- 1Center of Rehabilitation Medicine, Yueyang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2School of Rehabilitation Science, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Shulan International Medical College, Zhejiang Shuren University, Hangzhou, China
Arthritis is a group of highly prevalent joint disorders, and osteoarthritis (OA) and rheumatoid arthritis are the two most common types. The high prevalence of arthritis causes severe burdens on individuals, society and the economy. Currently, the primary treatment of arthritis is to relieve symptoms, but the development of arthritis cannot be effectively prevented. Studies have revealed that the disrupted balance of enzymes determines the pathological changes in arthritis. In particular, the increased levels of matrix metalloproteinases and the decreased expression of endogenous antioxidant enzymes promote the progression of arthritis. New therapeutic strategies have been developed based on the expression characteristics of these enzymes. Biomaterials have been designed that are responsive when the destructive enzymes MMPs are increased or have the activities of the antioxidant enzymes that play a protective role in arthritis. Here, we summarize recent studies on biomaterials associated with MMPs and antioxidant enzymes involved in the pathological process of arthritis. These enzyme-related biomaterials have been shown to be beneficial for arthritis treatment, but there are still some problems that need to be solved to improve efficacy, especially penetrating the deeper layer of articular cartilage and targeting osteoclasts in subchondral bone. In conclusion, enzyme-related nano-therapy is challenging and promising for arthritis treatment.
Introduction
Arthritis, which is a group of musculoskeletal diseases, is one of the leading causes of disability in the elderly population (Woolf and Pfleger, 2003). Osteoarthritis (OA) and rheumatoid arthritis (RA) are the most prevalent types of arthritis and affected 344 million people and 13 million people, respectively, globally in 2019 (Cieza et al., 2021). OA is characterized by joint degeneration, especially in the knee, and involves multiple joints, such as the hand, hip, knee and foot. A large-scale survey in the United Kingdom in 2017 showed that the prevalence of OA in adults was 10.7% (Swain et al., 2020-06). The increases in obesity and the ageing population contribute to the prevalence of OA (Briggs et al., 2020-10). RA is an immunization-induced systemic disease characterized by synovial inflammation and joint destruction, and the prevalence of RA is 0.5–1.0% in the US (Palmer et al., 2019).
OA and RA are both inflammatory joint diseases that involve joint and synovial destruction and immune cell infiltration (Zhang et al., 2019-03) and is associated with joint pain, swelling, and limited movement, resulting in a decline in physical function, increased dependence and reduced quality of life. Furthermore, the prevalence of OA and RA is expected to increase significantly as the global population ages. The treatment of arthritis is often a long and complex process due to irreversible damage and the risk of comorbidities, resulting in extremely high medical and economic burdens on society, and these burdens continue to increase globally (Briggs et al., 2020-10).
To date, there is no effective cure for OA or RA. The current interventions include medications, physical therapy, and surgical intervention, all of which are aimed at alleviating symptoms and reducing joint damage and disability. Medications for OA, including topical, oral and intra-articular (IA) injectable drugs, are palliative and limited to controlling symptoms of joint swelling, pain and stiffness (Tschon et al., 2020-06). A randomized clinical trial has even shown that IA corticosteroids may accelerate the destruction of articular cartilage (McAlindon et al., 2017-05). Currently, non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs) and disease-modifying anti-rheumatic drugs (DMARDs) are mainly used in the clinical treatment of RA. The targets of traditional DMARDs are not clear, and approximately 30%–50% of patients respond poorly to these drugs (Sparks, 2019). As a result of the poor bioavailability and short half-lives of anti-rheumatic drugs, prolonged repeated use can cause serious adverse reactions such as vomiting, drug resistance and bone marrow suppression.
Physical therapy for OA and RA includes weight loss, moderate exercise and knee joint distraction. Knee joint distraction can improve symptoms and promote tissue repair in severe knee joint degeneration, but there is frequent infection during the follow-up (Jansen and Mastbergen, 2022-01; van der Woude et al., 2017-01). When conservative treatment is not feasible for end-stage arthritis, surgical intervention, such as total joint replacement, can be considered, but this treatment strategy is related to persistent postsurgical pain and infection (Wylde et al., 2011-03; Chung et al., 2021-11).
Currently, new therapeutic strategies and drugs primarily alleviate symptoms to treat arthritis, and critically unsolved problems, such as how to restore abnormal cellular function in arthritis, should be considered. Cellular activity depends on various proteins, and some of these proteins are important enzymes for physiological and pathological processes. Herein, we summarized the essential enzymes that are involved in pathological changes in arthritis.
Arthritis-related enzymes
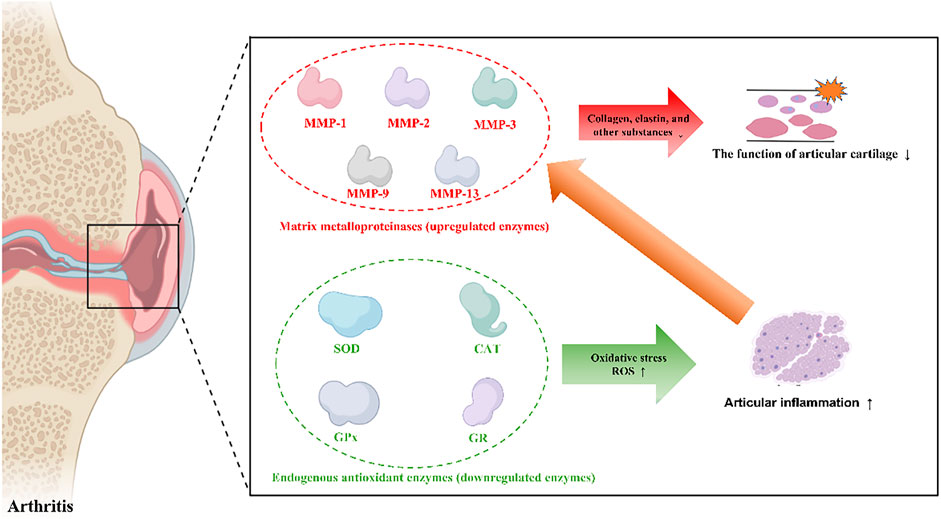
The pathological changes in OA and RA are mainly characterized by cartilage destruction and synovial inflammation (Trachana et al., 2019; Scherer et al., 2020). Cell metabolism is often regulated by different enzymes, and abnormal levels of enzymes are typically associated with the occurrence of various diseases. In cartilage, different matrix metalloproteinases (MMPs) are responsible for destroying chondrocytes by degrading collagen and proteoglycans.
evidence that these inflammatory mediators are involved in the pathogenesis of both OA and RA (Malemud, 2017; van Dalen et al., 2017). Neutrophils, monocytes and macrophages infiltrate cartilage and synovial tissue after inflammation occurs, releasing various inflammatory factors and chemokines, which cause an increase in MMPs.
The destruction or degradation of articular cartilage is regulated by MMPs, which are a family of proteolytic enzymes that hydrolyse extracellular matrix (ECM). Different types of MMPs are involved in degrading proteoglycans and collagens, which are the main components of ECM in cartilage, especially MMP-1, MMP-2, MMP-3, MMP-9 and MMP-13 (Itoh, 2017; Mehana et al., 2019). MMPs can degrade collagen, elastin, and other substances in the ECM of articular cartilage that maintain the structure of cartilage and ultimately destroy the integrity of ECM structure and function.
Under pathological conditions, the expression level of MMP-1 was significantly increased in OA and RA, and this factor degraded ECM collagen and mediated cartilage destruction (Wang et al., 2020a). In cartilage and synovium, MMP-1 expression increased steadily during the progression of OA in a rabbit model of anterior cruciate ligament transection (ACLT) (Wu et al., 2008). MMP-1 could lead to the degeneration of primary collagen (type Ⅱ collagen) in cartilage, and this effect was irreversible (Macdonald et al., 2018).
The development of OA and RA is associated with the increased secretion and activity of MMP-2 in synovial cells and the joints of RA patients, respectively (Kim et al., 2011; Galasso et al., 2012). Furthermore, MMP-2-sensitive peptide was shown to be specifically released in inflammatory joints in vitro and in vivo, which might be an important approach for drug-targeted treatment of RA (Yu et al., 2022).
Significantly increased levels of MMP-3 in the serum of OA patients were positively correlated with the severity of knee OA and RA in patients (Ma et al., 2014; Georgiev et al., 2018; Pengas et al., 2018). Furthermore, serum MMP-3 levels were closely correlated with disease activity scores, suggesting that serum MMP-3 levels could be used as an indicator of structural damage and monitor disease progression (Galil et al., 2016; Tuncer et al., 2019).
MMP-9 was also positively correlated with disease severity in OA patients (Lipari and Gerbino, 2013). A meta-analysis showed that MMP-2 and MMP-9 protein expression levels were significantly higher in the OA group than in the control group, indicating that MMP-2 and MMP-9 are involved in the pathogenesis of OA (Zeng et al., 2015). Multiple studies have shown that the expression of MMP-9 in synovial fluid and synovial cells of RA patients is increased (Silosi et al., 2015; Ma et al., 2019). The degree of inflammation in RA patients correlated with Toll-like receptor 2 (TLR2) expression in peripheral blood monocytes. The increased expression of TLR2 led to the increased expression of MMP-9 (Chen et al., 2015). MMP-9 could participate in the synovial cell-mediated inflammatory response and the degeneration of ECM, especially proteoglycans, which might directly cause joint destruction (Metzger et al., 2012).
