
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 28 February 2023
Sec. Supramolecular Chemistry
Volume 11 - 2023 | https://doi.org/10.3389/fchem.2023.1148073
This article is part of the Research TopicMetal-organic frames (MOF) - Based Materials and Their ApplicationsView all 5 articles
Robust DUT-67 was synthesized by the hydrothermal method and characterized by powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA). To systematically study the removal of Cr(VI) ion by DUT-67, single-factor, competition ion, material regeneration, kinetic, and thermodynamic experiments were designed. The experimental results show that DUT-67 had a maximum removal rate of 96.1% and a maximum adsorption capacity of 105.42 mg g−1 with material regeneration and outstanding selective adsorption. In addition, the process of removal of the Cr(VI) ion from an aqueous solution by DUT-67, which accorded with the pseudo-second-order kinetics model and Langmuir model, was studied, and its adsorption mechanism was reasonably explained by the theoretical calculation.
Chromium pollution due to the highly toxic Cr(VI) ion, which is easily absorbed by the human body through a variety of pathways, causes long-term harm to the environment and poses a great threat to human health, causing damage to the body, hoarse throats, perforation of the nasal septum, emphysema, lung sclerosis, and other diseases (Liu et al., 2020; Bell et al., 2022; Shen et al., 2022). Therefore, strict checks on the emission standards of Cr(VI) are necessary, requiring effective treatment of industrial waste with Cr(VI) ions (Wang et al., 2013). So far, the removal methods of the Cr(VI) ion mainly include flotation, precipitation, reverse osmosis, vaporization, bio-treatment and chemical oxidation, adsorption, ion exchange, and a combination of these methods (Gao et al., 2019; Li et al., 2021). The adsorption method was considered the most direct, simple, and effective method to treat the industrial waste with the Cr(VI) ion, both in industry and research (Schoenecker et al., 2012; Wang et al., 2016).
In recent years, porous materials such as large-pore resin, activated carbon, metal–organic frameworks (MOFs), natural zeolite, silica gel, molecular sieve, and covalent organic frameworks have significantly advanced in the field of adsorption and they interact favorably during separation and purification (Peng, et al., 2018; Wychowaniec et al., 2022). Metal–organic frameworks have high porosity, large surface area, high stability, and simple preparation when compared with the traditional porous materials (Bai et al., 2016; Zhang et al., 2021). In addition, the Cr(VI) ion in the nano-pore or nano-cage of MOFs can interact with active sites, facilitating the efficient removal of the Cr(VI) ion. So far, only few MOFs have high stability, such as ZIFs, MILs, and UiO-X (Wang et al., 2016; Zhang et al., 2021), which have been applied in the removal of the Cr(VI) ion. Although some progress on the highly stable porous MOF materials for the removal of the Cr(VI) ion has been achieved (Li et al., 2013; Zhang et al., 2015; Shen et al., 2022), the design of highly stable MOFs and application in the efficient removal of the Cr(VI) ion from wastewater remain a challenging work.
In this work, robust DUT-67 was used for the removal of the Cr(VI) ion from an aqueous solution, and the single-factor, competition ion, material regeneration, kinetic, and thermodynamic experiments were performed. In addition, the removal process by DUT-67 was analyzed via dynamic and thermodynamic modeling. The removal process of the Cr(VI) ion by DUT-67 was explored, and the adsorption mechanism was speculated through theoretical calculation.
2,5-Thiophenedicarboxylic acid (H2TDC) and ZrCl4 were used to prepare DUT-67 using the hydrothermal method, and its structure, morphology, and thermal stability were characterized by PXRD, SEM, and TGA, respectively. The PXRD results show that the characteristic peaks of the experimental and simulated DUT-67 are consistent, indicating the successful preparation of DUT-67. In addition, the characteristic peak positions of the activated and reused samples of DUT-67 are unchanged, indicating the high-temperature activated and reused samples of DUT-67 still maintain the crystal state (Figure 1A). The morphology of DUT-67 is observed as a white powder with regular morphology but uneven size (Figure 1B and Supplementary Figure S1). The TGA results showed that DUT-67 lost the guest solvent molecules between 25°C and 300°C, while the frameworks began to decompose after 350°C, indicating that DUT-67 exhibits high thermal stability (Supplementary Figure S2). DUT-67 can exist stably in concentrated HCl for 3 days, which indicates the chemical stability (Bon et al., 2013) of DUT-67. Therefore, DUT-67 exhibits both high thermal stability and chemical stability.
