
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 22 May 2023
Sec. Medicinal and Pharmaceutical Chemistry
Volume 11 - 2023 | https://doi.org/10.3389/fchem.2023.1200490
This article is part of the Research Topic Computational Drug Discovery of Medicinal Compounds for Cancer Management View all 16 articles
 Libo Hua1
Libo Hua1 Farah Anjum2*
Farah Anjum2* Alaa Shafie2
Alaa Shafie2 Amal Adnan Ashour3
Amal Adnan Ashour3 Abdulraheem Ali Almalki2
Abdulraheem Ali Almalki2 Ali Abdullah Alqarni3
Ali Abdullah Alqarni3 Hamsa Jameel Banjer2
Hamsa Jameel Banjer2 Sarah Abdullah Almaghrabi4,5
Sarah Abdullah Almaghrabi4,5 Shan He6,7,8
Shan He6,7,8 Nenggui Xu1*
Nenggui Xu1*Glycogen synthase kinase-3 (GSK3β), a serine/threonine protein kinase, has been discovered as a novel target for anticancer drugs. Although GSK3β is involved in multiple pathways linked to the etiology of various cancers, no specific GSK3β inhibitor has been authorized for cancer therapy. Most of its inhibitors have toxicity effects therefore, there is a need to develop safe and more potent inhibitors. In this study, a library of 4,222 anti-cancer compounds underwent rigorous computational screening to identify potential candidates for targeting the binding pocket of GSK3β. The screening process involved various stages, including docking-based virtual screening, physicochemical and ADMET analysis, and molecular dynamics simulations. Ultimately, two hit compounds, BMS-754807 and GSK429286A, were identified as having high binding affinities to GSK3β. BMS-754807 and GSK429286A exhibited binding affinities of −11.9, and −9.8 kcal/mol, respectively, which were greater than that of the positive control (−7.6 kcal/mol). Further, molecular dynamics simulations for 100 ns were employed to optimize the interaction between the compounds and GSK3β, and the simulations demonstrated that the interaction was stable and consistent throughout the study. These hits were also anticipated to have good drug-like properties. Finally, this study suggests that BMS-754807 and GSK429286A may undergo experimental validation to evaluate their potential as cancer treatments in clinical settings.
Cancer, a hyperproliferative condition, is characterized by excessive cell division and, eventually, metastasis. Protein kinases are essential regulators of many biological processes and are targets for a wide range of human disorders (Greten and Grivennikov, 2019). Glycogen synthase kinase-3 (GSK3β), a serine/threonine protein kinase, has been discovered as a novel target for anticancer drugs. It was originally thought to be the most significant enzyme involved in the metabolism of glycogen, but it is now largely recognized as a regulator of various cellular processes, such as the activity of several metabolic and signaling proteins when it phosphorylates (Cohen and Frame, 2001; Dickey et al., 2011). It promotes tumor cell survival in several cancers by using different pro-survival pathways regulated by NF-κB (Zhang et al., 2014; Saud et al., 2016), Hh/Gli (Trnski et al., 2015), mTOR (Pal et al., 2014), and STAT3 (Gao et al., 2017). GSK3β inhibitors are now being used to treat a variety of conditions, including Alzheimer’s disease, diabetes, and cancer (Klamer et al., 2010; Medina and Avila, 2010; Zeng et al., 2014). Several inhibitors of GSK3β have been developed and progressed to early-stage clinical trials for various types of cancer (Sahin et al., 2019). One such inhibitor is Tideglusib, which was initially developed to target tau phosphorylation in Alzheimer’s disease. However, studies have shown that it can increase proapoptotic proteins in murine models of human neuroblastoma, indicating its potential efficacy in cancer treatment (Mathuram et al., 2016). Another GSK3β inhibitor, LY2090314, has demonstrated antiproliferative properties in preclinical studies involving melanoma and neuroblastoma. This ATP-competitive inhibitor has shown promising results in clinical trials for cancer treatment (Palomo and Martinez, 2017; Kunnimalaiyaan et al., 2018). Additionally, Solasodine, a naturally occurring aglycone of glycoalkaloid, has been shown to inhibit the GSK-3 pathway and induce apoptosis in various types of malignancies (Zhuang et al., 2017). Although GSK3β is involved in multiple pathways linked to the etiology of various cancers, no specific GSK3β inhibitor has been authorized for cancer therapy.