MMP-13 is a crucial enzyme leading to the degradation of collagen types I, II and III and the cartilage proteoglycan aggrecans and is considered a significant factor in the pathogenesis of OA (Fosang et al., 1996). MMP-13 attracted much attention due to its obvious overexpression in the articular cartilage of OA patients, but it was almost undetectable in normal adult tissues (Kaneva, 2022). Interfering with the expression of MMP-13 in a surgically induced OA model could efficiently alleviate OA severity (Hoshi et al., 2017). Given its critical role in ECM degradation, MMP-13 has been a promising target in OA treatment (Hu and Ecker, 2021). K/BxN serum-induced arthritis increases MMP-13 expression in C57BL/6 mice, and MMP-13-deficient (MMP-13−/−) mice exhibit reduced inflammation and joint destruction (Singh et al., 2013). In addition, MMP-13 was also associated with the progression of RA, providing crucial predictive information about future structural damage and severity in early RA patients (Tatematsu et al., 2018).
2 Endogenous antioxidant enzymes linked with arthritis
Apart from the direct effect of MMPs on ECM degradation in cartilage and promoting the progression of arthritis, endogenous antioxidants such as superoxide dismutases (SODs), glutathione peroxidase (GPx), catalase (CAT), and glutathione reductase (GR) also affect the occurrence of arthritis by scavenging intracellular reactive oxygen species (ROS) and alleviating cellular oxidative stress.
ROS are key signalling molecules in the progression of inflammatory diseases (Mittal et al., 2014). Under inflammatory conditions, the oxidative stress induced by macrophages, monocytes, and neutrophils leads to the formation of interendothelial junctions, accelerating the crossing of the endothelial barrier and ultimately promoting inflammation (You et al., 2018).
The levels of intra-articular ROS (including H2O2, O2−, OH−, and HOCl) are significantly increased in OA patients, while ROS are maintained at low levels in normal articular tissue (Lepetsos and Papavassiliou, 2016; Yao et al., 2019). The overproduction of ROS causes overoxidation, protein carbonylation, and DNA damage and is considered the primary mechanism of chondrocyte loss and tissue damage (Hosseinzadeh et al., 2016). The associated ROS, including nitric oxide (NO), superoxide anion (O2−) and hydrogen peroxide (H2O2), are present in the articular cavities of RA patients in large quantities (Datta et al., 2014). When the local inflammatory response in RA joints is accelerated and ROS levels exceed physiological tolerance, they not only damage proteins, lipids, and nucleic acids but also act as important endogenous signalling regulators that amplify the synovial inflammatory response (Bala et al., 2017; Phull et al., 2018). Li et al. found that ROS significantly promoted the proliferation of RA synovial fibroblasts and the production of inflammatory factors and that inhibiting ROS significantly downregulated the inflammatory factors secreted by RA synovial fibroblasts, ultimately improving RA conditions (Li et al., 2018). Therefore, a potent antioxidant compound that can reduce ROS in inflammatory cells may be a key factor in the treatment of chronic inflammatory diseases.
ROS clearance is regulated by SODs, GPx, CAT and GR (He et al., 2017). CAT and GPx are involved in the decomposition of intracellular hydrogen peroxide and maintain normal ROS levels to reduce toxic reactions. SOD can catalyse O2− into O2 and H2O2. GR catalyses the reduction of glutathione disulfide (GSSG) to the sulfhydryl form of glutathione (GSH), which plays an important role in the tissue oxidative stress response (Deponte, 2013). The levels of SOD, CAT and other antioxidant enzymes in OA chondrocytes were significantly lower than those in normal chondrocytes, indicating that insufficient antioxidant capacity might cause cartilage damage (Zhuang et al., 2018). Unlike the expression pattern of other antioxidant enzymes, the expression of GR was increased in arthritis (Meshkibaf et al., 2019; Idzik et al., 2022).
The proliferation and activation of osteoclasts (OCs) are key factors leading to bone damage and bone metabolism disorders in RA (Auréal et al., 2020). Recent studies have shown a close correlation between bone destruction and oxidative stress in the pathogenesis of RA. ROS promote osteoclast differentiation (Gamal et al., 2018). Decreased expression of SOD, CAT and GPx was found in the ankle joints of RA rats (Ren et al., 2019a). ROS-induced peroxidation is inhibited by antioxidant enzymes, among which superoxide dismutase 3 (SOD3) is the key enzyme that protects cells from oxidative stress (Nguyen et al., 2020). SOD3 reduced proinflammatory cytokines (IL-1β, IL-2, IL-4, and TNF-α) and the release of MMPs (MMP-2, MMP-3 and MMP-9), ultimately inhibiting inflammatory responses (Xie et al., 2021). Icariin protects synoviocytes induced by lipopolysaccharide (LPS) by inhibiting ferroptosis by activating the Xc/GPX4 axis (Luo and Zhang, 2021).
Considering the importance of MMPs and oxide reductase associated with ROS in the occurrence of arthritis, biomaterials that target endogenous enzymes have become a hot research topic in recent years. Next, we will introduce the application of biomaterials that are linked with these enzymes.
Nanotherapies that target enzymes in arthritis
Enzymes that play critical roles in arthritis pathology are categorized into two groups according to their expression characteristics: upregulated enzymes and downregulated enzymes, which are listed in Figure 1. Enzyme homeostasis is critical for the human body. Both the upregulated and downregulated expression of these enzymes disrupt the balance of cell metabolism and can cause diseases. Therefore, therapeutic strategies have been designed according to the expression of these enzymes. If the expression of these enzymes is upregulated, nanomaterials can respond and release an effective drug to inhibit pathological changes, or nanomaterials can be fabricated to simulate the effects of downregulated enzymes. Next, we described two different functional enzymes in arthritis treatment.
1 Nanomaterials associated with upregulated enzymes in arthritis treatments
It is well known that MMPs are the main destructive enzymes in chondrocytes that degrade ECM components, such as proteoglycans and collagen networks. Degradation of the ECM leads to functional destruction of chondrocytes and cartilage erosion. Therefore, MMPs have become an important molecular target for studies on the treatment of OA. In particular, MMP-13, a critical protease in chondrocytes, is responsible for the degradation of type II collagen and proteoglycans.
OA is a chronic inflammatory disease. Growing evidence reveals that the changes in the OA microenvironment include excessive inflammation and MMP overexpression (Latourte et al., 2017; Li et al., 2017; Stocco et al., 2019). The microenvironment is an important factor in maintaining joint homeostasis. Long-term inhibition of MMP enzymatic activity may lead to adverse reactions. Therefore, it is necessary to design materials that are highly selective for MMPs and can adapt to the changes in MMP levels in vivo. When MMP expression is upregulated, MMP-responsive nanoparticles (NPs) work, and they are inactive when MMPs are at low levels.
The increased expression of MMPs in inflamed tissues may be a promising breakthrough for arthritis therapy. A commercially available, Food and Drug Administration (FDA)-approved molecule known as triglycerol monostearate (TGMS) has been shown to be responsive to MMPs (Wen et al., 2019).