DUT-67 crystallizes in the Fm3̅m space group with a = 39.120 (5) Å, and the central metal zirconium ion is octa-coordinated by four carboxylate O atoms from TDC2- ligands and the remaining four O atoms from four independent coordinated hydroxyl groups or water molecules and six zirconium atoms and eight TDC2- ligands could form the nanocluster of [Zr6O6(OH)2 (tdc)4(CH3COO)2] (Supplementary Figure S3). In addition, DUT-67 shows a binodal 8-connected three-dimensional framework with reo underlying net (Bon et al., 2013), which has the cuboctahedral cage with the diameter of 14.2 Å and the octahedral cage with the diameter of 11.7 Å (Figures 1C, D, and Supplementary Figure S4). The N2 adsorption and desorption curves of DUT-67 at 77 K conform to type-I with a maximum adsorption of 261.6 cm3/g, and a calculated BET value of 1,031.9 m2/g (Bon et al., 2013) (Supplementary Figure S5).
To reach the optimal conditions for the adsorption of the Cr(VI) ion from aqueous solution by DUT-67, four single-factor optimization experiments using the pH values of the Cr(VI) ion aqueous solution, the initial dose of DUT-67, the initial concentration of the Cr(VI) ion, and temperature were designed. In addition, the competition ion, material regeneration, kinetic, and thermodynamic experiments were discussed in detail.
The experimental results show that the removal rate gradually increases when the pH is between 2 and 4, while the removal rate decreases at pH between 5 and 7 (Figure 2A). When the pH value is between 2 and 5, the Cr(VI) ion is dominated by HCrO4− and Cr2O72-; when the pH value exceeds 7, the Cr(VI) ion is dominated by CrO42- (Li et al., 2021). Thus, the Cr(VI) ion aqueous solution with the highest removal rate was the solution with a pH value of 4.01. When the dose of DUT-67 is less than 20 mg, the removal rate of the Cr(VI) ion increases, while the removal rate decreases when DUT-67 dose exceeds 20 mg (Figure 2B). It is possible that the amount of adsorbent exceeds the maximum amount in a given volume; hence, its adsorption effect does not increase and the adsorption process is inhibited (Li et al., 2021). Therefore, the optimal dosage of DUT-67 is 20 mg. When the initial concentration of the Cr(VI) ion exceeds 50 μg/ml, the removal rate of DUT-67 gradually decreases with the increase of Cr(VI) ion concentration. When the initial concentration of the Cr(VI) ion increases, the DUT-67 removal rate reaches equilibrium and gradually decreases (Figure 2C). Thus, at the Cr(VI) ion concentration of 50 μg/ml, the highest DUT-67 removal rate of 88.06% is achieved. When the temperature ranges from 25°C to 45°C, the removal rate negligibly changes. However, when the temperature is higher than 45°C, the removal rate gradually decreases (Figure 2D). The most suitable removal temperature is 45°C with a removal rate of 96.2%. As the removal of the Cr(VI) ion by DUT-67 is an endothermic reaction, the mass transfer rate of the Cr(VI) ion in the solution is accelerated in the process. In summary, the optimal removal conditions are as follows: the pH of the solution is 4.01, dose of DUT-67 is 20 mg, initial concentration is 50 μg/ml, and adsorption temperature is 45°C, resulting in a maximum removal rate of 96.1%.