GSK3β is a 433-residue protein with three different structural domains. The first 134 residues form a 7-strand beta-barrel in the N-terminal domain. Residues 135–151 form a brief linker connecting the N-terminal domain to the alpha-helical domain. Residues 152–342 comprise the alpha-helical domain, and residues 343–433 make up the C-terminal domain. The ATP-binding site is located between the N-terminal and alpha-helical domains (Jacobs et al., 2012).
Drug development is a multidisciplinary, costly, and time-taking process. Computer-assisted drug discovery (CADD) is a constructive approach to drug development that hastens the process and decreases expenses. By reducing the need for animal models in pharmacological research and aiding in the rational design of safe drug candidates, CADD supports medicinal chemists and pharmacologists throughout drug discovery (Paul et al., 2021). The use of CADD has proven crucial to several projects across a range of contexts and research environments. CADD has played a substantial role in the identification and optimization of successful compounds that have moved to further stages of the drug development pipeline or commercialization (Talele et al., 2010). CADD has limitations due to the accuracy of computational models, the scarcity of structural data, the limited chemical diversity, the complexity of drug targets, and the lack of experimental validation (Sliwoski et al., 2014).
To identify possible candidates for targeting the binding pocket of GSK3β, we employed computational approaches to screening a diverse library of therapeutically active potential candidates for targeting the binding pocket of GSK3β.
The GSK3β protein (PDB: 4AFJ) was retrieved from the PDB database (Gentile et al., 2012). Heteroatoms, water molecules, and co-crystallized ligands were extracted, and the protein was saved in.pdb format. The heteroatoms were proto-oncogene frat, a 30 amino acid short peptide, SO4, GOL, and SJJ. The clean protein was finally prepared using Discovery Studio 2021 for further studies.
The process of identifying new compounds with specific bioactivity has been transformed by virtual screening methods, which use computer simulations to assess large structure libraries against a biological target (Macalino et al., 2015). This study employed a library of 4,222 anti-cancer compounds (retrieved from https://www.selleckchem.com), including both FDA-approved drugs and naturally occurring substances. The collection included compounds that were cell-permeable, therapeutically active, and diverse in terms of their chemical structure. The compound library was downloaded in ‘sdf’ format and then processed in ‘PyRx 0.8’ program (Dallakyan and Olson, 2015). The compounds library was minimized utilizing ‘UFF’ force field as the energy minimization parameter and finally saved in the ‘pdbqt’ format for further analysis. The grid coordinates of the GSK3β were set as X = 104.048, Y = 26.822, and Z = −12.474. The best hits were carefully chosen based on the binding affinity and interaction analysis toward GSK3β.
The DataWarrior tool was used to predict the drug-likeness and physicochemical properties of the top ten compounds, and the ProTox-II (Banerjee et al., 2018) and pkCSM web servers (Pires et al., 2015) were used for ADMET and pharmacokinetic properties.
The GROMACS 2021.4 software and GROMOS96 43a1 force-field were used to perform MD simulations of three complexes: GSK3β-control, GSK3β-BMS-754807, and GSK3β-GSK429286A, all at 300 K (Pol-Fachin et al., 2009). The topology and force-field factors of the compounds were generated using the PRODRG server, and their atoms were combined in the complex topology files. Na+ and Cl− ions were introduced to neutralize the charges on the GSK3β protein complexes using the ‘gmx_genion’ module (0.15 M) (Schuttelkopf and van Aalten, 2004). The ‘particle-mesh Ewald’ method was employed to analyze the interactions of GSK3β with these selected compounds. The system (for MD simulation) was minimized employing the ‘steepest descent’ method (1,500 steps) and equilibrated over a 100-ps period at constant volume in two stages: NVT and NPT ensembles. The total simulation of 100 ns was conducted at 300 K. Trajectories were analyzed using GROMACS modules, and 3D models were created using VMD (Humphrey et al., 1996) and PyMOL (Yuan et al., 2017).
GSK3β dysfunction has been reported in various cancer types (Domoto et al., 2020), and has been identified as being at the crossroads of various biochemical pathways, including cancer-related pathways (Duda et al., 2020). Here in this study, a unique collection of 4,222 anti-cancer compounds were screened against the active pocket of GSK3β. Based on binding affinity, we chose the top ten screened compounds for further visual inspection and interaction studies (Table 1).