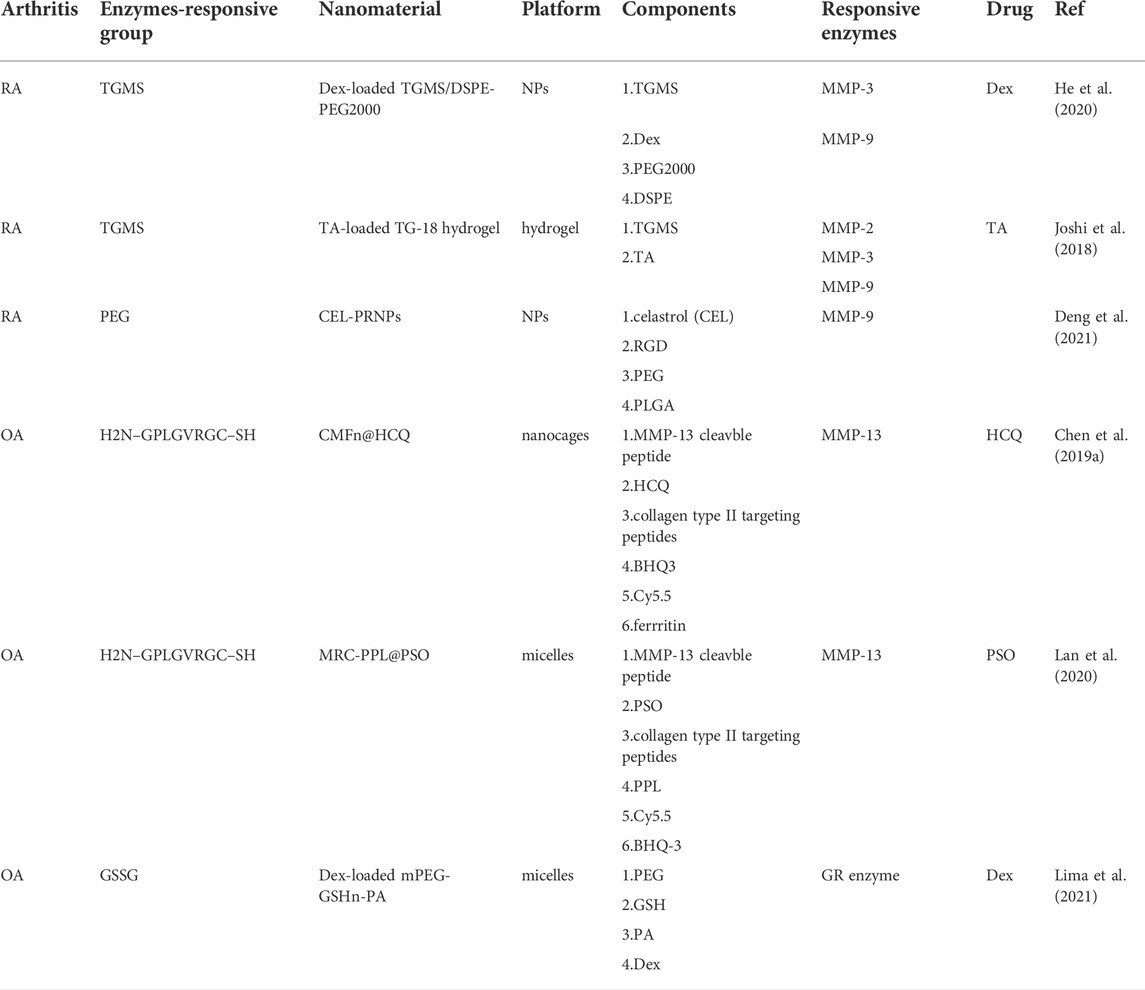
MMP-responsive PEGylated lipid NPs (TGMS/DSPE-PEG2000 NPs) can be produced through the coassembly of TGMS and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly (ethylene glycol) (DSPE-PEG2000). Dexamethasone (Dex)-loaded MMP-responsive NPs were obtained by loading TGMS/DSPE-PEG2000 NPs with Dex, and Dex can be rapidly released from the lipid NPs after TGMS is cleaved by MMP-3 and MMP-9. Dex-loaded MMP-responsive NPs significantly reduced the degree of joint swelling and inhibited the production of TNF-α and IL-1β in the joint (He et al., 2020).
In another study, the nanozyme-like role of the hydrogel form of TGMS(TG-18) was further confirmed in RA treatment. A hydrogel platform that exhibits disassembly and drug release controlled by the concentration of enzymes during arthritis flares was constructed. In this study, a triglycerol monostearate hydrogel (TG-18) loaded with the corticosteroid triamcinolone acetonide (TA) exhibited drug release in response to the increased activities of arthritis-related enzymes in vitro (MMP-2, MMP-3, MMP-9) or synovial fluid from patients with RA (Joshi et al., 2018).
In addition to synovial inflammation and joint swelling, obvious cartilage damage and bone erosion are often observed in RA. Synovial macrophages mediate joint inflammation once activated, and OCs are responsible for arthritic bone erosion and resorption of the bone matrix. Both OCs and synovial macrophages express high levels of αvβ3 integrin, which plays an important role in activated macrophage-dependent inflammation and OC-dependent bone resorption. Macrophages and OCs fail to undergo apoptosis in the RA joint, leading to persistent inflammation and joint destruction. Therefore, inducing OC and macrophage apoptosis in RA joints represents a promising strategy for advanced RA treatment. According to the characteristics of OCs and synovial macrophages, novel CEL-loaded PRNPs (CEL-PRNPs) were synthesized that contained celastrol (CEL), which can induce apoptosis in OCs and macrophages, RGD, which is a ligand of αvβ3 that targets OCs and inflammatory macrophages, and polyethylene glycol (PEG), which is cleaved by MMP-9. In an adjuvant-induced arthritis rat model, CEL-PRNPs efficiently reduced the number of OCs and inflammatory macrophages and relieved various symptoms, including ankle and paw swelling and bone erosion, in the inflamed joints of AIA rats with advanced arthritis (Deng et al., 2021).
To determine the inflammatory condition and investigate the therapeutic effects of MMP-responsive biomaterials, fluorescence imaging was considered for diagnosis and therapy.
Inflamed cartilage is characterized by MMP-13 overexpression and an acidic microenvironment. Therefore, MMP-13/pH-responsive ferritin nanocages (CMFn) loaded with an anti-inflammatory drug (hydroxychloroquine, HCQ), termed CMFn@HCQ, were constructed for OA imaging and therapy. CMFn is a marker for imaging diagnosis that emits light in response to MMP-13 overexpression. The intensity of CMFn light increases with the severity of OA. However, in normal joints, this compound emits no light. The release of HCQ causes an anti-inflammatory effect in OA joints to reduce synovial inflammation, and the retention time lasts up to 14 days (Chen et al., 2019a).
Cartilage-targeting C-PPL was created by grafting collagen type II-targeting peptides with the sequence WRYGRL onto the polymer poly (2-ethyl-2-oxazoline)-poly (ε-caprolactone) (PPL). Additionally, PPL was conjugated with a specific peptide substrate of the MMP-13 enzyme (H2N–GPLGVRGC–SH) that was labelled with a fluorescent dye (Cy5.5) and was subsequently coupled with the black hole quencher-3 (BHQ-3) that can quench Cy5.5 fluorescence to obtain an MMP-13-responsive and pH-sensitive polymer (MR-Cy5.5-BHQ-3-PPL). A cartilage-targeting and OA-specific theragnostic nanoplatform (MRC-PPL) was obtained by the self-assembly of C-PPL and MR-PPL. Finally, MRC-PPL was loaded with the traditional Chinese medicine psoralidin (PSO) to form MRC-PPL@PSO nano-micelles, which specifically target and protect cartilage (Lan et al., 2020). The synthesis and mechanism of MRC-PPL@PSO nano-micelles to treat OA are shown in Figure 2.
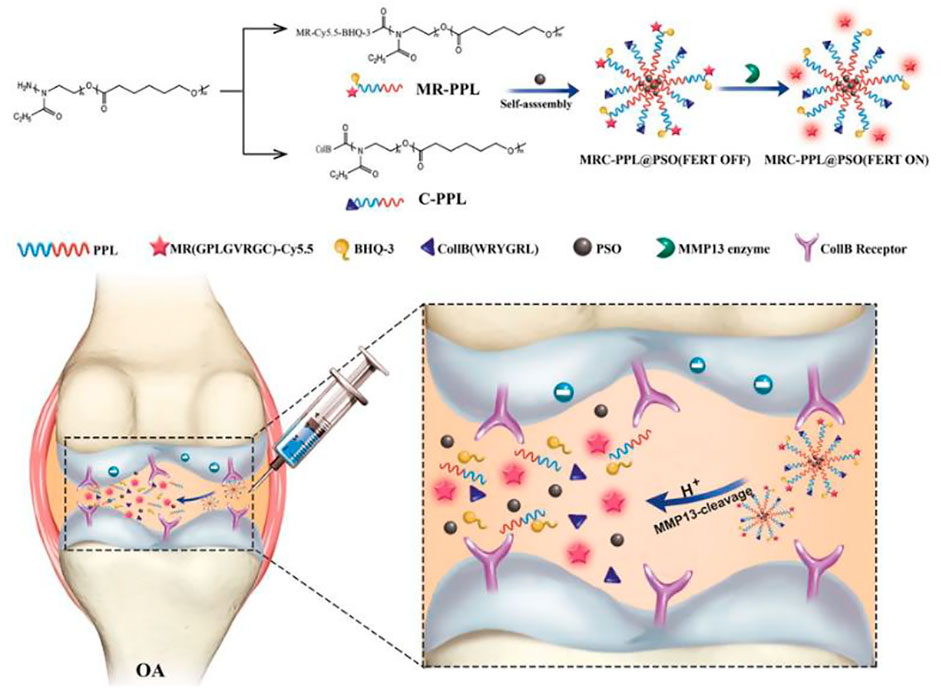
FIGURE 2. Schematic illustration of the synthesis and working mechanism of MMP-13 and pH responsive theranostic MRC-PPL@PSO nano-micelles for OA (Lan et al., 2020). Copyright, 2020, BMC.
In addition to MMP overexpression in arthritis tissue, the intrinsic properties of the OA microenvironment, especially synovial fluid, are also considered when designing novel nanomaterials. The increased activity of the GR enzyme was reported in the synovial fluid of RA and OA patients, and a selectively controlled drug release that is sensitive to the GR enzyme was designed for the treatment of arthritic diseases (Ostalowska et al., 2006; Sredzińska et al., 2009). Polymeric micelles were made of methoxypolyethylene glycol amine-glutathione-palmitic acid (mPEG-GSHn-PA) polymers. Dex was loaded into the cores of the polymeric micelles. The release of Dex was slow under physiological conditions, while the presence of the GR enzyme stimulated a burst release via a thiol−disulfide exchange between GSH and GSSG (Lima et al., 2021). The above biomaterials associated with MMPs are listed in Table 1.
