivatives and acylhydrazone derivatives selected with good antifungal activities.
Acylhydrazone (-CO-NH-N = CH-) core can be obtained by the condensation of a hydrazide with a ketone or an aldehyde, which is a particular type of Schiff base compound. As a result, acylhydrazone core has become one of the most extensively existed pharmacophores during the course of new drug research and creation due to their privileged structures and a large number of excellent bioactive properties, such as anticancer (Vilková et al., 2022), antiviral (Yang Z. et al., 2019), antibacterial (Zhou et al., 2018), anti-inflammatory (Hernández et al., 2012), antimalarial (Shaikh et al., 2021) and insecticidal (Ren et al., 2021) properties. Recently, it is worthy of attraction that some acylhydrazone derivatives have been highlighted as potential antifungal agents (Figure 1). For instance, compound B1 has been proved that it possesses potential antifungal activity against multiple Candida spp. (Backes et al., 2015). Compound B2 has displayed excellent activities against some fungi, such as Aspergillus fumigatus and Candida albicans, with minimum inhibitory concentration of 0.98 and 0.49 μg/ml, respectively, which is better than that of amphotericin B (Guilherme et al., 2019). Compound B3 has been demonstrated that it displayed a wide spectrum of antifungal activities against many clinically relevant fungal strains, such as C. albicans, C. krusei, C. krusei, C. parapsilosis, and A. fumigatus (Haranahalli et al., 2019). Compound B4 has exhibited excellent inhibition against P. brasiliensis and Candida spp. with minimum inhibitory concentration of 0.5 μg/ml (Rozada et al., 2020).
Thus, to improve the antifungal activities of 1,4-benzoxazin-3-one derivatives, we committed to introducing the bioactive acylhydrazone moiety to 1,4-benzoxazin-3-one skeleton, then, designing and synthesizing a serial of novel 1,4-benzoxazin-3-one derivatives with an active acylhydrazone moiety (Figure 2) and appraised for their in vitro antifungal activities against Gibberella zeae (G. zeae), Pellicularia sasakii (P. sasakii), Phytophthora infestans (P. infestans), Capsicum wilt (C. wilt) and Phytophthora capsica (P. capsica).
The methodology for the synthesis of the title compounds 5a–5s was presented in Scheme 1. The title compound 5 was obtained by the cyclization, substitution, hydrazinolysis and condensation reactions with 36%–53% yields over four steps. The structures of the title compound 5 were confirmed by 1H NMR, 13C NMR, 19F NMR and HRMS, and all analytical data were consistent with the assigned structures. The Supplementary Material contain the 1H NMR, 13C NMR, 19F NMR and HRMS spectra of the target compound 5 (Supplementary Figures S1–S66).
o-Amino phenol (3 g, 30 mmol), benzyl triethyl ammonium chloride (TEBA, 6.27 g, 30 mmol) and dichloromethane (20 ml) was added to a 100 ml reaction flask equipped with a magnetic stir bar. After the mixture was stirred for 10 min, sodium hydrogen carbonate (9.18 g, 108 mmol) was added step into mixture. The resultant mixture was allowed to stir at 0°C, and chloroacetyl chloride (3.66 g, 32.4 mmol) was added via syringe over a period of 15 min. Then, the reactant mixture was refluxed at 40°C for 6–8 h. After the reactants were consumed (TLC analysis), the resulting crude residue was poured into ice water (20 ml). The crude products were collected by filtration, washed with water, and recrystallized from methanol to obtain intermediate 2 (Benarjee et al., 2022).
The intermediate 2 (1.49 g, 10 mmol) and K2CO3 (1.38 g, 10 mmol) were added into a 100 ml reaction flask with 20 ml dimethylformamide (DMF). Then, ethyl bromoacetate (1.84 g, 11 mmol) was dropwise added via syringe and the reaction mixture was allowed to stir at rt for 12 h. After the reactants were consumed (TLC analysis), the resulting crude residue was extracted with ethyl acetate (3 × 20 ml). The organic layer was washed with water, dried over anhydrous Na2SO4, concentrated under reduced pressure to obtain intermediate 3 (Safakish et al., 2020).
Intermediate 3 was dissolved in 10 ml ethanol and heated at around 60°C about 10 min. Then, hydrazine hydrate (4 equiv.) was dropwise added via syringe to this mixture. The reaction mixture was refluxed for 6–8 h, and then allowed to pour into ice water. The crude residue was filtered to yield the intermediate 4 (Zhang et al., 2022).