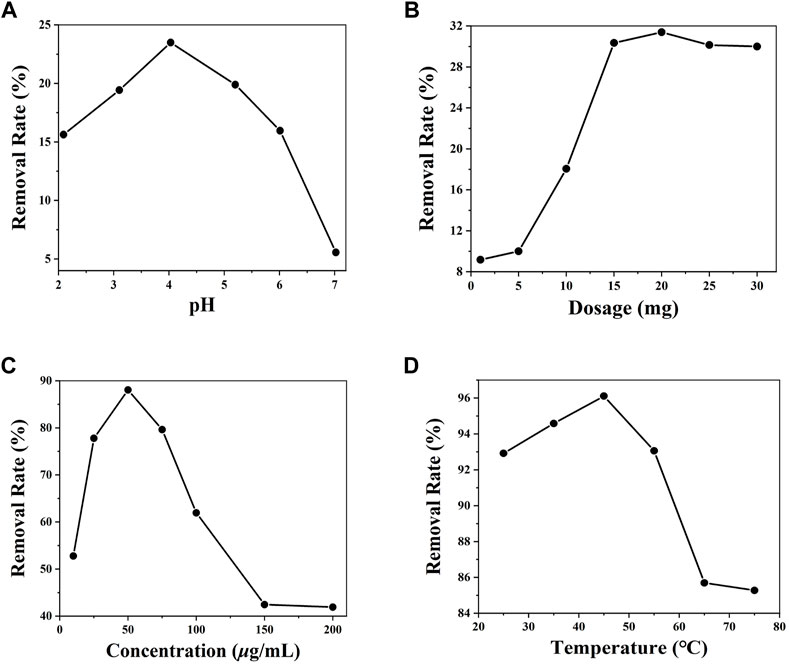
FIGURE 2. Influence of different pH (A), dosages (B), initial concentrations of the Cr(VI) ion, (C) and temperatures (D) on the removal by DUT-67.
Regarding industrial wastewater containing a large number of co-existing anions such as NO3−, CO32-, and SO42-, investigation of the effect of co-existing ions on the removal of the Cr(VI) ion is essential (Shen et al., 2022). To study this effect, different co-existing anions were added into an aqueous solution of the Cr(VI) ion. The results indicated that the co-existing ions had only weak effects on the removal of the Cr(VI) ion (Figure 3A). Thus, DUT-67 can maintain the removal rate for the Cr(VI) ion in the presence of co-existing ions. To study the recyclability of the material, the reusability of DUT-67 after the adsorption of the Cr(VI) ion was further investigated (Zheng et al., 2019; Shen et al., 2022). After six recycles, the removal rate of 80.1% was observed, and the DUT-67 framework maintained the crystal state (Figure 3B).
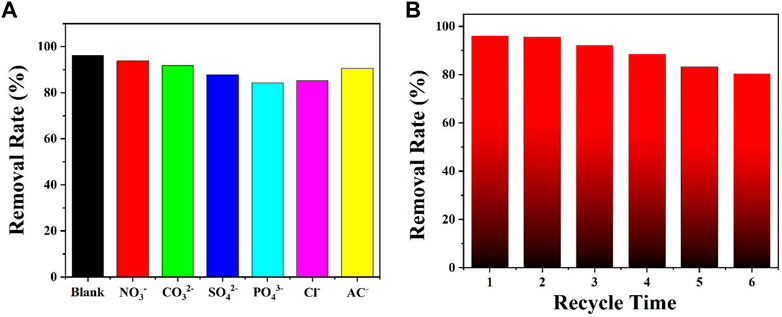
FIGURE 3. (A) Effect of co-existing ions on the removal of the Cr(VI) ion by DUT-67. (B) Recycling experiment on the removal of the Cr(VI) ion by DUT-67.
In addition, the adsorption equilibrium was gradually reached with the change of concentration and adsorption time, and the adsorption isotherm for the removal of the Cr(VI) ion by DUT-67 with the maximum adsorption capacity is 105.42 mg g−1 (Supplementary Figure S6), whose BET and the maximum adsorption capacity exhibited a moderate level compared with other reported MOFs (Supplementary Table S1).
Equations (3) and (4) (Supplementary Data Sheet 1) were used for fitting the pseudo-first-order kinetics and pseudo-second-order kinetics. The pseudo-first-order kinetics fitting curve is achieved by ln (qe-qt) and t, and the pseudo-second-order kinetics fitting curve is achieved by t/qt and t (Yang et al., 2019; Shen et al., 2022). The experimental results show that the R2 value of the pseudo-first-order kinetic model does not conform to the linear law, while the R2 value of the pseudo-second-order kinetic model is very close to 1 (Figure 4A and Supplementary Figure S7). This indicates that the adsorption process of the Cr(VI) ion by DUT-67 corresponds with the pseudo-second-order kinetic model with the rate-control process.