2 Nanomaterials associated with downregulated enzymes in arthritis treatments
Apart from the destruction of cartilage tissue induced by the increased expression of MMPs, the decreased expression of oxide reductase associated with ROS showed a similar effect on cartilage. To reduce the expression of oxide reductase, the strategy was to supply these enzymes directly or mimic the activities with special biomaterials.
Supplementation with antioxidant enzymes such as SOD has been shown to be effective in treating arthritis. Chitosan was chemically conjugated with SOD to generate the nanoparticle-like conjugate 6-O-2′-hydroxylpropyltrimethyl ammonium chloride chitosan-SOD (O-HTCC-SOD), which was superior to unmodified SOD in bioavailability, prolonged half-life and residence in the rat joint cavity. After IA injection of O-HTCC-SOD into rats with MIA-induced OA, mechanical allodynia was greatly reduced, and changes in the gross morphological and histological lesions of articular cartilage were dramatically inhibited (Wang et al., 2020b).
Although the nanosized conjugate O-HTCC–SOD has exhibited higher enzyme activity and superior membrane permeability to native SOD, natural enzymes are unstable, expensive and difficult to store. Currently, biomaterials called nanozymes have been designed to mimic the effects of these oxide reductases. Nanozymes are a specific kind of nanomaterial that have the activities of intrinsic enzymes and possess unique advantages, such as high efficiency, increased compatibility with specific environments, such as high temperatures and pH variations, cyclic use, and a large surface area, and these materials can be conjugated to multiple ligands to achieve multifunctionality. These features give rise to their promising applications in a variety of fields (Pirmohamed et al., 2010).
Recently, numerous nanomaterials with enzyme-like properties have been discovered for OA treatment, including metals, metal oxides, and carbon-based materials.
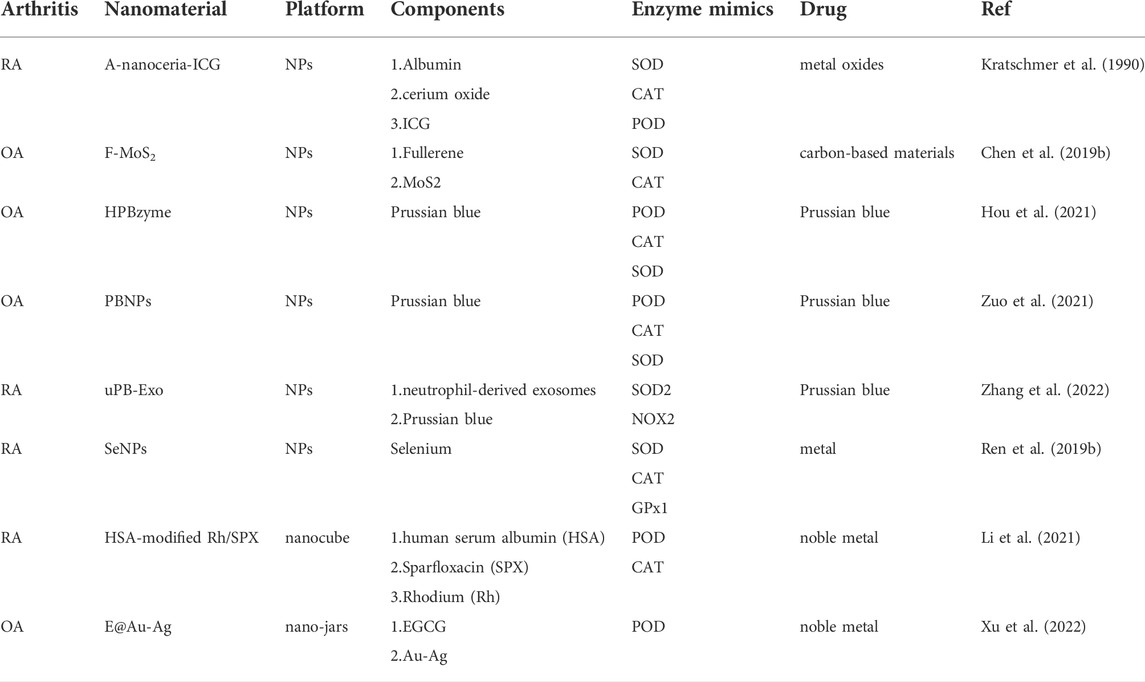
As a representative metal oxide, cerium oxide has been evaluated in RA treatment. Engineered cerium oxide (CeO2) nanoparticles (CeONPs), which are also known as nanoceria, have attracted much attention for exhibiting SOD−, CAT−, and oxidase-like activity (Heckert et al., 2008; Baldim et al., 2018; Kalashnikova et al., 2020). In reduction reactions, SOD catalyses O2•− into H2O2, which may undergo catalysis by CAT into H2O.
Given that albumin is a natural protein and scavenging receptors are widely distributed in the inflamed joints of RA, albumin-nanoceria NPs (A-nanoceria) were synthesized by connecting albumin to nanoceria and further conjugated with near-infrared, indocyanine green (ICG) dye. Enzymatic properties and ROS scavenging activities against a monocyte cell line and systemic targeting potential were evaluated in a collagen-induced arthritis (CIA) mouse model. Such a design has the advantages of targeting inflammation, assessing severity, and controlling inflammation with imaging guidance in RA (Kratschmer et al., 1990).
Moreover, carbon-based materials have also exhibited the activities of nanozymes in scavenging ROS. Fullerene (C60) is a spherical carbon molecule with a unique cage structure that functions as a free-radical scavenger. Apart from inhibiting ROS-induced catabolism in cartilage, fullerene also decreases friction on the cartilage surface and subsequently prevents the further development of cartilage degeneration. With these advantages, fullerene has been used to synthesize biomaterials for the treatment of arthritis. For example, fullerene-like MoS2 (F-MoS2) NPs are efficient lubricants and antioxidants for artificial synovial fluid. These NPs possess intrinsic dual-enzyme-like activity, mimicking SOD and CAT under physiological conditions (pH 7.4, 25°C) and regulating the ROS level in artificial synovial fluid containing HA (Chen et al., 2019b).
Prussian blue (PB) has been approved by the U.S. FDA as a commonly used dye and medicine due to its excellent biocompatibility and biosafety. The peroxidase, CAT, and SOD activities of PBzymes mediate the scavenging of •OH, •OOH, and H2O2, exhibiting outstanding anti-inflammatory and antioxidative bioactivities (Long et al., 2016; Zhang et al., 2016; Dacarro et al., 2018; Qin et al., 2018).
A hollow PBzyme (HPBzyme) with a mesopore structure and a high specific surface area was produced that could remodel the OA microenvironment by mitigating the inflammatory response, protecting against chondrocyte ECM degradation, and exhibiting therapeutic efficacy in vivo (Hou et al., 2021).
PB has also been integrated into other therapeutic approaches, such as exosomes and ultrasound, for arthritis treatment. Low-density ultrasound is a noninvasive biophysical treatment that can reduce joint swelling and inflammation in OA models (Iwabuchi et al., 2014). The combined therapeutic effects of PB and low-density ultrasound on animal OA by scavenging oxygen free radicals was investigated. It was found that this treatment could significantly remove ROS, alleviate ROS-induced apoptosis, and reduce the degeneration of articular cartilage (Zuo et al., 2021). Furthermore, neutrophil-derived exosomes engineered with ultrasmall PB nanoparticles (uPB-Exo) have been shown to be effective in treating RA. uPB-Exo selectively accumulated in activated fibroblast-like synoviocytes and acted as mimics of SOD2 and NOX2 in inflamed joints of RA in vivo, subsequently neutralizing proinflammatory factors, alleviating inflammatory synovitis and protecting against cartilage damage in an advanced RA mouse model (Zhang et al., 2022).
Selenium (Se) is an essential dietary nutrient and has been reported to have lower serum concentrations in RA patients than healthy individuals (Yu et al., 2016). Supplementation with Se is controversial in the treatment of arthritis is controversial due to its toxicity. Nanosized Se is known to have superior antioxidant effects and reduced toxicity (Malhotra et al., 2016). In a rat RA model, SeNPs exhibited potent anti-inflammatory effects and promoted the expression of CAT, SOD and GPX (Ren et al., 2019b).
Ultrasound, which is a noninvasive biophysical therapy and a common mode of sonodynamic therapy (SDT), can strongly penetrate inflammatory tissues and kill inflammatory cells, thus reducing synovial hyperplasia and minimizing oxidative damage to surrounding normal tissues. SDT is hampered by the hypoxic microenvironment of RA caused by fibroblast-like synoviocyte (FLS) proliferation. Rhodium NP (Rh) nanozymes with concave-cube shapes could compensate for the deficiency of ultrasound therapy by exhibiting the activities of POD and CAT, which generate O2 and •OH to alleviate hypoxia. In addition to its remarkable sonosensitive properties, the antibacterial drug sparfloxacin (SPX) can reside for a long time in joint tissues after systemic administration, which makes it possible to target the abnormal proliferation of FLSs in synovial tissue in the joint and block the development of RA. A small glycoprotein rich in cysteine known as SPARC is overexpressed in the synovial fluid and synovium from RA patients and increased in mice with CIA (Liu et al., 2019). SPARC has high affinity for human serum albumin (HAS) (Park et al., 2019). Therefore, HSA-modified Rh/SPX nanozyme was fabricated for RA treatment by combining the advantages and characteristics of these components (Li et al., 2021). The preparation of Rh-SPX/HSA and its related mechanisms in the treatment of RA are shown in Figure 3.