The drug-likeness and physicochemical attributes of these top 10 compounds were anticipated using the DataWarrior tool, which employs several parameters including LogP, LogS, H-bond donors and acceptors, relative PSA, and the presence of structures with particular pharmacological properties. Both GSK429286A and BMS-754807 retain an adequate range of drug-likeness properties (Table 2). GSK429286A was discovered to be a selective inhibitor of Rho-associated coiled-coil protein kinase 1 (ROCK1) and ROCK2. These kinases are involved in a several cellular activities, which include cell motility, contraction, and adhesion, and have been associated to cancer, cardiovascular disease, and neurological disorders (Kim et al., 2021). BMS-754807 efficiently and irreversibly inhibits both insulin receptor (IR) family kinases and the insulin-like growth factor 1 receptor (IGF-1R). These kinases have a high binding affinity for it (Ki, 2 nmol/L), which is important for controlling cell growth, survival, and metabolism. BMS-754807 is now in phase I clinical trials for the treatment of multiple types of human cancer (Carboni et al., 2009).
The robust stability of the hit compounds within the GSK3β active site was attributed to the presence of various Van der Waals and H-bonding interactions (Table 3). These interactions aided in the intercalation of the compounds into the active site, increasing their binding affinity and overall stability (Figures 1A–E). BMS-754807 interacted with several residues like Ile62, Gly63, Asn64, Gly65, Val70, Ala83, Lys85, Leu132, Tyr134, Val135, Pro136, Glu137, Thr138, Arg141, Lys183, Gln185, Asn186, Leu188, Cys199, and Asp200 and residues of GSK3β. The Van der Waals interaction involves a set of amino acid residues, namely, Gly63, Asn64, Gly65, Ala83, Lys85, Leu132, Tyr134, Val135, Pro136, Glu137, Thr138, Arg141, Lys183, Leu188, Cys199, and Asp200; while Gln185 and Asn186 residues were involved in H-bonding (Figure 1C). Further, GSK429286A interacted with Ile62, Gly63, Asn64, Ala83, Lys85, Glu97, Val110, Leu132, Tyr134, Val135, Pro136, Thr138, Arg141, Val70, Lys183, Gln185, Asn186, Leu188, Cys199, Asp200, and Phe201 residues of GSK3β. Gly63, Val70, Ala83, Lys85, Glu97, Val110, Leu132, Val135, Thr138, Lys183, Asn186, Leu188, and Phe201 residues participates in Van der Waals interactions; while Lys85, Pro136, and Asp200 residues were involved in H-bonding (Figure 1D). Several amino acid residues of the GSK3β have alrady been described to play an essential role in inhibitor binding. These residues include Ile62, Gly63, Asn64, Val70, Ala83, Lys85, Glu97, Leu132, Tyr134, Val135, Leu188, Cys199, Asp200, and Phe201 (Mishra et al., 2019). It is noteworthy that the compounds (BMS-754807, and GSK429286A) have been observed to bind with these GSK3β residues.
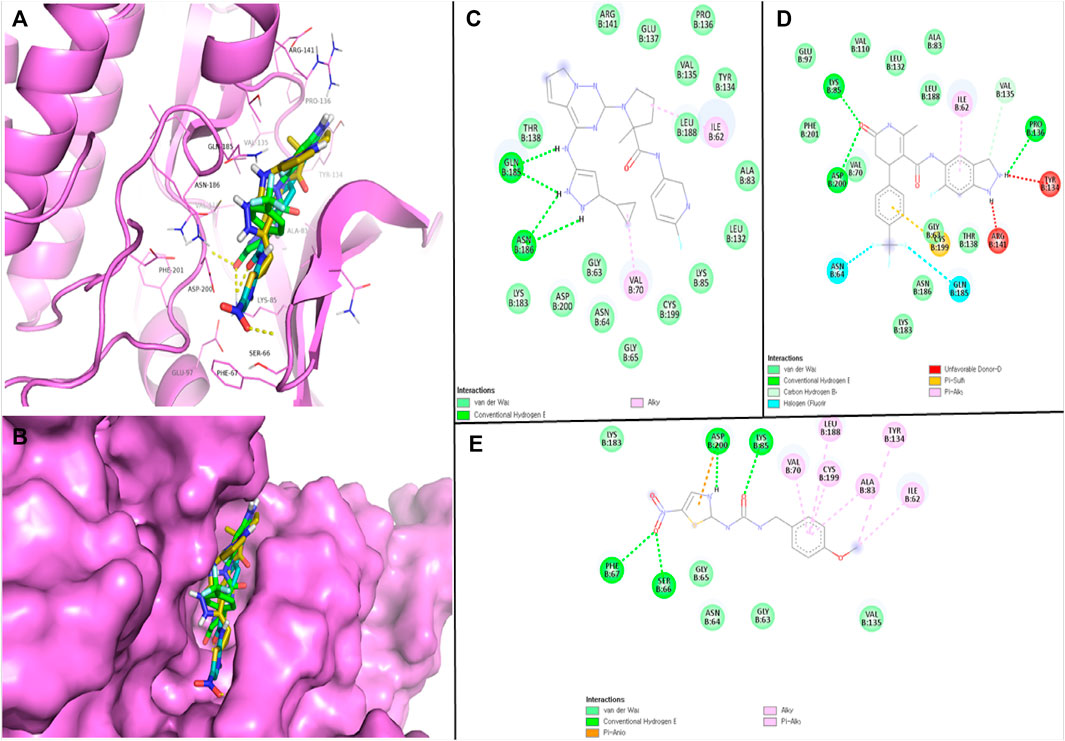
FIGURE 1. Visualization of hits and positive control in the GSK3β binding pocket (A, B); 2D view of GSK3β residues interacting with BMS-754807 (C), GSK429286A (D), and AR-AO-14418 (E).