Intermediate 4 was dissolved in 10 ml ethanol, and the solution was gently heated at around 60°C. Then, corresponding substituent aldehyde (1 equiv.) in 5 ml ethanol was dropwise added via syringe into this solution. The reaction mixture was refluxed for 2–4 h, and allowed to cool to room temperature. The crude residue was filtered and recrystallized from toluene to get the title compound 5.
The nuclear magnetic resonance (NMR) spectroscopy is a practical strategy to identify the cis-trans isomers in acylhydrazones (Lopes et al., 2013). The analysis of the 1H NMR spectra displayed that title compounds 5a–5s exist as Z/E isomers, and the ratio of Z/E isomers can be confirmed via 1H NMR based on the previously reported literature method (Palla, 1986). The chemical shift of 1H NMR spectrum of the title compounds 5a–5s show slightly narrow singlets for N–H group at 11.26–11.84 ppm. The characteristic singlets of imine hydrogens appear between 7.38 and 8.46 ppm with the cis-trans isomers, which control the Z/E isomers ratio of the title compound 5 synthesized from our approach is 3:1 according to previously reported literatures (Munir et al., 2021). The methylene moiety of 1,4-benzoxazin-3-one ring appeared at around 4.66 and 5.05 ppm also presents 3:1 Z/E isomers ratio, which is consistent with the Z/E isomers ratio of imine hydrogens. 13C NMR exhibited peaks at around 165.0 and 168.0 ppm for carbonyl groups of N-acylhydrazone and 1,4-benzoxazin-3-one ring portion. The signal of imine carbon is meant to appear at 142.8–145.1 ppm. For example, the signal of imine hydrogens of the title compound 5a appears at 8.06 and 8.24 ppm, with the Z/E isomers ratio of 3:1. The chemical shift of methylene moiety of 1,4-benzoxazin-3-one ring appeared at 5.08 and 4.67 ppm, also with the Z/E isomers ratio of 3:1. Finally, the ESI-HRMS spectrum revealed an obvious signal at 332.1006, which was assigned to the [M + Na]+ species of the target compound 5a.
All synthesized 1,4-benzoxazin-3-one derivatives in this work were screened for in vitro fungicidal activities against five types of plant pathogenic fungi including G. zeae, P. sasakii, P. infestans, C. wilt and P. capsica at the concentration of 50 μg/ml through the classical mycelium growth rate method according to the previously established procedure (see Supplementary Materials for full procedure details). Commercial agrochemicals hymexazol and carbendazim were used as positive fungicides against fungal strains. The obtained results are depicted in Table 1. The results of preliminary screening indicated that some of synthesized 1,4-benzoxazin-3-one derivatives also had moderate to good activities to inhibit the growth of G. zeae, P. sasakii, P. infestans and C. wilt fungal strains, but almost all the title compounds failed to exhibit effective inhibition effects against P. capsica in this work. Firstly, for G. zeae, compounds 5L and 5o exhibited excellent bioactive with the inhibitory rates 76.37% and 76.14%, respectively, which were superior to hymexazol (49.47%). Secondly, compounds 5L and 5q displayed stronger inhibitory activities against P. sasakii, with the inhibition rates of 64.38% and 73.32%, respectively, than that of hymexazol (60.70%). Meanwhile, compound 5r exhibited the excellent inhibitory activity against P. infestans, with the inhibition rate of 82.62%, which was better than hymexazol (72.86%) and carbendazim (58.57%). In addition, compound 5p had 71.33% inhibition rate against C. wilt, which was better than hymexazol (49.88%).
To further validate the antifungal activities of the title compounds, the EC50 values of some title compounds against G. zeae, P. sasakii, P. infestans, and C. wilt were tested and the result data were demonstrated in Table 2. Our results indicated that compounds 5L and 5o exhibited commendable in vitro antifungal activities against G. zeae, with the EC50 values of 20.06, and 23.17 μg/ml, respectively, which are nearly double effective than that of hymexazol (40.51 μg/ml). Meanwhile, compound 5q displayed outstanding inhibitory activity against P. sasakii, with the EC50 value of 26.66 μg/ml, respectively, which is better than hymexazol (32.77 μg/ml). Furthermore, compound 5r also had excellent inhibitory activity against P. infestans, with EC50 value of 15.37 μg/ml, which is double more efficient compared to that of carbendazim (34.41 μg/ml). Then, the EC50 values of compounds 5b and 5p against to C. wilt were calculated, just the EC50 of 5p (26.76 μg/ml) was slightly close to that of carbendazim (26.08 μg/ml).