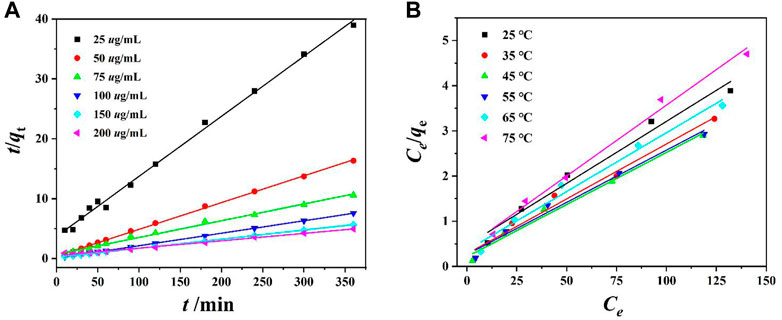
FIGURE 4. (A) Pseudo-second-order dynamic model fitting for Cr(VI) ion removal by DUT-67, and (B) Langmuir adsorption mode fitting for Cr(VI) ion removal by DUT-67.
Equations (5) and (6) were used for fitting of the Langmuir and Freundlich models. ce is plotted with ce/qe, to achieve the Langmuir isotherm, and the Freundlich isotherm is obtained by plotting lnce with lnqe (Yang et al., 2019; Shen et al., 2022). The R2 value of the Freundlich isotherm indicates a poor linear relationship, while the R2 value of the Langmuir isotherm demonstrates higher linearity (Figure 4B and Supplementary Figure S8). This indicates the thermodynamic process of Cr(VI) ion removal by DUT-67 conforms to the Langmuir model with the monolayer adsorption.
According to Equations (7) and 8), and 9), 1/T and lnK are regarded as the transverse coordinate and ordinate, respectively, and using them, the thermodynamic linear fitting curve is obtained (Wang et al., 2019; Shen et al., 2022). When the temperatures were 308, 318, and 328 K, the Gibbs free energy (△G) values were -0.135 kJ/mol, -0.524 kJ/mol, and -0.791 kJ/mol, respectively. The results indicated that the adsorption process of DUT-67 on the Cr(VI) ion from an aqueous solution is a spontaneous and exothermic process adsorption process. In summary, according to the kinetic and thermodynamic experiment, the process of removal of Cr(VI) ions from an aqueous solution by DUT-67 correlated with the pseudo-second-order kinetics model and Langmuir model, whose adsorption process belongs to weak chemical adsorption with a spontaneous and exothermic process (Babapour, et al., 2022; Valadi, et al., 2022; Wang, et al., 2022; Chen, et al., 2023; Yuan, et al., 2023).
To investigate the adsorption sites and the adsorption mechanism of the Cr(VI) ion in the pores of DUT-67, the adsorption locator module of Materials Studios 8.0 was used (Shen et al., 2022). The adsorption mechanism of the Cr(VI) ion by DUT-67 might be via the hydrogen bonding interactions between the O atom from the dichromate and H atom from the TDC2- ligand (Zheng et al., 2019; Li et al., 2021; Shen et al., 2022; Valadi, et al., 2022; Yuan, et al., 2023), the Cr(VI) atom from the dichromate, and the S atom from the TDC2- ligand (Figures 5A,B), which play crucial roles in the adsorption of the Cr(VI) ion by DUT-67.
Robust DUT-67 was prepared by the hydrothermal method and characterized by PXRD, TGA, and SEM. The effect of DUT-67 on the adsorption process of the Cr(VI) ion from an aqueous solution under different conditions was studied, and the competing ion, material regeneration, kinetic, and thermodynamic experiments were explored. The results led to the determination of the optimal adsorption conditions yielding a maximum removal rate of 96.1% and a maximum adsorption capacity of 105.42 mg g−1 with selective adsorption and material regeneration. In addition, the process of removal of Cr(VI) ions from an aqueous solution by DUT-67 correlated with the pseudo-second-order kinetics model and Langmuir model, and the adsorption mechanism of DUT-67 was reasonably explained. Therefore, DUT-67 can be regarded as a multifunctional material that can effectively remove Cr(VI) ions from the wastewater. This study provides a promising method for the separation and removal of Cr(VI) ions from wastewater in the future.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
YS wrote the original draft and guided the experimental operation; QY and YG designed and performed the experiments and data analysis; and JQ and QL reviewed and edited the article. All authors read and agreed to the published version of the manuscript.