N-(4-methoxybenzyl)-N0-(5-nitro-1,3-thiazol-2-yl) urea (AR-AO-14418) is a selective inhibitor of GSK3β (Bhat et al., 2003), and was used as a positive control in this study. In vitro, ARA014418 inhibited GSK3β without significantly inhibiting other kinases, reducing tau phosphorylation at Ser-396. In vivo, it induced antidepressant-like effects in rats by decreasing immobility time and both spontaneous and amphetamine-induced activity (Gould et al., 2004). AR-AO-14418 interacted with Ile62, Gly63, Asn64, Gly65, Ser66, Phe67, Val70, Ala83, Lys85, Tyr134, Val135, Lys183, Leu188, Cys199, and Asp200 residues of GSK3β (Figure 1E). Interestingly, Ile62, Gly63, Asn64, Val70, Ala83, Lys85, Tyr134, Val135, Lys183, Leu188, Cys199, and Asp200 GSK3β residues were observed to be common interacting residues with both the hits (BMS-754807, and GSK429286A) as well as the AR-AO-14418 (Figure 1C-1E). In addition, Lys85, and Asp200 were the common H-bonded residues with GSK429286A and the AR-AO-14418.
BMS-754807 oral toxicity prediction results were as follows: pLD50: 500 mg/kg, pToxicity Class: 4, average similarity: 39.97%, pAccuracy: 23%; while GSK429286A oral toxicity prediction results were as follows: pLD50: 50 mg/kg, pToxicity Class: 2, Average similarity: 45.44%, pAccuracy: 54.26%. BMS-754807 falls into the category of ‘harmful if swallowed’ while GSK429286A falls into the category of ‘fatal if swallowed’ as its LD50 value is greater than that of BMS-754807. In addition, multiple toxicity endpoints, including acute toxicity, hepatotoxicity, cytotoxicity, carcinogenicity, mutagenicity, immunotoxicity, etc., were within the acceptable range for these two compounds (Figure 2). Further, multiple pharmacokinetic properties including ADMET of both GSK429286A and BMS-754807 were predicted by the pkCSM web server, which results that both compounds are satisfactorily appropriate in several parameters of ADMET (Table 4).
MD simulation studies were carried out to evaluate complex stability. Protein stability can be measured using the root mean square deviation (RMSD), where a lower RMSD value indicates a more stable protein structure. GSK3β-control, GSK3β-BMS-754807, and GSK3β-GSK429286A had RMSD average values of 0.42, 0.31, and 0.35 nm, respectively. The RMSD plot revealed that GSK3β-BMS-754807 and GSK3β-GSK429286A complex showed more binding stability than the control compound. The ‘GSK3β-control’ complex showed high deviation from its original conformation, it showed that the active site pocket of GSK3β formed quite stable interaction with both of the selected compounds. In addition, the ligand RMSD exhibits GSK3β-BMS-754807 and GSK3β-GSK429286A high deviation, and interestingly, the GSK3β-control complex showed low deviation (Figure 3A-B).