For compounds 5a-5g (no substituents attached on 1,4-benzoxazin-3-one skeleton), only compound 5e showed better activity against P. infestans, with 3-Br attached aryl of acylhydrazone, the introduction of other substituents, including 4-F-Ph, 2-F-Ph, 3-F-Ph, 4-Br-Ph and 2-furan could not obviously affect the antifungal activities. Then, some of target compounds showed better activity against fungal strains, when introduction of methyl attached on 1,4-benzoxazin-3-one skeleton (for compounds 5h-5n), for example, compound 5L (3-Br attached aryl of acylhydrazone) exhibited bioactive with the inhibitory rates 76.37% and 64.38% against G. zeae and P. sasakii, respectively. However, target compounds (5o – 5t), with 6-Cl attached on 1,4-benzoxazin-3-one skeleton, exhibited better antifungal activity than other substituents attached on 1,4-benzoxazin-3-one skeleton, such as compound 5o, 5p, 5q and 5s were exhibited excellent bioactive with the inhibitory rates 76.14%, 71.33%, 73.32% and 82.62% against G. zeae, C. wilt, P. sasakii and P. infestans, with EC50 value of 23.17, 26.76, 26.66 and 15.37 μg/ml, respectively. Especially, compound 5s had the best activity against P. infestans fungal strain. The replacement of the benzene ring (5o) to alkyl such as ethyl (5t) group could not improve the antifungal activity.
In conclusion, a total of 19 original 1,4-benzoxazin-3-one derived compounds containing an acylhydrazone moiety were synthesized and estimated for their in vitro antifungal activities against five species of fungi. The preliminary antifungal evaluation of all the title compounds indicated that some of them had considerable activities against tested fungi. Among them, compounds 5L and 5o possessed commendable outstanding antifungal activity toward G. zeae with EC50 values of 20.06 and 23.17 μg/ml, respectively. Meanwhile, compound 5q showed outstanding inhibitory activity against P. sasakii, with EC50 value of 26.66 μg/ml. Additionally, compounds 5e and 5r had preeminent inhibitory activity against P. infestans, with EC50 values of 26.77 and 15.37 μg/ml, respectively. Our present work indicated that 1,4-benzoxazin-3-one derivatives with an acylhydrazone moiety could result in potential fungicide agents.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
WG and SY conducted most of the experiments in this work. WG and XH conducted the fungal activity test. XW contributed to some work in manuscript writing. CT conceptualized, directed the whole project and drafted the manuscript. All authors contributed to the article and approved the submitted version.
The work was performed under financial support by the Qiandongnan Science and Technology Plan Project (Qiandongnan kejichu [2021]17), the Youth Science and Technology Talent Growth Program of Guizhou Province’s Department of Education (Qian Jiaohe KY [2022]074) and the Qiandongnan Science and Technology Plan Project (Qiandongnan kehe J [2022]40).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2023.1233443/full#supplementary-material
Backes, G. L., Jursic, B. S., and Neumann, D. M. (2015). Potent antimicrobial agents against azole-resistant fungi based on pyridinohydrazide and hydrazomethylpyridine structural motifs. Bioorg. Med. Chem. 23 (13), 3397–3407. doi:10.1016/j.bmc.2015.04.040
Benarjee, V., Saritha, B., Hari Gangadhar, K., and Sailaja, B. B. V. (2022). Synthesis of some new 1,4-Benzoxazine-pyrazoles in water as EGFR targeting anticancer agents. J. Mol. Struct. 1265, 133188. doi:10.1016/j.molstruc.2022.133188
Bollu, R., Banu, S., Bantu, R., Reddy, A. G., Nagarapu, L., Sirisha, K., et al. (2017). Potential antimicrobial agents from triazole-functionalized 2H-Benzo[b] [1,4]oxazin-3(4H)-ones. Bioorg. Med. Chem. Lett. 27 (23), 5158–5162. doi:10.1016/j.bmcl.2017.10.061