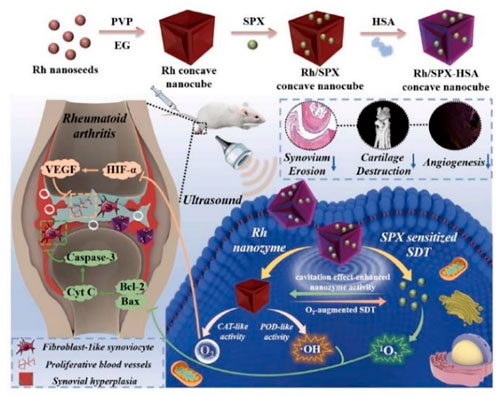
FIGURE 3. Schematic illustration of the synthesis and working mechanism of MMP-13 and pH responsive theranostic MRC-PPL@PSO nano-micelles for OA (Li et al., 2021). Copyright, 2021, Elsevier.
In addition to the combination of ultrasound and nanozymes to treat arthritis, a promising technique that combines near-infrared (NIR) with nanozymes for the treatment of OA was proposed. Epigallocatechin gallate (EGCG)-coated Au-Ag nanojars (E@Au-Ag) were produced based on the POD-like activity of Au-Ag and the scavenging of oxygen free radicals by EGCG, which is sensitive to NIR. These multifunctional enzyme-like nanomaterials can repair mitochondrial damage, promote cartilage migration, and reduce chondrocyte apoptosis (Xu et al., 2022). Biomaterials associated with antioxidant enzymes for arthritis treatment are listed in Table 2.
Discussion
OA and RA are both inflammatory diseases. RA is a systemic disease that affects joints all over the body, especially the overloaded knee joints, and affects normal movement (Radu and Bungau, 2021). OA is a local joint disease, which is common in patients with metabolic syndrome, trauma, and aging (Whittaker et al., 2021). In comparing OA and RA, a striking similarity in gene expression is found. For example, the increased levels of MMPs and the decreased expression of antioxidant enzymes occur in OA and RA, but the differences also exist. MMP-9 is the main enzyme that causes RA while MMP-13 is reported to be the most important enzyme for the development of OA. Meanwhile, in terms of pathological changes, the proliferation of synovial tissue and blood vessels in RA was more obvious than that in OA. Macrophages distributed in synovial tissue and osteoclasts from subchondral bone were the main sources of inflammation, ultimately leading to the destruction of cartilage. Therefore, chondrocytes, osteoclasts and macrophages have been the main targets for arthritis treatment with different biomaterials.
RA is a systemic inflammatory disease, and joint destruction is generally more intense than that in OA. Compared to IA injection, oral drug delivery for arthritis causes severe side effects. Recently, pain has been primarily controlled with corticosteroids and hyaluronic acid via IA injection. It is possible to deliver high drug concentrations directly to osteoarthritic joints through direct IA delivery. The administration of IA corticosteroids efficiently reduces articular pain and synovitis, but high concentrations of corticosteroids can also damage chondrocyte metabolism, causing changes in ECM composition and articular cartilage structure. A novel treatment for arthritis is urgently needed.
Enzymes are involved in various physiological reactions and participate in the proteolytic degradation of proteins and complex regulatory signalling pathways. Aberrant expression of these enzymes in the human body plays a critical role in pathological processes, especially inflammatory reactions. Different types of MMPs were upregulated by inflammatory factors and subsequently degrade the ECM. In addition to MMPs, ROS also participate in the development of arthritis. The generation of ROS is inhibited by endogenous antioxidants such as SOD, CAT, GPX, and heme oxygenase (HO-1). Despite the complex pathological process of arthritis, different types of arthritis including OA and RA share the common features: the increased levels of MMPs and the decreased expression of antioxidant enzymes. Hence, it is extremely feasible to design nanomaterials based on these enzymes as molecular targets for arthritis therapy.
Although nanomaterials have the advantages of high biocompatibility and bioavailability due to their structural and functional characteristics, the biosafety of nanomaterials cannot be ignored (Chen et al., 2021-09). Nanomaterials enter the body through ingestion, injection, inhalation and skin contact and subsequently accumulate in organs through blood flow, affecting the structure and function of organs (Ai et al., 2011-01). For arthritis treatment, intra-articular injection of enzyme-related biomaterials can guarantee the controlled release and targeted therapy without affecting other tissues or organs through blood circulation. Natural polymers are more suitable and safer for clinical application due to their biodegradation. Especially, hyaluronic acid from cartilage tissue has been commonly used for biomaterial. It is a promising strategy for arthritis treatment through discovering more biologically active materials from the human body in the future and combining them with drugs to regulate the expression of the enzymes mentioned above.
Given that cartilage and the synovium are affected in arthritis, various NPs that target these upregulated or downregulated enzymes mainly act on these sites, especially macrophages from the synovium and OCs from the subchondral bone. Both macrophages and osteoclasts are inflammatory cells with the same receptor on the surface of the membrane and release inflammatory factors. Therefore, biomaterials that target these inflammatory cells or chondrocytes are the current options for arthritis treatment. For the treatment of inflammatory arthritis, nano-drug delivery technologies that respond to subchondral enzymes are rare. There are technical challenges, such as how to penetrate the cartilage and reach the deep layer to target OCs that destroy the subchondral bone. Second, aside from MMPs and endogenous reductase, many enzymes are also involved in the pathological processes of arthritis. The expression of cyclooxygenase-2 (COX-2) in joints has also been linked to synovial inflammation in arthritis, and COX-2 inhibitors (celecoxib) have been frequently used and have shown therapeutic benefits in arthritis. Synergistic treatments targeting several enzymes may obtain better results. Finally, avoiding rapid clearance after IA injection is critical for maintaining drug concentrations and guaranteeing efficacy.
It should be noted that the current studies regarding enzyme-related biomaterials in the field of arthritis are not numerous; nanotherapies are extremely challenging and are also promising based on the molecular mechanism underlying arthritis.
Author contributions
Conceptualization, X-LX and D-FD; writing—original draft preparation, X-HL, J-YD; writing—review and editing, Z-HZ, -CW; visualization, Y-JS; All authors have read and agreed to publish the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (81902306, 82174406), the National Key R&D Program of China (Grant No. 2018YFC2001600) and Engineering Research Center of Traditional Chinese Medicine Intelligent Rehabilitation, Ministry of Education
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ai, J., Biazar, E., Jafarpour, M., Montazeri, M., Majdi, A., Aminifard, S., et al. (2011). Nanotoxicology and nanoparticle safety in biomedical designs. Int. J. Nanomedicine 6, 1117–1127. doi:10.2147/IJN.S16603
Auréal, M., Machuca-Gayet, I., and Coury, F. (2020). Rheumatoid arthritis in the view of osteoimmunology. Biomolecules 11 (1), 48. doi:10.3390/biom11010048
Bala, A., Mondal, C., Haldar, P. K., and Khandelwal, B. (2017). Oxidative stress in inflammatory cells of patient with rheumatoid arthritis: Clinical efficacy of dietary antioxidants. Inflammopharmacology 25 (6), 595–607. doi:10.1007/s10787-017-0397-1
Baldim, V., Bedioui, F., Mignet, N., Margaill, I., and Berret, J. F. (2018). The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce3+ surface area concentration. Nanoscale 10 (15), 6971–6980. doi:10.1039/c8nr00325d
Briggs, A. M., Shiffman, J., Shawar, Y. R., Åkesson, K., Ali, N., and Woolf, A. D. (2020). Global health policy in the 21st century: Challenges and opportunities to arrest the global disability burden from musculoskeletal health conditions. Best. Pract. Res. Clin. Rheumatol. 34 (5), 101549. doi:10.1016/j.berh.2020.101549