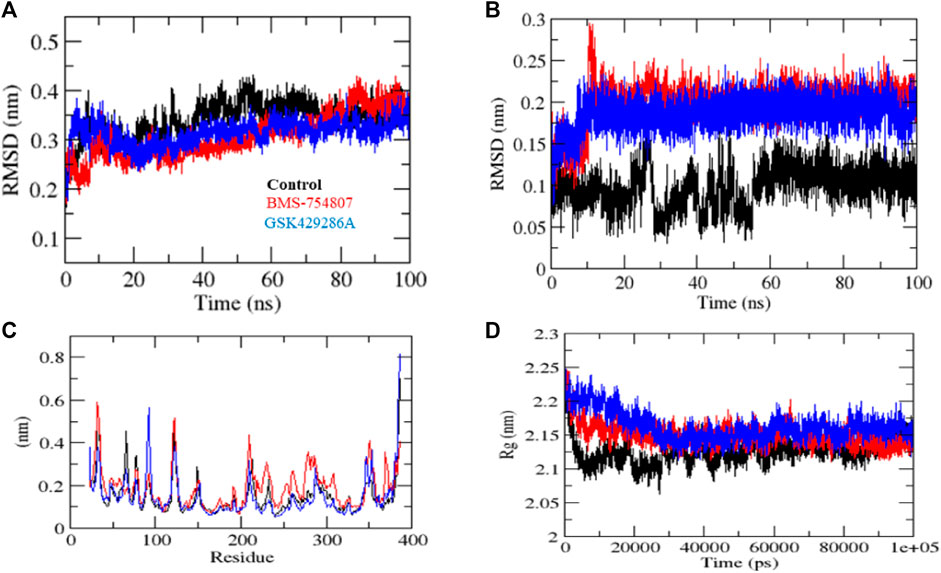
FIGURE 3. MD simulation studies of complexes. RMSD plot (A), RMSD plot of ligands (B), RMSF plot (C), and Rg plot (D) of GSK3β protein with the ligands.
The fluctuation of each residue during the simulation was averaged, and the root mean square fluctuation (RMSF) of GSK3β was calculated while binding to GSK3β-control, GSK3β-BMS-754807, and GSK3β-GSK429286A. These values were plotted against the residue numbers of GSK3β. The GSK3β-control and GSK3β-GSK429286A backbones presented steady fluctuations in the catalytic pocket of GSK3β, presumably due to different orientations and the GSK3β-BMS-754807 complex indicated high fluctuation in region 230–260 residues (Figure 3C). On the other hand, GSK3β-control, and GSK3β-GSK429286A complexes showed the least overall fluctuations.
By measuring the distribution of atoms around the axis of a protein, the radius of gyration (Rg) provides insight into the compactness profile of a complex in a biological system. The GSK3β-control, GSK3β-BMS-754807 and GSK3β-GSK429286A complexes had average Rg values of 2.13, 2.15, and 2.17 nm, respectively. Rg plot showed lesser compactness in GSK3β-control and GSK3β-BMS-754807 than GSK3β-GSK429286A complexes. It contingent that after binding, these compounds make GSK3β stable, due to GSK3β showing less Rg trajectories (Figure 3D). Among both compounds, BMS-754807 showed better stability in the catalytic pocket of GSK3β.
The Solvent-accessible surface area (SASA) of a protein refers to the portion of its surface area that interacts with its surrounding solvent molecules. The average SASA values for GSK3β-control, GSK3β-BMS-754807, and GSK3β-GSK429286A complexes were plotted during the 100 ns simulation. The SASA values for the GSK3β-control, GSK3β-BMS-754807, and GSK3β-GSK429286A complexes were 170.51, 178.10, and 180.42 nm2, respectively (Figure 4A). Further, GSK3β-control and GSK3β-BMS-754807 overlapped each other in 2D projection analysis, whereas GSK3β-GSK429286A showed a different pattern (Figure 4B). SASA exploration indicated that upon binding of control, BMS-754807, surface exposure has been reduced and the GSK429286A compound increases the surface area of solvent accessibility. Further, hydrogen bond analysis was performed of the docked complexes. To evaluate the stability of the docked complexes, 100 ns simulations of GSK3β-control, GSK3β-BMS-754807, and GSK3β-GSK429286A were conducted in the presence of a solvent environment. The control and BMS-754807 compound showed an average 2–7 H-bond with GSK3β protein whereas, the GSK429286A compound showed 2–6 H-bond. It inferred that the BMS-754807 compound showed more stable interaction and might work as a potential drug against the GSK3β protein (Figure 4C-E).
Next, Mean square displacement (MSD) was determined. It found that the GSK3β-BMS-754807 complex had a higher displacement than the control and the GSK3β-GSK429286A complex (Figure 5A). The Gibbs’ free energy (GFE) landscape was computed using GROMACS analysis modules, and the first (PC1) and second (PC2) eigenvectors were projected to generate a Comparable GFE contour map, where darker blue shades indicate lower energy levels. During the simulations, the global minima of GSK3β fluctuated due to the binding of the complexes to the GSK3β protein. The GSK3β-control and GSK3β-GSK429286A were showing similar projections; and GSK3β-BMS-754807 was showing dissimilar global minima, demonstrating that BMS-754807 global minima drastically changed during the simulation (Figure 5B-D). The above results suggested that the BMS-754807 compound might be used as a possible drug for the GSK3β protein.