Fisher, M. C., Hawkins, N. J., Sanglard, D., and Gurr, S. J. (2018). Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 360 (6390), 739–742. doi:10.1126/science.aap7999
Gleńsk, M., Gajda, B., Franiczek, R., Krzyżanowska, B., Biskup, I., and Włodarczyk, M. (2016). In vitro evaluation of the antioxidant and antimicrobial activity of DIMBOA [2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one]. Nat. Prod. Res. 30 (11), 1305–1308. doi:10.1080/14786419.2015.1054284
Guilherme, F. D., Simonetti, J. E., Folquitto, L. R. S., Reis, A. C. C., Oliver, J. C., Dias, A. L. T., et al. (2019). Synthesis, chemical characterization and antimicrobial activity of new acylhydrazones derived from carbohydrates. J. Mol. Struct. 1184, 349–356. doi:10.1016/j.molstruc.2019.02.045
Haranahalli, K., Lazzarini, C., Sun, Y., Zambito, J., Pathiranage, S., McCarthy, J. B., et al. (2019). SAR studies on aromatic acylhydrazone-based inhibitors of fungal sphingolipid synthesis as next-generation antifungal agents. J. Med. Chem. 62 (17), 8249–8273. doi:10.1021/acs.jmedchem.9b01004
Hernández, P., Cabrera, M., Lavaggi, M. L., Celano, L., Tiscornia, I., Rodrigues da Costa, T., et al. (2012). Discovery of new orally effective analgesic and anti-inflammatory hybrid furoxanyl N-acylhydrazone derivatives. Bioorg. Med. Chem. 20 (6), 2158–2171. doi:10.1016/j.bmc.2012.01.034
Hietala, P. K., Virtanen, A. I., Norén, B., Levitin, N. E., and Westin, G. (1960). Precursors of benzoxazolinone in rye plants. II. Precursor I, the glucoside. Acta Chem. Scand. 14, 502–504. doi:10.3891/acta.chem.scand.14-0502
Konda, S., Raparthi, S., Bhaskar, K., Munaganti, R. K., Guguloth, V., Nagarapu, L., et al. (2015). Synthesis and antimicrobial activity of novel benzoxazine sulfonamide derivatives. Bioorg. Med. Chem. Lett. 25 (7), 1643–1646. doi:10.1016/j.bmcl.2015.01.026
Lopes, A. B., Miguez, E., Kummerle, A. E., Rumjanek, V. M., Fraga, C. A., and Barreiro, E. J. (2013). Characterization of amide bond conformers for a novel heterocyclic template of N-acylhydrazone derivatives. Molecules 18 (10), 11683–11704. doi:10.3390/molecules181011683
Munir, R., Javid, N., Zia-Ur-Rehman, M., Zaheer, M., Huma, R., Roohi, A., et al. (2021). Synthesis of novel N-acylhydrazones and their C-N/N-N bond conformational characterization by NMR spectroscopy. Molecules 26 (16), 4908. doi:10.3390/molecules26164908
Palla, G., Predieri, G., Domiano, P., Vignali, C., and Turner, W. (1986). Conformational behaviour and E/Z isomerization of N-acyl and N-aroylhydrazones. Tetrahedron 42 (13), 3649–3654. doi:10.1016/S0040-4020(01)87332-4
Piao, Z.-T., Guan, L.-P., Zhao, L.-M., Piao, H.-R., and Quan, Z.-S. (2008). Synthesis of novel 7-benzylamino-2H-1,4-benzoxazin-3(4H)-ones as anticonvulsant agents. Eur. J. Med. Chem. 43 (6), 1216–1221. doi:10.1016/j.ejmech.2007.08.006
Ren, Z., Lv, M., Sun, Z., Li, T., Zhang, S., and Xu, H. (2021). Regioselective hemisynthesis and insecticidal activity of C8-Hydrazones/acylhydrazones/sulfonylhydrazones coumarin-type derivatives of osthole. Bioorg. Med. Chem. Lett. 40, 127962. doi:10.1016/j.bmcl.2021.127962
Rozada, A. M. F., Rodrigues-Vendramini, F. A. V., Goncalves, D. S., Rosa, F. A., Basso, E. A., Seixas, F. A. V., et al. (2020). Synthesis and antifungal activity of new hybrids pyrimido[4,5-d]pyridazinone-N-acylhydrazones. Bioorg. Med. Chem. Lett. 30 (14), 127244. doi:10.1016/j.bmcl.2020.127244
Safakish, M., Hajimahdi, Z., Vahabpour, R., Zabihollahi, R., and Zarghi, A. (2020). Novel benzoxazin-3-one derivatives: Design, synthesis, molecular modeling, anti-HIV-1 and integrase inhibitory assay. Med. Chem. 16 (7), 938–946. doi:10.2174/1573406415666190826161123