Chen, H., Qin, Z., Zhao, J., He, Y., Ren, E., Zhu, Y., et al. (2019). Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials 225, 119520. doi:10.1016/j.biomaterials.2019.119520
Chen, L., Huang, Q., Zhao, T., Sui, L., Wang, S., Xiao, Z., et al. (2021). Nanotherapies for sepsis by regulating inflammatory signals and reactive oxygen and nitrogen species: New insight for treating COVID-19. Redox Biol. 45, 102046. doi:10.1016/j.redox.2021.102046
Chen, T., Zou, H., Wu, X., Chen, Y., Situ, B., Zheng, L., et al. (2019). Fullerene-like MoS2 nanoparticles as cascade catalysts improving lubricant and antioxidant abilities of artificial synovial fluid. ACS Biomater. Sci. Eng. 5 (6), 3079–3088. doi:10.1021/acsbiomaterials.9b00372
Chen, Z., Su, L., Xu, Q., Katz, J., Michalek, S. M., Fan, M., et al. (2015). IL-1R/TLR2 through MyD88 divergently modulates osteoclastogenesis through regulation of nuclear factor of activated T cells c1 (NFATc1) and B lymphocyte-induced maturation protein-1 (Blimp1). J. Biol. Chem. 290 (50), 30163–30174. doi:10.1074/jbc.M115.663518
Chung, H. K., Wen, S. H., Chang, W. C., and Liu, K. L. (2021). Acute surgical site infection after total knee arthroplasty in patients with rheumatoid arthritis versus osteoarthritis. Sci. Rep. 11 (1), 22704. doi:10.1038/s41598-021-02153-x
Cieza, A., Causey, K., Kamenov, K., Hanson, S. W., Chatterji, S., and Vos, T. (2021). Global estimates of the need for rehabilitation based on the global burden of disease study 2019: A systematic analysis for the global burden of disease study 2019. Lancet 396 (10267), 2006–2017. doi:10.1016/S0140-6736(20)32340-0
Dacarro, G., Taglietti, A., and Pallavicini, P. (2018). Prussian blue nanoparticles as a versatile photothermal tool. Molecules 23 (6), 1414. doi:10.3390/molecules23061414
Datta, S., Kundu, S., Ghosh, P., Ghosh, A., Chatterjee, M., and De, S. (2014). Correlation of oxidant status with oxidative tissue damage in patients with rheumatoid arthritis. Clin. Rheumatol. 33 (11), 1557–1564. doi:10.1007/s10067-014-2597-z
Deng, C., Zhang, Q., He, P., Zhou, B., He, K., Sun, X., et al. (2021). Targeted apoptosis of macrophages and osteoclasts in arthritic joints is effective against advanced inflammatory arthritis. Nat. Commun. 12 (1), 2174. doi:10.1038/s41467-021-22454-z
Deponte, M. (2013). Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochimica Biophysica Acta - General Subj. 1830 (5), 3217–3266. doi:10.1016/j.bbagen.2012.09.018
Fosang, A. J., Last, K., Knäuper, V., Murphy, G., and Neame, P. J. (1996). Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett. 380 (1-2), 17–20. doi:10.1016/0014-5793(95)01539-6
Galasso, O., Familiari, F., De Gori, M., and Gasparini, G. (2012). Recent findings on the role of gelatinases (matrix metalloproteinase-2 and -9) in osteoarthritis. Adv. Orthop. 2012, 1–7. doi:10.1155/2012/834208
Galil, S. M., El-Shafey, A. M., Hagrass, H. A., Fawzy, F., and Sammak, A. E. (2016). Baseline serum level of matrix metalloproteinase-3 as a biomarker of progressive joint damage in rheumatoid arthritis patients. Int. J. Rheum. Dis. 19 (4), 377–384. doi:10.1111/1756-185X.12434
Gamal, R. M., Hammam, N., Zakary, M. M., Abdelaziz, M. M., Razek, M. R. A., Mohamed, M. S. E., et al. (2018). Telomere dysfunction-related serological markers and oxidative stress markers in rheumatoid arthritis patients: Correlation with diseases activity. Clin. Rheumatol. 37 (12), 3239–3246. doi:10.1007/s10067-018-4318-5
Georgiev, T., Ivanova, M., Kopchev, A., Velikova, T., Miloshov, A., Kurteva, E., et al. (2018). Cartilage oligomeric protein, matrix metalloproteinase-3, and coll2-1 as serum biomarkers in knee osteoarthritis: A cross-sectional study. Rheumatol. Int. 38 (5), 821–830. doi:10.1007/s00296-017-3887-y
He, L., Fan, D., Liang, W., Wang, Q., and Fang, J. (2020). Matrix metalloproteinase-responsive PEGylated lipid nanoparticles for controlled drug delivery in the treatment of rheumatoid arthritis. ACS Appl. Bio Mat. 3 (5), 3276–3284. doi:10.1021/acsabm.0c00242
He, L., He, T., Farrar, S., Ji, L., Liu, T., and Ma, X. (2017). Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. biochem. 44 (2), 532–553. doi:10.1159/000485089
Heckert, E. G., Karakoti, A. S., Seal, S., and Self, W. T. (2008). The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 29 (18), 2705–2709. doi:10.1016/j.biomaterials.2008.03.014
Hoshi, H., Akagi, R., Yamaguchi, S., Muramatsu, Y., Akatsu, Y., Yamamoto, Y., et al. (2017). Effect of inhibiting MMP13 and ADAMTS5 by intra-articular injection of small interfering RNA in a surgically induced osteoarthritis model of mice. Cell Tissue Res. 368 (2), 379–387. doi:10.1007/s00441-016-2563-y
Hosseinzadeh, A., Kamrava, S. K., Joghataei, M. T., Darabi, R., Shakeri-Zadeh, A., Shahriari, M., et al. (2016). Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J. Pineal Res. 61 (4), 411–425. doi:10.1111/jpi.12362
Hou, W., Ye, C., Chen, M., Gao, W., Xie, X., Wu, J., et al. (2021). Excavating bioactivities of nanozyme to remodel microenvironment for protecting chondrocytes and delaying osteoarthritis. Bioact. Mat. 6 (8), 2439–2451. doi:10.1016/j.bioactmat.2021.01.016
Hu, Q., and Ecker, M. (2021). Overview of MMP-13 as a promising target for the treatment of osteoarthritis. Int. J. Mol. Sci. 22 (4), 1742. doi:10.3390/ijms22041742
Idzik, M., Poloczek, J., Skrzep-Poloczek, B., Chelmecka, E., Jochem, J., and Stygar, D. (2022). General rehabilitation Program after knee or hip replacement significantly influences erythrocytes oxidative stress markers and serum ST2 levels. Oxid. Med. Cell. Longev. 2022, 1358858. doi:10.1155/2022/1358858
Itoh, Y. (2017). Metalloproteinases in rheumatoid arthritis: Potential therapeutic targets to improve current therapies. Prog. Mol. Biol. Transl. Sci. 148, 327–338. doi:10.1016/bs.pmbts.2017.03.002
Iwabuchi, Y., Tanimoto, K., Tanne, Y., Inubushi, T., Kamiya, T., Kunimatsu, R., et al. (2014). Effects of low-intensity pulsed ultrasound on the expression of cyclooxygenase-2 in mandibular condylar chondrocytes. J. Oral Facial Pain . Headache 28 (3), 261–268. doi:10.11607/ofph.1156
Jansen, M. P., and Mastbergen, S. C. (2022). Joint distraction for osteoarthritis: Clinical evidence and molecular mechanisms. Nat. Rev. Rheumatol. 18 (1), 35–46. doi:10.1038/s41584-021-00695-y
Joshi, N., Yan, J., Levy, S., Bhagchandani, S., Slaughter, K. V., Sherman, N. E., et al. (2018). Towards an arthritis flare-responsive drug delivery system. Nat. Commun. 9 (1), 1275. doi:10.1038/s41467-018-03691-1
Kalashnikova, I., Chung, S. J., Nafiujjaman, M., Hill, M. L., Siziba, M. E., Contag, C. H., et al. (2020). Ceria-based nanotheranostic agent for rheumatoid arthritis. Theranostics 10 (26), 11863–11880. doi:10.7150/thno.49069
Kaneva, M. K. (2022). Neutrophil elastase and its inhibitors-overlooked players in osteoarthritis. FEBS J. 289 (1), 113–116. doi:10.1111/febs.16194
Kim, K. S., Choi, H. M., Lee, Y. A., Choi, I. A., Lee, S. H., Hong, S. J., et al. (2011). Expression levels and association of gelatinases MMP-2 and MMP-9 and collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of patients with arthritis. Rheumatol. Int. 31 (4), 543–547. doi:10.1007/s00296-010-1592-1
Kratschmer, W., Lamb, L. D., Fostiropoulos, K., and Huffman, D. R. (1990). Solid C60: A new form of carbon. Nature 347, 354–358. doi:10.1038/347354a0
Lan, Q., Lu, R., Chen, H., Pang, Y., Xiong, F., Shen, C., et al. (2020). MMP-13 enzyme and pH responsive theranostic nanoplatform for osteoarthritis. J. Nanobiotechnology 18 (1), 117. doi:10.1186/s12951-020-00666-7
Latourte, A., Cherifi, C., Maillet, J., Ea, H. K., Bouaziz, W., Funck-Brentano, T., et al. (2017). Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 76 (4), 748–755. doi:10.1136/annrheumdis-2016-209757