Several small-molecule inhibitors of GSK3β, such as CHIR-99021, CHIR-98014, SB216763, SB415286, AR-A011418, CG701338, and CG202796, have been utilized in preclinical studies involving cell and animal models to investigate the potential involvement of GSK3β in cancer pathogenesis (Walz et al., 2017). These compounds, however, are primarily classified as “toolkit compounds” due to a lack of adequate ADMET properties required for advancement as drug candidates to clinical trials. To date, clinical trials on GSK3β inhibitors, including tideglusib and LY2090314, have demonstrated their tolerability, indicating that concerns about GSK3β inhibition causing widespread metabolic toxicity were not justified. LY2090314 has a suboptimal pharmacokinetic profile, so the lack of toxicity observed could be attributed to inadequate systemic exposure (Zamek-Gliszczynski et al., 2013). The selected compounds in this study demonstrated promising drug-like properties and have been proposed to inhibit cancer progression via their interaction with GSK3β.
In this study, an insilico screening approach was employed to investigate potential anti-cancer compounds targeting the GSK3β protein. BMS-754807 and GSK429286A were discovered to have high binding affinity and stability to the GSK3β protein. The favorable interactions were found to be attributed to various Van der Waals forces and H-bonding interactions. Additionally, both compounds exhibited promising drug-like properties. These findings provide a basis for further investigation in vitro and in vivo to develop potent GSK3β inhibitors for cancer management.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conceptualization, FA and NX; formal analysis, AA, SH, and HB; methodology, LH, AS, and AA; original draft preparation, LH and FA; review and editing, AA, SA, FA, and NX. All authors read and approved the final manuscript. All authors contributed to the article and approved the final version.
This work was supported by Discipline Collaborative Innovation Team Program of Double First-class and High-level Universities for Guangzhou University of Chinese Medicine—No. 2021XK01.
The researchers would like to acknowledge Deanship of Scientific Research, Taif University for funding this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Banerjee, P., Eckert, A. O., Schrey, A. K., and Preissner, R. (2018). ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic acids Res. 46, W257–W263. doi:10.1093/nar/gky318
Bhat, R., Xue, Y., Berg, S., Hellberg, S., Ormo, M., Nilsson, Y., et al. (2003). Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J. Biol. Chem. 278, 45937–45945. doi:10.1074/jbc.m306268200
Carboni, J. M., Wittman, M., Yang, Z., Lee, F., Greer, A., Hurlburt, W., et al. (2009). BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol. Cancer Ther. 8, 3341–3349. doi:10.1158/1535-7163.mct-09-0499
Cohen, P., and Frame, S. (2001). The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2, 769–776. doi:10.1038/35096075
Dallakyan, S., and Olson, A. J. (2015). Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 1263, 243–250. doi:10.1007/978-1-4939-2269-7_19
Dickey, A., Schleicher, S., Leahy, K., Hu, R., Hallahan, D., and Thotala, D. K. (2011). GSK-3β inhibition promotes cell death, apoptosis, and in vivo tumor growth delay in neuroblastoma Neuro-2A cell line. J. Neurooncol 104, 145–153. doi:10.1007/s11060-010-0491-3
Domoto, T., Uehara, M., Bolidong, D., and Minamoto, T. (2020). Glycogen synthase kinase 3β in cancer biology and treatment. Cells 9, 1388. doi:10.3390/cells9061388
Duda, P., Akula, S. M., Abrams, S. L., Steelman, L. S., Martelli, A. M., Cocco, L., et al. (2020). Targeting GSK3 and associated signaling pathways involved in cancer. Cells 9, 1110. doi:10.3390/cells9051110