This work was funded by the National Natural Science Foundation of China (21861044), Yunnan Province High-level Talent Training Support Program “Youth Top Talent” Project (2020), Yunnan Province Young and Middle-aged Academic and Technical Leaders Reserve Talent Project (202105AC160060), and Special Basic Cooperative Research Programs of Yunnan Provincial Undergraduate Universities’ Association (202101BA070001-042 and 202101BA070001-031).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2023.1148073/full#supplementary-material
Babapour, M., Dehghani, M. H., Alimohammadi, M., Moghadam Arjmand, M., Salari, M., Rasuli, L., et al. (2022). Adsorption of Cr(VI) from aqueous solution using mesoporous metal-organic framework-5 functionalized with the amino acids: Characterization, optimization, linear and nonlinear kinetic models. J. Mol. Liq. 345, 117835. doi:10.1016/j.molliq.2021.117835
Bai, Y., Dou, B., Xie, H., Rutledge, W., Li, J. R., and Zhou, H. C. (2016). Zr-Based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 45, 2327–2367. doi:10.1039/C5CS00837A
Bell, J., Ma, X., McDonald, T., Huang, C. H., and Sharma, V. K. (2022). Overlooked role of chromium(V) and chromium(IV) in chromium redox reactions of environmental importance. ACS ES&T Water 2 (6), 932–942. doi:10.1021/acsestwater.1c00409
Bon, V., Senkovska, I., Baburin, A., and Kaskel, S. (2013). Zr-And Hf-based metal-organic frameworks: Tracking down the polymorphism. Cryst. Growth Des. 13, 1231–1237. doi:10.1021/cg301691d
Chen, P., Wang, Y. L., Zhuang, X. Q., Liu, H., Liu, G., and Lv, W. (2023). Selective removal of heavy metals by Zr-based MOFs in wastewater: New acid and amino functionalization strategy. J. Environ. Sci. 124, 268–280. doi:10.1016/j.jes.2021.10.010
Gao, Q., Xu, J., and Bu, H. (2019). Recent advances about metal-organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 378, 17–31. doi:10.1016/j.ccr.2018.03.015
Li, X. X., Xu, H. Y., Kong, F. Z., and Wang, R. (2013). A cationic metal-organic framework consisting of nanoscale cages: Capture, separation and luminescent probing of Cr2O72– through a single-crystal to single-crystal process. Angew. Chem. Int. Ed. 52, 13769–13773. doi:10.1002/anie.201307650
Li, X., Zhong, B., Xie, H., Yabo, X., and Jianrong, L. (2021). Recent advances in adsorptive removal of Cr(VI) ions by metal-organic frameworks. Chin. J. Inorg. Chem. 37, 385–400. doi:10.11862/CJIC.2021.068
Liu, Z., Li, J., Zheng, Y., Song, Y., Shi, Z., Lin, Z., et al. (2020). Different pathways for Cr(III) oxidation: Implications for Cr(VI) reoccurrence in reduced chromite ore processing residue. Environ. Sci. Technol. 54, 11971–11979. doi:10.1021/acs.est.0c01855
Peng, G., Huang, L., Zhang, X., Kang, C., Chen, S., Song, L., et al. (2018). A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 9, 187. doi:10.1038/s41467-017-02600-2
Schoenecker, M., Carson, G., Jasuja, H., Flemming, C. J. J., and Walton, K. S. (2012). Effect of water adsorption on retention of structure and surface area of metal-organic frameworks. Ind. Eng. Chem. Res. 51, 6513–6519. doi:10.1021/ie202325p
Shen, Q., Duan, R., Qian, J., and Li, Q. (2022). Preparation of highly stable DUT-52 materials and adsorption of dichromate ions in aqueous solution. ACS Omega 7 (19), 16414–16421. doi:10.1021/acsomega.2c00373