Lepetsos, P., and Papavassiliou, A. G. (2016). ROS/oxidative stress signaling in osteoarthritis. Biochimica Biophysica Acta - Mol. Basis Dis. 1862 (4), 576–591. doi:10.1016/j.bbadis.2016.01.003
Li, H., Wang, D., Yuan, Y., and Min, J. (2017). New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. Ther. 19 (1), 248. doi:10.1186/s13075-017-1454-2
Li, W., Song, Y., Liang, X., Zhou, Y., Xu, M., Lu, Q., et al. (2021). Mutual-reinforcing sonodynamic therapy against Rheumatoid Arthritis based on sparfloxacin sonosensitizer doped concave-cubic rhodium nanozyme. Biomaterials 276, 121063. doi:10.1016/j.biomaterials.2021.121063
Li, Z., Chen, C., Zhu, X., Li, Y., Yu, R., and Xu, W. (2018). Glycyrrhizin suppresses RANKL-induced osteoclastogenesis and oxidative stress through inhibiting NF-κB and MAPK and activating AMPK/Nrf2. Calcif. Tissue Int. 103 (3), 324–337. doi:10.1007/s00223-018-0425-1
Lima, A. C., Reis, R. L., Ferreira, H., and Neves, N. M. (2021). Glutathione reductase-sensitive polymeric micelles for controlled drug delivery on arthritic diseases. ACS Biomater. Sci. Eng. 7 (7), 3229–3241. doi:10.1021/acsbiomaterials.1c00412
Lipari, L., and Gerbino, A. (2013). Expression of gelatinases (MMP-2, MMP-9) in human articular cartilage. Int. J. Immunopathol. Pharmacol. 26 (3), 817–823. doi:10.1177/039463201302600331
Liu, L., Hu, F., Wang, H., Wu, X., Eltahan, A. S., Stanford, S., et al. (2019). Secreted protein acidic and rich in cysteine mediated biomimetic delivery of methotrexate by albumin-based nanomedicines for rheumatoid arthritis therapy. ACS Nano 13 (5), 5036–5048. doi:10.1021/acsnano.9b01710
Long, J., Guari, Y., Guérin, C., and Larionova, J. (2016). Prussian blue type nanoparticles for biomedical applications. Dalton Trans. 45 (44), 17581–17587. doi:10.1039/c6dt01299j
Luo, H., and Zhang, R. (2021). Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the Xc-/GPX4 axis. Exp. Ther. Med. 21 (1), 72. doi:10.3892/etm.2020.9504
Ma, J. D., Jing, J., Wang, J. W., Yan, T., Li, Q. H., Mo, Y. Q., et al. (2019). A novel function of artesunate on inhibiting migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Res. Ther. 21 (1), 153. doi:10.1186/s13075-019-1935-6
Ma, J. D., Zhou, J. J., Zheng, D. H., Chen, L. F., Mo, Y. Q., Wei, X. N., et al. (2014). Serum matrix metalloproteinase-3 as a noninvasive biomarker of histological synovitis for diagnosis of rheumatoid arthritis. Mediat. Inflamm. 2014, 1–10. doi:10.1155/2014/179284
Macdonald, C. D., Falconer, A. M. D., Chan, C. M., Wilkinson, D. J., Skelton, A., Reynard, L., et al. (2018). Cytokine-induced cysteine- serine-rich nuclear protein-1 (CSRNP1) selectively contributes to MMP1 expression in human chondrocytes. PLoS One 13 (11), e0207240. doi:10.1371/journal.pone.0207240
Malemud, C. J. (2017). Matrix metalloproteinases and synovial joint pathology. Prog. Mol. Biol. Transl. Sci. 148, 305–325. doi:10.1016/bs.pmbts.2017.03.003
Malhotra, S., Welling, M. N., Mantri, S. B., and Desai, K. (2016). In vitro and in vivo antioxidant, cytotoxic, and anti-chronic inflammatory arthritic effect of selenium nanoparticles. J. Biomed. Mat. Res. 104 (5), 993–1003. doi:10.1002/jbm.b.33448
McAlindon, T. E., LaValley, M. P., Harvey, W. F., Price, L. L., Driban, J. B., Zhang, M., et al. (2017). Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: A randomized clinical trial. JAMA 317 (19), 1967–1975. doi:10.1001/jama.2017.5283
Mehana, E. E., Khafaga, A. F., and El-Blehi, S. S. (2019). The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 234, 116786. doi:10.1016/j.lfs.2019.116786
Meshkibaf, M. H., Maleknia, M., and Noroozi, S. (2019). Effect of curcumin on gene expression and protein level of methionine sulfoxide reductase A (MSRA), SOD, CAT and GPx in Freund's adjuvant inflammation-induced male rats. J. Inflamm. Res. 12, 241–249. doi:10.2147/JIR.S212577
Metzger, I. F., Sandrim, V. C., and Tanus-Santos, J. E. (2012). Endogenous nitric oxide formation correlates negatively with circulating matrix metalloproteinase (MMP)-2 and MMP-9 levels in black subjects. Mol. Cell. Biochem. 360 (1-2), 393–399. doi:10.1007/s11010-011-1079-8
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P., and Malik, A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20 (7), 1126–1167. doi:10.1089/ars.2012.5149
Nguyen, N. H., Tran, G. B., and Nguyen, C. T. (2020). Anti-oxidative effects of superoxide dismutase 3 on inflammatory diseases. J. Mol. Med. 98 (1), 59–69. doi:10.1007/s00109-019-01845-2
Ostalowska, A., Birkner, E., Wiecha, M., Kasperczyk, S., Kasperczyk, A., Kapolka, D., et al. (2006). Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthr. Cartil. 14 (2), 139–145. doi:10.1016/j.joca.2005.08.009
Palmer, J. S., Monk, A. P., Hopewell, S., Bayliss, L. E., Jackson, W., Beard, D. J., et al. (2019). Surgical interventions for symptomatic mild to moderate knee osteoarthritis. Cochrane Database Syst. Rev. 7, CD012128. doi:10.1002/14651858.CD012128.pub2
Park, C. R., Jo, J. H., Song, M. G., Park, J. Y., Kim, Y. H., Youn, H., et al. (2019). Secreted protein acidic and rich in cysteine mediates active targeting of human serum albumin in U87MG xenograft mouse models. Theranostics 9 (24), 7447–7457. doi:10.7150/thno.34883
Pengas, I., Eldridge, S., Assiotis, A., McNicholas, M., Mendes, J. E., and Laver, L. (2018). MMP-3 in the peripheral serum as a biomarker of knee osteoarthritis, 40 years after open total knee meniscectomy. J. Exp. Orthop. 5 (1), 21. doi:10.1186/s40634-018-0132-x
Phull, A. R., Nasir, B., Haq, I. U., and Kim, S. J. (2018). Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem. Biol. Interact. 281, 121–136. doi:10.1016/j.cbi.2017.12.024
Pirmohamed, T., Dowding, J. M., Singh, S., Wasserman, B., Heckert, E., Karakoti, A. S., et al. (2010). Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 46 (16), 2736–2738. doi:10.1039/b922024k
Qin, Z., Li, Y., and Gu, N. (2018). Progress in applications of prussian blue nanoparticles in biomedicine. Adv. Healthc. Mat. 7 (20), e1800347. doi:10.1002/adhm.201800347
Radu, A. F., and Bungau, S. G. (2021). Management of rheumatoid arthritis: An overview. Cells 10 (11), 2857. doi:10.3390/cells10112857
Ren, S. X., Zhan, B., Lin, Y., Ma, D. S., and Yan, H. (2019). Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 gene expression in a rheumatoid arthritis rat model. Med. Sci. Monit. 25, 991–1000. doi:10.12659/MSM.912545
Ren, S. X., Zhan, B., Lin, Y., Ma, D. S., and Yan, H. (2019). Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 gene expression in a rheumatoid arthritis rat model. Med. Sci. Monit. 25, 991–1000. doi:10.12659/MSM.912545
Scherer, H. U., Häupl, T., and Burmester, G. R. (2020). The etiology of rheumatoid arthritis. J. Autoimmun. 110, 102400. doi:10.1016/j.jaut.2019.102400
Silosi, I., Cojocaru, M., Foia, L., Boldeanu, M. V., Petrescu, F., Surlin, P., et al. (2015). Significance of circulating and crevicular matrix metalloproteinase-9 in rheumatoid arthritis-chronic periodontitis association. J. Immunol. Res. 2015, 1–6. doi:10.1155/2015/218060
Singh, A., Rajasekaran, N., Hartenstein, B., Szabowski, S., Gajda, M., Angel, P., et al. (2013). Collagenase-3 (MMP-13) deficiency protects C57BL/6 mice from antibody-induced arthritis. Arthritis Res. Ther. 15 (6), R222. doi:10.1186/ar4423
Sparks, J. A. (2019). Rheumatoid arthritis. Ann. Intern. Med. 170 (1), ITC1–ITC16. doi:10.7326/AITC201901010
Sredzińska, K., Galicka, A., Porowska, H., Sredziński, Ł., Porowski, T., and Popko, J. (2009). Glutathione reductase activity correlates with concentration of extracellular matrix degradation products in synovial fluid from patients with joint diseases. Acta Biochim. Pol. 56 (4), 635–640. doi:10.18388/abp.2009_2496