Gao, S., Li, S., Duan, X., Gu, Z., Ma, Z., Yuan, X., et al. (2017). Inhibition of glycogen synthase kinase 3 beta (GSK3β) suppresses the progression of esophageal squamous cell carcinoma by modifying STAT3 activity. Mol. Carcinog. 56, 2301–2316. doi:10.1002/mc.22685
Gentile, G., Merlo, G., Pozzan, A., Bernasconi, G., Bax, B., Bamborough, P., et al. (2012). 5-Aryl-4-carboxamide-1, 3-oxazoles: Potent and selective GSK-3 inhibitors. Bioorg. Med. Chem. Lett. 22, 1989–1994. doi:10.1016/j.bmcl.2012.01.034
Gould, T. D., Einat, H., Bhat, R., and Manji, H. K. (2004). AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int. J. Neuropsychopharmacol. 7, 387–390. doi:10.1017/s1461145704004535
Greten, F. R., and Grivennikov, S. I. (2019). Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 51, 27–41. doi:10.1016/j.immuni.2019.06.025
Humphrey, W., Dalke, A., and Schulten, K. (1996). Vmd: Visual molecular dynamics. J. Mol. Graph 14 (33-38), 33–38. doi:10.1016/0263-7855(96)00018-5
Jacobs, K. M., Bhave, S. R., Ferraro, D. J., Jaboin, J. J., Hallahan, D. E., and Thotala, D. (2012). GSK-3β: A bifunctional role in cell death pathways. Int. J. Cell Biol. 2012, 930710. doi:10.1155/2012/930710
Kim, S., Kim, S. A., Han, J., and Kim, I. S. (2021). Rho-kinase as a target for cancer therapy and its immunotherapeutic potential. Int. J. Mol. Sci. 22, 12916. doi:10.3390/ijms222312916
Klamer, G., Song, E., Ko, K. H., O'brien, T. A., and Dolnikov, A. (2010). Using small molecule GSK3946; inhibitors to treat inflammation. Curr. Med. Chem. 17, 2873–2881. doi:10.2174/092986710792065090
Kunnimalaiyaan, S., Schwartz, V. K., Jackson, I. A., Clark Gamblin, T., and Kunnimalaiyaan, M. (2018). Antiproliferative and apoptotic effect of LY2090314, a GSK-3 inhibitor, in neuroblastoma in vitro. BMC cancer 18, 560–568. doi:10.1186/s12885-018-4474-7
Macalino, S. J., Gosu, V., Hong, S., and Choi, S. (2015). Role of computer-aided drug design in modern drug discovery. Arch. Pharm. Res. 38, 1686–1701. doi:10.1007/s12272-015-0640-5
Mathuram, T. L., Ravikumar, V., Reece, L. M., Karthik, S., Sasikumar, C. S., and Cherian, K. M. (2016). Tideglusib induces apoptosis in human neuroblastoma IMR32 cells, provoking sub-G0/G1 accumulation and ROS generation. Environ. Toxicol. Pharmacol. 46, 194–205. doi:10.1016/j.etap.2016.07.013
Medina, M., and Avila, J. (2010). Glycogen synthase kinase-3 (GSK-3) inhibitors for the treatment of Alzheimer's disease. Curr. Pharm. Des. 16, 2790–2798. doi:10.2174/138161210793176581
Mishra, H., Kesharwani, R. K., Singh, D. B., Tripathi, S., Dubey, S. K., and Misra, K. (2019). Computational simulation of inhibitory effects of curcumin, retinoic acid and their conjugates on GSK-3 beta. Netw. Model. Analysis Health Inf. Bioinforma. 8, 3–7. doi:10.1007/s13721-018-0177-x
Pal, K., Cao, Y., Gaisina, I. N., Bhattacharya, S., Dutta, S. K., Wang, E., et al. (2014). Inhibition of GSK-3 induces differentiation and impaired glucose metabolism in renal cancer. Mol. Cancer Ther. 13, 285–296. doi:10.1158/1535-7163.mct-13-0681
Palomo, V., and Martinez, A. (2017). Glycogen synthase kinase 3 (GSK-3) inhibitors: A patent update (2014-2015). Expert Opin. Ther. Pat. 27, 657–666. doi:10.1080/13543776.2017.1259412
Paul, D., Sanap, G., Shenoy, S., Kalyane, D., Kalia, K., and Tekade, R. K. (2021). Artificial intelligence in drug discovery and development. Drug Discov. Today 26, 80–93. doi:10.1016/j.drudis.2020.10.010
Pires, D. E., Blundell, T. L., and Ascher, D. B. (2015). pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 58, 4066–4072. doi:10.1021/acs.jmedchem.5b00104
Pol-Fachin, L., Fernandes, C. L., and Verli, H. (2009). GROMOS96 43a1 performance on the characterization of glycoprotein conformational ensembles through molecular dynamics simulations. Carbohydr. Res. 344, 491–500. doi:10.1016/j.carres.2008.12.025