Valadi, F. M., Shahsavari, S. Y., Akbarzadeh, E., and Gholami, M. R. (2022). Preparation of new MOF-808/chitosan composite for Cr(VI) adsorption from aqueous solution: Experimental and DFT study. Carbohydr. Polym. 288, 119383. doi:10.1016/j.carbpol.2022.119383
Wang, H., Liu, L., Demir, K., Chen, J. P., and Li, K. (2016). Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 45, 5107–5134. doi:10.1039/C6CS00362A
Wang, H., Wang, S., Wang, S. X., Zhang, L., Zhou, Y., and Yang, F. (2022). Efficient and selective removal of Cr(VI) by the modified UiO-66-NH2 with phenothiazine-N-rhodanine from aqueous solution: Performance and mechanisms. Microporous Mesoporous Mater. 336, 111834. doi:10.1016/j.micromeso.2022.111834
Wang, W., Huang, J., Yang, Y., Hui, Y., Ge, Y., Larssen, T., et al. (2013). First report of a Chinese PFOS alternative overlooked for 30 years: Its toxicity, persistence, and presence in the environment. Environ. Sci. Technol. 47, 10163–10170. doi:10.1021/es401525n
Wang, X., Liu, W., Fu, F., Yi, X. H., Wang, P., Zhao, C., et al. (2019). Simultaneous Cr(VI) reduction and Cr(III) removal of bifunctional MOF/Titanate nanotube composites. Environ. Pollut. 249, 502–511. doi:10.1016/j.envpol.2019.03.096
Wychowaniec, K., Saini, H., Scheibe, B., Dubal, D. P., Schneemann, A., and Jayaramulu, K. (2022). Hierarchical porous metal-organic gels and derived materials: From fundamentals to potential applications. Chem. Soc. Rev. 51, 9068–9126. doi:10.1039/D2CS00585A
Yang, F., Shu, F., Zhuang, X., Li, Y., and Gu, J. (2019). Metal-organic frameworks bearing dense alkyl thiol for the efficient degradation and concomitant removal of toxic Cr(VI). Langmuir 35, 16226–16233. doi:10.1021/acs.langmuir.9b03057
Yuan, D. H., Shang, C. Y., Cui, J., Zhang, W., and Kou, Y. (2023). Removal of Cr(VI) from aqueous solutions via simultaneous reduction and adsorption by modified bimetallic MOF-derived carbon material Cu@MIL-53(Fe): Performance, kinetics, and mechanism. Environ. Res. 216, 114616. doi:10.1016/j.envres.2022.114616
Zhang, Q., Yu, J. C., Cai, J. F., Zhang, L., Cui, Y., Yang, Y., et al. (2015). A porous Zr-cluster-based cationic metal-organic framework for highly efficient Cr2O72– removal from water. Chem. Commun. 51, 14732–14734. doi:10.1039/C5CC05927E
Zhang, S., Wang, J., Zhang, Y., Ma, J., Huang, L., Yu, S., et al. (2021). Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut. 291, 118076. doi:10.1016/j.envpol.2021.118076
Keywords: DUT-67, highly efficient, removal of the Cr(VI) ion, aqueous solution, highly stable
Citation: Shen Y, Yang Q, Gao Y, Qian J and Li Q (2023) Robust DUT-67 material for highly efficient removal of the Cr(VI) ion from an aqueous solution. Front. Chem. 11:1148073. doi: 10.3389/fchem.2023.1148073
Received: 19 January 2023; Accepted: 14 February 2023;
Published: 28 February 2023.
Edited by:
Liming Fan, North University of China, ChinaReviewed by:
Linfeng Liang, Shanxi University, ChinaCopyright © 2023 Shen, Yang, Gao, Qian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingsong Yang, ODEyNjIwNTgzQHFxLmNvbQ==; Jinjie Qian, amluamllcWlhbkB3enUuZWR1LmNu; Qipeng Li, cXBsaUB6dHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.