Stocco, E., Barbon, S., Piccione, M., Belluzzi, E., Petrelli, L., Pozzuoli, A., et al. (2019). Infrapatellar fat pad stem cells responsiveness to microenvironment in osteoarthritis: From morphology to function. Front. Cell Dev. Biol. 7, 323. doi:10.3389/fcell.2019.00323
Swain, S., Sarmanova, A., Mallen, C., Kuo, C. F., Coupland, C., Doherty, M., et al. (2020). Trends in incidence and prevalence of osteoarthritis in the United Kingdom: Findings from the clinical practice research datalink (CPRD). Osteoarthr. Cartil. 28 (6), 792–801. doi:10.1016/j.joca.2020.03.004
Tatematsu, N., Waguri-Nagaya, Y., Kawaguchi, Y., Oguri, Y., Ikuta, K., Kobayashi, M., et al. (2018). Mithramycin has inhibitory effects on gliostatin and matrix metalloproteinase expression induced by gliostatin in rheumatoid fibroblast-like synoviocytes. Mod. Rheumatol. 28 (3), 495–505. doi:10.1080/14397595.2017.1350332
Trachana, V., Mourmoura, E., Papathanasiou, I., and Tsezou, A. (2019). Understanding the role of chondrocytes in osteoarthritis: Utilizing proteomics. Expert Rev. Proteomics 16 (3), 201–213. doi:10.1080/14789450.2019.1571918
Tschon, M., Salamanna, F., Martini, L., Giavaresi, G., Lorenzini, L., Calzà, L., et al. (2020). Boosting the intra-articular efficacy of low dose corticosteroid through a biopolymeric matrix: An in vivo model of osteoarthritis. Cells 9 (7), 1571. doi:10.3390/cells9071571
Tuncer, T., Kaya, A., Gulkesen, A., Kal, G. A., Kaman, D., and Akgol, G. (2019). Matrix metalloproteinase-3 levels in relation to disease activity and radiological progression in rheumatoid arthritis. Adv. Clin. Exp. Med. 28 (5), 665–670. doi:10.17219/acem/94065
van Dalen, S. C., Blom, A. B., Slöetjes, A. W., Helsen, M. M., Roth, J., Vogl, T., et al. (2017). Interleukin-1 is not involved in synovial inflammation and cartilage destruction in collagenase-induced osteoarthritis. Osteoarthr. Cartil. 25 (3), 385–396. doi:10.1016/j.joca.2016.09.009
van der Woude, J. A., Wiegant, K., van Heerwaarden, R. J., Spruijt, S., Emans, P. J., Mastbergen, S. C., et al. (2017). Knee joint distraction compared with total knee arthroplasty: A randomised controlled trial. Bone Jt. J. 99-B (1), 51–58. doi:10.1302/0301-620X.99B1.BJJ-2016-0099.R3
Wang, M., Zhou, Y., Huang, W., Zeng, Y., and Li, X. (2020). Association between matrix metalloproteinase-1 (MMP-1) protein level and the risk of rheumatoid arthritis and osteoarthritis: A meta-analysis. Braz. J. Med. Biol. Res. 54 (2), e10366. doi:10.1590/1414-431X202010366
Wang, Z., Zhang, R., Yan, X., and Fan, K. (2020). Structure and activity of nanozymes: Inspirations for de novo design of nanozymes. Mat. TodayKidlingt. 41, 81–119. doi:10.1016/j.mattod.2020.08.020
Wen, Y., Chen, X., Zhu, X., Gong, Y., Yuan, G., Qin, X., et al. (2019). Photothermal-chemotherapy integrated nanoparticles with tumor microenvironment response enhanced the induction of immunogenic cell death for colorectal cancer efficient treatment. ACS Appl. Mat. Interfaces 11 (46), 43393–43408. doi:10.1021/acsami.9b17137
Whittaker, J. L., Truong, L. K., Dhiman, K., and Beck, C. (2021). Osteoarthritis year in review 2020: Rehabilitation and outcomes. Osteoarthr. Cartil. 29 (2), 190–207. doi:10.1016/j.joca.2020.10.005
Woolf, A. D., and Pfleger, B. (2003). Burden of major musculoskeletal conditions. Bull. World Health Organ. 81 (9), 646–656. Epub 2003 Nov 14. doi:10.1590/S0042-96862003000900007
Wu, H., Du, J., and Zheng, Q. (2008). Expression of MMP-1 in cartilage and synovium of experimentally induced rabbit ACLT traumatic osteoarthritis: Immunohistochemical study. Rheumatol. Int. 29 (1), 31–36. doi:10.1007/s00296-008-0636-2
Wylde, V., Hewlett, S., Learmonth, I. D., and Dieppe, P. (2011). Persistent pain after joint replacement: Prevalence, sensory qualities, and postoperative determinants. Pain 152 (3), 566–572. doi:10.1016/j.pain.2010.11.023
Xie, Z., Hou, H., Luo, D., An, R., Zhao, Y., and Qiu, C. (2021). ROS-dependent lipid peroxidation and reliant antioxidant ferroptosis-suppressor-protein 1 in rheumatoid arthritis: A covert clue for potential therapy. Inflammation 44 (1), 35–47. doi:10.1007/s10753-020-01338-2
Xu, S., Chang, L., Zhao, X., Hu, Y., Lin, Y., Chen, Z., et al. (2022). Preparation of epigallocatechin gallate decorated Au-Ag nano-heterostructures as NIR-sensitive nano-enzymes for the treatment of osteoarthritis through mitochondrial repair and cartilage protection. Acta Biomater. 144, 168–182. doi:10.1016/j.actbio.2022.03.038
Yao, Y., Zhang, H., Wang, Z., Ding, J., Wang, S., Huang, B., et al. (2019). Reactive oxygen species (ROS)-responsive biomaterials mediate tissue microenvironments and tissue regeneration. J. Mat. Chem. B 7 (33), 5019–5037. doi:10.1039/c9tb00847k
You, Z., Sun, J., Xie, F., Chen, Z., Zhang, S., Chen, H., et al. (2018). Novel iron oxide–cerium oxide core–shell nanoparticles as a potential theranostic material for ROS related inflammatory diseases. J. Mat. Chem. B 6 (30), 4937–4951. doi:10.1039/c8tb00022k
Yu, C., Liu, H., Guo, C., Chen, Q., Su, Y., Guo, H., et al. (2022). Dextran sulfate-based MMP-2 enzyme-sensitive SR-A receptor targeting nanomicelles for the treatment of rheumatoid arthritis. Drug Deliv. (Lond). 29 (1), 454–465. doi:10.1080/10717544.2022.2032482
Yu, N., Han, F., Lin, X., Tang, C., Ye, J., and Cai, X. (2016). The association between serum selenium levels with rheumatoid arthritis. Biol. Trace Elem. Res. 172 (1), 46–52. doi:10.1007/s12011-015-0558-2
Zeng, G. Q., Chen, A. B., Li, W., Song, J. H., and Gao, C. Y. (2015). High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 14 (4), 14811–14822. doi:10.4238/2015.November.18.46
Zhang, L., Qin, Z., Sun, H., Chen, X., Dong, J., Shen, S., et al. (2022). Nanoenzyme engineered neutrophil-derived exosomes attenuate joint injury in advanced rheumatoid arthritis via regulating inflammatory environment. Bioact. Mat. 18, 1–14. doi:10.1016/j.bioactmat.2022.02.017
Zhang, S., Ren, Q., Qi, H., Liu, S., and Liu, Y. (2019). Adverse effects of fine-particle exposure on joints and their surrounding cells and microenvironment. ACS Nano 13 (3), 2729–2748. doi:10.1021/acsnano.8b08517
Zhang, W., Hu, S., Yin, J. J., He, W., Lu, W., Ma, M., et al. (2016). Prussian blue nanoparticles as multienzyme mimetics and reactive oxygen species scavengers. J. Am. Chem. Soc. 138 (18), 5860–5865. doi:10.1021/jacs.5b12070
Zhuang, C., Wang, Y., Zhang, Y., and Xu, N. (2018). Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. Int. J. Biol. Macromol. 115, 281–286. doi:10.1016/j.ijbiomac.2018.04.083
Zuo, D., Tan, B., Jia, G., Wu, D., Yu, L., and Jia, L. (2021). A treatment combined prussian blue nanoparticles with low-intensity pulsed ultrasound alleviates cartilage damage in knee osteoarthritis by initiating PI3K/Akt/mTOR pathway. Am. J. Transl. Res. 13 (5), 3987–4006. doi:10.21203/rs.3.rs-77010/v1
Keywords: biomaterials, nano-therapy, arthritis, matrix metalloproteinases, endogenous antioxidant enzymes
Citation: Liu X-H, Ding J-Y, Zhu Z-H, Wu X-C, Song Y-J, Xu X-L and Ding D-F (2022) Recent advances in enzyme-related biomaterials for arthritis treatment. Front. Chem. 10:988051. doi: 10.3389/fchem.2022.988051
Received: 06 July 2022; Accepted: 21 July 2022;
Published: 16 August 2022.
Edited by:
Xiangzhao Ai, Shanghai Jiao Tong University, ChinaReviewed by:
Magali Cucchiarini, Saarland University Medical Center, GermanyTao Feng, Northwestern Polytechnical University, China
Copyright © 2022 Liu, Ding, Zhu, Wu, Song, Xu and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Ling Xu, eml5YW8xOTg4QHpqdS5lZHUuY24=; Dao-Fang Ding, ZGluZ2Rhb2ZhbmdAc2h1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Xin-Hao Liu
Xin-Hao Liu Jia-Ying Ding
Jia-Ying Ding Zhi-Heng Zhu
Zhi-Heng Zhu Xi-Chen Wu
Xi-Chen Wu Yong-Jia Song
Yong-Jia Song Xiao-Ling Xu
Xiao-Ling Xu Dao-Fang Ding
Dao-Fang Ding