Sahin, I., Eturi, A., De Souza, A., Pamarthy, S., Tavora, F., Giles, F. J., et al. (2019). Glycogen synthase kinase-3 beta inhibitors as novel cancer treatments and modulators of antitumor immune responses. Cancer Biol. Ther. 20, 1047–1056. doi:10.1080/15384047.2019.1595283
Saud, S. M., Li, W., Gray, Z., Matter, M. S., Colburn, N. H., Young, M. R., et al. (2016). Diallyl disulfide (DADS), a constituent of garlic, inactivates NF-κB and prevents colitis-induced colorectal cancer by inhibiting GSK-3β. Cancer Prev. Res. (Phila) 9, 607–615. doi:10.1158/1940-6207.capr-16-0044
Schuttelkopf, A. W., and Van Aalten, D. M. (2004). Prodrg: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D. Biol. Crystallogr. 60, 1355–1363. doi:10.1107/s0907444904011679
Sliwoski, G., Kothiwale, S., Meiler, J., and Lowe, E. W. (2014). Computational methods in drug discovery. Pharmacol. Rev. 66, 334–395. doi:10.1124/pr.112.007336
Talele, T. T., Khedkar, S. A., and Rigby, A. C. (2010). Successful applications of computer aided drug discovery: Moving drugs from concept to the clinic. Curr. Top. Med. Chem. 10, 127–141. doi:10.2174/156802610790232251
Trnski, D., Sabol, M., Gojevic, A., Martinic, M., Ozretic, P., Musani, V., et al. (2015). GSK3β and Gli3 play a role in activation of Hedgehog-Gli pathway in human colon cancer — targeting GSK3β downregulates the signaling pathway and reduces cell proliferation. Biochim. Biophys. Acta 1852, 2574–2584. doi:10.1016/j.bbadis.2015.09.005
Walz, A., Ugolkov, A., Chandra, S., Kozikowski, A., Carneiro, B. A., O'halloran, T. V., et al. (2017). Molecular pathways: Revisiting glycogen synthase kinase-3β as a target for the treatment of cancer. Clin. Cancer Res. 23, 1891–1897. doi:10.1158/1078-0432.ccr-15-2240
Yuan, S., Chan, H. S., and Hu, Z. (2017). Using PyMOL as a platform for computational drug design. Wiley Interdiscip. Rev. Comput. Mol. Sci. 7, e1298. doi:10.1002/wcms.1298
Zamek-Gliszczynski, M. J., Abraham, T. L., Alberts, J. J., Kulanthaivel, P., Jackson, K. A., Chow, K. H., et al. (2013). Pharmacokinetics, metabolism, and excretion of the glycogen synthase kinase-3 inhibitor LY2090314 in rats, dogs, and humans: A case study in rapid clearance by extensive metabolism with low circulating metabolite exposure. Drug Metab. Dispos. 41, 714–726. doi:10.1124/dmd.112.048488
Zeng, J., Liu, D., Qiu, Z., Huang, Y., Chen, B., Wang, L., et al. (2014). GSK3β overexpression indicates poor prognosis and its inhibition reduces cell proliferation and survival of non-small cell lung cancer cells. PLoS One 9, e91231. doi:10.1371/journal.pone.0091231
Zhang, J. S., Herreros-Villanueva, M., Koenig, A., Deng, Z., De Narvajas, A. A., Gomez, T. S., et al. (2014). Differential activity of GSK-3 isoforms regulates NF-κB and TRAIL- or TNFα induced apoptosis in pancreatic cancer cells. Cell Death Dis. 5, e1142. doi:10.1038/cddis.2014.102
Keywords: glycogen synthase kinase-3, cancer, virtual screening, molecular dynamics, drug-likeness
Citation: Hua L, Anjum F, Shafie A, Ashour AA, Almalki AA, Alqarni AA, Banjer HJ, Almaghrabi SA, He S and Xu N (2023) Identifying promising GSK3β inhibitors for cancer management: a computational pipeline combining virtual screening and molecular dynamics simulations. Front. Chem. 11:1200490. doi: 10.3389/fchem.2023.1200490
Received: 05 April 2023; Accepted: 05 May 2023;
Published: 22 May 2023.
Edited by:
Khurshid Ahmad, Yeungnam University, Republic of KoreaReviewed by:
Anshul Tiwari, Vanderbilt University, United StatesCopyright © 2023 Hua, Anjum, Shafie, Ashour, Almalki, Alqarni, Banjer, Almaghrabi, He and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farah Anjum, ZmFyYWhhbmp1bUB0dS5lZHUuc2E=; Nenggui Xu, bmd4dTgwMThAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.