Shaikh, I., Jadeja, R. N., Patel, R., Mevada, V., and Gupta, V. K. (2021). 4-Acylhydrazone-5-Pyrazolones and their zinc(II) metal complexes: Synthesis, characterization, crystal feature and antimalarial activity. J. Mol. Struct. 1232, 130051. doi:10.1016/j.molstruc.2021.130051
Śmist, M., Kwiecień, H., and Krawczyk, M. (2016). Synthesis and antifungal activity of 2H-1,4-benzoxazin-3(4H)-one derivatives. J. Environ. Sci. Health, Part B 51 (6), 393–401. doi:10.1080/03601234.2016.1142744
Sonia, G., Thachil, K. K., Parameswaran, M. K., and Kochupappy, R. T. (2014). Synthesis of some benzoxazinyl pyrazolone arylidenes as potent antimicrobials and antioxidants. Med. Chem. Res. 23 (3), 1320–1326. doi:10.1007/s00044-013-0719-9
Vilková, M., Hudáˇcová, M., Palušeková, N., Jendželovský, R., Fedoroˇcko, P., Kožurková, M., et al. (2022). Acridine based N-acylhydrazone derivatives as potential anticancer agents: Synthesis, characterization and ctDNA/HSA spectroscopic binding properties. Molecules 27 (9), 2883. doi:10.3390/molecules27092883
Wang, D.-W., Zhang, H., Yu, S.-Y., Zhang, R.-B., Liang, L., Wang, X., et al. (2021). Discovery of a potent thieno[2,3-d]pyrimidine-2,4-dione-based protoporphyrinogen IX oxidase inhibitor through an in silico structure-guided optimization approach. J. Agric. Food Chem. 69 (47), 14115–14125. doi:10.1021/acs.jafc.1c05665
Yang, J., Guan, A., Li, Z., Zhang, P., and Liu, C. (2020). Design, synthesis, and structure–activity relationship of novel spiropyrimidinamines as fungicides against pseudoperonospora cubensis. J. Agric. Food Chem. 68 (24), 6485–6492. doi:10.1021/acs.jafc.9b07055
Yang, T., Shi, X., Guo, L., Gu, S., Zhang, W., Xu, G., et al. (2019). Design, synthesis, and antitumor activity of novel paeonol derivatives containing the 1,4-benzoxazinone and 1,2,3-triazole moieties. J. Chem. Res. 43 (7-8), 241–247. doi:10.1177/1747519819857479
Yang, Z. B., Li, P., and He, Y. J. (2019). Design, synthesis, and bioactivity evaluation of novel isoxazole-amide derivatives containing an acylhydrazone moiety as new active antiviral agents. Molecules 24 (20), 3766. doi:10.3390/molecules24203766
Ylijoki, K. E. O., and Kündig, E. P. (2011). The preparation of 2H-1,4-Benzoxazin-3-(4H)-ones viapalladium-catalyzed intramolecular C–O bond formation. Chem. Commun. 47 (38), 10608–10610. doi:10.1039/C1CC14209G
Zamani, L., Khabnadideh, S., Zomorodian, K., Sakhteman, A., Gholami, A., Rezaei, Z., et al. (2021). Docking, synthesis, antifungal and cytotoxic activities of some novel substituted 4H-Benzoxazin-3-one. Polycycl. Aromat. Compd. 41 (2), 347–367. doi:10.1080/10406638.2019.1584575
Zhang, J., Yang, R., Li, L., Liu, J., Liu, Y., Song, H., et al. (2022). Design, synthesis and bioactivity study of novel tryptophan derivatives containing azepine and acylhydrazone moieties. Molecules 27 (19), 6700. doi:10.3390/molecules27196700
Keywords: 1,4-benzoxazin-3-one, acylhydrazone, phytopathogenic fungi, antifungal activity, median effective concentration
Citation: Tang C, Guo W, Yang S, Hu X, Chen X and Wang X (2023) Design, synthesis and antifungal activity of novel 1,4-benzoxazin-3-one derivatives containing an acylhydrazone moiety. Front. Chem. 11:1233443. doi: 10.3389/fchem.2023.1233443
Received: 02 June 2023; Accepted: 04 July 2023;
Published: 20 July 2023.
Edited by:
Jiwen Zhang, Northwest A&F University, ChinaReviewed by:
Rajendra Rohokale, University of Florida, United StatesCopyright © 2023 Tang, Guo, Yang, Hu, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenghao Tang, Y2h0YW5nMTEyMkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.